Abstract
Background
Despite curative intent resection in patients with non-small cell lung cancer (NSCLC), recurrence leading to mortality remains too common. Melatonin has shown promise for the treatment of patients with lung cancer; however, its effect following cancer resection has not been studied. We evaluated if melatonin taken after complete resection reduces lung cancer recurrence and mortality, or impacts quality of life (QOL), symptomatology or immune function.
Methods
Participants received melatonin (20 mg) or placebo nightly for one year following surgical resection of primary NSCLC. The primary outcome was two-year disease-free survival (DFS). Secondary outcomes included five-year DFS, adverse events, QOL, fatigue, sleep, depression, anxiety, pain, and biomarkers assessing for immune function/inflammation. This study is registered at https://clinicaltrials.gov NCT00668707.
Findings
709 patients across eight centres were randomized to melatonin (n = 356) versus placebo (n = 353). At two years, melatonin showed a relative risk of 1·01 (95% CI 0·83–1·22), p = 0·94 for DFS. At five years, melatonin showed a hazard ratio of 0·97 (95% CI 0·86–1·09), p = 0·84 for DFS. When stratified by cancer stage (I/II and III/IV), a hazard reduction of 25% (HR 0·75, 95% CI 0·61–0·92, p = 0·005) in five-year DFS was seen for participants in the treatment arm with advanced cancer (stage III/IV). No meaningful differences were seen in any other outcomes.
Interpretation
Adjuvant melatonin following resection of NSCLC does not affect DFS for patients with resected early stage NSCLC, yet may increase DFS in patients with late stage disease. Further study is needed to confirm this positive result. No beneficial effects were seen in QOL, symptoms, or immune function.
Funding
This study was funded by the Lotte and John Hecht Memorial Foundation and the Gateway for Cancer Research Foundation.
Panel Research In Context.
Evidence before this study
Despite many advances in the treatment of NSCLC, recurrence leading to mortality remains common. Melatonin has shown promise for the treatment of various cancers, including NSCLC. A systematic review and meta-analysis of human RCTs was conducted by our research team in 2005 assessing for relative risks of mortality at one year. Studies that were included used melatonin as either sole treatment or as adjunct treatment and involved patients of any age, sex, or cancer stage. The results of our meta-analysis showed a relative risk reduction of 34% in the treatment arm. Similar results were obtained when the population was restricted to those with NSCLC. Despite favourable outcomes, there is a lack of rigorous evidence supporting melatonin in the treatment of NSCLC. At the time of our systematic review, there were no blinded or placebo-controlled clinical trials evaluating the effect of melatonin on recurrence and mortality in patients with NSCLC.
Added value of this study
This is the first randomized, placebo-controlled, blinded phase III clinical trial evaluating the effect of melatonin on recurrence and mortality in patients with resected NSCLC. Our results provide evidence against the use of melatonin in the prevention of recurrence and mortality in patients with early stage resected NSCLC, and potential evidence supporting its use in patients with late stage resected NSCLC. We also present evidence against the use of melatonin for chemo- and radiotherapy side effects, quality of life, fatigue, sleep, depression, anxiety, and pain at a dose of 20 mg in this population.
Implications of all available evidence
Taken together, evidence suggests the use of melatonin at a dose of 20 mg for patients with early stage NSCLC is not warranted. The results of our study outweigh those previously conducted. There is potential evidence to support its use in patients with late stage resected NSCLC; however, confident conclusions cannot be made based on the results of our study and other available evidence. Another placebo-controlled, blinded RCT designed around patients with late stage resected NSCLC is needed to confirm these results.
Alt-text: Unlabelled box
1. Introduction
Lung cancer accounts for the greatest incidence of cancer and cancer mortality worldwide. According to a 2018 report by the International Agency for Research on Cancer, lung cancer was responsible for 1·7 million deaths yearly, more than double that of any other cancer [1]. Non-small-cell lung cancers (NSCLC) comprise the vast majority of cases, accounting for 80% of lung cancer diagnoses [2]. Surgical resection, possibly followed by adjuvant platinum-based chemotherapy, remains the standard of care, offering the best long-term care for patients with resectable disease who can tolerate surgery [3]. Continual advances in the characterization of NSCLC have also led to targeted immunotherapies with the goal of prolonging survival for those with locally advanced or metastatic lung cancer [4]. Despite optimal therapy, however, recurrence occurs in too high a proportion of patients [5].
Melatonin is a natural health product that has shown promise for the treatment of various cancers. It has demonstrated anticancer activity in the laboratory, in observational studies, and in numerous randomized clinical trials [6], [7], [8]. We completed a systematic review and meta-analysis in 2005 assessing for relative risks of mortality at one year (n = 9 clinical trials) involving multiple solid tumours and found a relative risk reduction of 34% (RR: 0·66 (95% CI: 0·59–0·73), p < 0·001) [8]. Similar results were obtained after restricting the meta-analysis to trials in NSCLC (RR: 0·68 (95% CI: 0·54–0·85), p < 0·001). There is also evidence to suggest potential synergistic effects when melatonin is combined with chemotherapy [9,10], and antitoxic effects of melatonin on chemotherapy have been observed in some clinical studies [11,12]. In a recent update of this review we found that the addition of melatonin alongside chemotherapy significantly reduced the incidence and/or severity of asthenia, leucopenia, nausea and vomiting, hypotension, and thrombocytopenia [13]. Confirmation and validation of these results are needed from independent and methodologically strong clinical research.
