Abstract
Auranofin, an FDA-approved arthritis drug, has recently been repurposed as a potential antimicrobial agent; it performed well against many Gram-positive bacteria, including multidrug resistant strains. It is, however, inactive toward Gramnegative bacteria, for which we are in dire need of new therapies. In this work, 40 auranofin analogues were synthesized by varying the structures of the thiol and phosphine ligands, and their activities were tested against ESKAPE pathogens. The study identified compounds that exhibited bacterial inhibition (MIC) and killing (MBC) activities up to 65 folds higher than that of auranofin, making them effective against Gram-negative pathogens. Both thiol and the phosphine structures influence the activities of the analogues. The trimethylphosphine and triethylphosphine ligands gave the highest activities against Gramnegative and Gram-positive bacteria, respectively. Our SAR study revealed that the thiol ligand is also very important, the structure of which can modulate the activities of the AuI complexes for both Gram-negative and Gram-positive bacteria. Moreover, these analogues had mammalian cell toxicities either similar to or lower than that of auranofin.
Graphical Abstract
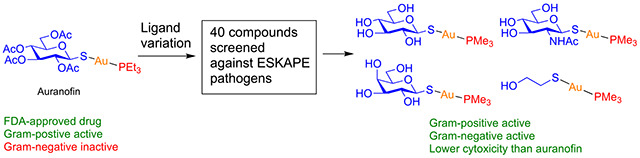
INTRODUCTION
The increasing prevalence of resistance to the majority of existing antibiotics has generated a pressing global healthcare crisis.1 To undermine the actions of antibiotics, bacteria have developed powerful resistance mechanisms, including mutational alterations of target proteins, loss of membrane porins,2 production of enzymes that degrade antibiotics,3–5 and overexpression of efflux pumps that drive antibiotics out of the bacterium.6–8 Certain highly resistant bacteria have acquired multiple mechanisms against all available antibiotics.9–11 The situation is especially dire for Gram-negative bacteria. The recent additions of antibiotics to the clinical pipeline have been limited to treating Gram-positive infections, and there have been no new classes of clinically approved antibiotics for Gram-negative bacteria since the discovery of quinolones in 1968.12 Untreatable antimicrobial resistance (AMR) is rapidly emerging in, for example, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii strains that are resistant to all commonly used antibiotics, including fluoroquinolones, β-lactams, macrolides, aminoglycosides, and tetracyclines, contributing to the majority of deaths caused by hospital-acquired infections. To combat AMR, new strategies are being developed, such as the use of efflux inhibitors,13 bacteriophages,14 and nanoparticles15–17 as antibacterial agents. However, these endeavors are often associated with lengthy and costly development processes; therefore, other avenues are needed. One such route is drug repurposing, which is the redirecting of known antiinflammatory drugs, anticancer drugs, and other drugs into antimicrobial use, providing viable candidates and potentially low cost ways to fight against AMR.18–20
Auranofin is a gold compound consisting of a tetraacetylated thioglucose and a triethylphosphine coordinated to AuI (1, Figure 1). The medicinal use of gold compounds can be traced back to the 19th century in treating tuberculosis, syphilis, and inflammatory rheumatoid diseases.21,22 Early studies showed that coordination of phosphine to AuI gave nonionic complexes that had high lipid solubility.23 In the search for better antiarthritic drugs, Sutton et al. at Smith Kline and French Laboratories discovered 2,3,4,6-tetra-O-acetyl-1-thio-β-d-glucopyranosato-S-(triethylphosphine) gold (i.e., auranofin), which gave excellent therapeutic efficacy and high serum gold concentration.24 Auranofin was subsequently approved by the FDA in 1985 as an oral anti-inflammatory aid in the treatment of rheumatoid arthritis.25,26 Its safety has been well-documented, with no reported concerns in terms of carcinogenicity, serious side effects, or other long-term safety issues.27 Auranofin has shown broad activity toward a number of thiol-dependent reductases that are essential for maintaining intracellular levels of free thiols and redox homeostasis. Inhibition of these enzymes by auranofin decreases cellular reducing capacity and weakens the defense against oxidative stress. Its broad activity has sparked increasing interest to repurpose this drug as potential therapy for other diseases.28 For example, its anticancer activity has been reported for different types of tumors, including melanoma and leukemia,29,30 and auranofin compares well with the currently marketed drug cisplatin.31,32 Auranofin has also shown strong activity against parasites that cause malaria33 and severe diarrhea,34 as well as invasive fungi that are particularly difficult to target.35,36 Auranofin is currently undergoing a phase II clinical trial for recurrent epithelial ovarian cancer.37 The results from an NIH-sponsored phase I trial to characterize the pharmacokinetics and safety of auranofin in healthy volunteers against the parasites Entamoeba histolytica and Giardia intestinalis supported the safety of auranofin as a broad-spectrum antiparasitic drug.25,38
Figure 1.
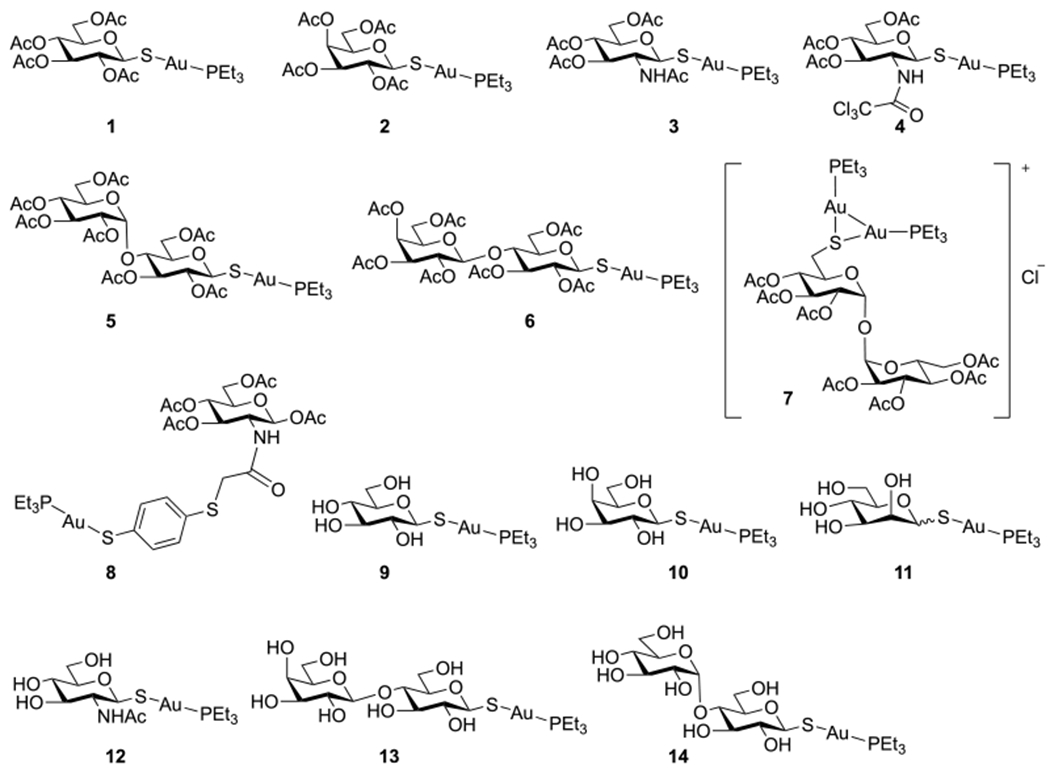
Auranofin (1) and Group 1 analogues with protected and unprotected thio sugar ligands (2–14).
The precise molecular mechanism of auranofin’s mode of action is still unclear. It is generally believed that upon administration of auranofin, the tetraacetylated thioglucose ligand is rapidly displaced by blood thiols.39 The AuI or [(PEt3)Au]+ species emanating from auranofin then bind to the redox-active selenocysteine or cysteine residues in thioredoxin reductase (TrxR) or other reductases.40 X-ray structure analyses, however, are inconclusive. In some cases, no gold was found in the active sites of, for example, E. histolytica TrxR41 or Schistosoma mansoni thioredoxin-glutathione reductase.40 In the case of Leishmania infantum trypanothione reductase, the tetraacetylated thioglucose was also found at the trypanothione binding site in addition to AuI, hinting at a dual mechanism of inhibition.42
Auranofin has also been assessed for its antimicrobial activity against different bacteria, where it showed no activity toward Gram-negative bacteria but performed well compared with current antibiotics against many Gram-positive bacteria, including multidrug resistant strains, and Mycobacterium tuberculosis.18,43–47 The source of this activity was postulated as the inhibition of the bacterial TrxRs, leading to lower intracellular thiol concentrations, disruption of the bacterial thiol-redox homeostasis, and weakening of the bacterial defense against oxidative stress.43 Other mechanisms have also been proposed, including disruption of selenium metabolism by forming a stable Au–Se adduct in the case of Clostridium difficile45 and inhibition of cell wall, DNA, protein, and toxin syntheses in the case of methicillin-resistant Staphylococcus aureus (MRSA).48 The thiophilic nature of auranofin, which may react with other cysteine-containing enzymes, and the suggested multiple modes of action argue well for the potential of auranofin against drug-resistant bacteria and in preventing the emergence of resistance. In fact, studies have shown the inability of M. tuberculosis43 and S. aureus48 to generate auranofin-resistant mutants.
The ineffectiveness of auranofin toward Gram-negative bacteria was suggested to be the result of their glutathione system, glutathione/glutathione reductase, being able to compensate for the loss of reducing capability in the Trx–TrxR system caused by auranofin.43 An alternative hypothesis was also proposed, suggesting that the outer membranes of Gram-negative bacteria were an effective barrier to auranofin.48 This argument was supported by results showing that Gramnegative strains could be made susceptible to auranofin by coadministration with a membrane permeable agent like polymyxin B.48,49
There are limited structure–activity relationship (SAR) studies on auranofin.24,49,50 These and other investigations generally conclude that the thioglucose ligand is not essential, and the pharmacophore is likely either AuI or the [(PEt3)Au]+ in complex with the target. In this study, we synthesized 40 auranofin analogues by varying the structures of the thiol and phosphine ligands and tested their antimicrobial activities against Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter cloacae, the six ESKAPE pathogens that have shown growing multidrug resistant virulence and cause two-thirds of all clinical infections,51 as well as a control strain of Gram-negative E. coli. A preliminary in vitro toxicity study was also carried out on A549 cells. Our results demonstrate the following: (1) Auranofin can be made active toward Gramnegative pathogens by manipulating the ligands coordinated to AuI. Although auranofin itself has no activity against Gramnegative pathogens, some analogues become active even for P. aeruginosa. (2) The thiol ligand is important for the activity. (3) The analogues showed mammalian cell toxicity either similar to or lower than that of auranofin.
RESULTS
Design and Synthesis of Auranofin Analogues.
Auranofin is a monomeric linear complex consisting of a Au(I) core coordinated with a peracetylated thioglucose and a triethylphosphine ligand (1, Figure 1). Gold is a late transition metal with the most common oxidation states of +1 (aurous AuI) and +3 (auric AuIII). Approved gold drugs, including sodium aurothiomalate and auranofin, are AuI compounds, probably because of the high toxicity of AuIII complexes (e.g., HAuCl4).27 AuI is a soft acid.52 AuI salts coordinated with relatively hard bases like chloride are unstable and generally disproportionate into metallic gold and AuIII in the presence of water.27 As such, the most stable AuI complexes and nanoclusters have soft ligands like thiols and phosphines. The roles of the phosphine and thiol ligands were investigated by Sutton et al., who studied the in vivo antiarthritic activities of 13 auranofin and analogues prepared by combining different alkylphosphine ligands (Me, Et, i-Pr, and n-Bu) with either Cl or a thio glucose ligand.24 It was concluded that the phosphine ligand played a major role in the activity of the gold complex, whereas the nonphosphine ligand had less of an effect. Triethylphosphine (PEt3), the ligand in auranofin, gave the best therapeutic efficacy and the highest serum gold concentration. The authors also showed that the nonphosphine ligand could modulate the cytotoxicity, such that the acetylated thio glucose ligand rendered auranofin less toxic than triethylphosphine gold chloride (rat acute oral LD50 values of 265 and 79.0 mg/kg, respectively), which was further confirmed by other studies.53
Here, we conducted an SAR study by varying the structures of the thiol and the phosphine ligands on auranofin. Three groups of compounds were initially synthesized: Group 1 compounds are analogues with different thio sugar structures (2–14), Group 2 compounds are analogues with aromatic or aliphatic thiols (15–23), and Group 3 compounds are analogues with varying phosphine structures (24–36). Subsequent antibacterial activity studies of these compounds led to the identification of structural features that were then applied to the design and synthesis of analogues 37–40.
In Group 1 (Figure 1), the peracetylated thioglucose (Glc(Ac)4) ligand in auranofin was replaced by peracetylated galactose (Gal(Ac)4, 2), N-acetylglucosamine (GlcNAc(Ac)3; 3, 4, and 8), maltose (Mal(Ac)7, 5), lactose (Lac(Ac)7, 6), and trehalose (Tre(Ac)7, 7). Deacetylated analogues of Glc (9), Gal (10), Man (11), GlcNAc (12), Lac (13), and Mal (14) were also included to test the impact of lipophilicity on the activities of the analogues.
Compounds 1–6 were synthesized from β-glycosyl thiols 1c–6c and Et3PAuCl according to reported methods (Scheme 1).54–57 Briefly, α-glycosyl halides 1a–6a were treated with KSAc to give peracetylated thioglycosides 1b–6b through a straightforward SN2 reaction. S-Deacetylation of the anomeric thioacetates 1b–6b by transthioesterification using dithiothreitol (DTT) afforded the glycosyl thiols 1c–6c, which were then treated with Et3PAuCl under mild basic conditions for 1–1.5 h to give auranofin, 1, and analogues 2–6 in good to excellent yields.
Scheme 1. Synthesis of Auranofin, 1, and Analogues 2–6a.
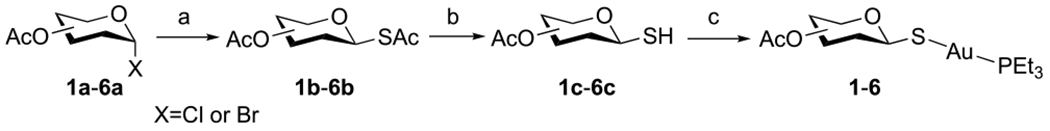
aReagents and conditions: (a) KSAc, acetone, RT, 3–5 h (82–90%). (b) DTT, NaHCO3, DMF, RT, 1–1.5 h (90–95%). (c) Et3PAuCl, K2CO3, DCM/H2O, 0 °C to RT, 1–1.5 h (61–94%).
The synthesis of the Tre(Ac)7 analogue, 7, follows the procedure shown in Scheme 2. Reaction of d-trehalose 7a with benzaldehyde dimethyl acetal catalyzed by TsOH gave a mixture of trehalose, trehalose benzylidene, and trehalose dibenzylidene, which was purified after acetylation to give peracetylated trehalose benzylidene 7b in 40% overall yield. The N-bromosuccinimide (NBS)-mediated cleavage of benzylidene acetal 7b gave the 6-bromo trehalose derivative, 7c, in 89% yield. Bromide 7c was then converted to thioacetate 7d through the iodide intermediate in an overall 89% yield. Deprotection of the benzoyl group followed by acetylation afforded 7e (72%, two steps), which was then S-deacetylated via DTT-mediated transthioesterification to afford the thiol derivative 7f in 89% yield. Direct coupling of 7f with Et3PAuCl gave only the dinuclear gold complex 7. Even after reduction of the amount of Et3PAuCl, no mononuclear gold(I) complex was observed. Stronger bases like DBN gave similar results, whereas NaOMe and NaOH removed the acetyl groups instead. The formation of the dinuclear gold structure was supported by NMR and high-resolution mass spectrometry (HRMS). The integrations of peaks at 1.80 and 1.17 ppm in the 1H NMR (Figure S34), assigned to CH2 and CH3 in PEt3, respectively, corresponded to two PEt3 ligands in the complex. The 31P NMR gave a single peak at 34.66 ppm (Figure S36), whereas for all other triethylphosphine mononuclear gold complexes synthesized in this work, the chemical shifts of P in PEt3 appeared at 36–39 ppm instead. The structure was further supported by HRMS, where the highest intensity peak at 1281.2744 corresponded to the molecular ion of 7. The thiolate in 7f, formed from a less acidic thiol at C-6, is more nucleophilic than those thiolates formed from more acidic anomeric thiols at C-1 in compounds 1–6, allowing the C-6 thiolate to react further with Et3PAuCl to form dinuclear gold complex 7. There are similar reports in the literature on the formation of dinuclear gold complexes, such as that from the reaction of a mononuclear gold(I) complex bearing a cysteine-containing dipeptide ligand with [Au(OTf)(PPh3)].58
Scheme 2. Synthesis of Analogue 7a.
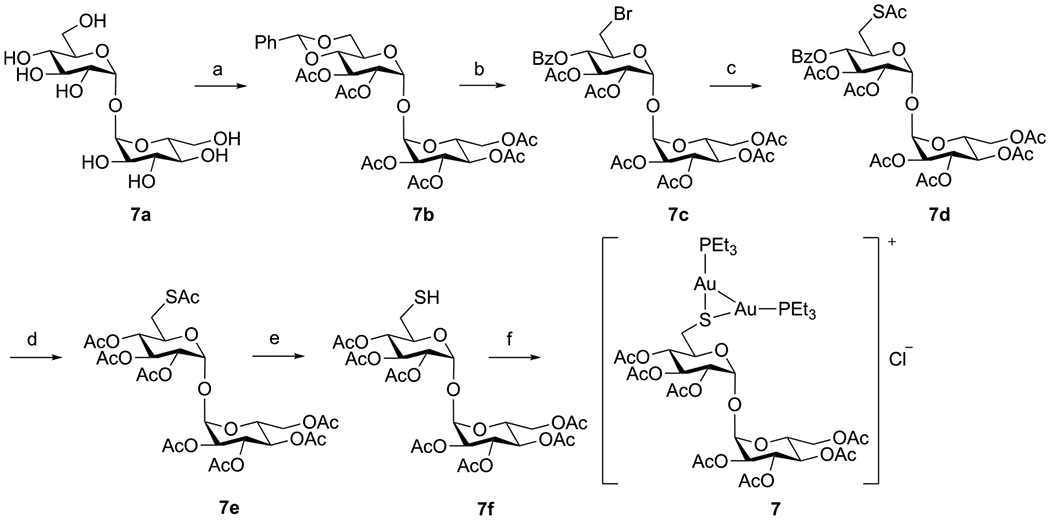
aReagents and conditions: (a) (i) PhCH(OMe)2, TsOH, DMF; (ii) Ac2O, Et3N, DMF (40% over two steps). (b) NBS, CaCO3, CCl4 (89%). (c) (i) KI, DMF, 60 °C; (ii) KSAc, DMF, RT, 8 h (87%). (d) (i) NaOMe, MeOH, RT; (ii) Ac2O, pyridine, 0 °C to RT (72%). (e) DTT, NaHCO3, DMF, RT, 24 h (93%). (f) Et3PAuCl, DBU, DCM, RT (62%).
