Abstract
Obesity-induced endoplasmic reticulum (ER) stress contributes to low-grade chronic inflammation in adipose tissue and may cause metabolic disorders such as diabetes mellitus and dyslipidemia. Identification of high serpina A1 (alpha-1 antitrypsin, A1AT) expression in mouse adipose tissue and adipocytes prompted us to explore the role of A1AT in the inflammatory response of adipocytes under ER stress. We aimed to determine the role of A1AT expression in adipocytes with ER stress during regulation of adipocyte homeostasis and inflammation. To this end, we chemically induced ER stress in A1AT small interfering RNA-transfected differentiating adipocytes using thapsigargin. Induction of CCAAT-enhancer-binding protein homologous protein (CHOP), an ER stress marker, by thapsigargin was lower in A1AT-deficient SW872 adipocytes. Thapsigargin or the proinflammatory cytokine tumor necrosis factor (TNF)α increased basal expression of cytokines such as interleukin (IL)-1β and IL-8 in both SW872 and primary omental adipocytes. This thapsigargin- or TNFα-induced expression of proinflammatory genes was increased by A1AT deficiency. These findings indicate that adipose A1AT may suppress the ER stress response to block excessive expression of proinflammatory factors, which suggests that A1AT protects against adipose tissue dysfunction associated with ER stress activation.
Keywords: Alpha-1 antitrypsin, Endoplasmic reticulum stress, Adipocyte, Proinflammatory factor
Highlights
-
•
Bip and CHOP expression responded to chemical ER stressor fluctuates in A1AT-silenced adipocytes.
-
•
Chemical ER stressor- and TNFα-induced proinflammatory factor expression is increased by silencing of adipose A1AT expression.
-
•
A1AT may protect against adipose tissue dysfunction through ER stress activation.
Abbreviations:
- A1AT
alpha-1 antitrypsin
- ER
endoplasmic reticulum
- GRP78/Bip
glucose-regulated protein 78
- CHOP
CCAAT-enhancer-binding protein homologous protein
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
1. Introduction
Sepsis is a major cause of death in intensive care units (ICUs) due to organ damage and an uncontrolled host response to infection [1,2]. The symptoms of sepsis are related to acute inflammation, which is characterized by high systemic concentrations of inflammatory cytokines [3]. Various medical treatments for sepsis have been attempted to date, but none improve the survival rate of patients significantly [[2], [3], [4]]. High sensitivity to postoperative sepsis is exhibited by obese patients, especially those with visceral obesity [5]. Obesity is now known to be a chronic inflammatory disorder and is characterized by high concentrations of inflammatory cytokines and chemokines secreted by adipocytes [6]. In particular, patients with visceral obesity exhibit low-grade adipose inflammation, aberrant adiponectin secretion, and insulin resistance. In addition, high mortality and a disruption of appropriate immune responses during sepsis occurs in mice fed a high-fat diet for a long period of time [7]. Therefore, more detailed knowledge of the mechanisms involved in visceral fat inflammation may provide useful therapeutic targets for septic patients.
Endoplasmic reticulum (ER) stress-mediated adipocyte dysfunction may, at least in part, mediate sepsis-related mortality [8]. Immune cells, including macrophages and T cells, play crucial roles in adipose tissue inflammation [9]. Adipose tissue macrophages secrete proinflammatory cytokines such as tumor necrosis factor (TNF)α, which plays a major role in induction of ER stress. ER stress, which is caused in part by macrophage infiltration into adipose tissue, may contribute to functional impairment of adipocytes in visceral fat. Our previous study showed that macrophages that infiltrate omental adipose tissue during cecal ligation and puncture (CLP)-induced sepsis may increase adipocyte apoptosis [10]. In addition, obese adipose tissue is exposed to greater oxidative stress, which may underlie ER stress [11,12], as may the pathophysiological changes associated with remodeling of adipose tissue in obesity [13]. Excessive or persistent ER stress in adipose tissue eventually results in cellular senescence and apoptosis, along with chronic inflammation and consequent dysfunction of the tissue, which may lead to the development of type 2 diabetes.
