Abstract
Background
The single nucleotide polymorphism (SNP) rs1893217 within the gene locus encoding PTPN2 represents a risk factor for inflammatory bowel disease (IBD). Our previous work demonstrated reduced PTPN2 activity and subsequently increased inflammatory signaling upon presence of SNP rs1893217. The naturally occurring polyamine spermidine reduces pro-inflammatory signaling via induction of PTPN2 activity; however, the effect of SNP rs1893217 on the anti-inflammatory potential of spermidine is still unknown. Here, we investigated how presence of SNP rs1893217 affects treatment efficacy of spermidine and whether it might serve as a potential biomarker for spermidine treatment.
Methods
Human T84 (wild-type [WT] for PTPN2 SNP rs1893217) and HT29 (heterozygous for PTPN2 SNP rs1893217) intestinal epithelial cells (IECs) were treated with several polyamines from the putrescine-spermidine pathway. T84 and HT29 IECs, THP-1 monocytes (WT and transfected with a lentiviral vector expressing PTPN2 SNP rs1893217) and genotyped, patient-derived peripheral blood mononuclear cells were challenged with IFN-γ and/or spermidine.
Results
Among the analyzed polyamines, spermidine was the most efficient activator of PTPN2 phosphatase activity, regardless of the PTPN2 genotype. Spermidine suppressed IFN-γ-induced STAT1 and STAT3 phosphorylation, along with decreased mRNA expression of ICAM-1, NOD2, and IFNG in IECs and monocytes. Of note, these effects were clearly more pronounced when the disease-associated PTPN2 C-variant in SNP rs1893217 was present.
Conclusions
Our data demonstrate that spermidine is the most potent polyamine in the putrescine-spermine axis for inducing PTPN2 enzymatic activity. The anti-inflammatory effect of spermidine is potentiated in the presence of SNP rs1893217, and this SNP might thus be a useful biomarker for possible spermidine-treatment in IBD patients.
Spermidine is the most potent protein tyrosine phosphatase nonreceptor type 2 (PTPN2) activator within the putrescine-spermine pathway, and presence of an autoimmunity-associated variant in PTPN2 potentiates the anti-inflammatory effect of spermidine, thus providing a valuable biomarker for treatment-response to spermidine.
Introduction
The two major forms of inflammatory bowel disease (IBD), namely ulcerative colitis (UC) and Crohn’s disease (CD), share key features in their pathogenesis, such as excessive innate and adaptive immune responses, a dysfunctional intestinal epithelial barrier, and alterations in the microbiota.1, 2 Although significant advances in developing novel treatment strategies have been made in the past few years, current treatment options are still limited, and many patients lose response or develop severe side effects. Therefore, novel, personalized therapeutic approaches are urgently needed.3
Besides immunological and environmental factors, genetic predisposition is an important risk factor for developing IBD.4 Genome wide association studies (GWASs) found more than 240 confirmed single nucleotide polymorphisms (SNPs) that contribute to the relative risk to develop IBD, which—among others—are located in genes that encode for proteins involved in the recognition of bacterial products, production of antimicrobial factors, immune cell activation, and epithelial barrier function.5–8 One of those risk genes is protein tyrosine phosphatase nonreceptor type 2 (PTPN2).6, 9
The IBD-associated single nucleotide polymorphisms (SNPs) within the PTPN2 gene locus are SNP rs2542151, rs7234029, rs478582,10 and rs1893217.6, 11, 12 It has been demonstrated that these variants are also associated with other inflammatory disorders, including rheumatoid arthritis, type 1 diabetes, and psoriasis.11–16 On a molecular level, PTPN2 plays a central role in controlling inflammatory signaling cascades9, 11, 17, 18 and maintaining intestinal barrier functions.18–20 PTPN2 acts as a negative regulator of pro-inflammatory cytokine signaling and counteracts IFN-γ-induced epithelial damage.11, 19 Important cellular targets of PTPN2 include janus kinases (JAKs), signal transducers and activator of transcription (STAT) molecules,11, 12, 21, 22 epidermal growth factor receptor (EGFR), mitogen-activated protein kinases (MAPKs), and Src.9, 17, 23–25 Moreover, loss of PTPN2 results in reduced autophagy.9 Presence of SNP rs1893217 leads to a dysfunctional PTPN2 protein, resulting in increased activation of STAT1 and STAT3 and an exacerbated inflammatory response to IFN-γ and TNF.18, 20 These important anti-inflammatory roles of PTPN2 might explain why presence of loss-of-function variants in the PTPN2 gene promotes the development of intestinal inflammation.11, 12 Given this important anti-inflammatory and barrier-maintaining role of PTPN2, PTPN2 activation might be a promising novel approach for the treatment of IBD. In line with this, we have previously demonstrated a negative regulation of IFN-γ-induced signaling by pharmacological activation of PTPN2 using the naturally occurring polyamine spermidine.26, 27 Spermidine treatment resulted in attenuated STAT1, STAT3, and p38 activation, reduced secretion of the pro-inflammatory cytokines IL-6 and MCP1, and reduced IFN-γ-induced intestinal barrier defects.26–28
Spermidine is present in all cells of the body, but especially high contents are found in tissues with a high turnover rate, such as the intestinal epithelium.29, 30 In addition to endogenous spermidine production, spermidine is also biosynthesized by gut bacteria, and it is commonly present in human diet.31 On a functional level, spermidine is involved in fundamental cellular processes, including cell growth and development by promotion of RNA translation, reduction of RNA degradation, and promoting DNA and RNA synthesis. Spermidine further regulates DNA and protein synthesis by modifying DNA condensation and post-translational protein modifications.29, 32–35 Other polyamines, including spermine and putrescine, and several acetylated derivatives that are generated within the polyamine synthesis pathway are also essential factors for cellular homeostasis/growth and influence various metabolic reactions.33 Besides this direct effect of spermidine on host cell metabolism, spermidine also affects intestinal microbes and their susceptibility to environmental stress/antibiotics.36 Therefore, spermidine might crucially affect the composition of the intestinal microbiome. Due to a reduction in the rate of normal endogenous synthesis of polyamines in IBD patients,34 polyamine supplementation—and specifically spermidine administration—might be a promising therapeutic option for IBD.
