Abstract
Working memory (WM) problems are frequently present in people with multiple sclerosis (MS). Even though hippocampal damage has been repeatedly shown to play an important role, the underlying neurophysiological mechanisms remain unclear. This study aimed to investigate the neurophysiological underpinnings of WM impairment in MS using magnetoencephalography (MEG) data from a visual‐verbal 2‐back task. We analysed MEG recordings of 79 MS patients and 38 healthy subjects through event‐related fields and theta (4–8 Hz) and alpha (8–13 Hz) oscillatory processes. Data was source reconstructed and parcellated based on previous findings in the healthy subject sample. MS patients showed a smaller maximum theta power increase in the right hippocampus between 0 and 400 ms than healthy subjects (p = .014). This theta power increase value correlated negatively with reaction time on the task in MS (r = −.32, p = .029). Evidence was provided that this relationship could not be explained by a ‘common cause’ confounding relationship with MS‐related neuronal damage. This study provides the first neurophysiological evidence of the influence of hippocampal dysfunction on WM performance in MS.
Keywords: hippocampus, magnetoencephalography, multiple sclerosis, n‐back, theta, working memory
This study is the first to provide neurophysiological evidence of impaired hippocampal WM processing in MS that can be related to WM performance. Our data suggests that impaired theta oscillatory processes in the right hippocampus, supposedly underlying WM encoding, lead to slower reaction times on the task. We also provide evidence that this relationship cannot be accounted for by general MS‐related neural damage (specifically hippocampal and white matter damage).
1. INTRODUCTION
Cognitive impairment is a significant burden for people with multiple sclerosis (PwMS), having an impact on their employment status, mental health and social and vocational activities. About 43–70% of the 2.3 million multiple sclerosis (MS) patients worldwide (Multiple Sclerosis International Federation, 2013) are estimated to suffer from cognitive impairment (Peyser, Rao, LaRocca, & Kaplan, 1990; Rao, Leo, Bernardin, & Unverzagt, 1991) together with the strong physical problems that are caused by the inflammatory demyelination and neurodegeneration that characterise this disorder. Particularly information processing speed (Deluca, Chelune, Tulsky, Lengenfelder, & Chiaravalloti, 2004; Van Schependom et al., 2015) and working memory (WM; Chiaravalloti & DeLuca, 2008; D'Esposito et al., 1996) but also attention and visuospatial abilities have been shown to be affected in PwMS, even in persons with only mild physical problems (Ruchkin et al., 1994).
Multiple functional magnetic resonance imaging (fMRI) studies have investigated WM in MS using the n‐back task, a task in which a subject has to respond when an item in a sequence is the same as the n‐th item before. By increasing n, the WM load or the number of items to be retained in WM is increased. Those studies have reported relatively heterogenous findings of possibly compensatory mechanisms in different regions (Forn et al., 2007; Penner, Rausch, Kappos, Opwis, & Radü, 2003; Sweet, Rao, Primeau, Durgerian, & Cohen, 2006; Sweet, Rao, Primeau, Mayer, & Cohen, 2004; Wishart et al., 2004). The most common finding for these studies was increased activation in the prefrontal cortex in PwMS (Forn et al., 2007; Sweet et al., 2004). One study also found a decreased activation in the right hippocampus in PwMS (Sweet et al., 2004). A decreased activation of the hippocampi was also found in an fMRI study of episodic memory in cognitively impaired compared to cognitively preserved MS patients (Hulst et al., 2015). While these fMRI studies were able to point to some specific brain regions possibly involved in WM impairment in MS, they have the disadvantage of low temporal resolution which makes it difficult to draw conclusions about which specific WM processes or mechanisms are disturbed in MS.
Electroencephalography (EEG) n‐back studies in healthy subjects reported P300 event‐related potentials (ERPs) along the central and parietal electrodes, with amplitudes decreasing with increasing levels of memory load (Ahonen, Huotilainen, & Brattico, 2016; Causse, Peysakhovich, & Fabre, 2016; Dong, Reder, Yao, Liu, & Chen, 2015; Scharinger, Soutschek, Schubert, & Gerjets, 2017; for review see Kok, 2001). Covey, Shucard, and Shucard (2017) have found that the amplitude of this P300, but also an earlier P100 component, were lower in PwMS than healthy subjects. Such P300 amplitude difference was also already described in an early study, albeit below statistical significance (Ruchkin et al., 1994). Another study found the latency of the N200 and P300 component to be larger in PwMS than healthy subjects, with n‐back specific cognitive training leading to a shorter N200 and P300 latency in PwMS (Covey, Shucard, Benedict, Weinstock‐Guttman, & Shucard, 2018). A recent magnetoencephalography (MEG) study was able to identify the right inferior temporal and parahippocampal gyrus and the left inferior temporal gyrus as the sources of the n‐back M300, the magnetic counterpart of the P300 (Costers et al., 2020).
Besides the involvement of ERPs described above, specific frequency band oscillations and their interactions have been shown to be essential for WM function. Theta band oscillations have been thought to be essential for WM activity, organising different WM sub‐processes through theta synchronisation (Roux & Uhlhaas, 2014) and encoding the temporal information of WM items in theta oscillatory cycles (Hsieh, Ekstrom, & Ranganath, 2011; Lisman & Idiart, 1995; Lisman & Jensen, 2013; Roberts, Hsieh, & Ranganath, 2013). In a recent study, causal evidence was provided for the claim that frontal theta oscillations were excitatory by nature and responsible for prioritising relevant information during WM (Riddle, Scimeca, Cellier, Dhanani, & D'Esposito, 2020). This study also provided causal evidence for the inhibitory influence of parietal alpha band oscillations to suppress WM representations. The function of alpha oscillations during WM has already previously been shown to be modulatory, through mechanisms of inhibition and release from inhibition. This was based on observations of increased neural firing correlating with decreases in alpha power in monkeys (Haegens, Nácher, Luna, Romo, & Jensen, 2011).
During the n‐back task, multiple EEG studies in healthy subjects have extensively described increases in frontal theta band power (Dong et al., 2015; Jensen & Tesche, 2002; Kawasaki, Kitajo, & Yamaguchi, 2010) and decreases in occipital‐parietal alpha or beta band power compared to baseline (Chen & Huang, 2016; Dong et al., 2015; for review see Roux & Uhlhaas, 2014). A recent MEG study also suggested the orbitofrontal cortex to be the source of this increase in frontal theta power (Costers et al., 2020). Another source of theta power increase was found to be the right hippocampus. While the measurability of the hippocampus using MEG has been heavily disputed, recent evidence has led to more acceptance in the field (see Pu, Cheyne, Cornwell, & Johnson, 2018 for review; see Supporting Information for a discussion on the topic of measuring hippocampal source activity using MEG). The strongest alpha and beta band power decreases compared to baseline were observed at later timepoints in the right hippocampus and occipital fusiform gyri, involved in letter recognition (Devlin, Jamison, Gonnerman, & Matthews, 2006; James, James, Jobard, Wong, & Gauthier, 2005; Joseph, Gathers, & Piper, 2003; Pernet, Celsis, & Démonet, 2005), suggesting that these regions together enable successful letter encoding and storage in WM. The hippocampus has been repeatedly shown to be involved in the encoding of memory information. A recent study showed using theta‐burst transcranial magnetic stimulation (TMS) that hippocampal theta processes play a role in the encoding of information in episodic memory (Hermiller, Chen, Parrish, & Voss, 2020). This finding was in line with previous studies claiming the involvement of hippocampal theta oscillations in the encoding of temporal information of WM items through observations of cross‐frequency coupling using intracranial EEG (iEEG) (Axmacher et al., 2010). Multiple other studies have been able to establish the involvement of the hippocampus in WM, through intracranial observations in rodents and humans (Axmacher et al., 2007; for review see Leszczynski, 2011) and hippocampal lesioning studies (Fortin, Agster, & Eichenbaum, 2002; Kesner, Gilbert, & Barua, 2002). The findings from the recent MEG study mentioned above also add to the evidence (Costers et al., 2020). In MS, multiple studies have reported relationships between WM performance and hippocampal atrophy or microstructural damage (Benedict, Ramasamy, Munschauer, Weinstock‐Guttman, & Zivadinov, 2009; Koenig et al., 2014, 2019; Longoni et al., 2015; Planche et al., 2017; Preziosa et al., 2016; Sacco et al., 2015; Sicotte et al., 2008). We hypothesise that MS‐related hippocampal neuronal damage could lead to impaired theta oscillatory responses that are required for successful WM or episodic memory performance. However, no neurophysiological evidence for the role of the hippocampus has been reported yet.
We investigated the neurophysiological underpinnings of WM impairment in MS by analysing MEG n‐back data of 79 MS patients and 38 healthy controls (HCs). We performed an ERF and time‐frequency analysis using the regions‐of‐interest (ROI) reported using the HC data (Costers et al., 2020). In addition to a more traditional group timeseries analysis based on comparing group means, we employed an approach considering individual maximum ERFs amplitudes or power changes within defined time windows. This was motivated by observations of a larger inter‐individual latency variation in the MS group when compared with HCs (e.g., see Covey et al., 2017). Performing an analysis based on group means could be biased when there is a large difference in heterogeneity between both groups. For example, the mean amplitude of an ERP in a sample with large latency variation would be smaller than in a group with the same individual data but a smaller latency variation (e.g., see Ouyang, Sommer, & Zhou, 2016).
