Abstract
In the open metabolic system, redox-related signaling requires continuous monitoring and fine-tuning of the steady-state redox set point. The ongoing oxidative metabolism is a persistent challenge, denoted as oxidative eustress, which operates within a physiological range that has been called the ‘Homeodynamic Space’, the ‘Goldilocks Zone’ or the ‘Golden Mean’. Spatiotemporal control of redox signaling is achieved by compartmentalized generation and removal of oxidants. The cellular landscape of H2O2, the major redox signaling molecule, is characterized by orders-of-magnitude concentration differences between organelles. This concentration pattern is mirrored by the pattern of oxidatively modified proteins, exemplified by S-glutathionylated proteins. The review presents the conceptual background for short-term (non-transcriptional) and longer-term (transcriptional/translational) homeostatic mechanisms of stress and stress responses. The redox set point is a variable moving target value, modulated by circadian rhythm and by external influence, summarily denoted as exposome, which includes nutrition and lifestyle factors. Emerging fields of cell-specific and tissue-specific redox regulation in physiological settings are briefly presented, including new insight into the role of oxidative eustress in embryonal development and lifespan, skeletal muscle and exercise, sleep-wake rhythm, and the function of the nervous system with aspects leading to psychobiology.
Keywords: Redox biology, Steady-state, Homeodynamics, Redox landscape, Hydrogen peroxide, Oxidative stress
1. Introduction
Maintenance of redox homeostasis is a continuously ongoing challenge. Constant surveillance is a hallmark for establishment and maintenance of redox homeostasis, which is more precisely called ‘homeodynamics’ because of its underlying dynamic nature [1]. Recent research progress in redox biology revealed a redox architecture of physiological function [2], which is organized according to a set of principles denoted as the ‘Redox Code’ [3]. One of these principles is that of activation/deactivation cycles of redox metabolism, especially involving H2O2, which with other molecular signaling agents supports spatiotemporal sequencing in differentiation and life cycles of cells and organs [3]. The wider field of redox signaling has been reviewed extensively (see, for example, Refs. [[4], [5], [6], [7], [8]]).
Cellular redox dynamics is intimately linked to sophisticated structural events at the molecular and cellular level. The latter is illustrated by the continual reshaping, at a seconds-timescale, of the cristae at the mitochondrial inner membrane, revealed by super-resolution nanoscopy [9]. Such reshaping, in turn, is linked to changes in mitochondrial supercomplex formation (see Ref. [10]). The underlying molecular monitoring events include redox parameters, and these precede the structural changes at timescales considerably shorter than the seconds range. H2O2 contributes to stability of the mitochondrial redox network [11], and there is an integrated redox network at the level of the cell and its organelles for monitoring homeostasis [12,13]. Maintenance is achieved by constant monitoring redox activity in ‘oxidative eustress’ [14].
Here, we start with the conceptual background on redox homeostasis, characterizing the metabolic steady-state. This is followed by a generalized snapshot of the cellular redox landscape with focus on H2O2, the central redox signaling metabolite [[15], [16], [17], [18]]. Short-term (non-transcriptional) and longer-term (transcriptional/translational) mechanisms will be outlined. Examples of emerging redox research areas will be presented, focusing on diurnal (circadian) rhythm, sleep-wake cycles, embryonal development and lifespan, skeletal muscle and exercise, and some aspects of the nervous system which touch on molecular relationships in psychobiology.
2. Conceptual: open system and maintenance of steady-state
Von Bertalanffy [19] pioneered the biophysics of flow-equilibrium, which is called ‘steady-state’: “Living systems are open systems, maintaining themselves in exchange of materials with environment, and in continuous building up and breaking down of their components” [19]. The open metabolic system requires continuous monitoring of inflow and outflow to minimize deviation from the steady-state set point. In redox regulation, this refers to physiological oxidative stress, or oxidative eustress (see Ref. [20]). The physiological range of excursions from the set point has been called the ‘Homeodynamic Space’ [21], the ‘Goldilocks Zone’ [22] and the ‘Golden Mean’ [23].
Furthermore, the ever-changing metabolic conditions require appropriate adjustment of the steady-state set point, i.e. the target value of reduction-oxidation in the spatiotemporal context. This is epitomized by Selye's adaptive stress concept [24]. Prigogine [25] analyzed time structure and fluctuations, which he called ‘dissipative structures’ in non-equilibrium thermodynamics. In biology, the idea of Claude Bernard's ‘milieu intérieur’ [26] has found attraction in the terms ‘resilience’ and ‘allostasis’. Resilience denotes the ability to return to the original condition, to bounce back, whereas allostasis refers to the achievement of stability through change to a new set point [27], which is a contradictio in adjecto. A related term is ‘adaptive homeostasis’, formulated by Davies [28].
Thus, mechanisms for maintenance of homeostasis can be divided into reactive (feedback, counterregulation) and predictive (feedforward, anticipatory) modes.
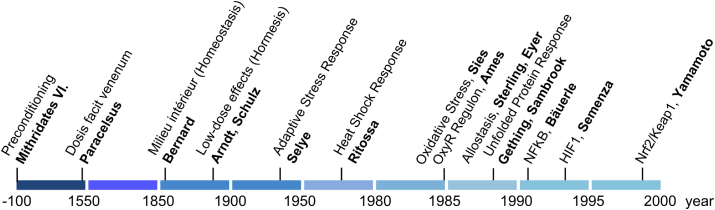
As for the latter, the capability of preconditioning in response to endogenous and exogenous (‘exposome’) cues, summarized under the terms ‘hormesis’ [29] and more specified ‘mitohormesis’ [30], is important for adaptive stress responses. Fig. 1 gives a timeline of the concepts of stress and stress responses.
Fig. 1.
Timeline of concepts of stress and adaptive stress responses. Mithridates VI [132] and Paracelsus [133] had early insight that the dose matters in deciding beneficial versus harmful outcome. Bernard's concept of the ‘milieu intérieur’ [26] received the name ‘homeostasis’ [134], and the Arndt-Schulz [135] rule received the name ‘hormesis’ [136]. The 20th century brought the adaptive stress syndrome [24], heat shock response [137], oxidative stress [138], OxyR [139], allostasis [27], unfolded protein response [140], and the major mammalian master regulators NF-kB [141], HIF1 [142], and Nrf2/Keap1 [143]. From Ref. [20].
Selye introduced the distinction between eustress and distress in 1975 [31], and oxidative eustress in molecular terms has become of interest in recent years to signify physiological, not harmful, oxidative stress [17], [20], [32a], [32], [33], [34], [35], [36].
