Comorbid type 2 diabetes poses a great challenge to the global control of tuberculosis. Here, we assessed the efficacy of metformin (MET), an antidiabetic drug, in mice infected with a very low dose of Mycobacterium tuberculosis. In contrast to diabetic mice, infected nondiabetic mice that received the same therapeutic concentration of MET presented with significantly higher disease burden. This warrants further studies to investigate the disparate efficacy of MET against tuberculosis in diabetic and nondiabetic individuals.
KEYWORDS: tuberculosis, diabetes, metformin, host-directed therapy
ABSTRACT
Comorbid type 2 diabetes poses a great challenge to the global control of tuberculosis. Here, we assessed the efficacy of metformin (MET), an antidiabetic drug, in mice infected with a very low dose of Mycobacterium tuberculosis. In contrast to diabetic mice, infected nondiabetic mice that received the same therapeutic concentration of MET presented with significantly higher disease burden. This warrants further studies to investigate the disparate efficacy of MET against tuberculosis in diabetic and nondiabetic individuals.
INTRODUCTION
Tuberculosis (TB) remains one of the deadliest infectious diseases, with an estimated annual mortality of 1.5 million and nearly 1.7 billion latently infected people worldwide (1). Whereas infections with drug-susceptible Mycobacterium tuberculosis, the causative agent of TB, can be treated with long-term antibiotic therapy, emergence of drug-resistant strains and increasing incidence of comorbid conditions, such as diabetes mellitus (DM), pose a great challenge to TB eradication (2). It is estimated that 463 million people are currently living with diabetes (3) and have a 3-fold-increased risk of developing active TB (4), and a strong association with multidrug-resistant (MDR) TB has been demonstrated (5). Poor treatment adherence, clinical complications, and continuous exposure to conventional anti-TB monotherapy often lead to drug tolerance and resistance (6). In addition to the evaluation of new and existing repurposed anti-TB drugs, there has been increased interest in nonantimicrobial host-directed therapies, which often target host immune responses with the potential to shorten and improve treatment duration and therapeutic efficacy against all forms of TB (7).
