Abstract
Aim
Preclinical studies suggest treatment of metabolic acidosis may slow chronic kidney disease (CKD) progression. This systematic review aimed to summarize evidence from randomized controlled trials (RCTs) concerning the benefits and risks of bicarbonate therapy on kidney outcomes.
Methods
Medline, EMBASE, and Cochrane databases were searched for RCTs with ≥3 months’ follow-up in patients with CKD (estimated glomerular filtration rate [eGFR] ≤60 ml/min per 1.73 m2 and/or proteinuria) comparing the effects of sodium bicarbonate with placebo/no study medication on kidney outcomes. The primary outcome was change from baseline to last measurement in kidney function measured as either eGFR or creatinine clearance. Treatment effects were summarized using random-effects meta-analysis.
Results
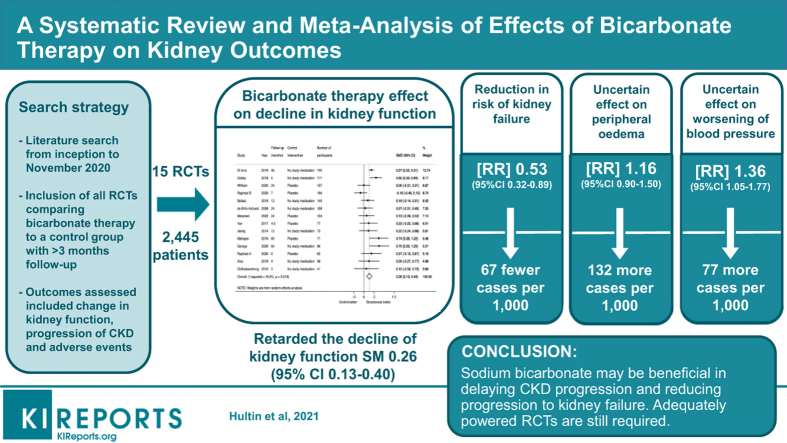
Fifteen trials (2445 participants, median follow-up 12 months) were eligible for inclusion. Compared with placebo or no study medication, sodium bicarbonate retarded the decline in kidney function (standardized mean difference [SMD]: 0.26; 95% confidence interval [CI]: 0.13–0.40; I2 = 50%, low certainty evidence), and reduced the risk of end-stage kidney failure (risk ratio [RR]: 0.53; 95% CI 0.32–0.89; I2 = 69%, low certainty evidence). The effect of sodium bicarbonate on proteinuria (SMD: −0.09; 95% CI −0.27 to 0.09; I2 = 28%, very low certainty evidence), systolic blood pressure (weighted mean difference [WMD]: −0.57 mm Hg; 95% CI −2.32 to 1.18; I2 = 0%, low certainty evidence), all-cause death (RR: 0.81; 95% CI: 0.39–1.68; I2 = 30%; very low certainty evidence) and edema (RR: 1.16; 95% CI: 0.90–1.50; I2 = 28%; low certainty evidence) were uncertain.
Conclusion
Sodium bicarbonate may slow CKD progression. Adequately powered randomized trials are required to evaluate the benefits and risks of sodium bicarbonate in CKD.
Keywords: acidosis, bicarbonate therapy, chronic kidney disease, clinical trial, dialysis, kidney function tests, kidney replacement therapy
Graphical abstract
Metabolic acidosis, defined as serum bicarbonate <22 mmol/l, varies in prevalence from 2% to 13% in chronic kidney disease (CKD) stage 3, increasing to 19% to 37% in CKD stage 4.1,2 It is one of the most common metabolic complications of CKD, reflecting the declining ability of the kidney to maintain acid-base homeostasis. As kidney function declines, the kidney loses its ability to excrete hydrogen ions and to generate bicarbonate to offset metabolic acid generation.3 Western diets further increase net endogenous acid production, thereby compounding the problem.4
Preclinical and clinical studies have shown that metabolic acidosis is associated with progression of CKD and all-cause mortality.5, 6, 7, 8 The Chronic Renal Insufficiency Cohort (CRIC) Study involving 3939 participants with CKD stages 2 to 4 showed that a 1 mmol/l increase in serum bicarbonate concentration was associated with a 3% lower risk of kidney failure or >50% reduction in eGFR.9 In another study involving 1781 individuals with CKD stages 2 to 4 who were either randomized, or screened but not randomized in the Modification of Diet in Renal Disease Study, the lowest quartile of serum bicarbonate concentration was associated with increased risks of progression to kidney failure (hazard ratio [HR]: 2.22, 95% confidence interval [CI]: 1.83–2.68) and all-cause mortality (HR: 1.39; 95% CI: 1.07–1.18) compared with the highest quartile of serum bicarbonate concentration.10 Although the mechanism by which metabolic acidosis may contribute to the progression of CKD remains uncertain, the presence of acidosis has been associated with multiple physiological abnormalities with potential nefarious consequences on the kidneys, including increased ammoniagenesis in residual nephrons leading to complement pathway activation and progressive tubular injury,11 impaired cardiac structure and function (including cardiac fibrosis, diastolic impairment, and heart failure),12 increased endothelin levels,13 chronic inflammation,13 decreased albumin synthesis and protein catabolism,14 insulin resistance,15 muscle wasting, and increased bone resorption and decreased bone formation.16
Due to these observed associations of metabolic acidosis with adverse outcomes, the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guidelines for the Evaluation and Management of CKD suggest correction of acidosis with oral bicarbonate supplementation in people with CKD and serum bicarbonate <22 mmol/l to maintain serum bicarbonate within the normal range, unless contraindicated (level 2B evidence).17 Concerns over bicarbonate supplementation relate to the effect of salt load and fluid retention as well as its contribution to polypharmacy, although large-scale trials are lacking.18 Therefore, we conducted this systematic review of RCTs to evaluate benefits and harms of bicarbonate therapy in CKD with respect to kidney outcomes.
Materials and Methods
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.19 The protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO 2017 CRD42017054546) (available at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017054546).
