Abstract
The global coronavirus 19 (COVID-19) pandemic and associated lockdown restrictions resulted in the majority of sports competitions around the world being put on hold. This includes the National Basketball Association, the UEFA Champions League, Australian Football League, the Tokyo 2020 Olympic Games, and regional competitions. The mitigation strategies in place to control the pandemic have caused disruption to daily schedules, working environments, and lifestyle factors. Athletes rely on regular access to training facilities, practitioners, and coaches to maintain physical and mental health to achieve maximal performance and optimal recovery. Furthermore, participation in sport at any level increases social engagement and promotes better mental health. It is, therefore, critical to understanding how the COVID-19 pandemic and associated lockdown measures have affected the lives of athletes. We surveyed elite and sub-elite athletes (n = 565) across multiple sports. Significant disruptions were reported for all lifestyle factors including social interactions, physical activity, sleep patterns, and mental health. We found a significant increase in total sleep time and sleep latency, as well as a delay in mid-sleep times and a decrease in social jetlag. Training frequency and duration significantly decreased. Importantly, the changes to training and sleep-related factors were associated with mental health outcomes. With spikes in COVID-19 cases rising around the world and governments reinstituting lockdowns (e.g. United Kingdom; Melbourne, Australia; California, USA) these results will inform messaging and strategies to better manage sleep and mental health in a population for whom optimal performance is critical.
Keywords: COVID-19, pandemic, athletes, elite, sleep, mental health, chronotype, lockdown, training, exercise, sports psychology
Statement of Significance.
Athletes are constantly working to optimise their physical and mental health to enhance recovery and performance. We found that training frequency and duration decreased during COVID-19 lockdown, which was associated with higher depression, anxiety, and stress symptoms. When presented with greater flexibility, athletes shifted the times of day in which they trained, particularly avoiding evening hours. Sleep duration increased during lockdown, as did the time taken to get to sleep. Outdoor light exposure decreased and time on screens increased. Changes were associated with poorer mental health outcomes. Finally, the outcomes were more prominent in “night owls” highlighting the importance of considering individual differences. These findings provide critical messaging for managing athletes during the on-going COVID-19 pandemic and associated lockdown restrictions.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Infection can spread through person to person contact and/or via airborne transmission [1]. In March 2020, Australia declared a state of emergency and imposed Stage 3 lockdown restrictions in an attempt to contain the disease. Infection control measures such as travel bans, social distancing, and self-isolation were put in place to protect those at risk of contracting COVID-19. This caused widespread disruption to the sports industry including changes or cancellations to training schedules for athletes and associated competitions. The impact of this disruption on lifestyle factors, sleep, and mental health in athletes is currently unknown.
Athletes are constantly trying to optimize physical and mental health to maximize performance. Key factors in achieving this include maintaining engagement, appropriate training load, and conditioning [2]. Adhering to prescribed training loads is critical to ensure appropriate physiological adaptations [3]. Overtraining can cause premature fatigue, decline in performance, emotional instability, and decreased motivation [4], whilst undertraining is associated with increased injury risk [5] and mood disturbances through lack of engagement [6]. Mental health and sleep have also been identified as critical components to consider in athlete management and rehabilitation [7, 8]. Furthermore, there is a growing body of evidence to suggest that sleep disturbance and mental health concerns are closely intertwined in athlete populations [9, 10]. It is, therefore, crucial for athletes and teams to find the right balance between intensity, volume, and frequency of training as well as develop strategies to manage physical recovery and psychological health.
Sleep deprivation and sleep disturbance result in decreased performance [11], impaired recovery [12], and increased injury risk [13]. Endogenous diurnal variations in physiological and behavioral rhythms are driven by the circadian system and show similar peaks for the majority of individuals. However, individual differences have been identified and are most readily observed in the predisposition for earlier or later sleep patterns. These differences are often termed as one’s chronotype [14]. Misalignment between these rhythms and the external environment often occurs as a consequence of differences in sleep timing between workdays and free days, and has been termed “social jetlag” [15]. Both later chronotype and larger magnitude of social jetlag are associated with poorer mental health [16, 17]. This is of particular importance for athletes, since achieving peak performance requires both mental and physical components. Furthermore, mental health concerns are very prevalent in this population [8], despite participation in sport being generally associated with psychosocial health benefits [18].
The aim of this study was to investigate how training and sleep-related factors have changed in a diverse group of athletes during COVID-19 lockdown and if these changes are associated with mental health symptoms. Compared to pre-COVID, it was hypothesized that time in bed and total sleep time (TST) will increase, whilst chronotype (mid-sleep) will delay and social jetlag will decrease during lockdown. We predicted larger changes in sleep outcomes for athletes with a preference for evening compared to morning.
Methods
Participants
The study protocol was approved by the Monash University Human Research Ethics Committee. Individuals were invited to participate in the study through online platforms including Twitter, Facebook, and directly through sport team contacts. Athletes were classified as anyone who regularly participated in a sport at an international, national, regional, club, or social level (Table 1). The survey was administered online via Qualtrics (Provo, UT, USA) with data collected between May 1 and June 1, 2020. Questions related to the COVID-19 pandemic and associated lockdown restrictions were based on the behavior “over the past month.” Therefore, the survey was administered after individuals had been in lockdown for at least 1 month. A total of 565 athletes consented to take part in the survey. Participants were excluded based on location (outside of Australia), occupation (retired), diagnosed sleep disorder or COVID-19, reported use of a sleep medication, and full-time shift workers. A total of 440 participants met all inclusion criteria (252 female, mean age 26.4 ± 8.7 years). Due to insufficient reporting of data, 41 participants were excluded from the analysis, leaving a total of 399 in the analysis sample (Table 1). Of the final sample, 41.2% were classified as elite athletes (international, national, and state/regional standard) and 58.8% as sub-elite athletes (club and social standard). A further 24 participants did not respond to sleep specific questions and were therefore not included in any analysis on diurnal preference (N = 375).