Metformin (MET; 1,1-dimethylbiguanide) is a widely prescribed AMP-activated protein kinase (AMPK)-activating antidiabetic drug found to be associated with reduced TB risk among diabetic patients (8, 9). MET was previously shown to inhibit intracellular growth of M. tuberculosis isolates and improve the efficacy of the first-line anti-TB drug isoniazid (INH) in young nondiabetic C57BL/6 mice (9). However, in a recent experiment, MET failed to enhance the potency of a current anti-TB treatment regimen in young BALB/c mice (10), implying the need to resolve the discrepant findings between experiments and, more important, to investigate the true impact of MET on TB in the context of diabetes. In this study, we sought to simultaneously evaluate the protective efficacy of MET alone and in combination with INH against TB using a robust model of murine type 2 diabetes (T2D) and age-matched nondiabetic control mice (11).
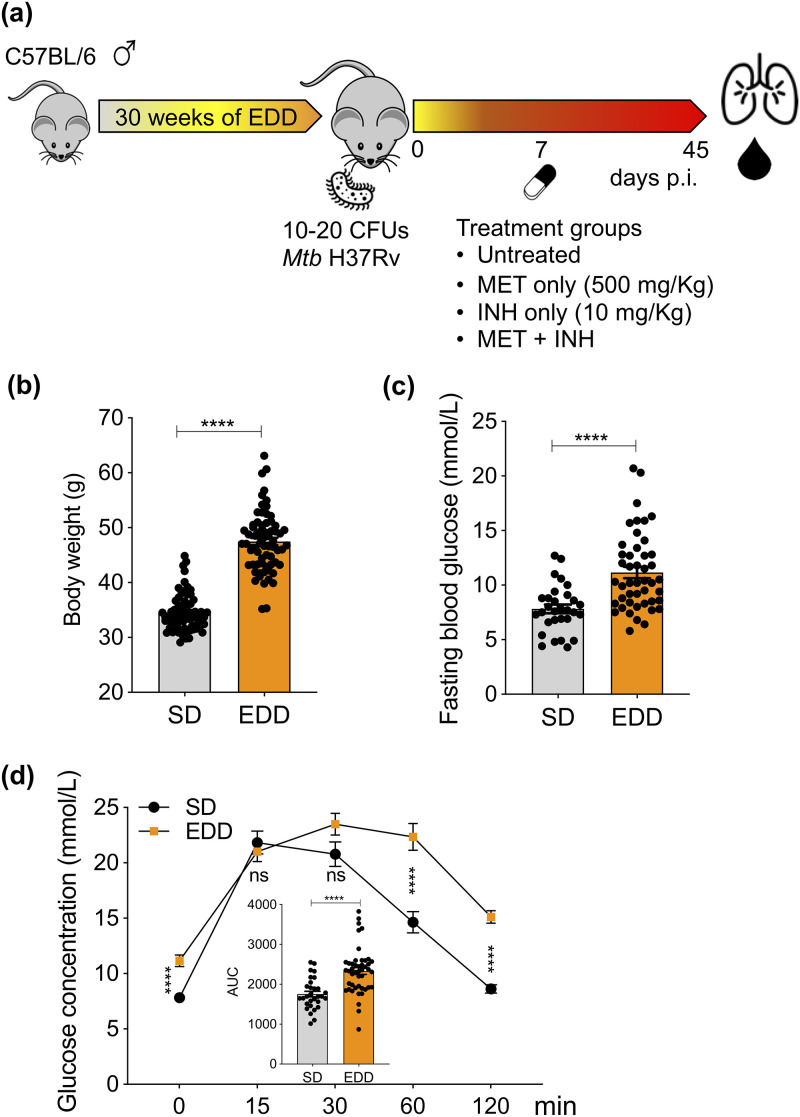
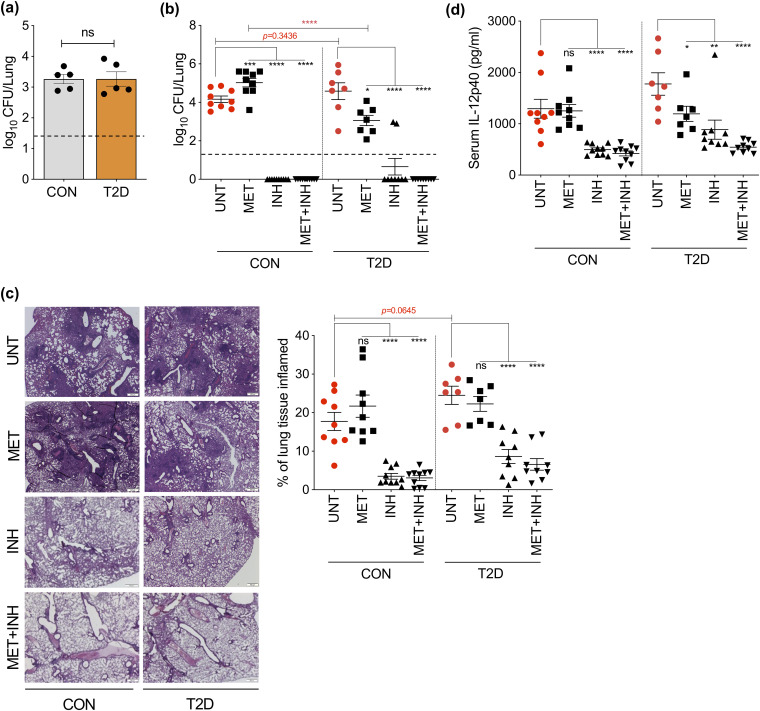
All animal experiments were approved by the animal ethics committee (A2400) of James Cook University, Australia. Specific-pathogen-free C57BL/6 male mice were randomly divided into two groups for dietary interventions. One group was given ad libitium access to an energy-dense diet (EDD; 23% fat, 19% protein, 50.5% dextrose, and 7.5% fiber), and the second group received an isometric quantity of standard rodent diet. After 30 weeks of diet intervention (Fig. 1a), mice fed EDD presented with significantly increased body weight (Fig. 1b), elevated fasting blood glucose levels (Fig. 1c), and impaired glucose tolerance, reflected by area under the curve (Fig. 1d), which are hallmark features of human T2D (11). To investigate whether MET can restrict the growth of M. tuberculosis isolates and enhance the efficacy of the first-line anti-TB drug INH, we infected nondiabetic control and T2D mice with a very low aerosol dose (10 to 20 CFU) of M. tuberculosis H37Rv using a Glas-Col inhalation exposure system to closely mimic human M. tuberculosis infection (Fig. 1a). To enumerate organ bacterial loads, aseptically removed lung tissues from M. tuberculosis-infected mice were homogenized in sampling bags containing 1 ml of sterile phosphate-buffered saline/0.05% Tween 80. Serial dilutions of tissue homogenates were plated on 10% oleic acid-albumin-dextrose-catalase-enriched 7H11 agar plates supplemented with 10 μg/μl cycloheximide and 20 μg/ml ampicillin. Colonies were counted after a 3- to 4-week incubation at 37°C. Mice that showed both CFU on 7H11 agar plates and M. tuberculosis bacilli by Ziehl-Neelsen stain were included (5 mice were excluded from analysis; see Fig. S1 and S2 in the supplemental material). CFU recovered from lung tissue 7 days postinfection (p.i.) revealed that control and T2D mice carried a similar bacterial burden before treatment (Fig. 2a). Treatments were initiated by administering MET and INH alone or in combination (MET+INH) to mice in drinking water, delivering ∼500 and ∼10 mg/kg, respectively (Fig. 1a). MET 1.25 mg/ml in drinking water delivers ∼250 mg/kg to mice (12).