Search Strategy, Study Selection, and Data Extraction
Studies were eligible for inclusion if they (i) were RCTs; (ii) included adults or children with CKD (eGFR ≤60 ml/min per 1.73 m2 and/or proteinuria for a period of at least 3 months); (iii) compared oral bicarbonate therapy with placebo, or no study medication; (iv) followed participants for at least 3 months post-randomization; and (v) reported any of the following outcomes: changes in eGFR, creatinine clearance, serum creatinine, or proteinuria from baseline to the end of study; or initiation of kidney replacement therapy. The presence of metabolic acidosis was not an inclusion criterion. Trials involving dialysis-dependent kidney failure patients or kidney transplant recipients were excluded. Trials evaluating interventions other than sodium bicarbonate, such as fruit and vegetable-based diet and veverimer were not eligible. Potentially relevant studies were identified initially in January 2018 using highly sensitive electronic searches of Medline, EMBASE, and the Cochrane Central Register of Controlled Trials with English language restriction (Supplementary Table S1 for complete search strategy). Reference lists of relevant reviews were also searched. The literature search was updated in November 2020. If a trial included 2 or more groups of the same experimental intervention, data from these groups were combined so that each trial arm was included only once in the respective analyses. If multiple secondary publications of the same data set were identified, the most complete data were used. Only data from the first phase of randomized crossover trials were eligible to reduce the risk of a carryover effect of interventions between treatment periods. Missing, incomplete, or unpublished data from the clinical trials were requested from the investigators.
The following data were extracted using a standardized form: patient demographic details, study design and conduct, outcomes (baseline and end-of-study values of eGFR, creatinine clearance, serum creatinine, proteinuria, blood pressure, serum bicarbonate, doubling of serum creatinine, rapid decline of kidney function as defined by respective investigators, and progression to kidney failure), and adverse events. The methodological quality of each included study was assessed using the risk of bias assessment tool developed by the Cochrane Bias Methods Group.20 Jadad scoring was applied to all included studies as a continuous variable in addition to stratifying trials into low quality (score <3), moderate quality (score =3), and high quality (score >3).21 The following 6 items were assessed: (i) random sequence generation; (ii) allocation concealment; (iii) blinding of participants, investigators, and outcome assessors; (iv) incomplete outcome data; (v) selective outcome reporting; and (vi) any other bias (e.g., insufficient rationale, study design). Data extraction was carried out independently by 2 authors (SH and SVB). Disagreements were resolved via consultation with 2 other authors (NDT and DWJ).
Outcomes Assessed
The primary outcome assessed was change in kidney function (eGFR or creatinine clearance as reported) from baseline to last measurement or end of follow-up. The secondary outcomes assessed included changes in eGFR, creatinine clearance, serum creatinine, proteinuria, blood pressure, serum bicarbonate, progression to kidney failure, rapid decline of kidney function, all-cause mortality, adverse events, including heart failure, or worsening blood pressure.
Statistical Analysis
For each study, the mean difference in treatment effect on continuous outcomes from baseline to last measurement between treatment groups was calculated, together with the 95% CI. Mean differences in treatment effects across all studies were summarized as WMDs and 95% CIs. Mean differences in treatment effects on change in kidney function and proteinuria were reported as SMDs due to substantial variations in the methods by which kidney function was reported (GFR and creatinine clearance), and proteinuria was measured (albumin-creatinine ratio on spot urinalysis or 24-hour urine collection). For dichotomous outcomes, the results were expressed as RR with 95% CI. Studies with no reported dichotomous outcomes were excluded from analysis. Treatment effects were obtained by random-effects model using the DerSimonian and Laird method.22 Heterogeneity across the studies was estimated using the Cochrane's Q and I2 statistic.23 I2 values of 25%, 50%, and 75% corresponded to low, moderate, and high levels of heterogeneity. Meta-regression was conducted to investigate whether the following variables were a source of statistical heterogeneity: control intervention (placebo or no medication), duration of follow-up (<12 months or ≥12 months), and trial quality assessed by the Jadad score.24 If sufficient data were available, subgroup analyses were conducted according to the control intervention (placebo or no medication), duration of follow-up (<12 months or ≥12 months), and trial quality. The potential for small study effects (publication bias) was assessed by testing funnel plot asymmetry using Egger’s test for continuous outcomes25 and modified Harbord’s test for dichotomous outcomes.26 All analyses were conducted using Stata/MP metan package (version 15.1; StataCorp, College Station, TX). The certainty of evidence across trials was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.27
Results
Study Selection and Description
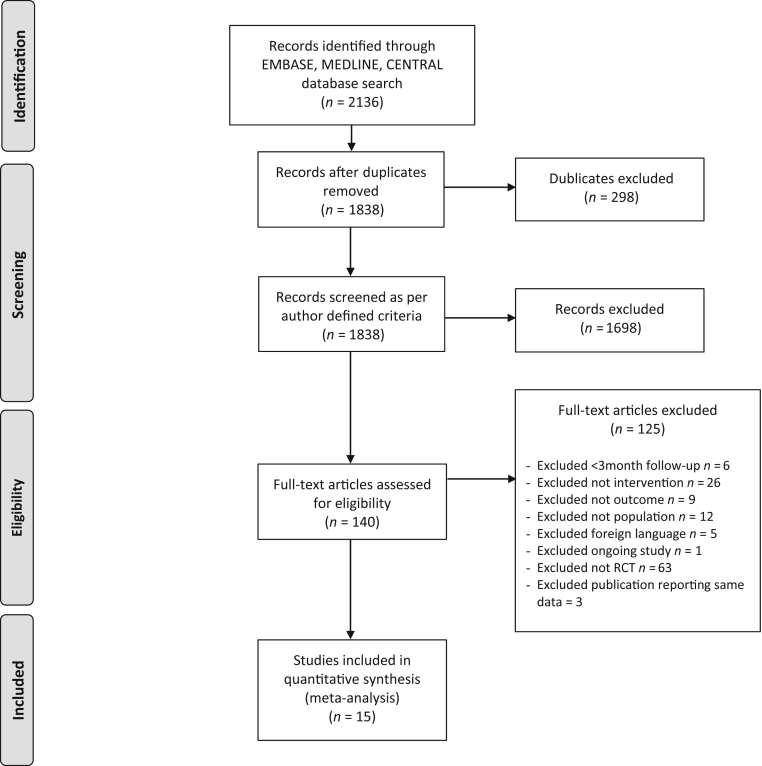
Fifteen trials involving 2445 participants (median sample size 84, range 40–795 patients; median follow-up 12 months, range 3–60 months) were included in the systematic review (Figure 1, Table 1). All included trials involved adult patients only. One trial comparing oral bicarbonate therapy to alkaline diet with fruits and vegetables was considered for inclusion, but excluded as both interventions were used to correct metabolic acidosis.28 One additional trial comparing bicarbonate supplementation with a high versus low serum bicarbonate target was considered for inclusion but was excluded because of the absence of a control group with placebo or no study medication.29 For 2 RCTs with more than 1 publication, data were extracted from the most recent publication.28,30, 31, 32 Except for 2 trials enrolling patients with eGFR 60 to 90 with albuminuria and patients with eGFR 15 to 89 ml/min, respectively,33,34 all enrolled participants had eGFR values <60 ml/min per 1.73 m2. Of these, 8 trials enrolled participants with CKD stages 3 or 4,18,29,35, 36, 37, 38, 39, 40, 41 3 trials enrolled participants with CKD stages 4 or 5,42, 43, 44 1 trial enrolled participants with CKD stages 3 to 5,45 and 1 trial reported an inclusion criterion as serum creatinine <5 mg/dl (442 μmol/l).46 One trial did not report an inclusion criterion based on serum bicarbonate concentration.46 Of the remaining trials, all except one33 included participants with metabolic acidosis with a median serum bicarbonate concentration of 20.6 mmol/l (range 16–26 mmol/l). Two trials included only participants with diabetes mellitus,34,35 whereas 2 trials excluded participants with diabetes mellitus.33,38 Median age was 61 years (range 40.5–73.9 years). By design, sodium bicarbonate was the interventional agent in all trials. However, 3 trials had an additional third intervention: fruit and vegetable diet, sodium chloride, and N-acetyl-cysteine in 1 trial each.33,38,39 Because the aim of this review was to compare sodium bicarbonate with placebo or no study medication, data from these trial arms were not included. Six trials were placebo-controlled studies.33,34,39,41,44,46
Figure 1.
