Abstract
Although the number of Ultrasound (US) imaging studies investigating the fascial layers are becoming more numerous, the majority tend to use different reference points and terminology to describe their findings. The current work set out to compare macroscopic and microscopic data of specimens of the fascial layers of the thigh with US imaging findings. Specimens of the different fascial layers of various regions of the thigh were collected for macroscopic and histological analyses from three fresh cadavers and compared with in vivo US images of the thighs of 20 healthy volunteers. The specimens showed that the subcutaneous tissue of the thigh is made up of three layers: a superficial adipose layer, a membranous layer/superficial fascia, and a deep adipose layer. The deep fascia is composed of an aponeurotic fascia, which envelops all the thigh muscles and is laterally reinforced by the iliotibial tract and an epimysial fascia, which is specific for each muscle. The morphometric measurements of the thickness of the superficial fascia were different (anterior: 153.2 ± 39.3 µm; medial: 128.4 ± 24.7 µm; lateral: 154 ± 28.9 µm; and posterior: 148.8 ± 33.2 µm) as were those of the deep fascia (anterior: 556.8 ± 176.2 µm; medial: 820.4 ± 201 µm; lateral: 1112 ± 237.9 µm; and posterior: 730.4 ± 186.5 µm). The US scans showed a clear picture of the superficial adipose tissue, the superficial fascia, and the deep adipose tissue, as well as the deep fasciae. The epimysial and aponeurotic fasciae of only some topographic areas could be independently identified. The US imaging findings confirmed that the superficial and deep fascia have different thicknesses, and they showed that the US measurements were always larger with respect to those produced by histological analysis (p < 0.001) probably due to shrinkage during the processing. The posterior region (level 1) of the superficial fascia had, for example, a mean thickness of 0.56 ± 0.12 mm at US, while the histological analysis showed that it was 148.8 ± 33.2 µm. Showing a similar pattern, the thickness of the deep fascia was as follows: 1.64 ± 0.85 mm versus 730.4 ± 186.5 µm. Study results have confirmed that US can be considered a valid, non‐invasive instrument to evaluate the fascial layers. In any event, there is a clear need for a set of standardised protocols since the thickness of the fascial layers of different parts of the human body varies and the data obtained using inaccurate reference points are not reproducible or comparable. Given the inconsistent terminology used to describe the fascial system, it would also be important to standardise the terminology used to define its parts. The difficulty in distinguishing between the epimysial and aponeurotic/deep fascia can also impede data interpretation.
Keywords: fascia lata, imaging, musculo‐skeletal ultrasound, superficial fascia, thickness
Although the number of Ultrasound (US) imaging studies investigating the fascial layers are becoming more numerous, the majority tend to use different reference points and terminology to describe their findings. The current work set out to compare macroscopic and microscopic data of specimens of the fascial layers of the thigh with US imaging findings. Specimens of the different fascial layers of various regions of the thigh were collected for macroscopic and histological analyses from three fresh cadavers and compared with in vivo US images of the thighs of 20 healthy volunteers.

1. INTRODUCTION
Although there are only a few studies that have compared anatomical and sonographic data to verify if Ultrasound (US) imaging can accurately and reliably delineate the fascial planes, the technique is becoming increasingly popular (Fede et al., 2018). Some studies investigating US examination of the fasciae (Fede et al., 2018; Langevin et al., 2011; Pirri et al, 2019; Stecco et al., 2014; Wilke et al., 2019) have demonstrated its accuracy in measuring the thickness of the subcutaneous and perimuscular areas at a lower cost than that of other noninvasive methods (Stecco et al., 2011). Langevin et al. (2011) also investigated gliding between muscles and the adjacent fascial layers and between the various fascial layers. A review by Fede et al. (2018) reported that the ultrasound data collected for the same fascia differed depending on the ultrasonographer, something that may be linked to the intrinsic difficulty of evaluating the fasciae, to intra‐individual variability in performing the examination, and/or to intra‐individual anatomic variability.
According to standard anatomy textbooks and the Terminologia Anatomica, the superficial fascia is considered all of the subcutaneous adipose tissue. In clinical practice, instead, the term is used to indicate the fibrous component, while the superficial adipose tissue (SAT) and the Deep adipose tissue (DAT) refer to the adipose tissue on either side of the fibrous component. In the early 19th century, Antonio Scarpa and Petrus Camper, respectively, called the superficial fascia the fibrous component and the fibro‐fatty component, which is the body part corresponding to the SAT.
Knowledge about the fasciae has become increasingly relevant in connection to regional anaesthesiology, given the growing interest in fascial plane, interfascial and nerve blocks. Knowing the exact thickness of a patient's fasciae reduces, in fact, the risk of nerve damage during a surgical procedure and it makes it possible to predict the effect of compartment models of anaesthetic drugs (Hotta, 2019).
The current study's first aim was to compare macro‐ and microscopic data and ultrasound imaging findings to verify if the fascial layers of the thigh can be accurately investigated and measured by US. The thigh was chosen as the reference region because it has a superficial fascia, an aponeurotic fascia lata and an epimysial fascia that are all clearly distinguishable (Stecco, 2015). Its second aim was to propose a US scanning protocol to assess the fascial layers of the thigh for ultrasonographers.
2. MATERIALS AND METHODS
2.1. Macroscopic study
Anatomical studies were conducted on three non‐embalmed cadavers (mean age 60 ± 5 years; 2 males, 1 female) without documented or anatomical evidence of thigh pathologies made available by the ‘Body Donation Programme’ of the Institute of Anatomy of the University of Padova (Porzionato et al., 2012).
The left thigh of each cadaver was dissected following the protocol described here. A longitudinal cutaneous incision was performed along the midline from the inguinal ligament of Poupart to the patella of the knee. Initially, the skin alone was cut and raised medially and laterally in order to examine the subcutaneous tissue. The incision was then deepened until the honeycomb structure of the membranous layer (also called superficial fascia) became visible. The superficial fascia was used as the dissection plane and followed laterally and medially, cranially and caudally, up to the posterior area of the thigh, and the skin with the superficial adipose tissue was removed. The deep part of the superficial fascia was then identified as the dissection plane and the adipose tissue was removed, exposing the plane of the deep fascia over the muscles of the various regions of the thigh.
