Abstract
Secondary metabolites enable plants to protect themselves from herbivorous insects. Among these, cucurbitacin B (cuc-B) is a bitter-tasting compound with promising pharmacological potential. Dietary exposure to cuc-B lowered the hemolymph glucose levels of Drosophila melanogaster fed with a high carbohydrate diet, which is homologous to high blood glucose in humans, and its effect was comparable to that of metformin, a well-known glucose-lowering drug. Furthermore, cuc-B reduced tissue sugar levels and glycogen levels, as well as triacylglycerol levels. Our results thus highlight the potential applicability of this compound to treat chronic metabolic diseases such as diabetes and obesity. Additionally, we analyzed sleep quality and taste-associative memory enhancement after cuc-B and metformin treatment. Both supplements increased nighttime bout length and metformin increased memory consolidation. Therefore, discarded shell of Cucurbitaceae could be processed into health supplements.
Keywords: cucurbitacin B, hypoglycemia, memory, metformin, sleep
INTRODUCTION
Plants produce a variety of natural compounds and release phytochemicals or secondary metabolites that directly regulate growth and development, provide protection from herbivorous animals, and perform specialized functions against environmental stressors (Molyneux et al., 2007). Some phytochemicals are known to have critical roles in the human body and are therefore considered essential nutrients. However, the health benefits of much of these compounds remain uncharacterized.
Terpenes are a major phytochemical class that possesses desirable properties for use in food products, cosmetics, and pharmaceuticals (Thimmappa et al., 2014). Cucurbitacin is a terpene that can be extracted from Cucurbitaceae species such as cucumber, gourd, melon, and watermelon (Kim et al., 2020). Cucurbitacins can be classified into 12 main groups according to their side chains (Chen et al., 2005), and are known to regulate insect growth by inhibiting metamorphosis in Drosophila melanogaster larvae and Helicoverpa armigera, to cite a few examples (Zou et al., 2018). Additionally, however, cucurbitacins reportedly possess several therapeutic properties such as anti-inflammatory, anti-atherosclerosis, and anti-diabetic effects (Kaushik et al., 2015), as well as cytotoxic properties that inhibit cancer proliferation (Chen et al., 2012; Pulito et al., 2013).
Most of these secondary metabolites have a bitter taste, and secondary plant metabolites such as caffeine, aristolochic acids, L-canavanine, saponins, and nicotine deter insect predation (Kim et al., 2010; Lee et al., 2009; 2012; Rimal and Lee, 2019; 2020; Sang et al., 2019). Natural cucurbitacins constitute a group of triterpenoid substances that are well-known for their bitterness and toxicity. Some plant materials such as flavonoids, terpenes, alkaloids, phenols, and other related compounds have insect repellent or insecticidal effects against phytophagous insects (Adeyemi, 2010). Cucurbitacin also has a bitter taste, and our recent study revealed that one of the gustatory receptors, GR33a, is necessary to detect and avoid cucurbitacin B (cuc-B) (Rimal et al., 2020).
Similar to vertebrates, circulating sugar provides the energy required to power flight muscles due to its fast availability (Wigglesworth, 1949). During periods of high energy demand, glycogen in tissues are broken down to glucose and converted into the disaccharide trehalose, which is the primary sugar in the hemolymph (i.e., a fluid that is analogous to blood in vertebrates). In animals, every sugar is broken down to glucose, which is a major source of ATP in the brain. In flies, trehalose is a major body sugar used for flight and locomotion. Continuous supply of glucose or trehalose in the cells is necessary for energy production and homeostasis. In general, lipids from food are absorbed through enterocytes in the gut and converted to triglycerides, after which they are supplied to peripheral tissues in the form of lipoproteins for energy production. Excess glucose in systemic circulation is converted to glycogen and fat to serve as a stored energy source. Hyperglycemia, a symptom of diabetes, is a condition where body glucose levels exceed normal levels, whereas the opposite condition is known as hypoglycemia.
Metformin is used as a first-line medication to treat many metabolic diseases such as type II diabetes (particularly in overweight people) with minimal cardiovascular side effects (Bailey, 2017). Moreover, this drug is also used to treat polycystic ovary syndrome and gestational diabetes (Rowan et al., 2008). In animals, metformin has been administered orally to lower blood glucose levels in hyperglycemic conditions. Metformin reduces glucose concentration in three ways: (1) it reduces sugar uptake or absorption in the gut (Ikeda et al., 2000); (2) it minimizes hepatic glucose production (Duca et al., 2015) via control of gluconeogenesis, a process through which glucose is produced from non-carbohydrate sources (Madiraju et al., 2014). Metformin is generally considered an excellent complementary treatment to insulin due to its ease of administration, lack of hypoglycemic effects after therapy, and its therapeutic value for both type I and II diabetes patients (Hundal et al., 2000; Vella et al., 2010). Additionally, metformin treatment significantly reduces visceral fat mass accumulation (Lin et al., 2000). Metformin also does not alter basal fatty acid metabolism and prevents insulin-mediated effects on fatty acid oxidation and integration into triacylglycerol (TAG) (Collier et al., 2006). In this study, fruit flies were dietarily exposed to cuc-B to test whether it may serve to treat metabolic conditions such as obesity and enhance sleep and learning and memory, as well as to assess how these effects compare to those of metformin.
MATERIALS AND METHODS
Fly stocks
The w 1118 line was used as the “wild-type” control; the Gr33a 1 mutant fly line was described in detail in our previous study (Moon et al., 2009).
Chemical sources
Sucrose (CAS No. 57-50-1, Cat No. S9378), sulforhodamine B (CAS No. 3520-42-1, Cat No. 230162), amyloglucosidase (CAS No. 9032-08-0, Cat No. A1602), metformin (CAS No. 1115-70-4, Cat No. D150959), porcine kidney trehalase (CAS No. 9025-52-9, Cat No. T8778), and cucurbitacin B (CAS No. 6199-67-3, Cat No. PHL82226) were purchased from Sigma-Aldrich (USA) (Oliveira et al., 2020). Brilliant Blue FCF (CAS No. 3844-45-9, Cat No. 027-12842) was purchased from Wako Pure Chemical Industries (Japan).
Ingestion assay
The ingestion assay was carried out as previously described (Sang et al., 2019) with slight modifications. Male flies (3-5 day old) were allowed to feed on standard cornmeal with 0.1% (w/v) Brilliant Blue FCF with or without the indicated chemical. After feeding, the flies were stored in the freezer. Six male flies were transferred to 1.5 ml ep-tubes filled with 200 μl PBST (1X PBS [phosphate-buffered saline] with 0.2% Triton X-100). The flies were then thoroughly ground, after which an additional 800 μl PBST was added. The tube was centrifuged for 5 min at ~16,000g at 4°C. The supernatant was then loaded into a cuvette for spectrophotometry. The samples in the cuvettes were measured at 630 nm, and PBST was used as the blank control. The ingestion index (I.I.) was calculated based on the difference in optical density (OD) between the standard cornmeal (control)-diet group and the treated-diet groups. The ingestion index was calculated as follows: (ODchemical-diet – ODcontrol-diet) / (ODcontrol-diet). Each experiment was performed four times.