PRISMA flow diagram showing selection of studies.
Table 1.
Summary of studies included in the systematic review
| Study (reference no.) | Inclusion criteria | n | Experimental intervention | Control intervention | Jadad score | Male sex, % | Age, y | Diabetes mellitus, % | Baseline kidney function | Baseline proteinuria | Baseline serum bicarbonate, mmol/l | Follow-up, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mathur 2006 (46) | Serum creatinine <4 mg/dl | 40 | Sodium bicarbonate 1.2 mEq/kg/d; target serum bicarbonate 22–26 mmol/l | Placebo | 3 | 63 | 40.5 | NR | Serum creatinine 2.9 mg/dl | NR | 19.4 | 3 |
| de Bristo-Ashurst 2009 (42) | CrCl 15 to 30 ml/min per 1.73 m2, serum bicarbonate 16–20 mmol/l | 134 | Sodium bicarbonate 600 mg thrice daily; target serum bicarbonate ≥23 mmol/l | No study medication | 3 | 52 | 54.8 | 36 | CrCl 20.4 ml/min per 1.73 m2 | 1.75 g/d | 19.9 | 24 |
| Mahajan 2010 (33) | Hypertension, urine ACR 200–2000 mg/g; eGFR 60–90 ml/min per 1.73 m2, serum bicarbonate >24.5 mmol/l | 80 | Sodium bicarbonate 0.5 mEq/kg/d | Placebo | 1 | 48 | 51.3 | 0 | eGFR 75.5 ml/min per 1.73 m2 | Urine ACR 421 mg/g | 26.1 | 60 |
| Disthabanchong 2010 (36) | eGFR ≤60 ml/min per 1.73 m2, serum bicarbonate ≤22 mmol/l | 44 | Sodium bicarbonate 1.8 to 3.6 g/d; target serum bicarbonate 21–43 mmol/l | No study medication | 2 | 48 | 62.8 | 49 | eGFR 18.8 ml/min per 1.73 m2 | NR | 20.9 | 3 to 4 |
| Jeong 2014 (43) | eGFR <30 ml/min per 1.73 m2, serum bicarbonate <22 mmol/l | 80 | Sodium bicarbonate 1000 mg thrice daily; target serum bicarbonate >22 mmol/l | No study medication | 1 | 71 | 54.6 | 26 | eGFR 16.9 ml/min per 1.73 m2 | NR | 18.7 | 12 |
| Bellasi 2016 (35) | eGFR 15–44 ml/min per 1.73 m2, serum bicarbonate <24 mmol/l | 145 | Sodium bicarbonate 0.5 mmol/kg twice daily; target serum bicarbonate 24–28 mmol/l | No study medication | 3 | 57 | 65.5 | 100 | CrCl 33.5 mL/min | NR | 21.4 | 12 |
| Yan 2017 (39) | eGFR 15–59 ml/min per 1.73 m2, serum bicarbonate 16–20 mmol/l, non-thyroid illness syndrome | 84 | Sodium bicarbonate 1–2 g/d; target serum bicarbonate 22–27 mmol/l | Placebo | 3 | 58 | 53.1 | 39 | eGFR 18.8 ml/min per 1.73 m2 | NR | 16.3 | 5 |
| Dubey 2018 (37) | eGFR 15–59 ml/min per 1.73 m2, serum bicarbonate <22 mmol/l | 188 | Sodium bicarbonate 0.5 mEq/kg/d; target serum bicarbonate 24–26 mmol/l | No study medication | 3 | 71 | 50.2 | 15 | eGFR 30.6 ml/min per 1.73 m2 | NR | 18.1 | 6 |
| Alva 2019 (40) | eGFR 15–30 ml/min per 1.73m2, serum bicarbonate 10–20 mmol/l | 67 | Sodium bicarbonate 1.8 g/d; target serum bicarbonate >23 mmol/l | No study medication | 2 | 71 | 72.6 | 3 | eGFR 21.8 ml/min per 1.73 m2 | NR | 16.7 | 9 |
| DiLorio 2019 (45) | eGFR 15–59 ml/min per 1.73m2, serum bicarbonate 18–24 mmol/l | 795 | Sodium bicarbonate up escalated by 25%/wk; target serum bicarbonate 24–28 mmol/l | No study medication | 3 | 62 | 67.6 | 30.7 | eGFR 33.4 ml/min per 1.73 m2 | Urine ACR 208 mg/g | 21.7 | 36 |
| Goraya 2019 (30) | eGFR 30–59 ml/min per 1.73 m2, urine ACR >200 mg/g; hypertension, serum bicarbonate 22–24 mmol/l | 72 | Sodium bicarbonate 0.3 mEq/kg/d | No study medication | 1 | 44 | 53.8 | 0 | eGFR 42.6 ml/min per 1.73 m2 | Urine ACR 316 mg/g | 23 | 60 |
| Witham 2020 (44) | eGFR 15–30 ml/min per 1.73 m2, serum bicarbonate <2 mmol/l, age >60 y | 300 | Sodium bicarbonate 500–1000 mg thrice daily; target serum >22mmol/l | Placebo | 5 | 57 | 73.9 | 50.5 | eGFR 18.9 ml/min per 1.73 m2 | Urine ACR 79.9 mg/g | 20.4 | 24 |
| Melamed 2020 (41) | eGFR 15–59 ml/min per 1.73m2, serum bicarbonate 20–26 mEq/l | 149 | Sodium bicarbonate 0.4 mEq/l/kg/d | Placebo | 5 | 54 | 61 | 62 | eGFR 36.2 ml/min per 1.73m2 | NR | 24 | 24 |
| Raphael 2020 (34) | eGFR 15–89 ml/min per 1.73m2, urine ACR <30 mg/g, serum bicarbonate 22–28 mEq/l | 74 | Sodium bicarbonate 0.5 mEq/kg/d in 2 divided doses | Placebo | 5 | 97 | 72 | 100 | eGFR 51 ml/min per 1.73 m2 | Urine ACR 121 mg/g | 24 | 6 |