The circulating serine protease inhibitor alpha-1 antitrypsin (A1AT), which is encoded by the SERPINA1 gene, is an acute phase protein that is principally synthesized in hepatocytes and is present at high circulating concentrations in inflammatory diseases. Recent studies show that a reduction in local A1AT protein expression may exacerbate the inflammatory response in endometriosis-like lesions in mice [14] and that A1AT administration improves the survival rate of mice with peritonitis and sepsis [15]. Furthermore, A1AT may reduce organ damage in a serine protease activity-independent fashion [[16], [17]]. Therefore, in the present study, we determined the effect of A1AT knockdown on ER stress-induced adipokine production in cultured adipocytes to investigate whether adipose A1AT is involved in the abnormal expression of ER stress-induced inflammatory adipokines and proinflammatory factors.
2. Materials and methods
2.1. Cell culture
The human liposarcoma cell line SW872 and human primary omental adipocytes were cultured in Dulbecco's-modified Eagle's medium (DMEM/F12, Fujifilm Wako Pure Chemical Corp., Osaka, Japan) containing 10% fetal bovine serum plus antibiotics, or in Omental adipocyte medium (Zen-Bio., Research Triangle Park, NC), respectively. After the cells were grown to sub-confluency, the media were replaced by differentiation-inducing medium containing 3-isobutyl-1-methylxanthine and a PPARγ agonist (Omental adipocyte differentiation medium: OM-DM, Zen-Bio), which was diluted twice in DMEM/F12, for 4 days to induce cell differentiation. Nontargeting control small interfering RNA (siRNA; 20 pmoL/well; Qiagen, Mississauga, Ontario, Canada) or siRNA targeting A1AT (20 pmoL/well; MISSION esiRNA, human SERPINA; Sigma-Aldrich) was transfected into the adipocytes for 24 h to reduce A1AT expression; the adipocytes were then treated with the ER stress inducer, thapsigargin, which inhibits the ER Ca2+-ATPase inhibitor, or TNFα for 24 h.
2.2. Oil Red O staining
Cells were stained with Oil Red O solution according to a standard protocol. Briefly, after fixation with 4% paraformaldehyde for 10 min at room temperature (RT), cells were incubated with 3 mg/mL Oil Red O (Sigma-Aldrich, Tokyo, Japan) in 60% isopropanol for 30 min at RT. After removing this solution, the cells were washed with 60% isopropanol and subsequently with PBS, and staining was evaluated using KEYENCE BZ X810 microscopy to assess the accumulation of lipid droplets during adipocyte differentiation.
2.3. RNA isolation and real time RT-PCR analysis
RNA was extracted from adipocytes using ISOGEN-II (NIPPON Gene, Tokyo, Japan) according to the manufacturer's protocol. Expression of genes encoding proinflammatory cytokines (interleukin [IL]1B, IL6, and C-X-C motif chemokine ligand 8: CXCL8) and related proteins (prostaglandin-endoperoxide synthase 2: PTGS2 and NLR family pyrin domain containing 3: NLRP3) was measured by quantitative real time RT-PCR using the iScript One-Step RT-PCR kit and SYBR green (Bio-Rad Laboratories, Hercules, CA. USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the reference gene. The primers used are listed in Table 1. PCRs were performed on an iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories), and the Comparative Ct method was used to compare mRNA expression levels between samples.
Table 1.
