Abstract
Keloid scar formation arises from a disorganized fibro-proliferative collagen response that extends beyond the original wound margins due to excessive production of extracellular matrix. Despite treatment options for keloid scars including medical and surgical therapies such as intralesional steroid injection and surgical excision, the recurrence rate remains high. Herein we consolidate recently published narrative reviews, systematic reviews, and meta-analyses to provide an overview of updated treatment recommendations for keloidal scar formation. PubMed access to the MEDLINE database was used to investigate updates regarding keloid incidence and treatment. More than 100 articles were reviewed. Keloid management remains a multimodal approach. There continues to be no gold standard of treatment that provides a consistently low recurrence rate; however the increasing number of available treatments and synergistic combinations of these treatments (i.e., laser-based devices in combination with intralesional steroids, or 5-fluorouracil in combination with steroid therapy) is showing favorable results. Future studies could target the efficacy of novel treatment modalities (i.e., autologous fat grafting or stem cell-based therapies) for keloid management. This review article provides updated treatment guidelines for keloids and discusses insight into management to assist patient-focused, evidence-based clinical decision-making.
Introduction
Keloid, meaning “crab’s claw,” was derived from Greek to describe its characteristic clinical presentation.1–3 Historically, the earliest-known keloid scarring was reported around 1700 CE Egypt in the Smith Papyrus.4 The term was first introduced into modern medical literature in 1814.5 Later that century, a medical textbook published, “In regards to treatment, we are almost helpless. It is pretty certain to reappear after excision, even though the incisions be carried far into the healthy skin”.6 Today, despite various treatment options, keloid scarring continues to escape the normal process of wound healing and remains recalcitrant.7,8
From a clinical perspective, keloids appear as elevated, firm bosselated papules and ill-defined plaques accompanied by variation in color, including erythematous, violaceous, or brown pigmentation.9 In contrast to normal and hypertrophic scarring, keloids extend beyond the borders of original injury and fail to regress.8,9 Given their composition of haphazardly branched and septal disorganized type I and III collagen bundles, keloids are often symptomatic with accompanying pain and pruritus.10 Following disruption of skin integrity resulting from superficial and deep injuries, keloids can form within months to years later,9 causing cosmetic deformation, functional impairment, psychological distress, and poor quality of life.10,11 A variety of epidermal to dermal insults are implicated including iatrogenic surgical incisions, burns, trauma wounds, body piercings, insect bites, folliculitis, chickenpox, herpes zoster infection, vaccinations, and acne.3 Keloid formation has also been observed following facial dermabrasion in patients on isotretinoin therapy.12 Indeed, apart from the hairless tissue of palms and soles, keloid scar distribution occurs without topographic discrimination, including on the cornea.13
Due to the complexity and mechanical forces at work during the wound healing process, the exact pathophysiology of keloid formation is still undetermined. Risk factors include personal or family history of keloids, skin of color ethnic groups, pregnancy, puberty and skin injuries overlying osteogenic surfaces.14 The incidence of keloid scaring in the Hispanic and African American population is 4.5 to 16%.15 In African Americans, there is a significant association between keloid scars, obesity, and hypertension.16,17 In a study examining systemic medical conditions and keloid formation, obesity was present in 28.57% of the keloid population as compared to 10.98% for the general population (P < 0.001).17 Hypertension was present in 44.29% of the keloid population as compared to 15.75% of the general population (P < 0.001).2
Despite a firm understanding of the risk factors for keloid formation, the lack of animal models limits investigational studies into the precise mechanism of keloid formation.18 The wound healing process that leads to tissue repair and regeneration proceeds in four time sensitive phases: (1) hemostasis, (2) inflammation, (3) proliferation, and (4) remodeling.19 The early phase of wound healing leads to the recruitment of inflammatory cells, epithelial cells and fibroblasts, which relocate into the wound matrix and contribute to scar remodeling. Specifically, fibroblasts and myofibroblasts create a collagen-containing extracellular matrix (ECM) that is in a delicate balance of synthesis and degradation.20 An imbalance associated with collagen production and ECM degradation therefore contributes to scar formation.20 A pro-inflammatory microenvironment triggered by dysregulated levels of three TGF-β isoforms (TGF-β1, TGF-β2, TGF-β3) and other cytokines secreted by the type 2 T-helper cell (Th2) immune response (IL-4, IL-5, IL-10, IL-13) has been postulated to play a role in keloid formation.10,21 Furthermore, increased elastin and fibrillin-1 expression has been noted in scar elasticity.22 Recent studies suggest that keloid fibroblasts displayed different actin filament stiffness and force generation as compared to normal fibroblasts, which may delineate keloid extension beyond original wound margin.2,23 Keloid fibroblasts also produce excess ECM when grown on a stiff substrate,2 partly informing the increased risk of keloid formation in high-tension body surfaces, such as chest and upper back.2
Despite the fact that keloid formation is classified as a benign dermal growth, it can behave like a malignant tumor in regards to invasion and hyper-proliferation. In contrast to a keloid, a hypertrophic scar behaves differently and contains mainly type III collagen arranged parallel to an epidermal surface. Studies suggest a greater genetic predisposition in keloids compared to hypertrophic scars.24 Four single-nucleotide polymorphisms (SNPs) across three loci on chromosome bands 1q41, 3q22.3–23, and 15q21.3 associated with keloid pathogenesis have been identified, although the mechanisms by which these SNPs contribute to keloid formation is poorly understood.25 MicroRNA-21 signaling pathways also contribute to keloid formation.24 Furthermore, epigenetic signaling involving histone modification, regulatory RNA alterations, and DNA methylation are also implicated in keloid pathogenesis.26
Keloidal pathophysiology therefore remains multimodal and complex. There are a wide range of therapies used in treating keloids with various degrees of success graded by time to recurrence. Keloid treatment can be classified into medical and surgical interventions as well as a combination including topical agents, intralesional injections, radiation and laser therapy.27, 28 Numerous clinical trials have reported the effectiveness of various treatments on keloid scarring. Yet variability in quality and other limitations render it difficult to ascertain intra-trial comparison. To date, the gold standard of treatment continues to vary across academic institutions and independent private practitioners.7 Herein, we consolidate recently published narrative reviews, systematic reviews, and meta-analyses to provide an overview of updated treatment recommendations for keloidal scar formation.
Methods
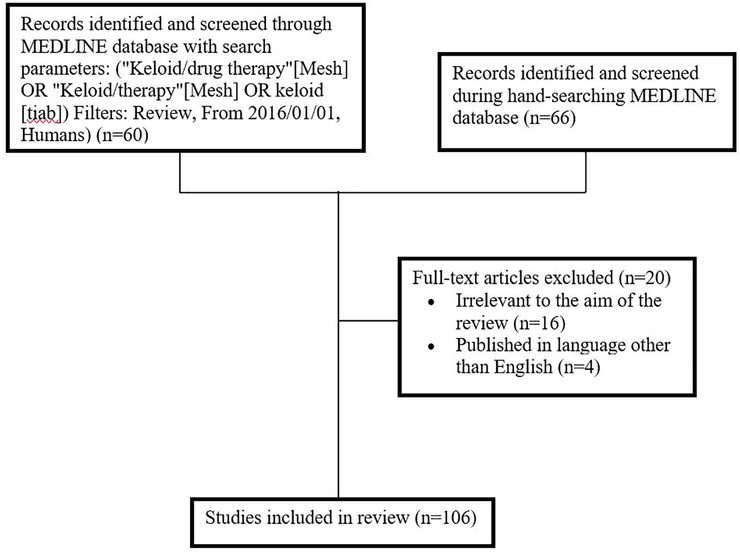
This review explores current therapies available to treat keloids. The PubMed search engine was used to access the MEDLINE database in order to search for studies pertaining to keloids. The following search phrases were used to refine results: “Keloid/drug therapy” OR “Keloid/therapy” OR “keloid”. The article type was limited to review, publication dates were limited to January 1, 2016, to January 1, 2019, and species was limited to human. A total of 60 articles were identified with the above search criteria. Screening based on English language (n=4) and relevance (n=16) excluded 20 articles (Figure 1).
