Abstract
Background
Ultra-processed food consumption and obesity have been highlighted as an important relationship to public health. We aimed to evaluate the association between ultra-processed food consumption and body fat from 6 to 11 years of age.
Methods
We assessed the association between ultra-processed food consumption (from food frequency questionnaires) and body fat (measured by air displacement plethysmography) between 6 and 11 years of age among participants of the Pelotas-Brazil 2004 Birth Cohort. The NOVA classification was used to classify foods according to the processing degree. Body fat was evaluated relative to the height using fat mass index (FMI). Generalized estimating equations were used to answer the main research question and mediation analyses were run to assess the direct and indirect effect of ultra-processed food in body fat.
Results
At fully adjusted analysis, an increase of 100 g in contribution from ultra-processed food to daily food intake at between 6 and 11 years of age was associated with a gain of 0.14 kg/m² in FMI in the same period; 58% of the total effect of ultra-processed food intake at 6 years (in grams) over the change in FMI from 6 to 11 years was mediated by its calorie content.
Conclusions
Ultra-processed food consumption was associated with an increase in body fat from childhood to early adolescence, and this association was not just due to the effect of ultra-processed food on calorie content.
Keywords: Ultra-processed food, body fat, childhood, adolescence, cohort studies
Key Messages
Ultra-processed food consumption has been linked to obesity and several health-related outcomes; however, literature is scarce regarding children and adolescents.
This prospective and longitudinal study used data from the Pelotas-Brazil 2004 Birth Cohort, including the 6- and 11-year follow-ups.
An increase of 100 g in contribution from ultra-processed food to daily food intake between 6 to 11 years of age was associated with a gain of 0.14 kg/m² in fat mass index in the same period.
Mediation analysis showed that the role of food processing in body fat accumulation is, possibly, beyond its calorie content.
Introduction
Obesity in childhood and adolescence is an important issue in public health, taking into consideration its rising prevalence globally1 and that obese individuals in early life tend to remain obese in the life course, affecting health in the medium and long term.2 In low- and middle-income countries, overweight prevalence in those early stages of life increased between 1980 and 2013 from 8.1% to 12.9% in boys and from 8.4% to 13.4% in girls.1
In the same period in which worldwide increase in obesity was observed, changes in food patterns were noted, including the replacement of basic traditional foods by ready-to-eat products3,4 currently classified as ultra-processed food, which contain substances for industrial use only, as colouring and flavoring.5 In Brazil, household availability of these products has increased, leading to the rise of energy density, fat and sugar in the diet and to the decrease of fibre and protein intake.6
The relationship between household availability or consumption of ultra-processed food and obesity has been highlighted in the literature,7–10 with a 26% higher risk of developing overweight among adults with high consumption of ultra-processed foods.10 Asfaw et al. pointed out that an increase of 10 percentage points in the energy contribution from these products resulted in an addition of 4.2% in the body mass index (BMI) among 10-year-olds or older individuals.9 The same association was not found in studies that evaluated adolescents only, possibly due to lack of power.8,11,12
The role of ultra-processed food in health has been discussed currently not only in relation to its high energy density.13 High palatability, packages with large portion sizes and persuasive marketing are characteristics that lead to a higher consumption of these foods5. Besides, their structure and degree of processing seem to lead to a lower satiety and a higher glycaemic response.14 Despite its importance, studies evaluating the effect of ultra-processed food consumption on obesity among children and adolescents are scarce, mainly by longitudinal designs. Furthermore, most of the literature uses BMI to define the outcome which, despite being a method of easy applicability and of low cost, does not allow discriminating the body volume and body fat. The excess of fat mass is related to a higher risk of premature morbidity and mortality, due to metabolic dysregulation, regardless of body weight.15
In this way, we aimed to evaluate the association between ultra-processed food consumption and body fat between 6 and 11 years of age, among participants of a birth cohort.
Materials and Methods
This was an observational study with a prospective and longitudinal design, using data from the 2004 Pelotas-Brazil Birth Cohort. All newborns to mothers residing in the urban area of Pelotas or Jardim América (neighbourhood adjoining Pelotas, belonging to the municipality of Capão do Leão) were eligible to the cohort. Deliveries that occurred in all of the city’s maternity hospitals in the year 2004 were eligible to the study. Mothers of 4231 children born alive accepted to participate and were interviewed soon after the delivery (refusal rate less than 1%) and their newborns were examined (perinatal study). Methodological details of the cohort are described in other articles.16,17
Data from the perinatal study and from the 6- and 11-year follow-ups, carried out in 2010–11 and 2015, were used in the current analyses. A total of 98 children died between birth and 11 years of age. Response rates for 6- and 11-year follow-ups were 90.2% and 86.6%, respectively. Trained interviewers applied standard and pre-codified questionnaires in the perinatal and follow-up interviews. The follow-ups were carried out in a clinic structured for the research.16,17
Body fat
Body fat data were obtained by air displacement plethysmography (BODPOD®) that provides a direct estimation of body fat in kilograms. Values from the equipment were converted into an index relative to the height. Fat mass index (FMI) was the outcome of interest and was calculated by dividing the value of body fat in kilograms by the height in metres squared, using in continuous format. Body weight was measured with a high-precision scale with 0.01-kg resolution (model BWB-627-A, Tanita, Tokyo, Japan). A Harpenden® portable stadiometer with maximum height of 2.06 metres and 1 mm of accuracy was used to measure height. Anthropometric measurements were run by standardized anthropometrists. Standard equations were used to define body fat by air displacement plethysmography (Wells et al., 2011 and Lohman, 1989) at 6- and 11-year follow-ups, respectively.18,19
Dietary assessment
Participants’ food consumption was investigated by quantitative food frequency questionnaires (FFQ) which contained 54 and 88 items at 6- and 11-year follow-ups, respectively, evaluating food habits of the 12 months preceding the interview. For each food item, it was questioned how many times it was consumed in a day, week, month or year and if the intake was usually lower than, equal to or higher than the average portion. Average portion sizes, based on domestic measures according to the Table for Assessment of Food Intake in Household Measures,20 were presented to the participant verbally and with the help of images. At 6-year follow-up, mothers answered the FFQ alone and at 11-year follow-up, with the help of participants.
