Abstract
Background.
The National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE), collected alongside clinician-reported CTCAE, enables comparison of patient and clinician reports on treatment toxicity.
Methods.
In a multi-site study of women receiving chemotherapy for early-stage breast cancer, symptom reports were collected on the same day from patients and their clinicians for 17 symptoms; their data were not shared with each other. Proportions of moderate, severe or very severe patient-reported symptom severity were compared with clinician-rated toxicity grades 2, 3 or 4. Patient-clinician agreement was assessed via Kappa statistics. Chi-square tests investigated whether patient characteristics were associated with patient-clinician agreement.
Results.
Among 267 women, median age was 58 (range 24–83) and 26% were non-white. There was moderate scoring agreement (Kappa 0.413 – 0.570) for 53% of symptoms, fair agreement for 41% (Kappa range 0.220 – 0.378), and slight agreement for 6% (Kappa 0.188). For example, for fatigue, patient-reported and clinician-rated percentages were 22% vs 8% severe or very severe, 41% vs 46% moderate, 32% vs 39% mild, and 6% vs 7% none. Clinician severity scores were lower for non-white compared to white patients for peripheral neuropathy, nausea, arthralgia and dyspnea.
Conclusion.
Although clinician reporting of symptoms is common practice in oncology, there is suboptimal agreement with the gold standard of patient self-reporting. These data provide further evidence supporting the integration of patient-reported outcomes into oncological clinical research and clinical practice to improve monitoring of symptoms as well as timely intervention on symptoms.
Clinical Trial Registration:
INTRODUCTION
The National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Events (CTCAE)1 is the long-standing standard approach for collection and reporting of adverse events (AEs) in oncology research2. Of the approximately 800 AEs included in the CTCAE item library, approximately 10% correspond to symptoms, such as nausea and sensory neuropathy. However, CTCAE items are recorded by clinical research staff rather than by patients. In response to growing evidence of the value of patient-reported symptom severity as a complement to clinician-assessed toxicity3, the NCI supported the development of a patient-reported outcome (PRO) version of the CTCAE, called the PRO-CTCAE4,5, which became publicly available in April 2016. Like CTCAE, PRO-CTCAE provides single-item measures for patient-reported symptom “severity”, but also includes items for “interference with usual or daily activities” and “frequency” of some symptoms.
The development of PRO-CTCAE held the promise of improved understanding of patient and clinician toxicity reports for multiple symptoms simultaneously and at multiple time points during chemotherapy, if PRO-CTCAE and CTCAE reports were completed in real time during the same clinic visit6. This would enable more rigorous analyses of convergence and divergence in patient and clinician perspectives on important clinical endpoints such as quality of life and function7. The PRO-CTCAE could also facilitate collaborative reporting on symptoms that are not asked about routinely, through a process in which patient-reported toxicity forms are made readily available to the treating clinician during routine clinic visits8.
Within a growing body of literature documenting discrepancies between patient- and clinician-reported toxicities8, studies making paired comparisons of health care provider-assessed CTCAE and patient-reported PRO-CTCAE (or patient-tested precursors to the PRO-CTCAE) have been conducted in patients receiving chemotherapy for head and neck cancer9, genitourinary cancer10, and lung cancer10,11, patients receiving radiotherapy12, and patients receiving chemotherapy and/or radiation therapy5, with four of these studies collecting data at more than one time point during treatment5,9,11,12. Additional studies have compared CTCAE toxicity grades with validated symptom measures such as the European Organization of Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30)13–15 or other study-specific symptom reports16, with one of these studies collecting data at multiple time points during treatment14. Some of the afore-mentioned studies included women with early breast cancer within a mixed sample of adults with cancer; however, findings were not reported separately for each type of cancer5,13,16 or had a specific focus on early-stage breast cancer.
In this study, we conducted an analysis among women with early-stage breast cancer in which we compared clinician-reported (CTCAE) and patient-reported (PRO-CTCAE) severity for 17 symptoms collected at multiple time points throughout chemotherapy. We have previously reported that patient-assessed symptom severity for these 17 symptoms varies significantly among four chemotherapy regimens commonly used in current clinical practice17, confirming the importance of continuous symptom monitoring throughout treatment. We have also reported that there is minimal agreement between patient- and clinician-reported severity scores for chemotherapy-induced peripheral neuropathy associated with these chemotherapy regimens18. In the current study, we compare patient and clinician reports for all 17 symptoms, and we identify factors that may be associated with patient-clinician consensus or divergence13.
PATIENTS AND METHODS
Study participants
This is a secondary analysis of data on a sample of women recruited into one of three prospective non-randomized studies of a walking intervention for patients receiving (neo)adjuvant chemotherapy for early breast cancer (Stage 0–3) (American Joint Committee on Cancer staging, 7th edition) (ClinicalTrials.gov identifiers NCT02167932, NCT02328313, and NCT03761706). Patients were ages 21 years or older and recruited prior to starting chemotherapy regimens that were selected by clinicians in consultation with their patients. Patients provided written informed consent, and the studies were approved by the University of North Carolina Lineberger Comprehensive Cancer Center Protocol Review Committee and the Institutional Review Boards for each study site.
