Abstract
Polyphasic myodegeneration potentially causes severe physiological and metabolic disorders in the breast muscle of fast-growing broiler chickens. To date, the etiology of recent muscle myopathies, such as the white striping (WS) phenotype, is still unknown. White striping–affected breast meats compromise the water holding capacity and predispose muscle to poor vascular tone, leading to the deterioration of meat qualities. Herein, this review article provides insight on the complexities around chicken breast myopathies: (i) the etiologies of WS occurrence in chicken; (ii) the metabolic changes that occur in WS defect in pectoralis major; and (iii) the interactions between breast muscle physiology and vascular tone. It also addressed the effects of nutritional supplements on muscle myopathies on chicken breast meats. Moreover, the review explored breast muscle biology focusing on the early preparation of satellite and vascular cells in fast-growth chicken breeds. Transcriptomics and histological analyses revealed poor vascularity in breast muscle of fast growth chickens. Thus, we suggest in ovo feeding of nutrients promoting vascularization and satellite cells replenishment as a potential strategy to enhance endothelium-derived nitric oxide availability to promote vascularization in the pectoralis major muscle region.
Key words: pectoralis major muscle, vascular tissue, satellite cell, hypertrophy, myodegeneration
Introduction
Genetic selection has helped the poultry industry to breed fast-growing broiler chickens over the decades. Poultry producers can also meet the demand for chicken meats by improving the feed's nutritional composition (Tallentire et al., 2018). Likewise, the modern production practices significantly reduced the time required for commercial birds to reach market sized weight. All these are subject to contributions from selective breeding and feeding technologies (Petracci et al., 2015). However, in the last few decades, some striking unintended consequences of fast growth rate are on the rise, including but are not limited to the occurrences of mild and severe muscular myopathies (Petracci et al., 2013).
Most recently, muscular defects such as white striping (WS) and wooden breast (WB) of chicken have been reported as 2 of the emerging pectoral major muscle (PMM) myopathies, and they pose higher economic risks on meat quality (Sihvo et al., 2014). Although, there are concerns about oxidative stress and improper handling; however, according to Kuttappan et al. (2013a; 2012c), Vitamin E supplements or preslaughter handling did not influence the appearance of WS phenotype. Therefore, poultry scientists are currently exploring ways to unravel WS and WB etiologies (Trocino et al., 2015). In fact, it is suggested that both myopathies exhibit similar molecular alterations but with distinct phenotypic characteristics (Malila et al., 2018). The causes of the WS defect are focused on the rapid growth of muscular tissues, vasodilation, hypoxia, and lipidosis in the pectoralis major region (Kuttappan et al., 2013a; Mudalal et al., 2015; Dalle Zotte et al., 2017; Lilburn et al., 2019).
Here, we reviewed the meat quality and genetic factors associated with muscle myopathies in broiler chickens. Moreover, considering the hypertrophic muscle development in fast-growing commercial broiler chickens, we speculate that the satellite cells (SC) are unable to keep up with the normal muscle growth and repair. Thus, we suggest that the enhancement of SC could support the cell's regenerative properties. Furthermore, strategies that increase muscle fiber numbers could likely reduce muscle hypertrophy stress. For example, hyperplasia increases the ratio of capillary density to muscle fibers in broiler chickens (Harthan et al., 2014); therefore, we propose that in ovo feeding of L-arginine might positively affect the relaxation of capillary tissue vasodilation in the PMM of broiler chickens.
Meat Quality, Consumers Acceptability, and Economic Impact of White Striping Breast Muscle Defect
Customer choice toward poultry meat is based on its nutritional profile and the relatively low price compared with other meats. The nutritional profile of chicken meat was reported to contribute to human health (Mir et al., 2017). Nevertheless, consumers are more concerned about the quality of meat available in the market and are willing to pay extra to eat safe, healthy, and nutritious breast fillet products. Factors such as meat color and tenderness influence consumer preference. For instance, research showed that consumers would purchase fewer breast fillets with an increase in WS phenotype severity (Kuttappan et al., 2012b). High fat deposits and increased drip loss are among other quality concerns that have been reported to correspond with WS and WB phenotypes (Mazzoni et al., 2015; Huang and Ahn, 2018). These increasing concerns are global problems, especially in the US, Europe, and China. Annually, the poultry industry in the United States of America reports a loss of over $200 million because of muscle-related defects (Bunge, 2019). The other problem is that the United States Department of Agriculture (USDA) recommended in 2017 that abnormal fillets be downgraded and used for processed foods (Qin et al., 2013; USDA-FSIS, 2017).
Growth Rate and Incidence of White Striping in Modern Broilers Chicken
The growth rate of broiler chickens has recorded significant improvement in the last 50 yr. Broiler chickens' weight at the market age has reached over a 200% increase between 1957 and 2001 (Havenstein et al., 2003). Compared with local breeds, modern fast growth broilers show a significantly higher feed to growth ratio (FCR), which explains the improvement in carcass yield. The carcass of commercial broilers (Ross 308) showed a relative increase in muscular weight than unselected genetically enhanced chickens (Havenstein et al., 2003). Thus, the focus is now on carcass yield with particular interests in increasing breast meat, thigh, and wings, constituting up to 35% of the body mass. In agreement with the study above, fast-growing birds have become more economical and sustainable than the older breeds of chickens (Zuidhof et al., 2014). Over the years, high energy feed helps achieve higher body weight and better FCR. Unfortunately, high energy feed changes the energy dynamics required for physical and metabolic activities in the body. For instance, a comparative study on broilers with fresh and abnormal fillets demonstrates the effect of growth rate on the progression of the muscular disorder and thereby suggested that increased growth rate, as seen in heavy birds, results in higher degrees of white stripes in the fillets (Kuttappan et al., 2012a).
More than 55% of broilers' breast meat was recorded to have a WS phenotype, and this appearance becomes more severe with higher slaughter age (Kuttappan et al., 2013a, 2013b). Bechtel showed that broiler muscle fibers undergo hypertrophy, whereas the blood capillaries tissue simultaneously decreases over time (Bechtel, 1986). The theory supporting Bechtel data was that chicken breast muscle (i.e., PMM) is composed mainly of type IIb fiber, which has lower capillary density and makes the PMM more vulnerable to muscle myopathies (Verdiglione and Cassandro, 2013). In related research, physical examinations of chicken fillets were reported to have 74% of WS on the meat surface and 97% damaged muscle when examined histologically (Trocino et al., 2015). The WS incidence increased by an average of 8.7 to 75% between 2012 and 2017 (Huang and Ahn, 2018), whereas its severity has a varying intensity in different species of broiler chickens. A study confirmed the thickness variations of white stripes to be about 25.0 and 7.4% in Ross 308 and Cobb 500 birds, respectively, with a higher percentage in heavy-sized than the medium-sized broiler chickens (Lorenzi et al., 2014). Kuttappan et al., therefore, designed a classification system that ranges between normal, moderate, and severe scores based on the visual appearance of the white stripe on the breast fillet samples (Kuttappan et al., 2012a). Accordingly, the grading system has helped to solve the disparity in categorizing the WS thickness.
In summary, muscle myopathies such as WS occur because of the increasing carcass yield of hybrid chickens selected for meat purposes. On this premise, the subsequent sections will provide insights into histology, omics, autophagy, and mechanisms that characterized WS-affected breast muscle in comparison to other muscle defects. We also review recent reports on the possible metabolic disorders such as nutritional deficiencies, oxidative stress, and genetic factors that may have contributed to the alteration in the muscle composition.
Characterization of white striping phenotype
Myopathies in the muscle are any neuromuscular diseases arising from defects in the structural composition of muscle fibers. Like other tissues, the muscle is vulnerable to degenerations and pathosis, in which WS and WB are linked with muscular disorders in broiler chickens. Specifically, muscle myopathies are characterized by necrosis and lipidosis, as seen in WS (Sihvo et al., 2014), whereas the WB is distinct by the hardening of the extracellular matrix cells (Velleman, 2020). The etiology of WS is still undefined; however, the rapid rate of muscle development in modern birds is suggested to overwhelm the supporting biological mechanisms like the SC and blood vessel system, which cause abnormalities in the cellular and molecular activities in the muscle (Velleman, 2015). On this note, it is necessary to discuss the features of typical breast muscles affected by WS.
Histology of Muscle Affected by White Striping Disorder
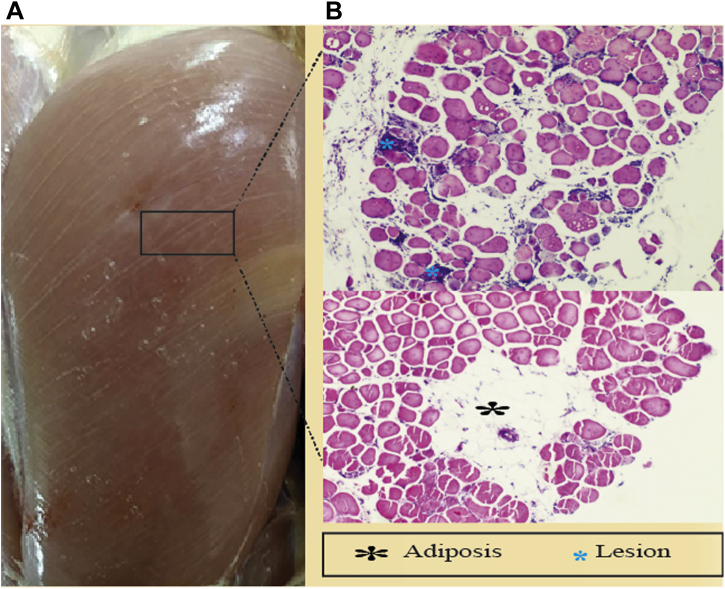
Macroscopically (Figure 1), white striation parallel to the direction of the muscle fibers characterizes the WS myopathy (Petracci and Cavani, 2012) seen on the ventral side of the pectoralis major (Rehfeldt et al., 2011). White striping can be categorized as a polyphasic myodegeneration (Zambonelli et al., 2016). Under a severe condition, WS-affected muscles show fatty deposits, and it becomes unappealing. Histological data prove that WS-affected fillet had abnormal fiber development with gross lesion condition, which compromises the muscle composition (Mazzoni et al., 2015). For example, perimysial and endomysial tissues of abnormal muscles lose their cross-sectional structure leading to fibrosis in severe WB conditions (Velleman et al., 2017; Velleman, 2020).
