ABSTRACT
La-related proteins (LARPs) share a La motif (LaM) followed by an RNA recognition motif (RRM). Together these are termed the La-module that, in the prototypical nuclear La protein and LARP7, mediates binding to the UUU-3ʹOH termination motif of nascent RNA polymerase III transcripts. We briefly review La and LARP7 activities for RNA 3ʹ end binding and protection from exonucleases before moving to the more recently uncovered poly(A)-related activities of LARP1 and LARP4. Two features shared by LARP1 and LARP4 are direct binding to poly(A) and to the cytoplasmic poly(A)-binding protein (PABP, also known as PABPC1). LARP1, LARP4 and other proteins involved in mRNA translation, deadenylation, and decay, contain PAM2 motifs with variable affinities for the MLLE domain of PABP. We discuss a model in which these PABP-interacting activities contribute to poly(A) pruning of active mRNPs. Evidence that the SARS-CoV-2 RNA virus targets PABP, LARP1, LARP 4 and LARP 4B to control mRNP activity is also briefly reviewed. Recent data suggests that LARP4 opposes deadenylation by stabilizing PABP on mRNA poly(A) tails. Other data suggest that LARP1 can protect mRNA from deadenylation. This is dependent on a PAM2 motif with unique characteristics present in its La-module. Thus, while nuclear La and LARP7 stabilize small RNAs with 3ʹ oligo(U) from decay, LARP1 and LARP4 bind and protect mRNA 3ʹ poly(A) tails from deadenylases through close contact with PABP.
Abbreviations: 5ʹTOP: 5ʹ terminal oligopyrimidine, LaM: La motif, LARP: La-related protein, LARP1: La-related protein 1, MLLE: mademoiselle, NTR: N-terminal region, PABP: cytoplasmic poly(A)-binding protein (PABPC1), Pol III: RNA polymerase III, PAM2: PABP-interacting motif 2, PB: processing body, RRM: RNA recognition motif, SG: stress granule.
KEYWORDS: Deadenylation, poly(A) phasing, pabp, pam2, mlle, ccr4
Introduction
The family of La-related proteins (LARPs) share a ‘La-module’ consisting of two tandem RNA-binding domains: a La motif (LaM) and an RNA recognition motif (RRM)[1]. Although RRM domains are present in hundreds of genes in vertebrates, the LaM is rare and the La-module is found only in the five subfamilies of LARP proteins: 1, 3, 4, 6 and 7. In humans, there are seven LARP genes: LARP1, 1B, 3, 4, 4B, 6 and 7 (1B and 4B arose by relatively recent gene duplications of 1 and 4) [1,2]. Among many potential isoforms, one major mRNA species represents each LARP examined in the cells tested [3].
Diagrams of the seven LARPs are shown in Fig. 1. The ancestral LARP is the nuclear La protein (aka LARP3) that binds the oligo(U) 3ʹends of precursor-tRNAs and other nascent RNA polymerase III (Pol III) transcripts and protects these from digestion by 3ʹ exonucleases [4–6] (reviewed in [7,8]). LARP7 is nuclear and a closely related La paralog that binds and protects a subset of RNAs with 3ʹ oligo(U) [9–11]. LARPs 1, 4 and 6 are highly divergent and reside in the cytoplasm. Evidence of LARP4 and LARP1 in mRNA 3ʹ poly(A) tail metabolism became apparent more recently [12,13]. While LARP1 has been well characterized as an mRNA translational repressor [14–18] whereas LARP4 (and 4B) promotes mRNA translation [12,19], recent data suggest that the La-module related activities of these LARPs may be more similar than was initially apparent. LARPs 1 and 4 both exhibit 3ʹ poly(A) tail length protection-mRNA stabilization associated with their La-modules and dependent on interactions with cytoplasmic poly(A)-binding protein (PABP, aka PABPC1). For both, PABP-binding is mediated by the PABP-interacting motif-2 (PAM2) adjacent to the La-module.
Figure 1.
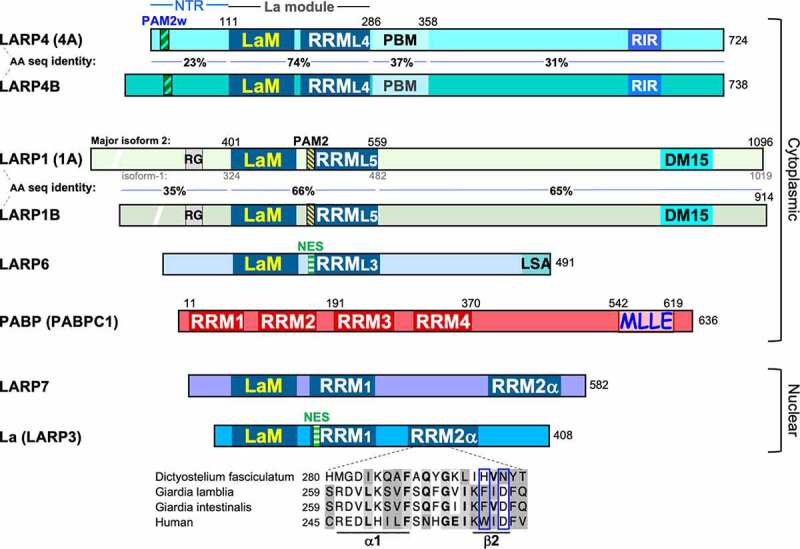
Linear schematic features of the human proteins relevant to this review. LARPs 4 and 4B are distinct gene products, as are LARPs 1 and 1B. For each of these pairs, the % amino acid (AA) sequence identity of different subregions is indicated. For LARP1 the numbering is shown for isoform 2, a dominantly expressed form [3] (the isoform 1 numbering is also indicated; isoforms −1 and −2 differ in their N-terminal regions only and are identical beginning with aa 68 of the former). The PAM2 sequence FSQLLNCPEFVP [22] begins towards the end of the interdomain linker between the La motif (LaM) and predicted RRM of LARP1 and continues through the predicted β1 strand of the RRM [1]. This PAM2 is extended on its N-terminus by a key residue, F419 relative to that previously noted [14]. Curiously, this PAM2 resides in a very similar relative position as a nuclear export sequence (NES) in the β1 strand of the LARP6 RRM [23–25]. A functionally mapped NES in the α1 helix of the La protein La-module RRM is also shown [45]. It should be noted that LARP6b and c homologs in plants have functional PAM2 sequences positioned similarly to LARPs 4 and 4B, whereas human LARP6 lacks a PAM2 [26]. An excerpt of a multiple sequence alignment is shown under the human La schematic RRM2α (the C-terminal RRM has also been referred to as RRM2 and xRRM, see text). These aligned sequences were obtained as the only full length La protein homologs in the single-cell organisms indicated; demarcation of the secondary structure elements corresponding to α1 and β2 under the sequence were from Jacks et al [68].; the vertical rectangles indicate conservation of RNP3 residues (F/Y/W/H)-x-(D/Q/E/N) [71,73]. Abbreviations are as follows. NTR: N-terminal region, LaM: La motif, RRM: RNA recognition motif, PBM: PABP binding motif, RIR: RACK1 interacting region, PAM2: PABP interacting motif-2, RG: conserved region consisting of eight arginine-glycine repeats [2], DM15: 5ʹTOP motif binding region, NES: nuclear export sequence, MLLE: mademoiselle domain
PAM2 motifs bind to a conserved binding site on the MLLE domain of PABP [20]. A summary of proteins containing PAM2 motifs is presented in Table 1. The PAM2 motif of LARP4 is unique with a tryptophan at position-10 that contributes to both poly(A) RNA and PABP binding [12,21]. The PAM2 within the La-module of LARP1 identified by Fonsesca et al. [14]. was recently characterized and the importance of an N-terminal residue for high affinity binding to PABP demonstrated [22]. Consideration of LARPs 1 and 4 as factors that bind and protect mRNA 3ʹ poly(A), dependent on PABP albeit by different mechanisms [22], is the objective of this review.
Table 1.
PAM2 proteins, alignment of their PAM2 sequences, and their properties
| Protein | PAM2 sequences1 | Affinity for PABP MLLE (Kd, μM)2 | Structure (PDB) | Another PABP-binding region | Properties/Function | SG3 or PB4 | Refs |
|---|---|---|---|---|---|---|---|
| position: | 1 3 7 * | 3.8 | Translation inhibition of 5ʹTOP mRNAs and stabilization, poly(A) length protection | SG | [14,22] | ||
| LARP1 | FSQLLNCPEFVP | ||||||
| LARP1B | FSQLIDCPEFVP | [22] | |||||
| eRF3 C | FVPNVHAAEFVP | 3.12 | 3KUJ | Translation termination | [99,113] | ||
| NFX1 | FKFNTDAAEFIP | Regulates telomerase levels | [27] | ||||
| PAIP1 | SKLSVNAPEFYP | 1.4 | 3NTW | yes, PAM1 | Translation enhancement | [28,29] | |
| PAIP2 | SNLNPNAKEFVP | 0.2 | 1JGN/3KUS/3KUT | yes, PAM1 | Translation inhibition | SG | [99,113] |
| PAIP2B | SNLNPDAKEFIP | yes, PAM1 | Translation inhibition | SG | [30] | ||
| USP10 | STLNPQAPEFIL | 26 | Ubiquitin specific peptidase-10, protein stability | SG | [144] | ||
| Tob2 265 | SQLSPNAKEFVY | Associates with CCR4-CAF1-NOT1 deadenylation complex | [138,144] | ||||
| Tob1 265 | SALSPNAKEFIF | [98] | |||||
| ATXN-2 | STLNPNAKEFNP | 0.7 | 3KTR | Translation regulation, deficiency associated with spinocerebellar ataxia | SG | [106,144] | |
| LARP4B | SELNPNAEVWGA | 3PTH | yes, PBM | mRNA stabilization, translation | SG | [37,100,178] | |
| LARP4 | TGLNPNAKVWQE | 22 | 3PKN | yes, PBM | mRNA stabilization, 3ʹ poly(A) length protection, opposes deadenylation | SG | [12] |
| TTC3/TPRD | LQLNPAAREFKP | 5.4 | Neurodegenerative down syndrome | [144] | |||
| eRF3 N | RKLNVNAKPFVP | 3.92 | 3KUI | Translation termination | [99,113] | ||
| Tob2 125 | SSFNPDAQVFVP | 16 | Associates with CCR4-CAF1-NOT1 deadenylation complex | PB | [138,144] | ||
| Tob1 125 | NSFNPEAQVFMP | [98] | |||||
| PAN3 | QTPNPTASEFIP | 40 | Deadenylase subunit | PB | [87,145] | ||
| TNRC6A (GW182) | .....WPPEFRPGEPWKGY | yes | Deadenylation, miRISC assembly | ||||
| TNRC6B | .....WPPEFQPGVPWKGI | yes | |||||
| TNRC6C | .....WPPEFHPGVPWKGL | 6 | 2X04/3KTP | yes | Deadenylation, miRISC assembly | PB | [31,32,106] |
1The PAM2 consensus positions are indicated by numbers above the aligned sequences except position-10 which is indicated by an asterisk. Position-10 is the most important for high affinity binding to the MLLE of PABP [113] (reviewed in [91])(see text). Position-3 typically has leucine (L), is sometimes phenylalanine (F) or proline (P), but in LARP1 and 1B is glutamine (Q). Leucine-3 was found to be the second most important for high affinity binding to the MLLE of PABP [113]. Position-10 is always F except in LARP4 and 4B in which it is tryptophan (W).
2The two overlapping PAM2s of eRF3, -C and -N, together provide a Kd of 1.0 μM.
3 Stress granule (SG).
4 Processing body (PB).
Working on RNA 3ʹ ends is a tradition in the LARP family
Although La and LARP7 differ from LARP1 and 4, an overview of their activities and functions is fitting. The La-modules of LARP7 and La are relatively conserved as compared to the other LARPs (reviewed in [33]). A unique feature of La and LARP7 is the presence of a second RRM domain, designated RRM2α, which will be described in the next section. The prototypic La-module recognizes UUU-3ʹOH in a sequence- and length-dependent manner [5,6] and protects the RNA from 3ʹ exonucleolytic digestion [34–36] (reviewed in [33,37]). Many precursor-tRNAs (and pre-mRNAs) are degraded in wild-type cells by a nuclear surveillance system that detects transcriptional and other errors [38]. La is known to protect pre-tRNAs from the degradative activities of 3ʹ-directed nuclear surveillance while also serving as a chaperone that can assist structurally challenged pre-tRNAs in their correct folding [35,39–42] (reviewed in [33,37]). As a molecular chaperone [43] with nuclear retention activity [44–46], La also prolongs the time window of protection for maturation events that stabilize local folds for formation of the correct tRNA structure.
