Abstract
Importance: Rural-dwelling Latinos are an underresourced population in need of accessible and effective wellness programs.
Objective: To evaluate patients’ long-term health-related outcomes after lifestyle intervention.
Design: An uncontrolled pilot trial assessing change in health from pretreatment to long-term follow-up (12 mo after intervention completion, no contact) and from posttreatment to long-term follow-up.
Setting: Rural, community-based primary care.
Participants: Latino and Hispanic safety-net primary care patients, ages 50 to 64 yr.
Intervention: A culturally tailored, 4-mo lifestyle intervention co-led by occupational therapy practitioners and Latino community health workers that features telehealth and in-home sessions covering topics such as healthy eating and navigating health care.
Outcomes and Measures: Self-reported and physiological outcomes: symptom–well-being (primary), stress, sleep disturbance, social satisfaction, physical activity, patient activation, blood pressure, and weight. Exit interviews addressed health experiences and intervention impact on participants’ lives.
Results: Participants (N = 27) demonstrated clinically significant pretreatment to long-term follow-up benefits in all symptom–well-being dimensions (Cohen’s d ≥ 0.8, p ≤ .004), with additional gains from posttreatment to long-term follow-up (d ≥ 0.4, p ≤ .05). Significant improvements from pre- to posttreatment in systolic blood pressure, stress, and social role and activity satisfaction were maintained at long-term follow-up. No changes were observed in weight, physical activity, or diastolic blood pressure. Participants described the intervention’s sustained positive effect on their wellness.
Conclusions and Relevance: A lifestyle intervention led by occupational therapy practitioners and community health workers in a primary care context has potential to achieve long-term health benefits in rural-dwelling, late-midlife Latinos.
What This Article Adds: This study reveals that rural, late-midlife Latinos showed long-lasting improvements in psychological and physical health after finishing a program that helped them make healthy lifestyle choices. This finding supports the unique contribution of occupational therapy in primary care settings.
The prevalence of multiple chronic conditions in older adulthood is staggering: 81% of older Americans live with two or more chronic diseases (Buttorff et al., 2017). To combat the societal and individual burden of multimorbidity, practitioners have increased efforts to enhance public health through provision of high-quality, patient-centered preventive care and disease self-management (Leland et al., 2017). Occupational therapy has the opportunity to establish a strong presence in this health promotion movement, particularly given its roots in client-centered practice. For instance, studies are now emerging that demonstrate the distinct value of lifestyle-centric occupational therapy in a primary care context (e.g., Pyatak et al., 2019), but more research is warranted.
In tandem with the spotlight on patient-centeredness and self-management is the resurrection of home-based care reminiscent of days when doctors made house calls (Boling et al., 2013). The renewed demand for this treatment model stems from considerations ranging from people’s barriers to accessing services to an increasing desire to age in place. Although in-person, home-based care is effective in improving multiple health outcomes (Szanton et al., 2016), the fiscal strain that this model could impose threatens its uptake in traditional primary care. Consequently, interventions that produce sustained health outcomes are imperative to help justify rekindling in-home programs. Alternate modes of delivering treatment at home (e.g., extending care through community health workers [CHWs], using telehealth) are also gaining traction as feasible ways to reduce health disparities and to reach underresourced groups.
Rural-dwelling Latinos represent a population that could benefit greatly from targeted, accessible, and effective wellness programs. The prevalence of certain chronic health problems (e.g., Type 2 diabetes, hypertension) and disability is markedly higher among Latinos versus other ethnic groups (Samper-Ternent et al., 2012). Residence in rural regions can compound this issue, with rural-living Latinos being at higher risk for poor health than Latino urbanites (Koopman et al., 2006). Rural-dwelling Latinos are further burdened with significant psychosocial stressors such as food insecurity or racism in small communities, and they face limited access to health care services (Sano et al., 2011; Stacciarini et al., 2015).
In 2016–2017, we conducted a pilot study to examine the feasibility of, and to obtain a preliminary estimate of the efficacy of, ¡Vivir Mi Vida! (Live My Life!, or ¡VMV!), a culturally tailored lifestyle intervention for late-midlife (ages 50–64 yr), rural-living Latinos, integrated into primary care services (Schepens Niemiec et al., 2018, 2019). ¡VMV! is an iteration of the Well Elderly Lifestyle Redesign® program, a preventive occupational therapy intervention for community-living older people (Clark et al., 1997). We observed positive health changes in psychosocial and clinical outcomes. Given the importance of identifying occupational therapy approaches in primary care that have the potential for long-term impact and sustainability, the purpose of the current study was to evaluate the health status and experiences of ¡VMV! participants 1 yr after the conclusion of the intervention.
Method
The University of Southern California institutional review board approved all study activities. We used an uncontrolled clinical trial to assess change in health outcomes during a 12-mo, no-contact period that followed intervention receipt. In addition, we conducted brief exit interviews with participants at the final assessment regarding their health experiences over the past year.
Participants and Setting
All participants who enrolled in the ¡VMV! pilot study (N = 40) were eligible for the long-term follow-up (LTFU) study. Detailed descriptions of the original cohort and participant selection criteria are available elsewhere (Schepens Niemiec et al., 2019). Briefly, participants were ages 50 to 64 yr, self-identified as Latino or Hispanic, and were patients enrolled in the Antelope Valley Community Clinic health system.
