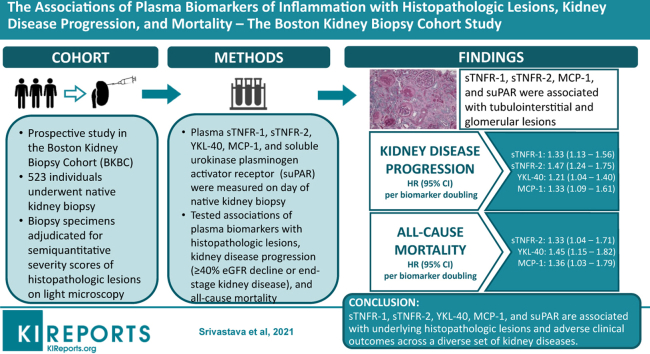
Abstract
Background
Soluble tumor necrosis factor receptor (sTNFR)-1, sTNFR-2, YKL-40, monocyte chemoattractant protein (MCP)-1, and soluble urokinase plasminogen activator receptor (suPAR) have emerged as promising biomarkers of inflammation but have not been evaluated across diverse types of kidney diseases.
Methods
We measured these plasma biomarkers in 523 individuals enrolled into a prospective, observational cohort study of patients undergoing clinically indicated native kidney biopsy at 3 tertiary care hospitals. Two kidney pathologists adjudicated biopsy specimens for semiquantitative scores of histopathology. Proportional hazard models tested associations between biomarkers and risks of kidney disease progression (composite of ≥40% estimated glomerular filtration rate [eGFR] decline or end-stage kidney disease [ESKD]) and death.
Results
Mean eGFR was 56.4±36 ml/min per 1.73 m2 and the median proteinuria (interquartile range) was 1.6 (0.4, 3.9) g/g creatinine. The most common primary clinicopathologic diagnoses were proliferative glomerulonephritis (29.2%), nonproliferative glomerulopathy (18.1%), advanced glomerulosclerosis (11.3%), and diabetic kidney disease (11.1%). sTNFR-1, sTNFR-2, MCP-1, and suPAR were associated with tubulointerstitial and glomerular lesions. YKL-40 was not associated with any histopathologic lesions after multivariable adjustment. During a median follow-up of 65 months, 182 participants suffered kidney disease progression and 85 participants died. After multivariable adjustment, each doubling of sTNFR-1, sTNFR-2, YKL-40, and MCP-1 was associated with increased risks of kidney disease progression, with hazard ratios ranging from 1.21 to 1.47. Each doubling of sTNFR-2, YKL-40, and MCP-1 was associated with increased risks of death, with hazard ratios ranging from 1.33 to 1.45. suPAR was not significantly associated with kidney disease progression or death.
Conclusions
sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR are associated with underlying histopathologic lesions and adverse clinical outcomes across a diverse set of kidney diseases.
Keywords: biomarker, fibrosis, histopathology, inflammation, kidney biopsy, kidney disease
Graphical abstract
Chronic kidney disease (CKD) is not a single entity but rather a heterogeneous condition with a wide spectrum of underlying etiologies, pathologic and clinical manifestations, and variable rates of progression. eGFR and albuminuria are the 2 primary clinical indicators used for CKD definition, staging, and prognosis.1 However, eGFR and albuminuria do not provide specificity regarding the underlying pathobiology and are limited in predicting risk of adverse outcomes.2 Kidney biopsies provide information on histopathologic lesions, which are strong predictors of outcomes in patients with CKD,3 but kidney biopsies are invasive procedures with risks.4 Emerging data suggest that inflammatory mechanisms in response to underlying kidney injury may contribute to the development and propagation of histopathologic lesions, which could lead to subsequent kidney disease progression.5, 6, 7, 8 Investigation of the associations of novel biomarkers of inflammation with underlying histopathologic lesions may enable noninvasive identification of individuals at high risk of kidney disease progression.
Prior studies on plasma biomarkers of inflammation in kidney disease and kidney disease progression have largely been restricted to individuals with diabetes or common forms of CKD without biopsy confirmation of cause.8, 9, 10, 11, 12, 13 We measured plasma biomarkers of inflammation (sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR) in samples from the Boston Kidney Biopsy Cohort, a prospective cohort study of individuals undergoing native kidney biopsy. We hypothesized that higher levels of these biomarkers of inflammation are associated with distinct kidney histopathologic lesions and subsequent adverse clinical outcomes.
Materials and Methods
Study Population
The Boston Kidney Biopsy Cohort is a prospective, observational cohort study of individuals undergoing native kidney biopsy at 3 tertiary care hospitals in Boston, MA. Details of the study design have been previously described.3 The study includes adults (≥18 years of age) who underwent a clinically indicated native kidney biopsy between September 2006 and June 2016. Exclusion criteria included the inability to provide written consent, severe anemia, pregnancy, and enrollment in competing studies. Participants provided blood and urine samples on the day of kidney biopsy. We included 523 participants who had available plasma samples for biomarker measurements. The Partners Human Research Committee (the Brigham and Women’s Hospital Institutional Review Board) approved the study protocol, which is in accordance with the principles of the Declaration of Helsinki.
Exposures, Blood Collection, and Laboratory Assays
The primary exposures were plasma concentrations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR. Blood samples were collected from participants on the day of biopsy in EDTA-containing vacutainers and were processed, aliquoted, and stored at −80°C within 4 hours of collection. Measurements of biomarkers were made in 523 participants for sTNFR-1 and sTNFR-2, 490 participants for YKL-40, 522 participants for MCP-1, and 498 participants for suPAR. Plasma samples were diluted 5-fold using sample diluent buffer (0.1 mol/l of HEPES, 0.1 mol/l of NaCl, 0.1% TWEEN 20, and 1% BSA; pH 7.4; filter sterilized). Plasma biomarkers were measured using multiplex microbead-based enzyme-linked immunosorbent assays on a Luminex platform. Recombinant proteins (Bio-Techne, Minneapolis, MN) were incubated with microbeads coupled with capture antibodies (Bio-Techne) for 1 hour on an orbital shaker, washed 3 times with 100 μl of phosphate-buffered saline–Tween buffer, and incubated with corresponding secondary antibodies (Bio-Techne) for 1 hour. After incubation, plates were washed with phosphate-buffered saline–Tween buffer and incubated with streptavidin-phycoerythrin for another 15 minutes. Beads were then washed again with phosphate-buffered saline–Tween buffer, and fluorescence was measured on a BioPlex 200 (Bio-Rad) analyzer. Samples were measured in duplicates with coefficients of variation <15% across duplicates.
