Abstract
The tropism of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a virus responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic, toward the host cells is determined, at least in part, by the expression and distribution of its cell surface receptor, angiotensin-converting enzyme 2 (ACE2). The virus further exploits the host cellular machinery to gain access into the cells; its spike protein is cleaved by a host cell surface transmembrane serine protease 2 (TMPRSS2) shortly after binding ACE2, followed by its proteolytic activation at a furin cleavage site. The virus primarily targets the epithelium of the respiratory tract, which is covered by a tightly regulated airway surface liquid (ASL) layer that serves as a primary defense mechanism against respiratory pathogens. The volume and viscosity of this fluid layer is regulated and maintained by a coordinated function of different transport pathways in the respiratory epithelium. We argue that SARS-CoV-2 may potentially alter evolutionary conserved second-messenger signaling cascades via activation of G protein-coupled receptors (GPCRs) or by directly modulating G protein signaling. Such signaling may in turn adversely modulate transepithelial transport processes, especially those involving cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial Na+ channel (ENaC), thereby shifting the delicate balance between anion secretion and sodium absorption, which controls homeostasis of this fluid layer. As a result, activation of the secretory pathways including CFTR-mediated Cl− transport may overwhelm the absorptive pathways, such as ENaC-dependent Na+ uptake, and initiate a pathophysiological cascade leading to lung edema, one of the most serious and potentially deadly clinical manifestations of COVID-19.
Keywords: CFTR, COVID-19, ENaC, GPCR, SARS-CoV-2
The variable clinical manifestations are characteristic of the ongoing global outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)(1). The majority of the infected patients are asymptomatic or report mild symptoms and recover completely. Approximately 2/3 of the patients with severe COVID-19, however, develop pneumonia, with ∼25% requiring mechanical ventilation, which in turn is associated with up to 80% of the mortality (2); demographically, these are usually older patients with comorbid conditions. Both the expression level of angiotensin-converting enzyme 2 (ACE2), the entry point of SARS-CoV-2 into cells, in the respiratory epithelia (3, 4) and the extent of the resulting inflammatory response (5) are suspected to determine the rapid deterioration of patients and the development of an acute respiratory distress syndrome (6). SARS-CoV-2 shows a gradient infectivity or replication efficiency from the proximal to distal airways that parallels ACE2 expression (7), with ciliated cells being the most ACE2-expressing cells. In upper airways, the ciliated cells dominate the nasal epithelium and show a high rate of infectivity whereas alveolar type II (ATII) epithelial cells are preferentially targeted by the virus in the distal airways (7). In line with competitive binding of SARS-CoV-2 to endogenous or exogenous ACE2, human recombinant soluble ACE2 reduced SARS-CoV-2 load in cell cultures and organoids (8), and the encouraging treatment of a patient with severe COVID-19 with intravenous infusion of soluble ACE2 was reported recently (9). Overall, ACE2 expression in airway epithelia is, however, not high (10) and is not only proposed to serve as an entry portal for the virus (3, 4) but also fulfills an important protective role against lung injury (11, 12). Furthermore, interventions which supposedly increase ACE2 expression in animal models (13), such as angiotensin receptor blockers (ARBs), have not been found to be associated with higher mortality in severe COVID-19 (14).
Taken together, the variable expression pattern of ACE2 in respiratory epithelia and its complex role in lung physiology and pathophysiology indicate that ACE2 (or TMPRSS2) expression levels and patterns may not fully reflect the susceptibility of cells to the virus but rather that other factor(s) may, at least in part, play a role in severe COVID-19 lung disease. Here, we consider the possibility that failure of the primary defense mechanism of the respiratory tract, the mucociliary clearance (MCC) and a disruption in alveolar liquid clearance (15, 16), may present one of the key factors in the pathophysiology of COVID-19 lung disease.