3. Spatiotemporal controls
Cellular organization is characterized by subcellular compartmentation and gradients. Interestingly, the fast-acting non-transcriptional signaling agents occur at about nM concentration; H2O2, Ca2+ and pH are shown in Table 1. (It may be pointed out that pH = 7 signifies [H+] = 100 nM H+.) Other important signaling entities such as .NO [37] and H2S [38] also occur at nM physiological concentration, constituting a ‘reactive species interactome’ [39,40]. Furthermore, there is redox signaling by reactive electrophiles for maintenance of the nucleophilic tone [23,41].
Table 1.
Fast-acting signaling agents H2O2and Ca2+occur at nanomolar concentration. Proton concentration [H+] is at similar concentration. Columns are in nanomolar [nanomol/L] and as p = –log10 [mol/L], to illustrate the similar physiological ranges for H2O2, Ca2+ and H+. Numbers refer to generic resting cells, ranges not shown; exact quantification in subcellular organelles is yet to be obtained.
| H2O2 |
Ca2+ |
H+ |
||||
|---|---|---|---|---|---|---|
| nM | (pH2O2) | nM | (pCa) | nM | (pH) | |
| ‘Overall cellular’a | 10 | (8) [128,129] | 100 | (7) | 100 | (7) |
| Cytosol | 0.1 | (10) [53] | <100 | (>7) | 100 | (7.0) |
| Mitochondrial matrix | 4 | (8.4) [54] | <100 | (>7) | 40 | (7.4) |
| Endoplasmic reticulum | 700 | (6.2) [52] | 500,000 | (3.3) | 60 | (7.2) |
| Golgi | 300 | (6.5) [130] | 300,000 | (3.5) | 400 | (6.4) |
| Peroxisome | ? | <100 | (>7) | 7 | (8.2) [69] | |
| Lysosome | ? | 300,000 | (3.5) [131] | 3200 | (5.5) | |
For rough orientation only: considerable subcellular variation; Ca2+ in various spaces was arbitrarily set to <100 nM.
‘Buffering’ of [Ca2+] relies on Ca2+ stores in the endoplasmic reticulum and the mitochondrial matrix (see Ref. [42]), and buffering of [H+] relies on the action of carbonic anhydrases and respiration. In contrast, there is no ‘buffering store’ of H2O2 within the cell. Here, control of [H2O2] relies on swift fine-tuning of enzymatic synthesis and degradation of H2O2 as well as on gradient control. A role of mitochondria as ‘ROS stabilizing device’ has been postulated [43], and this extends also to control by extramitochondrial sources such as NADPH oxidases [44].
H2O2 and Ca2+ are reciprocally interconnected in numerous signaling processes (see Refs. [[45], [46], [47], [48]]). As listed in Table 2, an illustrative example is that of the transient receptor potential (TRP) channels, which act as biosensors for redox environmental stimuli, being activated by H2O2, .NO and electrophiles [49]. These channels facilitate Ca2+ influx, triggering cellular responses. The receptor has a highly reactive cysteine (C621 in TRPA1), the modification of which leads to the opening of the gate [50]. Another example is the suppression of store-operated calcium entry upon oxidation of cysteine 313 in stromal interaction molecule 2 (STIM2), which gates calcium channels [51].
Table 2.
Selected examples of physiological oxidative stress (eustress) in life processes. Cysteine positions attributed to the effects are given. These entries refer to the selected examples discussed in text, as representative of many more in the literature.
3.1. Spatial: cellular redox landscape
The concentration pattern of H2O2 across cells is depicted in Fig. 2 according to currently available information, which is still limited in terms of calibrated numbers rather than color scales from imaging (see Table 1). The extremes go from the lumen of the endoplasmic reticulum, approaching the μM H2O2 range [52], down to the cytosol on the lower end, for which as low as 80 pM H2O2 has been calculated [53]. The mitochondrial matrix is estimated to contain around 4 nM H2O2 [54]. Little is known about the respective numbers for peroxisomes and lysosomes. Peroxisomal matrix H2O2 concentration was found to be considerably higher than mitochondrial matrix H2O2 in experiments with roGFP2-Orp1, but assigning numbers to the peroxisomal data is difficult [55]. Fig. 2 gives a rough orientation; clearly, there are subcellular microdomains [56] or nanodomains [57] with variant steady-state distribution of H2O2, indicating that the landscape is diversified and subtly readapted upon receiving cues. Thus, a more refined landscape would be considerably more sophisticated due to the intricate inter-organelle relationships known under the name of ‘contactology‘ [[58], [59], [60], [61], [62]].
Fig. 2.
‘Landscape’ of H2O2 across the cell.
Generalized overview of estimated concentrations of H2O2 in subcellular spaces. Color code is from light blue (80 pM) to blue-green (4 nM), green (ca. 20 nM), brown (300 nM), and red (700 nM) (see Table 1 for References).
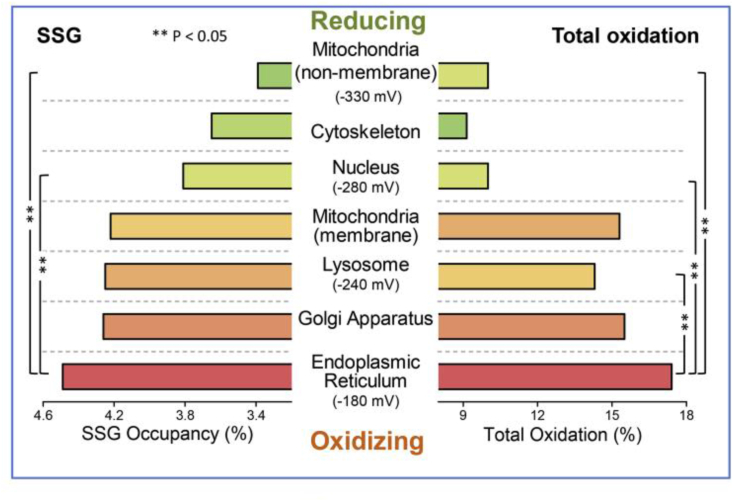
It is noteworthy that the ‘landscape’ of H2O2 resembles the pattern of protein S-glutathionylation: the average occupancy of proteins by S-glutathionylation was recently shown to be highest in the endoplasmic reticulum and lowest in the mitochondrial matrix [63]. The subcellular distribution of S-glutathionylated proteins (Fig. 3) thus resembles that of H2O2 concentration shown in Fig. 2. It will be of interest to compare this pattern to other types of oxidative posttranslational modifications of proteins (oxPTM), such as the predominantly mitochondrially located Coenzyme A, named S-CoAlation [64], and others like S-persulfhydration, S-acylation, S-nitrosation or S-palmitoylation of redox-sensitive proteins. Functional perspectives of the role of these oxPTMs are being elucidated, e.g. as given for S-glutathionylation [65,66] or S-CoAlation [67]. A further level of refinement concerns the reactivity of protein cysteine thiols: the pKa of glutathione persulfide (GSSH) is about 3.5 units lower than that of glutathione (GSH) [68], making GSSH much more reactive than GSH at physiological pH. Likewise, at the more basic pH of 8.2 at the peroxisomal matrix [69], thiolate chemistry will be considerably more prevalent than at neutrality. Thus, peculiarities of reactive species and of location can make for more than 1000-fold differences in reactivity, illustrating relationships between redox chemistry and compartmental pH.