Considering the potential of spermidine to activate PTPN2 and the close molecular similarity between spermidine and other naturally occurring polyamines within the human body, we here investigated whether other polyamines, namely spermine, putrescine, N1-acetylspermidine, N1-acetylspermine, and N8-acetylspermidine, might be even more potent PTPN2 activators and could thus potentially be used as novel therapeutics in IBD.
In addition, although the anti-inflammatory potential of spermidine treatment/PTPN2 activation in the context of intestinal inflammation has been demonstrated, it is not known how presence of PTPN2 variants affect the ability of spermidine to activate PTPN2 and/or reduce inflammation. Therefore, we investigated how presence of SNP rs1893217 affects PTPN2 activation upon treatment with spermidine.
METHODS
Cell Lines
In a screen of several commonly used intestinal epithelial cell (IEC) lines for SNP rs1893217 in PTPN2 using commercially available TaqMan based genotyping assays (Thermo Fisher Scientific, Waltham, Massachusetts, USA), we found that T84 cells (ATCC, Old Town, Manassas, Virginia, USA) were homozygous for the PTPN2 major (wild type [WT], referred to as TT) allele, whereas HT29 cells (ATCC) were heterozygous (CT) for SNP rs1893217 in PTPN2 (data not shown). Both cell lines were cultured in 75 cm2 polystyrene flasks to a confluency of 75%–80%. The T84 cells were grown in a 1:1 ratio of Dulbecco’s Modified Eagle Medium (Thermo Fisher Scientific) and F12 Nutrient Mixture Ham (Thermo Fisher Scientific) with 4.5 g/L D-glucose, 4.5 g/L L-glutamine, and 10% fetal calf serum. The HT29 cells were cultured in Dulbecco’s Modified Eagle Medium (Thermo Fisher Scientific) with 1 g/L D-glucose, 1 g/L L-glutamine, and 1% nonessential amino acids solution (Thermo Fisher Scientific), supplemented with 10% fetal calf serum, at 37°C in 10% CO2. The THP-1 cells were obtained from ATCC and transfected with a lentiviral vector expressing SNP rs1893217, as described previously.9
Patient Samples
Whole blood samples were obtained from Crohn’s disease (CD) and ulcerative colitis (UC) patients from the Swiss IBD cohort and from healthy volunteers. Supplementary Table S1 summarizes characteristics of all included subjects. All patients, including healthy controls (HCs), signed informed consent before study inclusion. To isolate peripheral blood mononuclear cells (PBMCs), the blood was diluted 1:2 in Dulbecco’s Phosphate Buffer Saline (PBS, Sigma-Aldrich, St. Louis, Missouri, USA) and overlaid on Ficoll (Histopaque-1077, Sigma-Aldrich) before density gradient centrifugation. Peripheral blood mononuclear cells were collected from the interphase, washed twice in PBS, and resuspended in RPMI Medium 1640 (Thermo Fisher Scientific) or frozen in fetal calf serum with 10% dimethyl sulfoxide (DMSO, Sigma-Aldrich).
Cell Stimulation
The T84, HT29, THP-1, and peripheral blood mononuclear cells were seeded on 12-well culture plates (TPP, Buchs, Switzerland) at a density of 1.0 × 106 cells per well and left to adhere for 24 hours. The cells were then treated with 0.1 μM, 1 μM, 10 μM, and 100 μM spermidine (Sigma-Aldrich), spermine tetrahydrochloride (Sigma-Aldrich), putrescine dihydrochloride (Sigma-Aldrich), N1-acetylspermidine hydrochloride (Cayman Chemicals, Ann Arbor, Michigan, USA), N1-acetylspermine trihydrochloride (Sigma-Aldrich), or N8-acetylspermidine (Cayman Chemical) for 30 minutes for protein collection and for 24 hours for mRNA isolation. In some experiments, cells were stimulated with IFN-γ (100 ng/mL, Sigma-Aldrich). In each experiment, a group of untreated cells was included as a reference control. For Transepithelial electrical resistance (TEER) measurements, the cells were grown for 9 days to allow the formation of tight junctions before the addition of spermidine and/or IFN-γ and TEER measuring using a chopstick electrode.
Protein Isolation
The HT29, T84, THP-1, and PBMCs were washed twice with PBS before lysis in mammalian protein extraction reagent (M-PER, ThermoFisher Scientific) containing cOmplete mini protease inhibitor cocktail (Roche) for 30 minutes on ice. Cell lysates where then centrifuged, and protein-containing supernatant was collected. Protein content was measured at 280 nm wavelength using a NanoDrop ND1000 spectrophotometer (Marshal Scientific, Hampton, New Hampshire, USA).
Western Blotting
Equal amounts of proteins were loaded on to 10% sodium dodecyl sulphate polyacrylamide gels and separated by electrophoresis (SDS-PAGE) and then transferred on to nitrocellulose membranes (0.45 µm pore size, Membrane Solution, Auburne, Washington, USA). Further, membranes were blocked in 3% milk powder (Carl Roth, Karlsruhe, Germany) and 1% bovine serum albumin (BSA, PAN Biotech, Regensburg, Germany) and incubated with primary antibodies and corresponding horseradish peroxidase (HRP)–labeled secondary antibodies. Detection was performed using the WesternBright (Advansta, San Jose, California, USA) or WesternBright Sirius (Advansta) ECL kit. Pictures were taken with a Fusion Solo S imager (Vilber Lourmat, Paris, France) using the Vision Capture Fusion Solo 3 v16.15 software. Densitometry measurements were performed using NIH ImageJ software. For phosphorylated STAT1 and STAT3, normalization was conducted to total STAT1 and STAT3 protein, respectively. The following antibodies were used for protein detection: anti-PTPN2 (MAB1930, R&D), anti-STAT-1 (9172S, Cell Signaling, Danvers, Massachusetts, USA), anti-pSTAT1 (7649S, Cell Signaling), anti-STAT3 (9139S, Cell Signaling), anti-pSTAT3 (9131S, Cell Signaling), and β-actin (MAB1501, EMD Milipore).