RNA association with La is transient and its dissociation can be critical to the RNA maturation pathways for tRNAs, the spliceosomal U6 snRNA, and 7SK RNA (related to P-TEFb [9–11], below) [43,47,48] (reviewed in [49,50]). Pre-U6 snRNA undergoes multiple enzymatic 3ʹ end processing activities and involving La protein before acquiring its mature form as a core component of the spliceosome [51–53] (reviewed in [54]). Unlike nascent pre-tRNAs and pre-U6, the nascent 7SK RNA does not undergo 3ʹ processing but is likely transferred as UUU-3ʹOH from La to LARP7 (see [49]). Also, unlike pre-tRNAs, the 5ʹ ends of 7SK, U6 and some other Pol III transcripts are not removed by processing but are capped with a monomethyl moiety on the gamma (γ) phosphate (ppp-CH3), which decreases the affinity for La [49,54,55] and presumably facilitates movement along their maturation pathways [49,54].
The La-module of LARP7 binds UUU-3ʹOH while its RRM2α stably binds a hairpin specific to 7SK RNA. This enables LARP7 to function in controlling P-TEFb (positive transcription elongation factor-b) [9–11] which activates elongation of Pol II that is paused near the transcription start site and regulates a large number of mRNA as well as snRNA and snoRNA transcripts [56] (see [49]). Cellular levels of P-TEFb activity are negatively regulated by binding to 7SK RNA [57]. LARP7 is a stably bound subunit of 7SK RNP which serves as a reservoir of P-TEFb activity, the latter of which can dissociate and bind on cue [9–11]. LARP7 levels determine 7SK RNA levels.
LARP7 also associates albeit transiently with metazoan U6 snRNA and thereby directs its 2′-O-methylation by a sequence-specific subfamily of box C/D snoRNPs [58,59] (reviewed in [49]). In this capacity, LARP7 binds U6 via its La-module and simultaneously binds the guide-snoRNA via its RRM2α. This allows it to function as a strand-annealing chaperone by assisting the complementary base pairing of the U6 and snoRNA [58].
La and LARP7 are involved in RNA 3ʹ end metabolism and may have co-existed in an ancient nucleated organism [60]. Other LARPs diverged within and beyond their La-modules [1,2]. LARPs 1 and 4 are highly divergent, yet their La-modules appear to exhibit surprisingly similar activities in RNA 3ʹ poly(A) tail metabolism [22].
La-module evolution
The milestone analysis of LARPs revealed that while their LaMs were highly conserved, they were segregated into five families on the basis of their RRM domains [1]. The RRM is considered an RNA-binding platform with ‘extreme structural versatility,’ comprised of a core of secondary structure elements with variations and extensions [61]. More than 500 high-resolution RRM structures have been resolved, ~20% of which include a bound RNA [62]. Several atypical RRMs subtypes with distinctive features have evolved [63]. Different RRMs can exhibit unique RNA binding specificities, e.g., individual members of the LARP7 family (below).
Sequence alignments and predicted secondary structure elements of the LARPs [1], benefited from high-resolution RRM structures including unexpected features of La protein bound to UUU-3ʹOH [6,64–66]. The RRMs in La and LARP7 La-modules were considered classic, whereas those in LARPs 6, 4 and 1 were variably divergent, termed RRM-L3, -L4, and -L5, respectively [1]. Further analysis suggested that the LaM and RRM coevolved with the various LARPs [1]. High-resolution structures for LARPs 4, 6, and 7 later emerged and while not all secondary structure elements aligned with or matched the initial predictions, the models generally fit. A suitable example is that despite strong evolutionary conservation of the La-modules of La and LARP7, a structure of the latter revealed only three β strands in its RRM1 [67] instead of the canonical four as in La (see [33]).
Phylogenetics indicate a La gene duplication in an ancestral eukaryote and that some alleles had one RRM and others had two. The C-terminal RRMs of LARP7 and La were named RRM2 [68–70] but have since been referred to as RRM2α [8,33] because of the most prominent feature of this atypical RRM, its α3 helical extension (Fig. 1). Two types of genuine La protein and two types of LARP7 exist in representatives of ancient eukaryotes, each with and without an RRM2 [1,2]. This suggests that an intragenic RRM duplication accompanied a gene duplication and raises the possibility that the two RRMs could have exchanged places during La-module evolution. Yeast La proteins lack RRM2 [8] and their La-module RRMs were noted to exhibit characteristics of RRM2α [45,71].
Certain atypical features of RRM2α appear to have differentially evolved in lineages of La and LARP7 homologs, so much so that it is referred to as xRRM in the latter [71,72]. RRM2α is unique to LARPs, found only in La and LARP7 homologs which include the telomerase RNA-associated p43 and p65 in ciliates [73–75] and Pof8 in S. pombe [60,72,76]. Because ciliates contain huge numbers of linear minichromosomes with telomeres, they require high levels of telomerase RNA which is synthesized by Pol III [77,78], the polymerase specialized for high output of short transcripts [79]. The La-module of p65 binds UUU-3ʹOH and its xRRM binds to a hairpin on the Tel-RNA with high affinity and sequence-specificity [80].
The α3 extension of a classic RRM was discovered as an integral element that lies across the RRM β-sheet surface of the human La RRM2 [68]. Multiple additional features of this atypical RRM were also noted: i) a five stranded β-sheet, unprecedented at the time, ii) lack of aromatic residues at conserved positions in the β3 and β1 strands of the RNP1 and RNP2 motifs, and iii) enrichment of acidic residues in the strands [68]. Structures of p65 and LARP7 bound to their different sequence-specific RNAs revealed these features along with a key RNP3 motif on β2 and a conserved arginine on β3 that now appear distinctive of xRRM [71,73,74,80]. RNP3 is a short sequence motif, often including an acidic residue, on the β2 strand that participates with α3 in RNA binding [71].
Distinctive among the RRM2α domains is that the α3 helices of LARP7 and p65 RRMs are partially unstructured in the absence of RNA but become extended upon binding their sequence-specific ligands, and mutations that prevent this decrease their high affinity RNA binding (reviewed in [72]). Differences in the amino acid composition of α3 in p65 and LARP7 appear to contribute to their RNA-binding sequence specificity [72]. Thus, differential α3 features contribute to high affinity and recognition-specificity for their different cognate RNAs, whereas Pof8 and La protein appear to be towards the other end of the α3 helix length spectrum [72]. Consistent with previous perspectives [33], the RRM2α domains exhibit distinctive and interesting features in their various host proteins.
Some atypical features of La RRM2α may be discerned by sequence alignment and structure prediction. Sequence conservation of RNP3 on β2 of La RRM2α extends from human to flies (R.M., unpublished, see [72,74]). Sequence evidence of α3 in the La RRM2α of Giardia, Dictyostelium and other deep rooted eukaryotes was reported [8,33]. Evidence of an RNP3 motif is also found in La proteins of some Giardia and Dictyostelium species (Fig. 1).
Features of RRM2α of La and LARP7 were recently examined, including the nature of the β strand-α3 interactions [72]. Notably, the La RRM2α, which bears a relatively short α3 (in absence of RNA) and lacks a β3-arginine that may otherwise contribute to RNA binding, is consistent with its quite low affinity for RNA and reported broad substrate recognition ([72] and refs therein). Although La requires its RRM2α for binding to hepatitis C virus domain IV RNA, no sequence-specificity could be found [81]. Also, despite its evolutionary conservation and apparent correlation of the presence of an RRM2α in La protein and the essentiality of that La protein for viability in the species examined (see [8]) no sequence-specific RNA ligand of any La RRM2α has been identified.
Additional evidence that the RRM1 of the prototypic La-module and the RRM2α share evolutionary roots is the very unusual asymmetric function of their β-sheets. The β2 strand is the major component of the β-sheet for RNA binding by RRM1 [6,64] and by the RRM2α/xRRM [73,80]. Neither RRM1 of prototypic La and LARP7 nor the RRM2α /xRRM of LARP7 and p65 interact with their RNA ligands through their β-sheet. Instead, they principally use the β2 strand to interact with RNA [6,71,73]. In RRM2α, the α3 helix lies across much of the β-sheet surface, forcing RNA interactions towards the edge defined by the β2 strand and its RNP3 amino acids [80].
The idea that the two RRMs could have exchanged during early LARP evolution is consistent with the observation that RRMs L3-L5 are deficient in RNP1 and RNP2 conserved residues [1]. The LARP4 La-module RRM lacks RNP1 and RNP2 conserved residues and binds RNA with low affinity [21]. Although LARP6 La-module RRM contains RNP1 and RNP2 residues, they appear blocked from RNA binding by contacts with a nearby α-helical element, somewhat reminiscent of RNP1 and RNP2 position interactions with α3 in xRRM [72].
While La and LARP7 are highly related and appear to have evolved in parallel, they provide different types of function. La interacts transiently with its RNA ligands and is not part of a stable RNP, whereas LARP7 homologs bind stably to RNAs and other proteins as part of stable RNPs. LARPs 1 and 4 appear much more divergent, yet exhibit similar type activity, each via stable binding to PABP involved in mRNA 3ʹ poly(A) related functions. We review PABP next, the central factor and integrator of mRNA translation, deadenylation and decay activities.
PABP helps form and stabilize translation complexes
PABP is a multifaceted factor that promotes efficient translation by facilitating multiple steps in the process [82],[83],[84],([85],and refs therein). It contributes to stable assembly of the pre-initiation complex comprised of cap-dependent translation initiation factors. It is also centrally involved with various factors in the stabilization and decay of mRNAs as well as translation termination [86]. In the latter case, interaction with translation termination factor, eRF3, was linked to mRNA stability through PABP [87].
PABP binds poly(A) with its four RRMs in an ordered manner; multiple PABP molecules oligomerize on poly(A) in a length-dependent manner with a footprint of ~27 nucleotides [88–90]. PABP-PABP interactions stabilize its binding to poly(A) [88]. Following the four RRM domains, PABP contains a mademoiselle (MLLE) domain (aka PABC) to which various PAM2-containing proteins can dock [91] (Figs. 1 & 2).
Figure 2.
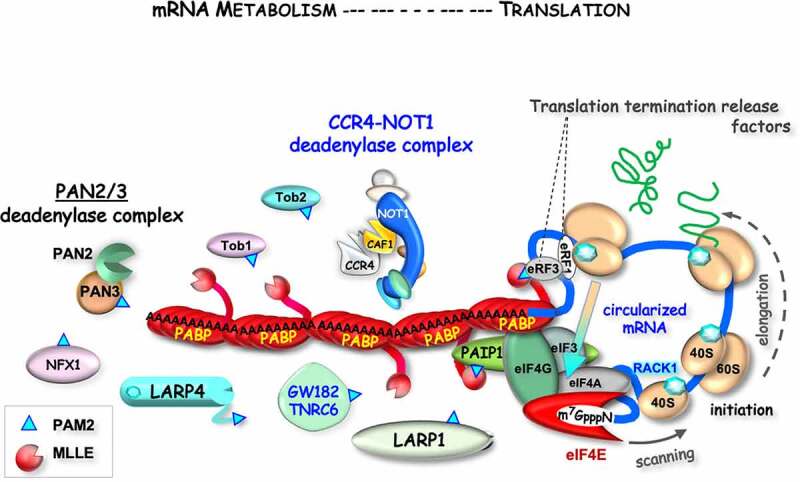
Schematized cartoon model of closed-loop mRNP-poly(A)-oligomerized-PABP. The 3ʹ end metabolism and translation activities of a closed-loop mRNP with a poly(A) tail of ~150 nucleotides bound by five PABP molecules is depicted. The 40S and 60S ribosome subunits and other factors are not to scale. Several of the known factors with a PAM2 motif, the latter depicted as small blue triangle, are shown. The MLLE domain tethered to RRM4 of PABP which binds to the PAM2 peptides is shown, as well as factors associated with the circularized mRNA according to the closed-loop model of high efficiency translation [87,91,107,108,115,190,192]. The figure depicts a fixed poly(A) length although as discussed, as deadenylation occurs a limiting number of MLLE-PABP binding sites may focus competing PAM2 proteins, for example for mRNA pruning [111]. Note that PAM2 proteins known to reside proximal to the translating mRNA, e.g., eRF3 tend to exhibit relative high affinity for MLLE (Table 1), whereas PAN2/3 and Tob2, known to work more distally have lower affinity
An example of an intricate PABP pre-initiation complex network follows. Eukaryotic initiation factor eIF4G interacts via the backside of the β-sheet RNA-binding surface of PABP RRM2 [92,93] while the β-sheet is bound to the mRNA poly(A) tail. In the pre-initiation complex, eIF4G is bound to eIF4E, the 5ʹ m7Gppp-cap binding protein (reviewed in [94]) a central regulatory point factor in health and disease [95–97]. Thus, PABP bridges the 5ʹ-end and the 3ʹ-poly(A) of an mRNA. Note that because this PABP-eIF4G interaction does not involve the MLLE, the latter is free to interact with PAM2-proteins. Other proteins increase the potential for interconnecting contacts in ‘closed-loop’ mRNA-ribonucleoproteins (mRNPs) which are recognized as efficiently translated (Fig. 2, right side). Moreover, the multiple PABPs on the poly(A) tail each with a MLLE domain is a potential PAM2–protein interaction site (Fig. 2, left).