Compound 8 was synthesized from the d-glucosamine hydrochloride 8a (Scheme 3). Reaction with p-anisaldehyde gave the Schiff base glucosamine imine 8b, which was then peracetylated to afford 8c. Subsequent hydrolysis in HCl/acetone gave peracetylated glucosamine hydrochloride 8d, which reacted with chloroacetic anhydride to afford 8e. A final substitution by 1,4-benzenedithiol followed by coupling with Et3PAuCl gave gold complex 8 in 96% yield.
Scheme 3. Synthesis of Analogue 8a.
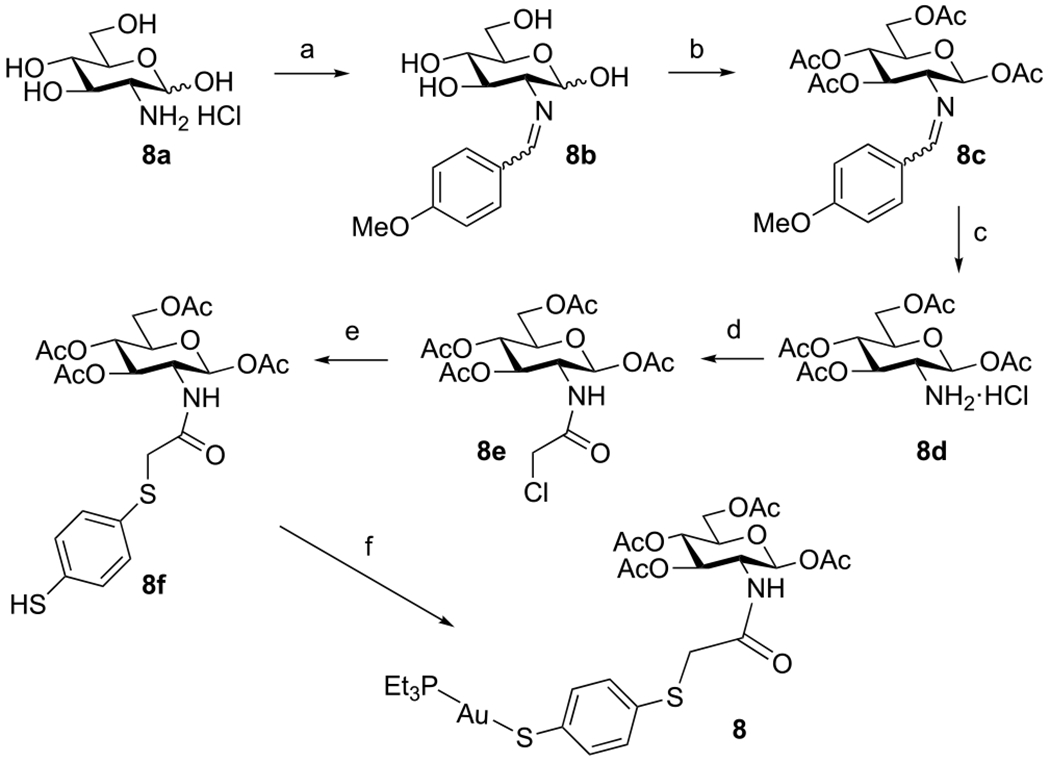
aReagents and conditions: (a) p-Anisaldehyde, NaOH, H2O, RT, 1 h (80%). (b) Ac2O, pyridine, RT, overnight (81%). (c) 5 M HCl, acetone, reflux, 30 min (86%). (d) Chloroacetic anhydride, pyridine, DCM, 0 °C to RT (89%). (e) 1,4-Benzenedithiol, TEA, DCM, 16 h (91%). (f) Et3PAuCl, DBU, DCM, RT, 1.5 h (96%).
The analogues containing unprotected thio sugar ligands (9–14) were synthesized by treating the corresponding peracylated thio sugar analogues with NaOMe/MeOH followed by Et3PAuCl in a one-pot reaction (Scheme 4).
Scheme 4. Synthesis of Analogues 9–14a.
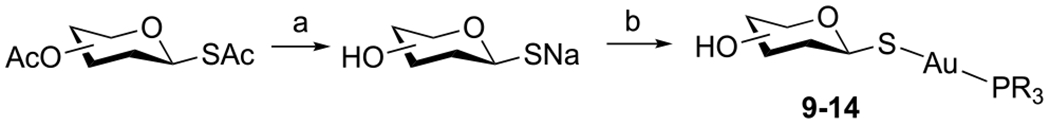
aReagents and conditions: (a) NaOMe, MeOH, RT, 2–4 h. (b) Et3PAuCl, MeOH, 1–2 h, 57–83% over two steps.
Analogues 15–23 in Group 2 are compounds with the thio sugar replaced by an aromatic or aliphatic thiol ligand (Figure 2). Both electron-donating and electron-withdrawing groups were included to test the electronic effect of the thiol ligand on the antimicrobial activity. For the synthesis of gold(I) complexes with aromatic thiol ligands (15, 16, and 18–20), the corresponding thiol was treated with Et3PAuCl in DCM in the presence of DBU, and the product was obtained after straightforward purification by column chromatography (condition a, Scheme 5). Analogues with aliphatic thiol ligands (21–23) were prepared by reacting the corresponding thiol with Et3PAuCl in the presence of one equivalent of NaOMe in methanol (condition b, Scheme 5). Analogue 17 was also synthesized following this method and was purified by recrystallization.
Figure 2.
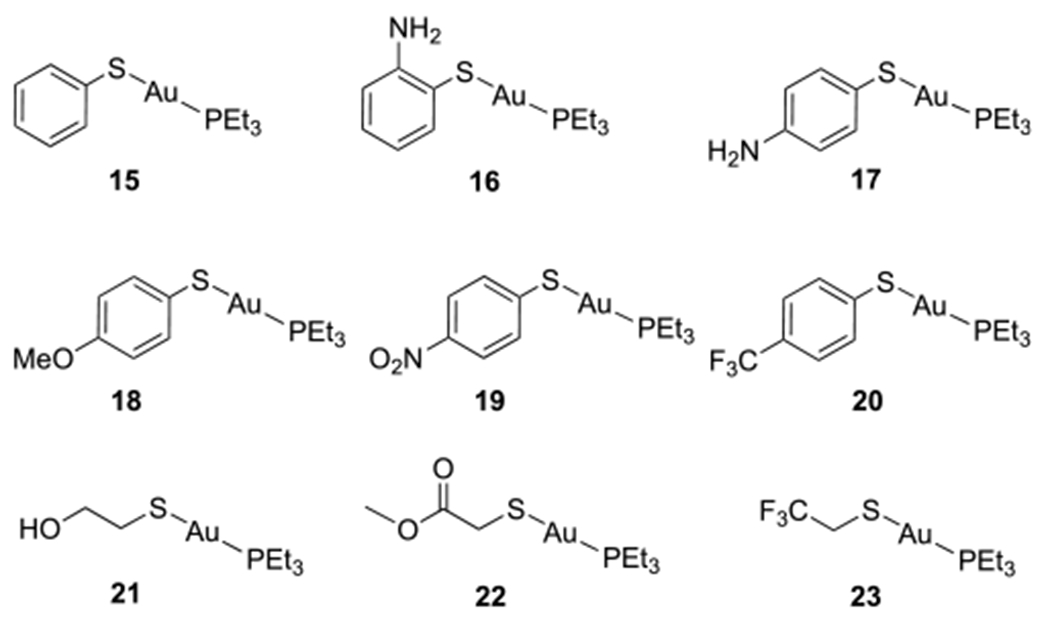
Group 2 analogues with aromatic and aliphatic thiol ligands.
Scheme 5. Synthesis of Group 2 Analogues 15–23 with Aromatic and Aliphatic Thiol Ligandsa.
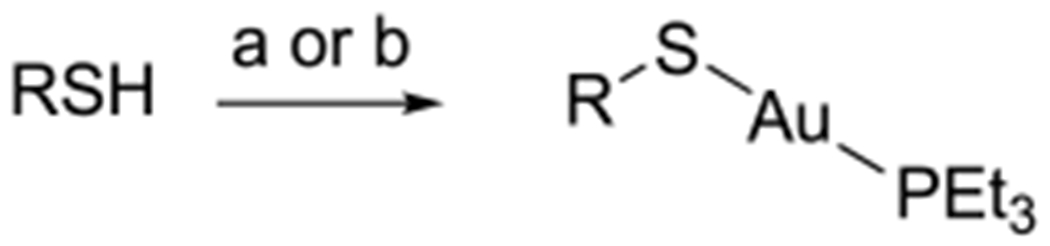
aReagents and conditions: (a) For compounds 15, 16, 18–20: Et3PAuCl, DBU, DCM, RT, 1–2 h (62–91%). (b) For compounds 17, 21–23: Et3PAuCl, NaOMe, MeOH, RT, 1–2 h (59–92%).
Analogues 24–36 in Group 3 are compounds with different phosphine structures (Figure 3). Aliphatic phosphines with varying chain lengths (methyl, ethyl, and n-butyl) and aromatic phosphines with either electron-donating (OMe) or electron-withdrawing (F or CF3) groups were prepared as both gold chloride and gold thio sugar complexes. AuCl was generated in situ by reducing HAuCl4 with 2,2′-thiodiethanol, which was treated immediately with a cold solution of 1.05 equiv of the corresponding triphosphine in EtOH/DCM to give triphosphine gold chlorides 24–30 (reaction a, Scheme 6).24
Figure 3.

Group 3 analogues with different phosphine ligand structures.
Scheme 6. Synthesis of Auranofin Analogues with Different Phosphine Ligand Structuresa.
aReagents and conditions: (a) 2,2′-Thiodiethanol, H2O/EtOH/DCM, 0 °C to RT, 2 h (72–94%). (b) 1c, DBU, toluene, RT, 2 h (83–90%).
Auranofin analogues 31–36 were prepared straightforwardly in high yields by treating the corresponding triphosphine gold chlorides (24 and 26–30) with peracetylated thioglucose 1c (reaction b, Scheme 6)
In Vitro Antimicrobial Activities.
The antimicrobial activities of auranofin and its analogues were tested against ESKAPE pathogens, including four Gram-negative strains (A. baumannii, P. aeruginosa, E. cloacae, and K. pneumonia) and two Gram-positive strains (S. aureus and E. faecium). A quality control strain, E. coli ATCC 25922, was included in all assays and validated with ciprofloxacin and teicoplanin in every trial. The results are presented in Tables 1–3 (in μM) and in Tables S1–S3 (in μg/mL) as minimal inhibitory concentrations (MICs), the lowest concentration that inhibits the growth of the bacteria, and minimal bactericidal concentrations (MBCs), the lowest concentration that kills the bacteria. Auranofin (1) showed potent activities against both Gram-positive strains, S. aureus and E. faecium, with MICs (MBCs) of 0.03 (0.06) and 0.12 (0.25) μg/mL, respectively (Table S1). It showed weak activity against A. baumannii and E. coli, with MIC/MBC values of 32 and 16 μg/mL, respectively. Auranofin lacks significant activity against P. aeruginosa, E. cloacae, and K. pneumonia. These results are in agreement with previous reports that consistently showed the anti-Gram-positive-only activity of auranofin.18,43,44,47,48,59,60
Table 1.
MIC/MBC (μM) Valuesa of Group 1 Analogues with Varying Thio Sugar Structures
| A. baumannii NCTC 13420 | P. aeruginosa NCTC 13437 | E. cloacae NCTC 13405 | K. pneumoniae ATCC 700603 | S. aureus JE2 (USA300) | E.faecium ATCC 700221 | E. coli ATCC 25922 | log P | |
|---|---|---|---|---|---|---|---|---|
| 1 | 47 (47) | 377 (377) | 189 (189) | 377 (377) | 0.04 (0.09) | 0.2/0.09 (0.4) | 24 (24) | 0.56 |
| 2 | 47 (94) | 377 (377) | 377 (377) | >377 | 0.04 (0.09) | 0.2/0.4 (0.4) | 24 (24) | 0.38 |
| 3 | 24 (47) | 189 (189) | 47 (94) | 189 (189) | 0.04/0.09 (0.09) | 0.09 (0.4) | 12 (12) | 0.26 |
| 4f | 82 (164) | >328 | >328 | >328 | 0.01 (0.01) | 0.08 (0.2) | 21 (21) | 1.01 |
| 5f | 33 (33) | 132 (132) | 66 (66) | >265 | 0.008 (0.06) | 0.03/0.06 (0.1) | 17 (17) | 0.45 |
| 6g | 66 (132) | 132 (132) | 265 (265) | >265 | 0.03 (0.1) | 0.1 (0.3) | 33 (33) | 1.04 |
| 7c | 12 (12) | >194 | 24 (24) | >194 | 0.003/0.006 (0.006) | 0.05 (0.09) | 24 (24) | −0.28 |
| 8f | 76/19 (19) | >303 | 152 (303) | >303 | 0.04/0.009 (0.1) | 0.04/0.07 (0.3) | 152/323 (152) | −0.36 |
| 9 | 63/16 (251) | 502 (502) | 125 (125) | 502 (502) | 0.06/0.1 (0.5) | 0.1 (2) | 31 (31) | −0.81 |
| 10 | 31 (31) | 251 (251) | 63 (63) | 251 (251) | 0.02 (0.1) | 0.1/0.2 (0.5) | 16 (16) | −0.73 |
| 11 | 31 (63) | 502 (502) | 125 (251) | 502 (502) | 0.02/0.06 (0.1) | 0.1/0.06 (0.5) | 16 (16) | −0.59 |
| 12 | 15/29 (116) | 464 (464) | 116 (232) | 464 (464) | 0.02 (0.05) | 0.05/0.1 (0.5) | 7/4 (7) | −0.89 |
| 13 | 24/48 (190) | 381 (381) | 190 (190) | 381 (381) | 0.02/0.05 (0.09) | 0.09 (0.4) | 24 (24) | −1.81 |
| 14 | 24 (24) | 381 (381) | 190 (190) | >381 | 0.09/0.04 (0.09) | 0.09/0.2 (0.4) | 12 (12) | −1.69 |
Assays were repeated twice. Only one value is presented unless both data are shown.
Lowest precipitation concentration at 4 μg/mL.
Lowest precipitation concentration at 8 μg/mL.
Lowest precipitation concentration at 16 μg/mL.
Lowest precipitation concentration at 32 μg/mL.
Lowest precipitation concentration at 64 μg/mL.
Lowest precipitation concentration at 128 μg/mL.
Table 3.
MIC/MBC (μM) Valuesa of Group 3 Analogues with Trialkyl- or Triaryl-phosphine Ligands
| A. baumannii NCTC 13420 | P. aeruginosa NCTC 13437 | E. cloacae NCTC 13405 | K. pneumoniae ATCC 700603 | S. aureus JE2 (USA300) | E. faecium ATCC 700221 | E. coli ATCC 25922 | log P | |
|---|---|---|---|---|---|---|---|---|
| 24 | 7/13 (13) | 52 (52) | 3 (3) | 7/13 (13) | 0.1/0.2 (1.6) | 0.4 (3) | 7 (7) | 0.16 |
| 25 | 11/23 (23) | 92 (183) | 23 (23) | 46 (46) | 0.001/0.02 (0.2) | 0.09/0.2 (0.7) | 23 (23) | 1.74 |
| 26e | 74/147 (147) | 74 (74) | >589 | 147 (147) | 2 (4) | 5 (5) | >589 | >3.99 |
| 27d | 16/129 (518) | >518 | >518 | >518 | 1/2 (4) | 4 (4) | >518 | >3.94 |
| 28c | >438 | >438 | >438 | >438 | 2 (3) | 3 (3) | >438 | >4.09 |
| 29d | 29/58 (233) | >467 | >467 | >467 | 1/ (4) | 2/4 (4) | >467 | >4.04 |
| 30e | >366 | >366 | >366 | >366 | 3/1 (11) | >366 | >366 | >3.94 |
| 31g | 6/13 (101) | 101 (101) | 3 (3) | 13 (13) | 0.1 (0.2) | 0.2/0.4 (0.8) | 6 (6) | 0.35 |
| 1 | 47 (47) | 377 (377) | 189 (189) | 377 (377) | 0.04 (0.09) | 0.2/0.09 (0.4) | 24 (24) | 0.56 |
| 32e | 84 (>336) | >336 | >336 | >336 | 3 (3) | 3/5 (3) | >336 | >4.32 |
| 33c | >311 | >311 | >311 | >311 | 1/2 (2) | 5 (5) | >311 | >3.87 |
| 34c | >281 | >281 | >281 | >281 | 1 (2) | 2/4 (4) | >281 | >3.03 |
| 35c | >292 | >292 | >292 | >292 | 1 (2) | 2/5 (5) | >292 | >3.04 |
| 36e | >249 | >249 | >249 | >249 | 4 (8) | 31 (62) | >249 | >3.55 |
Assays were repeated twice. Only one value is presented, unless both data are shown.
Lowest precipitation concentration at 4 μg/mL.
Lowest precipitation concentration at 8 μg/mL.
Lowest precipitation concentration at 16 μg/mL.
Lowest precipitation concentration at 32 μg/mL.
Lowest precipitation concentration at 64 μg/mL.
Lowest precipitation concentration at 128 μg/mL.
