Summary
25-hydroxy vitamin D (25 OHD) deficiency and secondary hyperparathyroidism have been seen after metabolic and bariatric surgery, but data are lacking on the bone health outcomes of adolescent sleeve gastrectomy (SG). The purpose of this study was to examine bone-related nutrition after SG, compared to laparoscopic adjustable gastric band (LAGB), and trend bone turnover markers following SG. This is an observational study of 197 adolescents who underwent LAGB (n = 98) or SG (n = 99). Bone health labs were collected at baseline and 6 and/or 12 months after LAGB or SG, with additional analysis of bone turnover markers in the SG group. Calcium and 25 OHD levels increased at 6 and 12 months after LAGB and SG, with no difference between the surgeries. Parathyroid hormone levels decreased only in the SG group. SG patients had increased osteocalcin and carboxy-terminal cross-linking telopeptide of type 1 collagen (CTX) at 6 and 12 months post-SG, although CTX decreased between 6 and 12 months. Excess weight loss at 6 months predicted the rise in CTX, but the changes in osteocalcin and CTX could not be attributed to 25 OHD deficiency, hypocalcemia or hyperparathyroidism. Patients had improved 25 OHD levels post-surgery, which may be secondary to stringent vitamin supplementation guidelines. However, there were marked increases in bone turnover markers following SG. More studies are needed to evaluate the effects of SG on adolescent bone health and to correlate the early changes in bone turnover with bone mineral density and fracture risk.
Keywords: adolescence, bariatric surgery, bone health
1 |. INTRODUCTION
In the United States, the prevalence of obesity in children and adolescents was 26.4% between 2015 and 2016, with extreme obesity (body mass index [BMI] ≥ to 120 of the 95th percentile or ≥ 35) equal to 7.9%. Adolescents with obesity have increased vascular and metabolic risk (hypertension, coronary artery disease, type 2 diabetes mellitus and dyslipidemia).1 Metabolic and bariatric surgery is becoming an increasingly popular method of treatment for severe obesity. Based on the American Academy of Pediatrics guidelines, bariatric surgery may be offered to adolescent patients ≥13-years-old with a BMI ≥35 kg/m2 with major comorbidities or a BMI ≥40 kg/m2 with or without other comorbidities.2 Due to the surgical risks and nutritional deficits associated with Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG) increased from 13% of the bariatric surgery cases performed from 2005 to 2009 to 83% of the cases in 2014 to 2015. Laparoscopic adjustable gastric banding (LAGB) has fallen out of favour due to complications and weight regain, and SG is now the primary bariatric surgery performed in paediatric patients.3
Although worse nutritional deficiencies are expected after more invasive malabsorptive procedures, like RYGB, SG involves removal of up to 80% of the stomach and has a risk of nutrient malabsorption, likely related to calorie restriction, food intolerance and insufficient supplementation.4 Both RYGB and SG are known to also affect gut hormones, gut microbiota and neurohormonal signalling from the gut and adipocytes, although it is unclear at this time if these alterations also impact nutritional absorption, independent of calorie restriction.5 Nutritional deficiencies, most commonly 25-hydroxy vitamin D (25 OHD) deficiency, are common prior to bariatric surgery in patients with obesity.6 25 OHD and calcium supplementation is recommended after bariatric surgery in all patients, with poor compliance noted in adolescents.7 Following bariatric surgery, 25 OHD deficiency and secondary hyperparathyroidism have been seen to varying degrees depending on the type of surgery.8 The mechanism of secondary hyperparathyroidism post-operatively is not completely understood, but appears to be partially attributed to 25 OHD deficiency and calcium malabsorption. This results in osteoclast differentiation and survival, ultimately leading to reabsorption of the bone matrix and reduced bone mass.9
Bone is remodelled during adolescence and adulthood, with bone loss occurring when bone reabsorption is increased relative to bone formation. Numerous studies in adults following RYGB have indicated a trend of increased bone reabsorption markers, with smaller increases in markers of bone formation.9 Osteocalcin is a protein secreted by osteoblasts and is a marker of bone formation, while carboxy-terminal cross-linking telopeptide of type 1 collagen (CTX) is formed in type-1 collagen degradation and is a marker of bone reabsorption.10 An increase in bone turnover markers has been shown to predict negative bone mineral density (BMD) and bone microarchitectural changes, resulting in increased fracture risk.11
SG is now the primary form of bariatric surgery performed in adolescent patients, but data are lacking on the long-term effects on bone health. In order to better evaluate bone health after adolescent SG, we examined bone health labs (calcium, parathyroid hormone [PTH], 25 OHD) following SG, as compared to the purely restrictive LAGB procedure. We also examined the changes in bone turnover makers (osteocalcin and CTX) in the first year after SG. We hypothesized that there would be greater negative changes in bone health labs after SG, as compared to LAGB. Bone turnover makers were only obtained in the SG cohort, and we hypothesized that bone turnover makers would increase from baseline in the 6 to 12 months after the SG procedure.
