Abstract
Dickkopf-1 (DKK1), broadly expressed by tumor cells from human multiple myeloma (MM) and other cancers but absent from most normal tissues, may be an ideal target for immunotherapy. Our previous studies have shown that DKK1 (peptide)-specific cytotoxic T lymphocytes can effectively lyse primary MM cells in vitro. To develop DKK1-based vaccines that can be easily and inexpensively made and used by all patients, we identified a DKK1 long peptide (LP), DKK13-76-LP, that contains 74 amino acids and epitopes that can potentially bind to all major MHC class I and II molecules. Using HLA-A*0201- and HLA-DR*4-transgenic mouse models, we found that DKK1-specific CD4+ and CD8+ T-cell responses, detected by DKK1 short peptide (P20 and P66v)-HLA-A*0201 tetramer staining and cytotoxic assay for CD8+ T cells or by carboxyfluorescein diacetate succinimidyl ester (CSFE) dilution and IFN-g secretion for CD4+ T cells, respectively, can be induced in vivo by immunizing mice with the DKK13-76-LP. In addition, DKK13-76-LP also induced anti-DKK1 humoral immunity in the transgenic mice and the DKK1 antibodies were functional. Finally, DKK13-76-LP stimulated human blood T cells ex vivo to generate DKK1-specific CD4+ and CD8+ T-cell responses from 8 out of 10 MM patients with different MHC backgrounds. The generated DKK1-specific CD8+ cells efficiently lysed autologous MM cells from these patients. Thus, these results confirm the immunogenicity of the DKK13-76-LP in eliciting DKK1-specific CD4+ and CD8+ T-cell responses in vitro and in vivo, and suggest that the DKK13-76-LP can be used for immunotherapy of MM and other cancers.
Introduction
Dickkopf-1 (DKK1) is highly expressed in tumor cells of multiple myeloma (MM) and other cancer types,1,2 but is absent from normal tissues and organs, with the exception of the placenta and prostate.3,4 Our previous studies have shown that DKK1 (peptide)-specific cytotoxic T lymphocytes (CTL) can effectively lyse primary myeloma cells in vitro, confirming that DKK1 may be a good tumor-associated antigen. We explored the efficacy of a murine DKK1 DNA vaccine in the murine MOPC-21 myeloma model and showed that active vaccination using the DKK1 vaccine was not only able to protect mice from developing myeloma, but was also therapeutic against established myeloma. Mechanistic studies revealed that the DKK1 vaccine elicited strong DKK1- and tumor-specific CD4+ and CD8+ immune responses.5 Thus, our studies provide a strong rationale for targeting DKK1 for immunotherapy in myeloma patients.
There has been substantial progress in the clinical use of long peptide (LP) therapeutic vaccination in recent years.6,7 Melief et al. reported an LP vaccine encompassing a CTL epitope and possessing immunotherapeutic potential. Disis et al. reported that vaccination with a CTL-epitope LP derived from human epidermal growth factor receptor 2 (EGFR2, better known as HER2) generated robust and persistent tumor-specific T-cell immunity in patients with metastatic breast cancer.8 Recent clinical studies using a telomerase-derived LP encompassing CTL-epitopes (GV1001) showed an increase in survival of cancer patients when given in combination with radio- and chemotherapy.9 The success of LP therapeutic vaccines can be attributed to the fact that it could induce close collaboration between cells of the innate immune system, in particular antigen-presenting dendritic cells (DC) and cells of the adaptive immune system, especially CD4+ T-helper (Th) cells and CD8+ CTL.10 LP must be taken up and processed by antigen-presenting cells (APC) before they are presented. Professional APC, such as DC, can manage pools of LP and are capable of properly excising multiple HLA class I and II peptide epitopes for presentation at the cell surface.11-13 Therefore, injection of LP will ensure the induction of both CD4+ and CD8+ T cells to available epitopes, each of which can contribute to the anti-tumor response. CD8+ CTL exert key cytotoxicity to tumor cells. CD4+ T cells are necessary elements of cellular immunity for priming tumor-specific CTL and influencing the differentiation and expansion of tumor antigen-specific CTL. Thus, an ideal peptide vaccine for cancer immunotherapy may be optimally composed of a single LP spanning epitopes for both Th cells and CTL.
In this study, we identified a human DKK1-derived LP (DKK13-76-LP) and explored its potential as a vaccine to induce human DKK1-specific CD4+ Th and CD8+ CTL responses. We found that DKK13-76-LP successfully induced Th1-cell responses in individuals expressing several common HLA allelic variants, including HLA-A, HLA-B, HLA-C, HLA-DR alleles, and that an efficient cross-presentation of the DKK13-76-LP also induced DKK1-specific CTL response.
Methods
Patients and samples
Peripheral blood samples from patients with MM and healthy donors were used. This study was approved by the Institutional Review Board of the Cleveland Clinic, and informed consent was obtained in accordance with the Declaration of Helsinki.
