Abstract
Background
Metformin is proposed to have chemopreventive effect of various cancer currently. However, the anti-cancer effect of metformin for diabetic patients with hepatocellular carcinoma (HCC) undergoing liver resection remains unclear. The aim of our cohort study was to assess whether metformin influence the recurrence of HCC.
Methods
We retrospectively enrolled 857 HCC patients who received primary resection from April 2001 to June 2016. 222 patients were diagnosed with diabetes mellitus (DM) from medical record. Factors influence the overall survival (OS) and recurrence-free survival (RFS) were analyzed by multivariate analysis.
Results
During the follow-up period (mean, 75 months), 471 (54.9%) patients experienced recurrence, and 158 (18.4%) patients died. Multivariate analysis revealed that DM (p = 0.015), elevated AST (p = 0.006), hypoalbuminemia (p = 0.003), tumor number (p = 0.001), tumor size (p < 0.001), vascular invasion (p <0.001), high Ishak fibrosis score (p <0.001), hepatitis B (p = 0.014), hepatitis C (p = 0.001) were independent predictors for RFS. In diabetic patients, only HbA1c>9% (p = 0.033), hypoalbuminemia (p = 0.030) and vascular invasion (p = 0.001) were independent risk factors for HCC recurrence; but the metformin use revealed no significance on recurrence. DM is a risk factor of HCC recurrence after resection. Adequate DM control can reduce the recurrence of HCC. However, the use of metformin does not reduce the risk of HCC recurrence in diabetic patient after initial resection. Hence, metformin may not have protective influences on HCC recurrence in diabetic patients who undergo initial liver resection.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide nowadays, and its incidence is approximately 850,000 new cases per year [1–3]. HCC is often considered to be linked to multiple risk factors, such as infections with hepatitis B virus (HBV) or hepatitis C virus (HCV), alcohol abuse, and metabolic syndrome [4]. Metabolic factor such as obesity and diabetes are associated with increased mortality rates of several cancers [5, 6] and diabetes is also reported as a risk factor for liver, pancreatic, renal, and colon cancers [7, 8]. Therefore, therapeutic intervention for diabetes may lead to prevention of HCC recurrence and may improve survival of diabetic HCC patient after hepatectomy.
Metformin is one of the most frequently prescribed antihyperglycemic drugs and is used as the first-line therapy for type 2 diabetes mellitus (T2DM) in Taiwan. Many previous studies have showed an anticancer effect from metformin in several cancer types with T2DM comorbidity [9, 10]. Patient received curative hepatectomy might ask whether metformin can prevent the recurrence of HCC and have better outcomes. However, the anticancer effect has not been noted in all cancers and remains controversial. Little is known for the anticancer effects of metformin on HCC recurrence and mortality recently.
We therefore evaluated the associations between metformin use and the risk of HCC recurrence and mortality among diabetic patients with HCC after curative resection.
Patients and methods
Patients
We reviewed a total of 2103 patients who were diagnosed with HCC and underwent surgical resection between January 2001 and June 2016 at Kaohsiung Chang Gung Memorial Hospital. This hospital is a tertiary referral center that covers the southern part of Taiwan. We excluded 234 patients with prior HCC treatment, 918 patients with BCLC stage B or C, 94 patients received liver transplantation after resection. Finally, we recruited 857 patients with BCLC stage 0 or A HCC who underwent primary curative resection. Among them, 222 patients were diagnosed with DM from medical record, and 136 patients used metformin as anti-DM treatment (Fig 1). This study complies with the standards of the Declaration of Helsinki and current ethical guidelines, and approval was obtained from the Ethics Committee of Chang Gung Memorial Hospital. The requirement for informed consent was waived by the IRB (IRB number: 201901103B0).
Fig 1. Patient selection flow diagram.
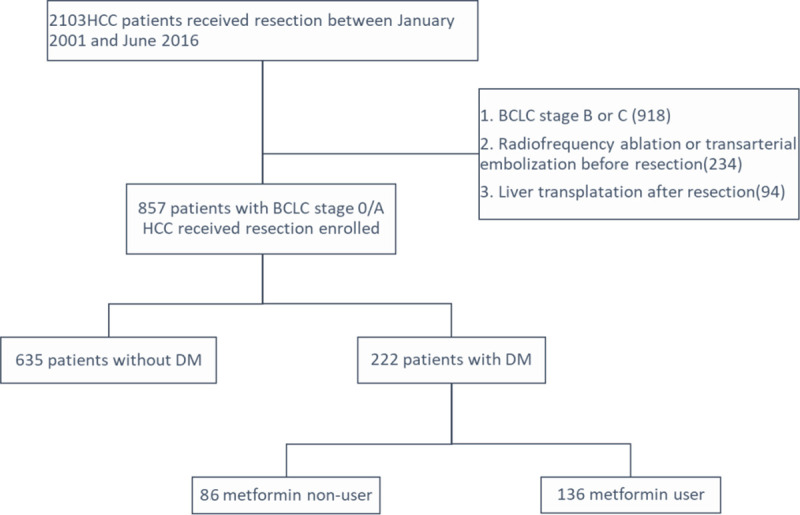
HCC was defined according to the results of imaging studies and biochemical assays, and the diagnosis was confirmed using histopathology. The HCC diagnosis was based on the criteria of the practice guidelines of the European Association for the Study of the Liver (EASL) or the American Association for the Study of Liver Disease (AASLD) [11, 12]. Patients were included in the T2DM group if they had ≧1 diagnosis of T2DM as noted by an ICD-10 code in the medical record or the usage of anti-diabetic medication for more than 3 months.
Drug exposure
Drug exposure was defined as receiving OHAs in the same class for at least three months during the follow-up period. All patients treated with metformin were categorized as ‘‘metformin users,” whereas use of other drugs including sulfonylurea, thiazolidinedione, insulin or other OHAs were categorized as ‘‘non-metformin users.” In patients treated with combination therapies, those prescribed metformin for more than 3 months were categorized as metformin users.
Assessments and follow-up evaluation
The baseline demographics, serum biochemistry, tumor burden and anti-DM therapy were comprehensively recorded before any forms of definite treatment. The diagnosis of cirrhosis, grade of steatosis and Ishak fibrosis score were documented by resected non-tumor pathologic report. The HCC stage was defined according to the Barcelona Clinic Liver Cancer (BCLC) guidelines. Tumor differentiation was determined using the Edmondson grading system. The follow-up ended on November 30, 2019. OS was defined as the interval between the dates of surgery and death or last observation. Patients were followed up at the 1st month after liver resection, followed by every 3 months in the first year and every 3–6 months in subsequent years. Routine tests such as serum AFP levels, serum biochemistry, and abdominal ultrasound were performed at every follow-up. Liver computed tomography or magnetic resonance image were performed at the 1st month after liver section and every 12 months or recurrence was suspected clinically.