The airway surface liquid (ASL) is a thin layer of fluid that covers the respiratory tract (17) which consists of two components (18). The first part, a mucous layer (ML), traps and clears airways of inhaled harmful agents and sits on top of the second component, the periciliary layer (PCL), which provides the appropriate setting for cilia beating. The appropriate volume and viscosity of the fluid layer, an important determinant of the proper functioning of the MCC (18), is achieved through coordinated processes of secretion and absorption that are orchestrated by a synchronized operation of several transepithelial transport pathways (17). Among the numerous epithelial transporters, cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial Na+ channel (ENaC) are recognized as the key players that determine ASL balance. CFTR functions as an ATP and protein kinase A (PKA)-dependent Cl− channel (19–21) and serves as a passageway for Cl− secretion whereas ENaC fulfills a key role in Na+ reabsorption (22, 23), with a well-described reciprocal relationship between these two proteins (24–28). In the airways, they regulate the ASL and play an important role in MCC (18). In the alveolar space, CFTR and ENaC participate in the regulation of the volume of the hypophase (a thin layer of the alveolar lining fluid) to maintain a thin air-liquid interface while preventing alveolar edema formation. Impaired ENaC function in ATII cells, as seen in acute lung injury, causes a reversal of alveolar chloride and fluid flux from absorptive to secretory mode (29–31), resulting in alveolar edema. Hence, any process that increases Cl− secretion or decreases Na+ reabsorption will interfere with MCC and alveolar fluid clearance, thus potentially impairing primary defense in the airways while concomitantly causing flooding of the alveolar space (30, 31).
An elaborate set of adaptor proteins (20, 32, 33) connect CFTR and ENaC to each other and serve as cellular signaling hub connecting these channels to intracellular signaling networks that regulate cellular homeostasis. Central to the regulation of this signaling hub are G protein-coupled receptors (GPCRs), the largest superfamily of receptors in humans (34), which regulate almost all known physiological processes (35, 36). GPCRs primarily function through signal transduction/propagation cascades (34, 37). Located at the cell surface, ligand-bound GPCRs transduce exogenous signals that activate GTP-binding “G” proteins which in turn activate effector proteins (such as adenylyl cyclase and phospholipases) and second messengers (e.g., calcium or cAMP) (34, 37). CFTR activity is regulated via the cAMP/PKA pathway (38) which is typically induced via Gαs-coupled GPCRs that stimulate adenylyl cyclase (AC), thereby raising cAMP levels and stimulating PKA (39). These endogenous signaling pathways are, however, frequently exploited by invading pathogens (40). For example, cholera toxin (from Vibrio cholerae) causes ADP-ribosylation of the α subunit of the stimulatory G protein, which in turn activates adenylate cyclase with a subsequent increase in cAMP that stimulates CFTR and triggers the excessive chloride secretion and fluid loss in the gut that are characteristics of cholera (41).
Two specific GPCRs are mainly involved in CFTR regulation and robustly expressed in airways, namely, A2B adenosine receptors and the β2 adrenergic receptors (39, 42). Under physiological conditions, the adenosine-CFTR regulation system is critical for the protection of mucosal airway surfaces (43) and alveolar surface layer (ASL) regulation (44). Viruses, however, are well recognized for their ability not only to exploit GPCRs to enter the host cells but also to use their intracellular signaling pathways for survival and replication (40). Based on this general concept, it is conceivable that SARS-CoV-2 may also potentially compromise GPCRs signaling and this effect may, at least in part, contribute to the pathophysiology of pulmonary edema. A simple and tempting speculation would be that SARS-CoV-2 may hijack the same GPCR signaling pathway that is used by cholera toxin (41)—namely, ADP-ribosylation of Gαs—to activate CFTR (Fig. 1) and turn on Cl− secretion, ultimately leading to lung edema in patients with COVID-19 (48, 49). At this point, however, this hypothesis seems unlikely as several reports suggest that SARS-CoV-2 in fact reverses cellular ADP-ribosylation to allow the virus to evade immune detection (51–53). Whether or not G proteins are involved in this response remains to be determined.
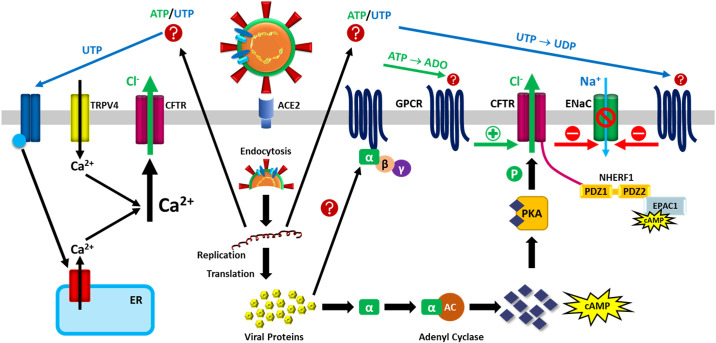
Figure 1.