Primers for real-time PCR analyses.
| Name (Accession No.) | Sequence | Product length (bp) | ||
|---|---|---|---|---|
| GAPDH | F: 5'- | AGCCACATCGCTCAGACA | -3′ | 66 |
| (NM_002046.7) | R: 5'- | GCCCAATACGACCAAATCC | -3′ | |
| IL1B | F: 5'- | TGATGGCTTATTACAGTGGCAATG | -3′ | 140 |
| (NM_000576.3) | R: 5'- | GTAGTGGTGGTGGGAGATTCG | -3′ | |
| IL6 | F: 5'- | CAGGAGCCCAGCTATGAACT | -3′ | 85 |
| (NM_000600.5) | R: 5'- | AGCAGGCAACACCAGGAG | -3′ | |
| CXCL8 | F: 5'- | AAGCATACTCCAAACCTTTCCA | -3′ | 123 |
| (NM_000584.4) | R: 5'- | CCAGACAGAGCTCTCTTCCA | -3′ | |
| NLRP3 | F: 5'- | GAGAGACCTTTATGAGAAAGCA | -3′ | 85 |
| (NM_183395.3) | R: 5'- | GCATATCACAGTGGGATTCGAA | -3′ | |
| PTGS2 | F: 5'- | CAGCACTTCACGCATCAGTT | -3′ | 128 |
| (NM_000963.4) | R: 5'- | CGCAGTTTACGCTGTCTAGC | -3′ | |
F: Forward, R: Reverse.
2.4. Western blot analysis
SW872 and primary omental adipocytes were lysed in RIPA buffer (Nacalai Tesque, Inc., Kyoto, Japan) according to the manufacturer's instructions. Equal amounts of lysate protein were separated using SDS-PAGE (SuperSep Ace, Fujifilm Wako Pure Chemical Corp.) and transferred onto polyvinylidene fluoride membranes. The membranes were blocked for 2 h and incubated overnight at 4 °C with primary antibodies against glucose-regulated protein 78 (Bip) (Cell Signaling Technology, Tokyo, Japan), CCAAT-enhancer-binding protein homologous protein (CHOP) (Cell Signaling Technology), A1AT (R & D System, Minneapolis, MN), or GAPDH (Sigma-Aldrich). The membranes were then washed with tris-buffered saline containing 0.1% Tween-20 and incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit or mouse antibodies (0.5 μg/mL; Vector Laboratories, Burlingame, CA). Immunoreactive bands were detected using Enhanced chemiluminescence reagent (Perkin Elmer Life Science, Inc., Boston, MA). The relative band intensity was assessed by densitometric analysis of digitalized autographic images using Scion image software (Scion Corp., Fredrick, MD) and normalized to GAPDH.
2.5. IL-6 ELISA
The concentration of IL-6 in the medium was quantified by ELISA (Human IL-6 Simple Step ELISA Kit, Abcam, Tokyo, Japan), according to the manufacturer's instructions.
2.6. Statistical analysis
