Abstract
Lessons Learned
A PHY906 and capecitabine combination could be effective as a salvage therapy for patients with hepatocellular carcinoma (HCC) previously treated with multiple systemic therapies.
This traditional Chinese medicine formulation can work with Western cancer chemotherapeutic agents to improve clinical outcomes or alleviate side effects for patients with advanced HCC.
Background
This study aimed to evaluate efficacy and safety of capecitabine combined with a PHY906 (a pharmaceutical‐grade formulation of four traditional Chinese herbs) in the treatment of advanced hepatocellular carcinoma (HCC) in Asian patients who were positive for hepatitis B virus (HBV).
Methods
This study was an open‐label, phase II safety and efficacy clinical trial of PHY906 and capecitabine in patients with advanced HCC. Patients received 750 mg/m2 capecitabine b.i.d. 14 days plus 800 mg of PHY906 b.i.d. on days 1–4 and days 8–11 every 21‐day cycle. The primary endpoint was 6‐month survival rate, and secondary endpoints were progression‐free survival, overall survival, disease control rate, and safety.
Results
Thirty‐nine subjects completed the study with a 46.2% stable disease rate. The median progression‐free survival was 1.5 months, and median overall survival (mOS) was 6 months with a 51.3% 6‐month survival rate. The most common adverse events included lower hemoglobin, diarrhea, pain, abdomen (not otherwise specified), fatigue, increased aspartate aminotransferase, and bilirubin. Patients who (a) had not received previous chemotherapies or targeted therapy or (b) had lower starting alpha‐fetoprotein (AFP) levels or (c) had HBV infection showed better clinical outcome.
Conclusion
Our data showed that PHY906 increases the therapeutic index of capecitabine by enhancing its antitumor activity and reduces its toxicity profile in advanced HCC.
Keywords: PHY906, Capecitabine, Hepatocellular carcinoma, Chinese herbal medicine
Discussion
In 2007, sorafenib was approved by the U.S. Food and Drug Administration (FDA). Results from two phase III clinical trials indicated that sorafenib increased mOS from 7.9 months to 10.7 months (in the U.S. SHARP trial) and from 4.2 months to 6.5 months (in the Asia‐Pacific trial). One potential explanation for the difference between the two populations was the etiology of the underlying hepatitis, with HBV‐positive HCC more prevalent in Asian countries. Any regimens capable of increasing the therapeutic index of current therapies among HBV‐positive patients with HCC would benefit the global HCC population.
YIV‐906 (PHY906) was developed as an orphan drug for treating patients with advanced liver cancer. In March 2018, the FDA granted YIV‐906 orphan drug designation for the indication of HCC. Based on the encouraging safety profile and the median overall survival from previous U.S. and Taiwan studies of YIV‐906 and capecitabine combination therapy and a phase I YIV‐906 and sorafenib combination therapy, an ongoing phase II randomized placebo‐controlled study investigating the combination of YIV‐906 and sorafenib (Nexavar, Bayer, Leverkusen, Germany) in HBV‐positive patients with advanced hepatocellular carcinoma is being conducted by Yiviva Inc. at 22 study sites in the U.S., China, Hong Kong, and Taiwan. The goal is to seek approval in the U.S. and China for YIV‐906 as a prescription drug for first‐line (sorafenib), second‐line (PD‐1), or third‐line (capecitabine) therapy.
In this study, the combination of PHY906 plus capecitabine was found to have an mOS of 6 months with a 6‐month survival rate of 51% among 39 patients assessed by intention to treat. Results indicated that patients who were systemic therapy naïve, including chemotherapy (n = 7), thalidomide, or everolimus treatments, could have better clinical outcome than those who have received multiple prior systemic therapies, with mOS of 9.2 and 5.45 months, respectively. Interestingly, patients with lower starting AFP also showed better mOS (9.2 months). In addition, 27 patients were treated with at least two cycles of study drug, whereas 12 patients had fewer than two cycles of treatment. A subgroup analysis was performed comparing these 27 evaluable patients with nonevaluable patients (fewer than two cycles of treatment, n = 12). The data indicated an mOS of 8.4 months versus 1.8 months (Fig. 1A; p = .0084).
Figure 1.
