Abstract
Recently, we reported high antibacterial efficiency of Loranthus acaciae (LA) against different standard strains of bacteria including Methicillin-Resistant Staphylococcus aureus (MRSA). Therefore, this study aimed to confirm the effectiveness of LA against clinically isolated Staphylococcus aureus (SA) including β-lactamase producer (Blac) and MRSA. Forty-eight SA isolates collected from various clinical samples were used in this study. Antibiotics susceptibility profile was determined for twenty different antibiotics using automated Microscan Walkaway 96 Plus system as recommended by Clinical and Laboratory Standards Institute (CLSI) guidelines. This system also identified β-lactamase producers and MRSA. In the meantime, LA ethanolic extract was fractionated using liquid–liquid fraction method to hexane, dichloromethane DCM and methanol 80% fractions. Antimicrobial activities of LA extract and fraction were performed with agar well diffusion method for all SA isolates, MIC and MBC were also recorded. Phytochemical screening for various phyto-constituent classes of LA ethanolic extract was determined. Out of 48 SA isolates, Cefoxitin-positive MRSA represent 31 (64.6%), Blac 17 (35.4%), and 41 (85.4%) were multidrug-resistant SA, which was resistant at least to one antibiotic from three different categories. All isolates were resistant to ampicillin and penicillin. Antimicrobial activities of LA extract and fractions revealed that ethanol extract was active against all isolated SA with inhibition zone ranged from 33 ± 2.00 to 25 ± 3.05 mm. While DCM exhibited the largest inhibition zone range from 37 ± 3.00 to 33 ± 2.00 mm. This study is first of its kind conforming the high antibacterial activity of LA against SA isolated from a different source of infection. The study concluded that LA extract and fractions are active and give positive result for all isolated SA. Therefore, suitable pharmacological formulation of LA extract as a promising antibacterial agent for the treatment of SA infection should be given extreme priority.
Keywords: β-lactamase enzyme, MRSA, Plicosepalus acaciae, Loranthus acaciae, Multi-drug resistant, Antimicrobial activities
Abbreviations: LA, Loranthus acaciae; MRSA, methicillin-resistant Staphylococcus aureus; SA, Staphylococcus aureus; Blac, β-lactamase producer; DCM, Dichloromethane; MIC, minimum inhibitory concentration; MBC, bactericidal concentration; CLSI, clinical and laboratory standards institute; ATCC, American type culture collection; MeOH, methanol
1. Introduction
Staphylococcus aureus SA is a common pathogen that is widely identified both in healthy populations and also isolated from several infections (Al-Amery et al., 2019, Beyene et al., 2017). Globally, the emergence of antimicrobial resistance particularly to methicillin has increased, leading to higher morbidity and mortality due to methicillin-resistant S. aureus (MRSA) infections (Okwu et al., 2019, Arshad et al., 2017, Moglad et al., 2020a, Elboshra et al., 2020). Antibiotic resistance can be defined as the inability of antibiotic to show its effects against some bacteria to which they were once sensitive. This resistance of bacteria towards antibiotic can be developed through misuse or overuse of antibiotics. As a result, the cases of antibiotic resistance is growing, resulting in considerable increase in morbidity and mortality rate due to infectious diseases (Chew et al., 2018). Centre of Disease Control and World Health Organization have stated the problem of antibiotic resistance as dramatically increasing since we are now living in the “post –antibiotic era” (Michael et al., 2014). S. aureus has developed some kind of resistance against the β-lactam antibiotics, such as penicillin, oxacillin, methicillin, and amoxicillin (Appelbaum, 2007). There are two primary mechanisms for development of this resistance. (1) The expression of β-lactamase enzymes (BLAC) resulting in resistance of penicillin and amoxycillin and (2) production penicillin with low affinity binding protein PBP-2a encoded by mecA gene resulting in resistance of higher β-lactam antibiotics (MRSA) (Ventola, 2015). This situation rings alarm for discovering new antibiotic as the treatment choices for infected patients are enormously constricted at the moment (Freire-Moran et al., 2011). Therefore, there is a need to search for the unconventional sources of active drug. Herbal medicines as an alternative therapeutics approach for governing common infections have gained wide acceptance throughout the world. High cost of synthetic compounds and the emergence of multiple drug-resistant strains of bacteria has engaged researchers to find new therapeutic agents from medicinal plants (Okwu et al., 2019).