Figure 1.
The histomorphology of white striping myopathy. (A) Phenotypic features of White striping in fast-growing broiler chickens. (B) Microscopic image revealing the myodegeneration in the breast muscle of fast-growing broiler chickens.
The significant increase of the muscle fiber diameters in heavy type chickens reduces the interstitial space for capillary tissue networks, thus, causing a lower supply of oxygenated blood (Velleman and Clark, 2015). In this context, reduced vasodilation is suggested to contribute to the genesis of altered capillary tissue in the breast fillets. This hypothesis aligns with previous studies that reported fewer capillary cells in WS-affected muscles (Alnahhas et al., 2015, 2016). Another study also confirmed disruption in the supply of oxygenated blood and reduction of metabolic waste removal in the breast muscles (Papah et al., 2018). In addition, the continuous enlargement in muscle fiber size also impairs regenerative myogenesis (Mutryn et al., 2015; Daughtry et al., 2017). The perturbations consequently trigger oxidative stress and necrosis in the breast muscle fibers (Mutryn et al., 2015). In fact, WS shares many metabolic similarities with WB, such as interstitial inflammatory infiltrates, fibrosis, and altered cell regeneration (Petracci et al., 2019). These reasons could support the idea that the phlebitis-like disorders found in PMM are the underlying causative pathomechanism of the WS myopathy.
Omics Profiling of White Striping
As previously described, the WS syndrome causes reduced space for the network of blood capillary tissues because of the larger fiber sizes in fast-growing broilers (Velleman and Clark, 2015). Considering the hypoxia in WS meat, Greene et al. (2019) reported the downregulation of oxygen transport proteins (hemoglobin subunits and hemerythrin) in the abnormal muscle. Upregulations of hypoxia-inducible factor subunits (HIF-1α and HIF-2α) combined with downregulations of oxygenated blood-transport proteins indicate that HIF-1α and HIF-2α are adaptive mechanisms for low oxygen supply in abnormal muscles (Nakazawa et al., 2016; Greene et al., 2019). HIF-1α, a transcriptional factor in hypoxia condition, modulates the gene expressions of glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase 1, and lactate dehydrogenase A proteins (Kaluz et al., 2008; Marin-Hernandez et al., 2009; Malila et al., 2019). These alterations in glycolysis and gluconeogenesis support the notion that HIF-1α activation is essential for anaerobic metabolism in breast myopathies (Malila et al., 2019). The modulation of metabolic enzymes has pointed out by Lake and Abasht (2020) is probably not the main cause of WS and WB; however, changes of these enzymes could reprogram the cellular activities for limited oxygen supply conditions in affected breast muscle (Malila et al., 2019).
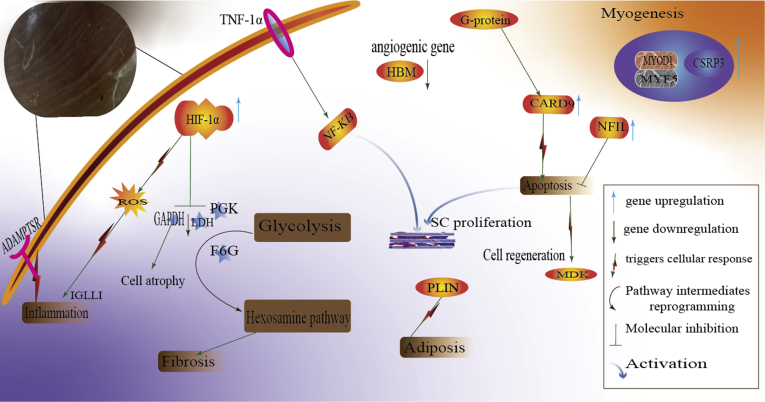
The RNA-Seq analysis of myopathic muscles revealed several differentially expressed genes depend on HIF-1 transcription (Malila et al., 2019; Marchesi et al., 2019). HIF-1 transcription regulates angiogenesis, myogenesis, vasodilation, and apoptosis (Marchesi et al., 2019). Among the 20 most-affected genes (e.g., CRH, MDK, PTX3, COQ10 B, IGLL1) that were differentially expressed are genes involved in hypoxia, amino acid (AA) metabolism, muscle tissue regeneration, and inflammatory activities (Marchesi et al., 2019). The study also confirmed the downregulation of angiogenesis transcription genes (such as bone morphogenetic protein-4, reticulon-4, and fibroblast growth factor-1) (Marchesi et al., 2019). We could deduce from their analyses the significant impacts that the altered genes have on the development of capillary tissue damage, contributing to polyphasic myodegeneration in WS-affected muscles (Figure 2). Under this premise, it is pertinent to investigate other molecular factors linked to muscular distress.
Figure 2.
Putative mechanisms of polyphasic myodegeneration in fast-growing broilers. In a white striping affected breast muscle, multiple cellular processes target satellite cell proliferation, oxidative stress alleviation, and enzymatic reprogramming to repair the damaged muscles. The dysfunction of these mechanisms purportedly causes the myopathic defect in the pectoralis major muscle in broiler chickens. Abbreviations: HIF, hypoxia-inducible factor; ROS, reactive oxygen species; SC, satellite cell; GADPH; glyceraldehyde-3-phosphate dehydrogenase, GK, phosphoglycerate kinase 1; LDH, lactate dehydrogenase; NFIL, nuclear factor interleukin.
Autophagy and Muscle Protein Turnover
Autophagy is among the biological processes activated as innate cellular response mechanisms in damaged cells (such as apoptosis). During metabolic disorders, reactive oxidative stress causes oxidative damage and triggers cellular apoptosis (Schwartzman and Cidlowski, 1993; Chen et al., 2016). In an attempt to mitigate the oxidative damage in muscle, evidence showed that autophagy systems are deployed as repair mechanisms in regulating muscular protein degradations induced by oxidation (Rabinowitz and White, 2010). The autophagy process is critical to maintaining cellular integrity by ensuring a balance between cellular synthesis and death (Ohsumi, 2001). However, the synthesis and degradation imbalance of protein cells in muscular tissue could contribute to degenerative damage seen in WS or WB poultry meat. This notion suggests that rapid growth outpaces the protein turnover mechanisms in broiler chickens (Vignale et al., 2017; Tallentire et al., 2018).
Avian species express autophagic genes that are tissue specific (Piekarski et al., 2014). Some of the autophagy-related genes highlighted in previous studies include LC3, Beclin 1, Bcl2, and Bax (Kabeya et al., 2000; Marino and Lopez-Otin, 2004). An enhanced LC3-II/LC3-I ratio expression was revealed to alleviate induced oxidative stress in the broiler's muscular cells (Chen et al., 2017). Their results proved that autophagy markers were induced by oxidative stress. Stimulation of autophagy is important as an innate strategy deployed to engulf degraded cells in the breast muscle. The sequences of mRNA transcription obtained from breast muscles of high feed efficiency birds (Rehfeldt et al., 2011) showed upregulations of both the mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPKα1) genes (Piekarski-Welsher et al., 2018). Surprisingly, the upregulation of both genes contradicts their functions, as mTOR and AMPKα1 regulate protein synthesis and inhibit energy usage, respectively.
The RNA seq database for the high feed efficiency broilers documented the upregulation of an autophagy pathway–related gene (Bottje et al., 2017), meaning that fast growth broilers exhibit autophagic enrichment genes. During metabolic distress in the muscle, the autophagy mechanism was triggered to restore homeostatic conditions in the cell (Slupecka et al., 2014; Jokl and Blanco, 2016; Cui et al., 2020). Indeed, these studies support the idea that muscular disorders emanate from the impairment in cell regeneration. Consequently, we suggest that impairment of the autophagy mechanism in breast muscle cells may potentially contribute to deficiency in protein turnover, lipidosis, and fibrosis linked with chicken meat myopathic tissues.
White Striping and Wooden Breast Degeneration Response Mechanisms
The PMM is exclusively composed of type IIb fibers. This implies that the primary source of ATP synthesis in the pectoralis major is via anaerobic glycolytic metabolism. Phosphoglucose isomerase, lactate dehydrogenase, and GNE genes in the glycolytic pathway were altered in WS-affected breast muscles (Zambonelli et al., 2016). On this account, muscle degeneration in WS-affected breast meat could result from the progressive inhibition of the genes encoding the enzymes that regulate anaerobic glycolysis (Pampouille et al., 2019). Lactate dehydrogenase, the key enzyme that regulates pyruvate conversion to lactate, was reportedly downregulated in a chronic hypoxia condition (Kuttappan et al., 2017). The downregulation of lactate dehydrogenase and phosphofructokinase indicate that the anaerobic glycolysis pathway is impaired (Mutryn et al., 2015).
On the other hand, pathways of the glycolytic intermediates are also changed (Zambonelli et al., 2016), leading to lipolysis and fibrosis (Soglia et al., 2015), as evident in WB-affected muscles. Because of these reasons, we still need to determine if the shift in the pathways of the substrate could potentially reduce the energy production in the type II fibers of the PM.
Muscle hypertrophy is favored by upregulations of growth factors like IGF-1 (Musarò et al., 2001; Sandri, 2008; Schiaffino et al., 2013) and myogenic factors like MYC (Pampouille et al., 2019). Because muscle development, degeneration, and repair take place simultaneously, it depicts crosstalk events regulated by multiple mechanisms controlled by the interactions between noncoding RNA and messenger RNA (Petracci et al., 2013). The interaction between MiRNA and mRNA induces differential expression of FHL2 (a postinflammatory gene) during the late developmental phase of chicken muscle growth (Li et al., 2019). A dysregulated interaction between MiRNA and mRNA will have considerable effects on muscle qualities (Khatri et al., 2018). Enhanced transcription of mRNA and protein translation in fast-growing birds is suggested to contribute to the degenerative WS phenotype. Therefore, elucidating the associated MiRNA and mRNA interaction will provide a better understanding of the mechanistic etiology of myopathic muscle in fast-growth chickens.