Table 1.
Summary of Demographic Data
| Variable | Descriptive statistics | Values | |
|---|---|---|---|
| Age (years) | Mean (SEM) | 26.5 (0.4) | |
| Median | 24.0 | ||
| Lower 95% CI of mean | 25.6 | ||
| Upper 95% CI of mean | 27.3 | ||
| Height (cm) | Mean (SEM) | 174.6 (0.5) | |
| Median | 174.0 | ||
| Lower 95% CI of mean | 173.6 | ||
| Upper 95% CI of mean | 175.7 | ||
| Weight (kg) | Mean (SEM) | 73.3 (0.7) | |
| Median | 71.0 | ||
| Lower 95% CI of mean | 71.9 | ||
| Upper 95% CI of mean | 74.7 | ||
| Frequency no. [%] | Total | 399 [100] | |
| Gender | Female | 227 [56.9] | |
| Male | 170 [42.6] | ||
| Other | 2 [0.4] | ||
| Standard | Elite | International | 21 [5.3] |
| National | 98 [24.6] | ||
| State/regional | 45 [11.3] | ||
| Sub-elite | Club | 175 [43.9] | |
| Social | 60 [15.0] | ||
| Sport* | Australian football | 109 [27.3] | |
| Field Hockey | 67 [16.8] | ||
| Netball | 59 [14.8] | ||
| Soccer | 42 [10.5] | ||
| Basketball | 39 [9.8] | ||
| Athletics/running | 30 [7.5] | ||
| Racketsports | 28 [7.0] | ||
| Watersports | 26 [6.5] | ||
| Cricket | 25 [6.3] | ||
| Volleyball | 15 [3.8] | ||
| Other† | 99 [24.8] | ||
| Employment | Full-time employment | 128 [29.1] | |
| Part time employment | 110 [25.0] | ||
| Self-employed | 5 [1.1] | ||
| Student | 116 [26.4] | ||
| Temporarily stood down (furloughed) | 40 [9.1] |
*Sporting categories were included if more than 10 participants selected the category. Frequencies exceed 399 [100] due to athletes who play multiple sports.
†Other sports include any sport represented by less than 10 athletes. Racketsports consisted of Tennis, Squash, and Badminton. Watersports included Swimming, Rowing, Water Polo, Surfing, Sailing, Canoe Polo, Dragon Boat, and Surf Life Saving.
Survey
The survey contained a combination of validated scales and additional expert-designed questions. Validated abbreviated versions of the original tools were used where possible. These included the Ultra-Short Munich Chronotype Questionnaire (µMCTQ) to estimate chronotype and social jetlag using mid-sleep times [19], the Single Daytime Sleepiness Item to assess daytime sleepiness [20]. Mental health was measured using the Patient Health Questionnaire-4 (PHQ-4) to examine depression and anxiety [21] and the Perceived Stress Scale-4 (PSS-4) [22]. The Morningness–Eveningness Questionnaire item 19 (MEQ19) was used to explore diurnal preference [23]. Members of the research team, who are also practitioners in elite sport, were involved in designing additional athlete-specific questions. These were derived from a tool currently under development by the lead author (Athlete Chronometric Evaluation©, ACE) and modified to quantify changes as a result of the COVID-19 pandemic and associated lockdown measures. Questions included details about training (frequency of individual vs. team sessions, timing and duration), sleep (time in bed, TST, sleep need, sleep onset latency) and general lifestyle changes. Participants were asked to provide their answers based on a “typical” month at baseline, that is, before the COVID-19 pandemic (pre-COVID), and over the past month (during lockdown). Lifestyle factors (social life, work/studies, physical activity, productivity, mood, sleeping patterns, mental health, nutrition/diet, and family life) were assessed using a 5-point Likert scale ranging from “extremely disrupted” to “extremely improved.” Substance use (units of caffeine/alcohol), and light exposure (daylight, indoor light, electronic light from screens) were assessed by asking participants if these factors had increased, remained the same or decreased compared with their typical behavior pre-COVID.
Data cleaning and analysis
Total sleep time was used to further screen data (required range 2–16 h; no data were excluded). Mid-sleep time and social jetlag variables were calculated using the µMCTQ as detailed by Blume, Schmidt, and Cajochen [24]. Degree of change in mid-sleep was calculated as the difference in mid-sleep pre-COVID compared to during lockdown for both workdays and free days. Sports-specific questions on duration (three categories; <1 h, 1–2 h, longer than 2 h) and timing (five categories; 05:00–08:00, 08:00–12:00, 12:00–16:00, 16:00–19:00, 19:00–23:00) of training were used to create frequency tables to quantify lockdown-induced changes. Questions with polynomial responses (lifestyle factors, substance use, and light exposure) were recoded to dichotomous to create frequency tables.
