Abstract
Corynebacterium diphtheriae is the leading cause of pharyngeal diphtheria, a respiratory disease characterized by formation of a pseudomembrane at the site of infection. Although the outbreaks of C. diphtheriae infections are rare nowadays, the emergence of multidrug-resistant C. diphtheriae strains is one of the significant public health concerns worldwide. While C. diphtheriae has been studied for more than a century, and diphtheria toxin and pili have been identified as major virulence factors, little is known about factors involved in bacterial colonization and development of disease. Here, we describe the utilization of Caenorhabditis elegans as a cost-effective, versatile model of infection to evaluate C. diphtheriae virulence. We provide detailed protocols for nematode synchronization and evaluation of nematode survival and formation of deformed anal region (Dar) induced by C. diphtheriae infection. These protocols will permit future high-throughput screenings of virulence factors in C. diphtheriae and advance our knowledge of C. diphtheriae pathogenesis.
Keywords: Corynebacterium diphtheriae, Caenorhabditis elegans, survival assay, intestinal colonization, Deformed anal region (Dar), virulence, infection, synchronization
INTRODUCTION
Guinea pigs are often used as an in vivo model for diphtheria toxin (DT) in C. diphtheriae, as mouse cells lack a functional cell surface receptor, EGF-like growth factor, for the toxin (Naglich, Metherall, Russell, & Eidels, 1992). As a result, naïve mice are insensitive to DT even at high doses of the toxin (Saito et al., 2001); when the human EFG factor is expressed in mice, these transgenic mice now become susceptible to DT (Saito et al., 2001). Regardless, guinea pigs and EGF-transgenic mice are prohibitively expensive for large-scale in vivo studies. Here, we describe in detail procedures that utilize the non-pathogenic nematode C. elegans as a cost-effective, versatile animal model of infection for C. diphtheriae to evaluate virulence factors as previously reported (Melissa M. Broadway, 2010; M. M. Broadway et al., 2013).
Basic Protocol 1 describes a procedure to synchronize the worms, whereas Basic Protocols 2 and 3 describe assays to evaluate nematode survival by corynebacterial infection, bacterial colonization, and the formation of deformed anal region (Dar) phenotype induced by corynebacterial colonization.
CAUTION
C. diphtheriae is a Biosafety Level 2 (BSL-2) pathogen. Therefore, appropriate guidelines and regulations for the use and handling of this pathogen are required.
BASIC PROTOCOL 1 – Synchronization of the nematodes
Infections occurring in different stages of the worms’ development may lead to different responses. To correct this, synchronization of the worms is important to consider before beginning a C. elegans assay. This protocol is modified from previously published procedure (Y. W. Chen, Ko, Chen, & Chen, 2018a, 2018b).
Materials
C. elegans N2 (gravid-adult stage)
Sterile deionized water
15 mL Centrifuge tube
ENGM agar plate (see Reagents and Solutions)
Escherichia coli OP50 (Caenorhabditis Genetics Center (CGC))
LB broth (Fisher, BP1426–2)
10–15% NaOCl (Fisher, cat. no. 60–014-90)
5M KOH (Fisher, NC9231992)
M9 buffer (see Reagents and Solutions)
3.5 cm Culture Dish (VWR, cat. no.10861–586)
Centrifuge (Thermo Scientific, Sorvall™ Legend™ X1)
Bacteria Incubator (New Brunswick, Innova 44)
Worm Incubator (VWR, Signature™ B.O.D. cat. no. 35960–056)
Stereo microscope (Olympus, SZ-6145TR)
Nematode synchronization
Collect about 2,000 gravid-adult stage worms from a plate and transfer to a 15-mL tube by washing with 10 mL sterile deionized water.
Harvest the worms by centrifugation at 500 × g for 1 min.
Remove the supernatant and repeat the washing step 3 times.
Keep the worms in 3.5 mL deionized water.
Add 1 mL NaOCl (10–15%) and 0.5 mL 5M KOH into the tube, and mix evenly by shaking to lyse the worm bodies for no more than 6 min.
