Abstract
Background
Chronic total occlusion (CTO) in a non-infarct-related artery (IRA) in patients with acute coronary syndrome (ACS) is associated with a poor prognosis. However, whether the prognostic impact of non-IRA CTO differs according to left ventricular ejection fraction (LVEF) is unclear.
Methods and results
A total of 2060 consecutive acute myocardial infarction (AMI) patients who underwent primary percutaneous coronary intervention (PCI) were classified into 2 groups according to their LVEF (reduced EF: LVEF < 50%, preserved EF: LVEF ≥ 50%) and further subdivided according to the presence of concomitant non-IRA CTO. In the reduced EF group, patients with CTO had a higher 1-year all-cause death rate (20.3% vs. 34.3%, P = 0.001) and major adverse cardiac event rate (MACE: 19.6% vs. 39.6%, P < 0.001) compared to those without CTO, but they were similar between patients with and without CTO in the preserved EF group. Non-IRA CTO was an independent predictor of all-cause death (HR 1.58, 95% CI 1.06–2.33, P = 0.02) and MACE (HR 1.67, 95% CI 1.14–2.46, P = 0.009) only in the reduced EF group. In addition, the outcomes of successful CTO-PCI seemed to be similar to those without CTO in the reduced EF group.
Conclusions
CTO in a non-IRA may contribute to a poor prognosis only in AMI patients with reduced LVEF.
Keywords: Left ventricular ejection fraction, Acute myocardial infarction, Chronic total occlusion, Prognosis
1. Introduction
Acute myocardial infarction (AMI) is one of the major causes of morbidity and mortality through the world. Several recent studies have highlighted a fall in acute and long-term mortality following ST-elevation myocardial infarction (STEMI) in parallel with greater use of reperfusion therapy, primary percutaneous coronary intervention (PCI), modern antithrombotic therapy, and secondary prevention [1], [2]. However, the mortality of STEMI has not yet improved to a satisfactory level, with 1-year mortality of approximately 7–12% in Western countries and Japanese registries [3], [4], [5], [6], [7]. Reduced LVEF is well known to be associated with high AMI mortality, and it is associated with increased cardiovascular risk in the early and long-term periods [8], [9]. On the other hand, chronic total occlusion (CTO) in a non-infarct-related artery (IRA) in patients with acute coronary syndrome (ACS) is associated with a poor prognosis, as reported in several recent studies of ACS [10], [11], [12], [13], [14]. However, there are limited data on whether the prognostic impact of non-IRA CTO differs according to left ventricular ejection fraction (LVEF). Accordingly, the purpose of this study was to clarify the effect of LVEF on the prognostic impact of non-IRA CTO in patients with AMI and to evaluate whether staged PCI of the non-IRA CTO improves patient outcomes.
2. Methods
2.1. Study population
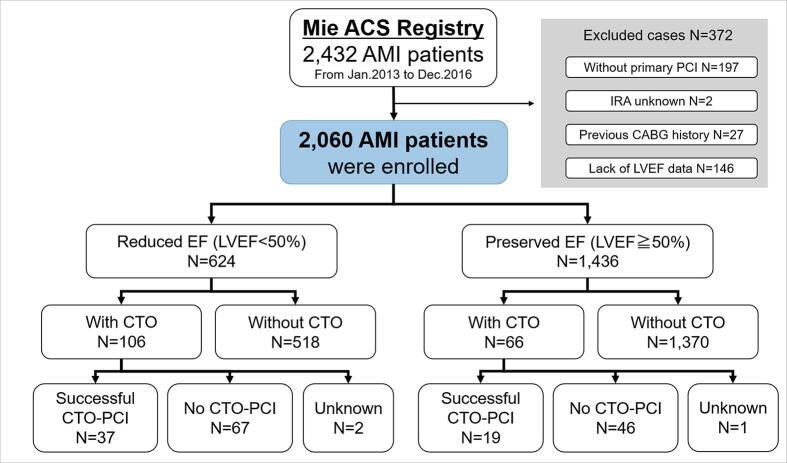
The Mie ACS Registry is a prospective, ongoing, multicenter registry in Japan [15], [16] (https://upload.umin.ac.jp/cgi-openbin/icdr_e/ctr_view.cgi?recptno=R000041031 Unique identifier: UMIN000036020). In principle, all 15 participating centers registered ACS patients based on the research protocol. Details of participating facilities are provided in Supplementary Table 1. A total of 2432 consecutive patients with AMI between January 2013 and December 2016 were evaluated using the data from the Mie ACS Registry, and the following subjects were excluded: patients who did not undergo primary PCI (N = 197, 8.1%); patients with a lack of data for IRA (N = 2, 0.1%) or for LVEF (N = 146, 6.0%); and patients who had a previous history of coronary artery bypass grafting (CABG) (N = 27, 1.1%). Finally, a total of 2060 AMI patients who underwent primary PCI were included in the current analysis (Fig. 1).
Fig. 1.
Patient flow chart. ACS: acute coronary syndrome; AMI: acute myocardial infarction; CAG: coronary angiography; PCI: percutaneous coronary intervention; IRA: infarct-related artery; CABG: coronary artery bypass grafting; LVEF: left ventricular ejection fraction; CTO: chronic total occlusion.
This registry study conformed to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Mie University Graduate School of Medicine and each participating hospital ethics committee (Reference number 2881). All patients gave their “opt-out” informed consent.
