Abstract
Rising trends in the incidence of cancer in low- and middle-income countries (LMICs) add to the existing challenges with communicable and noncommunicable diseases. While breast and colorectal cancer incidence rates are increasing in LMICs, the incidence of cervical cancer shows a mixed trend, with rising incidence rates in China and sub-Saharan Africa and declining trends in the Indian subcontinent and South America. The increasing frequencies of unhealthy lifestyles, notably less physical activity, obesity, tobacco use, and alcohol consumption are causing a threat to health care in LMICs. Also, poorly developed health systems tend to have inadequate resources to implement early detection and adequate basic treatment. Inequalities in social determinants of health, lack of awareness of cancer and preventive care, lack of efficient referral pathways and patient navigation, and nonexistent or inadequate health care funding can lead to advanced disease presentation at diagnosis. This article provides an overview of opportunities to address cancer control in LMICs, with a focus on tobacco control, vaccination for cervical cancer, novel tools to assist with early detection, and screening for breast and other cancers.
INTRODUCTION
Recent reports on the global burden of cancer estimate 18.1 million new cases and 9.6 million cancer deaths worldwide in 2018 (Fig. 1).1 As the burden of communicable disease and cardiovascular disease continues, the incidence of cancer is on the rise in LMICs.2 Regardless of the geographic area, social determinants of health (SDH) seem to play a crucial role in cancer control and its outcomes across the globe.3 Even in higher-income nations, factors related to SDHs play a vital role in obtaining cancer screening, including sociodemographic factors, health literacy, cultural influences, lack of beliefs in preventive health, socioeconomic status, and lack of access to health care.4 Also, patients’ attitudes, beliefs (including fear of cancer), and an inadequate number of facilities equipped with cancer screening tools all prevent timely screening.5 Unfortunately, limited allocation of resources to health care and a lack of implementation of government policies ultimately affect prevention and the disease burden. Although the incidence of cancer is increasing in LMICs, the cost of cancer is limiting its affordability.6 Therefore, a focus on cancer prevention, early detection, and use of novel and cost-effective methods of screening are invaluable in lowering cancer incidence and the related financial burden on a country’s economy. Herein, we describe the global burden of cancers related to tobacco use and other common cancers of the cervix, breast, and gastrointestinal tract, with an emphasis on potential opportunities for cancer prevention in LMICs.
FIGURE 1.
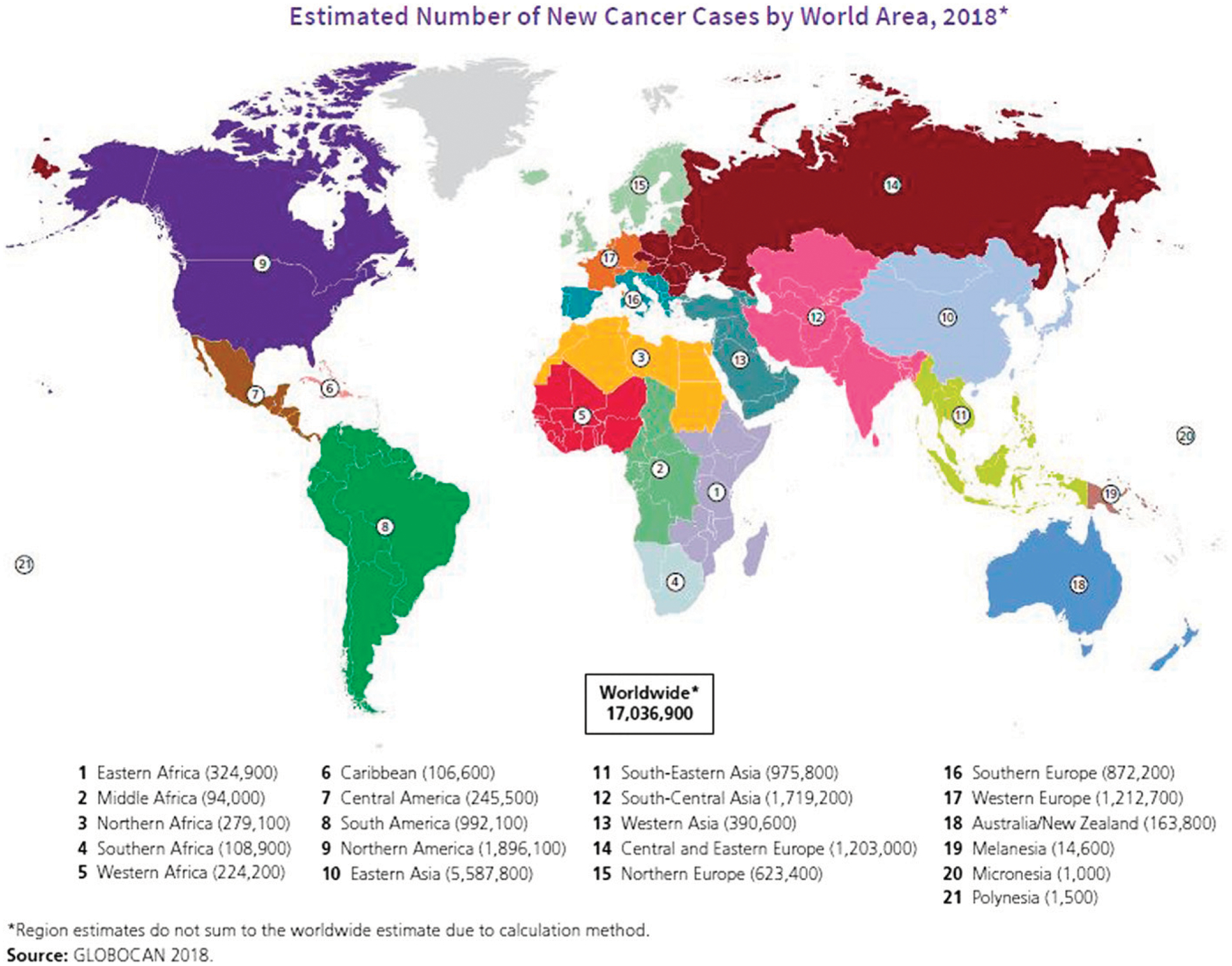
Estimated Number of New Cancer Cases by World Area, 2018
ADDRESSING THE BARRIERS TO SCREENING FOR THE MOST COMMON CANCERS
Tobacco Use in LMICs
Tobacco use continues to be the largest preventable risk factor for the main noncommunicable diseases, including cancer.7,8 Cigarette smoking increases the risk of at least 12 cancers, including acute myeloid leukemia and cancers of the bladder, colon and rectum, esophagus, kidney, larynx, liver, lung, oral cavity and pharynx, pancreas, stomach, and uterine cervix.7–9 More than 24% of all cancer deaths globally are attributable to tobacco use.7,9 Currently, more than 1 billion people in the world use tobacco products, and if nothing is done to help them quit and to prevent young people from starting, more than half of these people will succumb to premature deaths from tobacco-related diseases. In 2019 alone, the estimated death toll from tobacco-related disease was more than 8 million.8 The economic burden is also staggering, approximately $2 trillion (in 2016 purchasing power parity) from both higher health care costs and lost productivity.10 Moreover, the burden is increasing in LMICs, where the majority of tobacco users now live.