Figure 1.
Flowchart of study identification in literature review.
Results
I. Medical Therapies
Triamcinolone acetonide
Triamcinolone acetonide (TAC; Kenalog), an intralesional corticosteroid injection, remains the most commonly used, first-line choice treatment for keloid scarring.29 Corticosteroids affect multiple key pathways in the formation of keloids by decreasing inflammation during the wound healing process.29 It also suppresses collagen and glycosaminoglycan synthesis, reduces fibroblast development and augments collagen degradation.29 This treatment can be used as a monotherapy on a mature keloid or as an adjuvant to surgical excision or laser therapy. Response to TAC injections varies widely with a reported 50% to 100% regression.30 One year post- treatment, the recurrence rate is an estimated 33%.30 After 5 years, the recurrence rate increases to 50%.30 TAC is injected at a dosage of 10 to 40 mg/ml into the mid-dermis every 4 to 6 weeks until the scar has resolved.29 Side effects include skin atrophy and hypopigmentation.
Triamcinolone acetonide in combination with 5-Fluorouracil
The addition of 5-fluorouracil (5-FU) to TAC is postulated to lower the side effect profile due to a decreased dose requirement of each agent. Several studies have reported on this combination treatment, however, the number of reported cases is low.31 Ren et al. report the effectiveness of intralesional TAC alone compared to TAC in combination with 5-FU in a systematic review and meta-analysis.31 While hypertrophic scars were not analyzed separately from keloid scars, the combination of TAC and 5-FU was more effective than TAC alone. Effectiveness was measured in terms of patient assessment after treatment (odds ratio (OR), 2.92; 95% CI, 1.63 to 5.22, P<0.001), observer assessment following treatment (OR, 4.03; 95% CI, 1.40 to 11.61; P<0.01), scar height after treatment (mean differences (MD), −0.14; 95% CI, −0.23 to –0.05; P<0.01), and erythema score (MD, −0.20; 95% CI, −0.34 to –0.06; P<0.01).31 Data from Alexandrescu et al. support these findings.32
Mitomycin C
Mitomycin C (MMC), a derivative of Streptomyces caespitosus, is an antibiotic agent with anti-neoplastic and anti-proliferative activities, which has been used as a topical agent following keloid surgical excision. By inhibiting DNA, RNA and protein synthesis, MMC prevents cell division and fibroblast proliferation.27 In a meta-analysis, Shin and colleagues determined the recurrence rate for topical MMC to be 16.5% (95% CI, 7.9 to 31.1). Treatment consisted of 1 mg/mL MMC applied to the surgical wound for 3–5 minutes every 3 weeks.27 No adverse effects at the 1 mg/mL dose were noted.
Bleomycin
Bleomycin is a cytotoxic agent that induces sclerosis and is commonly used in the treatment of various malignancies.33 The first reported use of this intralesional treatment for keloids was in 1996 and achieved a 47% remission rate.30 Illustrating the difficulty of assessing treatment efficacy, subsequent studies report widely varying degrees of recurrence. The lowest recurrence rate noted for this treatment is 0% at a mean duration of 19 months follow up.34 However, another group reported recurrence rates in a Vietnamese population of 3.8%, 15.4%, 45.5% and 50% at 6, 12, 15 and 18 months follow-up, respectively.35 The wide range of recurrence rates based on follow-up time is also seen in intralesional TAC treatment as discussed previously. In a study comparing intralesional bleomycin to TAC in patients with Fitzpatrick skin types III to V, both treatments were comparable with no significant difference in efficacy between the two groups.36 However, there was a high rate of bleomycin induced hyperpigmentation (71.4%).36 A meta-analysis to standardize the efficacy of intralesional bleomycin is warranted.
Imiquimod
Imiquimod 5% cream (Aldara) is a topical immunomodulatory treatment, which increases expression of tissue necrotic factor alpha (TNF-α), gamma and alpha interferons (IFN-γ and α), and interleukin 1, 6, 8, 12. It also acts as a Toll-like receptor (TLR) agonist. A meta-analysis of topical 5% imiquimod cream applied for 6–8 weeks post-keloid excision showed that the rate of recurrence was 24.7% (95% CI, 3.2 to 76.4); variable outcomes were reported.27
Botulinum A
Use of botulinum toxin-A (BoNT-A) in keloid treatment is based on its ability to reduce muscle tension and thereby wound tension.37 BoNT-A has been reported to decrease TGF-β expression, reduce fibroblast proliferation and alter collagen activity during pathologic scar formation.38 A meta-analysis of treating keloids with BoNT-A remains to be conducted, however, Schlessinger and colleagues reviewed several small studies that have been performed to date.39 Efficacy of BoNT-A in keloid treatment is not definitive with studies showing mixed results.39 Interestingly, a meta-analysis of randomized controlled trials has been conducted on hypertrophic scars in the maxillofacial area and neck, which showed a statistically significant difference in scar width, patient satisfaction and visual analysis scores in the treatment of hypertrophic scars.40 While BoNT-A has been reported to be effective in hypertrophic scar prevention, its use in keloid treatment remains uncertain.
Interferons
Immune-response modifiers, interferon alpha and gamma (IFN-α and IFN-γ), are observed at decreased concentrations in keloids.41 Interferons exert anti-viral, anti-proliferative and anti-fibrotic properties.42 Indeed, IFN-γ and IFN-α antagonize TGF-β stimulated collagen metabolism in vitro.43 Treating keloids with intralesional interferon injections have had varying levels of success with adverse reactions, including systemic flu-like symptoms, pain at the injection site, edema and erythema reported.42,44 IFN-α−2b did not demonstrate efficacy in treating keloids.45 Al-Khawajah et al. reported that cases that withdrew due to local pain (n=7 out of 22 patients) during injection and due to severe systemic symptoms following the first injection (n=2 out of 22 patients).45 Other studies demonstrate more favorable results. Berman et al. found that intralesional IFN-α−2b injections into a keloid resulted in a 41% reduction in area.46 In a larger study (n=124) on interferon injections following keloid excision, IFN-α−2b demonstrated a lower recurrence rate (18.7%) compared to TAC (58.5%) and excision alone (51.2%).47
Onion extract (Allium cepa)
Onion extract (Allium cepa) has been shown to significantly improve dermal collagen organization in animal model scars.48 Additionally, the derivative of Allium cepa, quercetin, displays anti-proliferative and anti-histamine effects.48 The majority of trials to date tested this treatment on non-keloidal scars. In a study by Wananukul and colleagues, 10% onion extract in silicone derivative gel significantly decreased the incidence of hypertrophic scar formation from median sternotomy.49 However, no significant difference in keloid incidence between treatment and placebo groups was observed.49 The use of onion extract may be better suited for combination therapy. When intralesional TAC alone was compared to TAC with onion extract to treat keloid and hypertrophic scars, the TAC with onion extract group demonstrated a statistically significant improvement in pain-sensitiveness, pruritus, and elevation at week 20.50 Although, no significant difference in erythema or induration was seen between treatment groups.50 An additional study examining keloid and hypertrophic scars compared the following three treatment arms: onion extract alone, silicone gel sheet alone, and combination onion extract and silicon sheet.51 While onion extract alone was more effective in improving scar color, the silicone gel sheet was more effective in reducing scar height.51 The combination of these treatments provided the best response.51
Laser-based devices