Results are expressed as the mean ± SEM. Data were compared using the Tukey-Kramer test. P < 0.05 was considered to represent statistical significance using R software (ver.3.6.2).
3. Results
3.1. Effects of A1AT knockdown on thapsigargin-induced ER stress markers in cultured SW872 adipocytes
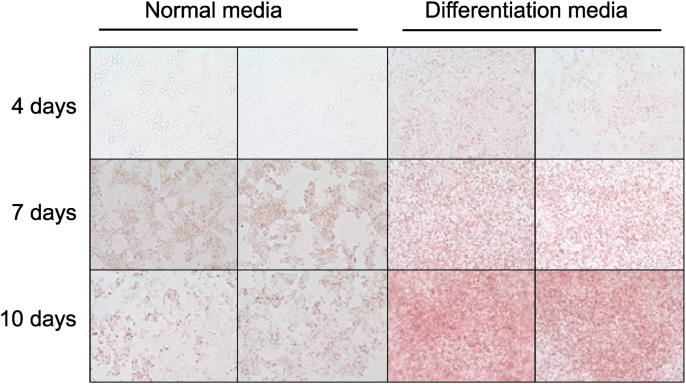
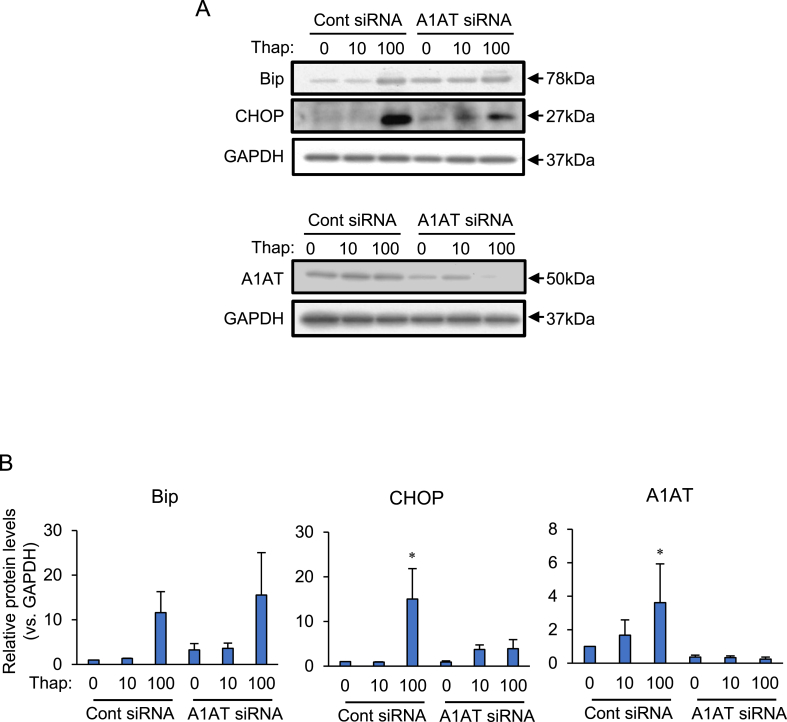
Culture in differentiation medium increased the number of SW872 adipocytes that contained lipid droplets. The volume of the lipid droplets increased for 10 days during the period of culture (Fig. 1). Expression of the ER stress markers Bip and CHOP was analyzed after knocking down A1AT in differentiating SW872 cells (Fig. 2). The basal protein level of A1AT was low, but treatment with the ER stress activator thapsigargin (100 nM) increased this, and also significantly increased expression of CHOP. Knocking down A1AT expression suppressed thapsigargin (100 nM)-induced CHOP expression. Expression of Bip and CHOP in thapsigargin (10 nM)-treated cells tended to be higher, but not statistically significant in A1AT-knockdowned cell than those in control cells. The expression of Bip in thapsigargin (100 nM)-treated cells was similar between in control cells and A1AT-knockdowned cells, suggesting that ER stress response reached saturation point.
Fig. 1.
Effect of the differentiation media on lipid accumulation in adipocyte SW872.
SW872 cells (2×104 cells) were cultured for 4, 7, or 10 days in normal media or differentiation media. Accumulation of lipid droplets during adipocyte differentiation was visualized with Oil-Red O staining. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Effects of alpha-1 antitrypsin (A1AT) knockdown on expression of ER stress markers, Bip, and CHOP in SW872 cells.
SW872 cells (2×104 cells) were transfected for 24 h with A1AT siRNA (20 pmol) and then treated for 24 h with thapsigargin (Thap: 10 or 100 nM). The protein levels of glucose- regulated protein 78 (Bip), CCAAT-enhancer-binding protein homologous protein (CHOP), and alpha-1 antitrypsin (A1AT) were measured by immunoblotting. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. Representative results (A) and densitometric analyses of n = 3 independent experiments (B) are shown.