Measures
From chemotherapy initiation through end of chemotherapy, patients completed a patient-reported symptom form for 17 symptoms. These symptoms were selected a priori for their observed frequency in the treatment of patients with early breast cancer. In two studies (NCT02167932, NCT02328313), the reporting form was the validated patient-reported symptom monitor (PRSM)19. The PRSM was a pre-cursor to the PRO-CTCAE, and was used because PRO-CTCAE was not publicly available when these two studies were initiated. The PRSM pre-cursor was developed by investigators who were also involved with the development of PRO-CTCAE4,20 and has an analogous structure and response scale to the PRO-CTCAE (Appendix 1), employing single-item measures of symptom severity on a 5-point scale with response options from none/no symptom to very severe21. In addition, patients reported symptom “interference with doing things you usually do” using a single-item measure, with similar 5-point response options from not at all to very much. When PRO-CTCAE became publicly available, it was used as the reporting form for the third study (NCT03761706). Depending on their chemotherapy infusion schedule over 4 to 8 total cycles, patients completed symptom reports every other week or every third week. Patients with weekly infusion schedules (mostly paclitaxel) completed symptom reports every other week, to avoid over-reporting in this cohort compared to the rest of the sample. Patients completed symptom reports during their chemotherapy infusion, which was after they had seen their oncology clinician.
On the same day that patients completed symptom reports, their oncology clinician (MD, Nurse Practitioner, or Physician Assistant) was asked to complete a CTCAE study form to rate the same set of 17 symptoms. The patient reports were not available to their clinicians. For comparison with patient-reported scores, CTCAE response options were standardized across symptoms as follows: 0=none, 1=mild, 2=moderate, 3=severe, and 4=disabling22,23. We matched patient-reported “none” with CTCAE grade 0, “mild” with grade 1, “moderate” with grade 2, and “severe/very severe” with grade 3/421,24, consistent with a previously developed mapping algorithm22,23.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics, breast cancer diagnosis and treatment, adverse events, and patient- and clinician-reported symptom scores. Because clinicians were not always available to complete reports, only data points from days where both the patient and the clinician reported are included. The essential metric for our study was the maximum score for each symptom at any time during the measurement period (start to end of chemotherapy), which is the approach used in clinical trials when reporting treatment toxicity. Proportions of moderate/severe/very severe patient-reported symptom “severity” and “interference” were compared with clinician-rated toxicity grades 2/3/4 for all 17 symptoms combined at each level of severity (none, mild, moderate, severe/very severe) for each symptom individually.
Agreement between patient- and clinician-reported dichotomized maximum scores was assessed by reporting simple Kappa coefficients for each symptom25. Dichotomization was “low” for none or mild and “high” for moderate, severe or very severe. A priori interpretation of the Kappa statistic used standard rating criteria26: < 0.0 less than chance agreement, 0.01 – 0.20 slight agreement, 0.21 – 0.40 fair agreement, 0.41 – 0.60 moderate agreement, 0.61 – 0.80 substantial agreement, and 0.81 – 0.99 almost perfect agreement. The same method was used to compare patient-reported symptom “interference with things you usually like to do” with clinician toxicity grade. Chi-square tests were conducted to investigate whether patient characteristics were associated with patient-clinician agreement on maximum severity scores for each symptom individually.
RESULTS
Patient Characteristics
In a sample of 267 women, median age was 58 years (range 24 – 83), of whom 26% were non-white. Breast cancer was distributed across stages I, II and III and across four different common chemotherapy regimens (Table 1). A total of 1203 same-day paired reports were considered in our analysis, and the maximum symptom score for each patient was the unit of analysis. For patients receiving AC-T (doxorubicin/cyclophosphamide followed or preceded by paclitaxel/Taxol) or AC-TC (doxorubicin/cyclophosphamide plus paclitaxel/carboplatin), the median number of reports was 6, for TC (docetaxel/cyclophosphamide plus/minus anti-HER2 therapy) it was 3, for TCH (docetaxel/carboplatin plus anti-HER2 therapy) it was 5, and for all regimens it was 4.
Table 1.
Study Participant Characteristics (N=267)
| Variable | |
|---|---|
| Age | 58 (SD 13) Range 24 – 83 |
| Race | |
| Not white | 70 (26%) |
| White | 197 (74%) |
| Education | |
| High school or less | 38 (14%) |
| More than high school | 227 (86%) |
| Married | |
| No | 116 (44%) |
| Yes | 149 (56%) |
| Body Mass Index (BMI) – mean SD | 30 (SD 7) Range 17 – 65 |
| Underweight (<18.5) | 3 (1%) |
| Normal (18.5 to <25) | 72 (27%) |
| Overweight (25 to <30) | 83 (31%) |
| Obese I (30 or above) | 109 (41%) |
| Menopausal status at breast cancer diagnosis | |
| Pre-menopausal | 81 (31%) |
| Post-menopausal | 183 (69%) |
| Breast cancer stage | |
| I | 67 (25%) |
| II | 133 (50%) |
| III | 67 (25%) |
| Breast cancer phenotype | |
| HR negative/HER-2 negative | 78 (29%) |
| HR negative/HER-2 positive | 34 (13%) |
| HR positive/HER-2 negative | 120 (45%) |
| HR positive/HER-2 positive | 34 (13%) |
| Breast cancer surgery | |
| None | 7 (3%) |
| Lumpectomy | 126 (48%) |
| Mastectomy | 127 (49%) |
| Anti-HER-2 therapy | 67 (25%) |
| Chemotherapy timing | |
| Neoadjuvant | 103 (39%) |
| Adjuvant | 159 (60%) |
| Both | 1 (1%) |
| Chemotherapy regimens -- drug combinations | |
| AC-T (doxorubicin/cyclophosphamide followed or preceded by paclitaxel/Taxol) | 82 (31%) |
| AC-TC (doxorubicin/cyclophosphamide plus paclitaxel/carboplatin) | 19 (7%) |
| TC (docetaxel/cyclophosphamide; N=3 plus anti-HER-2 therapy) | 70 (27%) |
| TCH (docetaxel/carboplatin plus anti-HER-2 therapy) | 41 (16%) |
| Other | 51 (19%) |
HR=hormone receptor. HER-2: Human Epithelial Growth Factor receptor 2.
