Abstract
Glycosylation is a fundamental co-translational and/or post-translational modification process where an attachment of sugars onto either proteins or lipids can alter their biological function, subcellular location and modulate the development and physiology of an organism. Glycosylation is not a template driven process and as such produces a vastly larger array of glycan structures through combinatorial use of enzymes and of repeated common scaffolds and as a consequence it provides a huge expansion of both the proteome and lipidome. While the essential role of N- and O-glycan modifications on mammalian glycoproteins is already well documented, we are just starting to decode their biological functions in plants. Although significant advances have been made in plant glycobiology in the last decades, there are still key challenges impeding progress in the field and, as such, holistic modern high throughput approaches may help to address these conceptual gaps. In this snapshot, we present an update of the most common O- and N-glycan structures present on plant glycoproteins as well as (1) the plant glycosyltransferases (GTs) and glycosyl hydrolases (GHs) responsible for their biosynthesis; (2) a summary of microorganism-derived GHs characterized to cleave specific glycosidic linkages; (3) a summary of the available tools ranging from monoclonal antibodies (mAbs), lectins to chemical probes for the detection of specific sugar moieties within these complex macromolecules; (4) selected examples of N- and O-glycoproteins as well as in their related GTs to illustrate the complexity on their mode of action in plant cell growth and stress responses processes, and finally (5) we present the carbohydrate microarray approach that could revolutionize the way in which unknown plant GTs and GHs are identified and their specificities characterized.
Keywords: Arabidopsis, glycosyltransferases, plant protein glycosylation, glycan arrays, O-glycosylation, N-glycosylation, glycosyl hydrolases, glycan functions
Introduction
In the model plant Arabidopsis thaliana, approx. 10–15% of the genome is devoted to construction, dynamic architecture, sensing functions, and metabolism of the plant cell wall (Cosgrove, 2005). The major components of plant cell walls include a complex composite of polysaccharide networks, lignin (secondary walls) together with minor amounts (generally less than 10%) of N- and/or O-glycosylated proteins (Somerville et al., 2004; Cosgrove, 2005; Albenne et al., 2009; Ellis et al., 2010; Lamport et al., 2011; Zielinska et al., 2012). Protein glycosylation, through co- and/or post-translational modification, results in addition of glycans (mono-/oligo-/polysaccharides and GPI anchors) that influence a protein’s stability, location and functional properties (Lerouge et al., 1998; Nagashima et al., 2018). While N-glycan synthesis in the endoplasmic reticulum (ER) is relatively well conserved in eukaryotes, N-glycan processing and O-glycan biosynthesis in the Golgi apparatus (GA) are kingdom-specific and result in different oligosaccharide structures attached to glycoproteins in plants and mammals (Gomord et al., 2010). The prasinophytes situated at the base of the green plant lineage feature a much simpler set of N-glycan elaborations (Ulvskov et al., 2013) which may represent either the primordial eukaryotic N-glycosylation machinery or be the result of gene loss. Following initial processing steps in the ER, the N-glycans show differences in the maturation steps in the GA. Interestingly, plant N-glycans differ from their animal counterparts by the following: (1) the complete absence of sialic acid, (2) the core Fuc residues (where present) are α(1 → 3) rather than α(1 → 6)-linked to the reducing GlcNAc, and (3) the core β-mannosyl residue is often substituted with Xylβ(1 → 2) (Sturm, 1995). In contrast to N-glycosylation, the primary mechanism for O-glycosylation in plants is unique among eukaryotes and is via attachment to the hydroxyl group of the imino acid hydroxyproline (Hyp/O; in mammalian systems this type of glycosylation is to hydroxylysine) and less commonly to the hydroxyl group of serine [Ser; e.g., in extensins (EXTs) (Kieliszewski, 2001)]. This O-linked glycosylation determines the molecular properties and biological functions of members of the Hyp-rich glycoprotein (HRGP) superfamily and some secreted small peptides (e.g., CLE for CLAVATA3/Endosperm surrounding region). In addition, in plants there is a complete absence of GalNAc-Ser/Thr in secreted glycoproteins that is common in mammalian secreted glycoproteins and whilst there are also some other forms of O-glycosylation they are less common (e.g., Ser-O-GlcNAc on cytoplasmic and nuclear proteins). This overview should be read in conjunction with more focused reviews recently published by Seifert (2020) and Silva et al. (2020) to gain a comprehensive coverage of the structure, function and biosynthesis of arabinogalactan-proteins (AGPs).
N-Glycan Processing Pathway in Plants: Glycosyltransferases (GTs) and Glycosyl Hydrolases (GHs)
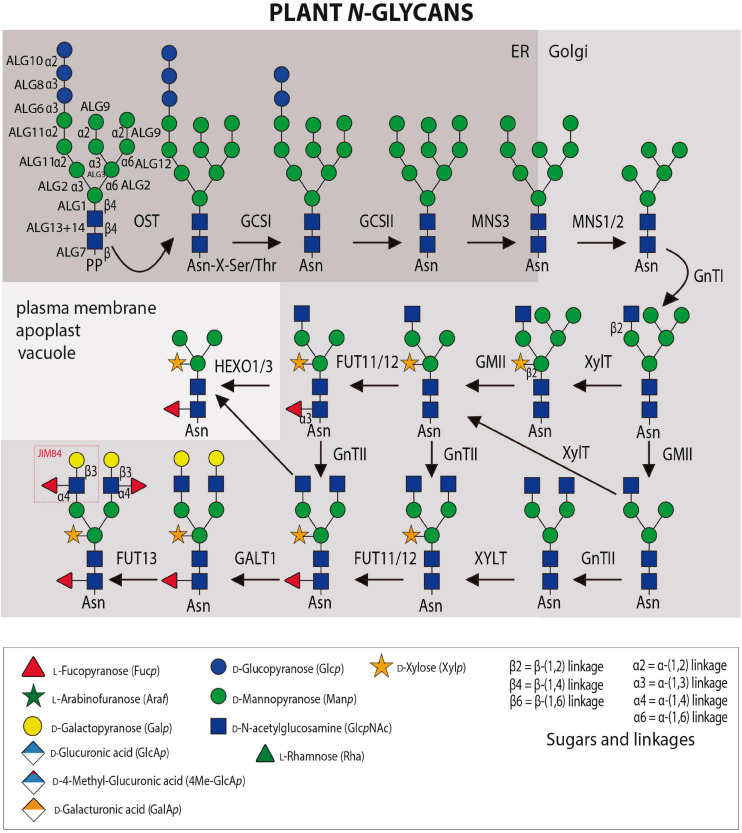
Asparagine (N)-linked glycosylation is a major co- and post-translational modification of proteins entering the secretory pathway. The initial step of N-glycosylation is the en bloc transfer of a preassembled oligosaccharide (Glc3Man9GlcNAc2) from a lipid carrier, dolicholpyrophosphate (PP-Dol) to selected Asn residues primarily in the canonical sequence Asn-X-Ser/Thr (X≠Pro) within nascent polypeptides, although some non-consensus sequences have been reported (Figure 1; Strasser, 2016). The lipid-linked oligosaccharide precursor is assembled in a stepwise manner by Asn-linked glycosylation (ALG) enzymes. The final step at the cytosolic side of the ER is catalyzed by ALG11 that transfers two consecutive α-(1 → 2) Man residues to the lipid-linked oligosaccharide. The resulting Man5GlcNAc2-PP-Dol is then transported across the ER membrane by a flippase-like protein and used as substrate in the ER lumen by the three mannosyltransferases ALG3, ALG9, ALG12 and the three glucosyltransferases (ALG6, ALG8, and ALG10) from Dol-P donors. The multi-subunit oligosaccharyltransferase (OST) complex catalyzes the transfer of the assembled oligosaccharide to the nascent polypeptide in the lumen of the ER with all subsequent steps restricted to the lumen of either the ER or GA. In the ER, the three Glc residues are sequentially trimmed by α-glucosidase I (GCSI) and II (GCSII) and a single α-(1 → 2)-Man residue is removed from the middle branch of the oligomannosidic N-glycan by the ER-α-mannosidase I (MNS3) to form the Man8GlcNAc2 structure (Liebminger et al., 2009). In the GA, the Golgi α-mannosidase I (MNS1/MNS2) cleaves off three additional Man residues and generates the acceptor substrate for N-acetylglucosaminyltransferase I (GnTI) that initiates complex-type N-glycan biosynthesis (von Schaewen et al., 1993; Strasser et al., 1999a, b). The product of GnTI can be either further trimmed by Golgi α-mannosidase II (GMII) or serve as a substrate for β-(1 → 2)-xylosyltransferase (XylT). N-acetylglucosaminyltransferase II (GnTII) transfers the second GlcNAc residue to complex-type N-glycans and core α-(1 → 3)-fucosyltransferase (FUT11/FUT12) attaches a α-(1 → 3)-linked Fuc to the innermost GlcNAc residue. The core fucosylation linkage is different from mammalian complex N-glycans which have a α-(1 → 6)-Fuc linked to the innermost GlcNAc residue (Strasser et al., 2004). The resulting structure (also termed GnGnXF) with two terminal GlcNAc residues, a β-(1 → 2)-linked Xyl and a core α-(1 → 3)-linked Fuc is the most prevalent complex-type N-glycan in plants (Wilson et al., 2001; Zeng et al., 2018). Truncated (paucimannosidic) N-glycans are generated in post-Golgi compartments either by the vacuolar β-N-acetylhexosaminidase 1 (HEXO1) or by HEXO3 which resides mainly in the plasma membrane/apoplast (Liebminger et al., 2011). The most elaborate complex-type N-glycans are generated in the trans Golgi by β-(1 → 3)-galactosyltransferase 1 (GALT1) and α-(1 → 4)-fucosyltransferase (FUT13) (Strasser et al., 2007b). The resulting Lewis A structure [α-L-Fucp-(1 → 4)- β-D-Galp-(1 → 3)- β-D-GlcpNAc-R] is ubiquitously found in plants (Fitchette-Lainé et al., 1997; Wilson et al., 2001; Zeng et al., 2018), but present only on a small number of secretory glycoproteins.
FIGURE 1.
N-glycosylation and N-glycan maturation steps in plants. Several Asn-linked glycosylation (ALG) enzymes catalyze the assembly of the dolichol pyrophosphate (PP)-linked oligosaccharide in the cytosol and ER. The multi-subunit oligosaccharyltransferase (OST) complex transfers the oligosaccharide to accessible Asn-residues of nascent proteins. N-glycan processing enzymes: α-glucosidase I (GCSI), α-glucosidase II (GCSII), ER α-mannosidase I (MNS3), Golgi α-mannosidase I (MNS1/MNS2), β-(1 → 2)-N-acetylglucosaminyltransferase I (GnTI), β-(1 → 2)-N-acetylglucosaminyltransferase II (GnTII), β-(1 → 2)-xylosyltransferase, core α-(1 → 3)-fucosyltransferase (FUT11/FUT12), β-(1 → 3)-galactosyltransferase 1 (GALT1), and α-(1 → 4)-fucosyltransferase (FUT13). Terminal GlcNAc residues can be removed by β-hexosaminidases (HEXO1/3). GHs and GTs are listed in Tables 1, 2 while probes against N-glycans are listed in Table 3.
Impaired N-glycosylation due to either a defective OST complex or a blocked Glc removal from the transferred oligosaccharide results in lethality in Arabidopsis (Boisson et al., 2001; Gillmor et al., 2002; Koiwa et al., 2003; Lerouxel et al., 2005). Distinct oligomannosidic N-glycans in the ER are critical for ER quality control and ER-associated degradation (ERAD) (Jin et al., 2007; Hong et al., 2012; Hüttner et al., 2014). For example, the biogenesis of the Arabidopsis EF-Tu receptor (EFR) is dependent on Glc trimming and reglucosylation of oligomannosidic N-glycans and association with the lectin chaperones calnexin/calreticulin (Li et al., 2009; Lu et al., 2009). Mutant misfolded variants of BRASSINOSTEROID INSENSITIVE1 (BRI1) that expose a terminal α-(1 → 6)-linked Man on the oligomannosidic N-glycan are recognized by the lectin OS9 and sent to ERAD (Hong et al., 2008, 2012; Hüttner et al., 2012). Knockout of the three α-mannosidases (MNS1–MNS3) involved in trimming of oligomannosidic N-glycans to Man5GlcNAc2 causes a severe root development phenotype (Liebminger et al., 2009). GnTI-deficient mutants (cgl1) which completely lack complex N-glycans and display primarily Man5GlcNAc2, on the other hand, do not show any growth or morphological phenotype in Arabidopsis (von Schaewen et al., 1993). However, a salt sensitivity phenotype has been described for cgl1 and other Arabidopsis N-glycan processing mutants that completely lack complex N-glycans or specific modifications in the GA and there are links to a role in cell wall formation (Kang et al., 2008). The β - (1→4)-endoglucanase KORRIGAN1, one of the potential glycoprotein candidates playing a role in these processes, does not require complex N-glycans for its activity (Liebminger et al., 2013), but there are other unknown factors that require GnTI and affect KORRIGAN1 function (Rips et al., 2014). Notably, an Arabidopsis mutant lacking complex N-glycans due to a deficiency in the UDP-GlcNAc transporter 1 (UGNT1) does not show a salt sensitivity phenotype (Ebert et al., 2018). Apart from Arabidopsis, complex N-glycan deficient rice and Lotus japonicus mutants have been characterized which display severe defects in growth and reproduction (Fanata et al., 2013; Strasser, 2014; Harmoko et al., 2016; Pedersen et al., 2017). Collectively, while the oligomannosidic N-glycans play a role in ER-quality control, the potential suite of biological functions of complex-type and paucimannosidic N-glycans on glycoproteins is still largely unknown and the underlying mechanisms remain to be elucidated for the described phenotypes in Arabidopsis and other plant species.
The biological function of the β-N-acetylhexosaminidase (HEXOs), especially HEXO3 acting at the plasma membrane/apoplast is unknown. In addition to HEXOs, it is possible that other GHs (Table 1) liberate monosaccharides from complex N-glycans either on a specific group of glycoproteins or in specific cell-types. Golgi-localized enzymes such as the recently characterized exo-β-(1 → 3)-galactosidases (Nibbering et al., 2020) may to some extent hydrolyze the Gal transferred by GALT1 directly in the Golgi (Table 2). Similarly, either Nicotiana benthamiana BGAL1 or another GH with β-(1 → 3/4)-galactosidase activity could modify Lewis A structures in the apoplast (Kriechbaum et al., 2020). Plant α-(1 → 3/4)-fucosidases that can cleave off Fuc residues from Lewis A structures have been identified in several plant species (Zeleny et al., 2006; Rahman et al., 2016; Kato et al., 2018). The only Arabidopsis GH29 α-(1 → 3/4)-fucosidase, AtFUC1, acts in the glycan degradation pathway in the vacuole and hydrolyses primarily the core α-(1 → 3)-linked Fuc. Consistent with the described substrate specificity, the AtFUC1-deficient mutant displayed slightly higher levels of Lewis A containing complex N-glycans. The degradation pathway for oligomannosidic and complex N-glycans in the vacuole involves several GHs whose substrate specificities are already well characterized (Léonard et al., 2009; Ishimizu, 2015; Kato et al., 2018). Apart from exo-glycosidases, plants have endo-glycosidases such as peptide-N-glycanase A (PNGase A) that is active on small glycopeptides and hydrolyzes complex N-glycans with core α-(1 → 3)-linked Fuc (Tretter et al., 1991; Altmann et al., 1998). Certain plant tissues such as the maize endosperm harbor an endo-glycanase (ENGase) that is active on oligomannosidic N-glycans and cleaves within the chitobiose core (Rademacher et al., 2008). Single GlcNAc residues or chitobiose at N-glycosylation sites have been detected on plant proteins (Ishimizu et al., 1999; Kim et al., 2013; Xu et al., 2016). How abundant those truncated glycans are and whether they have specific functions or represent intermediates of degradation pathways remains to be shown.