Fig. 3.
Subcellular distribution of average protein S-glutathionylation (SSG occupancy) and total oxidation. Analysis of the redox proteome of macrophages. From Ref. [63].
3.2. Temporal: the redox set point is a moving target
The temporal response can be divided into the seconds range for immediate redox response, and to longer timeframe response. Monitoring at the short time range, the sources of H2O2 such as NADPH oxidases, mitochondrial respiratory chain complexes and H2O2-generating enzymes (see Ref. [8]) are turned on instantly in response to metabolic and physical cues. This is without transcriptional/translational activation of gene expression, allowing for tight control of the steady-state set point by feedback loops. An illustrative example is that of the rapid initiation of wound healing, which involves H2O2, Ca2+ and ATP as transcription-independent damage signals operating instantly upon demand [70].
The longer timeframe redox response is on the hours range and beyond, based on transcriptional/translational activation, permitting feedforward as well as feedback regulation. One major feature here is diurnal rhythm, the circadian response pattern. Transcriptional/translational feedback loops (TTFL) form the backbone of the mammalian circadian clock [71,72]. Peroxiredoxins are conserved markers of circadian rhythm [73], and peroxiredoxins have an emerging role as redox relay hubs [74]. The rhythmic expression of peroxiredoxin-6 is cooperatively controlled by the clock protein Bmal1 and Nrf2[75]. The diurnal oscillations of H2O2, which exert redox control of the CLOCK protein, are influenced by the adaptor protein p66Shc, mediating the changes of the H2O2 set point over the day [76]. Thus, oscillations of H2O2 blend into the coupled network of circadian clocks [77], making the H2O2 set point a dynamic moving target.
The circadian rhythm widely impinges on physiology. The molecular basis of redox influence on sleep-wake patterns is beginning to be unraveled. In Drosophila, the Kv potassium channel ß-subunit was found to couple mitochondrial oxidative events to sleep [78]. Also in Drosophila, a bidirectional relationship between sleep and oxidative stress was observed [79]; an increase in sleep in wild-type flies increased their resistance to oxidative stress, while diminishing oxidative stress in neurons shortened sleep, which led the authors to the slogan: “sleep clears ROS, ROS promote sleep” [79]. Sleep loss leads to ‘ROS’ accumulation, and death from sleep loss has been attributed to oxidative stress in the gut [80]. This brief glance at an emerging research field may suffice here.
3.3. Gradient control: role of peroxiporins
The concentration of H2O2 in blood plasma was estimated to be 1–5 μM [81]. Thus, there is a steep gradient across the plasma membrane from outside towards the H2O2 concentrations inside cells, which is in the nM range (Table 1). Arguably, one could consider the μM extracellular H2O2 concentration to functionally serve as an ‘H2O2 store’, which can be tapped into on demand. Likewise, there are substantial H2O2 gradients between subcellular organelles and the cytosol. Several aquaporins (AQP3, AQP5, AQP8, AQP9, AQP11) facilitate transmembrane diffusion of H2O2, for which they are more specifically called ‘peroxiporins’ (see Ref. [82]). Work on AQP8 revealed a gating mechanism involving cysteine persulfidation (RSSH), suggesting H2O2 gradient control by peroxiporins in a redox-dependent manner [83]. AQP11 is localized in the endoplasmic reticulum membrane. It was found that AQP11 efficiently transports H2O2 to the cytosol, making it a potential regulator of endoplasmic reticulum-based redox signaling [84]. Diurnal aquaporin expression has been found early on in plants [85]. Whether peroxiporins undergo circadian rhythm in mammalian cells seems not to have been examined in detail, but AQP3 in the epidermis has been found to undergo such rhythm [86].
4. Functional: cell- and tissue-specific redox regulation; emerging fields
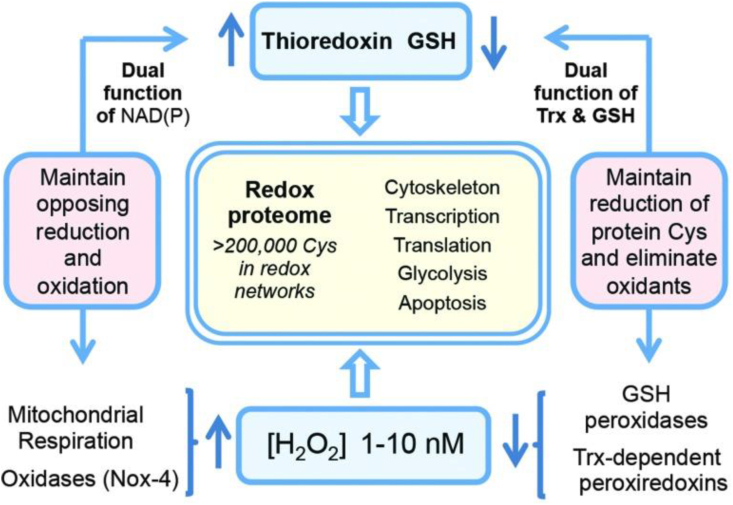
Cells and tissues engage in their specialized physiology with redox regulation playing essential roles. In particular, protein-cysteine redox networks within each tissue underlie tissue-specific biology. Fig. 4 recalls a general overview of the redox proteome, which is organized through kinetically controlled thiol switches [3,87,88]. Specificity is conferred by protein-cysteine thiolate reactivity and electrostatic gating [89], [89a] Quantitative mapping of the mouse cysteine proteome in vivo using a method called ‘Oximouse’ has opened new perspectives [89]. Assaying this ‘landscape’ revealed that redox regulation of specific proteins is highly tissue-specific. Oximouse redox networks help identifying new pathways of redox regulation (see Fig. 3 in Ref. [89]). Pathways for sensing and responding to H2O2 at the endoplasmic reticulum have been identified [90].
Fig. 4.
The redox proteome is organized through kinetically controlled thiol switches.
From Ref. [3].
Selected processes requiring oxidative eustress are now presented in this section, referring to recent literature on these emerging fields, without attempting full coverage of each of these rapidly developing topics. In a recent review [8], other important topics were addressed, including immune system, inflammation and wound repair, the cardiovascular system, insulin sensitivity and pathogenesis of diabetes, aging, and cancer.