The results of Group 1 compounds (i.e., analogues with varying thio sugar structures, Figure 1) are shown in Table 1. Changing Glc(Ac)4 in auranofin (1) to Gal(Ac)4 (2) did not cause much difference in activity against any of the strains. The GlcNAc analogue, 3, showed 2–4 times increases in activity against all Gram-negative strains and E. faecium. Substituting the N-acetyl group of GlcNAc with an N-trichloroacetyl group (4) completely diminished the enhancement in the activities against all Gram-negative strains, and the GlcNAc analogue with an aromatic linker (8) showed similar results. However, compound 4 showed excellent activity against S. aureus, lowering the MIC/MBC of auranofin by up to 4 times. The disaccharide maltose analogue, 5, also enhanced the activity against S. aureus, E. faecium, P. aeruginosa, and E. cloacae by 2–4 times. This was, however, not observed for the other disaccharide lactose analogue, 6, which showed no improvement against Gram-positives and was worse against all Gramnegative strains. The trehalose analogue, 7, increased the activity against E. cloacae by 8-fold and was 15 times better than auranofin against S. aureus, with an MIC/MBC of 6 nM, perhaps because of the presence of two AuPEt3+ in 7.58 The deacetylated auranofin analogue, 9, had similar activity to that of auranofin (1) against all strains. Among the other deacetylation analogues, compound 10 showed 3 times enhancement in activity against E. cloacae, and compound 12 was 2–3 times better than auranofin against S. aureus and E. coli.
Table 2 lists the MIC/MBC values of the Group 2 analogues, which contained aromatic and aliphatic thiol ligands (15–23, Figure 2). Among the compounds bearing aromatic thiol ligands (15–20), those with electron-donating groups on their phenyl rings (16–18) were generally more active than auranofin. Compound 17, with a p-aminophenyl group, showed the highest activity enhancement of up to 10 times over that of auranofin against all strains. The electron-withdrawing groups (NO2 and CF3), on the other hand, made analogues 19 and 20 completely inactive toward all Gram-negative strains tested. Compound 19 is, however, very active against S. aureus, lowering the MIC/MBC of auranofin by up to 10 times. Replacing the thio sugar in auranofin with an aliphatic thiol ligand generally increased the activity (21–23). For example, compound 21 had an MIC 19 times lower than that of auranofin against E. cloacae as well as against K. pneumoniae, and it showed activity against P. aeruginosa with a MIC/MBC of 41 μM, which was 9 times better than that of auranofin. For E. faecium, the activities of most analogues were similar to that of auranofin, with the exception of compounds 16 and 17, which had MICs more than 7 times lower than that of auranofin. With the exception of compound 17, all other analogues showed higher activities than that of auranofin against S. aureus.
Table 2.
MIC/MBC (μM) Valuesa of Group 2 Analogues with Aromatic or Aliphatic Thiol Ligands
| A. baumannii NCTC 13420 | P. aeruginosa NCTC 13437 | E. cloacae NCTC 13405 | K. pneumoniae ATCC 700603 | S. aureus JE2 (USA300) | E. faecium ATCC 700221 | E. coli ATCC 25922 | log P | |
|---|---|---|---|---|---|---|---|---|
| 1 | 47 (47) | 377 (377) | 189 (189) | 377 (377) | 0.04 (0.09) | 0.2/0.09 (0.4) | 24 (24) | 0.56 |
| 15f | 19/75 (>603) | 302 (302) | 75 (603) | >603 | 0.02 (0.6) | 0.6 (1) | 151 (151) | 2.20 |
| 16g | 18/36 (18) | 291 (291) | 18/73 (36/73) | 146 (146) | 0.009 (0.6) | 0.02 (0.1) | 36 (36) | 0.61 |
| 17d | 18 (18) | 146 (146) | 36 (36) | 36 (36) | 0.07/0.02 (0.07) | 0.02 (0.1) | 9 (9) | 1.36 |
| 18f | 35 (35) | 282 (282) | 141 (141) | 141 (141) | 0.02 (0.3) | 0.02/0.1 (0.3) | 35 (35) | 2.01 |
| 19e | >546 | >546 | >546 | >546 | 0.0004/0.02 (0.02) | 0.06/0.1 (0.5) | >546 | >3.28 |
| 20e | 520 (>520) | >520 | >520 | >520 | 0.02/0.1 (0.2) | 0.02 (0.2) | >520 | >3.18 |
| 21e | 10 (82) | 41 (41) | 5/10 (82) | 20 (20) | 0.03 (0.03) | 0.2/0.3 (0.6) | 10 (10) | 1.19 |
| 22d | 19 (76) | 305 (305) | 38 (152) | 76 (76) | 0.005/0.02 (0.02) | 0.1/0.3 (0.6) | 76 (76) | 1.75 |
| 23g | 37/74 (149) | 149 (149) | 37 (149) | 149 (149) | 0.009 (0.1) | 0.6 (1) | 74 (74) | 2.08 |
Assays were repeated twice. Only one value is presented, unless both data are shown.
Lowest precipitation concentration at 4 μg/mL.
Lowest precipitation concentration at 8 μg/mL.
Lowest precipitation concentration at 16 μg/mL.
Lowest precipitation concentration at 32 μg/mL.
Lowest precipitation concentration at 64 μg/mL.
Lowest precipitation concentration at 128 μg/mL.
The compounds in Group 3 are analogues with trialkylphosphines of varying chain lengths (24–26, 31, and 32) and triarylphosphines with different electronic properties (27–30 and 33–36, Figure 3); the MIC/MBC values are shown in Table 3. For the Gram-negative strains, the activity generally decreased with increasing alkyl chain length (24 > 25 > 26 and 31 > 1 > 32). Replacing triethylphosphine with trimethylphosphine resulted in a drastic enhancement in activity. For example, the trimethylphosphine analogue 31 improved the activity of auranofin against all Gram-negatives tested. The MICs of 31 against E. cloacae and K. pneumoniae were 60- and 30-fold lower, making it active against these two strains, whereas auranofin is inactive for both. On the other hand, the tri-n-butylphosphine ligand completely diminished the activity of the resulting analogue, 32, against all Gram-negatives. Aromatic phosphine ligands were also detrimental. Analogues with triarylphosphine ligands having either electron-withdrawing or electron-donating groups (33–36) showed almost no activity against the Gram-negative strains.
For the two Gram-positive strains, replacing triethylphosphine in auranofin with trimethylphosphine (31) had almost no impact on activity. The longer tri-n-butylphosphine ligand decreased the activity by more than an order of magnitude (32 vs 1 and 26 vs 25), as did all triarylphosphine ligands (27–30 and 33–36).
These results demonstrated that the Gram-negative activity of auranofin can be effectively modulated by altering the thiol and phosphine ligands. The following conclusions can be drawn from these data with regard to Gram-negative activities: (1) Deprotected thio sugars do not decrease the activity (e.g., 9–14, Table 1). It was reported that following oral administration, auranofin metabolized to yield deacetylated glucose.61 (2) Among the nonsugar thiol ligands, mercaptoethanol showed the best activity enhancement against Gram-negatives (21, Table 2). (3) Trimethylphosphine is a substantially better ligand than triethylphosphine against Gram-negatives (24 and 31, Table 3). On the basis of these observations, analogues 37–40 were designed (Figure 4). Compounds 37–39, which have trimethylphosphine ligands and deprotected Glc, Gal, and GlcNAc thio sugars, respectively, were prepared following reaction b in Scheme 4, except that Me3PAuCl was used instead of Et3PAuCl. All three compounds showed substantial enhancement in activity compared with that of auranofin against almost all Gramnegative strains (Table 4). Aside from the MIC against P. aeruginosa, the MICs against A. baumannii, K. pneumoniae, and E. coli were lowered by 3–12-fold, and that against E. cloacae was lowered by up to 44-fold. The highest activity was observed for compound 38, the deacetylated Gal and trimethylphosphine analogue, which reached MIC/MBC values of 4.5 and 34 μM against E. cloacae and K. pneumoniae, bacteria that are otherwise unaffected by auranofin.
Figure 4.
Auranofin analogues with trimethylphosphines and deprotected thio sugars (37–39) or mercaptoethanol ligands (40).
Table 4.
MIC/MBC (μM) Valuesa of Analogues with Trimethylphosphine Ligands
| A. baumannii NCTC 13420 | P. aeruginosa NCTC 13437 | E. cloacae NCTC 13405 | K. pneumoniae ATCC 700603 | S. aureus JE2 (USA300) | E. faecium ATCC 700221 | E. coli ATCC 25922 | log P | |
|---|---|---|---|---|---|---|---|---|
| 1 | 47 (47) | 377 (377) | 189 (189) | 377 (377) | 0.04 (0.09) | 0.2/0.09 (0.4) | 24 (24) | 0.56 |
| 31g | 6/13 (101) | 101 (101) | 3 (3) | 13 (13) | 0.09 (0.2) | 0.2/0.4(0.8) | 6(6) | 0.35 |
| 37 | 17/4 (17) | >547 | 4/9 (17) | 34 (34) | 0.3/0.5 (1) | 0.3/0.5 (0.5) | 9 (9) | −1.63 |
| 38 | 17/9 (17) | >547 | 4(4) | 34 (34) | 0.3 (0.3) | 0.3/0.5 (0.5) | 9 (9) | −1.88 |
| 39 | 16/8 (16) | >503 | 8/16 (16) | 31 (126) | 0.5 (0.5) | 0.2/0.5 (0.5) | 8/31 (31) | −2.03 |
| 40f | 6/3 (23) | 23/91 (91) | 3 (3) | 11 (23) | 0.3 (0.7) | 0.3 (0.3) | 1/6 (11) | −0.15 |
Assays were repeated twice. Only one value is presented, unless both data points are shown.
Lowest precipitation concentration at 4 μg/mL.
Lowest precipitation concentration at 8 μg/mL.
Lowest precipitation concentration at 16 μg/mL.
Lowest precipitation concentration at 32 μg/mL.
Lowest precipitation concentration at 64 μg/mL.
Lowest precipitation concentration at 128 μg/mL.
Analogue 40, with the best phosphine ligand (trimethylphosphine) and the best thiol ligand (mercaptoethanol), was synthesized following condition b in Scheme 5 using Me3PAuCl instead of Et3PAuCl. As expected, this compound had the highest activity against all five Gram-negative strains (Table 4). The best activity enhancement was seen for E. cloacae, against which it was 65-fold more active than auranofin, with an MIC/MBC of 2.9 μM.
When comparing the activities of all trimethylphosphine analogues and auranofin (Table 4), it can be seen that trimethylphosphine was the best ligand for Gram-negatives. Deacetylation of the thio glucose on 31 lowered the activity of the resulting analogue, 37, against most Gram-negatives, but the activity could be recovered by using the small polar ligand mercaptoethanol. This was not the case for the Gram-positives, against which 37–40 showed almost no change in activity.
Cytotoxicity.
The cytotoxicity was next tested against A549 human alveolar epithelial cancer cells, and the results are presented in Figure 5 and Table S5. Except for compounds 32–35, all other analogues had toxicities either similar to or lower than that of auranofin. As anticipated, the deprotected thio sugar analogues 9–14 and 37–39 were 3–10 times less toxic compared with auranofin, 1 (IC50 = 11.3 ± 1.9 μM). Analogues 31 and 40, which showed the largest improvement in activity against Gram-negative strains, had lower toxicity (IC50 = 20.6 ± 3.6 and 35.1 ± 8.9 μM, respectively) than auranofin.
Figure 5.
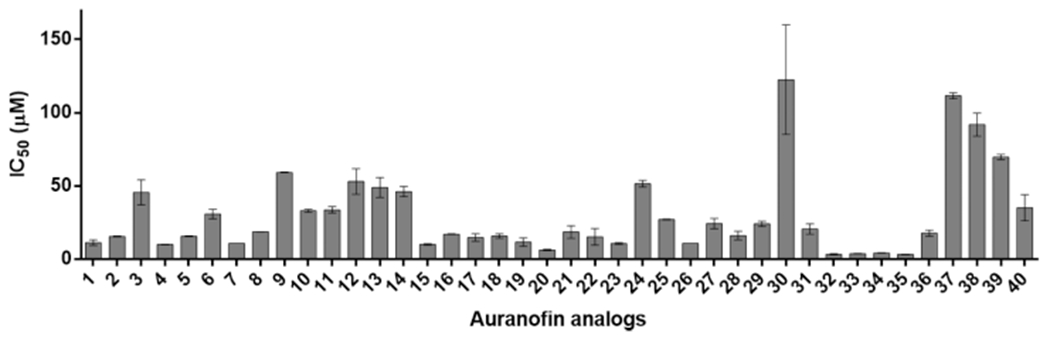
IC50 (μM) values of auranofin and analogues against A549 cells. The data are presented as means ± SEM from two independent experiments.
DISCUSSION AND CONCLUSIONS
Synthesis of AuI Complexes.
Auranofin can be straightforwardly synthesized from peracetylated thio glucose and Et3PAuCl using K2CO3 as the base in the biphasic medium DCM/H2O.55 Analogues 2–6 were synthesized following the same protocol (Scheme 1). However, this method is not general. For example, in the synthesis of 32–36, which contain more nonpolar triphosphines, the reactions required much longer times, and the yields were unsatisfactory, probably because side reaction formed byproducts like phosphine oxide. We then developed a single-phase reaction using DBU as the base in an organic solvent. This method worked well for most analogues (1–6, 8, 15–16, 18–20, and 31–36). The reactions were completed within 2 h, and the products could be purified straightforwardly by column chromatography. For 1–6 and 31–36, toluene was the preferred solvent instead of DCM (Scheme 6), as it was found that the thio sugars reacted with DCM to form the corresponding glycosylthiomethyl chlorides.62 Aromatic thiols were inert to DCM under the same conditions; thus analogues 15–16 and 18–20 were prepared in DBU/DCM (condition a, Scheme 5). However, this method failed for compound 17, for which TLC indicated the formation of an unstable compound. In this case, a strong base, NaOMe, was used, and compound 17 was successfully synthesized in 92% yield. This condition works well for substrates that can tolerate strong bases like NaOMe and are soluble in MeOH, such as for the cases of 9–14 (Scheme 4) and 21–23 (condition b, Scheme 5).
Selection of Bacteria.
ESKAPE pathogens were chosen as they are responsible for the majority of nosocomial infections and also because they provide a good scope of strains including both Gram-negative and Gram-positive species. A. baumannii NCTC 13420 was originally identified in 24 hospitals in the United Kingdom during 2000–2003; it produces OXA-51-like β-lactamases and is highly resistant to ampicillin, piperacillin, piperacillin/tazobactam, ceftazidime, cefotaxime, gentamicin, and ciprofloxacin.63 P. aeruginosa NCTC 13437 produces VIM-10 metallo-carbapenemase and Vietnamese extended-spectrum β-lactamase (VEB-1).64 Wild-type strain E. cloacae NCTC 13405 was originally named E. cloacae 684 and was isolated from patients at the London Hospital from 1982 to 1983.65 This strain has inducible AmpC β-lactamase and is resistant to ampiriHin/amoxirillin and cefoxitin. K. pneumoniae ATCC 700603 was isolated from the urine of a hospitalized patient in Richmond, Virginia, in 1994, and it produces SHV-18 extended spectrum β-lactamase (ESBL).66 JE2 (USA300) is a methicillin-resistant S. aureus (MRSA) strain that is also resistant to β-lactams, ciprofloxacin, tetracycline, macrolides (erythromycin), lincosamides (clindamycin), streptogramin B, and mupirocin.67 E. faecium ATCC 700221, a positive fecal vancomycin-resistant enterococcus (VRE) isolate, is resistant to several antibiotics, including vancomycin and teicoplanin.68 The quality control strain, Gram-negative E. coli ATCC 25922, was included in all assays and validated with ciprofloxacin and teicoplanin.
In Vitro Antimicrobial Activities.
Several conclusions can be drawn from the SAR studies. (1) Our data are consistent with previous reports that auranofin is active against Grampositive bacteria but inactive toward Gram-negative bacteria. (2) Auranofin can be made active toward Gram-negative bacteria by changing the ligands coordinated to AuI. Although auranofin itself has no activity against Gram-negative pathogens, some analogues become active even for P. aeruginosa. (3) The thiol ligand is important for activity in addition to modulating the toxicity. For instance, replacing tetraacetylated thioglucose with 2-mercaptoethanol (21) increased the activity of auranofin by 5–19 times for all Gram-negative pathogens while decreasing the cytotoxicity by 1.6 times. (4) The vast majority of the analogues showed mammalian cell toxicity either similar to or lower than that of auranofin (Figure 5).
Gram-Positive Activities.
Among the thio sugar analogues (Figure 1), the TreNAc7 ligand (7) showed the highest activity enhancement of 7-fold and an MIC/MBC value of 6 nM against S. aureus (Table 1). Also analogues 16, 19, 22, and 23 showed high activity against S. aureus, with MICs in the nanomolar range (Table 2). The best compounds for E. faecium are analogues 16–18, which have electron-donating groups on their benzene thiol ligands; they show 8-fold better activity than auranofin (Table 2). Triethylphosphine appears to be the best phosphine ligand for the two Gram-positive strains. Replacing triethylphosphine with trimethylphosphine (31) did not improve the activity (Table 3). Tri-n-butylphosphine and all triarylphosphines drastically decreased the activity (Table 3).
Gram-Negative Activities.
For the Gram-negative strains, there was a clear preference for aliphatic over aromatic phosphines. Analogues bearing triarylphosphines with either electron-withdrawing or electron-donating groups showed no activity against Gram-negatives (33–36, Table 3). The AuI chloride complexes (27–30) gave similar results. Among the aliphatic phosphine ligands, there was a clear preference for the smallest trimethylphosphine. Analogues prepared by replacing triethylphosphine with trimethylphosphine led to sizable improvements in activity (31, 37–39, and 40). Increasing the alkyl chain length to n-butyl drastically decreased the activity against Gram-negatives, except for that toward P. aeruginosa (26 vs 25 and 31 vs 1, Table 3). In the work of Sutton et al. that described the discovery of auranofin, a small SAR study was conducted where the alkyl group on trialkylphosphine R3P was varied to include methyl, ethyl, isopropyl, and n-butyl.24 Unfortunately, the antiarthritic activities and the plasma gold concentrations were only measured on the chloride complexes R3PAuCl, from which triethylphosphine was identify as the best phosphine ligand.
Auranofin is slightly active against A. baumannii, with an MIC/MBC of 47 μM. Changing the thio sugar structure had very little impact on the activity (2–14), and the activities of most analogues with aliphatic or aromatic thiols were little changed as well (15–23). Analogues with trimethylphosphine ligands showed the best activity against A. baumannii, with MIC values of 13 and 5.7 μM for 31 and 40, respectively.
