Graphical Abstract
ORIGIN
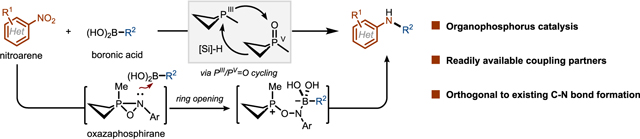
The preparation of (hetero)arylamines has been significantly advanced by developments in transition metal catalyzed Csp2–N coupling (e.g., Buchwald-Hartwig, Chan-Lam, and Ullmann couplings). As a complement to these strategies, our group recently demonstrated the reductive Csp2–N coupling of arylboronic acids and nitro compounds—initially nitro(hetero)arenes, and more recently nitromethane—by an organophosphorus-catalyzed method operating via PIII/PV=O redox cycling.
REACTION MECHANISM
The PIII/PV=O catalyzed reductive Csp2–N coupling method is predicated on a rapid reduction of the precatalyst phosphetane oxide 1·[O] to the corresponding phosphetane 1. Experimental kinetic (A) and spectroscopic (B) studies of the reaction of nitrobenzene and phenylboronic acid show that 1 is indeed the catalyst resting state, and that the overall two-fold reduction of the nitro substrate evolves via two distinct PIII/PV=O cycles. In the first cycle, a (3+1) cheletropic addition of nitrobenzene to phosphetane 1 gives cycloadduct Int-1 in an overall turnover limiting step (C). Int-1 undergoes a retro-(2+2) fragmentation to liberate nitrosobenzene (Int-2) and phosphetane oxide 1·[O], which is rapidly re-reduced to 1 by the hydrosilane reductant. In the second cycle, Int-2 undergoes facile (2+1) addition with PIII resting state 1 to provide oxazaphosphirane Int-3. Similar intermediates have been proposed in the literature previously, but we are the first to obtain spectroscopical data of this highly reactive intermediate (D). Int-3 serves as a product-determining branching point. In the absence of an arylboronic acid, Int-3 evolves with loss of 1·[O] to give an arylnitrene intermediate, as exemplified by the formation of carbazole from 2-nitrobiphenyl through Cadogan-type cyclization. However, in the presence of an arylboronic acid, oxazaphosphirane Int-3 is diverted to betaine Int-4, which undergoes 1,2-metallate rearrangement to form the desired Csp2–N bond and releases the product arylamine upon workup (E). Rapid deoxygenation of phosphetane oxide 1·[O] mediated by hydrosilane again closes the second deoxygenation cycle and regenerates the PIII phosphetane resting state 1.

IMPORTANCE
The intermolecular reductive Csp2–N coupling of nitroarenes and boronic acids represents a practical and robust organophosphorus-catalyzed method complementary to established transition metal-based Csp2–N coupling techniques. The mechanism emphasizes the biphilic character of phosphetanes in catalytic reductive O-atom transfer via PIII/PV=O redox cycling, providing a framework for further catalyst design and ongoing reaction development in the Csp2–N coupling space.
LITERATURE
- 1.Nykaza TV et al. (2018) Intermolecular Reductive C–N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis. J. Am. Chem. Soc 140, 15200–15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li G et al. (2020) An Improved PIII/PV=O-Catalyzed Reductive C–N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- and Product-Determining Steps. J. Am. Chem. Soc 142, 6786–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nykaza TV et al. (2018) Biphilic Organophosphorus-Catalyzed Intramolecular Csp2 −H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations. J. Am. Chem. Soc 140, 3103–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nykaza TV et al. (2020) PIII/PV=O-Catalyzed Cascade Synthesis of N-Functionalized Azaheterocycles. Angew. Chem., Int. Ed 59, 4505–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G et al. (2020) PIII/PV-Catalyzed Methylamination of Arylboronic Acids (Esters) By Reductive C–N Coupling of Nitromethane as a Methylamine Surrogate. J. Am. Chem. Soc 142, 16205–16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bariwal J et al. (2013) C–N Bond Forming Cross-coupling Reactions: an overview. Chem. Soc. Rev 42, 9283–9303. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Castillo P et al. (2016) Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev 116, 12564–12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West MJ et al. (2019) Mechanistic Development and Recent Applications of the Chan–Lam Amination. Chem. Rev 119, 12491–12523. [DOI] [PubMed] [Google Scholar]
- 9.Guo H et al. (2018) Phosphine Organocatalysis. Chem. Rev 118, 10049–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadogan JIG et al. (1965) The Reactivity of Organophosphorus Compounds. Part XIX. Reduction of Nitro-Compounds by Triethyl Phosphite: A Convenient New Route to Carbazoes, Indoles, Indazoles, Triazoles, and Related Compounds. J. Chem. Soc 4831–4837. [Google Scholar]