4.1. Development, lifespan
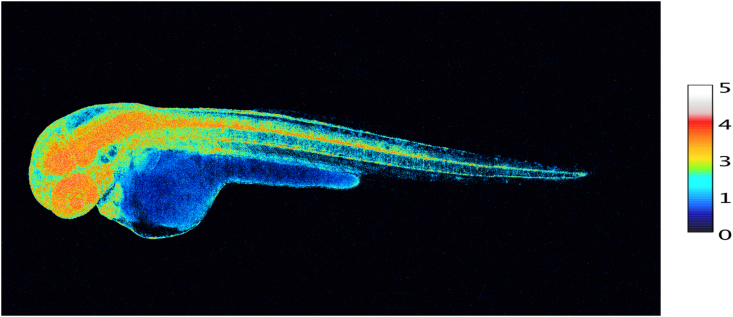
During embryogenesis and throughout development, redox signaling contributes to cell fate decisions and organogenesis. Development of an embryo entails considerable changes in redox state [91]. H2O2 is involved in morphogenesis and cell differentiation [[92], [93], [94]], accompanied by changes in glutathione utilization [95]. Fig. 5 provides a snapshot of the pattern of H2O2 concentrations in the various organs of a zebrafish embryo at 48 h post fertilization, using the genetically encoded probe, Hyper7. At this stage, H2O2 concentrations are particularly high in the developing nervous system (brain, retina and spinal cord) as well as in the heart. A role of NADPH oxidases in neuronal development has been substantiated [96]. H2O2 and the homeoprotein Engrailed synergize to shape the optical tectum, contributing to embryonal patterning in zebrafish [97], which extends earlier findings of the role of H2O2 on axonal growth cone pathfinding [98]. Information on the role of ‘ROS’ in axonal growth has also been obtained in regeneration studies. After nerve injury, NADPH oxidase-2 is released from macrophages into exosomes, which are incorporated into the injured axons via endocytosis [99]. The authors propose the signaling pathway of NOX2-PI3K-p-Akt for axonal regeneration [99].
Fig. 5.
Snapshot of diverse concentrations of H2O2 in various organs of the intact developing zebrafish embryo at 48 h post fertilization.
100 ng/μL of HyPer7 mRNA was injected in 1-cell stage zebrafish embryos.
Scale bar, 100 μm. Embryo H2O2 imaging was performed essentially as described in Ref. [97]. H2O2 concentration is correlated to the YFP500/YFP420 excitation ratio of HyPer7. Photo taken by M. Thauvin, kindly provided by Prof. Sophie Vriz, Paris.
A molecular link between early-life events, ROS-sensitive epigenetic marks, stress resistance and lifespan has been identified [100]. These effects, observed in C. elegans and in HeLa cells, were attributed to a global ROS-mediated decrease in a particular developmental histone modification: trimethylation of lysine 4 in histone 3 (H3K4me3) was diminished, causing increased stress resistance [100].
Fate and functions of stem cells are tightly linked to redox homeostasis, deciding between quiescence, self-renewal and differentiation (see Refs. [101], [101a]. Furthermore, the role of oxidants in reproduction is being appreciated [102]. The role of oxidants in assisted reproduction needs to be further elucidated, in order to preserve redox signaling while minimizing oxidative damage [103].
4.2. Skeletal muscle: exercise as eustress
H2O2 is a key signal in skeletal muscle physiology and adaptation to exercise [[104], [105], [106], [107]]. Both short-term immediate response to physical activity [108] and longer-term remodeling and adaptation [106] involve redox signaling by H2O2. 2-Cys peroxiredoxin-2 was found to be rapidly and reversibly oxidized in response to contractile activity, identifying this protein as an effector in muscle redox signaling [109]. Other effector molecules, likely other reactive peroxidases, will come into play in muscle adaptation [106], and the attenuated exercise response in older individuals may be explained by diminished transient oxidation of effectors such as peroxiredoxin-2[110]. Extracellular superoxide dismutase (SOD3) has been implicated in dampening oxidative challenge during exercise [111]. The loss of muscle mass (cachexia) in cancer is related to altered redox homeostasis [112].
4.3. Oxidative eustress, the nervous system and psychobiology
The brain utilizes redox signals for many functions, the hypothalamus being the ‘master orchestrator’ [113]. There is redox crosstalk with metabolic signaling at the neuron-astrocyte interface [114], and a tight relationship exists between astrocytes and neurons in patterning selenoproteins, which constitute part of redox control [115]. Synaptic plasticity [116], synaptic pruning [117] and glutamate receptor activation [118] are only a few more of many examples of functional use of oxidants in the nervous system, establishing oxidative eustress as pivotal.
The molecular links between psychobiology and redox biology in stress research are moving into focus of mind-body science [119]. A key observation was the distinction of eustress (‘good stress’) from distress (‘bad stress’) in anticipatory cortisol reactivity, and that moderate stress enhances resilience [120]. Interestingly, in critical incident stress training, even decreased salivary cortisol was observed in periods of self-assessed improved performance, i.e. psychologically denoted ‘eustress’ [121].
The relationship between psychological stress and mitochondrial function has found attention [122]. A mitochondrial health index (MHI) sensitive to mood and caregiving stress has been established [123]. MHI integrates human leukocyte-derived information on nuclear and mitochondrial DNA-encoded parameters, which reflects the respiratory chain capacity per unit of mitochondrial content. It consists of succinate dehydrogenase/citrate synthase and cytochome c oxidase/mitochondrial DNA copy number, respectively. It was found that MHI was correlated to mood parameters [123,124], which were assessed according to protocols for psychological stress and symptoms for depression and anxiety. Mitochondrial dysfunction is closely related to the manifestation of depression [125], and oxidative stress was found to be involved in the linking of psychosocial stress (social isolation, loneliness, effort-reward-imbalance) to cardiovascular disease [126,127].
5. Concluding remarks
The main direction of electron flow in aerobic living systems is catabolic, oxidative. However, anabolic metabolism also occurs, reductive: synthetic pathways are driven by reducing equivalents obtained from redox reactions (see Ref. [3]). Relevant for the present context, the main direction of electron flow is associated with a persistent oxidative challenge, characterized as oxidative eustress. The converse departure from the redox set point is towards reduction. Consequently, such process would be called ‘reductive eustress’, as would occur, for example, at hypoxia or with increased metabolic reducing conditions.
In this sense, the concept of physiological ‘resting state’ would be a misnomer, because at that state there is no ‘rest’; instead, it is continuous testing and correcting towards homeostatic balance, i.e. homeodynamics. The ‘resting state’ can be likened to the ‘stand-by’ or ‘idling’ position in an automobile, which is ready to accelerate, rather than to ‘motor off’, the shutdown of the engine. The three now classical major redox-responsive molecular switches epitomize this fact: the NFkB, Nrf2/Keap1, HIF systems operate on constant alert by coupling oxidant and electrophile status to their level of activation.