2 |. METHODS
2.1 |. Patients
Adolescents aged 13 to 20 who underwent LAGB or SG between 2006 and 2018 were included for retrospective analysis. Patients were initially referred to the Center for Adolescent Bariatric Surgery (CABS) at Columbia University Irving Medical Center (CUIMC) and received nutrition and exercise counselling prior to surgery. As outlined by Goldberg et al, from 2006 to 2010, patients were offered LAGB as the surgical procedure. Beginning in 2010, a gradual transition to SG began, and since 2011, patients were offered almost exclusively SG.12 The same surgeon performed all surgeries. Eligible patients were adolescents between the ages of 13 and 20 years who had a BMI ≥40 or ≥35 kg/m2 and at least one comorbidity. To ensure appropriate skeletal maturity, analysis was limited to patients ≥13-years-old and Tanner stage ≥3 with a bone age (BA) ≥14-years-old. Due to the independent effects of diabetes mellitus on bone health, patients with a HbA1C >7% were excluded from the analysis. This study was approved by the Institutional Review Board at CUIMC. All patients and their parents or legal guardian gave written informed consent and assent for on-going collection of data prospectively in the bariatric programme prior to participation.
2.2 |. Clinical assessments
Anthropomorphic measurements and fasting morning bone health labs (calcium, 25 OHD and PTH level) were obtained by a paediatric endocrinologist prior to bariatric surgery (T0). The T0 labs were collected prior to patients starting a liver reduction diet or recommended vitamin supplementation. Tanner stage was documented by a single paediatric endocrinologist (IF). Race and ethnicity data were self-reported and obtained from the medical record. BA was performed in patients <18-years-old at the time of pre-operative assessment and calculated by comparing the hand and wrist bones to the Greulich and Pyle Atlas, separately for males and females.13 Patients were advised to return for endocrinology evaluation at 3, 6 and 12 months post-operatively. The data from the 6 (range 3–8.99) and 12 (range 9–14.99) month follow-up visits were entered into a database. Excess weight loss (EWL) was calculated with the ideal body weight for a BMI at the 85th percentile for age, height and sex.14 Additional analysis of bone turnover markers was performed in the SG group, as osteocalcin and CTX were not collected in the LAGB group. All patients were advised to take vitamin D3 before and after bariatric surgery. Patients self-reported if they were taking vitamin D3 supplementation at T0. Compliance at T6 and T12 is unknown, although supplementation was increased as needed based on the 25 OHD laboratory values. Patients were started on calcium supplementation and bariatric multivitamins post-operatively, although compliance is also unknown.
2.3 |. Multivitamin/mineral supplement protocol
Vitamin D3 supplementation dose was based on the 25 OHD level. For 25 OHD levels of <10, 10 to 19, and 20 to 30 ng/mL, patients were started on 50 000 international units vitamin D3 supplementation weekly, bimonthly or monthly, respectively. Calcium supplementation was started postoperatively, at the recommended daily value.15
2.4 |. Assessment of biochemical markers
Calcium values were analysed at the Clinical Chemical Laboratory of CUIMC through colorimetric methodology. The majority of 25 OHD values were obtained by high-pressure liquid chromatography/tandem mass spectrometry at Esoterix laboratory (Calabasas, California). The remainder 25 OHD values were analysed by immunochemilumininometric assay (ICMA) at CUIMC. 25 OHD levels under 20 ng/mL were considered deficient, while levels 21 to 30 ng/mL were considered insufficient. PTH levels were analysed by ICMA at Esoterix, while CTX and osteocalcin were analysed through Electrochemiluminescent Immunoassay at ARUP laboratory (Salt Lake City, Utah).
2.5 |. Statistical analysis
Data from this retrospective observational study were imported to SAS v 9.4 (SAS Institute, Cary, NC). As greater than 10% of the data values were missing, the missing data mechanism was assessed using the logistic regression method for all outcome variables.16 None of the variables met statistical criteria, P < .10, for non-ignorability. The methods of Rubin (1976, 1996) were used: SAS Proc MI used the Markov chain Monte Carlo method (MCMC) to generate five datasets with multiply imputed data for the monotone missing data pattern.17,18 Multiply imputed datasets were analysed with linear mixed models for repeated measures for fixed effects using an AR (1) covariance structure, and SAS Proc MIANALYZE was used to summarize the analyses from the multiple imputation datasets and generate the estimates of the model effects.19,20 The fixed effects of surgical procedure, time and their interaction were used to compare the time course of change in LAGB vs SG surgeries; and the single fixed effect of time to assess the time course of change in bone turnover markers within the SG surgery group. We examined the associations between variable values at baseline with the same variable and other variables at different times with Spearman correlations; and the association between calcium, PTH and 25 OHD at the same and different times with Spearman correlations overall, by surgical procedure, and by sex within each surgical procedure, as well as with osteocalcin and CTX only in the SG group. Stepwise multiple regression, P < .20 to enter and P < .05 to stay, was used to explore predictors (sex, EWL, calcium change, PTH change and 25 OHD change) of change over time in osteocalcin and CTX, in separate models. Results are reported as means and SD, unless otherwise noted.