However, there is encouraging news for public health: Tobacco control has a well-developed package of evidence-based interventions that are proven to work. These interventions are enshrined internationally in the Framework Convention on Tobacco Control (FCTC), the world’s first public health treaty under the auspices of the World Health Organization (WHO).11 Coming into force in 2005, the treaty now has 181 parties, and it undergirds the global approach to tobacco control. In most LMICs, the main provisions of the FCTC are barely implemented, which leaves plenty of room for improvement.
The most effective provision is the increase in excise taxes on tobacco products. The mechanism is simple: When governments raise taxes, companies raise prices to maintain profits, and higher prices lead to lower consumption. Typically, a 10% increase in tobacco prices leads to a 4% to 8% decrease in consumption.12 This decreased consumption comes in large part from young people not initiating tobacco use. In fact, tax research unequivocally demonstrates two important dynamics: Young people and those in lower socioeconomic groups are more affected by changes in prices. Thus, lower-income people typically reap a disproportionate amount of the reward from these policies.
The other major benefit of tobacco excise tax increases is the increased revenue for government. Because tobacco products are typically inelastic (the decline in consumption is less than the increase in price), governments typically get the double benefit of higher revenue and reduced consumption. Nearly three dozen countries report investing these new tobacco tax revenues in health care, including two, Algeria and Mauritania, using the resources to address cancer specifically.11
A second effective provision is graphic warning labels on tobacco packaging. Research consistently shows that educating users, particularly young people, through packaging promotes quitting and reduces initiation.13 It is inexpensive because governments place the financial burden on the tobacco companies while retaining full control over the messages and images on the package. In recent years, many countries have moved a step further to plain, standardized packaging of tobacco products, which removes all colors and logos. Because these characteristics have been proven to appeal especially to children and youth, their removal is having demonstrable positive public health effects.14
A third proven intervention is the banning of tobacco industry marketing.15 It is well documented that one key part of the tobacco industry’s success in attracting and maintaining customers is their relentless marketing. Removing their ability to communicate to the public undermines their efforts to find new customers and maintain existing ones. The challenge is that the industry finds many creative ways to communicate. For example, much tobacco marketing is done now through point-of-sale displays. Another major strategy is price discounting. Governments must be vigilant in addressing the tobacco industry’s marketing innovations.
A fourth provision is smoke-free laws that prohibit smoking in public and workplaces. Exposure to second-hand smoke causes substantial morbidity and mortality annually, including an estimated 1.2 million deaths.8 Approximately one-third of females and one-fifth of males are exposed to second-hand smoke. Smoke-free laws not only protect nonsmokers, especially children, but they de-normalize smoking, and it is well established that smokers are more likely to quit and youth are less likely to start when smoking is rarely observed in public.16
An important fifth intervention is the use of mass media campaigns to inform the public. Whereas incorporating learning about the dangers of tobacco use in school curricula is important and useful, targeted mass media campaigns are proving to be a better investment value. Young people are particularly responsive to social media sources, from which they typically get most of their information.17
Consumption of Smokeless Tobacco
Although smoking remains the most common and deadly form of tobacco use, smokeless tobacco use is a major cancer risk factor for a number of cancers, most notably those of the head and neck.18 Globally, more than 350 million people use a wide variety of smokeless tobacco products.19 Though most smokeless users live in South and Southeast Asia (> 80%), the prevalence of smokeless tobacco use is above 10% in nearly 30 countries around the globe, and use is particularly concentrated in lower socioeconomic groups and increasingly among youth.20 Recent research suggests that the accessibility and affordability of these products is exacerbating this growing public health challenge. Most troubling is the lack of policy progress to tackle it, wherein interventions such as taxation and youth access lag behind other tobacco control efforts.21
There are three main barriers to successful tobacco control. First, the tobacco industry spends billions of dollars to market its deadly products. The messages of “cool” and “youthful” are ubiquitous and require perseverance by tobacco control proponents to counter. At the same time, the tobacco industry actively lobbies policy makers against public health provisions and even aggressively litigates regulations and policies that seek to reduce consumption. Last, weak governance, particularly in many LMICs, continues to be a major obstacle. Too often, governments are slow to react or do not make any effort to protect citizens from the obvious dangers posed by tobacco use. The FCTC has strong provisions in its Article 5.3 that help governments to insulate themselves from the pressure and influence of the tobacco industry, most notably by actively keeping the industry away from policy making.22
Cervical Cancer Prevention and Control
Cervical cancer is an important public health problem among middle-aged women in LMICs, where screening programs do not exist or are ineffective, and a high risk of HIV infection exists. It is well established that persistent infection with one of the 13 high-risk types of HPV may lead to the development of high-grade cervical precancerous lesions such as cervical intraepithelial neoplasia grade 3 (CIN 3) and grade 2 (CIN 2) or adenocarcinoma in situ, which if left undetected or untreated may lead to the development of invasive cervical cancer.23 Among the high-risk types, HPV 16 and HPV 18 cause 70% to 75% of cervical cancers and 50% to 60% of cervical precancerous lesions.24,25 This knowledge has led to the development of two major approaches to cervical cancer prevention: HPV vaccination to prevent HPV infection and testing for high-risk HPV to facilitate the detection of cervical cancer precursor lesions.
