Abstract
As the poultry industry recedes from the use of antibiotic growth promoters, the need to evaluate the efficacy of possible alternatives and the delivery method that maximizes their effectiveness arises. This study aimed at expounding knowledge on the effect of the delivery method of a probiotic product (Bacillus subtilis fermentation extract) on performance and gut parameters in broiler chickens. A total of 450 fertile eggs sourced from Cobb 500 broiler breeders were randomly allotted to 3 groups: in ovo probiotic (n = 66), in ovo saline (n = 66), and noninjection (n = 200) and incubated for 21 d. On day 18.5 of incubation, 200 μL of either probiotic (10 × 106 cfu) or saline was injected into the amnion. At hatch, chicks were reallotted to 6 new treatment groups: in ovo probiotic, in ovo saline, in-feed antibiotics, in-water probiotic, in-feed probiotics, and control (corn-wheat-soybean diet) in 6 replicate cages and raised for 28 d. Of all hatch parameters evaluated, only percentage pipped eggs was found significant (P < 0.05) with the noninjection group having higher percentage pipped eggs than the other groups. Treatments did not affect the incidence of necrotic enteritis on day 28 (P > 0.05). Irrespective of the delivery method, the probiotic treatments had no significant effect on growth performance. The ileum villus width of the in ovo probiotic treatment was 18% higher than the in ovo saline group (P = 0.05) but not statistically higher than other groups. The jejunum villus height was 23% higher (P = 0.000) in the in ovo probiotic group than in the control group. There was no effect of treatment on total cecal short-chain fatty acid concentration and cecal gut microbiota composition and diversity (P > 0.05), although few unique bacteria differential abundance were recorded per treatment. Conclusively, although probiotic treatments (irrespective of the delivery route) did not affect growth performance, in ovo delivery of the probiotic product enhanced intestinal morphology, without compromising hatch performance and gut homeostasis.
Key words: in ovo, probiotics, delivery routes, performance, broiler chickens
Introduction
Antibiotic growth promoters (AGPs) have been used subtherapeutically to improve bird performance and health in the poultry industry for almost 8 decades (Fallis, 2013; Gadde et al., 2017). This trend is now receiving strong criticism as a result of concerns of antimicrobial resistance, antibiotic residues, and food safety hazards (Muaz et al., 2018; Wales and Davies, 2019). In the light of the foregoing, the poultry industry is thus faced with the challenge of developing urgent alternatives to AGP, potent against economically important poultry diseases such as necrotic enteritis (NE), colibacillosis, salmonellosis, and so forth.
Probiotics are one of such alternatives being experimented. Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (FAO/WHO, 2001). These organisms help improve bird performance by modulating a favorable gut microflora in the host (Mountzouris et al., 2009; Cao et al., 2013; Zhang et al., 2014), improving feed conversion and digestive efficiency (Jin et al., 2000; Afsharmanesh and Sadaghi, 2014; Zhang and Kim, 2014), and producing antimicrobial substances (Fayol-Messaoudi et al., 2005; Corr et al., 2007) and several other benefits. Probiotics can achieve these positive effects because they successfully colonize the gastrointestinal tract of the host (Lan et al., 2003). Examples of probiotic bacteria in current use in broiler chicken production include Enterococcus, Lactobacillus, Lactococcus, Bifidobacterium, and Bacillus species (Patterson and Burkholder, 2003).
Several probiotic delivery routes exist, but conventional in-feed supplementation is the most commonly used. Other possible delivery routes include in-water supplementation, spray method, litter delivery, and more recently, in ovo delivery. The efficacy of probiotics has been inconsistent in the literature because of several limitations that characterize these delivery routes (Applegate et al., 2010; Ajuwon, 2016). During heat treatment, in-feed probiotics could be subjected to potential heat inactivation and instability (Ducatelle et al., 2014). In-water probiotic delivery will depend on the precision of chick watering devices, while also posing potential water quality risks. In ovo technology which involves the direct inoculation of bioactive substances to the developing embryo to elicit superior lifelong effects (Oladokun and Adewole, 2020), offers the opportunity to address some of these identified limitations. In addition, with in ovo technology, lesser quantities of bioactive substance are reported to be needed than in conventional delivery routes (Bednarczyk et al., 2016; Tavaniello et al., 2018). Furthermore, in ovo technology has been proffered as a solution to the perinatal nutritional stresses associated with a shift from yolk feeding to exogenous feeding, long hatchery window (24–36 h), and time-consuming hatchery activities that chicks often encounter (Noy and Uni, 2010). This technology has also been shown to be useful to stimulate the colonization of the embryonic gut with beneficial microbiota, among other potential advantages (reviewed by Oladokun and Adewole, 2020).
This study, therefore, sought to evaluate the effect of the delivery route (in-water vs. in-feed vs. in ovo) of a probiotic product (Bacillus subtilis fermentation extract) on growth performance, intestinal morphology, cecal short-chain fatty acid (SCFA) concentration, and cecal microbiota in broiler chickens.
Material and methods
The experiment was carried out at the hatchery facility of the Agricultural Campus of Dalhousie University and the broiler rearing facility of the Atlantic Poultry Research Center, Dalhousie Faculty of Agriculture. All experimental procedures were approved by the Animal Care and Use Committee of Dalhousie University, in accordance with guidelines of the Canadian Council on Animal Care (CCAC, 2009).
Eggs and Incubation
A total of 450 fertile eggs (Cobb 500) with average weight 64 ± 0.2 g (mean ± SE) were sourced from a commercial breeder flock in Nova Scotia and incubated in a ChickMaster single-stage incubator (ChickMaster G09, Cresskill, NJ) under standard conditions (37.5°C and 55% RH) for 21 d, in the aforementioned hatchery facility. Eggs were arranged in 6 replicate trays inside the incubator, each tray containing 75 eggs. The eggs were candled on day 17, and infertile eggs were disposed of. On day 18.5 of incubation, eggs were randomly allotted to 3 experimental groups: the in ovo probiotic group (66 eggs) injected with 200 μL of B. subtilis fermentation extract (each egg recieved 10 × 106 cfu of the bacterium/200 μL saline diluent), in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl) (66 eggs), and the control (CTRL) group—noninjection (200 eggs). Eggs were placed in a single incubator in such a way that all treatment groups were evenly distributed across all the trays. The probiotic solution was prepared for 100 eggs by dissolving 0.1 g of the B. subtilis product into 20 mL of 0.9% saline. The B. subtilis product was obtained from a commercial source (Probiotech International, St. Hyacinth, QC, Canada) at a concentration of 10 × 109 cfu/g.
Injection Procedure
Eggs were injected according to the procedure described by Tako et al. (2004) with some modifications. The amnion was the site of injection. Eggs were disinfected by swabbing the blunt ends with cotton balls soaked in 70% ethanol, a small hole was then punched into the shell at the center of the air-cell (the blunt end) using an 18-gauge needle. The injected bioactive substance was delivered to the amnion of each egg using a self-refilling injector (Socorex ultra 1810.2.05005, Ecublens, Switzerland) equipped with a 22-gauge needle at a 45-degree angle. After in ovo injection, eggs were sealed with sterile paraffin. However, in ovo delivery of bioactive substances could be manual or automated, with the automated method capable of inoculating as much as 35,000–70,000 eggs per hour (depending on the type) (Schijns et al., 2014); the manual method was used in the present study only to confirm the efficacy of our inoculated bioactive substance under experimental conditions. In any case, the in ovo technology has been reported to offer several advantages over conventional delivery routes (recently reviewed by Oladokun and Adewole (2020)).
Bird Rearing Conditions and Diets
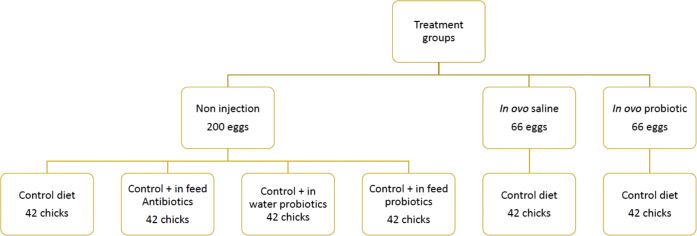
On day 21, unhatched eggs were counted and opened to check for the cause of embryo death. As presented in Figure 1, hatchlings were weighed and randomly reallotted to 6 new treatment groups. Birds in the initial noninjection group were randomly allocated into 4 groups (there were 42 birds per group) consisting of (1) chicks fed a basal corn-soybean meal-wheat–based diet (CTRL); (2) chicks fed CTRL + 0.05% bacitracin methylene disalicylate (in-feed antibiotics); (3) chicks fed CTRL + B. subtilis fermentation extract at a concentration of 0·025 g/L of drinking water (in-water probiotic containing 2.5 × 108 cfu of B. subtilis/L of drinking water); and (4) chicks fed 0.005% B. subtilis fermentation extract (in-feed probiotic containing 5 × 108 cfu/kg of feed). The initial in ovo saline and in ovo probiotic groups were placed on the control diet to form treatments 5 (in ovo saline treatment with 42 birds) and 6 (in ovo probiotic treatment with 42 birds), respectively, and raised in the previously mentioned broiler-rearing facility. Birds were allocated to 36 cages with 6 replicate cages of each treatment, comprising 7 birds per cage. Each cage was 0.93 m × 2.14 m in dimension. Broiler chickens were reared for 28 d under uniform controlled environmental conditions in line with recommendations of Cobb Broiler Management Guide (Cobb-Vantress, 2020). Room temperature was set at 31°C on day 0 and gradually reduced to 23°C on day 28, and relative humidity ranged between 45 and 55%. Dietary treatments, ingredients, and nutritional composition are presented in Table 1. The probiotic diet was prepared by premixing 0.005% B. subtilis fermentation extract with preground corn and adding the premix with the formulation thereafter. Diets were fed as mash during the starter phase and fed as pellets during the grower phase. All cages were equipped with water troughs, which were being monitored and replenished daily. Diets met or exceeded the NRC (1994) nutritional requirements for broiler chickens. Birds were provided feed and water ad libitum during a starter phase (0–14 d) and grower phase (15–28 d).
Figure 1.
Treatment structure in the hatchery and broiler barn.
Table 1.