Immunoprecipitation and Phosphatase Activity Assay
For immunoprecipitation of PTPN2, protein lysates were precleared with 50% (v/v) protein G-Sepharose 4 Fast Flow beads (Ge Healthcare Life Science) and supernatants subsequently incubated with anti-PTPN2 antibody (Merck) overnight at 4°C. Sepharose 4 Fast Flow beads were then added to each sample and left to incubate for 1 hour at 4°C. Pellets containing beads-antibody-antigen complex were washed 3 times with ice-cold PBS and resuspended in reaction buffer from the EnzChek-Phosphatase assay Kit (ThermoFisher Scientific).
PTPN2 phosphatase activity was measured using the EnzChek-Phosphatase assay Kit (ThermoFisher Scientific) according to the manufacturer’s instructions. Each sample was applied in triplicate to a 96-well black plate and mixed with 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP), which emits a fluorescent signal at 562 nm upon dephosphorylation. Fluorescence was measured on a microplate reader (Synergy HT and Synergy H1) using Gene5 V1.11 software. For each of the immunoprecipitates, Western blotting was performed to account for equal protein loading.
RNA Isolation and RT-PCR
Before mRNA isolation, cells were washed twice with PBS and snap frozen in RLT buffer (Qiagen) supplemented with 40 mM 1,4-dithiothreitol (DTT, Sigma-Aldrich), and RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA concentration was quantified using a Nanodrop ND1000 spectrophotometer (Marshal Scientific) at a wavelength of 260 nm.
Complementary DNA (cDNA) synthesis was performed using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s instructions. Real-time polymerase chain reaction (PCR) was performed using FAST qPCR MasterMix for Taqman Assays (Thermo Fisher Scientific) on a Fast HT7900 Real-Time PCR system using SDS Software (Thermo Fisher Scientific) or on a QuantStudio 6 System (Thermo Fisher Scientific). Measurements were performed in triplicates, human ACTB was used as endogenous control, and results were analyzed by the ΔΔCT method. The used gene expression assays were all obtained from Thermo Fisher Scientific.
Statistical Analysis
All data are presented as means with standard deviation (SD). Statistical analyses were performed using GraphPad Prism 6 (Graph Pad Software, La Jolla, CA) by using analysis of variance (ANOVA), followed by Tukey post hoc test. Statistical significance was considered when P < 0.05.
RESULTS
Polyamines Have Distinct Effects on PTPN2 Activity in Intestinal Epithelial Cells
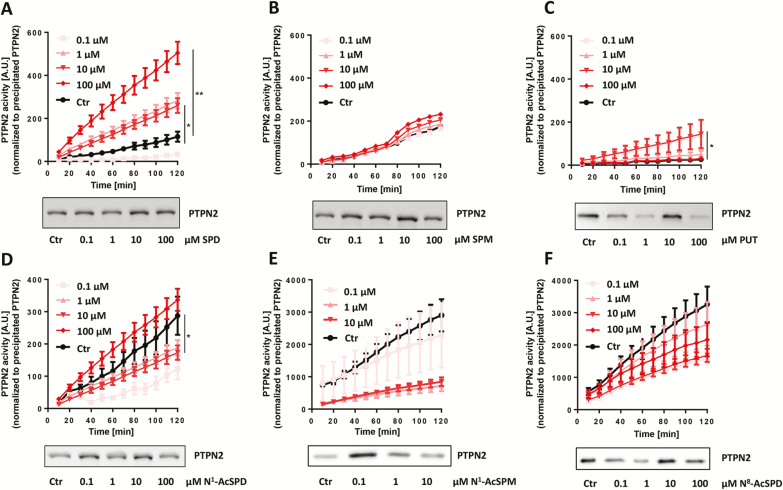
We initially determined how naturally occurring polyamines affect PTPN2 activation in human intestinal epithelial cells. For this aim, we used HT29 cells, which are heterozygous for the disease-associated C-variant in SNP rs1893217, and T84 cells, which are homozygous for the wild-type (T) variant, and treated them with 0.1 μM, 1 μM, 10 μM, and 100 μM of spermidine, spermine, putrescine, N1-acetylspermidine, N1-acetylspermine, or N8-acetylspermidine for 30 minutes. In HT29 cells, increased PTPN2 activity was observed when spermidine was applied at concentrations higher than 1 μM, with the most significant increase at a concentration of 100 μM (P < 0.01, Fig. 1A). Treatment with the closely related polyamine spermine, on the other hand, did not result in significant changes in enzymatic PTPN2 activity (Fig. 1B), whereas treatment with putrescine significantly enhanced PTPN2 activity at a concentration of 10 μM only, albeit to a lesser extent than spermidine (P < 0.05, Fig. 1C). Because polyamine metabolism involves acetylation of spermidine and spermine, we next evaluated whether acetylated polyamines would also affect PTPN2 enzymatic activity. Though treatment with 1 µM and 10 µM of N1-acetylspermidine resulted in decreased PTPN2 activity in HT29 cells (P < 0.05, Fig. 1D), treatment with N1-acetylspermine or N8-acetylspermidine did not show any effects compared with untreated controls (Fig. 1E, F). In T84 cells, which are homozygous for the WT PTPN2 variant, only spermidine treatment resulted in increased PTPN2 enzymatic activity (P < 0.001, Supplementary Fig. S1A–F). Those data demonstrate that spermidine is likely the polyamine with the most potent PTPN2-activatory capacity in human cells and that acetylation of spermidine clearly prevents PTPN2 activation.
Figure 1.
Effect of polyamine treatment on PTPN2 activity in HT29 cells. HT29 cells were treated for 30 minutes with (A) spermidine, (B) spermine, (C) putrescine, (D) N1-acetylspermidine, (E) N1-acetylspermine, and (F) N8-acetylspermidine at the indicated concentrations. Depicted are phosphatase activity levels of PTPN2-immunoprecipitates normalized to precipitated PTPN2 protein level and representative Western blot pictures of PTPN2 immunoprecipitates. Data represent means (n = 3) and standard deviations. Analysis of variance ANOVA followed by post hoc Tuckey; asterisks indicate significant differences between the treatment groups and control (*P < 0.05, **P < 0.01).