Droso-X assay
Ingestion amount was quantified with a Droso-X system (Scitech Korea, Korea), which was housed in an incubator (25°C, 60% humidity). Afterward, a mixture of 3% sucrose, 0.0125 mM brilliant blue dye, and the indicated concentration of chemicals was injected into a glass tube (Cat. No. 53432-706; VWR International, USA) with a syringe (KOVAX-SYRINGE 1 ml 26G; KOREA VACCINE, Korea) and needle (Cat No. 90025; Hamilton, Switzerland). Each cuvette housed 3- to 4-day-old male flies and was physically isolated to prevent the fruit flies from ingesting the solution before the experiment was recorded. The experiment was conducted for 2 h from 9 am to 11 am. The Droso-X recorded the amount of consumed solution using the DROSO X&XD software (Scitech Korea, Korea). Ingestion amount(X h) was calculated as (solutions amount(0 h) – solutions amount(X h)). Each experiment was performed more than 10 times.
Glucose measurements in adult hemolymph
Hemolymph glucose levels were measured as described previously (Dus et al., 2011). Ten males (3-5 days old) were used for hemolymph collection. Male flies were punctured in the thorax using a fine injection needle (Cat No. 90025) and placed shoulder down to prevent leakage from the genital tract into 0.5 ml tubes whose bases had been punctured with a 21-gauge needle. The tubes were placed within 1.5 ml microfuge tubes and centrifuged at ~2,800g for 5 min at 4°C. To 14.5 μl PBS, 0.5 μl of hemolymph was added and placed at 70°C for 5 min. Afterward, 100 μl of glucose reagent (G3293 VER; Sigma-Aldrich) was added and incubated at 37°C for 12 h. A commercial glucose (HK) assay kit (GAHK-20-1KT; Sigma-Aldrich) was then used to perform the assays and total glucose was measured at 340 nm. Glucose levels were quantified by comparing the values with a glucose standard curve.
Trehalose and glucose measurements in tissue
Quantification of trehalose and glucose levels in whole fly extracts were performed as described previously (Meunier et al., 2007). Briefly, 10 males (3-5 days old) were weighed and homogenized in 250 μl of 0.25 M Na2CO3 buffer and incubated in a water bath at 95°C for 5 min to inactivate all enzymes. Next, 150 μl of 1 M acetic acid and 600 μl of 0.25 M sodium acetate (pH 5.2) were added, and the solution was centrifuged (10 min, 12,500g, 24°C). Overnight, 200 μl of each supernatant was incubated at 37°C with 2 μl porcine kidney trehalase (T8778 UN; Sigma-Aldrich) to convert trehalose into glucose. Then, 100 μl of this solution was added to 1 ml of glucose hexokinase solution (GAHK-20; Sigma-Aldrich) and incubated for 20 min at 37°C. Glucose levels were quantified at 340 nm. Glucose concentrations were quantified using a glucose standard curve.
Glycogen measurements
Glycogen levels in whole fly extracts were measured as described in a previous study (Dus et al., 2011). Briefly, 5 males (3-5 day old) were weighed and homogenized in 100 μl of ice-cold phosphate buffered saline (1X PBS). The enzymes were then inactivated by incubating the homogenates at 70°C for 5 min, after which the samples were centrifuged at 12,500g for 3 min at 4°C. Then, 20 μl of the supernatants were transferred to 1.5 ml tubes and diluted 1:3 in 1X PBS. Afterward, 1.5 μl of amyloglucosidase suspension was diluted in 998.5 μl 1X PBS, and 20 μl of the diluted amyloglucosidase solution was added to 20 μl aliquots of each test sample (and to glycogen standards) to convert the glycogen into glucose. The samples and glycogen standards were incubated at 37°C for 60 min. A commercial glucose (HK) assay reagent (G3293 VER) was then used to measure total glucose at 340 nm. To determine the glycogen concentrations, the glucose levels in the test samples were compared with a standard curve derived from converting glycogen standards to glucose.
TAG level measurements
TAG level quantification was performed as described previously (De Truchis et al., 2007) using a LiquiColor Triglyceride Test kit (Cat No. 2100-225; Stanbio Laboratory, Germany) with some modifications. Samples from 10 male flies were weighed and crushed in 1 ml of PBST (1X PBS and 0.2% Triton X-100). The homogenate was centrifuged at ~9,500g for 3 min. Afterward, 100 μl of the supernatant was added to 1 ml of Stanbio LiquiColor Triglyceride Test kit reagent or 1 ml of deionized water, which was used as a baseline. The mixture was incubated at 37°C for 15 min, after which absorbance was recorded at 500 nm and compared with a standard calibration curve.
Sleep assay
Sleep assays were conducted as described previously with slight modifications (Vanderheyden et al., 2013). Young flies (1-3 day old) were placed in standard cornmeal food vials mixed with or without each indicated chemical for 6 days in the same incubator below. Drosophila activity monitor systems (DAMs) (Hundal et al., 2000) were used to automatically monitor fly activity in 1-min time bins. Activity was recorded whenever a fly crossed an infrared beam in the middle of the recording tube. The flies cannot perceive the infrared beam. Flies that remain immobile for longer than 5 min are considered to be asleep. The program was set at a 12 h/12 h light/dark cycle at 25°C. The aforementioned light/dark photoperiod was interpreted as daytime or nighttime. A male fly was inserted in each glass tube containing 1% sucrose in 1% agarose at one end. The DAMs were kept equipped with glass tubes in the incubator 18 h before the recording. These data were recorded for 2 days and converted to an average value for 2 days. Latency is the time it takes to fall asleep after the lights are turned off. Bout length was defined as the duration of uninterrupted sleep time. Bout number was defined as the number of uninterrupted sleep episodes. Sleep time, bout length, maximum bout length, bout number, and activity number were separately calculated during daytime and nighttime. Sixty flies were analyzed for each experiment.