Li et al. demonstrated that Forkhead Box O transcription (FOXO) signaling pathways are crucial for muscle fiber hypertrophy (Li et al., 2019). During muscle growth, FOXO3 targets several myoblast differentiation genes upregulated by AKT, an upstream gene in the FOXO pathway (Xue et al., 2017). It is not surprising that FOXO3 could maintain homeostasis through negative feedback loops because of the metabolic changes by the intermediates of both Akt and mTOR pathways (Chen et al., 2010; Stefanetti et al., 2018). Moreover, FOXO3 proteins enhance SC proliferation via the notch pathway during the regeneration in muscle cells (Stefanetti et al., 2018). However, a proteomics study revealed upregulations of mTOR and eIF-2 signaling pathways in WS-affected breast muscles (Kuttappan et al., 2017). Under such conditions, WS breast muscles could be triggered by the ubiquitin–proteasome system leading to protein degradation in the muscle fibers. Aberrant protein synthesis in hypertrophic muscles could cause posttranslation modification of E3 ubiquitin-protein ligase 1 in chickens with muscular dystrophy (Matsumoto et al., 2008). Besides, there is a possibility that the intermediates of the P13k/Akt pathway are stimulated because this pathway is involved in an increased protein translation in severe myopathic breast muscles (Deng et al., 2014). Such multifactorial mechanisms make it more challenging to unravel the cause of the abnormal phenotype appearing on chicken breast muscles.
Inflammation functions as one of the biological responses that inhibit oxidative damage caused by excess reactive compounds in tissues. As mentioned in previous studies, cytokines molecules related to muscle myopathies include IL-6, TNF-α, and NK-kB (Lundberg et al., 1995; Salomonsson and Lundberg, 2006). Cytokines regulate both proinflammatory and antiinflammatory cellular responses and may trigger the alleviation of muscle myopathies. Most cytokines produced by monocytes, macrophages, neutrophils, and many other immune cells in the endothelium (Goetz et al., 2004) complicate the pathogenesis of idiopathic inflammatory myopathies (Scheurich et al., 1987; Seko et al., 1993). The genes that encode the inflammatory cytokines in the abnormal breast muscle mediate cellular activities like proliferation and apoptosis. Data from WS-affected breast meat showed that upregulation of nuclear factor interleukin 3–coding genes demonstrates the effort of the tissue to alleviate apoptotic and necrotic cell activities (Zambonelli et al., 2016). Cytokines like IL-6 and TNF-α might play significant roles in myopathic cell regeneration in the breast muscle tissue. For example, the differential expression of TNFAIP6, an isoform of TNF-α in fast-growing muscles, might degrade the linkage between the extracellular matrix component and cytoskeleton caused by the matrix metalloproteinase expression (Pampouille et al., 2019). TNF-α also mediates MuRF1 protein expression (linked with muscle necrosis and disorder activities) (Minetti et al., 2011).
NK-kB transcription cascade enhances the signals for distressed muscles by increasing the inflammatory lesions in the muscle tissues. Therefore, it is interesting to know that cytokine/NK-kB signaling affects cell growth and regeneration in dystrophic muscles. There is an interplay among the cytokines linked with muscle myopathies, most importantly IL-6, TNF-α, and NK-kB, where they initiate SC growth and proliferation, as well as myoblast differentiation in damaged muscles (Guerci et al., 2012; Yang and Hu, 2018). The development of SC proliferation in the damaged myofibers is an indicator for the initiation of cell regeneration. Another significant cytokine, that is IL-4, participates in alleviating hypertrophic-related defects in the muscles (Guerci et al., 2012). Hypertrophic muscles may use cytokine molecules as a signal for damaged tissue, which leads to regenerative processes in WS-affected muscles (Pampouille et al., 2019).
Recent efforts to alleviate white striping and wooden breast myopathy
Different efforts, such as nutritional supplements and managerial practices, have improved carcass quality and production performance in broiler chickens. Above all, AA are the main component for protein synthesis in the body. This relation has inspired several studies to focus on AA's role in WS and WB development. Also, AA is critical to major metabolic pathways that regulate muscular growth and development (Wu, 2009). Considering the reports that underlying factors of WS and WB could originate from polyphasic degenerative activities in the breast muscle, modern fast growth birds may require changes in the feed composition. Improper adjustments could significantly affect the birds' performance, although most researchers believe that nutritional abnormalities do not cause WS and WB disorders. However, this review provides an overview of nutritional effects on WS/WB myopathies (Table 1).
Table 1.
Effect of supplements on breast muscle myopathies.
| Supplements | Concentration | Effects | Deduction | References |
|---|---|---|---|---|
| Glutamine | 1% | Exacerbate wooden breast (WB) and White striping (WS) in fillets—raises blood H2CO3, CO2, and decreases O2 | Hypoxia leads to fatty acid and Ca accumulations | (Livingston et al., 2018) |
| Arginine | 0.25% | |||
| Digestible amino acid | 15% reduction at grower's phase | Reduced average woody breast score without final BW decrease | Allows satellite cells to recuperate from fast growth in early stage | (Bodle et al., 2018) |
| Ascorbate | 94.4 mg/kg | Alleviate WB score by 32% | It relieves the overactivation of de novo ascorbate biosynthesis, thus, limits H2O2 by-products accumulation | (Abasht et al., 2016; Bodle et al., 2018) |
| Magnesium | 3000 mg/kg (optibreast®) | Decrease WB and WS scores by half | Protect against peroxidation in tissues | (Estevez and Petracci, 2019) |
| Upregulate catalase activities in plasma | ||||
| Vitamin E | 50 mg/kg | Increased plasma Vitamin E | WS/WB not related to nutritional deficiency | (Guetchom et al., 2012) |
| No significant alleviation of muscle damage | ||||
| Vitamin E | (15, 50, 100, 200, 400) mg/kg | No significant correlation in any of the dietary levels | WS occurrence is peculiar to heavy fillet weight | (Kuttappan et al., 2012c) |
| DL-α-tocopherol acetate | 200 IU/kg | Reduces the severity of WS and wooden breast at growers' phase | Alluded to early Vitamin E inclusion in the diets | (Wang et al., 2020) |
| Omega-3 | 3:1 ratio of n-6/n-3 | Decreased the growth performance index of the birds |
Nutritional Supplements
Amino Acids
Although there are limited reports on the intervention of dietary supplements to mitigate the muscular defect caused by enlarged muscle fibers, no study has concluded that WS/WB is related to nutritional deficiencies. Instead of glutamine and arginine supplements to alleviate the muscle defects in a previous study, they improved both the BW and FCR of the experimental broilers, contributing to the WS condition exacerbation (Livingston et al., 2018). From their results, the dietary supplement had an indirect effect on the development of muscle defects in heavy breed chickens. On the contrary, Bodle et al. (2018) decreased the AA ratio in the diet fed to broilers during the grower phase and aimed to replenish SC development from the rapid growth at the starter phase (Powell et al., 2014). Their report demonstrated that feeding lower AA at the grower's stage could possibly alleviate WB because of reduced SC activities (Bodle et al., 2018). Thus, it is okay to deduce that the PMM progressive degeneration may be reduced by minimizing the feeds' nutritional content at the grower's phase.
The implication of hypoxic conditions in myopathic breast muscle is equivocally agreed upon by researchers (Malila et al., 2019). In a previous trial, dietary arginine supplements improved hypoxia in the broiler muscles (Bodle et al., 2018). According to their result, Bodle et al. (2018) suggested that WB severity may be reduced by supplementing a higher ratio of arginine in broilers' diet without any significant negative impact on the overall breast meat yield. This could be because of the effect of arginine on the capillary vasodilation (Bautista-Ortega and Ruiz-Feria, 2010). On the contrary, TRIM63/MuRF1 upregulations involved in the atrophy of muscle cells was suggested to decrease the weight gain in broiler chickens fed modified methionine diets (Bodine and Baehr, 2014; Sachs et al., 2019). In a similar report on the modification of feed regimen, higher lysine supplement increased WS and WB occurrence in boilers (Cruz et al., 2016). Lysine is an essential amino acid for broiler growth performance and proper muscle development (Sterling et al., 2006). The explanations here emphasized that muscle outgrows its physiological system like vascular tone, therefore leading to muscular damage. Altogether, these results indicated that AA has no direct effect on muscle defects.
Minerals
Another notable disorder in myopathic muscles is the swelling of the tissues resulting from an intracellular build-up of inflammation, which induces osmotic dysregulations (Livingston et al., 2019). In a previous study, potassium's role in electrolyte balance was investigated in broiler chickens (Livingston et al., 2019). The study concluded that decreased WB incidence in birds fed potassium supplement might result from improved vascular circulation and hydrogen residue reduction in the muscle cells. Similarly, Magnesium supplement also significantly reduced the appearance of WS and WB phenotypes in broiler chickens (Estevez and Petracci, 2019). Their result corroborates with the study that showed magnesium deficiency exacerbates lipid peroxidation linked with muscular myopathy (Liu et al., 2007). Accumulation of these oxidative alterations confirmed excess free radical formation, oxidative degradation, and damage to cells and tissues as apparent in muscle fiber myopathies (Abasht et al., 2016). For example, a metabolomic study on WS muscle samples suggested an increase in calcium level results from the accumulation of unsaturated fatty acid in a deprived oxygen environment (Boerboom et al., 2018). Therefore, minerals imbalance is potentially a consequence of myodegeneration in broiler breast muscles.
Antioxidants
In myopathic muscles, hypertrophy and hypoxia cause tissue injuries and oxidative stress, respectively (Mutryn et al., 2015; Kuttappan et al., 2017; Malila et al., 2019). According to the omics report, there is little doubt that hypoxia could potentially be a cause of WS/WB in chicken breasts (Malila et al., 2019). This proposition suggests that hypoxia could predispose muscle to reactive oxygen species (ROS) accumulations in muscle tissue cells (Malila et al., 2019). During the onset of WS, increased secretion of antioxidant markers was observed to decrease free radical molecule accumulation (Salles et al., 2019). On the contrary, the glutathione S-transferase and glutathione peroxidase activities were reduced in severe WS-affected breast muscles (Malila et al., 2019). Indeed, the increase in free radicals in the pectoral muscle contributes to WS in chicken (Zambonelli et al., 2016; Salles et al., 2019). Excess ROS accumulation exacerbates oxidative damage in muscle cell lesions, DNA, and protein denaturation. Similarly, it could further aggravate inflammatory molecules, which might initiate the appearance of WS and WB on chicken fillets (Powers et al., 2010). Besides, broiler chickens are most vulnerable to increased oxidative stress because their meats contain a relatively higher polyunsaturated fatty acid found in WS/WB–affected muscles (Decker et al., 2010; Abasht et al., 2016). Nevertheless, it is still uncertain if oxidative stress is the cause or the effect of WS and WB phenotype in fast-growth type chickens (Zambonelli et al., 2016; Boerboom et al., 2018).
