Abstract:
Objective
To assess the association between meniscal volume, its change over time and the development of knee OA after 30 months in overweight/obese women.
Methods
Data from the PRevention of knee Osteoarthritis in Overweight Females study were used. This cohort included 407 women with a BMI ≥ 27 kg/m2, free of OA-related symptoms. The primary outcome measure was incident OA after 30 months, defined by one out of the following criteria: medial or lateral joint space narrowing (JSN) ≥ 1.0 mm, incident radiographic OA [Kellgren and Lawrence (K&L) ≥ 2], or incident clinical OA. The secondary outcomes were either of these items separately. Menisci at both baseline and follow-up were automatically segmented to obtain meniscal volume and delta-volumes. Generalized estimating equations were used to evaluate associations between the volume measures and the outcomes.
Results
Medial and lateral baseline and delta-volumes were not significantly associated to the primary outcome. Lateral meniscal baseline volume was significantly associated to lateral JSN [odds ratio (OR) = 0.87; 95% CI: 0.75, 0.99], while other measures were not. Medial and lateral baseline volume were positively associated to K&L incidence (OR = 1.32 and 1.22; 95% CI: 1.15, 1.50 and 1.03, 1.45, respectively), while medial and lateral delta-volume were negatively associated to K&L incidence (OR = 0.998 and 0.997; 95% CI: 0.997, 1.000 and 0.996, 0.999, respectively). None of the meniscal measures were significantly associated to incident clinical OA.
Conclusion
Larger baseline meniscal volume and the decrease of meniscal volume over time were associated to the development of structural OA after 30 months in overweight and obese women.
Keywords: meniscal volume, knee osteoarthritis, MRI
Rheumatology key messages
Medial and lateral baseline volume were positively associated to K&L incidence, while medial and lateral delta- volume were negatively associated to K&L incidence.
Lateral meniscal baseline volume was associated to lateral JSN.
Introduction
The diagnosis of OA is mainly based on symptoms and radiographic features. Since 1986, ACR criteria have been used to classify knee OA [1]. More recently, MRI has been shown to have a higher sensitivity in detecting structural knee OA, especially when compared with Kellgren and Lawrence (K&L) grading on weight-bearing posterior-anterior flexed knee radiographs [2]. Several studies indicated that MRI is able to detect early OA features in asymptomatic persons without radiographic knee OA [3, 4]. Radiographic abnormalities in OA have been described extensively, including joint space narrowing (JSN), sclerosis of subchondral bone and the presence of osteophytes. Compared with the surrogate measurement of JSN on radiographic images, MRI enables direct evaluation of the cartilage, which is the main abnormality in OA. Therefore, the MRI holds promise as an alternative to radiography in the evaluation of joint structure [5], although until now there has been no consensus or a standardized scoring system for knee OA, especially in quantitative MRI-based measurement.
It is widely accepted that a strong causal relationship between meniscal damage and structural progression of OA exists [6]. A meniscal pathway to knee OA was implicated by a loss of meniscal function due to damage or extrusion, leading to increased biomechanical stress in the knee joint. This stress results in damage such as cartilage loss, subchondral bone changes, bone marrow lesions and synovitis, eventually resulting in symptomatic OA [7]. In view of this significant pathway in the pathogenesis of OA, it is important to assess the presence of meniscal pathologies, especially when studying early-stage knee OA.
To better understand the meniscal changes, previous studies described meniscal constructs such as volume, extrusion, thickness (height) and tibial coverage [8–10]. In a recent study, we confirmed an independent association between meniscal extrusion and the development of knee OA in overweight and obese women [11]. However, extrusion was scored semi-quantitatively using MRI Osteoarthritis Knee Score (MOAKS) [12], which does not consider the absolute sizes of both tibial plateau and meniscus, and the percentage of tibial cartilage covered by the meniscus.
The quantification of meniscal volume has been explored by segmentation of MRI images to obtain 3D volumetric morphometry. However, until now, there are still conflicting results on the association between meniscal volume and incident knee OA [13–15]. In this study, we therefore evaluated the association between both baseline meniscal volume and its longitudinal change and incident knee OA among middle-aged, overweight and obese women. By quantitatively analysing meniscal volume for those who are at high risk for OA development, we tried to determine whether meniscal volume could be a biomarker for incident knee OA.