The right thigh of each cadaver was dissected following the protocol described here. Three full‐thickness specimens from the skin to the muscle plane were taken of the proximal, middle and distal parts of the four compartments: that is of the anterior, lateral, posterior and medial parts. The samples were 60 mm long and 30 mm thick, that is, double the length of the US probe (which was 30 mm long and 15 mm wide), in order to obtain optimal samples to examine the microscopic anatomy and thickness of the various subcutaneous layers. The anatomic integrity was always carefully respected.
2.2. Histological analysis
The 12 full‐thickness specimens collected from the right thigh were mounted on cardboard to avoid deformation artefacts, fixed in 10% formalin solution and embedded in paraffin; the investigator was careful to obtain full‐thickness sections from the skin to the muscle. Ten‐μm‐thick sections were stained with Azan‐Mallory (Figure 1) following a protocol described elsewhere (Stecco et al., 2007). All the preparations were observed under a DM4500‐B light microscope (Leica Microsystems, Wetzlar, Germany) and recorded in full colour (24 bits) by a digital camera (DFC 480, Leica Microsystems). Morphometric measurements of the superficial and deep fasciae (fascia lata) of the full‐thickness specimens were recorded and analysed using Image J software (Schneider et al., 2012). The mean values and standard deviations of the thickness measurements were calculated.
Figure 1.
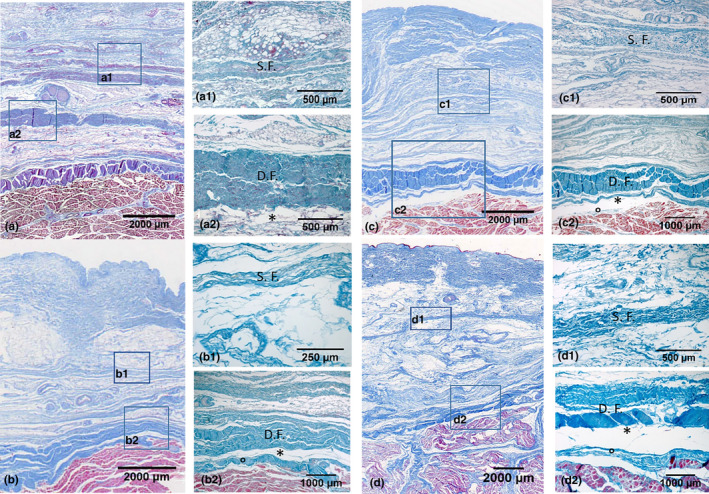
Histological images (Azan‐Mallory staining) of the anterior (a, a1, a2), medial (b, b1, b2), lateral (c, c1, c2) and posterior (d, d1, d2) regions at the Ant1, Med 1, Lat 1 and Post 1 levels. S.F., Superficial Fascia; D.F., Deep Fascia. *= Loose connective tissue. °= Epimysium
2.3. Ultrasound imaging
Twenty healthy volunteers (13 males and 7 females; mean age 30.45 ± 11.22 years) were recruited from personnel of the University of Padova. Subjects younger than 18, pregnant, suffering from any chronic skin condition (eczema, psoriasis, etc.), severe lower extremity trauma, a collagen disorder (scleroderma, mixed connective tissue disorder, etc.) or a chronic medical condition requiring medication were excluded.
A standardised protocol was developed for the bilateral assessment of the fascial layers (superficial fascia and fascia lata) of the thigh. All the US images were acquired using a 6‐15 MHz linear array probe (Sonosite Edge II, Fujifilm) by a physiatrist/ultrasonographer who had 4 years experience in carrying out musculoskeletal US and was specialised in fascial anatomy. The speed of sound of the US system was c = 1540 m/s, which is conventionally used by diagnostic US systems. The US, which was set to B‐mode, depicted a depth of 30 mm. The ultrasonographer used suitable amounts of gel to produce adequate scans and to reduce surface pressure on the skin. The probe was placed on the skin as gently as possible in an effort to avoid tissue compression, but at the same time as steadily as possible to maintain adequate contact between the probe and skin for coherent images. To eliminate the influence of possible thickness variations, three equidistant points per image/levels for the layers were measured and the values were averaged and analysed. The ultrasonographer followed the protocol carefully to ensure that each point of the superficial fascia and of the fascia lata of the thigh was quantified in the same way.
The US beam was kept perpendicular to the fascial layers which seem to be prone to anisotropy artefacts. The power and overall gain of the ultrasound machine were adjusted to optimise the visualisation of the fascial planes and to obtain the best possible views and scans (Derchi et al., 2007).
The ultrasonographer used the short‐axis approach because it is the best one to visualise the fascial layers using a landmark‐guided approach and to minimise anisotropy (Ahn & Kaptchuk, 2011). An appropriate protocol, described below, was followed for each region:
-
The anterior region (Figure 2): The patient was in a relaxed supine position with the right lower limb in a neutral position.
Anterior 1 (Ant 1): The probe, which was placed axially on the anterior superior iliac spine (ASIS), was moved downwards from it for 5 cm following the line connecting it to the upper border of the patella. The sartorius tendon was located medially: the rectus femoris muscle was beneath it, with the tensor fasciae latae lateral to it and the iliopsoas medial to it (Figure 2).
Anterior 2 (Ant 2): The probe was moved downwards following the rectus femoris and vastus intermedius muscles until the sartorius was no longer visible; the vastus medialis and vastus lateralis, respectively, appear medially and laterally.
Anterior 3 (Ant 3): The probe was moved downwards and the image at the point before the muscular bellies of the quadriceps unite to form the quadricipital tendon was acquired.
-
The medial region (Figure 2): The patient was supine with the right hip abducted, semiflexed and externally rotated; the knee was semiflexed (frog leg position).
Medial 1 (Med 1): The probe was placed axially on the proximal/antero‐medial thigh, keeping the femoral artery and vein laterally and the adductor muscles medially. The vastus medialis was no longer visible (Figure 2).
Medial 2 (Med 2): The probe was moved downwards following the gracilis, stopping just proximal to the crossing of the sartorius. The femoral vessels and saphenous nerves were used as the landmarks. The sartorius, vastus medialis, adductor magnus, adductor longus and gracilis were all visible.
Medial 3 (Med 3): The probe was placed over the vastus medialis muscle at the level of Hunter's canal. The quadriceps tendon was maintained laterally and the sartorius muscle medially.
-
The posterior region (Figure 2) The patient was prone with the right lower limb in a neutral position.
Posterior 1 (Post 1): The probe was placed axially at the level of the ischial tuberosity maintaining the sciatic nerve laterally (Figure 2).