Statistical analysis
Statistical analyses were performed using IDM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Experimental values of non-continuous variables are expressed as the median ± interquartile range (IQR). The chi-squared test is used as appropriate to evaluate the significance of differences in data and multiple comparison in groups. The relationship between recurrence-free survival (RFS), OS were analyzed using Kaplan–Meier survival curves and the log-rank test, and p<0.050 was considered statistically significant. Factors that were significant in the univariate analysis (p <0.05) were included in a multivariate analysis using a Cox forward stepwise variable selection process of the estimated OS and RFS.
Results
Baseline characteristics of the study patients
Table 1 presents the baseline characteristics of the study cohort. The mean follow-up time was 75 months. The sample comprised 670 men and 187 women, and the median age was 60 years at enrollment. As shown in Table 1, 635(74%) patients are non-diabetic and 222(26%) patients are diabetic. Compared to patients without DM, patients with DM were significantly older (p <0.001), lower serum bilirubin at baseline (p <0.001), higher prevalence of hypertension (p <0.001), higher BMI (p <0.001), higher grade of steatosis (p = 0.003), higher prevalence of HCV infection(p = 0.005), lower percentage of HBV infection (p< 0.001) but had a higher percentage of recurrence (p = 0.019). Overall, patients with DM had higher rates of death (28.4%) than subjects without DM (14.9%, p < 0.001).
Table 1. Comparison of clinical and pathological characteristics between patients with DM or not before hepatectomy.
| Total (n = 857) | DM (n = 222) | Non DM (n = 635) | P value | |
|---|---|---|---|---|
| Age (years; median, IQR) | 60(52~66) | 62(57~68) | 58.9(50~66) | <0.001 |
| Age (>60 years), n (%) | 449 (52.4%) | 146 (65.8%) | 303 (47.7%) | <0.001 |
| Male, n (%) | 670 (78.2%) | 170 (76.6%) | 500 (78.7%) | 0.502 |
| Bilirubin (g/dL; median, IQR) | 0.8(0.6~1.0) | 0.7(0.5~0.9) | 0.8(0.6~1) | <0.001 |
| Albumin (g/dL; median, IQR) | 3.7(3.2~4.09) | 3.66(3.10~4.09) | 3.70(3.30~4.09) | 0.177 |
| AST (U/L; median, IQR) | 35(26~51) | 35(27~52.5) | 34(26~50) | 0.316 |
| ALT (U/L; median, IQR) | 37(26~61) | 37(25~64) | 37(26~59) | 0.714 |
| Creatinine (mg/dL; median, IQR) | 0.86(0.73~1.02) | 0.87(0.7~1.1) | 0.86(0.74~1) | 0.228 |
| AFP (>200ng/mL), n (%) | 158 (18.4%) | 37 (16.7%) | 121 (19.1%) | 0.462 |
| Liver cirrhosis, n (%) | 400 (46.7%) | 115 (51.8%) | 285 (44.9%) | 0.075 |
| Tumor size (>2cm), n (%) | 643 (75%) | 175 (78.8%) | 468 (73.7%) | 0.137 |
| Tumor number (single: multiple) | 780: 77 | 199: 23 | 581: 54 | 0.405 |
| Child-Pugh grade (A: B) | 786: 71 | 201: 21 | 585: 50 | 0.461 |
| Micro/Macrovascular invasion, n (%) | 320 (37.3%) | 89 (40.1%) | 231 (36.4%) | 0.325 |
| Histological grade (well: moderate: poor) | 110: 714: 22 | 22: 189: 9 | 88: 525: 13 | 0.265 |
| Hypertension, n (%) | 321(37.5%) | 137(61.7%) | 184(28.9%) | <0.001 |
| Smoking, n (%) | 242(28.2%) | 65(29.3%) | 177(27.9%) | 0.699 |
| BMI | 24.4(22.2~26.8) | 25.39(22.83~28) | 24.16(22.04~26.28) | <0.001 |
| the grade of steatosis (<5%:5–33%: >33%) | 405:307:39 | 91:107:11 | 314:200:28 | 0.003 |
| Ishak fibrosis score (0–3:4–6) | 273:419 | 69:119 | 204:300 | 0.366 |
| HBV, n% | 484(56.5%) | 95(42.8%) | 389(61.3%) | <0.001 |
| HCV, n% | 300(35%) | 95(42.8%) | 205(32.3%) | 0.005 |
| Recurrence, n (%) | 471 (55%) | 137 (61.7%) | 334 (52.6%) | 0.019 |
| Death, n (%) | 158 (18.4%) | 63 (28.4%) | 95 (14.9%) | <0.001 |
AFP = α-fetoprotein
The chemopreventive effect of medication in diabetic patients with HCC after curative resection
Among those who with diabetic, 136 patients are metformin users and eighty-six are non-metformin users. Kaplan-Meier analysis reveals no statistically significant in overall survival and recurrence free survival between metformin group and non-metformin group (Fig 2A and 2B). Poor DM control (defined as HbA1C> 9%) would lead to higher recurrence rate of HCCs (p = 0.011) and a trend of poor overall survival (p = 0.142) (Fig 3A and 3B).
Fig 2.