Forbat and colleagues reviewed the effectiveness of light-, laser-, and energy-based devices in the management of keloid scars.28 Laser-based devices can be divided into ablative and non-ablative categories. Ablative lasers remove the epidermal layer and include erbium-doped yttrium aluminium garnet (Er: YAG) and carbon dioxide (CO2) lasers. Non-ablative lasers target the dermis and include potassium titanyl phosphate (KTP), pulsed dye (PDL) and neodymium-doped yttrium garnet (Nd:YAG) lasers.28,52 For CO2 laser treatments, keloid recurrence was noted between 2 weeks and 3 years post-laser. Treatment with the Er:YAG laser resulted in a 22% recurrence rate at 8 months post-laser. Nd:YAG laser exhibited recurrence rates that differed based on keloid site at 6 months post-laser; 52.9% recurrence for anterior chest, 35.7% recurrence for upper arms and 25% for scapula keloids. While this data is promising for the use of multiple laser sub-categories, additional randomized controlled trials are warranted to determine the effectiveness. Khansa et al. reported a similar conclusion that laser-based devices produced variable results.53 Higher Fitzpatrick skin types are at a greater risk of adverse effects from laser therapy, and thus the efficacy of laser-based devices to treat keloids in skin of color ethnic groups is not widely studied.28 In one study evaluating the 1064 nm Nd:YAG laser on patients with Fitzpatrick skin types I to VI, post-inflammatory pigmentation changes were not observed.54 The Nd:YAG laser may therefore be an acceptable option for patients with darker skin types IV to VI.54
Laser-based devices in combination with TAC
Pulsed-dye laser (PDL) is a non-ablative laser that can attenuate keloidal pain and pruritus53 but is limited in its ability to decrease scar size. PDL alone appears to downregulate TGF-β1 expression with no impact on type I collagen formation.53,55 Therefore, PDL in combination with TAC may provide a synergistic effect as corticosteroids also contribute to reducing collagen synthesis and increasing collagen degradation.29,53 Combination of these modalities yielded a 60% improvement in height, 40% improvement in erythema and 75% improvement in pruritus; however the sample size was small (n=7).56
Improved results with the CO2 and Nd:YAG lasers in combination with TAC have also been noted.30 Specifically, Stucker et al. demonstrated that intralesional TAC halts early recurrences of keloids treated with the CO2 laser.57 Another study using CO2 laser with intralesional TAC over 6 months noted a significant increase in recurrence of keloids in patients who did not follow-up regularly for TAC injections.58 Kumar et al. noted an additive effect of TAC and Nd:YAG laser; keloids that persisted following Nd:YAG laser therapy were subsequently treated with intralesional TAC, resulting in complete resolution over 18 months to 5 years of follow-up.59
Cryotherapy
Traditional cryotherapy involves using freeze-thaw cycles to damage scar tissue. Consequent damage to the surrounding skin surface gave rise to a more targeted approach of intralesional cryotherapy in which a specialized needle probe freezes the scar from the inside.60,61 A comprehensive review of eight studies found this approach favorable in reducing scar volume, pain, and pruritis.62 However, persistent hypopigmentation in Fitzpatrick 4–6 skin types was observed and recurrence rates varied widely between 0% to 24%.62
II. Surgical Therapy
Excision
Excising keloids without secondary intervention generally results in a poor outcome. Excision alone results in a recurrence rate of greater than 50%.63 While certain wound closure techniques reduce tension at the wound site, no data supports whether a specific wound closure technique reduces the likelihood of keloid recurrence. However, a tensionless closure following excision is considered important in reducing scar hypertrophy and scar widening.64
III. Combined Medical and Surgical Therapies
Triamcinolone acetonide following excision
A recent meta-analysis reported whether triamcinolone intralesional injection following surgical excision prevented the recurrence of keloids.65 Researchers analyzed 4 studies comprising 254 patients and found that surgery combined with TAC was not effective in lowering the rate of keloid recurrence with a pooled risk difference of 0.06 (95% CI, −0.16 to 0.28; P, not significant).65 However, the location of the keloid may be associated with the success of TAC following excision. Ear keloids exhibited increased responsiveness to TAC following excision.66 In a meta-analysis examining the use of TAC following excision of ear keloids, the recurrence rate was 15.4% (95% CI, 9.4 to 24.1%; P<0.001) proving to have similar efficacy as radiotherapy following excision.66
5-Fluorouracil following excision
5-Fluorouracil (5-FU) is an anti-neoplastic pyrimidine analog that has an inhibitory effect on fibroblasts.65 Shin and Kim conducted a meta-analysis on whether 5-FU prevents keloid recurrence following surgical excision.65 Following analysis of 2 studies with 107 total patients, keloid recurrence was statistically lower in patients treated with 5-FU (risk ratio, 0.18; 95% CI, 0.04 to 0.75; P=0.02).65 Most studies injected 50 to 150 mg of 5-FU to the excision border and wound bed following keloid removal.65
Radiotherapy following excision
Use of radiotherapy for keloid treatment was first described in 1906.5 Radiotherapy disrupts the normal wound healing process and can therefore be administered directly to a mature keloid or following surgical excision to prevent keloid re-formation. Mankowski and colleagues conducted a meta-analysis systematic review on radiation-based treatments involving 72 studies and 9048 keloids.63 Their data demonstrated that post-excisional radiotherapy was more effective in preventing recurrence than radiotherapy alone (22% and 37% recurrence rate, respectively, p=0.005).63 However, radiation as a monotherapy may be recommended when treating elderly patients for whom surgical removal may not be a viable option due to location or size. Additionally, radiotherapy applied to mature keloid has been reported to reduce pain and pruritus.67 In comparing radiation modalities, post-operative brachytherapy had the lowest recurrence rate of 15%, compared to a 23% recurrence rate for x-ray and 23% recurrence rate for electron beam radiation.63 In a separate meta-analysis examining ear keloids, radiotherapy following surgical excision proved to be effective with a recurrence rate of 14.0% (95% CI, 9.6 to 19.9%; P < 0.001).66
Furthermore, several studies have reported that excision followed by radiation is safe and practical.1,63 The most common side effect reported for radiotherapy treatment was alterations in skin pigmentation including erythema, transient hyperpigmentation, hyperpigmentation, hypopigmentation, and unspecified pigmentation changes with a total occurrence of 32.5%.63 Mankowski and colleagues also found that chest keloids have the highest recurrence rate.63
Pressure therapy following excision
Pressure therapy involves the use of specialized garments or devices, such as Zimmer splints or magnets to apply a prolonged state of pressure to the skin.68,69 It is hypothesized that the added pressure modifies wound tension and causes localized hypoxia.68,70 A meta-analysis examining trials using pressure garments to prevent scar formation in burn patients did not report a difference in global scar assessment between pressure garment treated scars and non-pressure treated scars.71 However, several observational studies using pressure therapy following surgical excision for ear keloids demonstrate favorable results with recurrence rate of 6.7% to 10.6%.68,69,72 One trial reported a 0% recurrence rate, however, a corticosteroid injection was combined with pressure therapy following surgical excision, and the study population was small (n=7).73
Topical silicone following excision
Silicone for prevention of keloids is a pragmatic form of at-home treatment. Its exact mechanism of action is yet to be elucidated; however postulated explanations include decreased skin stretching, occlusion, and hydration.7,74 While early studies of silicone gel sheeting suggested high success rates in treating keloids and hypertrophic scars, meta-analyses conclude silicone is not an effective treatment modality for the prevention of keloids.30,75,76 Hsu and colleagues reviewed the effectiveness of silicone for prevention of keloid scarring in patients with new wounds by evaluating 10 trials that were designed with a treatment and placebo arm.74 Topical silicone did not provide a significant difference in the prevention of keloids in patients who have a history of abnormal scarring.74 Furthermore, Cochrane systematic review analyzing 20 clinical trials concluded that there is weak evidence supporting the use of silicone gel sheeting as a means of prevention of abnormal scarring, however improvements in scar color and thickness were observed.77 Silicone can be applied to the skin in the form of a silicone gel sheet or a topical silicone gel for 12–24 hours per day with twice daily washing for a minimum of 1 month.78