Taste-associative memory assay
Taste-associative memory was assessed as described before (Poudel and Lee, 2018). Young flies (1-3 day old) were placed in standard cornmeal food vials mixed with or without each indicated chemical for 6 days. First, the flies were starved for 18 h. On the morning of the experiment (8 am), more than 15 flies were fixed onto a glass slide using nail polish and then anesthetized with ice. The flies were then kept at 25°C in a 60% humidified incubator to recover for at least 1 h. The experiments were divided into three different phases. The first phase was the pretest, in which the flies were stimulated with 500 mM sucrose on the leg. Only flies that exhibited positive proboscis extension to this stimulus were used for the next phases. This was followed by the training phase, where the flies were stimulated with 500 mM sucrose on the leg, after which they were presented with an aversive stimulus in the labellum (50 mM caffeine). Training was repeated 15 times for each fly. The training data were separated into three bins each consisting of five trials. Once training was completed, the flies were stimulated with 500 mM sucrose on the leg at different time intervals (0, 5, 15, 30, 45, and 60 min), and the proboscis extension response fraction was measured. All genotypes were assessed on the same day.
Statistical analyses
The reported P values correspond to Student t-test comparisons with appropriate genetic controls. Statistical analysis was performed using Origin 8 software (OriginPro; OriginLab Corporation, USA). Single-factor analysis of variance (ANOVA) coupled with Scheffe’s post hoc test was used to compare multiple datasets. Asterisks indicate statistical significance (*P < 0.05, **P < 0.01).
RESULTS AND DISCUSSION
Gr33a mutants ingest more cuc-B
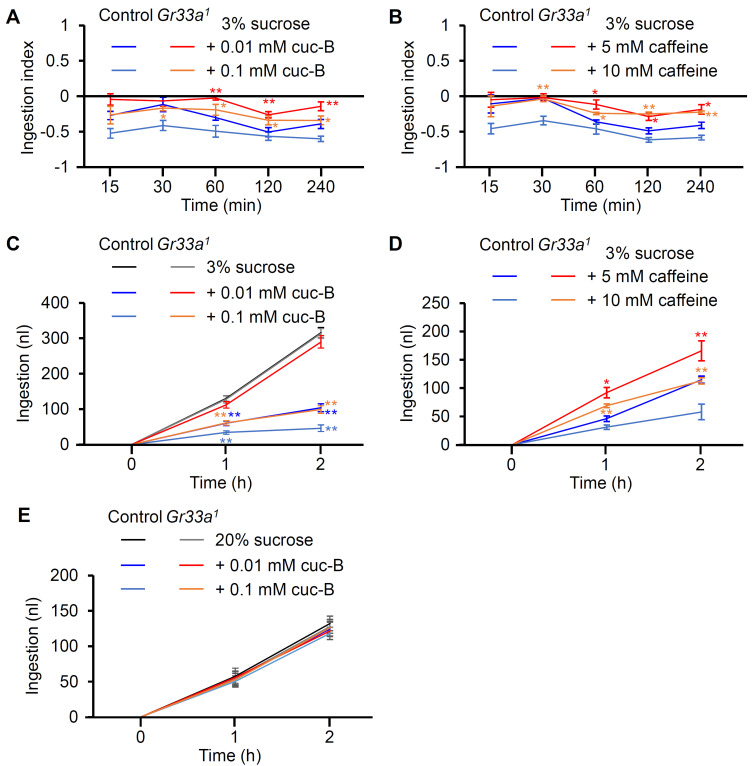
Cuc-B is a bitter-tasting compound, which is sensed by GR33a in the labellum (i.e., the insect equivalent of a tongue), in addition to many other plant metabolites such as caffeine and nicotine, and synthetic bitter compounds such as DEET (N,N-Diethyl-m-toluamide) and denatonium (Lee et al., 2009; 2010; Rimal and Lee, 2019; Rimal et al., 2020). Gr33a 1 mutants do not avoid the aforementioned bitter compounds and therefore ingest more cuc-B-treated cornmeal than wild-type flies. Therefore, the flies were allowed to feed on standard cornmeal or cuc-B-treated cornmeal with blue dye, after which we measured the OD of their abdomens at regular intervals from 15 to 240 min (Fig. 1A and Materials and Methods section in detail). A negative ingestion index indicates an avoidance of cuc-B-treated food. The difference in the amount of consumption between Gr33a 1 mutants and control flies varied depending on compound concentration, but were significantly different after 60 min (Fig. 1A). Caffeine consumption rates were also assessed in Gr33a 1 mutants, as this mutation also dampens the capacity of the flies to sense caffeine (Fig. 1B) (Moon et al., 2009). Similar to the cuc-B feeding assay, Gr33a 1 mutants exhibited an increase in caffeine ingestion compared to their wild-type counterparts.
Fig. 1. Cuc-B and caffeine ingestion assay with control and Gr33a1 male flies.
(A) Comparison between 0.01 mM or 0.1 mM cuc-B-treated cornmeal ingestion at each time point (n = 4). (B) Comparison between 5 mM or 10 mM caffeine-treated cornmeal ingestion at each time point (n = 4). (C) Ingestion of 3% sucrose combined without or with 0.01 mM or 0.1 mM cuc-B, as determined by the Droso-X assay (n = 12-22). (D) Ingestion of 3% sucrose with 5 mM or 10 mM caffeine (n = 10-11). (E) Ingestion of 20% sucrose combined with 0.01 mM or 0.1 mM cuc-B, as determined by the Droso-X assay (n = 10-11). All error bars indicate the SEM. Asterisks indicate statistical significance (*P < 0.05, **P < 0.01), as determined by Student’s t-test comparisons with appropriate genetic controls.
The Droso-X assay was conducted to quantify food ingestion; this computer-assisted approach enables the automatic quantification of feeding amounts (Supplementary Fig. S1 and Materials and Methods section in detail). A glass tube containing 3% sucrose and different concentrations of cuc-B or caffeine was introduced to a cuvette where a single fly was inserted. The 3% sucrose feeding amount of the control and Gr33a 1 mutant was almost similar (Fig. 1C). However, the feeding amount of the Gr33a 1 mutant was significantly higher than that of the control flies in the cuc-B and caffeine feeding trials (Figs. 1C and 1D). Furthermore, we also found that food consumption was inversely correlated with the concentration of cuc-B or caffeine (Figs. 1C and 1D). In these cases, 3% sucrose presumably inhibited the perception of the bitter compounds. To test it, we measured ingestion amount by feeding 20% sucrose and found that 20% sucrose completely masked the bitterness of cuc-B (Fig. 1E).