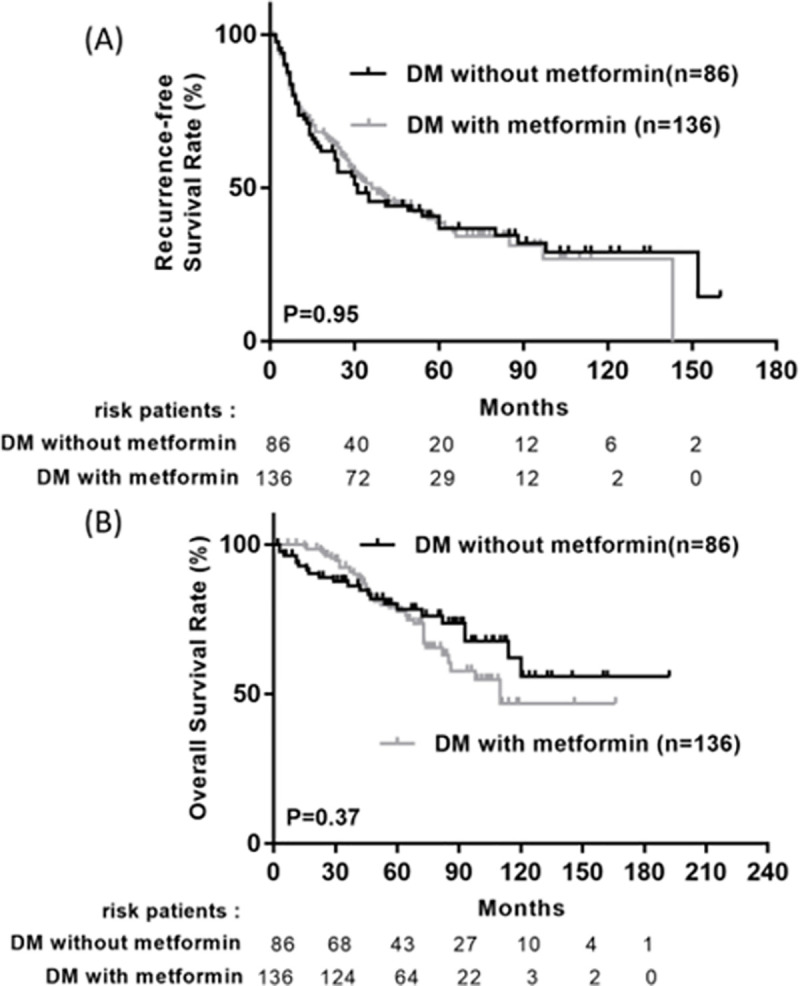
Cumulative recurrence-free survival (A) and overall survival (B) between patients with and without metformin use in diabetic patients.
Fig 3.
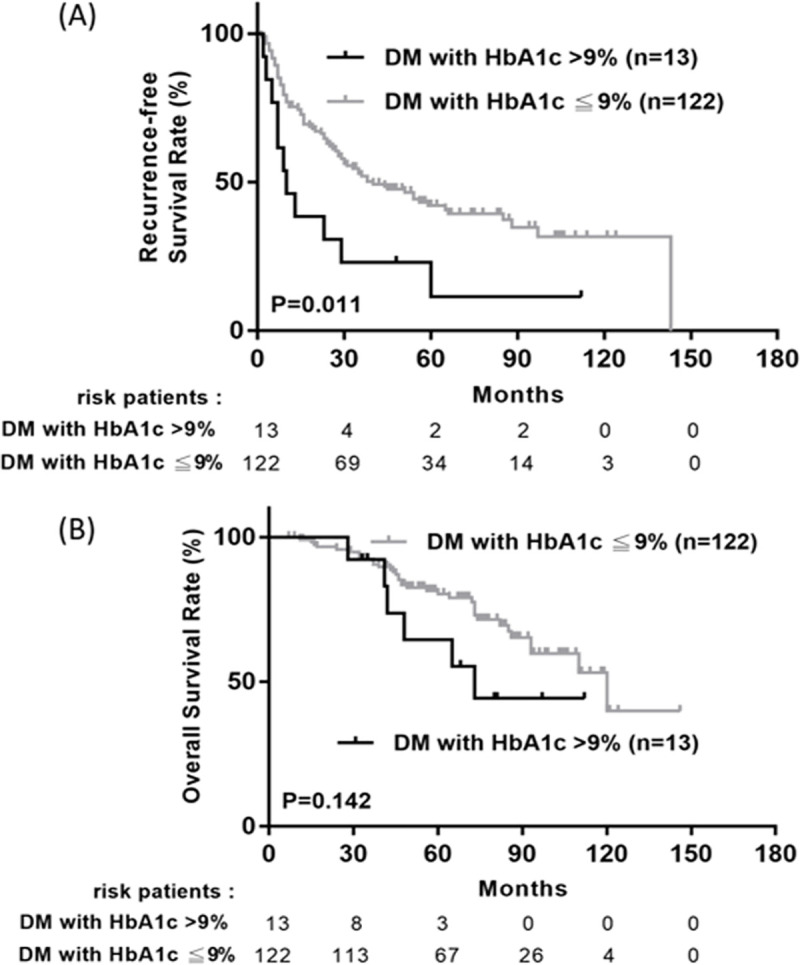
Cumulative recurrence-free survival (A) and overall survival (B) between diabetic with HbA1c>9% and those with HbA1C≦9%.
Independent factors for HCC recurrence
As shown in Table 2, age, diabetes mellitus (DM), hypertension, AST, ALT, thrombocytopenia, hypoalbuminemia, liver cirrhosis, Child Pugh grade, tumor number, tumor size, histology stage, vascular invasion, higher Ishak fibrosis score, hepatitis C were significantly associated with HCC recurrence in univariate analysis. In the multivariate analysis, DM (hazard ratio [12], 1.357; 95% CI, 1.061–1.735; p = 0.015), AST>40 (hazard ratio [12], 1.356; 95% CI, 1.090–1.687; p = 0.006), albumin≦3 (HR, 1.460; 95% CI, 1.140–1.869; p = 0.003), tumor number >1 (HR, 1.731; 95% CI, 1.236–2.425; p = 0.001), tumor size (HR, 1.298; 95% CI, 1.170–1.441; p < 0.001), and vascular invasion (HR, 1.631; 95% CI, 1.302–2.041; p < 0.001), Ishak fibrosis score >3 (HR, 1.730; 95% CI, 1.356–2.208; p < 0.001), hepatitis B (HR, 1.561; 95% CI, 1.093–2.229; p = 0.014), hepatitis C (HR, 1.866; 95% CI, 1.308–2.662; p = 0.001) remained independent predictive factors for RFS. The overall survival and RFS were significantly higher among patient without diabetic compared with T2DM patients during the follow-up period (Fig 4A and 4B).