| Raphael 2020 (18) | eGFR 20–44 ml/min per 1.73 m2 or eGFR 45–59 with urine ACR >50 mg/g, serum bicarbonate 20–28 mEq/l | 192 | Sodium bicarbonate 0.8 mEq/kg/d (high dose) or 12 mEq/d (low dose) | Placebo | 5 | 68 | 66 | 54 | eGFR 35 ml/min per 1.73 m2 | NR | 24 | 7 |
ACR, albumin-creatinine ratio; CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; NR, not reported
Risk of Bias
Figure 2 summarizes the risk of bias assessment (individual study bias assessment included in Supplementary Table S3). Random sequence generation and allocation concealment were reported using low-risk methods in 8 and 6 trials, respectively. Blinding of participants and investigators to the allocated intervention was reported in 7 and 5 trials, respectively. In general, risk of bias was higher in earlier trials, with trials published after 2019 reporting using known and lower risk bias methods. Similarly, placebo-controlled trials were more prevalent after 2019.
Figure 2.
Risk of bias assessment of the included studies according to the Cochrane Collaboration tool.
Kidney Outcomes
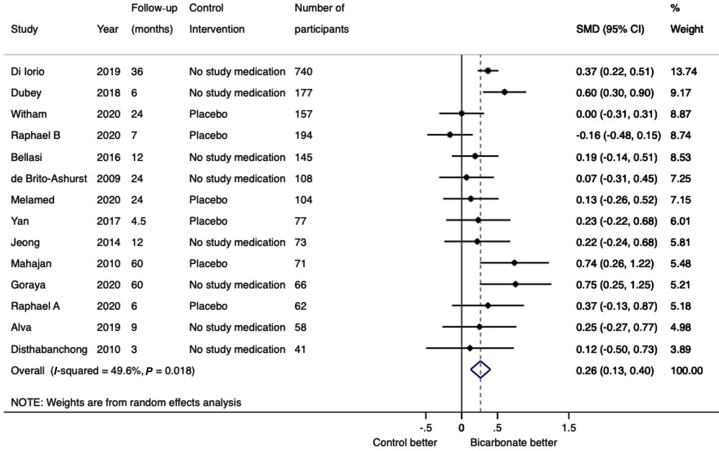
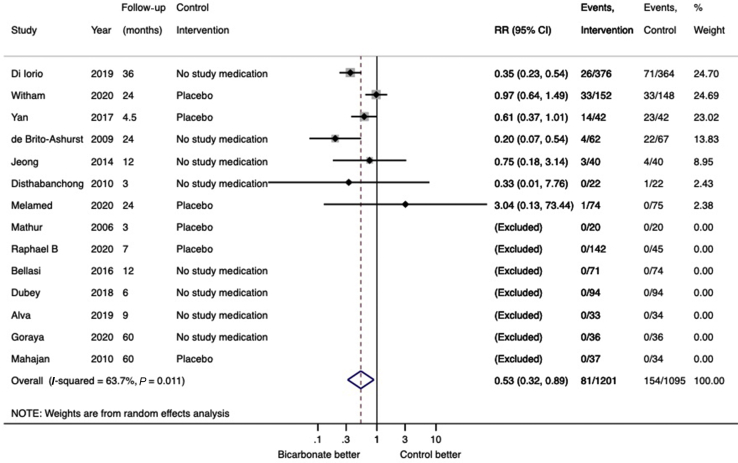
Eleven trials (1089 participants) reported data on eGFR and 3 trials (993 participants) reported data on creatinine clearance. Compared with placebo or no study medication, change in kidney function (eGFR or creatinine clearance) from baseline to trial completion was in favor of sodium bicarbonate (14 trials, 2082 participants, SMD: 0.26; 95% CI: 0.13–0.40; heterogeneity I2 = 50%, Figure 3) (low certainty evidence, Supplementary Table S2). Subgroup analysis demonstrated that heterogeneity was attributable to trial quality assessed by Jadad scoring (interaction P value 0.03) but was not modified by the control intervention (interaction P value 0.22) or follow-up duration (interaction P value 0.45) (Supplementary Figures S1–S3). Cumulative meta-analysis demonstrates stable and statistically significant point estimate of effect from 2016 onward (Supplementary Figure S4). When analyzed separately, both change in eGFR (11 trials, 1080 participants, WMD: 2.63; 95% CI: 0.70–4.55; heterogeneity I2=66%; Supplementary Figure S5) (very low certainty evidence) and creatinine clearance (3 trials, 993 participants, WMD: 5.78 ml/min; 95% CI: 3.56–7.99; heterogeneity I2 = 0%; low certainty evidence) (Supplementary Figure S6) were in favor of sodium bicarbonate. Subgroup analysis demonstrated that heterogeneity was attributable to the control intervention (interaction P value 0.04) and follow-up duration (interaction P value 0.03), but not by Jadad score (interaction P value 0.19) (Supplementary Figures S7–S9). Sodium bicarbonate had an uncertain effect on change in serum creatinine from baseline (6 trials, 559 participants, WMD: −0.20 mg/dl; 95% CI: −0.46 to 0.06 mg/dl; heterogeneity I2 = 56%; very low certainty evidence) (Supplementary Figure S10). For the outcome of progression to kidney failure, there were no reported events in 7 of the 14 trials that reported data on this outcome. In the remaining 7 trials (1526 participants), compared with the control arm, treatment with sodium bicarbonate reduced the risk of progression to kidney failure (RR: 0.53; 95% CI: 0.30–0.89; heterogeneity I2 = 64%; low certainty evidence) (Figure 4). Subgroup analysis demonstrated that heterogeneity was attributable to the control intervention (interaction P value 0.04), but not to follow-up duration (interaction P value 0.73) or Jadad score (interaction P value 0.24) (Supplementary Figures S11–S13). For the outcome of rapid decline of kidney function (defined as an annual decline in GFR or CrCl >3 ml/min per 1.73 m2), there were no reported events of rapid decline of kidney function in 4 of the 7 trials that reported data on this outcome. In the remaining 3 trials (1062 participants), treatment with sodium bicarbonate reduced the risk of rapid kidney function decline (RR: 0.32; 95% CI: 0.20–0.52; heterogeneity I2 = 58%; low certainty evidence) (Supplementary Figure S14).