Lipophilicity appears to play a critical role in the case of P. aeruginosa. For example, the trimethylphosphine and deprotected thio sugar analogues 37–39 (log P = −1.63 to −2.03, Table 4) improved the activities by 3–44-fold over that of auranofin against all Gram-negative strains except for P. aeruginosa, against which the activity was completely lost. Acetyl-protected analogue 31 recovered the activity of 37 against P. aeruginosa by 4 times. It appears that P. aeruginosa is more sensitive to the thiol than the phosphine ligand. Among the analogues that show activity against P. aeruginosa (21, 24, and 40), none of them bear a thio sugar ligand. The best performing analogue is compound 21, which has a mercaptoethanol ligand (MIC/MBC at 41 μM) and is 9 times better than auranofin (Table 2). Increasing the chain length of trialkylphosphine from methyl to ethyl to n-butyl led to drastic decreases in activity for all other Gram-negative strains, whereas in the case of P. aeruginosa, the changes were minimal (24–26 and 40 vs 21). The MIC decreased only 2 times from 24 (methyl) to 25 (ethyl), and the activity increased from 25 (ethyl) to 26 (n-butyl, Table 3).
Among the Gram-negative strains tested, E. cloacae appears to be the most affected by the structures of the thio sugar and the phosphine ligands. The structure of the phosphine ligand showed the largest impact on the activity toward E. cloacae. By replacing triethylphosphine in auranofin with trimethylphosphine, the MIC/MBC of the resulting analogue, 31, reached 3.1 μM, which is 61-fold lower than that of auranofin. Compound 38, which combines a trimethylphosphine and a deprotected Gal ligand, has similar activity. Most thiol ligands impact the activity in a positive way, except for aromatic thiols with strong electron-withdrawing groups (19 and 20), which completely diminish the activity. The best thiol ligand, mercaptoethanol, combined with the best phosphine ligand, trimethylphosphine, yielded analogue 40, which lowered the MIC/MBC of auranofin by 65-fold to 2.9, μM.
Auranofin is ineffective against K. pneumoniae, with an MIC/MBC of 377 μM. Changing the thio sugar structure has no impact on the activity (Table 1). Replacing the thio sugar with an aliphatic or an aromatic thiol ligand yielded a number of analogues that showed good activities (Table 2). The p-aminobenzene thiol (17) lowered the MIC/MBC of auranofin by 10-fold to 36 μM. The mercaptoethanol ligand (21) lowered the MIC/MBC further to 20, μM. Trimethylphosphine is again the best phosphine ligand. All trimethylphosphine analogues have good activities against K. pneumoniae, with the best candidate, 40, having an MIC of 11 μ/mL, which is 34-fold lower than that of auranofin.
Lipophilicity.
For Group 1 compounds with thio sugar ligands, the deprotected thio sugar (9–14, Table 1) did not impact the activities of the resulting analogues against either Gram-negative or Gram-positive strains, even though the log P increased by more than one unit, implying that lipophilicity is unlikely to be the key factor for in vitro activity. Among the Group 2 compounds with aromatic or aliphatic thiol ligands, the analogues with moderate log P values (1–2) exhibited better activity, whereas analogues 19 and 20, with high log P values (>3), had no activity against the Gram-negative strains (Table 2). In the trialkyl- and triaryl-phosphine series of Group 3 compounds, the in vitro activity correlated well with the lipophilicity. Analogues with high log P values (>4), including the tri-n-butylphosphine analogue and all the triarylphosphine analogues, showed either decreased or no activity (Tables 3 and 4). The deprotected thio sugar analogues 37–39 exhibited good activity against all Gram-negative strains except for P. aeruginosa. Compounds that are active against P. aeruginosa are moderately lipophilic with positive log P values of <2.
In summary, repurposing of the arthritis drug auranofin as an antimicrobial has shown that this drug has good activity against Gram-positive but not Gram-negative bacteria, for which we are in dire need of new therapies because of the increasing prevalence of antimicrobial resistance and the lack of new drugs to treat them. In this work, we demonstrated that antiGram-negative activity can be enabled by modulating the structures of the thiol and phosphine ligands on auranofin. These analogues with a variety of ligand structures were efficiently synthesized under mild conditions in good to high yields. We also demonstrated that both thiol and the phosphine structures influence the activities of the analogues. Trimethylphosphine gives the highest activities against Gramnegative bacteria, whereas triethylphosphine was the best ligand for Gram-positives. Our SAR study revealed that the thiol ligand is very important and that its structure can modulate the activities of the AuI complexes for both Gramnegative and Gram-positive bacteria. Importantly, the active analogues had either similar or lower mammalian cell toxicities compared with that of auranofin, paving the way for clinical translation of this class of compounds.
EXPERIMENTAL SECTION
Materials.
All reagents and solvents were used as received from Sigma-Aldrich or Fisher Scientific. All reactions were monitored by thin layer chromatography (TLC) using TLC plates precoated with TLC silica gel 60 F254 (Merck KGaA). Spots on TLC were visualized with ultraviolet light either directly or after staining with 5% H2SO4 solution in ethanol. NMR spectra were recorded on a Bruker Avance Spectrospin DRX500 spectrometer (1H and 13C NMR), a Bruker Avance Spectrospin DPX200 spectrometer (13C, 19F and 31P NMR), or a JEOL ECZ 400 MHz NMR (31P NMR). 1H NMR and 13C NMR signals are referenced to either the nondeuterated residual solvent peaks or the tetramethyl silane peak (TMS, δ 0.00 ppm). CF3COOH (−76.55 ppm) was used as the external standard in 19F NMR. For 31P NMR, a freshly prepared Ph3P solution (0.1 M in CDCl3, −6.00 ppm) or 85% H3PO4 (0.00 ppm) was used as the external standard for samples in CDCl3 or D2O, respectively. HRMS spectra were collected at the University of Illinois at Urbana–Champaign Mass Spectroscopy Facility. Purities of compounds 1–40 were determined by absolute qNMR following the “general guidelines for quantitative 1D 1H NMR (qHNMR) experiments,” provided by the Journal of Medicinal Chemistry. The detailed calculations can be found in the Supporting Information. Purities of ≥95% were obtained for all compounds. The purity of each compound is included at the end of the corresponding synthetic procedure, and detailed calculations can be found in the Supporting Information (pp S158–S197).
General Procedure A for the Synthesis of Thioacetates 1b–6b (Step a, Scheme 1).
To a solution of glycosyl halides 1a–6a (1.0 equiv) in acetone (0.3 M), potassium thioacetate (2.0 equiv) was added. The reaction mixture was stirred at RT for 3—5 h, after which the mixture was concentrated on a rotovap. Water was then added to the residue, and the mixture was extracted with ethyl acetate three times. The combined organic phase was washed with brine and dried over Na2SO4. After removal of the solvent under vacuum at RT, the residue was purified by silica gel chromatography to give thioacetates 1b–6b.
General Procedure B for the Synthesis of 1c–6c (Step b, Scheme 1).
S-Deacetylation was conducted following a reported method.55 Briefly, DMF (10 mL) was added to a mixture of thioacetates 1b–6b (1.5 mmol), NaHCO3 (0.15 mmol), and dithiothreitol (DTT, 2.5 mmol). The reaction mixture was stirred at RT until TLC showed the full consumption of the starting material. The mixture was poured into 100 mL of water and extracted with toluene (80 mL × 3). The combined organic phase was washed with water (100 mL), and the solvent was removed. The crude product was used in the next step without further purification.
General Procedure C for the Synthesis of Auranofin and Analogues 1–6 (Step c, Scheme 1).
To a solution of chloro(triethylphosphine)gold(I) (1.0 mmol) and thiols (1.05 mmol) in DCM (4 mL), a solution of K2CO3 (1.2 mmol in 4 mL of water) was added dropwise at 0 °C. The mixture was stirred at RT for 1 h, poured into 30 mL of water, and extracted with DCM (30 mL × 3). The combined organic phase was dried on MgSO4 and filtered. After removal of the solvent, the residue was purified by column chromatography to give 1–6.
General Procedure D for the Synthesis of Auranofin Analogues 8 (Step f, Scheme 3), 15, 16, 18–20 (Step a, Scheme 5), and 31–36 (Step b, Scheme 6).
To a solution of chloro(triethylphosphine)gold(I) (1.0 mmol) and thiols (1.05 mmol) in toluene or DCM (4 mL), DBU (1.2 mmol in 1 mL of toluene or DCM) was added dropwise. The mixture was stirred at RT for 2 h. The mixture was concentrated, after which the residue was purified by column chromatography to give auranofin analogues 8, 15, 16, 18–20, and 31–36.
General Procedure E for the Synthesis of Auranofin Analogues 9–14 (Step b, Scheme 4), 17, 21–23, (Step b, Scheme 5), and 37–40 (Step b, Scheme 6).
To a solution of thioacetate (0.5 mmol) in methanol (12 mL), NaOMe (0.6 mmol in 1 mL of methanol) was added dropwise at 0 °C. The mixture was stirred at RT for 1–4 h, after which chloro(triphosphine)gold(I) (1.0 mmol) was added, and the mixture was stirred for another 1 h. The solvent was removed, and the residue was purified by column chromatography to give auranofin analogues 9–14, 17, 21–23, and 37–40.
General Procedure F for the Synthesis of Chloro-(triphosphine) Gold(I) 24–30 (Scheme 6).
Thiodiglycol (5 mmol) was added dropwise to a solution of gold acid chloride trihydrate (2.5 mmol, in 6 mL of water). When the bright orange-yellow solution was almost colorless, it was cooled to 0 °C, and triphosphine (2.63 mmol, in DCM/EtOH) was added dropwise. The reaction was stirred at RT for 2 h. DCM (20 mL ) and 20 mL of water were added to the mixture, and the aqueous layer was extracted by DCM twice. To remove trace metal impurities in the solution, the combined organic layers were filtered, and 20 mL of EtOH/H2O (2:1, v/v) was added to this filtrate. The solution was slowly concentrated on a rotovap to remove the DCM. A large amount of precipitate formed, which was collected by filtration, washed with cold EtOH/H2O (1:2, v/v), air-dried, and vacuum-dried to give the desired product.
(2,3,4,6-Tetra-O-acetyl-1-thio-β-D-glucopyranosato)-(triethylphosphine) Gold(I) (1, Auranofin).
This compound was synthesized from compound 1b (450 mg, 1.11 mmol) according to general procedure B to give 400 mg of 1c, which was used to directly prepare compound 1, according to general procedure C. The crude was purified by flash column chromatography (ethyl acetate/hexanes = 4:3) to give 1 as a colorless, viscous solid (676 mg, 90% over two steps). 1H NMR (500 MHz, CDDl3) δ 5.11–4.99 (m, 3H, H-1, H-3, H-4), 4.94–4.87 (m, 1H, H-2), 4.17 (dd, J = 12.2, 4.8 Hz, 1H, H-6a), 4.3 (dd, J = 12.2, 2.3 Hz, 1H, H-6b), 3.66 (ddd, J = 9.6, 4.7, 2.3 Hz, 1H, H-5), 2.01 (s, 3H, OAc), 1.98 (s, 3H, OAc), 1.94 (s, 3H, OAc), 1.91 (s, 3H, OAc), 1.79 (dq, J = 10.0, 7.6 Hz, 6H, P(CH2CH3)3, 1.15 (dt, J = 18.5, 7.6 Hz, 9H, P(CH2CH3).3 The NMR spectrum was consistent with the published data.55 Purity 95% by qNMR.
(2,3,4,6-Tetra-O-acetyl-1-thio-β-D-galacotopyranosato)-(triethylphosphine) Gold(I) (2).
This compound was synthesized from compound 2b (300 mg, 0.824 mmol) according to general procedure B to give 2c (quant), which was directly used to prepare compound 2 according to general procedure C. The product was purified by flash column chromatography (ethyl acetate/hexanes = 4:3) to give 1 as a colorless, viscous solid (481 mg, 96% over two steps). 1H NMR (500 MHz, CDCl3) δ 5.38 (dd, J = 3.5, 1.1 Hz, 1H, H-4), 5.16 (t, J = 9.5 Hz, 1H, H-2), 5.11 (d, J = 9.4 Hz, 1H, H-1), 4.94 (dd, J = 9.6, 3.5 Hz, 1H, H-3), 4.11 (d, J = 6.8 Hz, 2H, H-6a, H-6b), 3.92 (td, J = 6.8, 1.1 Hz, 1H, H-5), 2.07 (s, 3H, OAc), 2.05 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.93 (s, 3H, OAc), 1.82 (dq, J = 9.9, 7.6 Hz, 6H, CH2CH3), 1.20 (dt, J = 18.4, 7.6 Hz, 9H, CH2CH3). 13C NMR (50 MHz, CDCl3) δ 170.56, 170.31, 170.31, 169.89, 83.79, 74.62, 74.32, 72.23, 68.02, 61.62, 21.33, 20.84, 20.74, 20.74, 18.16 (d, 1JCP = 33.4 Hz, P(CH2CH3)3), 9.07 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 37.57. Purity 95% by qNMR.
(3,4,6-Tri-O-acetyl-2-N-acetyl-1-thio-β-D-glucopyranosato)-(triethylphosphine) Gold(I) (3).
This compound was synthesized from 3b (203 mg, 0.501 mmol) according to modified general procedure B using ethyl acetate as extraction solvent instead of toluene. The crude product of 3c was further purified by flash column chromatography (ethyl acetate/hexanes = 4:1) to give 3c as a colorless, viscous solid (167 mg, 92%). Following general procedure C from 3c (110 mg, 0.303 mmol) and purification using flash column chromatography (ethyl acetate/acetone = 200:1), product 3 was obtained as a colorless, viscous solid (194 mg, 94%). 1H NMR (500 MHz, CDCl3) δ 5.73 (d, J = 7.8 Hz, 1H, NH), 5.14 (d, J = 9.8 Hz, 1H, H-1), 5.06 (t, J = 9.5 Hz, 1H, H-3), 5.02 (t, J = 9.5 Hz, 1H, H-4), 4.19 (dd, J = 12.2, 4.9 Hz, 1H, H-6a), 4.06 (dd, J = 12.1, 2.3 Hz, 1H, H-6b), 4.03 (t, J = 9.6 Hz, 1H, H-2), 3.68 (ddd, J = 9.6, 4.8, 2.3 Hz, 1H, H-5), 2.01 (s, 3H, OAc), 1.96 (s, 3H, OAc), 1.96 (s, 3H, OAc), 1.93 (s, 3H, NHAc), 1.82 (dq, J = 9.9, 7.6 Hz, 6H, P(CH2CH3)3), 1.17 (dt, J = 18.4, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (50 MHz, CDCl3) δ 170.98, 170.89, 169.80, 169.62, 84.26, 76.04, 74.92, 69.03, 63.05, 60.43, 23.73, 20.87, 20.83, 20.74, 17.91 (d, 1JCP = 33.4 Hz, P(CH2CH3)3), 8.97 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 36.93. HRMS (ESI) m/z: [M + Na]+ calcd for C20H35AuNO8PSNa 700.1379; found 700.1380. Purity 95% by qNMR.
(3,4,6-Tri-O-acetyl-2-N-trichloroactyl-1-thio-β-D-glucopyranosato)(triethylphosphine) Gold(I) (4).
Only the procedures for the conversion of 4b to 4 are described here. The detailed procedures for the synthesis of 4b and the other intermediates can be found in the Supporting Information. Compound 4b (260 mg, 0.511 mmol), DTT (118 mg, 0.767 mmol), and NaHCO3 (5 mg, 0.051 mmol) were added to 3 mL of DMF. The mixture was stirred at RT for 1.5 h and diluted with 30 mL of toluene and 30 mL of water. The aqueous layer was further extracted by toluene twice. The combined organic layer was dried over Na2SO4. After removal of the solvent, the crude product was dissolved in 4 mL of DCM; then Et3PAuCl (162 mg, 0.464 mmol) was added. The reaction was brought to 0 °C; then 2 mL of cold K2CO3 solution (35 mg. 0.255 mmol) was added dropwise. The mixture was further stirred at RT for 1.5 h; then it was diluted with 20 mL of DCM and 20 mL of brine. The aqueous layer was further extracted by DCM once more. The combined organic phase was dried over MgSO4. After removal of the solvent, the residue was purified by column chromatography (ethyl acetate/hexanes = 1:1) to afford 4 as colorless, viscous solid (242 mg, 61% over two steps). 1H NMR (500 MHz, CDCl3) δ 7.02 (d, J = 9.3 Hz, 1H, NH), 5.28 (d, J = 9.7 Hz, 1H, H-1), 5.23 (t, J = 9.8 Hz, 1H, H-3), 5.12 (t, J = 9.7 Hz, 1H, H-4), 4.24 (dd, J = 12.3, 5.0 Hz, 1H, H-6a), 4.16–4.03 (m, 2H, H-2, H-6b), 3.79 (ddd, J = 10.1, 5.0, 2.3 Hz, 1H, H-5), 2.07 (s, 3H, OAc), 2.03 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.88 (dq, J = 9.9, 7.7 Hz, 6H, P(CH2CH3)3), 1.22 (dt, J = 18.3, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 170.85, 170.85, 169.61, 161.52, 92.90, 83.85, 76.22, 74.05, 69.12, 63.03, 62.00, 20.91, 20.78, 20.77, 17.92 (d, 1JCP = 33.1 Hz, P(CH2CH3)3), 9.01 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 37.58. Purity 97% by qNMR.
[2,3,4,6-Tetra-O-acetyl-α-D-glucopyranosyl(1→4)-2,3,6-tri-O-acetyl-1-thio-β-D-glucopyranosato](triethylphosphine) Gold(I) (5).
This compound was synthesized from 5c (260 mg, 0.398 mmol) according to general procedure C and purified by flash column chromatography (ethyl acetate/hexanes = 2:3) to give 5 as a colorless, viscous solid (370 mg, 96%). 1H NMR (500 MHz, CDCl3) δ 5.36 (d, J = 4.0 Hz, 1H, H-1′), 5.31 (dd, J = 10.4,9.6 Hz, 1H, H-3′), 5.13 (t, J = 9.1 Hz, 1H, H-3), 5.12 (d, J = 9.6 Hz, 1H, H-1), 5.00 (t, J = 9.9 Hz, 1H, H-4′), 4.81 (dd, J = 10.5, 4.0 Hz, 1H, H-2′), 4.77 (t, J = 9.4 Hz, 1H, H-2), 4.31 (dd, J = 12.1, 2.7 Hz, 1H, H-6a), 4.20 (dd, J = 12.0, 4.2 Hz, 1H, H-6b), 4.16 (dd, J = 12.4, 3.7 Hz, 1H, H-6’a), 4.00 (dd, J = 12.4, 2.3 Hz, 1H, H-6’b), 3.98–3.93 (t, J = 9.3 Hz, 1H, H-4), 3.91 (ddd, J = 10.3, 3.4, 2.6 Hz, 1H, H-5’), 3.66 (ddd, J = 9.7, 4.2, 2.9 Hz, 1H, H-5), 2.08 (s, 3H, OAc), 2.05 (s, 3H, OAc), 2.00 (s, 3H, OAc), 2.00 (s, 3H, OAc), 1.97 (s, 3H, OAc), 1.94 (s, 3H, OAc), 1.92 (s, 3H, OAc), 1.80 (dq, J = 9.9, 7.6 Hz, 6H, CH2CH3), 1.16 (dt, J = 18.5, 7.6 Hz, 9H, CH2CH3). 13C NMR (50 MHz, CDCl3) δ 170.68, 170.68, 170.57, 170.30, 170.03, 169.92, 169.49, 95.58, 82.76, 78.17, 77.16, 75.96, 73.46, 69.96, 69.44, 68.43, 68.11, 64.06, 61.53, 21.21, 21.05, 21.01, 20.65, 20.65, 20.65, 20.65, 18.02 (d, 1JCP = 33.6 Hz, P(CH2CH3)3)), 8.98 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 36.88. HRMS (ESI) C32H50AuO17PS m/z: [M + H]+ 967.2245; found 967.2233. Purity 96% by qNMR.