Antioxidants, both biological and synthetic, prevent oxidative damage in adipose tissues and the gut immune system (Surai, 2007). Vitamin E represents an essential functional component in poultry feed and a potent antioxidant against lipid peroxidation in both plasma cells and skeletal muscle tissues (Smet et al., 2008; Gao et al., 2010; Guetchom et al., 2012). However, according to studies, antioxidants have negligible effects on excess free radical reduction in the muscle cells. Important antioxidants like vitamins C, E, and omega have been studied, but their results are inconsistent in mitigating the myopathy caused by oxidative stress. For instance, Kuttappan et al. (2012c) study showed an increased Vitamin E level did not decrease WS appearance in abnormal breast muscles. This might happened because WS is not directly linked with vitamin deficiency (Kuttappan et al., 2012c; Guetchom et al., 2012). On the contrary, Vitamin E (200 IU/kg) supplement in starter diets reduced two-third of WS severe and moderate scores from starter to growers' phase, whereas the WB severity score was alleviated only at the starters phase (Wang et al., 2020). The disparity in these studies could be from the difference in strains of experimental birds, management practices, or the accuracy level during grading of the phenotypic scores. From Wang et al. (2020) report, Vitamin E supplements could increase metabolic activities in broiler chickens at the starter phase. It might further alleviate excess ROS caused by oxidative stress, possibly through its scavenging properties (Miller et al., 1993; Voljč et al., 2011; Mutryn et al., 2015). In all, the bioavailability of the vitamin E in muscle depends on the vascular tone (Velleman et al., 2003; Petracci et al., 2019; Velleman, 2019; Wang et al., 2020), and it may be argued that capillary tissue damage could also its availability in breast muscles (Rocha et al., 2016; Papah et al., 2017).
Even though Bodle et al. (2018) reported that vitamin C could alleviate WB, on the contrary, the treatment did not decrease the appearance of WS in the same study. From their study, Vitamin C detoxified oxygen-derived free radicals only in a severe degree (WB). On the other hand, in the less severe condition like WS, it is evident that vitamin C supplement has no impact on its appearance in breast fillets. The explanation for the observed disparity could be because the required level of vitamin C is biologically synthesized in normal metabolic condition, and WB disorder occurs at the severe stage of hypoxic stress (Xiang et al., 2002), whereas white stripes phenotype starts to show on meat during the onset of these 2 breast muscle disorders (Kuttappan et al., 2017). This notion means that the effect of Vitamin C supplement against breast muscle myopathy can only be seen at the later degenerative stage of muscle myopathy (Bodle et al., 2018).
Broilers pectoralis muscle composition
Skeletal muscle is among the most abundant tissues in the body. Moreover, skeletal muscle growth, like any other tissue, also depends on protein turnover (Greising et al., 2012; Anthony, 2016). Therefore, this section provides insight into muscle growth and repair systems relating to myopathies in broiler chickens. It also addressed the prospect of SC in maintaining muscle integrity.
Muscle Development and Breast Muscle Myopathy
Myogenesis in broiler chickens involves the embryonic proliferation and differentiation of mesodermal cells to form multinucleated primary muscle fibers. After primary myofiber formation, the secondary muscle fibers get attached to the adjacent of the primary myofibers. Thus, these muscular cell syntheses make up the hyperplasia phase, and it biologically terminates at the ends of the incubation stage. Generally, it is assumed that hyperplasia decline till the early posthatch stage (Smith, 1963).
The fusion of SC and adjacent muscle fibers serve as the basis of increased genetic material in postnatal muscles (Daughtry et al., 2017). Note, the muscle fiber diameter increased to more than five-folds in heavy chicken type (Dalle Zotte et al., 2017). However, the physiology of skeletal muscle can only sustain the overgrowth to a certain threshold. As a result, it becomes a challenge for vascular cells to adjust to hypertrophic muscle in the later phase of chicken breast muscle growth (Petracci et al., 2013). For this reason, the skeletal muscle compositions are vulnerable to muscular damage, as seen in abnormal muscles. Muscle defects occur because of altered muscle components in WS-affected breast muscles (Pampouille et al., 2019). From a previous study, creatine kinase, domicile in the muscle fiber, was found at an increased level in the plasma sample of heavy strain turkey breeds (Wilson et al., 1990). Histological data showed a near-complete absence of connecting tissue spacing between the perimysial and endomysial fascicles (Wilson et al., 1990; Velleman, 2015). These data support the hypothesis that SC replenishment is impaired to compensate for myofiber degradation in myopathic muscles.
Satellite Cells and Muscle Regeneration
The myopathies in chicken breast muscles occur because of the suppression of SC mediating the cell regenerative process, a peculiar feature of hypertrophied muscle (Daughtry et al., 2017). Satellite cell, known to be the precursor for myogenesis, supports muscular growth (Rehfeldt et al., 2011). Satellite cells also function as regenerative cells during muscular injury. Unfortunately, in vitro analysis indicates that SC lose their abilities to proliferate and differentiate with age and hypertrophy, leading to myodegeneration (Daughtry et al., 2017). The expression of the paired box protein (PAX7) gene supports the idea that SC activations participate in muscle repair against myopathy (Daughtry et al., 2017). PAX7 gene has a specific role as a proliferating SC (Zammit et al., 2004) and is required to maintain muscle homeostasis by regulating myf5 and myoD expressions (Relaix et al., 2005).
Satellite cells are, in part, regulated by the physiological state of muscles. In a homeostatic condition, SC is found to be quiescent. However, when homeostasis is disturbed in hypertrophic muscle, SC proliferates and differentiates to support muscle repair. For SC regeneration to occur, vascular cells and other extrinsic factors like nutrients regulate its regenerative power. Evidence showed that SC proliferation relies on the vascular tone in the muscle (Christov et al., 2007; Rhoads et al., 2009). However, owing to the sparse density of mitochondrial in the breast (a fast-twitch) muscle, vascularization decreases in hypertrophic muscles. Accretion of muscle fibers also reduces the available space for connective tissue, contributing to poor vascularization. In all, increased muscular repair and poor vascular tone impede the SC proliferation to support muscular regeneration in fast-twitch muscle growth (Velleman, 2015). The inability of SC to proliferate might cause necrotic lesions (Velleman et al., 2018). Therefore, we believe that the impaired SC compromises the biological mechanisms for fast growth in chicken under hypertrophic conditions.
A possible way to enhance the SC proliferation in hypertrophic muscle is by identifying potential molecular markers linked to muscular tissues. Most of the isolated genes from abnormal muscles implicate adiposis, muscle fiber regeneration, and repair processes (Petracci et al., 2013). Gene upregulations such as CSRP3 and platelet-derived growth factor receptor genes (PDGFRα) in myopathic breast muscles (Zambonelli et al., 2016; Pampouille et al., 2019) indicated that muscle regeneration processes were activated in response to the histological changes in breast muscles. During muscle repair activities in abnormal muscles, the PDGFRα gene helps muscle cells differentiate into fibroblasts and adipocytes. Another important marker in the muscle is the collagen type VI α 3-chain. Collagen type VI α 3-chain is a collagen molecular marker for extracellular matrix, and any perturbation in their expressions may cause necrosis as apparent in WS-affected muscles (Pampouille et al., 2019; Papah and Abasht, 2019). Such genes play critical roles in preserving the structural integrity of muscle tissues. Over-expression of the proteoglycan gene (IMPG2) peculiar to collagen was also reported to be a causative agent of fibrosis in WS-affected breast muscles (Zambonelli et al., 2016). These perturbations proved that alterations in the cell repair gene expressions could aggravate progressive muscular tissue degenerations (Spence et al., 2002).
Satellite cell differentiation, a multipotential cell population, also relies on muscle fiber types and myogenic expression changes in abnormal muscle cells. Muscle switching from type IIb to type I fibers is suggested as one of the many responsive mechanisms in myopathic muscles (Mutryn et al., 2015). The presence of type I fiber in the PM region indicates that regeneration occurs in dystrophic muscles. The switching process could help relieve muscular stress because type-I fibers contain a higher proportion of SC numbers. For example, myoglobin—a globular protein that carries oxygen to oxidative muscles—was expressed in a high proportion in WB-affected breast meat (Mutryn et al., 2015). Not only myoglobin but also troponin I and myosin binding protein C1 were reportedly upregulated in the damaged muscle caused by WB myopathy. For these reasons, type I fibers' presence may be justified because they contain more capillary cells and thus relieve the stress from the rapid growth of the pectoralis region. This idea aligns with fiber-type switching previously linked with pale soft exudative muscle in turkey (Malila et al., 2013). In our opinion, we suggest that the focus of future research should be on strategies that can extend the threshold of SC in fast-growth type chickens.
Muscle Vascularization
The PMM primarily consists of fast-twitch fibers characterized by large muscle fiber diameter and metabolic stress (Hoving-Bolink et al., 2000; MacRae et al., 2006; Listrat et al., 2016). Indeed, the myofiber size determines the available space for total vascular density within the muscular region (Joiner et al., 2014; Radaelli et al., 2017). During hypertrophy, the insufficient space of blood vessels further exacerbates oxidative stress in muscles. Histologically, the early stage of WB samples was characterized by inflammation of the blood vessels that increased progressively over time (Papah et al., 2017; Sihvo et al., 2017). During muscular damage, angiogenesis promotes SC's ability to proliferate (Yin et al., 2013). On the contrary, poor vascularity, as seen in both WS and WB muscles, cannot support SC regenerations during muscle repair processes (Luque et al., 1995; Christov et al., 2007; Rhoads et al., 2009). Studies have shown that the fate of SC and capillary cells are intertwined (Luque et al., 1995; Christov et al., 2007; Rhoads et al., 2009), such that SC stimulates vascular cell development in the muscle region (Rhoads et al., 2009). However, when the muscle fiber is enlarged, it constricts the blood vessels' vascularity and affects SC response (Siller, 1985). Accordingly, it is possible to argue that impaired vascularity predisposes WS-affected breast muscles to polyphasic myodegenerations (Hoving-Bolink et al., 2000; Berri et al., 2007), meaning that the solution to enhanced muscle vascularity combined with SC proliferation could potentially solve the concerns of alleviating WS-affected breast muscles.