Hypothetical concept for the dysregulation of lung epithelial ion and fluid transport by SARS-CoV-2. SARS-CoV-2 binds to ACE2, is endocytosed into the cell, and uses the host cellular machinery for RNA replication and translation resulting in the production of viral proteins. These viral proteins may potentially interact with G proteins, triggering the classic GPCR pathway and activating CFTR. Activation of CFTR in turn can inhibit ENaC. Secondary mediators may in parallel or alternatively activate GPCRs from the outside (45, 46). For example, ATP may serve as secondary mediator and engage A1AR [(green arrow) which in turn stimulates CFTR; activation of CFTR in turn can inhibit ENaC]. UTP can engage P2Y6R (blue arrow) and inhibit amiloride sensitive ion transport via ENaC. In addition, UTP can lead to purinergic-mediated calcium mobilization from intracellular stores, which may combine with the influx of calcium via TRPV4 (acting as a mechano- and/or chemosensor) to increase intracellular calcium levels that can in turn activate CFTR; this activation of CFTR can cause chloride and fluid secretion, and inhibition of sodium reabsorption via ENaC (ENaC is not depicted in the left side of the figure). The potential link between TRPV4 and CFTR (47) could involve a Ca2+-dependent activation of adenylyl cyclase and the classical PKA pathway, whereas tyrosine phosphorylation can cause PKA-independent CFTR activation (47–49). NHERF1 anchors CFTR (via ezrin, not shown) to the actin cytoskeleton. Increased levels of cAMP allow PKA and EPAC1 to promote opening and stabilization of CFTR at the plasma membrane (50), again causing chloride and fluid secretion. ACE2, angiotensin converting enzyme 2; cAMP, cyclic AMP; CFTR, cystic fibrosis transmembrane conductance regulator; ENaC, epithelial Na+ channel; EPAC1, exchange protein directly activated by cAMP; ER, endoplasmic reticulum; GPCR, G protein coupled-receptor; NHERF1, Na+/H+-exchanger regulatory factor isoform 1; PDZ, postsynaptic density-95 discs large, zona occludens-1 (PDZ) domain-containing protein; PKA, protein kinase A; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TRPV4, transient receptor potential vanilloid 4.
In addition to the established cAMP/PKA activation pathway, a second regulatory pathway for transepithelial ion transport acts through EPAC1 (the exchange protein directly activated by cAMP) and stabilizes CFTR at the plasma membrane by inhibiting its endocytosis (50). This pathway is also cAMP-dependent, with EPAC1 acting as an alternative cAMP effector (to PKA) and interacting with CFTR via the Na+/H+-exchanger regulatory factor isoform 1 (NHERF1) (32, 50). Importantly, EPAC1 plays an important role in the regulation of Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV viral replication (54), suggesting that both viruses exploit a cAMP-dependent signaling pathway for infection of and replication in human host cells. Notably, this pathway also links CFTR and NHERF to lysophosphatidic acid receptor 2 (LPA2) (42). LPA2 is a GPCR (55) and its stimulation by LPA inhibits CFTR activity; accordingly, in vitro disruption of the association between LPA2 and NHERF leads to activation of CFTR (56). Interestingly, in mice, LPA markedly decreased CFTR-mediated intestinal fluid secretion induced by cholera toxin (56). At present, little is known about lipid signaling in general and lysophosphatidic acid specifically in the context of SARS-CoV-2 infection.
In a recent perspective, Kuebler et al. (57) argued that the calcium channel, the transient receptor potential vanilloid 4 (TRPV4), may play an important role in COVID-19 clinical progression from mild symptoms to severe disease. This hypothesis is based on the rationale that activation of TRPV4 results in impaired barrier function of the alveolocapillary membrane, which is the hallmark of acute respiratory distress syndrome (ARDS) (6, 57). This pathway may be equally relevant to transepithelial ion transport, as TRPV4 stimulation activates CFTR via elevated intracellular calcium levels (47). It thus seems possible that the activation of both channels may synergistically contribute to the development of pulmonary edema in SARS-CoV-2 infection via independent and/or interdependent pathways.
