Abstract
Background
Whether social determinants of health are associated with survival in the context of pediatric oncology–targeted immunotherapy trials is not known. We examined the association between poverty and event-free survival (EFS) and overall survival (OS) for children with high-risk neuroblastoma treated in targeted immunotherapy trials.
Methods
We conducted a retrospective cohort study of 371 children with high-risk neuroblastoma treated with GD2-targeted immunotherapy in the Children’s Oncology Group trial ANBL0032 or ANBL0931 at a Pediatric Health Information System center from 2005 to 2014. Neighborhood poverty exposure was characterized a priori as living in a zip code with a median household income within the lowest quartile for the cohort. Household poverty exposure was characterized a priori as sole coverage by public insurance. Post hoc analyses examined the joint effect of neighborhood and household poverty using a common reference. All statistical tests were 2-sided.
Results
In multivariable Cox regressions adjusted for disease and treatment factors, household poverty–exposed children experienced statistically significantly inferior EFS (hazard ratio [HR] = 1.90, 95% confidence interval [CI] = 1.28 to 2.82, P = .001) and OS (HR = 2.79, 95% CI = 1.63 to 4.79, P < .001) compared with unexposed children. Neighborhood poverty was not independently associated with EFS or OS. In post hoc analyses exploring the joint effect of neighborhood and household poverty, children with dual-poverty exposure (neighborhood poverty and household poverty) experienced statistically significantly inferior EFS (HR = 2.21, 95% CI = 1.48 to 3.30, P < .001) and OS (HR = 3.70, 95% CI = 2.08 to 6.59, P < .001) compared with the unexposed group.
Conclusions
Poverty is independently associated with increased risk of relapse and death among neuroblastoma patients treated with targeted immunotherapy. Incorporation of social and environmental factors in future trials as health-care delivery intervention targets may increase the benefit of targeted therapies.
Childhood cancer exemplifies the successes of modern medicine—almost incurable 60 years ago, 80% of children diagnosed today will survive at least 5 years (1,2). In the 21st century, a majority of children with cancer will be treated on a clinical trial if one is available (2), an approach to care delivery that facilitates evaluation of targeted therapies (3). Modern pediatric oncology trials aim to identify children for whom current therapeutic approaches are suboptimal (1), focusing on refining biological and response-based risk classification to improve outcomes (1). Missing from this paradigm of discovery and care has been consideration of nonbiological factors as outcome predictors or intervention targets.
Social determinants of health, including poverty, contribute substantially to health outcomes in the United States (4–6). It has been postulated that disparities in access to innovative therapies have the potential to increase preexisting disparate outcomes (7). Whether targeted therapies equitably improve survival outcomes for those patients who successfully access them has not been investigated. We posit that pediatric oncology provides an ideal population within which to investigate the association of poverty and targeted therapy outcomes given its high reliance on standardized clinical trial–based care delivery (2) that facilitates the ability to control for tumor biology and treatment variables.
One in 5 US children with cancer lives in poverty (8, 9), and childhood cancer remains a leading cause of death (10). Population-based pediatric cancer studies have begun to parse the relative contributions of socioeconomic status (SES) and biology and suggest that SES statistically significantly mediates (11) previously described racial and ethnic survival disparities (12–16). Such data are compelling but offer little insight into the question of whether clinical trials of targeted therapy lead to similar outcomes regardless of SES. Addressing this question is essential to ensure that therapeutic advances translate into improved health outcomes for all patients.
Neuroblastoma is the most common extracranial solid tumor in childhood (10), and high-risk disease defined by clinical factors and tumor biology is associated with relapse and poor survival (10, 17, 18). In 2011, a Children’s Oncology Group (COG) trial of targeted immunotherapy following intensive multi-modality therapy for high-risk neuroblastoma (HR NBL) demonstrated the most clinically significant event-free survival (EFS) improvement in decades (19). This trial cohort provides a logical population in which to explore the question of whether nonbiological variables, such as poverty, add prognostic value beyond known outcome predictors in the targeted immunotherapy trial setting (20). We sought to identify the association between poverty and EFS and overall survival (OS) for children with HR NBL treated on COG-targeted immunotherapy trials.
Methods
Data Sources
COG is a National Cancer Institute–supported clinical trials cooperative group conducting pediatric trials in North America, Europe, Australia, and New Zealand (2, 21). The COG ANBL0032 phase III clinical trial enrolled HR NBL patients beginning in October 2001 (19). Participation required at least a partial response (PR) to multi-agent induction chemotherapy and primary tumor resection. Participants must have additionally received consolidation therapy with autologous stem cell transplantation (ASCT) and external beam radiotherapy without disease progression. Patients were randomly assigned to receive 6 months of standard of care isotretinoin or isotretinoin plus targeted immunotherapy with the monoclonal antibody dinutuximab (19). Isotretinoin was administered orally in the outpatient setting. Dinutuximab and cytokines were given intravenously and subcutaneously during 5 inpatient cycles. Children randomly assigned to immunotherapy experienced statistically significantly improved EFS and randomization was stopped early (19), with all patients enrolled after 2009 assigned to immunotherapy. The ANBL0931 trial was designed to support US Food and Drug Administration registration of dinutuximab through collection of detailed safety and toxicity data at a limited number of participating centers (22). Eligibility criteria and the regimen administered were identical to those of the ANBL0032 immunotherapy arm (19, 22). Tumor biology data were collected for patients concurrently enrolled in the COG biology study ANBL00B1. For this analysis, ANBL0032 and ANBL0931 provided data on patient characteristics, tumor histology and biology, and disease outcome.
Insurance and zip code–linked US Census data were provided by the Pediatric Health Information System (PHIS) database, which includes administrative data from 45 US pediatric hospitals (23).
ANBL0032/0931 were approved by COG institutions’ local review boards. Patients provided written informed consent and assent for trial enrollment and future research use of data. PHIS data are deidentified and considered exempt from human subjects research review.