There is conclusive evidence that cervical precancerous lesions can be prevented by HPV vaccination, and cervical cancer can be prevented with early detection and treatment of precancerous lesions. Given the effective and affordable interventions for preventing cervical cancer, and the fact that implementation of both prevention and early detection can lead to avoidance of cervical cancer, WHO has called on member states to commit and implement a global initiative to scale up preventive, screening, and treatment interventions to eliminate cervical cancer as a public health problem in the 21st century.26
Global burden of cervical cancer
The estimated age-standardized incidence rate of cervical cancer globally is about 13.1 per 100,000 women (range, 2 to 75 per 100,000 women), based on cancer data from 185 countries.27 There were approximately 570,000 new cases of cervical cancer and 311,000 cervical cancer deaths in 2018.27 Cervical cancer was the fourth most common cancer in women, ranking after breast cancer (2.1 million cases), colorectal cancer (0.8 million), and lung cancer (0.7 million). It was the leading cause of cancer-related death in women in eastern, western, middle, and southern Africa. The highest incidence was reported from Eswatini (former Swaziland), with approximately 6.5% of women developing cervical cancer before age 75. More than one-third of the global burden of cervical cancer was contributed by China (106,000 cases; 48,000 deaths) and India (97,000 cases; 60,000 deaths). Globally, the average age at diagnosis of cervical cancer was age 53, ranging from age 44 (Vanuatu) to age 68 (Singapore). Cervical cancer ranked among the top three cancers affecting women younger than age 45 in 146 (79%) of 185 countries assessed.
Certain countries in West Asia and Maghreb with low incidence of cervical cancer (fewer than 5 in 100,000) have a low frequency of sexually transmitted infections and low prevalence of HPV infection, probably because of socioeconomic factors that reduce risky sexual behaviors.28 A recent report hypothesized that overtransmission of a protective genetic variant, TP53 codon 72 proline allele, may also be responsible for the low risk in the West Asian population.29 On the other hand, the high incidence of cervical cancer in sub-Saharan Africa, Latin America, and South Asia seems to be caused by increased background risk attributable to high rates of HPV persistence and HIV transmission.28,30 Because the burden of cervical cancer in sub-Saharan Africa is substantial and is increasing, there is a great need for improved prevention to reduce morbidity and mortality from this disease.31
Information on cancer trends over time enables dynamic planning for changing patterns of cancer at the national level. In Asia, a disturbing pattern is the increasing trend in cervical cancer incidence rates and the narrowing urban-rural differences in incidence after several years of falling rates in China32,33 and in sub-Saharan Africa.34,35 The annual percentage increase in age-standardized incidence rates is about 9.2% in China.33 In a pooled analysis of several prevalence studies, the overall prevalence of high-risk HPV infection was reported to range from 15% to 20% in China.36,37 On the other hand, cervical cancer incidence rates have been declining with a concomitant increase in breast cancer, particularly in urban populations in India, possibly because of socioeconomic development, reduced parity, and increasing age at marriage.38
Cervical cancer in immunocompromised women
Precancerous cervical lesions and cervical cancer are more aggressive and progressive in immunocompromised patients. Given the high prevalence of precancerous cervical lesions among HIV-positive women, prevention measures are extremely important in HIV-positive women.39
Currently bivalent (targeting HPV 16 and 18), quadrivalent (targeting HPV6, 11, 16, and 18), and nonavalent (targeting HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58) prophylactic vaccines effective in HPV-naive patients have been licensed in many countries, and new HPV vaccines are undergoing evaluation in India and China. The Indian vaccine is expected to be licensed in 2021. The WHO recommends that HPV vaccination be included in national immunization programs as part of a comprehensive strategy to prevent cervical cancer. It recommends two doses 6 or 12 months apart for 9- to 14-year-old girls and three doses over a 6-month period for those aged 15 and older and for immunocompromised patients irrespective of age.40 There is recent evidence that even a single dose of HPV vaccine might protect against cervical neoplasia.41 HPV vaccination has been followed by sustained protection against CIN2 or CIN3 and adenocarcinoma in situ associated with HPV 16/18 infection in young women who were not initially infected with high-risk HPV or HPV 16/18, and the occurrence of severe adverse events or adverse pregnancy outcomes was not significantly higher in recipients of HPV vaccines.32
Primary prevention of cervical cancer through HPV vaccination is a cost-effective preventive measure. HPV vaccination is being implemented in a number of sub-Saharan African countries with high vaccine coverage, improved community sensitization, and strong commitment from national governments (e.g., Rwanda, Botswana, Zambia), despite various challenges.42 HPV vaccination has been associated with negative media coverage and negative advocacy in a number of countries.43,44 Denmark provides an excellent example of how a country grappled with negative media coverage of HPV vaccination and the steadying impact of action by national authorities.43,44 These important national experiences provide valuable leads for successful vaccination implementation for countries beginning to plan for vaccine introduction.
Screening for cervical cancer
Cervical cancer can be prevented by screening for cervical precancerous lesions and treating them by screening with cytology or visual inspection with 3% to 5% acetic acid (VIA) or HPV testing. Cytology screening traditionally has been the cornerstone of cervical screening in high-income countries, where it has been followed by substantial decline in incidence and mortality.
The difficulty in implementing cytology or HPV screening in LMICs has led to the implementation of VIA screening and treatment of VIA-positive women with cryotherapy or thermal ablation.5 The real-time result of screening with VIA allows a single-visit approach combining screening and treatment, thereby maximizing compliance to treatment. Given the feasibility of introducing VIA screening in public health services, several sub-Saharan African countries have implemented it. However, a major challenge with VIA screening has been the difficulties in quality assurance and in maintaining a consistent screening performance in the long run.
The fact that HPV testing is more sensitive, accurate, and reproducible in detecting high-grade lesions and more effective in preventing future invasive cancers than screening with liquid-based or conventional cytology, particularly in the post-HPV vaccination scenario, has led to increasing implementation of HPV screening in national programs.45 Recent European guidelines strongly recommend primary HPV testing over cytology screening. The possibility of doing HPV testing by using self-collected cervical cell specimens facilitates reaching women who otherwise would not participate in screening. The high negative predictive value and the possibility of longer screening intervals for HPV-negative women are other advantages of HPV screening. It has been shown that even a single round of VIA or HPV screening has been followed by significant reduction in cervical cancer mortality in low-income countries.46,47
Elimination of cervical cancer
The goal set by WHO to reduce the incidence rate of cervical cancer to less than 4 in 100,000 women globally by vaccinating 90% of girls by age 15, screening 70% of women aged 35 to 45, and treating at least 90% of precancerous lesions detected by screening requires substantial investment by national governments. A judicious combination of HPV vaccination, screening and diagnosis, and effective treatment of early invasive cancer and political commitment and allocation of adequate resources are critical for elimination in LMICs.