Table 2. Univariate and multivariate analyses for recurrence in BCLC 0/A patients with HCC after curative hepatectomy.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | Comparison | N (%) | HR (95%CI) | P value | HR (95%CI) | P value |
| Age (years) | > 60 | 449 (52.4) | 1.333 (1.110–1.599) | 0.002 | ||
| ≦ 60 | 408 (47.6) | |||||
| Sex | Male | 670 (78.2) | 1.012 (0.812–1.263) | 0.913 | ||
| Female | 187 (21.8) | |||||
| DM | Yes | 222 (25.9) | 1.449 (1.186–1.770) | <0.001 | 1.357 (1.061–1.735) | 0.015 |
| No | 635 (74.1) | |||||
| Hypertension | Yes | 321 (37.5) | 1.350 (1.072–1.171) | 0.011 | ||
| No | 536 (62.5) | |||||
| Smoking | Yes | 242 (28.2) | 1.149 (0.864–1.528) | 0.340 | ||
| No | 615 (71.8) | |||||
| Alcohol | Yes | 182 (21.2) | 0.948 (0.691–1.300) | 0.741 | ||
| No | 675 (78.8) | |||||
| BMI | >25 | 360 (43.5) | 1.033 (0.821–1.299) | 0.784 | ||
| ≦25 | 468 (56.5) | |||||
| AST(U/L) | >40 | 320 (37.4) | 1.727 (1.439–2.072) | <0.001 | 1.356 (1.090–1.687) | 0.006 |
| ≦40 | 536 (62.6) | |||||
| ALT (U/L) | >40 | 372 (43.7) | 1.469 (1.225–1.762) | <0.001 | ||
| ≦40 | 479 (56.3) | |||||
| Total bilirubin | per mg/dL | 0.986 (0.604–1.610) | 0.956 | |||
| Serum creatinine | per mg/dL | 0.978 (0.912–1.049) | 0.533 | |||
| AFP (ng/mL) | > 200 | 158 (19) | 1.053 (0.829–1.337) | 0.673 | ||
| ≦ 200 | 674 (81) | |||||
| Platelet (109/L) | ≦ 150 | 439 (52) | 1.515 (1.261–1.819) | <0.001 | ||
| >150 | 406 (48) | |||||
| Albumin (g/dL) | ≦3 | 160 (18.8) | 1.770 (1.425–2.199) | <0.001 | 1.460 (1.140–1.869) | 0.003 |
| >3 | 689 (81.2) | |||||
| Liver cirrhosis | Yes | 400 (46.7) | 1.673 (1.395–2.007) | <0.001 | ||
| No | 457 (53.3) | |||||
| Child-Pugh grade | B | 71 (8.3) | 1.409 (1.002–1.943) | 0.036 | ||
| A | 786 (91.7) | |||||
| Tumor no. | Multiple | 77 (9) | 1.727 (1.306–2.284) | <0.001 | 1.731 (1.236–2.425) | 0.001 |
| Single | 780 991) | |||||
| Tumor size (cm) | per centimeter | 1.245 (1.155–1.800) | 0.001 | 1.298 (1.170–1.441) | <0.001 | |
| Histology stages | poor | 22 (2.6) | 2.561 (1.598–4.106) | <0.001 | ||
| well +moderate | 824 (97.4) | |||||
| Vascular invasion* | Yes | 320 (37.3) | 1.582 (1.314–1.903) | <0.001 | 1.631 (1.302–2.041) | <0.001 |
| No | 537 (62.7) | |||||
| Grade of steatosis | <5% (Reference) | 405 (53.9) | ||||
| 5–33% | 307 (40.9) | 1.129 (0.895–1.425) | 0.307 | |||
| >33% | 39 (5.2) | 0.895 (0.502–1.596) | 0.707 | |||
| Ishak fibrosis score | 4–6 | 419 (60.5) | 1.787 (1.416–2.256) | <0.001 | 1.730 (1.356–2.208) | <0.001 |
| 0–3 | 273 (39.5) | |||||
| Hepatitis B | Yes | 484 (56.5) | 1.148 (0.957–1.377) | 0.136 | 1.561 (1.093–2.229) | 0.014 |
| No | 373 (43.5) | |||||
| Hepatitis C | Yes | 300 (35) | 1.422 (1.182–1.711) | <0.001 | 1.866 (1.308–2.662) | 0.001 |
| No | 557 (65) | |||||
| Surgery | minor | 602 (70.2) | 0.889 (1.689–1.147) | 0.364 | ||
| major | 255 (29.8) | |||||
| Conventional | 757 (88.3) | 0.728 (0.518–1.023) | 0.067 | |||
| laparoscopic | 100 (11.7) | |||||
*2 patients are macrovascular invasion.
Fig 4. Long-term outcomes between patients with and without diabetes mellitus.
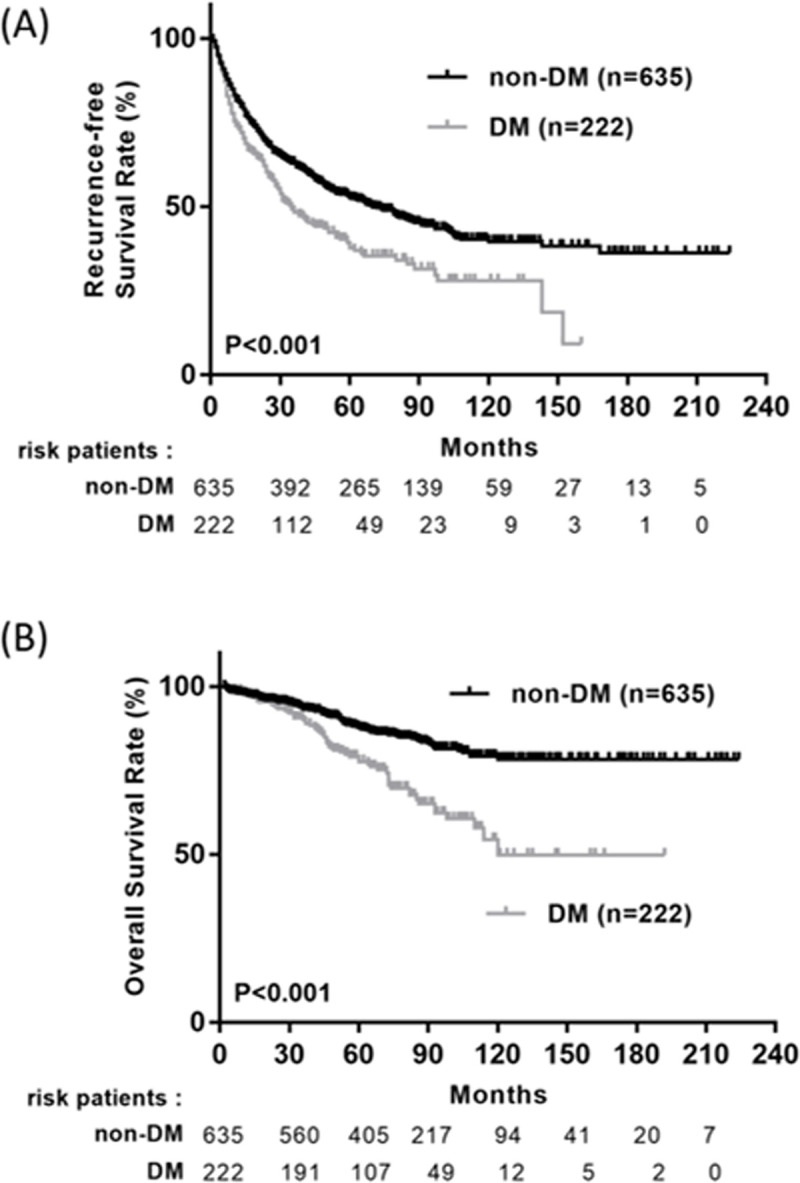
(A) Recurrence-free survival; (B) Overall survival.