Add 10 mL deionized water to stop lysis once the eggs are released from the worms.
Collect the worm eggs by centrifugation at 1,200 × g for 1 min.
Remove as much supernatant as possible.
Wash the eggs 3 times with 15 mL M9 buffer.
Transfer the eggs to a 3.5-cm dish with 1 mL M9 buffer and incubate eggs at 20oC for at least 12 hours. After the incubation, the eggs will hatch to worm larva (the larva stage L1). The L1 worms can be used for seeding on an ENGM plate in the next 3 days.
Randomly take 10 μL of L1 worms from three sites within the 3.5-cm dish and count the numbers under a stereo microscope (ocular lens, 10X; objective lens, 2–4X).
-
Seed at most 10,000 L1 stage worms on a 9-cm ENGM plate fully spread with E. coli OP50.
Inoculate 2-ml LB broth cultures of E. coli OP50 from a fresh colony and grow with shaking (160 rpm) at 37 oC overnight.
Spread 0.5 mL overnight cultured E. coli OP50 broth on a 9-cm ENGM plate.
Incubate the plate at 37oC overnight.
Cool the plate to room temperature and seed at most 10,000 L1 stage worms on the plate.
Place the plate upside down in the worm incubator at 20oC for 44 hours to grow the N2 worms from L1 to L4 stage.
Use the synchronized L4 stage worms in the following assays.
BASIC PROTOCOL 2 – A nematode survival assay for C. diphtheriae infection
In this assay, the survival of C. elegans L4 worms fed on C. diphtheriae is monitored and calculated every day. By comparing with the survival of the nematodes fed on E. coli OP50, the virulence potential of C. diphtheriae can be determined (M. M. Broadway et al., 2013).
Materials
E. coli OP50 (Caenorhabditis Genetics Center (CGC))
C. diphtheriae NCTC13129 or any C. diphtheriae strains
Heart Infusion Broth (HI broth) (BD, cat. no. 238400)
C. elegans N2 (L4 stage)
Brain Heart Infusion (BHI) agar (BD, cat. no. 211059)
Spectrophotometer (GE Healthcare, Ultrospec™ 7000)
Shaker/Incubator (New Brunswick, Innova 44)
Worm Incubator (VWR, Signature™ B.O.D. cat. no. 35960–056)
Stereo microscope (Olympus, SZ-6145TR)
Procedure
Inoculate 2-ml HI broth cultures of each strain, E. coli OP50 or C. diphtheriae NCTC13129, from a fresh colony and grow with shaking (160 rpm) at 37oC for 16 hours.
Measure the optical density at a wavelength of 600 nm (OD600) of the bacterial cultures and adjust the culture with HI broth to OD600 of ~ 2.0.
Spot and spread 100 μL of each bacterial culture onto 6-cm BHI agar plates; incubate the plates at room temperature overnight.
-
Using a worm-picker, randomly pick and transfer 30 synchronized L4 stage worms onto an BHI agar plate with growing C. diphtheriae NCTC13129 or E. coli OP50.
For synchronizing worms, please see Basic Protocol 1 above.
Place BHI plates containing infected worms upside down at 25oC.
-
Transfer the living worms to a newly prepared BHI agar plate with bacteria (see Step 3) and count the numbers of live, dead, and sensor worms every day until the last worm is dead.
Note: Transfer worms to a freshly-prepared BHI agar plate (see Step 3) every day for the maintenance of infection, even with infertile agents such as glp-4 worms or chemical FuDR.