Global Burden of Breast Cancer
Breast cancer continues to be the most common cancer in women both in developed and developing countries. According to the GLOBOCAN 2018 estimates, WHO reports 626,679 women lost their lives to breast cancer in 2018.48 Although the incidence of breast cancer is higher in high-income countries, with 74.4 per 100,000 in Western Europe, compared with 34.4 per 100,000 in Asia, the overall incidence is increasing in developing countries.49 In 2018, among the 2.1 million cases of female breast cancers diagnosed worldwide, approximately 911,000 cases (rate of 34 per 100,000) were reported from Asia. Correspondingly, the overall outcomes of breast cancer vary with survival rates of 80% or more in North America, compared with 40% or less in low-income countries, demanding continued efforts across the continuum of cancer care in LMICs.49
Epidemiology, prevention, and early detection of breast cancer
The rising incidence of breast cancer among the younger women in LMICs is concerning. Whereas the median age of breast cancer for women in developed countries is 63, it is more common among younger women between age 45 and 50 and in India, Mexico, and Arab countries.50 Given that the majority of LMICs have no screening guidelines in place, high-risk screening and chemoprevention options seem unfeasible. In this context, other preventive and cost-effective measures are optimal: mass cancer awareness and education camps, community health workers and navigator-based care delivery models, clinical breast examination (CBE), mobile technology, novel screening methods, addressing other risk factors such as obesity, and lifestyle modifications.51–53 Although breast cancer prevention trials incorporating high-risk surveillance with imaging and risk reduction strategies with prophylactic mastectomy have shown success in BRCA gene mutation carriers, the uptake of these services is not cost-effective in LMICs.54–56
Despite the lack of collective agreement on the benefits of CBE, it continues to be an important tool and a standard of practice along with screening mammography.57,58 Although the success of screening mammography with a 19% reduction in mortality for women age 40 to 70 appears promising, overdiagnosis continues to be a challenge.59 Mammography is a potential option for screening, but the majority of LMICs lack screening guidelines and equipped facilities for cancer screening. Eventually, many patients succumb to advanced stages of disease at diagnosis and high mortality rates. Also, incomplete treatments and poor adherence continue to add to the burden at a societal level. To explore the use of triennial CBE in reducing the rate of advanced breast cancer and mortality, Sankaranarayanan et al53 conducted a cluster randomized clinical trial in Trivandrum, India. Inthistrial, 115,652 women age 30 to 69 in 275 clusters were randomly assigned to receive CBE or no screening. CBE was performed by trained community health workers, who functioned as liaisons between the healthy women and physicians. This study demonstrated higher numbers of early-stage breast cancers in the CBE group compared with the control group (18.8 and 8.1 per 100,000 women, respectively).53
Mobile mammography: a potential tool to improve access and screening in LMICs
Previous studies reported mobile mammographic units as valuable screening tools in improving access for those with transportation barriers.51,60–68 However, the utility of and adherence to screening mammography on fixed versus mobile units depended on sociodemographic factors and women’s perceived risk of breast cancer.61,64 Whereas the users of fixed mammography units were noted to be from stable socioeconomic backgrounds, tended to be insured, and were adherent to screening,60,61,64 mobile units’ users tended to be from underserved communities with transient lifestyles and living situations and were less adherent.65 Vang et al67,68 reported critical gaps and the challenges of the mobile units, such as the quality, incomplete imaging, higher recalls, limited use of newer technology (e.g., film-screen, full-field digital, tomosynthesis), and the lack of diagnostic imaging. Despite the expanding use of mobile mammographic units in several parts of the world, such as sub-Saharan Africa,62 the association with sociodemographic factors and the recall rates continue to raise challenges for screening adherence.66 Rodríguez-Cuevas et al66 conducted a mobile mammography program in Mexico in 2005 and 2006 for women older than age 40. Among the 96,828 women submitted to mammography, only 1% of mammograms in the Breast Imaging Reporting and Data System were 0, 4, or 5. Among women with abnormal mammograms, 27.7% had breast cancer (ductal carcinoma in situ, 2.1%; stage I, 29.4%; stage II, 42.2%), and older age (age 50 and older) was found to be the only risk factor for having an abnormal mammogram. In LMICs with existing infrastructure, multidisciplinary evaluation of mobile mammographic units as a potential cost-effective opportunity will serve as a new avenue for screening.
Head and Neck Cancers
Head and neck cancers (including cancers of the lip and oral cavity, larynx, oropharynx, hypopharynx, sinuses, and salivary glands) contribute 4.9% and 4.7%, respectively, to the global cancer incidence and mortality.69 An estimated 76% of head and neck cancer cases and 84% of head and neck cancer deaths occur in LMICs,69 and high rates of tobacco consumption, including smokeless tobacco, are a crucial concern for increasing rates of these cancers.70 Although head and neck cancers are largely preventable, substantial morbidity, mortality, and financial burden could be avoided by early detection.71–73 Head and neck cancers impose catastrophic economic burden on families of patients in LMICs.74 One study of the economic effects of head and neck cancers in South Asia estimated that US$16.9 billion was lost on account of these cancers every year in the region.75 Another study conducted by Patterson et al reported an anticipated global cumulative loss of US$535 billion during the years 2018 to 2030 due to head and neck cancers.76 Surgery remains the treatment of choice for these cancers in LMICs since chemotherapy is very expensive and radiation facilities are limited.77,78 Even surgical care is fairly limited and usually not specialized to handle head and neck cancers.79
Liver Cancer
LMICs together contributed 83% of the global annual incidence of liver cancers and 82% of the global annual mortality from liver cancers in 2018, with China contributing more than 50% of these.80 Poor survival rates make liver cancer the seventh most common cause of cancer mortality worldwide (after lung, breast, prostate, colon, non-melanoma skin cancers, and stomach cancers).
Chronic infection with hepatitis B and hepatitis C viruses, alcohol, tobacco, aflatoxins, diabetes, and obesity are all associated with the disease. Cirrhosis is usually a precursor to liver cancer.81–83 Hepatitis B vaccination, universal safety precautions for preventing transmission of blood-borne infections, blood product safety, supply of sterile needles and syringes to injectable drug users, and safe sex practices have been shown to reduce the transmission of hepatitis B and C.84,85 Observational studies claim that coffee, statins, metformin, and aspirin have a protective effect against liver cancer.86–89 Coffee even finds a mention in the 2018 clinical practice guidelines of the European Association for the Study of the Liver.90 Several LMICs have incorporated hepatitis B vaccines into their national infant immunization programs, and some of these countries have reported declining rates of liver cancer.91 Sonography is the standard surveillance test recommended by the American Association for the Study of Liver Diseases, the European Association for the Study of the Liver, and the Asia Pacific Association for the Study of the Liver.91–93 Serum alpha-fetoprotein is sometimes used as an adjunct to sonography.94
Esophagus, Stomach, and Colorectal Cancers
LMICs contributed 75% of the global annual incidence and mortality from esophageal, stomach, and colorectal cancers combined in 2018.80 Contrast radiography and upper endoscopy have been used for screening stomach cancers. Observational studies suggest that the screening has contributed to early detection and mortality reduction, but there are no data from large randomized trials.95–103 The value of screening asymptomatic people for gastric cancer is controversial even in areas with a high incidence of gastric cancer.104 There are only limited data suggesting that such an approach may decrease the incidence of gastric cancer in high-incidence areas.105–108 Therefore, routine radiographic, endoscopic, and Helicobacter pylori screenings of asymptomatic healthy people are not recommended.