2.2. Definitions
All patients were classified into 2 groups according to their LVEF (reduced EF: LVEF < 50%, preserved EF: LVEF ≥ 50%), and then patients in each group were further divided into 2 subgroups according to the presence of non-IRA CTO (Fig. 1). The LVEF cut-off value of 50% was based on the definition of preserved ejection fraction in the Japanese and ACCF/AHA guidelines for heart failure [17], [18]. The diagnosis of AMI was based on the third universal definition of MI [19]. Non-IRA CTO was defined as complete obstruction of the non-infarct-related coronary vessel with Thrombolysis In Myocardial Infarction (TIMI) flow grade of 0 or 1 with an estimated duration of occlusion > 3 months, or presence of collateral flow with Rentrop grade of 1 or more based on a comprehensive judgement taking into account various examinations [20]. Only CTO of major epicardial coronary arteries or a major side branch with diameter ≥ 2 mm and judged to be subtending significant myocardial territory was evaluated. The differentiation between CTO and subacute or acute occlusions was made based on the morphology of the occlusion (absence of fresh thrombus, presence of bridge, epicardial, or septal collaterals) and a possible history of prior MI. Significant coronary artery disease was defined as ≥ 75% stenosis in a main epicardial coronary artery in at least one angiographic evaluation view. Coronary flow on angiography was determined based on the definition of TIMI flow grade [21]. The grading of collateral flow to the CTO segment was determined according to Rentrop grade [22]. Successful PCI was defined as residual stenosis < 30% and final TIMI flow grade > 2. As a general rule, LVEF was measured by transthoracic echocardiography with the biplane method of discs (i.e., according to the modified Simpson’s rule) mostly within a week after the onset of the index myocardial infarction [23].
2.3. Follow-up and outcomes
Outcome data were collected via patient interviews at the outpatient clinic, hospital chart reviews, or telephone interviews with the patient or close relatives, and the clinical events were recorded in a web-based system. Patients who were lost to follow-up were censored using data from the last contact. All patients were followed-up for 1-year for all-cause death and the major adverse cardiac event (MACE) rate, defined as the rate of the composite of cardiac death, myocardial infarction, CABG, and re-hospitalization because of unstable angina or heart failure.
2.4. Statistical analysis
Continuous variables with normal distributions are expressed as means ± standard deviation (SD) according to the distribution of the data. Categorical variables are expressed as percentages (%) unless otherwise indicated. Baseline characteristics were compared with the chi-squared test or Fisher’s exact test for categorical variables, and with Student’s or Welch’s t-test after testing for a normal distribution of continuous variables. Event analyses for all-cause death or MACE are displayed using Kaplan-Meier survival curves, and they were compared with the log-rank test. Hazard ratios (HRs) of each clinical outcome were calculated by Cox proportional hazards regression analysis adjusted by age, male sex, diabetes mellitus, anemia, CKD, and the Killip score. A multivariate Cox proportional hazards model was also used to investigate the independent predictors of all-cause death and MACE, which was constructed by adjusting for clinically relevant factors with P-values ≤ 0.01 on univariate analysis (Supplementary Table 2). The following variables were used as adjusted covariates in this model: CTO in a non-IRA, age, anemia (hemoglobin < 11 g/dL), CKD (eGFR ≤ 60 mL/min/1.73 m2), previous history of stroke and Killip classification. Diabetes mellitus was added as adjusted covariate in this model for 1-year MACE. To reduce the selection bias in this observation study, we performed 1:3 nearest neighbor within caliper (= 0.20) matching. The propensity-score was based on the probability of non-IRA CTO and estimated using a multivariable logistic regression model which included the following variables: age, sex, hypertension, diabetes mellitus, hemoglobin, CKD, history of MI, prior PCI, history of stroke, LAD as IRA and the Killip score. Significance was defined as a P-value < 0.05, and all statistical analyses were performed using EZR (version 1.40), which is a graphical user interface for R (version 3.5.2).
3. Results
The patients’ average age was 68.5 years, 78.2% were male, and the mean LVEF was 55.7 ± 12.4%. The median time from the diagnosis of the index AMI to echocardiography was 5 days (IQR 2–15 days). LVEF was determined with the M-mode tracing or the 2D-guided linear measurement method in case the biplane method of discs was not performed. Non-IRA CTO was seen in 8.3% of all 2060 patients. There were 624 (30.3%) patients with reduced LVEF (the reduced EF group), and 1436 (69.7%) patients had preserved LVEF (the preserved EF group). There were 106 patients with CTO and 518 patients without CTO in the reduced EF group, and 66 patients with CTO and 1370 patients without CTO in the preserved EF group (Fig. 1). Of the 106 patients with CTO in the reduced EF group, 37 underwent successful staged PCI for the CTO (CTO-PCI), and 67 were not attempted or failed CTO-PCI (not attempted PCI: 61 patients, failed PCI: 6 patients) during the follow-up period. On the other hand, of 66 patients with CTO in the preserved EF group, 19 underwent successful staged CTO-PCI, and 46 were not attempted or failed CTO-PCI (not attempted PCI: 43 patients, failed PCI: 3 patients).
3.1. Patients’ characteristics
Table 1 shows the patients’ characteristics by with and without CTO in each LVEF group. In the reduced EF group, patients with CTO had a significantly higher prevalence of diabetes, CKD, non-STEMI, and Killip classification 3 or 4, and they had significantly larger LV dimensions and lower LVEF than those without CTO. Patients with CTO had a similar IRA distribution, and they were more likely to undergo mechanical support with intra-aortic balloon pumping (IABP) than those without CTO in both EF groups. When comparing CTO patients in both EF groups, CTO patients with reduced EF tended to have a lower prevalence of a well-developed collateral circulation to the CTO segment (Rentrop grade 3) and a higher prevalence of collaterals only supplied from the IRA compared with those in CTO patients with preserved EF (20.8% vs. 37.9%, P = 0.39; 34.0% vs. 25.8%, P = 0.02, respectively). Supplementary Table 3 shows the characteristics of the propensity-score matched patients. Baseline and angiographic characteristics of patients with CTO were not much different between the no CTO-PCI and the successful CTO-PCI groups (Supplementary Table 4).
Table 1.