Based on previous EEG research (Covey et al., 2017), we hypothesised that we would observe a lower M300 amplitude in the right and left inferior temporal gyrus in PwMS. Looking at the results reported by fMRI studies in MS (Forn et al., 2007; Penner et al., 2003; Sweet et al., 2004) that coincide with the regions reported in the n‐back HC study using MEG (Costers et al., 2020), we expected group differences in the prefrontal regions (Forn et al., 2007; Penner et al., 2003; Sweet et al., 2004), the right fusiform gyrus and right hippocampus (Sweet et al., 2004). Considering the excitatory nature of theta oscillations and the inhibitory nature of alpha oscillations during n‐back WM, as recently shown by (Riddle et al., 2020), and their observation in those regions in (Costers et al., 2020) this would translate in a larger increase of theta power in the prefrontal cortex and a larger decrease in alpha power in the right fusiform gyrus in MS patients. As the hippocampus in the previous HC study (Costers et al., 2020) showed an early increase in theta power followed by a decrease in alpha power, we hypothesised to observe a smaller increase in theta power and/or a larger decrease of alpha power in MS patients compared to HCs.
2. METHODS AND MATERIALS
2.1. Participants
We analysed data from 79 MS patients and 38 HCs aged between 18 and 65 years performing a visual verbal n‐back task in a MEG scanner. All MS patients were recruited at the National MS Center Melsbroek using the following inclusion criteria: MS diagnosis according to the revised McDonald criteria (Polman et al., 2011) and an Expanded Disability Status Scale (EDSS) (Kurtzke, 1983) score smaller or equal to six at the time of recruitment. Exclusion criteria were having had a relapse or corticosteroid treatment in the 6 weeks preceding the study as well as having a pacemaker, dental wires, psychiatric disorders or epilepsy. All subjects had normal or corrected vision.
The MS sample had a mean age of 47.9 ± 9.6 years, education level of 13.9 ± 2.7 years and consisted of 72.2% females (see Table 1). The mean disease duration of the MS sample was 15.1 ± 7.9 years and consisted of 87.3% or 69 relapsing–remitting MS (RRMS), 8.9% or seven primary‐progressive MS (PPMS), 2.5% or two secondary‐progressive MS (SPMS) and 1.3% or one clinically isolated syndrome (CIS) patients. The MS patients had a median EDSS score of 3 (inter‐quartile range (IQR): [2–4]). Of the 79 MS patients, 19 (24.1%) were treated with benzodiazepines, five (6.3%) were treated with known painkillers, six (7.6%) with muscle relaxants, five (6.3%) with anti‐depressants, seven (8.9%) with anti‐epileptic drugs and three (3.8%) MS patients were treated with sleep medication.
TABLE 1.
Demographic and clinical characteristics of the MS and HC sample
| MS (n = 79) | HC (n = 38) | |
|---|---|---|
| Age (mean yrs ± SD) | 47.9 ± 9.6 | 48.0 ± 12.0 |
| Education level (mean yrs ± SD) | 13.9 ± 2.7 | 15.0 ± 2.0 |
| Gender (% female) | 72.2% (n = 57) | 60.5% (n = 23) |
| Disease duration (mean yrs ± SD) | 15.1 ± 7.9 | |
| EDSS (median, IQR) | 3, [2–4] | |
| Benzodiazepine use (%) | 24% (n = 19) | |
| Type of MS (%) | ||
| CIS | 1.3% (n = 1) | |
| RRMS | 87.3% (n = 69) | |
| PPMS | 8.9% (n = 7) | |
| SPMS | 2.5% (n = 2) |
Abbreviations: CIS, clinically isolated syndrome; EDSS, Expanded Disability Status Scale; HC, healthy controls; MS, multiple sclerosis; PPMS, primary progressive MS; RRMS, relapsing–remitting MS; SPMS, secondary progressive MS; yrs, years.
The subjects in the HC sample had a mean age of 48.0 ± 12.0 years, an education level of 15.0 ± 2.0 years and was comprised of 60.5% females. Years of education was counted from the start of primary school in Belgium (e.g., 12 years = finished middle school). The age of the HC sample was not significantly different from the MS sample (z = 0.23, p = .82), but the MS sample had a significantly higher education level (z = 2.32, p = .02), although these p‐values were not corrected for multiple comparisons. There was no significant difference in gender between both groups, assessed through a Chi‐Squared test: X 2 (1, N = 117) = 1.60, p = .21. All participants provided written informed consent. Ethical approval for the study was provided by the ethics committees of the National MS Center Melsbroek and the University Hospital Brussels (Commissie Medische Ethiek UZ Brussel, B.U.N. 143201423263, 2015/11).
2.2. Paradigm
We employed a visual verbal version of the n‐back paradigm with three conditions or levels of WM load (0, 1 and 2‐back). The task was split into four blocks per condition, with a total of 12 blocks which were presented in a pseudo‐random order. Every block consisted of 20 stimuli, for a total of 240 stimuli and respectively 25, 23 and 28 target trials per condition. Subjects were asked to respond with a button press using their right hand when the letter that appeared on the screen was the letter X (0‐back), the same letter as the one before in the sequence (1‐back) or the same letter as two letters before (2‐back). The paradigm is illustrated in Figure 1. The letter stimuli had a size of 6 by 6.5 cm and were projected on a screen 72 cm from the front of the MEG helmet. Each stimulus was presented on the screen for 1 s, with a 2.8 s inter‐trial interval. The instructions for the specific conditions were presented at the start of every block for 15 s. The onset of the visual stimuli was measured using a photodiode. Importantly, we also included non‐target trials in our analysis as the cognitive processes we are interested in are the stimulus processing, WM encoding and storage which is performed in every single trial. An additional reason was the resultant substantial increase of the number of trials included in the analyses. Trials during which an incorrect button press was recorded were removed from the analyses. Per subject, we removed spurious outlier reaction times using a conservative cut‐off of three or more scaled median absolute deviations. All participants performed practice trials before starting the task and a minimal understanding of the task was ensured, otherwise participants were asked to perform the practice trials again. Importantly, we only analysed MEG data from the 2‐back condition in order to limit the complexity of the results and because of the limited involvement of important WM processes such as updating and manipulating WM information in the 0 and 1‐back condition. For more details on the differences in MEG activity between the different n‐back conditions in HCs, see Costers et al. (2020).
FIGURE 1.
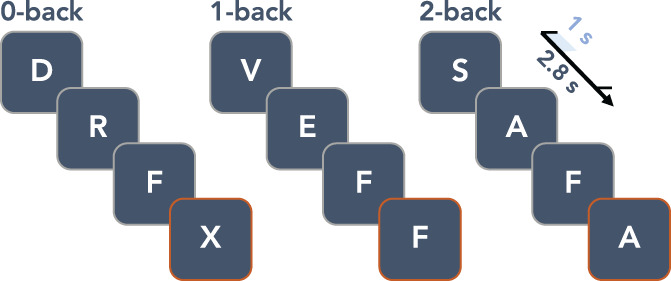
Illustration of the visual verbal n‐back paradigm. The inter‐trial interval was 2.8 s and stimuli were presented for 1 s on the screen. Target stimuli which required a response are highlighted with an orange border
2.3. Data acquisition
The MEG acquisition for the first 40 subjects (of which 13 or 32.5% HCs) was performed using an Neuromag VectorView™ system, while the last 77 subjects (of which 25 or 32.5% HCs) were scanned using an Neuromag™ TRIUX system (MEGIN Oy, Croton Healthcare, Helsinki, Finland) due to a system update at the CUB Hôpital Erasme (Brussels, Belgium). Both whole‐head systems use 306 channels, of which 204 planar gradiometers and 102 magnetometers, and are placed in a lightweight magnetically shielded room (MSR, Maxshield™, MEGIN Oy, Croton Healthcare, Helsinki, Finland). The characteristics of the MSR have been described elsewhere (De Tiège et al., 2008). MEG data were recorded at a 1,000 Hz sampling frequency with a band‐pass filter of [0.1–330] Hz. Participants were instructed to sit as still as possible during the acquisition and were seated in an upright position with their head positioned to the back of the MEG helmet. An electrocardiogram (ECG) and horizontal and vertical electrooculogram (EOG) was recorded to be used in offline artefact rejection. Four head position indicator coils were also attached to the left and right forehead and mastoid to track head movement. In order to allow qualitative co‐registration with the subjects' 3D T1‐weighted anatomical magnetic resonance image (MRI, see below for details) we registered a minimum of 400 points on the scalp and nose using Polhemus FASTTRAK 3D digitizer (Polhemus, Colchester, VT) together with three fiducials (nasion, left and right preauricular), to obtain the subjects' head shape.
The MR image was collected using a 3 Tesla Philips Achieva scanner (Amsterdam, Netherlands) located at the Universitair Ziekenhus Brussel (Jette, Belgium). The sequence for the 3D T1‐weighted images had the following parameters: TR = 4.939 ms, FA 8°, 230 × 230 mm2 FOV, 310 sagittal slices; resulting in a 0.53 by 0.53 by 0.5 mm3 resolution. This image was affinely coregistered to the MNI152 atlas.
We also acquired neuropsychological data to relate to neurophysiological outcomes. We performed the Symbol Digit Modalities Test (SDMT) and California Verbal Learning Test II (CVLT‐II). Both are part of the Brief International Cognitive Assessment for MS (BICAMS) (Benedict, Amato, et al., 2012) which was validated in a Belgian Dutch‐speaking population (Costers et al., 2017). For the CVLT‐II we only used the learning trials as described in the BICAMS guidelines. In addition, subjects also performed a Nine‐Hole Peg Test (Mathiowetz et al., 1985) to control for possible effects of finger dexterity on reaction times. Participants were timed from the moment they touched the first peg until they put the last peg in the dish. The test was performed twice with both hands and trials were restarted when a peg was dropped from the table.