Declaration of competing interest
I declare no conflict of interest.
Acknowledgements
Helpful discussions with Wilhelm Stahl are gratefully acknowledged. The author's research was funded over the years by Deutsche Forschungsgemeinschaft (DFG), Bonn, and by National Foundation for Cancer Research (NFCR), Bethesda, MD, USA.
References
- 1.Lloyd D., Aon M.A., Cortassa S. Why homeodynamics, not homeostasis? Sci. World J. 2001;1:133–145. doi: 10.1100/tsw.2001.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santolini J., Wootton S.A., Jackson A.A., Feelisch M. The Redox architecture of physiological function. Curr. Opin. Physiol. 2019;9:34–47. doi: 10.1016/j.cophys.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones D.P., Sies H. The redox code. Antioxidants Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Autreaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 5.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 6.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 9.Kondadi A.K., Anand R., Hänsch S. Cristae undergo continuous cycles of membrane remodelling in a MICOS-dependent manner. EMBO Rep. 2020;21 doi: 10.15252/embr.201949776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker N., Patel J., Khacho M. Linking mitochondrial dynamics, cristae remodeling and supercomplex formation: how mitochondrial structure can regulate bioenergetics. Mitochondrion. 2019;49:259–268. doi: 10.1016/j.mito.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Millare B., O'Rourke B., Trayanova N. Hydrogen peroxide diffusion and scavenging shapes mitochondrial network instability and failure by sensitizing ROS-induced ROS release. Sci. Rep. 2020;10:15758–71308. doi: 10.1038/s41598-020-71308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y., Lu Y., Saredi J. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020;37:101696. doi: 10.1016/j.redox.2020.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wojtovich A.P., Berry B.J., Galkin A. Redox signaling through compartmentalization of reactive oxygen species: implications for health and disease. Antioxidants Redox Signal. 2019;31:591–593. doi: 10.1089/ars.2019.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sies H. Oxidative eustress and distress: introductory remarks. In: Sies H., editor. Oxidative Stress: Eustress And Distress. Academic Press; London: 2020. pp. 3–12. [Google Scholar]
- 15.Forman H.J., Maiorino M., Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee S.G., Woo H.A. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H(2)O(2), and protein chaperones. Antioxidants Redox Signal. 2011;15:781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 17.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox. Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone J.R., Yang S. Hydrogen peroxide: a signaling messenger. Antioxidants Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 19.von Bertalanffy L. The theory of open systems in physics and biology. Science. 1950;111:23–29. doi: 10.1126/science.111.2872.23. [DOI] [PubMed] [Google Scholar]
- 20.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 21.Rattan S.I. Molecular gerontology: from homeodynamics to hormesis. Curr. Pharmaceut. Des. 2014;20:3036–3039. doi: 10.2174/13816128113196660708. [DOI] [PubMed] [Google Scholar]
- 22.Alleman R.J., Katunga L.A., Nelson M.A., Brown D.A., Anderson E.J. The "Goldilocks Zone" from a redox perspective-Adaptive vs. deleterious responses to oxidative stress in striated muscle. Front. Physiol. 2014;5:358. doi: 10.3389/fphys.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ursini F., Maiorino M., Forman H.J. Redox homeostasis: the Golden Mean of healthy living. Redox. Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 25.Prigogine I. Time, structure, and fluctuations. Science. 1978;201:777–785. doi: 10.1126/science.201.4358.777. [DOI] [PubMed] [Google Scholar]
- 26.Holmes F.L. Claude Bernard, the milieu intérieur, and regulatory physiology. Hist. Philos. Life Sci. 1986;8:3–25. [PubMed] [Google Scholar]
- 27.Sterling P., Eyer J. Allostasis. A new paradigm to explain arousal pathology. In: Fisher S., Reason J., editors. vols. 629–649. Wiley; New York: 1988. (Handbook Of Life Stress, Cognition And Health). [Google Scholar]
- 28.Davies K.J. Adaptive homeostasis. Mol. Aspect. Med. 2016;49:1–7. doi: 10.1016/j.mam.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabrese E.J. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Ristow M., Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp. Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Selye H. Stress and distress. Compr. Ther. 1975;1:9–13. [PubMed] [Google Scholar]
- 32.Niki E. [Eustress and distress] Nihon Yakurigaku Zasshi. 2007;129:76–79. doi: 10.1254/fpj.129.76. [DOI] [PubMed] [Google Scholar]
- 32a.Li G., He H. Hormesis, allostatic buffering capacity and physiological mechanism of physical activity: a new theoretic framework. Med. Hypotheses. 2009;72:527–532. doi: 10.1016/j.mehy.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Okegbe C., Sakhtah H., Sekedat M.D., Price-Whelan A., Dietrich L.E. Redox eustress: roles for redox-active metabolites in bacterial signaling and behavior. Antioxidants Redox Signal. 2012;16:658–667. doi: 10.1089/ars.2011.4249. [DOI] [PubMed] [Google Scholar]
- 34.Sanchis-Gomar F. Physical exercise as an epigenetic modulator: eustress, the "positive stress" as an effector of gene expression. J. Strength Condit Res. 2012;26:3469–3472. doi: 10.1519/JSC.0b013e31825bb594. [DOI] [PubMed] [Google Scholar]
- 35.Yan L.J. Positive oxidative stress in aging and aging-related disease tolerance. Redox. Biol. 2014;2C:165–169. doi: 10.1016/j.redox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niki E. Oxidative stress and antioxidants: distress or eustress? Arch. Biochem. Biophys. 2016;595:19–24. doi: 10.1016/j.abb.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Landar A., Darley-Usmar V.M. Nitric oxide and cell signaling: modulation of redox tone and protein modification. Amino Acids. 2003;25:313–321. doi: 10.1007/s00726-003-0019-7. [DOI] [PubMed] [Google Scholar]
- 38.Olson K.R. Reactive oxygen species or reactive sulfur species: why we should consider the latter. J. Exp. Biol. 2020;223:jeb196352. doi: 10.1242/jeb.196352. [DOI] [PubMed] [Google Scholar]