Patients’ characteristics.
| Reduced EF |
Preserved EF |
|||||
|---|---|---|---|---|---|---|
| With CTO N = 106 | Without CTO N = 518 | P-value | With CTO N = 66 | Without CTO N = 1,370 | P-value | |
| Baseline characteristics | ||||||
| Age, y | 68.8 ± 12.1 | 71.0 ± 13.0 | 0.09 | 70.6 ± 11.5 | 67.4 ± 12.5 | 0.03 |
| Male, % | 85.8 | 77.8 | 0.08 | 77.3 | 78.0 | 1.00 |
| Body mass index, kg/m2 | 23.4 ± 3.6 | 22.9 ± 3.8 | 0.27 | 23.7 ± 3.3 | 23.7 ± 3.6 | 1.00 |
| Hypertension, % | 61.3 | 62.1 | 0.97 | 69.7 | 65.3 | 0.54 |
| Diabetes, % | 46.2 | 32.5 | 0.01 | 45.5 | 29.6 | 0.01 |
| Dyslipidemia, % | 46.2 | 44.7 | 0.85 | 54.5 | 50.9 | 0.65 |
| Current smoker, % | 36.8 | 28 | 0.09 | 30.3 | 32.8 | 0.78 |
| Hemodialysis, % | 3.8 | 1.9 | 0.27 | 1.5 | 1.3 | 0.59 |
| CKD, % | 61.3 | 45.8 | 0.005 | 37.9 | 33.7 | 0.51 |
| Anemia, % | 12.3 | 14.3 | 0.71 | 7.6 | 6.6 | 0.80 |
| Previous MI, % | 17.9 | 13.2 | 0.26 | 9.1 | 5.3 | 0.29 |
| Previous HF, % | 4.7 | 4.3 | 0.80 | 4.5 | 0.7 | 0.02 |
| Previous PCI, % | 15.1 | 10.8 | 0.28 | 12.1 | 7.4 | 0.24 |
| Previous stroke, % | 10.4 | 6.4 | 0.21 | 7.6 | 4.4 | 0.36 |
| LVDd, mm | 54.7 ± 7.0 | 52.2 ± 6.3 | 0.003 | 49.3 ± 5.8 | 48.1 ± 5.2 | 0.10 |
| LVDs, mm | 44.0 ± 7.6 | 40.7 ± 6.3 | <0.001 | 32.6 ± 5.5 | 31.7 ± 4.9 | 0.21 |
| LVEF, % | 37.0 ± 8.3 | 40.8 ± 7.3 | <0.001 | 61.6 ± 7.8 | 61.9 ± 7.6 | 0.78 |
| eGFR, mL/min/1.73 m2 | 56.0 ± 23.0 | 62.1 ± 24.8 | 0.02 | 67.4 ± 26.5 | 68.1 ± 23.0 | 0.84 |
| Presentation of index AMI | ||||||
| STEMI, % | 71.0 | 83.6 | 0.005 | 65.6 | 79.8 | 0.01 |
| Killip class ≥ III, % | 41.3 | 28.1 | 0.01 | 10.8 | 6.0 | 0.20 |
| Peak creatinine phosphokinase, U/L | 4258 ± 5259 | 3557 ± 3462 | 0.19 | 2598 ± 3733 | 2217 ± 2297 | 0.41 |
| Hospital stay, days | 24.7 ± 32.0 | 19.1 ± 17.8 | 0.08 | 17.0 ± 18.1 | 14.2 ± 12.1 | 0.22 |
| Angiographic and procedural characteristics | ||||||
| Infarct-related artery | ||||||
| RCA/LMT/LAD/LCX | 29.2/1.9/55.7/13.2 | 24.9/4.6/56.4/14.1 | 0.56 | 33.3/1.5/43.9/21.2 | 42.0/0.8/42.8/14.5 | 0.22 |
| PCI procedure | ||||||
| POBA | 6.6 | 9.3 | 0.49 | 6.1 | 6.9 | 1.00 |
| BMS use | 12.3 | 12.4 | 1.00 | 1.5 | 11.8 | 0.005 |
| DES use | 81.1 | 76.8 | 0.40 | 92.4 | 80.1 | 0.02 |
| Thrombectomy | 59.0 | 67.0 | 0.15 | 50.8 | 68.2 | 0.005 |
| Distal protection | 11.7 | 8.2 | 0.36 | 6.2 | 10.6 | 0.30 |
| ECMO use | 14.2 | 3.9 | <0.001 | 0.0 | 0.4 | 1.00 |
| IABP use | 47.2 | 23.0 | <0.001 | 19.7 | 7.2 | <0.001 |
| Initial TIMI flow grade 0 | 54.3 | 63.2 | 0.11 | 41.5 | 55.5 | 0.04 |
| Final TIMI flow grade 3 | 91.0 | 82.1 | 0.04 | 97.0 | 93.2 | 0.31 |
| Details of CTO in a non-IRA | ||||||
| Number of CTOs per patients | 1.1 ± 0.3 | – | NA | 1.1 ± 0.3 | – | NA |
| Location of CTO | ||||||
| RCA/LAD/LCX | 51.9/17.9/40.6 | – | NA | 34.8/30.3/45.5 | – | NA |
| Collateral from only IRA | 34.0 | – | NA | 25.8 | – | NA |
| Rentrop grade 3 | 20.8 | – | NA | 37.9 | – | NA |
Categorical variables are expressed as percentages unless otherwise indicated. Continuous variables are shown as means ± SD. EF: ejection fraction; CTO: chronic total occlusion; CKD: chronic kidney disease; MI: myocardial infarction; HF: heart failure; PCI: percutaneous coronary intervention; LVDd: left ventricular end-diastolic diameter; LVDs: left ventricular end-systolic diameter; LVEF: left ventricular ejection fraction; eGFR: estimated glomerular filtration rate; AMI: acute myocardial infarction; STEMI: ST-elevation myocardial infarction; RCA: right coronary artery; LMT: left main trunk; LAD: left anterior descending artery; LCX: left circumflex artery; POBA: percutaneous old balloon angioplasty; BMS: bare metal stent; DES: drug-eluting stent; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pumping; TIMI: thrombolysis in myocardial infarction; IRA: infarct-related artery; NA: not applicable.