Posterior 2 (Post 2): The probe was moved downwards following the sciatic nerve which lies between the medial and lateral hamstring muscles. The point where the biceps muscle crosses the sciatic nerve was considered our landmark.
Posterior 3 (Post 3): The probe was moved downwards until the apex of the popliteal fossa was reached; medially the semitendinosus (ST) and the semimembranosus (SM) and laterally the biceps femoris (BF) were visualised so that the images of the fasciae above the hamstring muscle could be acquired.
-
The lateral region (Figure 2): The patient was placed in the left lateral decubitus position so that the right limb could be assessed.
Lateral 1 (Lat 1): The probe was placed transversally to the greater trochanter facets. The gluteus medius and minimus tendons could be differentiated from the iliotibial tract (IT). The latter is a well‐defined hyperechoic layer overlying the gluteus medius tendon (Figure 2).
Lateral 2 (Lat 2): The probe was moved downwards following the iliotibial band as far as the gluteal fold. The iliotibial band was clearly evident at that level, although there was no clear border with the deep fascia covering the vastus lateralis.
Lateral 3 (Lat 3): The probe was moved downwards along the iliotibial band. US images of the midline connecting the greater trochanter with the lateral epicondyle were acquired.
Lateral 4 (Lat 4): The probe was moved downwards as far as the knee joint. The linea aspera and lateral epicondyle near the lateral collateral ligament were used as landmarks.
Figure 2.
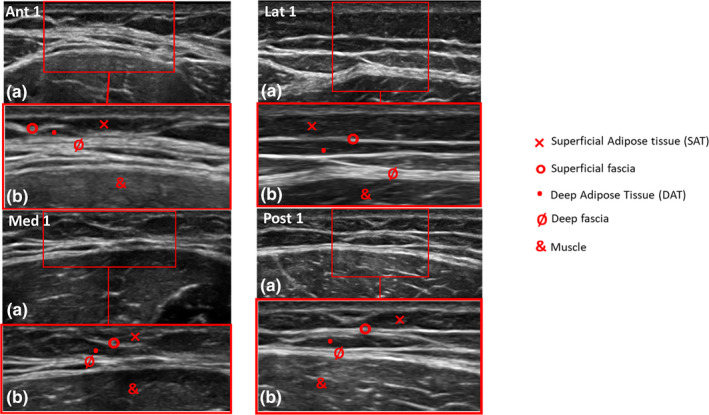
Ultrasound (US) images of the anterior, medial, lateral and posterior regions at the Ant 1, Med 1, Lat 1 and Post 1 level. (a) US imaging. (b) US imaging detailed
The images of each region/level were frozen and acquired at the end of each assessment; the fascial thickness was measured using Image J software. Each image was divided into three regions; three points in each of these with the clearest view were measured and averaged. Finally, the average of the three regions was calculated.
2.4. Data processing and statistics
The mean value, standard deviation (SD) and standard error (SE) of the US and histological thickness measurements were considered the representative estimators of the parameter. Normal distributions were tested using the Kolmogorov‐Smirnov test. The student's t‐test was used for each anatomical region of the fascia to compare the histological estimates of the fascia thickness with the US‐based measurements. The correlations existing between the values obtained by histology and US imaging were also analysed. The differences in the histology and the US‐based estimates of thicknesses across the regions/levels were compared using one‐way analysis of variance (ANOVA) followed by Bonferroni's test for multiple comparisons. All the analyses were performed using the GraphPad PRISM 3.03 (GraphPad Software Inc., San Diego, CA), and a p < 0.05 was considered the threshold for statistical significance.
3. RESULTS
3.1. Macroscopic examination
A first layer of adipose tissue SAT was identified under the dermis of all the cadavers. The layer was formed by large fat lobes encased between fibrous septa in a honeycomb‐like structure. The septa (retinacula cutis superficialis) appeared to be clearly defined. The fat lobes were organised in single or multiple layers, depending on the fat content and the subject's SAT thickness. No clear borders of the layer could be identified, either caudally or cranially; the retinacula cutis became more densely packed only at the level of the inguinal ligament dividing the subcutaneous compartment of the thigh from the abdominal one. Once the SAT was removed, there was a fibrous layer with a membranous appearance, apparently continuous and well organised macroscopically. Based on our knowledge, this layer was the superficial fascia, also called the membranous layer of the subcutaneous tissue. It could be followed as a dissection plane from the inguinal ligament to the patella of the knee in all directions. It did not appear to be uniform in thickness: it was a well‐defined white layer of the upper thigh that lost consistency in the middle level of the regions where it presented as a much thinner translucid collagen layer through which the adipose tissue could be seen. This fibrous layer split in the medial region in order to envelop the great saphenous vein. In the posterior region, it lost its fibrous appearance and was filled with fat tissue, assuming a honeycomb aspect. It was also more organised at the proximal with respect to the distal one. Lastly, it was difficult to dissect the superficial fascia around the knee where it fused with the deep fascia. After the superficial fascia was removed, another layer of adipose tissue was found, the DAT. Its fat lobes were smaller, flatter and less well‐defined than those of the SAT. It tended to become progressively smaller in its fat component near the knee joint, whereas the network of collagen fibres (retinacula cutis profunda) became stronger and more tightly packed. Removing it gave direct access to the plane of the superficial aspect of the deep fascia, which is usually called fascia lata in this area. The fascia lata covers all the muscles and appears as a thick, whitish layer of connective tissue, similar to an aponeurosis. Laterally, the fascia lata is reinforced by the iliotibial tract (ITT), which appears as a longitudinal, fibrous band, completely continuous with the surrounding fascia. The iliotibial tract could not be separated from the deep fascia by dissection, and the medial and lateral borders of the ITT could only be defined by cutting these continuities, thus, creating an anatomical artefact. Over the femoral triangle, the fascia lata is pierced by vessels and nerves, for example, the great saphenous vein, becoming very porous, the reason why it is called the cribriform fascia.
The fascia lata was generally easily separable from the underlying muscles due to the presence of loose connective tissue which created an uninterrupted sliding plane between the fascia lata and the muscle belly. Only distally some muscular bundles of the vastus medialis and lateralis muscles were inserted directly into the inner side of the fascia lata. A few strong intermuscular septa started from the inner surface of the fascia lata and extended between the muscle bellies, dividing the thigh into various compartments. Lastly, although thin and totally adhering to it, each muscle was surrounded by its epimysium, which appeared as a further fibrous layer.