Indeed, several viruses that cause respiratory infections have been shown to dysregulate ion transport in the airspaces (45,46, 58–60). Specifically, influenza virus (61, 62), respiratory syncytial virus (RSV) (63), and SARS-CoV (64) inhibit ENaC function in a protein kinase C (PKC)-dependent manner. Furthermore, the influenza M2 protein, which functions as a proton- conducting transmembrane channel, decreases ENaC activity by targeting the channel for ubiquitin-mediated proteasomal degradation (65). These findings indicate that the influenza virus interferes with the absorptive functions of ENaC and may thus contribute to pulmonary edema formation (45). In addition to ENaC dysregulation, the influenza M2 protein also promotes CFTR ubiquitination (66) while impairing CFTR maturation; the latter effect is likely caused by M2 protein functioning as a proton channel and increasing vesicular acidification. It is conceivable that SARS-CoV-2 may similarly exploit the PKC-dependent pathway and inhibit both CFTR and ENaC functions; in this case, only ENaC inhibition would contribute to lung edema formation. The severity of lung disease in patients with COVID-19, however, suggests that SARS-CoV-2 rather engages the classic GPCR pathway, resulting in a combined CFTR activation and ENaC inhibition.
P2 receptors and purinergic signaling play an important role in alveolar homeostasis and contribute to several lung pathologies, including ARDS and ventilator-induced lung injury (67–70). P2 receptor activation primarily depends on ATP (and most likely UTP) that is present in the alveolar hypophase at very low concentrations (71) but increases in mechanically induced lung injury (70) and some viral infections (45, 72). Binding of extracellular nucleotides activates ionotropic (P2X) and metabotropic (P2Y) purinoreceptors, which—depending on the receptor involved—in turn trigger second messenger responses and signaling cascades (67, 70). These responses can be complex and diverse, as a considerable variety of P2X and P2Y receptors are expressed on both resident and nonresident populations of lung cells including alveolar type I and type II cells, endothelial cells, macrophages, and neutrophils (70). Several studies point to the possibility that some viruses including influenza virus A and RSV can regulate CFTR and ENaC- mediated transport pathways via secondary viral mediators (46). Specifically, ATP and UTP, probably released into the alveolar space via volume-regulated anion channels (73–77), pannexin 1 channel (78–80), or other ATP release mechanisms (67, 70, 81), can serve as secondary mediators of viruses and engage A1AR and P2Y6R, two GPCRs that are abundantly expressed on respiratory and/or alveolar epithelial cells (46). Increased UTP levels inhibit amiloride-sensitive ion transport via P2Y6R, whereas elevated levels of ATP cause increased CFTR-mediated Cl− secretion via the A1AR receptor; in combination, these differential effects will synergize to cause extensive alveolar edema (46). It is conceivable that SARS-CoV-2, replication of which requires high levels of ATP (82), similarly uses such secondary mediators which in turn will regulate epithelial ion and fluid transport in an auto- or paracrine fashion (Fig. 1). This signaling cascade may act in parallel to the classic intracellular pathways. A combination of both effects may create a vicious cycle that keeps ENaC consistently inhibited and CFTR active, thus accounting for the development of severe ARDS in COVID-19. The picture is further complicated by the fact that the disturbed balance of pro- and anti-inflammatory cytokines (83–86) caused by SARS-CoV-2 (5) may further dysregulate ion-transporting pathways and contribute to lung edema in COVID-19 (85).
At present, these discussions are by nature hypothetical. In the absence of appropriate tools to measure transepithelial ion and fluid transport in patients with COVID-19 or adequate animal models, the functional consequences of SARS-CoV-2 infection on the homeostasis of the epithelial lining fluid in the airways and alveolar space remain speculative. That notwithstanding, the clinical presentation of severe COVID-19 and the precedent of other respiratory viruses—including SARS-CoV and MERS—hijacking GPCR signaling pathways and dysregulating ENaC and CFTR point to a very real possibility for a similar scenario in SARS-CoV-2 infection. The resulting imbalance between absorptive and secretory pathways in the airspace may relevantly contribute to the formation (and/or lack of resolution) of alveolar edema, thus, mimicking the effects of cholera toxin in intestinal epithelial cells (41). The functional significance of these arguments and their implications in the pathophysiology of COVID-19 require further study (87) but may be critical (88) in managing the respiratory pathology that drives severe and ultimately, fatal COVID-19.
GRANTS
This work was supported, in whole or in part, by MBRU-COM Internal Grant Awards MBRU-CM-RG2018-04 and MBRU-CM-RG2018-05 (to M.U. and B.K.B.), Sandoq Al Watan Research & Development Grant SWARD-F2018-002 (to M.U. and B.K.B.), AlMahmeed Collaborative Research Award 2018 (to M.U. and B.K.B.), and Al Jalila Foundation Grant AJF201763 (to M.U.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.K.B. conceived and designed research; R.A.H. and M.U. prepared figure; R.A.H., W.M.K., M.U., and B.K.B. drafted manuscript; R.A.H., E.C-B., W.M.K., M.U., and B.K.B. edited and revised manuscript; R.A.H., E.C-B., W.M.K., M.U., and B.K.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Firuz B. Berdiev for editing the manuscript.