3 |. RESULTS
3.1 |. Baseline characteristics
A total of 291 adolescents who underwent SG or LAGB and provided their consent/assent were screened for analysis. 12 patients were excluded for age <13 years and/or HbA1C >7%. Of the 279 patients remaining, 197 adolescents who underwent LAGB (n = 98) or SG (n = 99) and had follow-up bone health labs at 6 and/or 12 months post-operatively were included in the study. 176 patients (88 LAGB and 88 SG) had 6 month follow-up, and 123 patients (65 LAGB and 58 SG) had 12 month follow-up. The other 82 patients underwent surgery and did not have follow-up bone health labs, but were included for baseline data and imputed analysis. They did not differ from the remaining patients in age, sex or baseline BMI. Of these 82 patients, 62 had follow-up with the bariatric surgeon or nutrition team, and there were no known post-operative complications (Table 1).
TABLE 1.
Demographic information and baseline clinical characteristics
| LAGB (N = 139) | SG (N = 140) | ||||
|---|---|---|---|---|---|
| Females | Males | Females | Males | ||
| N = 95 | N = 44 | N = 99 | N = 41 | ||
| Age at surgery (years) | 17.04 ± 1.22 | 16.93 ± 1.24 | 16.70 ± 1.25 | 16.64 ± 1.24 | |
| Ethnicity N (%) | Non-Hispanic | 55 (57.9) | 23 (52.2) | 41 (41.4) | 18 (44) |
| Hispanic | 30 (31.6) | 14 (31.8) | 56 (56.6) | 23 (56.1) | |
| Unknown | 10 (10.5) | 7 (15.9) | 2 (2.0) | 0 (0) | |
| Race N (%) | African American | 15 (15.8) | 10 (22.7) | 24 (24.2) | 6 (14.6) |
| Caucasian | 58 (61.0) | 20 (45.5) | 61 (61.6) | 24 (58.5) | |
| Asian | 0 (0) | 0 (0) | 1 (1.0) | 1 (2.4) | |
| Pacific Islander/Hawaiian | 1 (1.1) | 1 (2.3) | 0 (0) | 1 (2.4) | |
| Native American | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Other | 5 (5.3) | 5 (11.4) | 1 (1.0) | 1 (2.4) | |
| Unknown | 16 (16.8) | 8 (18.2) | 12 (12.1) | 8 (19.5) | |
| BMI at time of surgery (kg/m2) | 46.84 ± 8.22 | 49.11 ± 7.51 | 47.65 ± 7.74 | 50.14 ± 12.58 | |
| Baseline Ca (mg/dL) | 9.31 ± 0.33 | 9.43 ± 0.34 | 9.34 ± 0.43 | 9.53 ± 0.50 | |
| Baseline 25 OHD (ng/mL) | 24.05 ± 10.86 | 18.96 ± 7.61 | 20.21 ± 8.30 | 18.97 ± 8.15 | |
| Baseline PTHa (pg/mL) | 26.58 ± 13.52 | 29.07 ± 13.51 | 43.21 ± 30.88 | 42.31 ± 25.61 | |
| %EWL at 6 moa | 20.92 ± 21.40 | 16.77 ± 13.67 | 47.85 ± 16.89 | 49.14 ± 22.06 | |
| %EWL at 12 moa | 25 ± 27.48 | 22.25 ± 17.18 | 62.35 ± 20.97 | 64.10 ± 28.60 | |
Note: Data listed as mean and SD, unless otherwise noted. Groups were similar at baseline (T0) in terms of age, race, ethnicity, sex, and baseline BMI. Baseline calcium and 25 OHD levels did not differ, although PTH levels were higher in the SG group. Excess weight loss (EWL) differed between LAGB and SG, with the SG group having significantly greater EWL at 6 and 12 months.
Abbreviations: 25 OHD, 25-hydroxy vitamin D; BMI, body mass index; LAGB, laparoscopic adjustable gastric banding; PTH, parathyroid hormone; SG, sleeve gastrectomy.
Between LAGB and SG differences, P < .001.
The LAGB and SG groups were similar at T0 in terms of race, ethnicity, sex, age and starting BMI. Prior to surgery, mean BMI was 47.97 ± 8.76 kg/m2 (range 35.3–100.6) and mean age was 16.8 ± 1.2 years (range 13.4–19.6).