Spermidine Is the Most Potent Polyamine to Activate PTPN2
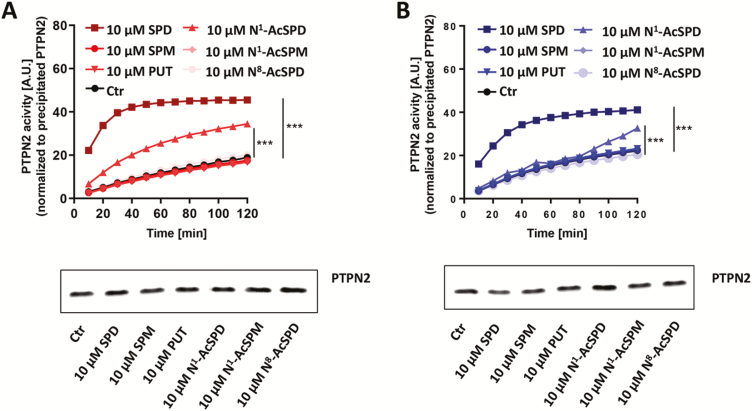
Subsequently, we directly compared the capacity of different polyamines to activate PTPN2. The T84 and HT29 cells were treated with 10 μM of spermidine, spermine, putrescine, N1-acetylspermidine, N1-acetylspermine, and N8-acetylspermidine for 30 minutes. In HT29 and T84 cells, spermidine was again the most effective activator of PTPN2 (P < 0.001) phosphatase activity among all tested polyamines, but N1-acetylspermidine was also able to significantly increase PTPN2 activity (P < 0.001, each; Fig. 2A, B), although to a much lower extent.
Figure 2.
Spermidine is the most potent activator of PTPN2 in HT29 and T84 cells. A, HT29 cells and (B) T84 cells were treated for 30 minutes with 10 µM of spermidine, spermine, putrescine, N1-acetylspermidine, N1-acetylspermine, or N8-acetylspermidine as indicated. Depicted is PTPN2 activity normalized to PTPN2 protein content and representative pictures of Western blot from PTPN2 precipitates. Data represent means (n = 3) and standard deviations. Analysis of variance ANOVA followed by post hoc Tuckey; asterisks indicate significant differences between the treatment groups and control (***P < 0.001).
Treatment With Polyamines Promotes Protein Expression of PTPN2 in Intestinal Epithelial Cells
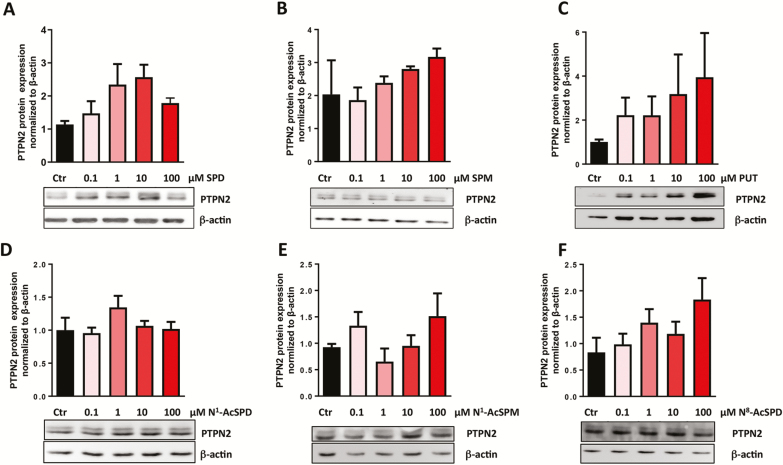
To evaluate whether treatment with polyamines influences protein expression of PTPN2 in HT29 and T84 IEC, the cells were treated with 0.1 μM, 1 μM, 10 μM, and 100 μM of spermidine, spermine, putrescine, N1-acetylspermidine, N1-acetylspermine, and N8-acetylspermidine for 24 hours. Spermidine treatment resulted in a dose dependent increase of PTPN2 protein expression in HT29 cells (Fig. 3A), which is in line with findings of Penrose et al.27 Although there was a clear trend of enhanced PTPN2 protein levels in spermine-treated and putrescine-treated HT29 cells, this did not reach significance (Fig. 3B, C). When assessing the role of acetylated polyamines on PTPN2 protein expression, we observed that neither N1-acetylspermidine nor N1-acetylspermine affected PTPN2 protein levels (Fig. 3D, E), whereas N8-acetylspermidine increased PTPN2 expression (Fig. 3F). Nevertheless, none of these effects reached statistical significance. Of note in T84 cells, none of the investigated polyamines affected PTPN2 protein levels (Supplementary Fig. S2A–F). Overall, in our hands PTPN2 activity did not directly correlate with PTPN2 expression, neither in HT29 nor in T84 cells and regardless of the used polyamine.
Figure 3.
Effect of polyamine treatment on PTPN2 protein expression in HT29 cells. HT29 cells were treated for 24 hours with (A) spermidine, (B) spermine, (C) putrescine, (D) N1-acetylspermidine, (E) N1-acetylspermine, and (F) N8-acetylspermidine. Depicted are representative Western blot pictures for PTPN2 and β-actin, as well as densitometry measurements of PTPN2 normalized to β-actin and untreated controls. Data represent means (n = 3) for each treatment. Analysis of variance ANOVA followed by post hoc Tuckey.
Spermidine Downregulates STAT1 and STAT3 Phosphorylation More Effectively in Cells Carrying the C-Variant in PTPN2
To investigate how the presence of the C-variant within the PTPN2 gene locus affects the potential of spermidine to suppress pro-inflammatory signaling cascades, we challenged T84 and HT29 cells for 30 minutes with IFN-γ in the presence or absence of spermidine. Again, there was no difference in PTPN2 protein levels between cell types and after treatment for 30 minutes (Fig. 4A). Consistent with our previous results, the enzymatic activity of PTPN2 was clearly increased upon spermidine administration and/or IFN-γ treatment (Fig. 4B). Although in T84 cells spermidine and IFN-γ cotreatment only slightly enhanced PTPN2 activity when compared with each single treatment, the additive effect was much more pronounced in HT29 cells (P < 0.001; Fig. 4B).