Ingredients and composition of experimental diets1 (as-fed basis, percentage, unless otherwise stated).
| Item | Starter |
Grower |
||||
|---|---|---|---|---|---|---|
| Control diet | Antibiotic diet | Probiotic diet | Control diet | Antibiotic diet | Probiotic diet | |
| Corn | 51.08 | 50.97 | 51.08 | 44.32 | 44.22 | 44.31 |
| Soybean meal-46.5 | 41.44 | 41.45 | 41.44 | 36.48 | 36.49 | 36.48 |
| Animal/vegetable fat | 2.93 | 2.97 | 2.93 | 4.59 | 4.63 | 4.60 |
| Wheat | - | - | - | 10.00 | 10.00 | 10.00 |
| Limestone | 1.80 | 1.80 | 1.80 | 1.65 | 1.65 | 1.65 |
| Dicalcium phosphate | 1.24 | 1.24 | 1.24 | 1.06 | 1.06 | 1.06 |
| DL-Methionine premix2 | 0.59 | 0.59 | 0.59 | 0.53 | 0.53 | 0.53 |
| Lysine HCl | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 |
| Vitamin–mineral premix3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.41 | 0.41 | 0.41 | 0.37 | 0.37 | 0.37 |
| Pellet binding agent4 | - | - | - | 0.50 | 0.50 | 0.50 |
| BMD 110G5 | - | 0.05 | - | - | 0.05 | - |
| Bacillus subtilis | - | - | 0.005 | - | - | 0.005 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated composition | ||||||
| ME (kcal/kg) | 3,000 | 3,000 | 3,000 | 3,100 | 3,100 | 3,100 |
| CP | 23.00 | 23.00 | 23.00 | 21.50 | 21.50 | 21.50 |
| Calcium | 0.96 | 0.96 | 0.96 | 0.87 | 0.87 | 0.87 |
| Available phosphorus | 0.48 | 0.48 | 0.48 | 0.44 | 0.44 | 0.44 |
| Sodium | 0.19 | 0.19 | 0.19 | 0.18 | 0.18 | 0.18 |
| Digestible lysine | 1.28 | 1.28 | 1.28 | 1.16 | 1.16 | 1.16 |
| Digestible methionine + cysteine | 0.95 | 0.95 | 0.95 | 0.87 | 0.87 | 0.87 |
| Analyzed composition | ||||||
| DM | 89.23 | 90.94 | 90.85 | 87.10 | 88.01 | 86.83 |
| CP | 22.77 | 22.40 | 24.16 | 21.72 | 21.63 | 21.87 |
| Crude fat | 5.06 | 5.23 | 5.17 | 6.77 | 6.56 | 6.35 |
| Calcium | 1.13 | 1.31 | 1.04 | 0.89 | 0.95 | 0.89 |
| Total phosphorus | 0.65 | 0.65 | 0.62 | 0.55 | 0.58 | 0.57 |
| Sodium | 0.19 | 0.20 | 0.33 | 0.15 | 0.17 | 0.21 |
Basal diet (NC); antibiotic diet containing NC + 0.05% bacitracin methylene disalicylate (BMD); probiotic diet containing NC + 0.005% Bacillus subtilis.
Supplied/kg premix: DL-Methionine, 0.5 kg; wheat middlings, 0.5 kg.
Starter vitamin–mineral premix contained the following per kg of diet: 9,750 IU vitamin A; 2,000 IU vitamin D3; 25 IU vitamin E; 2.97-mg vitamin K; 7.6-mg riboflavin; 13.5-mg Dl Ca-pantothenate; 0.012-mg vitamin B12; 29.7-mg niacin; 1.0-mg folic acid, 801-mg choline; 0.3-mg biotin; 4.9-mg pyridoxine; 2.9-mg thiamine; 70.2-mg manganese; 80.0-mg zinc; 25-mg copper; 0.15-mg selenium; 50-mg ethoxyquin; 1543-mg wheat middlings; 500-mg ground limestone. Grower vitamin–mineral premix contained the following per kg of diet: 9,750 IU vitamin A; 2,000 IU vitamin D3; 25 IU vitamin E; 2.97-mg vitamin K; 7.6-mg riboflavin; 13.5-mg Dl Ca-pantothenate; 0.012-mg vitamin B12; 29.7-mg niacin; 1.0-mg folic acid, 801-mg choline; 0.3-mg biotin; 4.9-mg pyridoxine; 2.9-mg thiamine; 70.2-mg manganese; 80.0-mg zinc; 25-mg copper; 0.15-mg selenium; 50-mg ethoxyquin; 1,543-mg wheat middlings; 500-mg ground limestone.
Pel-stik: Uniscope, Inc., Johnstown, CO.
Bacitracin methylene disalicylate (providing 55 mg/kg mixed feed); Alpharma, Inc., Fort Lee, NJ.
Hatch Parameters and Chick Quality
Hatched chicks were counted and weighed individually. Hatchability was calculated as the percentage of hatched chicks to incubated eggs, per replicate. The stage of egg embryonic death was classified as pipped (death occurring after the chick had made the piping hole) and late dead (chicks fully formed, but dead without pipping), and the ensuing counts were expressed as a percentage of fertile eggs and recorded. Hatched chick BW/initial egg weight ratio was also determined and recorded. Chick navel quality was evaluated by adapting the scoring method by Reijrink et al. (2009). Navel quality was scored 1—when the navel was completely closed and clean, scored 2—when the navel was discolored (i.e., when the navel color differs from the chick's skin color) with a maximum 2-mm opening, and scored 3—when the navel was discolored and with more than a 2-mm opening.
Growth Performance Parameters and Sampling
Growth performance parameters—feed intake, and average BW were measured on a pen basis at 7, 14, 21, and 28 d of age. The ADFI, ADG, and feed conversion ratio (FCR) were subsequently calculated from obtained data. The FCR was calculated as the amount of feed consumed per unit BW gain (BWG). Mortality was recorded daily and used to correct for feed consumption. On day 28, 2 birds per pen (12 replicate birds per treatment group) were randomly selected and euthanized by electrical stunning and exsanguination. After euthanasia of birds, the intestinal segments the jejunum (1.5-cm length midway between the point of entry of the bile ducts and Meckel's diverticulum) and ileum (1.5-cm length midway between Meckel's diverticulum and the ileocecal junction) were excised and fixed in neutral buffered formalin (10%) for further histomorphological processing (Awad et al., 2009). The digesta samples from each pair of the cecum of the euthanized bird were mixed and subsampled, a portion was stored in biofreeze kits (Alimetric Diagnostics, Espoo, Finland) for SCFA concentration measurement and the other held in RNase and DNase free tubes, immediately frozen in liquid nitrogen, and later stored at −80°C for subsequent gut microbiota analysis.
Incidence of NE was evaluated on small intestinal segments of euthanized birds using the lesion scoring guide by Shojadoost et al. (2012), with slight modifications. This scoring guide was as follows: NE score 0—no gross lesions present; NE score 1—no obvious ulcers in the mucosa, but the entire mucosal surface is covered with a layer of loosely adherent fibrin; NE score 2—excavated ulcer of the mucosa with acute, bright red hemorrhage within the ulcer bed and scant crusting of fibrin around the periphery; NE score 3—excavated ulcer of the mucosa with dark green-black pigment within the ulcer bed and scant crusting of fibrin over the surface; NE score 4—excavated ulcers of the mucosa, with periphery covered by thick, tightly adherent layers of fibrin, necrotic tissue, and inflammatory cells; NE score 5—mucosa covered by large, confluent plaques of fibrin, necrotic tissue, and inflammatory cells to the point of extending over broad regions of the intestinal mucosa.
Intestinal Morphology Measurement
Fixed jejunum and ileum tissue samples were further subjected to microtomy processing. This involved slicing into 3 sections and dehydration by increasing alcohol concentration from 0 to 100%. Tissue slices were infused with xylene and fixed in paraffin wax. Tissue section (0.5 μm thick) was cut with a microtome (Leica RM 2145, Leica Microsystems, Wetzlar, Germany) and mounted on a glass slide, followed by staining (Drury and Wallington, 1980) and morphometric measurements. Morphometric measurements included the villus height (from the base of the intestinal mucosa to the tip of the villus excluding the intestinal crypt), villus width (halfway between the base and the tip), crypt depth (from the base upward to the region of transition between the crypt and villi), and total mucosa thickness (villus height + crypt depth) (Ozdogan et al., 2014). Ten measurements of each component per slide were carried out using an image processing and analysis system (ImageJ, WI).
SCFA Concentration and Total Eubacteria Quantification
Cecal samples were submitted to Alimetrics Diagnostics AD19024-1(Espoo, Finland) for both SCFA concentration and total Eubacteria quantification. Acids quantified were acetic, propionic, butyric, valeric, isobutyric, 2-methylbutyric, isovaleric, and lactic acids in 6 replicates per treatment.
Gut Microbiota Analysis
Genomic DNA was extracted from 70–90 mg of cecal digesta samples obtained from 12 replicate birds per treatment group using Quick-DNA Fecal and Soil Microbe 96 Kit (CAT: D6011, Zymo Research, Orange County, CA) with slight modification to manufacturer's protocols. BashingBead buffer (400 μL), beta-mercaptoethanol, and genomic lysis buffer (0.5% v/v) were added to cecal samples in a 96-well plate bead beater, followed by centrifugation (10,000 × g, 2 × 5 min) to ensure cell lysis. BashingBead Lysis Rack (0.1 and 0.5 mm) was also centrifuged (4,700 × g, 5 min), after which 250-μL supernatant was transferred to a 96-well plate. Genomic lysis buffer (750 μL) was further added to the filtrate in the 96-well plate, followed by mixing and centrifugation (4,700 × g for 5 min). 500 μL from each well was transferred to the wells of a Silicon-A Plate, followed by centrifugation (4,700 × g for 5 min). Flow through from the collection plate was discarded. 200-μL DNA prewash buffer and 500-μL genomic DNA wash buffer were added to the wells of the Silicon-A plate; this was followed by concurrent centrifugation (3,000 × g for 5 min). 150-μL prep solution was added to the wells of a prepared Silicon-A HRC plate mounted on an elution plate; this was then incubated at room temperature for 5 min and centrifuged (3,500 × g for 5 min). Finally, 100 μL of the DNA elution buffer was added directly to the matrices on the Silicon-A Plate, followed by centrifugation (3,500 × g for 7 min) to elute the DNA extract. The efficiency of the DNA extraction protocol was confirmed by visual assessment on a 1% agarose gel. Extracted DNA concentration and purity were determined by spectrophotometry (Nanodrop ND1000; Thermo Scientific). Universal 16S primers, 515 F (5′- GTGCCAGCMGCCGCGGTAA) and 806R (5′GACTACHVGGGTWTCTAAT) targeting the V4 variable region of the 16S rRNA gene were used to prepare amplicon libraries and sequencing (paired ends 250 bp) was carried out on an Illumina MiSeq system at McGill University and Genome Quebec Innovation Center (Montreal, Canada). Amplicon analysis was carried out following Dada2 analysis methods (Callahan et al., 2016) at the Canadian Centre for Computational Genomics (C3G, Montreal, Canada) (Bourgey et al., 2019).