[2,3,4,6-Tetra-O-acetyl-β-D-galactopyranosyl(1→4)-2,3,6-tri-O-acetyl-1-thio-β-D-glucopyranosato](triethylphosphine) Gold(I) (6).
This compound was synthesized from 6c (300 mg, 0.460 mmol) according to general procedure C and purified by flash column chromatography (ethyl acetate/hexanes = 2:3) to give 6 as a colorless, viscous solid (370 mg, 83%). 1H NMR (500 MHz, CDCl3) δ 5.32 (dd, J = 3.4, 0.9 Hz, 1H, H-4′), 5.14–5.04 (m, 3H, H-3, H-1, H-2′), 4.92 (dd, J = 10.4, 3.5 Hz, 1H, H-3′), 4.87 (t, J = 9.4 Hz, 1H, H-2), 4.46 (d, J = 7.9 Hz, 1H, H-1′), 4.37 (dd, J = 11.9, 1.6 Hz, 1H, H-6′a), 4.17–4.00 (m, 3H, H-6a, H-6b, H-6′b), 3.84 (t, J = 8.0 Hz, 1H, H-5′), 3.80 (t, J = 9.6 Hz, 1H, H-4), 3.62 (ddd, J = 9.9, 5.1, 1.7 Hz, 1H, H-5), 2.13 (s, 3H, OAc), 2.08 (s, 3H, OAc), 2.06 (s, 3H, OAc), 2.04 (s, 3H, OAc), 2.02 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.94 (s, 3H, OAc), 1.83 (dq, J = 9.8, 7.6 Hz, 6H, P(CH2CH3)3), 1.19 (dt, J = 18.4, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (50 MHz, CDCl3) δ 170.69, 170.48, 170.30, 170.18, 170.07, 169.97, 169.22, 101.17, 82.94, 77.36, 76.98, 76.80, 74.61, 71.22, 70.66, 69.26, 66.73, 63.35, 60.91, 21.33, 21.09, 21.09, 20.74, 20.74, 20.74, 20.62, 18.10 (d, J = 33.2 Hz), 9.03 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 37.35. Purity 96% by qNMR.
Compound 7 (Scheme 2).
Only the procedures for the conversion of 7e to 7 are described here. The detailed procedures for the synthesis of 7e and the other intermediates can be found in the Supporting Information. Compound 7f was prepared from compound 7e (278 mg, 0.400 mmol) according to general procedure B (24 h) and purified by flash column chromatography (ethyl acetate/hexanes = 2:3) to give 7f as a viscous solid (260 mg, quant). Compound 7 was from 7f (260 mg, 0.400 mmol), following general procedure C, and was purified using flash column chromatography (ethyl acetate/hexanes = 3:1) to give compound 7 as a colorless, viscous solid (230 mg, 60%). 1H NMR (500 MHz, CDCl3) δ 5.68 (d, J = 3.8 Hz, 1H, H-1), 5.51–5.44 (m, 2H, H-3, H-3), 5.25 (d, J = 3.9 Hz, 1H, H-1), 5.15 (dd, J = 10.3, 3.8 Hz, 1H, H-2), 4.99 (m, 3H, H-2, H-4, H-4), 4.16 (dd, J = 12.3, 6.3 Hz, 1H, H-6a), 4.04–3.97 (m, 2H, H-6b, H-5), 3.95–3.88 (m, 1H, H-5), 2.98 (dd, J = 13.7, 2.1 Hz, 1H, H-6a), 2.88 (dd, J = 13.7, 8.5 Hz, 1H, H-6b), 2.21 (s, 3H, OAc), 2.06 (s, 3H, OAc), 2.04 (s, 3H, OAc), 2.02 (s, 3H, OAc), 2.02 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.80 (dq, J = 10.0, 7.6 Hz, 12H, P(CH2CH3)3), 1.17 (dt, J = 18.5, 7.6 Hz, 18HP(CH2CH3)3). 13C NMR (50 MHz, CDCl3) δ 170.60, 170.12, 170.09, 170.00, 170.00, 169.94, 169.75, 91.80, 91.50, 73.07, 72.09, 70.58, 70.34, 69.04, 68.87, 68.34, 62.10, 21.47, 20.91, 20.85, 20.73, 20.73, 20.73, 20.67, 18.19 (d, J = 34.6 Hz), 9.00 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 34.66. HRMS (ESI) m/z: [M - Cl]+ calcd for C38H65Au2O17P2S 1281.2744; found 1281.2732. Purity 95% by qNMR.
[1,3,4,6-Tetra-O-acetyl-2-((4-sulfanylbenzenethiolato)-(triethylphosphine)gold(I)) Acetamido-2-Deoxy-β-D-glucopyranose (8) (Scheme 3).
Only the last step, 8f → 8, is described here. The detailed procedures for the synthesis of 8f and intermediates 8a–8e can be found in the Supporting Information. To a solution of compound 8f (110 mg, 0.208 mmol) and Et3PAuCl (73 mg, 0.208 mmol) in DCM (6 mL), DBU (48 mg, 0312 mmol) was added. The reaction mixture was stirred for 1.5 h. After concentration in a rotovap, the crude was purified by flash column chromatography (ethyl acetate/DCM = 1:1 to 2:1) to give the product as a colorless, viscous solid (169 mg, 96%). 1H NMR (500 MHz, CDCl3) δ 7.46–7.39 (m, 6H, Ar–H), 7.04 (d, J = 9.3 Hz, 1H, NH), 7.01–6.97 (m, 6H. Ar–H), 5.77 (d, J = 8.8 Hz, 1H, H-1), 5.31 (dd, J = 10.6, 9.3 Hz, 1H, H-3), 5.09 (t, J = 9.6 Hz, 1H, H-4), 4.27 (dd, J = 12.5, 4.6 Hz, 1H. H-6a), 4.20 (dt, J = 10.5, 9.1 Hz, 1H, H-2), 4.11 (dd, J = 12.4, 2.2 Hz, 1H, H-6b), 3.85 (ddd, J = 10.0, 4.5, 2.3 Hz, 1H, H-5), 3.49 (q, J = 16.5 Hz, 2H, SCH2), 2.08 (s, 3H, OAc), 2.02 (s, 3H, OAc), 1.97 (s, 3H, OAc), 1.91 (s, 3H, OAc), 1.87 (dq, J = 9.9, 7.6 Hz, 6H, P(CH2CH3)3), 1.22 (dt, J = 18.6, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 170.75, 170.67, 169.43, 169.30, 168.97, 142.54, 133.31 (2C), 128.86 (2C) 127.92, 92.13, 72.72, 72.10, 68.24, 61.77, 53.20, 38.54, 20.83, 20.72, 20.60, 20.57, 18.14 (d, J = 33.9 Hz, 3C, P(CH2CH3)3)], 9.08 (s, P(CH2CH3)3). Purity 95% by qNMR.
(1-Thio-β-D-glucopyranosato)(triethylphosphine) Gold(I) (9) (Scheme 4).69
This compound was prepared from compound 1b (200 mg, 0.492 mmol) according to general procedure E and purified by silica gel chromatography (DCM/methanol = 10:1 to 6:1) to give compound 9 (208 mg, 83%) as a colorless, viscous solid. 1H NMR (500 MHz, D2O) δ 4.87 (d, J = 9.1 Hz, 1H, H-1), 3.81 (dd, J = 12.3, 2.1 Hz, 1H, H-6a), 3.60 (dd, J = 12.3, 6.0 Hz, 1H, H-6b), 3.47–3.28 (m, 3H), 3.20 (t, J = 9.1 Hz, 1H), 1.89 (dq, J = 9.8, 7.7 Hz, 6H, P(CH2CH3)3), 1.14 (dt, J = 18.5, 7.6 Hz, 9H, P(CH2CH3)3). Purity 98% by qNMR.
(1-Thio-β-D-galatopyranosato)(triethylphosphine) Gold(I) (10) (Scheme 4).
This compound was prepared from compound 1b (307 mg, 0.755 mmol) according to general procedure E and purified by silica gel chromatography (DCM/methanol = 10:1 to 6:1) to give compound 10 as a colorless, viscous solid (284 mg, 74%). 1H NMR (500 MHz, D2O) δ 4.90 (d, J = 9.0 Hz, 1H, H-1), 3.98 (d, J = 3.3 Hz, 1H, H-4), 3.80–3.66 (m, 3H, H-3, H-5, H-6a), 3.62 (dd, J = 9.6, 3.4 Hz, 1H, H-6b), 3.54 (t, J = 9.3 Hz, 1H, H-2), 1.97 (dq, J = 9.8, 7.6 Hz, 6H, P(CH2CH3)3), 1.23 (dt, J = 18.7, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (50 MHz, D2O) δ 85.39, 79.42, 76.96, 74.03, 69.54, 61.53, 17.78 (d, J = 33.9 Hz, P(CH2CH3)3), 8.98 (s, P(CH2CH3)3). 31P NMR (81 MHz, D2O) δ 37.75. Purity 96% by qNMR.
(1-Thio-D-mannopyranosato)(triethylphosphine) Gold(I) (11) (Scheme 4).
This compound was prepared from 2,3,4,6-tetra-O-acetyl-1-S-acetyl-α-D-mannopyranose70–73 (283 mg, 0.696 mmol) following general procedure E and purified by silica gel chromatography (DCM/methanol = 10:1 to 6:1) to give compound 11 as a colorless, viscous solid (277 mg, 78%). 1H NMR (400 MHz, D2O, α/β = 7.4:1) δ 5.73 (d, J = 1.2 Hz, 1H, H-1α), 4.72 (d, J = 1.5 Hz, 1H, H-1β), 4.31 (dd, J = 9.6, 3.3 Hz, 1H, H-3α), 4.17 (ddd, J = 9.8, 4.8, 2.7 Hz, 1H, H-5α), 3.99 (dd, J = 3.2, 1.6 Hz, 1H, H-2α), 3.89 (td, J = 3.6, 1.8 Hz, 1H, H-5β), 3.82–3.71 (m, 2H, H-6aα, H-6bα), 3.64 (t, J = 9.8 Hz, 1H, H-4), 1.92 (dq, J = 9.8, 7.6 Hz, 6H), 1.16 (dt, J = 18.4, 7.6 Hz, 9H). 13C NMR (101 MHz, D2O, α-anomer) δ 82.77, 76.37, 72.58, 70.16, 67.30, 60.91, 17.30 (d, J = 33.9 Hz, P(CH2CH3)3), 8.49 (s, P(CH2CH3)3). 31P NMR (162 MHz, D2O) δ 37.72. Purity 97% by qNMR.
(2-Acetamido-2-deoxy-1-thio-β-D-glucopyranosato)-(triethylphosphine) Gold(I) (12) (Scheme 4).
This compound was prepared from compound 3b (310 mg, 0.765 mmol) according to general procedure E and purified by silica gel chromatography (DCM/methanol = 5:1) to give compound 12 as a colorless, viscous solid (295 mg, 70%). 1H NMR (500 MHz, D2O) δ 5.10 (d, J = 9.7 Hz, 1H), 3.90 (dd, J = 12.3, 1.8 Hz, 1H), 3.77 (t, J = 9.5 Hz, 1H), 3.71 (dd, J = 12.3, 5.4 Hz, 1H), 3.54–3.40 (m, 2H), 2.08 (s, 3H), 1.98 (dq, J = 15.6, 7.6 Hz, 10H), 1.23 (dt, J = 18.6, 7.6 Hz, 16H). 13C NMR (50 MHz, D2O) δ 173.92, 83.48, 80.51, 76.16, 70.72, 62.18, 61.75, 23.04, 17.75 (d, J = 33.9 Hz, P(CH2CH3)3), 9.07 (s, P(CH2CH3)3). 31P NMR (81 MHz, D2O) δ 37.93. Purity 97% by qNMR.
[β-D-Galactopyranosyl-(1→4)-β-D-glucopyranosato]-(triethylphosphine) Gold(I) (13) (Scheme 4).
This compound was prepared from compound 6b (402 mg, 0.579 mmol) according to general procedure E and purified by silica gel chromatography (DCM/methanol = 5:1 to 3:1) to give compound 13 as a colorless, viscous solid (243 mg, 62%). 1H NMR (500 MHz, D2O) δ 5.00 (d, J = 9.2 Hz, 1H), 4.47 (d, J = 7.8 Hz, 1H), 4.02–3.92 (m, 2H), 3.78 (m, 4H), 3.70 (dd, J = 10.0, 3.3 Hz, 1H), 3.67–3.54 (m, 4H), 3.36 (t, J = 8.8 Hz, 1H), 1.98 (dq, J = 9.7, 7.8 Hz, 6H, P(CH2CH3)3), 1.24 (dt, J = 18.8, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (50 MHz, D2O) δ 103.36, 84.90, 79.71, 79.42, 79.24, 75.84, 75.78, 73.00, 71.41, 69.02, 61.47, 61.07, 17.82 (d, J = 34.5 Hz, P(CH2CH3)3), 9.06 (s, P(CH2CH3)3). 31P NMR (81 MHz, D2O) δ 39.39. Purity 95% by qNMR.
(1-Thio-β-D-maltosato(triethylphosphine) Gold(I) (14) (Scheme 4).
This compound was prepared from compound 5b (407 mg, 0.586 mmol) according to general procedure E and purified by silica gel chromatography (DCM/methanol = 5:1 to 3:1) to give compound 14 as a colorless, viscous solid (252 mg, 64%). 1H NMR (500 MHz, D2O) δ 5.41 (d, J = 3.8 Hz, 1H), 4.98 (d, J = 9.2 Hz, 1H), 3.98–3.85 (m, 2H), 3.82–3.66 (m, 5H), 3.65–3.56 (m, 3H), 3.44 (t, J = 9.5 Hz, 1H), 3.34 (t, J = 9.2 Hz, 1H), 1.99 (dq, J = 15.8, 7.7 Hz, 6H), 1.24 (dt, J = 18.7, 7.6 Hz, 9H). 13C NMR (50 MHz, D2O) δ 100.06, 84.94, 80.0, 79.05, 77.92, 77.63, 73.34, 73.14, 72.18, 69.82, 61.75, 60.99, 17.85 (d, J = 34.0 Hz, P(CH2CH3)3), 9.08 (s, P(CH2CH3)3). 31P NMR (81 MHz, D2O) δ 38.39. Purity 96% by qNMR.
(Benzenethiolato)(triethylphosphine) Gold(I) (15) (Condition a, Scheme 5).
This compound was prepared from thiophenol (35 mg, 0.314 mmol) according to general procedure E and purified by flash column chromatography (ethyl acetate/hexanes = 2:3) to afford the product as a pale yellow oil (104 mg, 78%). 1H NMR (500 MHz, CDCl3) δ 7.53 (d, J = 7.8 Hz, 2H), 7.07 (t, J = 7.7 Hz, 2H), 6.96 (t, J = 7.3 Hz, 1H), 1.83 (dq, J = 10.1, 7.7 Hz, 6H, P(CH2CH3)3), 1.20 (dt, J = 18.5, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 141.92, 132.44, 127.94, 123.27, 18.10 (d, J = 33.1 Hz, P(CH2CH3)3), 9.07 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 36.42. Purity 96% by qNMR.
(2-Aminobenzenethiolato)(triethylphosphine) Gold(I) (16) (Condition a, Scheme 5).
This compound was prepared from 2-aminobenzenethiol (36 mg, 0.285 mmol) according to general procedure D using DCM as the solvent, and the crude was purified by flash column chromatography (ethyl acetate/hexanes = 1:2) to afford the product as a light orange solid (78 mg, 62%). 1H NMR (500 MHz, CDCl3) δ 7.51 (dd, J = 7.7, 1.5 Hz, 1H, Ar–H), 6.86 (td, J = 7.7, 1.5 Hz, 1H, Ar–H), 6.66 (dd, J = 7.8, 1.4 Hz, 1H, Ar–H), 6.55 (td, J = 7.5, 1.4 Hz, 1H, Ar–H), 4.09 (bs, 2H, NH2), 1.80 (dq, J = 9.9, 7.6 Hz, 6H, P(CH2CH3)3), 1.17 (dt, J = 18.4, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 147.03, 135.32, 125.31, 124.15, 117.61, 114.23, 18.13 (d, J = 33.1 Hz, P(CH2CH3)3), 9.4 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 38.20. Purity 98% by qNMR.
(4-Aminobenzenethiolato)(triethylphosphine) Gold(I) (17) (Condition b, Scheme 5).
To a solution of 4-aminothiophenol (45 mg, 0.359 mmol) and NaOMe (100 μL, 25 wt % in MeOH) in cold MeOH (4 mL), gold triethylphosphine chloride (120 mg, 0.342 mmol) in DCM/MeOH (1:1, v/v) was added dropwise. The reaction was stirred for 1 h. The solvent was removed by rotovap, and water was added to the mixture. The mixture was extracted by DCM twice. The combined organic phase was washed with brine and dried over Na2SO4. The solution was concentrated; then toluene (1 mL) was added. The remaining DCM was removed by rotovap, after which 3 mL of hexanes was added. The solution was kept at −20 °C, after which yellow crystals formed. The supernatant was pipetted out, and the crystals were washed with hexanes and dried in a vacuum to afford the product as yellow crystals (138 mg, 92%). 1H NMR (500 MHz, CDCl3) δ 7.31–7.23 (m, 2H, Ar–H), 6.53–6.44 (m, 2H, Ar–H), 3.49 (s, 2H, NH2), 1.81 (dq, J = 9.8, 7.6 Hz, 6H, P(CH2CH3)3), 1.18 (dt, J = 18.4, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 142.80, 133.37, 128.36, 115.47, 18.03 (d, J = 32.9 Hz, P(CH2CH3)3), 8.99 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 37.40. Purity 98% by qNMR.