48.3% of patients were 25 OHD deficient (62 LAGB patients and 71 SG patients) and 34.2% were insufficient (44 LAGB patients and 50 SG patients). 11.5% of the LAGB group and 45% of the SG group reported vitamin D3 supplementation at T0 (P < .001).
T0 calcium and PTH were correlated (r = −0.27, P = .015), as was T0 25 OHD and PTH (r = −0.21, P = .063).
3.2 |. Bone health labs after surgery
No difference was found between the sexes, so males and females were analysed together. Calcium and 25 OHD levels did not differ significantly at T0 between the groups. Levels increased from T0 to 6 months (T6) and T0 to 12 months (T12) after LAGB and SG. There was no statistical difference in the change in calcium and 25 OHD levels between the two surgeries. PTH levels differed at T0 (P < .001) and decreased in the SG group between T0 and T6 (P = .021) and T0 and T12 (P < .001). Between T0 and T12, the decrease in PTH levels in the SG group was greater than the decrease in the LAGB group (P = .007) (Table 2).
TABLE 2.
Bone health labs change after LAGB vs SG
| LAGB | SG | Groupa time P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| T0 | Change T0 to T6 | Change T0 to T12 | T0 | Change T0 to T6 | Change T0 to T12 | T0–T6 | T0–T12 | |
| Calcium (mg/dL) | 9.35 ± 0.33 | +0.060a | +0.072a | 9.39 ± 0.46 | +0.068a | +0.131b | .809 | .107 |
| 25 OHD (ng/mL) | 22.44 ± 10.20 | +3.602b | +4.078b | 19.86 ± 8.24 | +5.168b | +4.483b | .051 | .613 |
| PTH (pg/mL) | 27.41 ± 13.51 | −0.089 | −1.622 | 42.95 ± 29.37 | −3.164a | −6.842b | .112 | .007a |
Note: Data listed as mean and SD, unless otherwise noted. Calcium and 25 OHD increased in both groups from T0 to T6 and T12. PTH decreased significantly in the SG group from T0 to T6 and T12. The groups differed only in the change in PTH from T0 to T12.
Abbreviations: 25 OHD, 25-hydroxy vitamin D; BMI, body mass index; LAGB, laparoscopic adjustable gastric banding; PTH, parathyroid hormone; SG, sleeve gastrectomy.
P < .05.
P < .001.
3.3 |. Bone turnover marker analysis
For the SG patients, osteocalcin and CTX increased from T0 to T6 and T0 to T12 (Table 3). Osteocalcin did not statistically change between T6 and T12, while CTX decreased between T6 and T12 (P = .002). Correlation analysis showed that the increases in osteocalcin and CTX trended towards an association at T6 (rho = 0.20913, P = .0533), and were positively correlated at T12 (rho = 0.37550, P = .0004). The increases in osteocalcin and PTH between T0 and T6 were correlated (rho = 0.242, P = .0248), while the increase in CTX between T0 and T6 and EWL at T6 was correlated (rho = 0.21438, P = .0475). Stepwise multiple regression was performed for the 43 SG patients with calcium, PTH, 25 OHD, osteocalcin and CTX data at T6. For each additional percent of EWL, CTX at T6 increased by 5.38 ± 2.47 pg/mL (P = .0349). Only 14 patients had complete data at T12, and there were no predictors of osteocalcin or CTX change retained in the model. There was no difference in EWL and thus no perceived selection bias in the patients included for regression analysis, as compared to the other patients with 6 and/or 12 month follow-up (Tables 3 and 4, Figure 1).
TABLE 3.
Bone turnover marker changes following SG
| T0 | Change T0 to T6 | Change T6 to T12 | Change T0 to T12 | |
|---|---|---|---|---|
| Osteocalcin (ng/mL) | 23.66 ± 13.38 | +7.971b | +0.590 | +8.561b |
| CTX (pg/mL) | 614.37 ± 267.71 | +353.489b | −49.592a | +303.897b |
Note: Data listed as mean and SD, unless otherwise noted. Osteocalcin and CTX increased significantly from T0 to T6 and T0 to T12, although not between T6 and T12.
Abbreviations: CTX, carboxy-terminal cross-linking telopeptide of type 1 collagen; SG, sleeve gastrectomy.
P < .05.
P < .001.
TABLE 4.
Correlations between bone turnover markers and bone health labs at 6 and 12 months
| CTX change | Osteocalcin change | |||
|---|---|---|---|---|
| T0–T6 | T0–T12 | T0–T6 | T0–T12 | |
| PTH change | 0.14021 | 0.09734 | 0.24200a | 0.18255 |
| 25 OHD change | −0.05637 | −0.01334 | −0.10183 | −0.06036 |
| Calcium change | 0.02394 | −0.12958 | −0.14844 | 0.01447 |
| EWL | 0.21438a | 0.08598 | −0.17379 | −0.03992 |
Note: The osteocalcin increase between T0 and T6 correlated with an increase in PTH, although there were no significant correlations between the changes in 25 OHD and calcium with the increase in bone turnover markers. EWL and CTX change between T0 and T6 were correlated.