2.4. Data pre‐processing and source projection
The first step of data pre‐processing was using the temporal extension of the signal‐space separation algorithm implemented in the Maxfilter™ software (version 2.2 with default parameters; MEGIN Oy, Croton Healthcare, Helsinki, Finland) to reduce external noise and correct for head movements. All the next steps of pre‐processing were performed using Oxford's Software Library (OSL; Oxford Centre for Human Brain Activity, U.K., https://ohba-analysis.github.io/osl-docs/), a software library designed for analysing MEG data using functions from SPM12 (Welcome Trust Centre for Neuroimaging, University College London) and Fieldtrip (Oostenveld, Fries, Maris, & Schoffelen, 2011). Using OSL, the data were converted to SPM format and downsampled to 250 Hz. Independent component analysis with automatic artefactual component rejection was then performed using OSL's AFRICA function. Rejections of components were based on correlations with EOG and ECG recordings (>.5) and kurtosis (>20). The resulting signal and rejected components were manually checked for every participant. Next, data were high‐pass filtered at 0.1 Hz, co‐registered with individual MRIs, and epoched (see below). Using an auto‐kick detection in the eigenspectra we identified the number of principal components in the data which was reduced after Maxfiltering. Within those PCs the minimum eigenvalue was used to normalise the different sensor types. We rejected bad channels and trials using generalised extreme studentised deviate tests (GESDs) (Rosner, 1983), with a cut‐off of significance at .05. We excluded an average of 5.9 ± 6.7 and 4.5 ± 5.7% of channels in the, respectively, MS and HC sample. An average of 5.2 ± 4.6 (MS) and 5.9 ± 4.7% (HC) of trials were abstained from analysis. The minimum amount of trials included for a subject was 53, the median over the whole sample was 75 trials. Finally, an average of 3.8 ± 2.2 (MS) and 3.2 ± 1.2 (HC) components were rejected. Source reconstruction was performed using a linearly constrained minimum variance beamformer that combines information over both magneto‐ and gradiometers and employs a Bayesian principal component analysis to regularise the data covariance matrix estimation and account for the reduction in dimensionality due to Maxfiltering (Woolrich, Hunt, Groves, & Barnes, 2011). This approach uses a data‐driven estimate of the data covariance matrix that automatically trades‐off between the signal‐to‐noise and spatial resolution (Woolrich et al., 2011). A single‐shell forward model in MNI space was used, with a projection on a 5 mm dipole grid.
2.5. Parcellation
We parcellated the data using a downsampled version of the Harvard‐Oxford (sub‐)cortical Atlas. The probability‐based atlas with 1 mm3 resolution was thresholded at 25% probability and then downsampled using FSL's FLIRT function to 5 mm3 resolution. After this, the downsampled atlas was again thresholded at 25% probability to produce an unweighted parcellation atlas. In OSL, the parcel timeseries were calculated by extracting the first principal component from all voxel timeseries in the parcel. We selected the ROI—frequency band pairs based on the findings in the HC analyses (Costers et al., 2020) (see Figure 2 for schematic representations of the parcellation atlases). Important to note is that we included ROIs found in HCs to be a local maximum in any of the three n‐back conditions, while we only analyse data from the 2‐back condition in this study. This was done in order to avoid not including relevant ROIs which could have been narrowly missed as a local maximum in the 2‐back condition. For the theta band we included the precuneus, right frontal orbital, left frontal orbital, right middle frontal, right thalamus, right hippocampus and right inferior temporal. For the alpha band, we included the right thalamus, right hippocampus, right temporal occipital fusiform, left occipital fusiform, left hippocampus and left superior parietal. As we assumed focality of the sources of ERFs, based on observations in the HCs (Costers et al., 2020), we used spherical ROIs with a diameter of 12 mm around the voxel of maximum effect on group level for our ERF analysis. We used anatomical parcellations for the analysis of time‐frequency effects because these were less focal, making the interpretation and relation to previous findings easier. We included only the ROIs reported in (Costers et al., 2020) assumed to be relevant for cognition, being the precuneus and the right and left inferior temporal gyrus, thus excluding the cerebellum and sensorimotor network from these analyses. The coordinates for these three ROIs were [10 −75 52], [40 −20 −32] and [−40 0 47] mm in MNI space.
FIGURE 2.
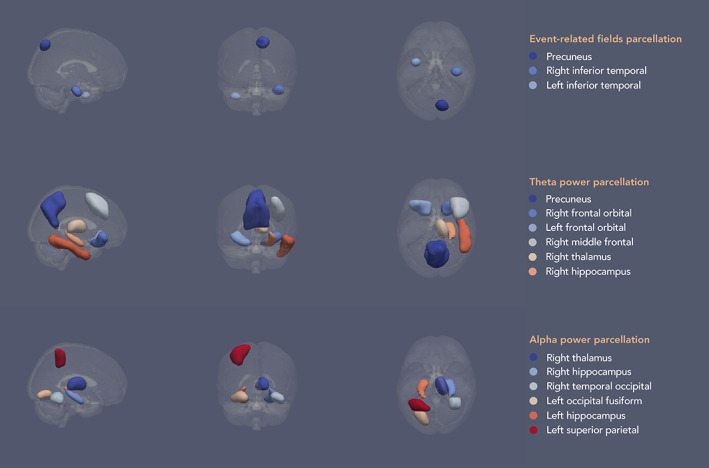
Schematic of the parcellation atlases for the different analyses based on findings in healthy controls (Costers et al., 2020). The parcels in the schematic have been smoothed solely for illustrative purposes. Parcellation atlases using 5 mm3 voxels were used for the analyses
2.6. Event‐related field analysis
OSL was used to perform source reconstruction, parcellation and first‐level analysis. For the ERF analysis, data was filtered between 0.1 and 40 Hz and epoched from [−200 to 800] ms relative to stimulus onset. Baseline correction using the time window −200 ms until stimulus presentation was performed in Fieldtrip using a traditional subtraction technique, after which subject‐level and group‐level analysis was performed. As mentioned before, we performed two types of analyses. A more traditional group EFR analysis using MaxStat correction (Nichols & Holmes, 2002) over all parcels and timepoints (see Section 2.9), and an analysis based on individual maximum ERF amplitudes. For our maximum peak value group analysis we based the time windows of interest on visual inspection of the results from the HC sample, resulting in a time window of [50–250] ms for the precuneus, [300–700] ms for the right inferior temporal and [150–400] ms for the left inferior temporal gyrus.
2.7. Time‐frequency analysis
For time‐frequency analysis, the data was filtered between 0.1 and 80 Hz. In order to avoid edge artefacts, epochs were initially kept at −1.25 s to +1.55 s relative to stimulus onset. The fixed inter‐trial interval was 2.8 s, so there was no overlap of epochs. Time‐frequency analysis was performed using the Morlet wavelet method (6 cycles) as implemented in Fieldtrip. We used a 0.5 Hz frequency resolution in the theta [4–8] Hz and alpha [8–13] Hz band over which the data was then averaged. Baseline normalisation was performed using decibel conversion (dB = 10*log10[power/baseline]) with a −500 to −100 ms baseline period, after which data was epoched to −200 to 800 ms to remove edge artefacts. Subject‐level and group‐level analysis were performed in Fieldtrip. As mentioned before, we performed a group analysis using MaxStat correction (Nichols & Holmes, 2002) over all timepoints and parcels (see Section 2.9). Importantly, this correction was performed per band. The minimum or maximum peak value of theta power increase and alpha power decrease relative to baseline was calculated using the, respectively, time windows [0–400] ms and [200–600] ms.
2.8. Correlations with performance, clinical and volumetric data
We calculated Pearson correlations between the maximum peak values that showed significant group differences, namely the maximum theta power value in the right hippocampus, and performance on the task (accuracy and reaction time) and clinical parameters (disease duration and EDSS) in the MS sample. In order to gain more insight into our findings and avoid possible confounders we also correlated the maximum theta power increase values in the right hippocampus to the normalised volume of the right hippocampus and scores on the California Verbal Learning Test‐II (CVLT‐II). The CVLT‐II is a verbal WM task which has shown high sensitivity for MS (Strober et al., 2009). In addition, we performed post‐hoc correlations between median reaction time and Symbol Digit Modalities Test (SDMT) score, a test for information processing speed in MS (Benedict, Smerbeck, et al., 2012), and normalised white matter volume in order to look into the effects of possible confounders.
The volumetric parameters (normalised white matter volume and normalised right hippocampal volume) were calculated from MR images of MS patients using icobrain (Jain et al., 2015; Version 3.1; formerly known as MSmetrix). To calculate white matter volume, the procedure consists of skull‐stripping the T1‐weighted input image and registering the MNI atlas to the image. Lesions are then filled after outlier detection in both the T1 and Fluid Attenuated Inversion Recovery (FLAIR) image. More details can be found in Jain et al. (2015). The procedure to calculate hippocampal volume is described in detail in Struyfs et al. (2020). In short, a multi‐atlas segmentation approach in applied on the T1‐weighted image to generate a probabilistic segmentation for the left and right hippocampus, which is then used as a prior in an intensity‐based maximum likelihood algorithm to produce the definitive segmentation.