The reported frequency of consumption of each item of the FFQ was first converted into annual consumption. For this, at the 6-year follow-up, the frequencies were multiplied by 1, 12, 52 or 365.25, if the reported unit was year, month, week or day, respectively. At 11-year follow-up, the possible answers represented a consumption of, respectively, zero, 12, 52, 104, 260, 365.25, 730.5 and 1826.25 times a year.21 Then, using the daily frequency consumption and reported portion size, the quantity in grams of each food was calculated. Macronutrients (carbohydrates, proteins and lipids) were defined for each food item, based on the consumed value in grams and on the Brazilian Food Composition Table (TACO)22 or on the United States Department of Agriculture (USDA) Nutrient Database for Standard Reference,23 when not available in TACO. Energy values in kilocalories for each item were obtained multiplying carbohydrates and proteins by 4 kilocalories and lipids by 9 kilocalories.24 Finally, the total daily energy intake was calculated grouping kilocalories from carbohydrates, proteins and lipids from all food items.
Ultra-processed foods (main exposure)
The FFQs items were distributed in the four groups proposed by the NOVA classification (unprocessed or minimally processed foods, processed culinary ingredients, processed foods and ultra-processed foods).5. Totals of 18 and 26 food items were classified as ultra-processed at 6- and 11-year follow-ups, respectively (Supplementary Table S1, available as Supplementary data at IJE online). For this study, the main exposure was daily ultra-processed food consumption in grams from 6 to 11 years of age.
Covariables
Potential confounders included maternal characteristics: age (≤24, 25–34, ≥35 years), schooling (0–4, 5–8, 9–11, ≥12 completed years of study) and self-reported skin colour (white, brown/yellow/indigenous or black); characteristics of the child at birth: sex (male or female) and low birthweight (<2500 g); and characteristics of the participant at 6- and 11-year follow-ups: screen time (watching TV ≥2 h/day or daily use of videogame or computer), ratio between daily energy intake and expenditure, consumption from sources other than ultra-processed food (in grams: the sum of the three other food processing groups—unprocessed or minimally processed foods, processed culinary ingredients and processed foods) and total daily energy intake.
In order to reduce misclassification bias from under- or over-reporting inherent to the use of FFQ, an energy intake/expenditure ratio was applied in the adjustment, according to Leech et al. (2018).25 This method has been used in other studies in the field.26,27 For this, energy intake was the total daily energy intake, and energy expenditure was estimated by equations proposed by the Institute of Medicine (IOM),28 considering sex, nutritional status and level of physical activity. Nutritional status was classified by BMI in low and normal weight (BMI-for-age < +1 z-score) or overweight (BMI-for-age ≥ +1 z-score).29 At 6-year follow-up, the level of physical activity was evaluated by the Netherlands Physical Activity Questionnaire.30 At 11-year follow-up, the level of physical activity was determined by accelerometers (GENEActiv; ActivInsights, Kimbolton, UK and Actigraph® GT3X) used by the participants for about 6 days with a 24-h protocol, and the raw data were analysed with R-package GGIR.31Moderate-to-vigorous intensity physical activity data were used (cut-off point of 100 mG, an acceleration threshold corresponding to the walk; and 10-min bout).31 For both follow-ups, quartile cut-offs were set to classify participants as very low active, low active, active and very active, according to the levels defined by the IOM.28
Data analyses
Data were collected and entered directly in software Pendragon and REDCap (Research electronic data capture).32 The statistical package Stata version 12.1 was used to run the analyses. Daily consumption of ultra-processed food between 6 and 11 years of age was described in grams, calories, percent grams (% grams) and percent calories (% calories). Generalized estimating equations (GEE) were used to evaluate the association between ultra-processed food consumption in grams and FMI from 6 to 11 years of age, taking the repeated nature of the measures and the correlation between data into consideration. For this, a minimal adjustment model had been previously defined by linear regression, when quality parameters and residual normality were assessed. The minimal adjustment model included maternal skin colour, age and schooling, participant sex, birthweight, screen time and energy intake/expenditure ratio at 6- and 11-year follow-ups. Interaction between the main exposure and sex, age at menarche (for girls) and level of physical activity was tested but no evidence of effect modification was found.