Tobacco, alcohol, malnutrition, and HPV are identified as risk factors for squamous cell carcinoma of the esophagus.109 Long standing gastroesophageal reflux disease, with Barrett esophagus as a usual precursor, is associated with increased risk of esophageal adenocarcinoma.110–112 Benefits of screening for Barrett esophagus have been debated, without a firm consensus. The logistics of implementing such a screening strategy and the subsequent management of patients diagnosed with Barrett esophagus present enormous challenges in LMICs. Overall, the support for radiographic and endoscopic esophageal screening has been modest.113
Fecal occult blood test (FOBT) screening for colorectal cancers have shown mortality reduction.114 Sigmoidoscopy, colonoscopy, and double-contrast barium enema are used to triage FOBT screening positives. Thailand implemented a successful pilot project demonstrating the feasibility of conducting an organized FOBT colorectal cancer screening program in LMICs, setting an example for other LMICs.115
DISCUSSION
The incidence and mortality of cancer are increasing in LMICs; however, more than 70% of the commonly occurring cancers in LMICs have evidence-based prevention, screening, and early detection interventions. LMICs should therefore invest in low-cost cancer awareness, prevention, screening, and early detection programs that are locally feasible. Large investments in human resources would be needed for such programs.
Effective tobacco control measures should top the health agenda in LMICs. Several LMICs already have WHO FCTC-based legislation in place, and they should ensure tougher enforcement.116,117 Smokeless tobacco use, which is very common in South Asia, is under-researched, and investments should be made in developing evidence-based interventions for smokeless tobacco control. Although increasing taxes and pack warnings118 on tobacco products are effective evidence-based strategies, additional challenges exist because the majority of tobacco products sold in LMICs are nonpackaged (e.g., single cigarettes) and hence escape regulatory controls. Banning tobacco advertising and marketing (including surrogate advertising and marketing), enacting and enforcing tough smoke-free laws, and promoting effective mass media and social media campaigns targeting teenagers and youth would yield optimal returns on investments.
As studies of single-dose HPV vaccination continue to accumulate data,41,119–121 two-dose nonavalent HPV vaccination as the standard of care (at least 6 months apart and before the 15th birthday) for girls (and boys if economically feasible) should be next on the list of LMIC health priorities.122 Along with HPV vaccination, population-based cervical screening with VIA/HPV testing at 5-year intervals between the ages of 30 and 65 (two or three lifetime screens)123,124 would help LMICs meet the WHO goal of global cervical cancer elimination. Despite the Global Alliance for Vaccines and Immunization subsidies of HPV vaccine, LMICs are still slow in the rollout of HPV vaccines, because of the costs and logistics associated with HPV vaccine delivery. International health agencies must work together to make HPV vaccination affordable in LMICs.
Because the age cohorts for breast cancer in women are much younger in LMICs, mammography as a population-based screening tool is likely to result in more harm than benefit. Based on the current evidence, breast self-examination, CBE, and sonography also cannot be recommended for population-based breast cancer screening in LMICs.125 Breast cancer control programs in LMICs should therefore be currently limited to awareness programs with increased facilities to diagnose and treat symptomatic referrals.
Screening and early detection of liver cancer continue to be challenging in LMICs. Although liver sonography has been used with some success in Asian countries, it is not feasible in African countries. Therefore, all LMICs should adopt a universal hepatitis B vaccination policy for infants. Additionally, blood safety, universal safety precautions, and safe sex practices should be promoted, given the additional health benefits. Esophagus, stomach, and colorectal cancers are also increasing in LMICs, but these could be attributed to a combination of variables including, diet, physical activity, increasing lifespan, and improved diagnosis of the disease and disease registration processes. Radiographic and endoscopic screening for these cancers, recommended in some high-income countries, is not feasible in LMICs because of inadequate numbers of service providers and necessary infrastructure. Therefore, focusing on tobacco and alcohol control, and FOBT screening where feasible, might be the best strategies for LMICs.
CONCLUSION
Cancer incidence in LMICs is on the rise, requiring resources and implementation of cost-effective prevention measures. In addition, the increasing prevalence of obesity and lack of physical activity among people living in urban areas in LMICs will eventually result in sharp increases in noncommunicable diseases, including cancers. Investing in programs to aggressively address this problem is necessary now to prevent this future catastrophe.
PRACTICAL APPLICATIONS.
Cancer incidence in LMICs is rapidly increasing, demanding the dedication of more resources and the implementation of cost-effective prevention measures.
Cancer awareness and education through community health workers and navigators can improve cancer prevention, screening, and early detection in low-resource settings.
Government support for comprehensive tobacco control programs—including higher taxes, graphic warnings, marketing bans, and accessible and free tobacco cessation programs—will reduce multiple tobacco-related cancers.
Prophylactic HPV vaccines have a significant impact in reducing the risk of cervical cancer if deployed successfully to young girls (ages 9 to 14).