Evaluation of Histopathology
Methods to evaluate and score histopathologic lesions were previously described.3 Briefly, kidney biopsies were adjudicated under light microscopy by 2 experienced kidney pathologists who provided semiquantitative scores of kidney inflammation, fibrosis, vascular sclerosis, and tubular injury (Supplementary Table S1). Of the 13 histopathologic lesions, all were scored during study sessions except for grades of global or segmental glomerulosclerosis, which were taken from the biopsy report, because they were each calculated as a percentage of the total number of glomeruli. We limited statistical analyses on histopathologic lesions to those with adjudicated cases (n=456, 87%) except for analyses of global or segmental glomerulosclerosis because they were taken from the biopsy report. We combined endocapillary glomerular inflammation, extracapillary cellular crescents, focal glomerular necrosis, and fibrocellular crescents into a single dichotomous variable named “glomerular inflammation” because of the relatively low prevalence and limited range of severity for each lesion in this cohort. All participants’ charts were reviewed alongside the histopathologic evaluations to provide the final primary clinicopathologic diagnosis.
Clinical Outcomes
The 2 primary outcomes were kidney disease progression and all-cause mortality. Kidney disease progression was defined as ≥40% decline in eGFR or ESKD (dialysis or kidney transplantation). eGFR during follow-up was obtained from the electronic medical record. The secondary endpoint was progression to ESKD. ESKD status was confirmed by reviewing the electronic medical record and linkage with the United States Renal Data System database.14 Mortality status was confirmed with the Social Security Death Index. Participants were followed up until the occurrence of death, voluntary study withdrawal, loss to follow-up, or February 1, 2020.
Covariates
We collected participant information at the biopsy visit by self-report or from the electronic medical record, including demographics (age, sex, race), medical history (hypertension, diabetes mellitus, systemic lupus erythematosus, systemic vasculitis, hepatitis B, hepatitis C, malignancy, human immunodeficiency virus non-kidney solid organ transplant), medication lists (angiotensin converting enzyme inhibitor, angiotensin II receptor blocker, mineralocorticoid receptor antagonist, calcium channel blocker, beta-blocker, corticosteroids, other immunosuppressive medications), reason for native kidney biopsy (proteinuria, hematuria, nephrotic syndrome, nephritis syndrome, abnormal eGFR), serum creatinine, and proteinuria. All data were stored at the REDCap electronic data capture tools hosted at Partners Health Care.15 We used the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation to calculate the eGFR.16 We obtained serum creatinine from the electronic medical record on the day of biopsy. In participants for whom this was unavailable, we measured serum creatinine in blood samples collected on the day of biopsy. We obtained spot urine protein-to-creatinine ratio or urine albumin-to-creatinine ratio from the date of kidney biopsy up to 3 months before biopsy from the electronic medical record. If a participant did not have either of these values, we measured urine albumin-to-creatinine ratio from urine collected on the day of kidney biopsy. Serum and urine creatinine were measured using a Jaffe-based method, and urine albumin was measured by an immunoturbidometric method.
Statistical Analysis
Descriptive statistics were summarized as count with percentages for categorical variables and mean ± standard deviation or median with interquartile range for continuous variables. For skewed data distributions, we performed natural logarithmic transformation as appropriate. Plasma biomarkers were examined as continuous variables (log base 2). We used Spearman correlation coefficients to determine associations between continuous variables and each biomarker. To evaluate associations of plasma biomarkers with histopathologic lesions, we used Wilcoxon rank sum or Kruskal-Wallis tests for 2-group and multiple group comparisons, respectively. To calculate percentage differences of plasma biomarkers by histopathologic lesion, we used multivariable-adjusted linear regression models.
For the outcome of kidney disease progression (time to either ≥40% eGFR decline or ESKD), we treated the data as interval censored because the exact date of eGFR decline is not known.3 We evaluated the association between each plasma biomarker and subsequent kidney disease progression using a nonparametric survival function for interval-censored data with differences assessed by the general log-rank test.17,18 We modeled the data using Cox proportional hazards regression models that accommodated interval-censored time to event.19,20 Traditional Cox proportional hazards regression models were used for the outcomes of ESKD and mortality. All proportional hazards models were first fit without adjustment and then stratified by site with multivariable adjustment for covariates, including age, sex, race, baseline eGFR, baseline natural log-transformed proteinuria, and primary clinicopathologic diagnosis. Because levels of biomarkers may differ by the underlying etiology of kidney disease, we adjusted for primary clinicopathologic diagnosis. We tested for statistical interaction between each plasma biomarker and primary clinicopathologic diagnosis (glomerulopathy vs. other diagnoses) for each outcome through multiplicative interaction terms. To test the predictive value of the plasma biomarkers of inflammation, we calculated c-statistics for the outcomes of ESKD and death using a base model that was stratified by study site and included age, sex, baseline natural log-transformed proteinuria, primary clinicopathologic diagnosis, and baseline eGFR) and also for the model that further included the 5 plasma biomarkers of inflammation.21,22 We performed a sensitivity analysis using subdistribution hazards models that acknowledged the competing risk of death.23 Because medications that suppress the immune system may alter levels of plasma biomarkers of inflammation or the risk of adverse clinical outcomes, we repeated the outcome analysis after further adjustment for the use of immunosuppressive medications or corticosteroids. We confirmed no violations of the proportional hazards assumption through assessment of Schoenfeld residuals. Complete case analysis was used for the analyses because there was less than 5% missing data. All statistical tests were 2-sided, and P values <0.05 were considered significant. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC), and Stata, version 15.0 (StataCorp, College Station, TX).