Due to the different number of food items in the FFQ from the 6- and the 11-year follow-up and in order to improve comparability between follow-ups, the final full adjusted analyses included the control for consumption of other than ultra-processed foods (a sum of the three other groups—unprocessed or minimally processed foods, processed culinary ingredients and processed foods). Also, although the total caloric intake represents a possible mediator, we ran another model including it into the minimal adjustment as a sensitivity analysis to evaluate the effect of being ultra-processed and not the effect of its calorie content.
To investigate the direct and indirect role of food processing degree in body fat, a mediation analysis was run using G-computation.33 First, the pure natural direct effect (NDE) of ultra-processed food consumption (in grams) at 6 years of age over the change in body fat from 6 to 11 years of age was calculated. Then, the pure natural indirect effect (NIE) (the effect of ultra-processed food at 6 years of age over the change in body fat from 6 to 11 years of age that was mediated by the calorie content of ultra-processed foods) was estimated. These effects were obtained taking into consideration the interaction between exposure (ultra-processed food consumption in grams) and the mediating variable (ultra-processed food consumption in calories). For theoretical reasons (differences between boys and girls during childhood and adolescence related to body composition),34 all the analyses were stratified by sex.
The Ethics Committee of the Faculty of Medicine of the Federal University of Pelotas approved the perinatal study and the 6- and 11-year follow-ups (of. 4.06.01.116, of. 35/10 and of. 889.753, respectively). Mothers or legal caregivers signed a consent term, authorizing the child’s participation in all follow-up stages. At 11-year follow-up, the cohort participants also signed an agreement term.
Results
At 6 and 11 years of age, 3128 and 3454 participants, respectively, had information on FFQ and body fat and were included in the analyses. In perinatal interview, mean (standard deviation: SD) maternal age was 26.1 (6.8) years, almost half of the mothers (41.4%) had 5–8 years of schooling and 73.0% self-declared white skin colour. Among the newborns, 51.9% were male and the prevalence of low birthweight was 10% (Table 1). There was no difference in the distribution of the 6- and 11-year follow-up samples compared with the original sample in terms of the above-described variables (Table 1).
Table 1.
Description of the original sample and participants assessed at 6 and 11 years of age in the 2004 Pelotas Birth Cohort (n = 4231)
| Variables | Original sample n (%) | 6 years (n = 3424) | P-valueb | 11 years (n = 3514) | P-valueb |
|---|---|---|---|---|---|
| Maternal characteristics | |||||
| Age (years)a | 26.1 (6.8) | 26.1 (6.8) | 0.702 | 26.3 (6.9) | 0.276 |
| Age (years) | 0.905 | 0.539 | |||
| ≤24 | 1947 (46.1) | 1560 (45.6) | 1576 (44.9) | ||
| 25–34 | 1717 (40.6) | 1398 (40.8) | 1448 (41.2) | ||
| ≥35 | 563 (13.3) | 464 (13.6) | 488 (13.9) | ||
| Skin colour | 0.736 | 0.898 | |||
| White | 3088 (73.0) | 2512 (73.4) | 2561 (72.9) | ||
| Black/brown/yellow/indigenous | 1141 (27.0) | 912 (26.6) | 953 (27.1) | ||
| Schooling (years of school)a | 8.1 (3.5) | 8.2 (3.4) | 0.452 | 8.2 (3.4) | 0.447 |
| Schooling (years of school) | 0.729 | 0.639 | |||
| 0–4 | 654 (15.6) | 499 (14.7) | 519 (14.9) | ||
| 5–8 | 1731 (41.4) | 1429 (42.2) | 1447 (41.6) | ||
| 9–11 | 1381 (33.0) | 1122 (33.1) | 1183 (34.0) | ||
| ≥12 | 420 (10.0) | 340 (10.0) | 332 (9.5) | ||
| Cohort member characteristics | |||||
| Sex | 0.963 | 0.855 | |||
| Male | 2195 (51.9) | 1775 (51.8) | 1816 (51.7) | ||
| Female | 2034 (48.1) | 1649 (48.2) | 1698 (48.3) | ||
| Low birthweight (<2500 g) | 0.100 | 0.102 | |||
| Yes | 423 (10.0) | 304 (8.9) | 313 (8.9) |
Mean (standard deviation).
In relation to the original sample.
At 6 years of age, the average weight, height and FMI among boys were 25.1 kg, 121.5 cm and 3.9 kg/m2, respectively; and 24.8 kg, 120.2 cm and 4.5 kg/m2, respectively, among girls. For the 11-year follow-up, the average weight, height and FMI for boys were 41.7 kg, 144.8 cm and 5.4 kg/m2, respectively; and for girls, 43.1 kg, 146.5 cm and 5.5 kg/m2, respectively (Table 2).
Table 2.