In subgroup of diabetic patients, higher HbA1c, AST>40(U/L), ALT>40(U/L), thrombocytopenia, hypoalbuminemia, liver cirrhosis and vascular invasion were significantly associated with RFS in univariate analysis. In the multivariate analysis, HbA1C >9% (HR, 2.095; 95% CI, 1.061–4.139; p = 0.033), hypoalbuminemia (HR, 1.883; 95% CI, 1.065–3.330; p = 0.030), vascular invasion (HR, 2.586; 95% CI, 1.473–4.537; p = 0.001) remained independent predictive factors for RFS (Table 3).
Table 3. Univariate and multivariate analysis for recurrence in BCLC 0/A patients with DM after curative hepatectomy.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | Comparison | N (%) | HR (95%CI) | P value | HR (95%CI) | P value |
| Age (years) | > 60 | 146 (65.8) | 1.173 (0.830–1.658) | 0.365 | ||
| ≦ 60 | 76 (34.2) | |||||
| Sex | Male | 170 (76.6) | 1.011 (0.676–1.513) | 0.957 | ||
| Female | 52 (23.4) | |||||
| HbA1C(%) | >9 | 13 (9.6) | 2.220 (1.174–4.196) | <0.014 | 2.095 (1.061–4.139) | 0.033 |
| ≦ 9 | 122 (90.4) | |||||
| Metformin | Yes | 136 (61.3) | 0.991 (0.696–1.410) | 0.958 | ||
| No | 86 (38.7) | |||||
| Sulfonylurea | Yes | 103 (46.4) | 0.994 (0.708–1.395) | 0.971 | ||
| No | 119 (53.6) | |||||
| Insulin | Yes | 28 (12.6) | 1.591 (0.984–2.572) | 0.058 | ||
| No | 194 (87.4) | |||||
| Hypertension | Yes | 137 (60.9) | 1.186 (0.775–1.813) | 0.432 | ||
| No | 85 (39.1) | |||||
| Smoking | Yes | 65 (29.3) | 1.384 (0.847–2.261) | 0.195 | ||
| No | 157 (70.7) | |||||
| Alcohol | Yes | 54 (24.3) | 0.735 (0.423–1.278) | 0.276 | ||
| No | 168 (75.7) | |||||
| BMI (kg/m2) | >25 | 119 (54.3) | 0.829 (0.531–1.296) | 0.411 | ||
| ≦25 | 100 (45.7) | |||||
| AST(U/L) | >40 | 93 (40.1) | 1.899 (1.354–2.663) | <0.001 | ||
| ≦40 | 128 (59.9) | |||||
| ALT(U/L) | >40 | 101 (46.3) | 1.660 (1.181–2.335) | 0.004 | ||
| ≦40 | 117 (53.7) | |||||
| Total bilirubin | per mg/dL | 0.657 (0.348–1.238) | 0.194 | |||
| Serum creatinine | per mg/dL | 0.990 (0.805–1.219) | 0.928 | |||
| AFP (ng/mL) | > 200 | 37 (17.3) | 1.415 (0.908–2.204) | 0.125 | ||
| ≦ 200 | 177 (82.7) | |||||
| Platelet (109/L) | ≦ 150 | 110 (50) | 1.585 (1.126–2.232) | 0.008 | ||
| > 150 | 110 (50) | |||||
| Albumin (g/dL) | ≦3 | 47 (21.6) | 1.717(1.155–2.554) | 0.008 | 1.883 (1.065–3.330) | 0.030 |
| >3 | 171 (88.4) | |||||
| Liver cirrhosis | Yes | 115 (51.8) | 1.457 (1.038–2.045) | 0.029 | ||
| No | 107 (48.2) | |||||
| Child-Pugh grade | B | 21 (9.5) | 1.326 (0.732–2.403) | 0.352 | ||
| A | 201 (90.5) | |||||
| Tumor no. | Multiple | 23 (10.4) | 1.383 (0.831–2.301) | 0.212 | ||
| Single | 199 (89.6) | |||||
| Tumor size (cm) | per centimeter | 1.214 (0.796–1.851) | 0.368 | |||
| Histology stages | Poor | 9 (4.1) | 1.630 (0.760–3.493) | 0.209 | ||
| well + moderate | 211 (95.9) | |||||
| Vascular invasion | Yes | 89 (40.1) | 1.588 (1.130–2.232) | 0.008 | 2.586 (1.473–4.537) | 0.001 |
| No | 133 (59.9) | |||||
| Hepatitis B | Yes | 95 (42.8) | 1.371 (0.979–1.920) | 0.066 | ||
| No | 127 (57.2) | |||||
| Hepatitis C | Yes | 95 (42.8) | 1.061 (0.753–1.496) | 0.753 | ||
| No | 127 (57.2) | |||||
| Steatosis grade | <5% (Reference) | 91 (43.5) | ||||
| 5–33% | 107 (52.2) | 1.659 (1.042–2.643) | 0.033 | |||
| >33% | 11 (4.3) | 0.459 (0.107–1.971) | 0.295 | |||
| Ishak fibrosis score | 4–6 | 119 (63.3) | 1.297 (0.841–1.998) | 0.239 | ||
| 0–3 | 69 (36.7) | |||||
| Surgery | major | 59 (26.6) | 0.846 (0.508–1.411) | 0.522 | ||
| minor | 163 (73.4) | |||||
| Conventional | 186 (83.8) | 0.732 (0.421–1.271) | 0.267 | |||
| laparoscopic | 36 (16.2) | |||||
Patterns of recurrence
There are 471 patients with recurrence, 137 DM patients and 334 non-DM patients. The patterns of recurrence among DM patients vs. Non-DM patients are 115(83.9%) versus 273(81.7%) within the Milan criteria (p = 0.568); 133(97%) vs. 323(96.7%) with intrahepatic recurrence (p = 0.834) and 83(60.6%) vs. 193(57.8%) with early recurrence (p = 0.575). The recurrence patterns showed no statistically difference between DM and non-DM patients.