- 39.Cortese-Krott M.M., Kuhnle G.G.C. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxidants Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feelisch M., Akaike T., Griffiths K. Long-lasting blood pressure lowering effects of nitrite are NO-independent and mediated by hydrogen peroxide, persulfides, and oxidation of protein kinase G1α redox signalling. Cardiovasc. Res. 2020;116:51–62. doi: 10.1093/cvr/cvz202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parvez S., Long M.J.C., Poganik J.R., Aye Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 2018;118:8798–8888. doi: 10.1021/acs.chemrev.7b00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carafoli E., Krebs J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016;291:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mailloux R.J. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxid. Med. Cell Longev. 2018:7857251. doi: 10.1155/2018/7857251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schröder K. NADPH oxidases: current aspects and tools. Redox Biol. 2020;34:101512. doi: 10.1016/j.redox.2020.101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox. Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hempel N., Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. 2017;63:70–96. doi: 10.1016/j.ceca.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joseph S.K., Booth D.M., Young M.P., Hajnoczky G. Redox regulation of ER and mitochondrial Ca(2+) signaling in cell survival and death. Cell Calcium. 2019;79:89–97. doi: 10.1016/j.ceca.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madreiter-Sokolowski C.T., Thomas C., Ristow M. Interrelation between ROS and Ca(2+) in aging and age-related diseases. Redox Biol. 2020;36:101678. doi: 10.1016/j.redox.2020.101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakaguchi R., Mori Y. Transient receptor potential (TRP) channels: biosensors for redox environmental stimuli and cellular status. Free Radic. Biol. Med. 2020;146:36–44. doi: 10.1016/j.freeradbiomed.2019.10.415. [DOI] [PubMed] [Google Scholar]
- 50.Zhao J., Lin King J.V., Paulsen C.E., Cheng Y., Julius D. Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature. 2020;585:141–145. doi: 10.1038/s41586-020-2480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gibhardt C.S., Cappello S., Bhardwaj R. Oxidative stress-induced STIM2 cysteine modifications suppress store-operated calcium entry. Cell Rep. 2020;33:108292. doi: 10.1016/j.celrep.2020.108292. [DOI] [PubMed] [Google Scholar]
- 52.Gao C., Tian Y., Zhang R., Jing J., Zhang X. Endoplasmic reticulum-directed ratiometric fluorescent probe for quantitive detection of basal H2O2. Anal. Chem. 2017;89:12945–12950. doi: 10.1021/acs.analchem.7b03809. [DOI] [PubMed] [Google Scholar]
- 53.Lim J.B., Huang B.K., Deen W.M., Sikes H.D. Analysis of the lifetime and spatial localization of hydrogen peroxide generated in the cytosol using a reduced kinetic model. Free Radic. Biol. Med. 2015;89:47–53. doi: 10.1016/j.freeradbiomed.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Stein K.T., Moon S.J., Nguyen A.N., Sikes H.D. Kinetic modeling of H2O2 dynamics in the mitochondria of HeLa cells. PLoS Comput. Biol. 2020;16 doi: 10.1371/journal.pcbi.1008202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lismont C., Nordgren M., Brees C. Peroxisomes as modulators of cellular protein thiol oxidation: a new model system. Antioxidants Redox Signal. 2019;30:22–39. doi: 10.1089/ars.2017.6997. [DOI] [PubMed] [Google Scholar]
- 56.Trewin A.J., Bahr L.L., Almast A. Mitochondrial reactive oxygen species generated at the complex-II matrix or intermembrane space microdomain have distinct effects on redox signaling and stress sensitivity in Caenorhabditis elegans. Antioxidants Redox Signal. 2019;31:594–607. doi: 10.1089/ars.2018.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Booth D.M., Enyedi B., Geiszt M., Varnai P., Hajnoczky G. Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Mol. Cell. 2016;63:240–248. doi: 10.1016/j.molcel.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Csordas G., Weaver D., Hajnoczky G. Endoplasmic reticulum-mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 2018;28:523–540. doi: 10.1016/j.tcb.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan Y., Simmen T. Mechanistic connections between endoplasmic reticulum (ER) redox control and mitochondrial metabolism. Cells. 2019;8:1071. doi: 10.3390/cells8091071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prinz W.A., Toulmay A., Balla T. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 2020;21:7–24. doi: 10.1038/s41580-019-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva B.S.C., DiGiovanni L. Maintaining social contacts: the physiological relevance of organelle interactions. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118800. doi: 10.1016/j.bbamcr.2020.118800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoboue E.D., Sitia R., Simmen T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 2018;9:331–330033. doi: 10.1038/s41419-017-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duan J., Zhang T., Gaffrey M.J. Stochiometric quantification of the thiol redox proteome of macrophages reveals subcellular compartmentalization and susceptibility to oxidative perturbations. Redox Biol. 2020;36:101649. doi: 10.1016/j.redox.2020.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuchiya Y., Peak-Chew S.Y., Newell C. Protein CoAlation: a redox-regulated protein modification by coenzyme A in mammalian cells. Biochem. J. 2017;474:2489–2508. doi: 10.1042/BCJ20170129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mailloux R.J. Protein S-glutathionylation reactions as a global inhibitor of cell metabolism for the desensitization of hydrogen peroxide signals. Redox Biol. 2020;32:101472. doi: 10.1016/j.redox.2020.101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Musaogullari A., Chai Y.C. Redox regulation by protein S-glutathionylation: from molecular mechanisms to implications in health and disease. Int. J. Mol. Sci. 2020;21:8113. doi: 10.3390/ijms21218113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gout I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem. Soc. Trans. 2018;46:721–728. doi: 10.1042/BST20170506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benchoam D., Semelak J.A., Cuevasanta E. Acidity and nucleophilic reactivity of glutathione persulfide. J. Biol. Chem. 2020;295:15466–15481. doi: 10.1074/jbc.RA120.014728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dansen T.B., Wirtz K.W., Wanders R.J., Pap E.H. Peroxisomes in human fibroblasts have a basic pH. Nat. Cell Biol. 2000;2:51–53. doi: 10.1038/71375. [DOI] [PubMed] [Google Scholar]
- 70.Cordeiro J.V., Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat. Rev. Mol. Cell Biol. 2013;14:249–262. doi: 10.1038/nrm3541. [DOI] [PubMed] [Google Scholar]
- 71.Milev N.B., Rhee S.G., Reddy A.B. Cellular timekeeping: it's redox o'Clock. Cold Spring Harb. Perspect. Biol. 2018;10:a027698. doi: 10.1101/cshperspect.a027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edgar R.S., Green E.W., Zhao Y. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stöcker S., van Lear K., Mijuskovic A., Dick T.P. The conundrum of hydrogen peroxide signaling and the emerging role of peroxiredoxins as redox relay hubs. Antioxidants Redox Signal. 2018;28:558–573. doi: 10.1089/ars.2017.7162. [DOI] [PubMed] [Google Scholar]