TABLE 1.
Selected examples for carbohydrate GTs acting on N-glycans and O-glycans including type-II AGs on AGPs and EXTs from Arabidopsis thaliana or otherwise as indicated.
| Activity | CAZy family | Protein name | References |
| GTs in N-glycan processing | |||
| β-(1 → 2)-N-acetylglucosaminyltransferase I | 13 | GnTI/CGL1/GlcNAc-T1 | von Schaewen et al., 1993; Strasser et al., 1999a |
| β-(1 → 2)-N-acetylglucosaminyltransferase II | 16 | GnTII | Strasser et al., 1999b |
| β-(1 → 2)-xylosyltransferase | 61 | XYLT | Strasser et al., 2000 |
| α-(1 → 3)-fucosyltransferase | 10 | FUT11/FUT12 | Leiter et al., 1999 |
| β-(1 → 3)-galactosyltransferase 1 | 31 | GALT1 | Strasser et al., 2007b |
| α-(1 → 4)-fucosyltransferase | 10 | FUT13/FucTC | Wilson et al., 2001; Bakker et al., 2001 |
| P4Hs in AGPs/EXTs processing | |||
| Prolyl-4-hydroxylase | – | CrP4H1 | Koski et al., 2007, 2009; Velasquez et al., 2011 |
| P4H2,P4H5,P4H13 | Velasquez et al., 2015a | ||
| GTs in AGPs processing | |||
| Hyp-O-galactosyltransferase | 31 | GALT2-GALT6; HPGT1-HPGT3 (GALT15-GALT17) | Basu et al., 2013, 2015a, b; Ogawa-Ohnishi and Matsubayashi, 2015 |
| β-(1 → 3)-galactosyltransferase | 31 | GALT14 (KNS4/UPEX1) | Suzuki et al., 2017; Li et al., 2012 |
| GALT9 (At1g77810) | Qu et al., 2008 | ||
| GALT31A | Geshi et al., 2013; Ruprecht et al., 2020 | ||
| β-(1 → 6)-galactosyltransferase | 29 | GALT29A | Dilokpimol et al., 2014 |
| 31 | GALT31A | Knoch et al., 2014 | |
| β-glucuronosyltransferase | 14 | GlcAT14A-GlcAT14E | Knoch et al., 2013; Dilokpimol et al., 2014; Lopez-Hernandez et al., 2020; Zhang et al., 2020 |
| α-fucosyltransferase | 37 | FUT4 | Wu et al., 2010; Liang et al., 2013; Tryfona et al., 2014 |
| FUT6 | |||
| FUT7 | Ruprecht et al., 2020 | ||
| β-arabinosyltransferase | 77 | RAY1 | Gille et al., 2013 |
| GTs in EXTs processing | |||
| Hyp-O-arabinosyltransferase | 95 | HPAT1-HPAT3 | Ogawa-Ohnishi et al., 2013; Velasquez et al., 2015a |
| β-(1 → 2)-arabinosyltransferase | 77 | RRA1-RRA3 | Egelund et al., 2007; Velasquez et al., 2011 |
| β-(1 → 2)-arabinosyltransferase | 77 | XEG113 | Gille et al., 2009; Velasquez et al., 2011 |
| α-(1 → 3)-arabinosyltransferase | 47 | EXAD | Møller et al., 2017 |
| Serine-O-galactosyltransferase | 96 | SGT1/SerGT1 | Saito et al., 2014; Velasquez et al., 2015a |
In addition, prolyl-4-hydroxylases (P4Hs) are included. Please also see Showalter and Basu (2016) and Silva et al. (2020) for GTs acting in AGP/EXT O-glycan processing.
TABLE 2.
Selected examples for carbohydrate GHs and lyases acting on N-glycans, O-glycans including type-II AGs on AGPs and EXTs.
| Activity | CAZy family | Protein name | Species | References |
| GHs in N-glycan processing and degradation | ||||
| α-(1 → 2)-glucosidase I | 63 | GCSI/KNF-14 | A. thaliana | Boisson et al., 2001; Gillmor et al., 2002 |
| α-(1 → 3)-glucosidase II | 31 | GCSII/RSW3 | A. thaliana | Burn et al., 2002 |
| ER α-(1 → 2)-mannosidase | 47 | MNS3 | A. thaliana | Liebminger et al., 2009 |
| Golgi α-(1 → 2)-mannosidase I | 47 | MNS1/MNS2/GMI | A. thaliana | Liebminger et al., 2009 |
| Glycine max | Nebenführ et al., 1999 | |||
| Golgi α-(1 → 3/6)-mannosidase II | 38 | GMII/HGL1 | A. thaliana | Strasser et al., 2006 |
| α-(1 → 3/4)-fucosidase | 29 | FUC1 | Prunus dulcis (almond), A. thaliana | Zeleny et al., 2006; Kato et al., 2018 |
| β-(1 → 3/4)-galactosidase | 35 | BGAL1 | Nicotiana benthamiana | Kriechbaum et al., 2020 |
| β-hexosaminidases | 20 | HEXOs | A. thaliana | Strasser et al., 2007a; Liebminger et al., 2011 |
| Lytic enzymes acting on type-II AGs glycans of AGPs | ||||
| FvEn3GAL | 16 | FvEn3GAL | Flammulina velutipes | Kotake et al., 2011 |
| exo-β-(1 → 3)-galactanase | 43 | Il1,3Gal | Irpex lacteus | Tsumuraya et al., 1990; Kotake et al., 2009 |
| 43 | A. thaliana | Nibbering et al., 2020 | ||
| endo-β-(1 → 6)-galactanase | 30 | Tv6GAL | Trichoderma viride | Kotake et al., 2004 |
| 30 | Nc6GAL | Neurospora crassa | Takata et al., 2010 | |
| β-(1 → 3),(1 → 6)-galactanase | 35 | RsBGAL1 | Raphanus sativus | Kotake et al., 2005 |
| β-(1 → 3),(1 → 6)-galactanase | 35 | SlTBG1 | Solanum lycopersicum | Eda et al., 2014 |
| α-L-arabinofuranosidase | 54 | NcAraf1 | Neurospora crassa | Takata et al., 2010 |
| 3 | RsAraf1 | A. thaliana, Raphanus sativus (radish), and Bacteroides thetaiotaomicron | Kotake et al., 2006 | |
| Bacteroides thetaiotaomicron | 127 | Cartmell et al., 2018 | ||
| β-L-arabinopyranosidase | 27 | SaArap27A | Streptomyces avermitilis | Ichinose et al., 2009 |
| 27 | AtAPSE | A. thaliana | Imaizumi et al., 2017 | |
| β-glucuronidase | 79 | NcGlcAase | Neurospora crassa | Konishi et al., 2008 |
| 79 | AnGlcAase | Aspergillus niger | ||
| 79 | AtGUS2 | A. thaliana | Eudes et al., 2008 | |
| 4-5-anhydro-glucuronidase | 154 | Bacteroides thetaiotaomicron, Bacteroides thetaiotaomicron | Cartmell et al., 2018 | |
| 105 | Cartmell et al., 2018 | |||
| α-L-rhamnosidase | 28 | SaRha78A | Aspergillus niger | Martens-Uzunova et al., 2006 |
| 78 | Streptomyces avermitilis | Ichinose et al., 2013 | ||
| 106 | Sphingomonas paucimobilis | Miyata et al., 2005) | ||
| α-L-rhamno-glucurono lyase | PL27 | Bacteroides cellulosilyticus | Cartmell et al., 2018 | |
| exo-α-L-(1 → 2)-fucosidase | 95 | AfcA | Bifidobacterium bifidum | Katayama et al., 2004 |
| 95 | Aspergillus nidulans | Pogorelko et al., 2016 | ||
| ? | Xanthomonas manihotis | Wong-Madden and Landry, 1995 | ||
| Lytic enzymes acting on glycans in EXTs | ||||
| β-L-arabinofuranosidase | Bifidobacterium bifidum | Fujita et al., 2014 | ||
| β-L-(1 → 2)-arabinofuranosidase | 121 | HypBA2 | Bifidobacterium bifidum | Fujita et al., 2011 |
| β-L-(1 → 2)-arabinofuranosidase | 121 | XeHypBA2 | Xanthomonas euvesicatoria | Nakamura et al., 2018 |
| (XCV2729) | ||||
| α-L-(1 → 3)-arabinofuranosidase | 43 | XeHypAA (XCV2728) | Xanthomonas euvesicatoria | Nakamura et al., 2018 |
| Arabinofuranosidase-(1 → 4)-Hyp | 127 | XeHypBA1 (XCV2724) | Xanthomonas euvesicatoria | Nakamura et al., 2018 |
Please also see Silva et al. (2020) for GHs acting on type-II AG O-glycans of AGPs.
Oligomannosidic N-glycans are commonly detected with the lectin concanavalin A (ConA) derived from the jack-bean Canavalia ensiformis (von Schaewen et al., 1993). Complex and truncated N-glycans carrying β-(1 → 2)-linked Xyl and/or a core α-(1 → 3)-linked Fuc residues are detected with antibodies against horseradish peroxidase (HRP) (Wilson et al., 1998; Strasser et al., 2004). The Lewis A structure is specifically recognized by the monoclonal antibody (mAb) JIM84. Bacterial endo-β-N-acetylglucosaminidase H (Endo H) cleaves within the unsubstituted chitobiose core to release oligomannosidic N-glycans from glycoproteins (Tarentino et al., 1974). In contrast to the PNGase A from almond, PNGase F from Flavobacterium meningosepticum is inhibited by the presence of core α-(1 → 3)-linked Fuc (Tretter et al., 1991) and therefore only of limited use for the deglycosylation of plant glycoproteins decorated with complex N-glycans.
O-Glycans, GTs and GHs of Plant Glycoproteins
O-linked glycosylation defines the molecular properties and biological function of the HRGP superfamily and some secreted small hormone peptides (e.g., CLE-like peptides). The HRGP superfamily is traditionally divided into three major subgroups: AGPs, EXTs including the Leucine-Rich eXtensins (LRXs), and the repetitive Pro-rich proteins (PRPs) (Seifert and Roberts, 2007; Ellis et al., 2010; Tan et al., 2012; Hijazi et al., 2014; Johnson et al., 2017). However, the HRGP superfamily is better understood as a spectrum of molecules ranging from the highly glycosylated AGPs to the minimally O-glycosylated PRPs. Two major types of O-glycans are attached to Hyp (O) in plant glycoproteins. The first type includes unbranched chains of up to five arabinose (Ara) units added to clusters of Hyp residues in EXTs (Marzol et al., 2018) and small CLE-like peptides (Ohyama et al., 2009; Shinohara and Matsubayashi, 2013). The second type are complex type II arabino-3,6-galactans (AGs) which are attached to non-contiguous Hyp residues (AO/SO/TO/VO) on AGPs and AGP-like proteins (Johnson et al., 2017). Finally, a single Gal is linked to Ser mostly in EXTs and EXT-related proteins. The Hyp contiguity hypothesis proposes that the addition of these two main types of O-glycan is controlled by “glycomotifs” in the HRGP protein sequence (Kieliszewski, 2001). This hypothesis predicts that short arabino-oligosaccharides are added to contiguous Hyp3–5 residues in EXTs, whereas complex AGs are assembled on clustered but non-contiguous Hyp residues in AGPs (Shpak et al., 1999; Tan et al., 2010). The only exception to this rule is CLE-like peptides (e.g., Tob/Tom-HypSys, PSY1, CLV3, and CLE2), in which non-contiguous Hyp residues are arabinosylated (Ohyama et al., 2009; Shinohara and Matsubayashi, 2013). The extent of glycosylation of PRPs remains unclear with low levels of Ara residues presumably O-linked to Hyp (Bernhardt and Tierney, 2000).
Arabinogalactan-Proteins-O-glycans and GTs
Arabinogalactan-proteins are complex cell surface proteoglycans with type II AG glycan moieties attached at non-contiguous Hyp residues consisting of a β-(1 → 3)-galactan backbone substituted at C(O)6 with side chains of β-(1 → 6)-galactan of variable length decorated further with Ara, and less frequently also with Fuc, Rha, (O-methyl)glucuronic acid (4-O-MeGlcA) and Xyl (Figure 2). AGPs have been implicated in a diverse array of plant growth and development processes including hormone signaling, cell expansion and division, embryogenesis of somatic cells, differentiation of xylem, reproduction and responses to abiotic stress (Seifert and Roberts, 2007; Ellis et al., 2010; Ma et al., 2018). Recently, it was shown that perturbing an AG-peptide (AGP21) in Arabidopsis triggers aberrant root hair development by altering expression of the homeodomain protein GLABRA 2 (GL2) expression in a BIN2 (a Type-II GSK3-like kinase)-dependent manner, similar to the phenotype observed in plants with defective brassinosteroid signaling (Borassi et al., 2020). These results imply an interesting parallel between plant AGPs and animal heparin sulfate proteoglycans (HSPGs), which are important co-receptors in signaling pathways mediated by growth factors, including members of Wnt/Wingless, Hedgehog, transforming growth factor−β, and fibroblast growth factor family members (Lin, 2004). AGP4, AGP6, and AGP11 from Arabidopsis have been shown to be essential for reproduction, with AtAGP4 shown to play a critical role in synergid degeneration and prevention of more than one pollen tube being attracted to the embryo sac (Pereira et al., 2016). AG glycan structures have also been found to be involved in reproductive development in Torenia fournieri with a methyl-glucuronosyl arabinogalactan (AMOR) released from the ovule inducing the competency of the pollen tube to respond to ovular attractant peptides (Mizukami et al., 2016; Jiao et al., 2017). UPEX1/KNS4/GALT14, a galactosyltransferase (GALT) from Arabidopsis that generates the β-(1 → 3)-galactan backbone of type II AG, has been shown to be vital for normal pollen exine development as upex1/kns4/galt14 mutants display a collapsed pollen phenotype with reduced viability and fertility (Suzuki et al., 2017). The requirement for specific glycan structures on AGPs for Ca2+ signaling during development is supported by mutants in GlcAT14 members. AG glycans with reduced glucuronosylation were shown to have lower Ca2+ binding capacity (Lopez-Hernandez et al., 2020). Double/triple glcat mutants displayed developmental defects that could be suppressed by additional Ca+2 in growth media. Unique glycan structures on AGPs in seagrasses, that include a high content of terminating 4-O-methyl-GlcA residues, are proposed to strengthen Ca2+ binding and limit the effects of salt as an adaptation to the marine environment (Pfeifer et al., 2020). These few examples demonstrate the indispensable nature of AGPs to plant processes and the important function their O-glycan moieties play, although their mechanistic role continues to remain elusive and ill-defined as recently reviewed (Seifert, 2020).