Patient and clinician symptom severity scores
In Figure 1, proportions of patient-reported maximum severity scores ranging from none to severe/very severe and clinician toxicity grades from 0 to 3/4 are presented for individual symptoms. For example for fatigue, patient- compared to clinician-rated percentages were 22% vs 8% severe/very severe, 41% vs 46% moderate, 32% vs 39% mild, and 6% vs 7% none. This figure illustrates how proportions of patient-reported moderate and severe/very severe symptoms are consistently higher than clinician-rated toxicity grades 2 and 3/4.
Figure 1.
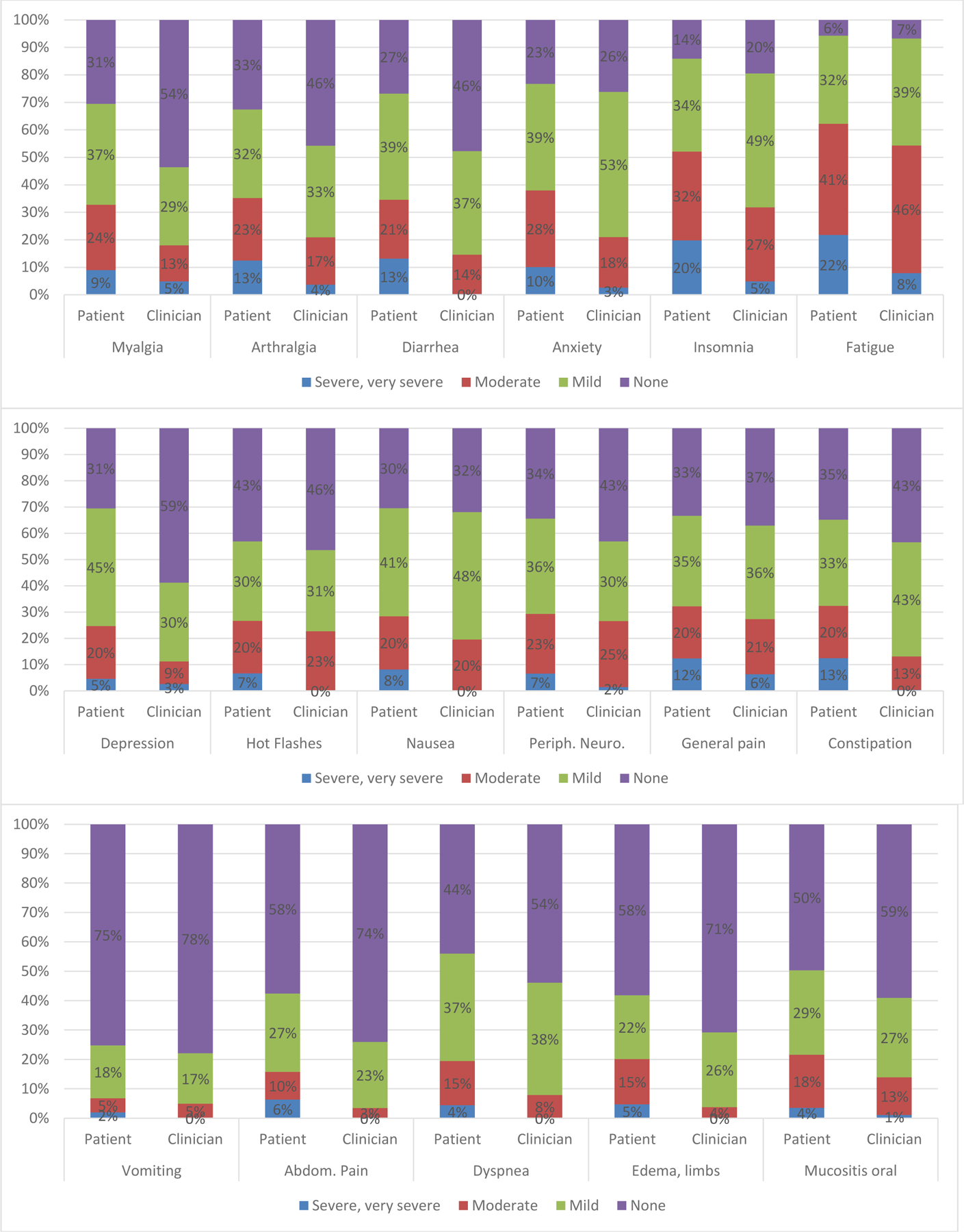
Maximum severity score at any time during chemotherapy – patient and clinician scores (percent)
Figure 2 illustrates the percent of patients who rated their symptom severity or interference as moderate, severe or very severe and clinicians rated their toxicity grade 2, 3 or 4. For example, in the far right grouping, 46% of patients rated more than four symptoms as moderate or worse severity; 34% of patients rated more than four symptoms as moderate or worse interference; and 27% of patients were rated by their clinician as having more than four symptoms graded 2, 3 or 4. In the far left group, 15% of patients rated none of their symptoms as moderate or worse severity, 27% of patients rated symptom interference as moderate or worse, and 19% of patients had none of their symptoms graded by their clinicians as 2 or higher.