PABP may integrate PAM2-associated activities via its MLLE domain
While PABP interacts with different proteins via its RRMs, it binds a larger number via its highly conserved MLLE which has a binding surface for a peptide motif known as PAM2 (Table 1)(see [86]), [91]. Alignment of >150 sequences representing multiple proteins revealed 12 amino acids comprising a PAM2 consensus [20,98]. Of the ~20 distinct PAM2-proteins, several are involved in mRNA processing and/or translation, including the deadenylase complex PAN2/PAN3 and TOB1/2 proteins that regulate the CCR4-NOT deadenylase complex (Table 1) [87,99]. Notably, PABP, LARPs 1, 4, 4B [12,100–102] and others also localize to stress granules (SGs) or processing bodies (PBs) (Table 1), in which mRNPs are isolated in a dormant state [103]. PBs contain deadenylated mRNPs, the translation repression and deadenylation protein 4E-T, PAN2/PAN3, CCR4-NOT-Tob1/2, GW182/TNRC6, and notably lack PABP [103–105].
Most PAM2 peptides interact with PABP in a similar way in which the conserved hydrophobic residues at positions 3 and 10 of their PAM2 sequence bind in two hydrophobic pockets separated on the surface of the MLLE [91]. However, the PAM2 or related peptides of some proteins, e.g., eRF3-C (the second of overlapping PAM2s in eRF3, see footnote 2 in Table 1) and GW182 bind MLLE in atypical ways [99,106]. As is reflected in the PAM2 sequence alignment in Table 1, the GW182 binding peptide is homologous only to the C-terminal part of the PAM2 consensus, and structures have shown that it interacts asymmetrically with MLLE relative to other PAM2 peptides. It engages only one of the hydrophobic pockets, leaving the other vacant, and uses its additional downstream residues that have no counterpart in other PAM2-MLLE interactions to bind an extended surface [106].
Binding affinities for PAM2 peptides and the MLLE span a 200-fold range in Kd, from 0.2 to 40 μM [91] (Table 1). Elegant studies provided evidence of competition among three PAM2 proteins in vivo with functional outcomes. This led to a model of competition for PABP between translation and mRNA deadenylation factors involving the translation termination release factor eRF3, CCR4-NOT and Pan2/Pan3 in human cells [87]. eRF3 contains overlapping PAM2 sequences that contribute to high affinity MLLE binding [99,107,108]. Further studies of eRF3 led to a model of stepwise assembly of a translation termination complex that is directed in part by PABP [109]. It should be expected that other PAM2 proteins also compete for the MLLE domain of PABP (also see [99]).
As noted above, PABP oligomerization on poly(A) can provide multiple docking sites for PAM2 proteins. This suggests potential for poly(A) length-dependent concentration of PABP-interacting proteins around mRNPs (Fig. 2), for example, in cell peripheral regions [110]. Such length-dependence might be relevant to an mRNP pruning model in which short poly(A) tails might limit less competitive PABP-interacting activities and concentrate others for efficient translation and/or protection of translational functions [111]. LARP4 contains a variant designated PAM2w that binds MLLE similarly to most PAM2 proteins but as will be reviewed below also contributes to poly(A) binding [12]. The PAM2 sequence of LARP1 is unique in multiple features and binds with relative high affinity (Table 1) [22]. The PAM2s of LARPs 1 and 1B differ at one position which is highly conserved in each of their lineages [22]. Curiously, the translation repressive proteins PAIPs 2 and 2B also differ in aspartic acid versus asparagine at position-6 (Table 1). The PAM2w sequences of LARPs 4 and 4B differ by 50% which are conserved in their lineages [2].
PAM2w in the LARP4 N-terminal region participates in and directs binding to poly(A) or PABP
LARP4 and LARP4B have a unique variant PAM2 sequence near their N-termini [37] known as PAM2w [12] which contains tryptophan in position-10 [12,100], where all other PAM2 sequences contain phenylalanine [98,112]. Phenylalanine-10 is considered the most important of the PAM2 amino acids for MLLE interaction based on binding affinities of systematic point mutated peptides (position-3 was the second most important), and because of severe binding deficiencies upon mutation of position-10 in various proteins [113] (reviewed in [91]). Nonetheless, the LARP4 and 4B PAM2w peptides were found to interact with the same residues on MLLE as other PAM2s, including tryptophan at position-10 [12,100]. PAM2s also reside near the N-termini of LARPs 6B and 6 C of plants104, [26] similar to LARP4 but contain phenylalanine at position-10 [12,100].
The principal determinant of poly(A) binding by LARP4 is the NTR (amino acids 1–111), of which PAM2w occupies positions 13–24, while the La-module contributes a minor role and this is mostly limited to interactions with the RRM [21]. Multiple analytical approaches indicate that the NTR contains disordered regions and transient helical and β-strand structural elements that were disrupted by PAM2w mutations, suggesting that such structure in the NTR is a required for poly(A) binding [21]. The NTR exists in an equilibrium state that supports rapid sampling of closed-open conformations with regard to interacting with the nearby La-module [21]. Most relevant is that maximal poly(A) binding to the NTR was dependent on W22 in position-10 of the PAM2w. A W22F substitution reduced poly(A) binding affinity more than 20-fold but did not significantly reduce binding to MLLE [21]. Thus, the LARP4-PAM2w can direct NTR binding to either the MLLE or poly(A) suggesting intricacy of LARP4, poly(A) and PABP dynamics [21]. That PAM2w uses Trp to engage MLLE similar to Phe in other PAM2s but in the context of LARP4 NTR which can uniquely bind poly(A) and with 10-fold higher affinity than for MLLE [21], suggests that this PAM2w is a determinant specialized for mRNA-poly(A)-PABP related function.
A unique PAM2 emerged in LARP1
PABP binding to LARP1 was attributed to a PAM2-like sequence that was identified by visual scrutiny [14]. However, at only eleven residues, lacking the invariant alanine at position-7 [91], and requiring a gap to align the critical L3 and F10 positions, the proposed sequence SQL-LNCPEFVP was very unusual [14]. Nonetheless, mutagenesis of the phenylalanine that was aligned with the PAM2 consensus position-10 decreased the amount of PABP that coimmunoprecipitated with LARP1 [14]. While this is important, this mutation does not exclude that this unusual sequence might represent an atypical/asymmetrical PAM2 such as the GW182 peptide [106] (Table 1).
A recent study characterized the PAM2 of LARP1 and identified an N-terminal Phe missing in the original identification [22]. The study showed the affinity of a LARP1-derived peptide was comparable to other PAM2 peptides. Point mutations of key residues in the LARP1 PAM2 demonstrated its importance in cellular assays of mRNA 3ʹ poly(A) tail protection and stabilization by full length LARP1 [22]. It was shown that a PAM2 mutation that impaired coimmunoprecipitation of cellular PABP with the LARP1 La-module that also impaired mRNA poly(A) protection-stabilization, did not affect oligo(A) binding by the recombinant, purified La-module [22].
As discussed above, LARPs 1 and 4 independently bind poly(A) and PABP. Another shared characteristic is association with the ribosome protein mRNAs, which are abundant, efficiently translated and tightly regulated [16,33,114]. As noted previously, LARP1 is a repressor whereas LARP4 promotes mRNA translation. We next discuss features relevant to these LARPs and translation before moving to deadenylation and decay.
Highly expressed and translated mRNAs have short poly(A) tails and other key features
Although de novo recruitment of 40S ribosomes to the start codon is considered a measure of translation efficiency [94], post-termination re-initiation in a closed-loop complex may also contribute to high efficiency [115] (Fig. 2). The closed-loop model involves the critical factors, m7Gppp-cap-eIF4E-eIF4G-PABP-poly(A) [116] although analysis in yeast emphasizes these are not exclusive to closed-loops [85]. LARPs 1 and 4 bind the principal factor PABP and involve interactions at both ends (including via the receptor for activated C kinase 1, RACK1 [12,19] below).
Poly(A) tail length is correlated with translation efficiency in early embryos, but not thereafter [117]. The poly(A) tails of the abundant ribosomal protein mRNAs are relatively short at steady state. The short poly(A) tails of such mRNPs are referred to as being maintained in a pruned state as pruning activities may be protective of the efficient translation and stability of these mRNPs [111]. Notable features of highly expressed, translated and closed-loop mRNPs include short ORFs and short poly(A) tails of 30–70 nucleotides [111,115,117]. mRNAs in this class often have short 5ʹUTRs which facilitate efficient de novo initiation [16]. Thus, a combination of features distinguish the ribosomal protein mRNAs: stability, short poly(A), high translation efficiency, favourable codon use, and also short 3ʹUTRs, the vast majority of which (human) are ≤100 nucleotides [118] (see [119] for UTR lengths), likely significant as 3ʹUTR length is associated with destabilizing elements that recruit deadenylases [120–122].
It is envisaged that mRNAs recruit abundant PABP in a poly(A) length-dependent manner (Figs. 2 & 3). As reviewed above, evidence exists of functional competition for PABP by PAM2 proteins [87,108] (see [123]). These observations prompt a model in which deadenylation-mediated pruning may be protective of translational function [111] as poly(A) shortening would tend to focus PAM2-associated activities to those with high affinity for PABP at the closed-loop, excluding those with low affinity (Table 1).
Figure 3.
