Abstract
Background and aims:
The relationship between obesity and arterial stiffness is complex, with a potential interaction by age (inverse association at younger age and positive at older age) and conflicting reports on the effect of lifestyle-based weight loss on arterial stiffness. Little is understood about post-bariatric surgery changes in arterial stiffness. This study aimed to examine post-bariatric surgery changes in arterial stiffness and identify factors associated with greater changes in arterial stiffness.
Methods:
In 72 patients (mean age 44.5 years, 72.2% female), we evaluated two arterial stiffness measures, cardio-ankle vascular index (CAVI) and heart-ankle pulse wave velocity (haPWV), one month prior to and 6 months after bariatric surgery. Another follow-up visit was conducted 12 months after bariatric surgery in a subset of 58 participants.
Results:
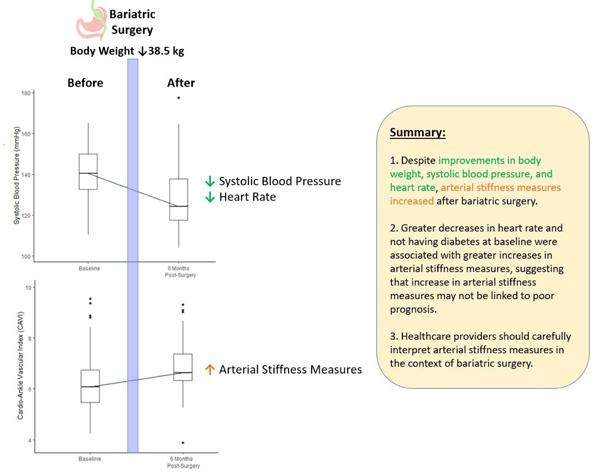
Six months after bariatric surgery, an evident decrease was seen in body mass index, heart rate, and systolic blood pressure. In contrast, both CAVI and haPWV significantly increased at 6 months (+0.64 [0.42, 0.87] and +0.24 [0.04, 0.44] m/s, respectively). Among 58 patients with relevant data, CAVI and haPWV remained elevated 12 months after bariatric surgery (+0.80 [0.53, 1.07] and +0.40 [0.17, 0.62] m/s, respectively). Being non-diabetic and having larger decreases in post-surgery heart rate were independently associated with greater increases in post-surgical CAVI.
Conclusions:
Arterial stiffness measures, CAVI and haPWV, were elevated after bariatric surgery despite other favorable cardiometabolic changes. Further studies are necessary to elucidate the underlying mechanism and prognostic implications of this elevation in arterial stiffness measures after bariatric surgery.
Keywords: arterial stiffness, cardio-ankle vascular index, bariatric surgery outcomes, obesity, pulse wave velocity
Graphical Abstract
1. Introduction
Increased arterial stiffness, characterized as the rigidity of arterial walls, is considered an early stage vascular phenotype in the pathophysiological development of atherosclerosis. Indeed, arterial stiffness is found to predate the manifestation of hypertension.1, 2 Arterial stiffness has also been linked to several traditional cardiovascular risk factors beyond hypertension including smoking, dyslipidemia, and diabetes.3 Moreover, in addition to its association with traditional risk factors, arterial stiffness is an independent predictor of both cardiovascular morbidity and mortality.4, 5
Obesity, an upstream condition of several traditional cardiovascular risk factors, has been found to demonstrate complex associations with arterial stiffness. For example, in children and younger adults, several studies have reported an inverse association between higher body mass index (BMI) and lower arterial stiffness measures.6–8 On the other hand, in older adults, a positive relationship between BMI and arterial stiffness has generally been recognized.7, 9 This observed phenomenon has been suggested to reflect differences in vascular response to obesity as a consequence of aging, with older adults losing the ability to adapt to the vascular burden related to obesity (e.g., increased blood volume).7
In addition, the contribution of weight loss to changes in arterial stiffness is poorly understood. Several studies have suggested that moderate weight reduction through lifestyle-change based programs may improve arterial stiffness. However, data regarding changes in arterial stiffness after bariatric surgery, the most potent weight-reduction treatment, are limited. Additionally, the few previous studies exploring this study question obtained conflicting results,10–12 warranting additional investigation.
Thus, using data from 72 morbidly obese patients, we sought to 1) investigate changes in arterial stiffness measures, cardio-ankle vascular index (CAVI) and heart-ankle pulse wave velocity (haPWV), before and after bariatric surgery and 2) identify factors associated with greater change in arterial stiffness measures after bariatric surgery.