Results
Baseline Characteristics
Baseline characteristics of the study cohort and median biomarker levels are shown in Table 1. The mean age was 52.8±16.6 years, 50.9% were women, and 63.7% were white. The most common primary clinicopathologic diagnoses were proliferative glomerulonephritis (29.2%), nonproliferative glomerulopathy (18.1%), advanced glomerulosclerosis (11.3%), and diabetic kidney disease (11.1%). The mean eGFR was 56.4±36.0 ml/min per 1.73 m2 and the median proteinuria (interquartile range) was 1.6 (0.4, 3.9) g/g creatinine. The median plasma levels (interquartile range) for each biomarker were as follows: sTNFR-1, 1012.7 (485.1, 2167.8) pg/ml; sTNFR-2, 6097.6 (3301.5, 12411.6) pg/ml; YKL-40, 53.5 (25.7, 98.6) ng/ml; MCP-1, 135.2 (95.3, 193.1) pg/ml; and suPAR, 3044.3 (1809.8, 6493.2) pg/ml. Supplementary Table S2 shows the plasma biomarker levels by primary clinicopathologic diagnosis.
Table 1.
Baseline characteristics of the Boston Kidney Biopsy Cohort (n = 523)
| Baseline characteristics | |
|---|---|
| Plasma biomarker concentrations, pg/ml | |
| sTNFR-1 | 1012.7 [485.1, 2167.8] |
| sTNFR-2 | 6097.6 [3301.5, 12411.6] |
| YKL-40 | 53.5 [25.7, 98.6] |
| MCP-1 | 135.2 [95.3, 193.1] |
| suPAR | 3044.3 [1809.8, 6493.2] |
| Clinical characteristics | |
| Age, yr | 52.8 ± 16.6 |
| Female | 266 (50.9) |
| Race | |
| White | 333 (63.7) |
| Black | 104 (19.9) |
| Other | 86 (16.4) |
| eGFR, ml/min per 1.73 m2 | 56.4 ± 36.0 |
| Serum creatinine, mg/dl | 1.5 [0.9–2.3] |
| Proteinuria, g/g creatinine | 1.6 [0.4, 3.9] |
| Reason for biopsya | |
| Proteinuria | 305 (58.3) |
| Hematuria | 127 (24.3) |
| Nephrotic syndrome | 69 (13.2) |
| Nephritic syndrome | 12 (2.3) |
| Abnormal eGFR | 268 (51.2) |
| Primary clinicopathologic diagnosisb | |
| Proliferative glomerulonephritis | 150 (29.2) |
| Nonproliferative glomerulopathy | 93 (18.1) |
| Advanced glomerulosclerosis | 58 (11.3) |
| Diabetic kidney disease | 57 (11.1) |
| Vascular disease | 44 (8.6) |
| Paraprotein-related disease | 42 (8.2) |
| Tubulointerstitial disease | 39 (7.6) |
| Other | 31 (6.0) |
| Comorbid conditions | |
| Diabetes mellitus | 120 (22.9) |
| Hypertension | 278 (53.2) |
| Systemic lupus erythematosus | 77 (14.7) |
| Systemic vasculitis | 13 (2.5) |
| Hepatitis B | 4 (0.8) |
| Hepatitis C | 10 (1.9) |
| Malignancy | 82 (15.7) |
| Human immunodeficiency virus | 5 (1.0) |
| Non-kidney solid organ transplant | 9 (1.7) |
| Medications | |
| ACEi/ARB | 242 (46.3) |
| MRA | 12 (2.3) |
| Calcium channel blockers | 137 (26.2) |
| Beta-blockers | 168 (32.1) |
| Immunosuppression | 94 (18.0) |
| Corticosteroids | 97 (18.5) |
| Immunosuppression or corticosteroids | 150 (28.7) |
| Clinical site | |
| Site 1 | 333 (63.7) |
| Site 2 | 118 (22.6) |
| Site 3 | 72 (13.8) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonist.
Data are presented as mean ± standard deviation, median [interquartile range], and count with frequencies (%) for binary and categorical variables.
Percentages do not add to 100 as there may have been more than 1 reason for kidney biopsy.
Nine individuals had insufficient tissue to make a clinicopathologic diagnosis; the “other diagnosis” category was composed of participants with minor abnormalities or relatively preserved parenchyma.
Correlations Between eGFR, Proteinuria, and Biomarkers
Table 2 shows associations of plasma biomarkers with eGFR, proteinuria, and each other. Plasma sTNFR-1, sTNFR-2, YKL-40, and suPAR had moderate to strong correlations with eGFR, and plasma MCP-1 had a weak correlation with eGFR. All biomarkers had positive correlations with proteinuria, but the correlation between suPAR and proteinuria was not significant. All plasma biomarkers had positive correlations with one another.
Table 2.
Spearman correlation coefficients between kidney function, proteinuria, and plasma biomarkers
| sTNFR-1 | sTNFR-2 | YKL-40 | MCP-1 | suPAR | |
|---|---|---|---|---|---|
| eGFR | –0.70 (<0.001) | –0.62 (<0.001) | –0.51 (<0.001) | –0.10 (0.02) | –0.46 (<0.001) |
| Proteinuria | 0.28 (<0.001) | 0.29 (<0.001) | 0.22 (<0.001) | 0.11 (0.01) | 0.07 (0.10) |
| sTNFR-1 | 1 | 0.86 (<0.001) | 0.58 (<0.001) | 0.19 (<0.001) | 0.45 (<0.001) |
| sTNFR-2 | 0.86 (<0.001) | 1 | 0.54 (<0.001) | 0.24 (<0.001) | 0.41 (<0.001) |
| YKL-40 | 0.58 (<0.001) | 0.54 (<0.001) | 1 | 0.15 (0.001) | 0.24 (<0.001) |
| MCP-1 | 0.19 (<0.001) | 0.24 (<0.001) | 0.15 (0.001) | 1 | 0.13 (0.005) |
| suPAR | 0.45 (<0.001) | 0.41 (<0.001) | 0.24 (<0.001) | 0.13 (0.005) | 1 |
eGFR, estimated glomerular filtration rate; MCP, monocyte chemoattractant protein; sTNFR, soluble tumor necrosis factor receptor, suPAR, soluble urokinase plasminogen activator receptor.