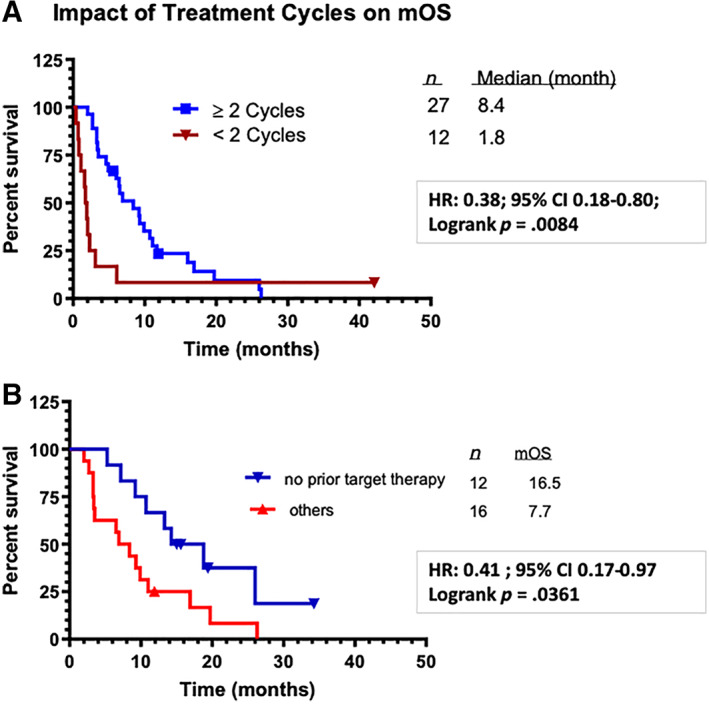
Kaplan‐Meier plots: percentage survival. (A): Impact of treatment cycles on the clinical outcomes. (B): Chemotherapy‐naïve evaluable patients with hepatocellular carcinoma and hepatitis B virus benefited most with PHY906 plus capecitabine drug treatment (combination of both U.S. and Taiwan studies).Abbreviations: CI, confidence interval; HR, hazard ratio; mOS, median overall survival.
In our previous study of PHY906/capecitabine in the U.S., better clinical outcomes were reported in evaluable Asian patients (who completed at least two cycles of treatment) than in the evaluable non‐Asian patients, with mOS of 16.5 and 6.9 months, respectively. By combining HBV‐positive, evaluable, Asian patients with HCC who were naïve to systemic therapy in both the Taiwan and the U.S. trials, the mOS was 16.5 months (Fig. 1B), suggesting that the PHY906/capecitabine combination may provide a survival benefit and has a tolerable safety profile for patients with HCC and HBV infection. This effect has also been observed in colon cancer, pancreatic cancer, and chemoradiation therapy.
Based on the encouraging safety profile and the mOS from previous studies, an ongoing phase II randomized placebo‐controlled study investigating the combination of PHY906 and sorafenib in HBV‐positive patients with advanced hepatocellular carcinoma is being conducted at 22 study sites in the U.S., China, Hong Kong, and Taiwan. The goal is to seek approval in the U.S. and China for PHY906 as a prescription drug for first‐line (sorafenib), second‐line (PD‐1), or third‐line (capecitabine) therapy.
Trial Information
| Disease | Hepatocellular carcinoma |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | One prior regimen |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Six‐month survival rate |
| Secondary Endpoints | Disease control rate (complete response/partial response + stable disease), progression‐free survival, overall survival, AFP reduction, change in quality of life, safety |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Drug 1 | |
| Generic/Working Name | PHY906, KD018, YIV‐906 |
| Trade Name | YIV‐906 |
| Company Name | Yiviva Inc. |
| Dose | 800 b.i.d. milligrams (mg) per day |
| Route | Oral (p.o.) |
| Schedule of Administration | Patients were initially treated for two 21‐day courses with PHY906 800 mg b.i.d. + capecitabine 750 mg/m2 b.i.d. according to the following schedule: capecitabine 14 days on treatment, days 1 through 14, and 7 days off treatment; PHY906 days 1 through 4 and days 8 through 11 of each course. Patients might remain on study beyond their initial two courses of treatment until tumor progression or unacceptable toxicity mandated their removal. |
| Drug 2 | |
| Generic/Working Name | Xeloda |
| Trade Name | Capecitabine |
| Company Name | Roche |
| Dose | 750 milligrams (mg) per squared meter (m2) |
| Route | Oral (p.o.) |
| Schedule of Administration | Patients were initially be treated for two 21‐day courses with PHY906 800 mg b.i.d. + capecitabine 750 mg/m2 b.i.d. according to the following schedule: capecitabine 14 days on treatment and 7 days off treatment and PHY906 days 1 through 4 and days 8 through 11 of each course. Patients might remain on study beyond their initial two courses of treatment until tumor progression or unacceptable toxicity mandated their removal. |
Patient Characteristics
| Number of Patients, Male | 32 |
| Number of Patients, Female | 7 |
| Stage | Stage II: 1 (2.6%); stage IIIA: 14 (35.9%); stage IIIB: 3 (7.7%); stage IIIC 4 (10.3%); stage IV 17 (43.6%) |
| Age | Median (range): 54 (32–75) years |
| Number of Prior Systemic Therapies | Median (range): 1 (0–3) |
| Performance Status: ECOG |
0 — 0 1 — 39 2 — 0 3 — 0 Unknown — 0 |
| Cancer Types or Histologic Subtypes |