Weight, height and fat mass index in the cohort participants at ages 6 and 11 years in the 2004 Pelotas Birth Cohort
| Variables | 6 years |
11 years |
||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Boys | ||||
| Weight (kg) | 1762 | 25.1 (5.9) | 1789 | 41.7 (11.4) |
| Height (cm) | 1896 | 121.5 (5.6) | 1809 | 144.8 (7.2) |
| Fat mass index (kg/m²) | 1739 | 3.9 (2.1) | 1788 | 5.4 (3.3) |
| Girls | ||||
| Weight (kg) | 1653 | 24.8 (6.1) | 1682 | 43.1 (12.2) |
| Height (cm) | 1757 | 120.2 (5.7) | 1696 | 146.5 (7.7) |
| Fat mass index (kg/m²) | 1635 | 4.5 (2.3) | 1679 | 5.5 (3.2) |
SD, standard deviation.
Table 3 presents median (interquartile range: IQR) daily food consumption at 6 and 11 years of age, according to the processing level of the food. Most of the amount of the food consumed per day was unprocessed or minimally processed: 1507 g (IQR 1134.2; 2002.5) at 6 years, and 1578.6 g (IQR 1142.0; 2238.8) at 11 years. At 6 and 11 years of age, median daily consumption of ultra-processed food was 948.6 (IQR 605.0; 1429.9) and 646.6 (IQR 395.4; 1030.6) grams, respectively (Table 3). Considering the percentage of daily energy contribution from the total energy intake, 44.2% (IQR 37.1; 51.5) and 52.3% (IQR 44.7; 60.2) was unprocessed or minimally processed at 6 and 11 years, respectively. Ultra-processed food provided 42.0% (IQR 34.6; 49.8) and 32.7% (IQR 25.1; 41.3) of the total daily calorie intake, respectively, at 6 and 11 years old (Table 3). Median energy intake/expenditure ratio was 2.2 (IQR 1.8; 2.9) and 1.6 (IQR 1.2; 2.4), respectively, at 6 and 11 years old.
Table 3.
Daily consumption (in grams/day, calories/day and percentage of daily energy contribution), according to the food processing level, at ages 6 and 11 years in the 2004 Pelotas Birth Cohort
| Variables | 6 years | 11 years | ||
|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | |
| Grams | 3424 | 3514 | ||
| Unprocessed or minimally processed foods | 1506.5 (1134.2; 2002.5) | 1578.6 (1142.0; 2238.8) | ||
| Processed culinary ingredients | 25.6 (0; 51.3) | 15.2 (2.8; 30.8) | ||
| Processed foods | 104.3 (54.3; 172.3) | 133.1 (79.4; 160.4) | ||
| Ultra-processed foods | 948.6 (605.0; 1429.9) | 646.6 (395.4; 1030.6) | ||
| Other sources (total)a | 1661.6 (1267.2; 2171.7) | 1734.7 (1259.2; 2413.9) | ||
| % grams | 3424 | 3514 | ||
| Unprocessed or minimally processed foods | 57.5 (47.9; 68.0) | 66.9 (58.0; 74.8) | ||
| Processed culinary ingredients | 0.7 (0; 1.9) | 0.6 (0.2; 1.1) | ||
| Processed foods | 3.9 (2.3; 6.1) | 4.9 (3.4; 7.0) | ||
| Ultra-processed foods | 36.2 (26.1; 46.8) | 27.0 (19.0; 35.8) | ||
| Other sources (total) | 63.8 (53.2; 73.9) | 73.0 (64.2; 81.0) | ||
| Calories | 3424 | 3514 | ||
| Unprocessed or minimally processed foods | 1444.3 (1130.1; 1865.6) | 1571.9 (1142.0; 2293.4) | ||
| Processed culinary ingredients | 102.3 (0; 204.6) | 63.4 (12.8; 128.5) | ||
| Processed foods | 294.1 (153.6; 482.5) | 380.1 (229.0; 463.5) | ||
| Ultra-processed foods | 1383.8 (1009.9; 1886.5) | 1004.2 (644.7; 1632.1) | ||
| Other sources (total) | 1904.3 (1509.5; 2433.3) | 2034.3 (1515.4; 2825.7) | ||
| Total calories | 3336. 1 (2671.3; 4225.3) | 3091.6 (2306.2; 4386.5) | ||
| % calories | 3424 | 3514 | ||
| Unprocessed or minimally processed foods | 44.2 (37.1; 51.5) | 52.3 (44.7; 60.2) | ||
| Processed culinary ingredients | 2.2 (0; 5.9) | 2.0 (0.5; 3.6) | ||
| Processed foods | 9.0 (5.5; 13.3) | 11.0 (7.5; 14.9) | ||
| Ultra-processed foods | 42.0 (34.6; 49.8) | 32.7 (25.1; 41.3) | ||
| Other sources (total) | 58.1 (50.2; 65.4) | 67.3 (58.7; 74.9) |
IQR, interquartile range.
Other sources are the sum of the three other food processing groups: unprocessed or minimally processed foods, processed culinary ingredients and processed foods.