P values are in parentheses.
Associations of Plasma Biomarkers With Histopathologic Lesions
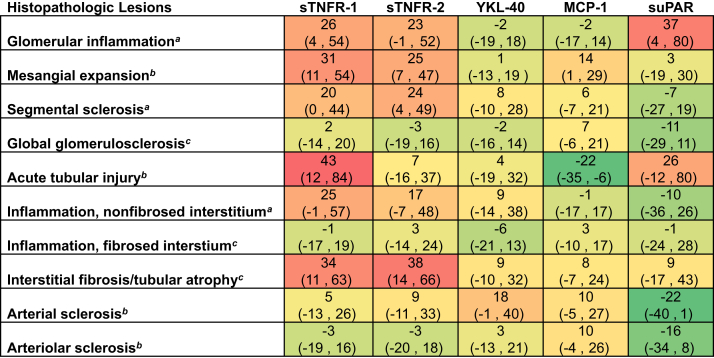
Supplementary Figures S1, S2, and S3 show differences in plasma biomarker concentrations across histopathologic lesions in the tubulointerstitial, glomerular, and microvascular compartments, respectively. Figure 1 shows adjusted differences in plasma biomarker concentrations across histopathologic lesions, using multivariable models adjusted for age, sex, race, and eGFR. Plasma sTNFR-1 levels were significantly higher with more severe acute tubular injury, interstitial fibrosis and tubular atrophy (IFTA), mesangial expansion, and the presence of glomerular inflammation compared with less severe lesions, respectively. Plasma sTNFR-2 levels were significantly higher with more severe IFTA, mesangial expansion, and the presence of segmental sclerosis compared with less severe lesions, respectively. Plasma YKL-40 was not significantly associated with any histopathologic lesions. Plasma MCP-1 levels were significantly higher with more severe mesangial expansion and lower with more severe acute tubular injury compared with less severe lesions, respectively. suPAR levels were significantly higher with the presence of glomerular inflammation compared with the absence of glomerular inflammation.
Figure 1.
Differences in sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR by histopathologic lesions. Models were fit using log-transformed biomarker as the outcome and each histopathologic lesion as the predictor variable. Percentage differences are derived from linear regression models of log base 2 sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR, respectively. Each individual model was adjusted for age, sex, race, and eGFR. Percentage differences in each biomarker were calculated by raising 2 to the power of the beta-coefficient, subtracting 1, and multiplying by 100 [(2β – 1) × 100)] for each respective histopathologic lesion from the linear regression model. eGFR, estimated glomerular filtration rate; MCP, monocyte chemoattractant protein; sTNFR, soluble tumor necrosis factor receptor, suPAR, soluble urokinase plasminogen activator receptor. Shading represents the magnitude of difference for each plasma biomarker by histopathologic lesions.
a Reference is absence of lesion.
b Reference is none or mild lesion severity.
c Reference is 0–25% of cortical volume affected.
Associations of Plasma Biomarkers With Kidney Disease Progression
During a median follow-up time of 59 months, 182 participants experienced kidney disease progression, and 124 participants progressed to ESKD. Table 3 shows the unadjusted and multivariable-adjusted associations between each plasma biomarker and kidney disease progression. sTNFR-1, sTNFR-2, YKL-40, and MCP-1 were each associated with increased risks of kidney disease progression, in fully adjusted models including proteinuria and eGFR. Each biomarker was also independently associated with progression to ESKD, but the association between YKL-40 and subsequent ESKD was confounded by eGFR and not significant in adjusted models. The associations of suPAR with kidney disease progression and ESKD were nominally higher but no longer significant after further adjustment for eGFR. There was no evidence of statistical interaction between plasma biomarkers and primary clinicopathologic diagnosis (glomerulopathy vs. other diagnoses) for either kidney disease progression or ESKD (P for interaction for kidney disease progression: sTNFR-1, 0.51; sTNFR-2, 0.93; YKL-40, 0.99; MCP-1, 0.50; suPAR, 0.13; P for interaction for ESKD: sTNFR-1, 0.64; sTNFR-2, 0.77; YKL-40, 0.34; MCP-1, 0.80; suPAR, 0.06) (Supplementary Table S3). Results from subdistribution hazards models that incorporated the competing risk of death yielded similar results, except that the association between suPAR and progression to ESKD (HR 1.12, 95% confidence interval [CI] 1.01–1.24) was statistically significant (Supplementary Table S4). Further adjustment for immunosuppressive medications did not qualitatively change the results (Supplementary Table S5). We tested the performance of the 5 plasma biomarkers of inflammation for predicting 5-year risk of ESKD in addition to the base model. The c-statistic of the base model was 0.85 (95% CI 0.82–0.89). The c-statistic of the model after addition of the 5 plasma biomarkers increased to 0.87 (95% CI 0.83–0.89; P = 0.17 for comparison with the c-statistic of the base model).
Table 3.
Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with adverse clinical outcomes
| Events | Events per 100 person-yearsa | Model 1, HR (95% CI) | Model 2, HR (95% CI) | Model 3, HR (95% CI) | |
|---|---|---|---|---|---|
| sTNFR-1 | |||||
| Kidney disease progressionb | 182 | 10.1 | 1.63 (1.47–1.81) | 1.58 (1.39–1.78) | 1.33 (1.13–1.56) |
| ESKD | 124 | 5.8 | 1.87 (1.65–2.11) | 1.88 (1.62–2.17) | 1.31 (1.07–1.60) |
| Mortality | 85 | 3.1 | 1.45 (1.25–1.68) | 1.37 (1.15–1.63) | 1.17 (0.94–1.46) |
| sTNFR-2 | |||||
| Kidney disease progressionb | 182 | 10.1 | 1.75 (1.55–1.97) | 1.79 (1.55–2.06) | 1.47 (1.24–1.75) |
| ESKD | 124 | 5.8 | 2.05 (1.75–2.39) | 2.17 (1.80–2.61) | 1.50 (1.18–1.90) |
| Mortality | 85 | 3.1 | 1.62 (1.35–1.95) | 1.53 (1.25–1.87) | 1.33 (1.04–1.71) |
| YKL-40 | |||||
| Kidney disease progressionb | 171 | 9.9 | 1.46 (1.30–1.65) | 1.41 (1.23–1.62) | 1.21 (1.04–1.40) |
| ESKD | 117 | 5.8 | 1.58 (1.37–1.84) | 1.59 (1.33–1.91) | 1.19 (0.99–1.44) |
| Mortality | 77 | 3.0 | 1.80 (1.48–2.19) | 1.57 (1.26–1.96) | 1.45 (1.15–1.82) |
| MCP-1 | |||||
| Kidney disease progressionb | 182 | 10.1 | 1.24 (1.06–1.45) | 1.23 (1.02–1.48) | 1.33 (1.09–1.61) |
| ESKD | 124 | 5.8 | 1.27 (1.05–1.54) | 1.25 (1.00–1.57) | 1.47 (1.16–1.88) |
| Mortality | 84 | 3.1 | 1.33 (1.05–1.69) | 1.32 (1.00–1.74) | 1.36 (1.03–1.79) |
| suPAR | |||||
| Kidney disease progressionb | 176 | 10.6 | 1.20 (1.12–1.29) | 1.17 (1.08–1.27) | 1.08 (0.99–1.19) |
| ESKD | 122 | 6.3 | 1.26 (1.17–1.37) | 1.25 (1.14–1.37) | 1.11 (0.99–1.25) |
| Mortality | 82 | 3.2 | 1.19 (1.07–1.32) | 1.15 (1.02–1.31) | 1.08 (0.94–1.24) |
CI, confidence interval; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HR, hazard ratio; MCP, monocyte chemoattractant protein; sTNFR, soluble tumor necrosis factor receptor, suPAR, soluble urokinase plasminogen activator receptor.
Model 1 is unadjusted. Model 2 is stratified by site and adjusted for age, sex, race, natural log transformed proteinuria, and primary clinicopathologic diagnosis. Model 3 is Model 2 and further adjusted for baseline eGFR.
HR per doubling of biomarker.
Approximate events per 100 person-years. For the composite outcome with interval censored data, if an event occurred, the time used is one-half of the interval width plus all of the time before the interval as the approximate exposure time (the exact time an event occurred is not known if a ≥40% decline in eGFR occurred.
Kidney disease progression defined as ≥40% eGFR decline or ESKD.
Associations of Plasma Biomarkers With All-Cause Mortality
During a median follow-up time of 65 months, 85 participants died. Table 3 shows the unadjusted and multivariable-adjusted associations between each plasma biomarker and subsequent risk of death. sTNFR-2, YKL-40, and MCP-1 were each associated with increased risks of death in adjusted models. sTNFR-1 and suPAR were both associated with increased risk of death, but the associations were confounded by eGFR and not statistically significant in fully adjusted models. There was no evidence of statistical interaction between each plasma biomarker and primary clinicopathologic diagnosis for all-cause mortality (P for interaction: sTNFR-1, 0.53; sTNFR-2, 0.86; YKL-40, 0.16; MCP-1, 0.39; suPAR, 0.29) (Supplementary Table S3). Further adjustment for immunosuppressive medications did not qualitatively change the results (Supplementary Table S5). We tested the performance of the 5 plasma biomarkers of inflammation for predicting the 5-year risk of death in addition to the base model. The c-statistic of the base model was 0.72 (95% CI 0.65–0.78). The c-statistic of the model after addition of the 5 plasma biomarkers increased to 0.76 (95% CI 0.70–0.82; P < 0.001 for comparison with c-statistic of base model).
Discussion
Inflammation is a key driver of many forms of kidney disease, most obviously in glomerulonephritis and interstitial nephritis, but also in common disorders including diabetic kidney disease and acute kidney injury (AKI).5, 6, 7, 8 In this study involving a diverse set of kidney diseases with biopsy confirmation and long-term clinical follow-up, we found strong associations of biomarkers of inflammation with histopathologic lesions as well as subsequent risks of kidney disease progression and death. The addition of plasma biomarkers of inflammation offered little to modest improvements in risk prediction for the outcomes of ESKD and death as judged by the change in c-statistic, respectively. Our findings provide evidence for the importance of inflammation across a wide variety of kidney diseases, with consistent findings for associations in both glomerulopathies and other kidney diseases. Our findings also highlight the potential roles for biomarkers in nephrology to estimate prognosis and noninvasively assess kidney histopathology. Further discussion of each biomarker illustrates and contextualizes our findings.
Tumor necrosis factor receptors (TNFRs) are activated by TNF-α, which has an essential role in inflammatory processes.24 TNFR-1 is expressed on nearly every cell in the body and TNFR-2 is primarily expressed in lymphocytes.24,25 After activation, TNFRs are shed from the cell surface into a soluble form in the bloodstream (sTNFR).26 Plasma sTNFR-1 and sTNFR-2 have been reported to be associated with tubulointerstitial and glomerular lesions in patients with glomerulopathies27, 28, 29 and glomerular lesions in patients with diabetes mellitus.30 However, recent work found minimal correlations between circulating sTNFR-1 and sTNFR-2 with glomerular or tubular expression of the corresponding genes and minimal mRNA expression of sTNFR-1 and sTNFR-2 in areas of glomerulosclerosis, IFTA, or lymphocyte infiltration in individuals with established diabetic kidney disease.8 After multivariable adjustment that included eGFR, we found that both sTNFR-1 and sTNFR-2 are associated with tubulointerstitial and glomerular lesions but follow slightly different patterns of injury. Most of the published literature on sTNFR-1 and sTNFR-2 has focused on diabetes or CKD without biopsy confirmation,11,12,31,32 with relatively little investigation in individuals with glomerulopathies.28,29 Our cross-sectional and prospective findings suggest that sTNFR-1 and sTNFR-2 merit further evaluation as biomarkers in glomerular diseases.