Hepatocellular carcinoma: 39 Hepatocellular carcinoma + HBV: 27 Hepatocellular carcinoma + hepatitis C virus: 7 Hepatocellular carcinoma + HBV + hepatitis C virus: 5 |
Primary Assessment Method
| Title | Response Assessment |
| Number of Patients Screened | 45 |
| Number of Patients Enrolled | 39 |
| Number of Patients Evaluable for Toxicity | 39 |
| Number of Patients Evaluated for Efficacy | 39 |
| Evaluation Method | RECIST 1.0 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 18 (46.2%) |
| Response Assessment PD | n = 20 (51.3%) |
| Response Assessment OTHER | n = 1 (2.6%) |
| (Median) Duration Assessments PFS | 1.50 months; confidence interval: 95% |
| (Median) Duration Assessments OS | 6.03 months |
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
| Diarrhea | 49 | 38 | 10 | 3 | 0 | 0 | 51 |
| Fatigue (asthenia, lethargy, malaise) | 51 | 31 | 18 | 0 | 0 | 0 | 49 |
| INR of prothrombin time | 62 | 33 | 5 | 0 | 0 | 0 | 38 |
| Bilirubin (hyperbilirubinemia) | 56 | 5 | 26 | 10 | 3 | 0 | 44 |
| Rash: hand‐foot skin reaction | 85 | 10 | 5 | 0 | 0 | 0 | 15 |
| Insomnia | 66 | 26 | 8 | 0 | 0 | 0 | 34 |
| Hyperpigmentation | 74 | 26 | 0 | 0 | 0 | 0 | 26 |
| Anorexia | 74 | 10 | 13 | 3 | 0 | 0 | 26 |
| Distension/bloating, abdominal | 71 | 5 | 21 | 3 | 0 | 0 | 29 |
| Nausea | 71 | 26 | 3 | 0 | 0 | 0 | 29 |
| Edema: limb | 74 | 18 | 8 | 0 | 0 | 0 | 26 |
| Alkaline phosphatase | 95 | 5 | 0 | 0 | 0 | 0 | 5 |
| ALT, SGPT | 66 | 21 | 5 | 8 | 0 | 0 | 34 |
| AST, SGOT | 51 | 5 | 18 | 18 | 8 | 0 | 49 |
| Sodium, serum‐low (hyponatremia) | 76 | 13 | 0 | 8 | 3 | 0 | 24 |
| Pain: abdomen NOS | 49 | 23 | 18 | 10 | 0 | 0 | 51 |
| Dyspnea (shortness of breath) | 73 | 21 | 3 | 3 | 0 | 0 | 27 |
| Platelets | 71 | 21 | 5 | 0 | 3 | 0 | 29 |
| Hemoglobin | 46 | 23 | 28 | 3 | 0 | 0 | 54 |
| Leukocytes (total WBC) | 81 | 8 | 8 | 0 | 3 | 0 | 19 |
| Lymphopenia | 77 | 0 | 8 | 15 | 0 | 0 | 23 |
| Neutrophils/granulocytes (ANC/AGC) | 91 | 3 | 3 | 0 | 3 | 0 | 9 |
Abbreviations: AGC, atypical glandular cells; ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; INR, international normalized ratio; NC/NA, no change from baseline/no adverse event; NOS, not otherwise specified; SGPT, serum glutamic pyruvic transaminase; SGOT, serum glutamic oxaloacetic transaminase; WBC, white blood cell.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Hepatocellular carcinoma (HCC) is a leading cause of death from cancer worldwide. The median survival time of patients with unresectable and recurrent HCC ranges from 3 to 7 months [1, 2, 3]. The etiology of the disease is multifactorial; hepatitis B virus (HBV) and C virus infections are strongly linked to its development [4, 5, 6, 7, 8]. Over the last few years, the number of cases of HCC has increased in the U.S., mainly because of hepatitis C virus infection. Worldwide, 55% of all HCC cases are reported from China, and more than 60% of HCC cases are associated with HBV infection [9, 10, 11, 12]. In most instances, HCC is associated with a background history of decompensated liver disease and cirrhosis. Usually patients with HCC present with advanced disease, whereby surgical resection and/or chemical embolism is not feasible; treatment options for such patients are limited [13, 14, 15, 16]. Inoperable HCC cases are mostly treated with sorafenib as first‐line treatment [17], and the efficacy of sorafenib has been evaluated in two large multicenter, randomized, double‐blind, placebo‐controlled phase III trials: the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial and a phase III trial conducted in the Asia‐Pacific region [18, 19]. Both trials demonstrated that sorafenib enhanced median overall survival (mOS) and time to tumor progression when compared with placebo. A noninferior alternative to sorafenib is lenvatinib, which received FDA approval for the first‐line treatment of unresectable HCC in 2018 [20]. Capecitabine, an