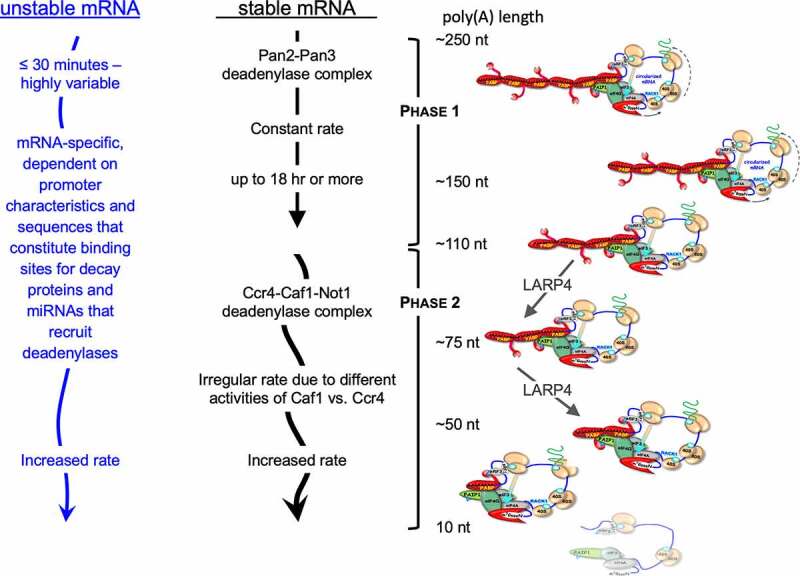
Schematic of model deadenylation pathways in mammalian cells. Model pathways for unstable mRNAs (left) and stable mRNAs (middle) derived from studies of reporter constructs and certain cellular mRNAs (see text). Classic stable mRNAs which have a paucity of destabilizing elements, follow a default pathway with biphasic deadenylation kinetics. The PAN2/3 initiates and carries out the first phase on nascent poly(A) tails of ~250 nucleotides with regular or constant rate depicted by straight downward arrow. After trimming to poly(A) lengths of 150–110 residues the mRNAs become preferred substrates of the CCR4-CAF1-NOT1 deadenylase complex CCR4-CNOT [.125,129] whose rate is irregular as depicted by the curved arrow. Unstable mRNAs, e.g., with AREs and/or miRNA binding sites in their 3ʹ UTR actively recruit deadenylases. Many mRNAs bear sequence elements or are engaged by other means that lead to active recruitment of the PAN2/3 and/or CCR4-NOT deadenylase complexes (see [134] and refs therein). The CCR4 and CAF1 deadenylase subunits of the CCR4-CNOT complex exhibit different types of deadenylase activity; CCR4 is active on poly(A) that is bound by PABP whereas CAF1 is active only on unbound substrate. Such differences as well as loss of cooperative interactions between PABP monomers and progressive instability on poly(A) contribute to the associated irregular and accelerating rates of deadenylation [88,129,143]. mRNA decay and translation rates are also coupled via codon content relative to tRNA activity [143]. Right: Schematized mRNP-PABP complexes with different poly(A) lengths. Diagonal green arrows depict poly(A) lengths representing those found by SM-PAT-seq profiling to be altered in cells in which LAReP4 was present or absent [149]. Two effects were observed; poly(A) length phasing of ribosomal protein-mRNAs suggested apparent impediment of conversion of mRNPs with presumed four PABPs to three PABPs in the presence of LARP4. Time-course profiling revealed apparent impediment of conversion of mRNPs with three PABPs to two PABPs in the presence of LARP4[149]
Model pathways of poly(A) deadenylation and mRNA decay
In their excellent review, Chen and Shyu note that poly(A) tails of ~250 nucleotides in mammalian cells (or 70–90 in yeast) are shortened by a ‘default’ pathway as soon as mRNAs enter the cytoplasm [124]. mRNAs with short UTRs contain few if any elements that recruit deadenylases and therefore maintain high stability [124].
Deadenylation is the initiating and rate-limiting step in mRNA decay and is regulated. Deadenylation occurs prior to decay of the mRNA body [124]. Features of biphasic deadenylation kinetics and mRNA decay are schematized in Fig. 3 which shows that the model pathways for stable and unstable mRNAs differ in the mechanisms of recruitment and relative timing of the deadenylases used [120,125]. According to current data and models, most mRNA decay becomes associated with deadenylation only when the poly(A) tails are short [126–128].
In classic work, Yamashita et al. showed a biphasic deadenylation pattern for stable β-globin mRNA [125]. Deadenylation in phase one is slow and synchronous, i.e., with fairly constant rate, but without mRNA decay [125] (see [124]). Using a reporter mRNA system, the PAN2-PAN3 deadenylase complex was attributed with the first phase of deadenylation, which is the trimming of long poly(A) tails down to ~150 nucleotides [125]. Deadenylation of stable mRNA in the second phase is processive with irregular rates (asynchronous), mediated by the CCR4 and CAF1 deadenylases of the CCR4-NOT complex and is associated with decay of the mRNA body [125] (see [127,129]). In this model pathway, the PAN2-PAN3 and CCR4-NOT deadenylase complexes are presumed to operate in default mode, i.e., are not actively recruited to the stable mRNA by ancillary factors (below).
For unstable mRNA, the first phase of deadenylation is rapid and decay is faster than for stable mRNA [125,130–132] (see [124]). Unstable mRNAs with sequence elements that bind proteins (e.g., TTP [133]) or engage micro-RNAs actively recruit CCR4-NOT deadenylase which leads to asynchronous deadenylation early in their lifetime. The deadenylation kinetics for many/most mRNAs are complex as they bear sequences or use other mechanisms that recruit the PAN2-PAN3 and/or CCR4-NOT deadenylases (see [134] and refs therein) and do not fit the classic model of stable or unstable reporter, the half-lives of which are typically ≥8 h and ≤75 min, respectively, [124,135,136].
The CCR4-NOT complex carries two types of deadenylase activities (described below) as well as several stable subunits [137], and can interact with the PAM2 proteins TOB1 and TOB2, enabling it with regulatory potential. Indeed, phosphorylation-dependent control of the PAM2-mediated PABP-binding activity of TOB2 has been shown to direct mRNA deadenylation by CCR4-NOT1 [138].
In summary, analyses that employed reporters driven by pulsed or Tet-off transcription as well as certain endogenous transcripts, followed by assays including gel mobility to monitor poly(A) length and decay have led to a broad view of deadenylation kinetics and decay for stable and unstable mRNAs [131,134-136].
Poly(A)-PABP faces up to deadenylases
Studies show that PABP is intricately involved in deadenylation. Workers reconstituted poly(A) mRNPs with PABP and deadenylases using human or yeast proteins [129,143]. The yeast studies included high resolution structural analysis that revealed the importance of the RRM4 of PABP and its C-terminal extension in cooperative oligomerization of PABP–PABP interactions while bound to poly(A) [88]. Unique bending of the poly(A)-PABP-oligomer creates a docking site for Pan2/3 with the poly(A) 3ʹ end threaded into its active site [88]. The yeast Pan2/3 deadenylates long poly(A)-PABP oligomers but becomes progressively less active as poly(A) is shortened and the number of bound PABPs is reduced, at which point the Caf1 and Ccr4 deadenylases can continue deadenylation [88]. Using human proteins, Yi et al. showed that CAF1 and CCR4 have differential activities, dependent on PABP-binding. The CAF1 subunit of the CCR4-NOT complex can only deadenylate naked poly(A) or after PABP dissociates. The CCR4 subunit can also do so and additionally appears capable of actively dislodging PABP from poly(A). Similar conclusions came from using yeast Ccr4 and Caf1 enzymes and PABP [143]. These experiments were done in the absence of PAM2 proteins.
One type of active role in deadenylation reflects that PABP can recruit deadenylases. PABP directly recruits CCR4 activity to the poly(A) 3ʹ end [129,143]. PABP can also recruit the CCR4-NOT1 deadenylase complex via the PAM2 proteins TOB1/2 [138,144–147], and the PAN2 deadenylase via its PAM2 protein partner PAN3 [87,134,145,148].
Webster et al. mapped PABP binding at the 5ʹ proximal region of poly(A), where its RRM4 meets the 3ʹ UTR sequence of the mRNA [143]. RRM4 could slide onto the UTR sequence with overlap binding on poly(A). The stability of this proximal mRNA-poly(A)-PABP was higher than PABP bound to poly(A) [143]. A consequent model predicts that the most proximal PABP-PABP dimers remaining after the poly(A) tail shortens to ~40-50 nucleotides might vary in dissociation kinetics in a sequence-specific, i.e., gene-specific manner (Fig. 3).
PABP stability is also dynamic at the poly(A) 3ʹ end. Its dissociation would appear to proceed in quantum steps that may correspond to dissociation of single RRM domains. The PABP molecule at the poly(A) 3ʹ end exhibits transitional dissociation states; the most 3ʹ distal of its RNA-binding domains, RRM1 can be dislodged or peeled off by CCR4 as if the poly(A) 3ʹ end can be digested away while under the RRM [143]. This mode of PABP dissociation from the poly(A) 3ʹ end is presumably the way deadenylation occurs when poly(A) is short.
An active role for PABP in poly(A) metabolism would also include recruitment of factors that oppose deadenylation, for example, such as LARP4 [114,149] and presumably LARP1 [22,114]. Altered phasing of mRNA poly(A) tails in LARP4 knock-out cells suggest that transitional states of 3ʹ-bound PABPs are sensitive to LARP4 stabilization [149] (Fig. 3, right). One can imagine that as different faces of the RRMs of PABP are revealed in their intermediate bound states [143], some may represent distinctive recruitment sites.
Critical transitions in poly(A) length may sensitize distinct PABP-binding states
Transcriptome-wide analyses point to PABP as contributing to the irregularity of deadenylation kinetics as mRNA poly(A) tails shorten to ≤150 nucleotides [129] (reviewed in [150]). This is reflected as ‘phasing’ in poly(A) sequencing profiles in which poly(A) tail length is plotted on the X-axis versus the fraction of total reads containing that length on the Y-axis. A simple phasing pattern appears as two-three peaks each reflecting a different number of PABP molecules associated with the peak poly(A) length [111]. A source of irregularity is a deadenylation rate difference related to how the CCR4 and CAF1 deadenylases respond to PABP.
As described, phased poly(A) length profiles are observed for a set of transcripts such as ribosomal protein mRNAs [111,149,150] (see [129]). Phasing reflects an in vivo state and is consistent with biochemical studies in which deadenylation rates accelerate as the number of bound PABPs decrease from three to one [129] (see [143]). This may result from the loss of intermolecular PABP-PABP contacts and RRM dissociation dynamics [88,143]. Accordingly, phasing was more prominent (and complex) after cellular CCR4 levels were decreased [129]. Gene-specific effects were also noted [129], suggesting 3ʹUTR sequence-specificity or involvement of transacting factors.
While phased poly(A) of relatively short length appear for ribosomal protein mRNAs, total mRNA poly(A) often resolves as one major peak (reviewed in [150]). In a study of mouse and human cells, poly(A) of ~75 nucleotides corresponding to three bound PABP proteins was most abundant [149]. As ~75 nucleotides poly(A) length appears to represent a majority in higher eukaryotes [150], it may reflect a dynamic state that is sensitive to regulation. For mRNPs in this state, loss of the 3ʹ PABP monomer might be expected to sensitize the remaining PABP-PABP dimer to dissociation and accelerated mRNA decay as reviewed above. Alternatively, such pruning may produce more efficiently translated ribosomal protein mRNPs [111,150]. Such fates may be programmed by 3ʹ UTRs.
LARP4 can alter the poly(A) phasing patterns of mRNAs
Transcriptome analysis by single molecule poly(A) tail-seq (SM-PAT-seq) confirmed effects of LARP4 on ribosomal protein mRNAs [149]. It revealed LARP4 as a general factor involved in mRNA poly(A) metabolism and led to insight into how its poly(A) 3ʹ length protection and mRNA stabilization activities are linked [149]. LARP4 slows deadenylation across a wide spectrum of poly(A) lengths [149]. Its prominent effects were resolved by comparing the poly(A) phasing of ribosomal protein mRNAs from LARP4 KO and wild-type cells, and their deadenylation kinetics during mRNA decay after transcription inhibition [149]. With regard to the latter, deadenylation of the peak mRNA poly(A) of ~75 nucleotides was observed to begin earlier in cells lacking LARP4 than in wild-type cells. The cumulative data suggested a model in which LARP4 is recruited to the poly(A) 3ʹ-PABP complex or somehow slowed deadenylation while, in its absence the poly(A) 3ʹ-PABP complex appeared to be more sensitive to dissociation by the CCR4-NOT1 deadenylases [88,129,143].
Although mechanisms remain unknown, the mutually exclusive poly(A) binding and PABP-binding by the LARP4 NTR seem likely involved[21]. The NTR binds poly(A) dependent on key residues in the PAM2w [21]. A proposed model is that LARP4 may stabilize PABP on short poly(A) in part by use of its poly(A) binding, and the resulting complex protects the RNA from deadenylases. We note again that the affinity of the LARP4 NTR is ~ 10-fold higher for poly(A) than the PAM2w affinity for the MLLE [21]. In any case, the mechanism is likely intricate because LARP4 also has another motif for PABP binding, the PBM [12] which remains relatively less well defined.
Northern blot mRNA mobility that monitors poly(A) length of reporters as well as endogenous mRNAs showed that LARP4 slows or generally decreases deadenylation and leads to mRNAs with longer poly(A) which are also more stable [114,149]. Time-resolved SM-PAT-seq analysis revealed that this activity should accurately be referred to as poly(A) 3ʹ length protection [22,114,149]. As described next, the mRNA mobility assays used to characterize poly(A) 3ʹ protection mRNA stabilization for LARP4 also show similar activity for LARP1 [22,114].