Among 137 DM patients with recurrence, we further divided them into the Metformin users (85 patients) vs. Non-Metformin users (52 patients). The recurrence patterns of these two groups are 70(82.4%) vs. 45(86.5%) within the Milan criteria (p = 0.517); 82(96.5%) vs. 51(98%) with intrahepatic recurrence (p = 0.588) and 48(56.5%) vs. 35(67.3%) with early recurrence (p = 0.208).
Independent factors for mortality
A total of 63 patients died during the follow-up period; 29 of them suffered from liver-related death: 26 died of HCC and 3 of complications associated with cirrhosis. Of the 34 patients with non-liver-related death, 25 died of sepsis, three of cardiovascular disease, two of malignancies other than HCC and four of DM complications. In the OS analysis, the multivariate Cox proportional hazards model revealed that insulin use (HR, 2.615; 95% CI, 1.424–4.804; p = 0.002), albumin≦3.5g/dL (HR, 2.215; 95% CI, 1.321–3.714; p = 0.003), vascular invasion (HR, 1.754; 95% CI, 1.066–2.886; p = 0.027) were independent risk factors associated with overall mortality. As regards to liver-related death, baseline AFP>200 (HR, 5.050; 95% CI, 1.694–15.056; p = 0.004) is poor independent factor.
Impact of DM and metformin on the outcomes of patients between patients with BCLC stage 0 and A
There are 124 patients categorized to BCLC stage 0 and 733 patients BCLC stage A. Kaplan-Meier plot of overall survival between BCLC 0 and A reveal p = 0.103 without statistically significance. However, the Kaplan-Meier plot of RFS between BCLC 0 and A showed there is statistically significance between these two groups with p<0.001 (S1 Fig). The BCLC 0 group had better RFS than BCLC A.
We further divided our study cohort into BCLC 0 and BCLC A. Kaplan-Meier plots revealed patients with DM had poor overall survival than those without DM in BCLC 0 group(p = 0.012), poor outcomes in RFS and OS in BCLC A (p< 0.001 and <0.001). Metformin wound not affect the outcomes in BCLC 0 and BCLC A (S2 and S3 Figs).
Discussion
Diabetes is associated with increased mortality rates of several cancers [5, 6] and reported as a risk factor for hepatocellular carcinoma [13]. Also, diabetic patients had higher recurrence rate and poor prognosis compared with those without DM after HCC treatment [14]. Therefore, whether DM management would be beneficial to the prognosis of HCC patients after curative hepatectomy is an important issue and needs further evaluation and studies.
Our study demonstrated that patients with diabetes mellitus have higher recurrence rate and poor overall survival rate after HCC resection compared with those without DM. The finding is identical to the result of Ikeda, Y. et al [14]. Diabetic patients have much more comorbidities, increased infection risk, difficult cell regeneration and wound healing, higher risk of cardiovascular events, weakened immune system and lead to poor overall survival. Also, hyperglycemia induces DNA damage and cytotoxicity, which contributed to carcinogenesis [15]. Furthermore, patients with noninsulin dependent diabetes mellitus are characterized by insulin resistance, compensatory hyperinsulinemia and increased growth factor production, which will interact with liver cells and stimulates mitogenesis or carcinogenesis [16, 17].
It is worth noting that in the present study, the use of Metformin is not significant in RFS and OS in diabetic HCC patients after curative resection (p>0.05) (Fig 3A and 3B). There is no statistically significant difference in the clinical and pathological characteristics between metformin and non-metformin user before received curative resection in our study cohort (S1 Table), including the level of glycohemoglobin (p = 0.627). Although many studies and systemic reviews showed the chemopreventive effect of metformin in several cancers as well as in HCC [18–22], some studies also demonstrated metformin doesn’t improve the survival in patients with hepatocellular carcinoma [23] and doesn’t reduce the risk of HCC in diabetic patients [24]. The reasons might be explained that although diabetes mellitus is a progressive disease accompanied by persistent chronic inflammation results from hyperglycemia or hyperinsulinemia, which play key roles in cancer cell activity, including its initiation, promotion, and progression [25], metformin can decrease insulin resistance but cannot directly reduce abnormal insulin secretion. In addition, hyperinsulinemia wound directly affect liver tissue and lead to the genesis of HCC but metformin wound not directly inhibit this pathway. Furthermore, DM results from chronic inflammation and can cause additional oxidative stress and lead to the HCC, but the anti-oxidative stress effect of metformin may be too weak to reverse this condition.
To show the dose-dependent relationship, we stratified the study population by metformin daily use level into three groups (non-users, 500–1000 mg, and >1000mg daily dose). The Kaplan-Meier survival analysis showed no statistically significances among non-users and different daily dosage of metformin use in RFS (p = 0.958) and OS (p = 0.355), respectively (S4 Fig). We further stratified the patients by overall metformin use levels into three groups (<90, 90–365, and >365 cDDD). Similarly, there were no significant differences in patients with different cDDD of metformin use in RFS (p = 0.284) as well as the OS (p = 0.606) (S5 Fig). This result implies that there was no dose-dependent relationship between the metformin use and HCC recurrence. However, such analysis was limited by the low number and heterogeneity of the study population. Thus, large, randomized trials in well-selected patients treated with different dosage are warranted to confirm the value of metformin in HCC recurrence.
We further divided our study cohort into BCLC 0 and BCLC A. DM was a poor factor for OS in BCLC 0 group(p = 0.012), and patients without DM had better RFS and OS in BCLC A (p< 0.001 and <0.001). Metformin wound not affect the outcomes in BCLC 0 and BCLC A. Patients in BCLC 0 group had better RFS (p< 0.001) than BCLC A. Therefore, a noninvasive diagnostic strategy to detect HCC at an early stage and to monitor HCC recurrence such as circulating tumor DNA (ctDNA) may provide better outcomes in early HCC patients.