Figure 3.
Forest plot showing the effect of bicarbonate therapy on change in kidney function (eGFR or creatinine clearance) from baseline to last measurement. CI, confidence interval; SMD, standardized mean difference.
Figure 4.
Forest plot showing the effect of bicarbonate therapy on progression to kidney failure. CI, confidence interval; RR, risk ratio.
There was no significant difference in change in proteinuria between the bicarbonate and control arms (6 trials, 633 participants, SMD: −0.09; 95% CI: −0.27 to 0.09; heterogeneity I2 = 16%; very low certainty evidence) (Supplementary Figure S15).
Other Outcomes
Compared with placebo or no study medication, treatment with sodium bicarbonate increased serum bicarbonate concentration (13 trials, 1814 participants, WMD: 2.59 mmol/l; 95% CI: 1.51–3.66 mmol/l; heterogeneity I2 = 95%; very low certainty evidence) (Supplementary Figure S16). Subgroup analysis demonstrated that heterogeneity was due to the control intervention (interaction P value 0.001), but not by follow-up duration (interaction P value 0.10) or Jadad score (interaction P value 0.58) (Supplementary Figures S17–S19). Treatment with bicarbonate had no significant effect on changes in systolic blood pressure (12 trials, 1932 participants, WMD: −0.57 mm Hg; 95% CI: −2.32 to 1.18 mm Hg; heterogeneity I2 = 0%; low certainty evidence) (Supplementary Figure S20), or diastolic blood pressure (10 trials, 1794 participants, WMD: 0.88 mm Hg; 95% CI: −0.61 to 2.38 mm Hg; heterogeneity I2 = 27%; low certainty evidence) (Supplementary Figure S21).
Adverse Events
There were no significant differences in the risks of worsening of blood pressure (5 trials, 1383 participants, RR: 1.36; 95% CI: 1.05–1.77; heterogeneity I2 = 58%; low certainty evidence) (Supplementary Figure S22), edema (6 trials, 1600 participants, RR: 1.16; 95% CI: 0.90–1.50; heterogeneity I2 = 28%; low certainty evidence) (Supplementary Figure S23), and change in weight (8 trials, 1535 participants, WMD: –0.11 kg; 95% CI: −0.93 to 0.70 kg; heterogeneity I2 = 0%; low certainty evidence) (Supplementary Figure S24) between the sodium bicarbonate and control groups. There were no death events in 3 of 9 trials (265 participants) that reported mortality data. In the remaining 6 trials (1648 participants) there was no difference in the risk of all-cause death between the bicarbonate and control groups (RR: 0.81; 95% CI: 0.39–1.68; heterogeneity I2 = 30%; very low certainty evidence) (Supplementary Figure S25). There were no reported events of hospitalization for heart failure in 2 trials (317 participants) that reported these data. In the remaining 3 trials (387 participants), there was no difference in the risk of hospitalization for heart failure between the sodium bicarbonate and control groups (RR: 1.19; 95% CI: 0.30–4.67; heterogeneity I2 = 9%; very low certainty evidence) (Supplementary Figure S26)
Test for Small Study Bias
No statistically significant evidence of small study bias was found (Funnel plots included in Supplementary Figures S27–S33).
Discussion
This systematic review demonstrated that bicarbonate therapy may slow the progression of CKD. Compared with placebo or no study medication, oral sodium bicarbonate treatment attenuated decline in kidney function, as assessed by eGFR or creatinine clearance and delayed progression to kidney failure. There were no observed differences in proteinuria or blood pressure. Although data on adverse events and mortality were reported in fewer trials, there was no appreciable difference reported in peripheral edema, hospitalization for heart failure, or all-cause mortality.
In view of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline suggesting correction of acidosis with oral bicarbonate supplementation in CKD, the results of the present systematic review are important.17 Our results are consistent with previous systematic reviews that also reported delayed CKD progression with bicarbonate treatment.47,48 The current systematic review, however, included more and recent trials with 2445 participants and examined a wide range of outcomes. The totality of evidence highlights the growing body of literature favoring correction of metabolic acidosis in CKD as a strategy to delay progression. The overall certainty of the evidence favoring this, however, was of low certainty.
Alongside the growing evidence of benefit, robust data on adverse events and side effects are required to determine the generalizability of our findings. There were significant differences in characteristics of included trials, including participant eligibility criteria, patient sex, comorbidities such as diabetes, stages of CKD, intervention in the control groups, and duration of follow-up. Although we investigated the source of statistical heterogeneity by conducting meta-regression, analyses were limited to only 3 study-level characteristics, as individual patient data were not available. Only 1 trial recruited elderly patients with CKD, and generalizability of our findings, the cost-benefit, and quality-of-life impact from pill burden across all patient cohorts and ages is uncertain and needs to be assessed in longer-term follow-up trials. Despite the heterogeneous study population in several included trials, generalizability of our findings was further limited by an underrepresentation of female, older, and more comorbid patients with CKD recruited into included trials. As there were no identified studies recruiting pediatric patients, generalizability of these results to children with CKD is uncertain. Adequately powered randomized trials are required to evaluate the harms and benefits of bicarbonate therapy on clinical outcomes in this patient population.
