Abstract
Spinal cord injury (SCI) always leads to functional deterioration due to a series of processes including cell death. In recent years, programmed cell death (PCD) is considered to be a critical process after SCI, and various forms of PCD were discovered in recent years, including apoptosis, necroptosis, autophagy, ferroptosis, pyroptosis and paraptosis. Unlike necrosis, PCD is known as an active cell death mediated by a cascade of gene expression events, and it is crucial for elimination unnecessary and damaged cells, as well as a defence mechanism. Therefore, it would be meaningful to characterize the roles of PCD to not only enhance our understanding of the pathophysiological processes, but also improve functional recovery after SCI. This review will summarize and explore the most recent advances on how apoptosis, necroptosis, autophagy, ferroptosis, pyroptosis and paraptosis are involved in SCI. This review can help us to understand the various functions of PCD in the pathological processes of SCI, and contribute to our novel understanding of SCI of unknown aetiology in the near future.
Keywords: pathological mechanisms, programmed cell death, spinal cord injury, therapy
Different types of programmed cell death (PCD) play different roles in spinal cord injury. PCD is an active cell death mediated by a cascade of gene expression events, and it is crucial for elimination unnecessary and damaged cells, as well as a defense mechanism. PCD consists of a series of activities, such as apoptosis, necroptosis, autophagy, ferroptosis, pyroptosis and paraptosis. This figure indicates how how apoptosis, necroptosis, autophagy, ferroptosis, pyroptosis and paraptosis are involved in spinal cord injury.
1. INTRODUCTION
Spinal cord injury (SCI) usually results in a large range of sensorimotor and autonomic nerve injury and remains a serious public health problem worldwide. SCI affects approximately 273 000 people in the United States, and there are some 12 000 new cases each year. 1 , 2 , 3 Therefore, SCI brings severe economy burdens and psychological pressure to patients. However, there are currently no effective therapies for SCI clinically, and an effective treatment is awaited. 4 , 5 , 6 This is due mainly to the molecular mechanisms of SCI remain elusive. The pathological process of SCI is known as a complex process, which can be classified into two phases: Primary injury is the direct mechanical damage of spinal cord tissue and includes demyelination and necrosis of neurons and axons; and the secondary injury is composed of a variety of pathophysiologic mechanisms, including local haemorrhage, ischaemia, oedema, ionic imbalance, free radical stress and inflammatory responses. 7 , 8 This complex pathological process of SCI may explain the difficulty in finding a suitable and effective therapy. Therefore, understanding the molecular mechanisms of SCI is critical for the development of therapeutic strategies.
Cell death is known as the final stage of cells and it can be resulted from cytotoxicity from exogenous or endogenous substances. 9 In 1842, cell death was first posed by Carl Vogt, and lots of molecules are considered to be involved in this irreversible process to support the maintenance of cellular homeostasis. 10 Cell death was initially divided into two types, necrosis and apoptosis. 11 Necrosis is considered as a passive and accidental cell death, which can be resulted from environmental perturbations and the large amounts of release of inflammatory cellular contents. 11 , 12 Apoptosis is considered as an active, programmed process of autonomous cellular dismantling without the release of cytoplasmic content to the extracellular space. 11 , 12 , 13 , 14 In recent years, various forms of programmed cell death (PCD) were discovered, including autophagy, necroptosis, pyroptosis and ferroptosis. 9 , 15 , 16 , 17 , 18 Unlike necrosis, PCD is known as an active cell death mediated by a cascade of gene expression events, commonly found in organisms. 15 , 16 PCD is important for elimination unnecessary and damaged cells, and it can also represent a defence mechanism. 19 , 20 , 21 As research has progressed, more and more researches showed that PCD was involved in central nervous system (CNS) development, neurodegenerative disorders, psychiatric disorders and CNS injury. 22
More recently, there still is a lack of the study on PCD in SCI, and increasing evidence shows that PCD play significant roles following SCI. 23 , 24 In this review, we summarized and explored the recent advances in the roles of PCD in SCI in order to know the molecular mechanisms underlying SCI.