Procedures and Assessment
As described in Schepens Niemiec et al.’s (2019) study, ¡VMV! was a culturally tailored lifestyle intervention for late-midlife Latino patients of a rural primary care clinic. The intervention was delivered by an occupational therapy practitioner–CHW team using a combination of telehealth occupational therapy and in-home sessions with a CHW. Overarching modular topics covered by the CHW and carefully linked to occupation by the occupational therapy practitioner included healthy eating and physical activity, health care navigation, chronic disease management, and mental well-being. An invitation to participate in LTFU was mailed to participants 12 mo after the intervention concluded along with an informed consent addendum detailing the added follow-up study components. For those who agreed to participate, a trained tester administered a battery of self-report (symptom–well-being outcome [SWO], stress, sleep disturbance, social satisfaction, physical activity, patient activation) and physiological (blood pressure, weight) in-home health measures; in addition, the tester conducted a brief structured exit interview to conclude the session (total visit was approximately 1.5 hr).
General procedures followed those used in the pilot study (Schepens Niemiec et al., 2019). The tester used for the LTFU study had participated in the original pilot study. She received a 2-hr refresher training on all assessment procedures, old and new. It is important to note that the tester had contact with participants only during the assessment periods (i.e., preintervention, postintervention, and LTFU testing).
A full description of instruments that overlapped with the original assessment battery (i.e., Measure Yourself Medical Outcome Profile [MYMOP2; Paterson, 1996], Satisfaction With Participation in Social Roles and Discretionary Social Activities–Short Form 7a [Cella et al., 2010], Pittsburgh Sleep Quality Index [Backhaus et al., 2002], Elo et al.’s [2003] Single Item Stress Index, and the International Physical Activity Questionnaire [Craig et al., 2003]) is available in Schepens Niemiec et al. (2018). The primary outcome assessment and new instruments included in the LTFU battery are described next.
SWO, the primary study outcome, was measured with the MYMOP2: a tool composed of an overall profile score and four symptom–well-being subscales. Participants identified one to two currently bothersome symptoms (Paterson, 1996). They rated each symptom’s severity and how much it interfered with daily activities. They also rated their overall well-being. At LTFU, participants evaluated the same symptoms identified at baseline and their impact on activities.
Four key alterations were made to the assessment protocol for LTFU. We added the 13-item Patient Activation Measure (PAM–13; Hibbard et al., 2005), a measure of patients’ engagement in their own health care process at postintervention and LTFU time points. This measure was included for its potential as a mediating mechanism of intervention effects. The PAM–13 is a short form that assesses one’s knowledge of and skills and confidence in health self-management (Hibbard et al., 2005). On a scale ranging from 0 to 100, patients are classified into one of four progressively higher activation categories (Level 1 indicates poor patient activation, and Level 4 indicates adequate patient activation).
We also added the short form of the Brief Pain Inventory (BPI; Keller et al., 2004) at LTFU as a result of several participants noting pain as a symptom of concern on the MYMOP2. The BPI comprises the Severity and Interference subscales. The Severity subscale measures the amount of pain, on a numerical scale ranging from 0 (no pain) to 10 (pain as bad as you can imagine), that a person has experienced at its “worst,” “least,” and “average” levels as well as present pain. The Interference subscale assesses how much a person’s pain interferes with everyday life, also on a scale ranging from 0 (does not interfere) to 10 (completely interferes). The BPI and its subscales are valid and reliable (de Andrés Ares et al., 2015; Keller et al., 2004).
For LTFU, to reduce testing burden, we removed the Block 2005 Food Frequency Questionnaire Spanish Version (Centers for Disease Control and Prevention, 2015) that was used to evaluate dietary intake; original administration took about 45 min. We replaced this instrument with the 25-item My Habits questionnaire (<10-min administration; Medina et al., 2007), which measures heart-healthy behaviors with three subscales: Salt Intake, Fat and Cholesterol Consumption, and Weight Control Behaviors. A 4-point Likert scale (1 = never, 4 = always) is used to answer “How often have you done the following over the past month?” Responses are summed and averaged for each subscale; higher scores represent healthier habits. Sample items from each respective subscale include “use herbs and spices instead of salt?” “cook ground meat and throw away the fat?” and “bake or broil foods instead of frying them?” The tool has acceptable reliability (Medina et al., 2007).
The final change to the assessment protocol was inclusion of a brief structured exit interview (approximately 10 min) conducted by the tester and designed to capture participants’ health experiences across the year and their description of ¡VMV!’s impact on their lives. Interviews were conducted in Spanish. To open the discussion, participants rated the item “Since the program ended, I used what I learned in ¡VMV! to actively try to improve my health” using a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree). For the structured exit interview questions and prompt guide, see Appendix A.
Data Analysis
We compared (1) differences between participants with versus without LTFU on baseline characteristics and (2) changes from pretest to posttest on outcome variables using Wilcoxon rank-sum tests or Fisher exact tests for continuous (age and body mass index) or categorical (all other) variables, respectively. Our primary outcomes were changes from pretest to LTFU and from posttest to LTFU, which we analyzed using Wilcoxon signed rank tests. We calculated effect sizes as Cohen’s d, defined as the mean change (pretest to LTFU or posttest to LTFU) divided by the initial standard deviation (at pretest or posttest, respectively); 95% confidence intervals were calculated for effect sizes.