Statistical Analysis
Data were analyzed as a completely randomized design. The normality of all data sets was ascertained by testing residuals by the Anderson-Darling test in Minitab statistical package (v.18.1). Data sets found to be normal including, performance data, navel score, SCFA concentrations, and gut morphology were subjected to one-way ANOVA in the same statistical package with experimental treatments as factor and the aforementioned data sets as variables. For hatchability parameters, hatching trays were the experimental units, and the pen was the experimental unit for growth performance parameters.
Data sets on total Eubacteria and relative operational taxonomic unit (OTU) taxa abundance (except for phylum Fimicutes and genus Ruminiclostridium) were natural log-transformed, whereas pipped eggs (%) were cube root transformed. After transformations, the data were equally subjected to ANOVA procedures in the same statistical package, with appropriately back-transformed data presented. Data sets found to be non-normal including late dead eggs (%), hatched chick BW/initial egg weight, NE scores, and mortality were subjected to a nonparametric Kruskal-Wallis test in the same statistical package, after failed transformation. Differences between significant means were tested using Tukey's honest significant difference test in the same statistical package. Analyzed data are presented as means ± SEM and probability values. Values were considered statistically different at P ≤ 0.05.
Gut Microbiota Statistical and Bioinformatics Analysis
Statistical analysis and visual exploration of bioinformatics data were carried out with the MicrobiomeAnalyst tool (Dhariwal et al., 2017). Data were filtered to a minimum count 2 and 10% prevalence in samples. Alpha diversity analysis was calculated based on the Shannon index. Significant differences in alpha diversity among different groups were calculated based on ANOVA, where a significant difference level was set at P < 0.05. Beta diversity was calculated based on the Bray-Curtis index, and statistical comparisons among groups were performed with permutational multivariate ANOVA. To determine differentially abundant taxa at different groups, MetagenomeSeq (Paulson et al., 2013) that uses the zero-inflated Gaussian fit model was used with an adjusted P value cutoff at 0.05.
Results
Hatch Performance
In this study, among all hatchability parameters, only percentage pipped eggs was found to be significantly (P < 0.05) different among the treatments (Table 2). Noninjected eggs recorded 98.75 and 57.84% more pipped eggs (%) than in ovo saline and in ovo probiotic treatments, respectively (Table 2). No difference (P > 0.05) among treatments was found for late dead eggs (%), hatchability, the average chick weight, and hatched chick BW to initial egg weight. Nonetheless, in ovo probiotic treatment had numerically higher average chick weight and hatched chick BW to initial egg weight relative to other treatments. In addition, chick navel quality was not significantly different across treatments, although in ovo probiotic treatment had the highest percentage of birds with a navel score 1 (27.96%), and in ovo saline treatment had the highest percentage of birds with a navel score range 2–3 (77.63%) (Supplementary Table 1).
Table 2.
Effect of in ovo delivery of Bacillus subtilis fermentation extract on hatch performance in broiler chickens.
| Hatch parameters | Treatments1 |
SEM | P value2 | ||
|---|---|---|---|---|---|
| Noninjection control | In ovo saline | In ovo probiotic | |||
| Pipped eggs (%) | 6.38a | 0.08b | 2.69a,b | 1.26 | 0.043 |
| Late dead eggs (%) | 1.39 | 0.00 | 0.00 | 4.423 | 0.584 |
| Hatchability (%) | 87.02 | 90.91 | 90.91 | 1.51 | 0.505 |
| Average chick weight (g) | 53.02 | 52.93 | 54.27 | 0.50 | 0.510 |
| Chick BW/initial egg weight (%) | 82.15 | 83.64 | 84.31 | 2.333 | 0.196 |
Treatment groups include noninjected eggs (control), in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and in ovo probiotic group injected with 200 μL of Bacillus subtilis fermentation extract (10 × 106 cfu); n = 6 replicate trays.
Means and median not sharing the same superscript differ significantly by Tukey's test (P ≤ 0.05).
Measure of variation about the median represented by the interquartile range.
Growth Performance
Results on evaluated growth parameters were not statistically significant between treatments (Table 3). During the starter phase (0–14 d), antibiotic treatment had the highest ADG and the lowest FCR compared with other treatments. In ovo probiotic treatment recorded the lowest ADFI and FCR of all treatments, during the grower phase (15–28 d).
Table 3.
Effect of Bacillus subtilis fermentation extract delivery route on growth performance in broiler chickens raised for 28 d.
| Growth performance parameters | Treatments1 |
SEM | P value2 | |||||
|---|---|---|---|---|---|---|---|---|
| Control | In-feed antibiotics | In-water probiotic | In-feed probiotic | In ovo saline | In ovo probiotic | |||
| Starter phase (0–14 d) | ||||||||
| ADFI (g/bird) | 25.4 | 23.3 | 23.2 | 23.6 | 26.2 | 27.7 | 0.84 | 0.582 |
| ADG (g/bird) | 16.9 | 20.9 | 18.0 | 18.3 | 19.0 | 16.0 | 0.51 | 0.086 |
| FCR3 | 1.53 | 1.15 | 1.33 | 1.36 | 1.40 | 1.75 | 0.07 | 0.254 |
| Mortality (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.004 | 0.818 |
| Grower phase (15–28 d) | ||||||||
| ADFI (g/bird) | 83.0 | 91.8 | 85.1 | 90.2 | 91.6 | 80.5 | 1.59 | 0.168 |
| ADG (g/bird) | 62.4 | 57.6 | 61.4 | 67.9 | 63.4 | 64.6 | 1.51 | 0.529 |
| FCR | 1.35 | 1.52 | 1.39 | 1.33 | 1.46 | 1.26 | 0.05 | 0.254 |
| Mortality (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.004 | 0.191 |
| Total trial period (0–28 d) | ||||||||
| ADFI (g/bird) | 56.3 | 59.7 | 56.9 | 58.7 | 58.9 | 55.1 | 0.96 | 0.739 |
| ADG (g/bird) | 49.9 | 51.6 | 50.4 | 54.1 | 52.6 | 50.2 | 0.84 | 0.709 |
| FCR | 1.13 | 1.09 | 1.13 | 1.09 | 1.12 | 1.10 | 0.02 | 0.935 |
| Mortality (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.004 | 0.750 |
Treatment groups include control (CTRL), in-feed antibiotic treatment containing CTRL + 0.05% bacitracin methylene disalicylate (BMD), CTRL diet + in-water probiotic (containing 0.025 g/L of Bacillus subtilis fermentation extract), in-feed probiotic containing CTRL + 0.005% Bacillus subtilis, in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and in ovo probiotics group injected with 200 μL of Bacillus subtilis fermentation extract (10 × 106 cfu) in n = 6 replicate pens of 7 birds each.
Significance was set at P ≤ 0.05.
FCR = Feed Conversion ratio.
Measure of variation about the median represented by the interquartile range.
Treatments had no significant effect on the intestinal NE lesion score in broiler chickens in this study (Supplementary Table 2). Based on the used NE scoring guide, no bird had an NE score of 4. All treatments, except in-water probiotics and in ovo probiotics, had 50% of birds with an NE score of 2. The CTRL treatment had the least number of birds with an NE score of 0 (25%); 50% of birds in other treatments had an NE score of 0.
Gut Morphology
The probiotic delivery route significantly (P ≤ 0.05) influenced the jejunum and ileum morphology of broiler chickens in this study (Table 4). In the jejunum, the villus height of the in ovo probiotic treatment was significantly higher (P < 0.001) than in-water probiotic, antibiotic, and CTRL treatments. The in ovo probiotic villus height was 23% higher (P < 0.001) than the CTRL treatment. The in ovo probiotic villus width in the ileum was also 18% wider (P < 0.001) than the in-feed treatment. The total mucosa thickness in the ileum of in ovo probiotic treatment was also 21% higher (P < 0.001) than the CTRL treatment. This was significantly different (P < 0.001) from in-water, antibiotic, and CTRL treatment. In the ileum, the villus height of the in ovo probiotic treatment was found the highest; this was 18% higher (P ≤ 0.05) than the in ovo saline treatment but not statistically different from other treatments.
Table 4.
Effect of Bacillus subtilis fermentation extract delivery route on the ileum and jejunum morphology in broiler chickens raised for 28 d.
| Gut morphology parameters (measured in mm) | Treatments1 |
SEM | P value2 | |||||
|---|---|---|---|---|---|---|---|---|
| Control | In-feed antibiotics | In-water probiotic | In-feed probiotic | In ovo saline | In ovo probiotic | |||
| Jejunum | ||||||||
| Villus height | 0.960c | 1.008b,c | 1.087b,c | 1.156a,b | 1.154a,b | 1.253a | 0.02 | 0.000 |
| Villus width | 0.220a | 0.221a | 0.223a | 0.178b | 0.192a,b | 0.218a | 0.00 | 0.001 |
| Crypt depth | 0.140 | 0.127 | 0.130 | 0.154 | 0.132 | 0.147 | 0.00 | 0.070 |
| Villus height:crypt depth | 8.115 | 9.681 | 9.365 | 9.967 | 9.843 | 11.023 | 0.32 | 0.203 |
| Total mucosa thickness | 1.100d | 1.135c,d | 1.217b,c,d | 1.310a,b | 1.286a,b,c | 1.399a | 0.02 | 0.000 |
| Ileum | ||||||||
| Villus height | 0.560 | 0.533 | 0.555 | 0.593 | 0.596 | 0.574 | 0.01 | 0.080 |
| Villus width | 0.196a,b | 0.205a,b | 0.193a,b | 0.199a,b | 0.174b | 0.213a | 0.00 | 0.052 |
| Crypt depth | 0.141 | 0.132 | 0.136 | 0.145 | 0.132 | 0.130 | 0.00 | 0.268 |
| Villus height:crypt depth | 4.320 | 4.270 | 4.379 | 4.461 | 4.799 | 4.731 | 0.09 | 0.352 |
| Total mucosa thickness | 0.701 | 0.665 | 0.692 | 0.738 | 0.728 | 0.704 | 0.01 | 0.087 |
Treatment groups include control (CTRL), in-feed antibiotics treatment containing CTRL + 0.05% bacitracin methylene disalicylate (BMD), CTRL diet + in-water probiotic (containing 0.025 g/L of Bacillus subtilis fermentation extract), in-feed probiotic containing CTRL + 0.005% Bacillus subtilis, in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and in ovo probiotic group injected with 200 μL of Bacillus subtilis fermentation extract (10 × 106 cfu) in n = 10 observations per treatment.
Means not sharing the same superscript differ significantly by Tukey's test (P ≤ 0.05).