3.2. Effect of A1AT knockdown on inflammatory cytokine expression during ER stress in SW872 cells
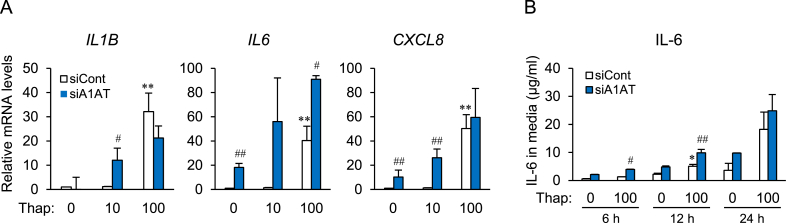
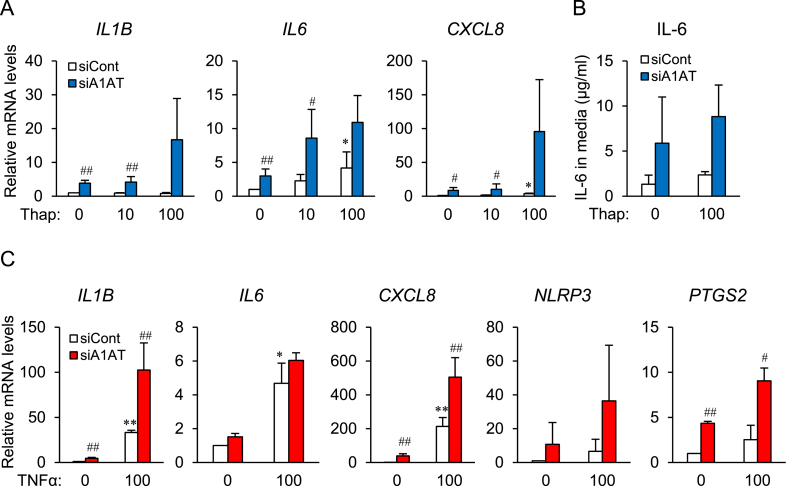
Thapsigargin (100 nM) treatment markedly increased expression of IL1B, IL6, and CXCL8 mRNA in control cells (Fig. 3A, white column). When A1AT siRNA-transfected cells were treated with thapsigargin (Fig. 3A, blue column), basal expression of IL6 and CXCL8 mRNA in untreated cells, and expression of IL1B and CXCL8 mRNA in thapsigargin (10 nM)-treated cells, increased significantly relative to that in control cells. A1AT knockdown increased IL6 expression in all groups (Fig. 3A). The IL6 concentration in control medium was higher 12 h after thapsigargin (100 nM) treatment, and increased significantly at 6 h and 12 h after knocking down A1AT (Fig. 3B).
Fig. 3.
Effect of alpha-1 antitrypsin (A1AT) knockdown on expression of ER stressor-induced proinflammatory factors in SW872 cells.
SW872 cells (2×104) were transfected for 24 h with A1AT siRNA (20 pmol) and then treated for 24 h with thapsigargin (Thap: 10 or 100 nM). Expression of mRNA encoding proinflammatory factors IL1B, IL6, IL8, and NLRP3 was quantified by real-time RT-PCR (A). A1AT-deficient SW872 cells were stimulated for 6 h, 12 h, or 24 h with Thap (100 nM) (B). The culture medium was collected and the concentration of IL-6 was measured by ELISA. Three independent experiments were performed. Results are expressed as the mean ± S.E.M. and compared using the Tuker-Kramer test. *p<0.05, **p<0.01 vs. Thap (0) in control siRNA (−)-transfected cells; #p<0.05, ##p<0.001 vs. control siRNA-transfected cells.
3.3. Effect of A1AT knockdown on TNFα-induced inflammatory cytokine expression in SW872 cells
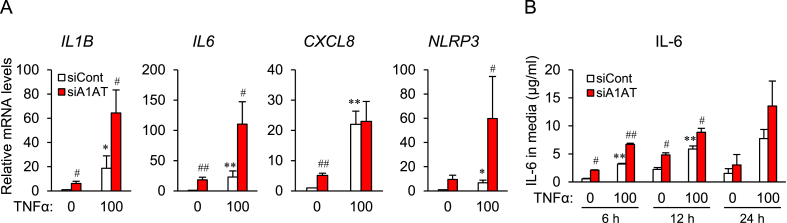
A1AT siRNA-transfected SW872 cells were treated with TNFα (Fig. 4), and this caused increases in expression of mRNA expression encoding IL1B, IL6, and CXCL8, and of the representative inflammasome NLRP3. Expression of IL1B and IL6 mRNA was significantly increased by A1AT knockdown (Fig. 4A). In addition, the concentration of IL-6 in the medium was increased by TNFα treatment, and further increased by A1AT knockdown (Fig. 4B).