Figure 2.
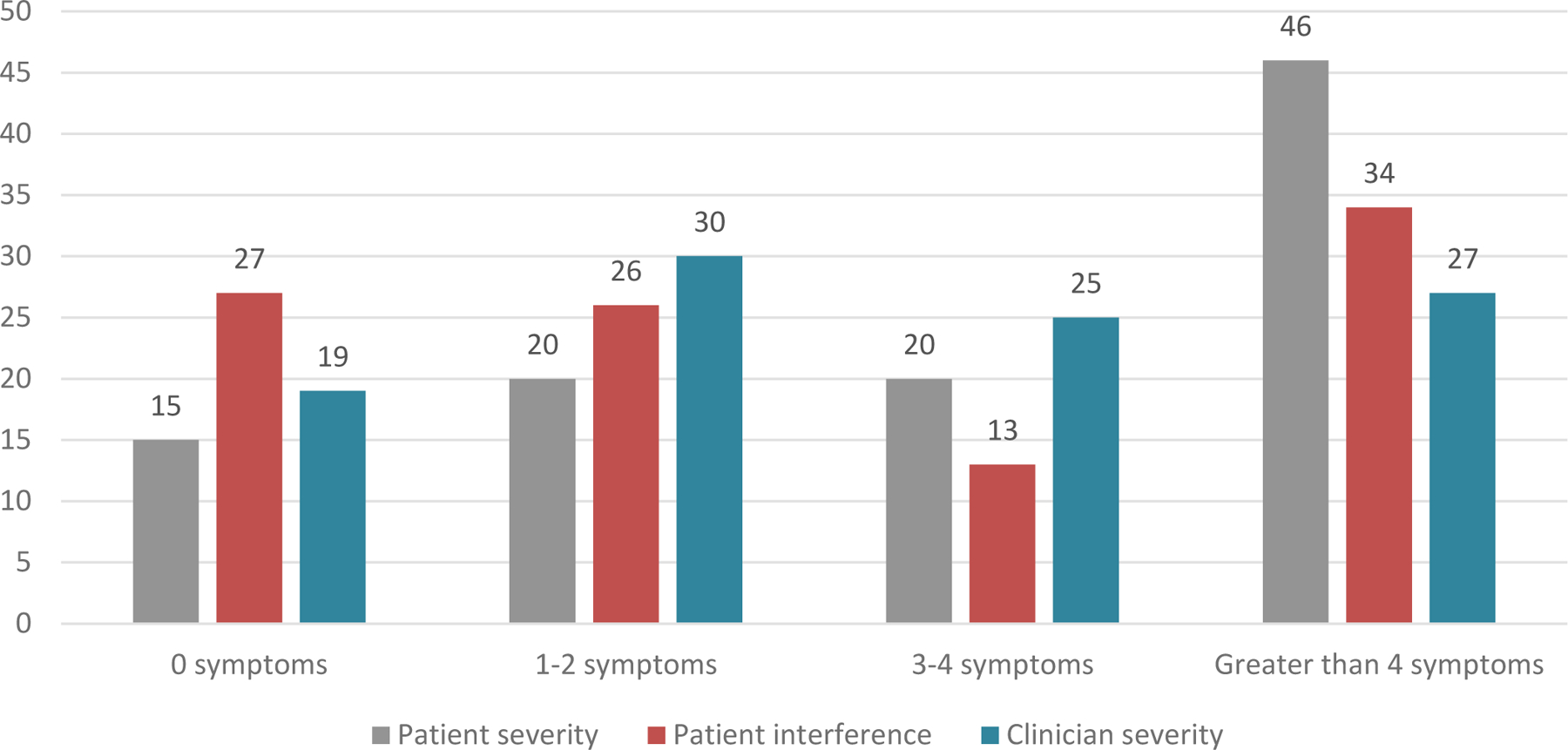
Patient-reported moderate/severe/very severe symptom severity and interference and clinician toxicity grade 2/3/4 (percent of patients)
Agreement of patient and clinician rated symptom severity
Table 2 shows the proportions of study participants where patients and clinicians agreed that symptom severity was “low” (none, mild) or “high” (moderate, severe/very severe). The table also shows where clinician maximum severity scores were higher than patient scores (clinician high/patient low), and where patient maximum scores were higher than clinician scores (patient high/clinician low). For example, for constipation, there was patient-physician agreement on “low” symptom severity for 65% of patients and agreement on “high” symptom severity for 11% of patients; however, for 3% of patients their clinicians rated symptom severity higher than their patients and, in turn, 21% of patients rated their constipation severity higher than their clinician.
Table 2.