Cecal SCFA Concentration
No significance (P > 0.05) was found for cecal SCFA concentration (micromolar) in this study (Table 5). Nonetheless, in ovo probiotic treatment had the numerically highest concentration of total SCFA and volatile fatty acids (VFA) compared with other treatments.
Table 5.
Effect of Bacillus subtilis fermentation extract delivery route on cecal short-chain fatty acid concentrations in broiler chickens raised for 28 d.
| Short-chain fatty acid concentration (μM) | Treatments1 |
SEM | P value2 | |||||
|---|---|---|---|---|---|---|---|---|
| Control | In-feed antibiotics | In-water probiotic | In-feed probiotic | In ovo saline | In ovo probiotic | |||
| Acetic acid | 47.4 | 55.0 | 43.5 | 48.7 | 49.5 | 52.7 | 2.44 | 0.832 |
| Propionic acid | 1.98 | 1.69 | 3.16 | 2.57 | 2.10 | 2.73 | 0.24 | 0.535 |
| Butyric acid | 13.7 | 12.4 | 11.5 | 7.15 | 10.1 | 13.4 | 0.82 | 0.184 |
| Valeric acid | 0.28 | 0.16 | 0.44 | 0.12 | 0.19 | 0.30 | 0.04 | 0.107 |
| Lactic acid | 2.05 | 3.23 | 4.93 | 2.52 | 2.13 | 4.66 | 0.63 | 0.664 |
| Branched-chain fatty acids | 0.40 | 0.07 | 0.30 | 0.33 | 0.16 | 0.22 | 0.04 | 0.321 |
| Volatile fatty acids | 63.8 | 69.4 | 58.9 | 58.9 | 62.0 | 69.4 | 3.05 | 0.865 |
| Total short-chain fatty acids | 65.8 | 72.6 | 63.8 | 61.4 | 64.2 | 74.1 | 2.92 | 0.790 |
Treatment groups include control (CTRL), in-feed antibiotics treatment containing CTRL + 0.05% bacitracin methylene disalicylate (BMD), CTRL diet + in-water probiotic (containing 0.025 g/L of Bacillus subtilis fermentation extract), in-feed probiotic containing CTRL + 0.005% Bacillus subtilis, in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and in ovo probiotic group injected with 200 μL of Bacillus subtilis fermentation extract (10 × 106 cfu) in n = 6 replicates per treatment.
Significance was set at P ≤ 0.05.
Cecal Microbiota
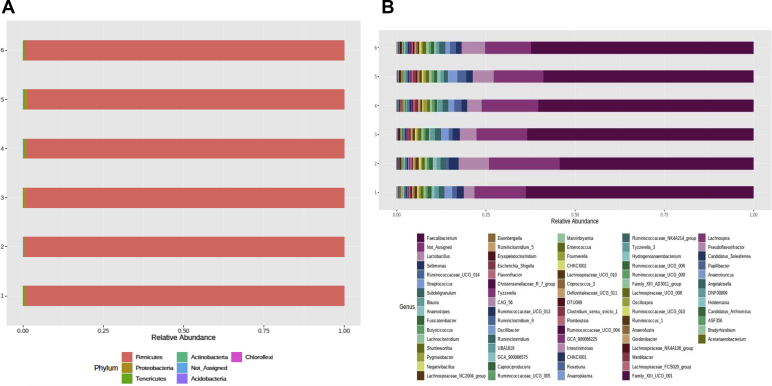
A total of 5,286,777 quality read counts were obtained, at an average of 73,427 counts per sample after quality filtering and demultiplexing. Information on the sequencing quality profile is presented in Supplementary Figure 1. A total of 805 OTU were identified at the 97% similarity level, belonging to a total of 5 phyla, 6 classes, 8 orders, 17 families, 57 genera, and 12 species. The relative abundance (percentage abundance) of different phyla and genera across treatment groups is presented in Figure 2. Bacteria composition at the family taxa is shown in Supplementary Figure 2. Treatment effects on major phyla and genera are presented in Table 6. Taxonomic analysis by ANOVA showed no difference for total Eubacteria counts across treatments (Table 6). Firmicutes represented >98% of identified phyla. No significant difference was recorded for all major phyla within treatments (Table 6). At the genus level, Ruminococcaceae_UCG-014 in the in-feed probiotic treatment tended (P = 0.07) to be 38% higher than the antibiotic treatment.
Figure 2.
Bacteria composition at the (A) phylum and (B) genus levels of broiler chickens with treatments groups 1—control (CTRL), 2—in-feed antibiotic treatment containing CTRL + 0.05% bacitracin methylene disalicylate, 3—CTRL diet + in-water probiotic (containing 0.025 g/L of Bacillus subtilis fermentation extract), 4—in-feed probiotic containing CTRL + 0.005% B. subtilis, 5—in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and 6—in ovo probiotic group injected with 200 μL of B. subtilis fermentation extract (10 × 106 cfu). The cecal content was collected from 28-day-old chickens. DNA was extracted from the cecal content, and relative abundances are shown as determined by Illumina sequencing and visualized with the web-based tool MicrobiomeAnalyst.
Table 6.
Effect of Bacillus subtilis fermentation extract delivery route on relative OTU abundance (specific phyla, genera, and total Eubacteria) in broiler chickens raised for 28 d.
| Item | Treatments1 |
SEM | P value2 | |||||
|---|---|---|---|---|---|---|---|---|
| Control | In-feed antibiotics | In-water probiotic | In-feed probiotic | In ovo saline | In ovo probiotic | |||
| Phylum (OTU) | ||||||||
| Firmicutes | 70,853 | 75,313 | 74,532 | 70,603 | 74,231 | 71,784 | 1,363 | 0.875 |
| Actinobacteria | 58.9 | 92.5 | 63.0 | 77.5 | 77.5 | 68.5 | 1.10 | 0.170 |
| Proteobacteria | 55.3 | 53.4 | 89.1 | 175.9 | 73.8 | 36.4 | 1.20 | 0.118 |
| Tenericutes | 105 | 34.3 | 74.4 | 72.5 | 67.4 | 96.7 | 1.20 | 0.295 |
| Genus (OTU) | ||||||||
| Ruminococcaceae_UCG-014 | 1,230 | 1,099 | 1,111 | 1,783 | 1,694 | 1,601 | 1.10 | 0.0733 |
| Ruminiclostridium_5 | 498 | 616 | 421 | 611 | 553 | 637 | 34.0 | 0.425 |
| Lactobacillus | 1,272 | 3,744 | 2,436 | 1,659 | 1,893 | 2,649 | 1.20 | 0.389 |
| Faecalibacterium | 45,528 | 41,070 | 47,618 | 42,992 | 44,195 | 44,954 | 1,501 | 0.878 |
| Subdoligranulum | 524 | 813 | 851 | 708 | 582 | 923 | 1.10 | 0.531 |
| Total Eubacteria (absolute copy number) | 1.71E+12 | 1.37E+12 | 1.96E+12 | 2.01E+12 | 1.73E+12 | 2.66E+12 | 1.10E+00 | 0.483 |
Treatment groups include control (CTRL), in-feed antibiotics treatment containing CTRL + 0.05% bacitracin methylene disalicylate (BMD), CTRL diet + in-water probiotics (containing 0.025 g/L of Bacillus subtilis fermentation extract), in-feed probiotics containing CTRL + 0.005% Bacillus subtilis, in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and in ovo probiotics group injected with 200 μL of Bacillus subtilis fermentation extract (10 × 106 cfu) in n = 12 observations per treatment, with the exception of total Eubacteria where n = 10 observations per treatment.
Significance was set at P ≤ 0.05.
Marginal significance at P < 0.07.
The differential abundance at different taxonomic levels by MetagenomeSeq (P < 0.05) is presented in Table 7. Order Rhizobiales and family Xanthobacteraceae were differentially significant (P < 0.001) in the CTRL treatment. Phylum Actinobacteria, class Coriobacteriia, order Coriobacteriales, and family Eggerthellaceae were all differentially significant (P < 0.001) in the in-feed antibiotic treatment. Family Streptococcaceae, genus Streptococcus, and an unknown species DNF0089 were significantly differentiated (P < 0.001) in the in-water probiotic treatment.
Table 7.
Effect of Bacillus subtilis fermentation extract delivery route on differentially abundant bacterial taxa between treatment groups.
| Taxa (log-transformed counts) | Treatments1 |
P value2 | False discovery rate (FDR) | |||||
|---|---|---|---|---|---|---|---|---|
| Control | In-feed antibiotics | In -water probiotics | In-feed probiotics | In ovo saline | In ovo probiotics | |||
| Phylum | Actinobacteria | 0.002 | 0.013 | |||||
| Class | Coriobacteriia | 0.001 | 0.014 | |||||
| Order | Coriobacteriales | 0.002 | 0.009 | |||||
| Rhizobiales | 0.002 | 0.009 | ||||||
| Family | Streptococcaceae | 0.000 | 0.000 | |||||
| Eggerthellaceae | 0.002 | 0.002 | ||||||
| Xanthobacteraceae | 0.007 | 0.045 | ||||||
| Genus | Streptococcus | 0.000 | 0.000 | |||||
| DNF0089 | 0.000 | 0.000 | ||||||
Treatment groups include control (CTRL), in-feed antibiotic treatment containing CTRL + 0.05% bacitracin methylene disalicylate (BMD), CTRL diet + in-water probiotics (containing 0.025 g/L of Bacillus subtilis fermentation extract), in-feed probiotics containing CTRL + 0.005% Bacillus subtilis, in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and in ovo probiotics group injected with 200 μL of Bacillus subtilis fermentation extract (10 × 106 cfu) in n = 12 observations per treatment.
Significance was set at P ≤ 0.05.
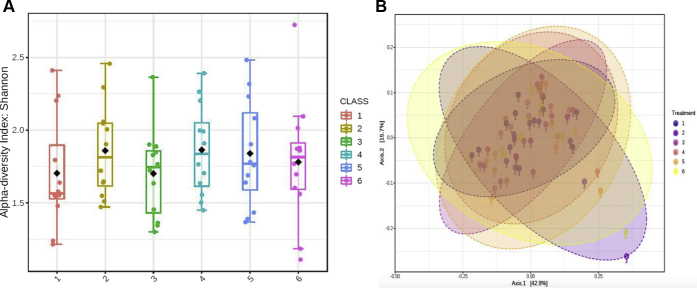
Analyzing the alpha diversity (specie richness) of the cecal content expressed as the number of observed OTU by the Shannon index showed similarity between treatments (Figure 3A). Numerically, the highest average Shannon index was in the antibiotic treatment 1.81 ± 0.09 (mean ± SE), whereas the lowest was 1.65 ± 0.11 in the CTRL treatment.
Figure 3.