oral 5‐fluorouracil prodrug approved for the treatment of metastatic colorectal and breast cancer, has been used off label to treat HCC and showed modest activity before any anti‐HCC drugs were approved [21, 22, 23, 24]. Studies also showed that capecitabine plus bevacizumab, or capecitabine plus bevacizumab/oxaliplatin in advanced HCC, were also effective and tolerable [25, 26]. The most common side effects associated with capecitabine are myelosuppression and skin toxicity, and the most limiting side effect is severe gastrointestinal (GI) toxicity. In contrast, common side effects associated with sorafenib include abdominal pain, anorexia, diarrhea, fatigue, hair loss, hand or foot skin reaction, nausea, rash or superficial skin shedding, and weight loss in patients with HCC [18, 19, 27, 28]. Among all side effects caused by sorafenib, 55% of recipients report diarrhea [29, 30]. Therefore, any agent that can alleviate the toxicity caused by HCC therapeutics without compromising the antitumor efficacy will provide an additive benefit. The FDA has approved several immunotherapies for HCC, including atezolizumab plus bevacizumab as first‐line treatment and nivolumab or pembrolizumab as second‐line treatments.
Traditional Chinese medicine has been used to treat a variety of diseases for centuries, especially for GI symptoms like nausea, vomiting, diarrhea, and abdominal spasms [31, 32, 33]. One traditional Chinese medicine formulation, PHY906 or YIV‐906, comprising a mixture of four herbs (Scutellaria baicalensis Georgi, Glycyrrhiza uralensis Fisch., Paeonia lactiflora Pall., and Ziziphus jujube Mill.), has been used for approximately 1,800 years for a variety of maladies, most notably severe gastrointestinal distress, for example, nausea, vomiting, diarrhea, and abdominal spasms. It is prepared under current Good Manufacturing Practice conditions and has been well characterized by both chemical and biological fingerprints. Multiple clinical batches of PHY906 have been documented to have more than 90% consistency using integration of chemical and biological fingerprints. Stability studies indicated that PHY906 capsules remained stable for at least 6 years at room temperature.
Notably, PHY906/YIV‐906 does not exhibit toxicities with other agents used for HCC chemotherapy in preclinical and clinical studies [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45]. In fact, in nearly all cases, the combination regimen was found to imply a better therapeutic outcome than the historical efficacy of the chemotherapeutic agent alone and did not exhibit toxicities [34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44]. More importantly, quality of life scores did not deteriorate significantly from baseline scores. For example, the mechanism of action in reducing CPT‐11–induced diarrhea and intestinal damage involves inhibition of several inflammatory processes, such as NF‐κB, COX‐2, IL‐6, iNOs, and promoting intestinal progenitor cell repopulation [35, 36]. In addition, the mechanism of enhancing antitumor agents are due to the activation of innate and adaptive immunity in the tumor tissue microenvironment [37, 39, 46, 47].
PHY906/capecitabine combination therapy resulted in limited deleterious side effects. Previous data from a U.S.‐based phase I/II clinical trial involving PHY906/capecitabine therapy revealed beneficial effects and reduced toxicities for the Asian subpopulation with an mOS of 16.5 months and no capecitabine‐induced grade 3/4 GI toxicities in advanced nonresectable patients (with HCC) with the PHY906 plus capecitabine combination therapy from a phase I/II study of PHY906 plus capecitabine in the U.S [49]. This study sought to validate similar effects of reduced chemotherapy‐induced gastrointestinal toxicity and enhanced antitumor activity for patient populations with HCC in Taiwan.