Loranthus acaciae Zucc. (LA) belongs to Loranthaceae family. LA is a semi-parasitic perennial plant that directly attaches to another host plant such as Acacia tree via haustoria (Moreno-Salazar et al., 2008). LA is widely spread in Sudan as well as Saudi Arabia. It has been used in traditional medicine to treat tonsilitis and otitis media and in treatment of hookworms infections, smallpox, and diarrhea (Noman et al., 2019). Numerous studies on biological activities of LA plant species indicated antidiabetic (Osadebe et al., 2004, Osadebe et al., 2010), potential anticancer (Sadik et al., 2003), wound healing, and vascular protective activities (Ameer et al., 2010). Some studies reported that bioactive phytochemicals compounds identified from LA are quercetin 3-O-β-D-glucopyranoside, catechin 7-O-gallate, quercetin 3-O-β-(6-O-galloyl)-glucopyranoside and catechin (Noman et al., 2019).
Our previous study reported significant antibacterial activities of LA methanolic extract towards standard ATCC Gram positive bacteria, and further study of fractionated active extract and investigating their activities against clinical isolates were recommended (Moglad et al., 2020b). Based on our previous data, the principal objective of this study is to evaluate the potential antibacterial activity of LA crude extract and fractions against isolated MRSA and Blac producing Staphylococcus aureus. This study is first of its kind.
2. Material and methods
2.1. Bacterial samples collection and identification
Forty-eight isolates of SA was collected from the Microbiology department of the Maternity and Children Hospital, Al Kharj, KSA. These were isolated from various clinical samples like pus, blood, nasal swabs, vaginal swabs, urine, and sputum. All the samples were inoculated on culture media like chocolate agar, 5% sheep blood agar and mannitol salt agar following standard microbiological procedures and incubated at 37 °C for 24–48 hrs. Identification of isolates was done using Gram stain and Catalase test. Confirmation of all the isolates was done using automated Microscan Walkaway 96 Plus system following the manufacturer’s instructions.
2.2. Antibiotic susceptibility patterns
Susceptibility of all isolates to antibiotics was determined using Microscan Positive BP combo 28 (PBC28) panel in automated Microscan Walkaway 96 Plus system. This provided Staphylococcus aureus identification and susceptibility results on one panel. PBC28 panel includes Cefoxitin screening to detect Methicillin-resistant Staphylococcus aureus (MRSA) with concentration of 4 µg/ml and Oxacillin in doubling dilutions from 0.25 − 2 µg/ml for MIC detection following latest CLSI guidelines. Compositions and concentrations of all the other antimicrobials of the PBC28 panel are given in table 1.
Table 1.
Compositions and concentrations of antimicrobials.
| Antimicrobials | PBC 28 (µg/ml) |
|---|---|
| Amoxicillin-Clavulanic acid | 4/2–8/4 |
| Ampicillin | 0.25, 4–8 |
| Azithromycin | 2–4 |
| Cefoxitin screen well | 4 |
| Ciprofloxacin | 1–2 |
| Clindamycin | 0.25–0.5, 2 |
| Daptomycin | 1–4 |
| Erythromycin | 0.5, 4 |
| Fosfomycin | 32 |
| Fusidic acid | 2, 16 |
| Gentamicin | 1, 4–8 |
| Gentamicin synergy | 500 |
| Imipenem | 4–8 |
| Inducible clindamycin | 4/0.5 |
| Levofloxacin | 1–4 |
| Linezoid | 2–4 |
| Moxifloxacin | 0.5–1 |
| Mupirocin | 4–8, 256 |
| Nitrofurantoin | 32–64 |
| Oxacillin | 0.25, 1–2 |
| Penicillin | 0.03–0.12, 8 |
| Rifampin | 1–2 |
| Streptomycin synergy | 1000 |
| Quinupristin Dalfopristin | 1–2 |
| Teicoplanin | 4–16 |
| Tetracycline | 4–8 |
| Trimethoprim-sulfametoxazole | 2/38–4/76 |
| Vancomycin | 0.5–16 |
The panels were run automatically following the manufacturer’s instructions. Quality control organisms used with Microscan panel were the primary ATCC 29213 Staphylococcus aureus strain recommended by Clinical and Laboratory Standards Institute (CLSI) for minimal antimicrobic susceptibility quality control testing and ATCC 43300 Staphylococcus aureus strain for confirmation of Cefoxitin susceptibility.