(4-Methoxybenzenethiolato)(triethylphosphine) Gold(I) (18) (Condition a, Scheme 5).
This compound was prepared from 4-methoxybenzenethiol (48 mg, 0.342 mmol) according to general procedure D using DCM as the solvent, and the crude was purified by flash column chromatography (ethyl acetate/hexanes = 1:2) to afford the product as a colorless, viscous solid (104 mg, 78%). 1H NMR (500 MHz, CDCl3) δ 7.44–7.38 (m, 2H, Ar–H), 6.71–6.64 (m, 2H, Ar–H), 3.73 (s, 3H, OMe), 1.83 (dq, J = 9.8, 7.6 Hz, 6H, P(CH2CH3)3), 1.20 (dt, J = 18.4, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 156.74, 133.56, 131.72, 113.85, 55.38, 18.18 (d, J = 33.0 Hz, P(CH2CH3)3), 9.09 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 38.79. Purity 99% by qNMR.
(4-Nitrobenzenethiolato)(triethylphosphine) Gold(I) (19) (Condition a, Scheme 5).
This compound was prepared from 4-nitrobenzenethiol (56 mg, 0.359 mmol) according to general procedure D using DCM as the solvent, and the crude was purified by flash column chromatography (ethyl acetate/hexanes = 1:4 to 1:2) to afford the product as a yellow solid (151 mg, 91%). 1H NMR (500 MHz, CDCl3) δ 7.95–7.88 (m, 2H, Ar–H), 7.63–7.57 (m, 2H, Ar–H), 1.92 (dq, J = 9.9, 7.6 Hz, 6H, P(CH2CH3)3), 1.24 (dt, J = 18.7, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 156.67, 143.86, 131.94, 122.99, 18.07 (d, J = 33.5 Hz, P(CH2CH3)3), 9.12 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 37.77. Purity 99% by qNMR.
(4-(Trifluoromethyl)benzenethiolato)(triethylphosphine) Gold(I) (20) (Condition a, Scheme 5).
This compound was prepared from 4-(trifluoromethyl)benzenethiol (61 mg, 0.342 mmol) according to general procedure D using DCM as the solvent, and the crude was purified by flash column chromatography (ethyl acetate/hexanes = 1:2) to give the product as a colorless, viscous solid (126 mg, 75%). 1H NMR (500 MHz, CDCl3) δ 7.63–7.58 (m, 2H, Ar–H), 7.31–7.26 (d, J = 8.1 Hz, 2H, Ar–H), 1.87 (dq, J = 9.9, 7.6 Hz, 6H, P(CH2CH3)3), 1.22 (dt, J = 18.6, 7.7 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 148.93, 132.22, 125.18 (q, 2JCF = 32.4 Hz), 124.88 (q, 1JCF = 270.72 Hz, CF3), 124.63 (q, 3JCF = 3.6 Hz), 18.18 (d, 1JCP = 33.4 Hz, P(CH2CH3)3), 9.13 (s, P(CH2CH3)3). 19F NMR (188 MHz, CDCl3) δ −60.60. 31P NMR (81 MHz, CDCl3) δ 39.08. Purity 101% by qNMR.
(2-Mercaptoethanolato)(triethylphosphine) Gold(I) (21) (Condition b, Scheme 5).
To a solution of 2-sulfanylethanol (24 μL, 0.342 mmol) in methanol (4 mL), NaOMe (20 mg, 25 wt % in methanol) was added. The solution was brought to 0 °C. A solution of Et3PAuCl (120 mg, 0.342 mmol) in methanol (2 mL) was added dropwise, and the reaction mixture was stirred for 1 h. The solution was concentrated, water was added, and the mixture was extracted by DCM twice. The combined organic phase was washed with brine and dried over Na2SO4 to give the product as a pale-yellow oil (134 mg, quant). 1H NMR (500 MHz, CD3OD) δ 3.68–3.59 (m, 2H, CH2OH), 2.98 (t, J = 7.5 Hz, 2H, CH2S), 1.94 (dq, J = 10.2, 7.6 Hz, 6H, P(CH2CH3)3), 1.23 (dt, J = 18.4, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CD3OD) δ 68.04, 31.24, 18.95 (d, 1JCP = 33.4 Hz, P(CH2CH3)3), 9.50 (s, P(CH2CH3)3). 31P NMR (81 MHz, CD3OD) δ 38.71. Purity 98% by qNMR.
(Methyl Mercaptoacetatato)(triethylphosphine) Gold(I) (22) (Condition b, Scheme 5).
This compound was prepared from methyl thioglycolate (38 mg, 0.359 mmol) according to general procedure E. After the reaction was completed, the mixture was concentrated. Water was added, and the mixture was extracted by DCM twice. The combined organic phase was washed with brine and dried over Na2SO4. The residue was purified by flash column chromatography (ethyl acetate/hexanes = 2:3) to afford the product as a colorless oil (85 mg, 59%). 1H NMR (500 MHz, CDCl3) δ 3.70 (s, 3H, OMe), 3.56 (s, 2H, MeOCOCH2), 1.84 (dq, J = 9.8, 7.7 Hz, 6H, P(CH2CH3)3), 1.22 (dt, J = 18.4, 7.7 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 175.69, 52.16, 29.15, 18.16 (d, 1JCP = 32.8 Hz, P(CH2CH3)3), 9.03 (s, P(CH2CH3)3). 31P NMR (81 MHz, CDCl3) δ 37.57. Purity 99% by qNMR.
(2,2,2-Trifluoroethanethiolato)(triethylphosphine) Gold(I) (23) (Condition b, Scheme 5).
This compound was prepared from (triethylphosphine)gold chloride (120 mg, 0.342 mmol) and 2,2,2-trifluoroethanethiol (44 mg, 0.377 mmol) according to general procedure C. The product was obtained as a colorless oil (135 mg, 92%) with satisfactory purity without column chromatography purification. 1H NMR (500 MHz, CDCl3) δ 3.43 (q, J = 10.1 Hz, 2H, CF3CH2), 1.85 (dq, J = 9.8, 7.6 Hz, 6H, P(CH2CH3)3), 1.21 (dt, J = 18.4, 7.6 Hz, 9H, P(CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 127.32 (d, J = 274.7 Hz, CF3), 30.33 (q, J = 32.2 Hz, CF3CH2), 18.13 (d, 1JCP = 33.2 Hz, P(CH2CH3)3), 9.00 (s, P(CH2CH3)3). 19F NMR (188 MHz, CDCl3) δ −65.55 (t, J = 10.0 Hz). 31P NMR (81 MHz, CDCl3) δ 38.87. Purity 102% by qNMR.
(Trimethylphosphine)gold Chloride (24) (Scheme 6).
This compound was prepared from gold acid chloride trihydrate (1.05 g, 2.62 mmol) and PMe3 (2.86 mL, 1 M in THF) according to general procedure F. The product was obtained as a white, amorphous solid (580 mg, 72%). 1H NMR (500 MHz, CDCl3) δ 1.63 (d, J = 11.3 Hz, 9H). 13C NMR (126 MHz, CDCl3) δ 16.25 (d, 1JCP = 40.3 Hz). 31P NMR (81 MHz, CDCl3) δ −9.94. The NMR spectra were in agreement with the published data.74 Purity 100% by qNMR.
(Triethylphosphine)gold Chloride (25) (Scheme 6).
This compound was prepared from gold acid chloride trihydrate (1.57 g, 3.99 mmol) and PEt3 (0.500 g, 4.25 mmol) according to general procedure F. The product was obtained as a white, amorphous solid (1.41 g, quant). 1H NMR (500 MHz, CDCl3) δ 1.87 (dq, J = 10.3, 7.6 Hz, 6H), 1.21 (dt, J = 19.0, 7.7 Hz, 9H). 1P NMR (81 MHz, CDCl3) δ 33.15. The NMR spectra were consistent with the published data.55 Purity 97% by qNMR.
(Tri-n-butylphosphine)gold Chloride (26) (Scheme 6).
This compound was prepared from gold acid chloride trihydrate (525 mg, 1.31 mmol) and tri-n-butylphosphine (287 mg, 1.40 mmol) according to general procedure F, with a slight modification. After extraction, the solvent was removed, and the residue was purified by column chromatography (ethyl acetate/hexanes = 1:3) to afford the product as a colorless oil (530 mg, 93%). 1H NMR (500 MHz, CDCl3) δ 1.85–1.74 (m, 2H), 1.63–1.50 (m, 2H), 1.46 (q, J = 7.3 Hz, 2H), 0.95 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 27.45, 25.77 (d, 1JCP = 36.1 Hz), 24.19 (d, 2JCP = 15.0 Hz), 13.75. 31P NMR (81 MHz, CDCl3) δ 22.38. The NMR spectra were consistent with the published data.75 Purity 100% by qNMR.
(Triphenylphosphine)gold Chloride (27) (Scheme 6).
This compound was prepared from gold acid chloride trihydrate (0.785 g, 1.99 mmol) and PPh3 (556 mg, 2.12 mmol) according to general procedure F. The product was obtained as a white, amorphous solid (960 mg, 97%). 1H NMR (500 MHz, CDCl3) δ 7.57–7.50 (m, 9H, Ar–H), 7.43–7.49 (m, 6H, Ar–H). 13C NMR (126 MHz, CDCl3) δ 134.30 (d, J = 13.8 Hz), 132.00 (d, J = 2.4 Hz), 129.35 (d, J = 11.8 Hz), 129.21 (d, J = 59.9 Hz). 31P NMR (81 MHz, CDCl3) δ 33.35. The NMR spectra were consistent with the published data.76 Purity 99% by qNMR.
(Tris(4-methoxyphenyl)phosphine)gold Chloride (28) (Scheme 6).
This compound was prepared from gold acid chloride trihydrate (525 mg, 1.31 mmol) and tris(4-methoxyphenyl)phosphine (500 mg, 1.35 mmol) according to general procedure F with slight modification. The precipitate was washed with EtOH instead of EtOH/H2O solution. The product was obtained as a white, amorphous solid (716 mg, 94%). 1H NMR (500 MHz, CDCl3) δ 7.57–7.35 (m, 6H, Ar–H), 7.04–6.86 (m, 6H, Ar–H), 3.84 (s, 9H, OMe). 13C NMR (126 MHz, CDCl3) δ 162.49 (d, J = 2.5 Hz), 135.71 (d, J = 15.3 Hz), 120.55 (d, J = 68.2 Hz), 114.88 (d, J = 13.0 Hz), 55.60. 31P NMR (81 MHz, CDCl3) δ 29.49. The NMR spectra were consistent with the published data.77 Purity 98% by qNMR.
(Tris(4-fluorophenyl)phosphine)gold Chloride (29) (Scheme 6).
This compound was prepared from gold acid chloride trihydrate (525 mg, 1.31 mmol) and tris(4-fluorophenyl)phosphine (443 mg, 1.37 mmol) according to general procedure F with slight modification. The precipitate was washed with EtOH instead of EtOH/H2O solution. The product was obtained as a white, amorphous solid (672 mg, 94%). 1H NMR (500 MHz, CDCl3) δ 7.63–7.48 (m, 6H, Ar–H), 7.21 (td, J = 8.5, 1.8 Hz, 6H, Ar–H). 13C NMR (126 MHz, CDCl3) δ 165.18 (dd, J = 255.7, 2.6 Hz), 136.34 (dd, J = 15.8, 8.9 Hz), 124.29 (dd, J = 65.2, 3.3 Hz), 117.04 (dd, J = 21.7, 13.3 Hz). 19F NMR (188 MHz, CDCl3) δ −102.96 (m). 31P NMR (81 MHz, CDCl3) δ 30.14 (s). The NMR spectra were consistent with the published data.78 Purity 97% by qNMR.
(Tris(4-trifluoromethylphenyl)phosphine)gold Chloride (30) (Scheme 6).
This compound was prepared from gold acid chloride trihydrate (525 mg, 1.31 mmol) and tris(4-trifluoromethylphenyl)-phosphine (647 mg, 1.37 mmol) according to general procedure F with slight modification. The precipitate was washed with EtOH instead of EtOH/H2O solution. The product was obtained as a white, amorphous solid (857 mg, 94%). 1H NMR (500 MHz, CDCl3) δ 7.79 (dd, J = 8.5, 2.1 Hz, 6H, Ar–H), 7.69 (dd, J = 12.8, 8.1 Hz, 6H, Ar–H). 13C NMR (126 MHz, CDCl3) δ 134.71 (qd, J = 33.4, 2.6 Hz), 134.69 (d, J = 14.7 Hz), 132.25 (d, J = 57.7 Hz), 126.63 (dq, J = 11.8, 3.6 Hz), 123.25 (q, J = 273.1 Hz, CF3). 19F NMR (188 MHz, CDCl3) δ −60.82. 31P NMR (81 MHz, CDCl3) δ 31.86. The 1H NMR, 19F NMR, and 31P NMR spectra were consistent with the published data.79 Purity 101% by qNMR.
(2,3,4,6-Tetra-O-acetyl-1-thio-β-D-glucopyranosato)-(trimethylphosphine) Gold(I) (31) (Scheme 6).
This compound was synthesized from trimethylphosphine gold chloride (85 mg, 0.274 mmol) and compound 1c (105 mg, 0.274 mmol) according to general procedure D using toluene as the solvent and purified by flash column chromatography (ethyl acetate/hexanes = 5:1) to afford the product as a colorless, viscous solid (145 mg, 83%). 1H NMR (500 MHz, CDCl3) δ 5.19–5.08 (m, 3H, H-1, H-3 and H-4), 4.98 (t, J = 9.3 Hz, 1H, H-2), 4.25 (dd, J = 12.2, 4.8 Hz, 1H, H-6a), 4.12 (dd, J = 12.1, 2.4 Hz, 1H, H-6b), 3.74 (ddd, J = 9.5, 4.7, 2.2 Hz, 1H, H-5), 2.08 (s, 3H, OAc), 2.07 (s, 3H, OAc), 2.02 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.61 (d, J = 10.8 Hz, 9H, PMe3). 13C NMR (126 MHz, CDCl3) δ 170.76, 170.24, 169.72, 169.65, 83.21, 77.30, 75.76, 74.40, 68.87, 62.83, 21.26, 20.91, 20.72, 20.69, 15.96 (d, J = 37.1 Hz). 31P NMR (81 MHz, CDCl3) δ −3.47. Purity 95% by qNMR.
(2,3,4,6-Tetra-O-acetyl-1-thio-β-D-glucopyranosato)(tri-n-butylphosphine) Gold(I) (32) (Scheme 6).
This compound was synthesized from tri-n-butylphosphine gold chloride (143 mg, 0.329 mmol) and compound 1c (126 mg, 0.345 mmol) according to general procedure D using toluene as the solvent and purified by flash column chromatography (ethyl acetate/hexanes = 1:2) to afford the product as a colorless, viscous solid (225 mg, 90%). 1H NMR (500 MHz, CDCl3) δ 5.18–5.06 (m, 3H, H-1, H-3 and H-4), 4.99 (t, J = 9.0 Hz, 1H, H-2), 4.24 (dd, J = 12.3, 4.9 Hz, 1H, H-6a), 4.10 (dd, J = 12.2, 2.3 Hz, 1H, H-6b), 3.72 (ddd, J = 8.6, 4.6, 2.0 Hz, 1H, H-5), 2.07 (s, 3H, OAc), 2.06 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.98 (s, 3H, OAc), 1.85–1.75 (m, 6H, P(CH2CH2CH2CH3)3), 1.65–1.53 (m, 6H, P(CH2CH2CH2CH3)3), 1.48 (h, J = 7.2 Hz, 6H, P-(CH2CH2CH2CH3)3), 0.96 (t, J = 7.2 Hz, 9H, P-(CH2CH2CH2CH3)3). 13C NMR (126 MHz, CDCl3) δ 170.72, 170.25, 169.53, 169.51, 83.34, 77.53, 75.73, 74.31, 69.00, 62.94, 27.23, 25.58 (d, J = 32.6 Hz), 24.16 (d, J = 14.4 Hz), 21.15, 20.83, 20.67, 20.64, 13.68. 31P NMR (81 MHz, CDCl3) δ 27.02. Purity 96% by qNMR.
(2,3,4,6-Tetra-O-acetyl-1-thio-β-D-glucopyranosato)-(triphenylphosphine) Gold(I) (33) (Scheme 6).
This compound was synthesized from triphenylphosphine gold chloride (258 mg, 0.521 mmol) and compound 1c (200 mg, 0.548 mmol) according to general procedure D using toluene as the solvent and purified by flash column chromatography (ethyl acetate/hexanes = 2:3 to 1:1) to afford the product as a colorless, viscous solid (377 mg, 88%). 1H NMR (500 MHz, CDCl3) δ 7.62–7.44 (m, 15H, Ar–H), 5.23–5.01 (m, 4H, H-1, H-2, H-3 and H-4), 4.22 (dd, J = 12.2, 4.8 Hz, 1H, H-6a), 4.12 (dd, J = 12.2, 2.4 Hz, 1H, H-6b), 3.76 (ddd, J = 9.8, 4.8, 2.4 Hz, 1H, H-5), 2.5 (s, 3H, OAc), 2.02 (s, 3H, OAc), 1.98 (s, 3H, OAc), 1.89 (s, 3H, OAc). 13C NMR (126 MHz, CDCl3) δ 170.88, 170.43, 169.70, 169.70, 134.36 (d, J = 14.2 Hz), 131.71 (d, J = 2.4Hz), 129.84 (d, J = 55.4 Hz), 129.28 (d, J = 11.5 Hz), 83.20, 77.73, 75.82, 74.34, 69.08, 62.97, 21.23, 20.83, 20.83, 20.78. 31P NMR (81 MHz, CDCl3) δ 36.93. The NMR spectra were consistent with the published data.80 Purity 96% by qNMR.
(2,3,4,6-Tetra-O-acetyl-1-thio-β-D-glucopyranosato)(tris(4-methoxyphenyl)phosphine) Gold(I) (34) (Scheme 6).