Abbreviations: 25 OHD, 25-hydroxy vitamin D; CTX, carboxy-terminal cross-linking telopeptide of type 1 collagen; EWL, excess weight loss; PTH, parathyroid hormone.
P < .05.
FIGURE 1.
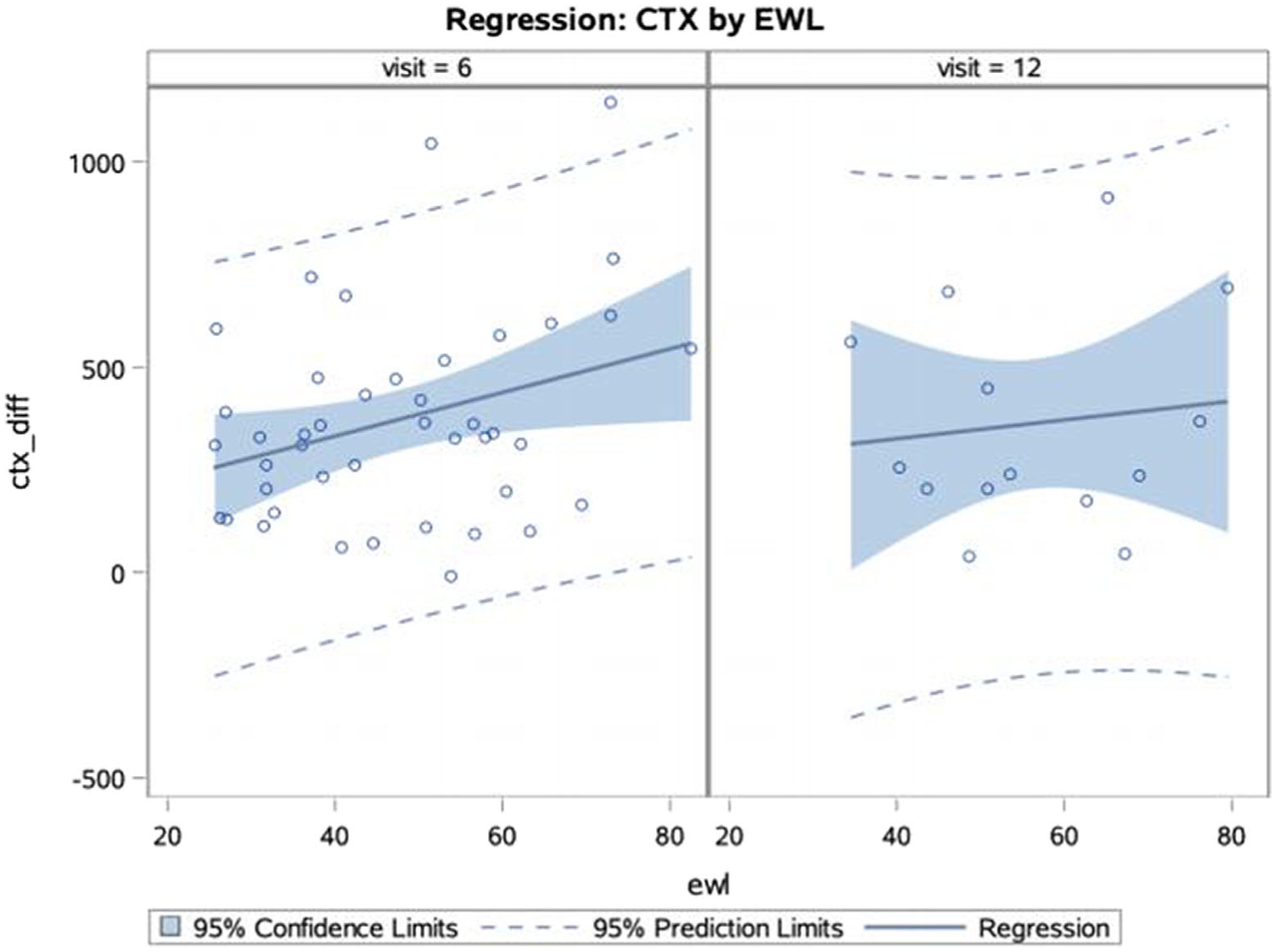
Regression of CTX difference (ctx_diff) by EWL at T6 and T12. At T6, the percent of EWL predicted the CTX difference (P = .0349). For each additional percent of EWL, CTX at 6 months increased by 5.38 ± 2.47 pg/mL. There was no association between EWL and CTX at T12. CTX, carboxy-terminal cross-linking telopeptide of type 1 collagen; ETX, excess weight loss
4 |. DISCUSSION
As predicted, our study showed a high prevalence of deficient or insufficient 25 OHD levels at baseline, with baseline 25 OHD levels <30 ng/mL in 76.2% of the LAGB group and 88.9% of the SG group. Previous work done by Censani et al on 236 patients at our centre who underwent bariatric surgery between March 2006 and June 2011 showed that only 18% of patients had a sufficient 25 OHD level > 30 ng/mL at baseline.6 Postoperatively, we found a significant rise in calcium and 25 OHD levels in both the LAGB and SG groups at T6 and T12, although the change in calcium was not clinically significant. Previous work in adults has found a high prevalence of 25 OHD deficiency before and after bariatric surgery, which has influenced the post-operative supplementation guidelines.15 In a recent Teen-Labs study, the frequency of 25 OHD deficiency defined by cut-off of 20.1 ng/mL in adolescent patients with obesity prior to SG was 19.4%, compared to 48.4% in our cohort. Of note, the Teen-Labs cohort is more racially homogenous (72% white and 93% non-Hispanic) than our sample.21 In addition, Elhag et al found an even higher prevalence (96.4%) of 25 OHD deficiency pre-operatively in a sample of adolescents in Qatar.22 In Teen-Labs, there was no significant change in 25 OHD levels after RYGB and SG, although levels declined after the first post-operative year.21 Other adolescent studies have also not found a significant change in 25 OHD and calcium levels in the 1 to 2 years after SG and RYGB.23–25 The stable or increasing levels of 25 OHD in our patients and other recent adolescent studies are likely secondary to more stringent supplementation guidelines post-operatively, although adherence at T6 and T12 is not known in our sample.
Following bariatric surgery, 25 OHD and calcium deficiencies have been associated with secondary hyperparathyroidism.26 It is not clear why our SG group had a significantly higher PTH at baseline, given the similar baseline BMI and 25 OHD and calcium levels between the groups. The PTH levels decreased in the SG group at both time points, with a greater decrease in PTH after SG compared to LAGB. Adult studies have found increasing secondary hyperparathyroidism post-operatively following RYGB.9,26 One adult study evaluating secondary hyperparathyroidism found no increase in PTH values at 1 year following SG or LAGB, although PTH levels were increased from baseline at 5 years after SG (P < .05).8 In terms of adolescent studies, Xanthakos et al found that PTH levels remained increased 5 years after RYGB, with no change in PTH levels after SG.21 Conversely, Elhag et al found the prevalence of secondary hyperparathyroidism, defined in their study as PTH >65 pg/mL, improved from 33.3% to 16.7% post-operatively, with no evidence of hypocalcemia following SG.22 In Santos et al, the PTH levels rose during the post-operative year from 47.32 to 63.17 pg/mL following RYGB.25 In comparison to RYGB, our data, like other adolescent SG data, does not show post-operative secondary hyperparathyroidism, which we presume is from a less invasive procedure and more consistent vitamin D3 and calcium supplementation. As such, the stable 25 OHD and calcium levels in our study were likely protective against the development of secondary hyperparathyroidism.