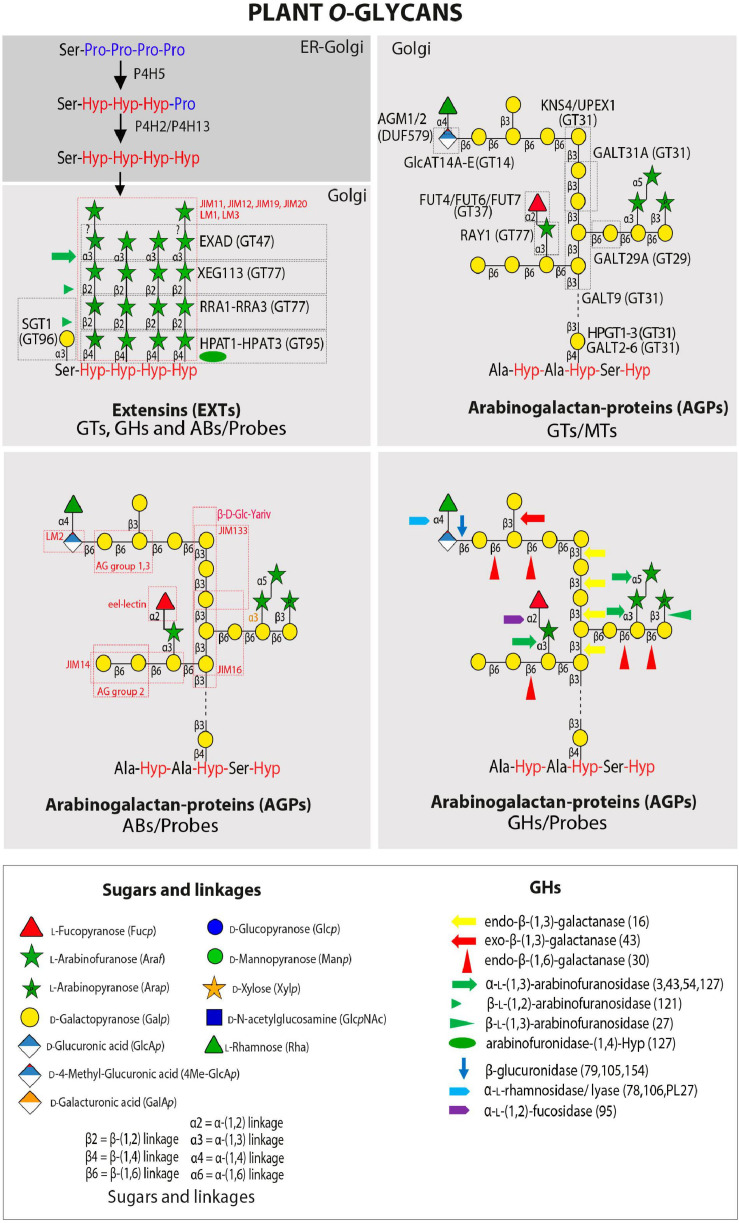
FIGURE 2.
Plant O-glycans. Schematic representation of an average carbohydrate structure of EXTs and AGPs with the GTs and GHs that have been characterized to date. Illustrated are the complex sugar side chains and the different linkages that are found in the sugar backbone. GHs and GTs are listed in Tables 1, 2 while probes recognizing specific epitopes in AGPs and EXTs are listed in Table 3. Number in brackets refer to GHs CAZY family. Please also see Silva et al. (2020) for GTs and GHs acting in AGP O-glycan processing.
In Arabidopsis, O-glycosylation of AGPs is initiated by a set of 8 Hyp-galactosyltransferases (Hyp-GALTs/HPGTs), which are members of the GT31 family1 (Lombard et al., 2014) and designated as GALT2-GALT6 and HPGT1-HPGT3 (also designated as GALT15-GALT17, respectively) (Basu et al., 2015a, b; Ogawa-Ohnishi and Matsubayashi, 2015) by different groups (Table 1 and Figure 2). These enzymes add a single Gal unit to Hyp residues. Other known GTs include β-(1 → 3)-GalTs also from the GT31 family such as GALT8, GALT9, KNS4/UPEX1/GALT14, (Qu et al., 2008; Li et al., 2012; Suzuki et al., 2017; Ruprecht et al., 2020) and β-(1 → 6)-galactosyltransferases such as GALT29A from GT29 (Geshi et al., 2013; Dilokpimol et al., 2014) although this activity is yet to be independently verified. Previously reported β-(1 → 6)-GalT activity for GALT31A (Geshi et al., 2013) has not been confirmed, rather it has been shown to possess β-(1 → 3)-GalT activity (Ruprecht et al., 2020). β-Glucuronosyltransferases including GlcAT14A-GlcAT14E from the GT14 family (Knoch et al., 2013; Dilokpimol et al., 2014; Lopez-Hernandez et al., 2020; Zhang et al., 2020) have been characterized as well as α-fucosyltransferases (FUT4, FUT6, and FUT7 from GT37) (Liang et al., 2013; Tryfona et al., 2014, Ruprecht et al., 2020) and a β-arabinosyltransferase (Reduced Arabinose Yariv1/RAY1 from GT77) (Gille et al., 2013). Two GlcA methyltransferases (AtAGM1 and AtAGM2) have also recently been identified (Temple et al., 2019). Collectively, this body of work highlights that robust data are required to confidently assign biochemical function(s) to GTs. Several other GTs involved in type II AG biosynthesis remain to be identified, including β-(1 → 6)-GalTs that elongate the side chains and other arabinosyltransferases, rhamnosyltransferases, and xylosyltransferases that decorate the non-reducing termini of the galactan chains as well as additional sugar modifying enzymes. Furthermore, structural characterization using NMR of artificial AGPs expressed in tobacco cell suspension cultures indicated kinks of β-(1 → 6)-linked Gal in the β-(1 → 3)-galactan backbone, suggesting the existence of additional GTs catalyzing the synthesis of this linkage (Tan et al., 2004, 2010). Please also see a recent review by Silva et al. (2020) that has reviewed the GTs acting in AGP O-glycan processing.
Variability of Type II AG O-Glycans
Compared to the relatively high degree of conservation of N-glycan structures, O-glycans attached to AGPs display a considerable degree of variation on every level (Figure 3 and references there in). There are variations between different species and tissues and in the same cell type at different stages of development. The common structural feature of type II AG that are O-linked to isolated Hyp residues on AGPs is a backbone of β-(1 → 3) Gal that contains β-(1 → 6) linked Gal side chains of variable length, although there are examples of β-(1 → 6) linked Gal backbones (Raju and Davidson, 1994; Dong and Fang, 2001). In some reports a β-(1 → 6) linked Gal is further β-(1 → 3) galactosylated forming a kink in the backbone (Churms et al., 1983; Bacic et al., 1987). Mostly however, the Gal side chains are modified by α-(1 → 3) linked L-Araf (Tryfona et al., 2010, 2012 and references therein). Additionally, the side branches can contain β-(1 → 6) linked GlcA or 4-O-MeGlcA. The L-Araf side groups are sometimes extended by one or two α-(1 → 3) linked L-Araf residues and terminated by either α-(1 → 3) linked L-Araf or α-(1 → 2) linked L-Fuc. In some cases, the L-Fuc is not the terminal sugar but further modified by β-(1 → 3) linked D-Xyl. While L-Araf incorporated in plant cell wall carbohydrates is predominantly found in its furanose form there have also been reports on L-Arap β-(1 → 3) linked to Araf or Galp as terminal sugars. Likewise, GlcAp and 4-O-methyl D-GlcAp are often found as terminal modifications of the galactan backbone but sometimes GlcA was found decorated by α-(1 → 4) linked L-Rha. In other cases, another β-(1 → 4) linked D-GlcA followed and terminated by β-(1 → 4) linked 4-O-methyl D-GlcAp were linked to this sugar. Another modification of D-GlcAp was α-(1 → 4) linked L-Rha as the first sugar of an extended heteropolymer resembling rhamnogalacturonan I. Besides this staggering multitude of structures attributed to AGP-linked type II AG, there exists variability in the degree of substitution of individual Hyp residues as well as the sizes of the individual glycans. This was demonstrated for artificial AGP-like fluorescent proteins that showed considerable variations in apparent molecular weight between different organs (Estevez et al., 2006). Moreover, the cell-type specific variation between type II AG structures is elegantly revealed by AGP-glycan specific monoclonal antibodies (mAbs) (Table 3).
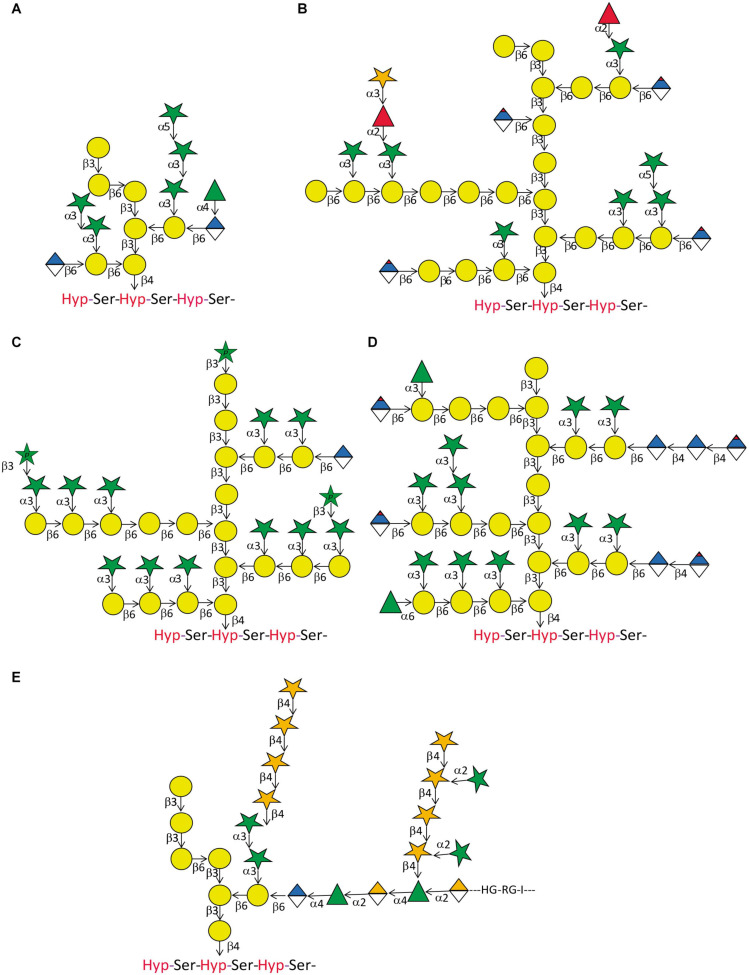
FIGURE 3.
Arabinogalactan-protein glycan variation. Five structures of type II glycans found on AGPs demonstrating common motifs and variations. (A) This relatively small glycan was produced on an artificial AGP recombinantly expressed in tobacco cell cultures by Tan et al. (2004). Note the β-(1 → 6) kink in the β-(1 → 3) galactan backbone. (B) This structure approximates the model described for AGP glycans purified from A. thaliana leaves (Tryfona et al., 2012). Note that the actual size of many of the glycans is probably much bigger than the structure displayed here. In a later study by the same group, the terminal modification of L-Fuc by D-Xyl was described (Tryfona et al., 2014). (C) Using the same tools of enzymatic degradation and mass spectrometry, this group also described the glycan-structure of wheat flour AGP (Tryfona et al., 2010). Again, we show an approximation of their model that should accommodate large variations in glycan size. A noteworthy feature of this glycan is the occurrence of terminally linked L-Arap. (D) AGP-glycans of the see grass Zostera marina are particularly rich in 4-Me-GlcAp (Pfeifer et al., 2020). (E) The partial glycan structure of the type II AG linked to an AGP named as APAP1 that is linked to both rhamnogalacturonan 1 (RG1) and arabinoxylan (AX) (Tan et al., 2013). Legend for sugar symbols is as per Figure 2.
TABLE 3.
Toolkit Abs/probes available to characterize N- and O-glycans.
| mAbs/reagent | Species origin | Tissue origin | Minimal epitope recognized | References |
| JIM4 | Daucus carota | Suspension cultured cells | β-GlcA-(1 → 3)-α-GalA-(1 → 2)-Rha | Knox et al., 1989; Yates et al., 1996 |
| JIM8 | Beta vulgaris | Suspension cultured cells | unknown | Pennell et al., 1991 |
| JIM13 | Daucus carota | Suspension cultured cells | β-GlcA-(1 → 3)-α-GalA-(1 → 2)-Rha | Knox et al., 1991; Yates et al., 1996 |
| JIM14 | Daucus carota | Suspension cultured cells | β-Gal-(1 → 6)-β-Gal-(1 → 6)-β-Gal-(1 → 6) | Knox et al., 1991; Yates et al., 1996; Ruprecht et al., 2017 |
| JIM15 | Daucus carota | Suspension cultured cells | unknown | Knox et al., 1991; Yates et al., 1996 |
| JIM16 | Daucus carota | Suspension cultured cells | β-Gal-(1 → 6) | Knox et al., 1991; Yates et al., 1996; Ruprecht et al., 2017 |
| β-Gal-(1 → 3)-β-Gal-(1 → 3)-β-Gal-(1 → 3) | ||||
| JIM84 | Daucus carota | Suspension cultured cells | α-L-Fucp-(1 → 4)-β-D-Galp-(1 → 3)-β-D-GlcpNAc-R | Horsley et al., 1993 |
| JIM101 | Gymnocolea inflata | Extracted AGPs | unknown | Pattathil et al., 2010 |
| JIM133 | Zinnia elegans | Tracheary element cell walls | β-Gal-(1 → 3)-β-Gal-(1 → 3)-β-Gal-(1 → 3) | Pattathil et al., 2010; Ruprecht et al., 2017 |
| LM2 | Oryza sativa | Suspension cultured cells | β-GlcA-(1 → 6)-β-Gal-(1 → 6)-β-Gal-(1 → 6)-β-Gal-(1 → 6) | Smallwood et al., 1996; Ruprecht et al., 2017 |
| LM14 | Arabidopsis thaliana | Mixed leaves, stems and roots | Unknown | Møller et al., 2008 |
| MAC204 | Pisum sativum | Peribacteroid membrane | Unknown | Bradley et al., 1988 |
| MAC207 | Pisum sativum | Peribacteroid membrane | β-GlcA-(1 → 3)-α-GalA-(1 → 2)-Rha | Pennell et al., 1989; Yates et al., 1996 |
| PN16.4B4 | Nicotiana glutinosa | Suspension cultured cells | Unknown | Norman et al., 1986; Pattathil et al., 2010 |
| β-Glc Yariv (synthetic dye) | β-Gal-(1 → 3)-β-Gal-(1 → 3)-β-Gal-(1 → 3)-β-Gal-(1 → 3)-β-Gal)n>5 | Yariv et al., 1967; Kitazawa et al., 2013; Paulsen et al., 2014 |
Arabinogalactan-Protein-Glycans Probes, Abs, and GHs
Characterization of AGP glycan structures is difficult due to the enormous diversity of protein backbones, the difficulty in extracting and purifying individual AGPs and the heterogeneity in their glycan moieties (Tan et al., 2012; Johnson et al., 2017). NMR techniques require significant amounts of relatively homogeneous samples and are therefore only rarely used on natural AGP glycans; for example, on the AGP glycans from pistils of Nicotiana alata (Gane et al., 1995). The most successful approaches include performing (i) linkage analyses of glycans in combination with partial chemical degradation of the polysaccharides (Pfeifer et al., 2020) and (ii) partial enzymatic degradation (GHs; Table 2) to analyze resulting oligosaccharides using carbohydrate gel electrophoresis (PACE) and MS fragmentation techniques (Tryfona et al., 2010, 2012). For specific detection of AGPs, β-Glc Yariv phenylglucoside reagent (1,3,5-tri-(4-β-D-glucopyranosyl-oxyphenylazo)-2,4,6-trihydroxybenzene) that recognizes and binds to β-(1 → 3)-Gal-linked oligosaccharides, six residues or longer, is used. In addition, numerous mAbs against AGP glycan epitopes are available (Table 3). A group of 36 AGP mAbs recognize a core set of Gal-β-(1 → 6)-Gal epitopes that are further sub-divided into three groups defined by side branches permitted and forbidden for mAb binding (Figure 2; Pattathil et al., 2010; Ruprecht et al., 2017). In addition, some mAbs recognize different epitopes, namely a β-(1 → 3)-trigalactosyl glycan (JIM133), a β-(1 → 6)-trigalactosyl glycan (JIM14) or a β-(1 → 6)-Gal branched β-(1 → 3)-trigalactosyl glycan (JIM16) while the terminal β-(1 → 6)-glucuronosyl modification is recognized by LM2. Furthermore, eel lectin binds to the terminal α-(1 → 2)-L-Fuc residue modification on AGPs. The binding epitopes of several AGP-specific mAbs remain to be characterized (e.g., JIM4, JIM8, JIM13, MAC207, and LM14) (Table 3).