2. Materials and methods
2.1. Study population and design
This study was conducted as part of the BARI-Heart Study that recruited 96 patients with morbid obesity undergoing clinical bariatric surgery, a mix of Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy, between 25 to 65 years of age in Meritus Health, Hagerstown, Maryland. The BARI-Heart Study planned to have two visits prior to bariatric surgery and two additional visits after surgery and assess cardiometabolic changes related to bariatric surgery. The pre-surgery visits were planned 3–6 months and one month prior to bariatric surgery. The post-surgery visits were scheduled 6 and 12 months after surgery. Since some participants missed the visit 3–6 months prior to surgery, the present study is based on the pre-surgery visits conducted one month prior to bariatric surgery as baseline and two post-surgery follow-up visits. Given the more complete data availability at 6-month follow-up and consistent results between 6- and 12-months post-surgery, we used 6-month post-surgery for our primary analysis. Participants who did not return to clinic for 6-month follow-up after bariatric surgery were excluded, resulting in a total of 72 participants for the final study population. Of these 72 participants, 56 fully completed the evaluation at 12 months after bariatric surgery. Written informed consent was collected from all participants. This study was approved by the Institutional Review Board of the Johns Hopkins School of Medicine.
2.2. Collection of baseline information
Prior to collection of baseline information, each patient received instructions about healthy diet before surgery. However, there were no significant changes in body weight from 6 months to 1 month prior to surgery. Baseline demographic information and clinical variables were collected as part of an interview with trained medical staff at the visit prior to bariatric surgery. Prior medical history of diabetes mellitus, atrial fibrillation, hypertension, and dyslipidemia was assessed by self-report.
BMI was calculated as weight in kilograms divided by height in meters squared.
2.3. Arterial stiffness and blood pressure measurements
We assessed CAVI and heart-ankle pulse wave velocity (haPWV) as measures of systemic arterial stiffness representing aorta and leg arteries. CAVI was measured twice bilaterally using a VaSera VS-1500 oscillometric device (Fukuda Denshi Co. Ltd., Tokyo, Japan) by trained technicians with participants in a supine position after 5 minutes of rest at each visit.13 Four blood pressure cuffs of appropriate sizes were wrapped around arms and ankles, with electrocardiogram electrodes placed on both upper arms and one microphone on the mid-sternum for phonocardiography. We considered CAVI as the primary arterial stiffness measure because CAVI was the arterial stiffness measure reported from the device and considered to be less influenced by blood pressure during arterial stiffness evaluation than PWV.13 haPWV was calculated from reported CAVI using the equation below13:
where Ps is systolic blood pressure, Pd is diastolic blood pressure, ∆P is Ps – Pd, ρ is blood density of 1.05 g/mL, and a and b are constants.14
For each CAVI and haPWV, we used the average of all measurements (i.e., bilateral duplicate) in the analysis. During the arterial stiffness evaluation, supine brachial systolic and diastolic blood pressures were also recorded.
2.4. Statistical analyses
Baseline characteristics were compared across tertiles of CAVI. Continuous and categorical variables were summarized as means and standard deviations and total counts and proportions, respectively. Boxplots were used to graphically display the distribution of heart rate, blood pressure, CAVI, and haPWV at each of the three study visits. Paired two-sample t-tests were used to compare within-subject changes in cardiac and vascular measures at baseline and each of the follow-up visits individually. Additional complete case analyses were performed by restricting the analysis only to individuals who had completed all three study visits. We also assessed whether 6-month CAVI changes were different by age category (<50 and ≥50 years), sex, and anti-hypertensive medication use subgroups with Welch’s t-tests.
Subsequently, we sought to identify factors associated with changes in CAVI following bariatric surgery. We examined several baseline characteristic (age, sex, medical history of diabetes, and atrial fibrillation) and 6-month changes in cardiometabolic parameters (heart rate, systolic and diastolic blood pressure, BMI, and N-terminal pro b-type natriuretic peptide [NT-proBNP]). We conducted univariable (each factor individually) and multivariable baseline-adjusted linear regressions with 6-month changes in CAVI as the outcome. These analyses were repeated in secondary analyses using 6-month changes in haPWV as the outcome.