A bilingual and bicultural coinvestigator (Martínez) reviewed the audio recording of each interview, in the language in which it was conducted (Spanish), for familiarization. Next, the investigator used live coding that involved simultaneous manual analysis while listening to each recording (Parameswaran et al., 2020), a procedure that minimizes misinterpretation of nuances such as cultural context and meaning in non-English language. Notes were taken on developing themes and key words or phrases to produce an initial coding schematic (Braun & Clarke, 2006; Parameswaran et al., 2020) using ATLAS.ti (Version 8.4.14; ATLAS.ti Scientific Software Development GmbH; Berlin, Germany) qualitative analysis software. Pertinent quotes were transcribed in Spanish if they helped to illustrate specific themes. The investigator listened to the recordings once more to complete the coding process. Next, candidate themes and transcribed quotes were translated to English for review by the principal investigator (Schepens Niemiec). The two investigators discussed this content to reach consensus.
Results
Findings from the original pre–postintervention assessment are reported in Schepens Niemiec et al. (2018). Of the 40 pilot study participants, 27 were available for reassessment, 11 were unable to be reached, and 2 declined to participate (1 because of illness, and 1 was not interested in participating). Participants who received LTFU (Table 1) did not differ significantly from nonparticipants on any baseline characteristics or pre- to posttreatment outcome changes (data not shown). A subset of outcomes originally reported (i.e., HbA1c, cholesterol, coronary heart disease risk, diabetes risk) were not included in this study because of limited available data primarily resulting from equipment failure.
Table 1.
Characteristics of Long-Term Follow-Up Study Participants (N = 27) at Baseline
| Characteristic | n (%) |
| Age, yr, M (SD) | 56.9 (4.8) |
| Sex: female | 26 (96.3) |
| Birthplace | |
| Mexico | 19 (70.4) |
| Central America | 7 (25.9) |
| Other | 1 (3.7) |
| Education level | |
| <High school | 17 (63.0) |
| High school degree | 7 (25.9) |
| Some college, business, or trade | 3 (11.1) |
| ≥College graduate | 0 |
| Household income,a $ | |
| ≤999/mo | 16 (59.3) |
| 1,000–1,999/mo | 10 (37.0) |
| Years in United States | |
| ≤20 | 7 (25.9) |
| >20 | 20 (74.1) |
| Relationship status | |
| Married or committed | 16 (59.3) |
| Single, widowed, or divorced | 11 (40.7) |
| Employed | |
| No | 20 (74.1) |
| Yesb | 7 (25.9) |
| Insurance | |
| Medi-Cal | 22 (81.5) |
| Private or other | 5 (18.5) |
| Emergency room visit past 12 moa | |
| No | 15 (55.6) |
| Yes | 11 (40.7) |
| Hospitalized past 12 moa | |
| No | 20 (74.1) |
| Yes | 6 (22.2) |
| Body mass index, kg/m2, M (SD) | 32.3 (6.9) |
Totals may not add to N = 27 because of missing data. bMean hours per week = 22.3.
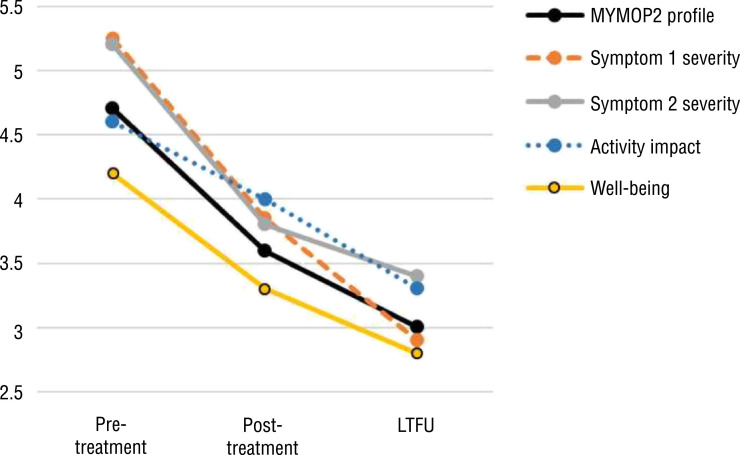
Participants demonstrated significant positive pretreatment to LTFU changes on all dimensions of the MYMOP2 (mean change score ≥ 0.8; Cohen’s d ≤ −0.8; p ≤ .004), with additional significant gains observed from posttreatment to LTFU (mean change score ≥ 0.7; d ≤ −0.4; p ≤ .05) for all but the Symptom 2 severity subscale (Figure 1). (As a note, pain was the most frequently identified symptom at baseline.) At least 75% of each pre- to postclinical improvement (whether significant or nonsignificant; i.e., for systolic blood pressure, diastolic blood pressure, stress, sleep, social roles satisfaction, and social activity satisfaction) was retained at LTFU (Table 2).
Figure 1.
Symptom well-being outcome as assessed at multiple time points using the MYMOP2 among participants with LTFU (N = 27)
Note. Lower scores indicate improvements in outcome. The MYMOP2 profile score is the mean of the four subscales. All change values were significant from pretreatment to LTFU (p ≤ .004) and from posttreatment to LTFU (p ≤ .05) except for Symptom 2 severity at posttreatment to LTFU (p = .2). Effect sizes were medium to large, ranging from −0.8 to −1.3 for pretreatment to LTFU and −0.4 to −0.7 for posttreatment to LTFU. LTFU = 1-yr long-term follow-up; MYMOP2 = Measure Yourself Medical Outcome Profile
Table 2.