Studies in paediatrics and adults have indicated an increase in bone turnover markers after RYGB, although no studies have examined bone turnover markers after adolescent SG. In a study in adults following RYGB, CTX levels were 196% of baseline at 2 years and remained 150% of baseline at 5 year follow-up (P < .001).27 Beamish et al showed a significant rise in CTX (mean difference [MD]: +0.62 ng/mL) and osteocalcin (MD: +25.4 ng/mL) in the first post-operative year following adolescent RYGB (P < .001). Both CTX (MD: −0.36 ng/mL) and osteocalcin (MD: −13.0 ng/mL) decreased between 1 and 2 year follow-up. At 2 years, CTX remained elevated from baseline in females (MD: +0.30 ng/mL, P = .006), while osteocalcin remained increased in both sexes (females MD: +14.5 ng/mL (P < .001) and males MD: +9.6 ng/mL (P = .057)).23 The few studies comparing RYGB and SG in adults have shown similar or increased changes in bone turnover after RYGB compared to SG.26 After SG in our adolescents, we found an increase in osteocalcin and CTX at T6, although CTX levels began to decrease between T6 and T12. Although the continued trajectory of osteocalcin and CTX in our sample is unknown, other studies have shown a persistent increase in bone turnover markers following RYGB surgery. Previous work in adolescents with obesity has demonstrated a decrease in bone turnover markers in late adolescence. We would thus not expect the significant increase in bone turnover markers seen in our study from time alone.28
The rise in PTH was correlated with the rise in osteocalcin between T0 and T6, which we infer is causative from PTH stimulating osteoclasts and increasing bone turnover. The changes in PTH, 25 OHD and calcium were not otherwise correlated with the changes in osteocalcin or CTX. In regression models, EWL predicted the increase CTX at T6, although calcium, PTH, and 25 OHD were not associated (P > .05) with the rise in CTX. None of the available measures predicted the change in osteocalcin. Other studies have proposed 25 OHD deficiency and secondary hyperparathyroidism postoperatively as one of the mechanisms for an increase in bone turnover markers. Although our results are limited by a low sample size, our data does not suggest a similar causation. Our findings are consistent with the hypothesis that neurohormonal changes and decreased excess weight drive the increase in bone turnover.26
Persistent increases in bone turnover markers and potential declines in BMD after SG may have important clinical implications for fracture risk in adolescent patients. This is particularly significant in adolescents, since bariatric surgery may lead to a decline in bone mineralization and BMD during peak bone mass accrual.29 Currently, there are inconclusive results on fracture risk after bariatric surgery, as fracture incidence appears to start increasing at least 2 to 5 years after surgery with few studies following patients longitudinally or through menopause.26,30–32 Adult guidelines recommend performing dual-energy X-ray absorptiometry scans for the lumbar spine and fem-oral sites at baseline and 2 years after bariatric surgery, although there are not screening guidelines in adolescents.33