3.2. Outcomes
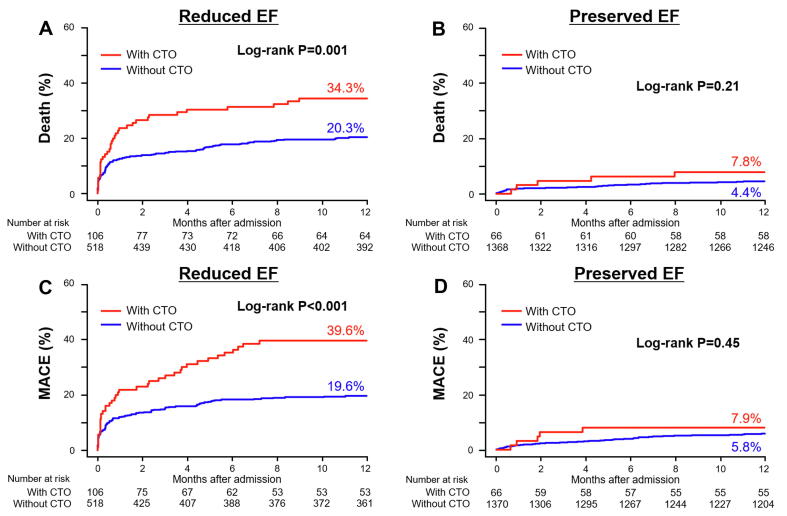
During the follow-up period, all-cause death occurred in 207 patients (10.0%), and 220 (10.7%) patients experienced a MACE. Fig. 2 shows the Kaplan-Meier curves for all-cause mortality and MACE for each EF group. In the reduced EF group, CTO patients had significantly higher all-cause mortality and MACE rates compared to those without CTO (P = 0.001 and P < 0.001 respectively) (Fig. 2A, C). On the other hand, there were no significant differences in both outcomes in the preserved EF group between patients with and without CTO (P = 0.21 and P = 0.45, respectively) (Fig. 2B, D). Table 2 summarizes the unadjusted and adjusted cumulative hazards (covariate variables: age, diabetes, anemia, CKD, and Killip classification) of the clinical outcomes of patients with CTO vs. without CTO during the 1-year follow-up period. In the reduced EF group, crude and adjusted HRs of all-cause death and cardiovascular death were significantly higher in patients with than without CTO. The findings of the Kaplan-Meier analysis showed the same tendency in propensity-score matched patients (Supplementary Fig. 1). Supplementary Table 5 summarized the adjusted hazards of the clinical outcomes of patients after propensity-score matching. The multivariate Cox regression analyses for 1-year all-cause death and MACE are summarized in Table 3. Anemia and the Killip score were the common prognostic factors for all-cause death in each EF group. However, CTO in a non-IRA was one of the independent prognostic factors only in the reduced EF group, but not in the preserved EF group. Similarly, CTO was also one of the independent prognostic factors of 1-year MACE only in the reduced EF group, but not in the preserved EF group.
Fig. 2.
Kaplan-Meier curves for the cumulative incidence of 1-year all-cause death (A, B) and MACE (C, D) in patients with and without CTO. A, C: Comparison in the reduced EF group; B, D: Comparison in the preserved EF group. MACE: major adverse cardiac event; EF: ejection fraction; CTO: chronic total occlusion.
Table 2.
Clinical outcomes of patients with and without CTO stratified by LVEF during 1-year follow-up.
| Variable | With CTO | Without CTO | Crude HR (95% CI) | P-value | Adjusted HR (95% CI) | P- value |
|---|---|---|---|---|---|---|
| Reduced EF group | ||||||
| All-cause death | 36 (34.0) | 104 (20.1) | 1.84 (1.26–2.68) | 0.002 | 1.63 (1.10–2.41) | 0.01 |
| Cardiovascular death | 28 (26.4) | 69 (13.3) | 2.10 (1.35–3.25) | <0.001 | 1.60 (1.01–2.55) | 0.04 |
| Non-fatal myocardial infarction | 2 (1.9) | 5 (1.0) | 2.23 (0.43–11.5) | 0.34 | 1.21 (0.22–6.58) | 0.82 |
| CABG | 6 (5.7) | 5 (1.0) | 6.34 (1.93–20.8) | 0.002 | 5.66 (1.33–24.0) | 0.02 |
| Unstable angina pectoris | 2 (1.9) | 1 (0.2) | 11.7 (1.06–129.1) | 0.04 | 16.8 (1.25–225.9) | 0.03 |
| Heart failure | 10 (9.4) | 24 (4.6) | 2.46 (1.18–5.14) | 0.02 | 1.95 (0.90–4.24) | 0.09 |
| Preserved EF group | ||||||
| All-cause death | 5 (7.6) | 62 (4.5) | 1.78 (0.71–4.42) | 0.22 | 1.24 (0.49–3.12) | 0.65 |
| Cardiovascular death | 2 (3.0) | 33 (2.4) | 1.29 (0.31–5.38) | 0.73 | 0.72 (0.17–3.08) | 0.65 |
| Non-fatal myocardial infarction | 0 (0.0) | 14 (1.0) | – | – | – | – |
| CABG | 2 (3.0) | 6 (0.4) | 7.09 (1.43–35.1) | 0.02 | 2.06 (0.24–18.1) | 0.51 |
| Unstable angina pectoris | 0 (0.0) | 13 (0.9) | – | – | – | – |
| Heart failure | 1 (1.5) | 24 (1.8) | 0.91 (0.12–6.72) | 0.93 | 0.62 (0.08–4.64) | 0.64 |
Adjusted risk was estimated by the multivariate Cox proportional hazards model. The following clinically relevant variables were used as adjusted covariates in this model: age, male sex, diabetes mellitus, anemia (hemoglobin < 11 g/dL), CKD (eGFR ≤ 60 mL/min/1.73 m2) and Killip classification. CABG: coronary artery bypass grafting; CI: confidence interval; HR: hazard ratio. Other abbreviations as in Table 1.
Table 3.