Future perspectives on in ovo feeding, satellite cells, and vascular tone
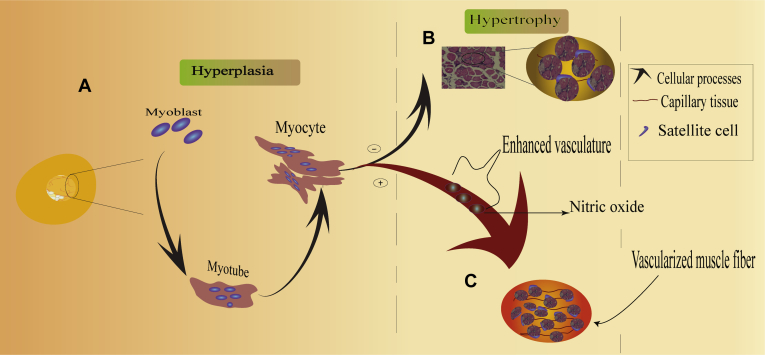
In line with other studies, we believed that poor vascular tone affects WS and WB developments in broiler chickens. Therefore, it is necessary to share ideas on possible ways to improve vasodilation in fast-growth type chickens. Nitric oxide (NO), an endothelium-derived relaxing factor, can be synthesized biologically from L-arginine (Vallance et al., 1989b) by nitric oxide synthases (Louis et al., 1987; Vallance et al., 1989a). Biologically, NO is a signaling molecule that dilates the blood vessel and relaxes smooth muscle cells, thus favoring increased blood flow in the blood vessels (Latchman, 1995). During vasodilation, NO metabolic role is to activate soluble guanylyl cyclase. Then, the soluble guanylyl cyclase triggers the activation of cGMP protein kinase. Subsequently, the protein kinase stimulates a cascade of metabolic processes that decrease intracellular calcium and promote breast muscle vascularity (Marchesi et al., 2019). According to this mechanism, NO might alleviate the poor vascularization in fast-growth chickens. Moreover, Klinger and Kadowitz had previously demonstrated that L-arginine stimulated the nitric oxide synthase pathway to enhance NO biosynthesis (Klinger and Kadowitz, 2017). On this principle, we may hypothesize that enhanced NO could alleviate WS phenotype (Figure 3) (Andriantsitohaina et al., 2012).
Figure 3.
Conceptualization of improved vascularity in fast-growing broiler chickens. (A) During the incubation phase, the number of muscle cells increases (hyperplasia) in ovo and then forms the myofibers. (B) The enlargement of muscle fibers in fast-growing chickens hinders the vasodilation of vascular tissues and thus disrupts the biological support mechanism of pectoralis major muscle. (C) Satellite cells proliferate synergistically with the development of blood capillaries. The secretion of endothelial-derived relaxing factors improves muscle vascularity. The enhancement of breast muscle vascularization suggests promoting satellite cell proliferation that can support the muscle growth pace in broiler chickens.
Nitric oxide low bioavailability has been previously linked with hypoxia-related ischemic diseases (Kevil and Patel, 2008; Napoli and Ignarro, 2009). Fortunately, L-arginine supplementation can biosynthesize endothelial nitric oxide synthase, an enzyme that contributes to the relaxation of the vascular tone in breast muscle fiber. L-arginine, a precursor of NO, reportedly relieved hypoxic conditions by improving vascular tone in broilers (Bautista-Ortega and Ruiz-Feria, 2010). A related study also showed that dietary arginine improved flow-dependent vasodilation and reduced pulmonary vascular resistance (Wideman et al., 1995). These results indicated that L-arginine could promote vasodilation and, in turn, increases blood pressure and blood flow. Another concern is the impairment of SC; however, muscle repair processes during degeneration have been suggested to resemble embryonic myogenesis (Yin et al., 2013). Therefore, by considering myogenesis at the early stage, SC can be improved to sustain hypertrophy in broiler chickens. On this account, enhanced SC proliferation in ovo could potentially support the growth pace in broiler chickens. This idea calls for the need to devise in ovo strategy to achieving efficient growth in broiler chickens.
In ovo feeding is designed to improve muscle development and poultry production efficiency (Zhao et al., 2017). In fact, in ovo injection of L-arginine has previously been used to enhance posthatch growth performance and regulate protein synthesis and deposition through the mTOR pathway and its downstream target proteins intermediate (Gao et al., 2017; Yu et al., 2018). The efficiency of in ovo injection of L-arginine has also been investigated in Japanese quail's physiological trait (Al-Daraji et al., 2012), small intestine immune barrier function, and lymphoid organ development (Gao et al., 2017). In a similar study, L-arginine in ovo injection stimulated the myogenin gene expression in posthatch chicken cultured tissue through the mediation of NO (Li et al., 2016). Therefore, we suggest that in ovo supplementation of L-arginine could be relevant in solving muscle myopathies by enhancing SC and angiogenesis during in ovo and early posthatch phases. Future studies should focus on new ways to design in ovo feeding to improve the meat quality and maintain the desired growth rate in broiler chickens.
Conclusion
Because of better feeding efficiency, fast-growing chickens have proven profitable on a commercial scale for meat products. Unfortunately, the growth rate of broiler chickens leads to muscle physiology perturbation; in other words, the rapid growth rate could not be sustained by the chicken biological system, especially the SC and vascular system. Consequently, some muscle defects, including growth-induced myopathies like WS phenotype, have become a great concern for broiler chicken farmers. Hence, this article provides an extensive review of possible breast muscle myopathy etiologies. This article, in fact, buttress research studies on muscle degeneration in the pectoralis major of fast-growing broilers, with the idea that early preparation of both vascular system and SC could potentially promote vasodilation and support processes of muscle regeneration, respectively. For these reasons, more studies should explore how nutritional strategies can help mitigate the defect in meat fillets. In particular, consideration should be given to in ovo feeding trials such as L-arginine in future research, as argued in this article.
Acknowledgments
The authors wish to appreciate the support from the National Key R&D Program of China (2017YFE0129900) and the Young Talent Supporting Program Funding of the College of Animal Science and Technology, China Agricultural University Education Foundation Grant (2017DKA002).
Disclosures
The authors declare no conflicts of interest.
References
- Abasht B., Mutryn M.F., Michalek R.D., Lee W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS One. 2016;11:e0153750. doi: 10.1371/journal.pone.0153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daraji H., Al-Mashadani A., Al-Mashadani W., Al-Hassani A., Mirza H. Effect of in ovo injection with l-arginine on productive and physiological traits of Japanese quail. South Afr. J. Anim. Sci. 2012;42:139–145. [Google Scholar]
- Alnahhas N., Berri C., Chabault M., Chartrin P., Boulay M., Bourin M.C., Le Bihan-Duval E. Genetic parameters of white striping in relation to body weight, carcass composition, and meat quality traits in two broiler lines divergently selected for the ultimate ph of the pectoralis major muscle. BMC Genet. 2016;17:61. doi: 10.1186/s12863-016-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnahhas N., Le Bihan-Duval E., Baéza E., Chabault M., Chartrin P., Bordeau T., Cailleau-Audouin E., Meteau K., Berri C. Impact of divergent selection for ultimate ph of pectoralis major muscle on biochemical, histological, and sensorial attributes of broiler meat1. J. Anim. Sci. 2015;93:4524–4531. doi: 10.2527/jas.2015-9100. [DOI] [PubMed] [Google Scholar]
- Andriantsitohaina R., Auger C., Chataigneau T., Etienne-Selloum N., Li H., Martinez M.C., Schini-Kerth V.B., Laher I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012;108:1532–1549. doi: 10.1017/S0007114512003406. [DOI] [PubMed] [Google Scholar]
- Anthony T.G. Mechanisms of protein balance in skeletal muscle. Domest. Anim. Endocrinol. 2016;56(Suppl):S23–S32. doi: 10.1016/j.domaniend.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista-Ortega J., Ruiz-Feria C. L-arginine and antioxidant vitamins e and c improve the cardiovascular performance of broiler chickens grown under chronic hypobaric hypoxia. Poult. Sci. 2010;89:2141–2146. doi: 10.3382/ps.2010-00764. [DOI] [PubMed] [Google Scholar]
- Bechtel P.J. 1st ed. Vol 2. Academic Press; Orlando, FL: 1986. Page 459 in Muscle as food. Food Science and Technology. [Google Scholar]
- Berri C., Le Bihan-Duval E., Debut M., Sante-Lhoutellier V., Baeza E., Gigaud V., Jego Y., Duclos M.J. Consequence of muscle hypertrophy on characteristics of pectoralis major muscle and breast meat quality of broiler chickens. J. Anim. Sci. 2007;85:2005–2011. doi: 10.2527/jas.2006-398. [DOI] [PubMed] [Google Scholar]
- Bodine S.C., Baehr L.M. Skeletal muscle atrophy and the e3 ubiquitin ligases murf1 and mafbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014;307:E469–E484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodle B.C., Alvarado C., Shirley R.B., Mercier Y., Lee J.T. Evaluation of different dietary alterations in their ability to mitigate the incidence and severity of woody breast and white striping in commercial male broilers. Poult. Sci. 2018;97:3298–3310. doi: 10.3382/ps/pey166. [DOI] [PubMed] [Google Scholar]
- Boerboom G., van Kempen T., Navarro-Villa A., Perez-Bonilla A. Unraveling the cause of white striping in broilers using metabolomics. Poult. Sci. 2018;97:3977–3986. doi: 10.3382/ps/pey266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottje W.G., Lassiter K., Dridi S., Hudson N., Kong B.W. Enhanced expression of proteins involved in energy production and transfer in breast muscle of pedigree male broilers exhibiting high feed efficiency. Poult. Sci. 2017;96:2454–2458. doi: 10.3382/ps/pew453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge J. Fast-growth chickens produce new industry woe: “Spaghetti meat”. The Wall Street Journal. 2019. https://www.wsj.com/articles/fast-growth-chickens-produce-new-industry-woe-spaghetti-meat-11552226401?mod=hp_lead_pos9 Accessed Mar. 2020.