Cohort
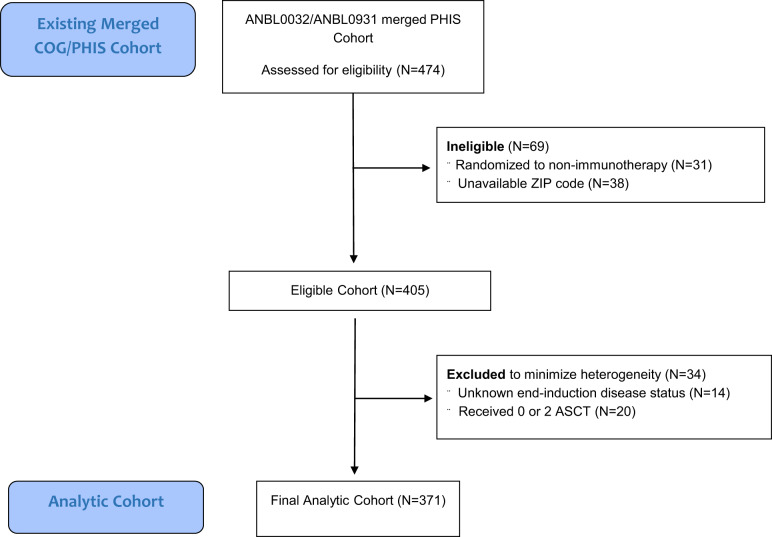
The study population was derived from a previously published cohort created by a data merge of patients enrolled in either ANBL0032 or ANBL0931 and treated at a PHIS center from 2005 to 2014 (24). The analytic cohort (Figure 1) included children randomly assigned or directly assigned to receipt of immunotherapy with available poverty exposure measures as detailed below. Children randomly assigned to isotretinoin alone were excluded. To minimize heterogeneity, the cohort was restricted to patients with available end-induction disease status and receipt of a single ASCT, resulting in an analytic cohort of 371 patients.
Figure 1.
Consort diagram of cohort creation. ASCT = autologous stem cell transplantation.
Poverty
Poverty was the primary exposure of interest and was characterized a priori at both the neighborhood level and household level. Neighborhood poverty was assigned by linkage of a child’s residential zip code at first trial-associated PHIS encounter to 2010 US Census median annual household income (25). Patients living in a zip code with a median household income within the lowest quartile for the cohort (median income ≤$35 916) were considered neighborhood poverty exposed (26). Household poverty was assigned based on insurance at first trial-associated PHIS encounter and dichotomized as sole coverage by public insurance (Medicaid or Children's Health Insurance Program [CHIP]) vs private or other (including commercial, dual commercial and public [eg, as a secondary insurer], military, and other insurers). Children with public insurance only were identified a priori as household poverty exposed (27, 28). Exploratory analyses examining the joint effect of neighborhood and household poverty using a common reference were performed post hoc.
Outcome
EFS was defined as time from ANLB0032/ANBL0931 study enrollment until first occurrence of relapse, progressive disease, secondary malignancy or death, or until last contact if no event occurred. OS was defined as time from study enrollment until death or last contact (19).
Covariates
Demographics
ANBL0032/ANBL0931 patient characteristics were provided by COG and include age at trial enrollment (<18 months or ≥18 months, based on known clinical risk criteria) (17), sex, ethnicity (Hispanic or non-Hispanic), and race (White, Black, other).
Tumor Biology and Treatment Variables
Tumor biology variables and staging were collected during the COG ANBL00B1 biology study if patients were enrolled (19). Staging was performed using the International Neuroblastoma Staging System (INSS) criteria in use at the time that ANBL0032 opened (29, 30). INSS staging was categorized as stage IIB, III, IV, IVS, or unknown and dichotomized in statistical models (stage IV or all other). Tumor MYCN status was categorized as amplified, nonamplified, or unknown. Tumor histology was categorized as favorable, unfavorable, or unknown per the International Neuroblastoma Pathology Classification system (31).
Treatment and disease response variables were provided by COG as collected on ANBL0032/ANBL0931. These include end-induction disease response before ASCT (complete response, very good PR [VGPR], or PR), trial (ANBL0032 or ANBL0931), and days from ASCT to trial enrollment, treating hospital, and treatment era (before or after 2009 publication of ANBL0032 immunotherapy outcomes).
Statistical Analysis
Patient demographics, tumor, and treatment characteristics were summarized for the overall cohort, by neighborhood poverty, and by household poverty using descriptive statistics. Association of characteristics between poverty groups was evaluated with χ2 and Wilcoxon rank sum tests for categorical and continuous variables, respectively. OS and EFS curves were plotted using Kaplan-Meier methods, and 2-year OS and EFS were estimated with 95% confidence intervals (CIs) where standard errors were calculated based on the Greenwood formula. EFS and OS were evaluated at 2 years to allow comparison with previously published ANBL0032 outcome data (19). Associations between poverty exposures (and covariates) and survival outcomes were evaluated with univariate Cox proportional hazard (PH) models. Those covariates associated with both exposure and outcome (based on P < .1 or a large enough effect size) were considered confounders and were retained in multivariable Cox models; tumor MYCN status was a priori specified for inclusion in models based on clinical pertinence. In addition, the final multivariable models included robust variance estimates (32) to account for potential hospital clustering, because treating hospital was considered a potential confounder but could not be adjusted for due to small numbers of patients per site.
We performed post hoc analyses exploring the independent and joint effects of neighborhood poverty and household poverty by creating a 4-category combined exposure variable with a common reference: dual-exposed neighborhood and household poverty, single-exposed neighborhood poverty, single-exposed household poverty, and dual-unexposed (reference group). Kaplan-Meier curve and multivariable Cox models were constructed. In all Cox models, PH assumptions were tested using Schoenfeld residuals and adding interaction terms between time and predictors (33, 34). All analyses were performed using SAS (version 9.2, SAS Institute, Inc, Cary, NC), and a 2-sided P less than .05 was considered statistically significant.
We performed a series of sensitivity analyses to assess the robustness of our primary analysis of poverty exposures and outcome (Supplementary Tables 1–5 available online). First, we performed a sensitivity analysis restricting the cohort to those treated post-2009 immunotherapy data publication to address potential heterogeneity in patient characteristics (N = 342). Second, we performed a sensitivity analysis censoring every patient at 2 years to address the differential length of follow-up between poverty exposure categories (N = 371). Third, to assess for selection bias due to exclusion of patients with missing end-induction disease status, we repeated analyses including these patients as either PR or VGPR (N = 385). Lastly, to address the large number of patients with unknown stage, we repeated analyses recategorizing unknown stage to stage IV based on clinical expectations (N = 385).
Results
Characteristics of Study Patients
The analytic cohort was comprised of 371 children (Table 1). Among survivors, median duration of follow-up was 1.97 years (interquartile range = 0.52-3.18 years). Ninety-three children (25.1%) lived in neighborhood poverty (median household income ≤$35 916, approximately 150% of the federal poverty level for a family of 4 in 2010) (35); 35.3% of children lived in household poverty as measured by US public insurance (Medicaid or CHIP) (Table 1). A total of 52 children (14.0%) were exposed to both household and neighborhood poverty, and 199 (53.6%) were exposed to neither. There were no statistically significant differences in INSS stage, end-induction disease response, tumor histology, tumor MYCN amplification status, or time from ASCT to trial enrollment by neighborhood or household poverty (Table 1). Children living in neighborhood poverty disproportionately lived in household poverty (55.9%), and children in household poverty were disproportionately Black (19.1%) and Hispanic (26.0%) (Table 1).
Table 1.