Figure 4.
Effect of spermidine treatment in IFN-γ treated HT29 and T84 cells. HT29 (red bars) and T84 cells (blue bars) were treated for 30 minutes with 100 ng/mL IFN-γ, 10 µM spermidine, or IFN-γ and spermidine as indicated. The graphs show (A) representative Western blots of PTPN2 levels, (B) PTPN2 enzymatic activity and Western blot from immunoprecipitation, and (C) representative Western blots and densitometry of STAT1 and STAT3 phosphorylation levels after 30 minutes. Data represent means (n = 4) and standard deviation. Analysis of variance ANOVA followed by post hoc Tuckey, asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between the treatment groups and control (4B) and between IFN-γ and IFN-γ with spermidine (4C).
As expected, treatment of T84 and HT29 cells with IFN-γ promoted phosphorylation of STAT1 and STAT3 molecules. This effect was almost completely prevented upon cotreatment with spermidine in HT29 cells (P < 0.001; Fig. 4C). In general, reduction of phosphorylated STAT1 and STAT3 was more pronounced in HT29 cells than in T84 cells (P < 0.05).
An important function of IECs is the formation of tight barriers, and it has been shown previously that loss of PTPN2 potentiates IFN-γ-induced barrier defects.27 To investigate how presence of SNP rs1893217 affects barrier permeability and whether this is affected by spermidine, we measured TEER in IECs grown on cell culture inserts and treated with IFN-γ, spermidine, or a combination. TEER was significantly lower in HT29 cells compared with T84, and IFN-γ further decreased TEER in both cell lines (Supplementary Fig. S3). Spermidine treatment on its own promoted TEER in HT29 cells but not in T84 cells, and consistent with previous reports,27 it was able to prevent the IFN-γ-induced drop in TEER in both cell lines almost completely (Supplementary Fig. S3). This indicates that spermidine treatment can partially rescue the lower TEER in HT29 cells and that it can prevent IFN-γ-induced barrier defects regardless of the PTPN2 genotype.
Reduced Expression of Pro-inflammatory Genes Upon Spermidine Treatment Is More Pronounced in Cells Carrying the PTPN2 C-Variant
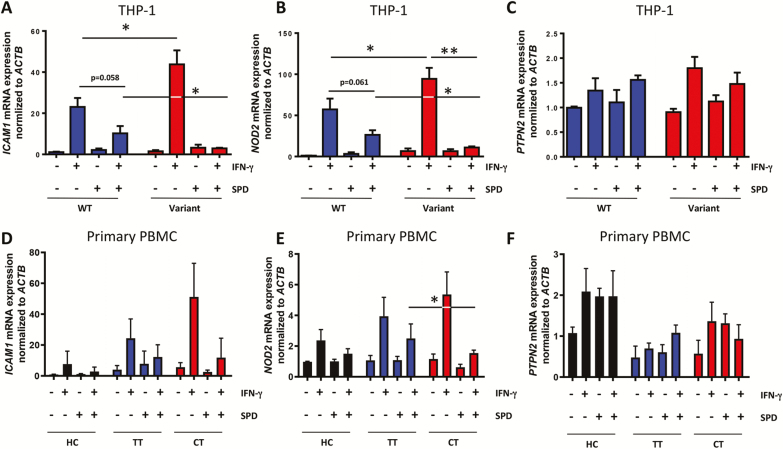
Because our data showed that spermidine treatment reduces the activity of downstream modulators of IFN-γ signaling, namely STAT1 and STAT3, more efficiently in carriers of the PTPN2 C-variant, we next investigated whether this effect is also observed with respect to the expression of IFN-γ target genes, such as intracellular adhesion molecule-1 (ICAM1), nucleotide-binding oligomerisation domain-containing protein 2 (NOD2), IFNG, and PTPN2. As expected, IFN-γ treatment promoted mRNA levels of ICAM1, NOD2, and IFNG in T84 and HT29 cells, whereas PTPN2 mRNA expression was not significantly altered (Fig. 5). Compared with IFN-γ-treated cells, co-administration of IFN-γ and spermidine resulted in significantly reduced mRNA levels of ICAM1 in HT29 cells (P < 0.001) but not in T84 cells, although there was a clear trend toward reduction (Fig. 5A). Moreover, in T84 and HT29 cells, expression of NOD2 (P < 0.05 and P < 0.001, respectively; Fig. 5B) and IFNG (P < 0.05 and P < 0.001, respectively; Fig. 5C) was down regulated. Again, this effect was more pronounced in HT29 cells when compared with T84 cells (P < 0.05, Fig. 5A–C). Summarized, these data indicate that presence of the C-allele in PTPN2 might render the cells more susceptible to the anti-inflammatory effects of spermidine.
Figure 5.
Effect of spermidine treatment on mRNA expression of IFN-γ target genes in HT29 and T84 cells. HT29 and T84 cells were treated for 24 hours with 100 ng/mL IFN-γ and /or 10 µM spermidine. The graphs show mRNA levels of (A) ICAM1, (B) NOD2, (C) IFNG and (D) PTPN2 in T84 (blue bars) and HT29 cells (red bars). Data represent means (n = 4) and standard deviations. Analysis of variance ANOVA followed by post hoc Tuckey; asterisks indicate significant differences between the treatment groups (*P < 0.05, ***P < 0.001).