YKL-40, also known as chitinase 3–like 1 (CHI3L1), is a glycoprotein produced by macrophages, neutrophils, and other local inflammatory cells.33 YKL-40 functions as an important mediator of inflammation after ischemia or reperfusion injury by activating phosphoinositide 3-kinase (PI3K)–dependent cell survival signaling via interleukin-13 receptor α2 (IL13Rα2) and stimulating macrophages.33,34 Through activation of chemokine receptor homologous molecule that is expressed on TH2 lymphocytes (CRTH2), YKL-40 is produced in response to ischemic AKI33 and promotes pro-fibrotic signaling in settings of sustained tissue injury and maladaptive repair.34 Prior studies from the cardiovascular literature suggested that YKL-40 is a growth factor for fibroblasts and promotes migration of vascular smooth muscles cells that can lead to tissue remodeling and subsequent vascular disease.35,36 We found higher levels of YKL-40 in individuals with more severe arterial sclerosis, but these associations were no longer significant after multivariable adjustment. Although a prior study identified an association between higher levels of YKL-40 and mortality in hemodialysis patients,37 this is the first study, to our knowledge, to demonstrate that higher plasma YKL-40 levels were independently associated with kidney disease progression and mortality in individuals with biopsy-confirmed kidney diseases.
MCP-1, also known as CCL2, is expressed by endothelial cells, macrophages, and fibroblasts and functions as a chemoattractant protein in response to tissue injury.38,39 Although much of the literature focuses on urinary MCP-1,39,40 less is known about plasma MCP-1 in the context of kidney disease.41 We identified significant associations of plasma MCP-1 with acute tubular injury and mesangial expansion. Our finding of an inverse relationship between plasma MCP-1 and acute tubular injury is inconsistent with prior animal and human studies that identified increased expression of MCP-1 in kidney tissue and higher levels of urinary MCP-1 with acute and chronic tubulointerstitial lesions.42, 43, 44 Additional studies in AKI cohorts are warranted to confirm or refute our finding. We also found that plasma MCP-1 was associated with kidney disease progression, ESKD, and death, which has not been reported previously to our knowledge.
suPAR is a marker of inflammation that is primarily expressed by endothelial and immune cells.45 suPAR has been suggested as a potential cause of focal segmental glomerulosclerosis through activation of podocyte β(3) integrin.46 While we did not identify significant associations of suPAR with segmental sclerosis, we found that higher levels of suPAR were associated with the presence of glomerular inflammation. Although suPAR levels have been associated with AKI,47 incident CKD,10 and kidney disease progression,13 the associations between suPAR and adverse clinical outcomes were heavily confounded by eGFR and not significant after further adjustment in the current study.
Our cross-sectional findings demonstrated that biomarkers of inflammation track with a number of histopathologic lesions. The reasons for these associations cannot be inferred from this study and may relate to other unmeasured confounding factors. Although the overlap of biomarker levels across histopathologic severity scores was substantial and do not permit accurate noninvasive estimates of specific lesions, multiplexed panels of a comprehensive set of inflammation biomarkers may enhance noninvasive histopathology assessment and represents an important area of investigation. The biomarkers we studied showed associations with future kidney disease progression and death, supporting their potential role as tools to identify high-risk individuals with kidney disease for enrollment in clinical trials.8,11,48,49 Biomarkers of inflammation could serve as a means of tracking response to therapy,50,51 particularly for therapies with anti-inflammatory effects52, 53, 54, 55, 56 that are increasingly being developed for a broad range of kidney disease, including AKI and diabetic kidney disease.
Strengths of our study include the diverse range of kidney diseases, adjudicated histopathologic scores on lesion severity, and prospective design with clinically important outcomes including ESKD and death. Our study has several limitations that warrant consideration as well. We included individuals who underwent clinically indicated native kidney biopsies, which do not represent the majority of cases of CKD and AKI, with a relative over-representation of glomerular diseases. Given the heterogeneity of the cohort, the number of individuals within each clinicopathologic diagnostic category was small, particularly for rare diseases. We did not have data on cause of death, which limited our ability to determine if higher levels of plasma biomarkers of inflammation are associated with specific causes of death. We did not account for therapy administered after the kidney biopsy, which could alter levels of biomarkers or an individual’s risk of the proposed outcomes, particularly in individuals requiring immunosuppressive therapy. Although we adjusted for a number of clinical predictors, there may be residual confounding from unmeasured confounders.
In conclusion, we found that plasma biomarkers of inflammation are associated with different patterns of histopathologic injury and are associated with adverse clinical outcomes across a variety of kidney disease etiologies. Our findings suggest that inflammation, a key pathologic driver of a number of kidney diseases, could be assessed noninvasively for estimation of risk, selecting patients for clinical trials, and tracking response to therapy. Future biomarker research in larger prospective cohorts and in randomized controlled trials are needed to identify the optimal biomarkers to improve clinical care and drug development in nephrology.