3.2. Microscopic/histological examination
All the subcutaneous layers of all the full‐thickness specimens were identified, although they presented different thicknesses and aspects depending on the area analysed. More specifically, from the surface going inwards, the following layers could be identified: the skin (epidermis and dermis), the SAT, the superficial fascia/membranous layer, the DAT, the aponeurotic/deep fascia, the epimysium (also called epimysial fascia) and the muscle.
Microscopically, the superficial fascia appears as a multilayered structure with a mean thickness of 146.6 ± 31.5 µm (Table 1 and Figure 3). It is thicker in the proximal portion of the thigh, but becomes very thin towards the knee, where it almost adheres to the deep fascia. The thickness of the superficial fascia also varies depending on the compartment: it is 153.2 ± 39.3 µm in the anterior compartment, 128.4 ± 24.7 µm in the medial one, 154.0 ± 28.8 µm in the lateral one and 148.8 ± 33.2 µm in the posterior one (Table 1). At some points, the superficial fascia includes fat lobules, small vessels and nerves.
Table 1.
Morphometrical analysis (µm) of the histological specimens
|
D.F. ANT |
S.F. ANT | D.F. MED | S.F. MED | D.F. LAT | S.F. LAT | D.F. POST | S.F. POST | |
|---|---|---|---|---|---|---|---|---|
| Number of values | 76 | 85 | 21 | 21 | 71 | 55 | 17 | 25 |
| Mean | 556.8 | 153.2 | 820.4 | 128.4 | 1112 | 154 | 730.4 | 148.8 |
| Std. Deviation | 176.2 | 39.3 | 201 | 24.7 | 237.9 | 28.9 | 186.5 | 33.2 |
| Std. Error | 20.2 | 4.3 | 43.9 | 5.4 | 28.2 | 3.9 | 45.2 | 6.6 |
D.F., deep fascia; S.F., superficial fascia; ANT, anterior; MED, medial; LAT, lateral; POST, posterior.
Figure 3.
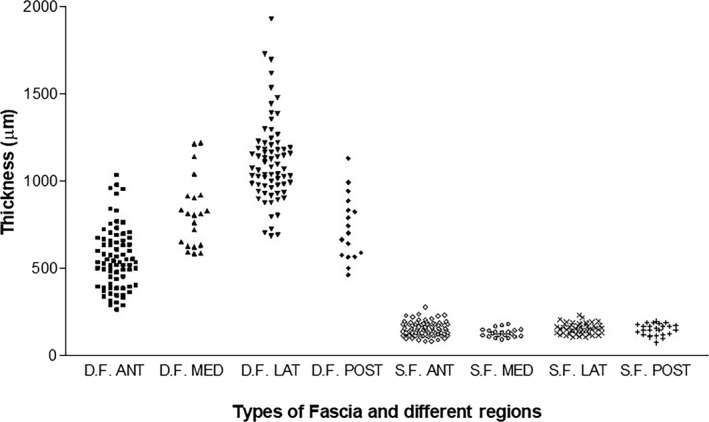
Histological thicknesses of the fascial layers of the thigh. D.F., Deep Fascia; S.F., Superficial Fascia; ANT, anterior; MED, medial; LAT, lateral; POST, posterior
The fascia lata appears as a fibrous layer of connective tissue with a mean thickness of 804.9 ± 200.4 µm. It progressively becomes thicker in the proximal/distal direction, but it is thin on the medial surface of the thigh (Table 1 and Figure 3). The thicknesses in the anterior region were as follows: 556.8 ± 176.2 µm, 820.4 ± 201 µm in the medial region, 1112 ± 237.9 µm in the lateral region and 730.4 ± 186.5 µm in the posterior one. The differences in the thickness in the four topographic regions were very significant from a statistical viewpoint (p < 0.001). From a microscopic viewpoint, the deep fascia appeared as a multilayered structure formed by two or three layers of densely packed collagen fibres with layers of loose connective tissue between them.
3.3. Ultrasound examination
3.3.1. Superficial fascia and deep fascia thickness
The fasciae appear as echogenic bands upon US imaging, contrasting very well with the surrounding tissues. More specifically, the deep fascia appears as a thin hyperechoic band adhering to the muscle. In short/axial/transversal scans, the superficial fascia of the thigh was easily identified in all the regions and levels analysed, appearing as linear, laminate, bi‐laminate or trilaminate hyper‐echoic layers. It was always clearly evident given the contrast within the context of the hypo‐echogenic subcutaneous adipose tissue. US thicknesses are shown in Table 2 and Figure 4. The differences between the various regions/levels were particularly pronounced (p < 0.001). The US thickness of the superficial fascia was different in the various regions of the transversal scans; they were statistically significant between the Med 3 versus the Lat 4 (p < 0.05), the Lat 1 versus the Lat 3 (p < 0.01), the Lat 1 versus the Lat 4 (p < 0.001), the Lat 3 versus the Post 1 (p < 0.01), the Lat 4 versus the Post 1 (p < 0.001) and the Lat 4 versus the Post 2 (p < 0.001) (Table 3). No differences, except with regard to the medial compartment, were found between the right and left sides (p > 0.05).
Table 2.
The ultrasound thicknesses (mm) of the superficial fascia in the various regions and levels of the thighs in the proximal‐distal direction
| Ant 1 | Ant 2 | Ant 3 | Med1 | Med 2 | Med 3 | Lat 1 | Lat 2 | Lat 3 | Lat 4 | Post 1 | Post 2 | Post 3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 0.50 | 0.51 | 0.49 | 0.49 | 0.52 | 0.52 | 0.56 | 0.49 | 0.45 | 0.42 | 0.56 | 0.54 | 0.50 |
| Std. Deviation | 0.10 | 0.12 | 0.14 | 0.13 | 0.11 | 0.10 | 0.12 | 0.13 | 0.15 | 0.12 | 0.12 | 0.12 | 0.11 |
| Std. Error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
Ant, anterior; Med, medial; Lat, lateral; Post, posterior. Number of values for each level = 40.
Figure 4.
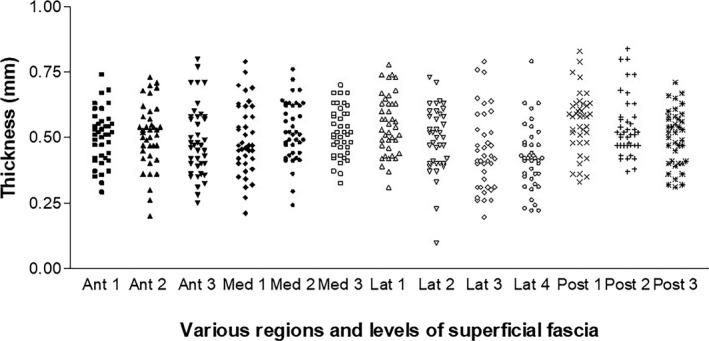
US thicknesses (mm) of the superficial fascia of the thigh in various regions and levels. Ant, anterior; Med, medial, Lat, lateral; Posterior, posterior
Table 3.