R (Version 3.6.1, Vienna, Austria: R Foundation for Statistical Computing) was used for data cleaning, manipulation and to conduct all analyses controlling for age. Exploratory analysis showed that the data structure did not meet parametric assumptions, therefore non-parametric tests were used where required. Frequencies are displayed as no. [%]. Descriptive statistics are presented as the mean ± standard error of the mean (SEM). Kruskal–Wallis rank sum tests were run for analyses that included categorical independent variables and pairwise comparisons were executed for the models that produced statistically significant results. Kendall–Theil Sen Siegel nonparametric linear regressions determined associations between numerical sleep and chronotype variables, and mental health variables. Where both dependent and independent variables were categorical in nature, Pearson’s chi-squared tests were run. Wilcoxon signed rank test with continuity correction assessed differences between sleep and chronotype variables pre-COVID and during lockdown. Daytime sleepiness was the only variable where a difference in the statistically significant outcome existed between genders. Statistical significance was set at p < 0.05. Where appropriate, Kruskal–Wallis χ 2 and β estimates are reported in addition to the p-values.
Results
Lifestyle factors, caffeine and alcohol use, and light exposure
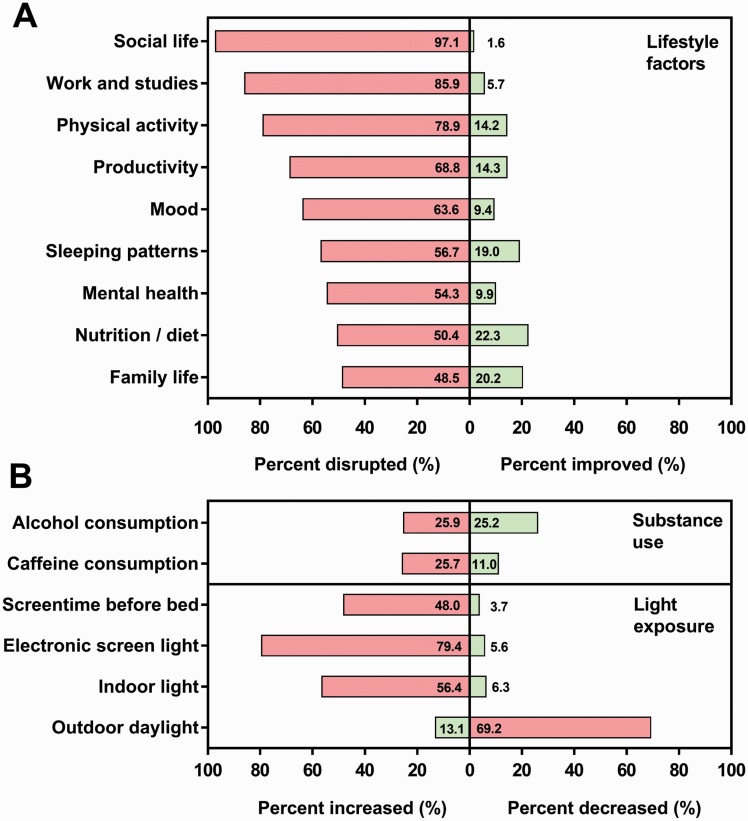
All lifestyle factors were disrupted in at least half of the sample (range 48.5%–97.1%). Physical activity was disrupted for 78.9% of participants, whilst 63.6% reported disrupted mood, 56.7% reported disrupted sleeping patterns, and 54.3% reported disrupted mental health. Average daily caffeine intake increased in approximately 25% of the sample, whilst alcohol intake showed an equal increase and decrease in approximately 25% of participants. Outdoor light exposure decreased in approximately 70% whilst time spent on screens increased in approximately 80%, 48.0% of which report an increase in exposure to screens directly before bed (Figure 1).
Figure 1.
Changes to lifestyle factors (A), substance use, and light exposure (B) in athletes as a result of the COVID-19 pandemic and associated lockdown restrictions. Frequency is shown as the percentage of sample (%). Health and lifestyle disruptions are represented as percent disrupted and improved. Substance use and light exposure are represented as percent increased and decreased. Bars with percentage values indicate negatively associated changes (red) and positively associated changes (green). Responses coded as “no change,” that is, not disrupted or improved, are not included so the total value of each category may not add up to 100.