An as yet incomplete list of GHs acting on various linkages in type II AGs are mainly known from various microbial sources (Table 3) and are used for their structural characterization. However, plant endogenous AGP-specific GHs have also been described. Two family GH43 exo-β-(1 → 3)-galactanases from Arabidopsis were shown to be required for controlling the apparent abundance of AGPs and their loss of function resulted in a sugar-conditional root expansion phenotype characteristic of many primary cell wall-defective mutants (Nibbering et al., 2020). Arabidopsis also has three close homologs encoding family 79 GHs. One member of this family named AtGUS2 was identified in a gel filtration fraction that showed O-β-glucuronidase activity in vitro (Eudes et al., 2008). A T-DNA insertion in this locus displayed abnormally short hypocotyls and overexpression of AtGUS2 enhanced both hypocotyl length and root length with purified AGPs displaying lower terminal-GlcA content. Finally, four Arabidopsis loci encode family 27 GHs named β-L-ARAPASE (APSE), and α-GALACTOSIDASE 1-3 (AGAL1-3) (Imaizumi et al., 2017). Although the majority of L-Ara found in plant carbohydrates is in its furanose form some examples of L-arabinopyranose (Arap) exist, one example being found in type II AGs (Tryfona et al., 2010). It was suggested that APSE and the AGALs act on these residues (Imaizumi et al., 2017), and apse agal3 mutants showed decreased β-L-arabinopyranosidase activity and increased levels of β-L-Arap, compared to wild type. Apart from a decrease in hypocotyl length, the apse agal3 mutants appeared phenotypically normal. Finally, a promiscuous α-L-arabinofuranosidase/β-D-xylosidase belonging to family GH3 has been purified and cloned from radish (Kotake et al., 2006]. However, since the Ara and Xyl residues exist in various carbohydrates it is presently unknown whether any of the fifteen Arabidopsis GH3 enzymes act as AGP-specific α-L-arabinofuranosidases. In addition, please see Silva et al. (2020) for GHs, both endogenous and heterologous, acting on type-II AG glycans of AGPs.
Extensins-Glycans, GTs, and GHs
Extensins are characterized by repetitive Ser-Hyp3–5 repeats, where the contiguous Hyp residues are substituted with up to 4–5 units of L-Araf with the following structure Hyp-O-(4 → 1)-β-L-Araf-(2 → 1)-β-L-Araf-(2 → 1)-β-L-Araf-(3 → 1)-α-L-term Araf; the linkage of the fifth Ara residue is not yet resolved (Velasquez et al., 2011; Møller et al., 2017), and the Ser is substituted with D-Gal as Ser-O-(1 → 3)-α-Galp (Saito et al., 2014) (Figure 2). EXTs and secreted signaling peptides require the conversion of specific peptidyl-proline residues to trans-4-Hyp by prolyl-4-hydroxylase (P4H) enzymes (Table 1). P4H enzymes are 2-oxoglutarate (2OG) dioxygenases that catalyze the formation of trans-4-Hyp from peptidyl-Pro (Koski et al., 2007, 2009). In root cells, P4H5 is the main P4H that initiates the hydroxylation of some Pro residues in EXTs, whereas P4H2 and P4H13 complete the hydroxylation on these contiguous Pro residues (Velasquez et al., 2011, 2015b). The first Araf is added by Hyp-O-β-arabinosyltransferase 1-3 (HPAT1-HPAT3), which belong to the GT95 family (Ogawa-Ohnishi et al., 2013). Reduced Residual Arabinose 1–3 (RRA1-RRA3) enzymes of the GT77 family are thought to transfer the second Araf (Egelund et al., 2007; Velasquez et al., 2011), while the third residue addition is catalyzed by Xyloglucanase113 (XEG113), which also belongs to the GT77 family (Gille et al., 2009). XEG113 was first identified in a screen of mutagenized Arabidopsis plants subjected to growth in liquid media in the presence of a xyloglucanase with xeg113 plants exhibiting more elongated hypocotyls than WT, providing genetic evidence that extensin arabinosylation is important for cell elongation (Gille et al., 2009). Finally, Extensin Arabinose Deficient (ExAD) transfers the fourth Araf residue with a α-(1 → 3) linkage. ExAD belongs to clade-E of the inverting GT47 family (Møller et al., 2017) (Table 1). The arabinosyltransferase that adds the fifth and final Ara unit has not yet been identified (Velasquez et al., 2011). On the other hand, O-arabinosylation with β-linked-L-arabinofuranosyltransferases at Hyp also takes place in the short signaling peptides of the CLE-like family using identical linkages/stereochemistry as used for the innermost three Araf residues found in the EXTs (Ito et al., 2006; Ohyama et al., 2009; Matsuzaki et al., 2010), suggesting that similar P4Hs and GTs might participate in these post-translational modifications. A single Serine-galactosyltransferase (SGT1/SerGT1) adds Gal to Ser in the repeated Ser-Hyp3–5 motif in EXTs (Saito et al., 2014). SerGT1 is the first example of a GT in the context of protein glycosylation with type-I membrane protein topology (i.e., N-terminal catalytic domain within the Golgi lumen) with no homology to known GTs, indicating that it is a novel plant-specific GT of the GT96 family (Table 1). Several EXT-specific mAbs are used to detect EXT epitopes but these epitopes remain to be structurally characterized (e.g., JIM11, JIM12, JIM19, JIM20, LM1) (see Table 3; Rydahl et al., 2018 and references therein).
Several GHs from different bacterial sources have been described that hydrolyze specific linkages within O-glycans of EXTs (Table 2). The GH127 enzyme from Xanthomonas euvesicatoria XeHypBA1 was described as a β-L-(1 → 2)-arabinofuranosidase (Nakamura et al., 2018) while two GH121 members, one from Bifidobacterium bifidum HypBA2 and XeHypBA2, were shown to hydrolyze the β-L-Araf-(2 → 1) linkages (Fujita et al., 2011, 2014; Nakamura et al., 2018). Finally, an α-L-(1 → 3)-arabinofuranosidase XeHypAA is able to hydrolyze β-L-Araf-(3 → 1)-α-L-Araf (Nakamura et al., 2018) (Table 2 and Figure 2). It is unclear if endogenous β-arabinofuranosidases are encoded by plant genomes, and if so whether they are secreted into the apoplast to regulate the length of EXTs O-glycans.
Decoding EXTs and Their O-Glycans Functions
It is already known that O-glycans increase HRGP solubility, resistance to proteolytic degradation and thermal stability (Shpak et al., 2001; Kieliszewski et al., 2011; Lamport et al., 2011; Seifert, 2020). EXTs are able to form, at least in vitro, a tridimensional covalent network through diTyr-linkages mediated by EXT peroxidases between individual EXT molecules and also via self-recognition and alignment of hydrophilic O-glycosylated Ser-(Hyp)3–4 repeats and hydrophobic peptide-cross-linking modules (Cannon et al., 2008). Thus, the ordered EXT monomer assembly in plant cell walls would involve a zipper-like endwise association via cross-linking at the ends of the molecules (Kieliszewski et al., 2011; Lamport et al., 2011). Recently, modeling experiments suggested that classical EXTs would be able to form a putative triple helix structure by lateral staggered alignment (Cannon et al., 2008) and diTyr cross-linking, similar to that present in collagen (Velasquez et al., 2015b; Marzol et al., 2018). It is also proposed that EXTs interact with pectins by a simple acid-base reaction forming a supramolecular ionic structure in the nascent cell wall (Valentin et al., 2010), which would serve as a framework for further cell wall deposition (Cannon et al., 2008; Lamport et al., 2011). In addition, covalent EXT-pectin cross-links were also suggested (Nuñez et al., 2009). However, it is unclear how EXT monomers are secreted and assembled into the glyco-network and how EXT and related glycoproteins-pectin interactions are controlled in a coordinated way during new cell wall formation.
Several mutants in O-glycosylation GTs of EXTs and related proteins (e.g., LRXs) have similarities to root hair-defective growth phenotypes (Velasquez et al., 2011; 2015b) and EXT content and their O-glycosylation levels were correlated with cotton fiber cell elongation (Guo et al., 2019), highlighting that O-glycans in EXTs affect EXT function during plant cell expansion. Furthermore, an in vitro study has revealed that both Ser-O-galactosylation and Hyp-O-arabinosylation determine the rate of EXT crosslinking and hence the efficiency of EXT network formation (Chen et al., 2015). Thus, correct arabinosylation of EXTs is essential for their in vivo functions. In addition, some of these mutants (e.g., rra2 and xeg113) showed enhanced susceptibility for specific root pathogens (Castilleux et al., 2020). The known roles of EXTs in cell wall assembly, cell shape and growth raises the question to the function of each individual EXT molecule (Hall and Cannon, 2002; Cannon et al., 2008; Velasquez et al., 2011). Although the Arabidopsis genome encodes several EXTs, so far only a single EXT mutant rsh (for root shoot hypocotyl-defective)/ext3) have a nearly lethal phenotype (Cannon et al., 2008). This finding suggests either the high redundancy or masked functions of EXTs in plant development, although their role in root hairs, pollen tubes and root growth are clear exceptions to this rule. Several EXT mutants (ext6-7/12-14/18) (Velasquez et al., 2011) and lrx1/2 mutants have aberrant root hair morphologies (Baumberger et al., 2001, 2003a, b; Ringli, 2010) and prp3 (Bernhardt and Tierney, 2000) display short root hairs. Characterization of multiple mutants for pollen LRXs (lrx8/9/10/11) indicates they are key components for proper polar growth as sentinels of cell wall integrity in these rapidly expanding cells (Fabrice et al., 2018; Wang et al., 2017; Sede et al., 2018; Herger et al., 2019) while the triple mutant lrx3/4/5 showed defects in cell expansion in root cells (Draeger et al., 2015), possibly mediated by abnormal vacuolar expansion (Dünser et al., 2019). Recently, a mechanism of action for LRXs was proposed based on LRX8 and LRX9 binding in the apoplast to the Rapid Alkalinization Factor 4-19 (RALF4 and RALF19) peptides as well as to the extracellular domains of some transmembrane receptors such as CrRLK1Ls (e.g., ANX1,2 and BUDS1,2) (Ge et al., 2017; Mecchia et al., 2017). In a similar manner, the extracellular LRX3/4/5-RALF22/23 together with CrRLK1L FERONIA (FER) are able to coordinate growth under salt conditions (Zhao et al., 2018, 2020) and LRX1/5-RALF1-FER in shoot and root growth (Dünser et al., 2019; Herger et al., 2020). It has been proposed that LRXs work together with CrRLK1Ls and RALF peptides to monitor the plant cell wall integrity status during cell growth (Ge et al., 2017; Mecchia et al., 2017; Dünser et al., 2019; Herger et al., 2020). Although the structural basis for the interaction between LRXs and RALFs peptides was recently established (Moussu et al., 2020), it is unclear how the O-glycans in the EXT domain of LRXs affects these protein-protein interactions. Since the EXT domain is variable among LRXs both in terms of length and motif (Baumberger et al., 2003a, b; Borassi et al., 2016), it is proposed that it has adapted to the specific cell wall architecture of the numerous tissues where they are located as putative cell wall integrity sensors (Baumberger et al., 2003a, b; Marzol et al., 2018; Sede et al., 2018; Herger et al., 2019).
Chemical Synthesis, Glycan Arrays and Technological Challenges
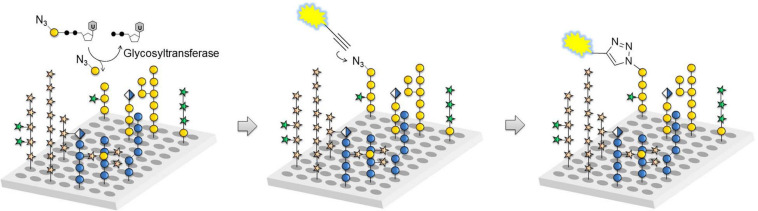
The tremendous heterogeneity of plant cell wall glycans such as the O-glycans in AGPs make the identification of the exact molecular structures that serve either as acceptors for GTs, substrates for GHs or epitopes for mAbs very challenging. There are basically two options to procure suitable oligosaccharide samples for biochemical assays used in GT functional studies. One possibility is purification of oligosaccharides from digests of natural polysaccharides or glycoproteins, which can provide a large number of oligosaccharides in acceptable time, but oftentimes with compromised purity and in limited quantities (Tan et al., 2012). The second possibility is chemical synthesis, which gives access to significant amounts of well-defined and pure oligosaccharides but is very time consuming (Kinnaert et al., 2017; Pfrengle, 2017). Automated glycan assembly (AGA) can significantly accelerate the process of chemical synthesis for a number a glycan classes (Seeberger, 2015). In AGA, protected monosaccharide building blocks are coupled in a stepwise manner to a linker-functionalized Merrifield resin, in a computer-controlled and automated manner. While many different complex oligosaccharides have been synthesized by AGA, only recently has it begun to be explored for synthesizing plant glycans, including AGP O-glycans (Bartetzko and Pfrengle, 2019). Chemically synthesized glycans as well as natural polysaccharides and isolated oligosaccharides can be printed as glycan arrays to obtain high-throughput platforms for analyzing plant cell wall-related enzymes and molecular probes such as mAbs (Møller et al., 2008; Pedersen et al., 2012). A recently developed glycan array equipped with chemically synthesized plant cell wall glycans, including many AGP glycan related substrates, has proven useful for the rapid characterization of a large number of cell wall glycan-directed mAbs (Ruprecht et al., 2017). The same glycan array has also aided in identifying acceptor substrates for GTs involved in AGP glycan biosynthesis such as GalT31A and FUT7 (Figure 4; Ruprecht et al., 2020). By extension, this technology has the potential to reveal the biochemical function of novel GTs that act in the O-glycosylation pathway of plant HRGPs and other glycoproteins.
FIGURE 4.
Glycan array assay for GT characterization. Glass slides equipped with plant cell wall-related oligosaccharides are incubated with azido-functionalized sugar nucleotides and GT candidates expressed in, for example human embryonic kidney (HEK) 293 cells. Any transferred monosaccharide is visualized by azide-alkyne cycloaddition reaction with a fluorescent dye to determine reactive acceptors (reprinted from Ruprecht et al., 2020).
Perspectives and Future Challenges in Plant Glycobiology
Major developments in nuclease-based gene editing, quantitative transcriptomics, metabolomics, and proteomics are now enabling high throughput approaches to explore plant protein and lipid glycosylation through analyzing and targeting enzymes involved in glycosylation processes. Although there has been significant progress in plant glycobiology, there are still many remaining fundamental questions to be addressed. Here, we attempt to highlight some selected aspects that are key to accelerating progress in this field:
-
•
In vivo N- and O-glycan mapping. The chemical reporter strategy known as bio-orthogonal click chemistry has arisen as a powerful methodology to investigate the dynamics and functions of non-genetically encoded biomolecules such as sialylated (Chang et al., 2009; Laughlin and Bertozzi, 2009; Mbua et al., 2013), fucosylated (Hsu et al., 2007; Laughlin and Bertozzi, 2009; Besanceney-Webler et al., 2011), and mucin-type O-linked glycans (Laughlin and Bertozzi, 2009; Baskin et al., 2010) in live cells and model organisms (Prescher and Bertozzi, 2005; Grammel and Hang, 2013). This approach relies on the labeling of specific sugars by feeding cells with a synthetic monosaccharide analog carrying a chemical reporter that is then reacted with a probe (e.g., a fluorophore suitable for fluorescent microscopy imaging) in living systems to locate/visualize the incorporated reporter. Despite the fast-growing number of examples of this potent method in animal cells, reports describing its use in plant biology are surprisingly few (Anderson et al., 2012; Dumont et al., 2016; Zhu et al., 2016; Zhu and Chen, 2017). In part, this is due to the capacity of these probes to penetrate the cell wall barrier and, in part, due to the limited diversity of sugar analogs available to replace the endogenous sugars that need to be transported into the plant cell, and incorporated into glycan structures by GTs in a similar manner. Other new technologies are being developed to directly perform imaging of single glycan molecules that are isolated by mass-selective, soft-landing electrospray ion beam deposition and imaged by low-temperature scanning tunneling microscopy (Wu et al., 2020). This generates glycan structures at the single-molecule and single cell levels to directly relate how molecular structure correlates with properties – a step forward toward cracking the “sugar code.”