- 75.Chhunchha B., Kubo E., Singh D.P. Clock protein Bmal1 and Nrf2 cooperatively control aging or oxidative response and redox homeostasis by regulating rhythmic expression of Prdx6. Cells. 2020;9:1861. doi: 10.3390/cells9081861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pei J.F., Li X.K., Li W.Q. Diurnal oscillations of endogenous H(2)O(2) sustained by p66(Shc) regulate circadian clocks. Nat. Cell Biol. 2019;21:1553–1564. doi: 10.1038/s41556-019-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finger A.M., Dibner C., Kramer A. Coupled network of the circadian clocks: a driving force of rhythmic physiology. FEBS Lett. 2020;594:2734–2769. doi: 10.1002/1873-3468.13898. [DOI] [PubMed] [Google Scholar]
- 78.Kempf A., Song S.M., Talbot C.B., Miesenbock G. A potassium channel beta-subunit couples mitochondrial electron transport to sleep. Nature. 2019;568:230–234. doi: 10.1038/s41586-019-1034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hill V.M., O'Connor R.M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaccaro A., Kaplan Dor Y. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell. 2020;181:1307–1328. doi: 10.1016/j.cell.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 81.Forman H.J., Bernardo A., Davies K.J. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 2016;603:48–53. doi: 10.1016/j.abb.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Bienert G.P., Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta. 2014;1840:1596–1604. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 83.Bestetti S., Medrano-Fernandez I., Galli M. A persulfidation-based mechanism controls aquaporin-8 conductance. Sci. Adv. 2018;4:eaar5770. doi: 10.1126/sciadv.aar5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bestetti S., Galli M., Sorrentino I. Human aquaporin-11 guarantees efficient transport of H(2)O(2) across the endoplasmic reticulum membrane. Redox Biol. 2020;28:101326. doi: 10.1016/j.redox.2019.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clarkson D.T., Carvajal M., Henzler T. Root hydraulic conductance: diurnal aquaporin expression and the effects of nutrient stress. J. Exp. Bot. 2000;51:61–70. [PubMed] [Google Scholar]
- 86.Matsunaga N., Itcho K. 24-hour rhythm of aquaporin-3 function in the epidermis is regulated by molecular clocks. J. Invest. Dermatol. 2014;134:1636–1644. doi: 10.1038/jid.2014.13. [DOI] [PubMed] [Google Scholar]
- 87.Go Y.M., Jones D.P. The redox proteome. J. Biol. Chem. 2013;288:26512–26520. doi: 10.1074/jbc.R113.464131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller C.G., Schmidt E.E. Sulfur metabolism under stress. Antioxidants Redox Signal. 2020;33:1158–1173. doi: 10.1089/ars.2020.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao H., Jedrychowski M.P. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell. 2020;180:968–983. doi: 10.1016/j.cell.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89a.Berndt C., Schwenn J.D., Lillig C.H. The specificity of thioredoxins and glutaredoxins is determined by electrostatic and geometric complementarity. Chem. Sci. 2015;6:7049–7058. doi: 10.1039/c5sc01501d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roscoe J.M., Sevier C.S. Pathways for sensing and responding to hydrogen peroxide at the endoplasmic reticulum. Cells. 2020;9:2314. doi: 10.3390/cells9102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Timme-Laragy A.R., Hahn M.E., Hansen J.M., Rastogi A., Roy M.A. Redox stress and signaling during vertebrate embryonic development: regulation and responses. Semin. Cell Dev. Biol. 2018;80:17–28. doi: 10.1016/j.semcdb.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oswald M.C.W., Garnham N., Sweeney S.T., Landgraf M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018;592:679–691. doi: 10.1002/1873-3468.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rampon C., Volovitch M., Joliot A., Vriz S. Hydrogen peroxide and redox regulation of developments. Antioxidants. 2018;7 doi: 10.3390/antiox7110159. antiox7110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilson C., Munoz-Palma E., Gonzalez-Billault C. From birth to death: a role for reactive oxygen species in neuronal development. Semin. Cell Dev. Biol. 2018;80:43–49. doi: 10.1016/j.semcdb.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 95.Rastogi A., Clark C.W., Conlin S.M., Brown S.E., Timme-Laragy A.R. Mapping glutathione utilization in the developing zebrafish (Danio rerio) embryo. Redox Biol. 2019;26:101235. doi: 10.1016/j.redox.2019.101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Terzi A., Suter D.M. The role of NADPH oxidases in neuronal development. Free Radic. Biol. Med. 2020;154:33–47. doi: 10.1016/j.freeradbiomed.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 97.Amblard I., Thauvin M. H(2)O(2) and Engrailed 2 paracrine activity synergize to shape the zebrafish optic tectum. Commun. Biol. 2020;3:536. doi: 10.1038/s42003-020-01268-7. 01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gauron C., Meda F., Dupont E. Hydrogen peroxide (H2O2) controls axon pathfinding during zebrafish development. Dev. Biol. 2016;414:133–141. doi: 10.1016/j.ydbio.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 99.Hervera A., De Virgilis F. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 2018;20:307–319. doi: 10.1038/s41556-018-0039-x. [DOI] [PubMed] [Google Scholar]
- 100.Bazopoulou D., Knoefler D. Developmental ROS individualizes organismal stress resistance and lifespan. Nature. 2019;576:301–305. doi: 10.1038/s41586-019-1814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tan D.Q., Suda T. Reactive oxygen species and mitochondrial homeostasis as regulators of stem cell fate and function. Antioxidants Redox Signal. 2018;29:149–168. doi: 10.1089/ars.2017.7273. [DOI] [PubMed] [Google Scholar]
- 101a.Prozorovski T., Schneider R., Berndt C., Hartung H.P., Aktas O. Redox-regulated fate of neural stem progenitor cells. Biochim. Biophys. Acta. 2015;1850:1543–1554. doi: 10.1016/j.bbagen.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 102.Almansa-Ordonez A., Bellido R., Vassena R., Barragan M., Zambelli F. Oxidative stress in reproduction: a mitochondrial perspective. Biology. 2020;9:269. doi: 10.3390/biology9090269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cobley J.N. Mechanisms of mitochondrial ROS production in assisted reproduction: the known, the unknown, and the intriguing. Antioxidants. 2020;9:E933. doi: 10.3390/antiox9100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cobley J.N., Close G.L., Bailey D.M., Davison G.W. Exercise redox biochemistry: conceptual, methodological and technical recommendations. Redox Biol. 2017;12:540–548. doi: 10.1016/j.redox.