In the whole sample and separately for boys and girls, ultra-processed food consumption was associated with FMI between 6 and 11 years of age. In crude analysis, for the whole sample and separately for boys and girls, higher consumption of ultra-processed food was associated with a lower FMI from 6 to 11 years of age. However, at the full adjusted analysis a daily increase of 100 grams in the contribution from ultra-processed food was associated with a gain of 0.14 kg/m² in FMI from 6 to 11 years. Even adjusting for total calorie intake (sensitivity analysis), consumption of ultra-processed food remained associated with FMI (Table 4).
Table 4.
Association between the consumption of ultra-processed foods (in grams) and fat mass index (kg/m²) between 6 and 11 years of age in the 2004 Pelotas Birth Cohort
| Ultra-processed food consumption | Fat mass index (kg/m²) |
|||||
|---|---|---|---|---|---|---|
| Total |
Boys |
Girls |
||||
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
| Crude analysis | −0.03 (−0.04;−0.02) | <0.001 | −0.04 (−0.06;−0.03) | <0.001 | −0.03 (−0.04;−0.02) | <0.001 |
| Minimal adjustmenta | 0.09 (0.07; 0.10) | <0.001 | 0.06 (0.05; 0.08) | <0.001 | 0.07 (0.05; 0.09) | <0.001 |
| Other food sources adjustmentb | 0.14 (0.13; 0.15) | <0.001 | 0.11 (0.09; 0.12) | <0.001 | 0.07 (0.05; 0.09) | <0.001 |
| Total calorie intake adjustmentc | 0.05 (0.04; 0.06) | <0.001 | 0.03 (0.01; 0.04) | <0.001 | 0.04 (0.02; 0.05) | <0.001 |
Grams are in the scale of 100 for better interpretation.
Minimal adjustment: skin colour, maternal age and schooling, birthweight and sex (perinatal), screen time and energy intake/expenditure ratio (6- and 11-year follow-ups).
The association with exposure in grams was adjusted by grams from other food sources than ultra-processed, in addition to the minimal adjustment (other food sources are the sum of the three other food processing groups).
The association with exposure in grams was adjusted by total calorie intake, in addition to the minimal adjustment.
In mediation analysis, 58.2% [0.07 (95% confidence interval: 0.05–0.10)] of the total effect of ultra-processed food consumption at 6 years (in grams) over the change in FMI from 6 to 11 years of age was mediated by its calorie content. The remaining 41.8% was the direct effect of ultra-processed food or the effect of variables that had not been measured (Figure 1).
Figure 1.
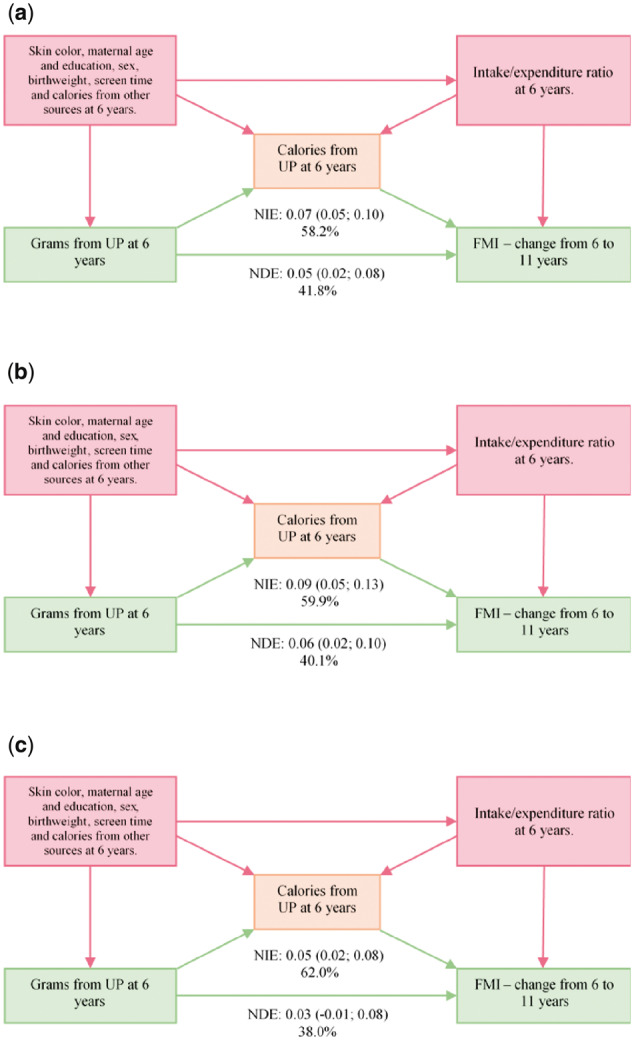
Direct and indirect effect of ultra-processed food consumption in grams at 6 years of age on the change in the fat mass index from 6 to 11 years of age. (a) Total sample; (b) boys; and (c) girls. UP, ultra-processed food; FMI, fat mass index; NDE, natural direct effect; NIE, natural indirect effect
Discussion
In summary, our study showed that ultra-processed food consumption from 6 years of age to early adolescence is positively associated with fat mass index in the same period. Additionally, the effect of ultra-processed food intake on body fat seems not to be exclusively due to its calorie content but also to other direct mechanisms. Ultra-processed food represents, currently, one of the main sources of food supply in high-, middle- and low- income countries, creating an obesogenic environment and collaborating in the increase in the burden of non-communicable chronic diseases.35
We found that 42% and 33% of the daily energetic value consumed at 6 and 11 years of age, respectively, was from ultra-processed food. Other Brazilian studies found similar percentages of about 38% for the childhood population (4–8 years old).36,37 There is no information from longitudinal or population-based surveys on the proportion of ultra-processed food consumption among Brazilian adolescents. In high-income countries, energy contribution from ultra-processed food was about 55% and 66% for children and adolescents (2–19 years old), in Canada and the USA, respectively.38,39 Also, as in the current study, other authors found a decrease in ultra-processed food consumption with increasing age.40,41
Our findings on the association between ultra-processed food consumption and body fat are in agreement with the literature. A systematic review showed that most of the published studies found a positive relationship between the consumption of these products and body fat, despite the fact that the studies had analysed only specific food (like soft drinks, salty snacks and ice cream) and did not apply the NOVA classification.42 A single study that used NOVA classification found an inverse association between ultra-processed food consumption and fat percentage (measured by bioelectrical impedance) or BMI.12 The lack of adjustment to energy intake/expenditure ratio was probably the reason for failure in detecting such association, as highlighted by the authors,12 as well as in other studies.26,27 We ran complementary analyses to check for the effect of ultra-processed food consumption over BMI gain from 6 to 11 years of age. In fully adjusted analysis, each increase of 100 g, 100 calories or 1 percentage point (in grams or calories) of contribution from ultra-processed food was associated with an increase of 0.20, 0.31, 0.06 and 0.11 kg/m² in BMI, respectively (Supplementary Table S2, available as Supplementary data at IJE online). Similar results were noted for boys and girls. Our findings are consistent with the literature, in spite of the absence of studies including this age range.8,10,43