This compound was synthesized from tris(4-methoxyphenyl)phosphine gold chloride (160 mg, 0.274 mol) and compound 1c (105 mg, 0.288 mmol) according to general procedure D using toluene as the solvent and purified by column chromatography (ethyl acetate/hexanes = 1:1) to afford the product as a colorless, viscous solid (224 mg, 89%). 1H NMR (500 MHz, CDCl3) δ 7.51–7.44 (m, 6H, Ar–H), 7.01–6.95 (m, 6H, Ar–H), 5.20–5.10 (m, 3H, H-1, H-3, H-4), 5.06 (t, J = 9.3 Hz, 1H, H-2), 4.22 (dd, J = 12.2, 4.8 Hz, 1H, H-6a), 4.13 (dd, J = 12.1, 2.5 Hz, 1H, H-6b), 3.84 (s, 9H, 3 × OMe), 3.77 (ddd, J = 9.7, 4.7 2.5 Hz, 2H, H-5), 2.05 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.98 (s, 3H, OAc), 1.91 (s, 3H, OAc). 13C NMR (50 MHz, CDCl3) δ 170.84, 170.34, 169.64, 169.58, 162.22, 135.70 (d, J = 15.1 Hz), 121.27 (d, J = 62.8 Hz), 114.79 (d, J = 12.5 Hz), 83.19, 77.41, 75.70, 74.32, 69.09, 62.99, 55.49, 55.49, 55.49, 21.23, 20.79, 20.79, 20.79. 31P NMR (81 MHz, CDCl3) δ 33.82. Purity 96% by qNMR.
(2,3,4,6-Tetra-O-acety/-1-thio-β-D-glucopyranosato)(tris(4-fluorophenyl)phosphine) Gold(I) (35) (Scheme 6).
This compound was synthesized from tris(4-fluorophenyl)phosphine gold chloride (144 mg, 0.262 mmol) and compound 1c (100 mg, 0.276 mmol) according to general procedure D using toluene as the solvent and purified by column chromatography (ethyl acetate/hexanes = 1:2) to afford the product as a colorless, viscous solid (198 mg, 86%). 1H NMR (500 MHz, CDCl3) δ 7.64–7.50 (m, 6H, Ar–H), 7.25–7.18 (m, 6H, Ar–H), 5.16 (d, J = 9.5 Hz, 1H, H-1), 5.15 (t, J = 9.3 Hz, 1H, H-3), 5.08 (dd, J = 9.9, 9.4 Hz, 1H, H-4), 5.03 (t, J = 9.3 Hz, 1H, H-2), 4.24 (dd, J = 12.2, 5.0 Hz, 1H, H-6a), 4.11 (dd, J = 12.2, 2.4 Hz, 1H, H-6b), 3.79–3.74 (m, 1H, H-5), 2.07 (s, 3H, OAc), 2.02 (s, 3H, OAc), 1.98 (s, 3H, OAc), 1.94 (s, 3H, OAc). 13C NMR (126 MHz, CDCl3) δ 170.69, 170.26, 169.77, 169.71, 163.99 (d, J = 2.4 Hz), 136.43 (dd, J = 16.0, 8.8 Hz), 125.37 (dd, J = 57.3, 3.5 Hz), 116.92 (dd, J = 21.6, 12.7 Hz), 83.31, 78.01, 75.83, 74.15, 69.02, 62.96, 21.19, 20.79, 20.73, 20.69. 19F NMR (188 MHz, CDCl3) δ −103.75. 31P NMR (81 MHz, CDCl3) δ 35.38. Purity 95% by qNMR.
(2,3,4,6-Tetra-O-acetyl-1-thio-β-D-glucopyranosato)(tris(4-(trifluoromethyl)phenylphosphine)) Gold(I) (36) (Scheme 6).
This compound was synthesized from tris(4-trifluoromethyl)-phenylphosphine gold chloride (183 mg, 0.262 mmol) and compound 1c (100 mg, 0.275 mmol) according to general procedure D using toluene as the solvent and purified by column chromatography (ethylacetate/hexanes = 1:3) to afford the product as a colorless, viscous solid (69 mg, 26%). 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 6.6 Hz, 6H, Ar–H), 7.71 (dd, J = 11.9, 8.1 Hz, 6H, Ar–H), 5.18 (d, J = 9.4 Hz, 1H, H-1), 5.15 (t, J = 9.3 Hz, 1H, H-3), 5.08 (t, J = 9.8 Hz, 1H, H-4), 5.04 (t, J = 9.3 Hz, 1H, H-2), 4.27 (dd, J = 12.3, 5.1 Hz, 1H, H-6a), 4.11 (dd, J = 12.3, 2.3 Hz, 1H, H-6b), 3.78 (ddd, J = 9.9, 5.0, 2.4 Hz, 1H, H-5), 2.08 (s, 3H, OAc), 2.03 (s, 3H, OAc), 1.97 (s, 3H, OAc), 1.94 (s, 3H, OAc). 13C NMR (50 MHz, CDCl3) δ 170.70, 170.29, 169.99, 169.75, 134.77 (d, J = 15.3 Hz), 134.52–132.25 (m), 126.49 (dq, J = 11.0, 3.5 Hz), 123.42 (q, J = 272.7 Hz, CF3), 83.50, 78.27, 76.06, 74.16, 69.02, 62.99, 21.27, 20.83, 20.73, 20.73. 19F NMR (188 MHz, CDCl3) δ –60.82. 31P NMR (81 MHz, CDCl3) δ 32.62. Purity 95% by qNMR.
(1-Thio-β-D-glucopyranosato)(trimethylphosphine) Gold(I) (37).
This compound was prepared from compound 1b (120 mg, 0.295 mmol) according to general procedure E and purified by silica gel chromatography (DCM/methanol = 10:1 to 5:1) to give the product as a colorless, viscous solid (70 mg, 57%). 1H NMR (500 MHz, D2O) δ 4.94 (d, J = 9.1 Hz, 1H), 3.93 (dd, J = 12.3, 1.7 Hz, 1H), 3.73 (dd, J = 12.3, 5.9 Hz, 1H), 3.53–3.42 (m, 3H), 3.28 (t, J = 8.9 Hz, 1H), 1.69 (d, J = 10.9 Hz, 9H, PMe3). 13C NMR (126 MHz, D2O) δ 84.54, 80.7, 79.30, 76.90, 70.08, 61.22, 15.11 (d, J = 37.7 Hz, PMe3). 31P NMR (162 MHz, D2O) δ −1.06. Purity 95% by qNMR.
(1-Thioβ-D-galatopyranosato)(trimethylphosphine) Gold(I) (38).
This compound was prepared from compound 2b (120 mg, 0.295 mmol) according to general procedure E and purified by silica gel chromatography (DCM/methanol = 10:1 to 5:1) to give compound 38 as a colorless, viscous solid (72 mg, 59%). 1H NMR (500 MHz, D2O) δ 4.91 (d, J = 9.1 Hz, 1H), 4.01 (d, J = 3.4 Hz, 1H), 3.83–3.70 (m, 3H), 3.65 (dd, J = 9.6, 3.4 Hz, 1H), 3.53 (t, J = 9.3 Hz, 1H), 1.69 (d, J = 10.0 Hz, 9H). 13C NMR (126 MHz, D2O) δ 85.15, 79.12, 76.53, 73.69, 69.16, 61.17, 15.10 (d, J = 38.3 Hz, PMe3). 31P NMR (162 MHz, D2O) δ −0.87. Purity 96% by qNMR.
(2-Acetamido-2-deoxy-1-thio-β-D-glucopyranosato)-(trimethylphosphine) Gold(I) (39).
This compound was prepared from compound 3b (150 mg, 0.370 mmol) according to general procedure E and purified by silica gel chromatography (DCM/methanol = 10:1 to 5:1) to give compound 39 as a colorless, viscous solid (126 mg, 67%). 1H NMR (500 MHz, D2O) δ 5.09 (d, J = 8.6 Hz, 1H), 3.92 (d, J = 12.1 Hz, 1H), 3.86–3.66 (m, 2H), 3.60–3.35 (m, 3H), 2.10 (s, 3H), 1.67 (d, J = 11.1 Hz, 9H, PMe3). 13C NMR (101 MHz, D2O) δ 173.62, 83.19, 80.24, 75.69, 70.09, 61.44, 61.14, 22.69, 15.06 (d, J = 37.7 Hz, PMe3). 31P NMR (162 MHz, D2O) δ −1.98. Purity 95% by qNMR.
(2-Mercaptoethanolato)(trimethylphosphine) Gold(I) (40).
To a solution of 2-sulfanylethanol (40 mg, 0.512 mmol) and Me3PAuCl (0.538 mmol, 166 mg) in MeOH, NaOMe (121 mg, 25 wt %, 0.563 mmol) was added. After being stirred at RT for 1.5 h, the solvent was removed. DCM (5 mL) and water (5 mL) were added, and the mixture was extracted by DCM twice. The combined organic phase was dried over Na2SO4 and filtered. After removal of the solvent, the desired product was obtained as a white solid (179 mg, 88%). 1H NMR (500 MHz, CDCl3) δ 3.73–3.62 (m, 2H, HOCH2CH2), 3.18–3.07 (m, 3H, HOCH2CH2 and OH), 1.60 (d, J = 10.5 Hz, 9H, PMe3). 13C NMR (126 MHz, CDCl3) δ 65.98, 32.24, 16.03 (d, 1JCP = 35.7 Hz, PMe3). 31P NMR (162 MHz, CDCl3) δ −0.13. Purity 100% by qNMR.
Antibacterial Assay.
The minimum inhibitory concentrations (MICs) and the minimum bactericidal concentrations (MBCs) were determined by the broth microdilution method, as described in the Clinical & Laboratory Standards Institute (CLSI) guidelines.81 All bacterial strains were cultured in cation-adjusted Muller–Hinton broth (CAMHB) up to log phase and then diluted to give a concentration of 7.5 × 105 CFU/mL. The gold complexes were dissolved in DMSO or water as stock solutions, which were then serially diluted 2-fold in a 96-well plate. Aliquots (100 μL) of the dilutions were mixed with equal volumes of the bacterial suspension in a 96-well plate (in triplicates). The plates were incubated at 37 °C for 18 h without shaking. Negative and positive controls were included in the same well plate, with wells having culture medium only and wells having bacterial solution only, respectively. The sample concentrations that gave inhibition values above 90% were determined as MICs, as calculated from absorbance values at 600 nm (OD600) obtained using a Tecan SPARK 10 M plate reader. To determine minimum bactericidal concentrations (MBCs), agar plates were treated with 5 μL of sample solutions from each well, and then incubated at 37 °C for 18 h without shaking. The sample concentrations that resulted in no colony formation on agar plates were determined as MBCs.
Cytotoxicity Assay.
Sample Preparation.
The compounds were dissolved in DMSO at 12.8 mg/mL as the stock solutions, except for compounds 9–14 and 37–39, which were directly dissolved in the culture medium at 12.8 mg/mL. The stock solutions were diluted with the culture medium to the testing concentration at 128 μg/mL (compounds 9–14 and 37–39 were diluted to 1280 μg/mL). The solutions were then serially diluted 2-fold in a 96-well plate and used in the cytotoxicity assay immediately.
Cell Culture and Cytotoxicity Assay.
A549 cells (ATCC CCL-185) were cultured in high glucose DMEM (Sigma-Aldrich, D6429) supplemented with 10% FBS, 1% penicillin–streptomycin solution (Sigma-Aldrich, P4333), 1% gentamicin solution (Sigma-Aldrich, G1272), and 1% amphotericin B solution (Sigma-Aldrich, A2942) at 37 °C in a humidified atmosphere containing 5% CO2. After cells reached 80–90% confluency, they were detached and counted by an Invitrogen Countess automated cell counter. Cells with viability over 90% were seeded at a density of 5 × 103 cells per well in 96-well cell culture plates. After overnight culture, the culture medium in each well was pipetted out; 80 μL of the compound solutions from each well of the 96-well plate prepared above were transferred to this 96-well plate containing the cells, and the plates were incubated for 20 h. After the addition of 8 μL of 110 μg/mL resazurin, the plates were incubated for a further 3 h at 37 °C in 5% CO2. The fluorescence intensity was measured using a Tecan M200 Infinite Pro microplate reader at an excitation wavelength of 560 nm and an emission wavelength of 590 nm. IC50 were calculated by curve fitting of the inhibition values versus the log of the concentration in GraphPad’s Prism. The cytotoxicity assays (in triplicate) were repeated twice using freshly prepared cells and compound solutions.
Log P Determination.
Calibration Curves.
Milli-Q Water (150 mL) was added to n-octanol (150 mL), and the mixture was stirred for 3 days to allow both phases to reach saturation. The gold standard solution (1000 ppm Au in hydrochloric acid, Fisher Scientific) was diluted to 1, 2, 3, 4, and 5 ppm with n-octanol-saturated water, and the absorbances of the resulting solutions were measured by flame atomic absorption spectroscopy (FAAS, Agilent Technology 200 Series AA, equipped with a gold HC Lamp-Au coded). The absorbance data were plotted against Au concentration, and the resulting calibration curve (curve A, Figure S142) was used for determining the concentrations of the analogues in the aqueous phase.
To construct the standard calibration curve of the Au concentration in the n-octanol phase, compound 40 was used. The Au concentration in compound 40 was first calibrated against the aqueous gold standard. For this, 0.440 mg of compound 40 was weighted on a Sartorius MC5 microbalance and was then added to 22 mL of n-octanol-saturated water. After the compound was fully dissolved, the absorbance of the solution was measured by FAAS, and the Au concentration was determined from calibration curve A to be 9.88 ppm. This data was then used to construct the standard calibration curve of the Au concentration in the n-octanol phase. For this, solutions of varying concentrations (0.99, 2.47, 4.94, 5.93, and 9.88 ppm) of compound 40 in water-saturated n-octanol were prepared. The absorbance was measured by FAAS, and the data were plotted against the Au concentration to give calibration curve B (Figure S143), which was used for determining the Au concentrations of the analogues in the n-octanol phase.
Determination of Au Concentrations in Auranofin and Analogues: Method 1.
Compound (0.5–1 mg) was weighed into a 2 mL Eppendorf tube containing 2 mL of n-octanol-saturated water. After being shaken for about 4 h, it was centrifuged at 6000 rpm for 3 min. The supernatant (1 mL) was transferred to a new tube containing 1 mL of water-saturated n-octanol, and the mixture was shaken for 1 h. After centrifugation at 6000 rpm for 5 min, the two layers were collected, and the gold concentration in each layer was determined by FAAS by comparing the absorbance to calibration curve A or B.
Determination of Au Concentrations in Auranofin and Analogues: Method 2.
This method was used for compounds 19, 20, 26–30, and 32–36, which gave very low absorbance reading on FAAS, likely because of the low solubilities of these compounds in n-octanol-saturated water. For Method 2, 0.6–1 mg of compound was weighed into a 2 mL Eppendorf tube containing 1 mL of n-octanol-saturated water and 1 mL of water-saturated n-octanol. After being shaken for 3–4 h, the mixture was centrifuged, and all the liquid was transferred to a 15 mL tube. The liquid was then diluted with 2 mL of n-octanol-saturated water and 2 mL of water-saturated n-octanol, and the mixture was shaken for another hour. After centrifugation at 6000 rpm for 5 min, the two layers were collected, and the gold concentration in each layer was determined by FAAS by comparing the absorbance to calibration curve A or B.
The log P value was calculated as follows: log P = log-[compound]oct/[compound]water.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health (NIH) for financial support (R21AI140418) and the Warwick Antimicrobial Screening Facility at the University of Warwick for help with the MIC/MBC determinations.
ABBREVIATIONS USED
- AMR
antimicrobial resistance
- MIC
minimal inhibitory concentration
- MBC
minimal bactericidal concentration
- SAR
structure–activity relationship
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- DCM
dichloromethane
- DMF
dimethylformamide
- DTT
dithiothreitol
- DBU
1,8-diazabicyclo-[5.4.0]undec-7-ene
- Et2O
diethyl ether
- RT
room temperature
- ATCC
American Type Culture Collection
- NCTC
National Collection of Type Cultures
- MRSA
methicillin-resistant Staphylococcus aureus
- M. tuberculosis
Mycobacterium tuberculosis
- A. baumannii
Acinetobacter baumannii
- P. aeruginosa
Pseudomonas aeruginosa
- E. cloacae
Enterobacter cloacae
- K. pneumonia
Klebsiella pneumonia
- S. aureus
Staphylococcus aureus
- E. coli
Escherichia coli
- E. faecium
Enterococcus faecium
- OXA
oxacillinases
- VEB
Vietnamese extended-spectrum β-lactamase
- VIM
Verona integron-mediated metallo-μ-lactamase
- AmpC
ampicillin class C
- ESBL
extended spectrum β-lactamase
- VRE
vancomycin-resistant enterococcus
- HRMS
high-resolution mass spectrometry
- Glc
D-glucose
- Gal
D-galactose
- GlcNAc
N-acetylglucosamine
- TLC
thin layer chromatography
- HRMS
high-resolution mass spectrometry
- TMS
tetramethylsilane
- CAMHB
cation-adjusted Muller–Hinton broth
- CFU
colony-forming unit
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.9b00550.
Synthesis of 1b–6b, 7d, and 8f; general procedure for qNMR; 1H NMR, 13C NMR, 31P NMR, 19F NMR, and HRMS spectra of intermediates and final products; purity assessments of compounds 1–40 by qHNMR, including methods of determination and calculation; FAAS calibration curves A and B for Au concentration determination; MIC/MBC tables; and cytotoxicity data of auranofin and analogues (PDF)
Molecular formula strings (CSV)
The authors declare no competing financial interest.