Calculate the survival percent as follows: [Number of living worms/ Initial number of total worms] x100%
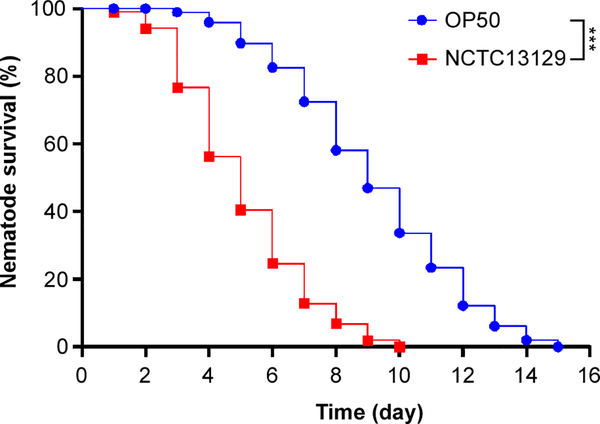
Graph and analyze survival curves using GraphPad Prism (Figure 1). Compare survival of worm fed on C. diphtheriae with survival of worms fed on E. coli.
Figure 1: Reduced survival of C. elegans infected with C. diphtheriae.
30 wild-type N2 worms were placed on BHI agar plates containing growing C. diphtheriae NCTC13129 (red squares) or E. coli OP50 (blue circles) and maintained at 25oC. Nematode survival was recorded daily. The results were presented as percentages and analyzed by GraphPad Prism; ***, P < 0.0001.
BASIC PROTOCOL 3 – Assays for bacterial colonization and formation of deformed anal region (Dar)
In this protocol, we evaluate the ability of C. diphtheriae to colonize the nematode’s intestine and to form Dar, a phenotype characterized by a swollen tail of the nematode originally observed in some genetic crosses (Brenner, 1974) and later detected in infection with Microbacterium nematophilum (Hodgkin, Kuwabara, & Corneliussen, 2000). For tracing bacterial colonization, we utilized corynebacteria harboring a recombinant plasmid expressing red fluorescent protein (pDsRed), which can be observed in the intestine of the transparent nematode by fluorescent microscopy.
Materials
Plasmid pDsRed; derivative of pCGL0482 (Reyes et al., 1991), expressing red fluorescent protein (Addgene, #68202)
E. coli OP50 (Caenorhabditis Genetics Center (CGC))
E. coli OP50 pDsRed (Ton-That Lab, UCLA)
C. diphtheriae NCTC13129 pDsRed (Ton-That Lab, UCLA)
Heart Infusion Broth (HI broth) (BD, cat. no. 238400)
C. elegans N2 (L4 stage)
Brain Heart Infusion (BHI) agar (BD, cat. no. 211059)
1% Sodium azide in M9 buffer
2% Agarose in M9 buffer
Glass slide and cover slide
Spectrophotometer (GE Healthcare, Ultrospec™ 7000)
Shaker/incubator (New Brunswick, Innova 44)
Worm incubator (VWR, Signature™ B.O.D. cat. no. 35960–056)
Stereo microscope (Olympus, SZ-6145TR)
Fluorescent microscope (NIKON, Eclipse Ts2)
Procedure
Inoculate individual 2-ml HI broth cultures of E. coli OP50, E. coli OP50 pDsRed or C. diphtheriae NCTC13129 pDsRed from fresh, single colonies. Culture with shaking (160 rpm) at 37oC for 16 hours.
Measure OD600 of the cultures and adjust them to OD600 of ~ 2.0 using fresh culture medium.
Spot and spread 100 μL of bacterial cultures on 6-cm BHI agar plates and incubate at room temperature overnight.
-
Randomly pick and transfer 30 synchronized L4 worms onto a BHI agar plate containing C. diphtheriae NCTC13129 pDsRed or E. coli OP50 pDsRed.
For synchronizing worms, please see Basic Protocol 1 above.
Place the BHI plates with the infected worms at 25oC for 24 hours.
Transfer the living worms to a newly prepared BHI agar plate spread with E. coli OP50 (see Step 3).
Grow the worms on BHI plates at 25oC for 48 hours.
-
Take images.
Randomly pick 10 worms onto a 2% agarose gel on slide individually.
Before taking images paralyze the worms with 1 μL of 1% sodium azide on agarose until the worms are not moving (usually less than 5 minutes).
Put a cover slip on worms (so as on the 2% agarose gel on slide).
Capture worm images using a bright field and DsRed/GFP/BFP filters from a fluorescent microscope.