Training/exercise frequency, duration, and timing
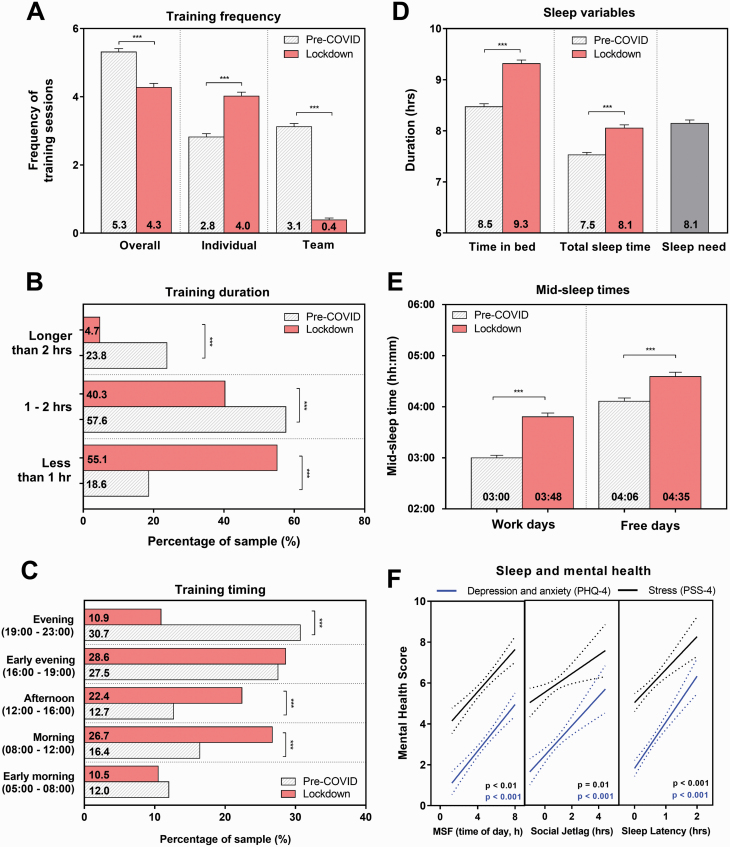
On average, there was a significant reduction in the number of training sessions per week during lockdown (p < 0.001). Training also moved toward more individual training sessions and away from team training sessions (Figure 2, A). There was a significant decrease in the duration of training sessions (χ 2 = 34.0, p < 0.001, Figure 2, B). Furthermore, those who indicated that they found exercising more difficult than before the lockdown chose to exercise for less time (χ 2 = 63.3, p < 0.001). Timing of training was also significantly different during lockdown, with athletes choosing to train earlier in the day compared to pre-COVID (χ 2 = 14.7, p < 0.01, Figure 2, C).
Figure 2.
Changes to training, sleep and mid-sleep times during lockdown and the relationship with mental health. Frequency of training sessions is shown in panel A, duration in panel B, and timing in panel C. Sleep patterns are shown in panel D, chronotype measured using mid-sleep times in panel E. Statistical significance is shown as p < 0.001***. The relationship between mental health (PHQ-4; red line, PSS-4; black line) and mid-sleep on free days (MSF), social jetlag and sleep latency are shown in panel F. Dotted lines show 95% confidence bands of the best fit line.
Sleep duration and timing
Time in bed (TIB) and TST significantly increased by 0.8 and 0.6 h, respectively during lockdown (both p < 0.001), and TST was no longer significantly different from self-reported sleep need (Table 2, Figure 2, D). Despite TST increasing during lockdown, athletes reported an increase in subjective daytime sleepiness (p = 0.01). In comparison to pre-COVID, both sleep onset and wake up times shifted later (Table 2). This combined change in sleep timing was represented by a significant delay in mid-sleep time by 48 min on workdays and 29 min on free days, accompanied by a 19-min reduction in social jetlag (all p < 0.001, Table 2, Figure 2, E). There was a 25% increase in sleep onset latency during lockdown which was found to be associated with time spent on screens before bed (Kruskal–Wallis χ 2 = 10.4, p < 0.01). Further investigation showed that individuals who spent less time on screens before bed compared to pre-COVID reported an average sleep onset latency of 22 min, whereas those who spent more time on screens before bed reported an average sleep latency of 37 min (p = 0.03).
Table 2.
Overall Changes to Sleep Duration, Timing and Mid-sleep on Workdays and Free Days during Lockdown and Mental Health Score Broken Down by Diurnal Preference
| Variable | Situation | Whole sample | Definite morning | Moderate morning | Moderate evening | Definite evening |
|---|---|---|---|---|---|---|
| Sample size no. [%] | NA | 375 [100] | 69 [18.4] | 127 [33.9] | 120 [32.0] | 59 [15.7] |
| Sleep need (h) | NA | 8.13 (0.07) | 7.72 (0.18) | 8.14 (0.11) | 8.32 (0.09) | 8.22 (0.19) |
| Time in bed (h) | Pre-COVID | 8.47 (0.06) | 8.23 (0.14) | 8.48 (0.10) | 8.55 (0.12) | 8.58 (0.15) |
| During lockdown | 9.32 (0.07)*** | 8.93 (0.15)*** | 9.28 (0.13)*** | 9.43 (0.11)*** | 9.64 (0.22)*** | |
| Total sleep time (h) | Pre-COVID | 7.52 (0.05) | 7.36 (0.13) | 7.60 (0.08) | 7.52 (0.08) | 7.52 (0.13) |
| During lockdown | 8.06 (0.06)*** | 7.82 (0.14)*** | 8.06 (0.11)*** | 8.15 (0.10)*** | 8.14 (0.19)** | |
| Sleep onset workdays (hh:mm) | Pre-COVID | 23:04 (00:03) | 22:35 (00:06) | 22:47 (00:05) | 23:14 (00:05) | 23:53 (00:09) |
| During lockdown | 23:49 (00:05)*** | 22:47 (00:10) | 23:28 (00:07)*** | 00:08 (00:08)*** | 01:08 (00:14)*** | |
| Wake up time workdays (hh:mm) | Pre-COVID | 06:55 (00:04) | 06:09 (00:06) | 06:45 (00:07) | 07:10 (00:06) | 07:43 (00:10) |
| During lockdown | 07:47 (00:05)*** | 06:49 (00:08)*** | 07:28 (00:07)*** | 08:10 (00:08)*** | 08:50 (00:14)*** | |
| Sleep onset free days (hh:mm) | Pre-COVID | 23:47 (00:04) | 23:01 (00:07) | 23:26 (00:07) | 00:03 (00:06) | 00:56 (00:10) |
| During lockdown | 00:24 (00:05)*** | 23:15 (00:10) | 00:00 (00:08)*** | 00:39 (00:08)*** | 02:04 (00:14)*** | |