Multivariate Cox regression analysis for predictors of 1-year all cause death and MACE.
| Reduced EF |
Preserved EF |
|||||
|---|---|---|---|---|---|---|
| Variable | Adjusted HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value |
| (A) 1-year all cause death | ||||||
| CTO in a non-IRA | 1.58 | 1.06–2.33 | 0.02 | 1.16 | 0.46–2.92 | 0.76 |
| Age | 1.02 | 1.00–1.04 | 0.03 | 1.07 | 1.04–1.10 | <0.001 |
| Anemia (Hb < 11 g/dL) | 1.77 | 1.20–2.61 | 0.004 | 2.14 | 1.18–3.87 | 0.012 |
| CKD | 1.64 | 1.07–2.50 | 0.02 | 1.71 | 0.97–3.00 | 0.06 |
| Previous stroke | 1.10 | 0.67–1.81 | 0.71 | 1.93 | 0.87–4.27 | 0.11 |
| Killip classification | 2.20 | 1.88–2.58 | <0.001 | 1.74 | 1.40–2.16 | <0.001 |
| (B) 1-year MACE | ||||||
| CTO in a non-IRA | 1.67 | 1.14–2.46 | 0.009 | 0.70 | 0.26–1.93 | 0.49 |
| Age | 1.00 | 0.99–1.02 | 0.83 | 1.05 | 1.02–1.07 | <0.001 |
| Diabetes mellitus | 1.37 | 0.97–1.95 | 0.07 | 1.92 | 1.22–3.00 | 0.005 |
| Anemia (Hb < 11 g/dL) | 1.26 | 0.81–1.95 | 0.31 | 2.29 | 1.30–4.03 | 0.004 |
| CKD | 2.05 | 1.33–3.13 | 0.001 | 1.37 | 0.85–2.22 | 0.20 |
| Previous stroke | 0.86 | 0.49–1.51 | 0.60 | 0.70 | 0.25–1.95 | 0.50 |
| Killip classification | 2.03 | 1.74–2.36 | <0.001 | 1.70 | 1.40–2.08 | <0.001 |
Multivariate Cox regression analysis for 1-year all-cause death was conducted with adjustment by CTO in a non-IRA, age, anemia (hemoglobin < 11 g/dL), CKD (eGFR ≤ 60 mL/min/1.73 m2), previous history of stroke and Killip classification. Diabetes mellitus was added as adjusted covariate in the model for 1-year MACE. CKD was defined as estimated GFR (eGFR) ≤ 60 mL/min/1.73 m2. MACE: major adverse cardiac event. Other abbreviations as in Table 1, Table 2.
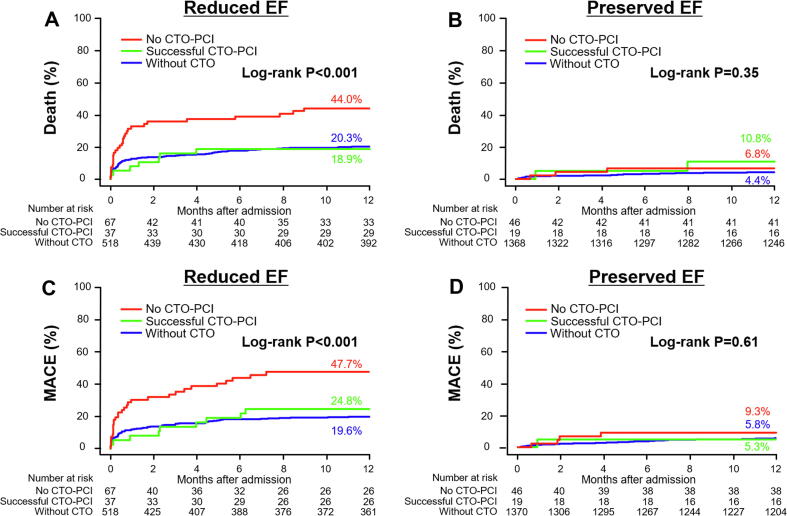
Whether successful staged PCI for the CTO during follow-up was associated with a better prognosis was also evaluated. Fig. 3 shows the Kaplan-Meier curves for all-cause death and MACE with comparisons among patients without CTO (without CTO), those with successful PCI for a CTO (successful CTO-PCI), and those who were not attempted or received failed PCI for CTO (no CTO-PCI). In the reduced EF group, successful CTO-PCI patients appeared to have lower 1-year all-cause death rates (18.9% vs 44.0%, HR 0.37, 95% CI 0.16 to 0.85, P = 0.02) and MACE rates (24.8% vs 47.7%, HR 0.42, 95% CI 0.20 to 0.88, P = 0.04) compared with no CTO-PCI patients, and similar 1-year mortality and MACE rates compared with patients without CTO (Fig. 3A, C). On the other hand, in the preserved EF group, no differences were seen in 1-year all-cause mortality and MACE rates among the three therapeutic subgroups. (Fig. 3B, D).
Fig. 3.
Kaplan-Meier curves for the cumulative incidence of 1-year all-cause death (A, B) and MACE (C, D) among the 3 sub-groups: without CTO, successful CTO-PCI, and no CTO-PCI. A, C: Comparison in the reduced EF group; B, D: Comparison in the preserved EF group. A: Pair-wise P-values in the post hoc analysis are: successful CTO-PCI vs. no CTO-PCI, P = 0.02; successful CTO-PCI vs. without CTO, P = 0.83; without CTO vs. no CTO-PCI, P < 0.001. C: Pair-wise P-values in the post hoc analysis are: successful CTO-PCI vs. no CTO-PCI, P = 0.04; successful CTO-PCI vs. without CTO, P = 0.54; without CTO vs. no CTO-PCI, P < 0.001. MACE: major adverse cardiac event; EF: ejection fraction; CTO: chronic total occlusion; PCI: percutaneous coronary intervention.
4. Discussion
This study investigated the prognostic relationship between non-IRA CTO and baseline LVEF in patients with AMI. This study had three important findings. First, CTO in a non-IRA was associated with a poor prognosis only in patients with reduced baseline EF, but not in those with preserved EF. Second, a CTO in a non-IRA was an independent prognostic factor for all-cause death and MACE only in the reduced EF group, but not in the preserved EF group. Third, successful PCI to a CTO in a non-IRA CTO was associated with improved prognosis only in patients with reduced EF. To the best of our knowledge, this is the first multicenter, observational study that evaluated the prognostic impact of a CTO in a non-IRA in patients with AMI stratified by baseline LVEF.