Agreement between patients and clinicians -- maximum symptom “severity” and ‘interference” scores at any time during treatment (percent) (N=267)
| Agreement on maximum symptom “severity” score | |||||
|---|---|---|---|---|---|
|
Symptom |
Agree “low” | Agree “high” | Clinician “high”, patient “low” | Patient “high”, clinician “low” |
Kappa* |
| Constipation | 65 | 11 | 3 | 21 | .329 |
| Diarrhea | 62 | 14 | 4 | 21 | .378 |
| Nausea | 65 | 14 | 7 | 14 | .437 |
| Vomiting | 90 | 2 | 4 | 5 | .220 |
| Mucositis oral | 74 | 10 | 5 | 12 | .447 |
| Fatigue, lack of energy | 31 | 48 | 7 | 14 | .570 |
| Aching joints/arthralgia | 60 | 17 | 5 | 18 | .455 |
| Aching muscles/myalgia | 62 | 13 | 6 | 20 | .363 |
| Peripheral neuropathy | 60 | 16 | 11 | 13 | .413 |
| Anxiety | 57 | 16 | 5 | 22 | .372 |
| Feeling sad, unhappy/depression | 73 | 10 | 2 | 15 | .444 |
| Insomnia | 43 | 27 | 5 | 25 | .416 |
| Dyspnea/light-headedness | 77 | 4 | 4 | 15 | .224 |
| Abdominal pain | 83 | 2 | 2 | 14 | .188 |
| Edema limbs | 79 | 4 | 1 | 17 | .245 |
| General pain | 60 | 20 | 8 | 12 | .523 |
| Hot flashes | 66 | 16 | 8 | 11 | .513 |
| Agreement on maximum symptom “interference” score | |||||
|
Symptom |
Agree “low” | Agree “high” | Clinician “high, patient “low” | Patient “high”, clinician “low” |
Kappa* |
| Constipation | 77 | 6 | 7 | 9 | .337 |
| Diarrhea | 70 | 11 | 6 | 13 | .426 |
| Nausea | 68 | 11 | 9 | 12 | .384 |
| Vomiting | 89 | 1 | 4 | 6 | .150 |
| Mucositis oral | 80 | 6 | 9 | 6 | .348 |
| Fatigue, lack of energy | 32 | 44 | 11 | 13 | .522 |
| Aching joints/arthralgia | 64 | 14 | 9 | 14 | .402 |
| Aching muscles/myalgia | 68 | 12 | 7 | 14 | .406 |
| Peripheral neuropathy | 64 | 10 | 18 | 8 | .256 |
| Anxiety | 68 | 13 | 9 | 11 | .441 |
| Feeling sad, unhappy/depression | 79 | 8 | 4 | 9 | .506 |
| Insomnia | 52 | 21 | 12 | 16 | .398 |
| Dyspnea/light-headedness | 78 | 4 | 4 | 12 | .275 |
| Abdominal pain | 85 | 3 | 1 | 11 | .265 |
| Edema limbs | 86 | 2 | 2 | 10 | .247 |
| General pain | 61 | 18 | 11 | 10 | .466 |
| Hot flashes | 71 | 8 | 16 | 5 | .311 |
“Low” = patient-reported none or mild; clinician rated toxicity grade 0 or 1
“High” = patient-reported moderate, severe or very severe; clinician-rated toxicity grade 2, 3 or 4
Kappa interpretation: < 0.0 less than chance agreement, 0.01 – 0.20 slight agreement, 0.21 – 0.40 fair agreement, 0.41 – 0.60 moderate agreement, 0.61 – 0.80 substantial agreement, and 0.81 – 0.99 almost perfect agreement.
All kappas were statistically significant (p=<.05)
Overall, there was “moderate” agreement (Kappa 0.413 – 0.570) on symptom severity for 9 of 17 (53%) symptoms, “fair” agreement on 7 symptoms (41%) (Kappa 0.220 – 0.372), and “slight” agreement on one symptom (6%) (Kappa 0.188). In the lower half of Table 2, we report the comparison of patient-reported symptom interference with clinician-reported severity. Again, we find “moderate” agreement (Kappa 0.402 – 0.522) on 7 of 17 symptoms (41%), “fair” agreement on 9 symptoms (53%) (Kappa 0.247 to 0.398), and “slight” agreement on one symptom (6%) (Kappa 0.150). All Kappa estimates were statistically significant (p<=.05)
Variables associated with patient-clinician agreement on symptom severity scores
Using three levels of agreement (agree, clinician high/patient low, and patient high/clinician low), associations with patient characteristics were explored (Appendix 2). The highest number of statistically significant associations – signifying differences between patient and clinician scores – was seen for race with regard to nausea (p=.05), arthralgia (p=.04), peripheral neuropathy (p=.04), and dyspnea (p=.05). These differences are further elucidated in Figure 3, which shows that in 21% of non-white patients as compared to 10% of white patients, clinicians rated peripheral neuropathy severity “low” when patients rated it “high” (p=.04). However, the reverse is shown for nausea where clinicians rated symptom severity “low” when patients rated it “high” in 15% of white compared to 12% of non-white patients (p=.05).
Figure 3.