Fig. 4.
Effect of alpha-1 antitrypsin (A1AT) knockdown on TNFα-induced proinflammatory factor expression in SW872 cells.
SW872 cells (2×104 cells) were transfected for 24 h with A1AT siRNA (20 pmol) and then treated for 24 h with TNFα (100 ng/ml). Expression of mRNA expression encoding proinflammatory factors IL1B, IL6, CXCL8, and NLRP3 was quantified by real-time RT-PCR (A). A1AT-deficient SW872 cells were treated for 6 h, 12 h, and 24 h with TNFα (100 ng/ml) (B). The culture medium was collected and the concentration of IL-6 was measured by ELISA. Three independent experiments were performed. Results are expressed as the mean ± S.E.M. *p<0.05, **p<0.01 vs. TNFα (0) in control siRNA (−)-transfected cells; #p<0.05, ##p<0.001 vs. control siRNA-transfected cells.
3.4. Effects of A1AT knockdown on ER stress- and TNFα-induced proinflammatory factor expression in primary omental adipocytes
In an attempt to corroborate the results obtained using human adipocyte cell line SW872, we also examined the effect of A1AT silencing on expression of proinflammatory factors in ER stressor- and TNFα-treated primary omental adipocytes (Fig. 5). Thapsigargin treatment increased expression of IL6 and CXCL8 mRNA, but only tended to increase IL-6 secretion. Treatment with A1AT siRNA increased basal expression of IL1B, CXCL8, and PTGS2 mRNA. Furthermore, TNFα significantly stimulated expression of IL1B, IL6, and CXCL8 mRNA, whereas expression of IL1B, CXCL8 mRNA, and that of PTGS2 was increased by A1AT siRNA.
Fig. 5.
Effect of alpha-1 antitrypsin (A1AT) knockdown on expression of ER stressor- and TNFα-induced proinflammatory factors in primary omental adipocytes.
Omental adipocytes (2×104 cells) were transfected for 24 h with A1AT siRNA and then treated for 24 h with thapsigargin (Thap: 10 or 100 nM) (A, B) and TNFα (100 ng/ml) (C). A, C: Expression of mRNA encoding IL1B, IL6, IL8, NLRP3, and PTGS2 was quantified by real-time RT-PCR. B: The concentration of IL-6 in the culture medium was measured by ELISA. Three independent experiments were performed. Results are expressed as the mean ± S.E.M. *p<0.05, **p<0.01 vs. Thap (0) or TNFα (0) in control siRNA-transfected cells; #p<0.05, ##p<0.001 vs. control siRNA-transfected cells.
4. Discussion
Systemic inflammation in severe sepsis causes organ damage because of local inflammation. Adipose tissue is an immunoregulatory organ with an endocrine function that produces proinflammatory cytokines such as TNFα and IL-6, and conversely anti-inflammatory adipokines such as adiponectin [[18], [19], [20]]. Regulation of adipose tissue cytokine production might represent a way of ameliorating systemic inflammation. Appropriate responses by adipocytes to sepsis are likely to be involved in protection against tissue damage. ER stress plays an important pathophysiological role in obesity-induced adipose tissue dysfunction [8,9,11]. ER stress induces low-grade chronic inflammation and is involved in development of metabolic diseases such as diabetes, dyslipidemia, and neurodegenerative diseases. The protein kinase RNA-like endoplasmic reticulum kinase (PERK) pathway is an ER stress sensor activated via the chaperone molecule Bip, resulting in higher expression of the downstream proapoptotic factor CHOP. The present study shows that functional changes associated with ER stress or TNFα stimulation are promoted by adipocyte A1AT deficiency. ER stress and TNFα stimulation increase inflammatory adipokine expression, which is further increased by low A1AT expression in both human SW872 cells and primary omental visceral adipocytes, as is basal expression of ER stress markers. These results suggest that endogenous A1AT deficiency may increase secretion of the proinflammatory adipokines that are released by adipose tissue during chronic inflammation.