Methods
For this study, data from the PRevention of knee Osteoarthritis in Overweight Females (PROOF) study [16] were used. Details regarding this study have been described previously (ISRCTN 42823086) [14]. In short, the original study was a randomized controlled trial in which the intervention groups received a weight-loss programme and/or glucosamine sulphate or placebo, to determine whether these interventions prevent the onset of knee OA. As both interventions proved to have no significant effects on OA development, data is here treated as a cohort, with additional adjustments for the randomized intervention groups. The PROOF study has been approved by the Medical Ethics Committee of Erasmus MC University Medical Center Rotterdam, the Netherlands.
Subjects
This cohort consisted of 407 overweight and obese women between 50 and 60 years old with a BMI ≥ 27 kg/m2. At baseline they were free of symptoms of knee OA according to the clinical criteria of the ACR [17] or other rheumatic diseases, were not being treated for knee complaints, were not using walking aids, had no contraindications for MRI, mastered the Dutch language, and did not use glucosamine [16, 18]. The participating women were recruited through their general practitioner. At both baseline and 30 months follow-up (FU) time, all subjects filled in a questionnaire on knee pain, physical activity level, quality of life, previous knee injuries, menopausal status and comorbidities. They also underwent a physical examination for Heberden’s nodes and measurement of body weight and height to calculate the BMI at baseline and FU.
MRI and radiography
MRI (1.5 T) was performed using the Philips Medical Systems (Model Intera), SIEMENS (Model Symphony and Model MAGNETOM ESSENZA) with a dedicated rigid knee coil for all knees at baseline and after 30 months FU. The protocol included coronal and sagittal non-fat suppressed proton density-weighted sequences (slice thickness 3.0 mm/slice gap 0.3 mm) and a sagittal 3D water selective sequence (WATS) with fat saturation (slice thickness 1.5 mm) with a coronal planar reconstruction, amongst other sequences [18]. Meniscal pathology, including extrusion and tears, was scored on the MR images by two trained readers and an experienced musculoskeletal radiologist, using the MOAKS scoring system [12, 19]. As previously published, the reliability of the scoring of the change in MOAKS features, determined by prevalence-adjusted bias-adjusted kappa (PABAK) statistics, showed ‘substantial’ to ‘nearly perfect’ agreement (range 0.77–0.88, observed agreement 89–94%) [19, 20].
Weight-bearing semi-flexed posterior-anterior knee radiographs of both knees were acquired with the metatarsophalangeal protocol [21] at baseline and after 30 months and scored according to the K&L criteria [22]. Joint space width and the medial knee alignment angle were measured on the radiographs for all knees. As previously described, reproducibility tests showed moderate agreement for KL grade (κ = 0.6) and good agreement for alignment (κ = 0.7) and minimal joint space width (κ = 0.7) [16].
Meniscus segmentation and volume quantification
The medial and lateral menisci from all knees at baseline and FU were segmented fully automatically in the coronal, proton density-weighted MRI scan, using in-house developed software that combines multi-atlas segmentation-by-registration with a high-dimensional voxel-based appearance model [23–25]. In this approach, the atlas was formed by 25 MRI scans from the PROOF data, which were manually segmented by using open-source ITK-SNAP software [26]. Manual segmentation of the menisci was performed on the coronal proton density sequence and was checked on the sagittal proton density and sagittal WATS images. Segmentation was done from anterior to posterior and performed on all slices where the meniscus was identifiable.
After the baseline and FU meniscal volumes were acquired from the segmentation, volume change over time (delta-volume) and relative volume change (relative delta-volume) were calculated. Delta-volumes were calculated by subtracting the baseline volume from the FU volume. The relative delta-volume was obtained by expressing the delta-volume as a percentage of the baseline volume, positive changes of volume over time signifying growth of meniscus, while negative changes signify shrinkage.