IV. New therapeutic developments
Verapamil, a calcium channel blocker, which alters fibroblast gene expression resulting in reduced collagen synthesis and increased collagenase, has been utilized in keloidal treatment.4 Studies using intralesional verapamil following surgical excision or alone reported a wide efficacy range from 1.4 to 48% recurrence.79 Its efficacy has been reported similar to TAC injections; high-quality trials are needed to define the role of verapamil in keloid treatment.80
Ultraviolet A1 (UV-A1) phototherapy in the spectral range of 340–400 nm induces increased collagenase activity.4 Evidence supports its use for fibrosing disorders, including localized scleroderma, lichen sclerosus et atrophicus and graft-versus-host disease.81 Only few studies to date have tested UV-A1 phototherapy in treating keloids in a total of 6 patients with mixed results.82–84
Other agents that may have a potential role in keloid treatment include tamoxifen and calmodulin inhibitors. Tamoxifen citrate is a non-steroidal anti-estrogen that downregulates TFG-β, fibroblast and collagen expression.85 Similarly, calmodulin inhibitors may also result in scar degradation and warrant further study.4
Angiotensin-converting enzyme inhibitors (ACEIs) affects wound healing by reducing collagen synthesis, TGF-β1 expression, and fibroblast proliferation.86 Topical enalapril significantly reduced the mean size of hypertrophic scars in a double-blinded clinical trial.87 One case report observed improvement in keloid scarring following oral enalapril.88 Topical captopril used in a separate case report on a post-burn keloid demonstrated improvement in the lesion and reduced redness, scaling, and itchiness.89
A new alternative to topical silicone is a combination of silicone oil with hypochlorous acid (HOCL); it is a gel or spray that can be applied twice daily.90 HOCL has an antimicrobial, antipruritic and anti-inflammatory role by increasing oxygenation and disrupting biofilm formation.90 Anecdotal reports suggest that HOCL gel performed better than 100% silicone gel in the management of keloid and hypertrophic scars.90
Apligraf® is a living, human, bi-layered skin substitute that promotes wound healing.91 FDA approved its use for venous leg ulcers and diabetic foot ulcers.91 The potential of using neodermal products for restructuring keloids continues to be an area of exploration with few case reports available in the literature and a pilot study with a small number of participants.92
Mammalian target of rapamycin (mTOR) is a potential therapeutic target for keloids.93 Research suggests mTOR plays a role in the regulation of collagen expression and decreases ECM deposition when inhibited.93 It is therefore postulated targeting mTOR with rapamycin therapy may block excess fibroproliferation leading to abnormal scarring.94 Indeed, rapamycin treatment of human fibroblasts in vitro blocks collagen synthesis pathways that are significantly increased in keloid scarring.94
TGF-β1, TGF-β2 and TGF-β3 isoforms appear to have interrelated roles in keloid pathogenesis.95 The TGF-β3 isoform in particular has been studied in clinical trials.95 Intradermal avotermin (recombinant TGF-β3) was administered prophylactically to improve scarring in three double-blind, placebo-controlled, phase I/II studies.96 However, it appears the phase 3 clinical trial in 2011 did not accomplish its endpoints leading the company to conclude avotermin may not provide significant benefit for scar revision.97 Efficacy of targeting other TGF-β isoforms remains to be further investigated.95
miRNAs (microRNA) are non-coding RNAs that silence genes at the post-transcriptional level.98 The expression of miRNAs in keloidal fibroblasts are expressed at different concentrations compared to normal fibroblasts.99 A growing body of research suggests specific miRNAs, such as miRNA-29 and miRNA-21–5p, appear to play key roles in keloid development providing additional targets for novel therapeutics.100,101
Discussion
Understanding fibro-proliferative process of keloid pathogenesis and achieving scar resolution through treatment modalities has behooved clinical investigators for decades. Herein we have categorized treatment options into medical, surgical or combination therapies. In this review, excision alone was the least effective method of treatment (Table 1). While TAC is a current mainstay of keloid management, it is not effective in lowering the rate of recurrence when administered following excision.65 However, effectiveness may depend on the location of the keloid as excision followed by TAC was effective for ear keloids.66 Anatomical location also influences the responsiveness to laser-based devices and pressure therapy. TAC appears to be effective in the management of mature keloids, however, repeated injections may be needed as the recurrence rate increased 27% from year 1 post-treatment to year 5 post-treatment30.
Table 1.
Efficacy of medical and surgical treatments on keloid scars
| Category | Treatment | Mean Recurrence Rate (%) | Mean Follow-up (months) | References |
|---|---|---|---|---|
| Medical | Triamcinolone acetonide | 33 | 12 | Morelli Coppola et al, 2018 30 |
| 50 | 60 | Morelli Coppola et al, 2018 30 | ||
| Er:YAG laser | 22 | 8 | Forbat et al, 2017 28 | |
| Nd:YAG laser | 25 to 52.9 | 6 | Rossi et al, 2013 42 | |
| Mitomycin C | 16.5 | ≥6 | Shin et al, 2017 27 Rossi et al, 2013 42 |
|
| Imiquimod | 24.7 | ≥6 | Shin et al, 2017 27 | |
| Bleomycin | 0 to 50 | 6 to 19 | Morelli Coppola et al, 2018 30 Saray et al, 2005 34 Hu et al, 2019 35 |
|
| Intralesional cryotherapy | 0 to 24 | 6 to 21.5 | van Leeuwen et al, 2015 51 | |
| Surgical | Excision | >50 | 6 to 12 | Mankowski et al, 2017 52 |
| Combined (Medical and Surgical) | Triamcinolone acetonide after excision | 15.4 | 12 to 35 | Shin et al, 2016 54, 55 |
| Brachytherapy after excision | 15 | 14.4 | Mankowski et al, 2017 52 | |
| X-ray after excision | 23 | 14.4 | Mankowski et al, 2017 52 | |
| Electron beam after excision | 23 | 14.4 | Mankowski et al, 2017 52 | |
| Pressure therapy after excision | 6.7 to 10.6 | 18 | Park et al, 2011 57 Park et al, 2013 61 |
Er:YAG, erbium-doped yttrium aluminium garnet; Nd:YAG, neodymium-doped yttrium garnet
Chemotherapeutics and immunomodulatory agents, 5-fluorouracil, mitomycin C, imiquimod, and bleomycin are each effective for keloid management (Table 1). Yet long term follow-up to 5 years post-treatment is lacking. Mitomycin C may reduce recurrence rates compared to imiquimod. In the meta-analysis conducted comparing mitomycin C to imiquimod, mitomycin C proved to have greater efficacy in terms of recurrence rates.27 Furthermore, TAC in combination with 5-fluorouracil was more effective in reducing hypertrophic and keloid scar recurrence compared to TAC alone.31,32 One mechanism could involve 5-fluorouracil inhibiting the expression of type I collagen gene that is induced by TGF-β; thereby inhibiting excess collagen synthesis similar to corticosteroids.102
Laser-based devices and forms of radiotherapy have also been effective in the treatment of keloids (Table 1). Efficacy varied by laser type. CO2 laser is least effective, while the Er:YAG laser resulted in a 22% keloid recurrence rate.28 The Nd:YAG laser shows varying results based on keloid location with a recurrence rate ranging from 25% for scapular keloids to 52.9% for keloids located on the anterior chest.28 Radiotherapy showed promising results following excision. Recurrence rates varied based on radiation type with electron beam and x-ray resulting in a 23% recurrence rate and brachytherapy resulting in a 15% recurrence rate.1,63,67 (Table 1)
Future areas of interest include the use of stem cells in preventing keloid formation. Certain stem cell types such as mesenchymal stem cells and umbilical cord blood stem cells can provide antioxidant effects and reduce inflammation during the wound healing process.103 However, their translation into clinical application continues to be limited due to unresolved concerns related to safety, potential tumorigenicity and effects on fibroblasts.103 Autologous fat grafting is another method that is being tested due to the lack of adequate results with the current treatments available.104 Silva and colleagues reviewed the studies of this treatment for hypertrophic scars and keloids.104 Due to the limited number of studies and hypertrophic scars being grouped with keloids, more data is needed to determine the efficacy of this therapy.