To our knowledge, this is the first study in adolescents to compare nutritional deficits after LAGB and SG and bone turnover markers after SG in an ethnically diverse population. Given the variability in the literature, additional studies are needed to ensure adequate calcium and 25 OHD levels pre- and post-operatively and to examine the long-term trend of bone turnover markers after SG. Further studies examining BMD, bone microarchitecture, and long-term fracture risk are also needed to determine if the changes seen in bone turnover are clinically relevant in the post-SG adolescent population.
We acknowledge limitations in our study. All patients were instructed to take vitamin D3 and calcium supplementation and self-reported their baseline compliance with vitamin D3, although true adherence is unknown. This was also a single site, retrospective study over 1 year, with a small subset of patients with complete 1 year data. The baseline data were collected after patients became enrolled in the CABS, so some patients may have started to lose weight through diet and exercise before the baseline data were collected. Furthermore, we had a significant proportion of missing data and utilized multiply imputed data for the analysis.
5 |. CONCLUSIONS
Ongoing supplementation of vitamin D3 and calcium may be protective against secondary hyperparathyroidism in adolescents post-SG. However, there were marked increases in bone turnover markers at T6 and T12 after SG. In regression analysis, EWL at T6 predicted the rise in CTX, but the changes in osteocalcin and CTX could not be attributed to 25 OHD deficiency, hypocalcemia or hyperparathyroidism. Our study indicates the importance of multidisciplinary follow-up after adolescent bariatric surgery to monitor for nutritional deficiencies and complications. More studies are needed to evaluate the effects of bariatric surgery on adolescent bone health and to correlate the early changes in bone turnover markers with BMD and bone microarchitecture in this patient population.
ACKNOWLEDGEMENTS
Dr Ilene Fennoy had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. This work was supported by a NIH NIDDK T32 DK 06552 in Pediatric Endocrinology (PI SE Oberfield). Ilene Fennoy conceived the initial study design. Alyson Weiner and Amanda Cowell performed the data collection. Donald J. McMahon performed the data analysis. Alyson Weiner drafted the manuscript. Rachel Tao and Jeffrey Zitsman allowed for acquisition of data. Sharon E. Oberfield performed a critical revision of the manuscript. All authors had approval of the submitted version. The members of this study would like to express their gratitude for the patients in the Center for Adolescent Bariatric Surgery.
Funding information
NIH NIDDK, Grant/Award Number: T32 DK 06552 Pediatric Endocrinology PI SEO
Abbreviations:
- 25 OHD
25-hydroxy vitamin D
- BA
bone age
- BMD
bone mineral density
- BMI
body mass index
- CABS
Center for Adolescent Bariatric Surgery
- CTX
carboxy-terminal cross-linking telopeptide of type 1 collagen
- CUIMC
Columbia University Irving Medical Center
- EWL
excess weight loss
- ICMA
immunochemilumininometric assay
- LAGB
laparoscopic adjustable gastric banding
- PTH
parathyroid hormone
- RYGB
Roux-en-Y gastric bypass
- SG
sleeve gastrectomy
- T0
baseline
- T12
12thinspacemonth follow-up
- T6
6nonbreakingspacemonth follow-up
Footnotes
CONFLICTS OF INTEREST
No conflict of interest was declared.