Secondary Outcomes at Multiple Time Points Among Participants With LTFU (N = 27)
| Health Outcome | M (SD) | Pretreatment vs. LTFU, n = 25–27a | Posttreatment vs. LTFU, n = 25–27a | ||||||
| Pretreatment | Posttreatment | LTFU | Changeb (SD) | p c | ES [95% CI] | Changeb (SD) | p c | ES [95% CI] | |
| Blood pressure | |||||||||
| Systolic | 128.9 (18.0) | 121.6 (18.1) | 121.6 (18.6) | −7.7 (16.8) | .05 | −0.4 [−0.8, −0.1] | −1.0 (16.5) | .46 | −0.1 [−0.4, 0.3] |
| Diastolic | 75.3 (11.1) | 74.1 (9.3) | 71.2 (9.5) | −4.2 (10.6) | .09 | −0.4 [−0.8, 0.0] | −3.1 (8.8) | .14 | −0.3 [−0.7, 0.0] |
| Weight, kg | 80.3 (17.4) | 80.3 (17.1) | 80.5 (18.0) | 0.1 (4.0) | .90 | 0.0 [−0.1, 0.1] | 0.2 (4.8) | .72 | 0.0 [−0.1, 0.1] |
| Stress | 2.9 (1.5) | 2.4 (1.3) | 2.4 (1.4) | 0.5 (1.3) | .06 | −0.3 [−0.6, 0.0] | 0 (1.3) | .95 | 0.0 [−0.4, 0.4] |
| Sleep disturbance | 8.4 (5.1) | 7.6 (4.9) | 5.8 (4.5) | −2.7 (4.6) | .005 | −0.5 [−0.9, −0.2] | −1.7 (3.2) | .004 | −0.4 [−0.6, −0.1] |
| Social roles satisfaction | 21.9 (7.8) | 25.9 (7.2) | 24.9 (8.2) | 3.0 (6.8) | .03 | 0.4 [0.1, 0.7] | −1.0 (5.0) | .38 | −0.1 [−0.4, 0.1] |
| Social activity satisfaction | 19.3 (6.2) | 22.6 (7.0) | 22.3 (7.8) | 3.0 (6.3) | .01 | 0.5 [0.1, 0.9] | −0.3 (5.4) | .72 | 0.0 [−0.3, 0.2] |
| Physical activity | 1.9 (0.8) | 1.8 (0.8) | 1.6 (0.8) | 0.4 (1.0) | .06 | −0.4 [−0.9, 0.0] | −0.2 (1.0) | .35 | −0.3 [−0.8, 0.2] |
Note. CI = confidence interval; ES = effect size (Cohen’s d ); LTFU = 1-yr long-term follow-up.
Sample size varies for each measure because of missing data. bChange values may not equal LTFU minus pretreatment or LTFU minus posttreatment because of missing data. cWilcoxon signed rank test.
Improved levels at posttreatment in systolic blood pressure, stress, and social satisfaction were maintained at LTFU. A nonsignificant decrease in sleep disturbance from pre- to posttreatment was supplemented with a further reduction from posttreatment to LTFU, such that pretreatment to LTFU (d = −0.5, p =.005) and posttreatment to LTFU (d = −0.4, p = .004) changes were statistically significant. Physical activity and weight did not change significantly from pretreatment to LTFU (d ≤ 0.0, p ≥ .06) or from posttreatment to LTFU (d ≤ 0.0, p ≥ .35). Patient activation, measured only at postintervention (M = 73.3, SD = 19.1) and LTFU (M = 74.6, SD = 22.1), did not change (d = 0.0, p = .83) but fell within the highest level of activation (i.e., >67; Insignia Health® scoring rules) in both instances. The sample reported the following pain characteristics and heart-healthy, food-related behaviors, measured only at LTFU: pain severity (M = 3.0, SD = 2.6), pain interference (M = 2.9, SD = 3.0), salt intake (M = 2.8, SD = 0.7), fat consumption (M = 2.8, SD = 0.6), and weight control behaviors (M = 3.1, SD = 0.6).
In response to the item “Since the program ended, I used what I learned in ¡VMV! to actively try to improve my health,” most respondents answered agree or strongly agree (mean rating = 4.5, SD = 0.7, range = 3–5). Analysis of subjective experiences across the year revealed four interrelated themes: (1) health-promoting knowledge and practices, (2) mental well-being, (3) impact on the broader community, and (4) looking toward the future (Appendix B). Respondents described meaningful improvements in their wellness-related knowledge, mental health, strength of social support networks, and ability to help others enhance their own well-being. They described that ¡VMV! increased their confidence in their ability to achieve better health and that the program gave them hope for their future.
After program conclusion, participants continued to set new goals to enrich their lives; 1 participant was learning a new language, something she said she would never have tried before ¡VMV!. Others shared their excitement for building on what they learned in ¡VMV! and discussed strategies they had adopted to overcome past barriers to health-related goals (e.g., exercising at a local gym instead of outside in hot weather).
At LTFU, all participants expressed deep gratitude for ¡VMV!: “I wasn’t expecting the program to change my life this much. It did something that even doctors or psychiatrists were not able to do.” They appreciated the personalized nature of ¡VMV! because the CHWs “visited like family members, not like strangers”; they also valued the attention that they were unaccustomed to receiving, as shown through statements such as “Thank you for focusing on older people like us. We can still change.” Participants reported using the intervention handouts and materials (e.g., measuring cups for portion control) even after a year had passed. Respondents voiced their desire for ¡VMV! to be longer so that they could continue to make progress toward their goals; respondents also wanted the program to be offered on a regular basis so that more members of their community could join and benefit from ¡VMV! in the same way.