Given that surgical resection is the standard of care for most patients with NSCLC [3]. there is a need for rigorous clinical research in this population. Melatonin is commonly used by the public and is recommended more than 50% of the time postoperatively for people with lung cancer by naturopathic doctors who focus on cancer care [14]. To date, there have been no randomized, placebo-controlled clinical trials evaluating the effects of adjuvant melatonin on recurrence and mortality in patients with resected cancers. If effective, melatonin could provide a safe, low-cost adjunct therapy for patients undergoing surgical resection to improve long-term risks of recurrence and mortality.
In this phase III study, we evaluated the effectiveness of adjuvant melatonin compared to a placebo in the prevention of cancer recurrence and mortality two years after surgical resection of NSCLC. We compared time to recurrence or mortality up to five years post-surgery and the effect on adverse events (AEs), quality of life (QOL), anxiety, depression, pain, sleep and fatigue.
In the lab, many mechanisms have been proposed for melatonin's anticancer effect. However, very little work has been done to explore a mechanistic pathway and serological surrogates for melatonin's effects in NSCLC patients [15]. We expanded the parameters of the AMPLCaRe study with a nested sub-study investigating the possibility that melatonin regulates mediators of systemic inflammation and immune activation. These markers have been shown to have good prognostic value in patients with NSCLC [16], [17], [18], [19], [20], [21], [22]. but it is not known to what extent they may be modulated by melatonin.
2. Methods
2.1. Design
The study was a two-arm, placebo-controlled, double blind, phase III randomized controlled trial. Consented patients were stratified by cancer stage and randomized to receive oral melatonin (20 mg) or placebo for one year post-surgery (Fig. 1). Participants were instructed to take the study product nightly approximately one hour before bedtime. Participants received questionnaires for QOL, anxiety, depression, pain, fatigue, and sleep, and were followed for AEs 24 months postoperatively. Participants were followed for recurrence and mortality up to 60 months postoperatively. This study followed all CONSORT guidelines for clinical trials.
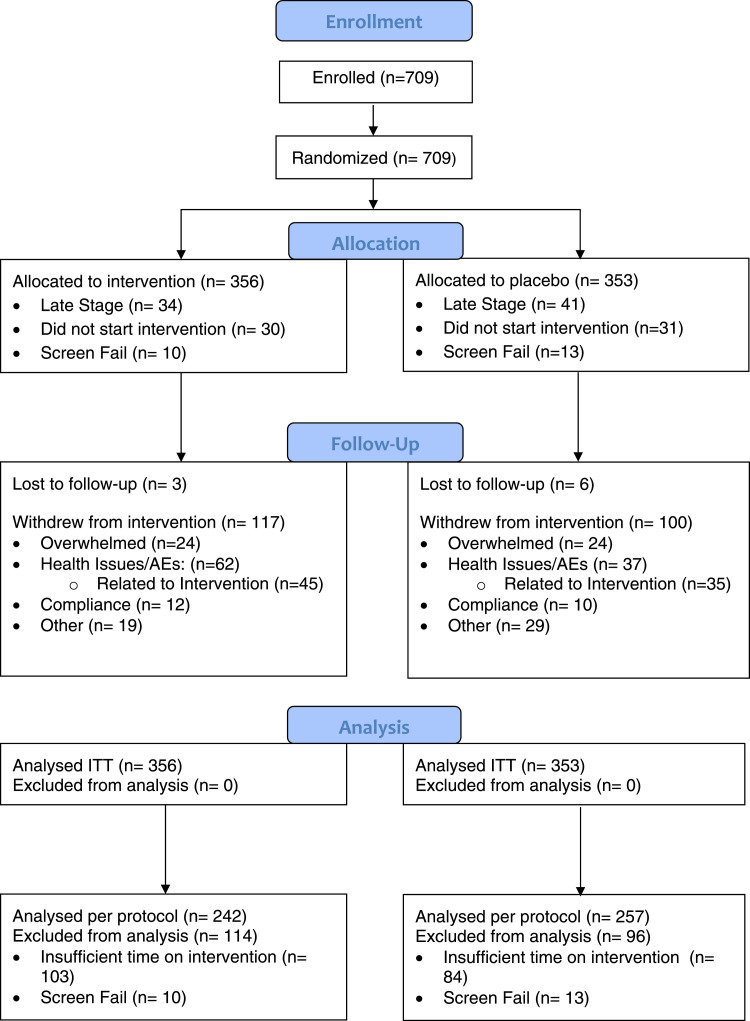
Fig. 1.
Consort Flow Diagram (Main Study). Includes all enrolled participants. ITT: intention to treat.
2.2. Participants
Patients were included in the study if they were adults with primary NSCLC eligible for complete surgical resection. Patients were excluded if they were already taking exogenous melatonin, had an incomplete resection (i.e., positive margins or synchronous lesions), or were pregnant or breastfeeding. Enrolment began in October 2007 and completed in September 2015. The last date of follow-up was November 30th, 2017. A total of 709 patients (356 melatonin, 353 placebo) were enrolled and randomized (Fig. 1).