9. Record bacterial colonization of the intestinal tract and formation of Dar.
10. Graph bar charts of intestinal colonization (Figure 2) and the Dar phenotype (Figure 3).
Figure 2: C. diphtheriae colonization in the intestine of C. elegans.
Wild-type N2 worms were infected with C. diphtheriae NCTC13129 harboring the vector pDsRed for 72 hours. Worms were imaged by a fluorescence microscope. (A) Shown is an overlapped image of a whole worm. (B-E) Enlarged images of (A) taken with bright field illumination (B) or red (λ 590 nm) (C), green (470 nm (D), or blue (385 nm) (E) channel. (F) A merged image of (C) to (E) shows auto-fluorescence in the C. elegans intestine. (G) A merged image of (B) to (E) shows that C. diphtheriae colonized the intestine (posterior midgut) and rectum (hindgut) of the worm. (H) Each point (percent) represents the occurrence of total DsRed signal in 10 worms measured in a single experiment; three independent experiments were performed; ***, P < 0.001. The scale bars in (A) and (B-G) indicate 100 mm and 25 mm, respectively.
Figure 3: C. diphtheriae induces Dar (deformed anal region) formation in C. elegans.
(A-B) Wild-type N2 worms were infected with E. coli OP50 (A) or C. diphtheriae NCTC13129 (B) for 72 hours; a distinctive swollen tail (Dar) is marked with a white arrowhead. (C) Each point (percent) represents the occurrence of Dar in 10 worms determined in a single experiment; three independent experiments were performed; ***, P < 0.001. The scale bars indicate 100 mm.
REAGENTS AND SOLUTIONS
ENGM agar plate
3 g NaCl
5 g bacterial peptone
1 g yeast extract
30 g agar
1 L of deionized water
Preparation
Autoclave at 121oC for 20 minutes
Wait until cooled to 55oC
Add 1 mL of 1 M CaCl2
Add 1 mL of 1 M MgSO4
Add 1 mL of 5% cholesterol in ethanol
Add 25 mL of worm phosphate buffer (see below)
Swirl to mix well.
Pour about 10 mL NGM into each 9 cm petri plate.
Leave plates at room temperature for 1 night and save at 4oC up to 1 month.
Warmup to room temperature before use.
Worm M9 buffer
1.50 g of KH2PO4
5.66 g of Na2HPO4
2.50 g of NaCl
500 mL of deionized water
Preparation
Autoclave at 121oC for 20 min.
Wait until cooling to room temperature.
Add 0.5 mL of sterile 1 M MgSO4 before first use.
Worm phosphate buffer
119.35 g of KH2PO4
21.43 g of K2HPO4
1 L of deionized water
Preparation
Adjust the pH value to pH=6.0 with KOH.
Autoclave at 121oC for 20 minutes.
Wait until cooling before use and save at room temperature.
COMMENTARY
BACKGROUND INFORMATION
The non-pathogenic nematode C. elegans has extensively been used in genetics, cell biology, and developmental biology (Ermolaeva & Schumacher, 2014), as well as investigation of cancer cell invasion (Stuelten, Parent, & Montell, 2018). C. elegans is a natural bacterivore, allowing researchers to manipulate the species and strains available as their food source to study bacterial colonization and pathogenicity. Due to the cost and scale, C. elegans has emerged as a facile and economical model host for the study of mechanisms of microbial pathogenesis and innate immunity (Sifri, Begun, & Ausubel, 2005). Ausubel’s group first used C. elegans as a model host to examine virulence mechanisms of the opportunistic human pathogen Pseudomonas aeruginosa (Mahajan-Miklos, Tan, Rahme, & Ausubel, 1999). This model permitted the screening of 3300 Tn phoA transposon clones for mutants reduced in virulence (Tan, Mahajan-Miklos, & Ausubel, 1999), a procedure that would not be possible with a rodent model of infection. Since then the nematode has widely been used as a model host for many other Gram-negative and Gram-positive pathogens, including Salmonella typhimurium (Aballay & Ausubel, 2001), Burkholderia pseudomallei (O’Quinn, Wiegand, & Jeddeloh, 2001), Aeromonas dhakensis (P. L. Chen et al., 2016; Y. W. Chen et al., 2020), enterohaemorrhagic E. coli O157:H7 (Chou et al., 2013), Enterococcus faecalis, Streptococcus pneumoniae, and Staphylococcus aureus (Garsin et al., 2001).