Two-sided p-values of less than 0.05 were considered statistically significant. All analyses were performed using Stata version 14.2 (StataCorp LP, College Station, Texas) and R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).15, 16
3. Results
3.1. Study population
Baseline characteristics of the 72 study participants are shown in Table 1. The average age of the study participants at baseline was 44.5 (SD 11.2) years with 52 (72.2%) females. The mean BMI was 47.1 (SD 7.4) kg/m2. A majority of participants had one or more pre-existing conditions: 26% with diabetes, 52% with hypertension, 13% with a history of atrial fibrillation, and 43% with dyslipidemia. Individuals at higher observed CAVI tertile at baseline tended to be older, have lower BMI, and have increased comorbid diabetes, hypertension, and dyslipidemia conditions (Table 1).
Table 1.
Study population characteristics by tertiles of baseline CAVI.
| Overall (N=72) |
Tertile 1 (N=24) |
Tertile 2 (N=24) |
Tertile 3 (N=24) |
|
|---|---|---|---|---|
| CAVI ranges | 4.3–5.7 | 5.7–6.4 | 6.5–9.6 | |
| Female (%) | 52 (72.2%) | 20 (83.3%) | 16 (66.7%) | 16 (66.7%) |
| White (%) | 62 (86.1%) | 20 (83.3%) | 20 (83.3%) | 22 (91.7%) |
| Age, years | 45 (11) | 36 (9) | 44 (9) | 54 (8) |
| Ever smoker (%) | 31 (43.1%) | 8 (33.3%) | 15 (62.5%) | 8 (33.3%) |
| Height, cm | 167 (11) | 163 (8) | 167 (11) | 170 (11) |
| Weight, kg | 131 (29) | 135 (32) | 132 (26) | 126 (29) |
| BMI, kg/m2 | 47.1 (7.4) | 50.7 (7.5) | 47.1 (6.5) | 43.6 (6.7) |
| Blood pressure | ||||
| Systolic, mmHg | 141 (12)a | 138 (13) | 140 (10) | 144 (13) |
| Diastolic, mmHg | 83 (9)a | 79 (7) | 85 (9) | 85 (10) |
| Heart rate, bpm | 68 (10)a | 69 (9) | 70 (11) | 66 (9) |
| Heart-ankle PWV, m/s | 6.6 (1.0) | 5.7 (0.3) | 6.5 (0.4) | 7.6 (1.0) |
| Pre-existing conditions | ||||
| Diabetes (%) | 19 (26.4%) | 2 (8.3%) | 6 (25.0%) | 11 (45.8%) |
| Hypertension (%) | 38 (52.8%) | 9 (37.5%) | 10 (41.7%) | 19 (79.2%) |
| Atrial fibrillation (%) | 9 (12.5%) | 5 (20.8%) | 1 (4.2%) | 3 (12.5%) |
| Dyslipidemia (%) | 30 (42.9%)b | 4 (17.4%) | 10 (43.5%) | 16 (66.7%) |
Values reported are mean (standard deviation) unless otherwise noted.
One missing observation (n = 71).
Two missing observations (n=70).
BMI = body mass index; bpm = beats per minute; CAVI = cardio-ankle vascular index; PWV = pulse wave velocity.
3.2. Changes in arterial stiffness measures after bariatric surgery
Average BMI decreased significantly from 47.1 kg/m2 before bariatric surgery to 34.8 kg/m2 six months after surgery. Heart rate was also found to decrease significantly six months following bariatric surgery (−11.5 [95% CI: −13.9, −9.2] beats per minute [bpm]) and remained decreased 12 months post-surgery (−12.4 [−14.7, −10.2] bpm; Figure 1A). Likewise, supine systolic blood pressure significantly declined six months after surgery (−12.3 [−15.7, −8.8] mmHg) and remained so 12 months following surgery (−12.3 [−16.0, −8.6] mmHg; Figure 1B). A similar pattern was observed for diastolic blood pressure (Figure 1C). Among participants with complete measures throughout the visits, the pattern of results was similar (Supplementary Figure 1).
Figure 1. Changes in heart and vascular function after bariatric surgery.