| Wake up time free days (hh:mm) | Pre-COVID | 08:26 (00:04) | 07:17 (00:07) | 08:10 (00:07) | 08:45 (00:06) | 09:40 (00:10) |
| During lockdown | 08:47 (00:05)*** | 07:27 (00:08) | 08:24 (00:08)** | 9:09 (00:08)*** | 10:27 (00:14)*** | |
| Mid-sleep workdays (hh:mm) | Pre-COVID | 03:00 (00:03) | 02:22 (00:05) | 02:46 (00:05) | 03:12 (00:04) | 03:48 (00:08) |
| During lockdown | 03:48 (00:05)*** | 02:48 (00:07)** | 03:28 (00:07)*** | 04:09 (00:07)*** | 04:59 (00:13)*** | |
| Mid-sleep free days (hh:mm) | Pre-COVID | 04:06 (00:04) | 03:09 (00:06) | 03:48 (00:06) | 04:24 (00:05) | 05:18 (00:08) |
| During lockdown | 04:35 (00:05)*** | 03:21 (00:08)* | 04:12 (00:07)*** | 04:54 (00:07)*** | 06:16 (00:13)*** | |
| Degree of change in mid-sleep (h) | Workdays | 0.81 (0.06) | 0.43 (0.12) | 0.70 (0.10) | 0.95 (0.11) | 1.18 (0.19) |
| Free days | 0.48 (0.05)*** | 0.19 (0.10)** | 0.40 (0.09)*** | 0.51 (0.09)*** | 0.96 (0.16) | |
| Social jetlag (h) | Pre-COVID | 1.11 (0.04) | 0.79 (0.08) | 1.03 (0.07) | 1.19 (0.07) | 1.50 (0.11) |
| During lockdown | 0.79 (0.04)*** | 0.55 (0.07)** | 0.73 (0.06)*** | 0.75 (0.09)*** | 1.28 (0.12) | |
| Sleep latency (min) | Pre-COVID | 23.72 (0.89) | 21.71 (1.84) | 21.36 (1.22) | 26.05 (1.79) | 26.42 (2.58) |
| During lockdown | 32.29 (1.33)*** | 28.68 (3.24)* | 28.84 (2.12)*** | 34.44 (2.36)*** | 39.56 (3.50)*** | |
| Daytime sleepiness | Pre-COVID | 3.48 (0.10) | 3.19 (0.22) | 3.44 (0.18) | 3.64 (0.17) | 3.59 (0.26) |
| During lockdown | 3.80 (0.12)** | 3.26 (0.25) | 3.51 (0.19) | 4.03 (0.21) | 4.61 (0.28)** | |
| Mental health (score) | PHQ-4 | 3.01 (0.13) | 2.81 (0.29) | 2.50 (0.22) | 3.25 (0.23) | 3.85 (0.37) |
| PSS-4 | 5.88 (0.14) | 5.74 (0.28) | 5.46 (0.25) | 6.03 (0.25) | 6.63 (0.40) |
Data is shown as mean (SEM) or no. [%]. Significant differences between the time points are shown in bold with a * denoting p < 0.05, ** denoting p < 0.01, and *** denoting p < 0.001.
Diurnal preference is associated with changes to sleep
When considering diurnal preference, all groups showed a significant delay in mid-sleep times, with the degree of change being higher on workdays compared to free days (p < 0.001, Table 2). Diurnal preference significantly predicted the degree of change in mid-sleep on workdays and free days (Kruskal–Wallis χ 2 = 14.6, p < 0.01 and χ 2 = 17.8, p < 0.001, respectively), with the magnitude of the delay increasing from definite morning to definite evening types. A similar pattern was seen in social jetlag and sleep latency, with highest values in evening types at both time points. The significant increase in daytime sleepiness was only observed for definite evening types (Table 2).
Changes to training and sleep are associated with mental health
Decreases in training frequency during lockdown were associated with increased reports of depression/anxiety and stress (both β estimate = −0.01, p < 0.001). Similarly, later mid-sleep times during lockdown showed a significant relationship with higher scores for depression, anxiety and stress on work and free days (all β estimate = 0.0, PHQ-4; p < 0.001, PSS-4; p < 0.01, Figure 2, F). The same was seen for social jetlag and sleep onset latency during lockdown, with higher social jetlag and latency being significantly correlated with poorer mental health (Figure 2, F).
Discussion
The COVID-19 pandemic is having on-going, widespread effects on the lifestyle of everybody around the world. Recent studies investigating changes to sleep as a result of COVID-19 lockdown have been conducted with members of the general public [24, 25], but currently there are no data available about the impact on sleep, mental health, and training of athletes. Our findings show significant changes to all training and sleep-related factors that were investigated. Importantly, these changes during lockdown were associated with increased depression, anxiety, and stress symptoms. These findings highlight a critical need to consider the impact of lockdowns on athletes and to develop strategies to maintain optimal sleep, mental health, and performance in this population.