In theory, obesity originates in the imbalance between energy intake and expenditure. However, some authors have pointed out ultra-processed food consumption as an obesity vector, not only due to its energy density, but also because of several other factors not related to the diet’s nutrient profile.13,14 A series of food additives are used in the ultra-processed manufacturing, monosodium glutamate among them. In animal models, high doses of this substance are toxic to neurons involved in the regulation of metabolic homeostasis, including secretion and action of insulin, leading to an increase in fasting blood glucose levels and severe visceral fat accumulation.44 Also, the higher the degree of food processing, the greater the potential to increase blood glucose levels and to decrease the satiety index, important mechanisms linked to the body fat accumulation.14,45 These mechanisms involve the action of the glucose-dependent insulinotropic polypeptide (GIP), an intestinal hormone that induces insulin release by pancreas after blood glucose increase. Considering that foods with a high glycaemic index promote a rapid increase in blood glucose and, consequently, a greater release of GIP, there is an early response of insulin, characterized by increased fat deposition and decreased fat oxidation. Additionally, GIP acts in the brain, increasing the release of neuropeptide Y (NPY), a hormone responsible for increasing appetite.45
Additionally, Zinöcker and Lindseth raised the discussion about the role of food processing degree in structural and behavioural changes in the human microbiome, this in turn leading to organism inflammation.46Ultra-processed food would provide readily accessible and more easily digestible substrates that can facilitate growth potential and changes of the gut microbiota.46 In rats, high-sugar diet increased gut inflammation and altered vagal gut-brain communication, with an increase in body fat accumulation.47
It is important to highlight some limitations in our study. Although the FFQ was built specifically to evaluate food consumption in the study population, it was not developed with the objective of evaluating the degree of food processing, which made it impossible to obtain additional information on the preparation of food. For this reason, food items from the FFQ were classified in a more conservative way: culinary preparations such as lasagna, for example, were considered as minimally processed food and breads (whole or white), considered as processed foods. There was a different number of food items in the 6- and 11-year FFQs. However, the extra food items in the 11-year follow-up were proportionally distributed among the four food processing groups. Besides, to improve the comparability between follow-ups, statistical control for grams from other food sources than ultra-processed was employed. Physical activity was measured differently at 6 and 11-year follow-ups; however, the questionnaire which was used at 6 years showed a good predictive value, comparing with accelerometers, and correlation coefficients were similar to those found in other questionnaires assessing physical activity. Considering that physical activity was used only for adjustment (energy intake/expenditure ratio), we believe this difference may not interfere in the overall results. Level of physical activity was also used as a potential confounder variable in the fully adjusted models but the results were the same (data not presented). Also, the absence of data from boys' pubertal stage was a limitation, although in girls we found no evidence of effect modification by the age of menarche. Participants included in the analyses were representative of deaths in all independent variables, except for low maternal education and low birthweight, both reflecting low socioeconomic status. Information from the same dataset showed that participants whose mothers reported lower education had a higher consumption of ultra-processed foods and, therefore, a higher fat mass index (data not presented). In this way, estimates of both ultra-processed food consumption and fat mass index, as well as the association between them, could be underestimated. On the other hand, there were no differential losses at 6- and 11-year follow-ups, when compared with the original sample.