Novel screening methods such as mobile mammographic units, detection of immunochemical fecal occult blood, and Papanicolaou test will assist in early detection of breast, colorectal, and cervical cancers, respectively.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Disclosures provided by the authors and data availability statement (if applicable) are available with this article at DOI https://doi.org/10.1200/EDBK_280625.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics: GLOBOCAN estimates of incidence and mortalityworldwidefor36cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Remais JV, Zeng G, Li G, et al. Convergence of non-communicable and infectious diseases in low- and middle-income countries. Int J Epidemiol. 2013; 42:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viswanath K, Emmons KM. Message effects and social determinants of health: its application to cancer disparities. J Commun. 2006;56 (suppl 1):S238–S264. [Google Scholar]
- 4.Marmot M. Health equity, cancer, and social determinants of health. Lancet Glob Health. 2018;6:s29. [Google Scholar]
- 5.Sankaranarayanan R Screening for cancer in low- and middle-income countries. Ann Glob Health. 2014;80:412–417. [DOI] [PubMed] [Google Scholar]
- 6.Fitzmaurice C, Abate D, Abbasi N, et al. ; Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. Epub 2019 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: Department of Health and Human Services. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 2014. [Google Scholar]
- 8.Stanaway JD, Afshin A, Gakidou E; GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wild CP, Weiderpass E, Stewart BW, (eds). World Cancer Report. Cancer Research for Cancer Prevention. Lyon, France: World Health Organization International Agency for Research on Cancer; 2020. [Google Scholar]
- 10.Drope JSN, Cahn Z, Drope J, et al. The Tobacco Atlas. Atlanta: American Cancer Society and Vital Strategies. The Tobacco Atlas Atlanta, GA: American Cancer Society and Vital Strategies; 2017. [Google Scholar]
- 11.World Health Organization. WHO Report on the Global Tobacco Epidemic 2017. Geneva, Switzerland: World Health Organization: 2017. [Google Scholar]
- 12.Chaloupka FJ. The economics of tobacco taxation. Presented at: 2009 Tobacco Summit; April 16, 2009; Fairbanks, AK. [Google Scholar]
- 13.O’Hegarty M, Pederson LL, Nelson DE, et al. Reactions of young adult smokers to warning labels on cigarette packages. Am J Prev Med. 2006;30:467–473. [DOI] [PubMed] [Google Scholar]
- 14.Wakefield M, Coomber K, Zacher M, et al. Australian adult smokers’ responses to plain packaging with larger graphic health warnings 1 year after implementation: results from a national cross-sectional tracking survey. Tob Control. 2015;24 (suppl 2):ii17–ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henriksen L. Comprehensive tobacco marketing restrictions: promotion, packaging, price and place. Tob Control. 2012;21:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly BC, Vuolo M, Frizzell LC, et al. Denormalization, smoke-free air policy, and tobacco use among young adults. Soc Sci Med. 2018;211:70–77. [DOI] [PubMed] [Google Scholar]
- 17.Freeman B, Potente S, Rock V, et al. Social media campaigns that make a difference: what can public health learn from the corporate sector and other social change marketers? Public Health Res Pract. 2015;25:e2521517. [DOI] [PubMed] [Google Scholar]
- 18.Niaz K, Maqbool F, Khan F, et al. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health. 2017; 39:e2017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha DN, Gupta PC, Kumar A, et al. The Poorest of Poor Suffer the Greatest Burden From Smokeless Tobacco Use: A Study From 140 Countries. Nicotine Tob. Res 2018;20:1529–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha DN, Kumar A, Bhartiya D, et al. Smokeless Tobacco Use Among Adolescents in Global Perspective. Nicotine Tob. Res 2017;19:1395–1396. [DOI] [PubMed] [Google Scholar]
- 21.Mehrotra R, Yadav A, Sinha DN, et al. Smokeless tobacco control in 180 countries across the globe: call to action for full implementation of WHO FCTC measures. Lancet Oncol. 2019;20:e208–e217. [DOI] [PubMed] [Google Scholar]
- 22.Prevent20. Home page. https://wecanprevent20.org/. Accessd March 24, 2020.
- 23.World Health Organization International Agency for Cancer Research. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2005;90. [Google Scholar]
- 24.Bzhalava D, Guan P, Franceschi S, et al. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology. 2013; 445:224–231. [DOI] [PubMed] [Google Scholar]
- 25.de Sanjose S, Quint WG, Alemany L, et al. ; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization: WHO director-general calls for all countries to take action to help end the suffering caused by cervical cancer. 2018. www.who.int/reproductivehealth/call-to-action-elimination-cervical-cancer/en/. Accessd March 24, 2020.
- 27.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020; 8:e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Global Health Observatory (GHO) data. 2019. www.who.int/gho/hiv/en/. Accessd March 24, 2020.
- 29.Alsbeih GA, Al-Harbi NM, Bin Judia SS, et al. Reduced rate of human papillomavirus infection and genetic overtransmission of TP53 72C polymorphic variant lower cervical cancer incidence. Cancer. 2017;123:2459–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010; 19:1893–1907. [DOI] [PubMed] [Google Scholar]
- 31.Vaccarella S, Laversanne M, Ferlay J, et al. Cervical cancer in Africa, Latin America and the Caribbean and Asia: regional inequalities and changing trends. Int J Cancer. 2017;141:1997–2001. [DOI] [PubMed] [Google Scholar]
- 32.Arbyn M, Xu L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev Vaccines. 2018;17:1085–1091. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Zheng R, Li X, et al. Trends of incidence rate and age at diagnosis for cervical cancer in China, from 2000 to 2014. Chin J Cancer Res. 2017;29:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chokunonga E, Borok MZ, Chirenje ZM, et al. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer. 2013; 133:721–729. [DOI] [PubMed] [Google Scholar]
- 35.Mboumba Bouassa RS, Prazuck T, Lethu T, et al. Cervical cancer in sub-Saharan Africa: a preventable noncommunicable disease. Expert Rev Anti Infect Ther. 2017;15:613–627. [DOI] [PubMed] [Google Scholar]
- 36.Li K, Li Q, Song L, et al. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer. 2019;125:1030–1037. [DOI] [PubMed] [Google Scholar]
- 37.Zhu B, Liu Y, Zuo T, et al. The prevalence, trends, and geographical distribution of human papillomavirus infection in China: the pooled analysis of 1.7 million women. Cancer Med. 2019;8:5373–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badwe RA, Dikshit R, Laversanne M, et al. Cancer incidence trends in India. Jpn J Clin Oncol. 2014;44:401–407. [DOI] [PubMed] [Google Scholar]
- 39.Weldegebreal F, Worku T. Precancerous cervical lesion among HIV-positive women in sub-Saharan Africa: a systematic review and meta-analysis. Cancer Contr. 2019;26:1073274819845872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Human papillomavirus vaccine, WHO position paper, May 2017: recommendations. Vaccine. 2017;35:5753–5755. [DOI] [PubMed] [Google Scholar]