The strengths of this review include comprehensive overview of the evidence, risk of bias assessment, use of the GRADE approach to assess the body of evidence, and subgroup analysis of outcomes with high heterogeneity. These strengths should be balanced against its limitations, which were largely due to the limitations of the included trials. These include clinical heterogeneity in participant characteristics (baseline kidney function, diabetes status, proteinuria, serum bicarbonate levels) and study-level characteristics (dosage of sodium bicarbonate, intervention in the control groups, duration of follow-up, methods of assessment of kidney function). Furthermore, subgroup analyses were limited to study-level variables including follow-up duration, control intervention, and study quality, with heterogeneity arising from patient-level characteristics not estimable without individual patient data. As the focus of this systematic review was on kidney outcomes, other outcomes, such as nutritional or inflammatory markers and mineral bone disease, quality of life or polypharmacy were not evaluated. This review also did not study non-bicarbonate treatment of metabolic acidosis, such as fruit and vegetable-based diets and veverimer.49,50
In conclusion, the available evidence showed that bicarbonate treatment may slow the progression of CKD in patients with moderate or advanced CKD. Past meta-analyses of the literature have concluded insufficient evidence to enable recommendation of bicarbonate supplementation without acidosis or to upgrade the evidence for supplementation as a strategy to delay CKD progression due to lack of high-quality RCTs addressing this issue.48,51 Since then, despite several new published RCTs, adequately high-powered RCTs are still required in light of low-quality evidence supporting this.
Disclosures
DWJ has previously received consultancy fees, research grants, speaker’s honoraria, and travel sponsorships from Baxter Healthcare and Fresenius Medical Care, consultancy fees from Astra Zeneca and AWAK, speaker’s honoraria and travel sponsorships from ONO, and travel sponsorships from Amgen. All the other authors declared no competing interests.
Acknowledgments
The authors gratefully thank Dr Sinee Disthabanchong (Ramathibodi Hospital, Mahidol University, Bangkok, Thailand), Dr Donald E. Wesson (Texas A&M College of Medicine, Scott and White Healthcare, Temple, Texas, USA), Professor Muhammad Yaqoob (William Harvey Research Institute and Barts and the London NHS Trust, London, United Kingdom), A/Professor Kalani Raphael (University of Utah, Salt Lake City, USA), and Professor Miles Witham (NIHR Newcastle Biomedical Research Centre, Newcastle University, United Kingdom) for providing requested data.
DWJ is supported by an Australian Government National Health and Medical Research Council (NHMRC) Practitioner Fellowship. SVB is supported by a John Chalmers Clinical Research Fellowship with the support of Servier from The George Institute for Global Health, Australia. These supporting organizations/agencies had no role in the design and conduct of the study, analysis and interpretation of the data, review and approval of the manuscript, and decision to submit the manuscript.
Author Contributions
Study design: SH, CH, NDT, KLC, DWJ, SVB; Literature search, study selection, and data extraction: SH, SVB; Statistical analysis and interpretation: SH, NDT, CH, KLC, SVB; Supervision: DWJ, SVB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Table S1. Electronic search strategy.
Table S2. Grade assessment of certainty of evidence.
Table S3. Individual study bias assessment.
Figure S1. Subgroup analysis of the effect of bicarbonate therapy on change in kidney function 1: according to the use of placebo or no study medication in the control arm.
Figure S2. Subgroup analysis of the effect of bicarbonate therapy on the change in kidney function 2: according to follow-up time.
Figure S3. Subgroup analysis of the effect of bicarbonate therapy on the change in kidney function 3: according to trial quality.
Figure S4. Cumulative metanalysis of the effect of bicarbonate therapy on the change in kidney function.
Figure S5. Effect of bicarbonate therapy on change in eGFR.
Figure S6. Effect of bicarbonate therapy on change in creatinine clearance.
Figure S7. Subgroup analysis of the effect of bicarbonate therapy on eGFR (ml/min per 1.73 m2) 1: according to the use of placebo or no study medication in the control arm.
Figure S8. Subgroup analysis of the effect of bicarbonate therapy on eGFR (ml/min per 1.73 m2) 2: according to follow-up time.
Figure S9. Subgroup analysis of the effect of bicarbonate therapy on eGFR (ml/min per 1.73 m2) 3: according to trial quality.
Figure S10. Effect of bicarbonate therapy on change in serum creatinine.
Figure S11. Subgroup analysis of the effect of bicarbonate therapy on progression to kidney failure 1: according to the use of placebo or no study medication in the control arm.
Figure S12. Subgroup analysis of the effect of bicarbonate therapy on the progression to kidney failure 2: according to follow-up time.
Figure S13. Subgroup analysis of the effect of bicarbonate therapy on the progression to kidney failure 3: according to trial quality.
Figure S14. Effect of bicarbonate therapy on rapid decline in kidney function.
Figure S15. Effect of bicarbonate therapy on change in proteinuria.
Figure S16. Effect of bicarbonate therapy on change in serum bicarbonate.
Figure S17. Subgroup analysis of the effect of bicarbonate therapy on serum bicarbonate (mmol/l) 1: according to the use of placebo or no study medication in the control arm.
Figure S18. Subgroup analysis of the effect of bicarbonate therapy on serum bicarbonate (mmol/l) 2: according to follow-up time.
Figure S19. Subgroup analysis of the effect of bicarbonate therapy on serum bicarbonate (mmol/l) 3: according to trial quality.
Figure S20. Effect of bicarbonate therapy on change in systolic blood pressure.
Figure S21. Effect of bicarbonate therapy on change in diastolic blood pressure.
Figure S22. Effect of bicarbonate therapy on worsening of blood pressure.
Figure S23. Effect of bicarbonate therapy on worsening of edema.
Figure S24. Effect of bicarbonate therapy on change in body weight.
Figure S25. Effect of bicarbonate therapy on all-cause mortality.
Figure S26. Effect of bicarbonate therapy on admissions for heart failure.
Figure S27. Funnel plot of effect of bicarbonate therapy on kidney function.
Figure S28. Funnel plot of effect of bicarbonate therapy on the progression to kidney failure.
Figure S29. Funnel plot of the effect of bicarbonate therapy on eGFR (ml/min per 1.73 m2).
Figure S30. Funnel plot of the effect of bicarbonate therapy on serum bicarbonate.
Figure S31. Funnel plot of the effect of bicarbonate therapy on weight (kg).
Figure S32. Funnel plot of the effect of bicarbonate therapy on systolic blood pressure.
Figure S33. Funnel plot of the effect of bicarbonate therapy on diastolic blood pressure.
Figure S34. Subgroup analysis of the effect of bicarbonate therapy on systolic blood pressure according to trial quality.
Figure S35. Subgroup analysis of the effect of bicarbonate therapy on diastolic blood pressure according to trial quality.
Figure S36. Subgroup analysis of the effect of bicarbonate therapy on change in weight according to trial quality.