- Chen C.C., Jeon S.M., Bhaskar P.T., Nogueira V., Sundararajan D., Tonic I., Park Y., Hay N. Foxos inhibit mtorc1 and activate akt by inducing the expression of sestrin3 and rictor. Dev. Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Chen K., Yuan S., Peng X., Fang J., Wang F., Cui H., Chen Z., Yuan J., Geng Y. Effects of aflatoxin b1 on oxidative stress markers and apoptosis of spleens in broilers. Toxicol. Ind. Health. 2016;32:278–284. doi: 10.1177/0748233713500819. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang L., Li J., Gao F., Zhou G. Hydrogen peroxide-induced change in meat quality of the breast muscle of broilers is mediated by ros generation, apoptosis, and autophagy in the nf-kappab signal pathway. J. Agric. Food Chem. 2017;65:3986–3994. doi: 10.1021/acs.jafc.7b01267. [DOI] [PubMed] [Google Scholar]
- Christov C., Chrétien F., Abou-Khalil R., Bassez G., Vallet G., Authier F.J., Bassaglia Y., Shinin V., Tajbakhsh S., Chazaud B., Gherardi R.K. Muscle satellite cells and endothelial cells: Close neighbors and privileged partners. Mol. Biology Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz R., Vieira S., Kindlein L., Kipper M., Cemin H., Rauber S. Occurrence of white striping and wooden breast in broilers fed grower and finisher diets with increasing lysine levels. Poult. Science. 2016;96:501–510. doi: 10.3382/ps/pew310. [DOI] [PubMed] [Google Scholar]
- Cui C., Han S., Tang S., He H., Shen X., Zhao J., Chen Y., Wei Y., Wang Y., Zhu Q., Li D., Yin A.H. The autophagy regulatory molecule csrp3 interacts with lc3 and protects against muscular dystrophy. Int. J. Mol. Sci. 2020;21:3. doi: 10.3390/ijms21030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Zotte A., Tasoniero G., Puolanne E., Remignon H., Cecchinato M., Catelli E., Cullere M. Effect of “wooden breast” appearance on poultry meat quality, histological traits, and lesions characterization. Czech J. Anim. Sci. 2017;62:51–57. [Google Scholar]
- Daughtry M.R., Berio E., Shen Z., Suess E.J.R., Shah N., Geiger A.E., Berguson E.R., Dalloul R.A., Persia M.E., Shi H., Gerrard D.E. Satellite cell-mediated breast muscle regeneration decreases with broiler size. Poult. Sci. 2017;96:3457–3464. doi: 10.3382/ps/pex068. [DOI] [PubMed] [Google Scholar]
- Decker E.A., Elias R.J., McClements D.J. Elsevier, Woodhead Publishing Limited; Cambridge: 2010. Oxidation in Foods and Beverages and Antioxidant Applications: Management in Different Industry Sectors. 085709033X. [Google Scholar]
- Deng H., Zheng A., Liu G., Chang W., Zhang S., Cai H. Activation of mammalian target of rapamycin signaling in skeletal muscle of neonatal chicks: effects of dietary leucine and age. Poult. Sci. 2014;93:114–121. doi: 10.3382/ps.2013-03287. [DOI] [PubMed] [Google Scholar]
- Estevez M., Petracci M. Benefits of magnesium supplementation to broiler subjected to dietary and heat stress: improved redox status, breast quality and decreased myopathy incidence. Antioxidants (Basel) 2019;8:10. doi: 10.3390/antiox8100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Lin H., Wang X., Song Z., Jiao H. Vitamin e supplementation alleviates the oxidative stress induced by dexamethasone treatment and improves meat quality in broiler chickens. J. Poult. Sci. 2010;89:318–327. doi: 10.3382/ps.2009-00216. [DOI] [PubMed] [Google Scholar]
- Gao T., Zhao M., Zhang L., Li J., Yu L., Lv P., Gao F., Zhou G. Effect of in ovo feeding of l-arginine on the hatchability, growth performance, gastrointestinal hormones, and jejunal digestive and absorptive capacity of posthatch broilers. J. Animal Science. 2017;95:3079–3092. doi: 10.2527/jas.2016.0465. [DOI] [PubMed] [Google Scholar]
- Goetz F.W., Planas J.V., MacKenzie S. Tumor necrosis factors. Developmental Comp. Immunol. 2004;28:487–497. doi: 10.1016/j.dci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Greene E., Flees J., Dadgar S., Mallmann B., Orlowski S., Dhamad A., Rochell S., Kidd M., Laurendon C., Whitfield H., Brearley C., Rajaram N., Walk C., Dridi S. Quantum blue reduces the severity of woody breast myopathy via modulation of oxygen homeostasis-related genes in broiler chickens. Front Physiol. 2019;10:1251. doi: 10.3389/fphys.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising S.M., Gransee H.M., Mantilla C.B., Sieck G.C. Systems biology of skeletal muscle: fiber type as an organizing principle. Wiley Interdiscip. Rev. Syst. Biol. 2012;4:457–473. doi: 10.1002/wsbm.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerci A., Lahoute C., Hébrard S., Collard L., Graindorge D., Favier M., Cagnard N., Batonnet-Pichon S., Précigout G., Garcia L. Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy. Cell Metabolism. 2012;15:25–37. doi: 10.1016/j.cmet.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Guetchom B., Venne D., Chénier S., Chorfi Y. Effect of extra dietary vitamin e on preventing nutritional myopathy in broiler chickens. J. Appl. Poult. Res. 2012;21:548–555. [Google Scholar]
- Harthan L.B., McFarland D.C., Velleman S.G. The effect of nutritional status and myogenic satellite cell age on Turkey satellite cell proliferation, differentiation, and expression of myogenic transcriptional regulatory factors and heparan sulfate proteoglycans syndecan-4 and glypican-1. Poult. Science. 2014;93:174–186. doi: 10.3382/ps.2013-03570. [DOI] [PubMed] [Google Scholar]
- Havenstein G., Ferket P., Qureshi M. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Science. 2003;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Hoving-Bolink A., Kranen R., Klont R., Gerritsen C., De Greef K. Fibre area and capillary supply in broiler breast muscle in relation to productivity and ascites. Meat Science. 2000;56:397–402. doi: 10.1016/s0309-1740(00)00071-1. [DOI] [PubMed] [Google Scholar]
- Huang X., Ahn D.U. The incidence of muscle abnormalities in broiler breast meat - a review. Korean J. Food Sci. Anim. Resour. 2018;38:835–850. doi: 10.5851/kosfa.2018.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K.S., Hamlin G.A., Lien A.R., Bilgili S.F. Evaluation of capillary and myofiber density in the pectoralis major muscles of rapidly growing, high-yield broiler chickens during increased heat stress. Avian Dis. 2014;58:377–382. doi: 10.1637/10733-112513-Reg.1. [DOI] [PubMed] [Google Scholar]
- Jokl E.J., Blanco G. Disrupted autophagy undermines skeletal muscle adaptation and integrity. Mamm. Genome. 2016;27:525–537. doi: 10.1007/s00335-016-9659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. Lc3, a mammalian homologue of yeast apg8p, is localized in autophagosome membranes after processing. EMBO Journal. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluz S., Kaluzová M., Stanbridge E.J. Regulation of gene expression by hypoxia: Integration of the hif-transduced hypoxic signal at the hypoxia-responsive element. J. Clinica Chim. Acta. 2008;395:6–13. doi: 10.1016/j.cca.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevil C.G., Patel R.P. Preserving vessel function during ischemic disease: new possibilities of inorganic nitrite therapy. Expert Review Cardiovascular Therapy. 2008;6:1175–1179. doi: 10.1586/14779072.6.9.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri B., Seo D., Shouse S., Pan J.H., Hudson N.J., Kim J.K., Bottje W., Kong B.C. Microrna profiling associated with muscle growth in modern broilers compared to an unselected chicken breed. BMC Genomics. 2018;19:683. doi: 10.1186/s12864-018-5061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger J.R., Kadowitz P.J. The nitric oxide pathway in pulmonary vascular disease. Am. J. Cardiol. 2017;120:S71–S79. doi: 10.1016/j.amjcard.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Kuttappan V., Brewer V., Apple J., Waldroup P., Owens C. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V., Brewer V., Mauromoustakos A., McKee S., Emmert J.L., Meullenet J., Owens C. Estimation of factors associated with the occurrence of white striping in broiler breast fillets. Poult. Sci. 2013;92:811–819. doi: 10.3382/ps.2012-02506. [DOI] [PubMed] [Google Scholar]
- Kuttappan V., Lee Y., Erf G., Meullenet J.-F., McKee S., Owens C. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Kuttappan V., Shivaprasad H., Shaw D., Valentine B., Hargis B., Clark F., McKee S., Owens C. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Bottje W., Ramnathan R., Hartson S.D., Coon C.N., Kong B.W., Owens C.M., Vazquez-Anon M., Hargis B. Proteomic analysis reveals changes in carbohydrate and protein metabolism associated with broiler breast myopathy. Poult. Sci. 2017;96:2992–2999. doi: 10.3382/ps/pex069. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Goodgame S., Bradley C., Mauromoustakos A., Hargis B., Waldroup P., Owens C. Effect of different levels of dietary vitamin e (dl-α-tocopherol acetate) on the occurrence of various degrees of white striping on broiler breast fillets. Poult. Sci. 2012;91:3230–3235. doi: 10.3382/ps.2012-02397. [DOI] [PubMed] [Google Scholar]
- Lake J.A., Abasht B. Glucolipotoxicity: a proposed etiology for wooden breast and related myopathies in commercial broiler chickens. Front Physiol. 2020;11:169. doi: 10.3389/fphys.2020.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman D.S. Lubert Stryer, WH Freeman; New York: 1995. Trends in Biochemical Sciences. 1995:0968-0004. [Google Scholar]
- Li Y., Chen Y., Jin W., Fu S., Li D., Zhang Y., Sun G., Jiang R., Han R., Li Z., Kang X., Li G. Analyses of microrna and mrna expression profiles reveal the crucial interaction networks and pathways for regulation of chicken breast muscle development. Front Genet. 2019;10:197. doi: 10.3389/fgene.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang Y., Willems E., Willemsen H., Franssens L., Buyse J., Decuypere E., Everaert N. In ovo l-arginine supplementation stimulates myoblast differentiation but negatively affects muscle development of broiler chicken after hatching. J. Anim. Physiol. Anim. Nutr. (Berl). 2016;100:167–177. doi: 10.1111/jpn.12299. [DOI] [PubMed] [Google Scholar]