Characteristics of study patients by neighborhood poverty and household poverty (N = 371)
| Patient, disease and treatment characteristics | Overall | Neighborhood poverty |
Household poverty |
||||
|---|---|---|---|---|---|---|---|
| Yes Median household income, Q1 (≤$35 916) |
No Median household income, Q2-Q4 (>$35 916) |
P b | Yes | No | P b | ||
| Total | 371 (100.0) | 93 (25.1) | 278 (74.9) | 131 (35.3) | 240 (64.7) | ||
| Length of follow-up, median (IQR), y | 1.97 (0.52-3.18) | 1.92 (0.53-2.66) | 2.06 (0.51-3.42) | .33 | 1.54 (0.38-2.58) | 2.18 (0.71-3.48) | .002 |
| Race, No (%) | .36 | <.001 | |||||
| Black | 38 (10.2) | 12 (12.9) | 26 (9.4) | 25 (19.1) | 13 (5.4) | ||
| White | 276 (74.4) | 64 (68.8) | 212 (76.3) | 77 (58.8) | 199 (82.9) | ||
| Other | 57 (15.4) | 17 (18.3) | 40 (14.4) | 29 (22.1) | 28 (11.7) | ||
| Ethnicity, No. (%) | .01 | <.001 | |||||
| Hispanic/Latino | 56 (15.1) | 22 (23.7) | 34 (12.2) | 34 (26.0) | 22 (9.2) | ||
| Not Hispanic/Latino | 315 (84.9) | 71 (76.3) | 244 (87.8) | 97 (74.1) | 218 (90.8) | ||
| Household poverty, No. (%) | <.001 | — | — | ||||
| Yes | 131 (35.3) | 52 (55.9) | 79 (28.4) | — | — | ||
| No | 240 (64.7) | 41 (44.1) | 199 (71.6) | — | — | ||
| Age, No. (%) | .82 | — | — | .69 | |||
| <18 mo | 29 (7.8) | 8 (8.6) | 21 (7.6) | 9 (6.9) | 20 (8.3) | ||
| ≥18 mo | 342 (92.2) | 85 (91.4) | 257 (92.5) | 122 (93.1) | 220 (91.7) | ||
| Sex, No. (%) | .40 | .74 | |||||
| Male | 221 (59.6) | 59 (63.4) | 162 (58.3) | 80 (61.1) | 141 (58.8) | ||
| Female | 150 (40.4) | 34 (36.6) | 116 (41.7) | 51 (38.9) | 99 (41.3) | ||
| Trial, No. (%) | .31 | .02 | |||||
| ANBL0032 | 318 (85.7) | 83 (89.3) | 235 (84.5) | 120 (91.6) | 198 (82.5) | ||
| ANBL0931 | 53 (14.3) | 10 (10.8) | 43 (15.5) | 11 (8.4) | 42 (17.5) | ||
| Treatment post-2009,a No. (%) | .15 | .01 | |||||
| Yes | 342 (92.2) | 89 (95.7) | 253 (91.0) | 127 (97.0) | 215 (89.6) | ||
| No | 29 (7.8) | 4 (4.3) | 25 (9.0) | 4 (3.1) | 25 (10.4) | ||
| Days from SCT to trial enrollment, median (IQR) | 85 (77-96) | 88 (79-98) | 85 (76-96) | .07 | 87 (78-98) | 85 (76-95) | .08 |
| End-induction disease response, No. (%) | .85 | .46 | |||||
| CR | 130 (35.0) | 31 (33.3) | 99 (35.6) | 43 (32.8) | 87 (36.3) | ||
| VGPR | 123 (33.2) | 33 (35.5) | 90 (32.4) | 41 (31.3) | 82 (34.2) | ||
| PR | 118 (31.8) | 29 (31.2) | 89 (32.0) | 47 (35.9) | 71 (29.6) | ||
| Tumor MYCN status, No. (%) | .30 | .40 | |||||
| Amplified | 131 (35.3) | 39 (41.9) | 92 (33.1) | 52 (39.7) | 79 (32.9) | ||
| Not amplified | 154 (41.5) | 34 (36.6) | 120 (43.2) | 52 (39.7) | 102 (42.5) | ||
| Unknown | 86 (23.2) | 20 (21.5) | 66 (23.7) | 27 (20.6) | 59 (24.6) | ||
| Tumor histology, No. (%) | .06 | .73 | |||||
| Unfavorable | 266 (71.7) | 67 (72.0) | 199 (71.6) | 97 (74.1) | 169 (70.4) | ||
| Favorable | 11 (3.0) | 6 (6.5) | 5 (1.8) | 4 (3.1) | 7 (2.9) | ||
| Unknown | 94 (25.3) | 20 (21.5) | 74 (26.6) | 30 (22.9) | 64 (26.7) | ||
| INSS stage, No. (%) | .22 | .79 | |||||
| IV | 258 (69.5) | 70 (75.3) | 188 (67.6) | 96 (73.3) | 162 (67.5) | ||
| III | 46 (12.4) | 9 (9.7) | 37 (13.3) | 15 (11.5) | 31 (12.9) | ||
| IIB | 9 (2.4) | 1 (1.1) | 8 (2.9) | 2 (1.5) | 7 (2.9) | ||
| IVS | 3 (0.8) | 2 (2.2) | 1 (0.4) | 1 (0.8) | 2 (0.8) | ||
| Unknown | 55 (14.8) | 11 (11.8) | 44 (15.8) | 17 (13.0) | 38 (15.8) | ||
ANBL0032 met early stopping rules in 2009 due to superior results associated with anti-GD2 immunotherapy. Thus all patients post-2009 received treatment recommendations reflecting this information. CR = complete response; IQR = interquartile range; INSS = International Neuroblastoma Staging System; PR = partial response; Q = quartile; SCT = stem cell transplantation; VGPR = very good partial response.
P values were from Wilcoxon rank sum tests for continuous variables and χ2 tests for categorical variables. All P values were 2-sided.
Associations Between Household and Neighborhood Poverty and Disease Outcome
In univariate analyses, household poverty–exposed children experienced statistically significantly inferior EFS compared with unexposed children (2-year EFS = 50.9% vs 75.7%; hazard ratio [HR] = 2.11, 95% CI = 1.41 to 3.15). OS was statistically significantly inferior for household poverty–exposed children vs unexposed children (2-year OS = 74.4% vs 90.9%, HR = 3.08, 95% CI = 1.76 to 5.39) and for neighborhood poverty–exposed children vs unexposed children (78.8% vs 87.7%, HR = 1.72, 95% CI = 0.96 to 3.09).
In multivariable analysis, household poverty–exposed children experienced statistically significantly inferior EFS compared with unexposed children (HR = 1.90, 95% CI = 1.28 to 2.82, P = .001) after adjusting for disease- and treatment-related covariates (Table 2). OS was also statistically significantly inferior in household poverty–exposed children (HR= 2.79, 95% CI = 1.63 to 4.79, P < .001) (Table 2; Figure 2).