Similar mRNA poly(A) tail protection and stabilization activities for LARPs 1 and 4
The mRNA mobility assays for mRNA poly(A) length protection activity were positive for LARPs 1, 4 and 4B and negative for LARPs, 6, 7 and La protein [114]. Further analysis localized the poly(A) protection and mRNA stabilization activity of LARP1 to a 304 amino acid La-module fragment that was shown to bind PABP by co-IP [22]. A 230 amino acid La-module fragment with different terminal extensions had been shown to bind poly(A) as a recombinant protein [18]. These two LARP1 La-module fragments exhibited different qualitative and quantitative degrees of poly(A) length protection and mRNA stabilization activities in HEK293 cells [22]. Importantly, theirs and LARP1 activities were decreased upon point mutations to the PAM222. In these assays, LARP1 exhibits activity on stable GFP mRNA and on β-globin-ARE unstable reporter mRNA, using LARP4 as a positive control [22]. Their dependency on PABP suggest that LARPs 1 and 4 harbour similar types of poly(A) length protection that confers mRNA stabilization.
Because the poly(A) length protection-mRNA stabilization occurred on standard reporter mRNAs, these activities were apparently uncoupled from the 5ʹTOP translation effects of LARP1 [114], and may be applicable to LARP1 activities that involve different sets of associated mRNAs [16,139–142,151–158]. Also however, the isolated La-module of LARP1 which lacks the 5ʹTOP-binding DM15 domain, was shown to exhibit PABP-dependent poly(A) length protection of endogenous TOP ribosomal protein-mRNAs as an ectopic protein in trans [22].
Converting 3' end protection activity to function: LARP4 contributes to immunity
SM-PAT-seq analysis included sets of mRNAs from interferon stimulated genes (ISGs), some of which have short half-lives [149]. ISG mRNAs accumulated to higher levels in response to LARP4 expression, dependent on its PABP interaction motifs [149].
Expression of LARP4 appears to be linked to a signalling branch in an innate immune pathway that involves the ARE in the 3ʹUTR of LARP4 mRNA that is recognized by TTP and negatively regulated by the proinflammatory cytokine, tissue necrosis factor-alpha (TNFα) [159]. TTP negatively regulates TNFα by binding the ARE in TNFα mRNA 3ʹUTR, recruits the CCR4-NOT1 deadenylase and mediates decay [160,161]. Exposure to TNFα led to a quick decline in the short-lived LARP4 [159]. In this pathway, a decrease in LARP4 would negate its stabilization of mRNA [114,149] and hypothetically contribute to tuning a balance of proinflammatory responses.
Investigations of another aspect of the immune system, involving a role for differential mRNA stability during activation of CD4+ T cells led to discovery of intron-retention (IR) as a mechanism involved [162]. IR is a prominent form of alternative splicing, and can have pivotal outcomes on fundamental processes [163]. With the goal to screen for factors involved in mRNA stability-mediated activation of CD4+ T cells, LARP4 emerged as the most influential RNA-binding protein [162]. This was substantiated by multiple approaches in human cells and confirmed using spleen-derived T cells isolated from LARP4 KO and WT mice [162].
SARS-CoV-2 may hijack or block LARP activities for mRNA 3ʹ poly(A) metabolism
Of the seven human LARPs, 1, 4, 4B, and 7 were found to bind SARS-CoV-2 proteins and/or RNAs [164,165] or by a different system [166]. As an RNA genome at the upper length limit, coronaviridae differ from smaller RNA viruses as all of their mRNAs are polyadenylated [167] and required for replication [168]. Gordon et al. found 332 HEK293T cell proteins stably bind the 26 proteins of SARS-CoV-2 [165]. LARP4B binds the virus transcription and replication factor, RNA-dependent RNA polymerase (RdRp, Nsp12). LARP7 binds Nsp8, a RdRp complex subunit [169,170]. LARP1, PABP and PABPC4 (very similar to PABP) are 3 of 15 host proteins that interact with nucleocapsid N-protein which binds viral RNA and also modulates the host immune response [165,171,172]. In the most severe cases of COVID-19 disease, an unbridled proinflammatory response creates a cytokine storm led by TNFα [173]. Most of the other N-interacting host proteins are involved in mRNA metabolism and the modulation of SGs [165], common among RNA viruses [103] and in innate immune control [174]. Also notable is that while PABP is targeted by viral proteases of several viruses(see refs in [175]), coronaviruses preserve intact 70 kDa PABP on their mRNA poly(A) [168].
Schmidt et al. isolated 104 human proteins that UV-crosslink to the SARS-CoV-2 RNA in infected cells [164]. CNBP, required for expression of certain pro-inflammatory cytokines, and LARP4 are the two host proteins most significantly enriched with the viral RNA, followed by others including PABP and LARP1 [164]. The importance of distinguishing between protein and RNA interactions was emphasized by the authors noting that only 10 of the 332 human proteins bound to SARS-CoV-2 proteins [165] also bound directly to the SARS-CoV-2 RNA [164]. In another approach, proximity labelling by Nsp2-biotinylase was used to identify LARPs 1, 4 and 7 among 513 host proteins involved in early viral RNA metabolism [166] which occurs in replicase and transcription complexes (RTCs) [176].
LARPs 7, 4B and 1 were among 40 host proteins that were differentially phosphorylated over multiple time points after SARS-CoV-2 infection [177]. Phosphorylation at multiple sites changed on these LARPs although most complexly for LARP1 [177]. CRISPR/Cas knock out of LARP1 led to an increase in SARS-CoV-2 replication, and its over-expression led to a decrease in viral infection [164]. These results would indicate LARP1 as an anti-coronavirus factor in HEK293T cells, consistent with its UV-crosslinking to the 5ʹ-cap pyrimidine-rich leader of the SARS-CoV-2 RNA [164] and its function as a repressor of mRNA translation [15,18]. Nonetheless, the complexity of its phosphorylation kinetics [177] may reflect effects on LARP1 activities during infection. Since LARP4 and 4B stabilize poly(A) mRNAs and promote translation, targeting of these factors along with PABP by the viral proteins and/or RNA may also have functional effects [13,16,19,22,114,149,159,178].
LARP1 function includes mRNA stabilization and likely in pathways other than for TOPs
Multiple aspects of LARP1-mediated mRNA stabilization remain unclear although it likely involves the La-module which can bind poly(A) with 40 nM Kd affinity [18] and itself confer mRNA poly(A) length protection and stabilization [22]. TOP mRNAs are the best known class of transcripts that are controlled by LARP1, the key factor that represses their translation in the absence of mTORC1 signalling [14–17,155,179,180]. In poor nutrient conditions, the DM15 domain of LARP1 binds the 5ʹTOP motif excluding eIF4E binding and averting mRNP assembly from entering into a pre-initiation complex [15]. When nutrients improve mTORC1 leads to release of the 5ʹTOP by the DM15 domain, eIF4E-5ʹcap binding and translation initiation [16]. Although ectopic expression of LARP1 stabilizes and knock-down leads to lower levels of TOP mRNAs including in nutrient-replete cells [13,14] the degree to which these mRNAs are stabilized/protected from decay during LARP1-mediated translational repression has not been thoroughly investigated..
Some data show that after starvation-mediated translational repression, TOP mRNA levels accumulate as their poly(A) tails lengthen over a course of ~12 h, dependent on LARP1. Upon nutritional repletion, the TOP mRNAs that had accumulated with long poly(A) tails were loaded on polysomes and the poly(A) quickly shortened thereafter [181]. How this TOP mRNA poly(A) lengthening is related to other LARP1-associated mRNA stabilization processes remains to be determined.
A screen for kinases and phosphatases based on the formation of SGs revealed that the protein kinase CDK1 robustly promotes TOP mRNA translation in a LARP1-dependent manner [139]. That study demonstrated CDK1-dependent phosphorylation of LARP1 (although not as a direct target), and that CDK1 can regulate TOP translation independently of mTOR. Moreover, inhibition of CDK1 and of TOP mRNA translation occurred while LARP1 maintained an association with TOP mRNAs [139].
The LARP1 interactome of regulatable TOP mRNAs is distinctly larger than initially thought [155]. Beyond this, LARP1 binds hundreds to thousands of mRNAs involved in various pathways including related to cancer [140,141,182,183]. Some of the mRNA sets exhibit sequence specificity and other features although these have not been as well defined nor characterized as the 5' TOP motif [101,140,154,157,184–186].
A link from LARP1 to mitochondrial DNA replication during oogenesis in Drosophila has been observed to exist. LARP1 was reported to be required for translation of mRNAs localized to the mitochondrial outer surface whose protein products function in mitochondrial DNA replication [186]. Phosphorylation of drLARP1 by PINK kinase inhibits an activity that promotes the translation of outer mitochondrial-located mRNAs [154]. Human LARP1 was found to be important for inner-mitochondrial translation required for oxidative phosphorylation (OXPHOS), groundbreaking and expanding the significance of the Drosophila link [153]. This functional link to energy production deepens the LARP1 connection to other aspects of mTORC1 metabolic control, and other factors [187].
LARP1 is conserved [2] and has been studied in a variety of organisms (reviewed in [33,142]). Although the yeast LARP1 homologs, Sro9p, Slf1p and spLARP [188] do not exhibit a DM15 domain [142], nor do yeast ribosomal protein mRNAs contain a 5ʹTOP motif, they are efficiently translated [85]. Slf1 is associated with an oxidative stress response and with cytoplasmic mRNAs that encode mitochondrial proteins [189]. Terminating ribosomes are proposed to efficiently reinitiate on closed-loop mRNPs critically involving the 40S ribosome-associated factor RACK1 [115,190]. That Sro9 and the RACK1 homolog Asc1 interact [191] is interesting because Asc1 can promote efficient translation of short ORF ribosomal protein mRNAs [115,192]. Sro9 and Slf1 associate with overlapping sets of mRNAs that include ribosomal protein mRNAs and although Slf1p is the less abundant it led to greater accumulation of its target mRNAs than Sro9 consistent with distinct stabilization activity [189]. The cumulative observations suggest conservation of LARP1-like activities.
The human LARP1 La-module can simultaneously bind poly(A) and pyrimidine-rich sequences comparable to TOPs but not requiring the m7G(5ʹ)ppp-cap which by contrast does contribute to DM15 binding [15,18]. Although part of a larger multimodular protein that functions in a signalling network, the relatively compact La-module with its PAM2 can confer PABP-dependent poly(A) length protection of ribosomal protein mRNAs and stabilization of reporter mRNAs as an ectopic protein expressed in trans [22]. As suggested by the different qualitative effects on poly(A) protection of this La-module bearing different extensions on its N- and C- termini [22], it might be expected that the activity of this unique La-module would be under regulatory control in the full length protein.
Concluding remarks
La and LARP7 are highly related nuclear proteins that bind and protect target RNAs with UUU-3ʹOH ends from exonucleases. Their La-modules bind RNA in a very similar manner that involves an unusual asymmetric use of their RRM1 domains. Both have a downstream RRM subtype unique to La and a diversity of LARP7 homologs with a similar unusual mode of asymmetric RNA recognition. Phylogenetics suggest the possibility that exchange between the two RRMs may have contributed to La-module evolution.
LARP4 and LARP1 contain divergent La-modules and other interaction regions but exhibit similar mRNA poly(A) 3ʹ protection-stabilization activities, and interact with PABP via PAM2 motifs [22]. Poly(A) tail profiling data suggests that LARP4 opposes deadenylation by interacting with PABP bound to 3ʹ poly(A). LARP 4 differs from LARP1 in RNA-binding characteristics, the position of its La-module relative to the PAM2, and in relative affinities for poly(A) and MLLE binding. The multiple differences suggest that these proteins likely act in mRNA poly(A) protection by employing distinct molecular, targeting and perhaps regulatory mechanisms [11–13,18,21,22,114]. Future insights into the basic mechanisms of deadenylation and the roles of PABP [88,111,129,143] as well as comparison of similarities and differences between LARP1 and LARP4, and contributions of other PAM2 proteins should provide a more integrated view of poly(A) metabolism, mRNA translation and decay.