2.3. Plant materials and extract preparation
Wild plant material was collected during March 2018 from South Kurdufan State, Sudan, after permission was obtained. The plant specimens were authenticated by Dr. Yahya Soliman, a plant taxonomist, the herbarium of the Medicinal and Aromatic Plants and Traditional Medicine Research Institute (MAPRI), National Center for Research, Khartoum-Sudan, and identified as Plicosepalus acacaciae (Zucc.) Wiem & Polhil (syn. Loranthus acaciae Zucc.). A voucher specimen (No. MAP/2018/10) deposited at MAPRI herbarium. The plant material was dried and ground into powder using mortar and pestle. One kilogram was soaked in 3 Liters of ethanol 96%. Extraction was carried out for three days using rotary evaporator apparatus, with daily filtration and evaporation of the solvent under reduced pressure (Moglad et al., 2020b).
2.4. Fractionation of ethanolic extract
Ethanolic extract was fractionated by liquid–liquid fractionation method (Handa et al., 2008). Briefly; twenty grams of extract were totally suspended in 500 ml distilled water, then partitioned with a same volume of n-hexane three times. Aqueous layer was lyophilized using freeze-drier machine. Then n-hexane was was partitioned with 90% MeOH:H2O, the n-hexane layer was separated and the concentration of MeOH layer was changed from 90% to 80% by addition of distilled water. Fractionation of this last layer was done using dichloromethane solvent.
2.5. Antibacterial activity of plant extract and fractions
2.5.1. Inoculum preparation
From a fresh subculture of all tested strains (overnight culture on nutrient agar media), bacterial suspension prepared using the direct colony suspension method; two to three colonies were dissolved in sterile normal saline solution (0.9%). The turbidity of suspension was compared with a 0.5 McFarland turbidity standard, to achieve a number of bacterial cell approximately 1–2 × 108 CFU/mL for each of the tested bacteria.
2.5.2. Antibacterial susceptibility test: Well diffusion method
Antibacterial activities of LA ethanolic extract and fractions were screened using the agar well diffusion method (Balouiri et al., 2016, Magaldi et al., 2004). Taking into consideration the recommendations by Eloff (Eloff, 2019) for avoiding pitfalls in antimicrobial activities screening. 200 µL of each isolated bacteria was consistently spread on the Muller and Hinton agar by sterile cotton swabs. Then 8 mm size wells were cut into the agar using a sterile Cork borer. Stock solution prepared from plant extract and fractions for antibacterial activities investigation using 100% dimethylsulfoxide (DMSO) at 100 mg/mL. Then the extract was diluted using methanol to obtain different concentrations of 10 mg/ml, 6 mg/ml, and 4 mg/ml. 100 μL of each extract and fractions were transferred into each of three wells on all plates, and methanol was used as a negative control. To dry the solution, lefted all the plates uncover in a sterile safety cabinet for 20 min (Dkhil et al., 2020). Vancomycin (30 µg) and gentamicin (10 µg) were used as standard antimicrobial drug. The result was recorded by measuring the size of the zone of inhibition after 24 h of incubation at 35 °C ± 2 °C. The Experiments were performed in duplicate and repeated three time independently, and the result is displayed as mean of inhibition zone ± standard deviation.