Conclusion
As keloid pathogenesis becomes better understood, additional mechanistic targets will be elucidated paving the way for more effective treatments. Further research into tumor growth and metastasis may provide further insight into keloid pathogenesis. Similarities between keloid scar formation, tumor growth, and metastasis have been observed, including over-expression of collagen triple helix repeat containing-1 (CTHRC1), fibroblast activation protein alpha (FAP-α), and dipeptidyl peptidase IV (DPPIV).105,106 For now, keloid management remains a multimodal approach. There continues to be no single treatment available that provides a consistently low recurrence rate. An increasing number of studies are examining the combination of existing treatments for a synergistic effect. This includes laser-based devices in combination with TAC, and 5-FU in combination with TAC. These combinations are providing favorable outcomes when compared to monotherapy.
Based on the most recent evidence, brachytherapy following excision provides the lowest rate of recurrence. For ear keloids, specifically, pressure therapy following excision is the most efficacious based on observational studies. However, in clinical practice, the patient and physician may opt to a minimally invasive, cost-effective treatment initially. In these circumstances, multiple TAC injections may be first line, followed by combination therapies if adequate results with TAC alone are not achieved. Treatment option may be limited to physician preference and resources available. Radiotherapy is limited to larger, well-funded medical establishments. Low cost and minimally invasive TAC injections may explain why TAC, while less effective than other modalities, is the most common form of treatment today.
There continues to be a need for randomized studies with larger sample sizes and longer follow-up time. Robust keloid patient registries could help assess additional risk factors, systemic medical conditions associated with keloid formation and effectiveness of various treatments. As discoveries in keloid pathogenesis unfold, targeted treatment can be designed to halt mechanistic checkpoints implicated in this type of scar formation. Keloids continue to have no gold standard of treatment; however the increasing number of treatments available and synergistic combinations of these treatments is showing favorable results. Future studies could target the efficacy of novel treatment modalities and the use of combination therapies for the management of keloids.
- The following is true about keloids:
- Keloids are benign fibroproliferative skin tumors growing beyond the site of original dermal injury
- Keloids are malignant fibroproliferative skin tumors growing beyond the site of original dermal injury
- Keloids are benign fibroproliferative skin tumors limited to the site of original dermal injury with high rates of recurrence
- Keloids are benign fibroproliferative skin tumors limited to the site of original dermal injury with low rates of recurrence
True or false: Given their composition of haphazardly branched and septal disorganized type I and III collagen bundles, keloids are often symptomatic with accompanying pain and pruritus.
- Which of the following is considered to be a risk factor associated with keloids?
- Personal or family history of keloids
- Skin of color ethnic groups
- Pregnancy
- Puberty
- Skin injuries overlying osteogenic surfaces
- All of the above
True or false: Pro-inflammatory microenvironment triggered by excess TGF-β and elevated production of certain cytokines has been postulated to play a role in keloid formation.
- What is the correct order of four time sensitive wound healing phases?
- (1) Inflammation (2) Hemostasis (3) Proliferation (4) Remodeling
- (1) Inflammation (2) Hemostasis (3) Remodeling (4) Proliferation
- (1) Hemostasis (2) Inflammation (3) Proliferation (4) Remodeling
- (1) Hemostasis (2) Inflammation (3) Remodeling (4) Proliferation
- How do corticosteroids (i.e. intralesional triamcinolone) affect keloid formation?
- Decrease inflammation during would healing process
- Increases collagen and glycosaminoglycan synthesis
- Reduces fibroblast development
- Augments collagen degradation
- A, C and D are correct
- A, B and C are correct
- Which of the following lasers is a potential option for keloid treatment in patients with darker skin types (Fitzpatrick IV to VI)?
- Ablative erbium-doped yttrium aluminium garnet laser (Er:YAG)
- Ablative yttrium-scandium-gallium-garnet laser (YSGG)
- Ablative carbon dioxide laser (CO2)
- Non-ablative neodymium-doped yttrium garnet laser (Nd:YAG)
True or false: Excising keloids without secondary intervention generally results in a good outcome with minimal recurrence rate.
- Recent studies have proposed that mesenchymal stem cells and umbilical cord blood stem cells affect wound healing and reduce scar formation. What is the proposed mechanism?
- Reduces antioxidant and anti-inflammatory effects during wound healing
- Improves antioxidant and anti-inflammatory effects during wound healing
- It is unknown
True or false: There continues to be no gold standard of keloid treatment that provides a consistently low recurrence rate.
Answers
1. A 6. E
2. True 7. D
3. F 8. False
4. True 9. B
5. C 10. True
Acknowledgments
Funding: This work was supported by the National Cancer Institute; grant CA215105.
Footnotes
Disclosures: The authors do not have any conflicts of interest and/or relevant financial relationships relevant to the work presented in this article.