Selection of HLA class I- and class II-binding peptides
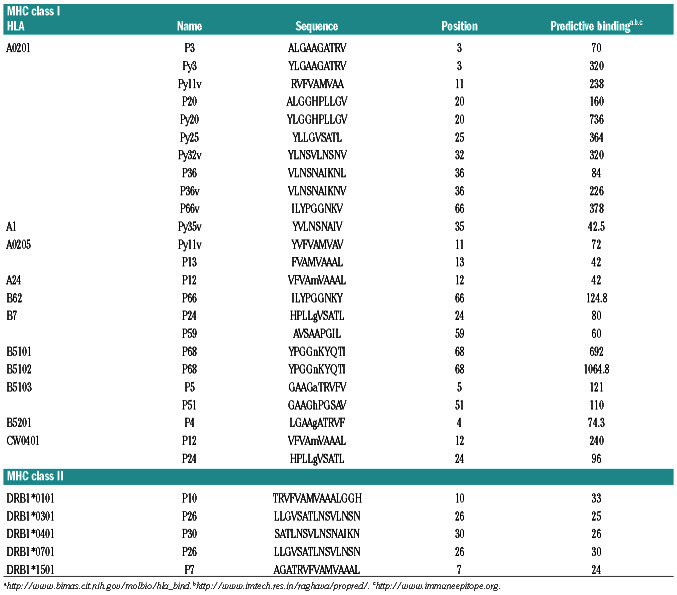
To predict possible promiscuous HLA class I and II-binding peptides on human DKK1, the amino acid sequence of the human DKK1 protein was analyzed by Immune Epitope Database (IEBD) recommended methods.14 The program identified a 74 amino acid LP, DKK13-76, that contains multiple peptide motifs (Figure 1) with high affinity for common and major MHC class I and class II molecules, representing 95% of humans.14
All peptides, including long and short MHC class I and class II binding peptides, were synthesized by Biosynthesis (Lewisville, TX, USA). The purity of synthetic peptides, confirmed by reversed-phase high-performance liquid chromatography and mass spectrometry, was over 98%. Synthetic peptides were dissolved in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO, USA), and stored at -20°C until use.
Generation of dendritic cells
Monocyte-derived mature DC were generated from human peripheral blood mononuclear cells (PBMC).11,15 The quality of DC was judged based on their expression of CD11c, CD40, CD80, CD86, and MHC class II molecules.16 Detailed information is provided in the Online Supplementary Appendix.
Determination of in vivo immunogenicity of DKK1 peptides
HLA-A*0201-transgenic (Tg[HLA-A2.1])17 and HLA-DR*4- transgenic mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA).18 Mice were maintained at the animal facility and studies were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic.
For immunization, peptides were diluted in phosphate buffered saline at room temperature, mixed, and emulsified with an equal volume of incomplete Freund's adjuvant (Sigma). Groups of three mice were immunized subcutaneously at the tail base with 100 L of emulsion containing 100 g of peptides. All of the mice were immunized at least three times. Two weeks after the immunization, mice were killed and splenocytes were isolated for in vitro studies. The same experiments were repeated three times.
Generation of DKK1-specific CD4+ and CD8+ T-cell responses
DKK1-specific T cells were generated from PBMC of HLAA *0201+ and HLA-DR*4+ blood donors and patients with MM by repeated stimulations of autologous T cells with DKK1 peptide- loaded mature DC. Further details are available in the Online Supplementary Appendix.
Cytotoxicity assay
The standard 7-AAD/CFSE Cell-Mediated Cytotoxicity Assay Kit was used to measure the cytolytic activity of T cells on target cells. Further details are available in the Online Supplementary Appendix.
Assessment of DKK1-specific T-cell responses
The frequency of peptide-specific, IFN-g-secreting CD4+ T cells was analyzed using 3x104 bulk CD4+ T cells stimulated with equal numbers of peptide-pulsed autologous PBMC or, alternatively, 5x104 bulk CD4+ T cells stimulated with 1x104 peptide-pulsed DC expressing HLA-DR or -DP molecules. Further details are available in the Online Supplementary Appendix.
Statistical analyses
Statistical analysis was performed with Student t-test. P<0.05 was considered statistically significant. Results are presented as mean ± standard deviation unless otherwise indicated.
Results
Identification of long peptide containing multiple T-cell epitopes on Dickkopf-1 protein
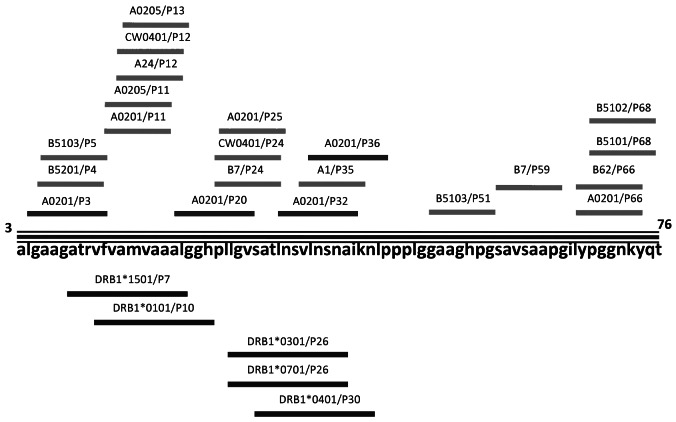
To identify LP comprising the most potential MHC class I and II binding epitopes on human DKK1 protein, we examined the amino acid sequence of DKK1 using the following websites: http://www.bimas.cit.nih.gov/molbio/hlabind/, http://www.imtech.res.in/raghava/propred/, www.immuneepitope.org to predict the epitopes. We focused on regions with multiple MHC class I and class II epitope binding prospects. As a result, we identified an LP, DKK13-76, that contains 74 amino acids and multiple epitopes that can potentially bind with all major MHC class I (e.g., HLA-A, B, or C) and class II molecules (e.g., HLADR1, -DR4, or -DR7) (Table 1 and Figure 1). DKK13-76-LP contains our previously identified HLA-A*0201-restricted T-cell epitopes DKK1-P20 and DKK1-P66v.19
Table 1.