REFERENCES
- 1.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3):e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong SC, Bolling CF, Michalsky MP, Reichard KW. Pediatric metabolic and bariatric surgery: evidence, barriers, and best practices. Pediatrics. 2019;144(6):e20193223. [DOI] [PubMed] [Google Scholar]
- 3.Inge TH, Coley RY, Bazzano LA, et al. Comparative effectiveness of bariatric procedures among adolescents: the PCORnet bariatric study. Surg Obes Relat Dis. 2018;14(9):1374–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damms-Machado A, Friedrich A, Kramer KM, et al. Pre- and postoperative nutritional deficiencies in obese patients undergoing laparoscopic sleeve gastrectomy. Obes Surg. 2012;22(6):881–889. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair P, Brennan DJ, le Roux CW. Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nat Rev Gastroenterol Hepatol. 2018;15(10):606–624. [DOI] [PubMed] [Google Scholar]
- 6.Censani M, Stein EM, Shane E, et al. Vitamin D deficiency is prevalent in morbidly obese adolescents prior to bariatric surgery. ISRN Obes. 2013;2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi AC, Zeller MH, Xanthakos SA, Jenkins TM, Inge TH. Adherence to vitamin supplementation following adolescent bariatric surgery. Obesity. 2013;21(3):E190–E195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei JH, Lee WJ, Chong K, et al. High incidence of secondary hyperparathyroidism in bariatric patients: comparing different procedures. Obes Surg. 2018;28(3):798–804. [DOI] [PubMed] [Google Scholar]
- 9.Corbeels K, Verlinden L, Lannoo M, et al. Thin bones: vitamin D and calcium handling after bariatric surgery. Bone Rep. 2018;8:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risteli L, Risteli J. Biochemical markers of bone metabolism. Ann Med. 1993;25(4):385–393. [DOI] [PubMed] [Google Scholar]
- 11.Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2016;20(6):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg HR, Chin VL, Zitsman JL, et al. Bariatric surgery in adolescents: is routine nutrient supplementation sufficient to avoid anemia following bariatric surgery? Nutr Clin Pract. 2017;32(4):502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed. Stanford, Calif: Stanford University Press; 1959. [Google Scholar]
- 14.Butte NE, Garza C, de Onis M. Evaluation of the feasibility of international growth standards for school-aged children and adolescents. Food Nutr Bull. 2006;27(4):169–174. [DOI] [PubMed] [Google Scholar]
- 15.Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the surgical weight loss patient 2016 update: micronutrients. Surg Obes Relat Dis. 2017;13(5):727–741. [DOI] [PubMed] [Google Scholar]
- 16.Heitjan DF. Ignorability and coarse data: some biomedical examples. Biometrics. 1993;49(4):1099–1109. [PubMed] [Google Scholar]
- 17.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–592. [Google Scholar]
- 18.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–489. [Google Scholar]
- 19.Schafer JL. Analysis of Incomplete Multivariate Data. 1st ed. New York, NY: Chapman and Hall/CRC; 1997. [Google Scholar]
- 20.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. [DOI] [PubMed] [Google Scholar]
- 21.Xanthakos SA, Khoury JC, Inge TH, et al. Nutritional risks in adolescents after bariatric surgery. Clin Gastroenterol Hepatol. 2019;18(5): 1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elhag W, El Ansari W, Abdulrazzaq S, Abdullah A, Elsherif M, Elgenaied I. Evolution of 29 anthropometric, nutritional, and cardiometabolic parameters among morbidly obese adolescents 2 years post sleeve gastrectomy. Obes Surg. 2018;28(2):474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beamish AJ, Gronowitz E, Olbers T, Flodmark CE, Marcus C, Dahlgren J. Body composition and bone health in adolescents after Roux-en-Y gastric bypass for severe obesity. Pediatr Obes. 2017;12(3):239–246. [DOI] [PubMed] [Google Scholar]
- 24.Misra M, Singhal V, Carmine B, et al. Bone outcomes following sleeve gastrectomy in adolescents and young adults with obesity versus non-surgical controls. Bone. 2020;134:115290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos D, Lopes T, Jesus P, et al. Bone metabolism in adolescents and adults undergoing Roux-en-Y gastric bypass: a comparative study. Obes Surg. 2019;29(7):2144–2150. [DOI] [PubMed] [Google Scholar]
- 26.Gagnon C, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018;2(3):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindeman KG, Greenblatt LB, Rourke C, Bouxsein ML, Finkelstein JS, Yu EW. Longitudinal 5-year evaluation of bone density and microarchitecture after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2018;103(11):4104–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geserick M, Vogel M, Eckelt F, et al. Children and adolescents with obesity have reduced serum bone turnover markers and 25-hydroxyvitamin D but increased parathyroid hormone concentrations - results derived from new pediatric reference ranges. Bone. 2020;132:115124. [DOI] [PubMed] [Google Scholar]
- 29.Gordon CM, Zemel BS, Wren TA, et al. The determinants of peak bone mass. J Pediatr. 2017;180:261–269. [DOI] [PubMed] [Google Scholar]
- 30.Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int. 2014;25(1):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousseau C, Jean S, Gamache P, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ. 2016;354:i3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2019;21(Suppl 1):S1–S27. [DOI] [PubMed] [Google Scholar]