Cuc-B suppresses hyperglycemia in hemolymph
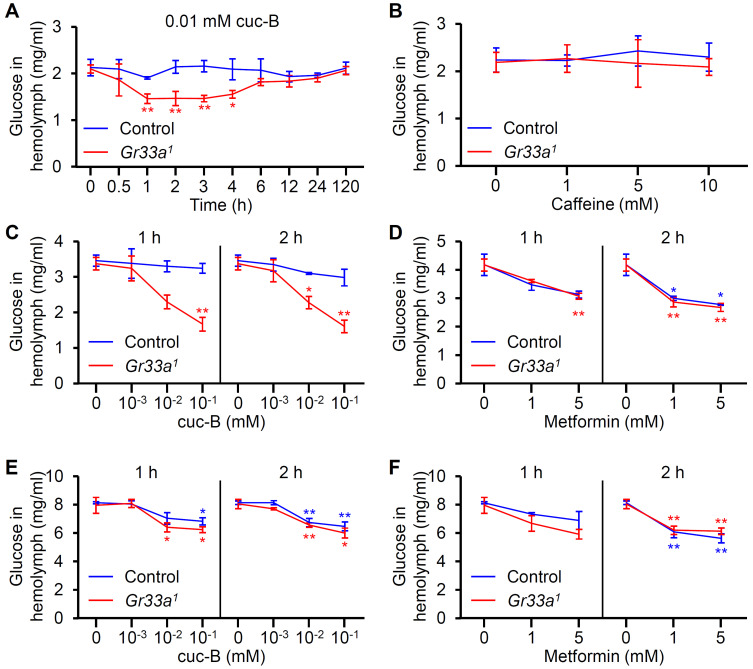
Cuc-B induces hypoglycemic effect in diabetic mice via bitter taste receptor signaling at enteroendocrine L-cells in mice (Kim et al., 2018). Hemolymph glucose levels in control and Gr33a 1 mutant male flies fed with cuc-B and caffeine were measured to assess the capacity of these compounds to alleviate hyperglycemia in Drosophila (Figs. 2A and 2B). Dietary cuc-B treatment generally reduced hemolymph glucose levels, albeit not significantly in the control (Fig. 2A). Surprisingly, the Gr33a 1 mutants exhibited 21.4% reductions in hemolymph glucose at 1 h post-feeding compared to 0 h and a 23.3% reduction compared to control flies at 1 h post-feeding (Fig. 2A). This suppression in the Gr33a 1 mutant persisted until 4 h and then slowly recovered from 6 h to the end of the trial. However, hypoglycemia induction was not detected 1 h after feeding 1 to 10 mM of caffeine to the control or Gr33a 1 mutants (Fig. 2B), indicating that the reduced glucose levels was specific to cuc-B.
Fig. 2. Effect of cuc-B, caffeine, and metformin treatment on hemolymph glucose levels.
(A) Hemolymph glucose levels in control and Gr33a 1 males after being fed with standard cornmeal mixed with 0.01 mM cuc-B (n = 4). (B) Hemolymph glucose levels in control and Gr33a 1 male flies after 1 h of being fed with standard cornmeal mixed with the indicated caffeine concentrations (n = 4). (C) Hemolymph glucose levels in control and Gr33a 1 flies 1 h and 2 h after being fed with 3% sucrose with the indicated cuc-B concentrations (n = 4). (D) Hemolymph glucose levels in control and Gr33a 1 flies 1 h and 2 h after being fed with 3% sucrose with the indicated metformin concentrations (n = 4). (E) Hemolymph glucose levels in control and Gr33a 1 flies 1 h and 2 h after being fed with 20% sucrose with the indicated cuc-B concentrations (n = 4-5). (F) Hemolymph glucose levels in control and Gr33a 1 flies 1 h and 2 h after being fed with 20% sucrose with the indicated metformin concentrations (n = 4-5). All error bars indicate the SEM. (A and B) Asterisks indicate statistical significance (*P < 0.05, **P < 0.01), as determined by Student’s t-test comparisons with appropriate genetic controls. (C-F) Single-factor ANOVA coupled with Scheffe’s analysis was used as a post hoc test to compare multiple datasets (*P < 0.05, **P < 0.01). Each point was compared with 0 mM of each tested compound for each genotype.
We hypothesized that this reduction in glucose levels was not detected in the controls due to inadequate cuc-B dosing. To test this, we fed the flies with low (3%) and high (20%) sucrose concentrations (Rovenko et al., 2015). Specifically, control and Gr33a 1 mutant flies were fed with 3% sucrose mixed with 0 to 0.1 mM cuc-B and measured hemolymph glucose levels at 1 h and 2 h post-feeding (Fig. 2C). The low-sugar diet (LSD) increased hyperglycemia by 60.6% compared to standard cornmeal (Figs. 2A and 2C). This induction was strongly suppressed in Gr33a 1 mutants fed with 0.01 to 0.1 mM cuc-B at 1 h and 2 h post-feeding (Fig. 2C). However, the LSD appeared to mask the effect of cuc-B in the control (Fig. 2C). We also assessed the effect of metformin on hemolymph glucose levels to compare it to that of cuc-B, as metformin is well known for its hypoglycemic action in animals (Ohadoma and Michael, 2011). Hemolymph glucose levels were significantly decreased in both control and Gr33a 1 mutant flies fed with 5 mM metformin at 1 h and 2 h (Fig. 2D). However, dietary exposure to 1 mM metformin only reduced glucose levels after 2 h (Fig. 2D). This indicated that metformin efficiently suppressed hyperglycemia in control and Gr33a 1 mutant flies. Afterward, we tested a high-sugar diet (HSD). HSD induced hyperglycemia, which was characterized by an almost 4-fold increase in hemolymph glucose levels (Figs. 2E and 2F). The HSD was enough to mask the bitterness of cuc-B, and therefore we detected a significant reduction in the glucose level of both control and Gr33a 1 mutant flies (Fig. 2E). This cuc-B-induced suppression was comparable to that of metformin (Figs. 2E and 2F).
Here, we demonstrated that the regulation of hemolymph glucose levels depended on how much cuc-B-treated food was consumed by the flies. Given that the Gr33a-GAL4 reporter is known to be expressed in the intestine and midgut enteroendocrine cells (Park and Kwon, 2011), including the peripheral neurons of the labellum and legs, hyperglycemia suppression might be positively controlled by bitter taste receptors in the intestinal cells similar to mice (Kim et al., 2018). However, there are several arguments against this explanation. First, if hemolymph sugar levels were mediated by GR33a in intestinal and midgut enteroendocrine cells, the glucose levels of wild-type flies should have been regulated based on the 0.01 mM cuc-B feeding time. Instead, the control flies showed normal hemolymph glucose levels. Second, cuc-B dosing in standard cornmeal or LSD was a major variable that reduced hyperglycemia only in the Gr33a 1 mutant but not in control flies. Interestingly, glucose levels were highly correlated with the amount of cuc-B ingested, and Gr33a 1 mutants ingested more cuc-B over a 1-h ingestion period than the controls. Third, when HSD induced hyperglycemia, the glucose-lowering effect of cuc-B was robust in both control and mutant flies. This strongly indicated that hyperglycemia suppression was specifically mediated by dietary cuc-B consumption, and therefore the potential hyperglycemia-suppressing role of Gr33a in the intestine and midgut enteroendocrine cells was likely very minor if at all present.