2.3. Setting
The primary site for enrolment and overall coordination of this study was The Ottawa Hospital (TOH), with the active engagement of the Ottawa Hospital Research Institute (OHRI). Through the collaboration of the Canadian Association of Thoracic Surgeons (CATS) AMPLCaRe Research Group, seven additional thoracic surgery centres were engaged, including: Surrey Memorial Hospital/Fraser Health Authority, Toronto's University Health Network, St. Joseph's Healthcare & McMaster University, London Health Sciences center, The Quebec Heart and Lung Institute, Kelowna General Hospital and Halifax's QEII Health Sciences center/Capital Health. Research personnel consisted of a central research team located at the Ottawa Hospital Research institute (OHRI) and the University of Ottawa, working with the Ottawa Integrative Cancer center (OICC). Study conduct was supported by site principal investigators, research coordinators, and nursing staff at each of the thoracic surgery units across Canada. Laboratory tests and analysis were done in Dr. Auer's OHRI laboratory.
2.4. Regulatory adherence
This study was approved by the Ottawa Health Sciences Research Ethics Board, Canadian College of Naturopathic Medicine's Research Ethics Board, and Health Canada by way of a No Objection Letter May 23rd, 2007. Registration for clinicaltrials.gov was submitted and made public on the website in April 2008. All participants signed an informed consent form prior to trial initiation after the study was explained to them by a research coordinator and all questions were answered.
2.5. Primary outcome
The primary outcome was two-year disease-free survival (DFS) measured by comparing the incidence of recurrence or mortality at two years post-surgery. Recurrence was determined through clinical examination by the participant's thoracic surgeon in addition to radiological evidence. Each site used their standard of care radiological assessments to determine recurrence, although minimal follow up included a mandated annual CT scan and clinical evaluation, as well as X-rays, PET scans, MRIs, and other imaging techniques as determined by the treating clinicians. If present, dates of recurrence and death were gathered through patient medical records. For those participants whose survival status could not be gathered through medical records, an obituary search was conducted.
2.6. Secondary outcomes
Secondary outcomes included assessing the impact of melatonin versus placebo on five-year DFS by comparing the time to recurrence or mortality. Additionally, the incidence of adverse effects caused by postoperative adjuvant chemotherapy and/or radiation and the impact on QOL, sleep, and fatigue was assessed in all participants. Participants at TOH were additionally assessed for anxiety, depression, and pain. AEs were collected by research coordinators through examination of participant diaries, interviews with participants, and chart review. AEs were classified based on severity and attribution to melatonin, surgery, chemotherapy, or radiation. QOL was assessed using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaires (Core-30 (QLQ-C30) and Lung Cancer-13 (QLQ-LC13) modules); [23] fatigue was assessed using the Multidimensional Fatigue Inventory 20 (MFI-20) [24] questionnaire; sleep was assessed using the Medical Outcomes Study (MOS) Sleep Survey; [25] depression was assessed using the Beck Depression Inventory 2 (BDI 2); [26] anxiety was assessed using the Beck Anxiety Inventory (BAI); [27] pain was assessed using the Brief Pain Inventory (BPI) [28].
2.7. Randomization & blinding
Randomization was conducted by the study pharmacist using a randomization list created by an independent statistician from the Ottawa Methods center. Participants were stratified into two groups dependent on their clinical cancer stage (I/II and III/IV) and randomized 1:1 using permuted blocks of four and six at each site. Participants, care providers, and researchers were blinded to the group assignment. Participants were provided pill bottles that were identical in appearance, except for the lot number, which allowed the pharmacy to identify melatonin and placebo. Both interventions consisted of small white capsules identical in appearance, smell and size.
2.8. Nested sub study
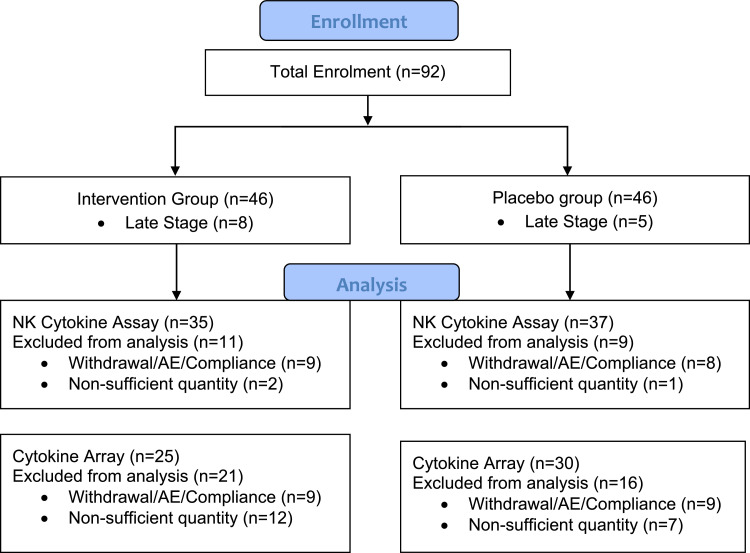
A subset of 92 participants from TOH were additionally enrolled in a blinded sub study evaluating the effect of melatonin versus placebo on mediators of systemic inflammation and immune activation. Participants were excluded if they withdrew between blood draws, experienced an AE around the time of their blood draw, or had poor compliance with the study product. 72 participants (35 melatonin, 37 placebo) were included in the analysis (Fig. 2). Participants underwent testing for Natural Killer (NK) cell function and phenotyping, as well as for blood levels of cytokines. Peripheral blood was drawn at baseline and six months following treatment initiation. Peripheral blood mononuclear cells were immediately separated by Ficoll Density centrifugation and cryopreserved in liquid nitrogen until testing. NK cell cytotoxicity was measured using either the Chromium-51 (Cr51) release assay or a flow cytometry fluorescence-based assay. In each assay the erythroleukemia cell line, K562, was used as targets. Measured cytokines included TGFβ, IL-2, TNFα, IFNα2, IL-4, IL-6, IL-10, IL-12 (p70), IL-15, IFNγ, GM-CSF, and VEGF.