References
- 1.Xu J, Yang E, Yu NZ, Long X. Radiation Therapy in Keloids Treatment: History, Strategy, Effectiveness, and Complication. Chinese medical journal. July 20 2017;130(14):1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu CK, Lin HH, Harn HI, Hughes MW, Tang MJ, Yang CC. Mechanical forces in skin disorders. Journal of dermatological science. June 2018;90(3):232–240. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa R Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. International journal of molecular sciences. March 10 2017;18(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mari W, Alsabri SG, Tabal N, Younes S, Sherif A, Simman R. Novel Insights on Understanding of Keloid Scar: Article Review. The journal of the American College of Clinical Wound Specialists. December 2015;7(1–3):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig RD, Pearson D. Early post-operative irradiation in the treatment of keloid scars. British journal of plastic surgery. October 1965;18(4):369–376. [DOI] [PubMed] [Google Scholar]
- 6.Morrow A A System of Genito-urinary Diseases, Syphilology and Dermatology: Appleton; 1894. [Google Scholar]
- 7.Del Toro D, Dedhia R, Tollefson TT. Advances in scar management: prevention and management of hypertrophic scars and keloids. Current opinion in otolaryngology & head and neck surgery. August 2016;24(4):322–329. [DOI] [PubMed] [Google Scholar]
- 8.Insalaco L, Saxon S, Spiegel JH. What is the role of intralesional corticosteroid injections for keloids before considering surgery? The Laryngoscope. March 2016;126(3):549–550. [DOI] [PubMed] [Google Scholar]
- 9.Trace AP, Enos CW, Mantel A, Harvey VM. Keloids and Hypertrophic Scars: A Spectrum of Clinical Challenges. American journal of clinical dermatology. June 2016;17(3):201–223. [DOI] [PubMed] [Google Scholar]
- 10.Ghazawi FM, Zargham R, Gilardino MS, Sasseville D, Jafarian F. Insights into the Pathophysiology of Hypertrophic Scars and Keloids: How Do They Differ? Advances in skin & wound care. January 2018;31(1):582–595. [DOI] [PubMed] [Google Scholar]
- 11.Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Archives of dermatological research. April 2006;297(10):433–438. [DOI] [PubMed] [Google Scholar]
- 12.Heppt MV, Kirchberger MC, Ruzicka T, Berking C, Heppt WJ. Indications and Use of Isotretinoin in Facial Plastic Surgery. Facial plastic surgery : FPS. February 2018;34(1):75–81. [DOI] [PubMed] [Google Scholar]
- 13.Gupta J, Gantyala SP, Kashyap S, Tandon R. Diagnosis, Management, and Histopathological Characteristics of Corneal Keloid: A Case Series and Literature Review. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). Sep-Oct 2016;5(5):354–359. [DOI] [PubMed] [Google Scholar]
- 14.Ferri FF. Ferri’s Clinical Advisor 2019. 1 ed: Elsevier; 2019. [Google Scholar]
- 15.Alster TS, Tanzi EL. Hypertrophic scars and keloids: etiology and management. American journal of clinical dermatology. 2003;4(4):235–243. [DOI] [PubMed] [Google Scholar]
- 16.Hinojosa JA, Pandya AG. The Importance of Patient Registries in Skin of Color. The journal of investigative dermatology. Symposium proceedings. October 2017;18(2):S31–s33. [DOI] [PubMed] [Google Scholar]
- 17.Adotama P, Rutherford A, Glass DA, 2nd. Association of keloids with systemic medical conditions: a retrospective analysis. International journal of dermatology. January 2016;55(1):e38–40. [DOI] [PubMed] [Google Scholar]
- 18.Marttala J, Andrews JP, Rosenbloom J, Uitto J. Keloids: Animal models and pathologic equivalents to study tissue fibrosis. Matrix biology : journal of the International Society for Matrix Biology. April 2016;51:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Frontiers in bioscience : a journal and virtual library. January 1 2004;9:283–289. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Jang YJ. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. International journal of molecular sciences. March 2 2018;19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiritsi D, Nystrom A. The role of TGFbeta in wound healing pathologies. Mechanisms of ageing and development. June 2018;172:51–58. [DOI] [PubMed] [Google Scholar]
- 22.Cohen BE, Geronemus RG, McDaniel DH, Brauer JA. The Role of Elastic Fibers in Scar Formation and Treatment. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. January 2017;43 Suppl 1:S19–s24. [DOI] [PubMed] [Google Scholar]
- 23.Harn HI, Wang YK, Hsu CK, et al. Mechanical coupling of cytoskeletal elasticity and force generation is crucial for understanding the migrating nature of keloid fibroblasts. Experimental dermatology. August 2015;24(8):579–584. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Zhang J, Lei Y, Lyu L, Zuo R, Chen T. MicroRNA-21 in Skin Fibrosis: Potential for Diagnosis and Treatment. Molecular diagnosis & therapy. December 2017;21(6):633–642. [DOI] [PubMed] [Google Scholar]
- 25.Glass DA, 2nd. Current Understanding of the Genetic Causes of Keloid Formation. The journal of investigative dermatology. Symposium proceedings. October 2017;18(2):S50–s53. [DOI] [PubMed] [Google Scholar]
- 26.He Y, Deng Z, Alghamdi M, Lu L, Fear MW, He L. From genetics to epigenetics: new insights into keloid scarring. Cell proliferation. April 2017;50(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin JY, Yun SK, Roh SG, Lee NH, Yang KM. Efficacy of 2 Representative Topical Agents to Prevent Keloid Recurrence After Surgical Excision. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. February 2017;75(2):401.e401–401.e406. [DOI] [PubMed] [Google Scholar]
- 28.Forbat E, Ali FR, Al-Niaimi F. Treatment of keloid scars using light-, laser- and energy-based devices: a contemporary review of the literature. Lasers in medical science. December 2017;32(9):2145–2154. [DOI] [PubMed] [Google Scholar]
- 29.Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloids: The paradigm of skin fibrosis - Pathomechanisms and treatment. Matrix biology : journal of the International Society for Matrix Biology. April 2016;51:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morelli Coppola M, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clinical, cosmetic and investigational dermatology. 2018;11:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren Y, Zhou X, Wei Z, Lin W, Fan B, Feng S. Efficacy and safety of triamcinolone acetonide alone and in combination with 5-fluorouracil for treating hypertrophic scars and keloids: a systematic review and meta-analysis. International wound journal. June 2017;14(3):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexandrescu D, Fabi S, Yeh LC, Fitzpatrick RE, Goldman MP. Comparative Results in Treatment of Keloids With Intralesional 5-FU/Kenalog, 5-FU/Verapamil, Enalapril Alone,Verapamil Alone, and Laser: A Case Report and Review of the Literature. Journal of drugs in dermatology : JDD. November 1 2016;15(11):1442–1447. [PubMed] [Google Scholar]
- 33.Horbach SE, Rigter IM, Smitt JH, Reekers JA, Spuls PI, van der Horst CM. Intralesional Bleomycin Injections for Vascular Malformations: A Systematic Review and Meta-Analysis. Plastic and reconstructive surgery. January 2016;137(1):244–256. [DOI] [PubMed] [Google Scholar]
- 34.Saray Y, Gulec AT. Treatment of keloids and hypertrophic scars with dermojet injections of bleomycin: a preliminary study. International journal of dermatology. September 2005;44(9):777–784. [DOI] [PubMed] [Google Scholar]
- 35.Huu ND, Huu SN, Thi XL, et al. Successful Treatment of Intralesional Bleomycin in Keloids of Vietnamese Population. Open Access Maced J Med Sci. 2019;7(2):298–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payapvipapong K, Niumpradit N, Piriyanand C, Buranaphalin S, Nakakes A. The treatment of keloids and hypertrophic scars with intralesional bleomycin in skin of color. Journal of cosmetic dermatology. March 2015;14(1):83–90. [DOI] [PubMed] [Google Scholar]