(A) Alpha diversity index showed no significant difference among treatments (ANOVA, P = 0.7619). The cecal content was collected from 28-day-old broiler chickens. The diamond shape represents the mean value in each group, and the whiskers indicate the minimum/maximum value. (B) Beta diversity measure of the effect of the Bacillus subtilis fermentation extract delivery route on cecal bacteria communities of broiler chickens raised for 28 d. Treatment groups include the following: 1—control (CTRL), 2—in-feed antibiotic treatment containing CTRL + 0.05% bacitracin methylene disalicylate, 3—CTRL diet + in-water probiotic (containing 0.025 g/L of B. subtilis fermentation extract), 4—in-feed probiotic containing CTRL + 0.005% B. subtilis, 5—in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and 6—in ovo probiotic group injected with 200 μL of B. subtilis fermentation extract (10 × 106 cfu) (PERMANOVA; P value < 0.128, F value = 1.3787, R-squared: 0.09457). Abbreviation: PERMANOVA, permutational multivariate ANOVA.
Beta diversity of cecal bacteria communities of the treatment groups are illustrated in the principal coordinates analysis plot based on the Bray-Curtis dissimilarity index (Figure 3B). Permutational multivariate ANOVA showed no significant differences in microbial community structure between treatments (R-squared 0.09, P > 0.05).
Discussion
In this study, the in ovo delivery of probiotics has been validated in broiler chickens, by comparing it with in-water and in-feed delivery routes. The probiotic used in this study was a B. subtilis fermentation extract. Bacillus subtilis is a spore-forming bacterium, with high resistance to temperature and harsh conditions (AFRC, 1989). These qualities make it a suitable probiotic candidate in poultry production.
Hatchability remains one of the most significant indicators of successful in ovo injection. In this study, we have successfully validated the in ovo delivery of B. subtilis fermentation extract through the amnion on day 18.5 of incubation, with no negative effect on embryo viability. All in ovo–injected eggs in our study recorded 91% hatchability and was not significantly different from the noninjected eggs that had 87% hatchability. These hatchability values are well in line with what is obtainable in commercial hatcheries. The patent of Uni and Ferket (2003) has previously proved that the inoculation of enteric modulators between day 17 and 19 of incubation through the amnion had no negative effect on hatchability because the injected substance is orally swallowed by the embryo in the amnion, after which it is made available to enteric tissues and other gut microbiota cells (Torshizi et al., 2010). Our results on hatchability are in conformation with the findings of Edens et al. (1997), Pender et al. (2016), Majidi-Mosleh et al. (2017), Khaligh et al. (2018), Beck et al. (2019), Skjøt-Rasmussen et al. (2019), and Alizadeh et al. (2020), which all reported no negative effect of the in ovo delivery of probiotics on hatchability. Contrarily, Tripplet et al. (2018) and El-Moneim et al. (2019) have both recently reported a negative effect of in ovo delivery of probiotics on hatchability. The disparity in the literature on the effect of in ovo delivery of probiotics on hatchability is attributable to several factors including the in ovo injection procedure, site of injection (air cell vs. amnion), inoculated dose, hatchery hygiene, and differing probiotic strain (Johnston et al., 1997; Bednarczyk et al., 2011; De Oliveira et al., 2014; Beck et al., 2019). In addition, noninjected eggs in this study recorded the highest percentage of pipped eggs (6.38), whereas in ovo probiotic treatment was intermediate (2.69) and the in ovo saline treatment had the least (0.08) percentage of pipped eggs. Previous researchers have reported no effect of probiotic inoculation on percentage pipped eggs (De Oliveira et al., 2014; Pender et al., 2016; Triplett et al., 2018). Factors affecting percentage pipped eggs include deficient hatching conditions (insufficient humidity and poor ventilation) (Willemsen et al., 2010), poor hatchery hygiene, and embryonic malposition within a particular region of the incubator, in response to gravity (Byerly and Olsen, 1937). Furthermore, treatments had no effect on navel quality in this study (Supplementary Table 1). Chick navel quality is often influenced by the rate of nutrient metabolism and yolk absorption during the late incubation period (Hamburger and Hamilton, 1951). Except for percentage pipped eggs, other hatchability parameters evaluated in this study, including, percentage late dead eggs, hatchability, average chick weight, hatched chick BW to initial egg weight, and chick navel quality elicited no significant treatment effect. These results suggest that the in ovo delivery of B. subtilis fermentation extract does not negatively impair embryo viability and hatch performance.
Furthermore, the use of probiotics, especially in the diet, as enteric gut modulators that ultimately elicit superior bird performance, continues to gain momentum in the poultry industry. No significant effect of treatment on all posthatch growth performance was recorded in this study. In conformation with our results, Majidi-Mosleh et al. (2017) have previously recorded no significant effect of amnion-delivered B. subtilis on performance parameters in a 42-d trial with broiler chickens, suggesting that probiotic supplementation in the late embryonic stage might not be sufficient enough to elicit superior performance effects. Subsequent in-feed supplementation of probiotics inoculated chicks to stimulate significant posthatch performance is an area that warrants further investigation. Knap et al. (2011) also reported no significant effect of orally delivered B. subtilis DSM17299 on the ADG and FCR in their study. Similarly, Santoso et al. (1999) found no significant effect of in-feed–delivered B. subtilis on the feed intake, BWG, and FCR. Olnood et al. (2015) also reported no significant effect of L. joshnsonii delivered in-feed or in-water, sprayed on litter, or orally gavaged on the feed intake, BWG, and FCR in broiler chickens. Chen et al. (2009) supplemented broiler feed with B. subtilis (106 cfu/g) and also recorded no significant effect on growth performance. On the contrary, other studies have reported positive effect of B. subtillis delivery on growth performance. Sen et al. (2012) reported a linear increase in the feed intake, BWG, and FCR with increasing in-feed delivery of B. subtilis LS 1-2. Jeong et al. (2014) also confirmed that the inclusion of B. subtilis spores significantly enhanced the ADG both in the starter and overall experimental periods, in their study. These inconsistencies in B. subtilis performance effect across several routes could be due to a variety of factors including viability, dosage, environmental stressors (Huang et al., 2004; Mountzouris et al., 2007), and sample size. Irrespective of the delivery route, probiotic treatments in this study (despite being nonsignificant) had at least 0.5% higher ADG than the CTRL treatment, over the 28-d trial. This insignificant performance effect might portend considerable economical gains, especially for large-scale commercial broiler producers. Indeed, more studies on the effect of probiotic delivery routes on broiler performance, especially in the commercial context, are needed.
In addition, no treatment effect on mortality and incidence of NE was found in this study. Several predisposing factors are reported to contribute to the growth and proliferation of Clostridium perfringens, the etiological agent of NE in broiler intestine. These include management conditions (including stress, alteration in feeding regimes, hatchery hygiene) and diet composition (especially barley- or wheat-containing diets as offered in the present study (Craven, 2000; Craven et al., 2001; Annet et al., 2002). Similarly, antibiotic withdrawal has also been associated with an increased incidence of NE (Wade and Keyburn, 2015). Aside from horizontal transmission (via contaminated feed and litter) of C. perfringens spores, vertical transmission from parent to progeny is also possible (Williams, 2002; Thanissery et al., 2010). These reasons make our assessment of NE in birds unchallenged with NE relevant, although we acknowledge that the bacteria distribution might not be uniform across treatments. Most experimented alternatives to AGP including organic acids, essential oils, synbiotics, prebiotics, and probiotics have all been reported to exhibit varying levels of pathogen exclusion activities, which often results in reduced incidence of NE (Finucane et al., 1999; Kaldhusdal, 2000). These activities are either direct or indirect via immunity boosting (Ao et al., 2012). With the CTRL treatment having the least number of birds with a desirable NE score of 0, it is plausible that all supplementations conferred birds with some sort of protection against NE.
Posthatch changes are more evident in the chicken's intestinal segments than other parts (Prabakar et al., 2016). In this study, beneficial effects of in ovo–delivered probiotics were observed both in the ileum and jejunum. The villus height, villus width, and total mucosa thickness were all numerically and, in most cases, significantly higher in the in ovo probiotic treatment. Intestinal morphological parameters, including the villus height, villus width, crypt depth, and villus length–to–crypt depth ratio are good indicators of gut health and the functional capacity of the intestine (Fasina and Olowo, 2013). The increased villus height, villus height–to–crypt depth ratio, and a decreased crypt depth are correlated with an increased epithelial turnover and increased digestive and absorptive functions (Fan et al., 1997; Xu et al., 2003; Munyaka et al., 2012; Shang et al., 2015). In agreement with our results, Sen et al. (2012) showed that the supplementation of B. subtilis LS 1-2 in broiler diets resulted in an increased villus height and villus height:crypt depth ratio in the duodenum and ileum at day 35. Li et al. (2018) also demonstrated that dietary cosupplementation of AGP and B. subtilis improved the intestinal morphology during the first 3 wk in pullets. A recent meta-analysis of 25 controlled trials also concluded that the supplementation of direct-fed microbials was associated with an increased villus height of the small intestine in broiler chickens (Heak et al., 2017). Improved digestive capacity, as evidenced by improved intestinal morphometric characteristics, would be expected to translate into improved feed conversion efficiency and ultimately significant improvement in growth performance. The smaller sample size used in the present study could have contributed to the lack of significant improvement in growth performance. Future studies on this type of product should use a larger sample size. In addition, the present study was conducted under a well-controlled management system with no sanitary challenge to disturb the intestinal health of the chickens.
The SCFA are the by-products of microbial fermentation in the cecum. They play important roles in bird's energy metabolism, intestinal functionality, and gut pathogen reduction (Van Der Wielen et al., 2000; Meimandipour et al., 2010). In the present study, no effect of treatment was recorded for the concentrations of total SCFA and individual fatty acids, although the in ovo probiotic treatment consistently recorded the highest concentration of total SCFA, VFA, and propionic acid concentrations. Meimandipour et al. (2010) have shown that the supplementation of Lactobacillus salivarius ssp. salicinius JCM 1230 and Lactobacillus agilis JCM 1048 can significantly increase propionate and butyrate concentrations using a 24-h simulated chicken cecum. Fujiwara et al. (2009) have also reported that 2% B. subtilis var. natto–fermented soybean supplementation tended to increase the total VFA and acetic acid concentration in chicks, especially when fed from day old, suggesting a linear age effect of B. subtilis supplementation on SCFA concentration. Because SCFA concentrations are associated with gut microbiota colonization, it is important to note that both Lactobacillus and Bacillus spp. differ in their capacities to colonize the gut. Although Lactobacillus and Enterococcus spp. are considered to be colonizing species, Bacillus spp. are considered free flowing and do not colonize the gut (Huyghebaert et al., 2011). To the best of our knowledge, this is the first study to report on the effect of in ovo–delivered B. subtilis on SCFA concentrations in broiler chickens, and more studies are thus needed to fully understand these effects.