Outcome measures
The primary outcome measure was the incidence of knee OA after 30 months, which was defined for each knee as at least one out of the following three criteria: (i) JSN in the medial or lateral compartment ≥ 1.0 mm; (ii) incident radiographic knee OA, defined by K&L ≥ 2 at FU, with baseline K&L < 2; or (iii) incident clinical knee OA according to the combined clinical and radiographic ACR criteria. The secondary outcomes were any of these items separately.
Statistics
Descriptive statistics were used for the baseline characteristics. To verify the reliability of the automated meniscus segmentation on MRI, we performed a 10-fold cross-validation [27] experiment on the atlas set of 25 MRI scans, comparing the automatic segmentations with the manual segmentations using the Dice similarity coefficient (DSC) [28]. The value of DSC ranges from 0, indicating no spatial overlap between the two segmentations, to 1, indicating perfect agreement [28]. The association between independent variables [baseline and (relative) delta-volumes] and both primary and secondary outcomes were analysed separately. These analyses were done by performing generalized estimating equations (GEE) in SPSS 25, which treated two knees within subjects as repeat measurement. The GEEs were adjusted for baseline meniscal volume of medial or lateral side (when using baseline volume as independent factor, using 100 mm3 as a unit), medial or lateral delta-volume (when using delta-volume as independent factor, using 100 mm3 as a unit), BMI, age, knee injury, knee alignment, postmenopausal status, Heberden’s nodes, meniscal pathologies, meniscal extrusion, osteophytes and cartilage defects at baseline. Also, to further understand the relationship between meniscal volume and meniscal extrusion, we analysed whether meniscal volume was a confounder for the previously published association between meniscal extrusion and OA development in the same cohort [11]. A P-value <0.05 was used to indicate statistical significance in all tests.
Results
Baseline and FU characteristics
A total of 407 women were eligible to participate in the PROOF study. First, 97 knees without MRI data at baseline were removed. In addition, knees with missing data for the primary outcome (n = 91) were excluded leaving 626 knees (338 subjects) for the final analysis. There were no statistically significant differences in baseline characteristics between included and excluded knees (data not shown). All baseline characteristics of the eligible sample are presented in Table 1.
Table 1.
Characteristics and features of the knee joint at baseline
| Characteristic variables | N (%) | Mean (s.d.) |
|---|---|---|
| Age at baseline (yr) | 814 (100) | 55.7 (3.2) |
| Baseline BMI (kg/m2) | 814 (100) | 32.4 (4.3) |
| Baseline self-report knee injury | 101 (12.4) | |
| Baseline cartilage defect | 411 (50.5) | |
| Baseline osteophyte | 474 (58.2) | |
| Heberden’s nodes | 216 (26.5) | |
| Knee varus alignment | 323 (39.7) | |
| Baseline postmenopausal | 550 (67.6) | |
| Meniscus pathologies without extrusion | 504 (61.9) | |
| Baseline medial volume (mm3) | 723 (88.8) | 1343.21 (320.50) |
| Baseline lateral volume (mm3) | 721 (88.6) | 1129.99 (263.17) |
| Baseline medial meniscal extrusion | 203 (24.9) | |
| Baseline lateral meniscal extrusion | 18 (2.2) | |
| K&L scores | 810 (100) | |
| K&L = 0 | 412 (50.9) | |
| K&L = 1 | 344 (42.5) | |
| K&L = 2 | 49 (6.0) | |
| K&L = 3 | 5 (0.6) | |
| Clinical knee OA | 32 (4.0) |
Baseline meniscal extrusion was defined as MOAKS ≥ 2, Heberden’s nodes was defined as a Heberden’s node in at least one hand. K&L: Kellgren and Lawrence.
One hundred and eleven knees (17.7%) developed knee OA according to the primary outcome after 30 months. Thirty-three knees (5.3%) developed medial JSN, 36 knees (5.8%) developed lateral JSN, 72 knees (11.6%) developed incident radiographic knee OA, and 49 knees (7.8%) developed incident clinical knee OA.
Meniscus segmentation
An example of meniscus segmentation was shown in Fig. 1. The cross-validation experiment on the atlas resulted in an average DSC of 0.75, which is in line with results reported in the literature for automated meniscus segmentation on 1.5 T MRI [29, 30].
Fig. 1.