Disclosure
AS reports personal fees from Horizon Pharma, PLC, AstraZeneca, and CVS Caremark. JVB is cofounder and holds equity in Goldfinch Bio, is coinventor on KIM-1 patents assigned to Partners Healthcare, received grant funding from Boehringer Ingelheim, and has received consulting income related to biomarkers from Biomarin, Aldeyra, Angion, Cadent, PTC, Praxis, Seattle Genomics, and Sarepta. SSW reports personal fees from Public Health Advocacy Institute, CVS, Roth Capital Partners, Kantum Pharma, Mallinckrodt, Wolters Kluewer, GE Health Care, GSK, Mass Medical International, Barron and Budd (vs. Fresenius), JNJ, Venbio, Strataca, Takeda, Cerus, Pfizer, Bunch and James, Harvard Clinical Research Institute (aka Baim), and grants and personal fees from Allena Pharmaceuticals. All the other authors declared no competing interests.
Acknowledgments
We thank the members of the laboratory of SSW for their invaluable assistance in the Boston Kidney Biopsy Cohort. This study was supported by National Institutes of Health (NIH) grant R01DK093574 (SSW). AS is supported by NIH grant K23DK120811 and core resources from the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY) P30DK114857. IMS is supported by the American Philosophical Society Daland Fellowship in Clinical Investigation. SSW is also supported by NIH grants UH3DK114915, U01DK085660, U01DK104308, R01DK103784, and R21DK119751. JVB was supported by NIH grants R37DK39773, R01DK072381, and UH3 TR002155. This work was conducted with support from Harvard Catalyst. The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, NIH Award UL1TR001102), and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers or the NIH.
Part of this work was presented as poster and oral presentation at the 2019 American Society of Nephrology Scientific Session on November 7 and 9 in Washington, District of Columbia.
Author Contributions
AS, IMS, and SSW were responsible for the concept and design of the study. AS, RP, and SSW adjudicated clinicopathologic diagnoses. IES and HGR were responsible for the adjudication of histopathology. VS and JVB measured the plasma biomarkers. AS, IMS, and SSW were responsible for the statistical analysis. All authors interpreted the data. AS, IMS, and SSW drafted the manuscript. All authors contributed to critical revisions of the manuscript for important intellectual content.
Footnotes
Table S1. Histopathologic scoring system for light microscopy.
Table S2. Absolute plasma biomarker concentrations by primary clinicopathologic diagnosis.
Table S3. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with adverse clinical outcomes by primary clinicopathologic diagnosis.
Table S4. Competing risk analyses for the associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with ESKD.
Table S5. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with adverse clinical outcomes after adjustment for immunosuppressive medications .
Figure S1. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with tubulointerstitial lesions.
Figure S2. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with glomerular lesions.
Figure S3. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with microvascular lesions.
Supplementary Material
Table S1. Histopathologic scoring system for light microscopy.
Table S2. Absolute plasma biomarker concentrations by primary clinicopathologic diagnosis.
Table S3. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with adverse clinical outcomes by primary clinicopathologic diagnosis.
Table S4. Competing risk analyses for the associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with ESKD.
Table S5. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with adverse clinical outcomes after adjustment for immunosuppressive medications .
Figure S1. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with tubulointerstitial lesions.
Figure S2. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with glomerular lesions.
Figure S3. Associations of sTNFR-1, sTNFR-2, YKL-40, MCP-1, and suPAR with microvascular lesions.
References
- 1.Tangri N., Grams M.E., Levey A.S. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315:164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall Y.N., Himmelfarb J. The CKD classification system in the precision medicine era. Clin J Am Soc Nephrol. 2017;12:346–348. doi: 10.2215/CJN.09310916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava A., Palsson R., Kaze A. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol. 2018;29:2213–2224. doi: 10.1681/ASN.2017121260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preda A., LC V.D., Van Oostaijen J. Complication rate and diagnostic yield of 515 consecutive ultrasound-guided biopsies of renal allografts and native kidneys using a 14-gauge Biopsy gun. Eur Radiol. 2003;13:527–530. doi: 10.1007/s00330-002-1482-3. [DOI] [PubMed] [Google Scholar]
- 5.Remuzzi G., Benigni A., Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H.J., Vaziri N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298:F662–F671. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 7.Manning R.D., Jr., Tian N., Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25:311–317. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 8.Niewczas M.A., Pavkov M.E., Skupien J. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019;25:805–813. doi: 10.1038/s41591-019-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathcke C.N., Persson F., Tarnow L. YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care. 2009;32:323–328. doi: 10.2337/dc08-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayek S.S., Sever S., Ko Y.A. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coca S.G., Nadkarni G.N., Huang Y. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol. 2017;28:2786–2793. doi: 10.1681/ASN.2016101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatraju P., Zelnick L., Shlipak M. Association of soluble TNFR-1 concentrations with long-term decline in kidney function: The Multi-Ethnic Study of Atherosclerosis. J Am Soc Nephrol. 2018;29:2713–2721. doi: 10.1681/ASN.2018070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo S., Coresh J., Tin A. Soluble urokinase-type plasminogen activator receptor in black Americans with CKD. Clin J Am Soc Nephrol. 2018;13:1013–1021. doi: 10.2215/CJN.13631217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden C., Driscoll A.K., Simon A.E. Linkage of NCHS population health surveys to administrative records from social security administration and Centers for Medicare Medicaid Services. Vital Health Stat. 2015;1(58):1–53. [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wellner J.A., Zhan Y. A hybrid algorithm for computation of the nonparametric maximum likelihood estimator from censored data. J Am Stat Assoc. 1997;92:945–959. [Google Scholar]
- 18.Zhao Q., Sun J. Generalized log-rank test for mixed interval-censored failure time data. Stat Med. 2004;23:1621–1629. doi: 10.1002/sim.1746. [DOI] [PubMed] [Google Scholar]