REFERENCES
- 1.Uddin M, Mustafa F, Rizvi TA, Loney T, Suwaidi HA, Al-Marzouqi AHH, Eldin AK, Alsabeeha N, Adrian TE, Stefanini C, Nowotny N, Alsheikh-Ali A, Senok AC. SARS-CoV-2/COVID-19: viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses 12: 526, 2020. doi: 10.3390/v12050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239–1242, 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280,2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedford J, Enria D, Giesecke J, Heymann DL, Ihekweazu C, Kobinger G, Lane HC, Memish Z, Oh MD, Sall AA, Schuchat A, Ungchusak K, Wieler LH. Strategic WHO, technical advisory group for infectious H. COVID-19: towards controlling of a pandemic. Lancet 395: 1015–1018, 2020. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, , et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182: 429–446,2020. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181: 905–913, 2020. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoufaly A, Poglitsch M, Aberle JH, Hoepler H, Seitz T, Traugott M, Grieb A, Pawelka P, Laferl L, Wenisch C, Neuhold S, Haider H, Stiasny K, Bergthaler A, Puchhammer-Stoeckl E, Mirazimi A, Montserrat N, Zhang H, Slutsky AS, Penninger JM. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med 8: 1154–1158, 2020. doi: 10.1016/s2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hikmet F, Mear L, Edvinsson A, Micke P, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol 16: e9610, 2020. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuba K, Imai Y, Rao S, Jiang C, Penninger JM. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med (Berl) 84: 814–820, 2006. doi: 10.1007/s00109-006-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimas J, Olvedy M, Ochodnicka-Mackovicova K, Kruzliak P, Cacanyiova S, Kristek F, Krenek P, Ochodnicky P. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med 19: 1965–1974, 2015. doi: 10.1111/jcmm.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo KB, Bhargav R, Salacup G, Pelayo J, Albano J, McCullough PA, Rangaswami J. Angiotensin converting enzyme inhibitors and angiotensin II receptor blockers and outcomes in patients with COVID-19: a systematic review and meta-analysis. Expert Rev Cardiovasc Ther 18: 919–930, 2020. doi: 10.1080/14779072.2020.1826308. [DOI] [PubMed] [Google Scholar]
- 15.Matthay MA, Clerici C, Saumon G. Invited review: active fluid clearance from the distal air spaces of the lung. J Appl Physiol (1985) 93: 1533–1541, 2002. doi: 10.1152/japplphysiol.01210.2001. [DOI] [PubMed] [Google Scholar]
- 16.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 17.Bartoszewski R, Matalon S, Collawn JF. Ion channels of the lung and their role in disease pathogenesis. Am J Physiol Lung Cell Mol Physiol 313: L859–L872, 2017. doi: 10.1152/ajplung.00285.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol 35: 116–129, 2007. doi: 10.1080/01926230601060025. [DOI] [PubMed] [Google Scholar]
- 19.Collawn JF, Matalon S. CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol 307: L917–L923, 2014. doi: 10.1152/ajplung.00326.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7: 426–436, 2006. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 21.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 22.Matalon S, Bartoszewski R, Collawn JF. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol 309: L1229–L1238, 2015. doi: 10.1152/ajplung.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossier BC, Baker ME, Studer RA. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev 95: 297–340, 2015. doi: 10.1152/physrev.00011.2014. [DOI] [PubMed] [Google Scholar]
- 24.Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst 5: 123–127, 2009. doi: 10.1039/b810471a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23: 146–158, 2004. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 26.Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 302: L1141–L1146, 2012. doi: 10.1152/ajplung.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 28.Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science 269: 847–850, 1995. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 29.Kaestle SM, Reich CA, Yin N, Habazettl H, Weimann J, Kuebler WM. Nitric oxide-dependent inhibition of alveolar fluid clearance in hydrostatic lung edema. Am J Physiol Lung Cell Mol Physiol 293: L859–L869, 2007. doi: 10.1152/ajplung.00008.2007. [DOI] [PubMed] [Google Scholar]