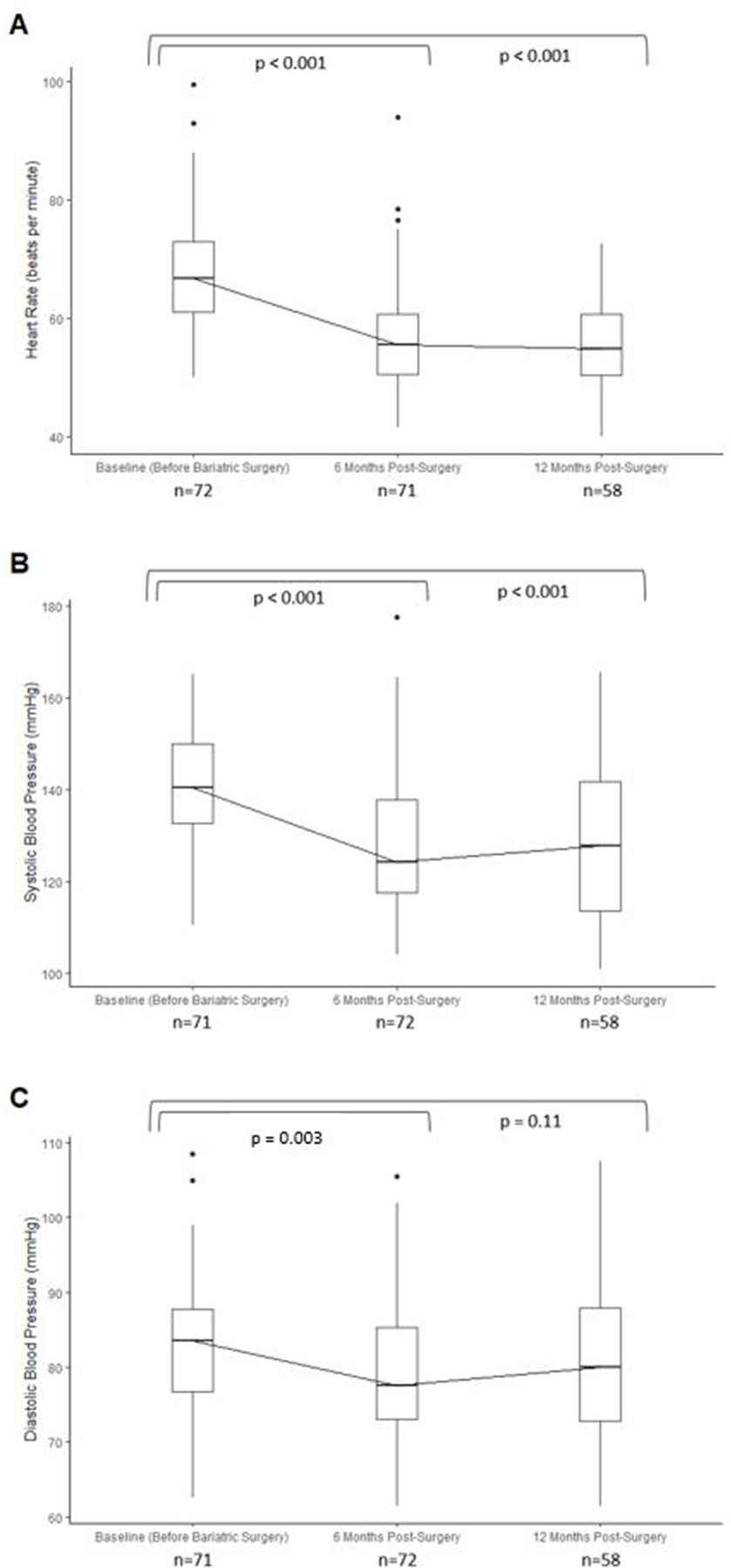
(A) Heart rate and (B) systolic blood pressure and (C) diastolic blood pressure *Sample size of non-missing observations denoted under each respective visit.
Despite overall favorable changes in BMI, blood pressure, and heart rate, arterial stiffness measures, CAVI and haPWV, significantly increased six months after bariatric surgery (+0.64 [0.42, 0.87] and +0.24 [0.04, 0.44], respectively) (Figure 2). These elevated arterial stiffness measurements were sustained 12 months after surgery (+0.80 [0.53, 1.07] and +0.40 [0.17, 0.62] m/s, respectively) (Figure 2 and Supplementary Figure 2). Results were consistent within subgroups of age, sex, and anti-hypertensive medication use (Supplementary Figure 3).
Figure 2. Changes in arterial stiffness measures after bariatric surgery.