- 37.Cohen JL, Scuderi N. Safety and Patient Satisfaction of AbobotulinumtoxinA for Aesthetic Use: A Systematic Review. Aesthetic surgery journal. May 1 2017;37(suppl_1):S32–s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin E, Koo E, Jagdeo J. The Cellular Response of Keloids and Hypertrophic Scars to Botulinum Toxin A: A Comprehensive Literature Review. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. February 2018;44(2):149–157. [DOI] [PubMed] [Google Scholar]
- 39.Schlessinger J, Gilbert E, Cohen JL, Kaufman J. New Uses of AbobotulinumtoxinA in Aesthetics. Aesthetic surgery journal. May 1 2017;37(suppl_1):S45–s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang DZ, Liu XY, Xiao WL, Xu YX. Botulinum Toxin Type A and the Prevention of Hypertrophic Scars on the Maxillofacial Area and Neck: A Meta-Analysis of Randomized Controlled Trials. PloS one. 2016;11(3):e0151627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viera MH, Vivas AC, Berman B. Update on Keloid Management: Clinical and Basic Science Advances. Advances in wound care. October 2012;1(5):200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trisliana Perdanasari A, Lazzeri D, Su W, et al. Recent developments in the use of intralesional injections keloid treatment. Archives of plastic surgery. November 2014;41(6):620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tredget EE, Wang R, Shen Q, Scott PG, Ghahary A. Transforming growth factor-beta mRNA and protein in hypertrophic scar tissues and fibroblasts: antagonism by IFN-alpha and IFN-gamma in vitro and in vivo. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. February 2000;20(2):143–151. [DOI] [PubMed] [Google Scholar]
- 44.Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plastic and reconstructive surgery. August 2002;110(2):560–571. [DOI] [PubMed] [Google Scholar]
- 45.al-Khawajah MM. Failure of interferon-alpha 2b in the treatment of mature keloids. International journal of dermatology. July 1996;35(7):515–517. [DOI] [PubMed] [Google Scholar]
- 46.Berman B, Duncan MR. Short-term keloid treatment in vivo with human interferon alfa-2b results in a selective and persistent normalization of keloidal fibroblast collagen, glycosaminoglycan, and collagenase production in vitro. Journal of the American Academy of Dermatology. October 1989;21(4 Pt 1):694–702. [DOI] [PubMed] [Google Scholar]
- 47.Berman B, Flores F. Recurrence rates of excised keloids treated with postoperative triamcinolone acetonide injections or interferon alfa-2b injections. Journal of the American Academy of Dermatology. November 1997;37(5 Pt 1):755–757. [DOI] [PubMed] [Google Scholar]
- 48.Saulis AS, Mogford JH, Mustoe TA. Effect of Mederma on hypertrophic scarring in the rabbit ear model. Plastic and reconstructive surgery. July 2002;110(1):177–183; discussion 184–176. [DOI] [PubMed] [Google Scholar]
- 49.Wananukul S, Chatpreodprai S, Peongsujarit D, Lertsapcharoen P. A prospective placebo-controlled study on the efficacy of onion extract in silicone derivative gel for the prevention of hypertrophic scar and keloid in median sternotomy wound in pediatric patients. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. November 2013;96(11):1428–1433. [PubMed] [Google Scholar]
- 50.Koc E, Arca E, Surucu B, Kurumlu Z. An open, randomized, controlled, comparative study of the combined effect of intralesional triamcinolone acetonide and onion extract gel and intralesional triamcinolone acetonide alone in the treatment of hypertrophic scars and keloids. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. November 2008;34(11):1507–1514. [DOI] [PubMed] [Google Scholar]
- 51.Hosnuter M, Payasli C, Isikdemir A, Tekerekoglu B. The effects of onion extract on hypertrophic and keloid scars. Journal of wound care. June 2007;16(6):251–254. [DOI] [PubMed] [Google Scholar]
- 52.Preissig J, Hamilton K, Markus R. Current Laser Resurfacing Technologies: A Review that Delves Beneath the Surface. Semin Plast Surg. 2012;26(3):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khansa I, Harrison B, Janis JE. Evidence-Based Scar Management: How to Improve Results with Technique and Technology. Plastic and reconstructive surgery. September 2016;138(3 Suppl):165s–178s. [DOI] [PubMed] [Google Scholar]
- 54.Rossi A, Lu R, Frey MK, Kubota T, Smith LA, Perez M. The use of the 300 microsecond 1064 nm Nd:YAG laser in the treatment of keloids. Journal of drugs in dermatology : JDD. November 2013;12(11):1256–1262. [PubMed] [Google Scholar]
- 55.Kuo YR, Wu WS, Jeng SF, et al. Suppressed TGF-beta1 expression is correlated with up-regulation of matrix metalloproteinase-13 in keloid regression after flashlamp pulsed-dye laser treatment. Lasers in surgery and medicine. January 2005;36(1):38–42. [DOI] [PubMed] [Google Scholar]
- 56.Connell PG, Harland CC. Treatment of keloid scars with pulsed dye laser and intralesional steroid. Journal of cutaneous laser therapy. September 2000;2(3):147–150. [DOI] [PubMed] [Google Scholar]
- 57.Stucker FJ, Shaw GY. An approach to management of keloids. Archives of otolaryngology--head & neck surgery. January 1992;118(1):63–67. [DOI] [PubMed] [Google Scholar]
- 58.Garg GA, Sao PP, Khopkar US. Effect of carbon dioxide laser ablation followed by intralesional steroids on keloids. Journal of cutaneous and aesthetic surgery. January 2011;4(1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar K, Kapoor BS, Rai P, Shukla HS. In-situ irradiation of keloid scars with Nd:YAG laser. Journal of wound care. May 2000;9(5):213–215. [DOI] [PubMed] [Google Scholar]
- 60.Goldenberg G, Luber AJ. Use of intralesional cryosurgery as an innovative therapy for keloid scars and a review of current treatments. The Journal of clinical and aesthetic dermatology. July 2013;6(7):23–26. [PMC free article] [PubMed] [Google Scholar]
- 61.van Leeuwen MC, Bulstra AE, van Leeuwen PA, Niessen FB. A new argon gas-based device for the treatment of keloid scars with the use of intralesional cryotherapy. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. December 2014;67(12):1703–1710. [DOI] [PubMed] [Google Scholar]
- 62.van Leeuwen MC, Bulstra AE, Ket JC, Ritt MJ, van Leeuwen PA, Niessen FB. Intralesional Cryotherapy for the Treatment of Keloid Scars: Evaluating Effectiveness. Plastic and reconstructive surgery. Global open. June 2015;3(6):e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mankowski P, Kanevsky J, Tomlinson J, Dyachenko A, Luc M. Optimizing Radiotherapy for Keloids: A Meta-Analysis Systematic Review Comparing Recurrence Rates Between Different Radiation Modalities. Annals of plastic surgery. April 2017;78(4):403–411. [DOI] [PubMed] [Google Scholar]
- 64.Watson D, Panuganti B. Treating Scars in the Auricle Region. Facial plastic surgery clinics of North America. February 2017;25(1):73–81. [DOI] [PubMed] [Google Scholar]
- 65.Shin JY, Kim JS. Could 5-Fluorouracil or Triamcinolone Be an Effective Treatment Option for Keloid After Surgical Excision? A Meta-Analysis. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. May 2016;74(5):1055–1060. [DOI] [PubMed] [Google Scholar]
- 66.Shin JY, Lee JW, Roh SG, Lee NH, Yang KM. A Comparison of the Effectiveness of Triamcinolone and Radiation Therapy for Ear Keloids after Surgical Excision: A Systematic Review and Meta-Analysis. Plastic and reconstructive surgery. June 2016;137(6):1718–1725. [DOI] [PubMed] [Google Scholar]
- 67.Ogawa R, Akaishi S, Kuribayashi S, Miyashita T. Keloids and Hypertrophic Scars Can Now Be Cured Completely: Recent Progress in Our Understanding of the Pathogenesis of Keloids and Hypertrophic Scars and the Most Promising Current Therapeutic Strategy. Journal of Nippon Medical School = Nippon Ika Daigaku zasshi. 2016;83(2):46–53. [DOI] [PubMed] [Google Scholar]
- 68.Park TH, Seo SW, Kim JK, Chang CH. Outcomes of surgical excision with pressure therapy using magnets and identification of risk factors for recurrent keloids. Plastic and reconstructive surgery. August 2011;128(2):431–439. [DOI] [PubMed] [Google Scholar]
- 69.Russell R, Horlock N, Gault D. Zimmer splintage: a simple effective treatment for keloids following ear-piercing. British journal of plastic surgery. September 2001;54(6):509–510. [DOI] [PubMed] [Google Scholar]