In the present study, capecitabine/PHY906 combination therapy resulted in only a few grade 3 and 4 drug‐related toxicities. In essence, this combination was well tolerated by patients in both the current Taiwan and previous U.S. HCC studies. The incidence of nausea and emesis was lower with the PHY906/capecitabine combination than with the capecitabine treatment alone. Moreover, only two patients (5.13%) discontinued treatment in the current combination because of adverse effects from capecitabine [18, 19, 23, 24]. Similar to the earlier trial in the U.S., toxicities were manageable with minimal grade 3 or 4 toxicities [48]. As in the previous U.S. trial, quality of life scores did not deteriorate significantly from baseline scores during the combination therapy of PHY906 and capecitabine. These observations concur with previous studies involving irinotecan‐based chemotherapy in colorectal cancer, gemcitabine‐refractory pancreatic cancer, and chemoradiation therapy in rectal cancer [37, 39, 46, 47, 49].
Sorafenib has been standard for HCC treatment. Based on results of the SHARP and Asia‐Pacific phase III studies, 95% of patients were classified as Child‐Pugh A and had no previous treatment. The mOS of patients enrolled in the SHARP and Asian studies was 10.7 and 6.5 months, respectively, whereas that of placebo was 7.9 and 4.2 months, respectively [18, 19]. The patients enrolled in the current study had a poorer prognosis; 90% were previously treated with chemotherapy or targeted therapy involving chemoembolization or radiation, and > 60% had had two prior treatments. The antitumor outcome (mOS, 6‐month‐ or 12‐month survival rate) in our Taiwan study (n = 39) was not as promising as that of U.S. study (n = 42). The combination regimen of PHY906 plus capecitabine was mainly used as the first‐line treatment in the U.S. study, whereas it was mainly used as a second‐ or third‐line treatment in the Taiwan study. Patients in the present Taiwan study were heavily pretreated with various procedures or regimens, including targeted therapies, chemotherapies, transarterial chemoembolization/percutaneous ethanol injection, surgery, radiation therapy, or a combination. The starting alpha‐fetoprotein (AFP) levels were relatively higher in Taiwan, with 33.3% of patients having AFP higher than 12,000 ng/mL, compared with the counterpart U.S. study (16.7%) [48].
In the Taiwan study, the PHY906/capecitabine combination increased the median overall survival time to 6 months, whereas the average survival time was around 3 months for patients with HCC whose previous treatments had failed. Patients who did not receive prior targeted therapy or chemotherapy, or who had lower starting AFP level, had a better clinical outcome. Because some of the patients did not finish two courses of combination therapy, additional analysis was done to compare the differences between patients who had fewer than two cycles of treatment (n = 12) and patients who completed at least two cycles of treatment (n = 27). The mOS difference between these two groups of patients was 1.8 and 8.4 months, respectively (p = .0084) (Fig. 1A). Interestingly our data also indicated that HBV‐positive evaluable patients (with two or more courses of combination therapy) had an mOS of 8.4 months. In our previous PHY906/capecitabine U.S. study, Asian patients (n = 10) had an mOS of 16.5 months, relative to 6.7 months for the non‐Asian counterpart (n = 10). Notably, patients in the group infected with HBV only (n = 9) did not reach 50% overall survival, whereas a median survival of 6.7 months was estimated for others (n = 11). The results implied that combination therapy might benefit Asian patients with HBV infection. By combining Asian HBV‐infected patients (with HCC) who (a) did not receive prior systemic therapy and (b) finished two or more cycles of combination treatment from the U.S. and Taiwan trials, the mOS was 16.5 months (Fig. 1B). These results support the notion that the PHY906/capecitabine combination therapy may provide a survival benefit with a tolerable safety profile in patients with advanced HCC. Moreover, Asian patients with HBV seem to have remarkable mOS in both previous and current HCC studies. These results suggest that PHY906/capecitabine combination therapy may provide a selective clinical advantage for patients with HCC and HBV infection.