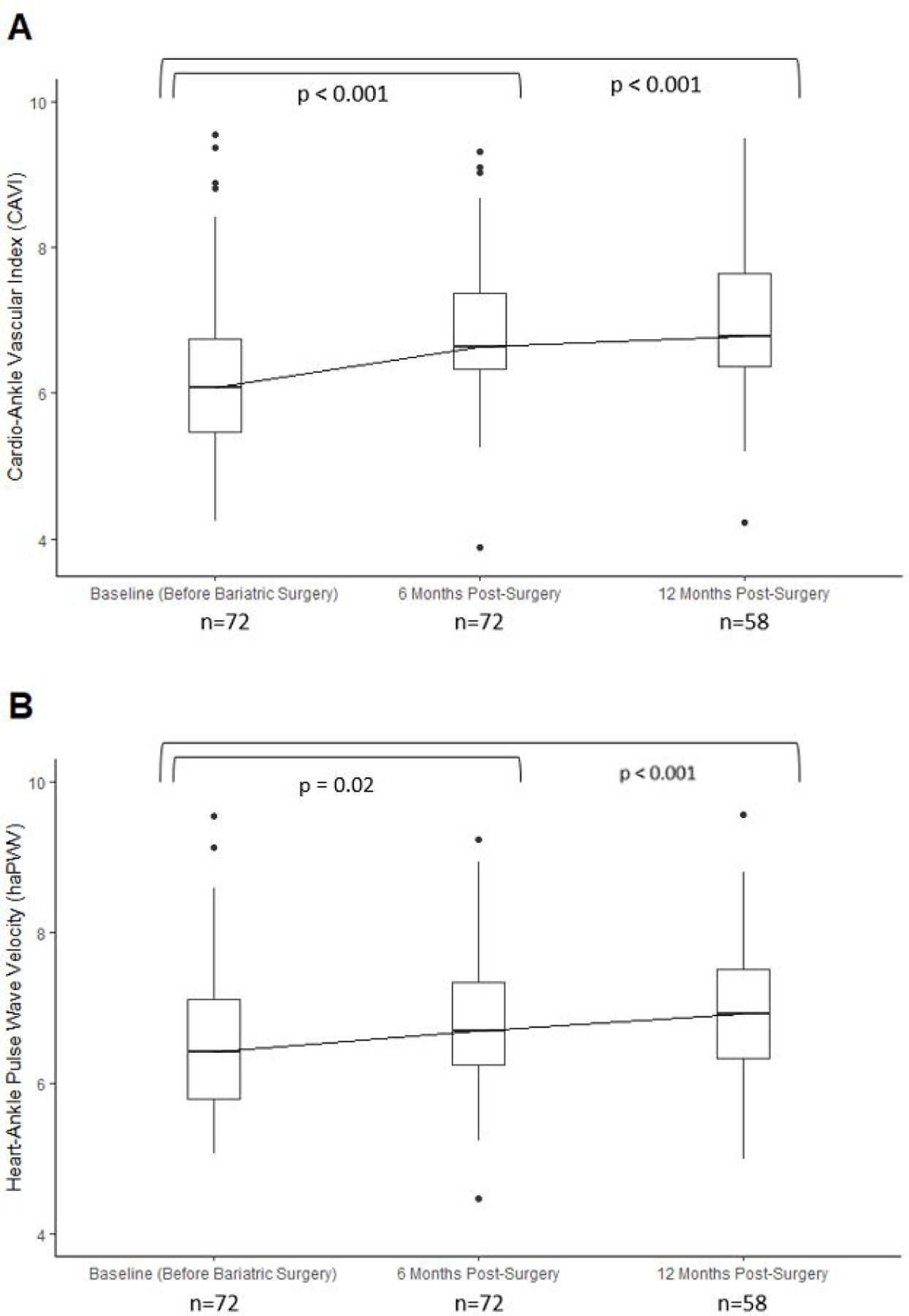
(A) CAVI (B) haPWV. *Sample size of non-missing observations denoted under each respective visit.