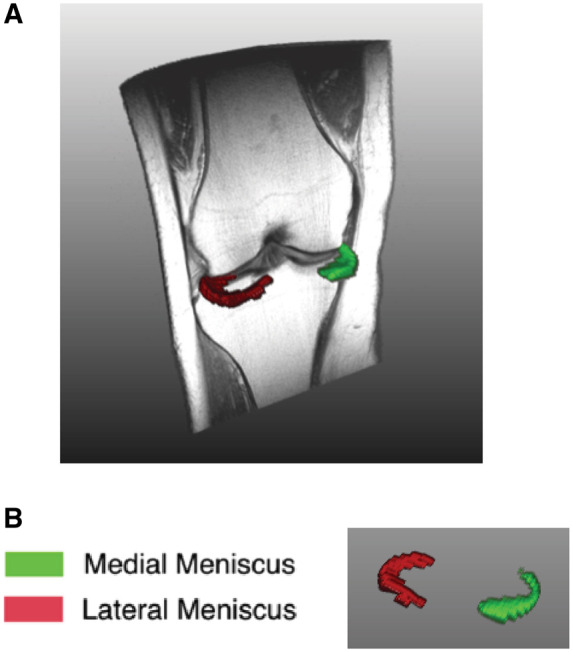
Example of meniscus segmentation
(A) 3D overview of one left knee and coronal view of meniscus segmentation. (B) 3D view of meniscus from segmentation (green: medial meniscus; red: lateral meniscus).
Baseline meniscal volume and knee OA development
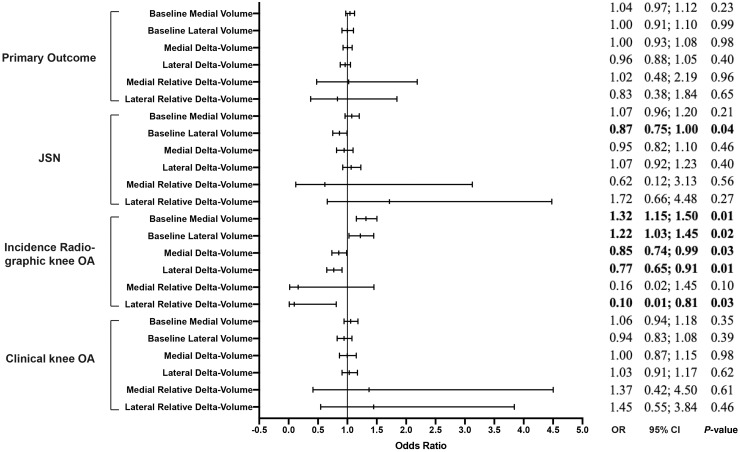
Baseline medial and lateral volume were not significantly associated to the primary outcome [odds ratio (OR)=1.04; 95% CI: 0.97, 1.12 and OR=1.00; 95% CI: 0.91, 1.10]. Lateral meniscal volume (not medial) was significantly associated to lateral JSN (OR =0.87; 95% CI: 0.75, 1.00). Baseline medial and lateral volume were both significantly associated with incident radiographic knee OA (OR=1.32; 95% CI: 1.15, 1.50 and OR=1.22; 95% CI: 1.03, 1.45). Additional adjustments for intervention groups did not result in significant changes of the results (data not shown). The associations between all baseline meniscal volumes and incident clinical knee OA were not statistically significant (see Fig. 2).
Fig. 2.
Association between baseline and delta meniscal volume and primary and secondary outcomes (baseline and 30 months)
All odds ratios are adjusted for meniscal volume, BMI, age, knee injury and knee alignment, postmenopausal status, Heberden’s nodes, meniscal pathologies, extrusion, osteophytes and cartilage defects at baseline. OR: odds ratio; JSN medial (lateral): medial (lateral) joint space narrowing. OR>1 signify larger volume at baseline or growth of volume during follow-up. OR<1 signify lower volume at baseline or shrinkage of volume during follow-up.