Discussion
Evidence supporting the long-term benefits of occupation-based intervention in older people is uncommon in the occupational therapy research literature (Nielsen et al., 2017) and is nonexistent in primary care research given the newness of occupational therapy’s presence in this setting (Metzler et al., 2012). Long-term appraisals of lifestyle interventions led by CHWs are likewise rare. ¡VMV! holds promise in filling these evidentiary gaps in both occupational therapy and CHW practice by demonstrating that initial psychosocial and physiological health gains could be maintained for 12 mo after conclusion of a jointly led occupational therapy practitioner–CHW intervention for late-midlife Latino patients in primary care.
The reductions in symptom severity and negative impact of symptoms on daily activity, as well as improvements in overall well-being, evidenced clinical significance. Positive change in SWO from pretreatment to LTFU was uniform (mean change ≥ 0.8) for all MYMOP2 subscales, with medium to large effect sizes. Guyatt et al. (1998) suggested that clinical meaningfulness on a 7-point rating scale, such as the MYMOP2, is indicated with mean change scores of >0.5 (small), >1.0 (moderate), and >1.5 (large). Using this barometer, we found that SWO changed in a small to moderate clinically significant way. Furthermore, the 95% confidence intervals for the effect sizes revealed clinically meaningful effects even at the low end of the intervals. These changes in SWO showed additional statistically significant improvements from posttest to LTFU in all but one MYMOP2 value. Moreover, secondary self-reported outcomes, including stress, sleep disturbance, and social satisfaction, were also maintained—and reduced in the case of sleep disturbance—during the no-contact period.
Systolic blood pressure reduction from baseline to postintervention was maintained over the subsequent year. With clinically meaningful change in systolic blood pressure defined as >2 mm Hg (Ketola et al., 2000), the observed reduction of nearly 8 mm Hg over 1 yr is an encouraging result supportive of cardiovascular disease risk reduction. Weight, however, was maintained for the duration of the original and LTFU studies, which was expected given that this was not a focus of the intervention. Because physical activity, a key factor in healthy lifestyle, also did not change over the study, a stronger focus on embedding physical activity into daily routine for future iterations of ¡VMV! may be warranted to enhance cardiometabolic outcomes.
Although a subset of secondary outcomes were measured only after intervention (i.e., patient activation [postintervention, LTFU] and pain characteristics and food-related behaviors [LTFU]), results help to characterize our sample. Participants’ engagement in their own health care at both postintervention and LTFU (M ≥ 73.3) appeared to be higher than levels reported for Latinos after a targeted patient activation intervention (M = 58.7; Deen et al., 2011). Mean pain severity and interference levels appeared low (∼3 on a 0–10 scale), which corresponds with SWO results. Food-related behaviors (range = 2.8–3.1 on a scale ranging from 1 [never] to 4 [always]) were on par with frequency reported (2.8) after implementation of the National Heart, Lung, and Blood Institute’s heart health curriculum by CHWs for 501 Latinos (Hurtado et al., 2014).
Overall findings from this LTFU study were consistent with the lasting health benefits that resulted from the Well Elderly Lifestyle Redesign program (Clark et al., 2001). In the Well Elderly study, approximately 90% of the therapeutic gains in self-rated health outcomes were retained for 6 mo in the absence of intervention. The stability of observed improvements over time in ¡VMV! echoed that of Well Elderly, with 75% of health outcome improvements sustained after 1 yr of no contact among participants who returned for LTFU. Although findings from ¡VMV! cannot be directly compared with those of the Well Elderly studies because of lack of a control group and distinctive features of the program (e.g., occupational therapy practitioner–CHW collaboration, late-midlife target age, rural adaptation), results are encouraging that ¡VMV! may have similar, sustained effects on older people’s psychosocial well-being. The unique combination of lifestyle-centric occupational therapy and culturally sensitive education and social support proffered by CHWs may have contributed to these enduring health changes. Patients were guided on how to embed healthy habits into their daily routines in practical ways. In doing so, ¡VMV! may have activated self-sustainable, healthy lifestyle practices with the necessary impetus for long-term change.
Interview responses supported the quantitative findings. ¡VMV! participants reported a deep-seated, long-term impact of the intervention, particularly in their health-related knowledge, health-promoting practices, and mental well-being. They felt that they could extend ¡VMV! to their friends, family, and community by sharing their knowledge and skills with others. Participants stressed that ¡VMV! had changed and continued to influence their outlook on health and wellness goals for the future. These findings are contrary to those of Lifestyle Matters (Chatters et al., 2017): a U.K.-adapted version of the Well Elderly Lifestyle Redesign approach that included a 24-mo qualitative follow-up study. Authors of the Lifestyle Matters LTFU study speculated that most participants were already well engaged in diverse activities, were highly resourceful, and were not at a point in their lives when a wellness intervention could have the most impact (i.e., during a time when life is demanding transition and change). ¡VMV! participants, however, may have experienced a lasting positive impression as a result of their traditionally underresourced sociocultural context or because the intervention was presented at an optimal time in their lives when change was crucial. As was true in the original pilot study (Schepens Niemiec et al., 2018), participants reaffirmed the need to extend program length to facilitate additional personal health gains.