Potential Dickkopf-1 peptides for different MHC molecules.
In vivo immunogenicity of the Dickkopf-13-76-long peptide in activating Dickkopf-1-specific CD8+ cytotoxic T lymphocytes
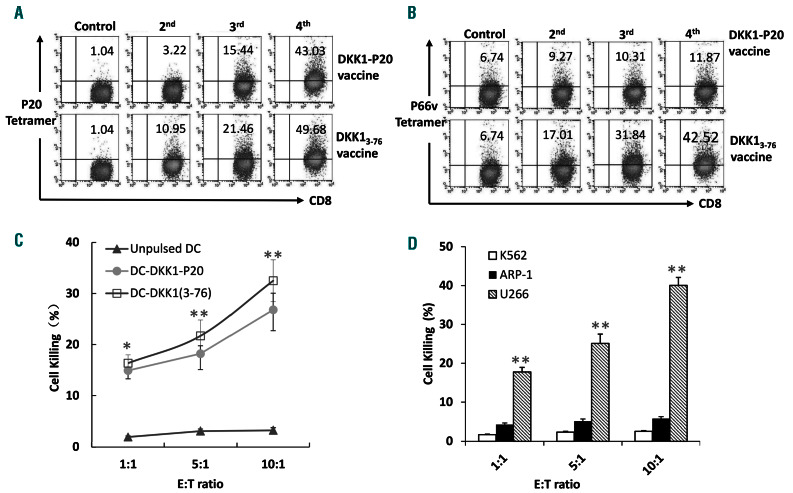
To assess the immunity of the DKK13-76-LP in inducing CD8+ CTL response in vivo, we used HLA-A*0201-transgenic mice and immunized them four times with either DKK13-76-LP or DKK1-P20 short peptide. The capacity of DKK13-76-LP to prime DKK1-specific CTL response was examined using HLA-A*0201-P20- (Figure 2A) or HLAA *0201-P66v tetramer staining (Figure 2B). The results clearly showed that mice immunized with DKK1-P20 short peptide (Figure 2A and B, top panels) had increased percentages of DKK1-P20 (P<0.01, compared with control), but not DKK1-P66v, tetramer+ CD8+ T cells in the spleen after each round of immunization, whereas mice immunized with DKK13-76-LP (Figure 2A and B, bottom panels) generated CD8+ T-cell response against both DKK1-P20 and DKK1-P66v (P<0.01, compared with control). Moreover, CD8+ T cells isolated from mice immunized with DKK13-76-LP were not only able to kill syngeneic DC pulsed with DKK13-76-LP, but also DC pulsed with DKK1-P20 short peptide (P<0.01, compared with controls) (Figure 2C). Furthermore, these CD8+ T cells also killed HLA-A*0201+ U266, but not HLA-A*0201 ARP-1 myeloma cells or K562 cells (to exclude natural killer [NK]-cell activity) (P<0.01, compared with controls) (Figure 2D). Hence, these results demonstrate that DC, after uptake of DKK13-76-LP, efficiently cross-present T-cell epitopes on the DKK13-76-LP and activate CTL specific for various Tcell epitopes, including DKK1-P20- and DKK1-P66v, in vivo.
In vivo immunogenicity of the Dickkopf-13-76-long peptide in activating Dickkopf-1-specific CD4+ T-helper cells and antibody production
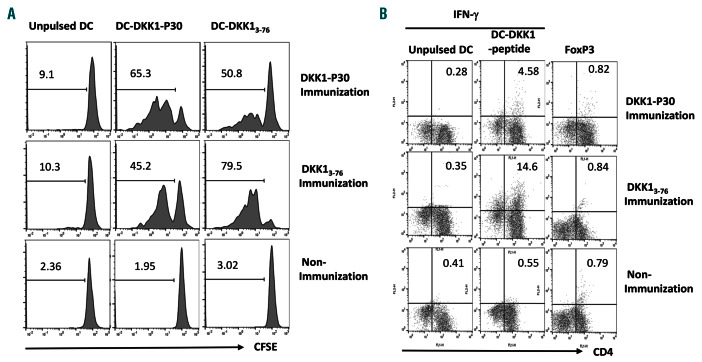
Next, we assessed whether DKK13-76-LP could also elicit DKK1-specific CD4+ Th cell response. HLA-DR*4-transgenic mice were available commercially and immunized four times with DKK13-76-LP or a HLA-DR*4-restricted and -binding DKK1-P30 short peptide (Table 1). CD4+ T-cell response was detected by CFSE dilution and IFN-g secretion. The results clearly showed that mice immunized with either DKK13-76-LP or DKK1 P30 short peptide had significantly higher percentages of proliferating CD4+ T cells in the spleen after ex vivo re-stimulation with DC pulsed, but not unpulsed, with DKK13-76-LP or DKK1-P30 short peptide (P<0.01, compared with non-immunized mice) (Figure 3A). Moreover, splenocytes isolated from DKK13-76-LP - or DKK1 P30-immunized mice contained significantly more CD4+ IFN-g-expressing cells after ex vivo re-stimulation with DKK13-76-LP - or DKK1 P30-, respectively, pulsed, but not unpulsed, DC than non-immunized mice (P<0.01, compared with those from non-immunized mice) (Figure 3B). Interestingly, the percentages of Foxp3+CD4+ T cells were similarly low in mice with or without peptide immunization (Figure 3B), indicating that DKK13-76-LP vaccination induced DKK1-specific CD4+ Tcell responses without promoting regulatory T-cell (Treg) formation in vivo. Taken together, these results demonstrate that the DKK13-76-LP is immunogenic in vivo to induce DKK1-specific CD4+ and CD8+ T-cell responses.