Sepsis is a life-threatening disorder that causes an uncontrolled host response, resulting in organ damage; indeed, it is the leading cause of death in ICUs. Prevention of postoperative infections and alleviation of septic symptoms are important clinical aims. Visceral obesity underlies the metabolic syndrome, which is a cluster of risk factors for cardiovascular disease that includes dyslipidemia, abnormal glucose metabolism, and high blood pressure [12]. We showed recently that A1AT is highly expressed in mouse adipose tissue and that its expression is reduced by CLP-induced sepsis (data not shown), which suggests involvement of adipose A1AT in abnormal production of proinflammatory adipokines. In the present study, A1AT expression was increased by treatment with the ER stressor thapsigargin, whereas low A1AT expression in cultured adipocytes caused alteration in ER stress-induced response of CHOP, which suggests that A1AT may be involved in the ER stress response. TNFα-stimulated proinflammatory cytokine expression was also higher in A1AT-deficient adipocytes.
These results are consistent with the possibility that dysregulation of the ER stress response exacerbates adipose tissue damage via increased production of proinflammatory adipokines, and that this dysregulation may be promoted by low A1AT expression. Thus, expression of A1AT in visceral adipocytes might be essential for prevention of ER stress- and TNFα-induced inflammatory cytokine expression; also, A1AT may be an endogenous suppressor of adipose tissue inflammation. Further studies are needed to dissect the mechanisms by which A1AT affects adipokine expression and ER stress response. Elucidation of these mechanisms and regulating A1AT expression may lead to identification of novel therapeutic targets for adipose tissue dysfunction-related disorders and systemic inflammatory diseases such as septic shock.
Author statement
Yukari Ando: Investigation, Writing-original draft. Akito Kuroda: Investigation, Validation, Data Curation. Kazuya Kusama Investigation, Validation, Data Curation. Takeshi Matsutani: Resources, Funding acquisition. Akihisa Matsuda: Resources, Methodology. Kazuhiro Tamura: Conceptualization, Supervision, Project administration, Writing-Review and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 17K10526. The funding source had no involvement in all research work including the study design and the writing of the report.
References
- 1.Martin G.S., Mannino D.M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda A., Jacob A., Wu R., Aziz M., Yang W.L., Matsutani T., Suzuki H., Furukawa K., Uchida E., Wang Novel therapeutic targets for sepsis: regulation of exaggerated inflammatory responses. J. Nippon Med. Sch. 2012;79:4–18. doi: 10.1272/jnms.79.4. [DOI] [PubMed] [Google Scholar]
- 3.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.L., Angus The third international consensus definitions for sepsis and septic shock (Sepsis-3) J. Am. Med. Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redl H., Schlag G., Bahrami S. Animal models of sepsis and shock: a review and lessons learned. Edwin A Deith. Shock. 1998;10:442–445. [PubMed] [Google Scholar]