Limitations
This LTFU study had several limitations. Sample size was small, and there was no control group. Consequently, results can neither be directly attributed to ¡VMV! nor widely generalized. A future large-scale randomized controlled trial with LTFU is warranted. Responses to self-report measures and interview questions may have been subject to bias. Social desirability is a source of bias associated with cultural norms among Latinos (Hopwood et al., 2009). The missing data for HbA1c, cholesterol, coronary heart disease risk, and diabetes risk left us with only blood pressure and weight as objective outcomes, and these outcomes showed mixed results. With use of MYMOP2 without a control group, regression to the mean is a concern because it asks about self-selected, currently troublesome symptoms. Finally, the measurement tools we selected for dietary intake changed from the original study to the LTFU study to reduce participant burden. This change precluded us from comparing dietary behaviors after intervention cessation.
Implications for Occupational Therapy Practice
The results of this study have the following implications for occupational therapy practice:
The observed tendency toward sustained positive outcomes is consistent with the American Occupational Therapy Association’s Vision 2025 to embrace diversity, accessibility, and collaborative care as well as its position on occupational therapy’s burgeoning role in health promotion through occupation (American Occupational Therapy Association, 2013, 2017).
¡VMV! included a final module that explicitly addressed preparation for postintervention continuance of healthy habits (Schepens Niemiec et al., 2019), an emphasis that could potentially be implemented in other occupational therapy contexts.
Although the results of the study are encouraging and consistent with prior research (e.g., Clark et al., 2001), they suggest a need for improved attention to factors that promote long-term intervention effects in alternate treatment populations or settings (e.g., older adults in poor health, assisted living facilities).
¡VMV! provides a model of how occupational therapy practitioners can successfully work in tandem with CHWs: a group that is an underused yet valuable resource in primary care and that creates a trusted bridge to the community (Hartzler et al., 2018).
Conclusion
This study is the first to demonstrate sustained treatment effects for this type of interdisciplinary collaboration in primary care, and it suggests the possibility of extending this model to alternate practice contexts. Our results bolster the prospect for occupational therapy’s cost-effectiveness because of the lack of any added expense associated with gains that endure after intervention completion. To better understand this potential for cost-efficient outcomes, future researchers could routinely incorporate extended follow-up intervals postintervention and subsequent cost-effectiveness analyses. Consistent documentation of long-term effects would boost occupational therapy practitioners’ awareness of the impact of treatment and add important information to the profession’s growing evidence base.
Acknowledgments
We thank the participants, as well as Antelope Valley Partners for Health, Antelope Valley Community Clinic, and the Southern California Clinical and Translational Science Institute, for their support of this project. This work was funded in part by the National Center for Medical and Rehabilitation Research and the National Institute of Neurological Disorders and Stroke (Grant K12-HD-055929 awarded to Stacey L. Schepens Niemiec) and by an internal award through the University of Southern California Occupational Science and Occupational Therapy Initiatives Program (awarded to Stacey L. Schepens Niemiec).
Appendix A. Structured Exit Interview Questions and Prompts
The following questions were used for the structured exit interview:
What do you remember most about the ¡Vivir Mi Vida! program?
Tell me about any healthy lifestyle changes that you made because of the program and that you are continuing to do today.
Since the program ended, what obstacles have you encountered while trying to maintain or improve your health?
The following potential prompts were used for the structured exit interview:
What about the program helped you to make these changes?
How could the program have been different to help you make healthy lifestyle changes?
How could the program have been different to help better prepare you for those obstacles?
Appendix B. Exit Interview Summary of Results
Table B.1.
Emergent Themes and Exemplar Quotes of the Structured Exit Interview
| Interview Theme | Example 1 | Example 2 | Example 3 |
| Health promoting knowledge and practices | “I learned to buy healthier foods, check nutrition labels, measure serving sizes, and reduce food portions.” | “When I go out to a get-together there are lots of foods available, but now I know what is good for me and what to stay away from.” | “I learned that I am the one that decides to be healthier, to exercise, and eat better.” |
| Mental well-being | “When I am anxious, I go out for a walk . . . It helps. I come back calm, more relaxed.” | “It [¡Vivir Mi Vida!] helped me get back to living . . . Now I want to live my life and spend time with others.” | “I felt depressed and like no one understood me. [Since ¡Vivir Mi Vida!] I have started to feel different. I feel better every day.” |
| Impact on the broader community | “My coworkers saw me eating better and would ask me about the program.” | “I think about how to [use what I learned to] help my family and my parents. I’m reconnecting with my friends and family.” | “I learned that being healthy isn’t just for me. It’s something to share with my family.” |
| Looking toward the future | “For me, [¡Vivir Mi Vida!] is about progressive learning. It is about applying the lessons and knowledge from the program.” | “The climate in Antelope Valley makes it hard to exercise outside. I plan to go to a gym to keep losing weight.” | “[Before ¡Vivir Mi Vida!] I didn’t want to leave my home. Now I want to learn English and find a place where I can learn computer skills.” |
Contributor Information
Stacey L. Schepens Niemiec, Stacey L. Schepens Niemiec, PhD, OTR/L, is Associate Professor of Research, Mrs. T. H. Chan Division of Occupational Science and Occupational Therapy, University of Southern California, Los Angeles; schepens@usc.edu
Cheryl L. P. Vigen, Cheryl L. P. Vigen, PhD, is Associate Professor of Research, Mrs. T. H. Chan Division of Occupational Science and Occupational Therapy, University of Southern California, Los Angeles.