The significant differences between the Ultrasound thicknesses of the superficial fascia in the various regions and levels of the thighs in proximal‐distal direction
| Region/level vs. Region/level | p‐value |
|---|---|
| Med 2 vs. Lat 4 | p < 0.05 |
| Med 3 vs. Lat 4 | p < 0.05 |
| Lat 1 vs. Lat 3 | p < 0.01 |
| Lat 1 vs. Lat 4 | p < 0.001 |
| Lat 3 vs. Post 1 | p < 0.01 |
| Lat 3 vs. Post 2 | p < 0.05 |
| Lat 4 vs. Post 1 | p < 0.001 |
| Lat 4 vs. Post 2 | p < 0.001 |
Ant, anterior; Med, medial; Lat, lateral; Post, posterior.
The fascia lata was also easily identified in all the compartments and levels analysed, appearing as linear, bi‐ and trilaminate hyper‐echogenic layers among hypo‐echogenic layers of loose connective tissue. The deep fascia was best visualised at the anterior compartment level where the deep fascia shows classic features: a clearly defined white layer bordered by two hypoechogenic lines (corresponding to the DAT superficially and loose connective tissue more deeply). Instead, in the posterior compartments, the deep fascia was thicker, but it often appears infiltrated by loose connective tissue and fat lobules creating a division between the collagen fibrous layers. Lastly, in the lateral region, the fascia lata appears very well organised and the longitudinal fibrous bundles of the iliotibial band are evident. The ilio‐tibial band always appeared in US imaging as a specific reinforcement of the fascia lata and not as an isolated structure. The US thicknesses of the fascia lata are shown in Table 4 and Figure 5. In some subjects and in some areas, the deep fascia could not be distinguished from the epimysium of the underlying muscles, probably because the loose connective tissue between them was too thin. In those cases, the thickness of the fascia lata registered includes the thickness of the epimysium. The differences between some regions/levels were statistically very significant (p < 0.001) (Table 5). The US thickness of the deep fascia was greatest in the lateral region (1.6 ± 0.52 mm). The posterior region was thicker (1.4 ± 0.55 mm) than the anterior one (1.34 ± 0.42 mm). The medial region was the thinnest (1 ± 0.33 mm). No differences were found between the right and left sides (p > 0.05).
Table 4.
The Ultrasound thicknesses (mm) of the deep fascia in the various regions and levels in the thighs in the proximal‐distal direction
| Ant 1 | Ant 2 | Ant 3 | Med 1 | Med 2 | Med 3 | Lat 1 | Lat 2 | Lat 3 | Lat 4 | Post1 | Post 2 | Post 3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 1.39 | 1.29 | 1.35 | 1.02 | 0.89 | 1.09 | 1.94 | 1.64 | 1.33 | 1.57 | 1.64 | 1.34 | 1.24 |
| Std. Deviation | 0.48 | 0.32 | 0.47 | 0.30 | 0.23 | 0.27 | 0.73 | 0.41 | 0.34 | 0.60 | 0.85 | 0.45 | 0.35 |
| Std. Error | 0.08 | 0.05 | 0.07 | 0.05 | 0.04 | 0.04 | 0.16 | 0.07 | 0.05 | 0.10 | 0.13 | 0.07 | 0.06 |
Ant, anterior; Med, medial; Lat, lateral; Post, posterior. Number of values for each level = 40.
Figure 5.
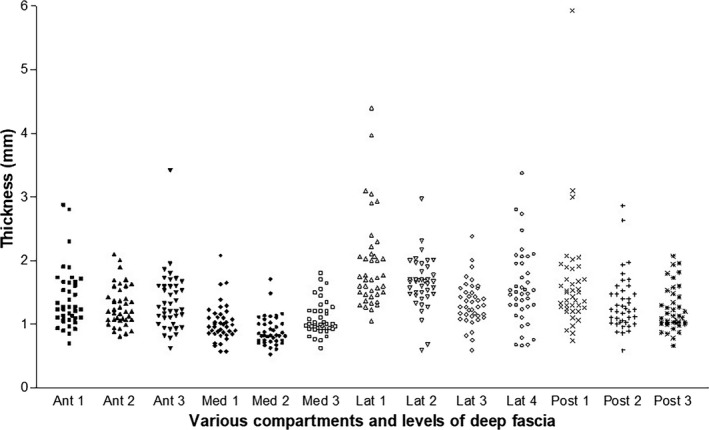
US thicknesses (mm) of the fascia lata in various regions and levels. Ant, anterior; Med, medial; Lat, lateral; Posterior, posterior
Table 5.
The significant differences between the Ultrasound thicknesses of the deep fascia in the various regions and levels of the thighs in the proximal‐distal direction
| Region/level vs. Region/level | p‐value |
|---|---|
| Ant 1 vs. Med 2 | p < 0.001 |
| Ant 1 vs. Lat 1 | p < 0.001 |
| Ant 2 vs. Med 2 | p < 0.05 |
| Ant 2 vs. Lat 1 | p < 0.001 |
| Ant 3 vs. Med 2 | p < 0.01 |
| Ant 3 vs. Lat 1 | p < 0.001 |
| Med 1 vs. Lat 1 | p < 0.001 |
| Med 1 vs. Lat 2 | p < 0.001 |
| Med 1 vs. Lat 4 | p < 0.001 |
| Med 1 vs. Post 1 | p < 0.001 |
| Med 2 vs. Lat 1 | p < 0.001 |
| Med 2 vs. Lat 2 | p < 0.001 |
| Med 2 vs. Lat 3 | p < 0.01 |
| Med 2 vs. Lat 4 | p < 0.001 |
| Med 2 vs. Post 1 | p < 0.001 |
| Med 2 vs. Post 2 | p < 0.01 |
| Med 3 vs. Lat 1 | p < 0.001 |
| Med 3 vs. Lat 2 | p < 0.001 |
| Med 3 vs. Lat 4 | p < 0.001 |
| Med 3 vs. Post 1 | p < 0.001 |
| Lat 1 vs. Lat 3 | p < 0.001 |
| Lat 1 vs. Post 2 | p < 0.001 |
| Lat 1 vs. Post 3 | p < 0.001 |
| Lat 2 vs. Post 3 | p < 0.05 |
| Post 1 vs. Post 3 | p < 0.05 |
Ant, anterior; Med, medial; Lat, lateral; Post, posterior.