- 30.Londino JD, Matalon S. Chloride secretion across adult alveolar epithelial cells contributes to cardiogenic edema. Proc Natl Acad Sci USA 110: 10055–10056, 2013. doi: 10.1073/pnas.1307480110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solymosi EA, Kaestle-Gembardt SM, Vadasz I, Wang L, Neye N, Chupin CJ, Rozowsky S, Ruehl R, Tabuchi A, Schulz H, Kapus A, Morty RE, Kuebler WM. Chloride transport-driven alveolar fluid secretion is a major contributor to cardiogenic lung edema. Proc Natl Acad Sci USA 110: E2308–E2316, 2013. doi: 10.1073/pnas.1216382110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunzelmann K. CFTR: interacting with everything? News Physiol Sci 16: 167–170, 2001. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Naren AP. Macromolecular complexes of cystic fibrosis transmembrane conductance regulator and its interacting partners. Pharmacol Ther 108: 208–223, 2005. doi: 10.1016/j.pharmthera.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol 53: 531–556, 2013. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilger D, Masureel M, Kobilka BK. Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol 25: 4–12, 2018. doi: 10.1038/s41594-017-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650, 2002. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 37.Magalhaes AC, Dunn H, Ferguson SS. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol 165: 1717–1736, 2012. doi: 10.1111/j.1476-5381.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monterisi S, Casavola V, Zaccolo M. Local modulation of cystic fibrosis conductance regulator: cytoskeleton and compartmentalized cAMP signalling. Br J Pharmacol 169: 1–9, 2013. doi: 10.1111/bph.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hentchel-Franks K, Lozano D, Eubanks-Tarn V, Cobb B, Fan L, Oster R, Sorscher E, Clancy JP. Activation of airway cl- secretion in human subjects by adenosine. Am J Respir Cell Mol Biol 31: 140–146, 2004. doi: 10.1165/rcmb.2004-0012OC. [DOI] [PubMed] [Google Scholar]
- 40.Sodhi A, Montaner S, Gutkind JS. Viral hijacking of G-protein-coupled-receptor signalling networks. Nat Rev Mol Cell Biol 5: 998–1012, 2004. doi: 10.1038/nrm1529. [DOI] [PubMed] [Google Scholar]
- 41.Barrett KE. Rethinking cholera pathogenesis- No longer all in the same “camp”. Virulence 7: 751–753, 2016. doi: 10.1080/21505594.2016.1212156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naren AP, Cobb B, Li C, Roy K, Nelson D, Heda GD, Liao J, Kirk KL, Sorscher EJ, Hanrahan J, Clancy JP. A macromolecular complex of beta 2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci USA 100: 342–346, 2003. doi: 10.1073/pnas.0135434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson MJ, Lee SL, Marklew AJ, Gilmore RC, Gentzsch M, Sassano MF, Gray MA, Tarran R. The cystic fibrosis transmembrane conductance regulator (CFTR) uses its C-terminus to regulate the A2B adenosine receptor. Sci Rep 6: 27390, 2016. doi: 10.1038/srep27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Wang W, Parker W, Clancy JP. Adenosine regulation of cystic fibrosis transmembrane conductance regulator through prostenoids in airway epithelia. Am J Respir Cell Mol Biol 34: 600–608, 2006. doi: 10.1165/rcmb.2005-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Londino JD, Lazrak A, Collawn JF, Bebok Z, Harrod KS, Matalon S. Influenza virus infection alters ion channel function of airway and alveolar cells: mechanisms and physiological sequelae. Am J Physiol Lung Cell Mol Physiol 313: L845–L858, 2017. doi: 10.1152/ajplung.00244.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolk KE, Lazarowski ER, Traylor ZP, Yu EN, Jewell NA, Durbin RK, Durbin JE, Davis IC. Influenza A virus inhibits alveolar fluid clearance in BALB/c mice. Am J Respir Crit Care Med 178: 969–976, 2008. doi: 10.1164/rccm.200803-455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genovese M, Borrelli A, Venturini A, Guidone D, Caci E, Viscido G, Gambardella G, di Bernardo D, Scudieri P, Galietta LJV. TRPV4 and purinergic receptor signalling pathways are separately linked in airway epithelia to CFTR and TMEM16A chloride channels. J Physiol 597: 5859–5878, 2019. doi: 10.1113/JP278784. [DOI] [PubMed] [Google Scholar]