Figure 1.
Dickkopf-1 (DKK1)3-76-long peptide (LP). Schematic presentation of DKK13-76-LP and epitopes for MHC class I and class II molecules.
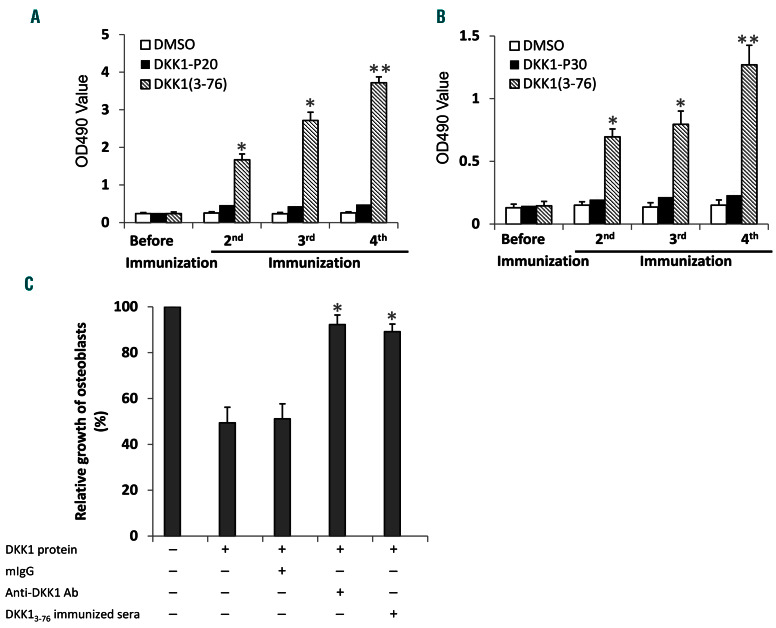
We also investigated whether the DKK13-76-LP could induce a DKK1-specific humoral immune response and examined whether there were DKK1-specific antibodies in the sera of DKK13-76-LP-immunized HLA-A*0201- or HLA-DR*4-transgenic mice. ELISA results showed that high titers of DKK1-specific IgG antibodies were detected in HLA-A*0201-transgenic mice immunized with DKK13-76-LP, but not with DKK1 P20 short peptide, and the titers of the antibodies increased after each cycle of immunization (P<0.01, compared with DMSO control) (Figure 4A). Similarly, DKK1-specific antibodies were also detected in the sera of HLA-DR*4-transgenic mice immunized with DKK13-76-LP but not the DKK1-P30 short peptide (P<0.01, compared with DMSO control) (Figure 4B).
Next, we determined whether the detected DKK1-specific antibodies in DKK13-76-LP-immunized mice were biologically functional. As DKK1 was shown to inhibit human osteoblast differentiation,20 we used an in vitro osteoblast culture system to determine the function of the immunized sera on osteoblast formation in the presence of recombinant human DKK1. The results showed that commercially obtained DKK1-specific antibodies and sera from DKK13-76-LP-immunized transgenic mice abolished DKK1-induced inhibition of human osteoblast differentiation (Figure 4C). These results indicate that the DKK13-76-LP encompasses naturally processed B-cell epitopes, and antibodies generated by DKK13-76-LP immunization are able to neutralize DKK1.