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gomez-Cabrera M.C., Arc-Chagnaud C., Salvador-Pascual A. Redox modulation of muscle mass and function. Redox Biol. 2020;35:101531. doi: 10.1016/j.redox.2020.101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jackson M.J., Stretton C., McArdle A. Hydrogen peroxide as a signal for skeletal muscle adaptations to exercise: what do concentrations tell us about potential mechanisms? Redox Biol. 2020;35:101484. doi: 10.1016/j.redox.2020.101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Margaritelis N.V., Paschalis V., Theodorou A.A., Kyparos A., Nikolaidis M.G. Redox basis of exercise physiology. Redox Biol. 2020;35:101499. doi: 10.1016/j.redox.2020.101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trinity J.D., Broxterman R.M., Richardson R.S. Regulation of exercise blood flow: role of free radicals. Free Radic. Biol. Med. 2016;98:90–102. doi: 10.1016/j.freeradbiomed.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stretton C., Pugh J.N., McDonagh B. 2-Cys peroxiredoxin oxidation in response to hydrogen peroxide and contractile activity in skeletal muscle: a novel insight into exercise-induced redox signalling? Free Radic. Biol. Med. 2020;160:199–207. doi: 10.1016/j.freeradbiomed.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jackson M.J. On the mechanisms underlying attenuated redox responses to exercise in older individuals: a hypothesis. Free Radic. Biol. Med. 2020;161:326–338. doi: 10.1016/j.freeradbiomed.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan Z., Spaulding H.R. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. 2020;32:101508. doi: 10.1016/j.redox.2020.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Penna F., Ballaro R., Costelli P. The redox balance: a target for interventions against muscle wasting in cancer cachexia? Antioxidants Redox Signal. 2020;33:542–558. doi: 10.1089/ars.2020.8041. [DOI] [PubMed] [Google Scholar]
- 113.Burdakov D. Reactive and predictive homeostasis: roles of orexin/hypocretin neurons. Neuropharmacology. 2019;154:61–67. doi: 10.1016/j.neuropharm.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 114.Vicente-Gutierrez C., Jimenez-Blasco D., Quintana-Cabrera R. Intertwined ROS and metabolic signaling at the neuron-astrocyte interface. Neurochem. Res. 2020;10–02965 doi: 10.1007/s11064-020-02965-9. [DOI] [PubMed] [Google Scholar]
- 115.Steinbrenner H., Sies H. Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch. Biochem. Biophys. 2013;536:152–157. doi: 10.1016/j.abb.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 116.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cobley J.N. Synapse pruning: mitochondrial ROS with their hands on the shears. Bioessays. 2018;40 doi: 10.1002/bies.201800031. [DOI] [PubMed] [Google Scholar]
- 118.Aizenman E., Loring R.H., Reynolds I.J., Rosenberg P.A. The redox biology of excitotoxic processes: the NMDA receptor, TOPA quinone, and the oxidative liberation of intracellular zinc. Front. Neurosci. 2020;14:778. doi: 10.3389/fnins.2020.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aschbacher K., Mason A.E. Eustress, distress, and oxidative stress: promising pathways for mind-body medicine. In: Sies H., editor. Oxidative Stress: Eustress and Distress. Academic Press; London, San Diego: 2020. pp. 583–617. [Google Scholar]
- 120.Aschbacher K., O'Donovan A. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38:1698–1708. doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Balters S., Geeseman J.W., Tveten A.K., Hildre H.P., Yu W., Steinert M. Mayday, Mayday Mayday. Using salivary cortisol to detect distress (and eustress!) in critical incident training. Int. J. Indus. Ergonom. 2020;78:102975. [Google Scholar]
- 122.Picard M., McEwen B.S., Epel E.S., Sandi C. An energetic view of stress: focus on mitochondria. Front. Neuroendocrinol. 2018;49:72–85. doi: 10.1016/j.yfrne.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Picard M., Prather A.A., Puterman E. A mitochondrial health index sensitive to mood and caregiving stress. Biol. Psychiatr. 2018;84:9–17. doi: 10.1016/j.biopsych.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Picard M., Trumpff C., Burelle Y. Mitochondrial psychobiology: foundations and applications. Curr. Opin. Behav. Sci. 2019;28:142–151. doi: 10.1016/j.cobeha.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Allen J., Romay-Tallon R., Brymer K.J., Caruncho H.J., Kalynchuk L.E. Mitochondria and mood: mitochondrial dysfunction as a key player in the manifestation of depression. Front. Neurosci. 2018;12:386. doi: 10.3389/fnins.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li H., Xia N. The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol. 2020;37:101585. doi: 10.1016/j.redox.2020.101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Siegrist J., Sies H. Disturbed redox homeostasis in oxidative distress: a molecular link from chronic psychosocial work stress to coronary heart disease? Circ. Res. 2017;121:103–105. doi: 10.1161/CIRCRESAHA.117.311182. [DOI] [PubMed] [Google Scholar]
- 128.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 129.Lyublinskaya O., Antunes F. Measuring intracellular concentration of hydrogen peroxide with the use of genetically encoded H(2)O(2) biosensor HyPer. Redox Biol. 2019;24:101200. doi: 10.1016/j.redox.2019.101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Samoylenko A., Hossain J.A. Nutritional countermeasures targeting reactive oxygen species in cancer: from mechanisms to biomarkers and clinical evidence. Antioxidants Redox Signal. 2013;19:2157–2196. doi: 10.1089/ars.2012.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Narayanaswamy N., Chakraborty K. A pH-correctable, DNA-based fluorescent reporter for organellar calcium. Nat. Methods. 2019;16:95–102. doi: 10.1038/s41592-018-0232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Valle G., Stanislao M., Facciorusso A., Carmignani M., Volpe A.R. Mithridates VI Eupator, father of the empirical toxicology. Clin. Toxicol. 2009;47:433. doi: 10.1080/15563650902899144. [DOI] [PubMed] [Google Scholar]
- 133.Borzelleca J.F. Profiles in toxicology - Paracelsus: herald of modern toxicology. Toxicol. Sci. 2000;53:2–4. doi: 10.1093/toxsci/53.1.2. [DOI] [PubMed] [Google Scholar]
- 134.Cannon W.B. Norton; New York: 1932. The Wisdom of the Body. [Google Scholar]
- 135.Oberbaum M., Gropp C. Update on hormesis and its relation to homeopathy. Homeopathy. 2015;104:227–233. doi: 10.1016/j.homp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 136.Southam C.M., Ehrlich J. Effects of extracts of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–524. [Google Scholar]
- 137.Ritossa F. A new puffing pattern introduced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- 138.Sies H. Oxidative stress: introductory remarks. In: Sies H., editor. Oxidative Stress. Academic Press; London: 1985. pp. 1–8. [Google Scholar]
- 139.Christman M.F., Morgan R.W., Jacobson F.S., Ames B.N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 140.Kozutsumi Y., Segal M., Normington K., Gething M.J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 141.Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Semenza G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 143.Itoh K., Chiba T., Takahashi S. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]