Longitudinal meniscal volume changes and knee OA development
All associations between meniscal delta-volume, relative delta-volume and the primary and secondary outcome measures are presented in Fig. 2. Neither medial nor lateral delta-volume were significantly associated with the primary outcome or medial/lateral JSN. Both medial and lateral delta-volume showed significant associations with incident radiographic knee OA (OR=0.85; 95% CI: 0.74, 0.99 and OR=0.77; 95% CI: 0.65, 0.91). Lateral relative delta-volume was significantly associated to incident radiographic knee OA (OR=0.10; 95% CI: 0.01, 0.81). The associations between all meniscal changes and incident clinical knee OA were not significant. Additional adjustments for intervention groups did not result in significant changes of the results (data not shown).
Meniscal extrusion
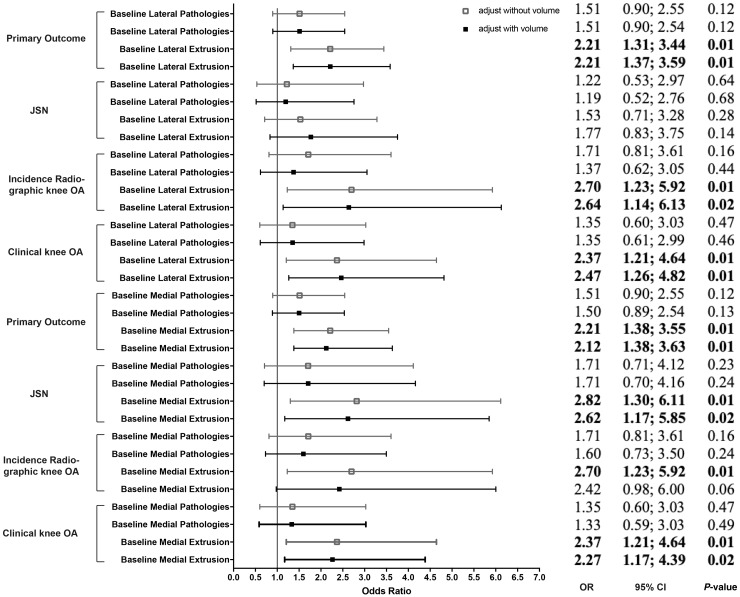
By comparing the association between meniscal extrusion and all outcomes with and without adjusting for baseline meniscal volume, we found the odds for OA development in knees with meniscal extrusion only changed marginally after additional adjustment for baseline meniscal volume (see Fig. 3).
Fig. 3.
Association between baseline meniscal extrusion and primary and secondary outcomes, with and without adjustment for meniscal volume (baseline and 30 months)
All odds ratios are adjusted for meniscal volume, BMI, age, knee injury and knee alignment, postmenopausal status, Heberden’s nodes, meniscal pathologies, extrusion, osteophytes and cartilage defects at baseline. OR: odds ratio; JSN medial (lateral): medial (lateral) joint space narrowing. Hollow square: adjusted without meniscal volume; solid square: adjusted with meniscal volume.
Discussion
In the present study we evaluated the association between the volume of the meniscus and its change over time and the development of knee OA in a high-risk group of overweight and obese women. We found that subjects with larger baseline volume (potentially suggestive for meniscus swelling) and a decrease of meniscal volume over time had a higher risk for incident radiographic knee OA. Only baseline lateral meniscal volume was associated with lateral JSN, while neither medial nor lateral meniscal volume were significantly related to incident clinical knee OA.
The meniscus is considered to be a protective structure by providing biomechanical support in a healthy knee joint. However, as our results indicate, both larger meniscal volume at baseline and the decrease of volume during FU may act as risk factors for the development of knee OA in overweight and obese women. Previously, Andrea et al. reported larger meniscal volume in the lateral meniscus body in knee OA subjects [13] and Wolfgang et al. found that menisci were thicker in OA knees and had a larger meniscal volume when compared with non-OA knees [8]. As individuals in the current study were free of clinical knee OA at baseline, the results suggest that swelling of the menisci may take place prior to the shrinkage of the menisci, along with the development of structural knee OA; similar to cartilage swelling that is reported to occur prior to cartilage degeneration [31, 32].