In most recent papers, a CTO in a non-IRA was reported to occur in approximately 8–12% of AMI patients [12], [24], comparable to the present result (8.3% of AMI patients). The prognostic importance of the presence of non-IRA CTO in patients with AMI is well recognized [10], [12]. However, few clinical studies have evaluated the prognostic relationship between CTO and baseline LVEF in patients with AMI, except for only one single-center study with a relatively small sample size [11]. The present results clearly showed that CTO was associated with a poor prognosis only in patients with reduced baseline EF, but not in those with preserved EF. Some previous studies reported that a history of previous MI and a worse Killip classification on admission were the independent predictors of mortality in AMI patients with CTO [24]. These results may suggest that the prognostic effect of CTO may differ depending on the degree of baseline LV systolic dysfunction. LVEF is a useful non-invasive index that can be easily obtained at the bedside by echocardiography during hospitalization, and it is well known to be an important prognostic factor in AMI patients treated with primary PCI [25]. Therefore, the present study, which demonstrated the risk stratification ability of baseline LVEF in AMI patients with concomitant non-IRA CTO, may have great clinical importance.
CTO patients with reduced EF may have a higher risk profile associated with a worse clinical outcome when compared to CTO patients with preserved EF or non-CTO patients. Indeed, these patients tended to have a higher prevalence of diabetes, history of previous MI, Killip class of 3 or 4 on admission, and CKD compared with patients in other groups. However, after adjustment for these differences in baseline characteristics, the presence of CTO remained a strong and independent predictor of all-cause mortality in the reduced EF group.
Another explanation for the poor prognosis of CTO patients with reduced EF could be that they are potentially at ‘double jeopardy’ from the AMI. Myocardium normally supplied by the CTO is partially maintained in a hibernating status supplied by collateral blood flow. Once the IRA occludes, poor collateral filling into CTO segments indicates broad ischemia, whereas fair collateral filling indicates ischemia in a single-vessel area of the IRA. A previous study reported that poor collateral filling of the CTO segment was an independent predictor of mortality in patients with STEMI [26]. Moreover, if the territory of CTO depends on collateral blood flow from the IRA, the acute ischemic area during the index AMI would be widely extended to a two-vessel area. This could result in increased infarct size, and decreased LVEF, leading to higher mortality. Previous research showed that collateral flow from the IRA was an independent predictor associated with all-cause mortality [27]. In the present study, CTO patients with reduced EF tended to have lower collateral filling of the CTO segment assessed by Rentrop grade, a higher prevalence of occluded IRA to supply the myocardium of a CTO, and higher peak creatine phosphokinase levels compared with those in CTO patients with preserved EF, supporting these hypotheses.
The present study suggested that the prognostic impact of a successful CTO-PCI concomitant with AMI might differ according to baseline LVEF. The clinical value of staged PCI for a CTO in AMI patients is not established due to the paucity of robust randomized data and conflicting results in several observational studies. Renato et al. investigated the prognostic effect of staged CTO-PCI in patients with STEMI treated with successful primary PCI with a concurrent CTO in an observational study; they showed that successful CTO-PCI was associated with significantly improved 3-year cardiac survival [28]. In contrast, the data of the CREDO Kyoto AMI registry showed that successful PCI to a CTO did not improve the 5-year survival rate [10]. Similarly, some recently reported randomized, clinical trials, such as the EXPLORE trial [29], showed no beneficial effect of CTO-PCI on the incidence of cardiovascular death or MACE.
In the CREDO-Kyoto AMI registry and the EXPLORE trial, the average LVEF values in patients in the CTO-PCI group were 48% and 44%, respectively, and the rate of patients with LVEF ≤ 40% was approximately only 26% and less than 50%, respectively [10], [29]. In contrast, in the study by Renato et al, the average LVEF was relatively low, at 37%, and more than 60% of all patients had reduced baseline LVEF of ≤ 40% [28]. Based on the present result, these differences in the proportion of patients with reduced baseline LVEF between the previous studies might partially contribute to the conflicting results. Accurate risk stratification is needed to select the patients who will potentially benefit from CTO-PCI. From this point of view, the present findings that suggest the usefulness of LVEF in risk stratification of patients with AMI with a concurrent CTO in a non-IRA may have important clinical implications.
However, our results do not necessarily recommend non-specific, LVEF-guided revascularization for a non-IRA CTO without evaluation of myocardial viability and ischemia in the CTO territory, nor deny the potential prognostic benefit of CTO-PCI for patients with preserved EF. The adequacy of PCI for CTO lesions is controversial because these procedures have lower success and higher complication rates and require longer procedural times, greater contrast volume use, radiation exposure and cost in comparison with PCI of nonocclusive lesions. Although there remains some conflicting data, there appears to be several increasing evidences of the effectiveness of functional evaluation of lesion significance in terms of ischemia and viability of the myocardial territory which supplied from CTO vessels, as the revascularization of non-viable myocardium or of a not relevant lesion was associated with immediate risk of an invasive procedure and no guarantee of longer-term benefit [30], [31], [32]. In the CTO cases of this study, the indication of revascularization of non-IRA CTO were decided by the each attending physicians based on the comprehensive consideration of comorbidities, myocardial ischemia and viability evaluated by such as stress nuclear, echocardiography, or magnetic resonance imaging and complexity of anatomic features of targeted vessels. However, such detailed information had not been collected in this registry, which was the limitation of this study.
The optimal revascularization timing of the remaining CTO is not well established except that multivessel PCI should not be performed in a non-infarct artery at the time of primary PCI in patients without hemodynamic compromise [33]. In the early acute phase of AMI, several problems such as effect of anti-thrombotic drugs, amount of contrast injected and acute myocardial inflammation of infarct area, could provoke high rate of vascular and bleeding complication and decrease recanalization success. The present study showed that the patients who underwent successful CTO-PCI within 30 days tended to have higher all-cause mortality compared to those over 30 days in reduced EF group (Supplementary Fig. 2). However, this comparison is hard to interpret because patients who have attempted CTO-PCI within 1-month have worse baseline characteristics and the number of cases were too small for such comparison (Supplementary Table 6). Large-scale well-designed trails are needed to confirm these findings.
4.1. Clinical implications
Baseline LVEF is a useful index that can stratify the prognostic risk of patients with AMI with a concurrent non-IRA CTO. In such patients, routine CTO-PCI should be avoided due to a lack of robust evidence. Based on appropriate and careful assessment of patients to select those who will benefit from CTO-PCI based on the LVEF, PCI for a non-IRA CTO may improve their clinical prognosis.