3.3.2. Comparison and correlation of histological and ultrasound thicknesses
The differences between the histological and ultrasound thicknesses of the superficial fascia and deep fascia were always highly significant (p < 0.001); the patterns in the thickness measurements were similar.
The correlation between the means thickness values (n = 8) obtained by histology and US (r = 0.918) was highly significant (p < 0.01) (Figure 6).
Figure 6.
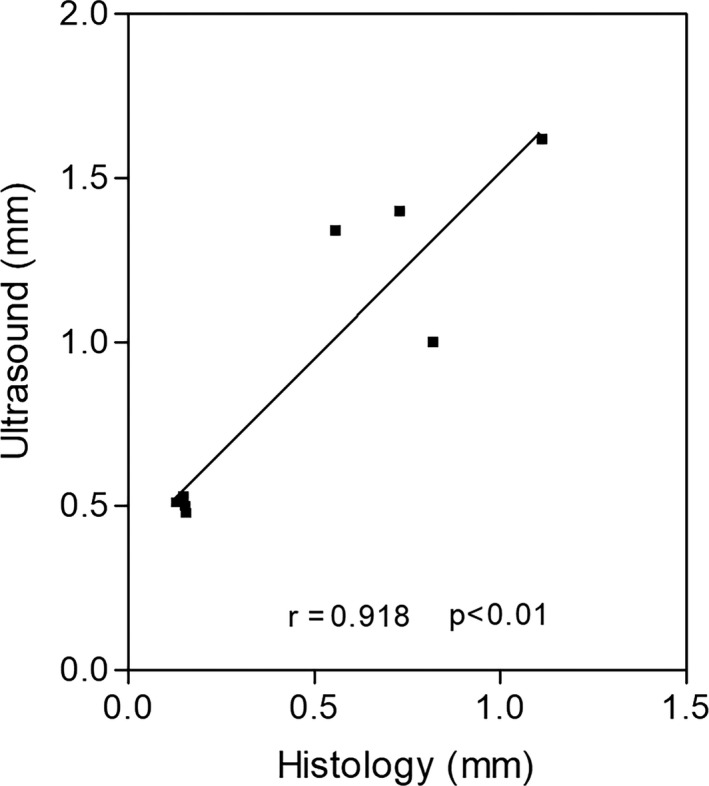
The correlation between the mean histological (from 20 to 40 measurements) and ultrasound (40 measurements) thicknesses (mm) of the superficial and deep fascia in various regions/levels (n = 8). Pearson (r) = 0.918; p < 0.01
4. DISCUSSION
Study findings showed that the superficial and deep fascia of the thigh could always be identified. But while the fascial layers can be viewed and assessed with US imaging, it is often the terminology utilized to refer to them that may lead to misinterpretation (Pirri et al., 2020). In the light of macroscopic and microscopic anatomy findings, it is important during US evaluation to look for the superficial fascia in the middle of the subcutaneous adipose tissue and then to identify the deep fascia closer to the muscles. It is, thus, possible to avoid the mistake, which has appeared in some articles in which the superficial fascia is confused with the superficial layer of the deep fascia (Galant et al., 1998). In reality, the two fasciae have different microscopic anatomy appearances and functions. The superficial fascia is connected to the subcutaneous structures, organising the fat tissue, but also enveloping the saphenous vein and other small subcutaneous vessels and nerves. The deep fascia, which is connected to the muscles, probably plays a key role in proprioception (Stecco et al, 2019).
Our histological findings for both the superficial fascia and fascia lata are consistent with those of other studies investigating fascial thickness (Abu‐Hijleh et al., 2006; Stecco et al., 2009). Study analysis showed that the superficial fascia is thinner than the deep fascia in all compartments and levels in both histology and US imaging findings. In addition, both the histological and US evaluations collected during our study show that the variations in the thicknesses of the various regions/levels of the superficial fascia and deep fascia of the thigh were particularly pronounced (p < 0.001), as clearly shown in Figures 3, 4 and 5. It seems worthwhile to define specific landmarks to measure these fasciae so that the results of various studies can be compared. In this regard, it bears mentioning that there are also wide intraindividual and intrasex differences (Müller et al., 2020; Sengeis et al., 2019).
Our results uncovered a wide discrepancy between the absolute thicknesses of the fasciae measured according to histological sections and US images (p < 0.001), but the patterns in the thickness measurements were similar. This is probably due to the fact that the treatment of histological sections involve tissue dehydration and thus shrinkage. As reported by other studies (e.g. Langevin et al., 2007), the tissue shrinkage that takes place during the preparation of connective tissue specimens for histological examination may explain these differences. According to published data, there are many variables that may cause shrinkage during tissue processing, that is, post mortem changes in the cadaver, ambient temperature, gender and formalin fixation prior to staining (Dauendorffer et al., 2009; Kerns et al., 2008).
The resolution of US images and the strong hyper‐echogenicity of the fasciae with respect to those of adipose tissue and muscles may, instead, lead to overestimated values. The accuracy of B‐mode US imaging depends on: the probe frequency (high‐frequency increases the resolution, but it also reduces image depth due to increased attenuation) using the appropriate setting of the US system and the skill and experience of the ultrasonographer (Müller et al., 2020). Acoustic impedance, which refers to the physical property of a tissue, depends on the density and the propagation speed of US waves in the tissue. The intensity of the reflected echo and the thickness of the hyper‐echogenic lines generated are proportional to the difference in the acoustic impedance between the different tissue interfaces (Abrahams et al, 2019). Müller et al. (2020) demonstrated that in subcutaneous tissue, changing the speed of sound did not impact the technical accuracy; rather biological features (such as furrowed borders and visco‐elastic deformations of adipose tissue) seemed to affect its reliability. In fact, in a phantom study, Abrahams et al (2019) demonstrated that US imaging of the abdominal wall could be used to measure the thickness of the parietal peritoneum. The authors concluded that the hyperechoic line generated by US represents the interface between two neighbouring tissues and not a separate morphological structure, meaning that the discrepancy between two assessments could be explained by different in vivo and in vitro conditions.