2.9. Statistics
For the analysis of the performance data, we used non‐parametric Wilcoxon‐rank sum tests using the Benjamini‐Hochberg procedure to control the false discovery rate (FDR) (Benjamini & Hochberg, 1995). For MEG data, our statistical analyses consisted of a non‐parametric maximum statistic (MaxStat) approach (Nichols & Holmes, 2002) based on group permutations correcting for multiple comparisons over all parcels and timepoints of the ERF timeseries or time‐frequency power timeseries. Group permutations were performed using random label swapping and Welch t‐tests for unequal variances. For a quality check and comparison with results reported in (Costers et al., 2020), we also performed single‐group maximum statistic permutation tests. In this case, a dependent t‐test was used in the MaxStat approach and the participants' data was permuted with zero values. This corresponds to a sign flipping procedure which is more standard but currently not implemented in Fieldtrip. All tests were two‐tailed, except the tests on performance data, performed in Fieldtrip with 5,000 permutations and alpha = 0.05. For the analyses of the ERF and time‐frequency maximum peak values, we also used a similar MaxStat approach but using a non‐parametric Wilcoxon Rank Sum test. All reported p‐values for correlations were controlled for multiple comparisons using FDR correction (Benjamini & Hochberg, 1995). Importantly, before all statistics we regressed out the effects of scanner type and benzodiazepines from the ERF or time‐frequency data. This was done by first regressing out scanner type over the whole sample at every datapoint ([parcel * frequency * timepoint] for time‐frequency analysis; [parcel * timepoint] for ERF analysis). We continued to work with the sum of the intercept and the residuals. Subsequently, the effect of benzodiazepines was regressed out, but only in the MS sample as no HCs reported the use of benzodiazepines.
3. RESULTS
3.1. Behavioural data
For the 0‐back condition the median reaction time for the HC sample was 423 ms (IQR: [367–505]) and for the MS sample 473 ms (IQR: [435–509]). For the 1‐back condition, the median reaction times for both samples were, respectively, 423 ms (IQR: [361–521]) and 483 ms (IQR: [449–538]). For the 2‐back the median reaction time for the HC sample was 511 ms (IQR: [403–575]) and 560 ms (IQR: [488–627]). The median accuracy for the 0‐back condition for the two groups were both 100% (IQR: [100–100]). For the 1‐back they were 100% (IQR: [91.3–100]) and 95.7% (IQR: [95.7–100]) for, respectively the HC and MS group. For the 2‐back the median accuracy for the HCs was 89.7% (IQR: [79.3–96.6]) and 82.76% (IQR: [55.2–93.1]) in MS. We observed a significant difference between the two groups in reaction time on the 0‐back (z = −2.50, p = .007), but not in accuracy (z = 0.79, p = .215). In the 1‐back condition we found both a significant difference between MS patients and HCs in reaction time (z = −2.81, p = .007) and accuracy (z = 2.81, p = .007). In the 2‐back condition we also found a significant group difference in reaction time (z = −2.61, p = 0.007) and accuracy (z = 2.60, p = 0.007). The performed tests were one‐tailed Wilcoxon rank sum tests, because of previous studies reporting a statistically significantly longer latency and worse accuracy on the n‐back task in MS patients (Covey et al., 2017; Covey, Zivadinov, Shucard, & Shucard, 2011; Parmenter, Shucard, Benedict, & Shucard, 2006). All p‐values mentioned above were corrected for multiple comparisons using false‐discovery rate control (Benjamini & Hochberg, 1995). In order to rule out any effects of fine motor skills on reaction times, we correlated the median reaction times with the Nine‐Hole Peg Test results from the dominant hand with which the task was performed. We found no significant correlation between both variables (r = .09, p = .32; Figure 3).
FIGURE 3.
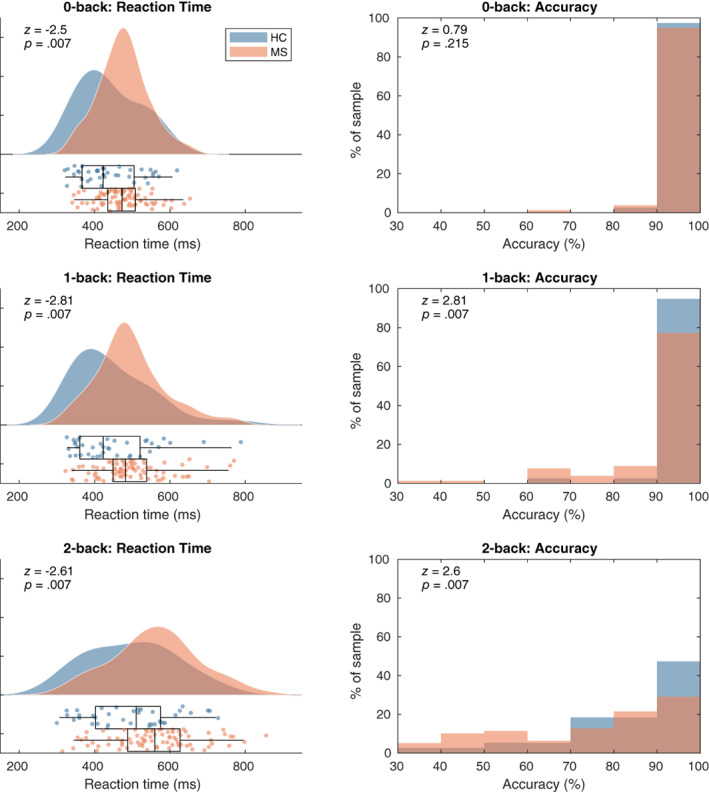
Left panels: reaction time kernel density distribution and boxplots for the three WM load conditions. Right panels: histograms of accuracies per subject. The two groups did not significantly differ in reaction time or accuracy. ERF, event‐related field; HC, healthy controls; MS, multiple sclerosis patients
3.2. Event‐related fields
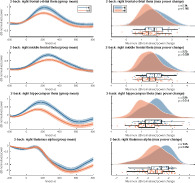
Results did not show significant ERF differences in the three ROIs between the MS and control sample assessed via a MaxStat permutation approach correcting over parcels and timepoints (see Figure 4, left column). See Figure A1 for the values of the t‐statistics at all timepoints. A statistical comparison of the maximum peak values for the ERFs in the precuneus (z = 1.14, p = .32), right inferior temporal (z = 1.28, p = .26) or left inferior temporal gyrus (z = 0.38, p = .72) also did not show any significant differences between the two groups (see Figure 4, right column). All p‐values were corrected for multiple comparisons (see Section 2.9).
FIGURE 4.
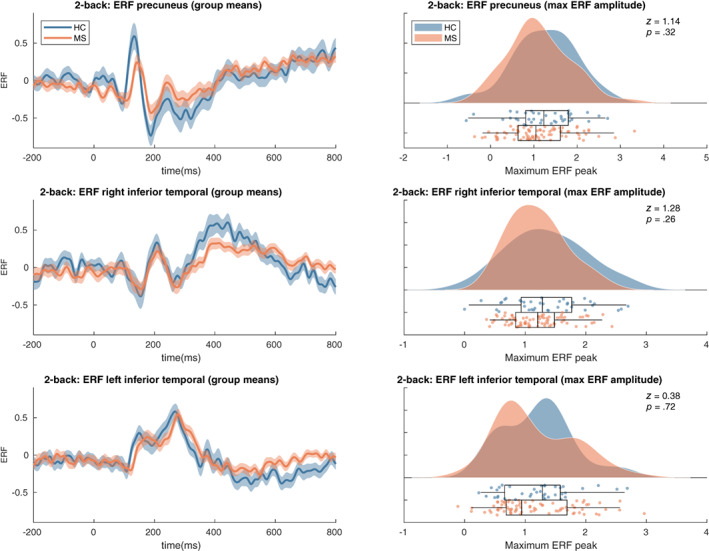
Event‐related field results. Left panels: shaded error bars (using standard error) of group mean ERFs in the selected ROIs. Right panels: raincloud plot of distribution of the maximum peaks of ERFs in the selected ROIs. See Figure A1 for t‐statistics of the single group and group difference tests on the ERF timeseries. HC, healthy controls
3.3. Time‐frequency results
We did not find group differences using the MaxStat approach over the complete timeseries of power changes relative to baseline in any of the 12 theta band or alpha band regions of interest (see Figure 5, left column). The analyses of group differences in the maximum power increase in the theta frequency band showed a significant difference in the right hippocampus (z = 2.98, p = .014) but not in the precuneus (z = 2.04, p = .21), right frontal orbital (z = 2.15, p = .16), left frontal orbital (z = 0.19, p = .99) or right thalamus (z = 1.96, p = .25) (see Figure 5, right column). In the alpha band no group differences were found in the maximum power decrease in the right thalamus (z = 0.195, p = .25), right hippocampus (z = 1.02, p = .86), right temporal occipital fusiform (z = 0.37, p = .99), left occipital fusiform (z = − 0.19, p = .99), left hippocampus (z = 0.21, p = .99) or left superior parietal (z = 1.17, p = .78). All p‐values were corrected for multiple comparisons (see Section 2.9).
FIGURE 5.
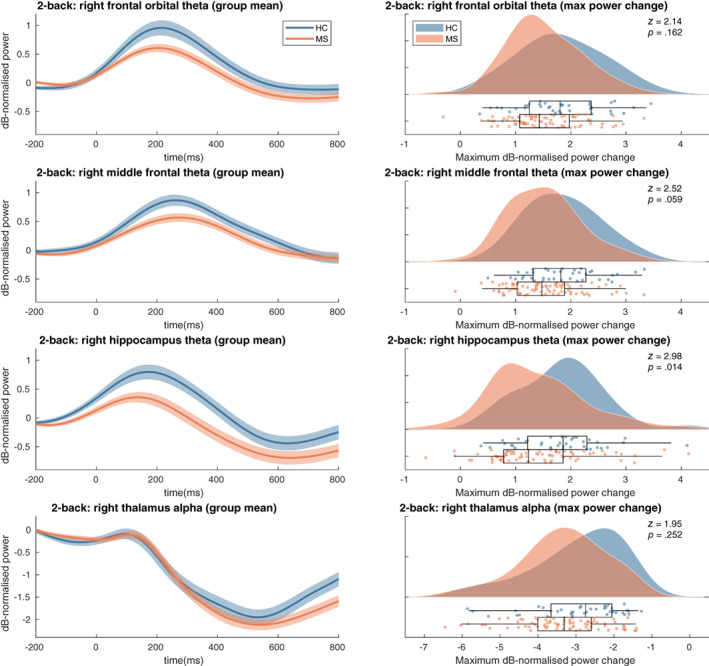
Time‐frequency max power changes results. Left panels: shaded error bars (using standard error) of group mean power changes in the selected ROIs. Right: raincloud plot of distribution of maximum power change values per group in selected ROIs. See Figure A1 for t‐statistics of the single group and group difference tests on the power change timeseries for all parcels. HC, healthy controls; MS, multiple sclerosis patients
3.4. Relationship with performance and clinical variables
In order to gain insight in the possible causes of impaired n‐back performance in MS, we investigated correlations of the parcel‐frequency pair that showed significant group differences (maximum theta band power change in the right hippocampus) and multiple variables such as performance on the task (median reaction time and accuracy), clinical variables (disease duration and EDSS) and score on the CVLT‐II verbal WM test (see Figure 6). We observed a significant correlation between the maximum theta power increase in the right hippocampus and the median reaction time (r = −.32, p = .029) implying that subjects with a higher maximum theta power increase report a shorter median reaction time.