Fig. 2.
Flow Diagram (Sub Study). Includes only those participants who were additionally enrolled in the sub-study.
2.9. Data quality
Study data was stored on a secure server at TOH in a password-protected file accessible only by delegated personnel. Ten percent of this data (71 participants) was doubly entered and checked for accuracy to the original dataset. All questionnaires were scored using algorithms provided by the owners of the respective questionnaires (Appendix 1·0). Five percent of case report forms from each site were chosen for remote data monitoring (by personnel not involved with data collection or entry) to check the accuracy to source documents. No concerns arose from any of these data checks.
2.10. Power calculations
Based on the results of our systematic review, we powered our trial to detect a relative risk reduction of one third. Assuming an outcome rate of death or recurrence of 30% at two years in the control arm, we required a sample of 294 per arm to obtain 80% power to detect a relative risk of 0·67. In order to account for up to 15% loss to follow-up, we inflated our target sample size to 346 per arm.
2.11. Statistical methods
The primary analysis was performed using an intention to treat approach. The primary outcome of two-year DFS was analyzed between treatment arms using unadjusted and adjusted logistic regressions. Adjustments included adjuvant chemotherapy, adjuvant radiation, and smoking history. Results were presented as relative risks. DFS up to five years post-surgery was compared using Kaplan-Meier curves and the log rank test, followed by a hazard ratio calculated using the Cox proportional hazard model adjusting for adjuvant chemotherapy, adjuvant radiation, and smoking history. A sub-analysis of DFS was additionally performed whereby participants were grouped based on randomization strata (stage I/II and III/IV). AEs were compared using the chi-square test and categorical baseline characteristics were assessed using frequency distributions. Change over time in questionnaire scores between arms was analyzed using mixed models. Between-arm least squares mean difference in change from randomization to 24 months post-surgery represented the effect of the intervention. NK cell cytotoxicity/phenotype and cytokine changes were compared within each group using the Wilcoxon signed-rank test and between groups using the Mann-Whitney U test.
In addition, a complementary ‘per-protocol’ analysis of each outcome was conducted whereby all participants who did not take the study product for at least three months or were screen fails (i.e., deemed ineligible post-randomization) were excluded (Fig. 1). Statistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute Inc, Cary, NC).
2.12. Role of funders
Financial support for this study was provided by the Lotte and John Hecht Memorial Foundation and the Gateway for Cancer Research Foundation. This study was investigator-led. The funders did not have any role in the design of the study, the collection, analysis, or interpretation of the data, the writing of this manuscript, or the decision to submit this manuscript for publication.
3. Results
3.1. Baseline data
A total of 709 patients (356 melatonin, 353 placebo) were randomized. Due to the randomization process, both groups were equally balanced and there were no clinically meaningful differences in their demographic characteristics, type of surgical operation, cancer type, stage of cancer, or preoperative comorbidities (Table 1). See Appendix 2.0, Table S7 for comorbidity data.
Table 1.
Baseline Characteristics.
| Melatonin N (%) | Placebo N (%) | |
| Age in years (Mean ± SD) | 67.2 ± 8.5 | 67.2 ± 8.6 |
| Male sex | 166 (46.6) | 145 (40.7) |
| Pre-operative Chemotherapy or Radiation Therapy | 8 (2.2) | 14 (3.9) |
| Current Smoker | 47 (13.2) | 52 (14.6) |
| Past Smoker | 279 (78.4) | 263 (73.9) |
| Never Smoked | 25 (7.0) | 31 (8.7) |
| Unknown | 5 (1.4) | 7 (2.0) |
| Histological Subtype (Non-Surgical N = 3) | ||
| Squamous Cell | 93 (26.2) | 72 (20.4) |
| Adenocarcinoma | 229 (64.5) | 239 (67.7) |
| Large Cell | 7 (2.0) | 15 (4.2) |
| Bronchoalveolar | 10 (2.8) | 8 (2.3) |
| Undifferentiated | 2 (0.6) | 3 (0.8) |
| Other | 14 (3.9) | 14 (4.0) |
| Operation Details (Non-Surgical: N = 3) | ||
| Incision Type | ||
| Open | 114 (32.2) | 122 (34.6) |
| VATS | 213 (60.2) | 212 (60.0) |
| VATS converted to open | 27 (7.6) | 19 (5.4) |
| Surgery Type | ||
| Pneumonectomy | 16 (4.5) | 21 (5.9) |
| Lobectomy | 290 (81.7) | 284 (80.5) |
| Segmentectomy | 18 (5.1) | 16 (4.5) |
| Wedge Resection | 31 (8.7) | 29 (8.2) |
| No Operation | 1 (0.3) | 1 (0.3) |
| Pathological Cancer Stage (Non-Surgical N = 3) | ||
| IA | 136 (38.3) | 120 (34.0) |
| IB | 109 (30.7) | 97 (27.5) |
| IIA | 40(11.3) | 57 (16.1) |
| IIB | 35 (9.9) | 35 (9.9) |
| IIIA | 31 (8.7) | 36 (10.2) |
| IIIB | 1 (0.3) | 2 (0.6) |
| IV | 2 (0.6) | 3 (0.8) |
Cancer stage as per the AJCC Lung Cancer TNM 7th edition. Melatonin N = 356; Placebo N = 353. VATS: video-assisted thoracoscopic surgery.