- Lilburn M.S., Griffin J.R., Wick M. From muscle to food: oxidative challenges and developmental anomalies in poultry breast muscle. Poult. Sci. 2019;98:4255–4260. doi: 10.3382/ps/pey409. [DOI] [PubMed] [Google Scholar]
- Listrat A., Lebret B., Louveau I., Astruc T., Bonnet M., Lefaucheur L., Picard B., Bugeon J. How muscle structure and composition influence meat and flesh quality. Scientific World J. 2016;2016:2356–6140. doi: 10.1155/2016/3182746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.X., Guo Y.M., Wang Z. Effect of magnesium on reactive oxygen species production in the thigh muscles of broiler chickens. Br. Poult. Sci. 2007;48:84–89. doi: 10.1080/00071660601148187. [DOI] [PubMed] [Google Scholar]
- Livingston M., Ferket P., Brake J., Livingston K. Dietary amino acids under hypoxic conditions exacerbates muscle myopathies including wooden breast and white stripping. Poult. Science. 2018;98:1517–1527. doi: 10.3382/ps/pey463. [DOI] [PubMed] [Google Scholar]
- Livingston M.L., Landon C.D., Barnes H.J., Brake J., Livingston K.A. Dietary potassium and available phosphorous on broiler growth performance, carcass characteristics, and wooden breast. Poult. Sci. 2019;98:2813–2822. doi: 10.3382/ps/pez015. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Mudalal S., Cavani C., Petracci M. Incidence of white striping under commercial conditions in medium and heavy broiler chickens in Italy. J. Appl. Poult. Res. 2014;23:754–758. [Google Scholar]
- Louis J.I., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri A.G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg I., Brengman J.M., Engel A.G. Analysis of cytokine expression in muscle in inflammatory myopathies, duchenne dystrophy, and non-weak controls. J. Neuroimmunol. 1995;63:9–16. doi: 10.1016/0165-5728(95)00122-0. [DOI] [PubMed] [Google Scholar]
- Luque E., Peña J., Martin P., Jimena I., Vaamonde R. Capillary supply during development of individual regenerating muscle fibers. Anatomia, Histologia, Embryologia. 1995;24:87–89. doi: 10.1111/j.1439-0264.1995.tb00016.x. [DOI] [PubMed] [Google Scholar]
- MacRae V.E., Mahon M., Gilpin S., Sandercock D.A., Mitchell M.A. Skeletal muscle fibre growth and growth associated myopathy in the domestic chicken (gallus domesticus) Br. Poult. Sci. 2006;47:264–272. doi: 10.1080/00071660600753615. [DOI] [PubMed] [Google Scholar]
- Malila Y., Chupraj J.U., Srimarut Y., Chaiwiwattrakul P., Uengwetwanit T., Arayamethakorn S., Punyapornwithaya V., Sansamur C., Kirschke C.P., Huang L., Tepaamorndech S., Petracci M., Rungrassamee W., Visessanguan W. Monitoring of white striping and wooden breast cases and impacts on quality of breast meat collected from commercial broilers (gallus gallus) Asian-australas J. Anim. Sci. 2018;31:1807–1817. doi: 10.5713/ajas.18.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malila Y., Tempelman R.J., Sporer K.R., Ernst C.W., Velleman S.G., Reed K.M., Strasburg G.M. Differential gene expression between normal and pale, soft, and exudative Turkey meat. Poult. Sci. 2013;92:1621–1633. doi: 10.3382/ps.2012-02778. [DOI] [PubMed] [Google Scholar]
- Malila Y., Thanatsang K., Arayamethakorn S., Uengwetwanit T., Srimarut Y., Petracci M., Strasburg G.M., Rungrassamee W., Visessanguan W. Absolute expressions of hypoxia-inducible factor-1 alpha (hif1a) transcript and the associated genes in chicken skeletal muscle with white striping and wooden breast myopathies. PLoS One. 2019;14:e0220904. doi: 10.1371/journal.pone.0220904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi J.A.P., Ibelli A.M.G., Peixoto J.O., Cantao M.E., Pandolfi J.R.C., Marciano C.M.M., Zanella R., Settles M.L., Coutinho L.L., Ledur M.C. Whole transcriptome analysis of the pectoralis major muscle reveals molecular mechanisms involved with white striping in broiler chickens. Poult. Sci. 2019;98:590–601. doi: 10.3382/ps/pey429. [DOI] [PubMed] [Google Scholar]
- Marin-Hernandez A., Gallardo-Perez J.C., Ralph S.J., Rodriguez-Enriquez S., Moreno-Sanchez R. Hif-1α modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem. 2009;9:1084–1101. doi: 10.2174/138955709788922610. [DOI] [PubMed] [Google Scholar]
- Marino G., Lopez-Otin C. Autophagy: molecular mechanisms, physiological functions and relevance in human pathology. Cell Mol Life Sci. 2004;61:1439–1454. doi: 10.1007/s00018-004-4012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Maruse H., Inaba Y., Yoshizawa K., Sasazaki S., Fujiwara A., Nishibori M., Nakamura A., Takeda S., Ichihara N., Kikuchi T., Mukai F., Mannen H. The ubiquitin ligase gene (wwp1) is responsible for the chicken muscular dystrophy. FEBS Lett. 2008;582:2212–2218. doi: 10.1016/j.febslet.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Mazzoni M., Petracci M., Meluzzi A., Cavani C., Clavenzani P., Sirri F. Relationship between pectoralis major muscle histology and quality traits of chicken meat. Poult. Sci. 2015;94:123–130. doi: 10.3382/ps/peu043. [DOI] [PubMed] [Google Scholar]
- Miller J.K., Brzezinska-Slebodzinska E., Madsen F.C. Oxidative stress, antioxidants, and animal function. J. Dairy Science. 1993;76:2812–2823. doi: 10.3168/jds.S0022-0302(93)77620-1. [DOI] [PubMed] [Google Scholar]
- Minetti G.C., Feige J.N., Rosenstiel A., Bombard F., Meier V., Werner A., Bassilana F., Sailer A.W., Kahle P., Lambert C. Gαi2 signaling promotes skeletal muscle hypertrophy, myoblast differentiation, and muscle regeneration. Sci. Signal. 2011;4:201. doi: 10.1126/scisignal.2002038. ra80-ra80. [DOI] [PubMed] [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- Musarò A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E.R., Sweeney H.L., Rosenthal N. Localized igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genetics. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Mutryn M.F., Brannick E.M., Fu W., Lee W.R., Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using rna-sequencing. BMC Genomics. 2015;16:399. doi: 10.1186/s12864-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa M.S., Keith B., Simon M.C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer. 2016;16:663–673. doi: 10.1038/nrc.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Ignarro L.J. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch. Pharm. Res. 2009;32:1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- Pampouille E., Hennequet-Antier C., Praud C., Juanchich A., Brionne A., Godet E., Bordeau T., Fagnoul F., Le Bihan-Duval E., Berri C. Differential expression and co-expression gene network analyses reveal molecular mechanisms and candidate biomarkers involved in breast muscle myopathies in chicken. Sci. Rep. 2019;9:14905. doi: 10.1038/s41598-019-51521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papah M.B., Abasht B. Dysregulation of lipid metabolism and appearance of slow myofiber-specific isoforms accompany the development of wooden breast myopathy in modern broiler chickens. Sci. Rep. 2019;9:17170. doi: 10.1038/s41598-019-53728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of wooden breast disease in modern broiler chickens. Avian Pathol. 2017;46:623–643. doi: 10.1080/03079457.2017.1339346. [DOI] [PubMed] [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Gene expression profiling of the early pathogenesis of wooden breast disease in commercial broiler chickens using rna-sequencing. PLoS One. 2018;13:e0207346. doi: 10.1371/journal.pone.0207346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Bonfiglio A., Cavani C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult. Sci. 2013;92:1670–1675. doi: 10.3382/ps.2012-03001. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. World’s Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Petracci M.F.S., Madruga M., Carvalho L., Ida Elza, Est'evez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. comprehensive reviews in food science and food safety. Inst. Food Technologists. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Piekarski-Welsher A., Greene E., Lassiter K., Kong B.C., Dridi S., Bottje W. Enrichment of autophagy and proteosome pathways in breast muscle of feed efficient pedigree male broilers. Front Physiol. 2018;9:1342. doi: 10.3389/fphys.2018.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski A., Khaldi S., Greene E., Lassiter K., Mason J.G., Anthony N., Bottje W., Dridi S. Tissue distribution, gender- and genotype-dependent expression of autophagy-related genes in avian species. PLoS One. 2014;9:e112449. doi: 10.1371/journal.pone.0112449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D.J., McFarland D.C., Cowieson A.J., Muir W.I., Velleman S.G. The effect of nutritional status and muscle fiber type on myogenic satellite cell fate and apoptosis. Poult. Sci. 2014;93:163–173. doi: 10.3382/ps.2013-03450. [DOI] [PubMed] [Google Scholar]
- Powers S.K., Duarte J., Kavazis A.N., Talbert E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiology. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N., Puolanne E., Ruusunen M.J. Department of Food and Environmental Sciences, University of Helsinki; Helsinki, Finland: 2013. The Utilization of Poultry Breast Muscle of Different Quality Classes. [Google Scholar]
- Rabinowitz J.D., White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaelli G., Piccirillo A., Birolo M., Bertotto D., Gratta F., Vascellari C., Xiccato M., Trocino G. and. Effect of age on the occurrence of muscle fiber degeneration associated with myopathies in broiler chickens submitted to feed restriction. Poult. Sci. 2017;96:309–319. doi: 10.3382/ps/pew270. [DOI] [PubMed] [Google Scholar]
- Rehfeldt C., Te Pas M.F., Wimmers K., Brameld J.M., Nissen P.M., Berri C., Valente L.M., Power D.M., Picard B., Stickland N.C., Oksbjerg N. Advances in research on the prenatal development of skeletal muscle in animals in relation to the quality of muscle-based food. I. Regulation of myogenesis and environmental impact. Animal. 2011;5:703–717. doi: 10.1017/S1751731110002089. [DOI] [PubMed] [Google Scholar]