The COVID-19 pandemic and associated lockdown led to a significant disruption to multiple lifestyle factors, which may have contributed to the reported increase in trouble falling asleep (sleep latency), increase in daytime sleepiness, and related associations with poorer mental health. One of the most prominent disruptions was in light exposure, with exposure to outdoor light reducing and time on screens increasing in the majority of the sample. A reduction in exposure to light during wakefulness has been shown to decrease slow-wave activity [26], and later timing in exposure to light increases sleep disturbance through increased awakenings and reduced slow-wave sleep accumulation [27]. When explored in more detail, those participants who increased screen time before bed during lockdown took significantly longer to fall asleep than those who reduced screen time before bed. The use of light-emitting devices immediately before bed is not recommended because of the potent effects of light on the human circadian system [28]. Exposure to light in the evening, even at low intensity, has an acute alerting effect, suppresses the release of the sleep-facilitating hormone melatonin, and delays the circadian clock, which when combined make it more difficult to fall asleep and reduces the quality of sleep [28]. Therefore, both the reduction of outdoor light and increase in screen time before bed may have contributed to the longer sleep latency observed.
Our study also showed that higher sleep onset latency was significantly associated with poorer mental health. Raising awareness and education about the implications of using screens before bed and increasing early exposure to daylight would be a simple strategy for sports organizations that could reduce sleep onset latency, thereby increasing sleep duration, sleep quality, and improve mental health of their athletes.
Our results show a significant change in the frequency, duration, and timing of training sessions for athletes during the lockdown. The assumed flexibility presented by the COVID-19 lockdown resulted in athletes choosing to train during the morning and afternoon more often, and in the evening less often. This could reflect early chronotypes having the ability to self-select when not constrained to fixed schedules. Since the timing of exercise has been shown to help facilitate circadian adaptation [29] and the time of peak performance is influenced by chronotype [14], these changes further support the need for taking into account individual (chronotype) factors when scheduling training and preparing for competition, especially within the elite sector.
During COVID-19 lockdown, athletes trained less frequently and for shorter periods of time, which could have contributed to the association with higher depression, anxiety, and stress scores. A key factor in this change is most likely motivation, that is, athletes uncertain of when competitions will recommence in the future, which could be reflected in the high percentage of the sample who reported disruptions to productivity and mood. An excessive reduction in training load can impact psycho-social engagement and could lead to detrimental training-induced anatomical and physiological adaptations (detraining) [3]. When not managed properly, these changes could result in increased injury risk when training resumes. In addition, social isolation and loneliness are associated with reduced physical activity [30] and an increase in depressive symptoms [31]. Although lockdown restrictions require social distancing, sporting organizations and teams should focus on maintaining training load, and provide remote opportunities to enhance social connectedness, motivation, and support [18]. However, in line with our findings showing that more screen time before bed increases sleep latency, careful consideration should be given to the timing of these activities to ensure scheduling is not too close to bedtime.
Sufficient sleep in all dimensions (quantity, quality, and timing) is important for athletes to maintain good mental health, maximize athletic performance, and avoid overtraining [2, 7]. Overall, we found that athletes spent longer time in bed and longer time asleep during lockdown compared to pre-COVID. This suggests that athletes may require more sleep than they typically get when restricted to usual schedules, which is further supported by no longer seeing a significant difference between TST and sleep need during lockdown. There was a significant shift in sleep onset and wake up times shown by a delay in mid-sleep, which was more pronounced on workdays compared to free days, leading to an overall reduction in social jetlag. The decrease in social jetlag was associated with better mental health. This indicates that, where possible, scheduling should be adapted to prevent a large discrepancy in sleep timings on workdays versus free days, in an effort to reduce social jetlag and thereby improve mental health. It also provides evidence to support the notion that sleep behaviors are modifiable, which may inform sleep health intervention strategies for athletes.
By considering the impact of diurnal preference on changes in sleep and mental health outcomes during the lockdown period, we showed that evening types are most affected. During the lockdown, definite evening types showed the greatest delay to sleep timing, the longest sleep latency and were the only group whose daytime sleepiness was significantly higher compared to pre-COVID. In addition, even though social jetlag decreased during the lockdown, it was still higher in definite evening types than all other groups compared to pre-lockdown. Around 35% of the general population are evening types [32], who are particularly vulnerable to disruption of sleep and higher social jetlag. This is due to a chronic and recurrent mismatch between the internal circadian system, sleep–wake behavior, and social schedules, which impacts physical and cognitive performance, general health, and wellbeing [14, 33]. Our results also show that all of these factors (later mid-sleep, higher social jetlag, and longer sleep latency) are associated with poorer mental health outcomes. The finding that these factors are more pronounced in definite evening types highlights a critical need to account for individual differences when managing athletes through changes to schedules. This could include increasing flexibility and using circadian interventions to shift circadian timing [33].
In addition to providing important insights into the effect of the COVID-19 pandemic and associated lockdown period on training, sleep, and mental health in athletes, the findings can be directly translated to off-season periods where athletes can choose when they train and have more flexibility over their sleeping patterns. The findings could also be applied to other sport-specific settings since varying schedules are a common occurrence for athletes who have to adapt from week to week and season to season.
Limitations of this study should be acknowledged. First, achieving optimal sleep and mental health is multifactorial so we are only able to investigate associations and not causation. Our findings suggest that there are multiple factors impacting mental health which may differ between individuals, for example, athletes who reduce their social jetlag are not necessarily the same athletes that reduce their training load. Additionally, the cessation of all competitions and supervised training prevented us from being able to assess performance so we cannot determine the impact of these changes on objective performance outcomes. We also did not collect TIB and TST separately on work and free days, which limits our ability to investigate build-up of sleep need. Future work should address this to provide more insight into accumulation of sleep debt during the week. Nonetheless, our findings provide some important insight into changes due to COVID-19 lockdown, and are similar to recent research into sleep and physical activity in other populations [24, 25, 34].