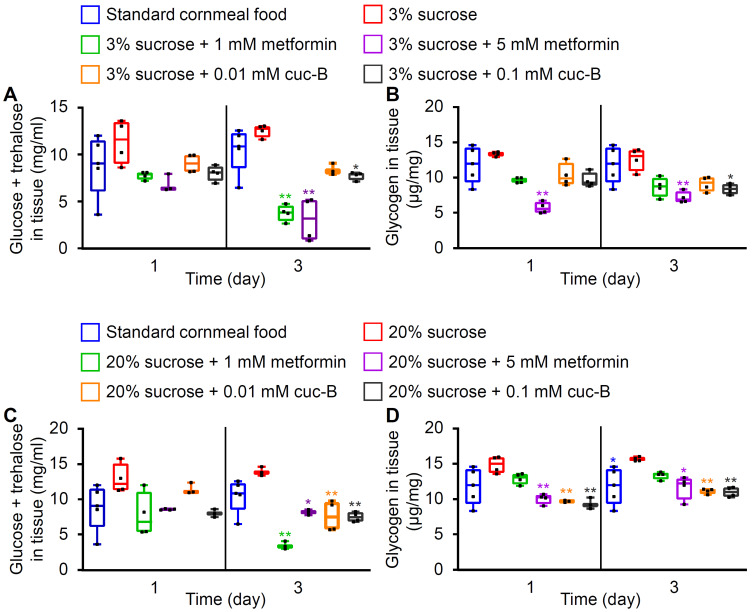
Cuc-B can reduce tissue sugar levels
Trehalose is a nonreducing disaccharide composed of two glucose units, which is the most abundant circulating carbohydrate in insects. Furthermore, circulating glucose can be transferred to tissues to be used as an energy source or be stored as glycogen. To test whether cuc-B and metformin affected tissue sugar levels, the flies were tested 1 day and 3 days after the LSD or HSD assays with or without cuc-B/metformin. In the LSD condition, 1 mM or 5 mM metformin efficiently suppressed tissue sugar levels at 3 days post-feeding (Fig. 3A). This suppression was also detectable in flies fed with 0.1 mM cuc-B feeding at 3 days (Fig. 3A). Moreover, 5 mM metformin or 0.1 mM cuc-B were sufficiently effective in reducing tissue glycogen levels at 3 days (Fig. 3B). The metformin effect was detected from day 1 (Fig. 3B). In the HSD condition, significant reductions of tissue sugar levels were detected in both cuc-B- or metformin-treated flies, with even lower concentrations after 3 days (Fig. 3C). Furthermore, glycogen reduction was investigated in flies treated with 5 mM metformin or 0.01 to 0.1 mM cuc-B at 1 day and 3 days post-feeding (Fig. 3D). Cuc-B was generally more effective than metformin to suppress glycogen levels.
Fig. 3. Measurement of tissue sugar levels.
(A and B) Comparison of sugar and glycogen levels in tissue after feeding a low sugar diet (LSD: 3% sucrose). (A) Glucose and trehalose levels in tissue and (B) glycogen levels in tissues of control flies after being fed with standard cornmeal, LSD, or LSD combined with the indicated chemicals (n = 4). (C and D) Comparison between sugar and glycogen levels in fly tissue after being fed with a high sugar diet (HSD: 20% sucrose). (C) Glucose and trehalose levels in tissue and (D) glycogen levels in tissues of control flies after being fed with standard cornmeal, HSD, or HSD combined with the indicated chemicals (n = 4). All error bars indicate the maximum or minimum values. Single-factor ANOVA coupled with Scheffe’s post hoc test was conducted to compare multiple datasets. Asterisks indicate significant differences (*P < 0.05, **P < 0.01) between (A and B) LSD or (C and D) HSD feeding.
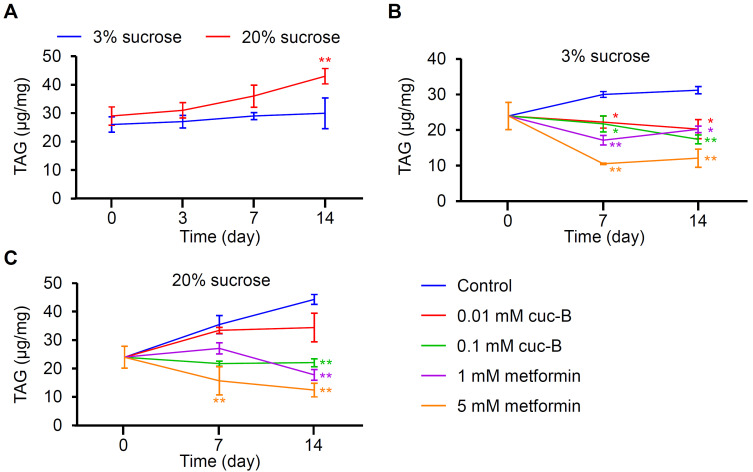
Cuc-B-treated diets reduced TAG storage in Drosophila
Excess dietary sugar can be stored in the form of TAG in fat bodies by de novo lipogenesis. Fat bodies serve as major energy reservoirs that mainly store TAG. Increased dietary sucrose in animals leads to obesity and metabolic syndromes. HSD-fed in mammal increases fat deposits in white adipose tissue, glucose intolerance, insulin resistance, pancreatic cell secretion, and hepatic lipid accumulation (Oliveira et al., 2020). To assess TAG accumulation in Drosophila, wild-type flies were fed with LSD or HSD for 14 days (Fig. 3A). The HSD began to induce higher TAG levels on day 7, but significant increases were only detected after 14 days (Fig. 4A). Obese humans usually suffer from high TAG serum and tissue levels. To test the effect of cuc-B and metformin on fat levels, the LSD was co-administered with 1 mM or 5 mM metformin (Fig. 4B). TAG levels were then measured at 7 days and 14 days. As expected, 1 mM metformin suppressed fat storage by 40.2% and 36.8% on days 7 and 14, whereas 5 mM metformin suppressed fat storage by 64.9% and 60.6% on days 7 and 14 (Fig. 4B). Furthermore, cuc-B treatment also reduced TAG levels, albeit less so than metformin treatment (Fig. 4B). A significant fat reduction was only detected with 0.1 mM cuc-B after 14 days. Next, the flies were fed with the HSD (Fig. 4C). TAG increases were clearly suppressed by 5 mM metformin dietary treatment or 0.1 mM cuc-B at 7 days and 14 days. However, 0.01 mM cuc-B did not effectively suppress TAG accumulation and 1 mM metformin was only effective after 14 days, but not 7 days (Fig. 4C). Overall, metformin reduced TAG levels in adult Drosophila, and the metformin concentrations assessed herein were deemed non-toxic (i.e., they did not affect survival and reproduction) (Slack et al., 2012). Nonetheless, cuc-B could also be used to efficiently suppress fat storage.