Mediation analysis showed an effect of ultra-processed food consumption (in grams) over FMI that was not mediated by its calorie content. Besides energy content and other mechanisms that can lead to body fat accumulation, part of this result may be due to limitation of FFQ in measuring absolute calories. As a result, the actual calorie-mediated effect could be greater than observed. Nevertheless, our results collaborate to strengthen the hypothesis of the causal relationship between ultra-processed food consumption and body fat.
Study strengths include meticulous methods applied in the cohort both for exposure and for outcome data collection and definition. The longitudinal study design, together with the longitudinal methodology of analysis (GEE and mediation analysis), increase the study robustness. Finally, the majority of the studies in the field had analysed ultra-processed food as a percentage of calorie contribution, a relative measure that depends on the calorie contribution from other food sources. Therefore, to allow comparability with other studies, we ran the analyses taking the exposure in calories, % grams and % calories; and a direct effect of ultra-processed food intake over the FMI was also observed for both boys and girls (Supplementary Table S3, available as Supplementary data at IJE online).
In conclusion, our study showed an important effect of ultra-processed food intake from childhood to early adolescence over the fat mass index change during the period. In addition, our results suggest that the role of food processing in body fat accumulation is beyond its calorie content. Measures to reduce ultra-processed food and to increase minimally processed natural food availability, purchase and consumption would contribute to prevent body fat increase and associated chronic non-communicable diseases.
Supplementary data
Supplementary data are available at IJE online.
Funding
This work was supported by the Wellcome Trust [grant number 086974/Z/08/Z] at the 6-year follow-up and by DECIT (Departamento de Ciência e Tecnologia) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico- Brazil CNPq [grant number 400943/2013-1] at the 11-year follow-up.
Supplementary Material
Acknowledgements
This study was conducted in a Post-Graduate Program supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001; and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brazil (CNPq).
Conflict of interest
None declared.
References
- 1. Ng M, Fleming T, Robinson M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh AS, Mulder C, Twisk JW, Van Mechelen W, Chinapaw MJ.. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 2008;9:474–88. [DOI] [PubMed] [Google Scholar]
- 3. Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr 2006;84:289–98. [DOI] [PubMed] [Google Scholar]
- 4. Popkin BM. Relationship between shifts in food system dynamics and acceleration of the global nutrition transition. Nutr Rev 2017;75:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Monteiro CA, Cannon G, Moubarac J-C, Levy RB, Louzada MLC, Jaime PC.. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr 2018;21:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martins APB , Levy RB, Claro RM, Moubarac JC, Monteiro CA.. Increased contribution of ultra-processed food products in the Brazilian diet (1987–2009). Rev Saúde Pública 2013;47:656–65. [DOI] [PubMed] [Google Scholar]
- 7. Canella DS, Levy RB, Martins APB. et al. Ultra-processed food products and obesity in Brazilian households (2008–2009. ). PLoS One 2014;9:e92752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Louzada M, Baraldi LG, Steele EM. et al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev Med 2015;81:9–15. [DOI] [PubMed] [Google Scholar]
- 9. Asfaw A. Does consumption of processed foods explain disparities in the body weight of individuals? The case of Guatemala. Health Econ 2011;20:184–95. [DOI] [PubMed] [Google Scholar]
- 10. Mendonça RD, Pimenta AM, Gea A. et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study, 2. Am J Clin Nutr 2016;104:1433–40. [DOI] [PubMed] [Google Scholar]
- 11. de Melo ISV, Costa C, dos Santos JVL, dos Santos AF, Florêncio T, Bueno NB.. Consumption of minimally processed food is inversely associated with excess weight in adolescents living in an underdeveloped city. PLoS One 2017;12:e0188401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunha DB, Costa THM, Veiga GV, Pereira RA, Sichieri R.. Ultra-processed food consumption and adiposity trajectories in a Brazilian cohort of adolescents: ELANA study. Nutr Diabetes 2018;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poti JM, Braga B, Qin B.. Ultra-processed food intake and obesity: what really matters for health—processing or nutrient content? Curr Obes Rep 2017;6:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct 2016;7:2338–46. [DOI] [PubMed] [Google Scholar]
- 15. Romero-Corral A, Somers VK, Sierra-Johnson J. et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 2009;ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santos IS, Barros AJ, Matijasevich A, Domingues MR, Barros FC, Victora CG.. Cohort Profile: The 2004 Pelotas (Brazil) Birth Cohort Study. Int J Epidemiol 2011;40:1461–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santos IS, Barros AJ, Matijasevich A. et al. Cohort Profile Update: 2004 Pelotas (Brazil) Birth Cohort Study. Body composition, mental health and genetic assessment at the 6 years follow-up. Int J Epidemiol 2014;43:1437af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells JC, Haroun D, Williams JE. et al. Evaluation of lean tissue density for use in air displacement plethysmography in obese children and adolescents. Eur J Clin Nutr 2011;65:1094–101. [DOI] [PubMed] [Google Scholar]