3.2. Primary outcome
For two-year disease-free survival (DFS), melatonin showed an adjusted relative risk of 1·01 (95% CI 0·83 – 1·22), p = 0·94 compared to a placebo. The per protocol analysis showed an adjusted relative risk of 1·12 (95% CI 0·96 – 1·32), p = 0·14. See Table 2 for event rates and unadjusted relative risks.
Table 2.
Two-Year Disease-Free Survival.
| Events (1) | Relative Risk (Unadjusted) RR (95% CI) | P Value | Relative Risk (Adjusted) (2) RR (95% CI) | P Value | ||
| Melatonin N (%) | Placebo N (%) | |||||
| Intention to Treat | 80 (22.5) | 85 (24.1) | 1.07 (0.81 – 1.41) | 0.64 | 1.01 (0.83 – 1.22) | 0.94 |
| Per Protocol | 58 (23.3) | 51 (20.4) | 1.24 (1.08 – 1.42) | 0.001 | 1.12 (0.96 – 1.32) | 0.14 |
Melatonin (N = 356); Placebo (N = 353).
(1) An event is defined as a recurrence or mortality (i.e., one or the other) within 2 years of surgery.
(2) Adjusted for adjuvant chemotherapy, adjuvant radiation, and baseline smoking status.
3.3. Secondary outcomes
Five-year median DFS was not reached in either arm. Melatonin showed a hazard ratio of 0·97 (95% CI 0·86–1·09), p = 0·84 for five-year DFS compared to placebo (Appendix 2.0, Figure S1). Fig. 3 shows the five-year DFS curves separated by cancer stage. Melatonin showed a hazard ratio of 0·97 (95% CI 0·85–1·11), p = 0·66 in the early stage group (I and II) and a hazard reduction of 25% (HR 0·75, 95% CI 0·61–0·92), p = 0·005 in the late stage group (III and IV). Five-year median DFS was not reached in the early stage group. In the late stage group, there was no difference in median DFS (Melatonin: 18.0 months [95% CI 9.4–26.6]; Placebo: 18.0 months [95% CI 2.2–23.8]). All per protocol analyses showed similar results (Appendix 2.0, Figure S1 and Table S9).
Fig. 3.
Five-Year Disease-Free Survival. Survival refers to the ratio of participants who have not experienced a recurrence or mortality at any given time. Stage I & II: Melatonin (n = 320); Placebo (n = 309). Stage III & IV: Melatonin (n = 34); Placebo (n = 41). Early Stage: Stage I & II; Late Stage: Stage III & IV. .
Of the 709 participants enrolled, 134 received adjuvant chemotherapy (66 melatonin, 68 placebo) and 43 received adjuvant radiation (22 melatonin, 21 placebo). Of these participants, 92 experienced at least one AE related to their chemotherapy (44 melatonin, 48 placebo, p = 0·62) and 13 experienced at least one AE related to their radiation (8 melatonin, 5 placebo, p = 0·37). There were no clinically or statistically significant differences in the number, severity, or seriousness of AEs between arms (data not shown).
There were no clinically or statistically significant differences between groups with regards to fatigue, QOL, or sleep at the one or two-year time points (Table 4). In the subset of participants from TOH, no clinically or statistically significant differences were seen regarding pain, depression, or anxiety (data not shown). Additionally, no clinically or statistically significant differences were seen in the per protocol analysis (Table S10, Appendix 2.0).
Table 4.
Questionnaire Scores.
| Interval | Difference in means (95% CI) | P Value | |
| Fatigue | 0–12M | −3.854 (−7.575 – −0.133) | 0.05 |
| 0–24M | −3.136 (−7.198 – 0.927) | 0.13 | |
| Quality of Life | |||
| Symptoms | 0–12M | 0.156 (−2.494 – 2.806) | 0.91 |
| 0–24M | 0.368 (−2.527 – 3.262) | 0.80 | |
| Functional | 0–12M | −1.537 (−4.692 – 1.618) | 0.34 |
| 0–24M | −0.703 (−4.150 – 2.743) | 0.70 | |
| Global Health | 0–12M | −3.829 (−8.087 – 0.428) | 0.08 |
| 0–24M | −3.800 (−8.451 – 0.850) | 0.11 | |
| LC13 | 0–12M | 1.246 (−1.047 – 3.539) | 0.29 |
| 0–24M | 0.637 (−1.875 – 3.150) | 0.62 | |
| Sleep | |||
| Sleep Adequacy | 0–12M | −3.536 (−8.986 – 1.195) | 0.20 |
| 0–24M | −4.057 (−9.975 – 1.862) | 0.18 | |
| Sleep Problems Index II | 0–12M | 1.215 (−1.920 – 4.350) | 0.48 |
| 0–24M | 1.440 (−1.968 – 4.847) | 0.40 | |
Scores represent a difference of means. Mean scores not shown. Fatigue scores calculated using the Multidimensional Fatigue Index 20 questionnaire. QoL calculated using the EORCT QLQ C30 and LC13 questionnaires. Sleep scores calculated using the MOS Sleep Survey. See Appendix 2.0 “Questionnaire Scoring Algorithms” for information on how questionnaires were scored. Melatonin (N = 356); Placebo (N = 353).