FIGURE 6.
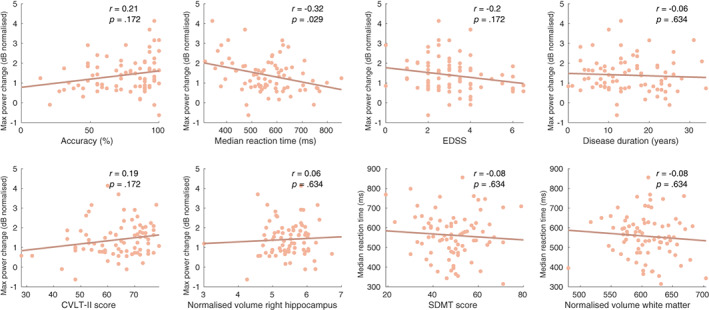
Correlations between the maximum theta power change value in the right hippocampus and performance measures, clinical parameters and volumetric measurements in the MS sample. The last two figures (bottom right) display correlations between the median reaction time and SDMT score and normalised white matter volume. CVLT‐II, California Verbal Learning Test II; EDSS, Extended Disability Status Scale; SDMT, Symbol Digit Modalities Test
Correlations between the maximum theta power increase in the right hippocampus and 2‐back accuracy (r = −.21, p = .172), EDSS score (r = −.2, p = .172), disease duration (r = −.06, p = .634) and CVLT‐II score (r = .19, p = .172) were not significant. We also explored the correlations mentioned above relevant for HCs but found none to be significant (see Figure A2).
3.5. Post‐hoc analysis
It could be that the relationship between the hippocampal theta response and reaction time in MS is confounded by general MS‐related disease progression (a ‘common cause’ confounder relationship; see Figure A3 for a visual illustration). Specifically, it could be that (1) subjects with more severe damage to the hippocampus show a decreased theta response and (2) those subjects also have more demyelination and damage to white matter tracts which would lead to slower information processing and consequently slower reaction times. To test hypothesis 1, we looked for a correlation between normalised right hippocampal volume, a surrogate marker for hippocampal damage, and the right hippocampal theta response which did not show to be strong nor significant (r = .06, p = .634). To test hypothesis 2, we correlated median reaction time with white matter volume and SDMT score, surrogate markers for white matter damage and the consequent decrease in conduction speed, which were also not strong nor significant (respectively r = −.08, p = .634 and r = −0.08, p = .634). Based on this, we concluded that our post‐hoc hypothesis of a confounding role of general MS‐related disease progression (specifically hippocampal damage and white matter damage) was false.
4. DISCUSSION
This study aimed to investigate the neurophysiological mechanisms of WM impairment in MS. We selected ROIs for an ERF and time‐frequency analysis based on results from our HC sample (Costers et al., 2020). A group means based approach was performed as well as an analysis based on individual maximum ERF amplitudes or power changes in the theta and alpha band. Finally, we related parameters that were significantly different between MS patients and HCs to performance, and clinical and anatomical volume parameters.
MS patients performed significantly worse in accuracy and reaction time on all conditions, except accuracy in the 0‐back condition. Only one of the previous n‐back fMRI studies (Forn et al., 2007; Penner et al., 2003; Sweet et al., 2004, 2006) was able to find significant differences in performance between the HC and MS sample (Wishart et al., 2004). A previous EEG study (Covey et al., 2017) and two neuropsychological n‐back studies (Covey et al., 2011; Parmenter et al., 2006) did also report prolonged reaction times during the n‐back task in MS. We found no group differences in the ERFs in the precuneus, right inferior temporal and left inferior temporal. Unlike a previous scalp EEG study (Covey et al., 2017), we did not find the amplitude of the P300‐resembling ERFs to be lower in the MS sample compared to the HCs. When comparing the ERF peak amplitudes, which we assume to be a more sensitive approach, we also did not observe a group difference. Possibly, the summative nature of potentials measured on the EEG scalp level leads to ERPs that are more sensitive to small but widespread changes. As an example, a similar effect of a lower P300 amplitude due to an increasing n‐back WM load has been repeatedly found at EEG scalp level (Ahonen et al., 2016; Causse et al., 2016; Dong et al., 2015; Scharinger et al., 2017) but has been failed to be reported at source level (Costers et al., 2020).
We did observe a significantly smaller maximum theta band power increase in the right hippocampus in MS patients between 0 and 400 ms. This finding is in line with our hypothesis based on a previous finding of decreased activation in the right hippocampus in MS patients in an n‐back fMRI study (Sweet et al., 2004). We based this hypothesis on recent evidence for the excitatory function of theta oscillations during WM (Riddle et al., 2020), assuming that a smaller increase in theta power would translate to the lower activations observed using fMRI. However, such translations of findings from fMRI to MEG are not always as straight‐forward and caution must be taken (see Hall, Robson, Morris, & Brookes, 2014 for review). As mentioned before, hippocampal theta oscillations during WM have been found to play a role in the encoding of temporal information of (working) memory information (Axmacher et al., 2010; Hermiller et al., 2020), which thus seems to be disturbed in MS patients. However, due to the parallel nature of WM processes during the n‐back task it is difficult to make any strong claims about the specific processes that reflect this impaired hippocampal theta oscillatory response. Future studies should explore other WM paradigms that are able to isolate specific WM processes such as the encoding of information to confirm this hypothesis.
Interestingly our data showed that that this increase in theta power in the right hippocampus had a strong negative correlation with reaction time on the task in MS patients. Thus, MS patients with a less strong hippocampal theta power increase responded slower to the 2‐back trials. This suggests that impaired WM function in MS could be related to disturbed theta oscillatory processes in the right hippocampus. A possible ‘common cause’ confounder for this relationship between the maximum theta power increase and reaction time could be general MS‐related neuronal damage (see Figure A3 for a visual illustration). In summary, it could be that two aspects of general MS‐related neural damage give rise to an artificial relationship between the hippocampal theta response and reaction time. Hippocampal damage, demyelination and synaptic abnormalities are frequent in MS (for review see Rocca et al., 2018) which could lead to an impaired hippocampal theta response as observed in our MS sample. MS patients with severe hippocampal damage would also have a higher change of damage to white matter tracts (and a subsequent decrease in conduction velocity and information processing speed), which could consequently lead us to observe an artificial relationship between reaction time and a hippocampal theta response. We performed post‐hoc tests evaluate these hypotheses. A correlation between the right hippocampal theta response and normalised right hippocampal volume, a surrogate marker of hippocampal damage, showed to be very small and non‐significant. The same was true for the correlation between the median reaction time and both SDMT score, a measure for information processing speed in MS, and normalised white matter volume, which should be surrogate markers for white matter damage. Based on this, we concluded that our post‐hoc hypothesis of a confounding role of general MS‐related neural damage and disease progression (specifically hippocampal damage and white matter damage) on the relationship between the right hippocampal theta power response and reaction time was false.
As mentioned before, it is not surprising to find the hippocampus to play an important role in MS‐related WM impairment. It is one of the brain regions most frequently affected by atrophy in MS, which can already be present in CIS (Planche et al., 2018). Hippocampal atrophy or microstructural damage has been repeatedly related to impaired (working) memory function in MS (Benedict et al., 2009; Koenig et al., 2014, 2019; Longoni et al., 2015; Planche et al., 2017; Preziosa et al., 2016; Sacco et al., 2015; Sicotte et al., 2008). For example, a recent study found CA1 atrophy at the time of CIS to be related to episodic verbal memory performance 1 year after CIS (Planche et al., 2018). Besides the findings of decreased activations during 2‐back trials by Sweet and colleagues (Sweet et al., 2004), only two fMRI studies have provided neurophysiological evidence for impaired hippocampal functioning in MS. Roosendaal and colleagues (Roosendaal et al., 2010) observed decreased resting‐state connectivity between the hippocampi and the cerebellum, the anterior cingulate gyrus, thalamus, and prefrontal cortex in MS patients. Increased connectivity between the left hippocampus and the right posterior cingulate was reported in MS patients during rest by Hulst and colleagues (Hulst et al., 2015). They also observed lower hippocampal activation during an episodic memory task in cognitively impaired versus cognitively preserved MS patients, which was related to memory status. The findings in this study are novel in that specific neurophysiological evidence of hippocampal WM dysfunction in MS, in this case disturbed theta oscillatory processes between 0 and 400 ms post‐stimulus, can be linked to impaired WM performance. Importantly, this finding, and a lack of such finding using an approach based on group means, highlights the importance of correctly characterising a person's neurophysiological response in a clinically heterogeneous disease as MS, instead of implicitly assuming that the same neural processes are active in all subjects at a certain timepoint.