- 19. Lohman TG. Assessment of body composition in children. Pediatr Exerc Sci 1989;1:19–30. [DOI] [PubMed] [Google Scholar]
- 20. Pinheiro ABV, Lacerda EMdA, Benzecry EH, Gomes MCdS, Costa VMd.. Tabela Para Avaliação de Consumo Alimentar em Medidas Caseiras [Table for Assessment of Food Intake in Household Measures]. 5th edn. Rio de Janeiro: Atheneu, 2005. [Google Scholar]
- 21. Schneider BC, dos Santos Motta JV, Muniz LC. et al. Design of a digital and self-reported food frequency questionnaire to estimate food consumption in adolescents and young adults: birth cohorts at Pelotas, Rio Grande do Sul, Brazil. Rev Bras Epidemiol 2016;19:419–32. [DOI] [PubMed] [Google Scholar]
- 22. Universidade Estadual de Campinas, Núcleo de Estudos e Pesquisas em Alimentação - NEPA. Tabela Brasileira de Composição de alimentos - TACO [Brazilian Food Composition Table]. 2nd edn. Campinas (SP), Brazil: UNICAMP, 2006. [Google Scholar]
- 23.United States Department of Agriculture. National Nutrient Database for Standard Reference. Beltsville, MD: Human Nutrition Research Center, Nutrient Data Laboratory, 2011
- 24. Merrill AL, Watt BK.. Energy Value of Foods: Basis and Derivation. Agriculture Handbook. Vol. 74. Washington, DC: US Agriculture Research Service, 1973. [Google Scholar]
- 25. Leech RM, Worsley A, Timperio A, McNaughton SA.. The role of energy intake and energy misreporting in the associations between eating patterns and adiposity. Eur J Clin Nutr 2018;72:142–47. [DOI] [PubMed] [Google Scholar]
- 26. Mendez MA, Popkin BM, Buckland G. et al. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am J Epidemiol 2011;173:448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Börnhorst C, Huybrechts I, Hebestreit A. et al. Diet–obesity associations in children: approaches to counteract attenuation caused by misreporting. Public Health Nutr 2013;16:256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J.. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bielemann RM, Reichert FF, Paniz VM, Gigante DP.. Validation of the Netherlands physical activity questionnaire in Brazilian children. Int J Behav Nutr Phys Act 2011;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. da Silva IC, van Hees VT, Ramires VV. et al. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol 2014;43:1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daniel RM, De Stavola BL, Cousens SN.. gformula: estimating causal effects in the presence of time-varying confounding or mediation using the g-computation formula. Stata J 2011;11:479–517. [Google Scholar]
- 34. Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab 2007;21:415–30. [DOI] [PubMed] [Google Scholar]
- 35. Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B.. Ultra‐processed products are becoming dominant in the global food system. Obes Rev 2013;14:21–28. [DOI] [PubMed] [Google Scholar]
- 36. de Almeida Fonseca PC, Ribeiro SAV, Andreoli CS. et al. Association of exclusive breastfeeding duration with consumption of ultra-processed foods, fruit and vegetables in Brazilian children. Eur J Nutr 2019;58:2887–88. [DOI] [PubMed] [Google Scholar]
- 37. Rauber F, Campagnolo P, Hoffman D, Vitolo M.. Consumption of ultra-processed food products and its effects on children's lipid profiles: a longitudinal study. Nutr Metab Cardiovasc Dis 2015;25:116–22. [DOI] [PubMed] [Google Scholar]
- 38. Moubarac J-C, Batal M, Louzada M, Steele EM, Monteiro C.. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 2017;108:512–20. [DOI] [PubMed] [Google Scholar]
- 39. Baraldi LG, Steele EM, Canella DS, Monteiro CA.. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open 2018;8:e020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marrón-Ponce JA, Sánchez-Pimienta TG, da Costa Louzada ML, Batis C.. Energy contribution of NOVA food groups and sociodemographic determinants of ultra-processed food consumption in the Mexican population. Public Health Nutr 2018;21:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vandevijvere S, De Ridder K, Fiolet T, Bel S, Tafforeau J.. Consumption of ultra-processed food products and diet quality among children, adolescents and adults in Belgium. Eur J Nutr 2019;58:3267–12. [DOI] [PubMed] [Google Scholar]
- 42. Costa CS, Del-Ponte B, Assunção MCF, Santos IS.. Consumption of ultra-processed foods and body fat during childhood and adolescence: A systematic review. Public Health Nutr 2018;21:148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nardocci M, Leclerc B-S, Louzada M-L, Monteiro CA, Batal M, Moubarac J-C.. Consumption of ultra-processed foods and obesity in Canada. Can J Public Health 2019;110:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bautista RJH, Mahmoud AM, Königsberg M, Guerrero N.. Obesity: Pathophysiology, monosodium glutamate-induced model and anti-obesity medicinal plants. Biomed Pharmacother 2019;111:503–16. [DOI] [PubMed] [Google Scholar]
- 45. Pfeiffer AF, Keyhani-Nejad F.. High glycemic index metabolic damage–a pivotal role of GIP and GLP-1. Trends Endocrinol Metab 2018;29:289–99.. [DOI] [PubMed] [Google Scholar]
- 46. Zinöcker M, Lindseth I.. The Western diet–microbiome-host interaction and its role in metabolic disease. Nutrients 2018;10:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sen T, Cawthon CR, Ihde BT. et al. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol Behav 2017;173:305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