Acknowledgments
We thank LARP Society members, M. Bayfield (York University), A. Berman (University of Pittsburgh), M. Sasi Conte (Kings College London) as well as E. Valkov (NCI, NIH) and M. Hafner (NIAMS, NIH) for helpful discussion. This work was supported by the DIR of the NICHD, NIH.
Funding Statement
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [ZIA HD000412-31 PGD].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Bousquet-Antonelli C, Deragon JM.. A comprehensive analysis of the La-motif protein superfamily. RNA. 2009;15:750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Deragon JM. Distribution, organization an evolutionary history of La and LARPs in eukaryotes. RNA Biol. 2020;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schwenzer H, Abdel Mouti M, Neubert P, et al. LARP1 isoform expression in human cancer cell lines. RNA Biol. 2020;1744320:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rinke J, Steitz JA. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982;29:149–159. [DOI] [PubMed] [Google Scholar]
- [5].Stefano JE. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3ʹ termini of RNA polymerase III transcripts. Cell. 1984;36:145–154. [DOI] [PubMed] [Google Scholar]
- [6].Teplova M, Yuan Y-R, Ilin S, et al. Structural basis for recognition and sequestration of UUU-OH 3ʹ-termini of nascent RNA pol III transcripts by La, a rheumatic disease autoantigen. Mol Cell. 2006;21:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wolin SL, Cedervall T. The La protein. Annu Rev Biochem. 2002;71:375–403. [DOI] [PubMed] [Google Scholar]
- [8].Blewett NH, Maraia RJ. La involvement in tRNA and other RNA processing events including differences among yeast and other eukaryotes. Biochim Biophys Acta. 2018;1861:361–372. [DOI] [PubMed] [Google Scholar]
- [9].He N, Jahchan NS, Hong E, et al. A La-related protein modulates 7SK snRNP integrity to suppress P-TEFb-dependent transcriptional elongation and tumorigenesis. Mol Cell. 2008;29:588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Markert A, Grimm M, Martinez J, et al. The La-related protein LARP7 is a component of the 7SK ribonucleoprotein and affects transcription of cellular and viral polymerase II genes. EMBO Rep. 2008;9:569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Krueger BJ, Jeronimo C, Roy BB, et al. LARP7 is a stable component of the 7SK snRNP while P-TEFb, HEXIM1 and hnRNP A1 are reversibly associated. Nucleic Acids Res. 2008;36:2219–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang R, Gaidamakov SA, Xie J, et al. LARP4 binds poly(A), interacts with poly(A)-binding protein MLLE domain via a variant PAM2w motif and can promote mRNA stability. Mol Cell Biol. 2011;31:542–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aoki K, Adachi S, Homoto M, et al. LARP1 specifically recognizes the 3ʹ terminus of poly(A) mRNA. FEBS Lett. 2013;587:2173–2178. [DOI] [PubMed] [Google Scholar]
- [14].Fonseca BD, Zakaria C, Jia JJ, et al. La-related protein 1 (LARP1) represses terminal oligopyrimidine (TOP) mRNA translation downstream of mTOR complex 1 (mTORC1). J Biol Chem. 2015;290:15996–16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lahr RM, Fonseca BD, Ciotti GE, et al. La-related protein 1 (LARP1) binds the mRNA cap, blocking eIF4F assembly on TOP mRNAs. eLife. 2017;6:e24146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fonseca BD, Lahr RM, Damgaard CK, et al. LARP1 on TOP of ribosome production. Wiley Interdiscip Rev RNA. 2018;9:e1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Philippe L, Vasseur JJ, Debart F, et al. La-related protein 1 (LARP1) repression of TOP mRNA translation is mediated through its cap-binding domain and controlled by an adjacent regulatory region. Nucleic Acids Res. 2018;46:1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Al-Ashtal HA, Rubottom CM, Leeper TC, et al. The LARP1 La-module recognizes both ends of TOP mRNAs. RNA Biol. 2019;1669404:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kuspert M, Murakawa Y, Schaffler K, et al. LARP4B is an AU-rich sequence associated factor that promotes mRNA accumulation and translation. RNA. 2015;21:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kozlov G, Trempe JF, Khaleghpour K, et al. Structure and function of the C-terminal PABC domain of human poly(A)-binding protein. Proc Natl Acad Sci U S A. 2001;98:4409–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cruz-Gallardo I, Martino L, Kelly G, et al. LARP4A recognizes polyA RNA via a novel binding mechanism mediated by disordered regions and involving the PAM2w motif, revealing interplay between PABP, LARP4A and mRNA. Nucleic Acids Res. 2019;47:4272–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mattijssen S, Kozlov G, Gaidamakov S, et al. The isolated La-module of LARP1 mediates 3ʹ poly(A) protection and mRNA stabilization, dependent on its intrinsic PAM2 binding to PABPC1. RNA Biol. 2020;1–15. DOI: 10.1080/15476286.2020.1860376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Weng H, Kim C, Valavanis C, et al. Acheron, an novel LA antigen family member, binds to CASK and forms a complex with Id transcription factors. Cell Mol Biol Lett. 2009;14:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Valavanis C, Wang ZH, Sun H, et al. Acheron, a novel member of the Lupus antigen family, is induced during the programmed cell death of skeletal muscles in the moth Manduca sexta. Gene. 2007;393:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Martino L, Pennell S, Kelly G, et al. Synergic interplay of the La motif, RRM1 and the interdomain linker of LARP6 in the recognition of collagen mRNA expands the RNA binding repertoire of the La module. Nucleic Acids Res. 2015;43:645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Merret R, Martino L, Bousquet-Antonelli C, et al. The association of a La module with the PABP-interacting motif PAM2 is a recurrent evolutionary process that led to the neofunctionalization of La-related proteins. RNA. 2013;19:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Katzenellenbogen RA, Egelkrout EM, Vliet-Gregg P, et al. NFX1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16 E6-expressing cells. J Virol. 2007;81:3786–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roy G, De Crescenzo G, Khaleghpour K, et al. Paip1 interacts with poly(A) binding protein through two independent binding motifs. Mol Cell Biol. 2002;22:3769–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Muñoz-Escobar J, Matta-Camacho E, Kozlov G, et al. The MLLE domain of the ubiquitin ligase UBR5 binds to its catalytic domain to regulate substrate binding. J Biol Chem. 2015;290:22841–22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Berlanga JJ, Baass A, Sonenberg N. Regulation of poly(A) binding protein function in translation: characterization of the Paip2 homolog, Paip2B. RNA. 2006;12:1556–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jinek M, Fabian MR, Coyle SM, et al. Structural insights into the human GW182-PABC interaction in microRNA-mediated deadenylation. Nat Struct Mol Biol. 2010;17:238–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huntzinger E, Braun JE, Heimstadt S, et al. Two PABPC1-binding sites in GW182 proteins promote miRNA-mediated gene silencing. Embo J. 2010;29:4146–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Maraia RJ, Mattijssen S, Cruz-Gallardo I, et al. The LARPs, La and related RNA-binding proteins: structures, functions and evolving perspectives. WIREs RNA. 2017;e1430. DOI: 10.1002/wrna.1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Maraia RJ, Kenan DJ, Keene JD, Eukaryotic transcription termination factor La mediates transcript release and facilitates reinitiation by RNA polymerase III. Mol Cell Biol. 1994. Mar;14(3):2147–58. doi: 10.1128/mcb.14.3.2147.PMID:8114745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yoo CJ, Wolin SL. The yeast La protein is required for the 3ʹ endonucleolytic cleavage that matures tRNA precursors. Cell. 1997;89:393–402. [DOI] [PubMed] [Google Scholar]
- [36].Lin-Marq N, Clarkson SG. Efficient synthesis, termination and release of RNA polymerase III transcripts in Xenopus extracts depleted of La protein. Embo J. 1998;17:2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bayfield MA, Yang R, Maraia RJ. Conserved and divergent features of the structure and function of La and La-related proteins (LARPs). Biochim Biophys Acta. 2010;1799:365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gudipati RK, Xu Z, Lebreton A, et al. Extensive degradation of RNA precursors by the exosome in wild-type cells. Mol Cell. 2012;48:409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Anderson J, Phan L, Cuesta R, et al. The essential Gcd10-Gcd14 nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12:3650–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chakshusmathi G, Kim SD, Rubinson DA, et al. A La protein requirement for efficient pre-tRNA folding. Embo J. 2003;22:6562–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kadaba S, Krueger A, Trice T, et al. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 2004;18:1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huang Y, Bayfield MA, Intine RV, et al. Separate RNA-binding surfaces on the multifunctional La protein mediate distinguishable activities in tRNA maturation. Nat Struct Mol Biol. 2006;13:611–618. [DOI] [PubMed] [Google Scholar]
- [43].Pannone B, Xue D, Wolin SL. A role for the yeast La protein in U6 snRNP assembly: evidence that the La protein is a molecular chaperone for RNA polymerase III transcripts. Embo J. 1998;17:7442–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Simons FH, Broers FJ, Van Venrooij WJ, et al. Characterization of cis-acting signals for nuclear import and retention of the La (SS-B) autoantigen. Exp Cell Res. 1996;224:224–236. [DOI] [PubMed] [Google Scholar]
- [45].Bayfield MA, Kaiser TE, Intine RV, et al. Conservation of a masked nuclear export activity of La proteins and its effects on tRNA maturation. Mol Cell Biol. 2007;27:3303–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Intine RV, Dundr M, Misteli T, et al. Aberrant nuclear trafficking of La protein leads to disordered processing of associated precursor tRNAs. Mol Cell. 2002;9:1113–1123. [DOI] [PubMed] [Google Scholar]
- [47].Bayfield MA, Maraia RJ. Precursor-product discrimination by La protein during tRNA metabolism. Nat Struct Mol Biol. 2009;16:430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Eichhorn K, Jackson SP. A role for TAF3B2 in the repression of human RNA polymerase III transcription in nonproliferating cells. J Biol Chem. 2001;276:21158–21165. [DOI] [PubMed] [Google Scholar]
- [49].Hasler D, Meister G, Fischer U. Stabilize and connect: the role of LARP7 in nuclear non-coding RNA metabolism. RNA Biol. 2020;1767952:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Maraia RJ, Lamichhane TN. 3ʹ processing of eukaryotic precursor tRNAs. Wires Rna. 2011;2:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lund E, Dahlberg JE. Cyclic 2ʹ,3ʹ-phosphates and nontemplated nucleotides at the 3ʹ end of spliceosomal U6 small nuclear RNA’s. Science. 1992;255:327–330. [DOI] [PubMed] [Google Scholar]
- [52].Terns MP, Lund E, Dahlberg JE. 3ʹ-end-dependent formation of U6 small nuclear ribonucleoprotein particles in Xenopus laevis oocyte nuclei. Mol Cell Biol. 1992;12:3032–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xue D, Rubinson DA, Pannone BK, et al. U snRNP assembly in yeast involves the La protein. Embo J. 2000;19:1650–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Didychuk AL, Butcher SE, Brow DA. The life of U6 small nuclear RNA, from cradle to grave. RNA. 2018;24:437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bhattacharya R, Perumal K, Sinha K, et al. Methylphosphate cap structure in small RNAs reduces the affinity of RNAs to La protein. Gene Expr. 2002;10:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Egloff S, Vitali P, Tellier M, et al. The 7SK snRNP associates with the little elongation complex to promote snRNA gene expression. Embo J. 2017;36:934–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang Z, Zhu Q, Luo K, et al. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature. 2001;414:317–322. [DOI] [PubMed] [Google Scholar]
- [58].Hasler D, Meduri R, Bąk M, et al. The alazami syndrome-associated protein LARP7 guides U6 small nuclear RNA modification and contributes to splicing robustness. Mol Cell. 2020;77:1014–31.e13. [DOI] [PubMed] [Google Scholar]
- [59].Wang X, Li ZT, Yan Y, et al. LARP7-mediated U6 snRNA modification ensures splicing fidelity and spermatogenesis in mice. Mol Cell. 2020;77:999–1013.e6. [DOI] [PubMed] [Google Scholar]