2.5.3. Determination of Minimum Inhibitory concentration (MIC)
MIC was done using broth dilution method as described by Magaldi et al. (Magaldi et al., 2004). From stock solution, a serial two-fold dilutions made to obtain different concentrations of extracts ranged from 50 mg/mL, 25 mg/mL, 12.5 mg/mL, 6.25 mg/mL, 3.125 mg/mL, and 1.56 mg/mL. Methanol was used as solvent and as negative control. 100 μL of each standardized bacterial suspension typically 1–2 × 108 CFU/mL inoculated into broth media containing extracts. . After incubation at 37 °C, turbidity was recorded, and Minimum Bactericidal Concentration (MBC) was evaluated. The test was triplicated.
2.6. Phytochemical screening
Phytochemical screening for the detection of saponins, coumarins, alkaloids, flavonoids, tannins, phytosterols, triterpenoids, cardiac glycosides and anthraquinone were carried out on ethanolic extract and investigated qualitatively by different standardized methods (Mujeeb et al., 2014, Ali et al., 2018).
2.7. Statistical analysis
SPSS Version 20.0 used to measure the different source frequency and antibiotics susceptibility profile. Antimicrobial activities of LA were analyzed by using a Microsoft excel version 2016. All experiments were performed in triplicates. The well diffusion experimental results were displayed as mean ± standard deviation.
3. Results
Forty-eight clinically isolated SA encompassed in the study. The highest frequency of samples were isolated from nasal swab 25 (52.1%) followed by pus 13 (27.1%) and the remaining from samples like tracheal aspirate 3 (6.2%), urine 2 (4.2%), sputum 2 (4.2%) and one each from blood, vaginal swab, and nail finger swab showed.
Among the 48 isolates, 17 (35.4%) were β-lactamase producer showing resistance to Penicillin and ampicillin and sensitive to β-lactam + β-lactamase inhibitor combination drugs like Amoxicillin-calavulanate. Whereas the remaining number of isolates 31 (64.6%) were positive to Cefoxitin screen test and also resistant to Oxacillin, thus identified as Methicillin-resistant Staphylococcus aureus (MRSA). These isolates are considered resistant to all β-lactam antibiotics like Cephalosporins, Penicillins, and Carbapenems. Thus in our study all the 48 isolates were shown resistant to Penicillin and Ampicillin. Majority of the isolates 41 (85.4%) showed simultaneous resistance to three or more than three different groups of antibiotics (Table 2). This once again emphasizes the extent of growing resistance to available antimicrobials and the urgent need for an alternative therapeutic approach showed.
Table 2.
Antibiotics susceptibility profile of isolated SA.
| Antibiotics |
Sensitive No (%) |
Resistant No (%) |
|---|---|---|
| Amoxicillin-Clavulanate | 17 (35.4%) | 31 (64.6%) |
| Ampicillin | 0 (0%) | 48 (100%) |
| Oxacillin | 17 (35.4%) | 31 (64.6%) |
| Azithromycin | 12 (25%) | 36 (75%) |