- 48.Billet A, Hanrahan JW. The secret life of CFTR as a calcium-activated chloride channel. J Physiol 591: 5273–5278, 2013. doi: 10.1113/jphysiol.2013.261909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billet A, Luo Y, Balghi H, Hanrahan JW. Role of tyrosine phosphorylation in the muscarinic activation of the cystic fibrosis transmembrane conductance regulator (CFTR). J Biol Chem 288: 21815–21823, 2013. doi: 10.1074/jbc.M113.479360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lobo MJ, Amaral MD, Zaccolo M, Farinha CM. EPAC1 activation by cAMP stabilizes CFTR at the membrane by promoting its interaction with NHERF1. J Cell Sci 129: 2599–2612, 2016. doi: 10.1242/jcs.185629. [DOI] [PubMed] [Google Scholar]
- 51.Alhammad YMO, Kashipathy MM, Roy A, Gagne JP, McDonald P, Gao P, Nonfoux L, Battaile KP, Johnson DK, Holmstrom ED, Poirier GG, Lovell S, Fehr AR. The SARS-CoV-2 conserved macrodomain is a highly efficient ADP-ribosylhydrolase enzyme (Preprint). bioRxiv, 2020. doi: 10.1101/2020.05.11.089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Claverie JM. A putative role of de-mono-ADP-ribosylation of STAT1 by the SARS-CoV-2 Nsp3 protein in the cytokine storm syndrome of COVID-19. Viruses 12: 646, 2020. doi: 10.3390/v12060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin MH, Chang SC, Chiu YC, Jiang BC, Wu TH, Hsu CH. Structural, biophysical, and biochemical elucidation of the SARS-CoV-2 nonstructural protein 3 macro domain. ACS Infect Dis 6: 2970–2978, 2020. doi: 10.1021/acsinfecdis.0c00441. [DOI] [PubMed] [Google Scholar]
- 54.Tao X, Mei F, Agrawal A, Peters CJ, Ksiazek TG, Cheng X, Tseng CT. Blocking of exchange proteins directly activated by cAMP leads to reduced replication of Middle East respiratory syndrome coronavirus. J Virol 88: 3902–3910, 2014. doi: 10.1128/JVI.03001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res 55: 1192–1214, 2014. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med 202: 975–986, 2005. doi: 10.1084/jem.20050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuebler WM, Jordt SE, Liedtke WB. Urgent reconsideration of lung edema as a preventable outcome in COVID-19: inhibition of TRPV4 represents a promising and feasible approach. Am J Physiol Lung Cell Mol Physiol 318: L1239–L1243, 2020. doi: 10.1152/ajplung.00161.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, Song W, Davis IC, Shrestha K, Schwiebert E, Sullender WM, Matalon S. Inhibition of Na+ transport in lung epithelial cells by respiratory syncytial virus infection. Am J Respir Cell Mol Biol 40: 588–600, 2009. doi: 10.1165/rcmb.2008-0034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song W, Liu G, Bosworth CA, Walker JR, Megaw GA, Lazrak A, Abraham E, Sullender WM, Matalon S. Respiratory syncytial virus inhibits lung epithelial Na+ channels by up-regulating inducible nitric-oxide synthase. J Biol Chem 284: 7294–7306, 2009. doi: 10.1074/jbc.M806816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem 280: 35751–35759, 2005. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen XJ, Seth S, Yue G, Kamat P, Compans RW, Guidot D, Brown LA, Eaton DC, Jain L. Influenza virus inhibits ENaC and lung fluid clearance. Am J Physiol Lung Cell Mol Physiol 287: L366–L373, 2004. doi: 10.1152/ajplung.00011.2004. [DOI] [PubMed] [Google Scholar]
- 62.Kunzelmann K, Beesley AH, King NJ, Karupiah G, Young JA, Cook DI. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc Natl Acad Sci USA 97: 10282–10287, 2000. doi: 10.1073/pnas.160041997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D IC, Lazarowski ER, Chen FP, Hickman-Davis JM, Sullender WM, Matalon S. Post-infection A77-1726 blocks pathophysiologic sequelae of respiratory syncytial virus infection. Am J Respir Cell Mol Biol 37: 379–386, 2007. doi: 10.1165/rcmb.2007-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji HL, Song W, Gao Z, Su XF, Nie HG, Jiang Y, Peng JB, He YX, Liao Y, Zhou YJ, Tousson A, Matalon S. SARS-CoV proteins decrease levels and activity of human ENaC via activation of distinct PKC isoforms. Am J Physiol Lung Cell Mol Physiol 296: L372–L383, 2009. doi: 10.1152/ajplung.90437.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lazrak A, Iles KE, Liu G, Noah DL, Noah JW, Matalon S. Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species. FASEB J 23: 3829–3842, 2009. doi: 10.1096/fj.09-135590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Londino JD, Lazrak A, Jurkuvenaite A, Collawn JF, Noah JW, Matalon S. Influenza matrix protein 2 alters CFTR expression and function through its ion channel activity. Am J Physiol Lung Cell Mol Physiol 304: L582–L592, 2013. doi: 10.1152/ajplung.00314.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol 9: 262–267, 2009. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 69.Schmid A, Clunes LA, Salathe M, Verdugo P, Dietl P, Davis CW, Tarran R. Nucleotide-mediated airway clearance. Subcell Biochem 55: 95–138, 2011. doi: 10.1007/978-94-007-1217-1_5. [DOI] [PubMed] [Google Scholar]