3.3. Factors associated with greater increase in CAVI
Table 2 describes factors associated with changes in CAVI six months after bariatric surgery. Among baseline characteristics, only diabetes was significantly associated with the degree of change in CAVI, with diabetic patients demonstrating less CAVI increases than those without diabetes (−0.61 [1.09, −0.12] in an unadjusted model). Among the change variables we modeled, only changes in heart rate showed a significant association with CAVI change. Specifically, a +0.19 [0.09, 0.30] greater increase in CAVI was observed for each 5 bpm decrease in heart rate 6 months following bariatric surgery. Changes in NT-proBNP (a measure of volume overload), systolic and diastolic blood pressure, and BMI were not associated with changes in CAVI. Baseline diabetic status and 6-month changes in heart rate remained significantly associated with observed changes in CAVI after adjusting for baseline characteristics (the “Adjusted” column in Table 2). Similar results were observed when we evaluated changes in haPWV, although diabetes did not reach statistical significance (Supplementary Table 1).
Table 2.
Association between participant characteristics and changes in CAVI six months after bariatric surgery
| Variable | Unadjusted | Adjusteda | |
|---|---|---|---|
| Baseline | Age | −0.01 (−0.03, 0.01) | −0.01 (−0.03, 0.01) |
| Female | 0.16 (−0.33, 0.66) | 0.07 (−0.42, 0.55) | |
| Diabetes | −0.61 (−1.09, −0.12) | −0.52 (−1.04, −0.01) | |
| Atrial fibrillation | 0.44 (−0.22, 1.10) | 0.32 (−0.34, 0.98) | |
| Six-month changes | Heart rate (per 5 bpm decrease) | 0.19 (0.09, 0.30) | 0.16 (0.05, 0.27) |
| Systolic blood pressure (per 10 mmHg decrease) | −0.07 (−0.22, 0.08) | -- | |
| Diastolic blood pressure (per 5 mmHg decrease) | −0.10 (−0.22, 0.02) | -- | |
| BMI (per 5 kg/m2 decrease) | 0.22 (−0.05, 0.47) | -- | |
| NT-proBNP change (per 10 pg/mL increase) | −0.01 (−0.03, 0.01) | -- | |
Adjusted by baseline age, sex, diabetes, and atrial fibrillation status.
BMI = body mass index; bpm = beats per minutec
Statistically significant values in bold.
4. Discussion
This is one of the largest studies examining changes in arterial stiffness measures after bariatric surgery. Despite favorable changes observed in cardiometabolic parameters (BMI, heart rate, systolic, and diastolic blood pressure), two arterial stiffness measures, CAVI and haPWV, were found to be significantly elevated after bariatric surgery. Similar results were observed across age and sex stratum. Non-diabetes was related to more CAVI increase than diabetes, and larger post-surgical heart rate decline was associated with a greater increase in CAVI. On the other hand, changes in blood pressure, BMI, and NT-proBNP were not associated with changes in CAVI.
Although our findings that arterial stiffness measures increase after bariatric surgery seem counterintuitive, a prior smaller-scale study demonstrated similar results using CAVI.12 Additionally, our observations seem to be in line with several other studies showing an inverse association between obesity and other arterial stiffness measures.6, 17, 18 Nonetheless, we also acknowledge other studies reporting conflicting results of no change10, 19 or decrease11, 20 in arterial stiffness after bariatric surgery. We are not sure about the exact reasons behind these heterogeneous observations across studies, but differences in study population (e.g., age, sex, and race), study design (types of bariatric surgery and duration of follow-up), and/or measures of arterial stiffness (types of PWVs and CAVI) may play a role.
These heterogeneous results seem to highlight the complex relationship between body fat, weight loss, and arterial stiffness. For example, Corden et al. reported that the BMI-PWV association is modified by age.7 Specifically, they observed that percent body fat was inversely associated with aortic PWV in younger and middle-aged adults but positively associated in older adults.7 Of note, our study participants were mainly middle-aged adults. They speculated that in younger adults with obesity, the body may compensate the increased vascular burden of obesity by elevating cardiac output and decreasing arterial stiffness. Notably, a few other studies have also demonstrated an inverse association between obesity and arterial stiffness in children.6, 21–23
The mechanisms underlying the observed decrease in post-bariatric surgery arterial stiffness measures are obscure. Despite the proposed explanation of compensatory mechanisms behind lower arterial stiffness in obesity, we did not observe an association between NT-proBNP and changes in arterial stiffness measures in our study. Additionally, we observed that a decline in heart rate was associated with a higher magnitude of CAVI elevation after bariatric surgery, which seems to deny the possibility that these elevated arterial stiffness measures result from sympathetic nervous system activation. Nonetheless, an increase in CAVI may reflect a compensation to maintain hemodynamics after reductions in heart rate and blood pressure. Another factor related to changes in arterial stiffness in our study was baseline diabetes status, with less CAVI increase in diabetics versus non-diabetics. An alternative potential mechanism may be related to the reduction of nitric oxide after bariatric surgery24 since nitric oxide is known to modulate endothelial function and arterial stiffness.25, 26
We are not certain about the prognostic implications of these changes in arterial stiffness measures after bariatric surgery. However, our observations do not support the notion that the elevation of post-bariatric surgery arterial stiffness measures would contribute to adverse outcomes, since non-diabetes and decreased heart rate, two potent protective factors (compared to diabetes and elevated heart rate, respectively), were associated with greater elevation of CAVI. Moreover, we should recognize that the changes in CAVI levels in our study fell mostly in the normal range. Thus, future prospective studies with longer follow-up are necessary to understand the prognostic value of changes in arterial stiffness measures after bariatric surgery.