We found that meniscal volume was not significantly related to the incidence of clinical knee OA. This may be because the FU period was only 30 months, when clinical complaints like pain may not be observed yet in people free of symptoms and disease at baseline [17]. Other studies also concluded that structural features of OA (e.g. osteophytes) were more reliable than clinical symptoms as an early indication of knee OA, as pain is more commonly seen in higher grades of OA [33, 34]. As individuals with more severe radiographic OA features show an increased risk for the presence of knee pain [35], it is important to identify individuals at increased risk for radiographic knee OA, for example using meniscal volume as a predictive biomarker.
As greater baseline meniscal volume and decrease of volume during FU were associated to the incidence of K&L ≥ 2, which is defined by the combination of definite osteophytes and possible JSN, but not to JSN alone, we could further hypothesize that meniscal volume is related to osteophyte formation. As a consequence of meniscal volume change, mechanical stresses or soluble growth factors like insulin-like growth factor-1, fibroblast growth factor and bone morphogenetic protein or transforming growth factor-β may activate compensatory cartilage repair, which then induces the osteophyte formation [36–38].
According to previous studies and our current results, meniscal volume and meniscal extrusion are both independently associated to incidence of radiographic knee OA [11, 39]. There are several theories suggesting that meniscal volume and extrusion are interrelated. Wenger et al. suggested that meniscal extrusion could coexist with change in meniscal volume, possibly because the extruded part of the meniscus potentially swells as it becomes unloaded outside the joint margin [13]. Another hypothesis is that a swollen meniscus at baseline may be more vulnerable to becoming extruded, owing to its larger size. The displacement of the meniscus caused by both meniscal extrusion and swelling may alter the knee load distribution capacities, which could lead to osteophyte formation and cartilage loss. However, further research is needed to test these hypotheses.
There are some strengths and limitations to our study. By using MRI, we confirmed a quantitative biomarker of meniscal volume to be associated with the incidence of radiographic knee OA. This measurement potentially provides a tool to detect knee OA in overweight women, especially in the early phase of the disease. Early detection may help intervention since pre-OA is suggested to be a modifiable disease process [40]. Also, the change in meniscal volume during FU has the potential to become a surrogate end point. Moreover, our analyses make use of automatic segmentations of the meniscus, instead of manual segmentations, as it means the segmentations are objective and repeatable, which would make it more suitable for future clinical use. One limitation is that three different scanners were used throughout the cohort. However, the scanner type was only associated to meniscal volume, which was the exposure in the GEE models. The adjustment for scanner type should therefore be unnecessary [41]. Although there were different treatment groups in this cohort, additional adjustment for the treatment groups did not significantly affect the results (data not shown). Another limitation was the FU time of only 30 months, which might be relatively short for evaluating a degenerative disease, especially in subjects without symptoms at baseline. In this study, we did not indicate a cut-off value for meniscal volume in subjects with high risk of knee OA. Once meniscal volume is indisputably proven as a biomarker for knee OA development, new initiatives on valuable cut-off scores should be undertaken.
Conclusion
As is known for cartilage volume, knees with higher baseline meniscal volume and a stronger decrease in meniscal volume over time are at increased risk for developing radiographic knee OA. Given the lack of a (reversed) association between meniscal measures and medial/lateral JSN, this suggests a relation with osteophyte growth, but this relation needs to be confirmed in future studies. Meniscal volume may function as a prognostic biomarker for future structural knee OA in overweight and obese women.
Acknowledgements
D.X. contributed to data analysis, interpretation, writing of the manuscript and final approval of the article. J.V. contributed to revision of the article. M.H., S.K. and E.O. contributed to analysis of MRIs and critical revision of the article in the methods part. F.W. contributed to the primary analysis of the data and interpretation. S.B.Z. contributed to the conception and design of the study including obtaining funding and revision of the article. J.R. contributed to the conception and design of the study, including data collection, analysis, results interpretation and critical revision of the article. Data are available on reasonable request. The research has previously been accepted as an abstract and poster presentation at the Osteoarthritis Research Society International (OARSI) World Congress 2019 and as an oral presentation at the International Workshop on Osteoarthritis Imaging (IWOAI) 2019.