While these findings should be confirmed by other studies, they could potentially lead to the development of new therapies for the improvement of WM impairment in MS. For example, while one recent TMS study was able to increase WM capacity by applying theta TMS stimulation to the prefrontal cortex or alpha frequency stimulation to the parietal cortex (Riddle et al., 2020) another study was able to improve episodic memory recollection by applying theta TMS stimulation to the hippocampal network during WM encoding (Hermiller et al., 2020). The short‐lived nature of WM theta processes however, as shown in Figure 4, should be taken into consideration as well as the succession by alpha oscillatory processes, as observed in the right hippocampus. Considering the inhibitory nature of alpha oscillations during WM processes, there seems to be only a small window of time during which hippocampal processes are necessary for n‐back WM activity. This would be a challenge for possible WM therapies in MS based on theta‐band TMS stimulation of the right hippocampus.
We did not find any group differences in theta band power changes in the selected ROIs (see Figure 2) using our MaxStat approach correcting over ROIs and all timepoints. A general trend in the theta band ROIs was that MS patients showed smaller increases in theta power compared to HCs, for example, in line with our hypothesis in the right hippocampus, but none of the group differences were significant. In the frontal ROIs this observation seems to be the opposite of our hypothesis of a stronger theta response in MS patients based on previous observations of increased activations in the prefrontal cortex reported by most fMRI studies (Forn et al., 2007; Penner et al., 2003; Sweet et al., 2004, 2006), and causal evidence for the excitatory role of frontal theta oscillations (Riddle et al., 2020). One fMRI study did report less activation in the prefrontal and specifically also in the middle frontal regions in MS patients (Wishart et al., 2004). More neurophysiological M/EEG studies are needed to elucidate the changes in frontal theta oscillations during WM in MS.
In all alpha band ROIs MS patients generally showed a stronger alpha power decrease relative to baseline compared to HCs, in line with our hypothesis in the right hippocampus, but none of the ROIs yielded significant group differences at any of the timepoints. In the right fusiform gyrus, this trend was also in line with our hypothesis which was based on causal evidence of the inhibitory role of alpha oscillations during WM (Riddle et al., 2020) and decreased activations in the right fusiform gyrus in MS patients reported during an n‐back fMRI study (Sweet et al., 2004). A possible explanation for these non‐significant findings could be that the MaxStat procedure which corrects for multiple comparisons over all parcels and timepoints is considered as being relatively conservative. We chose this technique because it allows clear inference about the location of found effects in time and space, in contrast to more sensitive methods such as threshold‐free cluster enhancement (Smith & Nichols, 2009). In our view, the main reason why individual maximum theta power increases in the right hippocampus showed to be significant, and a MaxStat approach correcting over ROIs and timepoints did not find such significant difference, is because of the amount of data reduction that is a consequence of working with individual maxima for every ROI compared to data consisting of 251 timepoints for every ROI.
5. LIMITATIONS
As mentioned before, we did not investigate MEG data from the 0‐back and 1‐back condition for two reasons (1) the supposed lack of involvement of crucial WM processes such as updating and manipulating WM information in 0 and 1‐back trials (2) the subsequent increase in the complexity of the results, as was illustrated by Costers et al. (2020). This consequentially keep us from claiming that the differences in hippocampal theta power in MS patients are specific to WM.
We also chose to focus on the ROIs based on a previous study (Costers et al., 2020) but it would be interesting if future studies investigated theta power differences between HCs and MS patients in other brain regions such as for example the left hippocampus, to investigate whether the effect is bilateral or lateralised. In context of this specific question concerning the hippocampi, it must be noted that the function of the right hippocampus, assumed to be involved in spatial memory processing, has been suggested to differ to that of the left hippocampus, which is assumed to be involved in episodic verbal memory (Ezzati et al., 2016).
6. CONCLUSION
This study is the first to provide neurophysiological evidence of impaired hippocampal WM processing in MS that can be related to WM performance. Our data suggests that impaired theta oscillatory processes in the right hippocampus, supposedly underlying WM encoding, lead to slower reaction times on the task. We also provide evidence that this relationship cannot be accounted for by general MS‐related neural damage (specifically hippocampal and white matter damage).
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Appendix S1: Supporting Information
Figure A1 t‐statistics of the single‐group and group‐difference tests on theta and alpha band power. Stars illustrate the significant timepoints calculated using MaxStat correction over parcels (per band) and timepoints.
Figure A2 Correlations between the maximum theta power change value in the right hippocampus and performance measures, neuropsychological scores and volumetric measurements in the healthy control sample. CVLT‐II: California Verbal Learning Test II; SDMT: Symbol Digit Modalities Test. All reported p‐values were corrected using FDR correction.
Figure A3 This illustrates the possible common cause confounding influence of general MS‐related disease progression, on the relationship between the right hippocampal theta response and reaction time in MS. Specifically, it could be that (1) subjects with more severe damage to the hippocampus are not able to generate a strong theta response and (2) those subjects also have more demyelination and damage to white matter tracts which would lead to slower information processing and consequently slower reaction times. To test hypothesis 1, we looked for a correlation between normalised right hippocampal volume, a surrogate marker for hippocampal damage, and the right hippocampal theta response which did not show to be strong nor significant To test hypothesis 2, we correlated median reaction time with white matter volume and SDMT score, surrogate markers for white matter damage and the consequent decrease in conduction speed, which were also not strong nor significant. Based on this, we concluded that our post‐hoc hypothesis of a confounding role of general MS‐related disease progression (specifically hippocampal damage and white matter damage) is false.
ACKNOWLEDGEMENT
The authors would like to thank the participants for their time and commitment to this study. The authors would also like to thank Ann Van Remoortel for her help with the recruitment of participants.
Costers L, Van Schependom J, Laton J, et al. The role of hippocampal theta oscillations in working memory impairment in multiple sclerosis. Hum Brain Mapp. 2021;42:1376–1390. 10.1002/hbm.25299
Funding information Fond Erasme pour la Recherche Médicale; Belgian Charcot Foundation; Fondation Philippe Wiener ‐ Maurice Anspach; Fonds De La Recherche Scientifique ‐ FNRS; Fonds Erasme; Fonds Wetenschappelijk Onderzoek, Grant/Award Numbers: 11B7218N, 12I1817N, 1501218N, 1805620N; Genzyme Universitaire Stichting België
DATA AVAILABILITY STATEMENT
The data for this study are not publicly available. Researchers interested in a collaboration on these data are welcome to contact the senior authors. Analysis scripts are available upon request from the corresponding author.
REFERENCES
- Ahonen, L. , Huotilainen, M. , & Brattico, E. (2016). Within‐ and between‐session replicability of cognitive brain processes: An MEG study with an N‐back task. Physiology and Behavior, 158, 43–53. 10.1016/j.physbeh.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Axmacher, N. , Henseler, M. M. , Jensen, O. , Weinreich, I. , Elger, C. E. , & Fell, J. (2010). Cross‐frequency coupling supports multi‐item working memory in the human hippocampus. Proceedings of the National Academy of Sciences, 107(7), 3228–3233. 10.1073/pnas.0911531107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher, N. , Mormann, F. , Fernández, F. , Cohen, M. X. , Elger, C. E. , & Fell, J. (2007). Sustained neural activity patterns during working memory in the human medial temporal lobe. Journal of Neuroscience, 27(29), 7807–7816. 10.1523/JNEUROSCI.0962-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict, R. H. , Amato, M. P. , Boringa, J. , Brochet, B. , Foley, F. , Fredrikson, S. , … Trojano, M. (2012). Brief International Cognitive Assessment for MS (BICAMS): International standards for validation. BMC Neurology, 12(1), 55. 10.1186/1471-2377-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict, R. H. , Ramasamy, D. , Munschauer, F. , Weinstock‐Guttman, B. , & Zivadinov, R. (2009). Memory impairment in multiple sclerosis: Correlation with deep grey matter and mesial temporal atrophy. Journal of Neurology, Neurosurgery and Psychiatry, 80, 201–206. 10.1136/jnnp.2008.148403 [DOI] [PubMed] [Google Scholar]