The use of insulin is a risk factor for poor OS and RFS. We noticed the study group of insulin user had higher mortality rate in diabetic patient after HCC resection (p = 0.001). The insulin group had higher glycohemoglobin level (8.25% vs 6.7%, p = 0.013), higher mortality rate (50% vs 25.3%, p = 0.007) and lower albumin level (3.3 vs 3.7, p = 0.012), showed in S2 Table. There is no statistically significant difference in age, gender, liver cirrhosis, Child Pugh grade, tumor size, tumor recurrence between these two study groups. Hyperglycemia contributed to the environment of hyperinsulinemia and increased the demand of insulin for sugar control, which led to a vicious cycle.
Adequate blood sugar control is a good factor for diabetic HCC patients with BCLC 0/A received curative resection. In our present study, patients with poor DM control (HbA1c> 9%) have higher HCC recurrence rate (p = 0.011). On the contrary, patients with diabetes under adequate blood sugar control had no difference in HCC recurrence and mortality compared with those without DM. These results indicated that adequate management of hyperglycemia led to reduction in the risk of HCC recurrence and improvement of overall survival. Hyperglycemia and hyperinsulinemia cause a chronic inflammation condition and lead to the genesis of cancer cell. If we can well control the blood sugar of diabetic patient, which would not lead to vicious course of hyperinsulinemia, cause chronic inflammation and oxidative stress. Hosokawa et al emphasized that inadequate maintenance of blood glucose in diabetic patients is a significant risk factor for recurrence of HCC and for poor survival after curative RFA therapy [26]. Therefore, we suggested diabetic patient should focus on adequate blood sugar maintenance rather than craving for the chemopreventive effect of metformin in HCC. There may be several mechanisms involved in the relationship between hyperglycemia and HCC recurrence. In animal study [27], high sugar content diet leads to the greatest liver tumor incidence. Diet-induced postpradial hyperglycemia and hyperinsulinemia significantly correlated with tumor incidence. Hyperglycemia promotes cancer cell proliferation [28–30] through accelerated cell cycle progression or through the production of reactive oxygen species. Iwasaki et al. confirmed that high glucose alone, as well as in combination with pro-inflammatory cytokines, could stimulate the nuclear factor Kappa-B-mediated transcription in hepatocytes in vitro [31]. The results support our finding, sugar control is the key point to avoid HCC recurrence and overall survival instead of the chemopreventive effect of metformin in diabetic patients. Second, the insulin user’s HbA1c level is higher than non-insulin user, and difficult sugar control, more diabetic complications and shorter survival rate. Also, the use of insulin contributes to hyperinsulinemia and attributes to carcinogenesis. It is compatible with our result, insulin users had poor prognosis after curative hepatectomy.
There are 484 patients with hepatitis B virus infection and 264 patients received nucleos(t)ide analogue (NUC). Also, there are 300 patients with HCV infection and 123 patients received HCV treatment. Kaplan-Meier plots revealed the treatment of HBV and HCV wound lead to better outcomes in OS and RFS.
We further stratified our study cohort based on the viral infection status. Multivariate analysis also revealed the treatment of HBV (HR, 0.601; 95% CI, 0.441–0.818; p = 0.001) and HCV (HR, 0.467; 95% CI, 0.328–0.664; p<0.001) are good independent factor for RFS in each group, the same results as previous study [32–34].
In our study cohort, posthepatectomy liver failure (PHLF) was defined according to by the International Study Group of Liver Surgery (ISGLS) definition [35]. The rate of posthepatectomy liver failure among our study cohort was 6.2% (53/857). 41 patients (41/635, 6.5%) without DM had post hepatectomy liver failure, and 5.4% with the diagnosis of DM had post hepatectomy liver failure. The p value between DM and non-DM is 0.576. As for the subgroup of metformin user and non-metformin user, the metformin user group had higher rates of post hepatectomy liver failure, 8.1% (11/136) vs. 1.2% (1/86) with p value = 0.026.
There are some possible limitations in our study. First, it is not a prospective study. However, we believed that the bias was small because patients were followed by the same physicians throughout the course of disease, with clinical and laboratory assessment and HCC screening using ultrasonography every 3–6 months. Second, the prevalence of DM in Taiwan is 6.6%, whereas it is up to 12.3% or more in the population of the Western countries [36]. Moreover, the access to medical professionals of blood sugar control is easy and affordable in Taiwan but medication nonadherence remains must not to be ignored.
In conclusion, DM is a risk factor of HCC recurrence after resection. Adequate blood sugar control is associated with the prognosis of diabetic patients with BCLC 0/A HCC after curative resection. However, the use of metformin does not reduce the risk of HCC recurrence in diabetic cohort after initial resection. Hence, we suggested diabetic patient with HCC after resection should go on adequate diet and/or medication control for blood sugar maintenance rather than craving for the chemopreventive effect of metformin in HCC. Further prospective randomized controlled study is required to validate our observation.
Supporting information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank Chih-Yun Lin and the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work.