Funding: This research was sponsored by the Netherlands Organisation for Health Research and Development (ZonMw 120520001), the China Scholarship Council (No. 201806380153) and the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 654248, CORBEL – shared services for life-science.
Disclosure statement: S.B.Z. reports grants from the Netherlands Organisation for Health Research and Development, the Dutch Arthritis Association, personal fees from Osteoarthritis Research Society International and consultancy fee from Infirst Healthcare. N.M. declares funds from the European Union’s Horizon 2020 research and innovation programme. The other authors have declared no conflicts of interest.
References
- 1. Zhang W, Doherty M, Peat G. et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis 2010;69:483–9. [DOI] [PubMed] [Google Scholar]
- 2. Schiphof D, Oei EH, Hofman A. et al. Sensitivity and associations with pain and body weight of an MRI definition of knee osteoarthritis compared with radiographic Kellgren and Lawrence criteria: a population-based study in middle-aged females. Osteoarthritis Cartilage 2014;22:440–6. [DOI] [PubMed] [Google Scholar]
- 3. Niu J, Felson DT, Neogi T. et al. Patterns of coexisting lesions detected on magnetic resonance imaging and relationship to incident knee osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Rheum 2015;67:3158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma L, Hochberg M, Nevitt M. et al. Knee tissue lesions and prediction of incident knee osteoarthritis over 7 years in a cohort of persons at higher risk. Osteoarthritis Cartilage 2017;25:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunter DJ, Zhang W, Conaghan PG. et al. Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthritis Cartilage 2011;19:589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma L, Eckstein F, Song J. et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum 2008;58:1716–26. [DOI] [PubMed] [Google Scholar]
- 7. Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A.. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol 2012;8:412–9. [DOI] [PubMed] [Google Scholar]
- 8. Wirth W, Frobell RB, Souza RB. et al. A three-dimensional quantitative method to measure meniscus shape, position, and signal intensity using MR images: a pilot study and preliminary results in knee osteoarthritis. Magn Reson Med 2010;63:1162–71. [DOI] [PubMed] [Google Scholar]
- 9. Bowers ME, Tung GA, Fleming BC, Crisco JJ, Rey J.. Quantification of meniscal volume by segmentation of 3T magnetic resonance images. J Biomech 2007;40:2811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siorpaes K, Wenger A, Bloecker K. et al. Interobserver reproducibility of quantitative meniscus analysis using coronal multiplanar DESS and IWTSE MR imaging. Magn Reson Med 2012;67:1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Voet JA, Runhaar J, van der Plas P. et al. Baseline meniscal extrusion associated with incident knee osteoarthritis after 30 months in overweight and obese women. Osteoarthritis Cartilage 2017;25:1299–303. [DOI] [PubMed] [Google Scholar]
- 12. Hunter DJ, Guermazi A, Lo GH. et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage 2011;19:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wenger A, Wirth W, Hudelmaier M. et al. Meniscus body position, size, and shape in persons with and persons without radiographic knee osteoarthritis: quantitative analyses of knee magnetic resonance images from the osteoarthritis initiative. Arthritis Rheum 2013;65:1804–11. [DOI] [PubMed] [Google Scholar]
- 14. Bloecker K, Guermazi A, Wirth W. et al. Tibial coverage, meniscus position, size and damage in knees discordant for joint space narrowing -- data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2013;21:419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dube B, Bowes MA, Kingsbury SR. et al. Where does meniscal damage progress most rapidly? An analysis using three-dimensional shape models on data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2018;26:62–71. [DOI] [PubMed] [Google Scholar]
- 16. Runhaar J, van Middelkoop M, Reijman M. et al. Prevention of knee osteoarthritis in overweight females: the first preventive randomized controlled trial in osteoarthritis. Am J Med 2015;128:888–95.e4. [DOI] [PubMed] [Google Scholar]