The chicken's gut is inhabited by numerous species of microorganisms, whose continuous interaction influences host performance and well-being. This is particularly true for the cecum, the posterior gut section with the highest bacteria diversity (Oakley et al., 2014). In this study, we observe that broiler chicken cecum microbiota is mainly composed of >95% members of phyla Firmicutes, Proteobacteria, Tenericutes, and Actinobacteria, irrespective of treatments (Figure 1A). This is to an extent consistent with results reported for breeder fecal microbiota (Trudeau et al., 2020), probiotic-supplemented chicken ceca (Wang et al., 2017), and Bacillus direct-fed microbial-supplemented broiler chicken ceca (Hernandez-Patlan et al., 2019). Although our results might be consistent with the relative percentage of microbes reported in these studies, it does not necessarily conform with the order of abundance reported. In addition, we did not record the presence of bacteria in the phylum Bacteroidetes. The resolution of the V4 region of the 16S rRNA gene sequenced in this study could have influenced this outcome. García-López et al. (2020) have recently shown that both the V3 and V3V4 hypervariable regions capture a broader spectrum of microbiota diversity than the V4 region. Although the V3 region offers the advantage of faster sequencing time and lower cost, the V3V4 region offers a higher taxonomic resolution at an increased cost (García-López et al., 2020). Increased abundance of Firmicutes has been associated with increased nutrient absorption and energy harvest from diets (Jumpertz et al., 2011). Phylum Proteobacteria on the other hand is made up of gram-negative bacteria implicated in some metabolic diseases, including gut–brain alterations and intestinal inflammation in rats (Maharshak et al., 2013; Vaughn et al., 2017). Tenericutes are also implicated in mycoplasma infection. In contrast, Actinobacterial species are reported to combat bacterial diseases and at the same time help convert feedstuff into fermentable microbial biomass (Anandan et al., 2016). It is important to note that the relative abundance of phylum Proteobacteria and Tenericutes ranged from 0.2 to 1.02% of the total OTU identified, justifying the homeostatic gut environment, as evidenced by noncompromised bird performance and health, across treatments, observed in our study. Order Rhizobiales and family Xanthobacteraceae were differentially significant in the CTRL treatment as compared with other treatments. Both bacteria are rarely found in animal species and have been reported in host fed nitrogen-deficient feedstuff (Stoll et al., 2007; Ikeda-Ohtsubo et al., 2018). This observation is surprising as our basal diet met or exceeded NRC (1994) CP requirement. Phylum Actinobacteria, class Coriobacteriia, order Coriobacteriales, and family Eggerthellaceae were all differentially significant in the in-feed antibiotic treatment as compared with other treatments. The functional roles of these bacterial communities include lipid metabolism and cholesterol metabolism (Martínez et al., 2013). They have also been linked to the pathologies of periodontitis, bacteremia, and other zoonotic diseases, especially Coriobacteriaceae and Eggerthella (Clavel et al., 2014; Pandit et al., 2018). This further emphasizes the cost-benefit effects of antibiotic use in poultry production. In addition, family Streptococcaceae, genus Streptococcus, and an unknown species DNF0089 were significantly differentiated in the in-water probiotic treatment; although Streptococcus has been associated with infections in poultry (Sekizaki et al., 2008), they are also capable of reducing gut pathogen load through competitive exclusion (Roto et al., 2015). However, more information on the specific strain of Streptococcus is needed, as the 2 main Streptococcus strains have been reported to have different functions (Fåk and Bäckhed, 2012). We also recorded no significant difference in bacterial alpha diversity among treatments (Figure 3A). Thibodeau et al. (2015) demonstrated that only extreme events that modify the number of ecological niches in different bacterial species can alter the alpha diversity. However, the ability of B. subtilis to enhance bacterial species richness has been reported (Li et al., 2016; Oh et al., 2017). Similarly, we recorded no significant effect of treatment on beta diversity in this study (Figure 3B). This suggests phylogenetic similarities between treatments. Except for treatment effect, which is nutrition, other possible factors or conditions shared by the birds could have influenced beta diversity. These shared factors include the local gastrointestinal condition, gut pH, and chick-rearing environment (Cisek and Binek, 2014; Oakley et al., 2014; Choi et al., 2015). Taken together, it is obvious that our probiotic treatment, irrespective of the delivery routes, did not inhibit microbiota-mediated homeostasis.
Conclusion
This study has successfully established the procedure for the in ovo delivery of B. subtilis in broiler chickens, recording 91% hatchability rate. Although, B. subtilis treatment (irrespective of the delivery route) had no significant effect on growth performance, in ovo delivery of the probiotic product enhanced the intestinal morphology, without compromising hatch and gut homeostasis.
Acknowledgments
The authors are grateful to Probiotech International for supplying the Bacillus subtilis fermentation extract, to Jing Lu for helping with injection and animal trial, and to Rojman Khomayezi for conducting the in ovo injection pretrials. Appreciation also goes to Sarah Macpherson and Krista Budgell for animal care and Jamie Fraser for diet preparation. The Canadian Center for Computational Genomics (C3G) is acknowledged for support with bioinformatic analysis. The Canadian Center for Computational Genomics (C3G) is a Genomics Technology Platform (GTP) supported by the Canadian Government through Genome Canada.
This project was funded by the Canadian Agricultural Partnership–Pan Atlantic program of Agriculture and Agri-Food Canada with Industry supports from chicken farmers of Nova Scotia and Atlantic Poultry Research Institute.
Disclosures
The authors declare no conflict of interest(s).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.10.063.
Supplementary Data
Supplementary Figure 1.
The mean quality score per treatment for forward and reverse reads. Treatment groups that include 1—control (CTRL), 2—in-feed antibiotic treatment containing CTRL + 0.05% bacitracin methylene disalicylate, 3—CTRL diet + in-water probiotic containing 0.025 g/L of Bacillus subtilis fermentation extract, 4—in-feed probiotic containing CTRL + 0.005% B. subtilis, 5—in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and 6—in ovo probiotic group injected with 200 μL of B. subtilis fermentation extract (10 × 106 cfu) are presented in different colors. Treatments were in 12 replicates (72 samples) (Bourgey et al., 2019).
Supplementary Figure 2.
Bacteria composition at the family taxa of broiler chickens with treatments groups: 1—control (CTRL), 2—in-feed antibiotic treatment containing CTRL + 0.05% bacitracin methylene disalicylate, 3—CTRL diet + in-water probiotic (containing 0.025 g/L of Bacillus subtilis fermentation extract), 4—in-feed probiotic containing CTRL + 0.005% B. subtilis, 5—in ovo saline group injected with 200 μL of physiological saline (0.9% NaCl), and 6—in ovo probiotic group injected with 200 μL of B. subtilis fermentation extract (10 × 106 cfu). The cecal content was collected from 28-day-old chickens. DNA was extracted from the cecal content, and relative abundances are shown as determined by Illumina sequencing and visualized with the web-based tool MicrobiomeAnalyst.
References
- AFRC, R. F Probiotics in man and animals. J. Appl. Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- Afsharmanesh M., Sadaghi B. Effects of dietary alternatives (probiotics, green tea powder, and Kombucha tea) as antimicrobial growth promoters on growth, ileal nutrient digestibility, blood parameters, and immune response of broiler chickens. Comp. Clin. Path. 2014;23:717–724. [Google Scholar]
- Ajuwon K.M. Toward a better understanding of mechanisms of probiotics and prebiotics action in poultry species. J. Appl. Poult. Res. 2016;25:277–283. [Google Scholar]
- Alizadeh M., Shojadoost B., Astill J., Taha-Abdelaziz K., Karimi S.H., Bavananthasivam J., Kulkarni R.R., Sharif S. Effects of in ovo inoculation of multi-strain lactobacilli on cytokine gene expression and Antibody-mediated immune responses in chickens. Front. Vet. Sci. 2020;7:105. doi: 10.3389/fvets.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandan R., Dharumadurai D., Manogaran G.P. An introduction to actinobacteria. In: Dhanasekaran D., Jiang Y., editors. Actinobacteria-Basics and Biotechnological Applications. Tech Publisher; London, UK: 2016. [Google Scholar]
- Annett C.B., Viste J.R., Chirino-Trejo M., Classen H.L., Middleton D.M., Simko E. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002;31:598–601. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- Ao Z., Kocher A., Choct M. Effects of dietary additives and early feeding on performance, gut development and immune status of broiler chickens challenged with clostridium perfringens. Asian-Australas J. Anim. Sci. 2012;25:541–551. doi: 10.5713/ajas.2011.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate T.J., Klose V., Steiner T., Ganner A., Schatzmayr G. Probiotics and phytogenics for poultry: Myth or reality? J. Appl. Poult. Res. 2010;19:194–210. [Google Scholar]
- Awad W.A., Ghareeb K., Abdel-Raheem S., Böhm J. Effects of dietary inclusion of probiotics and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–55. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Beck C.N., McDaniel C.D., Wamsley K.G.S., Kiess A.S. The potential for inoculating Lactobacillus animalis and Enterococcus faecium alone or in combination using commercial in ovo technology without negatively impacting hatch and post-hatch performance. Poult. Sci. 2019;98:7050–7062. doi: 10.3382/ps/pez441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk M., Stadnicka K., Kozłowska I., Abiuso C., Tavaniello S., Dankowiakowska A., Sławińska A., Maiorano G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal. 2016;10:1271–1279. doi: 10.1017/S1751731116000173. [DOI] [PubMed] [Google Scholar]