FIG 1.
Diet-induced model of murine T2D, M. tuberculosis (Mtb) infection, and treatments. (a) Six- to 8-week-old C57BL/6 male mice were fed EDD and standard rodent diet (SD; control mice) for 30 weeks to induce murine T2D. Following dietary intervention, mice were assessed for body weight (b), fasting blood glucose levels (c), and glucose tolerance (d). (d) Glucose tolerance test was performed by measuring glucose concentrations at 15, 30, 60, and 120 min after intraperitoneal glucose administration (2 g/kg) and calculating area under the curve (AUC). (a) Diabetic and nondiabetic control mice were infected with a very low aerosol dose (10–20 CFU) of M. tuberculosis H37Rv. Seven days p.i., MET (500 mg/kg), INH (10 mg/kg), and MET+INH were administered in drinking water. Results are presented as individual data points (b–d) and pooled data means ± SEM (d) from 2 pooled independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by Student's t test.
FIG 2.
Divergent effects of MET on control (CON) and T2D mice. (a) Seven days p.i., a group of mice were sacrificed and assessed for lung bacterial loads. Forty-five days p.i., treated and untreated mice from control and T2D groups were assessed for viable bacteria in lung (b), lung inflammation (c), and serum IL-12p40 levels (d). Results are presented as individual data points (a–d) and representative images (25×) (c) from 2 pooled independent experiments (n = 7–10 mice/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by Student's t test (b, c) and one-way analysis of variance followed by Dunnett’s multiple-comparison test (b–d). UNT, untreated.
Cumulative evidence suggests that MET prescription is associated with a significantly lower incidence of active TB among TB/DM comorbid patients (13). Reduced mortality, fewer pulmonary cavities, and rapid culture conversion are evidence of improved health outcome. In addition, Singhal and colleagues (9) reported that MET treatment was also associated with reduced latent TB infection (LTBI) prevalence and enhanced M. tuberculosis-specific T-cell responses determined by T-SPOT assay. However, no significant association between LTBI and TB/DM patients taking MET was found in a more recent study (14). Collectively, these retrospective studies indicate that MET has the propensity to improve the overall treatment outcome when used with existing anti-TB regimens in TB/DM comorbid patients. While an earlier time point (35 days p.i.) or reduced dose of INH (5 mg/kg) may have shown potential adjunct therapeutic effects of MET (9), MET did not further enhance the sterilizing effect of INH in our experimental settings (Fig. 2b). These discrepant findings may also be due to age-related mechanisms, such as differences in tertiary lymphoid structures (15), composition of age-associated B cells (16), altered cytokine/chemokine milieu (17, 18), or maturity of the immune system, compared with previous studies (6- to 8-week- versus 9-month-old mice). In addition to no bacterial persistence, both INH and MET+INH mice had significantly lower lung immunopathology, determined by hematoxylin and eosin staining (Fig. 2c and Fig. S1).
Therapeutic concentrations of MET significantly improved the disease outcome in our T2D mice (∼1.5-log bacterial reduction compared with untreated T2D mice) (Fig. 2b). Strikingly, in contrast to a previous report (9), MET-treated nondiabetic mice presented with augmented lung bacillary loads (Fig. 2b), increased lung immunopathology (Fig. 2c), and similar proinflammatory interleukin (IL)-12p40 levels to untreated control mice (Fig. 2d), indicating diminished TB immunity. The increased lung pathology in the MET group may be attributed to the AMPK-dependent neutrophil-derived matrix metalloproteinase-8 secretion in lungs (19). This disparate efficacy of MET in nondiabetic and T2D mice resulted in an ∼2-log difference in lung M. tuberculosis burden. Overall, serum cytokine/chemokine levels (measured using Bio-Plex Pro mouse cytokine 23-plex assay; Bio-Rad) were comparable between control and T2D mice (see Fig. S3a in the supplemental material), and all drug regimens seem to have downregulated a majority of analytes (Fig. S3b).