As modern probes can vary their frequency depending on scan depth, we decided to use 30 mm. While the frequency is automatically adapted by the system and is useful to optimise visualisation of the fasciae, it does not provide the real frequency during the recordings. We used a sound speed of 1540 m/s, which is conventionally present in diagnostic US machines, and which, in accordance with reports in the literature, is appropriate for soft tissue, while 1450 m/s is normally used for adipose tissue. It would probably be opportune to use different sound speeds in accordance with the different microscopic features of superficial and deep fasciae. Deep fasciae can be better assessed using a conventional sound speed as they are mainly constituted of fibrous components, but the same sound speed could be used for superficial fascia since adipose tissue is merged with it in subcutaneous tissue. The fascial shrinkage linked to high resolution of US probe could help to explain the discrepancy in thicknesses between histological and US imaging.
The fasciae showed different thicknesses in the proximal/distal directions: in the superficial fasciae, the thickness is greater in the medial and posterior regions, explaining the role of packing the fat in subcutaneous tissue and enveloping/protecting/supporting the most important peripheral nerves and vessels of the lower limb. For example, in the medial regions, the superficial fascia envelops the saphenous vein and becomes thicker in a proximal/distal direction, although the result is not statistically significant. The superficial fascia could be thought of as a kind of elastic stocking under graduated pressure that compresses more distally and less proximally in order to support the vessel wall and improve venous return.
As the superficial fascia is not a rigid fibrous layer as is the deep fascia, in fatter people it tends to thicken in order to contain all the infiltrated fat. That means that in topographic regions in which there is more fat, it tends to play a greater mechanical role.
The deep fasciae, which tend to be thicker laterally and posteriorly, play the important role of myofascial force transmission in these compartments (Stecco et al., 2013). They are also thicker in the proximal levels, compatibly with the role of the attachment and myofascial expansion of the thigh muscles (Stecco, 2015), decreasing in the middle level and increasing again in the distal level because it is reinforced by the knee retinacula.
The medial region is the thinnest area because mechanically the forces acting on the deep fascia are clearly less at this level. From a postural viewpoint, the lateral and posterior portions of the fascia lata are most affected by the load due to the bipedal position, and therefore the iliotibial tract, with its longitudinal arrangement, is the thicker part. The iliotibial tract serves an important postural function, allowing asymmetric standing (pelvic slouch), with the upward pull of the lower attachment of the IT tract locking the knee in hyperextension and creating a rigid support pillar (Evans, 1979). In humans, the posterior compartments tend to be tilted forward by gravity, so that it is mechanically more stimulated than the anterior compartment.
Study findings confirm that ultrasonography is an efficacious method to study the superficial and deep fasciae and their conformations in different regions, with the exception of the epimysial layer under the deep fascia, which is often difficult to analyse ultrasonographically, probably due to variability in the amount of loose connective tissue between the deep fascia and the epimysium of the underlying muscle. This is an interesting point, which should be further investigated since it may have implications for myofascial pain, and may explain the importance of loose connective tissue rich in hyaluronan acid in determining the functions of the fasciae in sliding (Stecco et al., 2011).
This is the first work to our knowledge to examine and compare the thicknesses of the fascial layers of the thigh using US and histology. Future longitudinal studies including larger numbers of patients will be able to contribute to our knowledge of the pathophysiology of different thickness patterns. US may also be able to uncover changes which are invisible during clinical inspection and unforeseen by current clinical practice. Finally, being able to define the specific structures involved in fascial dysfunctions would facilitate a more targeted approach to treatment.
5. LIMITATIONS OF THE STUDY
Since the study involved only a small number of healthy volunteers and given the qualitative limitations of the US assessments, it was impossible for us to analyse the prevalence of the US findings or to make any hypotheses on causes, prognostic significance or therapeutic implications. This is particularly true for some fascial layers such as the posterior and medial ones, which are described here for the first time. The differences in age between the cadavers and the healthy volunteers could be considered a study limitation because age may have an effect on fascial tissue. In addition, the US data are based on manually performed thickness measurements instead of semiautomatic ones. In clinical practice, an initial parameter/protocol of fascial assessment needs to be defined, just as its clinical relevance warrants further investigation. Finally, as US evaluation of fascial layers depends to a great extent on the ultrasonographer's skill and expertise, this could be considered a study limitation.
6. CONCLUSIONS
To conclude, study results confirm that US is a reliable tool for assessing the fascial layers, providing an excellent anatomical definition that accurately corresponds to histological findings. The wide variability found in the various regions and levels of the fasciae studied underlines the importance of establishing standardised landmarks to assess the fasciae and to make results comparable. The use of US imaging can open new doors to greater knowledge of fascial pathophysiology which will in turn make US an increasingly popular technology.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Carmelo Pirri: contributed to concept, dissections, ultrasound analysis, acquisition of data and drafting the manuscript. Caterina Fede: critical revision and histological examination. Lucia Petrelli: histological examinations. Diego Guidolin: critical revision and acquisition of data. Chenglei Fan: dissections. Raffaele De Caro: critical revision. Carla Stecco: contributed to concept, dissections, drafting manuscript and critical revision.
ACKNOWLEDGEMENTS
The authors are grateful to Dr Anna Rambaldo for technical assistance. We confirm that we have read the position of the Journal of Anatomy on issues involved in ethical publication and affirm that this report is consistent with the Journal’s guidelines.