Jenny Martínez, Jenny Martínez, OTD, OTR/L, BCG, is Associate Professor, Department of Occupational Therapy, Thomas Jefferson University, Philadelphia, PA..
Jeanine Blanchard, Jeanine Blanchard, PhD, OTR/L, is Project Manager, Mrs. T. H. Chan Division of Occupational Science and Occupational Therapy, University of Southern California, Los Angeles..
Mike Carlson, Mike Carlson, PhD, is Professor of Research, Mrs. T. H. Chan Division of Occupational Science and Occupational Therapy, University of Southern California, Los Angeles..
References
- American Occupational Therapy Association. (2013). Occupational therapy in the promotion of health and well-being. American Journal of Occupational Therapy, 67(6, Suppl.), S47–S59. 10.5014/ajot.2013.67S47 [DOI] [Google Scholar]
- American Occupational Therapy Association. (2017). Vision 2025. American Journal of Occupational Therapy, 71, 7103420010. 10.5014/ajot.2017.713002 [DOI] [PubMed] [Google Scholar]
- Backhaus, J., Junghanns, K., Broocks, A., Riemann, D., & Hohagen, F. (2002). Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research, 53, 737–740. 10.1016/s0022-3999(02)00330-6 [DOI] [PubMed] [Google Scholar]
- Boling, P. A., Chandekar, R. V., Hungate, B., Purvis, M., Selby-Penczak, R., & Abbey, L. J. (2013). Improving outcomes and lowering costs by applying advanced models of in-home care. Cleveland Clinic Journal of Medicine, 80(Suppl. 1), eS7–eS14. 10.3949/ccjm.80.e-s1.03 [DOI] [PubMed] [Google Scholar]
- Braun, V., & Clarke, V. (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3, 77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- Buttorff, C., Ruder, T., & Bauman, M. (2017). Multiple chronic conditions in the United States. RAND Corporation. 10.7249/TL221 [DOI] [Google Scholar]
- Cella, D., Riley, W., Stone, A., Rothrock, N., Reeve, B., Yount, S., . . . Choi, S. (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63, 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2015). National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm
- Chatters, R., Roberts, J., Mountain, G., Cook, S., Windle, G., Craig, C., & Sprange, K. (2017). The long-term (24-month) effect on health and well-being of the Lifestyle Matters community-based intervention in people aged 65 years and over: A qualitative study. BMJ Open, 7, e016711. 10.1136/bmjopen-2017-016711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, F., Azen, S. P., Carlson, M., Mandel, D., LaBree, L., Hay, J., . . . Lipson, L. (2001). Embedding health-promoting changes into the daily lives of independent-living older adults: Long-term follow-up of occupational therapy intervention. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 56, 60–63. 10.1093/geronb/56.1.P60 [DOI] [PubMed] [Google Scholar]
- Clark, F., Azen, S. P., Zemke, R., Jackson, J., Carlson, M., Mandel, D., . . . Lipson, L. (1997). Occupational therapy for independent-living older adults: A randomized controlled trial. JAMA, 278, 1321–1326. 10.1001/jama.1997.03550160041036 [DOI] [PubMed] [Google Scholar]
- Craig, C., Marshall, A., Sjöström, M., Bauman, A., Booth, M., Ainsworth, B., . . . Sallis, J. (2003). International Physical Activity Questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise, 35, 1381–1395. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- de Andrés Ares, J., Cruces Prado, L. M., Canos Verdecho, M. A., Penide Villanueva, L., Del Valle Hoyos, M., Herdman, M., . . . Velázquez Rivera, I. (2015). Validation of the Short Form of the Brief Pain Inventory (BPI–SF) in Spanish patients with non-cancer-related pain. Pain Practice, 15, 643–653. 10.1111/papr.12219 [DOI] [PubMed] [Google Scholar]
- Deen, D., Lu, W. H., Rothstein, D., Santana, L., & Gold, M. R. (2011). Asking questions: The effect of a brief intervention in community health centers on patient activation. Patient Education and Counseling, 84, 257–260. 10.1016/j.pec.2010.07.026 [DOI] [PubMed] [Google Scholar]
- Elo, A.-L., Leppänen, A., & Jahkola, A. (2003). Validity of a single-item measure of stress symptoms. Scandinavian Journal of Work, Environment and Health, 29, 444–451. 10.5271/sjweh.752 [DOI] [PubMed] [Google Scholar]
- Guyatt, G. H., Juniper, E. F., Walter, S. D., Griffith, L. E., & Goldstein, R. S. (1998). Interpreting treatment effects in randomised trials. BMJ, 316, 690–693. 10.1136/bmj.316.7132.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler, A. L., Tuzzio, L., Hsu, C., & Wagner, E. H. (2018). Roles and functions of community health workers in primary care. Annals of Family Medicine, 16, 240–245. 10.1370/afm.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard, J. H., Mahoney, E. R., Stockard, J., & Tusler, M. (2005). Development and testing of a short form of the Patient Activation Measure. Health Services Research, 40, 1918–1930. 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood, C. J., Flato, C. G., Ambwani, S., Garland, B. H., & Morey, L. C. (2009). A comparison of Latino and Anglo socially desirable responding. Journal of Clinical Psychology, 65, 769–780. 10.1002/jclp.20584 [DOI] [PubMed] [Google Scholar]