The mechanism underlying the function of PHY906 is multifactorial and could involve inhibition of multidrug‐resistant protein and CYP450, which may facilitate the uptake of chemotherapeutic drugs. Several pathways have been implicated in the mechanism of PHY906. The inhibition of tachykinin NK‐1, opiate δ receptors, and acetylcholinesterase could be reasons for the reduction of gastrointestinal toxicity [51]. Moreover, reports have shown that NF‐κB and matrix metalloproteases can be inhibited by PHY906. PHY606 may also affect the integrity of blood vessels and HIF‐α and Fos/Juk pathway. In mouse models, PHY906 was found to increase the inflammation in the tumor microenvironment through activation of M1 macrophages, resulting in tumor rejection [44]. Some or all of these mechanisms could play a critical role in PHY906 enhancement of antitumor properties when combined with other chemotherapeutic agents.
Based on previous studies, the Chinese herb medicine extract PHY906 is a formula that enhances antitumor activity and reduces chemotherapy‐induced gastrointestinal toxicity in hepatocellular cancer. Results from this study also suggest that PHY906 combination therapy could be an alternative to currently available treatment options for HCC. Further larger cohorts for phase II/III clinical studies involving PHY906 combination therapy are warranted. For future consideration, the trial design can be improved by using a double‐blind, randomized placebo control to reduce the potential bias. Moreover, the inclusion criteria can be redefined on the number of prior treatments to confirm whether PHY906 selectively benefits naïve patients with HCC or those receiving second, third, or multiple lines of treatment. The combination treatment options could also be redesigned and use FDA‐approved standard of care, such as sorafenib or lenvatinib instead of capecitabine, in the trial. Therefore, an ongoing study entitled “A Phase II Randomized Placebo‐Controlled Study Investigating the Combination of YIV‐906 and Sorafenib (Nexavar) in HBV(+) Patients with Advanced Hepatocellular Carcinoma” (ClinicalTrials.gov identifier: NCT04000737) was designed to resolve the previously mentioned issues. We plan to conduct a phase III study to combination therapy of PHY906 plus capecitabine as a third‐line therapy for Asian patients with HCC and HBV infection.
Disclosures
Shwu‐Huey Liu: Yiviva (E, OI [cofounder]), PHY906 (YIV‐906) patents (IP); Yung‐Chi Cheng: Yiviva (E, OI [cofounder], C/A, SAB, RF‐institutional), PHY906 (YIV‐906) patents (IP). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The authors thank National Health Research Institutes and TTY Biopharm Co., Ltd for conducting and sponsoring the study in Taiwan. The authors also thank TTY Biopharm Co., Ltd. for supporting this clinical trial in Taiwan between 2008 and 2012. Parts of this study were supported by Sino‐American Cancer Foundation. The authors wish to acknowledge An Lu for her assistance in the preparation of this manuscript.
Footnotes
- ClinicalTrials.gov Identifier: NCT00076609
- Sponsor: Yiviva Inc.
- Principal Investigator: Yun Yen
- IRB Approved: Yes
Contributor Information
Chun A. Changou, Email: austinc99@tmu.edu.tw.
Yun Yen, Email: yyen@tmu.edu.tw.
References
- 1. Llovet JM, Bustamante J, Castells A et al. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62–67. [DOI] [PubMed] [Google Scholar]
- 2. Di Bisceglie AM, Rustgi VK, Hoofnagle JH et al. Hepatocellular carcinoma. Ann Intern Med 1998;108:390–401. [DOI] [PubMed] [Google Scholar]
- 3. Yang JD, Hainaut P, Gores GJ et al. A global view of hepatocellular carcinoma: Trends, risk, prevention, and management. Nat Rev Gastroenterol Hepatol 2019;16:589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsukuma H, Hiyama T, Tanaka S et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 1993;328:1797–1801. [DOI] [PubMed] [Google Scholar]
- 5. Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol 2010;7:448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Omata M, Cheng AL, Kokudo N et al. Asia‐Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int 2017;11:317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schietroma I, Scheri GC, Pinacchio C et al. Hepatitis C virus and hepatocellular carcinoma: Pathogenetic mechanisms and impact of direct‐acting antivirals. Open Virol J 2018;12:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi J, Zhu L, Liu S et al. A meta‐analysis of case‐control studies on the combined effect of hepatitis B and hepatitis C virus infections in causing hepatocellular carcinoma in China. Br J Cancer 2005;92:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassan MM, Frome A, Patt YZ et al. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol 2002;35:266–269. [DOI] [PubMed] [Google Scholar]