Table 2.
EFS and OS adjusted for ethnicity, insurance, INSS stage, disease response, MYCN status, and hospital clustering (N = 371)
| Characteristics | Univariate analyses of outcome |
Multivariable analyses of outcomea |
||||||
|---|---|---|---|---|---|---|---|---|
| EFS |
OS |
EFS |
OS |
|||||
| HR (95% CI) | P b | HR (95% CI) | Pb | HR (95% CI) | P b | HR (95% CI) | P b | |
|
Child or sociodemographic characteristics |
.36 | |||||||
|
Neighborhood poverty |
||||||||
| No (Q2-4: >$35 916) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Yes (Q1:≤35 916) | 1.38 (0.89 to 2.12) | .15 | 1.72 (0.96 to 3.09) | .07 | 1.16 (0.79 to 1.70) | .46 | 1.25 (0.78 to 1.99) | |
| Household poverty | <.001 | |||||||
| No | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Yes | 1.99 (1.34 to 2.96) | <.001 | 3.08 (1.76 to 5.39) | <.001 | 1.90 (1.28 to 2.82) | .001 | 2.79 (1.63 to 4.79) | |
| Race | —c | — | — | — | ||||
| White | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Black | 1.09 (0.57 to 2.12) | .79 | 1.32 (0.56 to 3.14) | .56 | ||||
| Other | 0.79 (0.43 to 1.46) | .44 | 0.98 (0.44 to 2.20) | .96 | ||||
| Ethnicity | .09 | |||||||
| Non-Hispanic | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Hispanic | 1.55 (0.94 to 2.56) | .08 | 2.54 (1.37 to 4.72) | .003 | 1.20 (0.79 to 1.82) | .34 | 1.70 (0.92 to 3.14) | |
| Age | __ | __ | __ | __ | ||||
| ≥18 mo | 1.00 (Ref) | .79 | 1.00 (Ref) | .59 | ||||
| <18 mo | 0.90 (0.42 to 1.94) | 1.29 (0.51 to 3.24) | ||||||
| Sex, female | 0.73 (0.48 to 1.09) | .13 | 0.83 (0.48 to 1.46) | .52 | — | — | — | — |
| Tumor and treatment characteristics | ||||||||
| Trial | — | — | — | — | ||||
| ANBL0032 | 1.00 (Ref) | 1.00 (Ref) | ||||||
| ANBL0931 | 0.82 (0.49 to 1.39) | .47 | 1.01 (0.50 to 2.04) | .97 | ||||
| Treatment post-2009 | — | — | — | — | ||||
| Yes | 1.00 (Ref) | 1.00 (Ref) | ||||||
| No | 1.11 (0.57 to 2.14) | .76 | 1.16 (0.48 to 2.79) | .74 | ||||
| Days from SCT to trial enrollment | — | — | — | — | ||||
| INSS stage | 1 (0.99 to 1.01) | .43 | 0.99 (0.98 to 1.01) | .38 |
.42 .16 |
|||
| IIB/III/IVS | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| IV | 2.00 (1.00 to 4.01) | .049 | 2.23 (0.80 to 6.25) | .13 | 1.53 (0.84 to 2.77) | .17 | 1.53 (0.55 to 4.26) | |
| Missing stage | 2.66 (1.19 to 5.99) | .02 | 3.29 (1.01 to 10.7) | .048 | 2.20 (0.80 to 6.05) | .13 | 3.07 (0.65 to 14.54) | |
| Tumor MYCN |
.991 .964 |
|||||||
| Not amplified | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Amplified | 0.76 (0.47 to 1.21) | .24 | 0.94 (0.49 to 1.80) | .85 | 0.77 (0.54 to 1.09) | .14 | 1.00 (0.55 to 1.81) | |
| Missing | 1.17 (0.72 to 1.91) | .52 | 1.49 (0.76 to 2.94) | .25 | 0.96 (0.49 to 1.88) | .91 | 1.02 (0.40 to 2.64) | |
| Tumor histology | — | |||||||
| Favorable | 1.00 (Ref) | 1.00 (Ref) | — | — | — | |||
| Unfavorable | 2.23 (0.31 to 16.02) | .43 | 0.89 (0.12 to 6.51) | .91 | ||||
| Missing | 3.11 (0.42 to 22.79) | .27 | 1.58 (0.21 to 11.90) | .66 | ||||
| End-induction disease response |
.049 .07 |
|||||||
| CR | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| VGPR | 1.71 (1.03 to 2.83) | .04 | 2.09 (0.98 to 4.47) | .06 | 1.59 (0.95 to 2.66) | .08 | 1.89 (1.004 to 3.56) | |
| PR | 1.65 (0.99 to 2.75) | .06 | 2.47 (1.61 to 5.27) | .002 | 1.48 (0.74 to 2.93) | .27 | 2.27 (0.94 to 5.49) | |
Variables included in multivariable model: neighborhood poverty, household poverty, ethnicity, INSS stage, tumor MYCN, end-induction disease response, and robust variance estimates to account for potential hospital clustering. CI = confidence interval; CR = complete response; EFS = event-free survival; HR = hazard ratio; INSS = International Neuroblastoma Staging System; OS = overall survival; PR = partial response; Q = quartile; SCT = stem cell transplantation; VGPR = very good partial response.
P values were from Cox regression model and were 2-sided.
Empty cells reflect covariates not included in multivariable analyses as detailed in Methods.
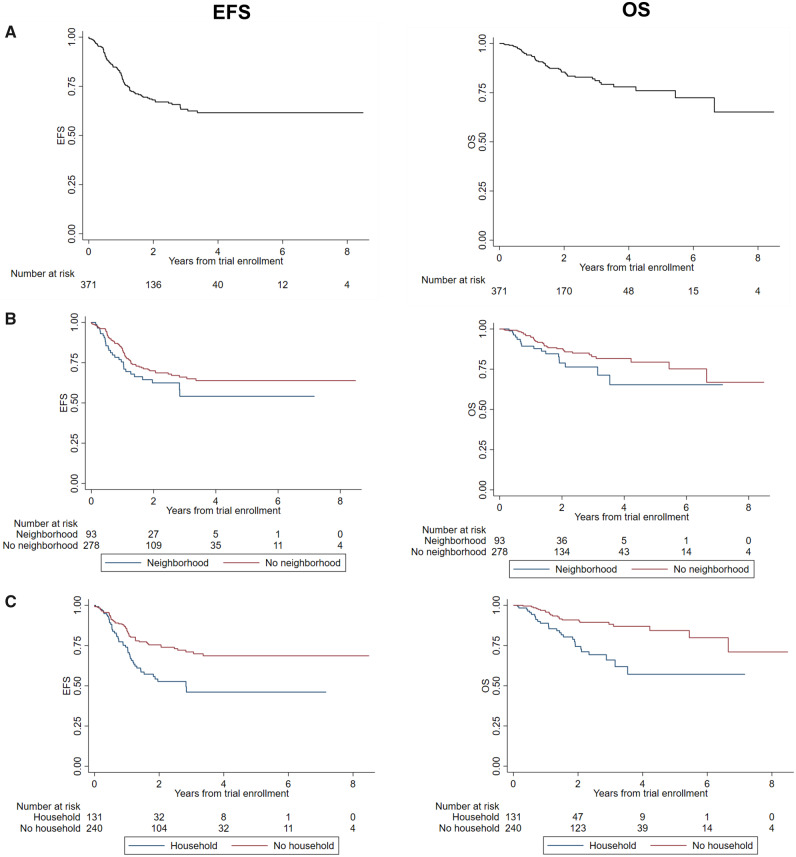
Figure 2.