There are a few other research and clinical implications of our study. Given conflicting results across previous studies, to better understand the complex relationship between obesity and arterial stiffness, it would be ideal to conduct a study evaluating various segment-specific PWVs before and after bariatric surgery in patients with a broad age range and with long-term follow-up of at least a few years to evaluate the prognostic value of changes in arterial stiffness. Also, the conflicting results regarding arterial stiffness outcomes after bariatric surgery suggest that healthcare providers should carefully interpret these measures amongst individuals who have undergone bariatric surgery.
There are several limitations in our study. Although it was one of the largest for this specific study question, the sample size was still small, limiting the types of analyses we could perform. Since the study population was predominantly white adults, another constraint lies in the generalizability of these findings beyond this race group. Another limitation was that haPWV was calculated from reported CAVI, rather than directly measured. Additionally, the lack of long-term CAVI measures past 12 months of follow-up after bariatric surgery limits the current study’s ability to gauge arterial stiffness outcomes in bariatric surgery patients beyond one year. Further studies may continue to assess whether the elevated measures of systemic arterial stiffness after bariatric surgery are abated or sustained on the long-term. Lastly, limited availability of variables curtailed our ability to explore other potential predictors of arterial stiffness change (such as menopausal status, bariatric surgery type, and inflammatory markers).
In conclusion, two measures of systemic arterial stiffness, CAVI and haPWV, were significantly elevated after bariatric surgery despite favorable changes in body weight, systolic blood pressure, and heart rate. Our study, together with previous studies, underscore the complex relationship between obesity and arterial stiffness. Thus, healthcare providers should carefully interpret any arterial stiffness measures among individuals with morbid obesity before and after bariatric surgery.
Supplementary Material
Highlights.
Arterial stiffness measures were elevated after bariatric surgery
Predictors of this elevation were post-surgery heart rate decrease and no diabetes
These predictors suggest this elevation may not be linked to poor prognosis
Arterial stiffness in bariatric surgery should be carefully interpreted
Acknowledgements:
The authors thank the participants and staff at the George W. Comstock Center for Public Health Research and Prevention.
Financial support:
F.W. received support from the NIH T32 institutional training grant (T32 HL007024). The BARI-Heart study was supported by the National Heart, Lung, and Blood Institute (NHLBI) National Institutes of Health (NIH) grant K23 HL12247. This specific study was also supported by Fukuda Denshi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests: K.M. received research funding and personal fees from Fukuda Denshi outside of the work. The other authors hve nothing to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
- 1.Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. September 5 2012;308(9):875–81. doi: 10.1001/2012.jama.10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. April 2008;51(14):1377–83. doi: 10.1016/j.jacc.2007.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis. July 2012;1(4)doi: 10.1258/cvd.2012.012016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato Y, Nagayama D, Saiki A, et al. Cardio-Ankle Vascular Index is Independently Associated with Future Cardiovascular Events in Outpatients with Metabolic Disorders. J Atheroscler Thromb. May 2016;23(5):596–605. doi: 10.5551/jat.31385 [DOI] [PubMed] [Google Scholar]
- 5.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. March 2010;55(13):1318–27. doi: 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- 6.Charakida M, Jones A, Falaschetti E, et al. Childhood obesity and vascular phenotypes: a population study. J Am Coll Cardiol. December 2012;60(25):2643–50. doi: 10.1016/j.jacc.2012.08.1017 [DOI] [PubMed] [Google Scholar]