- Bednarczyk M., Urbanowski M., Gulewicz P., Kasperczyk K., Maiorano G., Szwaczkowski T. Field and in vitro study on prebiotic effect of raffinose family oligosaccharides in chickens. Bull Vet. Inst. Pulawy. 2011;55:465–469. [Google Scholar]
- Bourgey M., Dali R., Eveleigh R., Chen K.C., Letourneau L., Fillon J., Michaud M., Caron M., Sandoval J., Lefebvre F., Leveque G. GenPipes: an open-source framework for distributed and scalable genomic analyses. Gigascience. 2019;8:giz037. doi: 10.1093/gigascience/giz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly T.C., Olsen M.W. Egg turning, pipping position and malpositions. Poult. Sci. 1937;16:371–373. [Google Scholar]
- Callahan B.J., Mcmurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. dada2: high-resolution sample inference from illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . 2009. Guide to the care and use of experimental animals. Accessed Nov. 2020. https://www.ccac.ca/en/CCAC_Programs/Guidelines_Policies/GUIDES/ENGLISH/toc_v1.htm. [Google Scholar]
- Cao G.T., Zeng X.F., Chen A.G., Zhou L., Zhang L., Xiao Y.P., Yang C.M. Effects of a probiotics, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013;92:2949–2955. doi: 10.3382/ps.2013-03366. [DOI] [PubMed] [Google Scholar]
- Chen K.L., Kho W.L., You S.H., Yeh R.H., Tang S.W., Hsieh C.W. Effects of Bacillus subtilis var. natto and Saccharomyces cerevisiae mixed fermented feed on the enhanced growth performance of broilers. Poult. Sci. 2009;88:309–315. doi: 10.3382/ps.2008-00224. [DOI] [PubMed] [Google Scholar]
- Choi K.Y., Lee T.K., Sul W.J. Metagenomic analysis of chicken gut microbiota for improving metabolism and health of chickens - a review. Asian-Australas. J. Anim. Sci. 2015;28:1217–1225. doi: 10.5713/ajas.15.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek A.A., Binek M. Chicken intestinal microbiota function with a special emphasis on the role of probiotics bacteria. Pol. J. Vet. Sci. 2014;17:385–394. doi: 10.2478/pjvs-2014-0057. [DOI] [PubMed] [Google Scholar]
- Clavel T., Lepage P., Charrier C. The Family Coriobacteriaceae. In: Rosenberg E., DeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. The Prokaryotes. Springer; Berlin, Heidelberg, Germany: 2014. pp. 201–238. [Google Scholar]
- Cobb-Vantress Cobb. 2020. https://www.cobb-vantress.com/
- Corr S.C., Li Y., Riedel C.U., O’Toole P.W., Hill C., Gahan C.G.M. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven S.E. Colonization of the intestinal tract by Clostridium perfringens and fecal shedding in diet-stressed and unstressed broiler chickens. Poult. Sci. 2000;79:843–849. doi: 10.1093/ps/79.6.843. [DOI] [PubMed] [Google Scholar]
- Craven S.E., Cox N.A., Stern, Mauldin J.M. Prevalence of Clostridium perfringens in commercial broiler hatcheries. Avian Dis. 2001;45:1050–1053. [PubMed] [Google Scholar]
- De Oliveira J.E., Van Der Hoeven-Hangoor E., Van De Linde I.B., Montijn R.C., Van Der Vossen J.M.B.M. In ovo inoculation of chicken embryos with probiotics bacteria and its effect on posthatch Salmonella susceptibility. Poult. Sci. 2014;93:818–829. doi: 10.3382/ps.2013-03409. [DOI] [PubMed] [Google Scholar]
- Dhariwal A., Chong J., Habib S., King I.L., Agellon L.B., Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury R., Wallington E. Carleton’s Histological Techniques. 5th ed. Oxford University Press; New York, NY: 1980. pp. 41–54. [Google Scholar]
- Ducatelle R., Eeckhaut V., Haesebrouck F., Van Immerseel F. A review on prebiotics and probiotics for the control of dysbiosis: present status and future perspectives. Animal. 2014;9:43–48. doi: 10.1017/S1751731114002584. [DOI] [PubMed] [Google Scholar]
- Edens F.W., Parkhurst C.R., Casas I.A., Dobrogosz W.J. Principles of Ex ovo competitive exclusion and in ovo administration of Lactobacillus reuteri. Poult. Sci. 1997;76:179–196. doi: 10.1093/ps/76.1.179. [DOI] [PubMed] [Google Scholar]
- El-Moneim A.E.M.E.A., El-Wardany I., Abu-Taleb A.M., Wakwak M.M., Ebeid T.A., Saleh A.A. Assessment of in ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal Histomorphometry, blood Hematological, and Biochemical parameters of broilers. Probiotics Antimicrob. Proteins. 2019;12:439–450. doi: 10.1007/s12602-019-09549-2. [DOI] [PubMed] [Google Scholar]
- Fåk F., Bäckhed F. Lactobacillus reuteri Prevents diet-induced Obesity, but not Atherosclerosis, in a strain dependent Fashion in Apoe-/- mice. PLoS One. 2012;7:10. doi: 10.1371/journal.pone.0046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallis A. The overuse of antibiotics in food animals threatens public health. J. Chem. Inf. Model. 2013;53:1689–1699. [Google Scholar]
- Fan Y.K., Croom J., Christensen V.L., Black B.L., Bird A.R., Daniel L.R., Mcbride B.W., Eisen E.J. Jejunal Glucose Uptake and Oxygen Consumption in Turkey Poults selected for Rapid growth. Poult. Sci. 1997;76:1738–1745. doi: 10.1093/ps/76.12.1738. [DOI] [PubMed] [Google Scholar]
- FAO/WHO Health and nutritional Properties of probiotics in food including powder Milk with live lactic acid bacteria. Argentina. Prev. 2001;5:1–10. [Google Scholar]
- Fasina Y.O., Olowo Y.L. Effect of a commercial yeast-based product (Maxigen®) on intestinal villi morphology and growth performance of broiler chickens. Int. J. Poult. Sci. 2013;12:09–14. [Google Scholar]
- Fayol-Messaoudi D., Berger C.N., Coconnier-Polter M.H., Liévin-Le Moal V., Servin A.L. pH-, lactic acid-, and non-lactic acid-dependent activities of probiotics lactobacilli against Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2005;71:6008–6013. doi: 10.1128/AEM.71.10.6008-6013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane M., Spring P., K N. Incidence of mannose-sensitive adhesions in enteric bacteria. Poult. Sci. 1999;78:139. [Google Scholar]
- Fujiwara K., Yamazaki M., Abe H., Nakashima K., Yakabe Y., Otsuka M., Ohbayashi Y., Kato Y., Namai K., Toyoda A., Miyaguchi Y., Nakamura Y. Effect of bacillus subtilis var. natto fermented soybean on growth performance, microbial activity in the caeca and cytokine gene expression of domestic meat type chickens. J. Poult. Sci. 2009;46:116–122. [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- García-López R., Cornejo-Granados F., Lopez-Zavala A.A., Sánchez-López F., Cota-Huízar A., Sotelo-Mundo R.R., Guerrero A., Mendoza-Vargas A., Gómez-Gil B., Ochoa-Leyva A. Doing more with less: a comparison of 16S hypervariable regions in Search of defining the Shrimp microbiota. Microorganisms. 2020;8:134. doi: 10.3390/microorganisms8010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Heak C., Sukon P., Kongpechr S., Tengjaroenkul B., Chuachan K. Effect of direct-fed microbials on intestinal villus height in broiler chickens: a systematic review and meta-analysis of controlled trials. Int. J. Poult. Sci. 2017;16:403–414. [Google Scholar]
- Hernandez-Patlan D., Solis-Cruz B., Pontin K.P., Hernandez-Velasco X., Merino-Guzman R., Adhikari B., López-Arellano R., Kwon Y.M., Hargis B.M., Arreguin-Nava M.A., Tellez-Isaias G., Latorre J.D. Impact of a Bacillus direct-fed microbial on growth performance, intestinal Barrier Integrity, necrotic enteritis lesions, and ileal microbiota in broiler chickens using a Laboratory challenge model. Front. Vet. Sci. 2019;6:108. doi: 10.3389/fvets.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.K., Choi Y.J., Houde R., Lee J.W., Lee B., Zhao X. Effects of lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poult. Sci. 2004;83:788–795. doi: 10.1093/ps/83.5.788. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Ikeda-Ohtsubo W., Brugman S., Warden C.H., Rebel J.M.J., Folkerts G., Pieterse C.M.J. How can We define “Optimal microbiota?”: a Comparative review of structure and functions of microbiota of animals, fish, and Plants in Agriculture. Front. Nutr. 2018;5:90. doi: 10.3389/fnut.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J.S., Kim I.H. Effect of Bacillus subtilis C-3102 spores as a probiotics feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult. Sci. 2014;93:3097–3103. doi: 10.3382/ps.2014-04086. [DOI] [PubMed] [Google Scholar]
- Jin L.Z., Ho Y.W., Abdullah N., Jalaludin S. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poult. Sci. 2000;79:886–891. doi: 10.1093/ps/79.6.886. [DOI] [PubMed] [Google Scholar]
- Johnston P.A., Liu H., O’Connell T., Phelps P., Bland M., Tyczkowski J., Kemper A., Harding T., Avakian A., Haddad E. Applications in in ovo technology. Poult. Sci. 1997;76:165–178. doi: 10.1093/ps/76.1.165. [DOI] [PubMed] [Google Scholar]
- Jumpertz R., Le D.S., Turnbaugh P.J., Trinidad C., Bogardus C., Gordon J.I., Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldhusdal M.I. Necrotic enteritis as affected by dietary ingredients. World Poult. 2000;16:42–43. [Google Scholar]
- Khaligh F., Hassanabadi A., Nassiri-Moghaddam H., Kalidari G. Effect of probiotics administration route and dietary nutrient Density on growth performance, gut health, and some Hematological variables in Healthy or Eimeria infected broiler chickens. Iran. J. Appl. Anim. Sci. 2018;8:305–315. [Google Scholar]
- Knap I., Kehlet A.B., Bennedsen M., Mathis G.F., Hofacre C.L., Lumpkins B.S., Jensen M.M., Raun M., Lay A. Bacillus subtilis (DSM17299) significantly reduces Salmonella in broilers. Poult. Sci. 2011;90:1690–1694. doi: 10.3382/ps.2010-01056. [DOI] [PubMed] [Google Scholar]
- Lan P.T.N., Binh L.T., Benno Y. Impact of two probiotics Lactobacillus strains feeding on fecal lactobacilli and weight gains in chicken. J. Gen. Appl. Microbiol. 2003;49:29–36. doi: 10.2323/jgam.49.29. [DOI] [PubMed] [Google Scholar]
- Li X., Wu S., Li X., Yan T., Duan Y., Yang X., Duan Y., Sun Q., Yang X. Simultaneous supplementation of bacillus subtilisand antibiotic growth promoters by stages improved intestinal function of pullets by altering gut microbiota. Front. Microbiol. 2018;9:2328. doi: 10.3389/fmicb.2018.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu Q., Huang Z., Lv L., Liu X., Yin C., Yan H., Yuan J. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016;120:195–204. doi: 10.1111/jam.12972. [DOI] [PubMed] [Google Scholar]