- 70.Wirsching E, Fauler M, Fois G, Frick M. P2 purinergic signaling in the distal lung in health and disease. Int J Mol Sci 21: 4973, 2020. doi: 10.3390/ijms21144973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patel AS, Reigada D, Mitchell CH, Bates SR, Margulies SS, Koval M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am J Physiol Lung Cell Mol Physiol 289: L489–L496, 2005. doi: 10.1152/ajplung.00074.2005. [DOI] [PubMed] [Google Scholar]
- 72.Atkinson SK, Morice AH, Sadofsky LR. Rhinovirus-16 increases ATP release in A549 cells without concomitant increase in production. ERJ Open Res 6: 00159-2020, 2020. doi: 10.1183/23120541.00159-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D IC, Lazarowski ER, Hickman-Davis JM, Fortenberry JA, Chen FP, Zhao X, Sorscher E, Graves LM, Sullender WM, Matalon S. Leflunomide prevents alveolar fluid clearance inhibition by respiratory syncytial virus. Am J Respir Crit Care Med 173: 673–682, 2006. doi: 10.1164/rccm.200508-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nilius B, Eggermont J, Droogmans G. The endothelial volume-regulated anion channel, VRAC. Cell Physiol Biochem 10: 313–320, 2000. doi: 10.1159/000016364. [DOI] [PubMed] [Google Scholar]
- 75.Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol 124: 513–526, 2004. doi: 10.1085/jgp.200409154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal 1: 311–328, 2005. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sabirov RZ, Okada Y. Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway. Biophys J 87: 1672–1685, 2004. doi: 10.1529/biophysj.104.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41: 525–534, 2009. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O'Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 286: 26277–26286, 2011. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diem K, Fauler M, Fois G, Hellmann A, Winokurow N, Schumacher S, Kranz C, Frick M. Mechanical stretch activates piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. FASEB J 34: 12785–12804, 2020. doi: 10.1096/fj.202000613RRR. [DOI] [PubMed] [Google Scholar]
- 82.Jang KJ, Jeong S, Kang DY, Sp N, Yang YM, Kim DE. A high ATP concentration enhances the cooperative translocation of the SARS coronavirus helicase nsP13 in the unwinding of duplex RNA. Sci Rep 10: 4481, 2020. doi: 10.1038/s41598-020-61432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peteranderl C, Sznajder JI, Herold S, Lecuona E. Inflammatory responses regulating alveolar ion transport during pulmonary infections. Front Immunol 8: 446, 2017. doi: 10.3389/fimmu.2017.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vadasz I, Lucas R. Editorial: Cytokine-ion channel interactions in pulmonary inflammation. Front Immunol 9: 2598, 2018. doi: 10.3389/fimmu.2018.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weidenfeld S, Kuebler WM. Cytokine-regulation of Na(+)-K(+)-Cl(-) cotransporter 1 and cystic fibrosis transmembrane conductance regulator-potential role in pulmonary inflammation and edema formation. Front Immunol 8: 393, 2017. doi: 10.3389/fimmu.2017.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wynne BM, Zou L, Linck V, Hoover RS, Ma HP, Eaton DC. Regulation of lung epithelial sodium channels by cytokines and chemokines. Front Immunol 8: 766, 2017. doi: 10.3389/fimmu.2017.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gentzsch M, Rossier BC. A pathophysiological model for COVID-19: critical importance of transepithelial sodium transport upon airway infection. Function (Oxf) 1: zqaa024, 2020. doi: 10.1093/function/zqaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eisenhut M, Shin JI. Pathways in the pathophysiology of coronavirus 19 lung disease accessible to prevention and treatment. Front Physiol 11: 872, 2020. doi: 10.3389/fphys.2020.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]