4.2. Limitations
This study has several potential limitations. First, because this was an observational study, unexpected and unmeasured confounders may have affected the outcomes. Second, the decisions to perform PCI for CTO and other therapeutic strategies were at the discretion of the attending physicians in each hospital. Therefore, the potential for selection bias cannot be excluded. Third, there was no information regarding medications before onset of the index AMI. Fourth, detailed data of the extent of myocardial viability and the extent of the ischemic area were not available in this study. Fifth, the timing of echocardiographic assessment of LVEF in the present study might have been too early to evaluate the prognostic association between LVEF and non-IRA CTO, which might have affected our results due to overestimate the prognostic risk of CTO. Previous study reported that the LVEF recovery after culprit lesion revascularization were shown in the nearly half of patients with AMI and most of such functional recovery occurred during the first month from index AMI [34]. Therefore, serial assessment of LVEF at acute phase and 1-month after the index infarction might be needed to evaluate the true prognostic importance of non-IRA CTO.
Finally, the sample size of the present study might be relatively small, and the follow-up duration might be relatively short to evaluate long-term outcomes and prognostic factors. When a power calculation was performed using the 1-year survival rate (56.0% in no CTO-PCI and 81.1% in successful CTO-PCI) in the reduced EF group, the statistical power of the survival analysis was slightly lower, 0.76, with type I error of 0.05 and type II error of 0.20 (CTO-PCI, N = 67; successful CTO-PCI, N = 37) in the present study. From this calculation, the minimum sample size required to achieve adequate statistical power would be at least 116 patients (no CTO-PCI N = 75, successful CTO-PCI N = 41) in the reduced EF group. Furthermore, in preserved EF group, the statistically differences for cumulative incidence of all-cause death and MACE may be small due to small sample sizes and beta error. To reduce these biases and the effect of confounding, we also performed Kaplan-Meier analysis comparing patients with or without CTO using the propensity-score matched data. In the preserved EF group, there were no significant differences in both outcomes. A well-designed, large-scale, prospective trial is needed to confirm the present findings.
5. Conclusions
A CTO in a non-IRA concomitant with AMI was associated with a poor prognosis only in patients with reduced LVEF, but not in patients with preserved LVEF. Moreover, successful PCI for a concurrent CTO in a non-IRA might be associated with improved all-cause mortality and MACE rates in the reduced EF group, but not in the preserved EF group.
Funding
This study was supported and funded by the Mie Cardiovascular and Renal Disease Network.
Declaration of Competing Interest
Kaoru Dohi received lecture fees from Otsuka Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Company Limited. Masaaki Ito received departmental research grant support from Bristol-Myers Squibb, Daiichi Sankyo Company Limited, Shionogi Co., Ltd., MSD K.K., Takeda Pharmaceutical Company Limited, and Otsuka Pharmaceutical Co., Ltd. Masaaki Ito received lecture fees from Daiichi Sankyo Company Limited and Takeda Pharmaceutical Company Limited. Other authors, including the first author, have no financial conflicts of interest to disclose concerning this study.
Acknowledgements
The authors would like to thank all participating facilities, the Mie ACS Registry co-investigators, Mie CCU Network Support Center, and Mie University Hospital Clinical Research Support Center.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100738.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Puymirat E., Simon T., Steg P.G., Schiele F., Gueret P., Blanchard D. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA. 2012;308:998–1006. doi: 10.1001/2012.jama.11348. [DOI] [PubMed] [Google Scholar]
- 2.Gale C.P., Allan V., Cattle B.A., Hall A.S., West R.M., Timmis A. Trends in hospital treatments, including revascularisation, following acute myocardial infarction, 2003–2010: a multilevel and relative survival analysis for the National Institute for Cardiovascular Outcomes Research (NICOR) Heart. 2014;100:582–589. doi: 10.1136/heartjnl-2013-304517. [DOI] [PubMed] [Google Scholar]
- 3.McManus D.D., Gore J., Yarzebski J., Spencer F., Lessard D., Goldberg R.J. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am. J. Med. 2011;124:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alabas O.A., Jernberg T., Pujades-Rodriguez M., Rutherford M.J., West R.M., Hall M. Statistics on mortality following acute myocardial infarction in 842 897 Europeans. Cardiovasc. Res. 2020;116:149–157. doi: 10.1093/cvr/cvz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen F., Butrymovich V., Kelbæk H., Wachtell K., Helqvist S., Kastrup J. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J. Am. Coll. Cardiol. 2014;64:2101–2108. doi: 10.1016/j.jacc.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita Y., Shiomi H., Morimoto T., Yaku H., Furukawa Y., Nakagawa Y. Cardiac and noncardiac causes of long-term mortality in ST-segment-elevation acute myocardial infarction patients who underwent primary percutaneous coronary intervention. Circ. Cardiovasc. Qual. Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.116.002790. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda S., Honda S., Takegami M., Nishihira K., Kojima S., Asaumi Y. Contemporary antiplatelet therapy and clinical outcomes of Japanese Patients With acute myocardial infarction- results from the Prospective Japan Acute Myocardial Infarction Registry (JAMIR) Circ. J. 2019;83:1633–1643. doi: 10.1253/circj.CJ-19-0145. [DOI] [PubMed] [Google Scholar]
- 8.Bosch X., Théroux P. Left ventricular ejection fraction to predict early mortality in patients with non-ST-segment elevation acute coronary syndromes. Am. Heart J. 2005;150:215–220. doi: 10.1016/j.ahj.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Syyli N., Hautamaki M., Antila K., Mahdiani S., Eskola M., Lehtimaki T. Left ventricular ejection fraction adds value over the GRACE score in prediction of 6-month mortality after ACS: the MADDEC study. Open Heart. 2019;6:e001007. doi: 10.1136/openhrt-2019-001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe H., Morimoto T., Shiomi H., Furukawa Y., Nakagawa Y., Ando K. Chronic total occlusion in a non-infarct-related artery is closely associated with increased five-year mortality in patients with ST-segment elevation acute myocardial infarction undergoing primary percutaneous coronary intervention (from the CREDO-Kyoto AMI registry) EuroIntervention. 2017;12:e1874–e1882. doi: 10.4244/EIJ-D-15-00421. [DOI] [PubMed] [Google Scholar]