- 70.Chrisostomidis C, Konofaos P, Chrisostomidis G, et al. Management of external ear keloids using form-pressure therapy. Clinical and experimental dermatology. May 2008;33(3):273–275. [DOI] [PubMed] [Google Scholar]
- 71.Anzarut A, Olson J, Singh P, Rowe BH, Tredget EE. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. January 2009;62(1):77–84. [DOI] [PubMed] [Google Scholar]
- 72.Park TH, Park JH, Kim JK, Seo SW, Rah DK, Chang CH. Analysis of 15 cases of auricular keloids following conchal cartilage grafts in an asian population. Aesthetic plastic surgery. February 2013;37(1):102–105. [DOI] [PubMed] [Google Scholar]
- 73.Bran GM, Brom J, Hormann K, Stuck BA. Auricular keloids: combined therapy with a new pressure device. Archives of facial plastic surgery. Jan-Feb 2012;14(1):20–26. [DOI] [PubMed] [Google Scholar]
- 74.Hsu KC, Luan CW, Tsai YW. Review of Silicone Gel Sheeting and Silicone Gel for the Prevention of Hypertrophic Scars and Keloids. Wounds : a compendium of clinical research and practice. May 2017;29(5):154–158. [PubMed] [Google Scholar]
- 75.Fulton JE Jr. Silicone gel sheeting for the prevention and management of evolving hypertrophic and keloid scars. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. November 1995;21(11):947–951. [DOI] [PubMed] [Google Scholar]
- 76.Katz BE. Silicone gel sheeting in scar therapy. Cutis. July 1995;56(1):65–67. [PubMed] [Google Scholar]
- 77.O’Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database of Systematic Reviews. 2013(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gold MH, McGuire M, Mustoe TA, et al. Updated international clinical recommendations on scar management: part 2--algorithms for scar prevention and treatment. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. August 2014;40(8):825–831. [DOI] [PubMed] [Google Scholar]
- 79.Abedini R, Sasani P, Mahmoudi HR, Nasimi M, Teymourpour A, Shadlou Z. Comparison of intralesional verapamil versus intralesional corticosteroids in treatment of keloids and hypertrophic scars: A randomized controlled trial. Burns : journal of the International Society for Burn Injuries. September 2018;44(6):1482–1488. [DOI] [PubMed] [Google Scholar]
- 80.Wang R, Mao Y, Zhang Z, Li Z, Chen J, Cen Y. Role of verapamil in preventing and treating hypertrophic scars and keloids. International wound journal. August 2016;13(4):461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gambichler T, Schmitz L. Ultraviolet A1 Phototherapy for Fibrosing Conditions. Front Med (Lausanne). 2018;5:237–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Asawanonda P, Khoo LSW, Fitzpatrick TB, Taylor CR. UV-A1 for Keloid. JAMA Dermatology. 1999;135(3):348–349. [DOI] [PubMed] [Google Scholar]
- 83.Hannuksela-Svahn A, Grandal OJ, Thorstensen T, Christensen OB. UVA1 for treatment of keloids. Acta dermato-venereologica. November 1999;79(6):490. [DOI] [PubMed] [Google Scholar]
- 84.Polat M, Kaya H, Sahin A. A New Approach in the Treatment of Keloids: UVA-1 Laser. Photomedicine and laser surgery. March 2016;34(3):130–133. [DOI] [PubMed] [Google Scholar]
- 85.Gragnani A, Warde M, Furtado F, Ferreira LM. Topical tamoxifen therapy in hypertrophic scars or keloids in burns. Archives of dermatological research. January 2010;302(1):1–4. [DOI] [PubMed] [Google Scholar]
- 86.Fang QQ, Wang XF, Zhao WY, et al. Angiotensin-converting enzyme inhibitor reduces scar formation by inhibiting both canonical and noncanonical TGF-beta1 pathways. Scientific reports. February 20 2018;8(1):3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mohammadi AA, Parand A, Kardeh S, Janati M, Mohammadi S. Efficacy of Topical Enalapril in Treatment of Hypertrophic Scars. World journal of plastic surgery. September 2018;7(3):326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iannello S, Milazzo P, Bordonaro F, Belfiore F. Low-dose enalapril in the treatment of surgical cutaneous hypertrophic scar and keloid--two case reports and literature review. MedGenMed : Medscape general medicine. December 20 2006;8(4):60. [PMC free article] [PubMed] [Google Scholar]
- 89.Ardekani GS, Aghaei S, Nemati MH, Handjani F, Kasraee B. Treatment of a postburn keloid scar with topical captopril: report of the first case. Plastic and reconstructive surgery. March 2009;123(3):112e–113e. [DOI] [PubMed] [Google Scholar]
- 90.Gold MH, Andriessen A, Dayan SH, Fabi SG, Lorenc ZP, Henderson Berg MH. Hypochlorous acid gel technology-Its impact on postprocedure treatment and scar prevention. Journal of cosmetic dermatology. June 2017;16(2):162–167. [DOI] [PubMed] [Google Scholar]
- 91.Towler MA, Rush EW, Richardson MK, Williams CL. Randomized, Prospective, Blinded-Enrollment, Head-To-Head Venous Leg Ulcer Healing Trial Comparing Living, Bioengineered Skin Graft Substitute (Apligraf) with Living, Cryopreserved, Human Skin Allograft (TheraSkin). Clinics in podiatric medicine and surgery. July 2018;35(3):357–365. [DOI] [PubMed] [Google Scholar]
- 92.Bidic SM, Dauwe PB, Heller J, Brown S, Rohrich RJ. Reconstructing large keloids with neodermis: a systematic review. Plastic and reconstructive surgery. February 2012;129(2):380e–382e. [DOI] [PubMed] [Google Scholar]
- 93.Ong CT, Khoo YT, Mukhopadhyay A, et al. mTOR as a potential therapeutic target for treatment of keloids and excessive scars. Experimental dermatology. May 2007;16(5):394–404. [DOI] [PubMed] [Google Scholar]
- 94.Wong VW, You F, Januszyk M, Gurtner GC, Kuang AA. Transcriptional profiling of rapamycin-treated fibroblasts from hypertrophic and keloid scars. Annals of plastic surgery. 2014;72(6):711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. International journal of burns and trauma. 2012;2(1):18–28. [PMC free article] [PubMed] [Google Scholar]
- 96.Ferguson MW, Duncan J, Bond J, et al. Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. Lancet (London, England). April 11 2009;373(9671):1264–1274. [DOI] [PubMed] [Google Scholar]
- 97.Gauglitz GG. Management of keloids and hypertrophic scars: current and emerging options. Clinical, cosmetic and investigational dermatology. 2013;6:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsai CH, Ogawa R. Keloid research: current status and future directions. Scars, burns & healing. Jan-Dec 2019;5:2059513119868659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li C, Bai Y, Liu H, et al. Comparative study of microRNA profiling in keloid fibroblast and annotation of differential expressed microRNAs. Acta biochimica et biophysica Sinica. August 2013;45(8):692–699. [DOI] [PubMed] [Google Scholar]
- 100.Zhang GY, Wu LC, Liao T, et al. A novel regulatory function for miR-29a in keloid fibrogenesis. Clinical and experimental dermatology. June 2016;41(4):341–345. [DOI] [PubMed] [Google Scholar]
- 101.Yan L, Wang LZ, Xiao R, et al. Inhibition of microRNA-21–5p reduces keloid fibroblast autophagy and migration by targeting PTEN after electron beam irradiation. Laboratory investigation; a journal of technical methods and pathology. March 2020;100(3):387–399. [DOI] [PubMed] [Google Scholar]
- 102.Bijlard E, Steltenpool S, Niessen FB. Intralesional 5-fluorouracil in keloid treatment: a systematic review. Acta dermato-venereologica. September 2015;95(7):778–782. [DOI] [PubMed] [Google Scholar]
- 103.Li Q, Zhang C, Fu X. Will stem cells bring hope to pathological skin scar treatment? Cytotherapy. August 2016;18(8):943–956. [DOI] [PubMed] [Google Scholar]
- 104.Silva VZ, Albacete AN, Horacio GS, et al. Evidences of autologous fat grafting for the treatment of keloids and hypertrophic scars. Revista da Associacao Medica Brasileira (1992). December 2016;62(9):862–866. [DOI] [PubMed] [Google Scholar]
- 105.Gal P, Varinska L, Faber L, et al. How Signaling Molecules Regulate Tumor Microenvironment: Parallels to Wound Repair. Molecules (Basel, Switzerland). October 26 2017;22(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Q, Yang Q, Sun H. Role of collagen triple helix repeat containing-1 in tumor and inflammatory diseases. Journal of cancer research and therapeutics. 2017;13(4):621–624. [DOI] [PubMed] [Google Scholar]