The use of C. elegans in many corynebacterial species has been documented (Ott et al., 2012). In C. diphtheriae, mutants lacking DT or pili confers a longer survival rate of C. elegans, as compared to the parental strain (M. M. Broadway et al., 2013). This defective phenotype has been confirmed with a guinea pig model of diphtheritic infection (Reardon-Robinson et al., 2015). Intriguingly, C. diphtheriae was found to induce Dar (deformed anal region) formation (Melissa M. Broadway, 2010). Currently, DT and sortase-assembled pili are the only two known major virulence factors that have been examined in animal models, although a few adhesins have recently been identified (Moreira, Mattos-Guaraldi, & Andrade, 2008; Peixoto, Rosado, Leite, Rosado, & Bourne, 2017). Therefore, the protocols described here provide guidance for setting up the C. elegans platform in studying C. diphtheriae pathogenesis.
CRITICAL PARAMETERS
Synchronizing worms is important to generate reliable and reproducible results in subsequent assays. Because mix-stage worms may have heterogeneous responses to a pathogen, using non-synchronized worms in an infection may not produce consistent results. Well documented in literature, N2 worms need 44 hours to grow from L1 to L4 stage at 20oC. However, different worm strains may have different growth rates. Therefore, growth rate and synchronization are two important parameters to be considered before performing any experiments.
Different C. diphtheriae strains may induce different phenotypic observations in C. elegans. For example, C. diphtheriae NCTC13129 was found to localize the pharynx and hind gut of C. elegans N2. C. diphtheriae ISS3319 and C. diphtheriae DSM 43988 both colonize in the midgut but not in the hindgut of the nematode (Ott et al., 2012), while C. diphtheriae CDC-E8392 colonizes in the midgut and hindgut in 48 hours but only in the midgut of C. elegans N2 after chase with E. coli OP50 (Antunes et al., 2016). Thus, it is important to include some controls, such as C. diphtheriae NCTC13129, to set up new experiments.
It is noteworthy that slight changes in incubating temperature may result in changes in survival rates. Therefore, worm incubators’ temperature should be frequently monitored. Additionally, minimize operating time when worms are not in the incubator.
TROUBLESHOOTING
Please see Table 1 for troubleshooting if problems arise.
TABLE 1:
TROUBLESHOOTING GUIDES
| Issues | Possible Cause | Solution |
|---|---|---|
| No eggs or dead L1 after synchronization steps | Over bleaching | Expose worms to NaOCl and KOH less than 6 min |
| No hatching | Osmolarity imbalance | Remake worm M9 buffer |
| Crinkling of worms | Osmolarity imbalance | Remake worm M9 buffer and related agarose |
| Failure of worm paralysis within 5 min | Incorrect concentration of sodium azide | Remake a new batch of 1% sodium azide in M9 buffer |
| Different sizes of corynebacterial colonies on agar plates | Contamination | 35 μg/ml nalidixic acid can be added to inoculating cultures to prevent contamination by Gram-negative bacteria |
STATISTICAL ANALYSIS
For Basic Protocol 2, we recommend using at least 30 worms per group. All experiments are performed at least three times independently. The survival is assessed using the Kaplan-Meier method, and is analyzed using log-rank tests. For Basic Protocol 3, we recommend using at least 10 worms per group for three independent experiments. The incidence of phenotype is analyzed using non-paired T-test. The statistical significance is set at P<0.05. (***: P<0.001; **: P<0.01; *: P<0.05).
UNDERSTANDING RESULTS
For Basic Protocol 1, approximately 25 worms are expected to hatch in 1 μl M9 buffer. After growing on an ENGM plate for 44 hours, nearly 95% of worms are ready at L4 stage.