- 17. Altman R, Asch E, Bloch D. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 18. Zhang F, Bierma-Zeinstra SM, Oei EH. et al. Factors associated with meniscal body extrusion on knee MRI in overweight and obese women. Osteoarthritis Cartilage 2017;25:694–9. [DOI] [PubMed] [Google Scholar]
- 19. Runhaar J, Schiphof D, van Meer B. et al. How to define subregional osteoarthritis progression using semi-quantitative MRI osteoarthritis knee score (MOAKS). Osteoarthritis Cartilage 2014;22:1533–6. [DOI] [PubMed] [Google Scholar]
- 20. Landsmeer MLA, de Vos BC, van der Plas P. et al. Effect of weight change on progression of knee OA structural features assessed by MRI in overweight and obese women. Osteoarthritis Cartilage 2018;26:1666–74. [DOI] [PubMed] [Google Scholar]
- 21. Buckland-Wright JC, Wolfe F, Ward RJ, Flowers N, Hayne C.. Substantial superiority of semiflexed (MTP) views in knee osteoarthritis: a comparative radiographic study, without fluoroscopy, of standing extended, semiflexed (MTP), and Schuss views. J Rheumatol 1999;26:2664–74. [PubMed] [Google Scholar]
- 22. Kellgren JH, Lawrence JS.. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Lijn F, de Bruijne M, Klein S. et al. Automated brain structure segmentation based on atlas registration and appearance models. IEEE Trans Med Imaging 2012;31:276–86. [DOI] [PubMed] [Google Scholar]
- 24. Fortunati V, Verhaart RF, van der Lijn F. et al. Tissue segmentation of head and neck CT images for treatment planning: a multiatlas approach combined with intensity modeling. Med Phys 2013;40:071905. [DOI] [PubMed] [Google Scholar]
- 25. Hansson NM, Oei EHG, Klein S. Evaluation of two multi-atlas cartilage segmentation models for knee MRI: data from the osteoarthritis initiative. Poster presentation at 9th International Workshop on Osteoarthritis Imaging (IWOAI), Oulu, Finland, 2016.
- 26. Yushkevich PA, Piven J, Hazlett HC. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31:1116–28. [DOI] [PubMed] [Google Scholar]
- 27. Gareth JD, Hastie T, Tibshirani R.. An introduction to statistical learning. 1st edn.New York: Springer-Verlag, 2013. [Google Scholar]
- 28. Zou KH, Warfield SK, Bharatha A. et al. Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol 2004;11:178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rahman MM, Durselen L, Seitz AM.. Automatic segmentation of knee menisci -- A systematic review. Artif Intell Med 2020;105:101849. [DOI] [PubMed] [Google Scholar]
- 30. Fripp J, Bourgat P, Engstrom C, Ourselin S, Crozier S, Salvado O. Automated segmentation of the menisci from MR images. In: Proceedings of the 6th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Boston, MA, 2009.
- 31. Ding C, Martel-Pelletier J, Pelletier JP. et al. Two-year prospective longitudinal study exploring the factors associated with change in femoral cartilage volume in a cohort largely without knee radiographic osteoarthritis. Osteoarthritis Cartilage 2008;16:443–9. [DOI] [PubMed] [Google Scholar]
- 32. Bank RA, Soudry M, Maroudas A, Mizrahi J, TeKoppele JM.. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum 2000;43:2202–10. [DOI] [PubMed] [Google Scholar]
- 33. Creamer P, Hochberg MC.. Why does osteoarthritis of the knee hurt–sometimes? Br J Rheumatol 1997;36:726–8. [DOI] [PubMed] [Google Scholar]
- 34. Eckstein F, Burstein D, Link TM.. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed 2006;19:822–54. [DOI] [PubMed] [Google Scholar]
- 35. Neogi T, Felson D, Niu J. et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ 2009;339:b2844–b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blom AB, van der Kraan PM, van den Berg WB.. Cytokine targeting in osteoarthritis. Curr Drug Targets 2007;8:283–92. [DOI] [PubMed] [Google Scholar]
- 37. Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB.. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis 2006;65:1414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawaguchi H. Endochondral ossification signals in cartilage degradation during osteoarthritis progression in experimental mouse models. Mol Cells 2008;25:1–6. [PubMed] [Google Scholar]
- 39. Raynauld JP, Martel-Pelletier J, Berthiaume MJ. et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther 2005;8:R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chu CR, Williams AA, Coyle CH, Bowers ME.. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther 2012;14:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schisterman EF, Cole SR, Platt RW.. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]