Survival among children with high-risk neuroblastoma receiving targeted immunotherapy on Children’s Oncology Group (COG) protocols ANBL0032 or ANBL0931 at a Pediatric Health Information System (PHIS) center. Data are shown for Kaplan-Meier estimates of event-free survival (EFS) and overall survival (OS) for overall cohort from time of trial enrollment. Trial enrollment occurred after completion of both induction and consolidation therapy A). Two-year estimates (95% confidence interval [CI]): EFS = 67.9% (95% CI = 61.9% to 73.2%); OS = 85.5% (95% CI = 80.6% to 89.3%). B) Data for EFS and OS = stratified by neighborhood poverty group. Two-year estimates (95% CI): EFS no neighborhood poverty = 70.0% (95% CI = 63.1% to 75.8%) vs neighborhood poverty = 61.6% (95% CI = 48.5% to 72.3%), log rank test P = .15; OS no neighborhood poverty = 87.7% (95% CI = 82.2% to 91.6%) vs neighborhood poverty = 78.8% (95% CI = 66.6% to 87.0%), log rank test P = .07. C) EFS and OS stratified by household poverty group. Two-year estimates (95% CI): EFS no household poverty = 75.7% (95% CI = 68.8% to 81.2%) vs household poverty = 50.9% (95% CI = 39.4% to 61.3%), log rank test (P < .001); OS no household poverty = 90.9% (95% CI = 85.6% to 94.3%) vs household poverty = 74.4% (95% CI = 63.4% to 82.5), log rank test P < .001.
No other covariates maintained statistical significance with EFS in the multivariable model. End-induction disease status remained associated with OS; children with VGPR experienced inferior OS (HR = 1.89, 95% CI = 1.004 to 3.56, P = .49) vs those with complete response. The magnitude of the hazard ratios for neighborhood poverty, Hispanic ethnicity, unknown INSS stage, and end-induction disease response remained relatively unchanged from univariate analyses, suggesting that reduced power may have limited detection of independent effects for these variables.
Exploratory Analysis of the Joint Effect of Neighborhood Level and Household Level Poverty on Outcomes
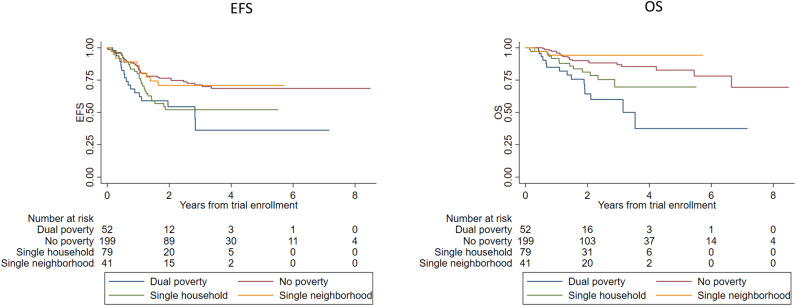
In post hoc exploratory analyses of the joint effect of neighborhood and household poverty exposures, statistically significant differences in EFS and OS were observed across the 4 exposure levels (log-rank P = .005 for EFS, P < .001 for OS; Figure 3). Two-year EFS was 76.5% (95% CI = 68.9% to 82.4%) for no poverty, 70.9% (95% CI = 52.5% to 83.3%) for single-neighborhood poverty, 52.1% (95% CI = 37.7% to 64.7%) for single-household poverty, and 54.5% (95% CI = 36.2% to 69.5%) for dual poverty, suggesting that household poverty exposure was associated with inferior EFS regardless of neighborhood poverty exposure. Two-year OS was 90.1% (95% CI = 83.8% to 94.0%) for no poverty, 94.3% (95% CI = 79.0% to 98.5%) for single-neighborhood poverty, 81.2% (95% CI = 67.6% to 89.6%) for single-household poverty, and 64.3% (95% CI = 45.0% to 78.3%) for dual-poverty, suggesting that the association of household poverty exposure and OS was stronger for patients concomitantly exposed to neighborhood poverty.
Figure 3.
Survival by combined poverty status among children with high-risk neuroblastoma receiving targeted immunotherapy on Children’s Oncology Group (COG) protocols ANBL0032 or ANBL0931 and treated at a Pediatric Health Information System (PHIS) center. Data are shown for Kaplan-Meier estimates of (A) event-free survival (EFS) from time of trial enrollment by combined neighborhood and household poverty, 2-year estimates: no poverty = 76.5% (95% CI = 68.9% to 82.4%), single-neighborhood poverty = 70.9% (95% CI = 52.5% to 83.3%), single-household poverty = 52.1% (95% CI = 37.7% to 64.7%), dual-poverty = 54.5% (95% CI = 36.2% to 69.5%), log-rank P value = .005; and B) overall survival (OS) from time of trial enrollment by combined neighborhood and household poverty, 2-year estimates: no poverty = 90.1% (95% CI = 83.8% to 94.0%), single-neighborhood poverty = 94.3% (95% CI = 79.0% to 98.5%), single-household poverty = 81.2% (95% CI = 67.6% to 89.6%), dual poverty = 64.3% (95% CI = 45.0% to 78.3%), log rank test P less than .001. Trial enrollment occurred after completion of both induction and consolidation therapy.
In multivariable analyses, EFS was statistically significantly inferior in dual poverty–exposed children (HR = 2.21, 95% CI = 1.48 to 3.30, P < .001) and single-household poverty–exposed children (HR = 1.88, 95% CI = 1.21 to 2.91, P = .005) compared with the unexposed group (Table 3). OS was inferior in dual poverty–exposed children (HR = 3.7, 95% CI = 2.08 to 6.59, P < .001). There was a trend toward inferior OS in single-household poverty–exposed children compared with the unexposed group that did not reach statistical significance (HR = 1.98, 95% CI = 0.93 to 4.21, P = .08; Table 3). Schoenfeld residuals and testing interaction terms between time and predictors suggest no evidence of PH assumption violations.