| Erythromycin | 16 (33.3%) | 32 (66.7%) |
| Moxifloxacin | 28 (58.3%) | 20 (41.7%) |
| Levofloxacin | 29 (60.4%) | 19 (39.6%) |
| Ciprofloxacin | 28 (58.3%) | 20 (41.7%) |
| Clindamycin | 18 (37.5) | 30 (62.5%) |
| Fusidic Acid | 20 (41.7%) | 28 (58.3%) |
| Gentamicin | 33 (86.8%) | 15 (13.2%) |
| Imipenem | 17 (35.4%) | 31 (64.6%) |
| Linezolid | 26 (54.2%) | 22 (45.8%) |
| Mupirocin | 33 (68.8%) | 15 (31.2%) |
| Pencillin | 0 (0%) | 48 (100%) |
| Rifampin | 45 (93.8%) | 3 (6.2%) |
| Teicoplanin | 45 (93.8%) | 3 (6.2%) |
| Tetracycline | 38 (79.2%) | 10 (20.8%) |
| Trimethoprim/ Sulfamethoxazole | 43 (89.6%) | 5 (10.4%) |
| Vancomycin | 30 (62.5%) | 18 (37.5) |
3.1. Antibacterial activity of LA extract and fractions
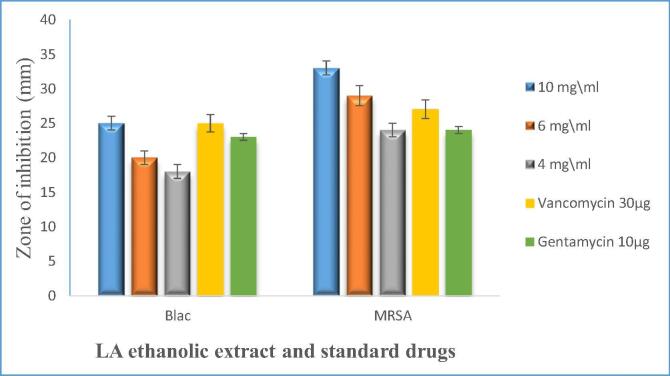
LA ethanolic extract was investigated against all isolated MRSA and β-lactamase producing S. aureus, the result revealed that the ethanolic extract is active against both BLAC producers and MRSA. The largest zone of inhibition obtained from LA was the ethanolic extract at the concentration of 10 mg/ml against MRSA (Fig. 1).
Fig. 1.
Comparison of antimicrobial activity of LA ethanol extract different concentration 10, 6, and 4 mg\ml against all isolated SA and standards antimicrobial drugs: Blac = β-lactamase producing S. aureus, MRSA = Methicillin-Resistant S. aureus. The Experiments were done in duplicate and repeated three time independently, and the result displayed as mean of inhibition zone ± standard deviation.
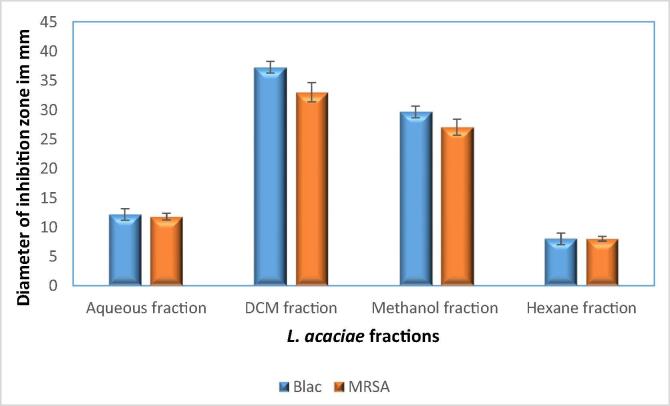
LA ethanol extract and fractions obtained using different solvent (Hexane, Dichloromethane DCM, methanol 80% and aqueous). Among fractions, the largest growth inhibition zone obtained from DCM fraction followed by Methanol 80%. While, Hexane fraction was inactive, and aqueous fraction gave intermediate zone of inhibition (Fig. 2).
Fig. 2.
Antimicrobial activities of LA fractions at concentration 10 mg\ml against all isolated SA. The Experiments were done in duplicate and repeated three time independently, and the result was displayed as mean of inhibition zone ± standard deviation.
3.2. Minimum Inhibitory concentration MIC and MBC
Different concentrations of extract were prepared using twofold dilution method to determine MIC against SA isolates. The lowest concentration of the extracts which fully inhibited bacterial growth was defined as MIC. MBC was recorded when no visible growth was seen on Manitol Salt agar inoculated from the first clear tube in the dilutions for all isolates. The result obtained indicated that MIC varies from 3.125 to 1.56 mg/ml, and MBC were 6.25 mg/ml.
3.3. Phytochemical screening
Primary phytochemical result showed that saponins, coumarins, flavonoids, tannins, phytosterols, triterpenoids, cardiac glycosides and anthraquinone were present in extract. While, Alkaloid was not present in ethanol extract.