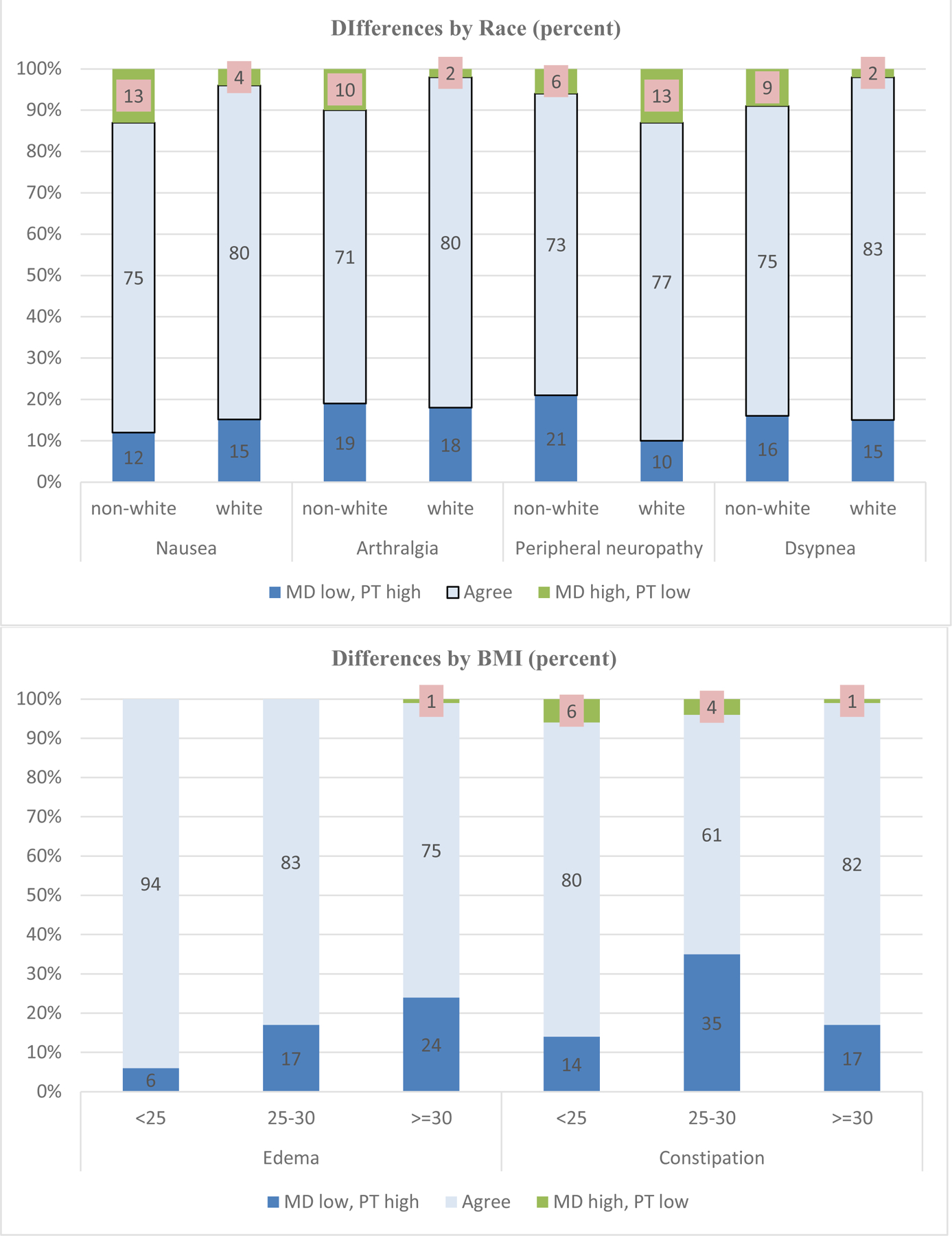
Patient-clinician congruence by race and Body Mass Index (BMI) (percent)
Similarly, Figure 3 presents significant differences by BMI, with clinician severity scores for constipation lower than patient scores for patients with BMI less than 25 (14%), BMI 25–30 (35%), and BMI 30 or higher (17%) (p=.003). BMI-related differences are also shown for edema (p=.005), with the rate of clinician under-reporting increasing with increasing BMI levels. Regarding marital status, clinician severity scores for peripheral neuropathy were lower for unmarried (19%) compared to married (8%) patients (p=.03). There were no significant differences for age, education or menopausal status.
DISCUSSION
Quality of life has been measured extensively in women with early breast cancer27, but few studies have administered single-item symptom assessments related to specific treatment- or disease-related adverse events at frequent intervals during active treatment (which is an emerging standard for adverse event monitoring in clinical trials)8, or have compared same-day patient and clinician reporting of this information. In our sample of women with early breast cancer, toxicity scores for 17 symptoms were collected longitudinally using single-item scales for patient-reported symptom severity and interference (PRO-CTCAE or PRSM) and clinician toxicity grades (CTCAE). Patients completed their form before seeing their oncologist, and clinicians completed their form after the visit. Scoring reports were not shared between patients and clinicians. Analysis was limited to patient-clinician scores that were collected on the same day (“paired”).
Across all 17 symptoms, clinician toxicity grades were lower than patient-reported severity scores, as seen in the proportion of symptoms where patients rated symptom severity high while clinicians rated toxicity low. This observation corroborates findings from an Italian study in women with early breast cancer that compared symptom questionnaires from patients at two time points (using a translation of CTCAE into Italian) to toxicity grades that were extracted and interpreted from clinician notes by research staff nurses28. In our study, we note higher congruence between patients and clinicians when symptoms severity was “low” and lower congruence when symptom severity was “high”, which is similar to what has been previously reported in other studies29. This observation is especially problematic when patients report “high” symptom severity while their clinician notes “low” toxicity, as observed with regard to insomnia in 25% of patients, anxiety in 22% of patients, constipation in 25% of patients, diarrhea in 21% of patients, and myalgia in 20% of patients. It was exceptional when clinicians rated symptom toxicity “high” when their patients rated it “low, as observed for peripheral neuropathy in 11% of patients, general pain and hot flashes in 8% of patients, and fatigue in 7% of patients.