Immunogenicity of the Dickkopf-13-76-long peptide in priming human Dickkopf-1-specific T cells ex vivo
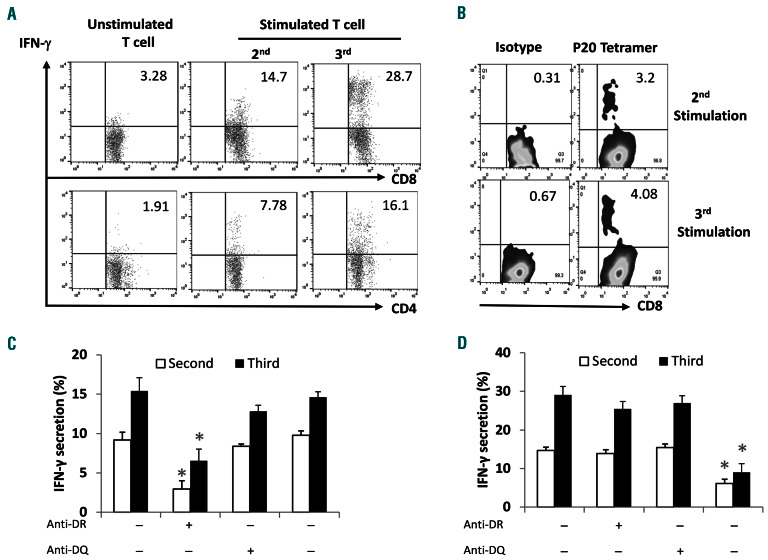
Next, we investigated whether DKK13-76-LP was able to induce human DKK1-specific T-cell responses ex vivo. Freshly prepared PBMC from healthy donors or myeloma patients were stimulated with DKK13-76-LP every week. The presence and frequency of DKK1-specific T cells were detected by flow cytometry. Figure 5A shows an increased frequency of DKK1-specific, IFN-g-secreting CD4+ and CD8+ cells in cultured T cells during in vitro (re)-stimulation with the LP. Figure 5B shows the percentages of HLAA *0201-DKK1-P20 tetramer+ CD8+ T cells in cultures after repeated stimulations with DKK13-76-LP. By using monoclonal antibodies (mAb) specific to HLA-DR or -DQ or HLA-ABC added to T-cell cultures before assay, we showed that MHC class II-restricted CD4+ T cells were the main IFN-g-secreting cells, because anti-HLA-DR mAb significantly reduced the percentage of IFN-g-secreting CD4+ cells (Figure 5C). Moreover, HLA-A2 antibody significantly reduced the percentages of IFN-g-secreting CD8+ T cells in cultures after repeated stimulations with DKK13-76-LP. The results showed that DKK13-76-LP-specific CD8+ T-cell responses were HLA*0201-restricted (Figure 5D). Taken together, the results demonstrate that human DKK1-specific CTL and Th1 cells can be induced by DKK13-76-LP ex vivo.
Figure 2.
Cross-presentation of Dickkopf-1 (DKK1)3-76-long peptide (LP) efficiently primes DKK1-specific CD8+ T cells in vivo. Shown are CD8+ T-cell responses induced in HLA-A*0201-transgenic mice after immunization with DKK13-76-LP or HLA-A*0201-restricted P20 short peptide. (A) DKK1-P20-specific tetramer staining showing the frequency of DKK1 P20-specific CD8+ T cells in the spleen of a HLA-A*0201-transgenic mouse. Representative results from one of three mice are shown. (B) DKK1-P66v-specific tetramer staining showing the frequency of DKK1 P66v-specific CD8+ T cells in the spleen of an HLA-A*0201-transgenic mouse. These results indicate that DKK13-76-LP was cross-presented and efficiently primed DKK1-P66v-specific CD8+ T cells in HLA-A*0201-transgenic mice. Representative results from one of three mice are shown. (C) Cytolytic activity of CD8+ T cells isolated from mice immunized with DKK13-76 LP against unpulsed dendritic cells (DC) or DC pulsed with DKK13-76 LP or P20 short peptide. Representative results of three experiments are shown. (D) Cytolytic activity of CD8+ T cells isolated from mice immunized with DKK13-76 LP against U266 and ARP-1 human myeloma cell lines, and K562. Representative results of three experiments are shown. *P<0.05; **P<0.01.
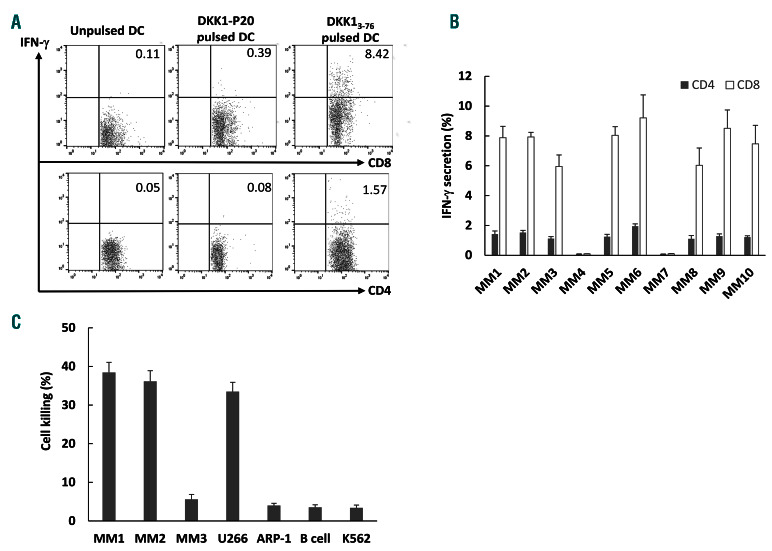
Finally, we determined whether the DKK13-76-LP could induce DKK1-specific HLA-A*0201-restricted and HLADR *4-restricted T-cell responses from MM patients. After 3-4 weeks of stimulating patient-derived PBMC with DKK13-76-LP in vitro, the frequency of DKK1-specifc CD8+ CTL and CD4+ Th1 cells was detected by intracellular IFN-g staining. Figure 6A is a representative flow cytometry analysis showing the percentages of IFN-g-secreting CD8+ and CD4+ T cells after re-stimulation with DKK13-76- LP from a MM patient (MM1). Low percentages of IFN-gsecreting CD8+ and CD4+ T cells were observed in T-cell cultures re-stimulated with unpulsed DC or DC pulsed with DKK1-P20 short peptide. Figure 6B shows the percentages of IFN-g-secreting CD4+ and CD8+ T cells from a total of ten patients with MM (with different MHC backgrounds) after a 4-week in vitro stimulation of blood T cells with DKK13-76-LP-pulsed autologous DC. Furthermore, we generated CD8+ T-cell lines from HLA-A*0201+ patients by in vitro repeated stimulations with DKK13-76-LP and these DKK1-specific T cells killed autologous patient (MM1) myeloma cells, primary myeloma cells from another HLA-A*0201+ patient (MM2), and HLA-A*0201+ myeloma cell line U266. No killing was observed on HLAA *0201- primary myeloma cells (MM3) or myeloma cell line ARP-1, normal B cells, or K562 cells (to exclude NKcell activity) (Figure 6C).