Table 3.
Post hoc analysis: EFS and OS according to combined neighborhood and household poverty exposure adjusting for ethnicity, disease response, INSS stage, MYCN, and hospital clustering (N = 371)
| Child or sociodemographic characteristics | EFS |
OS |
||
|---|---|---|---|---|
| HR (95% CI) | P a | HR (95% CI) | P a | |
| Neighborhood or household | ||||
| Unexposed poverty | 1.00 (Ref) | 1.00 (Ref) | ||
| Single neighborhood poverty | 1.13 (0.53 to 2.40) | .76 | 0.47 (0.1 to 2.24) | .34 |
| Single household poverty | 1.88 (1.21 to 2.91) | .005 | 1.98 (0.93 to 4.21) | .08 |
| Dual poverty exposed | 2.21 (1.48 to 3.30) | <.001 | 3.70 (2.08 to 6.59) | <.001 |
| Ethnicity | ||||
| Non-Hispanic | 1.00 (Ref) | 1.00 (Ref) | ||
| Hispanic | 1.20 (0.78 to 1.85) | .41 | 1.76 (0.92 to 3.35) | .09 |
| End-induction disease response | ||||
| CR | 1.00 (Ref) | 1.00 (Ref) | ||
| VGPR | 1.59 (0.95 to 2.66) | .08 | 1.91 (0.998 to 3.66) | .05 |
| PR | 1.48 (0.74 to 2.93) | .27 | 2.28 (0.94 to 5.54) | .07 |
| INSS stage | ||||
| IIB/III/IVS | 1.00 (Ref) | 1.00 (Ref) | ||
| IV | 1.53 (0.84 to 2.77) | .16 | 1.53 (0.57 to 4.14) | .40 |
| Unknown | 2.2 (0.82 to 5.94) | .12 | 3.50 (0.75 to 16.32) | .11 |
| Tumor MYCN | ||||
| Not amplified | 1.00 (Ref) | 1.00 (Ref) | ||
| Amplified | 0.77 (0.54 to 1.11) | .16 | 1.12 (0.60 to 2.10) | .71 |
| Unknown | 0.96 (0.49 to 1.88) | .89 | 0.912 (0.35 to 2.35) | .85 |
P values were from Cox regression model and were 2-sided. CI = confidence interval; CR = complete response; EFS = event-free survival; HR = hazard ratio; INSS = International Neuroblastoma Staging System; OS = overall survival; PR = partial response; VGPR = very good partial response.
Sensitivity Analyses
Results of the associations between poverty exposures and disease outcomes remained consistent with the primary study analyses in all sensitivity analyses (Supplementary Tables 1–5 available online).
Discussion
Poverty is independently associated with EFS and OS in a paradigmatic population of children receiving clinical trial–delivered targeted immunotherapy for cancer. Children exposed to household poverty as measured by public insurance experienced a 90% increased risk of an event and a 179% increased risk of death at 2 years compared with unexposed children, and this difference in risk was not explained by disease or treatment response characteristics. Post hoc multivariable analyses demonstrated that dual household and neighborhood poverty exposure conferred a striking 270% increased risk of death at 2 years compared with no poverty exposure, with a corresponding 22% absolute difference in 2-year EFS and 26% absolute difference in 2-year OS. This magnitude of effect is similar to that observed with key therapeutic interventions over the past several decades.
Poverty-related health outcome disparities are well documented outside the context of cancer-directed targeted therapy (5, 36) and offer possible explanations for the inferior EFS observed in household poverty–exposed children. First, child poverty leads to negative health consequences (5, 37), which may increase treatment-related complications and subsequent delays or reductions in planned therapy. Alternatively, nonadherence to recommended therapy is a recognized risk factor for cancer relapse (38–40) as well as morbidity and mortality in other chronic diseases (41–45). Although a majority of trial therapy was delivered in the inpatient setting, nonadherence to oral isotretinoin may have contributed to the inferior EFS observed. Finally, poverty-associated stress might affect innate immune responses to immunotherapy (46–48). These hypotheses are being investigated in ongoing work.
We observed a substantially greater poverty-associated decrement in OS than in EFS. These data suggest that not only do poor children experience excess relapse following targeted immunotherapy, but their access to life-prolonging relapse therapy may also be inferior. Disparities in access to specialized therapies—including invasive cardiac procedures, stem cell transplant, and proton beam radiotherapy—are well documented (49–52). In neuroblastoma, the targeted radiopharmaceutical meta-iodo-benzylguanidine has a 35%-40% response rate in relapsed or refractory neuroblastoma (53) but is only available at specialized centers. It is possible that such salvage therapy is out of reach for less-resourced families given the out-of-pocket costs and work disruptions associated with travel as well as the numerous challenges in obtaining insurance coverage for salvage therapies administered in other states.
There are important limitations to our data. We observed poverty-associated outcome disparities in a highly selected cohort restricted to clinical trial–enrolled patients treated at PHIS institutions and may have underestimated the true magnitude of disparities by focusing on a population of “best actors.” ANBL0032 and ANBL0931 restricted enrollment to patients with at least partial disease control following initial therapy. Thus, our data do not reflect outcomes for trial-ineligible patients, such as those who experienced inadequate disease control or treatment-related toxicities early in therapy. Compared with the previously published PHIS HR NBL population (23), our analytic cohort included fewer Black patients (8% vs 13%) and more privately insured patients (52% vs 46%), potentially limiting our ability to detect independent effects of race and ethnicity previously associated with higher risk of late-occurring events (15), though not 2-year outcomes. We used public insurance as a proxy for household poverty due to lack of parent-reported household poverty data. Although most children qualify for Medicaid or CHIP based on household income (54), a minority qualify based on disability. We may have misclassified children with public insurance from wealthier homes or those who had private or other insurance but were nonetheless living in low-income homes. We used zip code median household income quartiles to identify neighborhood poverty, a measure limited by its sample-dependent nature as well as the socioeconomic heterogeneity inherent in a zip code’s large population (55). We lacked data on other social determinants of health, including language, literacy, education, and social supports that may mediate the observed disparities (56). Finally, our data are specific to the United States and may not be generalizable to other countries.
These limitations notwithstanding, our data identify striking outcome disparities in the context of targeted immunotherapy trials, suggesting a critical need for further investigation. Intervening on poverty as a risk factor for relapse and death requires identification of intervention targets—either modifiable poverty measures or mechanisms linking poverty and outcome amenable to care delivery interventions. These gaps are being investigated in ongoing studies that aim to identify mechanistic links using parent-reported poverty measures, including both income and household material hardship (food, housing, heat, and transportation insecurities) (5, 37, 57). Concurrently, evaluations of interventions directly targeting household material hardship are being conducted (58–61).