We investigated patient-reported scores for “interference with what you usually like to do” and found them to be substantially lower than patient-reported symptom severity. We also compared patient-reported interference with clinician toxicity scores to see if this comparison yielded greater congruence, which it did not. In our final analysis of the data, we found that patient characteristics were by and large not associated with patient-clinician disagreement on severity scores. However, we did find that clinician under-estimation of certain symptoms was greater in non-white patients as compared to white patients. This finding warrants further research, but also reflects the larger literature documenting racial disparities in patient-provider communication30–33 as well as racial differences in symptom management experiences34.
We note that patients completed their form prior to seeing their oncologist. Clinicians completed their form after the visit; however, not always immediately after seeing the patient. It is possible that substantial time lag (which we did not measure) between seeing the patient and completing the form may have affected the clinician’s recall of the patient’s symptom severity. We also did not gather data on whether the clinician form was completed by an MD, NP or PA, and therefore did not analyze potential differences among clinicians.
Patient-centered care, which is crucial to high quality health care35, requires inclusion of the patient’s assessment of treatment toxicity. It is important to understand when and how patient and clinician perspectives diverge, and for which symptoms and patient characteristics. Our study points to the potential for racial disparities in symptom assessment by clinicians. The moderate or lower Kappa agreement across all 17 symptoms suggests challenges in effective patient-clinician communication about symptom experience across domains of symptom clusters (e.g., psycho-neurological, gastrointestinal, hormonal)36. Disagreement in scores tends to be at the high symptom severity end of the spectrum, with clinicians underestimating severity. Continuous symptom monitoring from both patients and clinicians, from pre-chemotherapy (to establish the patient’s baseline)7 through end-of-chemotherapy, provides an opportunity for early intervention on symptoms for which there are pharmaceutical remedies (e.g., anxiety, depression, insomnia) or non-pharmacological remedies (such as moderate exercise to mitigate fatigue37,38).
There is growing evidence that patients are willing and able to complete PRO-CTCAE items during a treatment-related clinic visit and after treatment has been completed39. Using nurses and nurse navigators, clinics could consider developing processes to record and review patient reported symptoms and consult with the oncologist for “real time” interventions to reduce symptom severity. These processes would likely improve the likelihood of treatment completion, potentially improve patient quality of life during chemotherapy to the extent toxicities are effectively managed, and enhance overall satisfaction with care11,40. Alternative payment models for oncology could facilitate the incorporation of patient-reported symptom assessment in quality metrics by providing reimbursement for these added responsibilities.
CONCLUSION
Although clinician reporting of symptoms is common practice in oncology, there is suboptimal agreement with the gold standard of patient self-reporting, particularly for non-white patients. These data provide further evidence supporting the integration of patient-reported outcomes into cancer research and clinical practice to improve symptom monitoring and guide timely interventions. This, in turn, would enable the timely identification of symptoms for which there are evidence-based interventions. Our findings support attention to patient-clinician interactions that are patient-centered and focus on quality of life as well as effective symptom with particular attention to cultural sensitivity32,41. Further research is needed to explore approaches to encouraging and enabling patient-provider communication on symptom severity in ways that are actionable in “real world” clinical practice.
Supplementary Material
Acknowledgements
We greatly appreciate the active support of oncology clinicians and their research staff at multiple sites and, most importantly, the breast cancer patients participating in our study. The sites are: Duke University Medical Center/Cancer Institute, Ohio State University Comprehensive Cancer Center, MD Anderson Cancer Center, UNC Rex Healthcare, and UNC Cancer Center. We also thank Tucker Brenizer, Erin O’Hare, Nicole Markowski, Nora Christopher, Emily Bell, Chad Wagoner, Will Pulley, Nancy Burns, and Amy Garrett for their unwavering commitment to study implementation best practices.
Funding:
This study was supported by the Breast Cancer Research Foundation (New York NY), Kay Yow Cancer Fund (Raleigh NC), and UNC Lineberger Comprehensive Cancer Center/University Cancer Research Fund (Chapel Hill NC).
Footnotes
Disclaimers. The authors do not have any conflict of interest disclaimers.