4. Discussion:
This study reported high prevalence of MRSA 31(64.6%), Blac 17(35.4%) and 41(85.4%) multidrug resistant SA in community and a high level of resistance to commonly used therapeutic agents. This alarming situation forced the researchers to find out an alternative medicine to treat SA infection. LA is one such medicinal plant which has been investigated for different biological activities for instance; antioxidant, anti-inflammatory, wound healing activities, anti-diabetic, and antibacterial activities (Moreno-Salazar et al., 2008, Noman et al., 2019). However, this is the first study reporting its antibacterial activity against clinically isolated SA. Furthermore, our results entirely agreed with other various studies that have stated the antimicrobial activities of LA against wide range of bacteria (Elegami et al., 2001, El-Shafei et al., 2018).
It is remarkable to observe that the susceptibility test of all SA strains shows only slight difference between LA ethanol extract, DCM, and methanol fractions. This means that probably there are numerous active components that might possibly cause synergistic multi-target effects to the antibacterial activity (Coutinho et al., 2009). This Synergistic multi-target properties of herbal medicine is combined with drugs (Efferth and Koch, 2011). These combination of components may work on different antibacterial targets synchronously, such as change the cell membrane permeability, decomposing the genetic materials, and inhibiting the efflux pump (Kim et al., 2013, Coutinho et al., 2009). A previous study stated that the existence of several ingredients might show greater activity inside the host (Efferth and Koch, 2011).
LA phytochemical screening showed the presence of saponins, coumarins, flavonoids, tannins, phytosterols, triterpenoids, cardiac glycosides and anthraquinone, which probably could be responsible for the antibacterial activities of LA. Another study reported that the presence of loranthin, flavanocoumarin, quercetin, catechin, rutin, methyl gallate and gallic acid in LA revealed a significant antibacterial activity (Badr et al., 2013). LA extract and fractions were active against all isolated SA, this may be because of Phytochemical compounds which may change the outer membrane permeability, and occupy the active sites and act as β-lactamase inhibitors, and inhibit multidrug resistance efflux pumps (Król et al., 2015, Mingeot-Leclercq and Décout, 2016).
Our recently published research investigated the acute toxicity of LA ethanol extract on mice, and the result revealed that LA extract is not toxic and the LD50 was more than 2 g/kg body weight orally (Moglad et al., 2020b).
5. Conclusion
From the above recorded evidence, our outcomes show that the prevalence of MRSA and Blac are increasing. LA extract and fractions exhibited significant antibacterial activity towards all isolated SA. Therefore, formulation of LA extract in suitable pharmacological dose should be given extreme priority for the treatment of SA diseases.
Funding
Not funded
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This publication was supported by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University, Alkharj, Saudi Arabia. I would also like to extend my appreciation to the Microbiology department of laboratory, Maternity and Children Hospital, Al Kharj, for providing the clinical isolates of Staphylococcus aureus. Also, I would like to thanks Miss Reem Osman for her help and support in the plant extraction procedure.