Discussion
In this study, we identified a 74aa DKK13-76-LP thast contains multiple epitopes for CD4+ and CD8+ T cells and explored the potential of using this LP for immunotherapy of human MM. We showed that DKK1-specific CTL, detected by DKK1 short peptide (P20 and P66v)-HLAA *0201 tetramer staining, and DKK1-specific Th cells, detected by IFN-g secretion and CSFE-dilution assay, can be induced by (cross)-presentation of DKK13-76-LP in vitro and in vivo. We also verified the presence of DKK1-specific Th1 responses in MM patients.
Recent studies evaluating the CTL repertoire of HPV-16 e6 and e7 oncogenic protein showed complete and lasting regression of end-stage cervical cancer patients after melanoma antigen A3 (MAGE-A3) vaccination with peptide21,22,29-31 After immunization, a new wave of antigenspecific CTL clones arose in the peripheral blood, providing solid evidence that the phenomenon of epitope spreading is critical to the development of effective anticancer immunity elicited by peptide vaccination. These results further implicate functional interactions between vaccine-induced CTL and malignant cells that facilitate the induction of large numbers of tumor-specific CTL, the cytolytic effector immune cells that subsequently destroy tumor cells.
Figure 3.
Dickkopf-1 (DKK1)3-76-long peptide (LP) efficiently induces DKK1-specific CD4+ T-cell response in vivo. Shown are CD4+ T-cell responses induced in HLADR *4-transgenic mice after immunization with DKK13-76 -LP or HLA-DR-restricted P30 short peptide. (A) Carboxyfluorescein succinimidyl ester (CFSE) dilution assay showing the percentages of proliferating CD4+ T cells from the spleen of mice after ex vivo re-stimulation with dendritic cells (DC) pulsed, but not unpulsed, with DKK13-76 LP or DKK1-P30 short peptide. Representative results from one of three independent experiments are shown. (B) Flow cytometry analysis showing the percentages of IFN-g-expressing or FoxP3+ CD4+ T cells from the spleen of mice after ex vivo re-stimulation with DC pulsed, but not unpulsed, with DKK13-76 LP or DKK1- P30 short peptide. Representative results from one of four experiments are shown.
Figure 4.
Dickkopf-1 (DKK1)3-76-long peptide (LP) induces DKK1-specific humoral immune response in vivo. Serum titers of DKK1-specific antibodies in mice after immunization with DKK13-76-LP or short peptide are shown. (A) ELISA showing the titers of DKK1-specific IgG antibodies in HLA-A*0201-transgenic mice immunized with DKK13-76 LP or P20. (B) ELISA showing the titers of DKK1-specific IgG antibodies in HLA-DR*4- transgenic mice immunized with DKK13-76 LP or P30. (C) Effect of DKK1 on differentiation of human osteoblasts in culture with the addition of control mouse IgG, anti-DKK1 antibody (R&D Systems) or serum from DKK13-76 LP vaccinated (after the 4th vaccination) HLA-A*0201-transgenic mice. Results of three experiments are shown. DMSO: dimethyl sulfoxide. *P<0.05; **P<0.01.
Figure 5.
Dickkopf-1 (DKK1)3-76-long peptide (LP) induces DKK1-specific human T cells in vitro. Fresh peripheral blood mononuclear cells (PBMC) derived from healthy donors (HLA-A0201+ or HLA-DR*4+) were stimulated with DKK13-76-LP plus IL-2 and IL-7 weekly in vitro. (A) Frequency of DKK1-specific CD4+ and CD8+ IFN-g-secreting cells detected by intracellular staining assay. (B) Percentages of HLA-A*0201-DKK1-P20 tetramer+CD8+ T cells in culture after second or third in vitro stimulation with DKK13-76-LP. (C) MHC class II-restriction of DKK13-76-LP-specific CD4+ T-cell response. PBMC stimulated with DKK13-76-LP for 1 week were re-stimulated with DKK13-76-LP in the presence of different monoclonal antibodies (mAb) specific for HLA-DR, -DQ or HLA-ABC. The results showed that HLA-DR antibody significantly reduced the percentages of IFN-g-secreting CD4+ T cells. (D) HLA-A*0201-restriction of DKK13-76-LP-specific CD8+ T-cell response. PBMC stimulated with DKK13-76-LP for 1 week were re-stimulated with DKK13-76-LP in the presence of different mAb specific for HLA-DR, -DQ or HLA-A2. The results showed that HLA-A*0201 antibody significantly reduced the percentages of IFN-g-secreting CD8+ T cells. Representative results of three experiments are shown. *P<0.05; **P<0.01.