- [60].Páez-Moscoso DJ, Pan L, Sigauke RF, et al. Pof8 is a La-related protein and a constitutive component of telomerase in fission yeast. Nat Commun. 2018;9:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Maris C, Dominguez C, Allain FH. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. Febs J. 2005;272:2118–2131. [DOI] [PubMed] [Google Scholar]
- [62].Corley M, Burns MC, Yeo GW. How RNA-Binding Proteins Interact with RNA: molecules and Mechanisms. Mol Cell. 2020;78:9–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Daubner GM, Cléry A, Allain FH. RRM-RNA recognition: NMR or crystallography … and new findings. Curr Opin Struct Biol. 2013;23:100–108. [DOI] [PubMed] [Google Scholar]
- [64].Kotik-Kogan O, Valentine ER, Sanfelice D, et al. Structural analysis reveals conformational plasticity in the recognition of RNA 3ʹ ends by the human La protein. Structure. 2008;16:852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Maraia RJ, Bayfield MA. The La protein-RNA complex surfaces. Mol Cell. 2006;21:149–152. [DOI] [PubMed] [Google Scholar]
- [66].Curry S, Conte MR. A terminal affair: 3ʹ-end recognition by the human La protein. Trends Biochem Sci. 2006;31:303–305. [DOI] [PubMed] [Google Scholar]
- [67].Uchikawa E, Natchiar KS, Han X, et al. Structural insight into the mechanism of stabilization of the 7SK small nuclear RNA by LARP7. Nucleic Acids Res. 2015;43:3373–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jacks A, Babon J, Kelly G, et al. Structure of the C-terminal domain of human La protein reveals a novel RNA recognition motif coupled to a helical nuclear retention element. Structure (Camb). 2003;11:833–843. [DOI] [PubMed] [Google Scholar]
- [69].Dock-Bregeon AC, Lewis KA, Conte MR. The La-related proteins: structures and interactions of a versatile superfamily of RNA-binding proteins. RNA Biol. 2019;1695712:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sommer G, Heise T. Role of the RNA-binding protein La in cancer pathobiology. RNA Biol. 2020;1792677:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Singh M, Choi CP, Feigon J. xRRM: A new class of RRM found in the telomerase La family protein p65. RNA Biol. 2013;10:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Basu R, Eichhorn CD, Cheng R, et al. Structure of S. pombe telomerase protein Pof8 C-terminal domain is an xRRM conserved among LARP7 proteins. RNA Biol. 2020;1836891:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Eichhorn CD, Yang Y, Repeta L, et al. Structural basis for recognition of human 7SK long noncoding RNA by the La-related protein Larp7. Proc Natl Acad Sci U S A. 2018;115:E6457–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Eichhorn CD, Chug R, Feigon J. hLARP7 C-terminal domain contains an xRRM that binds the 3ʹ hairpin of 7SK RNA. Nucleic Acids Res. 2016;44:9977–9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jiang J, Miracco EJ, Hong K, et al. The architecture of Tetrahymena telomerase holoenzyme. Nature. 2013;496:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mennie AK, Moser BA, Nakamura TM. LARP7-like protein Pof8 regulates telomerase assembly and poly(A)+TERRA expression in fission yeast. Nat Commun. 2018;9:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. [DOI] [PubMed] [Google Scholar]
- [78].Aigner S, Postberg J, Lipps HJ, et al. The Euplotes La motif protein p43 has properties of a telomerase-specific subunit. Biochemistry. 2003;42:5736–5747. [DOI] [PubMed] [Google Scholar]
- [79].Maraia RJ, Rijal K. Structural biology: A transcriptional specialist resolved. Nature. 2015;528:204–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Singh M, Wang Z, Koo BK, et al. Structural basis for telomerase RNA recognition and RNP assembly by the holoenzyme La family protein p65. Mol Cell. 2012;47:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Martino L, Pennell S, Kelly G, et al. Analysis of the interaction with the hepatitis C virus mRNA reveals an alternative mode of RNA recognition by the human La protein. Nucleic Acids Res. 2012;40:1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kahvejian A, Svitkin YV, Sukarieh R, et al. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 2005;19:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sachs AB, Davis RW. The poly(A) binding protein is required for poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell. 1989;58:857–867. [DOI] [PubMed] [Google Scholar]
- [84].Roy B, Jacobson A. The intimate relationships of mRNA decay and translation. Trends Genet. 2013;29:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Costello J, Castelli LM, Rowe W, et al. Global mRNA selection mechanisms for translation initiation. Genome Biol. 2015;16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Eliseeva IA, Lyabin DN, Ovchinnikov LP. Poly(A)-binding proteins: structure, domain organization, and activity regulation. Biochemistry (Mosc). 2013;78:1377–1391. [DOI] [PubMed] [Google Scholar]
- [87].Funakoshi Y, Doi Y, Hosoda N, et al. Mechanism of mRNA deadenylation: evidence for a molecular interplay between translation termination factor eRF3 and mRNA deadenylases. Genes Dev. 2007;21:3135–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Schafer IB, Yamashita M, Schuller JM, et al. Molecular Basis for poly(A) RNP Architecture and Recognition by the Pan2-Pan3 Deadenylase. Cell. 2019;177:1619–31 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Baer BW, Kornberg RD. Repeating structure of cytoplasmic poly(A)-ribonucleoprotein. Proc Natl Acad Sci U S A. 1980;77:1890–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Deo RC, Bonanno JB, Sonenberg N, et al. Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell. 1999;98:835–845. [DOI] [PubMed] [Google Scholar]
- [91].Xie J, Kozlov G, Gehring K. The “tale” of poly(A) binding protein: the MLLE domain and PAM2-containing proteins. Biochim Biophys Acta. 2014;1839:1062–1068. [DOI] [PubMed] [Google Scholar]
- [92].Safaee N, Kozlov G, Noronha AM, et al. Interdomain allostery promotes assembly of the poly(A) mRNA complex with PABP and eIF4G. Mol Cell. 2012;48:375–386. [DOI] [PubMed] [Google Scholar]
- [93].Imataka H, Gradi A, Sonenberg N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. Embo J. 1998;17:7480–7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hinnebusch AG. Structural insights into the mechanism of scanning and start codon recognition in eukaryotic translation initiation. Trends Biochem Sci. 2017;42:589–611. [DOI] [PubMed] [Google Scholar]
- [95].Topisirovic I, Svitkin YV, Sonenberg N, et al. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip Rev RNA. 2011;2:277–298. [DOI] [PubMed] [Google Scholar]
- [96].Ramon YCS, Castellvi J, Hummer S, et al. Beyond molecular tumor heterogeneity: protein synthesis takes control. Oncogene. 2018;37:2490–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Roux P, Topisirovic I. Signaling pathways involved in regulation of mRNA translation. Mol Cell Biol. 2018;38:e00070-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Albrecht M, Lengauer T. Survey on the PABC recognition motif PAM2. Biochem Biophys Res Commun. 2004;316:129–138. [DOI] [PubMed] [Google Scholar]
- [99].Kozlov G, Gehring K, Kursula P. Molecular basis of eRF3 recognition by the MLLE domain of poly(A)-binding protein. PLoS One. 2010;5:e10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Grimm C, Pelz JP, Schneider C, et al. Crystal structure of a variant PAM2 motif of LARP4B bound to the MLLE domain of PABPC1. Biomolecules. 2020;10:872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hopkins, K.C., Tartell, M.A., Herrmann, C., Hackett, B.A., Taschuk, F., Panda, D., Menghani, S.V., Sabin, L.R. and Cherry, S. (2015) Virus-induced translational arrest through 4EBP1/2-dependent decay of 5′-TOP mRNAs restricts viral infection. Proc Natl Acad Sci U S A, 112, E2920–2929 doi: 10.1073/pnas.1418805112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wilbertz JH, Voigt F, Horvathova I, et al. Single-molecule imaging of mRNA localization and regulation during the integrated stress response. Mol Cell. 2019;73:946–58.e7. [DOI] [PubMed] [Google Scholar]
- [103].Ivanov P, Kedersha N, Anderson P. Stress granules and processing bodies in translational control. Cold Spring Harb Perspect Biol. 2019;11:a032813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Aizer A, Kalo A, Kafri P, et al. Quantifying mRNA targeting to P-bodies in living human cells reveals their dual role in mRNA decay and storage. J Cell Sci. 2014;127:4443–4456. [DOI] [PubMed] [Google Scholar]
- [105].Räsch F, Weber R, Izaurralde E, et al. 4E-T-bound mRNAs are stored in a silenced and deadenylated form. Genes Dev. 2020;34:847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kozlov G, Safaee N, Rosenauer A, et al. Structural basis of binding of P-body-associated proteins GW182 and ataxin-2 by the Mlle domain of poly(A)-binding protein. J Biol Chem. 2010;285:13599–13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Uchida N, Hoshino S, Imataka H, et al. A novel role of the mammalian GSPT/eRF3 associating with poly(A)-binding protein in Cap/Poly(A)-dependent translation. J Biol Chem. 2002;277:50286–50292. [DOI] [PubMed] [Google Scholar]
- [108].Osawa M, Hosoda N, Nakanishi T, et al. Biological role of the two overlapping poly(A)-binding protein interacting motifs 2 (PAM2) of eukaryotic releasing factor eRF3 in mRNA decay. RNA. 2012;18:1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Beißel C, Neumann B, Uhse S, et al. Translation termination depends on the sequential ribosomal entry of eRF1 and eRF3. Nucleic Acids Res. 2019;47:4798–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Holt CE, Martin KC, Schuman EM. Local translation in neurons: visualization and function. Nat Struct Mol Biol. 2019;26:557–566. [DOI] [PubMed] [Google Scholar]
- [111].Lima SA, Chipman LB, Nicholson AL, et al. Short poly(A) tails are a conserved feature of highly expressed genes. Nat Struct Mol Biol. 2017;24:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kozlov G, Menade M, Rosenauer A, et al. Molecular determinants of PAM2 recognition by the MLLE domain of poly(A)-binding protein. J Mol Biol. 2010;397:397–407. [DOI] [PubMed] [Google Scholar]
- [113].Kozlov G, De Crescenzo G, Lim NS, et al. Structural basis of ligand recognition by PABC, a highly specific peptide-binding domain found in poly(A)-binding protein and a HECT ubiquitin ligase. Embo J. 2004;23:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Mattijssen S, Arimbasseri AG, Iben JR, et al. LARP4 mRNA codon-tRNA match contributes to LARP4 activity for ribosomal protein mRNA poly(A) tail length protection. Elife. 2017;6. DOI: 10.7554/eLife.28889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Thompson MK, Gilbert WV. mRNA length-sensing in eukaryotic translation: reconsidering the “closed loop” and its implications for translational control. Curr Genet. 2017;63:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Vicens Q, Kieft JS, Rissland OS. Revisiting the Closed-Loop Model and the Nature of mRNA 5ʹ-3ʹ Communication. Mol Cell. 2018;72:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Subtelny AO, Eichhorn SW, Chen GR, et al. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature. 2014;508:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Yoshihama M, Uechi T, Asakawa S, et al. The human ribosomal protein genes: sequencing and comparative analysis of 73 genes. Genome Res. 2002;12:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Majoros WH, Ohler U. Spatial preferences of microRNA targets in 3ʹ untranslated regions. BMC Genomics. 2007;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chen CA, Shyu AB. Emerging Themes in Regulation of Global mRNA Turnover in cis. Trends Biochem Sci. 2017;42:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Di Giammartino DC, Nishida K, Manley JL. Mechanisms and consequences of alternative polyadenylation. Mol Cell. 2011;43:853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Mayr C. What Are 3ʹ UTRs Doing? Cold Spring Harb Perspect Biol. 2019;11:a034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jerbi S, Jolles B, Bouceba T, et al. Studies on human eRF3-PABP interaction reveal the influence of eRF3a N-terminal glycin repeat on eRF3-PABP binding affinity and the lower affinity of eRF3a 12-GGC allele involved in cancer susceptibility. RNA Biol. 2016;13:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chen CY, Shyu AB. Mechanisms of deadenylation-dependent decay. Wiley Interdiscip Rev RNA. 2011;2:167–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Yamashita A, Chang TC, Yamashita Y, et al. Concerted action of poly(A) nucleases and decapping enzyme in mammalian mRNA turnover. Nat Struct Mol Biol. 2005;12:1054–1063. [DOI] [PubMed] [Google Scholar]