Availability of data and materials
The datasets used and/or analysed during the current study are included in this published article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Amery K., Elhariri M., Elsayed A., El-Moghazy G., Elhelw R., El-Mahallawy H., el Hariri M., Hamza D. Vancomycin-resistant Staphylococcus aureus isolated from camel meat and slaughterhouse workers in Egypt. Antimicrobial Resist. Infection Control. 2019;8:129. doi: 10.1186/s13756-019-0585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Khan M., Ullah I., Sajid M., Zahra Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement. Alternative Med. 2018;18 doi: 10.1186/s12906-018-2114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameer O.Z., Salman I.M., Siddiqui M.J., Yam M.F., Sriramaneni R.N., Mohamed A.J., Sadikun A., Ismail Z., Shah A.M., Asmawi M.Z. Pharmacological mechanisms underlying the vascular activities of Loranthus ferrugineus Roxb. in rat thoracic aorta. J. Ethnopharmacol. 2010;127:19–25. doi: 10.1016/j.jep.2009.09.057. [DOI] [PubMed] [Google Scholar]
- APPELBAUM, P.C., 2007. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin. Infect. Dis, 45 Suppl 3, S165–70. [DOI] [PubMed]
- Arshad N., Mehreen A., Liaqat I., Arshad M., Afrasiab H. In vivo screening and evaluation of four herbs against MRSA infections. BMC Complement. Alternat. Med. 2017;17 doi: 10.1186/s12906-017-2001-z. 498-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr J.M., Shaala L.A., Youssef D.T.A. Loranthin: A new polyhydroxylated flavanocoumarin from Plicosepalus acacia with significant free radical scavenging and antimicrobial activity. Phytochem. Lett. 2013;6:113–117. [Google Scholar]
- Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyene T., Hayishe H., Gizaw F., Beyi A.F., Abunna F., Mammo B., Ayana D., Waktole H., Abdi R.D. Prevalence and antimicrobial resistance profile of Staphylococcus in dairy farms, abattoir and humans in Addis Ababa, Ethiopia. BMC Research Notes. 2017;10:171. doi: 10.1186/s13104-017-2487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, Y.L., Mahadi, A.M., Wong, K.M., Goh, J.K., 2018. Anti-methicillin-resistance Staphylococcus aureus (MRSA) compounds from Bauhinia kockiana Korth. And their mechanism of antibacterial activity. 18, 70. [DOI] [PMC free article] [PubMed]
- Coutinho, H.D., Costa, J.G., Lima, E.O., Falcão-Silva, V.S., Siqueira, J.P., JR., 2009. Herbal therapy associated with antibiotic therapy: potentiation of the antibiotic activity against methicillin--resistant Staphylococcus aureus by Turnera ulmifolia L. BMC Complement Altern. Med., 9, 13. [DOI] [PMC free article] [PubMed]
- Dkhil M.A., Zreiq R., Hafiz T.A., Mubaraki M.A., Sulaiman S., Algahtani F., Abdel-Gaber R., Al-Shaebi E.M., Al-Quraishy S. Anthelmintic and antimicrobial activity of Indigofera oblongifolia leaf extracts. Saudi J. Biol. Sci. 2020;27:594–598. doi: 10.1016/j.sjbs.2019.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T., Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets. 2011;12:122–132. doi: 10.2174/138945011793591626. [DOI] [PubMed] [Google Scholar]
- El-Shafei G., Al-Hazmi B., Marghelani A., Al-Moalem D., Badr J., Moneib N. Antimicrobial activity of different extracts of Plicosepalus acacia. Records Pharmaceut. Biomed. Sci. 2018;1:47–51. [Google Scholar]
- Elboshra M.M.E., Hamedelnil Y.F., Moglad E.H., Altayb H.N. Prevalence and characterization of virulence genes among methicillin-resistant Staphylococcus aureus isolated from Sudanese patients in Khartoum state. New Microbes New Infect. 2020;38 doi: 10.1016/j.nmni.2020.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elegami A.A., Elnima E.I., Muddathir A.K., Omer M.E. Antimicrobial activity of Plicosepalus acaciae. Fitoterapia. 2001;72:431–434. doi: 10.1016/s0367-326x(01)00268-4. [DOI] [PubMed] [Google Scholar]
- ELOFF, J. N. 2019. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement Altern. Med., 19, 106. [DOI] [PMC free article] [PubMed]