For Basic Protocol 2, the survival rate of C. elegans infected with C. diphtheriae NCTC13129 is lower than with E. coli OP50 at every timepoint. The minimal/maximal survival days are around 5/10 days when C. elegans is infected with C. diphtheriae and 9/15 days with E. coli OP50, respectively.
For Basic Protocol 3, C. diphtheriae NCTC13129 harboring pDsRed colonizes the intestine (posterior midgut) and rectum (hindgut) of C. elegans in 72 hours; based on the DsRed signal, C. diphtheriae colonization of the worm is around 90%. On the other hand, red fluorescence from E. coli OP50 with pDsRed is hardly seen (colonization assumed to be 0%). Furthermore, almost 97% of the worms form Dar with C. diphtheriae infection in 72 hours, whereas no Dar phenotype is observed with E. coli OP50.
TIME CONSIDERATIONS
In Basic Protocol 1, it takes about 5 days to get synchronized L4 stage worms. In Basic Protocol 2, it takes approximately 3 days to prepare plates with bacterial lawn and 15 days to finish the survival assay. Finally, in Basic Protocol 3, it takes about 3 days to prepare plate with bacteria lawn and 3 days (72 hours) for colonization and Dar formation.
ACKNOWLEDGEMENTS
We would like to thank Melissa Broadway for technical assistance and Emily Peluso and Matthew Scheible for critical review and discussion of this manuscript. Work related to this manuscript was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under the award numbers DE025015 and DE017382 (to H.T.-T).
LITERATURE CITED
- Aballay A, & Ausubel FM (2001). Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc Natl Acad Sci U S A, 98(5), 2735–2739. doi: 10.1073/pnas.041613098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes CA, Clark L, Wanuske MT, Hacker E, Ott L, Simpson-Louredo L, … Burkovski A (2016). Caenorhabditis elegans star formation and negative chemotaxis induced by infection with corynebacteria. Microbiology, 162(1), 84–93. doi: 10.1099/mic.0.000201 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974). The genetics of Caenorhabditis elegans. Genetics, 77(1), 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadway MM (2010). Molecular Basis of Corynebacterium diphtheriae virulence and infection in the Caenorhabditis elegans model host. (Masters of Science MS), University of Texas Health Science Center at Houston, Houston, TX. (AVT 204597) [Google Scholar]
- Broadway MM, Rogers EA, Chang C, Huang IH, Dwivedi P, Yildirim S, … Ton-That H (2013). Pilus gene pool variation and the virulence of Corynebacterium diphtheriae clinical isolates during infection of a nematode. J Bacteriol, 195(16), 3774–3783. doi:/ 10.1128/JB.00500-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PL, Chen YW, Ou CC, Lee TM, Wu CJ, Ko WC, & Chen CS (2016). A Disease Model of Muscle Necrosis Caused by Aeromonas dhakensis Infection in Caenorhabditis elegans. Front Microbiol, 7, 2058. doi: 10.3389/fmicb.2016.02058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Ko WC, Chen CS, & Chen PL (2018a). Evaluating Virulence and Pathogenesis of Aeromonas Infection in a Caenorhabditis elegans Model. J Vis Exp(142). doi: 10.3791/58768 [DOI] [PubMed] [Google Scholar]
- Chen YW, Ko WC, Chen CS, & Chen PL (2018b). RIOK-1 Is a Suppressor of the p38 MAPK Innate Immune Pathway in Caenorhabditis elegans. Front Immunol, 9, 774. doi: 10.3389/fimmu.2018.00774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Yeh WH, Tang HJ, Chen JW, Shu HY, Su YC, … Chen PL (2020). UvrY is required for the full virulence of Aeromonas dhakensis. Virulence, 11(1), 502–520. doi: 10.1080/21505594.2020.1768339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Chiu HC, Kuo CJ, Wu CM, Syu WJ, Chiu WT, & Chen CS (2013). Enterohaemorrhagic Escherichia coli O157:H7 Shiga-like toxin 1 is required for full pathogenicity and activation of the p38 mitogen-activated protein kinase pathway in Caenorhabditis elegans. Cell Microbiol, 15(1), 82–97. doi: 10.1111/cmi.12030 [DOI] [PubMed] [Google Scholar]