Pirri C., Fede C., Petrelli L., Guidolin D., Fan C., De Caro R., Stecco C.. An anatomical comparison of the fasciae of the thigh: A macroscopic, microscopic and ultrasound imaging study. J. Anat. 2021;238:999–1009. 10.1111/joa.13360
DATA AVAILABILITY STATEMENT
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- Abrahams, A.C. , Dendooven, A. , van der Veer, J.W. , Wientjes, R. , Toorop, R.J. , Bleys, R.L. et al. (2019) Direct comparison of the thickness of the parietal peritoneum using peritoneal biopsy and ultrasonography of the abdominal wall in patients treated with peritoneal dialysis. Peritoneal Dialysis International, 39(5), 455–464. [DOI] [PubMed] [Google Scholar]
- Abu‐Hijleh, M.F. , Roshier, A.L. , Al‐Shboul, Q. , Dharap, A.S. and Harris, P.F. (2006) The membranous layer of superficial fascia: evidence for its widespread distribution in the body. Surgical and Radiologic Anatomy, 28(6), 606–619. [DOI] [PubMed] [Google Scholar]
- Ahn, A.C. and Kaptchuk, T.J. (2011) Spatial anisotropy analyses of subcutaneous tissue layer: potential insights into its biomechanical characteristics. Journal of Anatomy, 219(4), 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauendorffer, J.N. , Bastuji‐Garin, S. , Guéro, S. , Brousse, N. and Fraitag, S. (2009) Shrinkage of skin excision specimens: formalin fixation is not the culprit. British Journal of Dermatology, 160(4), 810–814. [DOI] [PubMed] [Google Scholar]
- Derchi, L. and Rizzatto, G. (2007) Technical requirements. In: Bianchi, S. and Martinoli, C. (Eds.) Ultrasound of the musculoskeletal system. Germany: Springer Verlag, pp. 3–16. [Google Scholar]
- Evans, P. (1979) The postural function of the iliotibial tract. Annals of the Royal College of Surgeons of England, 61(4), 271–280. [PMC free article] [PubMed] [Google Scholar]
- Fede, C. , Gaudreault, N. , Fan, C. , Macchi, V. , DeCaro, R. and Stecco, C. (2018) Morphometric and dynamic measurements of muscular fascia in healthy individuals using ultrasound imaging: a summary of the discrepancies and gaps in the current literature. Surgical and Radiologic Anatomy, 40(12), 1329–1341. [DOI] [PubMed] [Google Scholar]
- Galant, J. , Martí‐Bonmatí, L. , Soler, R. , Saez, F. , Lafuente, J. , Bonmatí, C. et al. (1998) Grading of subcutaneous soft tissue tumors by means of their relationship with the superficial fascia on MR imaging. Skeletal Radiology, 27(12), 657–663. [DOI] [PubMed] [Google Scholar]
- Hotta, K. (2019) Fascial plane blocks: anatomical structures that affect the spread of local anesthetic. Journal of Anesthesia, 33(4), 493–494. [DOI] [PubMed] [Google Scholar]
- Kerns, M.J.J. , Darst, M.A. , Olsen, T.G. , Fenster, M. , Hall, P. and Grevey, S. (2008) Shrinkage of cutaneous specimens: formalin or other factors involved? Journal of Cutaneous Pathology, 35(12), 1093–1096. [DOI] [PubMed] [Google Scholar]
- Langevin, H.M. , Fox, J.R. , Koptiuch, C. , Badger, G.J. , Greenan‐, A.C. , Naumann, N.A. et al. (2011) Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskeletal Disorders, 12, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin, H.M. , Rizzo, D.M. , Fox, J.R. , Badger, G.J. , Junru, W.u. , Konofagou, E.E. et al. (2007) Dynamic morphometric characterisation of local connective tissue network structure in humans using ultrasound. BMC Systems Biology, 5(1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, W. , Fürhapter‐Rieger, A. , Ahammer, H. , Lohman, T.W. , Meyer, N.L. , Sardinha, L.B. et al. (2020) Relative Body Weight and Standardised Brightness‐Mode Ultrasound Measurement of Subcutaneous Fat in Athletes: An International Multicentre Reliability Study, Under the Auspices of the IOC Medical Commission. Sports Medicine (Auckland, N.), 50(3), 597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirri, C. , Stecco, C. , Fede, C. , Macchi, V. and Özçakar, L. (2020) Ultrasound Imaging of the Fascial Layers: You See (Only) What You Know. Journal of Ultrasound in Medicine, 39(4), 827–828. [DOI] [PubMed] [Google Scholar]
- Pirri, C. , Todros, S. , Fede, C. , Pianigiani, S. , Fan, C. , Foti, C. et al. (2019) Inter‐rater reliability and variability of ultrasound measurements of abdominal muscles and fasciae thickness. Clinical Anatomy, 32(7), 948–960. [DOI] [PubMed] [Google Scholar]
- Porzionato, A. , Macchi, V. , Stecco, C. , Mazzi, A. , Rambaldo, A. , Sarasin, G. et al. (2012) Quality management of Body Donation Program at the University of Padova. Anatomical Sciences Education, 5(5), 264–272. [DOI] [PubMed] [Google Scholar]
- Schneider, C.A. , Rasband, W.S. and Eliceiri, K.W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9(7), 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengeis, M. , Müller, W. , Störchle, P. and Führhapter‐Rieger, A. (2019) Body weight and subcutaneous fat patterning in elite judokas. Scandinavian Journal of Medicine & Science In Sports, 29(11), 1774–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecco, A. , Gilliar, W. , Hill, R. , Brad, F. and Stecco, C. (2013) The anatomical and functional relation between gluteus maximus and fascia lata. Journal of Bodywork and Movement Therapies, 17(4), 512–517. [DOI] [PubMed] [Google Scholar]
- Stecco, A. , Macchi, V. , Masiero, S. , Porzionato, A. , Tiengo, C. , Stecco, C. et al. (2009) Pectoral and femoral fasciae: common aspects and regional specialisations. Surgical and Radiologic Anatomy, 31(1), 35–42. [DOI] [PubMed] [Google Scholar]
- Stecco, A. , Meneghini, A. , Stern, R. , Stecco, C. and Imamura, M. (2014) Ultrasonography in myofascial neck pain: randomised clinical trial for diagnosis and follow‐up. Surgical and Radiologic Anatomy, 36(3), 243–253. [DOI] [PubMed] [Google Scholar]
- Stecco, C. (2015) Functional atlas of the human fascial system, 1st edition. Edinburgh: Churchill Livingstone Elsevier. [Google Scholar]
- Stecco, C. , Gagey, O. , Belloni, A. , Pozzuoli, A. , Porzionato, A. , Macchi, V. et al. (2007) Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie, 91, 38–43. [DOI] [PubMed] [Google Scholar]
- Stecco, C. , Pirri, C. , Fede, C. , Fan, C. , Giordani, F. , Stecco, L. et al. (2019) Dermatome and fasciatome. Clinical Anatomy, 32(7), 896–902. [DOI] [PubMed] [Google Scholar]
- Stecco, C. , Stern, R. , Porzionato, A. , Macchi, V. , Masiero, S. , Stecco, A. et al. (2011) Hyaluronan within fascia in the etiology of myofascial pain. Surgical and Radiologic Anatomy, 33, 891–896. [DOI] [PubMed] [Google Scholar]
- Wilke, J. , Macchi, V. , De Caro, R. and Stecco, C. (2019) Fascia thickness, aging and flexibility: is there an association? Journal of Anatomy, 234(1), 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon request.