Reduction of STAT1 and STAT3 Phosphorylation Is More Pronounced in THP-1 Cells and IBD-derived PBMCs Carrying the PTPN2 C-Variant
To confirm that the enhanced anti-inflammatory effect of spermidine treatment we observed in HT29 vs T84 cells results from the presence of the PTPN2 C-allele and is not due to general differences between these two cell lines, we next assessed the effect of spermidine treatment in the same cell line with WT or variant PTPN2. Because CRISPR-Cas9 editing of the PTPN2 SNP rs1893217 locus resulted in several technical difficulties and an overall reduced cell viability in targeted cells, we used a lentiviral construct that overexpresses the disease-associated variant.However, lentiviral transfection of our IEC cell lines compromised cell viability; hence, we used the monocytic cell line THP-1 for these transfection studies. Levels of PTPN2 were similar in THP-1 cells that were transfected with a lentiviral construct expressing the C-allele (“variant”) in SNP rs1893217 as in THP-1 cells transfected with a lentiviral construct expressing the T-allele (“WT”; Fig. 6A). Of interest, without treatment and upon IFN-γ treatment, PTPN2 was less active in THP-1 cells expressing the variant allele when compared with cells expressing the WT allele (Fig. 6B). However, though spermidine induced a moderate increase in PTPN2 activation in WT transfected cells, this increase was significantly higher in variant transfected cells (Fig. 6B). Consistent with previous reports and our results in IEC cell lines, spermidine treatment resulted in lower phosphorylation of STAT1 and STAT3 (Fig. 6C). Of note, this effect was again more pronounced in variant transfected cells than in WT transfected cells. Summarized, this indicates that the pronounced anti-inflammatory effect of spermidine seems to be due to the presence of the C-allele and not due to differences between the cell lines. To further test the relevance of these findings in primary patient-derived cells, we next assessed how spermidine treatment affects inflammatory responses in PBMCs from healthy controls or IBD patients being WT (TT) or heterozygous (CT) for SNP rs1893217. Again, there was a clear trend toward increased STAT1 and STAT3 phosphorylation in cells from variant carriers, but they responded better to spermidine treatment (Fig. 6D, E).
Figure 6.
Spermidine downregulates phosphorylation of STAT1 and STAT3 more efficiently in IFN-γ treated THP-1 cells and IBD-patient derived PBMC carrying the PTPN2 C-variant. THP-1 cells were stably transfected with lentiviral constructs containing the T (“WT”) or the C (“variant”) allele in PTPN2 SNP rs1893217. After selection of clones with stable expression of the lentiviral DNA, the cells were treated for 30 minutes with 100 ng/mL IFN-γ and/or 10 µM spermidine. (A) Representative Western blots show the protein levels of PTPN2, (B) PTPN2 enzymatic activity and representative Western blot pictures from immunoprecipitations used for the activity assay, (C) representative Western blots and densitometry of STAT1 and STAT3 phosphorylation levels after 30 minutes. Data represent means (n = 3) and standard deviation. PBMC from healthy controls (HC, black bars, homozygous for the T allele, n = 5), or IBD patients homozygous for the T allele (TT, blue bars, n = 6) or heterozygous (CT, red bars, n = 5) for the PTPN2 SNP rs1893217 were treated for 30 minutes with 100 ng/mL IFN-γ and/or 10 µM spermidine. Representative Western blots and densitometry analysis show phosphorylation level of (D) STAT1 and (E) STAT3. Data represent means and standard deviations. Analysis of variance ANOVA followed by post hoc Tuckey; asterisks indicate significant differences (*P < 0.05, ***P < 0.001)
More Pronounced Reduction of IFN-γ Response Genes in THP-1 Cells and IBD Patient-derived PBMCs Expressing the PTPN2 C- Variant
To confirm the effect of spermidine treatment on IFN-γ response genes, we also analyzed the expression of pro-inflammatory genes downstream of IFN-γ signaling in THP-1 cells and patient-derived PBMCs. Similar to the effects observed in HT29 and T84 cells, we found that spermidine treatment reduced the expression of ICAM1 and NOD2 in WT transfected THP1 cells challenged with IFN-γ (Fig. 7A, B). Interestingly, transfection with the variant resulted in increased induction of these two genes upon IFN-γ treatment, whereas there was no difference in the expression levels of PTPN2 (Fig. 7C). Again, as observed in HT29 vs T84 cells, the effect of spermidine treatment was more pronounced in THP-1 cells transfected with the variant, and variant transfected cells treated with IFN-γ and spermidine showed clearly reduced levels of ICAM1 and NOD2 expression when compared with WT transfected THP-1 cells treated with IFN-γ and spermidine (Fig. 7A–C). Similar effects were observed in PBMCs from PTPN2-genotyped IBD patients, although the effects were less pronounced, likely due to high interpatient variability (Fig. 7D–F).
Figure 7.
Effect of spermidine treatment on mRNA expression of IFN-γ target genes in THP-1 cells and IBD patient-derived PBMC expressing the C-variant. THP-1 cells expressing the T (“WT”) or the C (“variant”) allele in SNP rs1893217 were treated for 24 hours with 100 ng/mL IFN-γ and/or 10 µM spermidine. The graphs show mRNA levels of (A) ICAM1, (B) NOD2, and (C) PTPN2. Data represent means and standard deviations (n = 5). PBMC from healthy controls (HC, black bars, homozygous for the T allele, n = 5), or from IBD patients homozygous for the T allele (TT, blue bars, n = 6) or heterozygous (CT, red bars, n = 5) for the PTPN2 SNP rs1893217 were treated for 24 hours with 100 ng/mL IFN-γ and/or 10 µM spermidine. The graphs show mRNA levels of (D) ICAM1, (E) NOD2 and (F) PTPN2. Data represent means and standard deviations. Analysis of variance ANOVA followed by post hoc Tuckey; asterisks indicate significant differences between the treatment groups (*P < 0.05, **P < 0.01).
Discussion
Our data demonstrate that among several naturally occurring polyamines in the human body, spermidine was the most potent activator of PTPN2 and exerted anti-inflammatory effects in intestinal epithelial cells, monocytes, and patient-derived PBMCs. We further show that spermidine is a potent activator of PTPN2 and suppresses IFN-γ-induced pro-inflammatory signaling cascades not only in cancer cell lines but also in monocytes. Interestingly, we demonstrated that the activation of PTPN2 using spermidine was more effective in cells carrying the C-variant in SNP rs1893217, a variant that results in reduced PTPN2 activity and predisposes to IBD, which underlines the potential clinical relevance of our findings.