- Hurtado, M., Spinner, J. R., Yang, M., Evensen, C., Windham, A., Ortiz, G., . . . Ivy, E. D. (2014). Knowledge and behavioral effects in cardiovascular health: Community Health Worker Health Disparities Initiative, 2007–2010. Preventing Chronic Disease, 11, 130250. 10.5888/pcd11.130250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, S., Bann, C. M., Dodd, S. L., Schein, J., Mendoza, T. R., & Cleeland, C. S. (2004). Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clinical Journal of Pain, 20, 309–318. 10.1097/00002508-200409000-00005 [DOI] [PubMed] [Google Scholar]
- Ketola, E., Sipilä, R., & Mäkelä, M. (2000). Effectiveness of individual lifestyle interventions in reducing cardiovascular disease and risk factors. Annals of Medicine, 32, 239–251. 10.3109/07853890009011767 [DOI] [PubMed] [Google Scholar]
- Koopman, R. J., Mainous, A. G., III, & Geesey, M. E. (2006). Rural residence and Hispanic ethnicity: Doubly disadvantaged for diabetes. Journal of Rural Health, 22, 63–68. 10.1111/j.1748-0361.2006.00009.x [DOI] [PubMed] [Google Scholar]
- Leland, N. E., Fogelberg, D. J., Halle, A. D., & Mroz, T. M. (2017). Occupational therapy and management of multiple chronic conditions in the context of health care reform. American Journal of Occupational Therapy, 71, 7101090010. 10.5014/ajot.2017.711001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina, A., Balcázar, H., Hollen, M. L., Nkhoma, E., & Mas, F. S. (2007). Promotores de salud: Educating Hispanic communities on heart-healthy living. American Journal of Health Education, 38, 194–202. 10.1080/19325037.2007.10598970 [DOI] [Google Scholar]
- Metzler, C. A., Hartmann, K. D., & Lowenthal, L. A. (2012). Defining primary care: Envisioning the roles of occupational therapy. American Journal of Occupational Therapy, 66, 266–270. 10.5014/ajot.2010.663001 [DOI] [PubMed] [Google Scholar]
- Nielsen, T. L., Petersen, K. S., Nielsen, C. V., Strøm, J., Ehlers, M. M., & Bjerrum, M. (2017). What are the short-term and long-term effects of occupation-focused and occupation-based occupational therapy in the home on older adults’ occupational performance? A systematic review. Scandinavian Journal of Occupational Therapy, 24, 235–248. 10.1080/11038128.2016.1245357 [DOI] [PubMed] [Google Scholar]
- Parameswaran, U. D., Ozawa-Kirk, J. L., & Latendresse, G. (2020). To live (code) or to not: A new method for coding in qualitative research. Qualitative Social Work, 19, 630–644. 10.1177/1473325019840394 [DOI] [Google Scholar]
- Paterson, C. (1996). Measuring outcomes in primary care: A patient generated measure, MYMOP, compared with the SF-36 Health Survey. BMJ, 312, 1016–1020. 10.1136/bmj.312.7037.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyatak, E., King, M., Vigen, C. L. P., Salazar, E., Diaz, J., Schepens Niemiec, S. L., . . . Shukla, J. (2019). Addressing diabetes in primary care: Hybrid effectiveness–implementation study of Lifestyle Redesign® occupational therapy. American Journal of Occupational Therapy, 73, 7305185020. 10.5014/ajot.2019.037317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samper-Ternent, R., Kuo, Y. F., Ray, L. A., Ottenbacher, K. J., Markides, K. S., & Al Snih, S. (2012). Prevalence of health conditions and predictors of mortality in oldest old Mexican Americans and non-Hispanic Whites. Journal of the American Medical Directors Association, 13, 254–259. 10.1016/j.jamda.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano, Y., Garasky, S., Greder, K. A., Cook, C. C., & Browder, D. E. (2011). Understanding food insecurity among Latino immigrant families in rural America. Journal of Family and Economic Issues, 32, 111–123. 10.1007/s10834-010-9219-y [DOI] [Google Scholar]
- Schepens Niemiec, S. L., Blanchard, J., Vigen, C. L. P., Martínez, J., Guzmán, L., Concha, A., . . . Carlson, M. (2018). Evaluation of ¡Vivir Mi Vida! to improve health and wellness of rural-dwelling, late middle-aged Latino adults: Results of a feasibility and pilot study of a lifestyle intervention. Primary Health Care Research and Development, 19, 448–463. 10.1017/S1463423617000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens Niemiec, S. L., Vigen, C., Blanchard, J., Martínez, J., Guzmán, L., & Carlson, M. (2019). A pilot study of the ¡Vivir Mi Vida! lifestyle intervention for rural-dwelling, late-midlife Latinos: Study design and protocol. OTJR: Occupation, Participation and Health, 39, 5–13. 10.1177/1539449218762728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacciarini, J. M., Smith, R., Garvan, C. W., Wiens, B., & Cottler, L. B. (2015). Rural Latinos’ mental wellbeing: A mixed-methods pilot study of family, environment and social isolation factors. Community Mental Health Journal, 51, 404–413. 10.1007/s10597-014-9774-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanton, S. L., Leff, B., Wolff, J. L., Roberts, L., & Gitlin, L. N. (2016). Home-based care program reduces disability and promotes aging in place. Health Affairs, 35, 1558–1563. 10.1377/hlthaff.2016.0140 [DOI] [PubMed] [Google Scholar]