- 41.Sankaranarayanan R, Joshi S, Muwonge R, et al. ; Indian HPV vaccine study group. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018;36:4783–4791. [DOI] [PubMed] [Google Scholar]
- 42.Black E, Richmond R. Prevention of cervical cancer in sub-Saharan Africa: the advantages and challenges of HPV vaccination. Vaccines (Basel). 2018;6:E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen PR, Schmidtblaicher M, Brewer NT. Resilience of HPV vaccine uptake in Denmark: decline and recovery. Vaccine. 2020;38:1842–1848. [DOI] [PubMed] [Google Scholar]
- 44.Sankaranarayanan R, Basu P, Kaur P, et al. Current status of human papillomavirus vaccination in India’s cervical cancer prevention efforts. Lancet Oncol. 2019;20:e637–e644. [DOI] [PubMed] [Google Scholar]
- 45.Maver PJ, Poljak M. Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect. Epub 2019 Sep 17. [DOI] [PubMed] [Google Scholar]
- 46.Sankaranarayanan R, Esmy PO, Rajkumar R, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. [DOI] [PubMed] [Google Scholar]
- 47.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. [DOI] [PubMed] [Google Scholar]
- 48.International Agency for Research on Cancer. World Health Organization. GLOBOCAN. http://gco.iarc.fr/. Accessed March 24, 2020. [Google Scholar]
- 49.Coleman MP, Quaresma M, Berrino F, et al. ; CONCORD Working Group. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9:730–756. [DOI] [PubMed] [Google Scholar]
- 50.Tfayli A, Temraz S, Abou Mrad R, et al. Breast cancer in low- and middle-income countries: an emerging and challenging epidemic. J Oncol. 2010; 2010:490631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamaraju S, DeNomie M, Visotcky A, et al. Increasing mammography uptake through academic-community partnerships targeting immigrant and refugee communities in Milwaukee. WMJ. 2018;117:55–61. [PubMed] [Google Scholar]
- 52.Kamaraju S, Olson J, DeNomie M, et al. Community breast health education for immigrants and refugees: lessons learned in outreach efforts to reduce cancer disparities. J Cancer Educ. 2019;34:1092–1096. [DOI] [PubMed] [Google Scholar]
- 53.Sankaranarayanan R, Ramadas K, Thara S, et al. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103:1476–1480. [DOI] [PubMed] [Google Scholar]
- 54.Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, et al. ; Hereditary Breast Cancer Clinical Study Group. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer. 2008;122:2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paluch-Shimon S, Cardoso F, Sessa C, et al. ; ESMO Guidelines Committee. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27 (suppl 5):v103–v110. [DOI] [PubMed] [Google Scholar]
- 56.Rosner BA, Colditz GA, Hankinson SE, et al. Validation of Rosner-Colditz breast cancer incidence model using an independent data set, the California Teachers Study. Breast Cancer Res Treat. 2013;142:187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2012: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2012;62:129–142. [DOI] [PubMed] [Google Scholar]
- 58.US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009; 151:716–726. [DOI] [PubMed] [Google Scholar]
- 59.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311:1327–1335. [DOI] [PubMed] [Google Scholar]
- 60.Katapodi MC, Lee KA, Facione NC, et al. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening:a meta-analytic review. Prev Med. 2004;38:388–402. [DOI] [PubMed] [Google Scholar]
- 61.Elewonibi BR, Thierry AD, Miranda PY. Examining mammography use by breast cancer risk, race, nativity, and socioeconomic status. J Immigr Minor Health. 2018;20:59–65. [DOI] [PubMed] [Google Scholar]
- 62.Apffelstaedt JP, Hattingh R, Baatjes K, et al. Results of a pilot programme of mammographic breast cancer screening in the Western Cape. S Afr Med J. 2014; 104:297–298. [DOI] [PubMed] [Google Scholar]
- 63.Brooks SE, Hembree TM, Shelton BJ, et al. Mobile mammography in underserved populations: analysis of outcomes of 3,923 women. J Community Health. 2013;38:900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen YR, Chang-Halpenny C, Kumarasamy NA, et al. Perspectives of mobile versus fixed mammography in Santa Clara County, California: a focus group study. Cureus. 2016;8:e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moyce SC, Schenker M. Migrant workers and their occupational health and safety. Annu Rev Public Health. 2018;39:351–365. [DOI] [PubMed] [Google Scholar]
- 66.Rodríguez-Cuevas S, Guisa-Hohenstein F, Labastida-Almendaro S. First breast cancer mammography screening program in Mexico: initial results2005–2006. Breast J. 2009;15:623–631. [DOI] [PubMed] [Google Scholar]
- 67.Scheel JR, Tillack AA, Mercer L, et al. Mobile versus fixed facility: Latinas’ attitudes and preferences for obtaining a mammogram. J Am Coll Radiol. 2018; 15:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vang S, Margolies LR, Jandorf L. Mobile mammography participation among medically underserved women: a systematic review. Prev Chronic Dis. 2018; 15:E140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.International Agency for Research on Cancer. Cancer Today. Cancer Fact Sheets https://gco.iarc.fr/today/fact-sheets-cancers. Accessed on March 18, 2020.
- 70.Asthana S, Labani S, Kailash U, et al. Association of Smokeless Tobacco Use and Oral Cancer: A Systematic Global Review and Meta-Analysis. Nicotine Tob. Res 2019;21:1162–1171. [DOI] [PubMed] [Google Scholar]
- 71.Sankaranarayanan R, Ramadas K, Thara S, et al. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncol. 2013;49:314–21. [DOI] [PubMed] [Google Scholar]
- 72.Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. The Lancet. 2005;365:1927–1933. [DOI] [PubMed] [Google Scholar]
- 73.Subramanian S, Sankaranarayanan R, Bapat B, et al. Cost-effectiveness of oral cancer screening: results from a cluster randomized controlled trial in India. Bull. World Health Organ 2009;87:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jan S, Kimman M, Peters SAE, et al. ; ACTION Study Group. Financial catastrophe, treatment discontinuation and death associated with surgically operable cancer in South-East Asia: Results from the ACTION Study. Surgery. 2015;157:971–982. [DOI] [PubMed] [Google Scholar]
- 75.Alkire BC, Bergmark RW, Chambers K, et al. Head and neck cancer in South Asia: Macroeconomic consequences and the role of the head and neck surgeon. Head Neck. 2016;38:1242–1247. [DOI] [PubMed] [Google Scholar]
- 76.Patterson RH, Fischman VG, Wasserman I, et al. Global Burden of Head and Neck Cancer: Economic Consequences, Health, and the Role of Surgery. Otolaryngol Head Neck Surg. 2020;162:296–303. [DOI] [PubMed] [Google Scholar]
- 77.Wilson BE, Jacob S, Yap ML, et al. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: a population-based study. Lancet Oncol. 2019;20:769–780. [DOI] [PubMed] [Google Scholar]
- 78.Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16:1153–1186. [DOI] [PubMed] [Google Scholar]
- 79.Meara JG, Leather AJM, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386:569–624. [DOI] [PubMed] [Google Scholar]
- 80.World Health Organization International Agency for Research on Cancer. Cancer Today. https://gco.iarc.fr/today/home. Accessed March 24, 2020.