-
•
GT activity characterization by glycan arrays. The use of glycan arrays equipped with oligosaccharide acceptors, in combination with expressed GT/GH candidates, may significantly accelerate the identification and characterization of further GTs/GHs responsible for plant glycosylation/modulation in the future. To enable rapid progress in this area, intensive research on the chemical and/or enzymatic synthesis of oligosaccharide acceptors and sugar nucleotide donors as well as on high-yielding production of active GT candidates in different expression systems is required. In this direction, a JBEI (The Joint BioEnergy Institute) GT Collection with almost 500 GTs from Arabidopsis and rice were cloned in-frame into Gateway technology compatible vectors to readily enable downstream applications (Lao et al., 2014). Either more collective resources from our laboratories (a major barrier for individual groups when research funding is scarce) or commercial intervention (which would require the same importance placed on plant biology as medical research where such resources are provided) are necessary to drive functional genomic approaches in plant glycobiology.
-
•
Structural diversity in N- and O-glycans present in plant glycoproteins. Although some progress has been made recently, the precise N-glycan composition of individual native plant glycoproteins from different cells or tissues is only partially known (Xu et al., 2016; Zeng et al., 2018). Future efforts will aim to obtain a more comprehensive picture on N-glycan composition within specific glycoproteins to identify distinct N-glycan structures that are causative for a specific phenotype. In the same vein, determining functional roles for individual HRGP O-glycoproteins has been hampered by our failure to directly characterize each of these complex O-glycan structures. Only few studies have been able to purify AGPs and analyze their glycan structural variations in detail (Tan et al., 2004; Tryfona et al., 2010, 2012, 2014; Pfeifer et al., 2020). Biochemical characterization needs to be linked to detailed functional studies (e.g., site-directed mutagenesis). In general, functional validation is experimentally much more complex as well as time-consuming compared to the biochemical quantitation of the O-glycosylation levels. Furthermore, small changes in O-glycosylation in AGPs/FLAs and in EXTs can result in either activation or inactivation of their in vivo functions and can have an effect on their subcellular localization targeting (Velasquez et al., 2015b; Xue et al., 2017; Borassi et al., 2020; Seifert, 2020), so the functional relevance of each event cannot directly be inferred from large-scale quantitative analysis. A dual convergent approach between both enzymology/biochemistry and genetics is required to address this important aspect of plant glycoprotein structural diversity at the single cell level.
-
•
Overcome functional genetic redundancy of plant glycoproteins. Addressing genetic redundancy and functional overlap might be achieved by using multiplex CRISPR-CAS9/genome editing/gene knock-out technology. Some recent reports have used this approach to overcome functional redundancy in AGPs (Moreira et al., 2020) and in GTs (e.g., GLCATs) acting on AGPs (Zhang et al., 2020). This might be extended to investigate their function in other plant species.
-
•
Plant glycoproteome-interactome. Finding new proteins associated with plant glycoproteins, plant GTs and GHs will expand our knowledge on the regulatory aspects of plant glycobiology. New techniques such as proximity labeling (e.g., APEX, TurboID, etc.) together with the existing tools for detecting in vivo protein-protein interactions (e.g., BiFC, TriFC, FRET, etc.) will allow us to improve our plant glycobiology interactome inventory. Deeper integration of the N-and O-glycosylation pathway into the broader context of plant cell biology and systems biology is necessary. We envisage the development of a broad atlas of glycomes across plant tissues and cell types to integrate protein glycosylation features into plant gene and protein databases.
Author Contributions
KJ, MD, CR, and FP analyzed the references, wrote the manuscript, and helped on the figures design. RS, GS, AB, and JE analyzed the references, supervised the project, and wrote the manuscript. All authors have read the manuscript and have approved this submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This work was supported by grants from the Austrian Science Fund (FWF) P31920-B22 to RS and P32332-B to GS, the German Research Council (DFG) PF850/1-1 and PF850/7-1 to FP, the Australia Research Council to the ARC Centre of Excellence in Plant Cell Walls (CE1101007) to AB, MD, and KJ, and ANPCyT (PICT2016-0132 and PICT2017-0066), Fondo Nacional de Desarrollo Científico y Tecnológico (1200010), and Instituto Milenio iBio – Iniciativa Científica Milenio, MINECON to JE.
References
- Albenne C., Canut H., Boudart G., Zhang Y., San Clemente H., Pont-Lezica R., et al. (2009). Plant cell wall proteomics: mass spectrometry data, a trove for research on protein structure/function relationships. Mol. Plant 2 977–989. 10.1093/mp/ssp059 [DOI] [PubMed] [Google Scholar]
- Altmann F., Paschinger K., Dalik T., Vorauer K. (1998). Characterisation of peptide-N4-(N-acetyl-beta-glucosaminyl)asparagine amidase A and its N-glycans. Eur. J. Biochem. 252 118–123. 10.1046/j.1432-1327.1998.2520118.x [DOI] [PubMed] [Google Scholar]
- Anderson C. T., Wallace I. S., Somerville C. R. (2012). Metabolic click-labeling with a fucose analog reveals pectin delivery, architecture, and dynamics in Arabidopsis cell walls. Proc. Natl. Acad. Sci. U.S.A. 109 1329–1334. 10.1073/pnas.1120429109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacic A., Churms S. C., Stephen A. M., Cohen P. B., Fincher G. B. (1987). Fine structure of the arabinogalactan-protein from Lolium multiflorum. Carbohydr. Res. 162 85–93. 10.1016/0008-6215(87)80203-3 [DOI] [Google Scholar]
- Bakker H., Schijlen E., de Vries T., Schiphorst W., Jordi W., Lommen A., et al. (2001). Plant members of the alpha1–>3/4-fucosyltransferase gene family encode an alpha1–>4-fucosyltransferase, potentially involved in Lewis(a) biosynthesis, and two core alpha1–>3-fucosyltransferases. FEBS Lett. 507 307–312. 10.1016/s0014-5793(01)02999-4 [DOI] [PubMed] [Google Scholar]
- Bartetzko M. P., Pfrengle F. (2019). Automated glycan assembly of plant oligosaccharides and their application in cell-wall biology. ChemBioChem 20 877–885. 10.1002/cbic.201800641 [DOI] [PubMed] [Google Scholar]
- Baskin J. M., Dehnert K. W., Laughlin S. T., Amacher S. L., Bertozzi C. R. (2010). Visualizing enveloping layer glycans during zebrafish early embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 107 10350–10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D., Liang Y., Liu X., Himmeldirk K., Faik A., Kieliszewski M., et al. (2013). Functional identification of a hydroxyproline-O-galactosyltransferase specific for arabinogalactan protein biosynthesis in Arabidopsis. J. Biol. Chem. 288 10132–10143. 10.1074/jbc.M112.432609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D., Tian L., Wang W., Bobbs S., Herock H., Travers A., et al. (2015a). A small multigene hydroxyproline-O-galactosyltransferase family functions in arabinogalactan-protein glycosylation, growth and development in Arabidopsis. BMC Plant Biol. 15:295. 10.1186/s12870-015-0670-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D., Wang W., Ma S., DeBrosse T., Poirier E., Emch K., et al. (2015b). Two hydroxyproline galactosyltransferases, GALT5 and GALT2, function in arabinogalactan-protein glycosylation, growth and development in Arabidopsis. PLoS One 10:e0125624. 10.1371/journal.pone.0125624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N., Doesseger B., Guyot R., Diet A., Parsons R. L., Clark M. A., et al. (2003a). Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice: a conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol. 131 1313–1326. 10.1104/pp.102.014928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N., Ringli C., Keller B. (2001). The chimeric leucine rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev. 15 1128–1139. 10.1101/gad.200201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N., Steiner M., Ryser U., Keller B., Ringli C. (2003b). Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J. 35 71–81. 10.1046/j.1365-313x.2003.01784.x [DOI] [PubMed] [Google Scholar]
- Bernhardt C., Tierney M. L. (2000). Expression of AtPRP3, a proline-rich structural cell wall protein from Arabidopsis is regulated by cell-type-specific developmental pathways involved in root hair formation. Plant Physiol. 122 705–714. 10.1104/pp.122.3.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besanceney-Webler C., Jiang H., Wang W., Baughn A. D., Wu P. (2011). Metabolic labeling of fucosylated glycoproteins in Bacteroidales species. Bioorg. Med. Chem. Lett. 21 4989–4992. 10.1016/j.bmcl.2011.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson M., Gomord V., Audran C., Berger N., Dubreucq B., Granier F., et al. (2001). Arabidopsis glucosidase I mutants reveal a critical role of N-glycan trimming in seed development. EMBO J. 20 1010–1019. 10.1093/emboj/20.5.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borassi C., Gloazzo Dorosz J., Ricardi M. M., Carignani Sardoy M., Pol Fachin L., Marzol E., et al. (2020). A cell surface arabinogalactan-peptide influences root hair cell fate. New Phytol. 227 732–743. 10.1111/nph.16487 [DOI] [PubMed] [Google Scholar]
- Borassi C., Sede A. R., Mecchia M. A., Salgado Salter J. D., Marzol E., Muschietti J. P., et al. (2016). An update on cell surface proteins containing extensin-motifs. J. Exp. Bot. 67 477–487. 10.1093/jxb/erv455 [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Wood E. A., Larkins A. P., Galfre G., Butcher G. W., Brewin N. J. (1988). Isolation of monoclonal antibodies reacting with peribacteriod membranes and other components of pea root nodules containing Rhizobium leguminosarum. Planta 173 149–160. 10.1007/BF00403006 [DOI] [PubMed] [Google Scholar]
- Burn J., Hurley U., Birch R., Arioli T., Cork A., Williamson R. (2002). The cellulose-deficient Arabidopsis mutant rsw3 is defective in a gene encoding a putative glucosidase II, an enzyme processing N-glycans during ER quality control. Plant J. 32 949–960. 10.1046/j.1365-313x.2002.01483.x [DOI] [PubMed] [Google Scholar]
- Cannon M. C., Terneus K., Hall Q., Tan L., Wang Y., Wegenhart B. L., et al. (2008). Self-assembly of the plant cell wall requires an extensin scaffold. Proc. Natl. Acad. Sci. U.S.A. 105 2226–2231. 10.1073/pnas.0711980105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell A., Munoz-Munoz J., Briggs J. A., Ndeh D. A., Lowe E. C., Basle A., et al. (2018). A surface endogalactanase in Bacteroides thetaiotaomicron confers keystone status for arabinogalactan degradation. Nat. Microbiol. 3 1314–1326. 10.1038/s41564-018-0258-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilleux R., Plancot B., Gügi B., Attard A., Loutelier-Bourhis C., Lefranc B., et al. (2020). Extensin arabinosylation is involved in root response to elicitors and limits oomycete colonization. Ann. Bot. 125 751–763. 10.1093/aob/mcz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. V., Chen X., Smyrniotis C., Xenakis A., Hu T., Bertozzi C. R., et al. (2009). Metabolic labeling of sialic acids in living animals with alkynyl sugars. Angew. Chem. Int. Ed. Engl. 48 4030–4033. 10.1002/anie.200806319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Dong W., Tan L., Held M. A., Kieliszewski M. J. (2015). Arabinosylation plays a crucial role in extension cross-linking In Vitro. Biochem. Insights 8 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churms S. C., Merrifield E.-H., Stephen A. M. (1983). Some new aspects of the molecular structure of Acacia senegal gum (gum arabic). Carbohydr. Res. 123 267–279. 10.1016/0008-6215(83)88483-3 [DOI] [Google Scholar]
- Cosgrove D. J. (2005). Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6 850–861. 10.1038/nrm1746 [DOI] [PubMed] [Google Scholar]
- Dilokpimol A., Poulsen C. P., Vereb G., Kaneko S., Schulz A., Geshi N. (2014). Galactosyltransferases from Arabidopsis thaliana in the biosynthesis of type II arabinogalactan: molecular interaction enhances enzyme activity. BMC Plant Biol. 14:90. 10.1186/1471-2229-14-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q., Fang J. N. (2001). Structural elucidation of a new arabinogalactan from the leaves of Nerium indicum. Carbohydr. Res. 332 109–114. 10.1016/s0008-6215(01)00073-8 [DOI] [PubMed] [Google Scholar]
- Draeger C., Ndinyanka Fabrice T., Gineau E., Mouille G., Kuhn B. M., Moller I., et al. (2015). Arabidopsis leucine-rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol. 15:155. 10.1186/s12870-015-0548-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M., Lehner A., Vauzeilles B., Malassis J., Marchant A., Smyth K., et al. (2016). Plant cell wall imaging by metabolic click-mediated labelling of rhamnogalacturonan II using azido 3-deoxy-d-manno-oct-2-ulosonic acid. Plant J. 85 437–447. 10.1111/tpj.13104 [DOI] [PubMed] [Google Scholar]
- Dünser K., Gupta S., Herger A., Feraru M. I., Ringli C., Kleine-Vehn J. (2019). Extracellular matrix sensing by FERONIA and leucine-rich repeat extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana. EMBO J. 38:e100353. 10.15252/embj.2018100353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B., Rautengarten C., McFarlane H. E., Rupasinghe T., Zeng W., Ford K., et al. (2018). A Golgi UDP-GlcNAc transporter delivers substrates for N-linked glycans and sphingolipids. Nat. Plants 4 792–801. 10.1038/s41477-018-0235-5 [DOI] [PubMed] [Google Scholar]
- Eda M., Ishimaru M., Tada T., Sakamoto T., Kotake T., Tsumuraya Y., et al. (2014). Enzymatic activity and substrate specificity of the recombinant tomato β-galactosidase 1. J. Plant Physiol. 171 1454–1460. 10.1016/j.jplph.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Egelund J., Obel N., Ulvskov P., Geshi N., Pauly M., Bacic A., et al. (2007). Molecular characterization of two Arabidopsis thaliana glycosyltransferase mutants, rra1 and rra2, which have a reduced residual arabinose content in a polymer tightly associated with the cellulosic wall residue. Plant Mol. Biol. 64 439–451. 10.1007/s11103-007-9162-y [DOI] [PubMed] [Google Scholar]
- Ellis M., Egelund J., Schultz C. J., Bacic A. (2010). Arabinogalactan-proteins: key regulators at the cell surface? Plant Physiol. 153 403–419. 10.1104/pp.110.156000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez J. M., Kieliszewski M. J., Khitrov N., Somerville C. (2006). Characterization of hydroxyproline rich oligopeptides with AGP- and extensin-motifs expressed in Arabidospsis: posttranslational modifications, in situ localization and phenotypic effects. Plant Physiol. 142 458–470. 10.1104/pp.106.084244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudes A., Mouille G., Thevenin J., Goyallon A., Minic Z., Jouanin L. (2008). Purification, cloning and functional characterization of an endogenous beta-glucuronidase in Arabidopsis thaliana. Plant Cell Physiol. 49 1331–1341. 10.1093/pcp/pcn108 [DOI] [PubMed] [Google Scholar]