Supplementary Material
Table S1. Electronic search strategy.
Table S2. Grade assessment of certainty of evidence.
Table S3. Individual study bias assessment.
Figure S1. Subgroup analysis of the effect of bicarbonate therapy on change in kidney function 1: according to the use of placebo or no study medication in the control arm.
Figure S2. Subgroup analysis of the effect of bicarbonate therapy on the change in kidney function 2: according to follow-up time.
Figure S3. Subgroup analysis of the effect of bicarbonate therapy on the change in kidney function 3: according to trial quality
Figure S4. Cumulative metanalysis of the effect of bicarbonate therapy on the change in kidney function.
Figure S5. Effect of bicarbonate therapy on change in eGFR.
Figure S6. Effect of bicarbonate therapy on change in creatinine clearance.
Figure S7. Subgroup analysis of the effect of bicarbonate therapy on eGFR (ml/min per 1.73 m2) 1: according to the use of placebo or no study medication in the control arm.
Figure S8. Subgroup analysis of the effect of bicarbonate therapy on eGFR (ml/min per 1.73 m2) 2: according to follow-up time.
Figure S9. Subgroup analysis of the effect of bicarbonate therapy on eGFR (ml/min per 1.73 m2) 3: according to trial quality.
Figure S10. Effect of bicarbonate therapy on change in serum creatinine.
Figure S11. Subgroup analysis of the effect of bicarbonate therapy on progression to kidney failure 1: according to the use of placebo or no study medication in the control arm.
Figure S12. Subgroup analysis of the effect of bicarbonate therapy on the progression to kidney failure 2: according to follow-up time.
Figure S13. Subgroup analysis of the effect of bicarbonate therapy on the progression to kidney failure 3: according to trial quality.
Figure S14. Effect of bicarbonate therapy on rapid decline in kidney function.
Figure S15. Effect of bicarbonate therapy on change in proteinuria.
Figure S16. Effect of bicarbonate therapy on change in serum bicarbonate.
Figure S17. Subgroup analysis of the effect of bicarbonate therapy on serum bicarbonate (mmol/l) 1: according to the use of placebo or no study medication in the control arm.
Figure S18. Subgroup analysis of the effect of bicarbonate therapy on serum bicarbonate (mmol/l) 2: according to follow-up time.
Figure S19. Subgroup analysis of the effect of bicarbonate therapy on serum bicarbonate (mmol/l) 3: according to trial quality.
Figure S20. Effect of bicarbonate therapy on change in systolic blood pressure.
Figure S21. Effect of bicarbonate therapy on change in diastolic blood pressure.
Figure S22. Effect of bicarbonate therapy on worsening of blood pressure.
Figure S23. Effect of bicarbonate therapy on worsening of edema.
Figure S24. Effect of bicarbonate therapy on change in body weight
Figure S25. Effect of bicarbonate therapy on all-cause mortality.
Figure S26. Effect of bicarbonate therapy on admissions for heart failure.
Figure S27. Funnel plot of effect of bicarbonate therapy on kidney function.
Figure S28. Funnel plot of effect of bicarbonate therapy on the progression to kidney failure.
Figure S29. Funnel plot of the effect of bicarbonate therapy on eGFR (ml/min per 1.73 m2).
Figure S30. Funnel plot of the effect of bicarbonate therapy on serum bicarbonate.
Figure S31. Funnel plot of the effect of bicarbonate therapy on weight (kg).
Figure S32. Funnel plot of the effect of bicarbonate therapy on systolic blood pressure.
Figure S33. Funnel plot of the effect of bicarbonate therapy on diastolic blood pressure.
Figure S34. Subgroup analysis of the effect of bicarbonate therapy on systolic blood pressure according to trial quality.
Figure S35. Subgroup analysis of the effect of bicarbonate therapy on diastolic blood pressure according to trial quality.
Figure S36. Subgroup analysis of the effect of bicarbonate therapy on change in weight according to trial quality.
References
- 1.Raphael K.L., Zhang Y., Ying J., Greene T. Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology (Carlton) 2014;19:648–654. doi: 10.1111/nep.12315. [DOI] [PubMed] [Google Scholar]
- 2.Eustace J.A., Astor B., Muntner P.M. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 2004;65:1031–1040. doi: 10.1111/j.1523-1755.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 3.Yaqoob M.M. Acidosis and progression of chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:489–492. doi: 10.1097/MNH.0b013e32833b64fa. [DOI] [PubMed] [Google Scholar]
- 4.Moore L.W., Byham-Gray L.D., Scott Parrott J. The mean dietary protein intake at different stages of chronic kidney disease is higher than current guidelines. Kidney Int. 2013;83:724–732. doi: 10.1038/ki.2012.420. [DOI] [PubMed] [Google Scholar]
- 5.Shah S.N., Abramowitz M., Hostetter T.H., Melamed M.L. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis. 2009;54:270–277. doi: 10.1053/j.ajkd.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramowitz M.K., Melamed M.L., Bauer C. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol. 2013;8:714–720. doi: 10.2215/CJN.08340812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harambat J., Kunzmann K., Azukaitis K. Metabolic acidosis is common and associates with disease progression in children with chronic kidney disease. Kidney Int. 2017;92:1507–1514. doi: 10.1016/j.kint.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Raphael K.L., Wei G., Baird B.C. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011;79:356–362. doi: 10.1038/ki.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobre M., Yang W., Chen J. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2013;62:670–678. doi: 10.1053/j.ajkd.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon V., Tighiouart H., Vaughn N.S. Serum bicarbonate and long-term outcomes in CKD. Am J Kidney Dis. 2010;56:907–914. doi: 10.1053/j.ajkd.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Dobre M., Rahman M., Hostetter T.H. Current status of bicarbonate in CKD. J Am Soc Nephrol. 2015;26:515–523. doi: 10.1681/ASN.2014020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell J.H., Wildenthal K., Johnson R.L., Jr. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972;1:375–389. doi: 10.1038/ki.1972.48. [DOI] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K., Mehrotra R., Fouque D., Kopple J.D. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial. 2004;17:455–465. doi: 10.1111/j.0894-0959.2004.17606.x. [DOI] [PubMed] [Google Scholar]
- 14.Ballmer P.E., McNurlan M.A., Hulter H.N. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest. 1995;95:39–45. doi: 10.1172/JCI117668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi S., Maesato K., Moriya H. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis. 2005;45:275–280. doi: 10.1053/j.ajkd.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Martin K.J., Gonzalez E.A. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol. 2007;18:875–885. doi: 10.1681/ASN.2006070771. [DOI] [PubMed] [Google Scholar]