Secondly, we were unable to foresee the COVID-19 pandemic and therefore had to gather the baseline data by retrospective self-report which could be subjected to recall bias. To minimize this possibility, we followed similar procedures to others who have conducted sleep research during the COVID-19 pandemic [24]. In addition, athletes are often subject to regular routines and are very familiar with their training schedules, which we would expect to result in more reliable and accurate recall of pre-COVID times and therefore reduce recall bias.
Thirdly, we studied athletes across multiple sports and standards, as each could be impacted differently. We used purposive sampling to ensure our sample was representative of the athlete population and to increase the generalizability of the findings. The mean age of our sample (26.5 years) is similar to that reported by the International Olympic Committee (25.7 years), and falls into the typical age range shown in a longitudinal analysis of professional athletes (23.4 ± 4 years) [35]. However, by combining the data set we limit the ability to investigate specific relationships between subgroups, such as elite versus sub-elite athletes and across sports. These questions should be explored in future research, especially since the distribution of chronotype can vary between different sports (Supplementary Figure S1). Finally, we obtained a cross-section view of athlete training, sleep, and mental health, which could be influenced by the type of participation (team vs. individual) [36] and time of year (in-season vs. out of season). The majority of athletes in our sample were involved in team sport (79%) which were predominately winter based, meaning lockdown occurred during the typical season. Although further investigations are required to explore this in more detail, we believe that by including a range of sports we increase the external validity of the findings to athletes and organizations across the industry.
Conclusion
In summary, a decrease in training frequency along with later mid-sleep time, higher social jetlag, greater sleep latency, and increased screen time before bed are all independently associated with poorer mental health outcomes. As lockdowns continue to be reinstated around the world, messaging should focus on increasing early outdoor daylight and decreasing time on screens before bed, which would likely improve sleep onset latency, help shift sleep timing earlier and reducing social jetlag. Where possible, athletes should be given flexibility over timing of training for greater consistency between work and free days, and an effort should be made to increase the frequency of training and social interaction. In addition, individual differences in sleep and chronobiology should be considered in health and rehabilitation programs for athletes, especially for those with an evening chronotype who may be more vulnerable to negative mental health consequences. These recommendations could also be meaningful to the general population, and provide insights for organizations to improve sleep and mental health beyond the COVID-19 pandemic. As we try to navigate this on-going disruption with the likelihood of further lockdowns, our findings provide crucial information for the sport industry, which could inform future research as well as programs and interventions to optimize sleep, training, and mental health in athletes.
Supplementary Material
Funding
E.R.F-C has received funding from the Department of Industry, Innovation and Science (Australian Government, #ICG000899 and #ICG001546), and is currently supported by a Science Industry Endowment Fund (SIEF) Ross Metcalf STEM+ Business Fellowship administered by the Commonwealth Scientific and Industrial Research Organisation (CSIRO).
Conflicts of interest statement. E.R.F-C and D.H. declare they are practitioners in elite sports. Monash University has an on-going industry collaboration with St Kilda Football Club. D.H. currently holds a position with St Kilda Football Club. S.M.W.R has served as a Program Leader for the CRC for Alertness, Safety, and Productivity, Australia and is the President of the Sleep Health Foundation.
Financial disclosure statement
E.R.F-C has received research support or consultancy fees from the Australian National Football League, Team Focus Ltd, British Athletics, Australian National Rugby League, Henley Business School and Collingwood Football Club which are not related to this paper. S.M.W.R. has received grants from Vanda Pharmaceuticals, Philips Respironics, Cephalon, Rio Tinto, BHP Billiton, and Shell; and has received equipment support and consultancy fees through his institution from Optalert, Compumedics, Teva Pharmaceuticals, and Circadian Therapeutics, which are not related to this paper. The authors declare there are no other competing financial interests.
Ethical approval and consent
The study protocol was approved by the Monash University Human Research Ethics Committee (MUHREC #24257). All participants provided electronic consent prior to involvement.
Data availability statement
De-identified participant data can be made available by contacting the corresponding author upon reasonable request. Reuse is only permitted following a written agreement from the corresponding author and primary Institution.
Author contributions
E.R.F-C., S.P.A.D., and S.W.R. designed the study. E.R.F-C and J.T. collected the data. E.R.F-C and D.H. analyzed the data with contributions from S.P.A.D. and S.W.R. E.R.F-C wrote the manuscript and created all the figures and tables. All authors commented on the manuscript.