- Fabrice T. N., Vogler H., Draeger C., Munglani G., Gupta S., Herger A. G., et al. (2018). LRX proteins play a crucial role in pollen grain and pollen tube cell wall development. Plant Physiol. 176, 1981–1992. 10.1104/pp.17.01374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanata W. I., Lee K. H., Son B. H., Yoo J. Y., Harmoko R., Ko K. S., et al. (2013). N-glycan maturation is crucial for cytokinin-mediated development and cellulose synthesis in Oryza sativa. Plant J. 73 966–979. 10.1111/tpj.12087 [DOI] [PubMed] [Google Scholar]
- Fitchette-Lainé A. C., Gomord V., Cabanes M., Michalski J. C., Saint Macary M., Foucher B., et al. (1997). N-glycans harboring the Lewis a epitope are expressed at the surface of plant cells. Plant J. 12 1411–1417. 10.1046/j.1365-313x.1997.12061411.x [DOI] [PubMed] [Google Scholar]
- Fujita K., Sakamoto S., Ono Y., Wakao M., Suda Y., Kitahara K., et al. (2011). Molecular cloning and characterization of a β-L-arabinobiosidase in Bifidobacterium longum that belongs to a novel glycoside hydrolase family. J. Biol. Chem. 286 5143–5150. 10.1074/jbc.M110.190512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Takashi Y., Obuchi E., Kitahara K., Suganuma T. (2014). Characterization of a novel β-L-arabinofuranosidase in Bifidobacterium longum. J. Biol. Chem. 289 5240–5249. 10.1074/jbc.M113.528711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane A. M., Craik D., Munro S. L. A., Howlett G. J., Clarke A. E., Bacic A. (1995). Structural analysis of the carbohydrate moiety of arabinogalactan-proteins from stigmas and styles of Nicotiana alata. Carbohydr. Res. 277 67–85. 10.1016/0008-6215(95)00197-2 [DOI] [PubMed] [Google Scholar]
- Ge Z., Bergonci T., Zhao Y., Zou Y., Du S., Liu M. C., et al. (2017). Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358 1596–1600. 10.1126/science.aao3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geshi N., Johansen J. N., Dilokpimol A., Rolland A., Belcram K., Verger S., et al. (2013). A galactosyltransferase acting on arabinogalactan protein glycans is essential for embryo development in Arabidopsis. Plant J. 76 128–137. [DOI] [PubMed] [Google Scholar]
- Gille S., Hänsel U., Ziemann M., Pauly M. (2009). Identification of plant cell wall mutants by means of a forward chemical genetic approach using hydrolases. Proc. Natl. Acad. Sci. U.S.A. 106 14699–14704. 10.1073/pnas.0905434106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille S., Sharma V., Baidoo E. E. K., Keasling J. D., Scheller H. V., Pauly M. (2013). Arabinosylation of a Yariv-precipitable cell wall polymer impacts plant growth as exemplified by the Arabidopsis glycosyltransferase mutant ray1. Mol. Plant 6 1369–1372. 10.1093/mp/sst029 [DOI] [PubMed] [Google Scholar]
- Gillmor C., Poindexter P., Lorieau J., Palcic M., Somerville C. (2002). Alpha-glucosidase I is required for cellulose biosynthesis and morphogenesis in Arabidopsis. J. Cell Biol. 156 1003–1013. 10.1083/jcb.200111093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomord V., Fitchette A. C., Menu-Bouaouiche L., Saint-Jore-Dupas C., Plasson C., Michaud D., et al. (2010). Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol. J. 8 564–587. 10.1111/j.1467-7652.2009.00497.x [DOI] [PubMed] [Google Scholar]
- Grammel M., Hang H. C. (2013). Chemical reporters for biological discovery. Nat.Chem. Biol. 9 475–484. 10.1038/nchembio.1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Hansen B. Ø, Moeller S. R., Harholt J., Mravec J., Willats W., et al. (2019). Extensin arabinoside chain length is modulated in elongating cotton fibre. Cell Surf. 5:100033. 10.1016/j.tcsw.2019.100033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Q., Cannon M. C. (2002). The cell wall hydroxyproline-rich glycoprotein RSH is essential for normal embryo development in Arabidopsis. Plant Cell 14 1161–1172. 10.1105/tpc.010477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmoko R., Yoo J. Y., Ko K. S., Ramasamy N. K., Hwang B. Y., Lee E. J., et al. (2016). N-glycan containing a core α1,3-fucose residue is required for basipetal auxin transport and gravitropic response in rice (Oryza sativa). New Phytol. 212 108–122. 10.1111/nph.14031 [DOI] [PubMed] [Google Scholar]
- Herger A., Dünser K., Kleine-Vehn J., Ringli C. (2019). Leucine-rich repeat extensin proteins and their role in cell wall sensing. Curr. Biol. 29 R851–R858. 10.1016/j.cub.2019.07.039 [DOI] [PubMed] [Google Scholar]
- Herger A., Gupta S., Kadler G., Franck C. M., Boisson-Dernier A., Ringli C. (2020). Overlapping functions and protein-protein interactions of LRR-extensins in Arabidopsis. PLoS Genet. 16:e1008847. 10.1371/journal.pgen.1008847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijazi M., Velasquez S. M., Jamet E., Estevez J. M., Albenne C. (2014). An update on post-translational modifications of hydroxyproline-rich glycoproteins: toward a model highlighting their contribution to plant cell wall architecture. Front. Plant Sci. 5:395. 10.3389/fpls.2014.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z., Jin H., Tzfira T., Li J. (2008). Multiple mechanism-mediated retention of a defective brassinosteroid receptor in the endoplasmic reticulum of Arabidopsis. Plant Cell 20 3418–3429. 10.1105/tpc.108.061879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z., Kajiura H., Su W., Jin H., Kimura A., Fujiyama K., et al. (2012). Evolutionarily conserved glycan signal to degrade aberrant brassinosteroid receptors in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 109 11437–11442. 10.1073/pnas.1119173109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley D., Coleman J., Evans D., Crooks K., Peart J., Satiat-Jeunemaître B., et al. (1993). A monoclonal antibody, JIM 84, recognizes the Golgi apparatus and plasma membrane in plant. J. Exp. Bot. 44 223–229. [Google Scholar]
- Hsu T. L., Hanson S. R., Kishikawa K., Wang S. K., Sawa M., Wong C. H. (2007). Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc. Natl. Acad. Sci. U.S.A. 104 2614–2619. 10.1073/pnas.0611307104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttner S., Veit C., Schoberer J., Grass J., Strasser R. (2012). Unraveling the function of Arabidopsis thaliana OS9 in the endoplasmic reticulum-associated degradation of glycoproteins. Plant Mol. Biol. 79 21–33. 10.1007/s11103-012-9891-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttner S., Veit C., Vavra U., Schoberer J., Liebminger E., Maresch D., et al. (2014). Arabidopsis class I α-mannosidases MNS4 and MNS5 are involved in endoplasmic reticulum-associated degradation of misfolded glycoproteins. Plant Cell 26 1712–1728. 10.1105/tpc.114.123216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose H., Fujimoto Z., Honda M., Harazono K., Nishimoto Y., Uzura A., et al. (2009). A beta-l-arabinopyranosidase from Streptomyces avermitilis is a novel member of glycoside hydrolase family 27. J. Biol. Chem. 284 25097–25106. 10.1074/jbc.M109.022723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose H., Fujimoto Z., Kaneko S. (2013). Characterization of an alpha-L-rhamnosidase from Streptomyces avermitilis. Biosci. Biotechnol. Biochem. 77 213–216. 10.1271/bbb.120735 [DOI] [PubMed] [Google Scholar]
- Imaizumi C., Tomatsu H., Kitazawa K., Yoshimi Y., Shibano S., Kikuchi K., et al. (2017). Heterologous expression and characterization of an Arabidopsis beta-L-arabinopyranosidase and alpha-D-galactosidases acting on beta-L-arabinopyranosyl residues. J. Exp. Bot. 68 4651–4661. 10.1093/jxb/erx279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimizu T. (2015). “Plant N-glycans and their degrading enzymes,” in Glycoscience: Biology and Medicine, eds Taniguchi N., Endo T., Hart G., Seeberger P., Wong C. H. (Tokyo: Springer; ), 10.1007/978-4-431-54841-6_69 [DOI] [Google Scholar]
- Ishimizu T., Mitsukami Y., Shinkawa T., Natsuka S., Hase S., Miyagi M., et al. (1999). Presence of asparagine-linked N-acetylglucosamine and chitobiose in Pyrus pyrifolia S-RNases associated with gametophytic self-incompatibility. Eur. J. Biochem. 263 624–634. 10.1046/j.1432-1327.1999.00499.x [DOI] [PubMed] [Google Scholar]
- Ito Y., Nakanomyo I., Motose H., Iwamoto K., Sawa S., Dohmae N., et al. (2006). Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313 842–845. 10.1126/science.1128436 [DOI] [PubMed] [Google Scholar]
- Jiao J., Mizukami A. G., Sankaranarayanan S., Yamguchi J., Itami K., Higashiyawma T. (2017). Structure-activity relation of AMOR sugar molecule that activates pollen-tubes for ovular guidance. Plant Physiol. 173 354–363. 10.1104/pp.16.01655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Yan Z., Nam K. H., Li J. (2007). Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of UGGT in ER quality control. Mol. Cell 26 821–830. 10.1016/j.molcel.2007.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. L., Cassin A. M., Lonsdale A., Bacic A., Doblin M. S., Schultz C. J. (2017). A motif and amino acid bias bioinformatics pipeline to identify hydroxyproline-rich glycoproteins. Plant Physiol. 174 886–903. 10.1104/pp.17.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Frank J., Kang C., Kajiura H., Vikram M., Ueda A., et al. (2008). Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus. Proc. Natl. Acad. Sci. U.S.A. 105 5933–5938. 10.1073/pnas.0800237105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T., Sakuma A., Kimura T., Makimura Y., Hiratake J., Sakata K., et al. (2004). Molecular cloning and characterization of Bifidobacterium bifidum 1,2-alpha-L-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J. Bacteriol. 186 4885–4893. 10.1128/JB.186.15.4885-4893.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Hayashi M., Kitagawa M., Kajiura H., Maeda M., Kimura Y., et al. (2018). Degradation pathway of plant complex-type N-glycans: identification and characterization of a key α1,3-fucosidase from glycoside hydrolase family 29. Biochem. J. 475 305–317. 10.1042/BCJ20170106 [DOI] [PubMed] [Google Scholar]
- Kieliszewski M. J. (2001). The latest hype on Hyp-O-glycosylation codes. Phytochemistry 57 319–323. 10.1016/s0031-9422(01)00029-2 [DOI] [PubMed] [Google Scholar]
- Kieliszewski M. J., Lamport D. T. A., Tan L., Cannon M. C. (2011). Hydroxyproline-rich glycoproteins: form and function. Annu. Plant Rev. 41 321–342. 10.1002/9781119312994.apr0442 [DOI] [Google Scholar]
- Kim Y. C., Jahren N., Stone M. D., Udeshi N. D., Markowski T. W., Witthuhn B. A., et al. (2013). Identification and origin of N-linked β-D-N-acetylglucosamine monosaccharide modifications on Arabidopsis proteins. Plant Physiol. 161 455–464. 10.1104/pp.112.208900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaert C., Daugaard M., Nami F., Clausen M. H. (2017). Chemical synthesis of oligosaccharides related to the cell walls of plants and algae. Chem. Rev. 117 11337–11405. 10.1021/acs.chemrev.7b00162 [DOI] [PubMed] [Google Scholar]
- Kitazawa K., Tryfona T., Yoshimi Y., Hayashi Y., Kawauchi S., Antonov L., et al. (2013). β-galactosyl Yariv reagent binds to the β-1,3-galactan of arabinogalactan proteins. Plant Physiol. 161 1117–1126. 10.1104/pp.112.211722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch E., Dilokpimol A., Geshi N. (2014). Arabinogalactan proteins: focus on carbohydrate active enzymes. Front. Plant Sci. 11:198. 10.3389/fpls.2014.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch E., Dilokpimol A., Tryfona T., Poulsen C. P., Xiong G., Harholt J., et al. (2013). A β-glucuronosyl transferase from Arabidopsis thaliana involved in biosynthesis of type II arabinogalactan has a role in cell elongation during seedling growth. Plant J. 76 1016–1029. 10.1111/tpj.12353 [DOI] [PubMed] [Google Scholar]
- Knox J. P., Day S., Roberts K. (1989). A set of cell surface glycoproteins forms an early marker of cell position, bu not cell type, in the root apical meristem of Daucus carota L. Development 106 47–56. [Google Scholar]
- Knox J. P., Linstead P. J., Peart J., Cooper C., Roberts K. (1991). Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1 317–326. 10.1046/j.1365-313x.1991.t01-9-00999.x [DOI] [PubMed] [Google Scholar]
- Koiwa H., Li F., McCully M. G., Mendoza I., Koizumi N., Manabe Y., et al. (2003). The STT3a subunit isoform of the Arabidopsis oligosaccharyltransferase controls adaptive responses to salt/osmotic stress. Plant Cell 15 2273–2284. 10.1105/tpc.013862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T., Kotake T., Soraya D., Matsuoka K., Koyama T., Kaneko S., et al. (2008). Properties of family 79 beta-glucuronidases that hydrolyze beta-glucuronosyl and 4-O-methyl-beta-glucuronosyl residues of arabinogalactan-protein. Carbohydr. Res. 343 1191–1201. 10.1016/j.carres.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Koski M. K., Hieta R., B€ollner C., Kivirikko K., I, Myllyharju J., Wierenga R. K. (2007). The active site of an algal prolyl 4-hydroxylase has a large structural plasticity. J. Biol. Chem. 282 37112–37123. 10.1074/jbc.m706554200 [DOI] [PubMed] [Google Scholar]
- Koski M. K., Hieta R., Hirsil€a M., R€onk€a A., Myllyharju J., Wierenga R. K. (2009). The crystal structure of an algal prolyl 4-hydroxylase complexed with a proline-rich peptide reveals a novel buried tripeptide binding motif. J. Biol. Chem. 284 25290–25301. 10.1074/jbc.m109.014050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake T., Dina S., Konishi T., Kaneko S., Igarashi K., Samejima M., et al. (2005). Molecular cloning of a β-galactosidase from radish that specifically hydrolyzes β-(1→3)− and →-(1→6)-galactosyl residues of arabinogalactan protein. Plant Physiol. 138 1563–1576. 10.1104/pp.105.062562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake T., Hirata N., Degi Y., Ishiguro M., Kitazawa K., Takata R., et al. (2011). Endo-beta-1,3-galactanase from winter mushroom Flammulina velutipes. J. Biol. Chem. 286 27848–27854. 10.1074/jbc.M111.251736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake T., Kaneko S., Kubomoto A., Haque M. A., Kobayashi H., Tsumuraya Y. (2004). Molecular cloning and expression in Escherichia coli of a Trichoderma viride endo-beta-(1–>6)-galactanase gene. Biochem. J. 377(Pt 3) 749–755. 10.1042/BJ20031145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake T., Kitazawa K., Takata R., Okabe K., Ichinose H., Kaneko S., et al. (2009). Molecular cloning and expression in Pichia pastoris of a Irpex lacteus exo-beta-(1–>3)-galactanase gene. Biosci. Biotechnol. Biochem. 73 2303–2309. 10.1271/bbb.90433 [DOI] [PubMed] [Google Scholar]
- Kotake T., Tsuchiya K., Aohara T., Konishi T., Kaneko S., Igarashi K., et al. (2006). An alpha-L-arabinofuranosidase/beta-D-xylosidase from immature seeds of radish (Raphanus sativus L.). J. Exp. Bot. 57 2353–2362. 10.1093/jxb/erj206 [DOI] [PubMed] [Google Scholar]