Fig. 4. Lipid (TAG) level measurement in control male flies.
(A) TAG level measurement at indicated time points after LSD or HSD administration (n = 4). (B) TAG level measurement at 0, 7, and 14 days after LSD administration with or without the indicated chemical (n = 4-5). (C) TAG level measurement at 0, 7, and 14 days after HSD administration with or without the indicated chemical (n = 4-5). All error bars indicate the SEM. (A) Asterisks indicate statistical significance (**P < 0.01), as determined by Student’s t-test comparisons between LSD and HSD. (B and C) Single-factor ANOVA coupled with Scheffe’s post hoc test was conducted to compare multiple datasets (*P < 0.05, **P < 0.01).
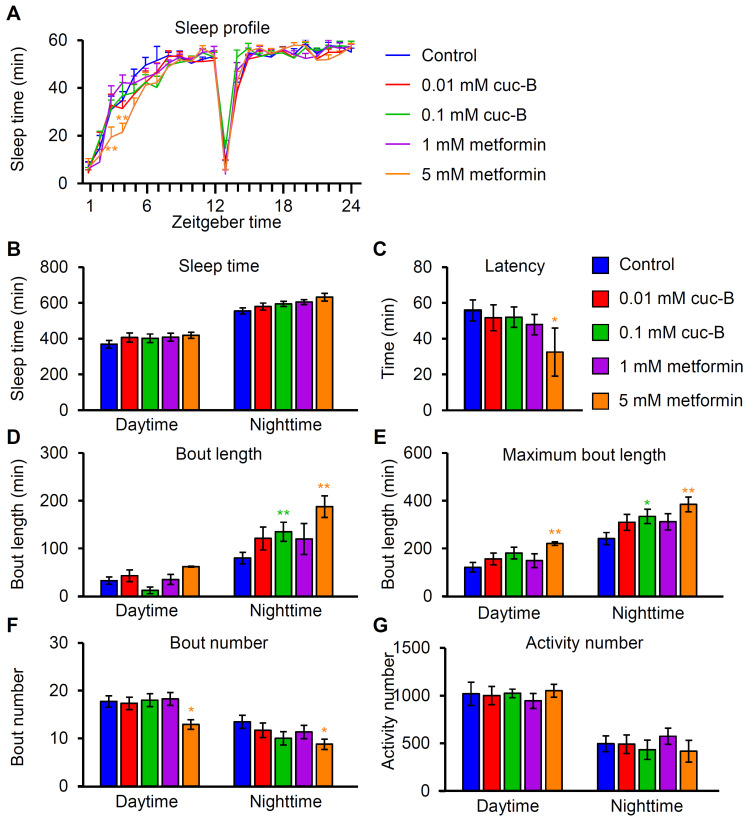
Cuc-B and metformin increase bout length
Metformin therapy might be associated with longer sleep times and better sleep efficiency in humans (Kajbaf et al., 2014). Furthermore, sleep quality can be modulated by a high sugar diet (Catterson et al., 2010). Therefore, we explored treatment with cuc-B and metformin.
Most animals choose a preferred location to become immobile at a particular time and remain relatively unresponsive to environmental stimuli. This sleep-like state occurs in fish, amphibians, and even some invertebrates such as flies (Campbell and Tobler, 1984; Hendricks et al., 2000; Shaw et al., 2000). Caffeine acts on the human adenosine receptor, thereby reducing sleep. In contrast, hydroxyzine (an H1 antagonist) increases sleep duration (Shaw et al., 2000). These observations suggest that sleep-modulatory drugs act by regulating neurotransmitter systems in humans and elicit similar behavioral effects in animal model organisms including flies. To determine whether cuc-B or metformin intake affects sleep quality, our study characterized the sleep time, latency, bout length, and bout number of male flies (Fig. 5).
Fig. 5. Sleep quality analysis after cuc-B or metformin dietary treatment in control male flies.
(A) Sleep profile after each treatment. (B) Total average sleep amount during daytime and nighttime. (C) Sleep latency at night. (D) Average bout length. (E) Average maximum bout length. (F) Average bout number. (G) Average activity number (n = 60). All error bars indicate the SEM. Single-factor ANOVA coupled with Scheffe’s post hoc test was conducted to compare multiple datasets (*P < 0.05, **P < 0.01).
Flies engage in regular short resting periods during the day and sleep for longer periods during the night. Flies were treated with cuc-B or metformin for 6 days before the sleep assay to show a long-term effect of each drug on sleep. The daily sleep profile and total sleep times were almost unaffected by the drug feedings during the daytime and nighttime except subtle decreases of sleep time on ZT (zeitgeber time) 3 and 4 by 5 mM metformin feeding (Figs. 5A and 5B). A decreased sleep latency at night was only detected in flies treated with 5 mM metformin (Fig. 5C). Moreover, 0.1 mM cuc-B and 5 mM metformin significantly increased nighttime bout length and maximum bout length (Figs. 5D and 5E), indicating that these compounds can enhance sleep quality by inducing deep sleep at night. Additionally, 5 mM metformin induced longer maximum sleep lengths during daytime resting periods (Fig. 5E). Notably, only the 5 mM metformin treatment decreased bout number in both daytime and nighttime (Fig. 5F). The decrease of bout number and increase of maximum bout length during the daytime may affect daytime activities. Therefore, we measured total beam crossings which indicate fly activities (Fig. 5G). We found that metformin treatment did not affect daytime activity. This indicates that cuc-B and metformin treatment can improve sleep quality, which highlights the potential of these compounds to be used as deep sleep-inducing supplements.