- Benedict, R. H. , Smerbeck, A. , Parikh, R. , Rodgers, J. , Cadavid, D. , & Erlanger, D. (2012). Reliability and equivalence of alternate forms for the symbol digit modalities test: Implications for multiple sclerosis clinical trials. Multiple Sclerosis (Houndmills, Basingstoke, England), 18(9), 1320–1325. 10.1177/1352458511435717 [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Browne, P. , Chandraratna, D. , Angood, C. , Tremlett, H. , Baker, C. , Taylor, B. V. , & Thompson, A. J. (2014). Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology, 83(11), 1022–1024. 10.1212/wnl.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causse, M. , Peysakhovich, V. , & Fabre, E. F. (2016). High working memory load impairs language processing during a simulated piloting task: An ERP and Pupillometry Study. Frontiers in Human Neuroscience, 10(May), 1–14. 10.3389/fnhum.2016.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , & Huang, X. (2016). Modulation of alpha and beta oscillations during an n‐back task with varying temporal memory load. Frontiers in Psychology, 6(JAN), 1–10. 10.3389/fpsyg.2015.02031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti, N. D. , & DeLuca, J. (2008). Cognitive impairment in multiple sclerosis. The Lancet Neurology, 7, 1139–1151. 10.1016/S1474-4422(08)70259-X [DOI] [PubMed] [Google Scholar]
- Costers, L. , Gielen, J. , Eelen, P. L. P. L. , Schependom, J. V. J. V. , Laton, J. , Van Remoortel, A. V. A. , … Nagels, G. (2017). Does including the full CVLT‐II and BVMT‐R improve BICAMS? Evidence from a Belgian (Dutch) validation study. Multiple Sclerosis and Related Disorders, 18, 33–40. 10.1016/j.msard.2017.08.018 [DOI] [PubMed] [Google Scholar]
- Costers, L. , Van Schependom, J. , Laton, J. , Baijot, J. , Sjøgård, M. , Wens, V. , … Nagels, G. (2020). Spatiotemporal and spectral dynamics of multi‐item working memory as revealed by the n‐back task using MEG. Human Brain Mapping, 41, 2431–2446. 10.1002/hbm.24955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey, T. J. , Shucard, J. L. , Benedict, R. H. , Weinstock‐Guttman, B. , & Shucard, D. W. (2018). Improved cognitive performance and event‐related potential changes following working memory training in patients with multiple sclerosis. Multiple Sclerosis Journal ‐ Experimental, Translational and Clinical, 4(1), 205521731774762. 10.1177/2055217317747626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey, T. J. , Shucard, J. L. , & Shucard, D. W. (2017). Event‐related brain potential indices of cognitive function and brain resource reallocation during working memory in patients with Multiple Sclerosis. Clinical Neurophysiology, 128(4), 604–621. 10.1016/j.clinph.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Covey, T. J. , Zivadinov, R. , Shucard, J. L. , & Shucard, D. W. (2011). Information processing speed, neural efficiency, and working memory performance in multiple sclerosis: Differential relationships with structural magnetic resonance imaging. Journal of Clinical and Experimental Neuropsychology, 33, 1129–1145. 10.1080/13803395.2011.614597 [DOI] [PubMed] [Google Scholar]
- D'Esposito, M. , Onishi, K. , Thompson, H. , Robinson, K. , Armstrong, C. , & Grossman, M. (1996). Working memory impairments in multiple sclerosis: Evidence from a dual‐task paradigm. Neuropsychology, 10(1), 51–56. 10.1037/0894-4105.10.1.51 [DOI] [Google Scholar]
- De Tiège, X. , de Beeck, M. O. , Funke, M. , Legros, B. , Parkkonen, L. , Goldman, S. , & Van Bogaert, P. (2008). Recording epileptic activity with MEG in a light‐weight magnetic shield. Epilepsy Research, 82(2–3), 227–231. 10.1016/j.eplepsyres.2008.08.011 [DOI] [PubMed] [Google Scholar]
- Deluca, J. , Chelune, G. J. , Tulsky, D. S. , Lengenfelder, J. , & Chiaravalloti, N. D. (2004). Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? Journal of Clinical and Experimental Neuropsychology, 26(4), 550–562. 10.1080/13803390490496641$16.00 [DOI] [PubMed] [Google Scholar]
- Devlin, J. T. , Jamison, H. L. , Gonnerman, L. M. , & Matthews, P. M. (2006). The role of the posterior fusiform gyrus in reading. Journal of Cognitive Neuroscience, 18(6), 911–922. 10.1162/jocn.2006.18.6.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, S. , Reder, L. M. , Yao, Y. , Liu, Y. , & Chen, F. (2015). Individual differences in working memory capacity are reflected in different ERP and EEG patterns to task difficulty. Brain Research, 1616, 146–156. 10.1016/j.brainres.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Ezzati, A. , Katz, M. J. , Zammit, A. R. , Lipton, M. L. , Zimmerman, M. E. , Sliwinski, M. J. , & Lipton, R. B. (2016). Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia, 93, 380–385. 10.1016/j.neuropsychologia.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forn, C. , Barros‐Loscertales, A. , Escudero, J. , Benlloch, V. , Campos, S. , Parcet, M. A. , & Ávila, C. (2007). Compensatory activations in patients with multiple sclerosis during preserved performance on the auditory n‐back task. Human Brain Mapping, 28(5), 424–430. 10.1002/hbm.20284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, N. J. , Agster, K. L. , & Eichenbaum, H. B. (2002). Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience, 5(5), 458–462. 10.1038/nn834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens, S. , Nácher, V. , Luna, R. , Romo, R. , & Jensen, O. (2011). α‐Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proceedings of the National Academy of Sciences of the United States of America, 108(48), 19377–19382. 10.1073/pnas.1117190108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, E. L. , Robson, S. E. , Morris, P. G. , & Brookes, M. J. (2014). The relationship between MEG and fMRI. NeuroImage, 102, 80–91. 10.1016/j.neuroimage.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Hermiller, M. S. , Chen, Y. F. , Parrish, T. B. , & Voss, J. L. (2020). Evidence for immediate enhancement of hippocampal memory encoding by network‐targeted theta‐burst stimulation during concurrent fMRI. Journal of Neuroscience, 40, 7155–7168. 10.1523/JNEUROSCI.0486-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, L. T. , Ekstrom, A. D. , & Ranganath, C. (2011). Neural oscillations associated with item and temporal order maintenance in working memory. Journal of Neuroscience, 31, 10803–10810. 10.1523/JNEUROSCI.0828-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulst, H. E. , Schoonheim, M. M. , Van Geest, Q. , Uitdehaag, B. M. J. , Barkhof, F. , & Geurts, J. J. G. (2015). Memory impairment in multiple sclerosis: Relevance of hippocampal activation and hippocampal connectivity. Multiple Sclerosis Journal, 21, 1705–1712. 10.1177/1352458514567727 [DOI] [PubMed] [Google Scholar]
- Jain, S. , Sima, D. M. , Ribbens, A. , Cambron, M. , Maertens, A. , Van Hecke, W. , … Smeets, D. (2015). Automatic segmentation and volumetry of multiple sclerosis brain lesions from MR images. NeuroImage: Clinical, 8, 367–375. 10.1016/j.nicl.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, K. H. , James, T. W. , Jobard, G. , Wong, A. C. N. , & Gauthier, I. (2005). Letter processing in the visual system: Different activation patterns for single letters and strings. Cognitive, Affective, & Behavioral Neuroscience, 5, 452–466. 10.3758/CABN.5.4.452 [DOI] [PubMed] [Google Scholar]
- Jensen, O. , & Tesche, C. D. (2002). Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience, 15(8), 1395–1399. 10.1046/j.1460-9568.2002.01975.x [DOI] [PubMed] [Google Scholar]
- Joseph, J. E. , Gathers, A. D. , & Piper, G. A. (2003). Shared and dissociated cortical regions for object and letter processing. Cognitive Brain Research, 17(1), 56–67. 10.1016/S0926-6410(03)00080-6 [DOI] [PubMed] [Google Scholar]
- Kawasaki, M. , Kitajo, K. , & Yamaguchi, Y. (2010). Dynamic links between theta executive functions and alpha storage buffers in auditory and visual working memory. European Journal of Neuroscience, 31(9), 1683–1689. 10.1111/j.1460-9568.2010.07217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner, R. P. , Gilbert, P. E. , & Barua, L. A. (2002). The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience, 116(2), 286–290. 10.1037/0735-7044.116.2.286 [DOI] [PubMed] [Google Scholar]
- Koenig, K. A. , Rao, S. M. , Lowe, M. J. , Lin, J. , Sakaie, K. E. , Stone, L. , … Phillips, M. D. (2019). The role of the thalamus and hippocampus in episodic memory performance in patients with multiple sclerosis. Multiple Sclerosis Journal, 25(4), 574–584. 10.1177/1352458518760716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig, K. A. , Sakaie, K. E. , Lowe, M. J. , Lin, J. , Stone, L. , Bermel, R. A. , … Phillips, M. D. (2014). Hippocampal volume is related to cognitive decline and fornicial diffusion measures in multiple sclerosis. Magnetic Resonance Imaging, 32, 354–358. 10.1016/j.mri.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok, A. (2001). On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology, 38(3), 557–577. 10.1017/s0048577201990559 [DOI] [PubMed] [Google Scholar]
- Kurtzke, J. F. (1983). Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology, 33(11), 1444–1452. 10.1212/wnl.33.11.1444 [DOI] [PubMed] [Google Scholar]
- Leszczynski, M. (2011). How does hippocampus contribute to working memory processing? Frontiers in Human Neuroscience, 5(NOVEMBER), 1–2. 10.3389/fnhum.2011.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman, J. E. , & Idiart, M. A. P. (1995). Storage of 7 ± 2 short‐term memories in oscillatory subcycles. Science, 267(5203), 1512–1515. 10.1126/science.7878473 [DOI] [PubMed] [Google Scholar]
- Lisman, J. E. , & Jensen, O. (2013). The Theta‐gamma neural code. Neuron, 77(6), 1002–1016. 10.1016/j.neuron.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoni, G. , Rocca, M. A. , Pagani, E. , Riccitelli, G. C. , Colombo, B. , Rodegher, M. , … Filippi, M. (2015). Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Structure and Function, 220, 435–444. 10.1007/s00429-013-0665-9 [DOI] [PubMed] [Google Scholar]
- Mathiowetz, V. , Kashman, N. , Volland, G. , Weber, K. , Dowe, M. , & Rogers, S. (1985). Grip and pinch strength: Normative data for adults. Archives of Physical Medicine and Rehabilitation. 66(2), 69–74. [PubMed] [Google Scholar]