- Kriechbaum R., Ziaee E., Grünwald-Gruber C., Buscaill P., van der Hoorn R. A. L., Castilho A. (2020). BGAL1 depletion boosts the level of β-galactosylation of N- and O-glycans in N. benthamiana. Plant Biotechnol. J. 18 1537–1549. 10.1111/pbi.13316 Epub 2020 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T., Kieliszewski M. J., Chen Y., Cannon M. C. (2011). Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 156 11–19. 10.1104/pp.110.169011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao J., Oikawa A., Bromley J. R., McInerney P., Suttangkakul A., Smith-Moritz A. M., et al. (2014). The plant glycosyltransferase clone collection for functional genomics. Plant J. 79 517–529. 10.1111/tpj.12577 [DOI] [PubMed] [Google Scholar]
- Laughlin S. T., Bertozzi C. R. (2009). Imaging the glycome. Proc. Natl. Acad. Sci. U.S.A. 106 12–17. 10.1073/pnas.0811481106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter H., Mucha J., Staudacher E., Grimm R., Glössl J., Altmann F. (1999). Purification, cDNA cloning, and expression of GDP-L-Fuc:Asn-linked GlcNAc alpha1,3-fucosyltransferase from mung beans. J. Biol. Chem. 274 21830–21839. 10.1074/jbc.274.31.21830 [DOI] [PubMed] [Google Scholar]
- Léonard R., Strasser R., Altmann F. (2009). Plant glycosidases acting on protein-linked oligosaccharides. Phytochemistry 70 318–324. 10.1016/j.phytochem.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Lerouge P., Cabanes-Macheteau M., Rayon C., Fischette-Lainé A. C., Gomord V., Faye L. (1998). N-glycoprotein biosynthesis in plants: recent developments and future trends. Plant Mol. Biol. 38 31–48. 10.1007/978-94-011-5298-3_2 [DOI] [PubMed] [Google Scholar]
- Lerouxel O., Mouille G., Andème-Onzighi C., Bruyant M. P., Séveno M., Loutelier-Bourhis C., et al. (2005). Mutants in DEFECTIVE GLYCOSYLATION, an Arabidopsis homolog of an oligosaccharyltransferase complex subunit, show protein underglycosylation and defects in cell differentiation and growth. Plant J. 42 455–468. 10.1111/j.1365-313X.2005.02392.x [DOI] [PubMed] [Google Scholar]
- Li B., Mock F., Wu P. (2012). Imaging the glycome in living systems. Methods Enzymol. 505 401–419. 10.1016/b978-0-12-388448-0.00029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhao-Hui C., Batoux M., Nekrasov V., Roux M., Chinchilla D., et al. (2009). Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc. Natl. Acad. Sci. U.S.A. 106 15973–15978. 10.1073/pnas.0905532106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Basu D., Pattathil S., Xu W. L., Venetos A., Martin S. L., et al. (2013). Biochemical and physiological characterization of fut4 and fut6 mutants defective in arabinogalactan-protein fucosylation in Arabidopsis. J. Exp. Bot. 64 5537–5551. 10.1093/jxb/ert321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebminger E., Grass J., Altmann F., Mach L., Strasser R. (2013). Characterizing the link between glycosylation state and enzymatic activity of the endo-β1,4-glucanase KORRIGAN1 from Arabidopsis thaliana. J. Biol. Chem. 288 22270–22280. 10.1074/jbc.M113.475558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebminger E., Hüttner S., Vavra U., Fischl R., Schoberer J., Grass J., et al. (2009). Class I alpha-mannosidases are required for N-glycan processing and root development in Arabidopsis thaliana. Plant Cell 21 3850–3867. 10.1105/tpc.109.072363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebminger E., Veit C., Pabst M., Batoux M., Zipfel C., Altmann F., et al. (2011). Beta-N-acetylhexosaminidases HEXO1 and HEXO3 are responsible for the formation of paucimannosidic N-glycans in Arabidopsis thaliana. J. Biol. Chem. 286 10793–10802. 10.1074/jbc.m110.178020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. (2004). Functions of heparan sulfate proteoglycans in cell signaling during development. Development 131 6009–6021. 10.1242/dev.01522 [DOI] [PubMed] [Google Scholar]
- Lombard V., Golaconda Ramulu H., Drula E., Coutinho P. M., Henrissat B. (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42 D490–D495. 10.1093/nar/gkt1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Hernandez F., Tryfona T., Rizza A., Yu X. L., Harris M. O. B., Webb A. A. R., et al. (2020). Calcium binding by arabinogalactan polysaccharides is important for normal plant development. Plant Cell 32 3346–3369. 10.1105/tpc.20.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Tintor N., Mentzel T., Kombrink E., Boller T., Robatzek S., et al. (2009). Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc. Natl. Acad. Sci. U.S.A. 106 22522–22527. 10.1073/pnas.0907711106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zeng W., Bacic A., Johnson K. L. (2018). Arabinogalactan-proteins, looking back through time and place. Annu. Plant Rev. 3 767–804. [Google Scholar]
- Martens-Uzunova E. S., Zandleven J. S., Benen J. A., Awad H., Kools H. J., Beldman G., et al. (2006). A new group of exo-acting family 28 glycoside hydrolases of Aspergillus niger that are involved in pectin degradation. Biochem. J. 400 43–52. 10.1042/BJ20060703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzol E., Borassi C., Bringas M., Sede A., Rodríguez Garcia D. R., Capece L., et al. (2018). Filling the gaps to solve the extensin puzzle. Mol. Plant 11 645–658. 10.1016/j.molp.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y., Ogawa-Ohnishi M., Mori A., Matsubayashi Y. (2010). Secreted peptide signals required for maintenance of root stem cell niche in Arabidopsis. Science 329 1065–1067. 10.1126/science.1191132 [DOI] [PubMed] [Google Scholar]
- Mbua N. E., Flanagan-steet H., Johnson S., Wolfert M. A., Boons G., Steet R. (2013). Abnormal accumulation and recycling of glycoproteins visualized in Niemann – Pick type C cells using the chemical reporter strategy. Proc. Natl. Acad. Sci. U.S.A. 110 10207–10212. 10.1073/pnas.1221105110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecchia M. A., Santos-Fernandez G., Duss N. N., Somoza S. C., Boisson-Dernier A., Gagliardini V., et al. (2017). RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358 1600–1603. 10.1126/science.aao5467 [DOI] [PubMed] [Google Scholar]
- Miyata T., Kashige N., Satho T., Yamaguchi T., Aso Y., Miake F. (2005). Cloning, sequence analysis, and expression of the gene encoding Sphingomonas paucimobilis FP2001 alpha-L -rhamnosidase. Curr. Microbiol. 51 105–109. 10.1007/s00284-005-4487-8 [DOI] [PubMed] [Google Scholar]
- Mizukami A. G., Inatsugi R., Jiao J., Kotake T., Kuwata K., Ootani K., et al. (2016). The AMOR arabinogalactan sugar chain induces pollen-tube competency to respond to ovular guidance. Curr. Biol. 26 1091–1097. 10.1016/j.cub.2016.02.040 [DOI] [PubMed] [Google Scholar]
- Møller I., Marcus S. E., Haeger A., Verhertbruggen Y., Verhoef R., Schols H., et al. (2008). High-throughput screening of monoclonal antibodies against plant cell wall glycans by hierarchical clustering of their carbohydrate microarray binding profiles. Glycoconj. J. 25 37–48. 10.1007/s10719-007-9059-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller S. R., Yi X., Vela’ squez S. M., Gille S., Hansen P. L., Poulsen C. P., et al. (2017). Identification and evolution of a plant cell wall specific glycoprotein glycosyl transferase, ExAD. Sci. Rep. 7:45341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira D., Pereira A. M., Lopes A. L., Coimbra S. (2020). The best CRISPR/Cas9 versus RNA interference approaches for arabinogalactan proteins’ study. Mol. Biol. Rep. 47 2315–2325. 10.1007/s11033-020-05258-0 [DOI] [PubMed] [Google Scholar]
- Moussu S., Broyart C., Santos-Fernandez G., Augustin S., Wehrle S., Grossniklaus U., et al. (2020). Structural basis for recognition of RALF peptides by LRX proteins during pollen tube growth. Proc. Natl. Acad. Sci. U.S.A. 117 7494–7503. 10.1073/pnas.2000100117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima Y., von Schaewen A., Koiwa H. (2018). Function of N-glycosylation in plants. Plant Sci. 274 70–79. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Yasukawa Y., Furusawa A., Fuchiwaki T., Honda T., Okamura Y., et al. (2018). Functional characterization of unique enzymes in Xanthomonas euvesicatoria related to degradation of arabinofurano-oligosaccharides on hydroxyproline-rich glycoproteins. PLoS One 13:e0201982. 10.1371/journal.pone.0201982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A., Gallagher L., Dunahay T., Frohlick J., Mazurkiewicz A., Meehl J., et al. (1999). Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol. 121 1127–1142. 10.1104/pp.121.4.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbering P., Petersen B. L., Motawia M. S., Jorgensen B., Ulvskov P., Niittyla T. (2020). Golgi-localized exo-beta1,3-galactosidases involved in cell expansion and root growth in Arabidopsis. J. Biol. Chem. 295 10581–10592. 10.1074/jbc.RA120.013878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman P. M., Wingate V. P. M., Fitter M. S., Lamb C. J. (1986). Monoclonal antibodies to plant plasma-membrane antigens. Planta 167 452–459. 10.1007/BF00391220 [DOI] [PubMed] [Google Scholar]
- Nuñez A., Fishman M. L., Fortis L. L., Cooke P. H., Hotchkiss A. T. (2009). Identification of extensin protein associated with sugar beet pectin. J. Agric. Food Chem. 57 10951–10958. 10.1021/jf902162t [DOI] [PubMed] [Google Scholar]
- Ogawa-Ohnishi M., Matsubayashi Y. (2015). Identification of three potent hydroxyproline O-galactosyltransferases in Arabidopsis. Plant J. 81 736–746. 10.1111/tpj.12764 [DOI] [PubMed] [Google Scholar]
- Ogawa-Ohnishi M., Matsushita W., Matsubayashi Y. (2013). Identification of three hydroxyproline O-arabinosyltransferases in Arabidopsis thaliana. Nat. Chem. Biol. 9 726–730. 10.1038/nchembio.1351 [DOI] [PubMed] [Google Scholar]
- Ohyama K., Shinohara H., Ogawa-Ohnishi M., Matsubayashi Y. (2009). A glycopeptide regulating stem cell fate in Arabidopsis thaliana. Nat. Chem. Biol. 5 578–580. 10.1038/nchembio.182 [DOI] [PubMed] [Google Scholar]
- Pattathil S., Avci U., Baldwin D., Swennes A. G., McGill J. A., Popper Z., et al. (2010). A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 153 514–525. 10.1104/pp.109.151985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen B. S., Craik D. J., Dunstan D. E., Stone B. A., Bacic A. (2014). The Yariv reagent: behaviour in different solvents and interaction with a gum arabic arabinogalactan-protein. Carbohydr. Polym. 106 460–468. 10.1016/j.carbpol.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Pedersen C. T., Loke I., Lorentzen A., Wolf S., Kamble M., Kristensen S. K., et al. (2017). N-glycan maturation mutants in Lotus japonicus for basic and applied glycoprotein research. Plant J. 91 394–407. 10.1111/tpj.13570 [DOI] [PubMed] [Google Scholar]
- Pedersen H. L., Fangel J. U., McCleary B., Ruzanski C., Rydahl M. G., Ralet M. C., et al. (2012). Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 287 39429–39438. 10.1074/jbc.M112.396598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell R. I., Janniche L., Kjellbom P., Scofield G. N., Peart J. M., Roberts K. (1991). Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 3 1317–1326. 10.1105/tpc.3.12.1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell R. I., Knox J. P., Scofield G. N., Selvendran R. R., Roberts K. (1989). A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J. Cell Biol. 108 1967–1977. 10.1083/jcb.108.5.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A. M., Nobre M. S., Pinto S. C., Lopes A. L., Costa M. L., Masiero S., et al. (2016). “Love is strong, and you’re so sweet”: JAGGER is essential for persistent synergid degeneration and polytubey block in Arabidopsis thaliana. Mol. Plant 9 601–614. 10.1016/j.molp.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Pfeifer L., Shafee T., Johnson K. L., Bacic A., Classen B. (2020). Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments. Sci. Rep. 10:8232. 10.1038/s41598-020-65135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrengle F. (2017). Synthetic plant glycans. Curr. Opin. Chem. Biol. 40 145–151. 10.1016/j.cbpa.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Pogorelko G. V., Reem N. T., Young Z. T., Chambers L., Zabotina O. A. (2016). Post-synthetic defucosylation of AGP by Aspergillus nidulans alpha-1,2-fucosidase expressed in Arabidopsis apoplast induces compensatory upregulation of alpha-1,2-fucosyltransferases. PLoS One 11:e0159757. 10.1371/journal.pone.0159757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescher J. A., Bertozzi C. R. (2005). Chemistry in living systems. Nat. Chem. Biol. 1 13–21. [DOI] [PubMed] [Google Scholar]
- Qu Y., Egelund J., Gilson P. R., Houghton F., Gleeson P. A., Schultz C. J., et al. (2008). Identification of a novel group of putative Arabidopsis thaliana beta-(1,3)-galactosyltransferases. Plant Mol. Biol. 68 43–59. 10.1007/s11103-008-9351-3 [DOI] [PubMed] [Google Scholar]
- Rademacher T., Sack M., Arcalis E., Stadlmann J., Balzer S., Altmann F., et al. (2008). Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly single-GlcNAc N-glycans. Plant Biotechnol. J. 6 189–201. 10.1111/j.1467-7652.2007.00306.x [DOI] [PubMed] [Google Scholar]
- Rahman M. Z., Fujishige M., Maeda M., Kimura Y. (2016). Rice α-fucosidase active against plant complex type N-glycans containing Lewis a epitope: purification and characterization. Biosci. Biotechnol. Biochem. 80 291–294. 10.1080/09168451.2015.1079479 [DOI] [PubMed] [Google Scholar]
- Raju T. S., Davidson E. A. (1994). Structural features of water-soluble novel polysaccharide components from the leaves of Tridax procumbens Linn. Carbohydr. Res. 258 243–254. 10.1016/0008-6215(94)84090-3 [DOI] [PubMed] [Google Scholar]
- Ringli C. (2010). The hydroxyproline-rich glycoprotein domain of the Arabidopsis LRX1 requires Tyr for function but not for insolubilization in the cell wall. Plant J. 63 662–669. 10.1111/j.1365-313x.2010.04270.x [DOI] [PubMed] [Google Scholar]
- Rips S., Bentley N., Jeong I. S., Welch J. L., von Schaewen A., Koiwa H. (2014). Multiple N-glycans cooperate in the subcellular targeting and functioning of Arabidopsis KORRIGAN1. Plant Cell 26 3792–3808. 10.1105/tpc.114.129718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht C., Bartetzko M. P., Senf D., Dallabernadina P., Boos I., Andersen M. C. F., et al. (2017). A synthetic glycan microarray enables epitope mapping of plant cell wall glycan-directed antibodies. Plant Physiol. 175 1094–1104. 10.1104/pp.17.00737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht C., Bartetzko M. P., Senf D., Lakhina A., Smith P. J., Soto M. J., et al. (2020). A glycan array-based assay for the identification and characterization of plant glycosyltransferases. Angew. Chem. Int. Ed. Engl. 59 12493–12498. 10.1002/anie.202003105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydahl M. G., Hansen A. R., Kracun S. K., Mravec J. (2018). Report on the current inventory of the toolbox for plant cell wall analysis: proteinaceous and small molecular probes. Front. Plant Sci. 9:581. 10.3389/fpls.2018.00581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F., Suyama A., Oka T., Yoko-O T., Matsuoka K., Jigami Y., et al. (2014). Identification of novel peptidyl serine O-galactosyltransferase gene family in plants. J. Biol. Chem. 30 20405–20420. 10.1074/jbc.m114.553933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sede A. R., Borassi C., Wengier D. L., Mecchia M. A., Estevez J. M., Muschietti J. P. (2018). Arabidopsis pollen extensins LRX are required for cell wall integrity during pollen tube growth. FEBS Lett. 592 233–243. 10.1002/1873-3468.12947 [DOI] [PubMed] [Google Scholar]