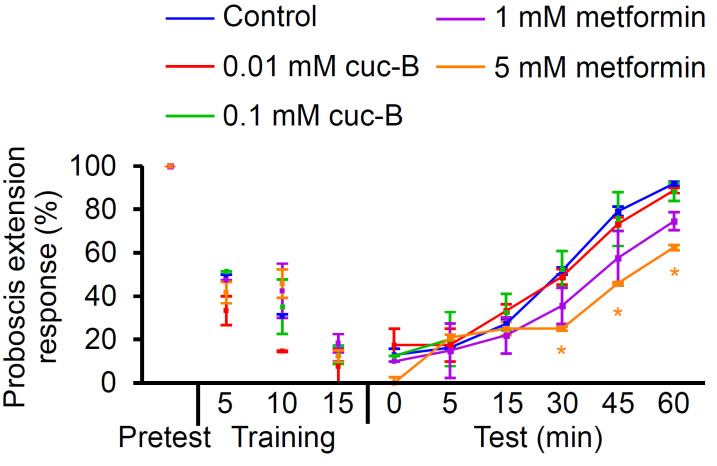
Metformin, but not cuc-B, improves taste-associative memory
Metformin promotes learning and memory in insulin-resistant rats (Pintana et al., 2012). There are many reports to worsen dementia by a high sucrose diet (Seetharaman, 2016; Yeh et al., 2020). Therefore, we treated control flies with metformin and cuc-B to investigate possible enhancement in memory. Flies can be trained to associate an attractive stimulus with an aversive stimulus (Kirkhart and Scott, 2015; Masek et al., 2015). Therefore, to assess the effects of cuc-B and metformin on associative memory, only flies that exhibited proboscis extension responses to sucrose (Rovenko et al., 2015) were selected for downstream behavioral assays. The flies were trained 15 times with two different stimuli. The presentation of sucrose to the leg served as an attractive stimulus, which was followed shortly thereafter with caffeine presentation to the proboscis as an aversive stimulus (Poudel and Lee, 2018). Control flies were then fed cuc-B-treated or metformin-treated cornmeal for 6 days, whereas untreated cornmeal was administered to the control group (Fig. 6). To detect improvements in taste-associative memory, the responses to sucrose at 0, 5, 15, 30, 45, and 60 min were evaluated. Flies are capable of recalling that an aversive stimulus is administered when they extend their proboscis and therefore learn to withdraw their proboscis when stimulated with sucrose in their legs. Administration of 5 mM metformin significantly reduced the proboscis extension reflex after 30 min, indicating an improved associative memory (Fig. 6). However, no improvements in learning and memory were observed in the cuc-B or control treatments (Fig. 6). These findings indicate that metformin, but not cuc-B, enhances taste-associative memory in wild-type flies.
Fig. 6. Effect of cuc-B and metformin on taste associative memory of control flies.
More than 10 flies were analyzed for learning and memory after being fed with each indicated chemical for 7 days (n = 4). All error bars indicate SEM. Single-factor ANOVA coupled with Scheffe’s post hoc test was conducted to compare multiple datasets (*P < 0.05).
Supplemental Materials
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
ACKNOWLEDGMENTS
This work was supported by grants to Y.L. from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A2B6004202) and Korea Environmental Industry and Technology Institute (KEITI) grant funded by the Ministry of Environment of Korea. S.D. was supported by the Global Scholarship Program for Foreign Graduate Students at Kookmin University in Korea.
Footnotes
AUTHOR CONTRIBUTIONS
J.S., S.D., and Y.L. conceived and designed the experiments. J.S. and S.D. performed the experiments. J.S., S.D., and Y.L. wrote the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Adeyemi M.H. The potential of secondary metabolites in plant material as deterents against insect pests: a review. Afr. J. Pure Appl. Chem. 2010;4:243–246. [Google Scholar]
- Bailey C.J. Metformin: historical overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- Campbell S.S., Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- Catterson J.H., Knowles-Barley S., James K., Heck M.M., Harmar A.J., Hartley P.S. Dietary modulation of Drosophila sleep-wake behaviour. PLoS One. 2010;5:e12062. doi: 10.1371/journal.pone.0012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.C., Chiu M.H., Nie R.L., Cordell G.A., Qiu S.X. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat. Prod. Rep. 2005;22:386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- Chen X., Bao J., Guo J., Ding Q., Lu J., Huang M., Wang Y. Biological activities and potential molecular targets of cucurbitacins: a focus on cancer. Anticancer Drugs. 2012;23:777–787. doi: 10.1097/CAD.0b013e3283541384. [DOI] [PubMed] [Google Scholar]
- Collier C.A., Bruce C.R., Smith A.C., Lopaschuk G., Dyck D.J. Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2006;291:E182–E189. doi: 10.1152/ajpendo.00272.2005. [DOI] [PubMed] [Google Scholar]
- De Truchis P., Kirstetter M., Perier A., Meunier C., Zucman D., Force G., Doll J., Katlama C., Rozenbaum W., Masson H. Reduction in triglyceride level with N-3 polyunsaturated fatty acids in HIV-infected patients taking potent antiretroviral therapy: a randomized prospective study. J. Acquir. Immune Defic. Syndr. 2007;44:278–285. doi: 10.1097/QAI.0b013e31802c2f3d. [DOI] [PubMed] [Google Scholar]
- Duca F.A., Côté C.D., Rasmussen B.A., Zadeh-Tahmasebi M., Rutter G.A., Filippi B.M., Lam T.K. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 2015;21:506. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus M., Min S., Keene A.C., Lee G.Y., Suh G.S. Taste-independent detection of the caloric content of sugar in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks J.C., Finn S.M., Panckeri K.A., Chavkin J., Williams J.A., Sehgal A., Pack A.I. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Hundal R.S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V., Inzucchi S.E., Schumann W.C., Petersen K.F., Landau B.R. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Iwata K., Murakami H. Inhibitory effect of metformin on intestinal glucose absorption in the perfused rat intestine. Biochem. Pharmacol. 2000;59:887–890. doi: 10.1016/s0006-2952(99)00396-2. [DOI] [PubMed] [Google Scholar]
- Kajbaf F., Fendri S., Basille-Fantinato A., Diouf M., Rose D., Jounieaux V., Lalau J.D. The relationship between metformin therapy and sleep quantity and quality in patients with Type 2 diabetes referred for potential sleep disorders. Diabet. Med. 2014;31:577–580. doi: 10.1111/dme.12362. [DOI] [PubMed] [Google Scholar]