- 7.Corden B, Keenan NG, de Marvao AS, et al. Body fat is associated with reduced aortic stiffness until middle age. Hypertension. June 2013;61(6):1322–7. doi: 10.1161/HYPERTENSIONAHA.113.01177 [DOI] [PubMed] [Google Scholar]
- 8.Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging. September 2008;28(5):287–93. doi: 10.1111/j.1475-097X.2008.00806.x [DOI] [PubMed] [Google Scholar]
- 9.Rider OJ, Tayal U, Francis JM, et al. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity (Silver Spring). December 2010;18(12):2311–6. doi: 10.1038/oby.2010.64 [DOI] [PubMed] [Google Scholar]
- 10.Streese L, Königstein K, Goricki L, et al. Short- and Long-Term Effects of Bariatric Surgery on Vascular Phenotype. Obes Surg. April 2019;29(4):1301–1308. doi: 10.1007/s11695-018-03679-2 [DOI] [PubMed] [Google Scholar]
- 11.Bäckdahl J, Andersson DP, Eriksson-Hogling D, et al. Long-Term Improvement in Aortic Pulse Wave Velocity After Weight Loss Can Be Predicted by White Adipose Tissue Factors. Am J Hypertens. 03 2018;31(4):450–457. doi: 10.1093/ajh/hpx201 [DOI] [PubMed] [Google Scholar]
- 12.Galkine A, Dzenkeviciute V, Sapoka V, et al. Effects of body weight reduction on arterial stiffness and endothelial function after bariatric surgery in morbidly obese patients: a 4-year clinical study. Acta Endocrinol (Buchar). 2018 Oct-Dec 2018;14(4):491–497. doi: 10.4183/aeb.2018.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. April 2006;13(2):101–7. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamamoto T, Tsuda S, et al. Coefficients in the CAVI Equation and the Comparison Between CAVI With and Without the Coefficients Using Clinical Data. J Atheroscler Thromb. May 2019;26(5):465–475. doi: 10.5551/jat.44834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- 16.StataCorp. 2011. Stata Statistical Software: Release 12 . College Station, TX: StataCorp LP. [Google Scholar]
- 17.Yang H, Zhao J, Deng X, et al. Pulse wave velocity is decreased with obesity in an elderly Chinese population. J Clin Hypertens (Greenwich). September 2019;21(9):1379–1385. doi: 10.1111/jch.13659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Won KB, Chang HJ, Niinuma H, et al. Inverse association between central obesity and arterial stiffness in Korean subjects with metabolic syndrome: a cross-sectional cohort study. Diabetol Metab Syndr. 2015;7:3. doi: 10.1186/1758-5996-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjevestad E, Hjelmesaeth J, Sandbu R, Nordstrand N. Effects of intensive lifestyle intervention and gastric bypass on aortic stiffness: a 1-year nonrandomized clinical study. Obesity (Silver Spring). January 2015;23(1):37–45. doi: 10.1002/oby.20880 [DOI] [PubMed] [Google Scholar]
- 20.Domienik-Karłowicz J, Lisik W, Rymarczyk Z, et al. The short-term effect of bariatric surgery on non-invasive markers of artery function in patients with metabolic syndrome. Diabetol Metab Syndr. 2015;7:76. doi: 10.1186/s13098-015-0076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lurbe E, Torro I, Garcia-Vicent C, Alvarez J, Fernández-Fornoso JA, Redon J. Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension. August 2012;60(2):550–5. doi: 10.1161/HYPERTENSIONAHA.112.194746 [DOI] [PubMed] [Google Scholar]
- 22.Dangardt F, Chen Y, Berggren K, Osika W, Friberg P. Increased rate of arterial stiffening with obesity in adolescents: a five-year follow-up study. PLoS One. 2013;8(2):e57454. doi: 10.1371/journal.pone.0057454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity (Silver Spring). January 2012;20(1):165–71. doi: 10.1038/oby.2011.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin LY, Lee WJ, Shen HN, et al. Nitric oxide production is paradoxically decreased after weight reduction surgery in morbid obesity patients. Atherosclerosis. February 2007;190(2):436–42. doi: 10.1016/j.atherosclerosis.2006.02.033 [DOI] [PubMed] [Google Scholar]
- 25.Kinlay S, Creager MA, Fukumoto M, et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. November 2001;38(5):1049–53. doi: 10.1161/hy1101.095329 [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. January 2002;105(2):213–7. doi: 10.1161/hc0202.101970 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