- 11.Mizuguchi Y., Takahashi A., Hashimoto S., Yamada T., Taniguchi N., Nakajima S. Impact of the presence of chronic total occlusion in a non-infarct-related coronary artery in acute myocardial infarction patients. Int. Heart J. 2015;56:592–596. doi: 10.1536/ihj.15-080. [DOI] [PubMed] [Google Scholar]
- 12.Claessen B.E., Dangas G.D., Weisz G., Witzenbichler B., Guagliumi G., Mockel M. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur. Heart J. 2012;33:768–775. doi: 10.1093/eurheartj/ehr471. [DOI] [PubMed] [Google Scholar]
- 13.Claessen B.E., van der Schaaf R.J., Verouden N.J., Stegenga N.K., Engstrom A.E., Sjauw K.D. Evaluation of the effect of a concurrent chronic total occlusion on long-term mortality and left ventricular function in patients after primary percutaneous coronary intervention. JACC Cardiovasc. Interv. 2009;2:1128–1134. doi: 10.1016/j.jcin.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Vallabhajosyula S., Prasad A., Gulati R., Barsness G.W. Contemporary prevalence, trends, and outcomes of coronary chronic total occlusions in acute myocardial infarction with cardiogenic shock. Int. J. Cardiol. Heart Vasc. 2019;24:100414. doi: 10.1016/j.ijcha.2019.100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda J., Kishi M., Kumagai N., Yamazaki T., Sakata K., Higuma T. Rural-urban disparity in emergency care for acute myocardial infarction in Japan. Circ. J. 2018;82:1666–1674. doi: 10.1253/circj.CJ-17-1275. [DOI] [PubMed] [Google Scholar]
- 16.Fukuoka S., Kurita T., Dohi K., Masuda J., Seko T., Tanigawa T. Untangling the obesity paradox in patients with acute myocardial infarction after primary percutaneous coronary intervention (detail analysis by age) Int. J. Cardiol. 2019;289:12–18. doi: 10.1016/j.ijcard.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsui H., Isobe M., Ito H., Ito H., Okumura K., Ono M. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure - digest version. Circ. J. 2019;83:2084–2184. doi: 10.1253/circj.CJ-19-0342. [DOI] [PubMed] [Google Scholar]
- 18.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Colvin M.M. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K., Alpert J.S., Jaffe A.S., Simoons M.L., Chaitman B.R., White H.D. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Stone G.W., Kandzari D.E., Mehran R., Colombo A., Schwartz R.S., Bailey S. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. Circulation. 2005;112:2364–2372. doi: 10.1161/CIRCULATIONAHA.104.481283. [DOI] [PubMed] [Google Scholar]
- 21.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N. Engl. J. Med. 312 (1985) 932-936. [DOI] [PubMed]
- 22.Rentrop K.P., Cohen M., Blanke H., Phillips R.A. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J. Am. Coll. Cardiol. 1985;5:587–592. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 23.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28(1–39):e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Shi G., He P., Liu Y., Lin Y., Yang X., Chen J. Evaluation of the effect of concurrent chronic total occlusion and successful staged revascularization on long-term mortality in patients with ST-elevation myocardial infarction. ScientificWorldJournal. 2014;2014:756080. doi: 10.1155/2014/756080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng V.G., Lansky A.J., Meller S., Witzenbichler B., Guagliumi G., Peruga J.Z. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Eur. Heart J. Acute Cardiovasc. Care. 2014;3:67–77. doi: 10.1177/2048872613507149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujii T., Nakano M., Ohno Y., Nakazawa G., Shinozaki N., Matsukage T. Collateral filling efficiency of comorbid chronic total occlusion segment on short-term mortality in ST-elevation myocardial infarction. Int. J. Cardiol. 2017;230:346–352. doi: 10.1016/j.ijcard.2016.12.107. [DOI] [PubMed] [Google Scholar]
- 27.Fujii T., Sakai K., Nakano M., Ohno Y., Nakazawa G., Shinozaki N. Impact of the origin of the collateral feeding donor artery on short-term mortality in ST-elevation myocardial infarction with comorbid chronic total occlusion. Int. J. Cardiol. 2016;218:158–163. doi: 10.1016/j.ijcard.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Valenti R., Marrani M., Cantini G., Migliorini A., Carrabba N., Vergara R. Impact of chronic total occlusion revascularization in patients with acute myocardial infarction treated by primary percutaneous coronary intervention. Am. J. Cardiol. 2014;114:1794–1800. doi: 10.1016/j.amjcard.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Henriques J.P., Hoebers L.P., Ramunddal T., Laanmets P., Eriksen E., Bax M. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J. Am. Coll. Cardiol. 2016;68:1622–1632. doi: 10.1016/j.jacc.2016.07.744. [DOI] [PubMed] [Google Scholar]
- 30.Bonow R.O., Maurer G., Lee K.L., Holly T.A., Binkley P.F., Desvigne-Nickens P. Myocardial viability and survival in ischemic left ventricular dysfunction. N. Engl. J. Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucciarelli-Ducci C., Auger D., Di Mario C., Locca D., Petryka J., O'Hanlon R. CMR guidance for recanalization of coronary chronic total occlusion. JACC Cardiovasc. Imaging. 2016;9:547–556. doi: 10.1016/j.jcmg.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Zimarino M., Curzen N., Cicchitti V., De Caterina R. The adequacy of myocardial revascularization in patients with multivessel coronary artery disease. Int. J. Cardiol. 2013;168:1748–1757. doi: 10.1016/j.ijcard.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 33.O'Gara P.T., Kushner F.G., Ascheim D.D., Casey D.E., Jr., Chung M.K., de Lemos J.A. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 34.Parodi G., Memisha G., Carrabba N., Signorini U., Migliorini A., Cerisano G. Prevalence, predictors, time course, and long-term clinical implications of left ventricular functional recovery after mechanical reperfusion for acute myocardial infarction. Am. J. Cardiol. 2007;100:1718–1722. doi: 10.1016/j.amjcard.2007.07.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.