- Seeberger P. H. (2015). The logic of automated glycan assembly. Acc. Chem. Res. 48 1450–1463. 10.1021/ar5004362 [DOI] [PubMed] [Google Scholar]
- Seifert G. J. (2020). On the potential function of type II arabinogalactan O-glycosylation in regulating the fate of plant secretory proteins. Front. Plant Sci. 11:563735. 10.3389/fpls.2020.563735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G. J., Roberts K. (2007). The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 58 137–161. 10.1146/annurev.arplant.58.032806.103801 [DOI] [PubMed] [Google Scholar]
- Shinohara H., Matsubayashi Y. (2013). Chemical synthesis of Arabidopsis CLV3 glycopeptide reveals the impact of hydroxyproline arabinosylation on peptide conformation and activity. Plant Cell Physiol. 54 369–374. 10.1093/pcp/pcs174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. M., Basu D. (2016). Extensin and arabinogalactan-protein biosynthesis: glycosyltransferases, research challenges, and biosensors. Front. Plant Sci. 7:814. 10.3389/fpls.2016.00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak E., Barbar E., Leykam J. F., Kieliszewski M. J. (2001). Contiguous hydroxyproline residues direct hydroxyproline arabinosylation in Nicotiana tabacum. J. Biol. Chem. 276 11272–11278. 10.1074/jbc.M011323200 [DOI] [PubMed] [Google Scholar]
- Shpak E., Leykam J. F., Kieliszewski M. J. (1999). Synthetic genes for glycoprotein design and the elucidation of hydroxyproline-O-glycosylation codes. Proc. Natl. Acad. Sci. U.S.A. 96 14736–14741. 10.1073/pnas.96.26.14736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J., Ferraz R., Dupree P., Showalter A. M., Coimbra S. (2020). Three decades of advances in arabinogalactan-protein biosynthesis. Front. Plant Sci. 11:610377. 10.3389/fpls.2020.610377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood M., Yates E. A., Willats W. G. T., Martin H., Knox J. P. (1996). Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 198 452–459. 10.1007/BF00620063 [DOI] [Google Scholar]
- Somerville C., Bauer S., Brininstool G., Facette M., Hamann T., Milne J., et al. (2004). Toward a systems approach to understanding plant cell walls. Science 306 2206–2211. 10.1126/science.1102765 [DOI] [PubMed] [Google Scholar]
- Strasser R. (2014). Biological significance of complex N-glycans in plants and their impact on plant physiology. Front. Plant Sci. 5:363. 10.3389/fpls.2014.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R. (2016). Plant protein glycosylation. Glycobiology 26 926–939. 10.1093/glycob/cww023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R., Altmann F., Mach L., Glössl J., Steinkellner H. (2004). Generation of Arabidopsis thaliana plants with complex N-glycans lacking beta1,2-linked xylose and core alpha1,3-linked fucose. FEBS Lett. 561 132–136. 10.1016/s0014-5793(04)00150-4 [DOI] [PubMed] [Google Scholar]
- Strasser R., Bondili J., Schoberer J., Svoboda B., Liebminger E., Glössl J., et al. (2007a). Enzymatic properties and subcellular localization of Arabidopsis beta-N-acetylhexosaminidases. Plant Physiol. 145 5–16. 10.1104/pp.107.101162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R., Bondili J. S., Vavra U., Schoberer J., Svoboda B., Glössl J., et al. (2007b). A unique beta1,3-galactosyltransferase is indispensable for the biosynthesis of N-glycans containing Lewis a structures in Arabidopsis thaliana. Plant Cell 19 2278–2292. 10.1105/tpc.107.052985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R., Mucha J., Mach L., Altmann F., Wilson I., Glössl J., et al. (2000). Molecular cloning and functional expression of beta1, 2-xylosyltransferase cDNA from Arabidopsis thaliana. FEBS Lett. 472 105–108. 10.1016/s0014-5793(00)01443-5 [DOI] [PubMed] [Google Scholar]
- Strasser R., Mucha J., Schwihla H., Altmann F., Glössl J., Steinkellner H. (1999a). Molecular cloning and characterization of cDNA coding for beta1,2N-acetylglucosaminyltransferase I (GlcNAc-TI) from Nicotiana tabacum. Glycobiology 9 779–785. 10.1093/glycob/9.8.779 [DOI] [PubMed] [Google Scholar]
- Strasser R., Schoberer J., Jin C., Glössl J., Mach L., Steinkellner H. (2006). Molecular cloning and characterization of Arabidopsis thaliana Golgi alpha-mannosidase II, a key enzyme in the formation of complex N-glycans in plants. Plant J. 45 789–803. [DOI] [PubMed] [Google Scholar]
- Strasser R., Steinkellner H., Borén M., Altmann F., Mach L., Glössl J., et al. (1999b). Molecular cloning of cDNA encoding N-acetylglucosaminyltransferase II from Arabidopsis thaliana. Glycoconj. J. 16 787–791. [DOI] [PubMed] [Google Scholar]
- Sturm A. (1995). N-glycosylation of plant proteins. New Compr. Biochem. 29(Pt A) 521–542. 10.1016/s0167-7306(08)60603-1 [DOI] [Google Scholar]
- Suzuki T., Narciso J. O., Zeng W., van de Meene A., Yasutomi M., Takemura S., et al. (2017). KNS4/UPEX1: a type II arabinogalactan β-(1,3)-galactosyltransferase required for pollen exine development. Plant Physiol. 173 183–205. 10.1104/pp.16.01385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata R., Tokita K., Mori S., Shimoda R., Harada N., Ichinose H., et al. (2010). Degradation of carbohydrate moieties of arabinogalactan-proteins by glycoside hydrolases from Neurospora crassa. Carbohydr. Res. 345 2516–2522. 10.1016/j.carres.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Tan L., Eberhard S., Pattathil S., Warder C., Glushka J., Yuan C., et al. (2013). An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25 270–287. 10.1105/tpc.112.107334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Qiu F., Lamport D. T., Kieliszewski M. J. (2004). Structure of a hydroxyproline (Hyp)-arabinogalactan polysaccharide from repetitive Ala-Hyp expressed in transgenic Nicotiana tabacum. J. Biol. Chem. 279 13156–13165. 10.1074/jbc.m311864200 [DOI] [PubMed] [Google Scholar]
- Tan L., Showalter A. M., Egelund J., Hernandez-Sanchez A., Doblin M. S., Bacic A. (2012). Arabinogalactan-proteins and the research challenges for these enigmatic plant cell surface proteoglycans. Front. Plant Sci. 3:140. 10.3389/fpls.2012.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Varnai P., Lamport D. T., Yuan C., Xu J., Qiu F., et al. (2010). Plant O-hydroxyproline arabinogalactans are composed of repeating trigalactosyl subunits with short bifurcated side chains. J. Biol. Chem. 285 24575–24583. 10.1074/jbc.m109.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr., Maley F. (1974). The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J. Biol. Chem. 249 818–824. 10.1016/s0021-9258(19)43002-0 [DOI] [PubMed] [Google Scholar]
- Temple H., Mortimer J. C., Tryfona T., Yu X., Lopez-Hernandez F., Sorieul M., et al. (2019). Two members of the DUF579 family are responsible for arabinogalactan methylation in Arabidopsis. Plant Direct 3:e00117. 10.1002/pld3.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V., Altmann F., März L. (1991). Peptide-N4-(N-acetyl-beta-glucosaminyl)asparagine amidase F cannot release glycans with fucose attached alpha 1-3 to the asparagine-linked N-acetylglucosamine residue. Eur. J. Biochem. 199 647–652. 10.1111/j.1432-1033.1991.tb16166.x [DOI] [PubMed] [Google Scholar]
- Tryfona T., Liang H. C., Kotake T., Kaneko S., Marsh J., Ichinose H., et al. (2010). Carbohydrate structural analysis of wheat flour arabinogalactan protein. Carbohydr. Res. 345 2648–2656. 10.1016/j.carres.2010.09.018 [DOI] [PubMed] [Google Scholar]
- Tryfona T., Liang H. C., Kotake T., Tsumuraya Y., Stephens E., Dupree P. (2012). Structural characterization of Arabidopsis leaf arabinogalactan polysaccharides. Plant Physiol. 160 653–666. 10.1104/pp.112.202309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryfona T., Theys T. E., Wagner T., Stott K., Keegstra K., Dupree P. (2014). Characterisation of FUT4 and FUT6 a-(1→2)-fucosyltransferases reveals that absence of root arabinogalactan fucosylation increases Arabidopsis root growth salt sensitivity. PLoS One 9:e93291. 10.1371/journal.pone.0093291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumuraya Y., Mochizuki N., Hashimoto Y., Kovac P. (1990). Purification of an exo-beta-(1-→3)-D-galactanase of Irpex lacteus (Polyporus tulipiferae) and its action on arabinogalactan-proteins. J. Biol. Chem. 265 7207–7215. 10.1016/s0021-9258(19)39100-8 [DOI] [PubMed] [Google Scholar]
- Ulvskov P., Paiva D. S., Domozych D., Harholt J. (2013). Classification, naming and evolutionary history of glycosyltransferases from sequenced green and red algal genomes. PLoS One 8:e76511. 10.1371/journal.pone.0076511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin R., Cerclier C., Geneix N., Aguié-Béhin V., Gaillard C., Ralet M. C., et al. (2010). Elaboration of extensin-pectin thin film model of primary plant cell wall. Langmuir 26 9891–9898. 10.1021/la100265d [DOI] [PubMed] [Google Scholar]
- Velasquez S. M., Marzol E., Borassi C., Pol-Fachin L., Ricardi M. M., Mangano S., et al. (2015a). Low sugar is not always good: impact of specific O-glycan defects on tip growth in Arabidopsis. Plant Physiol. 168 808–813. 10.1104/pp.114.255521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez S. M., Ricardi M. M., Dorosz J. G., Fernandez P. V., Nadra A. D., Pol-Fachin L., et al. (2011). O-glycosylated cell wall extensins are essential in root hair growth. Science 332 1401–1403. 10.1126/science.1206657 [DOI] [PubMed] [Google Scholar]
- Velasquez S. M., Ricardi M. M., Poulsen C. P., Oikawa A., Dilokpimol A., Halim A., et al. (2015b). Complex regulation of prolyl-4-hydroxylases impacts root hair expansion. Mol. Plant 8 734–746. 10.1016/j.molp.2014.11.017 [DOI] [PubMed] [Google Scholar]
- von Schaewen A., Sturm A., O’Neill J., Chrispeels M. J. (1993). Isolation of a mutant Arabidopsis plant that lacks N-acetyl glucosaminyl transferase I and is unable to synthesize Golgi-modified complex N-linked glycans. Plant Physiol. 102 1109–1118. 10.1104/pp.102.4.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. X., Wang K., Liu X. Y., Liu M., Cao N., Duan Y., et al. (2017). Pollen-expressed leucin-rich repeat extensins are essential for pollen germination and growth. Plant Physiol. 176 1993–2006. 10.1104/pp.17.01241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. B., Harthill J. E., Mullin N. P., Ashford D. A., Altmann F. (1998). Core alpha1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology 8 651–661. 10.1093/glycob/8.7.651 [DOI] [PubMed] [Google Scholar]
- Wilson I. B., Zeleny R., Kolarich D., Staudacher E., Stroop C. J., Kamerling J. P., et al. (2001). Analysis of Asn-linked glycans from vegetable foodstuffs: widespread occurrence of Lewis a, core alpha1,3-linked fucose and xylose substitutions. Glycobiology 11 261–274. 10.1093/glycob/11.4.261 [DOI] [PubMed] [Google Scholar]
- Wong-Madden S. T., Landry D. (1995). Purification and characterization of novel glycosidases from the bacterial genus Xanthomonas. Glycobiology 5 19–28. 10.1093/glycob/5.1.19 [DOI] [PubMed] [Google Scholar]
- Wu X., Delbianco M., Anggara K., Michnowicz T., Pardo-Vargas A., Bharate P., et al. (2020). Imaging single glycans. Nature 582 375–378. [DOI] [PubMed] [Google Scholar]
- Wu Y., Williams M., Bernard S., Driouich A., Showalter A. M., Faik A. (2010). Functional identification of two nonredundant Arabidopsis alpha(1,2)fucosyltransferases specific to arabinogalactan proteins. J. Biol. Chem. 285 13638–13645. 10.1074/jbc.M110.102715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S. L., Medzihradszky K. F., Wang Z. Y., Burlingame A. L., Chalkley R. J. (2016). N-glycopeptide profiling in Arabidopsis inflorescence. Mol. Cell. Proteomics 15 2048–2054. 10.1074/mcp.M115.056101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H., Veit C., Abas L., Tryfona T., Maresch D., Ricardi M. M., et al. (2017). Arabidopsis thaliana FLA4 functions as a glycan-stabilized soluble factor via its carboxy-proximal fasciclin 1 domain. Plant J. 91 613–630. 10.1111/tpj.13591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yariv J., Lis H., Katchalski E. (1967). Precipitation of arabic acid and some seed polysaccharides by glycosylphenylazo dyes. Biochem. J. 105 1C–2C. 10.1042/bj1050001c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates E. A., Valdor J. F., Haslam S. M., Morris H. R., Dell A., Mackie W., et al. (1996). Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6 131–139. 10.1093/glycob/6.2.131 [DOI] [PubMed] [Google Scholar]
- Zeleny R., Leonard R., Dorfner G., Dalik T., Kolarich D., Altmann F. (2006). Molecular cloning and characterization of a plant alpha1,3/4-fucosidase based on sequence tags from almond fucosidase I. Phytochemistry 67 641–648. 10.1016/j.phytochem.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Zeng W., Ford K. L., Bacic A., Heazlewood J. L. (2018). N-linked glycan micro-heterogeneity in glycoproteins of Arabidopsis. Mol. Cell. Proteomics 17 413–421. 10.1074/mcp.RA117.000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Held M. A., Showalter A. M. (2020). Elucidating the roles of three beta-glucuronosyltransferases (GLCATs) acting on arabinogalactan-proteins using a CRISPR-Cas9 multiplexing approach in Arabidopsis. BMC Plant Biol. 20:221. 10.1186/s12870-020-02420-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Jiang W., Zayed O., Liu X., Tang K., Nie W., et al. (2020). The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. Natl. Sci. Rev. 8:nwaa149. 10.1093/nsr/nwaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zayed O., Yu Z., Jiang W., Zhu P., Hsu C. C., et al. (2018). Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 115 13123–13128. 10.1073/pnas.1816991115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Chen X. (2017). Expanding the scope of metabolic glycan labeling in Arabidopsis thaliana. Chembiochem 18 1286–1296. 10.1002/cbic.201700069 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Wu J., Chen X. (2016). Metabolic labeling and imaging of N-linked glycans in Arabidopsis thaliana. Angew. Chem. Int. Ed. Engl. 55 9301–9305. 10.1002/anie.201603032 [DOI] [PubMed] [Google Scholar]
- Zielinska D. F., Gnad F., Schropp K., Wiśniewski J. R., Mann M. (2012). Mapping N-glycosylation sites across seven evolutionarily distant species reveals a divergent substrate proteome despite a common core machinery. Mol. Cell 46 542–548. 10.1016/j.molcel.2012.04.031 [DOI] [PubMed] [Google Scholar]