- Relaix F., Rocancourt D., Mansouri A., Buckingham M. A pax3/pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Rhoads R.P., Johnson R.M., Rathbone C.R., Liu X., Temm-Grove C., Sheehan S.M., Hoying J.B., Allen R.E. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am. Journal Physiology Cell Physiology. 2009;296:C1321–C1328. doi: 10.1152/ajpcell.00391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha D.M., Caldas A.P., Oliveira L.L., Bressan J., Hermsdorff H.H. Saturated fatty acids trigger tlr4-mediated inflammatory response. Atherosclerosis. 2016;244:211–215. doi: 10.1016/j.atherosclerosis.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Sachs N.J., Hampton A.R., Foster K.K., Pechanec M.Y., Henderson J.D., King A.J., Mienaltowski M.J. The effects of an alternative diet regimen with natural methionine ingredients on white striping breast myopathy in broiler chickens. Poult. Sci. 2019;98:413–421. doi: 10.3382/ps/pey327. [DOI] [PubMed] [Google Scholar]
- Salles G.B.C., Boiago M.M., Silva A.D., Morsch V.M., Gris A., Mendes R.E., Baldissera M.D., da Silva A.S. Lipid peroxidation and protein oxidation in broiler breast fillets with white striping myopathy. J. Food Biochem. 2019;43:e12792. doi: 10.1111/jfbc.12792. [DOI] [PubMed] [Google Scholar]
- Salomonsson S., Lundberg I.E. Cytokines in idiopathic inflammatory myopathies. Autoimmunity. 2006;39:177–190. doi: 10.1080/08916930600622256. [DOI] [PubMed] [Google Scholar]
- Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (tnf)-alpha: Induction of tnf receptors on human t cells and tnf-alpha-mediated enhancement of t cell responses. J. Immunol. 1987;138:1786–1790. [PubMed] [Google Scholar]
- Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- Schwartzman R.A., Cidlowski J.A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr. Rev. 1993;14:133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- Seko Y., Matsuda H., Kato K., Hashimoto Y., Yagita H., Okumura K., Yazaki Y. Expression of intercellular adhesion molecule-1 in murine hearts with acute myocarditis caused by coxsackievirus b3. J. Clinical Investigation. 1993;91:1327–1336. doi: 10.1172/JCI116333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Linden J., Airas N., Immonen K., Valaja J., Puolanne E. Wooden breast myodegeneration of pectoralis major muscle over the growth period in broilers. Vet. Pathol. 2017;54:119–128. doi: 10.1177/0300985816658099. [DOI] [PubMed] [Google Scholar]
- Siller W. Deep pectoral myopathy: a penalty of successful selection for muscle growth. Poult. Sci. 1985;64:1591–1595. doi: 10.3382/ps.0641591. [DOI] [PubMed] [Google Scholar]
- Slupecka M., Wolinski J., Gajewska M., Pierzynowski S.G. Enteral leptin administration affects intestinal autophagy in suckling piglets. Domest. Anim. Endocrinol. 2014;46:12–19. doi: 10.1016/j.domaniend.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Smet K., Raes K., Huyghebaert G., Haak L., Arnouts S., De Smet S. Lipid and protein oxidation of broiler meat as influenced by dietary natural antioxidant supplementation. Poult. Sci. 2008;87:1682–1688. doi: 10.3382/ps.2007-00384. [DOI] [PubMed] [Google Scholar]
- Smith J.H. Relation of body size to muscle cell size and number in the chicken. Poult. Sci. 1963;42:283–290. [Google Scholar]
- Soglia F., Mudalal S., Babini E., Di Nunzio M., Mazzoni M., Sirri F., Cavani C., Petracci M. Histology, composition, and quality traits of chicken pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2015;95:651–659. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Spence H.J., Chen Y.J., Winder S.J. Muscular dystrophies, the cytoskeleton and cell adhesion. Bioessays. 2002;24:542–552. doi: 10.1002/bies.10098. [DOI] [PubMed] [Google Scholar]
- Stefanetti R.J., Voisin S., Russell A., Lamon S. Recent Advances in Understanding the Role of Foxo3. Review. 2018;7:F1000. doi: 10.12688/f1000research.15258.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling K.G., Pesti G.M., Bakalli R.I. Performance of different broiler genotypes fed diets with varying levels of dietary crude protein and lysine. Poult. Sci. 2006;85:1045–1054. doi: 10.1093/ps/85.6.1045. [DOI] [PubMed] [Google Scholar]
- Surai P.F. World Poultry Science Association; Strasbourg, France: 2007. Pages 26–30 in Natural Antioxidants in Poultry Nutrition: New Developments. Proceedings of the 16th European Symposium on Poultry Nutrition. [Google Scholar]
- Tallentire C.W., Leinonen I., Kyriazakis I. Artificial selection for improved energy efficiency is reaching its limits in broiler chickens. Sci. Rep. 2018;8:1168. doi: 10.1038/s41598-018-19231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocino A., Piccirillo A., Birolo M., Radaelli G., Bertotto D., Filiou E., Petracci M., Xiccato G. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poult. Sci. 2015;94:2996–3004. doi: 10.3382/ps/pev296. [DOI] [PubMed] [Google Scholar]
- USDA-FSIS . Book-FSIS Notice 35-17. USDA; Washington, DC: 2017. Disposition instructions for woody breast and white striping poultry conditions. [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. The Lancet. 1989;334:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Nitric oxide synthesised from l-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovasc. Res. 1989;23:1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- Velleman S.G. Pectoralis major (breast) muscle extracellular matrix fibrillar collagen modifications associated with the wooden breast fibrotic myopathy in broilers. Front Physiol. 2020;11:461. doi: 10.3389/fphys.2020.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velleman S.G. Recent developments in breast muscle myopathies associated with growth in poultry. Annu. Rev. Anim. Biosci. 2019;7:289–308. doi: 10.1146/annurev-animal-020518-115311. [DOI] [PubMed] [Google Scholar]
- Velleman S.G. Relationship of skeletal muscle development and growth to breast muscle myopathies: a review. Avian Diseases. 2015;59:525–531. doi: 10.1637/11223-063015-Review.1. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Anderson J.W., Coy C.S., Nestor K.E. Effect of selection for growth rate on muscle damage during Turkey breast muscle development. Poult. Sci. 2003;82:1069–1074. doi: 10.1093/ps/82.7.1069. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L. Histopathologic and myogenic gene expression changes associated with wooden breast in broiler breast muscles. Avian Dis. 2015;59:410–418. doi: 10.1637/11097-042015-Reg.1. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L., Tonniges J.R. The effect of the wooden breast myopathy on sarcomere structure and organization. Avian Dis. 2018;62:28–35. doi: 10.1637/11766-110217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L., Tonniges J.R. Fibrillar collagen organization associated with broiler wooden breast fibrotic myopathy. Avian Dis. 2017;61:481–490. doi: 10.1637/11738-080217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Verdiglione R., Cassandro M. Characterization of muscle fiber type in the pectoralis major muscle of slow-growing local and commercial chicken strains. Poult. Sci. 2013;92:2433–2437. doi: 10.3382/ps.2013-03013. [DOI] [PubMed] [Google Scholar]
- Vignale K., Caldas J.V., England J.A., Boonsinchai N., Magnuson A., Pollock E.D., Dridi S., Owens C.M., Coon C.N. Effect of white striping myopathy on breast muscle (pectoralis major) protein turnover and gene expression in broilers. Poult. Sci. 2017;96:886–893. doi: 10.3382/ps/pew315. [DOI] [PubMed] [Google Scholar]
- Voljč M., Frankič T., Levart A., Nemec M., Salobir J. Evaluation of different vitamin e recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat. Poult. Science. 2011;90:1478–1488. doi: 10.3382/ps.2010-01223. [DOI] [PubMed] [Google Scholar]
- Wang J., Clark D.L., Jacobi S.K., Velleman S.G. Effect of vitamin e and omega-3 fatty acids early posthatch supplementation on reducing the severity of wooden breast myopathy in broilers. Poult. Sci. 2020;99:2108–2119. doi: 10.1016/j.psj.2019.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman J.R.F., Kirby Y.K., Ismail M., Bottje W.G., Moore R.W., Vardeman R.C. Supplemental l-arginine attenuates pulmonary hypertension syndrome (ascites) in broilers. Poult. Sci. 1995;74:323–330. doi: 10.3382/ps.0740323. [DOI] [PubMed] [Google Scholar]
- Wilson B.W., Nieberg P.S., Buhr R.J., Kelly B.J., Shultz F.T. Turkey muscle growth and focal myopathy. Poult. Sci. 1990;69:1553–1562. doi: 10.3382/ps.0691553. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Xiang R.P., Sun W.D., Wang J.Y., Wang X.L. Effect of vitamin c on pulmonary hypertension and muscularisation of pulmonary arterioles in broilers. Br. Poult. Sci. 2002;43(5 Suppl):705–712. doi: 10.1080/0007166021000025064. [DOI] [PubMed] [Google Scholar]
- Xue Q., Zhang G., Li T., Ling J., Zhang X., Wang J. Transcriptomic profile of leg muscle during early growth in chicken. PLoS One. 2017;12:e0173824. doi: 10.1371/journal.pone.0173824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Hu P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Translat. 2018;13:25–32. doi: 10.1016/j.jot.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L.L., Gao T., Zhao M.M., Lv P.A., Zhang L., Li J.L., Jiang Y., Gao F., Zhou G.H. Effects of in ovo feeding of l-arginine on breast muscle growth and protein deposition in post-hatch broilers. Animal. 2018;12:2256–2263. doi: 10.1017/S1751731118000241. [DOI] [PubMed] [Google Scholar]
- Zambonelli P., Zappaterra M., Soglia F., Petracci M., Sirri F., Cavani C., Davoli R. Detection of differentially expressed genes in broiler pectoralis major muscle affected by white striping–wooden breast myopathies. Poult. Science. 2016;95:2771–2785. doi: 10.3382/ps/pew268. [DOI] [PubMed] [Google Scholar]
- Zammit P.S., Golding J.P., Nagata Y., Hudon V., Partridge T.A., Beauchamp J.R. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biology. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Gao T., Zhang L., Li J., Lv P., Yu L., Gao F., Zhou G. Effects of in ovo feeding of creatine pyruvate on the hatchability, growth performance and energy status in embryos and broiler chickens. Animal. 2017;11:1689–1697. doi: 10.1017/S1751731117000374. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]