- 10. Makarova‐Rusher OV, Altekruse SF, McNeel TS et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016;122:1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davila JA, Morgan RO, Shaib Y et al. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population‐based study. Gastroenterology 2004;127:1372–1380. [DOI] [PubMed] [Google Scholar]
- 12. Di Bisceglie AM, Lyra AC, Schwartz M et al.; Liver Cancer Network. Hepatitis C‐related hepatocellular carcinoma in the United States: Influence of ethnic status. Am J Gastroenterol 2003;98:2060–2063. [DOI] [PubMed] [Google Scholar]
- 13. Lu M, Li J, Rupp LB et al. Hepatitis C treatment failure is associated with increased risk of hepatocellular carcinoma. J Viral Hepat 2016;23:718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Venook AP. Treatment of hepatocellular carcinoma: Too many options? J Clin Oncol 1994;12:1323–1334. [DOI] [PubMed] [Google Scholar]
- 15. Goldberg D, Ditah IC, Saeian K et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017;152:1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim D, Li AA, Perumpail BJ et al. Changing trends in etiology‐based and ethnicity‐based annual mortality rates of cirrhosis and hepatocellular carcinoma in the United States. Hepatology 2019;69:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Llovet JM. Clinical and molecular classification of hepatocellular carcinoma. Liver Transpl 2007;3(11 suppl 2):S13–S16. [DOI] [PubMed] [Google Scholar]
- 18. Llovet JM, Ricci S, Mazzaferro V et al.; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 19. Cheng A, Kang Y, Chen Z et al. Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: A phase III randomized, double‐blind, placebo‐controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- 20. Kudo M, Finn RS, Qin S et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: A randomized phase 3 non‐inferiority trial. Lancet 2018;391:1163–1173. [DOI] [PubMed] [Google Scholar]
- 21. Yang TS, Lin YC, Chen JS et al. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer 2000;89:750–756. [DOI] [PubMed] [Google Scholar]
- 22. Yang TS, Yang CH, Hsieh RK et al. Gemcitabine and doxorubicin for the treatment of patients with advanced hepatocellular carcinoma: A phase I‐II trial. Ann Oncol 2002;13:1771–1778. [DOI] [PubMed] [Google Scholar]
- 23. Patt YZ, Hassan MM, Aguayo A et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 2004;101:578–586. [DOI] [PubMed] [Google Scholar]
- 24. von Delius S, Lersch C, Mayr M et al. Capecitabine for treatment of advanced hepatocellular carcinoma. Hepatogastroenterology 2007;54:2310–2314. [PubMed] [Google Scholar]
- 25. Murer F, Pozzan C, Peserico G et al. Capecitabine in advanced hepatocellular carcinoma. Dig Liver Dis 2016;48:1260–1261. [DOI] [PubMed] [Google Scholar]
- 26. Hsu CH, Yang TS, Hsu C et al. Phase II study of bevacizumab + capecitabine in patients with advanced/metastatic hepatocellular carcinoma: Final report. J Clin Oncol 2008;26:4603a. [Google Scholar]
- 27. Sun W, Haller DG, Mykulowycz K et al. Combination of capecitabine, oxaliplatin with bevacizumab in treatment of advanced hepatocellular carcinoma (HCC): A phase II study. J Clin Oncol 2007;25(suppl 18):4574a. [Google Scholar]
- 28. Li Y, Gao ZH, Qu XJ. The adverse effects of sorafenib in patients with advanced cancers. Basic Clin Pharmacol Toxicol 2015;116:216–221. [DOI] [PubMed] [Google Scholar]
- 29. Hsu CH, Shen YC, Shao YY et al. Sorafenib in advanced hepatocellular carcinoma: Current status and future perspectives. J Hepatocell Carcinoma 2014;12:1:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Neil BH, Venook AP. Hepatocellular carcinoma: The role of the North American GI Steering Committee Hepatobiliary Task Force and the advent of effective drug therapy. The Oncologist 2007;12:1425–1432. [DOI] [PubMed] [Google Scholar]
- 31. Abou‐Alfa GK, Venook AP. The impact of new data in the treatment of advanced hepatocellular carcinoma. Curr Oncol Rep 2008;10:199–205. [DOI] [PubMed] [Google Scholar]