3.4. Sub study results
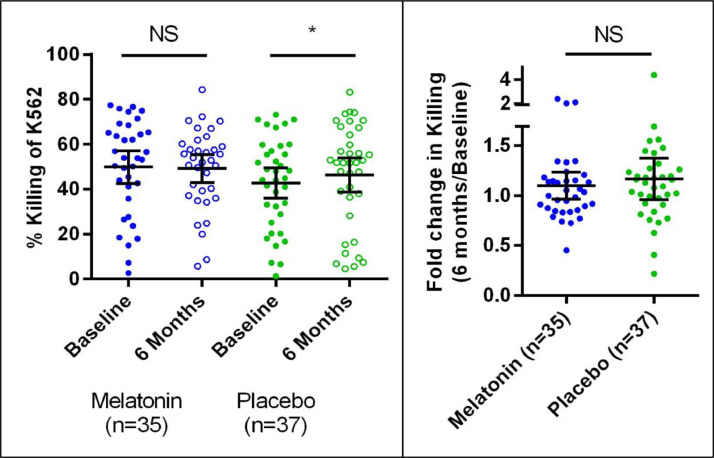
Fig. 4 shows the mean NK cell-mediated cytotoxicity changes from baseline to six months. Changes are as follows: Melatonin: 49.8 ± 3.6% to 49.2 ± 3.0%, an average change of −0·67% (95% CI −3.21–4.56), p = 0·95; Placebo: 42.7 ± 3.3% to 46.4 ± 3.8%, an average change of 3.63% (95% CI −0.15–7.42), p = 0·02. The mean fold change from baseline to six months was 1·10 (95% CI 0.97 – 1.23) in the melatonin group and 1·16 (95% CI 0.96–1.37) in the placebo group, p = 0·34. No clinically or statistically significant changes were seen in absolute measurements or fold changes of NK cell phenotypes (CD56+/CD3- NK cells) or of any measured cytokines (data not shown).
Fig. 4.
NK Cell cytotoxicity. Left: Raw cytotoxicity values at baseline and six months. Right: Fold changes between baseline and six months. Cytotoxicity was quantified as either the amount of Cr51 released into the supernatant by dying Cr51-labelled K562 cells or the amount of CP450-labelled K562 cells that stain positive for propidium iodide. Error bars represent mean and 95% confidence interval. NS = not statistically significant; * = statistically significant.
4. Discussion
In this multicenter double-blind randomized controlled trial, we compared the addition of adjuvant melatonin versus placebo for DFS, QOL, symptomatology, and immune function. A dose of 20 mg melatonin was chosen as it is commonly used in clinical practice [14] and studied in literature [6], [7], [8] for recurrence and mortality outcomes. For patients undergoing complete surgical resection of NSCLC, melatonin did not affect two-year or five-year DFS when compared to placebo. These results held when only participants who took the study product for at least three months were included. When stratified by cancer stage, a statistically significant hazard reduction in five-year DFS was seen in participants with late stage cancer (III/IV) but not in early stage cancer (I/II). No differences were seen in median DFS for those with late stage disease.
This was the first study to evaluate the effects of melatonin on recurrence and mortality in patients with surgically resected cancer. The positive result seen in participants with resected late stage disease is comparable to previous literature that found beneficial effects for melatonin in patients with late stage or metastatic cancers [6], [7], [8].
In addition to recurrence and mortality outcomes, we found that melatonin had no beneficial effects on QOL, sleep, anxiety, depression, pain, or fatigue and did not reduce AEs associated with chemotherapy or radiation in our population. All results held when only those who took the study product for at least three months were included. This contrasts with evidence that melatonin benefits sleep and QOL in cancer patients [29,30], and to literature on the beneficial effects of melatonin on AEs in patients receiving chemotherapy [11,12].
In our mechanistic sub-study we chose to focus on NK cell cytotoxicity because of the central role NK cells play in the formation of metastases [31] and prior reports of the beneficial effect of melatonin on NK cell number and function [32]. No clinically meaningful changes were seen with regards to NK cell cytotoxicity or phenotypes and no clinically or statistically significant changes were seen in the levels of 12 inflammatory cytokines. Although there was a statistically significant increase in cytotoxicity within the placebo group at six months, this was not clinically significant. This was the first study to evaluate the potential modulation of these markers by melatonin. Based on the above findings, we conclude that 20 mg of nightly melatonin has no effect on NK cell cytotoxicity or phenotype, and no effect on blood levels of inflammatory cytokines in this population.
This trial presented challenges and limitations, but none that discredit our findings. The dose of 20 mg melatonin was chosen to reflect common clinical use and previous clinical trials of melatonin for outcomes related to recurrence and mortality; however, melatonin is indicated and has the most positive research for sleep and quality of life at doses of 1–10 mg [33]. This could, in part, explain why no effects were seen on these outcomes despite previous research to the contrary. Melatonin is readily available over the counter; thus, participants were queried each 3 months while on the intervention to ensure they were not taking exogenous melatonin. We cannot be sure that participants did not take melatonin on their own volition, regardless of which group they were in, but based on participant responses, the issue of contamination appears to not have been a major limitation.
We would like to note that enrolment and follow-up period (2007–2017) for this study was long and the surgical standard of care has changed during this time, particularly a greater use of minimally invasive techniques [34,35]. There were no clinically meaningful differences in surgical techniques used (Table 1) and ongoing randomization through the course of enrolment would have nullified any other differences seen between treatment and placebo arms Table 3.
Table 3.
Chemotherapy and Radiotherapy Adverse Events.
| Melatonin N (%) | Placebo N (%) | P Value | |
| Attributed to Chemotherapy | 44 (66.7) | 48 (70.6) | 0.62 |
| Attributed to Radiation | 8 (36.4) | 5 (23.8) | 0.37 |
Number of participants who experienced adverse events related to chemotherapy and radiation therapy. Participants were included if they were given adjuvant chemotherapy or radiation and experienced an adverse event. Attribution required the adverse event to be at least possibly related to the chemotherapy or radiation under the discretion of the treating surgeon. Chemotherapy: Melatonin (N = 66); Placebo (N = 68). Radiotherapy: Melatonin (N = 22); Placebo (N = 21).