Among the investigated polyamines, besides spermidine, only putrescine and N1-acetylspermidine had a small impact on enzymatic activity of PTPN2 and, interestingly, exclusively upon presence of the C-variant. Because putrescine and N1-acetylspermidine are converted into spermidine within cells, the increase of PTPN2 activity upon administration of these two polyamines might either result from their physiologic conversion into spermidine, or they might have the capacity to activate PTPN2 themselves. However, further analysis would be necessary to clearly assess how these polyamines are converted into each other and which metabolites finally affect expression and activity of PTPN2. Notably, we observed a trend toward increased PTPN2 protein expression in spermidine, spermine, putrescine, and N8-acetylspermidine-treated IEC carrying the PTPN2 C-variant. This is partially in line with the studies performed by Penrose et al and our own previous work.26, 27 However in contrast to those studies, we did not observe an effect in PTPN2 WT cells, which might result from differences in experimental conditions (9 day trans-well cultures by Penrose et al vs 24 h in our study) and the cell type (THP-1 monocytes/PBMCs vs IEC cell lines).
Consistent with previous reports,26, 27 our data demonstrate that activation of PTPN2 upon spermidine treatment resulted in reduction of IFN-γ-induced pro-inflammatory signaling in IECs and monocytes. Interferon-γ promoted phosphorylation of STAT1 and STAT3 molecules, which leads to their translocation to the cell nucleus and triggers the transcription of pro-inflammatory genes. Consistent with those previous studies,26, 27 IFN-γ-induced STAT1 and STAT3 phosphorylation was reduced in all cells treated with spermidine; and in line with reduced STAT1/3 activation, IFN-γ-induced mRNA expression of the pro-inflammatory genes ICAM-1, NOD2, and IFNG was reduced upon spermidine co-administration. Though these effects were expected based on previous reports,26, 27 we here show for the first time that presence of the C-variant affects the cellular response to spermidine treatment. In particular, we show that presence of the C-allele renders the cells more responsive to the anti-inflammatory effects of spermidine. It will be of interest to confirm the effect of the C-variant for spermidine-mediated PTPN2 activation using CRISPR-Cas9 technologies to generate subclones from the very same cell line that are either homozygous for the T-allele, heterozygous, or homozygous for the C-allele.
Among patients from the Swiss IBD Cohort, about 30% are heterozygous PTPN2 SNP rs1893217 C-variant carriers.37 We have previously shown that presence of the C-variant leads to the development of a more severe disease course but also to a better response to treatment with anti-TNF antibodies.37 Here we demonstrated that in IFN-γ challenged cells expressing the PTPN2 C-variant, phosphorylation levels of STAT1 and STAT3 molecules were more pronounced than in cells expressing the T-variant, and a similar effect was observed when addressing mRNA levels of IFN-γ target genes, such as ICAM1, NOD2, and IFNG. This indicates that cells expressing the C-variant seem to be inherently more responsive to IFN-γ treatment, which is in line with previous reports demonstrating that presence of this variant leads to reduced PTPN2 function.9 When we co-administered spermidine, STAT activation and expression of IFN-γ response genes were significantly reduced, and these effects were again more pronounced in cells expressing the C-allele. These results clearly indicate that presence of SNP rs1893217 might be a good treatment predictor for spermidine supplementation.
In summary, our results show that among the polyamines of the putrescine-spermine pathway, spermidine is the most potent inducer of PTPN2 activation. Further, the anti-inflammatory effect of spermidine is more pronounced when SNP rs1893217 C-variant is present. This implicates that spermidine treatment may represent a novel therapeutic approach for IBD and that its application might be especially successful in patients carrying the PTPN2 C-variant, suggesting that SNP rs1893217 could serve as a biomarker for therapy response. However, additional, more patient-focused studies will be needed to confirm the in vivo anti-inflammatory potential of spermidine. A major concern regarding polyamine and especially spermidine supplementation is the role of spermidine in promoting cell growth and proliferation.38 Though enhancing cell growth during wound healing combined with anti-inflammatory effects is clearly beneficial for the treatment of chronic inflammatory diseases, ongoing use past the point of mucosal healing might potentially elevate the risk of prolonged proliferation, contributing to the onset of colorectal cancer. Therefore, further studies investigating the effect of spermidine during cancer development are needed to clarify the safety and the appropriate timing and duration of spermidine supplementation. Notably, long-term spermidine treatment prolonged the lifespan in several species without evidence of increased aberrant cell proliferation or the emergence of tumors,39, 40 and it induced cell death in cervical cancer cells,41 indicating that the growth-promoting and anti-apoptotic effect of spermidine is context-dependent, and its anti-inflammatory potential might be of more importance than the growth-promoting effect. Because IBD is not the only disease in which genetically mediated PTPN2 dysfunction might contribute to disease onset,6, 16 PTPN2 activation using spermidine might also open novel treatment options for patients suffering from other inflammatory disorders, including type 1 diabetes, rheumatoid arthritis, and multiple sclerosis.
Supplementary Material
Author Contribution: AN and MB contributed to performing experiments, data analysis and interpretation, and writing the first draft of the manuscript. SM, KB, LvdL, SL, and CG contributed to performing experiments and data analysis. MS and MRS contributed to conceived, designed, and supervised the study. All authors wrote, corrected, and approved the manuscript.
Supported by: This work was supported by grants from the Swiss National Science Foundation to MS (grant no. 314730-146204, grant no. 314730_166381 and grant no. CRSII3_154488/1) and to MRS (project no. P300PB_177932), the Crohn’s Colitis Foundation Litwin IBD Pioneer Program to MS, and the Stiftung Experimentelle Biomedizin to MS. The funding institutions had no role in study design and data interpretation.