Poor children with HR NBL treated uniformly with targeted immunotherapy are at increased risk of relapse and death compared with their nonpoor counterparts. That poverty is independently associated with inferior survival in the context of targeted therapy even after adjustment for known biological variables is sobering. Few advances in medicine have garnered the enthusiasm of the medical community and generated as much hope for improving outcomes as the application of targeted therapies in cancer. Indeed, the use of targeted immunotherapy resulted in the single greatest improvement in survival for children with HR NBL in decades (19). Poverty-associated outcome disparities in this context highlight the stubborn reality that increased understanding of tumor and host biology and the development of rational therapeutics may be necessary, but not sufficient, to achieve the cures we desire. Our data identify new pathways for investigation and intervention in the clinical trial context—namely the consideration of social and environmental factors as outcome predictors. Transformative improvements in outcome are most likely to be achieved if we expand our conceptual model of discovery and intervention beyond biology to include social determinants of health outcomes.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health grant numbers K07CA211847 (KB), U10 CA180899 (AN through the Children’s Oncology Group Statistics and Data Center), and U10CA180886 (National Clinical Trials Network (NCTN) Operations Center Grant, which supported the conduct of Children’s Oncology Group trials ANBL0031 and ANBL0931). It was additionally supported by Alex’s Lemonade Stand Foundation (RA and RB) and the St. Baldrick’s Foundation (clinical trial support of ANBL0032 and ANBL0931).
Notes
Role of the funder: No funders were involved in the design or conduct of this study, nor were they involved in analysis or interpretation of data.
Disclosures: KB: No relationships to disclose; YL: No relationships to disclose; LW: No relationships to disclose; KG: No relationships to disclose; YH: No relationships to disclose; BF: Research Funding (Merck, Pfizer), Advisory Role (Astellas); AD: Ownership of Stock (Pfizer), Research Funding (Ignyta and Merck), Consulting (Merck); TR: No relationships to disclose; MH: No relationships to disclose; AN: Consulting or Advisory Role (Novartis); TH: Research Funding (Seattle Genetics) and Other Relationship (Seattle Genetics); RA: Honoraria (Sigma-Tau), Expert Testimony (Wiggin and Dana), Travel, Accommodations, Expenses (Sigma-Tau); RB: No relationships to disclose.
Role of the authors: YL: Data curation; Formal analysis; Writing—review and editing. LEW: Writing—review and editing. KDG: Data curation; Formal analysis; Writing—review and editing. Y-SH: Data curation; Formal analysis; Writing—review and editing. BTF: Writing—review and editing. AVD: Data curation; Writing—review and editing. TR: Data curation; Writing—review and editing. MH: Data curation; Writing—review and editing. AN: Data curation; Investigation; Writing—review and editing. TOH: Writing—review and editing. RA: Conceptualization; Data curation; Funding acquisition; Resources; Writing—review and editing. RB: Conceptualization; Methodology; Writing—review and editing.
Acknowledgments: We thank the patients, investigators and Children’s Oncology Group institutions that were essential to contributing the data used for analysis in this study.
Previous presentations: Presented in part as a poster at the American Society of Clinical Oncology (ASCO) 2019 Annual Meeting; Chicago; IL. Bona K et al. J Clin Oncol 37, 2019 (suppl; abstr 10034).
Data Availability
The merged data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Smith MA, Reaman GH.. Remaining challenges in childhood cancer and newer targeted therapeutics. Pediatr Clin North Am. 2015;62(1):301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Leary M, Krailo M, Anderson JR, et al. Progress in childhood cancer: 50 years of research collaboration, a report from the Children's Oncology Group. Semin Oncol. 2008;35(5):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins FS, Varmus H.. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schroeder SA. We can do better--improving the health of the American people. N Engl J Med. 2007;357(12):1221–1228. [DOI] [PubMed] [Google Scholar]
- 5.Council On Community. Poverty and child health in the United States. Pediatrics. 2016;137(4):e20160339. [DOI] [PubMed] [Google Scholar]
- 6. Chetty R, Stepner M, Abraham S, et al. The association between income and life expectancy in the United States, 2001-2014. JAMA. 2016;315(16):1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ginsburg GS, Phillips KA.. Precision medicine: from science to value. Health Aff (Millwood). 2018;37(5):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Annie E. Casey Foundation. KIDS COUNT Data Center. https://datacenter.kidscount.org/data/tables/43-children-in-poverty-100-percent-poverty? loc=1&loct=1#detailed/1/any/false/871,870,573,869,36,868,867,133,38,35/any/321,322. Accessed October 1, 2018.
- 9. Macartney S. Child poverty in the United States 2009 and 2010: selected race groups and Hispanic origin American community survey briefs. US Census Bureau. http://www.census.gov/prod/2011pubs/acsbr10-05.pdf. Issued November 2011. Accessed December 18, 2012.
- 10.American Cancer Society. Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 11. Kehm RD, Spector LG, Poynter JN, et al. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer. 2018;124(20):4090–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Austin MT, Nguyen H, Eberth JM, et al. Health disparities are important determinants of outcome for children with solid tumor malignancies. J Pediatr Surg. 2015;50(1):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedrich P, Itriago E, Rodriguez-Galindo C, et al. Racial and ethnic disparities in the incidence of pediatric extracranial embryonal tumors. J Natl Cancer Inst. 2017;109(10):djx050. 10.1093/jnci/djx050. [DOI] [PubMed] [Google Scholar]
- 14. Gupta S, Wilejto M, Pole JD, et al. Low socioeconomic status is associated with worse survival in children with cancer: a systematic review. PLoS One. 2014;9(2):e89482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2011;29(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolfson J, Sun CL, Kang T, et al. Impact of treatment site in adolescents and young adults with central nervous system tumors. J Natl Cancer Inst. 2014;106(8):dju166. doi:10.1093/jnci/dju166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27(2):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ladenstein R, Potschger U, Pearson ADJ, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18(4):500–514. [DOI] [PubMed] [Google Scholar]
- 19. Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Irwin MS, Park JR.. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am 2015;62(1):225–256. [DOI] [PubMed] [Google Scholar]
- 21.Children's Oncology Group, About Us. https://www.childrensoncologygroup.org/index.php/research-collaborations. Accessed November 1, 2018. [Google Scholar]
- 22. Ozkaynak MF, Gilman AL, London WB, et al. A comprehensive safety trial of chimeric antibody 14.18 with GM-CSF, IL-2, and isotretinoin in high-risk neuroblastoma patients following myeloablative therapy: children's oncology group study ANBL0931. Front Immunol. 2018;9:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desai AV, Kavcic M, Huang YS, et al. Establishing a high-risk neuroblastoma cohort using the Pediatric Health Information System Database. Pediatr Blood Cancer. 2014;61(6):1129–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bagatell R, Fisher BT, Seif AE, Huang Y-S, et al. Evaluation of resources used during care of children with high-risk neuroblastoma (HR NBL) via merging of cooperative group trial data and administrative data. J Clin Oncol. 2014;32(15_suppl):10069–10069. [Google Scholar]
- 25.U.S. Census 2010 American Community Survey. East Brunswick, NJ: Data, Geolytics, Inc; 2010. http://geolytics.com/USCensus, Census2010ACS, Categories.asp
- 26. Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50(2):398–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser Family Foundation. Where are states today? Medicaid and CHIP eligibility levels for children and non-disabled adults. Washington, DC; 2013. https://kaiserfamilyfoundation.files.wordpress.com/2013/04/7993-03.pdf. Accessed November 1, 2018.