A high proportion of patients with early stage NSCLC prevented both groups from reaching median disease-free survival, prompting the secondary analysis stratifying by cancer stage. It is possible that reaching median survival through a longer follow-up period could increase the magnitude of the effect by increasing the number of events; however, this is unlikely due to the almost negligible effects observed. Furthermore, a high withdrawal rate may have introduced bias for an intention to treat analysis. This was at least partly addressed through the addition of the per-protocol analysis, which included only those who received a therapeutic dose of product and were not deemed screen fails.
In summary, this multi-site trial evaluating the effects of adjuvant melatonin on recurrence and mortality in patients with resected NSCLC showed no net beneficial effects when compared to a placebo. When the population was stratified by cancer stage, a 25% hazard reduction in five-year DFS was seen in participants with late stage NSCLC; however, this was accompanied by no change in median DFS. QOL, fatigue, sleep, anxiety, depression, pain, and AEs associated with chemo- and radiotherapy showed no clinical or statistical differences. In light of the results, we do not recommend the inclusion of adjuvant melatonin for patients with early stage NSCLC. Evidence suggests there may be a benefit for those with late stage disease; however, because of the mixed findings observed, we recommend a follow-up randomized controlled trial involving a larger population focusing on later stage resected lung cancer to clarify these results.
Declaration of Competing Interests
Dr. Villeneuve reports ‘other’ from Minogue Medical, outside the submitted work. All other authors have no conflicts of interest to disclose.
Acknowledgments
Funding
The authors would like to acknowledge the Lotte and John Hecht Memorial Foundation and Gateway for Cancer Research Foundation who provided financial support for this study.
Acknowledgments
We gratefully recognize the contributions of the late Dr. Bill Nelems and Dr. Jean Deslauriers in their support of this trial in their respective centers, the Kelowna General Hospital and Quebec Heart and Lung Institute. We recognize the contributions of Dr. Paula Ugalde and Dr. Dalilah Fortin as site principal investigators at the Quebec Heart and Lung Institute and the London Health Sciences center, respectively. We would also like to give thanks to the OICC research team members, the OHRI thoracic & intensive care research team members, the Canadian Association of Thoracic Surgeons, and the numerous research staff at each center that provided ongoing support throughout the trial.
Contributors
DS, RA, DEM, FS, RSS, SG, TW, CF, DF, TR, and AJES contributed to study design; AJES, DEM, FS, RSS, SG, PJV, ASA, KH, YS, and MP contributed to the recruitment of participants; LA, MAK, and LHT contributed to blood collection and analysis; ML, AF, ED, CA, LA, and MAK contributed to data collection and aggregation; DS, ML, LA, MAK, DF, TR, and AJES contributed to statistical analysis; all authors contributed to data interpretation and writing.
Data Sharing Statement
Datasets utilized in this study are available by request only. Please contact Dr. Dugald Seely for access to a dataset or a copy of the study protocol.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100763.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Blanchon F., Grivaux M., Asselain B., Lebas F.-.X., Orlando J.-.P., Piquet J. 4-year mortality in patients with non-small-cell lung cancer: development and validation of a prognostic index. Lancet Oncol. 2006 Oct;7(10):829–836. doi: 10.1016/S1470-2045(06)70868-3. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J., Chirieac L.R. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017 Apr;15(4):504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 4.Osmani L., Askin F., Gabrielson E., Li Q.K. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018;52:103–109. doi: 10.1016/j.semcancer.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller V.A. Optimizing therapy in previously treated non-small cell lung cancer. Semin Oncol. 2006 Feb;33:S25–S31. doi: 10.1053/j.seminoncol.2005.12.005. 1 Suppl 1. [DOI] [PubMed] [Google Scholar]
- 6.Lissoni P., Chilelli M., Villa S., Cerizza L., Tancini G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res. 2003 Aug;35(1):12–15. doi: 10.1034/j.1600-079x.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 7.Seely D., Wu P., Fritz H., Kennedy D a., Tsui T., JE Seely a. Melatonin as adjuvant cancer care with and without chemotherapy: a systematic review and meta-analysis of randomized trials. Integr Cancer Ther. 2012;11(4):293–303. doi: 10.1177/1534735411425484. [DOI] [PubMed] [Google Scholar]
- 8.Mills E., Wu P., Seely D., Guyatt G. Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J. Pineal Res. 2005;39:360–366. doi: 10.1111/j.1600-079X.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 9.McCulloch M., See C., Shu X.J., Broffman M., Kramer A., Fan W.Y. Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small-cell lung cancer: meta-analysis of randomized trials. J Clin Oncol. 2006;24(3):419–430. doi: 10.1200/JCO.2005.03.6392. [DOI] [PubMed] [Google Scholar]
- 10.St Mathijssen RHJ.Effects of. John's wort on irinotecan metabolism. CancerSpectrum Knowl Environ. 2002;94(16):1247–1249. doi: 10.1093/jnci/94.16.1247. [DOI] [PubMed] [Google Scholar]