REFERENCES
- (1).Global action plan on the antimicrobial resistance; World Health Organization, 2015. [DOI] [PubMed] [Google Scholar]
- (2).Delcour AH Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta, Proteins Proteomics 2009, 1794 (5), 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jacoby GA AmpC β-lactamases. Clin. Microbiol. Rev 2009, 22 (1), 161–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Cantón R; Coque TM The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol 2006, 9 (5), 466–475. [DOI] [PubMed] [Google Scholar]
- (5).Paterson DL; Bonomo R A. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev 2005, 18 (4), 657–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Nikaido H, Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 1994, 264 (5157), 382–388. [DOI] [PubMed] [Google Scholar]
- (7).Silver LL Challenges of antibacterial discovery. Clin. Microbiol. Rev 2011, 24 (1), 71–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Poole K, Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother 2005, 56 (1), 20–51. [DOI] [PubMed] [Google Scholar]
- (9).Snitkin ES; Zelazny AM; Thomas PJ; Stock F; Henderson DK; Palmore TN; Segre JA Tracking a hospital outbreak of carbapenem-resistant klebsiella pneumoniae with whole-genome sequencing. Sci. Transl Med 2012, 4 (148), No. 148ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Phillips L Infectious disease: TB’s revenge. Nature 2013, 493 (7430), 14–16. [DOI] [PubMed] [Google Scholar]
- (11).Brown ED; Wright GD Antibacterial drug discovery in the resistance era. Nature 2016, 529 (7586), 336–343. [DOI] [PubMed] [Google Scholar]
- (12).Lewis K Platforms for antibiotic discovery. Nat. Rev. Drug Discovery 2013, 12 (5), 371–387. [DOI] [PubMed] [Google Scholar]
- (13).Lomovskaya O; Bostian KA Practical applications and feasibility of efflux pump inhibitors in the clinic–a vision for applied use. Biochem. Pharmacol 2006, 71 (7), 910–918. [DOI] [PubMed] [Google Scholar]
- (14).Merril CR; Biswas B; Carlton R; Jensen NC; Creed GJ. ; Zullo S; Adhya S. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. U. S. A 1996, 93 (8), 3188–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Rai M; Yadav A; Gade A Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv 2009, 27 (1), 76–83. [DOI] [PubMed] [Google Scholar]
- (16).Hao N; Chen X; Jeon S; Yan M Carbohydrate-conjugated hollow oblate mesoporous silica nanoparticles as nanoantibiotics to target mycobacteria. Adv. Healthcare Mater 2015, 4 (18), 2797–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Xie S; Manuguri S; Proietti G; Romson J; Fu Y; Inge AK; Wu B; Zhang Y; Hall D; Ramstrom O; Yan M Design and synthesis of theranostic antibiotic nanodrugs that display enhanced antibacterial activity and luminescence. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (32), 8464–8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Cassetta MI; Marzo T; Fallani S; Novelli A; Messori L Drug repositioning: auranofin as a prospective antimicrobial agent for the treatment of severe staphylococcal infections. BioMetals 2014, 27 (4), 787–791. [DOI] [PubMed] [Google Scholar]
- (19).Younis W; Thangamani S; Seleem M Repurposing nonantimicrobial drugs and clinical molecules to treat bacterial infections. Curr. Pharm. Des 2015, 21 (28), 4106–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zheng W; Sun W; Simeonov A Drug repurposing screens and synergistic drug-combinations for infectious diseases. Br. J. Pharmacol 2018, 175 (2), 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hunt LB The figuirers of montpelier. Ambix 1979, 26, 221–223. [DOI] [PubMed] [Google Scholar]
- (22).Forestier J L’Aurothiopie dans les rthumatisme chronique. Bull. Mem. Soc. Med. Hop. Paris 1929, 53, 323–327. [Google Scholar]
- (23).Booth G Complexes of the transition metals with phosphines, arsines, and stibines. Adv. Inorg. Chem. Radiochem 1964, 6, 1–69. [Google Scholar]
- (24).Sutton BM; McGusty E; Walz DT; DiMartino MJ Oral gold. antiarthritic properties of alkylphosphinegold coordination complexes. J. Med. Chem 1972, 15 (11), 1095–1098. [DOI] [PubMed] [Google Scholar]
- (25).Nardon C; Pettenuzzo N; Fregona D Gold complexes for therapeutic purposes: an updated patent review (2010–2015). Curr. Med. Chem 2016, 23 (29), 3374–3403. [DOI] [PubMed] [Google Scholar]
- (26).Mjos KD; Orvig C Metallodrugs in medicinal inorganic chemistry. Chem. Rev 2014, 114 (8), 4540–4563. [DOI] [PubMed] [Google Scholar]
- (27).Kean WF; Hart L; Buchanan WW Auranofin. Rheumatology 1997, 36 (5), 560–572. [DOI] [PubMed] [Google Scholar]
- (28).Roder C; Thomson MJ Auranofin: repurposing an old drug for a golden new age. Drugs R&D 2015, 15 (1), 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Sachweh MC; Stafford WC; Drummond CJ; McCarthy AR; Higgins M; Campbell J; Brodin B; Arner ES; Lain S Redox effects and cytotoxic profiles of MJ25 and auranofin towards malignant melanoma cells. Oncotarget 2015, 6 (18), 16488–16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Fiskus W; Saba N; Shen M; Ghias M; Liu J; Gupta SD; Chauhan L; Rao R; Gunewardena S; Schorno K; Austin CP; Maddocks K; Byrd J; Melnick A; Huang P; Wiestner A; Bhalla KN Auranofin induces lethal oxidative and endoplasmic reticulum stress and exerts potent preclinical activity against chronic lymphocytic leukemia. Cancer Res. 2014, 74 (9), 2520–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Glisic BD; Djuran MI Gold complexes as antimicrobial agents: an overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. Dalton Trans 2014, 43 (16), 5950–5969. [DOI] [PubMed] [Google Scholar]
- (32).Marzano C; Gandin V; Folda A; Scutari G; Bindoli A; Rigobello MP Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radical Biol. Med 2007, 42 (6), 872–881. [DOI] [PubMed] [Google Scholar]
- (33).Sannella AR; Casini A; Gabbiani C; Messori L; Bilia AR; Vincieri FF; Majori G; Severini C New uses for old drugs. Auranofin, a clinically established antiarthritic metallodrug, exhibits potent antimalarial effects in vitro: mechanistic and pharmacological implications. FEBS Lett. 2008, 582 (6), 844–847. [DOI] [PubMed] [Google Scholar]
- (34).Miyamoto Y; Eckmann L Drug development against the major diarrhea-causing parasites of the small intestine, cryptosporidium and giardia. Front. Microbiol 2015, 6, 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wiederhold NP; Patterson TF; Srinivasan A; Chaturvedi AK; Fothergill AW; Wormley FL; Ramasubramanian AK; Lopez-Ribot JL Repurposing auranofin as an antifungal: in vitro activity against a variety of medically important fungi. Virulence 2017, 8 (2), 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Fuchs BB; RajaMuthiah R; Souza AC; Eatemadpour S; Rossoni RD; Santos DA; Junqueira JC; Rice LB; Mylonakis E Inhibition of bacterial and fungal pathogens by the orphaned drug auranofin. Future Med. Chem 2016, 8 (2), 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Clinic Mayo. Auranofin in treating patients with recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer; NCT01747798; U.S. National Library of Medicine, ClinicalTrials.gov, 2012. [Google Scholar]
- (38).Capparelli EV; Bricker-Ford R; Rogers MJ; McKerrow JH; Reed SL Phase I clinical trial results of auranofin, a novel antiparasitic agent. Antimicrob. Agents Chemother 2017, 61 (1), No. e01947–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Shaw CF Gold-based therapeutic agents. Chem. Rev 1999, 99 (9), 2589–2600. [DOI] [PubMed] [Google Scholar]
- (40).Angelucci F; Sayed AA; Williams DL; Boumis G; Brunori M; Dimastrogiovanni D; Miele AE; Pauly F; Bellelli A Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects. J. Biol. Chem 2009, 284 (42), 28977–28985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Parsonage D; Sheng F; Hirata K; Debnath A; McKerrow JH; Reed SL; Abagyan R; Poole LB; Podust LM X-ray structures of thioredoxin and thioredoxin reductase from Entamoeba histolytica and prevailing hypothesis of the mechanism of Auranofin action. J. Struct. Biol 2016, 194 (2), 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Ilari A; Baiocco P; Messori L; Fiorillo A; Boffi A; Gramiccia M; Di Muccio T; Colotti G A gold-containing drug against parasitic polyamine metabolism: the X-ray structure of trypanothione reductase from Leishmania infantum in complex with auranofin reveals a dual mechanism of enzyme inhibition. Amino Acids 2012, 42 (2–3), 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Harbut MB; Vilcheze C; Luo X; Hensler ME; Guo H; Yang B; Chatterjee AK; Nizet V; Jacobs WR Jr.; Schultz PG; Wang F Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (14), 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Hokai Y; Jurkowicz B; Fernandez-Gallardo J; Zakirkhodjaev N; Sanau M; Muth TR; Contel M Auranofin and related heterometallic gold(I)-thiolates as potent inhibitors of methicillin-resistant Staphylococcus aureus bacterial strains. J. Inorg. Biochem 2014, 138, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Jackson-Rosario S; Cowart D; Myers A; Tarrien R; Levine RL; Scott RA; Self WT Auranofin disrupts selenium metabolism in Clostridium difficile by forming a stable Au-Se adduct. JBIC, J. Biol. Inorg. Chem 2009, 14 (4), 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Jackson-Rosario S; Self WT Inhibition of selenium metabolism in the oral pathogen Treponema denticola. J. Bacteriol 2009, 191 (12), 4035–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Yanagawa A; Narikawa S; Kudo T; Shimada J; Mizushima Y; Endo T; Kusakari K Anti-bacterial and anti-fungal effect of several anti-rheumatic drugs. Ensho 1995, 15 (3), 261–264. [Google Scholar]
- (48).Thangamani S; Mohammad H; Abushahba MF; Sobreira TJ; Hedrick VE; Paul LN; Seleem MN Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep 2016, 6, 22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Marzo T; Cirri D; Pollini S; Prato M; Fallani S; Cassetta MI; Novelli A; Rossolini GM; Messori L Auranofin and its analogues show potent antimicrobial activity against multidrug-resistant pathogens: structure-activity relationships. ChemMedChem 2018, 13 (22), 2448–2454. [DOI] [PubMed] [Google Scholar]
- (50).Marzo T; Cirri D; Gabbiani C; Gamberi T; Magherini F; Pratesi A; Guerri A; Biver T; Binacchi F; Stefanini M; Arcangeli A; Messori L Auranofin, Et3PAuCl, and Et3PAuI are highly cytotoxic on colorectal cancer cells: a chemical and biological study. ACS Med. Chem. Lett 2017, 8 (10), 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Rice LB Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis 2008, 197 (8), 1079–1081. [DOI] [PubMed] [Google Scholar]
- (52).Pearson R G. Hard and soft acids and bases. J. Am. Chem. Soc 1963, 85 (22), 3533–3539. [Google Scholar]
- (53).Rush GF; Smith PF; Hoke GD; Alberts DW; Snyder RM; Mirabelli CK The mechanism of acute cytotoxicity of triethylphosphine gold (I) complexes: II. Triethylphosphine gold chloride-induced alterations in mitochondrial function. Toxicol. Appl Pharmacol 1987, 90 (3), 391–400. [DOI] [PubMed] [Google Scholar]
- (54).Pregosin PS; Becker ED 1H-NMR spectroscopy of gold drugs. High-resolution studies of β-d-thioglucosetetraacetate phosphine complexes of Au(I). Helv. Chim. Acta 1983, 66 (5), 1436–1440. [Google Scholar]
- (55).Shu P; Zeng J; Tao J; Zhao Y; Yao G; Wan Q Selective S-deacetylation inspired by native chemical ligation: practical syntheses of glycosyl thiols and drug mercapto-analogues. Green Chem. 2015, 17 (4), 2545–2551. [Google Scholar]
- (56).Razi MT; Sadler PJ; Hill DT; Sutton BM Proton, carbon-13, and phosphorus-31 nuclear magnetic resonance studies of (2,3,4,6-tetra-O-acetyl-1-thio-β-d-glucopyranosato-S)-(triethylphosphine)gold (auranofin), a novel antiarthritic agent. J. Chem. Soc., Dalton Trans 1983, No. 7, 1331–1334. [Google Scholar]
- (57).Wang J; Mi X; Wang J; Yang Y An efficient approach to chloro(organophosphine) gold(i) complexes for the synthesis of auranofin. Green Chem. 2017, 19 (3), 634–637. [Google Scholar]
- (58).Gutierrez A; Marzo I; Cativiela C; Laguna A; Gimeno MC Highly cytotoxic bioconjugated gold(I) complexes with cysteinecontaining dipeptides. Chem. - Eur. J 2015, 21, 11088–11095. [DOI] [PubMed] [Google Scholar]
- (59).Aguinagalde L; Díez-Martínez R; Yuste J; Royo I; Gil C; Lasa Í; Martín-Fontecha M; Marín-Ramos NI; Ardanuy C; Liñares J; García P; García E; Sánchez-Puelles JM Auranofin efficacy against MDR Streptococcus pneumoniae and Staphylococcus aureus infections. J. Antimicrob. Chemother 2015, 70 (9), 2608–2617. [DOI] [PubMed] [Google Scholar]
- (60).Sun W; Weingarten RA; Xu M; Southall N; Dai S; Shinn P; Sanderson PE; Williamson PR; Frank KM; Zheng W Rapid antimicrobial susceptibility test for identification of new therapeutics and drug combinations against multidrug-resistant bacteria. Emerging Microbes Infect. 2016, 5, No. e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Tepperman K; Finer R; Donovan S; Elder RC; Doi J; Ratliff D; Ng K Intestinal uptake and metabolism of auranofin, a new oral gold-based antiarthritis drug. Science 1984, 225 (4660), 430–432. [DOI] [PubMed] [Google Scholar]
- (62).Zhu X; Schmidt RR Glycosylthiomethyl chloride: A new species for S-neoglycoconjugate synthesis. Synthesis of 1-N-glycosylthiomethyl-1,2,3-triazoles. J. Org. Chem 2004, 69 (4), 1081–1085. [DOI] [PubMed] [Google Scholar]
- (63).Turton JF; Kaufmann ME; Warner M; Coelho J; Dijkshoorn L; van der Reijden T; Pitt TL A prevalent, multiresistant clone of Acinetobacter baumannii in Southeast England. J. Hosp Infect 2004, 58 (3), 170–179. [DOI] [PubMed] [Google Scholar]
- (64).Woodford N; Zhang J; Kaufmann ME; Yarde S; Tomas M d M; Faris C; Vardhan MS; Dawson S; Cotterill SL; Livermore DM. Detection of Pseudomonas aeruginosa isolates producing VEB-type extended-spectrum β-lactamases in the United Kingdom. J. Antimicrob. Chemother. 2008, 62 (6), 1265–1268. [DOI] [PubMed] [Google Scholar]
- (65).Yang Y; Livermore D; Williams R J. Chromosomal β-lactamase expression and antibiotic resistance in Enterobacter cloacae. J. Med. Microbiol. 1988, 25 (3), 227–233. [DOI] [PubMed] [Google Scholar]
- (66).Rasheed JK; Anderson GJ; Yigit H; Queenan AM; Doménech-Sánchez A; Swenson JM; Biddle JW; Ferraro MJ; Jacoby GA; Tenover FC Characterization of the extended-spectrum β-lactamase reference strain, Klebsiella pneumoniae K6 (ATCC 700603), which produces the novel enzyme SHV-18. Antimicrob. Agents Chemother. 2000, 44 (9), 2382–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Diep BA; Gill SR; Chang RF; Phan TH; Chen JH; Davidson MG; Lin F; Lin J; Carleton HA; Mongodin EF; Sensabaugh GF; Perdreau-Remington F Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006, 367 (9512), 731–739. [DOI] [PubMed] [Google Scholar]
- (68).Patel R; Uhl JR; Kohner P; Hopkins MK; Cockerill FR 3rd Multiplex PCR detection of vanA, vanB, vanC-1, and vanC-2/3 genes in enterococci. J. Clin. Microbiol. 1997, 35 (3), 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Pearson S; Scarano W; Stenzel MH Micelles based on gold-glycopolymer complexes as new chemotherapy drug delivery agents. Chem. Commun. 2012, 48 (39), 4695–4697. [DOI] [PubMed] [Google Scholar]
- (70).Deng L; Wang X; Uppalapati S; Norberg O; Dong H; Joliton A; Yan M; Ramstrom O Stereocontrolled 1-S-glycosylation and comparative binding studies of photoprobe-thiosaccharide conjugates with their O-linked analogs. Pure Appl. Chem. 2013, 85 (9), 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Norberg O; Lee IH; Aastrup T; Yan M; Ramstrom O Photogenerated lectin sensors produced by thiol-ene/yne photo-click chemistry in aqueous solution. Biosens. Bioelectron. 2012, 34 (1), 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Deng L; Norberg O; Uppalapati S; Yan M; Ramstrom O Stereoselective synthesis of light-activatable perfluorophenylazide-conjugated carbohydrates for glycoarray fabrication and evaluation of structural effects on protein binding by SPR imaging. Org. Biomol. Chem. 2011, 9 (9), 3188–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Wu B; Ge J; Ren B; Pei Z; Dong H Synthesis and binding affinity analysis of positional thiol analogs of mannopyranose for the elucidation of sulfur in different position. Tetrahedron 2015, 71 (23), 4023–4030. [Google Scholar]
- (74).Griffiths MBE; Koponen SE; Mandia DJ; McLeod JF; Coyle JP; Sims JJ; Giorgi JB; Sirianni ER; Yap GPA; Barry ST Surfactant directed growth of gold metal nanoplates by chemical vapor deposition. Chem. Mater. 2015, 27 (17), 6116–6124. [Google Scholar]
- (75).Chabanne L; Matas I; Patra SK; Manners I Organic-metalloblock copolymersvia photocontrolled living anionic ring-opening polymerization. Polym. Chem. 2011, 2 (11), 2651–2660. [Google Scholar]
- (76).Della Sala F; Kay ER Reversible control of nanoparticle functionalization and physicochemical properties by dynamic covalent exchange. Angew. Chem. Int. Ed. 2015, 54 (14), 4187–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Lu Y; Fu X; Chen H; Du X; Jia X; Liu Y An efficient domino approach for the synthesis of multisubstituted pyrroles via gold/silver-catalyzed amination/cycloisomerization of (Z)-2-En-4-yn-1-ols. Adv. Synth. Catal 2009, 351 (1–2), 129–134. [Google Scholar]
- (78).Harris RJ; Nakafuku K; Widenhoefer RA Kinetics and mechanism of the racemization of aryl allenes catalyzed by cationic gold(I) phosphine complexes. Chem. - Eur. J 2014, 20 (38), 12245–12254. [DOI] [PubMed] [Google Scholar]
- (79).Sutherland DR; Kinsman L; Angiolini SM; Rosair GM; Lee A-L Gold(I)-catalysed hydroarylation of 1,3-disubstituted allenes with efficient axial-to-point chirality transfer. Chem. - Eur. J 2018, 24 (27), 7002–7009. [DOI] [PubMed] [Google Scholar]
- (80).Gunatilleke SS; Barrios AM Inhibition of lysosomal cysteine proteases by a series of Au(I) complexes: A detailed mechanistic investigation. J. Med. Chem. 2006, 49 (13), 3933–3937. [DOI] [PubMed] [Google Scholar]
- (81).Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; Document M100-S25; Clinical and Laboratory Standards Institute, 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