- 17.Chapter 3: Management of progression and complications of CKD. Kidney Int Suppl. 2013;3:73–90. doi: 10.1038/kisup.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raphael K.L., Isakova T., Ix J.H. A randomized trial comparing the safety, adherence, and pharmacodynamics profiles of two doses of sodium bicarbonate in CKD: the BASE pilot trial. J Am Soc Nephrol. 2020;31:161–174. doi: 10.1681/ASN.2019030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J., Altman D.G. Assessing risk of bias in included studies. In: Higgins J., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons Ltd; Chichester, West Sussex, England: 2008. pp. 187–241. [Google Scholar]
- 21.Jadad A.R., Moore R.A., Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson S.G., Higgins J.P. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 25.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harbord R.M., Egger M., Sterne J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goraya N., Simoni J., Jo C.H., Wesson D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8:371–381. doi: 10.2215/CJN.02430312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kittiskulnam P., Srijaruneruang S., Chulakadabba A. Impact of serum bicarbonate levels on muscle mass and kidney function in pre-dialysis chronic kidney disease patients. Am J Nephrol. 2020;51:24–34. doi: 10.1159/000504557. [DOI] [PubMed] [Google Scholar]
- 30.Goraya N., Munoz-Maldonado Y., Simoni J., Wesson D.E. Fruit and vegetable treatment of chronic kidney disease-related metabolic acidosis reduces cardiovascular risk better than sodium bicarbonate. Am J Nephrol. 2019;49:438–448. doi: 10.1159/000500042. [DOI] [PubMed] [Google Scholar]
- 31.Goraya N, Munoz-Maldonado Y, Simoni J, Wesson DE. Treatment of chronic kidney disease-related metabolic acidosis with fruits and vegetables compared to NaHCO3 yields more and better overall health outcomes and at comparable five-year cost [e-pub ahead of print]. J Ren Nutr. https://doi.org/10.1053/j.jrn.2020.08.001, Accessed November 5, 2020. [DOI] [PubMed]
- 32.Withamo M.D., Bando M., Chongo H. Sodium bicarbonate to improve physical function in patients over 60 years with advanced chronic kidney disease: The BiCARB RCT. Health Technol Assess. 2020;24:1–120. doi: 10.3310/hta24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahajan A., Simoni J., Sheather S.J. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78:303–309. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]
- 34.Raphael K.L., Greene T., Wei G. Sodium bicarbonate supplementation and urinary TGF-beta1 in nonacidotic diabetic kidney disease: a randomized, controlled trial. Clin J Am Soc Nephrol. 2020;15:200–208. doi: 10.2215/CJN.06600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellasi A., Di Micco L., Santoro D. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016;17:158. doi: 10.1186/s12882-016-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Disthabanchong S., Treeruttanawanich A. Oral sodium bicarbonate improves thyroid function in predialysis chronic kidney disease. Am J Nephrol. 2010;32:549–556. doi: 10.1159/000321461. [DOI] [PubMed] [Google Scholar]
- 37.Dubey A.K., Sahoo J., Vairappan B. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: a randomized controlled trial. Nephrol Dial Transplant. 2020;35:121–129. doi: 10.1093/ndt/gfy214. [DOI] [PubMed] [Google Scholar]
- 38.Goraya N., Simoni J., Jo C.H., Wesson D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;86:1031–1038. doi: 10.1038/ki.2014.83. [DOI] [PubMed] [Google Scholar]
- 39.Yan W., Wang L., Huang T., Xu G. Treatment for non-thyroidal illness syndrome in advanced chronic kidney disease: a single-blind controlled study. J Nephrol. 2017;30:557–565. doi: 10.1007/s40620-016-0341-2. [DOI] [PubMed] [Google Scholar]
- 40.Alva S., Divyashree M., Kamath J. A study on effect of bicarbonate supplementation on the progression of chronic kidney disease. Indian J Nephrol. 2020;30:91–97. doi: 10.4103/ijn.IJN_93_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melamed M.L., Horwitz E.J., Dobre M.A. Effects of sodium bicarbonate in CKD stages 3 and 4: a randomized, placebo-controlled, multicenter clinical trial. Am J Kidney Dis. 2020;75:225–234. doi: 10.1053/j.ajkd.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Brito-Ashurst I., Varagunam M., Raftery M.J., Yaqoob M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong J., Kwon S.K., Kim H.Y. Effect of bicarbonate supplementation on renal function and nutritional indices in predialysis advanced chronic kidney disease. Electrolyte Blood Press. 2014;12:80–87. doi: 10.5049/EBP.2014.12.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clinical and cost-effectiveness of oral sodium bicarbonate therapy for older patients with chronic kidney disease and low-grade acidosis (BiCARB): a pragmatic randomised, double-blind, placebo-controlled trial. BMC Med. 2020;18:91. doi: 10.1186/s12916-020-01542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Iorio B.R., Bellasi A., Raphael K.L. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol. 2019;32:989–1001. doi: 10.1007/s40620-019-00656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathur R.P., Dash S.C., Gupta N. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Ren Fail. 2006;28:1–5. doi: 10.1080/08860220500461187. [DOI] [PubMed] [Google Scholar]
- 47.Susantitaphong P., Sewaralthahab K., Balk E.M. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol. 2012;35:540–547. doi: 10.1159/000339329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navaneethan S.D., Shao J., Buysse J., Bushinsky D.A. Effects of treatment of metabolic acidosis in CKD: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2019;14:1011–1020. doi: 10.2215/CJN.13091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bushinsky D.A., Hostetter T., Klaerner G. Randomized, controlled trial of TRC101 to increase serum bicarbonate in patients with CKD. Clin J Am Soc Nephrol. 2018;13:26–35. doi: 10.2215/CJN.07300717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wesson D.E., Mathur V., Tangri N. Veverimer versus placebo in patients with metabolic acidosis associated with chronic kidney disease: a multicentre, randomised, double-blind, controlled, phase 3 trial. Lancet. 2019;393:1417–1427. doi: 10.1016/S0140-6736(18)32562-5. [DOI] [PubMed] [Google Scholar]
- 51.Hu M.K., Witham M.D., Soiza R.L. Oral bicarbonate therapy in non-haemodialysis dependent chronic kidney disease patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Med. 2019;8:208. doi: 10.3390/jcm8020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.