- 11.Kukuruzovic R.H. Complementary medicines and therapies, surging ahead in popularity: how is conventional medicine responding? J Paediatr Child Health. 2005;41(1–2):21–22. doi: 10.1111/j.1440-1754.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 12.Kelly K.M. Complementary and alternative medical therapies for children with cancer. Eur J Cancer. 2004;40(14):2041–2046. doi: 10.1016/j.ejca.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Blask D., Sauer L., Dauchy R. Melatonin as a chronobiotic /anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2005;2(2):113–132. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 14.Seely D., Ennis J.K., McDonell E., Zhao L. Naturopathic oncology care for thoracic cancers: a practice survey. Integr Cancer Ther. 2018;17(3):793–805. doi: 10.1177/1534735418759420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mediavilla M D., Sanchez-Barcelo E J., Tan D X., Manchester L., Reiter R J. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr Med Chem. 2011;17(36):4462–4481. doi: 10.2174/092986710794183015. [DOI] [PubMed] [Google Scholar]
- 16.O'Dowd C., McRae L.A., McMillan D.C., Kirk A., Milroy R. Elevated preoperative C-reactive protein predicts poor cancer specific survival in patients undergoing resection for non-small cell lung cancer. J Thorac Oncol. 2010;5(7):988–992. doi: 10.1097/JTO.0b013e3181da78f9. [DOI] [PubMed] [Google Scholar]
- 17.De Meis E., Pinheiro V.R., Zamboni M.M., Guedes M.T.S., Castilho I.A.M., Martinez M.M.K. Clotting, immune system, and venous thrombosis in lung adenocarcinoma patients: a prospective study. Cancer Invest. 2009;27(10):989–997. doi: 10.3109/07357900903124464. [DOI] [PubMed] [Google Scholar]
- 18.Martín F., Santolaria F., Batista N., Milena A., González-Reimers E., Brito M.J. Cytokine levels (IL-6 and IFN-γ), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine. 1999;11(1):80–86. doi: 10.1006/cyto.1998.0398. [DOI] [PubMed] [Google Scholar]
- 19.Gupta D., Lis C.G. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9 doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones J.M., McGonigle N.C., McAnespie M., Cran G.W., Graham A.N. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer. 2006;53(1):97–101. doi: 10.1016/j.lungcan.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Meehan K.R., Zacharski L.R., Moritz T.E., Rickles F.R. Pretreatment fibrinogen levels are associated with response to chemotherapy in patients with small cell carcinoma of the lung: department of veterans affairs cooperative study 188. Am J Hematol. 1995;49(2):143–148. doi: 10.1002/ajh.2830490208. [DOI] [PubMed] [Google Scholar]
- 22.McMillan D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Vol. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. Current Opinion in Clinical Nutrition and Metabolic Care. [DOI] [PubMed] [Google Scholar]
- 23.Fayers P., Aaronson N., Bjordal K. EORTC QLQ-C30 scoring manual [Internet] Eortc. 2001:1–77. Available fromhttp://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:EORTC+QLQ-C30+Scoring+Manual#0. Access October 2020. [Google Scholar]
- 24.Smets E.M.A., Garssen B., Bonke B., De Haes J.C.J.M. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 25.Spritzer Karen, Hays R. A Manual for Use and Scoring; 2020. MOS sleep scale. Version 1.0 [Internet]. Available fromhttps://labs.dgsom.ucla.edu/hays/files/view/docs/surveys/sleep/sleepman-112603.pdf. Accessed October. [Google Scholar]
- 26.Beck A.T., Steer R.A., Brown G.K. Manual for the beck depression inventory-II. San Antonio. TX Psychol Corp. 1996:1–82. [Google Scholar]
- 27.Beck A.T., Steer R.A. Behaviour research and therapy. Manual for the Beck Anxiety Inventory. 1990;37:25–74. [Google Scholar]
- 28.Cleeland C. The brief pain inventory user guide. Br Pain Invent [Internet] 2009:3–4. Available from: http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/BPI_UserGuide.pdf. Accessed October 2020. [Google Scholar]
- 29.Del Fabbro E., Dev R., Hui D., Palmer L., Bruera E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double-blind placebo-controlled trial. J Clin Oncol. 2013;31(10):1271–1276. doi: 10.1200/JCO.2012.43.6766. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sookprasert A., Johns N.P., Pnunmanee A., Pongthai P., Cheawchanwattana A., Johns J. Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial. Anticancer Res. 2014;34(12):7327–7337. [PubMed] [Google Scholar]
- 31.López-Soto A., Gonzalez S., Smyth M.J., Galluzzi L. Control of Metastasis by NK Cells. Vol. 32, Cancer Cell. 2017. p. 135–54. [DOI] [PubMed]
- 32.Miller S.C., Pandi P.S.R., Esquifino A.I., Cardinali D.P., Maestroni G.J.M. The role of melatonin in immuno-enhancement: potential application in cancer. Int J Exp Pathol. 2006;87:81–87. doi: 10.1111/j.0959-9673.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferracioli-Oda E., Qawasmi A., Bloch M.H. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS ONE. 2013 May;(5):e63773. doi: 10.1371/journal.pone.0063773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batchelor T.J.P., Rasburn N.J., Abdelnour-Berchtold E. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (ERAS®) society and the European society of thoracic surgeons (ESTS) Eur J Cardiothorac Surg. 2019;55(1):91–115. doi: 10.1093/ejcts/ezy301. [DOI] [PubMed] [Google Scholar]
- 35.Onugha O., Ivey R., McKenna R. Novel techniques and approaches to minimally invasive thoracic surgery. Surg Technol Int. 2017;30:231–235. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.