- 19.Sun J. A non-parametric test for interval-censored failure time data with application to AIDS studies. Stat Med. 1996;15:1387–1395. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1387::AID-SIM268>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z., Sun J. Interval censoring. Stat Methods Med Res. 2010;19:53–70. doi: 10.1177/0962280209105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina M.J., D'Agostino R.B. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 22.Pencina M.J., D'Agostino R.B., Sr., D'Agostino R.B., Jr. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 23.Fine J., Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24.Al-Lamki R.S., Mayadas T.N. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int. 2015;87:281–296. doi: 10.1038/ki.2014.285. [DOI] [PubMed] [Google Scholar]
- 25.Chen G., Goeddel D. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 26.Bell J.H., Herrera A.H., Li Y. Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J Leukoc Biol. 2007;82:173–176. doi: 10.1189/jlb.0307193. [DOI] [PubMed] [Google Scholar]
- 27.Sonoda Y., Gohda T., Suzuki Y. Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh Y.J., An J.N., Kim C.T. Circulating tumor necrosis factor alpha receptors predict the outcomes of human IgA nephropathy: a prospective cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S.M., Yang S., Cha R.H. Circulating TNF receptors are significant prognostic biomarkers for idiopathic membranous nephropathy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavkov M.E., Weil E.J., Fufaa G.D. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int. 2016;89:226–234. doi: 10.1038/ki.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niewczas M.A., Gohda T., Skupien J. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavkov M.E., Nelson R.G., Knowler W.C. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015;87:812–819. doi: 10.1038/ki.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt I.M., Hall I.E., Kale S. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol. 2013;24:309–319. doi: 10.1681/ASN.2012060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montgomery T.A., Xu L., Mason S. Breast regression protein-39/chitinase 3-like 1 promotes renal fibrosis after kidney injury via activation of myofibroblasts. J Am Soc Nephrol. 2017;28:3218–3226. doi: 10.1681/ASN.2017010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langley S.R., Willeit K., Didangelos A. Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques. J Clin Invest. 2017;127:1546–1560. doi: 10.1172/JCI86924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kucukali Turkyilmaz A., Devrimsel G., Serdaroglu Beyazal M. The relationship between serum YKL-40 levels and arterial stiffness in patients with ankylosing spondylitis. Acta Reumatol Port. 2017;42:183–190. [PubMed] [Google Scholar]
- 37.Lorenz G., Schmalenberg M., Kemmner S. Mortality prediction in stable hemodialysis patients is refined by YKL-40, a 40-kDa glycoprotein associated with inflammation. Kidney Int. 2018;93:221–230. doi: 10.1016/j.kint.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Chow F.Y., Nikolic-Paterson D.J., Ozols E. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 39.Ix J.H., Katz R., Bansal N. Urine fibrosis markers and risk of allograft failure in kidney transplant recipients: a case-cohort ancillary study of the FAVORIT trial. Am J Kidney Dis. 2017;69:410–419. doi: 10.1053/j.ajkd.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadkarni G.N., Rao V., Ismail-Beigi F. Association of urinary biomarkers of inflammation, injury, and fibrosis with renal function decline: the ACCORD trial. Clin J Am Soc Nephrol. 2016;11:1343–1352. doi: 10.2215/CJN.12051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg J.H., Abraham A.G., Xu Y. Plasma biomarkers of tubular injury and inflammation are associated with CKD progression in children. J Am Soc Nephrol. 2020;31:1067–1077. doi: 10.1681/ASN.2019070723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grandaliano G., Gesualdo L., Ranieri E. Monocyte chemotactic peptide-1 expression in acute and chronic human nephritides: a pathogenetic role in interstitial monocytes recruitment. J Am Soc Nephrol. 1996;7:906–913. doi: 10.1681/ASN.V76906. [DOI] [PubMed] [Google Scholar]
- 43.Segerer S., Cui Y., Hudkin K. Expression of the chemokine monocyte chemoattractant protein-1 and its receptor chemokine receptor 2 in human crescentic glomerulonephritis. J Am Soc Nephrol. 2000;11:2231–2242. doi: 10.1681/ASN.V11122231. [DOI] [PubMed] [Google Scholar]
- 44.Eardley K.S., Zehnder D., Quinkler M. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–1197. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- 45.Mahdi F., Shariat-Madar Z., Todd R., III Expression and colocalization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood. 2001;97:2342–2350. doi: 10.1182/blood.v97.8.2342. [DOI] [PubMed] [Google Scholar]
- 46.Wei C., El Hindi S., Li J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayek S.S., Leaf D.E., Samman Tahhan A. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382:416–426. doi: 10.1056/NEJMoa1911481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamanouchi M., Skupien J., Niewczas M.A. Improved clinical trial enrollment criterion to identify patients with diabetes at risk of end-stage renal disease. Kidney Int. 2017;92:258–266. doi: 10.1016/j.kint.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parikh C.R., Liu C., Mor M.K. Kidney biomarkers of injury and repair as predictors of contrast-associated AKI: a substudy of the PRESERVE trial. Am J Kidney Dis. 2019;75:187–194. doi: 10.1053/j.ajkd.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heerspink H.J.L., Perco P., Mulder S. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62:1154–1166. doi: 10.1007/s00125-019-4859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mejia-Vilet J.M., Zhang X.L., Cruz C. Urinary soluble CD163: a novel noninvasive biomarker of activity for lupus nephritis. J Am Soc Nephrol. 2020;31:1335–1347. doi: 10.1681/ASN.2019121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navarro-Gonzalez J.F., Mora-Fernandez C., Muros de Fuentes M. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015;26:220–229. doi: 10.1681/ASN.2014010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demirjian S., Ailawadi G., Polinsky M. Safety and tolerability study of an intravenously administered small interfering ribonucleic acid (siRNA) post on-pump cardiothoracic surgery in patients at risk of acute kidney injury. Kidney Int Rep. 2017;2:836–843. doi: 10.1016/j.ekir.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pickkers P., Mehta R.L., Murray P.T. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA. 2018;320:1998–2009. doi: 10.1001/jama.2018.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuttle K.R., Brosius F.C., 3rd, Adler S.G. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a phase 2 randomized controlled clinical trial. Nephrol Dial Transplant. 2018;33:1950–1959. doi: 10.1093/ndt/gfx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridker P.M., MacFadyen J.G., Glynn R.J. Inhibition of Interleukin-1beta by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71:2405–2414. doi: 10.1016/j.jacc.2018.03.490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.