The potential role of MET as an adjunctive therapy for TB is exciting. However, current evidence for MET-induced anti-TB protection has come mostly from retrospectively evaluated studies involving TB/DM comorbid patients. Several studies have provided evidence for MET-induced reduction in proinflammatory threshold via possible inhibition of mammalian target of rapamycin (mTOR) (20–22) and/or perturbed gut microbiota (23–25). Whereas this immunomodulatory effect of MET can be favorable in certain high-risk populations, such as people with diabetes and chronic inflammation, further preclinical studies are warranted to exclude the plausibility of an excessive host anti-inflammatory response that may exert negative influence on the early control of M. tuberculosis infection and bacterial dissemination as observed in our nondiabetic mice. In the future, our long-term T2D mouse model will enable us to investigate the efficacy and optimum therapeutic concentration of MET against TB at various stages of diabetes development.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chris Wright, Lachlan Pomfrett, and Serrin Rowarth for assistance with PC3 and animal house operations.
This work was supported by the National Health and Medical Research Council (NHMRC) through a CJ Martin Biomedical Early Career Fellowship (grant APP1052764), a Career Development Fellowship (grant APP1140709), a New Investigator Project Grant (APP1120808), and an Australian Institute of Tropical Health and Medicine (AITHM) Capacity Building Grant (15031) to N.K. and A.K. H.D.S. was supported by an AITHM scholarship.
We declare no conflicts of interest.
N.K. and A.K. conceived the study. H.D.S., K.H., S.M.-H., C.R., and A.K. performed experiments. H.D.S. and A.K. performed data analysis. B.G., C.M.R., L.H., and N.K. assisted with troubleshooting and intellectual input. H.D.S. and A.K. wrote the initial manuscript. All coauthors commented extensively on the manuscript and approved it.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.WHO. 2019. Global tuberculosis report: 2019. World Health Organization, Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1. [Google Scholar]
- 2.Amberbir A. 2019. The challenge of worldwide tuberculosis control: and then came diabetes. Lancet Glob Health 7:e390–e391. doi: 10.1016/S2214-109X(19)30053-1. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. 2019. IDF diabetes atlas, 9th ed. IDF, Brussels, Belgium. http://www.diabetesatlas.org/. [Google Scholar]
- 4.Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. 2017. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS One 12:e0187967. doi: 10.1371/journal.pone.0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tegegne BS, Mengesha MM, Teferra AA, Awoke MA, Habtewold TD. 2018. Association between diabetes mellitus and multi-drug-resistant tuberculosis: evidence from a systematic review and meta-analysis. Syst Rev 7:161. doi: 10.1186/s13643-018-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie SH. 2002. Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrob Agents Chemother 46:267–274. doi: 10.1128/aac.46.2.267-274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zumla A, Chakaya J, Centis R, D'Ambrosio L, Mwaba P, Bates M, Kapata N, Nyirenda T, Chanda D, Mfinanga S, Hoelscher M, Maeurer M, Migliori GB. 2015. Tuberculosis treatment and management–an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med 3:220–234. doi: 10.1016/S2213-2600(15)00063-6. [DOI] [PubMed] [Google Scholar]
- 8.Lin SY, Tu HP, Lu PL, Chen TC, Wang WH, Chong IW, Chen YH. 2018. Metformin is associated with a lower risk of active tuberculosis in patients with type 2 diabetes. Respirology 23:1063–1073. doi: 10.1111/resp.13338. [DOI] [PubMed] [Google Scholar]
- 9.Singhal A, Jie L, Kumar P, Hong GS, Leow MK, Paleja B, Tsenova L, Kurepina N, Chen J, Zolezzi F, Kreiswirth B, Poidinger M, Chee C, Kaplan G, Wang YT, De Libero G. 2014. Metformin as adjunct antituberculosis therapy. Sci Transl Med 6:263ra159. doi: 10.1126/scitranslmed.3009885. [DOI] [PubMed] [Google Scholar]