- Ermolaeva MA, & Schumacher B (2014). Insights from the worm: the C. elegans model for innate immunity. Semin Immunol, 26(4), 303–309. doi: 10.1016/j.smim.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin DA, Sifri CD, Mylonakis E, Qin X, Singh KV, Murray BE, … Ausubel FM (2001). A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A, 98(19), 10892–10897. doi: 10.1073/pnas.191378698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Kuwabara PE, & Corneliussen B (2000). A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode C. elegans. Curr Biol, 10(24), 1615–1618. doi: 10.1016/s0960-9822(00)00867-8 [DOI] [PubMed] [Google Scholar]
- Mahajan-Miklos S, Tan MW, Rahme LG, & Ausubel FM (1999). Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell, 96(1), 47–56. doi: 10.1016/s0092-8674(00)80958-7 [DOI] [PubMed] [Google Scholar]
- Moreira LO, Mattos-Guaraldi AL, & Andrade AF (2008). Novel lipoarabinomannan-like lipoglycan (CdiLAM) contributes to the adherence of Corynebacterium diphtheriae to epithelial cells. Arch Microbiol, 190(5), 521–530. doi: 10.1007/s00203-008-0398-y [DOI] [PubMed] [Google Scholar]
- Naglich JG, Metherall JE, Russell DW, & Eidels L (1992). Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell, 69(6), 1051–1061. [DOI] [PubMed] [Google Scholar]
- O’Quinn AL, Wiegand EM, & Jeddeloh JA (2001). Burkholderia pseudomallei kills the nematode Caenorhabditis elegans using an endotoxin-mediated paralysis. Cell Microbiol, 3(6), 381–393. doi: 10.1046/j.1462-5822.2001.00118.x [DOI] [PubMed] [Google Scholar]
- Ott L, McKenzie A, Baltazar MT, Britting S, Bischof A, Burkovski A, & Hoskisson PA (2012). Evaluation of invertebrate infection models for pathogenic corynebacteria. FEMS Immunol Med Microbiol, 65(3), 413–421. doi: 10.1111/j.1574-695X.2012.00963.x [DOI] [PubMed] [Google Scholar]
- Peixoto RS, Rosado PM, Leite DC, Rosado AS, & Bourne DG (2017). Beneficial Microorganisms for Corals (BMC): Proposed Mechanisms for Coral Health and Resilience. Front Microbiol, 8, 341. doi: 10.3389/fmicb.2017.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon-Robinson ME, Osipiuk J, Jooya N, Chang C, Joachimiak A, Das A, & Ton-That H (2015). A thiol-disulfide oxidoreductase of the Gram-positive pathogen Corynebacterium diphtheriae is essential for viability, pilus assembly, toxin production and virulence. Mol Microbiol, 98(6), 1037–1050. doi: 10.1111/mmi.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes O, Guyonvarch A, Bonamy C, Salti V, David F, & Leblon G (1991). ‘Integron’-bearing vectors: a method suitable for stable chromosomal integration in highly restrictive corynebacteria. Gene, 107(1), 61–68. doi: 10.1016/0378-1119(91)90297-o [DOI] [PubMed] [Google Scholar]
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, … Kohno K (2001). Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol, 19(8), 746–750. doi: 10.1038/90795 [DOI] [PubMed] [Google Scholar]
- Sifri CD, Begun J, & Ausubel FM (2005). The worm has turned--microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol, 13(3), 119–127. doi:S0966-842X(05)00025-9 [pii] 10.1016/j.tim.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Stuelten CH, Parent CA, & Montell DJ (2018). Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat Rev Cancer, 18(5), 296–312. doi: 10.1038/nrc.2018.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MW, Mahajan-Miklos S, & Ausubel FM (1999). Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A, 96(2), 715–720. doi: 10.1073/pnas.96.2.715 [DOI] [PMC free article] [PubMed] [Google Scholar]