- Kaushik U., Aeri V., Mir S.R. Cucurbitacins-an insight into medicinal leads from nature. Pharmacogn. Rev. 2015;9:12. doi: 10.4103/0973-7847.156314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.H., Lee I.S., Park J.Y., Kim Y., An E.J., Jang H.J. Cucurbitacin b induces hypoglycemic effect in diabetic mice by regulation of amp-activated protein kinase alpha and glucagon-like peptide-1 via bitter taste receptor signaling. Front. Pharmacol. 2018;9:1071. doi: 10.3389/fphar.2018.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Lee Y., Akitake B., Woodward O.M., Guggino W.B., Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.C., Choi D., Cha A., Lee Y.G., Baek N.I., Rimal S., Sang J., Lee Y., Lee S. Critical enzymes for biosynthesis of cucurbitacin derivatives in watermelon and their biological significance. Commun. Biol. 2020;3:1–11. doi: 10.1038/s42003-020-01170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkhart C., Scott K. Gustatory learning and processing in the Drosophila mushroom bodies. J. Neurosci. 2015;35:5950–5958. doi: 10.1523/JNEUROSCI.3930-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kang M.J., Shim J., Cheong C.U., Moon S.J., Montell C. Gustatory receptors required for avoiding the insecticide L-canavanine. J. Neurosci. 2012;32:1429–1435. doi: 10.1523/JNEUROSCI.4630-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim S.H., Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Moon S.J., Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.Z., Yang S.Q., Chuckaree C., Kuhajda F., Ronnet G., Diehl A.M. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat. Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- Madiraju A.K., Erion D.M., Rahimi Y., Zhang X.M., Braddock D.T., Albright R.A., Prigaro B.J., Wood J.L., Bhanot S., MacDonald M.J. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P., Worden K., Aso Y., Rubin G.M., Keene A.C. A dopamine-modulated neural circuit regulating aversive taste memory in Drosophila. Curr. Biol. 2015;25:1535–1541. doi: 10.1016/j.cub.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier N., Belgacem Y.H., Martin J.R. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J. Exp. Biol. 2007;210:1424–1434. doi: 10.1242/jeb.02755. [DOI] [PubMed] [Google Scholar]
- Molyneux R.J., Lee S.T., Gardner D.R., Panter K.E., James L.F. Phytochemicals: the good, the bad and the ugly? Phytochemistry. 2007;68:2973–2985. doi: 10.1016/j.phytochem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Moon S.J., Lee Y., Jiao Y., Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohadoma S., Michael H. Effects of co—administration of methanol leaf extract of Catharanthus roseus on the hypoglycemic activity of metformin and glibenclamide in rats. Asian Pac. J. Trop. Med. 2011;4:475–477. doi: 10.1016/S1995-7645(11)60129-6. [DOI] [PubMed] [Google Scholar]
- Oliveira D.T.d., Fernandes I.d.C., Sousa G.G.d., Santos T.A.P.d., Paiva N.C.N.d., Carneiro C.M., Evangelista E.A., Barboza N.R., Guerra-Sá R. High-sugar diet leads to obesity and metabolic diseases in ad libitum-fed rats irrespective of caloric intake. Arch. Endocrinol. Metab. 2020;64:71–81. doi: 10.20945/2359-3997000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Kwon J.Y. A systematic analysis of Drosophila gustatory receptor gene expression in abdominal neurons which project to the central nervous system. Mol. Cells. 2011;32:375. doi: 10.1007/s10059-011-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintana H., Apaijai N., Pratchayasakul W., Chattipakorn N., Chattipakorn S.C. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91:409–414. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Poudel S., Lee Y. Impaired taste associative memory and memory enhancement by feeding omija in Parkinson’s disease fly model. Mol. Cells. 2018;41:646. doi: 10.14348/molcells.2018.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulito C., Sanli T., Rana P., Muti P., Blandino G., Strano S. Metformin: on ongoing journey across diabetes, cancer therapy and prevention. Metabolites. 2013;3:1051–1075. doi: 10.3390/metabo3041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimal S., Lee Y. Molecular sensor of nicotine in taste of Drosophila melanogaster. Insect Biochem. Mol. Biol. 2019;111:103178. doi: 10.1016/j.ibmb.2019.103178. [DOI] [PubMed] [Google Scholar]
- Rimal S., Sang J., Dhakal S., Lee Y. Cucurbitacin B activates bitter-sensing gustatory receptor neurons via gustatory receptor 33a in Drosophila melanogaster . Mol. Cells. 2020;43:530. doi: 10.14348/molcells.2020.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovenko B.M., Kubrak O.I., Gospodaryov D.V., Perkhulyn N.V., Yurkevych I.S., Sanz A., Lushchak V., Lushchak V.I. High sucrose consumption promotes obesity whereas its low consumption induces oxidative stress in Drosophila melanogaster. J. Insect Physiol. 2015;79:42–54. doi: 10.1016/j.jinsphys.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Rowan J.A., Hague W.M., Gao W., Battin M.R., Moore M.P. Metformin versus insulin for the treatment of gestational diabetes. N. Engl. J. Med. 2008;358:2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- Sang J., Rimal S., Lee Y. Gustatory receptor 28b is necessary for avoiding saponin in Drosophila melanogaster. EMBO Rep. 2019;20:e47328. doi: 10.15252/embr.201847328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharaman S. The influences of dietary sugar and related metabolic disorders on cognitive aging and dementia. In: Malavolta M., editor. Molecular Basis of Nutrition and Aging. Elsevier; San Diego: 2016. pp. 331–344. [Google Scholar]
- Shaw P.J., Cirelli C., Greenspan R.J., Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Slack C., Foley A., Partridge L. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS One. 2012;7:e47699. doi: 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappa R., Geisler K., Louveau T., O'Maille P., Osbourn A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014;65:225–257. doi: 10.1146/annurev-arplant-050312-120229. [DOI] [PubMed] [Google Scholar]
- Vanderheyden W.M., Gerstner J.R., Tanenhaus A., Yin J.C., Shaw P.J. ERK phosphorylation regulates sleep and plasticity in Drosophila. PLoS One. 2013;8:e81554. doi: 10.1371/journal.pone.0081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella S., Buetow L., Royle P., Livingstone S., Colhoun H., Petrie J. The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia. 2010;53:809–820. doi: 10.1007/s00125-009-1636-9. [DOI] [PubMed] [Google Scholar]
- Wigglesworth V. The utilization of reserve substances in Drosophila during flight. J. Exp. Biol. 1949;26:150–163. doi: 10.1242/jeb.26.2.150. [DOI] [PubMed] [Google Scholar]
- Yeh S.H.H., Shie F.S., Liu H.K., Yao H.H., Kao P.C., Lee Y.H., Chen L.M., Hsu S.M., Chao L.J., Wu K.W. A high-sucrose diet aggravates Alzheimer's disease pathology, attenuates hypothalamic leptin signaling, and impairs food-anticipatory activity in APPswe/PS1dE9 mice. Neurobiol. Aging. 2020;90:60–74. doi: 10.1016/j.neurobiolaging.2019.11.018. [DOI] [PubMed] [Google Scholar]
- Zou C., Liu G., Liu S., Liu S., Song Q., Wang J., Feng Q., Su Y., Li S. Cucurbitacin B acts a potential insect growth regulator by antagonizing 20‐hydroxyecdysone activity. Pest Manag. Sci. 2018;74:1394–1403. doi: 10.1002/ps.4817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.