- 28. Rudowitz R, Artiga S, Arguello R. Children's health coverage: Medicaid, CHIP and the ACA. The Kaiser Commission on Medicaid and the Uninsured; 2014. https://kaiserfamilyfoundation.files.wordpress.com/2014/03/8570-children_s-health-coverage-medicaid-chip-and-the-aca1.pdf. Accessed March 10, 2019.
- 29. Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–1477. [DOI] [PubMed] [Google Scholar]
- 30. Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6(12):1874–1881. [DOI] [PubMed] [Google Scholar]
- 31. Shimada H, Ambros IM, Dehner LP, et al. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86(2):349–363. [PubMed] [Google Scholar]
- 32. Lee EW, Wei LJ, Amato DA.. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Goel JAP, ed. Survival Analysis: State of the Art. Dordrecht, The Netherlands: Kluwer Academic; 1992:237–247. [Google Scholar]
- 33. Hosmer DW, Lemeshow S.. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. [DOI] [PubMed] [Google Scholar]
- 34. Hallqvist J, Ahlbom A, Diderichsen F, et al. How to evaluate interaction between causes: a review of practices in cardiovascular epidemiology. J Intern Med. 1996;239(5):377–382. [DOI] [PubMed] [Google Scholar]
- 35.2010 HHS Poverty Guidelines. Poverty thresholds for 2010 by size of family and number of related children under 18 years 2010. The poverty guidelines updated periodically in the Federal Register by the U.S. Department of Health and Human Services under the authority of 42 U.S.C. https://aspe.hhs.gov/2010-hhs-poverty-guidelines. Accessed January 3, 2012.
- 36. Bayer R, Galea S.. Public health in the precision-medicine era. N Engl J Med. 2015;373(6):499–501. [DOI] [PubMed] [Google Scholar]
- 37. Frank DA, Casey PH, Black MM, et al. Cumulative hardship and wellness of low-income, young children: multisite surveillance study. Pediatrics. 2010;125(5):e1115-e–1123.. [DOI] [PubMed] [Google Scholar]
- 38. Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol. 2012;30(17):2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34(21):2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306–311. [DOI] [PubMed] [Google Scholar]
- 41. Serper M, et al. Medication misuse, non-adherence, and clinical outcomes among liver transplant recipients. Liver Transpl. 2015;21(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aylward BS, Rausch JR, Modi AC.. An examination of 1-year adherence and persistence rates to antiepileptic medication in children with newly diagnosed epilepsy. J Pediatr Psychol. 2015;40(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim S, Shin DW, Yun JM, et al. Medication adherence and the risk of cardiovascular mortality and hospitalization among patients with newly prescribed antihypertensive medications. Hypertension. 2016;67(3):506–512. [DOI] [PubMed] [Google Scholar]
- 44. Molnar MZ, Gosmanova EO, Sumida K, et al. Predialysis cardiovascular disease medication adherence and mortality after transition to dialysis. Am J Kidney Dis. 2016;68(4):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duramad P, Tager IB, Holland NT.. Cytokines and other immunological biomarkers in children's environmental health studies. Toxicol Lett. 2007;172(1-2):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnson SB, Riley AW, Granger DA, et al. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. MacGillivray DM, Kollmann TR.. The role of environmental factors in modulating immune responses in early life. Front Immunol. 2014;5:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mitchell JM, Conklin EA.. Factors affecting receipt of expensive cancer treatments and mortality: evidence from stem cell transplantation for leukemia and lymphoma. Health Serv Res. 2015;50(1):197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Odei B, Boothe D, Frandsen J, et al. The role of radiation in all stages of nodular lymphocytic predominant Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2017;17(12):819–824. [DOI] [PubMed] [Google Scholar]
- 51. Quatromoni J, Jones R.. Inequalities in socio-economic status and invasive procedures for coronary heart disease: a comparison between the USA and the UK. Int J Clin Pract. 2008;62(12):1910–1919. [DOI] [PubMed] [Google Scholar]
- 52. Shen CJ, Hu C, Ladra MM, et al. Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer. 2017;123(20):4048–4056. [DOI] [PubMed] [Google Scholar]
- 53. Taggart DR, Han MM, Quach A, et al. Comparison of iodine-123 metaiodobenzylguanidine (MIBG) scan and [18F]fluorodeoxyglucose positron emission tomography to evaluate response after iodine-131 MIBG therapy for relapsed neuroblastoma. J Clin Oncol. 2009;27(32):5343–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Musumeci MB, Foutz J. Medicaid’s role for children with special health care needs: a look at eligibility, services, and spending. Henry J. Kaiser Family Foundation; 2018. https://www.kff.org/medicaid/issue-brief/medicaids-role-for-children-with-special-health-care-needs-a-look-at-eligibility-services-and-spending/. Accessed June 3, 2019.
- 55. Krieger N, Waterman P, Chen JT, et al. Zip code caveat: bias due to spatiotemporal mismatches between Zip codes and US census-defined geographic areas--the Public Health Disparities Geocoding Project. Am J Public Health. 2002;92(7):1100–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294(22):2879–2888. [DOI] [PubMed] [Google Scholar]
- 57. Bona K, London WB, Guo D, et al. Trajectory of material hardship and income poverty in families of children undergoing chemotherapy: a prospective cohort study. Pediatr Blood Cancer. 2016;63(1):105–111. [DOI] [PubMed] [Google Scholar]
- 58. Berkowitz SA, Hulberg AC, Standish S, et al. Addressing unmet basic resource needs as part of chronic cardiometabolic disease management. JAMA Intern Med. 2017;177(2):244–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garg A, Toy S, Tripodis Y, et al. Addressing social determinants of health at well child care visits: a cluster RCT. Pediatrics. 2015;135(2):e296–e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sege R, Preer G, Morton SJ, et al. Medical-legal strategies to improve infant health care: a randomized trial. Pediatrics. 2015;136(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ClinicalTrials.Gov. Pilot Feasibility of the Pediatric Cancer Resource Equity (PEDICARE) Intervention. Bethesda, MD: National Library of Medicine; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The merged data underlying this article will be shared on reasonable request to the corresponding author.