- 10.Dutta NK, Pinn ML, Karakousis PC. 2017. Metformin adjunctive therapy does not improve the sterilizing activity of the first-line antitubercular regimen in mice. Antimicrob Agents Chemother 61: e00652-17. doi: 10.1128/AAC.00652-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JL, Bridson TL, Alim MA, Rush CM, Rudd DM, Govan BL, Ketheesan N. 2016. Development of a diet-induced murine model of diabetes featuring cardinal metabolic and pathophysiological abnormalities of type 2 diabetes. Biol Open 5:1149–1162. doi: 10.1242/bio.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandel NS, Avizonis D, Reczek CR, Weinberg SE, Menz S, Neuhaus R, Christian S, Haegebarth A, Algire C, Pollak M. 2016. Are metformin doses used in murine cancer models clinically relevant? Cell Metab 23:569–570. doi: 10.1016/j.cmet.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, He JQ. 2020. Impacts of metformin on tuberculosis incidence and clinical outcomes in patients with diabetes: a systematic review and meta-analysis. Eur J Clin Pharmacol 76:149–159. doi: 10.1007/s00228-019-02786-y. [DOI] [PubMed] [Google Scholar]
- 14.Magee MJ, Salindri AD, Kornfeld H, Singhal A. 2019. Reduced prevalence of latent tuberculosis infection in diabetes patients using metformin and statins. Eur Respir J 53:1801695. doi: 10.1183/13993003.01695-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson HL, Smithey MJ, Surh CD, Nikolich-Zugich J. 2017. Functional and homeostatic impact of age-related changes in lymph node stroma. Front Immunol 8:706. doi: 10.3389/fimmu.2017.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubtsova K, Rubtsov AV, Cancro MP, Marrack P. 2015. Age-associated B cells: a T-bet-dependent effector with roles in protective and pathogenic immunity. J Immunol 195:1933–1937. doi: 10.4049/jimmunol.1501209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettiford JN, Jason J, Nwanyanwu OC, Archibald LK, Kazembe PN, Dobbie H, Jarvis WR. 2002. Age-related differences in cell-specific cytokine production by acutely ill Malawian patients. Clin Exp Immunol 128:110–117. doi: 10.1046/j.1365-2249.2002.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boukhvalova MS, Yim KC, Kuhn KH, Hemming JP, Prince GA, Porter DD, Blanco JC. 2007. Age-related differences in pulmonary cytokine response to respiratory syncytial virus infection: modulation by anti-inflammatory and antiviral treatment. J Infect Dis 195:511–518. doi: 10.1086/510628. [DOI] [PubMed] [Google Scholar]
- 19.Ong CW, Elkington PT, Brilha S, Ugarte-Gil C, Tome-Esteban MT, Tezera LB, Pabisiak PJ, Moores RC, Sathyamoorthy T, Patel V, Gilman RH, Porter JC, Friedland JS. 2015. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog 11:e1004917. doi: 10.1371/journal.ppat.1004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lachmandas E, Eckold C, Bohme J, Koeken V, Marzuki MB, Blok B, Arts RJW, Chen J, Teng KWW, Ratter J, Smolders EJ, Van den Heuvel C, Stienstra R, Dockrell HM, Newell E, Netea MG, Singhal A, Cliff JM, Van Crevel R. 2019. Metformin alters human host responses to Mycobacterium tuberculosis in healthy subjects. J Infect Dis 220:139–150. doi: 10.1093/infdis/jiz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, Kleinnijenhuis J, Lachmandas E, Goncalves LG, Belinha A, Cunha C, Oosting M, Joosten LAB, Matarese G, van Crevel R, Netea MG. 2016. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep 17:2562–2571. doi: 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachmandas E, Beigier-Bompadre M, Cheng SC, Kumar V, van Laarhoven A, Wang X, Ammerdorffer A, Boutens L, de Jong D, Kanneganti TD, Gresnigt MS, Ottenhoff TH, Joosten LA, Stienstra R, Wijmenga C, Kaufmann SH, van Crevel R, Netea MG. 2016. Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur J Immunol 46:2574–2586. doi: 10.1002/eji.201546259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elbere I, Kalnina I, Silamikelis I, Konrade I, Zaharenko L, Sekace K, Radovica-Spalvina I, Fridmanis D, Gudra D, Pirags V, Klovins J. 2018. Association of metformin administration with gut microbiome dysbiosis in healthy volunteers. PLoS One 13:e0204317. doi: 10.1371/journal.pone.0204317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma W, Chen J, Meng Y, Yang J, Cui Q, Zhou Y. 2018. Metformin alters gut microbiota of healthy mice: implication for its potential role in gut microbiota homeostasis. Front Microbiol 9:1336. doi: 10.3389/fmicb.2018.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumas A, Corral D, Colom A, Levillain F, Peixoto A, Hudrisier D, Poquet Y, Neyrolles O. 2018. The host microbiota contributes to early protection against lung colonization by Mycobacterium tuberculosis. Front Immunol 9:2656. doi: 10.3389/fimmu.2018.02656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.