- 81.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 (suppl 3):S206–S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parikh S, Hyman D. Hepatocellular cancer: a guide for the internist. Am J Med. 2007;120:194–202. [DOI] [PubMed] [Google Scholar]
- 83.Venook AP, Papandreou C, Furuse J, et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 (suppl 4):5–13. [DOI] [PubMed] [Google Scholar]
- 84.Franco E, Bagnato B, Marino MG, et al. Hepatitis B: epidemiology and prevention in developing countries. World J Hepatol. 2012;4:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franco E, Meleleo C, Serino L, et al. Hepatitis A: epidemiology and prevention in developing countries. World J Hepatol. 2012;4:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bravi F, Tavani A, Bosetti C, et al. Coffee and the risk of hepatocellular carcinoma and chronic live disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev. 2017;26:368–377. [DOI] [PubMed] [Google Scholar]
- 87.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:1808–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. [DOI] [PubMed] [Google Scholar]
- 89.Zhou YY, Zhu GQ, Liu T, et al. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci Rep. 2016; 6:33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 91.Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the South-East Asia region, 1992–2015. Vaccine. 2018;36:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 93.Tan CH, Low SC, Thng CH. APASL and AASLD consensus guidelines on imaging diagnosis of hepatocellular carcinoma: a review. Int J Hepatol. 2011; 2011:519783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang TS, Wu YC, Tung SY, et al. Alpha-fetoprotein measurement benefits hepatocellular carcinoma surveillance in patients with cirrhosis. Am J Gastroenterol. 2015;110:836–844. [DOI] [PubMed] [Google Scholar]
- 95.Choi KS, Jun JK, Suh M, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer. 2015;112:608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fukao A, Tsubono Y, Tsuji I, et al. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population-based case-control study. Int J Cancer. 1995;60:45–48. [DOI] [PubMed] [Google Scholar]
- 97.Hisamichi S, Sugawara N, Fukao A. Effectiveness of gastric mass screening in Japan. Cancer Detect Prev. 1988;11:323–329 [PubMed] [Google Scholar]
- 98.Inaba S, Hirayama H, Nagata C, et al. Evaluation of a screening program on reduction of gastric cancer mortality in Japan: preliminary results from a cohort study. Prev Med. 1999;29:102–106. [DOI] [PubMed] [Google Scholar]
- 99.Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol. 2006;13:221–228. [DOI] [PubMed] [Google Scholar]
- 100.Mizoue T, Yoshimura T, Tokui N, et al. ; Japan Collaborative Cohort Study Group. Prospective study of screening for stomach cancer in Japan. Int J Cancer.2003; 106:103–107. [DOI] [PubMed] [Google Scholar]
- 101.Murakami R, Tsukuma H, Ubukata T, et al. Estimation of validity of mass screening program for gastric cancer in Osaka, Japan. Cancer. 1990;65:1255–1260. [DOI] [PubMed] [Google Scholar]
- 102.Oshima A, Hirata N, Ubukata T, et al. Evaluation of a mass screening program for stomach cancer with a case-control study design. Int J Cancer. 1986; 38:829–833. [DOI] [PubMed] [Google Scholar]
- 103.Tsuji I, Fukao A, Sugawara N, et al. Cost-effectiveness analysis of screening for gastric cancer in Japan. Tohoku J Exp Med. 1991;164:279–284. [DOI] [PubMed] [Google Scholar]
- 104.Leung WK, Wu MS, Kakugawa Y, et al. ; Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. [DOI] [PubMed] [Google Scholar]
- 105.Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saito K, Arai K, Mori M, et al. Effect of Helicobacter pylori eradication on malignant transformation of gastric adenoma. Gastrointest Endosc. 2000;52:27–32. [DOI] [PubMed] [Google Scholar]
- 107.Uemura N, Mukai T, Okamoto S, et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639–642. [PubMed] [Google Scholar]
- 108.Wong BC, Lam SK, Wong WM, et al. ; China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. [DOI] [PubMed] [Google Scholar]
- 109.Glade MJ. Food, nutrition, and the prevention of cancer: a global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition. 1999;15:523–526. [DOI] [PubMed] [Google Scholar]
- 110.Wijnhoven BP, Tilanus HW, Dinjens WN. Molecular biology of Barrett’s adenocarcinoma. Ann Surg. 2001;233:322–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999; 340:825–831. [DOI] [PubMed] [Google Scholar]
- 112.Skacel M, Petras RE, Gramlich TL, et al. The diagnosis of low-grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol. 2000;95:3383–3387. [DOI] [PubMed] [Google Scholar]
- 113.Sami SS, Ragunath K, Iyer PG. Screening for Barrett’s esophagus and esophageal adenocarcinoma: rationale, recent progress, challenges, and future directions. Clin Gastroenterol Hepatol. 2015;13:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541–1549. [DOI] [PubMed] [Google Scholar]
- 115.Khuhaprema T, Sangrajrang S, Lalitwongsa S, et al. Organised colorectal cancer screening in Lampang Province, Thailand: preliminary results from a pilot implementation programme. BMJ Open. 2014;4:e003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Myers ML. The FCTC’s evidence-based policies remain a key to ending the tobacco epidemic. Tob Control. 2013;22 (suppl 1):i45–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shastri A, Shastri SS. Cancer screening and prevention in low-resource settings. Nat Rev Cancer. 2014;14:822–829. [DOI] [PubMed] [Google Scholar]
- 118.Francis DB, Mason N, Ross JC, et al. Impact of tobacco-pack pictorial warnings on youth and young adults: a systematic review of experimental studies. Tob Induc Dis. 2019;17:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brotherton JM, Budd A, Rompotis C, et al. Is one dose of human papillomavirus vaccine as effective as three? A national cohort analysis. Papillomavirus Res. 2019;8:100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sankaranarayanan R, Prabhu PR, Pawlita M, et al. ; Indian HPV Vaccine Study Group. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Whitworth HS, Gallagher KE, Howard N, et al. Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two-dose vaccination regimens: a systematic review of evidence from clinical trials. Vaccine. 2020;38:1302–1314. [DOI] [PubMed] [Google Scholar]
- 122.Bergman H, Buckley BS, Villanueva G, et al. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst Rev. 2019;11:CD013479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jeronimo J, Castle PE, Temin S, et al. Secondary prevention of cervical cancer: ASCO resource-stratified clinical practice guideline. J Glob Oncol. 2016; 3:635–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee H, Hur S, Jang H, et al. Cost-utility of a two-dose human papillomavirus vaccination programme added to cervical cancer screening compared with cervical cancer screening alone in Korea. Asian Pac J Cancer Prev. 2019;20:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lauby-Secretan B, Scoccianti C, Loomis D, et al. ; International Agency for Research on Cancer Handbook Working Group. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med. 2015;372:2353–2358. [DOI] [PubMed] [Google Scholar]