- 32. Liu SH, Chuang WC, Lam W et al. Safety surveillance of traditional Chinese medicine: Current and future. Drug Saf 2015;38:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu SH, Cheng YC. Old formula, new Rx: The journey of PHY906 as cancer adjuvant therapy. J Ethnopharmacol 2012;140:614–623. [DOI] [PubMed] [Google Scholar]
- 34. Chinese Botany. Vol. 7. 1st ed. Shanghai, China: Shanghai Science and Technology Publishing Company, 1999. [Google Scholar]
- 35. Liu SH, Jiang Z, Cheng YC. A Chinese medicine formulation, PHY‐906, can enhance the therapeutic index of CPT‐11 and other anticancer drugs against cancer in mice. Proc Am Assoc Cancer Res 2001;42:458a. [Google Scholar]
- 36. Lam W, Bussom S, Guan F et al. PHY906, a four‐herb Chinese medicine formula first described 1800 years ago, reduces irinotecan‐induced intestinal damage through anti‐inflammatory effects and by promotion of the repopulation of intestinal progenitor cells. Sci Transl Med 2010;2:45ra59. [DOI] [PubMed] [Google Scholar]
- 37. Wang E, Bussom S, Chen J et al. Interaction of a traditional Chinese medicine (PHY906) and CPT‐11 on the inflammatory process in the tumor environment. BMC Med Genomics 2011;11:4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kummar S, Copur MS, Rose M et al. A phase I study of the Chinese herbal medicine PHY906 as a modulator of irinotecan‐based chemotherapy in patients with advanced colorectal cancer. Clin Colorectal Cancer 2011;10:85–96. [DOI] [PubMed] [Google Scholar]
- 39. Lam W, Jiang Z, Guan F et al. The number of intestinal bacteria is not critical for the enhancement of antitumor activity and reduction of intestinal toxicity of irinotecan by the Chinese herbal medicine PHY906 (KD018). BMC Complement Altern Med 2014;15:14:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saif MW, Lansigan F, Ruta S et al. Phase I study of the botanical formulation PHY906 with capecitabine in advanced pancreatic and other gastrointestinal malignancies. Phytomedicine 2010;17:161–169. [DOI] [PubMed] [Google Scholar]
- 41. Sze DM, Chan GCF. Supplements for immune enhancement in hematologic malignancies. Hematology Am Soc Hematol Educ Program 2009;2009:313–319. [DOI] [PubMed] [Google Scholar]
- 42. Liu SH, Jiang Z, Gao W. PHY906, a Chinese herbal formulation enhances the therapeutic effect of cancer chemotherapy in human colorectal and liver cancer. Proc Am Soc Clin Oncol 2003;22:864a. [Google Scholar]
- 43. Liu SH, Foo A, Jiang Z et al. PHY906 as a broad‐spectrum enhancer in therapy: Clinical and preclinical results in hepatocellular carcinoma. Proc Am Assoc Cancer Res 2006;47:2142a. [Google Scholar]
- 44. Lam W, Jiang Z, Guan F et al. PHY906(KD018), an adjuvant based on a 1800‐year‐old Chinese medicine, enhanced the anti‐tumor activity of sorafenib by changing the tumor microenvironment. Sci Rep 2015;30:5:9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu SH, Jiang Z, Su TM et al. Developing PHY906 as a broad‐spectrum modulator of chemotherapeutic agents in cancer therapy. Cancer Res 2004;64(suppl 7):557a. [Google Scholar]
- 46. Rockwell S, Grove TA, Liu Y et al. Preclinical studies of the Chinese Herbal Medicine formulation PHY906 (KD018) as a potential adjunct to radiation therapy. Int J Radiat Biol 2013;89:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Farrell MP, Kummar S. Phase I/IIA randomized study of PHY906, a novel herbal agent, as a modulator of chemotherapy in patients with advanced colorectal cancer. Clin Colorectal Cancer 2003;2:253–256. [DOI] [PubMed] [Google Scholar]
- 48. Yen Y, So S, Rose M et al. Phase I/II study of PHY906/capecitabine in advanced hepatocellular carcinoma. Anticancer Res 2009;29:4083–4092. [PubMed] [Google Scholar]
- 49. Yen Y, Chen LT, Liu SH et al. A comparison of US and Taiwan phase II clinical trials on combination therapy of PHY906 plus capecitabine in hepatocellular carcinoma. Abstract presented at: 11th Meeting of the Consortium for Globalization of Chinese Medicine (CGCM); August 21–23, 2012; Macau.
- 50. Johung K, Kann B, Lacy J et al. Pilot trial of YIV‐906 with neoadjuvant chemoradiotherapy (CRT) in patients with locally advanced rectal cancer. Ann Oncol 2018;29(suppl 5):V90. [Google Scholar]
- 51. Liu SH, Jiang Z, Foo A et al. PHY906 in hepatocellular carcinoma. Proc Am Assoc Cancer Res 2007;48:1841a. [Google Scholar]