REFERENCES
- 1.National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) v.5, U.S. Department of Health and Human Services [Google Scholar]
- 2.Velikova G, Booth L, Smith AB, et al. : Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 22:714–24, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson TM, Ryan SJ, Bennett AV, et al. : The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer 24:3669–76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basch E, Reeve BB, Mitchell SA, et al. : Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 106, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dueck AC, Mendoza TR, Mitchell SA, et al. : Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 1:1051–9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kluetz PG, Kanapuru B, Lemery S, et al. : Informing the Tolerability of Cancer Treatments Using Patient-Reported Outcome Measures: Summary of an FDA and Critical Path Institute Workshop. Value Health 21:742–747, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Pe M, Dorme L, Coens C, et al. : Statistical analysis of patient-reported outcome data in randomised controlled trials of locally advanced and metastatic breast cancer: a systematic review. Lancet Oncol 19:e459–e469, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Basch E, Bennett A, Pietanza MC: Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J Natl Cancer Inst 103:1808–10, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Falchook AD, Green R, Knowles ME, et al. : Comparison of Patient- and Practitioner-Reported Toxic Effects Associated With Chemoradiotherapy for Head and Neck Cancer. JAMA Otolaryngol Head Neck Surg 142:517–23, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basch E, Iasonos A, McDonough T, et al. : Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7:903–9, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Basch E, Jia X, Heller G, et al. : Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst 101:1624–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moon DH, Chera BS, Deal AM, et al. : Clinician-observed and patient-reported toxicities and their association with poor tolerance to therapy in older patients with head and neck or lung cancer treated with curative radiotherapy. J Geriatr Oncol 10:42–47, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Laugsand EA, Sprangers MA, Bjordal K, et al. : Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes 8:104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fromme EK, Eilers KM, Mori M, et al. : How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol 22:3485–90, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Quinten C, Maringwa J, Gotay CC, et al. : Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst 103:1851–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirillo M, Venturini M, Ciccarelli L, et al. : Clinician versus nurse symptom reporting using the National Cancer Institute-Common Terminology Criteria for Adverse Events during chemotherapy: results of a comparison based on patient’s self-reported questionnaire. Ann Oncol 20:1929–35, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Nyrop KA, Deal AM, Shachar SS, et al. : Patient-reported toxicities during chemotherapy regimens in current clinical practice for early breast cancer. The Oncologist [accepted for publication December 2018], 2018 [DOI] [PMC free article] [PubMed]
- 18.Nyrop KA, Deal AM, Reeder-Hayes KE, et al. : Patient and clinician-reported chemotherapy-induced peripheral neuropathy (CIPN) in early breast cancer: current clinical practice. Cancer [Epub ahead of print], 2019 [DOI] [PubMed]
- 19.Stover A, Irwin DE, Chen RC, et al. : Integrating Patient-Reported Outcome Measures into Routine Cancer Care: Cancer Patients’ and Clinicians’ Perceptions of Acceptability and Value. EGEMS (Wash DC) 3:1169, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay JL, Atkinson TM, Reeve BB, et al. : Cognitive interviewing of the US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Qual Life Res 23:257–69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkinson TM, Hay JL, Dueck AC, et al. : What Do “None,” “Mild,” “Moderate,” “Severe,” and “Very Severe” Mean to Patients With Cancer? Content Validity of PRO-CTCAE Response Scales. J Pain Symptom Manage 55:e3–e6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basch E, Becker C, Rogak LJ, et al. : Development of a Composite Scoring Algorithm for the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) -- oral presentation. 26th Annual Meeting of the International Society for Quality of Life Research, 2019 [Google Scholar]
- 23.Basch E, Rogak LJ, Dueck AC: Methods for Implementing and Reporting Patient-reported Outcome (PRO) Measures of Symptomatic Adverse Events in Cancer Clinical Trials. Clin Ther 38:821–30, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basch E, Artz D, Dulko D, et al. : Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol 23:3552–61, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Altman DG: Practical Statistics for Medical Research. London, Chapman and Hall, 1991 [Google Scholar]
- 26.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics 33:159–174, 1977 [PubMed] [Google Scholar]
- 27.Lemieux J, Goodwin PJ, Bordeleau LJ, et al. : Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001–2009). J Natl Cancer Inst 103:178–231, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Montemurro F, Mittica G, Cagnazzo C, et al. : Self-evaluation of Adjuvant Chemotherapy-Related Adverse Effects by Patients With Breast Cancer. JAMA Oncol 2:445–52, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Atkinson TM, Reeve BB, Dueck AC, et al. : Application of a Bayesian graded response model to characterize areas of disagreement between clinician and patient grading of symptomatic adverse events. J Patient Rep Outcomes 2:56, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer NR, Kent EE, Forsythe LP, et al. : Racial and ethnic disparities in patient-provider communication, quality-of-care ratings, and patient activation among long-term cancer survivors. J Clin Oncol 32:4087–94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royak-Schaler R, Passmore SR, Gadalla S, et al. : Exploring patient-physician communication in breast cancer care for African American women following primary treatment. Oncol Nurs Forum 35:836–43, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Gonzales FA, Sangaramoorthy M, Dwyer LA, et al. : Patient-clinician interactions and disparities in breast cancer care: the equality in breast cancer care study. J Cancer Surviv, 2019 [DOI] [PMC free article] [PubMed]
- 33.Robertson-Jones TA, Tissue MM, Connolly M, et al. : Exploring Racial Differences in Patient Centeredness of Care (PCC) During Breast Cancer (BC) Chemotherapy Clinical Visits. 6:94–100, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Samuel CA, Schaal J, Robertson L, et al. : Racial differences in symptom management experiences during breast cancer treatment. Support Care Cancer 26:1425–1435, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute of Medicine Committee on Quality of Health Care in America: Crossing the Chasm: A New Health System for the 21st Century. Washington DC, National Academy Press, 2001 [Google Scholar]
- 36.Kim HJ, Barsevick AM, Tulman L, et al. : Treatment-related symptom clusters in breast cancer: a secondary analysis. J Pain Symptom Manage 36:468–79, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Brown JC, Huedo-Medina TB, Pescatello LS, et al. : Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol. Biomarkers Prev 20:123–133, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Cramp F, Byron-Daniel J: Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev, 2012 [DOI] [PMC free article] [PubMed]
- 39.Basch E, Pugh SL, Dueck AC, et al. : Feasibility of Patient Reporting of Symptomatic Adverse Events via the Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) in a Chemoradiotherapy Cooperative Group Multicenter Clinical Trial. Int J Radiat Oncol Biol Phys 98:409–418, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neugut AI, Hillyer GC, Kushi LH, et al. : A prospective cohort study of early discontinuation of adjuvant chemotherapy in women with breast cancer: the breast cancer quality of care study (BQUAL). Breast Cancer Res Treat 158:127–38, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Check DK, Chawla N, Kwan ML, et al. : Understanding racial/ethnic differences in breast cancer-related physical well-being: the role of patient-provider interactions. Breast Cancer Res Treat 170:593–603, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