- Freire-Moran L., Aronsson B., Manz C., Gyssens I.C., So A.D., Monnet D.L., Cars O. Critical shortage of new antibiotics in development against multidrug-resistant bacteria-Time to react is now. Drug Resist Updat. 2011;14:118–124. doi: 10.1016/j.drup.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Handa S.S., Khanuja S., Longo G., Rakesh D.D. Extraction technologies for medicinal and aromatic plants. Int. Centre Sci. High Technol. 2008:21–25. [Google Scholar]
- Kim K.S., Lim D.J., Yang H.J., Choi E.K., Shin M.H., Ahn K.S., Jung S.H., Um J.Y., Jung H.J., Lee J.H., Lee S.G., Jung S.K., Jang H.J. The multi-targeted effects of Chrysanthemum herb extract against Escherichia coli O157:H7. Phytother. Res. 2013;27:1398–1406. doi: 10.1002/ptr.4859. [DOI] [PubMed] [Google Scholar]
- Król, E., De Sousa Borges, A., Da Silva, I., Polaquini, C. R., Regasini, L. O., Ferreira, H., Scheffers, D.J., 2015. Antibacterial activity of alkyl gallates is a combination of direct targeting of FtsZ and permeabilization of bacterial membranes. Front. Microbiol., 6, 390. [DOI] [PMC free article] [PubMed]
- Magaldi, S., Mata-Essayag, S., Hartung de Capriles, C., Perez, C., Colella, M. T., Olaizola, C., Ontiveros, Y, 2004. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Diseases, 8, 39–45. [DOI] [PubMed]
- Michael C.A., Dominey-Howes D., Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front. Public Health. 2014;2 doi: 10.3389/fpubh.2014.00145. 145 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingeot-Leclercq M.-P., Décout J.-L. Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides. MedChemComm. 2016;7:586–611. [Google Scholar]
- Moglad E.H.O., Boon S.K.A., Ali H.T.O. Various medicinal plants: a promising treatment for multidrug-resistant bacteria isolated from wound infection. Int. J. Pharm. Sci. Res. 2020;11:839–843. [Google Scholar]
- Moglad, E. H., Hamad, A. M., Fatima, F., Devanathadesikan Seshadri, V., Naz, M., 2020b. Antimicrobial and wound healing activities of certain Sudanese medicinal plants. Saudi J. Biol. Sci., 27, 1766–1772. [DOI] [PMC free article] [PubMed]
- Moreno-Salazar S.F., Robles-Zepeda R.E., Johnson D.E. Plant folk medicines for gastrointestinal disorders among the main tribes of Sonora, Mexico. Fitoterapia. 2008;79:132–141. doi: 10.1016/j.fitote.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Mujeeb F., Bajpai P., Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/497606. 497606 497606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman, O. M., Mothana, R. A., Al-Rehaily, A. J., Al Qahtani, A. S., Nasr, F. A., Khaled, J. M., Alajmi, M. F., Al-Said, M. S., 2019. Phytochemical analysis and anti-diabetic, anti-inflammatory and antioxidant activities of Loranthus acaciae Zucc. Grown in Saudi Arabia. Saudi Pharm. J., 27, 724–730. [DOI] [PMC free article] [PubMed]
- Okwu M.U., Olley M., Akpoka A.O., Izevbuwa O.E. Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: a brief review. AIMS Microbiol. 2019;5:117–137. doi: 10.3934/microbiol.2019.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osadebe P.O., Okide G.B., Akabogu I.C. Study on anti-diabetic activities of crude methanolic extracts of Loranthus micranthus (Linn.) sourced from five different host trees. J. Ethnopharmacol. 2004;95:133–138. doi: 10.1016/j.jep.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Osadebe P.O., Omeje E.O., Nworu S.C., Esimone C.O., Uzor P.F., David E.K., Uzoma J.U. Antidiabetic principles of Loranthus micranthus Linn. parasitic on Persea americana. Asian Pacific J. Trop. Med. 2010;3:619–623. [Google Scholar]
- Sadik G., Islam R., Rahman M.M., Khondkar P., Rashid M.A., Sarker S.D. Antimicrobial and cytotoxic constituents of Loranthus globosus. Fitoterapia. 2003;74:308–311. doi: 10.1016/s0367-326x(03)00041-8. [DOI] [PubMed] [Google Scholar]
- Ventola, C.L., 2015. The antibiotic resistance crisis: part 1: causes and threats. P t, 40, 277–283. [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are included in this published article.