- [126].Yu S, Kim VN. A tale of non-canonical tails: gene regulation by post-transcriptional RNA tailing. Nat Rev Mol Cell Biol. 2020;21:542–556. [DOI] [PubMed] [Google Scholar]
- [127].Eisen TJ, Eichhorn SW, Subtelny AO, et al. The dynamics of cytoplasmic mRNA metabolism. Mol Cell. 2020;77:786–99 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Eisen TJ, Eichhorn SW, Subtelny AO, et al. MicroRNAs cause accelerated decay of short-tailed target mRNAs. Mol Cell. 2020;77:775–85 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Yi H, Park J, Ha M, et al. PABP cooperates with the CCR4-NOT complex to promote mRNA deadenylation and block precocious decay. Mol Cell. 2018;70:1081–8 e5. [DOI] [PubMed] [Google Scholar]
- [130].Shyu AB, Greenberg ME, Belasco JG. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989;3:60–72. [DOI] [PubMed] [Google Scholar]
- [131].Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221–231. [DOI] [PubMed] [Google Scholar]
- [132].Lai WS, Carballo E, Strum JR, et al. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19:4311–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Fabian MR, Mathonnet G, Sundermeier T, et al. Mammalian miRNA RISC recruits CAF1 and PABP to affect PABP-dependent deadenylation. Mol Cell. 2009;35:868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Chen CA, Zhang Y, Xiang Y, et al. Antagonistic actions of two human Pan3 isoforms on global mRNA turnover. RNA. 2017;23:1404–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Xu N, Loflin P, Chen CY, et al. A broader role for AU-rich element-mediated mRNA turnover revealed by a new transcriptional pulse strategy. Nucleic Acids Res. 1998;26:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Chen CY, Yamashita Y, Chang TC, et al. Versatile applications of transcriptional pulsing to study mRNA turnover in mammalian cells. RNA. 2007;13:1775–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Raisch T, Chang CT, Levdansky Y, et al. Reconstitution of recombinant human CCR4-NOT reveals molecular insights into regulated deadenylation. Nat Commun. 2019;10:3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Chen CA, Strouz K, Huang KL, et al. Tob2 phosphorylation regulates global mRNA turnover to reshape transcriptome and impact cell proliferation. RNA. 2020;26:1143–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Haneke K, Schott J, Lindner D, et al. CDK1 couples proliferation with protein synthesis. J Cell Biol. 2020;219. DOI: 10.1083/jcb.201906147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mura M, Hopkins TG, Michael T, et al. LARP1 post-transcriptionally regulates mTOR and contributes to cancer progression. Oncogene. 2015;34:5025–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Stavraka C, Blagden S. The La-related proteins, a family with connections to cancer. Biomolecules. 2015;5:2701–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Deragon JM, Bousquet-Antonelli C. The role of LARP1 in translation and beyond. Wiley Interdiscip Rev RNA. 2015;6:399–417. [DOI] [PubMed] [Google Scholar]
- [143].Webster MW, Chen YH, Stowell JAW, et al. mRNA deadenylation is coupled to translation rates by the differential activities of Ccr4-not nucleases. Mol Cell. 2018;70:1089–100 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Lim NS, Kozlov G, Chang TC, et al. Comparative peptide binding studies of the PABC domains from the ubiquitin-protein isopeptide ligase HYD and poly(A)-binding protein. Implications for HYD function. J Biol Chem. 2006;281:14376–14382. [DOI] [PubMed] [Google Scholar]
- [145].Siddiqui N, Mangus DA, Chang TC, et al. Poly(A) nuclease interacts with the C-terminal domain of polyadenylate-binding protein domain from poly(A)-binding protein. J Biol Chem. 2007;282:25067–25075. [DOI] [PubMed] [Google Scholar]
- [146].Ezzeddine N, Chen CY, Shyu AB. Evidence providing new insights into TOB-promoted deadenylation and supporting a link between TOB’s deadenylation-enhancing and antiproliferative activities. Mol Cell Biol. 2012;32:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Huang KL, Chadee AB, Chen CY, et al. Phosphorylation at intrinsically disordered regions of PAM2 motif-containing proteins modulates their interactions with PABPC1 and influences mRNA fate. RNA. 2013;19:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ezzeddine N, Chang TC, Zhu W, et al. Human TOB, an antiproliferative transcription factor, is a poly(A)-binding protein-dependent positive regulator of cytoplasmic mRNA deadenylation. Mol Cell Biol. 2007;27:7791–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Mattijssen S, Iben JR, Li T, et al. Single molecule poly(A) tail-seq shows LARP4 opposes deadenylation throughout mRNA lifespan with most impact on short tails. Elife. 2020;9. DOI: 10.7554/eLife.59186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nicholson AL, Pasquinelli AE. Tales of detailed poly(A) tails. Trends Cell Biol. 2019;29:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Berman AJ, Thoreen CC, Dedeic Z, et al. Controversies around the function of LARP1. RNA Biol. 2020;1733787:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Taha MS, Haghighi F, Stefanski A, et al. Novel FMRP interaction networks linked to cellular stress. Febs J. 2020. DOI: 10.1111/febs.15443. [DOI] [PubMed] [Google Scholar]
- [153].To TL, Cuadros AM, Shah H, et al. A compendium of genetic modifiers of mitochondrial dysfunction reveals intra-organelle buffering. Cell. 2019;179:1222–38.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Zhang Y, Wang ZH, Liu Y, et al. PINK1 inhibits local protein synthesis to limit transmission of deleterious mitochondrial DNA mutations. Mol Cell. 2019;73:1127–37.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Philippe L, van den Elzen AMG, Watson MJ, et al. Global analysis of LARP1 translation targets reveals tunable and dynamic features of 5ʹ TOP motifs. Proc Natl Acad Sci U S A. 2020;117:5319–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Cockman E, Anderson P, Ivanov P. TOP mRNPs: molecular Mechanisms and Principles of Regulation. Biomolecules. 2020;10:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Hong S, Freeberg MA, Han T, et al. LARP1 functions as a molecular switch for mTORC1-mediated translation of an essential class of mRNAs. Elife. 2017;6. DOI: 10.7554/eLife.25237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Meyuhas O, Kahan T. The race to decipher Top secrets of TOP mRNAs. Biochm Biophs Acta. 2015;1849:801–811. [DOI] [PubMed] [Google Scholar]
- [159].Mattijssen S, Maraia RJ. LARP4 is regulated by tumor necrosis factor alpha in a tristetraprolin-dependent manner. Mol Cell Biol. 2015;36:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. [DOI] [PubMed] [Google Scholar]
- [161].Fabian MR, Frank F, Rouya C, et al. Structural basis for the recruitment of the human CCR4-NOT deadenylase complex by tristetraprolin. Nat Struct Mol Biol. 2013;20:735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Tian Y, Zeng Z, Li X, et al. Transcriptome-wide stability analysis uncovers LARP4-mediated NFκB1 mRNA stabilization during T cell activation. Nucleic Acids Res. 2020;48:8724–8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Jacob AG, Smith CWJ. Intron retention as a component of regulated gene expression programs. Hum Genet. 2017;136:1043–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Schmidt N, Lareau C, Keshishian H, et al. The SARS-CoV-2 RNA-protein interactome in infected human cells. Nature Microbiol. 2020. Nat Microbiol. 2020 Dec 21. DOI: 10.1038/s41564-020-00846-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].V’Kovski P, Gerber M, Kelly J, et al. Determination of host proteins composing the microenvironment of coronavirus replicase complexes by proximity-labeling. Elife. 2019;8. DOI: 10.7554/eLife.42037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Sola I, Almazan F, Zuniga S, et al. Continuous and Discontinuous RNA Synthesis in Coronaviruses. Annu Rev Virol. 2015;2:265–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Spagnolo JF, Hogue BG. Host protein interactions with the 3ʹ end of bovine coronavirus RNA and the requirement of the poly(A) tail for coronavirus defective genome replication. J Virol. 2000;74:5053–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Gao Y, Yan L, Huang Y, et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Subissi L, Posthuma CC, Collet A, et al. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc Natl Acad Sci U S A. 2014;111:E3900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Surjit M, Lal SK. The SARS-CoV nucleocapsid protein: a protein with multifarious activities. Infect Genet Evol. 2008;8:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Totura AL, Baric RS. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr Opin Virol. 2012;2:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19:102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].McCormick C, Khaperskyy DA. Translation inhibition and stress granules in the antiviral immune response. Nat Rev Immunol. 2017;17:647–660. [DOI] [PubMed] [Google Scholar]
- [175].Sun D, Wang M, Wen X, et al. Cleavage of poly(A)-binding protein by duck hepatitis A virus 3C protease. Sci Rep. 2017;7:16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Knoops K, Kikkert M, Worm SH, et al. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bouhaddou M, Memon D, Meyer B, et al. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell. 2020;182:685–712.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Schaffler K, Schulz K, Hirmer A, et al. A stimulatory role for the La-related protein 4B in translation. RNA. 2010;16:1488–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Tcherkezian J, Cargnello M, Romeo Y, et al. Proteomic analysis of cap-dependent translation identifies LARP1 as a key regulator of 5ʹTOP mRNA translation. Genes Dev. 2014;28:357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Jia, J.J., Lahr, R.M., Solgaard, M.T., Moraes, B.J., Pointet, R., Yang, A.D., Celucci, G., Graber, T.E., Hoang, H.D., Niklaus, M.R. et al. (2021) mTORC1 promotes TOP mRNA translation through site-specific phosphorylation of LARP1. Nucleic Acids Res. doi: 10.1093/nar/gkaa1239 [DOI] [PMC free article] [PubMed]
- [181].Ogami K, Yuka Oishi TN, Kentaro S. Shin-ichi Hoshino LARP1 facilitates translational recovery after amino acid refeeding by preserving long poly(A)-tailed TOP mRNAs. bioRxiv Preprint Not Peer-reviewed. 2020. bioRxiv doi: 10.1101/716217 [DOI] [Google Scholar]
- [182].Blagden SP, Gatt M, Archambault V, et al. Drosophila LARP associates with poly(A)-binding protein and is required for male fertility and syncytial embryo development. Dev Biol. 2009;334:186–197. [DOI] [PubMed] [Google Scholar]
- [183].Merret R, Descombin J, Juan YT, et al. XRN4 and LARP1 are required for a heat-triggered mRNA decay pathway involved in plant acclimation and survival during thermal stress. Cell Rep. 2013;5:1279–1293. [DOI] [PubMed] [Google Scholar]
- [184].Nykamp K, Lee MH, Kimble JC. elegans La-related protein, LARP-1, localizes to germline P bodies and attenuates Ras-MAPK signaling during oogenesis. RNA. 2008;14:1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Burrows C, Latip NA, Lam SJ, et al. The RNA binding protein Larp1 regulates cell division, apoptosis and cell migration. Nucleic Acids Res. 2010;38:5542–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Zhang Y, Chen Y, Gucek M, et al. The mitochondrial outer membrane protein MDI promotes local protein synthesis and mtDNA replication. Embo J. 2016;35:1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Sobel SG, Wolin SL, Pfeffer SR. Two yeast La motif-containing proteins are RNA-binding proteins that associate with polyribosomes. Mol Biol Cell. 1999;10:3849–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Kershaw CJ, Costello JL, Castelli LM, et al. The yeast La related protein Slf1p is a key activator of translation during the oxidative stress response. PLoS Genet. 2015;11:e1004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Sengupta J, Nilsson J, Gursky R, et al. Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by cryo-EM. Nat Struct Mol Biol. 2004;11:957–962. [DOI] [PubMed] [Google Scholar]
- [191].Opitz N, Schmitt K, Hofer-Pretz V, et al. Capturing the asc1p/receptor for activated C kinase 1 (RACK1) microenvironment at the head region of the 40S ribosome with quantitative BioID in yeast. Mol Cell Proteomics. 2017;16:2199–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Thompson MK, Rojas-Duran MF, Gangaramani P, et al. The ribosomal protein Asc1/RACK1 is required for efficient translation of short mRNAs. Elife. 2016;5. DOI: 10.7554/eLife.11154 [DOI] [PMC free article] [PubMed] [Google Scholar]