- Maharshak N., Packey C.D., Ellermann M., Manick S., Siddle J.P., Young Huh E., Plevy S., Sartor R.B., Carroll I.M. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes. 2013;4:316–324. doi: 10.4161/gmic.25486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi-Mosleh A., Sadeghi A.A., Mousavi S.N., Chamani M., Zarei A. Ileal MUC2 gene expression and microbial population, but not growth performance and immune response, are influenced by in ovo injection of probiotics in broiler chickens. Br. Poult. Sci. 2017;58:40–45. doi: 10.1080/00071668.2016.1237766. [DOI] [PubMed] [Google Scholar]
- Martínez I., Perdicaro D.J., Brown A.W., Hammons S., Carden T.J., Carr T.P., Eskridge K.M., Walter J. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl. Environ. Microbiol. 2013;79:516–524. doi: 10.1128/AEM.03046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimandipour A., Shuhaimi M., Soleimani A.F., Azhar K., Hair-Bejo M., Kabeir B.M., Javanmard A., Muhammad Anas O., Yazid A.M. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poult. Sci. 2010;89:470–476. doi: 10.3382/ps.2009-00495. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Balaskas C., Xanthakos I., Tzivinikou A., Fegeros K. Effects of a multi-species probiotics on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella enteritidis. Br. Poult. Sci. 2009;50:467–478. doi: 10.1080/00071660903110935. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K. Evaluation of the efficacy of a probiotics containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007;86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Muaz K., Riaz M., Akhtar S., Park S., Ismail A. Antibiotic residues in chicken meat: Global prevalence, threats, and decontamination strategies: a review. J. Food Prot. 2018;81:619–627. doi: 10.4315/0362-028X.JFP-17-086. [DOI] [PubMed] [Google Scholar]
- Munyaka P.M., Echeverry H., Yitbarek A., Camelo-Jaimes G., Sharif S., Guenter W., House J.D., Rodriguez-Lecompte J.C. Local and systemic innate immunity in broiler chickens supplemented with yeast-derived carbohydrates. Poult. Sci. 2012;91:2164–2172. doi: 10.3382/ps.2012-02306. [DOI] [PubMed] [Google Scholar]
- Noy Y., Uni Z. Early nutritional strategies. Worlds. Poult. Sci. J. 2010;66:639–646. [Google Scholar]
- NRC . National Academies Press; Washington DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Oh J.K., Pajarillo E.A.B., Chae J.P., Kim I.H., Yang D.S., Kang D.K. Effects of Bacillus subtilis CSL2 on the composition and functional diversity of the faecal microbiota of broiler chickens challenged with Salmonella Gallinarum. J. Anim. Sci. Biotechnol. 2017;8:1. doi: 10.1186/s40104-016-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladokun S., Adewole D.I. In ovo delivery of bioactive substances: an alternative to the use of antibiotic growth promoters in poultry production—a review. J. Appl. Poult. Res. 2020;29:744–763. [Google Scholar]
- Olnood C.G., Beski S.S.M., Iji P.A., Choct M. Delivery routes for probiotics: effects on broiler performance, intestinal morphology and gut microflora. Anim. Nutr. 2015;1:192–202. doi: 10.1016/j.aninu.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdogan M., Topal E., Paksuz E.P., Kirkan S. Effect of different levels of crude glycerol on the morphology and some pathogenic bacteria of the small intestine in male broilers. Animal. 2014;8:36–42. doi: 10.1017/S1751731113001833. [DOI] [PubMed] [Google Scholar]
- Pandit R.J., Hinsu A.T., Patel N.V., Koringa P.G., Jakhesara S.J., Thakkar J.R., Shah T.M., Limon G., Psifidi A., Guitian J., Hume D.A., Tomley F.M., Rank D.N., Raman M., Tirumurugaan K.G., Blake D.P., Joshi C.G. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome. 2018;6:115. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Paulson J.N., Colin Stine O., Bravo H.C., Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods. 2013;10:1200–1202. doi: 10.1038/nmeth.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. Effects of in ovo supplementation of probiotics on performance and immunocompetence of broiler chicks to an Eimeria challenge. Benef. Microbes. 2016;7:699–705. doi: 10.3920/BM2016.0080. [DOI] [PubMed] [Google Scholar]
- Prabakar G., Pavulraj S., Shanmuganathan S., Kirubakaran A., Mohana N. Early nutrition and its importance in poultry: a review. Indian J. Anim. Nutr. 2016;33:245. [Google Scholar]
- Reijrink I.A.M., Meijerhof R., Kemp B., Graat E.A.M., van den Brand H. Influence of prestorage incubation on embryonic development, hatchability, and chick quality. Poult. Sci. 2009;88:2649–2660. doi: 10.3382/ps.2008-00523. [DOI] [PubMed] [Google Scholar]
- Roto S.M., Rubinelli P.M., Ricke S.C. An introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Front. Vet. Sci. 2015;2:28. doi: 10.3389/fvets.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso U., Ohtani S., Tanaka K., Sakaida M. Dried Bacillus subtilis Culture reduced Ammonia gas Release in poultry House. Asian-Australas. J. Anim. Sci. 1999;12:806–809. [Google Scholar]
- Schijns V.E., Van de Zande S., Lupiani B., Reddy S.M. Practical aspects of poultry vaccination. Avian Immunol. 2014;2:345–362. [Google Scholar]
- Sekizaki T., Nishiya H., Nakajima S., Nishizono M., Kawano M., Okura M., Takamatsu D., Nishino H., Ishiji T., Osawa R. Endocarditis in chickens caused by Subclinical infection of Streptococcus gallolyticus subsp. gallolyticus. Avian Dis. 2008;52:183–186. doi: 10.1637/8048-070307-Case. [DOI] [PubMed] [Google Scholar]
- Sen S., Ingale S.L., Kim Y.W., Kim J.S., Kim K.H., Lohakare J.D., Kim E.K., Kim H.S., Ryu M.H., Kwon I.K., Chae B.J. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Shang Y., Regassa A., Kim J.H., Kim W.K. The effect of dietary fructooligosaccharide supplementation on growth performance, intestinal morphology, and immune responses in broiler chickens challenged with Salmonella Enteritidis lipopolysaccharides. Poult. Sci. 2015;94:2887–2897. doi: 10.3382/ps/pev275. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjøt-Rasmussen L., Sandvang D., Blanch A., Nielsen J.M., Styrishave T., Schnabl J., Brockmann E., Beck C.N., Kiess A.S. Post hatch recovery of a probiotics Enterococcus faecium strain in the yolk sac and intestinal tract of broiler chickens after in ovo injection. FEMS Microbiol. Lett. 2019;366:1–5. doi: 10.1093/femsle/fnz078. [DOI] [PubMed] [Google Scholar]
- Stoll S., Gadau J., Gross R., Feldhaar H. Bacterial microbiota associated with ants of the genus Tetraponera. Biol. J. Linn. Soc. 2007;90:399–412. [Google Scholar]
- Tako E., Ferket P.R., Uni Z. Effects of in ovo feeding of carbohydrates and β-hydroxy-β- methylbutyrate on the development of chicken intestine. Poult. Sci. 2004;83:2023–2028. doi: 10.1093/ps/83.12.2023. [DOI] [PubMed] [Google Scholar]
- Tavaniello S., Maiorano G., Stadnicka K., Mucci R., Bogucka J., Bednarczyk M. Prebiotics offered to broiler chicken exert positive effect on meat quality traits irrespective of delivery route. Poult. Sci. 2018;97:2979–2987. doi: 10.3382/ps/pey149. [DOI] [PubMed] [Google Scholar]
- Thanissery R., McReynolds J.L., Conner D.E., Macklin K.S., Curtis P.A., Fasina Y.O. Evaluation of the efficacy of yeast extract in reducing intestinal Clostridium perfringens levels in broiler chickens. Poult. Sci. 2010;89:2380–2388. doi: 10.3382/ps.2010-00879. [DOI] [PubMed] [Google Scholar]
- Thibodeau A., Fravalo P., Yergeau E., Arsenault J., Lahaye L., Letellier A. Chicken Caecal Microbiome modifications induced by campylobacter Jejuni colonization and by a non-antibiotic feed additive. PLoS One. 2015;10:e0131978. doi: 10.1371/journal.pone.0131978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torshizi M.A.K., Moghaddam A.R., Rahimi S., Mojgani N. Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. Br. Poult. Sci. 2010;51:178–184. doi: 10.1080/00071661003753756. [DOI] [PubMed] [Google Scholar]
- Triplett M.D., Zhai W., Peebles E.D., McDaniel C.D., Kiess A.S. Investigating commercial in ovo technology as a strategy for introducing probiotics bacteria to broiler embryos. Poult. Sci. 2018;97:658–666. doi: 10.3382/ps/pex317. [DOI] [PubMed] [Google Scholar]
- Trudeau S., Thibodeau A., Côté J.-C., Gaucher M.-L., Fravalo P. Contribution of the broiler breeders’ fecal microbiota to the Establishment of the Eggshell microbiota. Front. Microbiol. 2020;11:666. doi: 10.3389/fmicb.2020.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z., Ferket P.R. 2003. Enhancement of development of Oviparous species by in ovo feeding, Yissum Research Development Company of Hebrew University of Jerusalem, North Carolina State University, assignee, Google Patents. United States Patent US 6:592–878. [Google Scholar]
- Van Der Wielen P.W.J.J., Biesterveld S., Notermans S., Hofstra H., Urlings B.A.P., Van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000;66:2536–2540. doi: 10.1128/aem.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn A.C., Cooper E.M., Dilorenzo P.M., O’Loughlin L.J., Konkel M.E., Peters J.H., Hajnal A., Sen T., Lee S.H., de La Serre C.B., Czaja K. Energy-dense diet triggers changes in gut microbiota, reorganization of gut-brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. (Wars) 2017;77:18–30. doi: 10.21307/ane-2017-033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Wales A., Davies R. 2019. Antimicrobial drug resistance in Salmonella and related organisms in poultry-what do we know about risk factors?, Proceedings of the 13th Turkey Science and Production Conference, Turkeytimes. [Google Scholar]
- Wang Y., Sun J., Zhong H., Li N., Xu H., Zhu Q., Liu Y. Effect of probiotics on the meat flavour and gut microbiota of chicken. Sci. Rep. 2017;7:1–3. doi: 10.1038/s41598-017-06677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman V., Tona K., Decuypere E. Delay in feed access and spread of hatch: Importance of early nutrition. Worlds Poult. Sci. J. 2010;66:177–188. [Google Scholar]
- Williams R.B. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. 2002;31:317–353. doi: 10.1080/03079450220148988. [DOI] [PubMed] [Google Scholar]
- Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang H., Shepherd M., Wen K., Li G., Yang X., Kocher J., Giri-Rachman E., Dickerman A., Settlage R., Yuan L. Probiotics and virulent human rotavirus modulate the transplanted human gut microbiota in gnotobiotic pigs. Gut Pathog. 2014;6:39. doi: 10.1186/s13099-014-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.F., Kim I.H. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Am. Hist. Rev. 2014;119:364–370. doi: 10.3382/ps.2013-03314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.