Disis et al. reported that vaccination with a herceptin-2 (HER-2/neu)-derived LP encompassing an HLA-A*0201- restricted CTL epitope elicited embedded CTL-epitope specific CD8+ T cells in cancer patients.8 They showed that tumor-specific CTL can be elicited in vivo via crosspresentation of HER-2/neu-derived LP. Such T-cell responses are considered to be crucial for tumor eradication and for generating long-term memory.23 With this premise in mind, we identified an immunogenic DKK13-76- LP that encompasses both Th epitopes and CTL-epitopes and demonstrated that cross-presentation of DKK13-76-LP induced priming and expansion of DKK1-specific CTL in vitro and in vivo. Vaccination with DKK13-76-LP can potentially elicit combined Th and CTL responses. A recent clinical trial showed that targeting Th cells with DC pulsed with both HLA class I and II-restricted epitopes effectively enhanced vaccine-specific immune responses and improved clinical outcome.24 DKK13-76-LP bolstered the induction of DKK1-P20-specific CTL derived from both healthy donors and MM patients in vitro. Thus, DKK13-76-LP administered in combination with DKK1-P20 immunotherapy may be able to augment the elicitation of antigen-specific CTL.
Based on the HLA-subtypes capable of antigen presentation from studies using healthy donors, DKK1-P20 and DKK13-76-LP are predicted to be useful in approximately 80% of the total population. We showed that DKK13-76-LP induced HLA-DP5-, HLA-DR8-, or HLA-DR15-restricted Th cells in healthy donors and also induced HLA-DR- or HLADQ- restricted Th cells in MM patients. However, these MM patients were negative for HLA-DP5, -DR8, or -DR15 alleles. We also showed that DKK13-76-LP induced HLA-DR15- or HLA-DQ-restricted Th cells in healthy donors. These results suggest that DKK13-76-LP may encompass Th cell epitopes not previously identified in experiments involving cells derived from healthy donors and DKK13-76-LP may be broadly useful in the majority of MM patients.
Weide et al. reported that the presence of circulating Th cells responding to melanoma antigens Melan-A or NY-ESO-1 has a strong independent prognostic impact on survival among chemotherapy-treated advanced melanoma patients.25 Another study has shown a possible synergy between the telomerase-specific Th responses with chemotherapy in lung cancer.26 The introduction of immunotherapy in clinical practice also emphasized the influence of immune responses on cancer prognosis and chemotherapy effectiveness.27,28 Politou et al.2 and Terpos et al.29,30 reported that serum concentration of DKK1 protein were increased in patients with MM and were correlated with severe bone disease. Autologous stem cell transplantation and chemotherapies with bortezomib, melphalan, dexamethasone and intermittent thalidomide significantly reduced serum DKK1 level and led to normalization of bone remodeling in relapsed myeloma. These pieces of evidence support the hypothesis that induction or augmentation of DKK1-specific Th1 cells by vaccination with DKK13-76-LP may improve the clinical outcome of cancer patients when combined with chemotherapy or other standard therapies.31,32 DKK13-76-LP-specific Th responses in MM patients may positively influence overall survival. The impact of DKK1-specific Th responses on clinical outcome will be evaluated in future studies.
Figure 6.
Dickkopf-1 (DKK1)3-76-long peptide (LP) induces DKK1-specific human Tcell responses from myeloma patients. Myeloma patient’s peripheral blood mononuclear cells were stimulated weekly with DKK13-76-LP plus IL-2 and IL-7 in vitro, and the frequency of DKK1-specific CD4+ and CD8+ IFN-g-secreting cells was detected by intracellular staining assay. (A) Representative results of T cells derived from a myeloma patient (MM1; HLA-A0201+ and HLA-DR*4+) and (B) summarized results from all ten myeloma patients at 4 weeks of in vitro stimulation. (C) Cytolytic activity of DKK1-specific T cells derived from MM1 against various target cells including autologous (MM1) or allogeneic (MM2 and MM3) plasma cells, myeloma cell lines, B cells, and K562 cells. MM1 and MM2 are HLA-A0201+; MM3 is HLA-A1+ and A32+; U266 is HLA-A0201+, ARP-1 is HLAA0201-. Representative results from one of three experiments are shown. DC: dendritic cells.
In conclusion, DKK13-76-LP provides a useful tool for propagation of both DKK1-specific Th1 cells and CTL, and may synergize with CTL-epitopes to enhance cancer cell killing. These findings provide a rationale for a clinical trial of DKK13-76-LP -based immunotherapy against a broad spectrum of cancer types, as DKK1 is widely expressed by human cancer cells.19
Supplementary Material
Funding Statement
Funding: This work was supported by Cleveland Clinic startup fund, VeloSano, the Leukemia and Lymphoma Society (6469-15), and the Multiple Myeloma Research Foundation.