References
- 1. Morawska L, et al. . Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. 2020;139:105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samuels C. Sleep, recovery, and performance: the new frontier in high-performance athletics. Phys Med Rehabil Clin N Am. 2009;20(1):149–59, ix. [DOI] [PubMed] [Google Scholar]
- 3. Mujika I. Intense training: the key to optimal performance before and during the taper. Scand J Med Sci Spor. 2010; 20: 24–31. [DOI] [PubMed] [Google Scholar]
- 4. Kuipers H. How much is too much? Performance aspects of overtraining. Res Q Exercise Sport. 1996; 67 (sup3): S-65–S-69. [DOI] [PubMed] [Google Scholar]
- 5. Gabbett TJ, et al. . If overuse injury is a “training load error,” should undertraining be viewed the same way? Br J Sports Med. 2016; 50(17): 1017–1018. . [DOI] [PubMed] [Google Scholar]
- 6. Scotto di Luzio S, et al. . Exploring the role of sport sense of community in perceived athlete burnout, sport motivation, and engagement. J Appl Sport Psychol. 2020; 32(5):513–528. [Google Scholar]
- 7. Kroshus E, et al. . Wake up call for collegiate athlete sleep: narrative review and consensus recommendations from the NCAA Interassociation Task Force on Sleep and Wellness. Br J Sports Med. 2019;53(12):731–736. [DOI] [PubMed] [Google Scholar]
- 8. Reardon CL, et al. . Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667–699. [DOI] [PubMed] [Google Scholar]
- 9. Grandner MA, et al. . Mental health in student athletes: associations with sleep duration, sleep quality, insomnia, fatigue, and sleep apnea symptoms. Athletic Training Sports Health Care. 2020. doi: 10.3928/19425864-20200521-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nabhan D, et al. . Expanding the screening toolbox to promote athlete health: how the US Olympic & Paralympic Committee screened for health problems in 940 elite athletes. Br J Sport Med. 2020: bjsports-2020–102756. doi: 10.1136/bjsports-2020-102756 [DOI] [PubMed] [Google Scholar]
- 11. Skein M, et al. . Intermittent-sprint performance and muscle glycogen after 30 h of sleep deprivation. Med Sci Sports Exerc. 2011;43(7):1301–1311. [DOI] [PubMed] [Google Scholar]
- 12. Skein M, et al. . The effect of overnight sleep deprivation after competitive rugby league matches on postmatch physiological and perceptual recovery. Int J Sports Physiol Perform. 2013;8(5):556–564. [DOI] [PubMed] [Google Scholar]
- 13. Milewski MD, et al. . Chronic lack of sleep is associated with increased sports injuries in adolescent athletes. J Pediatr Orthop. 2014;34(2):129–133. [DOI] [PubMed] [Google Scholar]
- 14. Facer-Childs E, et al. . The impact of circadian phenotype and time since awakening on diurnal performance in athletes. Curr Biol. 2015;25(4):518–522. [DOI] [PubMed] [Google Scholar]
- 15. Wittmann M, et al. . Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509. [DOI] [PubMed] [Google Scholar]
- 16. Antypa N, et al. . Chronotype associations with depression and anxiety disorders in a large cohort study. Depress Anxiety. 2016;33(1):75–83. [DOI] [PubMed] [Google Scholar]
- 17. Levandovski R, et al. . Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 2011;28(9):771–778. [DOI] [PubMed] [Google Scholar]
- 18. Eime RM, et al. . A systematic review of the psychological and social benefits of participation in sport for adults: informing development of a conceptual model of health through sport. Int J Behav Nutr Phys Act. 2013;10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghotbi N, et al. . The µMCTQ: an ultra-short version of the Munich ChronoType Questionnaire. J Biol Rhythms. 2020;35(1):98–110. [DOI] [PubMed] [Google Scholar]
- 20. Burkhalter H, et al. . Validation of a single item to assess daytime sleepiness for the Swiss Transplant Cohort Study. Prog Transplant. 2013;23(3):220–228. [DOI] [PubMed] [Google Scholar]
- 21. Kroenke K, et al. . An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–621. [DOI] [PubMed] [Google Scholar]
- 22. Cohen S, et al. . A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 23. Adan A, Almirall H. Horne & Östberg morningness-eveningness questionnaire: a reduced scale. Pers Indiv Differ. 1991; 12 (3): 241–253. [Google Scholar]
- 24. Blume C, et al. . Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol. 2020; 30(14): R795–R797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wright KP, et al. . Sleep in university students prior to and during COVID-19 stay-at-home orders. Curr Biol. 2020; 30(14): R797–R798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Korf EM, et al. . Blindfolding during wakefulness causes decrease in sleep slow wave activity. Physiol Rep. 2017; 5(7): e13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wams EJ, et al.. Linking light exposure and subsequent sleep: A field polysomnography study in humans. Sleep. 2017; 40 (12). doi: 10.1093/sleep/zsx165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang AM, et al. . Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112(4):1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barger LK, et al. . Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1077–R1084. [DOI] [PubMed] [Google Scholar]
- 30. Hawkley LC, et al. . Loneliness predicts reduced physical activity: cross-sectional & longitudinal analyses. Health Psychol. 2009;28(3):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith KJ, Victor C. Typologies of loneliness, living alone and social isolation, and their associations with physical and mental health. Ageing Soc. 2019; 39 (8): 1709–1730. [Google Scholar]
- 32. Roenneberg T, et al. . Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. [DOI] [PubMed] [Google Scholar]
- 33. Facer-Childs ER, et al. . Resetting the late timing of ‘night owls’ has a positive impact on mental health and performance. Sleep Med. 2019;60:236–247. [DOI] [PubMed] [Google Scholar]
- 34. Ong J, et al.. COVID-19 related mobility reduction: heterogenous effects on sleep and physical activity rhythms. Sleep. 2020. doi: 10.1093/sleep/zsaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoffman DT, et al. . Australian football league injury characteristics differ between matches and training: a longitudinal analysis of changes in the setting, site, and time span from 1997 to 2016. Orthop J Sports Med. 2019;7(4):2325967119837641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lastella M, et al. . Sleep/wake behaviours of elite athletes from individual and team sports. Eur J Sport Sci. 2015;15(2):94–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified participant data can be made available by contacting the corresponding author upon reasonable request. Reuse is only permitted following a written agreement from the corresponding author and primary Institution.