References
- 1. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. [DOI] [PubMed] [Google Scholar]
- 2. Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gomollón F, Dignass A, Annese V, et al. ; ECCO 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 4. Lees CW, Barrett JC, Parkes M, et al. . New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. [DOI] [PubMed] [Google Scholar]
- 5. Jostins L, Ripke S, Weersma RK, et al. . Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wellcome Trust Case Control C. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Lange KM, Moutsianas L, Lee JC, et al. . Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franke A, Balschun T, Karlsen TH, et al. ; IBSEN study group Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. [DOI] [PubMed] [Google Scholar]
- 9. Scharl M, Mwinyi J, Fischbeck A, et al. . Crohn’s disease-associated polymorphism within the PTPN2 gene affects muramyl-dipeptide-induced cytokine secretion and autophagy. Inflamm Bowel Dis. 2012;18:900–912. [DOI] [PubMed] [Google Scholar]
- 10. Sharp RC, Beg SA, Naser SA. Role of PTPN2/22 polymorphisms in pathophysiology of Crohn’s disease. World J Gastroenterol. 2018;24:657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spalinger MR, McCole DF, Rogler G, et al. . Protein tyrosine phosphatase non-receptor type 2 and inflammatory bowel disease. World J Gastroenterol. 2016;22:1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glas J, Wagner J, Seiderer J, et al. . PTPN2 gene variants are associated with susceptibility to both Crohn’s disease and ulcerative colitis supporting a common genetic disease background. Plos One. 2012;7:e33682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson SD, Sudman M, Ramos PS, et al. . The susceptibility loci juvenile idiopathic arthritis shares with other autoimmune diseases extend to PTPN2, COG6, and ANGPT1. Arthritis Rheum. 2010;62:3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Todd JA, Walker NM, Cooper JD, et al. ; Genetics of Type 1 Diabetes in Finland; Wellcome Trust Case Control Consortium Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smyth DJ, Plagnol V, Walker NM, et al. . Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359:2767–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cerosaletti K, Schneider A, Schwedhelm K, et al. . Multiple autoimmune-associated variants confer decreased IL-2R signaling in CD4+ CD25(hi) T cells of type 1 diabetic and multiple sclerosis patients. Plos One. 2013;8:e83811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scharl M, Hruz P, McCole DF. Protein tyrosine phosphatase non-receptor Type 2 regulates IFN-γ-induced cytokine signaling in THP-1 monocytes. Inflamm Bowel Dis. 2010;16:2055–2064. [DOI] [PubMed] [Google Scholar]
- 18. Scharl M, McCole DF, Weber A, et al. . Protein tyrosine phosphatase N2 regulates TNFα-induced signalling and cytokine secretion in human intestinal epithelial cells. Gut. 2011;60:189–197. [DOI] [PubMed] [Google Scholar]
- 19. Scharl M, Paul G, Weber A, et al. . Protection of epithelial barrier function by the Crohn’s disease associated gene protein tyrosine phosphatase n2. Gastroenterology. 2009;137:2030–2040.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spalinger MR, Kasper S, Chassard C, et al. . PTPN2 controls differentiation of CD4⁺ T cells and limits intestinal inflammation and intestinal dysbiosis. Mucosal Immunol. 2015;8:918–929. [DOI] [PubMed] [Google Scholar]
- 21. ten Hoeve J, de Jesus Ibarra-Sanchez M, Fu Y, et al. . Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol Cell Biol. 2002;22:5662–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simoncic PD, Lee-Loy A, Barber DL, et al. . The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol. 2002;12:446–453. [DOI] [PubMed] [Google Scholar]
- 23. Tonks NK Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. [DOI] [PubMed] [Google Scholar]
- 24. Mattila E, Pellinen T, Nevo J, et al. . Negative regulation of EGFR signalling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase TCPTP. Nat Cell Biol. 2005;7:78–85. [DOI] [PubMed] [Google Scholar]
- 25. van Vliet C, Bukczynska PE, Puryer MA, et al. . Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat Immunol. 2005;6:253–260. [DOI] [PubMed] [Google Scholar]
- 26. Morón B, Spalinger M, Kasper S, et al. . Activation of protein tyrosine phosphatase non-receptor type 2 by spermidine exerts anti-inflammatory effects in human THP-1 monocytes and in a mouse model of acute colitis. PLoS One. 2013;8:e73703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Penrose HM, Marchelletta RR, Krishnan M, et al. . Spermidine stimulates T cell protein-tyrosine phosphatase-mediated protection of intestinal epithelial barrier function. J Biol Chem. 2013;288:32651–32662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mattila E, Marttila H, Sahlberg N, et al. . Inhibition of receptor tyrosine kinase signalling by small molecule agonist of T-cell protein tyrosine phosphatase. BMC Cancer. 2010;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pegg AE Functions of polyamines in mammals. J Biol Chem. 2016;291:14904–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madeo F, Eisenberg T, Pietrocola F, et al. . Spermidine in health and disease. Science. 2018;359. [DOI] [PubMed] [Google Scholar]
- 31. Sugiyama Y, Nara M, Sakanaka M, et al. . Analysis of polyamine biosynthetic- and transport ability of human indigenous Bifidobacterium. Biosci Biotechnol Biochem. 2018;82:1606–1614. [DOI] [PubMed] [Google Scholar]
- 32. Bardócz S, Duguid TJ, Brown DS, et al. . The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73:819–828. [DOI] [PubMed] [Google Scholar]
- 33. Pegg AE Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weiss TS, Herfarth H, Obermeier F, et al. . Intracellular polyamine levels of intestinal epithelial cells in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:529–535. [DOI] [PubMed] [Google Scholar]
- 35. Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. [DOI] [PubMed] [Google Scholar]
- 36. Kwon DH, Lu CD. Polyamine effects on antibiotic susceptibility in bacteria. Antimicrob Agents Chemother. 2007;51:2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spalinger MR, Voegelin M, Biedermann L, et al. ; Swiss IBD Cohort Study Group The clinical relevance of the IBD-associated variation within the risk gene locus encoding protein tyrosine phosphatase non-receptor type 2 in patients of the Swiss IBD cohort. Digestion. 2016;93:182–192. [DOI] [PubMed] [Google Scholar]
- 38. Casero RA Jr, Murray Stewart T, Pegg AE. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat Rev Cancer. 2018;18:681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eisenberg T, Abdellatif M, Schroeder S, et al. . Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22: 1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Madeo F, Bauer MA, Carmona-Gutierrez D, et al. . Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy. 2019;15:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen Y, Zhuang H, Chen X, et al. . Spermidine‑induced growth inhibition and apoptosis via autophagic activation in cervical cancer. Oncol Rep. 2018;39:2845–2854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.