- Nichols, T. E. , & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping, 15, 1–25. 10.1002/hbm.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 1–9. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang, G. , Sommer, W. , & Zhou, C. (2016). Reconstructing ERP amplitude effects after compensating for trial‐to‐trial latency jitter: A solution based on a novel application of residue iteration decomposition. International Journal of Psychophysiology, 109, 9–20. 10.1016/j.ijpsycho.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Parmenter, B. A. , Shucard, J. L. , Benedict, R. H. B. , & Shucard, D. W. (2006). Working memory deficits in multiple sclerosis: Comparison between the n‐back task and the Paced Auditory Serial Addition Test. Journal of the International Neuropsychological Society., 12, 677–687. 10.1017/S1355617706060826 [DOI] [PubMed] [Google Scholar]
- Penner, I. K. , Rausch, M. , Kappos, L. , Opwis, K. , & Radü, E. W. (2003). Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. Journal of Neurology, 250, 461–472. 10.1007/s00415-003-1025-0 [DOI] [PubMed] [Google Scholar]
- Pernet, C. , Celsis, P. , & Démonet, J. F. (2005). Selective response to letter categorization within the left fusiform gyrus. NeuroImage, 28(3), 738–744. 10.1016/j.neuroimage.2005.06.046 [DOI] [PubMed] [Google Scholar]
- Peyser, J. M. , Rao, S. M. , LaRocca, N. G. , & Kaplan, E. (1990). Guidelines for neuropsychological research in multiple sclerosis. Archives of Neurology, 47, 94–97. 10.1001/archneur.1990.00530010120030 [DOI] [PubMed] [Google Scholar]
- Planche, V. , Koubiyr, I. , Romero, J. E. , Manjon, J. V. , Coupé, P. , Deloire, M. , … Tourdias, T. (2018). Regional hippocampal vulnerability in early multiple sclerosis: Dynamic pathological spreading from dentate gyrus to CA1. Human Brain Mapping, 39(4), 1814–1824. 10.1002/hbm.23970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planche, V. , Ruet, A. , Coupé, P. , Lamargue‐Hamel, D. , Deloire, M. , Pereira, B. , … Tourdias, T. (2017). Hippocampal microstructural damage correlates with memory impairment in clinically isolated syndrome suggestive of multiple sclerosis. Multiple Sclerosis, 23, 1214–1224. 10.1177/1352458516675750 [DOI] [PubMed] [Google Scholar]
- Polman, C. H. , Reingold, S. C. , Banwell, B. , Clanet, M. , Cohen, J. A. , Filippi, M. , … Wolinsky, J. S. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69, 292–302. 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preziosa, P. , Rocca, M. A. , Pagani, E. , Stromillo, M. L. , Enzinger, C. , Gallo, A. , … Filippi, M. (2016). Structural MRI correlates of cognitive impairment in patients with multiple sclerosis: A multicenter study. Human Brain Mapping, 37, 1627–1644. 10.1002/hbm.23125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu, Y. , Cheyne, D. O. , Cornwell, B. R. , & Johnson, B. W. (2018). Non‐invasive investigation of human hippocampal rhythms using magnetoencephalography: A review. Frontiers in Neuroscience, 12(APR), 1–16. 10.3389/fnins.2018.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, S. M. , Leo, G. J. , Bernardin, L. , & Unverzagt, F. (1991). Cognitive dysfunction in multiple sclerosis. 1. Frequency, patterns, and prediction. Neurology, 41(5), 685–691. [DOI] [PubMed] [Google Scholar]
- Riddle, J. , Scimeca, J. , Cellier, D. , Dhanani, S. , & D'Esposito, M. (2020). Causal evidence for a role of Theta and alpha oscillations in the control of working memory. Current Biology, 30, 1–7. 10.1016/j.cub.2020.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, B. M. , Hsieh, L. T. , & Ranganath, C. (2013). Oscillatory activity during maintenance of spatial and temporal information in working memory. Neuropsychologia, 51, 349–357. 10.1016/j.neuropsychologia.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca, M. A. , Barkhof, F. , De Luca, J. , Frisén, J. , Geurts, J. J. G. , Hulst, H. E. , … Yousry, T. A. (2018). The hippocampus in multiple sclerosis. The Lancet Neurology, 17(10), 918–926. 10.1016/S1474-4422(18)30309-0 [DOI] [PubMed] [Google Scholar]
- Roosendaal, S. D. , Hulst, H. E. , Vrenken, H. , Feenstra, H. E. M. , Castelijns, J. A. , Pouwels, P. J. W. , … Geurts, J. J. G. (2010). Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology, 255(2), 595–604. 10.1148/radiol.10091433 [DOI] [PubMed] [Google Scholar]
- Rosner, B. (1983). Percentage points for a generalized ESD many‐outlier procedure. Technometrics, 25(2), 165. 10.2307/1268549 [DOI] [Google Scholar]
- Roux, F. , & Uhlhaas, P. J. (2014). Working memory and neural oscillations: Alpha‐gamma versus theta‐gamma codes for distinct WM information? Trends in Cognitive Sciences, 18(1), 16–25. 10.1016/j.tics.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Ruchkin, D. S. , Grafman, J. , Krauss, G. L. , Johnson, R. , Canoune, H. , & Ritter, W. (1994). Event‐related brain potential evidence for a verbal working memory deficit in multiple sclerosis. Brain, 117(2), 289–305. 10.1093/brain/117.2.289 [DOI] [PubMed] [Google Scholar]
- Sacco, R. , Bisecco, A. , Corbo, D. , Della Corte, M. , d'Ambrosio, A. , Docimo, R. , … Bonavita, S. (2015). Cognitive impairment and memory disorders in relapsing–remitting multiple sclerosis: The role of white matter, gray matter and hippocampus. Journal of Neurology, 262, 1691–1697. 10.1007/s00415-015-7763-y [DOI] [PubMed] [Google Scholar]
- Scharinger, C. , Soutschek, A. , Schubert, T. , & Gerjets, P. (2017). Comparison of the working memory load in N‐back and working memory span tasks by means of EEG frequency band power and P300 amplitude. Frontiers in Human Neuroscience, 11(January), 1–19. 10.3389/fnhum.2017.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicotte, N. L. , Kern, K. C. , Giesser, B. S. , Arshanapalli, A. , Schultz, A. , Montag, M. , … Bookheimer, S. Y. (2008). Regional hippocampal atrophy in multiple sclerosis. Brain, 131, 1134–1141. 10.1093/brain/awn030 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , & Nichols, T. E. (2009). Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Strober, L. , Englert, J. , Munschauer, F. , Weinstock‐Guttman, B. , Rao, S. , & Benedict, R. H. B. (2009). Sensitivity of conventional memory tests in multiple sclerosis: Comparing the Rao brief repeatable neuropsychological battery and the minimal assessment of cognitive function in MS. Multiple Sclerosis (Houndmills, Basingstoke, England), 15(9), 1077–1084. 10.1177/1352458509106615 [DOI] [PubMed] [Google Scholar]
- Struyfs, H. , Sima, D. M. , Wittens, M. , Ribbens, A. , Pedrosa de Barros, N. , Phan, T. V. , … Smeets, D. (2020). Automated MRI volumetry as a diagnostic tool for Alzheimer's disease: Validation of icobrain dm. NeuroImage: Clinical, 26, 102243. 10.1016/j.nicl.2020.102243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet, L. H. , Rao, S. M. , Primeau, M. , Durgerian, S. , & Cohen, R. A. (2006). Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Human Brain Mapping, 27(1), 28–36. 10.1002/hbm.20163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet, L. H. , Rao, S. M. , Primeau, M. , Mayer, A. R. , & Cohen, R. A. (2004). Functional magnetic resonance imaging of working memory among multiple sclerosis patients. Journal of Neuroimaging, 14, 150–157. 10.1177/1051228403262695 [DOI] [PubMed] [Google Scholar]
- Van Schependom, J. , D'hooghe, M. B. M. B. , Cleynhens, K. , D'hooge, M. , Haelewyck, M. C. M. C. , De Keyser, J. , & Nagels, G. (2015). Reduced information processing speed as primum movens for cognitive decline in MS. Multiple Sclerosis Journal, 21(1), 83–91. 10.1177/1352458514537012 [DOI] [PubMed] [Google Scholar]
- Wishart, H. A. , Saykin, A. J. , McDonald, B. C. , Mamourian, A. C. , Flashman, L. A. , Schuschu, K. R. , … Kasper, L. H. (2004). Brain activation patterns associated with working memory in relapsing‐remitting MS. Neurology, 62, 234–238. 10.1212/01.WNL.0000103238.91536.5F [DOI] [PubMed] [Google Scholar]
- Woolrich, M. , Hunt, L. , Groves, A. , & Barnes, G. (2011). MEG beamforming using Bayesian PCA for adaptive data covariance matrix regularization. NeuroImage, 57(4), 1466–1479. 10.1016/j.neuroimage.2011.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Figure A1 t‐statistics of the single‐group and group‐difference tests on theta and alpha band power. Stars illustrate the significant timepoints calculated using MaxStat correction over parcels (per band) and timepoints.
Figure A2 Correlations between the maximum theta power change value in the right hippocampus and performance measures, neuropsychological scores and volumetric measurements in the healthy control sample. CVLT‐II: California Verbal Learning Test II; SDMT: Symbol Digit Modalities Test. All reported p‐values were corrected using FDR correction.
Figure A3 This illustrates the possible common cause confounding influence of general MS‐related disease progression, on the relationship between the right hippocampal theta response and reaction time in MS. Specifically, it could be that (1) subjects with more severe damage to the hippocampus are not able to generate a strong theta response and (2) those subjects also have more demyelination and damage to white matter tracts which would lead to slower information processing and consequently slower reaction times. To test hypothesis 1, we looked for a correlation between normalised right hippocampal volume, a surrogate marker for hippocampal damage, and the right hippocampal theta response which did not show to be strong nor significant To test hypothesis 2, we correlated median reaction time with white matter volume and SDMT score, surrogate markers for white matter damage and the consequent decrease in conduction speed, which were also not strong nor significant. Based on this, we concluded that our post‐hoc hypothesis of a confounding role of general MS‐related disease progression (specifically hippocampal damage and white matter damage) is false.
Data Availability Statement
The data for this study are not publicly available. Researchers interested in a collaboration on these data are welcome to contact the senior authors. Analysis scripts are available upon request from the corresponding author.