Abbreviations
- DM
diabetes mellitus
- HCC
hepatocellular carcinoma
- HbA1c
hemoglobin A1c
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. 10.1038/nrdp.2016.18 . [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. 10.3322/caac.21262 . [DOI] [PubMed] [Google Scholar]
- 3.Cho WR, Hung CH, Chen CH, Lin CC, Wang CC, Liu YW, et al. Ability of the post-operative ALBI grade to predict the outcomes of hepatocellular carcinoma after curative surgery. Sci Rep. 2020;10(1):7290. Epub 2020/05/01. 10.1038/s41598-020-64354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding XX, Zhu QG, Zhang SM, Guan L, Li T, Zhang L, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715–30. 10.18632/oncotarget.18382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. Epub 2003/04/25. 10.1056/NEJMoa021423 . [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. Epub 2008/02/19. 10.1016/S0140-6736(08)60269-X . [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91(6):542–7. Epub 1999/03/24. 10.1093/jnci/91.6.542 . [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166(17):1871–7. Epub 2006/09/27. 10.1001/archinte.166.17.1871 . [DOI] [PubMed] [Google Scholar]
- 9.Gandini S, Guerrieri-Gonzaga A, Puntoni M, Decensi A. Metformin and breast cancer risk. J Clin Oncol. 2013;31(7):973–4. Epub 2013/01/30. 10.1200/JCO.2012.46.3596 . [DOI] [PubMed] [Google Scholar]
- 10.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8(8):e71583. Epub 2013/08/13. 10.1371/journal.pone.0071583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36. Epub 2005/10/27. 10.1002/hep.20933 . [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–30. Epub 2001/10/11. 10.1016/s0168-8278(01)00130-1 . [DOI] [PubMed] [Google Scholar]
- 13.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–8. Epub 2004/02/06. 10.1053/j.gastro.2003.10.065 . [DOI] [PubMed] [Google Scholar]
- 14.Ikeda Y, Shimada M, Hasegawa H, Gion T, Kajiyama K, Shirabe K, et al. Prognosis of hepatocellular carcinoma with diabetes mellitus after hepatic resection. Hepatology. 1998;27(6):1567–71. Epub 1998/06/10. 10.1002/hep.510270615 . [DOI] [PubMed] [Google Scholar]
- 15.Lorenzi M, Montisano DF, Toledo S, Barrieux A. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest. 1986;77(1):322–5. Epub 1986/01/01. 10.1172/JCI112295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn CR, White MF. The insulin receptor and the molecular mechanism of insulin action. J Clin Invest. 1988;82(4):1151–6. Epub 1988/10/01. 10.1172/JCI113711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabor E. Tumor suppressor genes, growth factor genes, and oncogenes in hepatitis B virus-associated hepatocellular carcinoma. J Med Virol. 1994;42(4):357–65. Epub 1994/04/01. 10.1002/jmv.1890420406 . [DOI] [PubMed] [Google Scholar]
- 18.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62(4):606–15. Epub 2012/07/10. 10.1136/gutjnl-2011-301708 . [DOI] [PubMed] [Google Scholar]
- 19.Chan KM, Kuo CF, Hsu JT, Chiou MJ, Wang YC, Wu TH, et al. Metformin confers risk reduction for developing hepatocellular carcinoma recurrence after liver resection. Liver Int. 2017;37(3):434–41. Epub 2016/10/25. 10.1111/liv.13280 . [DOI] [PubMed] [Google Scholar]
- 20.Seo YS, Kim YJ, Kim MS, Suh KS, Kim SB, Han CJ, et al. Association of Metformin Use With Cancer-Specific Mortality in Hepatocellular Carcinoma After Curative Resection: A Nationwide Population-Based Study. Medicine (Baltimore). 2016;95(17):e3527. Epub 2016/04/29. 10.1097/MD.0000000000003527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26(5):858–65. Epub 2011/01/22. 10.1111/j.1440-1746.2011.06664.x . [DOI] [PubMed] [Google Scholar]
- 22.Fujita K, Iwama H, Miyoshi H, Tani J, Oura K, Tadokoro T, et al. Diabetes mellitus and metformin in hepatocellular carcinoma. World J Gastroenterol. 2016;22(27):6100–13. Epub 2016/07/29. 10.3748/wjg.v22.i27.6100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhat M, Chaiteerakij R, Harmsen WS, Schleck CD, Yang JD, Giama NH, et al. Metformin does not improve survival in patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20(42):15750–5. Epub 2014/11/18. 10.3748/wjg.v20.i42.15750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen HH, Lin MC, Muo CH, Yeh SY, Sung FC, Kao CH. Combination Therapy of Metformin and Statin May Decrease Hepatocellular Carcinoma Among Diabetic Patients in Asia. Medicine (Baltimore). 2015;94(24):e1013. Epub 2015/06/20. 10.1097/MD.0000000000001013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grotenhuis BA, Wijnhoven BP, van Lanschot JJ. Cancer stem cells and their potential implications for the treatment of solid tumors. J Surg Oncol. 2012;106(2):209–15. Epub 2012/03/01. 10.1002/jso.23069 . [DOI] [PubMed] [Google Scholar]
- 26.Hosokawa T, Kurosaki M, Tsuchiya K, Matsuda S, Muraoka M, Suzuki Y, et al. Hyperglycemia is a significant prognostic factor of hepatocellular carcinoma after curative therapy. World J Gastroenterol. 2013;19(2):249–57. Epub 2013/01/25. 10.3748/wjg.v19.i2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Healy ME, Lahiri S, Hargett SR, Chow JD, Byrne FL, Breen DS, et al. Dietary sugar intake increases liver tumor incidence in female mice. Sci Rep. 2016;6:22292. Epub 2016/03/01. 10.1038/srep22292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Ma Q, Li J. High glucose promotes cell proliferation and enhances GDNF and RET expression in pancreatic cancer cells. Mol Cell Biochem. 2011;347(1–2):95–101. Epub 2010/10/21. 10.1007/s11010-010-0617-0 . [DOI] [PubMed] [Google Scholar]
- 29.Okumura M, Yamamoto M, Sakuma H, Kojima T, Maruyama T, Jamali M, et al. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: reciprocal involvement of PKC-alpha and PPAR expression. Biochim Biophys Acta. 2002;1592(2):107–16. Epub 2002/10/16. 10.1016/s0167-4889(02)00276-8 . [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M, Patel NA, Taggart J, Sridhar R, Cooper DR. A shift from normal to high glucose levels stimulates cell proliferation in drug sensitive MCF-7 human breast cancer cells but not in multidrug resistant MCF-7/ADR cells which overproduce PKC-betaII. Int J Cancer. 1999;83(1):98–106. Epub 1999/08/17. . [DOI] [PubMed] [Google Scholar]
- 31.Shi DY, Xie FZ, Zhai C, Stern JS, Liu Y, Liu SL. The role of cellular oxidative stress in regulating glycolysis energy metabolism in hepatoma cells. Mol Cancer. 2009;8:32. Epub 2009/06/06. 10.1186/1476-4598-8-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. Jama. 2012;308(18):1906–14. Epub 2012/11/20. 10.1001/2012.jama.11975 . [DOI] [PubMed] [Google Scholar]
- 33.Wong GL, Tse YK, Chan HL, Yip TC, Tsoi KK, Wong VW. Oral nucleos(t)ide analogues reduce recurrence and death in chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2016;43(7):802–13. Epub 2016/02/05. 10.1111/apt.13548 . [DOI] [PubMed] [Google Scholar]
- 34.Lui FH, Moosvi Z, Patel A, Hussain S, Duong A, Duong J, et al. Decreased risk of hepatocellular carcinoma recurrence with direct-acting antivirals compared with no treatment for hepatitis C: a meta-analysis. Ann Gastroenterol. 2020;33(3):293–8. Epub 2020/05/10. 10.20524/aog.2020.0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713–24. Epub 2011/01/18. 10.1016/j.surg.2010.10.001 . [DOI] [PubMed] [Google Scholar]
- 36.International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels, Belgium: International Diabetes Federation; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.