References
- 1.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003; 349(26):2483-2494. [DOI] [PubMed] [Google Scholar]
- 2.Politou MC, Heath DJ, Rahemtulla A, et al. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer. 2006;119(7):1728-1731. [DOI] [PubMed] [Google Scholar]
- 3.Kohn MJ, Kaneko KJ, DePamphilis ML. DkkL1 (Soggy), a Dickkopf family member, localizes to the acrosome during mammalian spermatogenesis. Mol Reprod Dev. 2005;71(4):516-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65(17):7554-7560. [DOI] [PubMed] [Google Scholar]
- 5.Qian J, Zheng Y, Zheng C, et al. Active vaccination with Dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood. 2012; 119(1):161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwaveling S, Ferreira Mota SC, Nouta J, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169(1):350-358. [DOI] [PubMed] [Google Scholar]
- 7.Vambutas A, DeVoti J, Nouri M, et al. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a preclinical cottontail rabbit papillomavirus model. Vaccine. 2005;23(45):5271-5280. [DOI] [PubMed] [Google Scholar]
- 8.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neuspecific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009; 27(28):4685-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunsvig PF, Kyte JA, Kersten C, et al. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011;17(21):6847-6857. [DOI] [PubMed] [Google Scholar]
- 10.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8(5):351-360. [DOI] [PubMed] [Google Scholar]
- 11.Qian J, Wang S, Yang J, et al. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clin Cancer Res. 2005;11(24 Pt 1):8808-8815. [DOI] [PubMed] [Google Scholar]
- 12.Yi Q, Bergenbrant S, Osterborg A, et al. Tcell stimulation induced by idiotypes on monoclonal immunoglobulins in patients with monoclonal gammopathies. Scand J Immunol. 1993;38(6):529-534. [DOI] [PubMed] [Google Scholar]
- 13.Dabadghao S, Bergenbrant S, Anton D, He W, Holm G, Yi Q. Anti-idiotypic T-cell activation in multiple myeloma induced by Mcomponent fragments presented by dendritic cells. Br J Haematol. 1998;100(4):647-654. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Ponomarenko J, Zhu Z, et al. Immune epitope database analysis resource. Nucleic Acids Res. 2012;40(Web Server issue):W525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romani N, Reider D, Heuer M, et al. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196(2):137-151. [DOI] [PubMed] [Google Scholar]
- 16.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77-92. [DOI] [PubMed] [Google Scholar]
- 17.Alexander J, Oseroff C, Dahlberg C, et al. A decaepitope polypeptide primes for multiple CD8+ IFN-gamma and Th lymphocyte responses: evaluation of multiepitope polypeptides as a mode for vaccine delivery. J Immunol. 2002;168(12):6189-6198. [DOI] [PubMed] [Google Scholar]
- 18.Tangri S, Ishioka GY, Huang X, et al. Structural features of peptide analogs of human histocompatibility leukocyte antigen class I epitopes that are more potent and immunogenic than wild-type peptide. J Exp Med. 2001;194(6):833-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian J, Xie J, Hong S, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110(5):1587-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD. Antibodybased inhibition of DKK1 suppresses tumorinduced bone resorption and multiple myeloma growth in vivo. Blood. 2007; 109(5):2106-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbiere V, Chapiro J, Stroobant V, et al. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res. 2011; 71(4):1253-1262. [DOI] [PubMed] [Google Scholar]
- 22.Ribas A, Timmerman JM, Butterfield LH, Economou JS. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol. 2003; 24(2):58-61. [DOI] [PubMed] [Google Scholar]
- 23.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006; 24:519-540. [DOI] [PubMed] [Google Scholar]
- 24.Aarntzen EH, De Vries IJ, Lesterhuis WJ, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res. 2013;73(1):19-29. [DOI] [PubMed] [Google Scholar]
- 25.Weide B, Zelba H, Derhovanessian E, et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol. 2012;30(15):1835-1841. [DOI] [PubMed] [Google Scholar]
- 26.Godet Y, Fabre E, Dosset M, et al. Analysis of spontaneous tumor-specific CD4 T-cell immunity in lung cancer using promiscuous HLA-DR telomerase-derived epitopes: potential synergistic effect with chemotherapy response. Clin Cancer Res. 2012; 18(10):2943-2953. [DOI] [PubMed] [Google Scholar]
- 27.Kyte JA, Gaudernack G, Dueland S, Trachsel S, Julsrud L, Aamdal S. Telomerase peptide vaccination combined with temozolomide: a clinical trial in stage IV melanoma patients. Clin Cancer Res. 2011;17(13):4568-4580. [DOI] [PubMed] [Google Scholar]
- 28.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castrationresistant prostate cancer. N Engl J Med. 2010;363(5):411-422. [DOI] [PubMed] [Google Scholar]
- 29.Terpos E, Heath DJ, Rahemtulla A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135(5):688-692. [DOI] [PubMed] [Google Scholar]
- 30.Terpos E, Kastritis E, Roussou M, et al. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia. 2008;22(12):2247-2256. [DOI] [PubMed] [Google Scholar]
- 31.Ding ZC, Zhou G. Cytotoxic chemotherapy and CD4+ effector T cells: an emerging alliance for durable antitumor effects. Clin Dev Immunol. 2012;2012:890178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254-1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.