- 5.Lee J.G.H., Genga K.R., Pisitsak C., Boyd J.H., Leung A.K.K., RussellJA, Walley K.R. Survival benefit of a low ratio of visceral to subcutaneous adipose tissue depends on LDL clearance versus production in sepsis. Crit. Care. 2018;22:1985–1991. doi: 10.1186/s13054-018-1985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louise S., Margareta V., Maria E., Niklas A., Sylvie A., Karin O., Anna B., Mikael B., Johan B., Maria B., Staffan N., Jansson John-Olov. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PloS One. 2009;4 doi: 10.1371/journal.pone.0007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garfinkel B.P., Hotamisligil G.S. ER Stress promotes inflammation through Re-wIREd macrophages in Obesity. Mol. Cell. 2017;1833:731–733. doi: 10.1016/j.molcel.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T., Gao J., Ishigaki Y., Kondo K., Sawada S., Izumi T., Uno K., Kaneko K., Tsukita S., Takahashi K., Asao A., Ishii N., Imai J., Yamada T., Oyadomari S., Katagiri H. ER Stress protein CHOP mediates insulin resistance by modulating adipose tissue macrophage polarity. Cell Rep. 2017;18:2045–2057. doi: 10.1016/j.celrep.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 10.Matsutani T., Tamura K., Kutsukake M., Matsuda A., Tachikawa E., Uchida E. Impact of pioglitazone on macrophage dynamics in adipose tissues of cecal ligation and puncture-treated mice. Biol. Pharm. Bull. 2017;40:638–644. doi: 10.1248/bpb.b16-00883. [DOI] [PubMed] [Google Scholar]
- 11.Shan B., Wang X., Wu Y., Xu C., Xia Z., Dai J., Shao M., Zhao F., He S., Yang L., Zhang M., Nan F., Li J., Liu J., Liu J., Jia W., Qiu Y., Song B., Han J.J., Rui L., Duan S.Z. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol. 2017;18:519–529. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- 12.Yazıcı D., Sezer H. Insulin resistance, obesity and lipotoxicity. Adv. Exp. Med. Biol. 2017;960:277–304. doi: 10.1007/978-3-319-48382-5_12. [DOI] [PubMed] [Google Scholar]
- 13.Mihai A.D., Schröder M. Glucose starvation and hypoxia, but not the saturated fatty acid palmitic acid or cholesterol, activate the unfolded protein response in 3T3-F442A and 3T3-L1 adipocytes. Adipocyte. 2015;4:188–202. doi: 10.4161/21623945.2014.989728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K., Takashima H., Fumoto K., Kajihara T., Uchino S., Ishihara O., Yoshie M., Kusama K., Tachikawa E. Possible role of α1-antitrypsin in endometriosis-like grafts from a mouse model of endometriosis. Reprod. Sci. 2015;22:1088–1097. doi: 10.1177/1933719115570901. [DOI] [PubMed] [Google Scholar]
- 15.Kaner Z., Ochayon D.E., Shahaf G., Baranovski B.M., Bahar N., Mizrahi M., Lewis E.C. Acute phase protein α1-antitrypsin reduces the bacterial burden in mice by selective modulation of innate cell responses. J. Infect. Dis. 2015;211:1489–1498. doi: 10.1093/infdis/jiu620. [DOI] [PubMed] [Google Scholar]
- 16.Jonigk D., Al-Omari M., Maegel L., Müller M., Izykowski N., Hong J., Hong K., Kim S.H., Dorsch M., Mahadeva R., Laenger F., Kreipe H., Braun A., Shahaf G., Lewis E.C., Welte T., Dinarello C.A., Janciauskiene S. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15007–15012. doi: 10.1073/pnas.1309648110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pott G.B., Chan E.D., Dinarello C.A., Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J. Leukoc. Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reilly S.M., Saltiel A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda M., Sakaue H. Adipocyte death and chronic inflammation in obesity. J. Med. Invest. 2017;64:193–196. doi: 10.2152/jmi.64.193. [DOI] [PubMed] [Google Scholar]
- 20.Vendrell J., Maymo-Masip E. Tumor necrosis-like weak inducer of apoptosis as a proinflammatory cytokine in human adipocyte cells: up-regulation in severe obesity is mediated by inflammation but not hypoxia. J. Clin. Endocrinol. Metab. 2010;95:2983–2992. doi: 10.1210/jc.2009-2481. [DOI] [PubMed] [Google Scholar]