2. THE ROLE OF APOPTOSIS IN SPINAL CORD INJURY
As a type of PCD, apoptosis is the most common form of PCD, it was extensively studied and could be defined as programmed self‐killing simply. 25 Apoptosis has been considered as an immunologically silent form of PCD since cell corpses are cleared by phagocytes, and it can arise from the extrinsic (death receptor initiated) and the intrinsic (mitochondrial) pathways. 26 , 27 , 28 Excitotoxins, free radicals and inflammatory mediators are known as the factors that initiate cell death and stimulate necrosis or apoptosis. 29 Apoptosis is known as a naturally occurring physiological process and may play a key role in secondary SCI. 29 The secondary injury following SCI is considered to be due to the continuation of cellular destruction through apoptosis, and the long‐term neurological deficits following SCI might result from a large range of apoptosis of neurons and oligodendrocytes in the injured spinal cord, so a better understanding of apoptosis following SCI will lead to novel strategies for therapeutic interventions.
Apoptosis can be found in the injured spinal cord of animal models and humans. Injury‐induced apoptosis could be observed in neurons, astrocytes, oligodendroglia and microglia in rat spinal cord after injury, 30 and the apoptotic oligodendrocytes mainly located in the longitudinal tracts of the white matter. 31 Apoptosis in neurons was detected at 4 hours and peaked at 8 hours after injury; apoptosis in glial cells was detected at 4 hours after injury, and peaked at 24 hours after injury; apoptosis in oligodendrocytes was detected in the white matter at 24 hours and reached to the highest at 8 days after injury. 32 , 33 Apoptosis in microglia was relatively rare at 24 hours and 5 days, but gradually increased in numbers and peaked at 8 days post‐injury. 33 Furthermore, most of apoptotic cells could be observed in the rim of surviving tissue around the centre of the injured spinal cord, and this may help explain why the lesion area expand, progressively. 29 Apoptosis can also be detected in oligodendrocytes in white matter tracts showing Wallerian degeneration, which is a significant characteristic in chronic phase of SCI. 29 , 34
In recent years, more and more studies occur to investigate the role of apoptosis after SCI. Apoptosis is a caspase‐dependent cell death modality, and this programme was primarily regulated by the caspase family of cysteine proteases. 35 Upstream and downstream components of the caspase‐3 apoptotic pathway have proved to be activated after SCI in rats and play a vital role in the process of apoptosis. 36 Previous studies showed that activation of the Fas death receptor pathway played a key role in neuronal, oligodendrocyte and microglia apoptosis in animal models following SCI. 37 , 38 , 39 The interaction of Fas and Fas receptor (FasR) initiates apoptosis via the activation of the cysteine proteases resulting in proteolysis and DNA cleavage by effector caspases culminating in cell death. 40 , 41 Some key signalling pathways, including SIRT1/AMPK signalling pathway, Wnt/β‐catenin signalling pathway and E2F1/CDK1 pathway, have already proved to be involved in regulating apoptotic activity in SCI. 42 , 43 , 44 Furthermore, recent studies revealed that microRNAs (miRNAs) and long non‐coding RNAs (lncRNAs) may have an important effect on the pathogenesis of SCI including cell apoptosis. A previous study revealed that miR‐137 could inhibit apoptosis after SCI via targeting MK2. 45 Gu et al 46 found that knockdown of long coding RNA XIST played a critical role in inhibiting neuronal apoptosis after SCI by competitively binding miR‐494 and regulation on PTEN/AKT/mTOR pathway. Another study showed that PI3K/Akt/mTOR signalling pathway took part in the apoptosis of neurons after SCI and this pathway could induce apoptosis by activation of the mitochondrial pathway. 47
Having an in‐depth knowledge of the molecular and cellular mechanisms of apoptosis can help us screen out the specific drug targets. Liu et al used proteomics analysis to find a key protein, endoplasmic reticulum protein 29 (ERp29), which could regulate a group of genes related with cell survival and apoptosis including caspase and Erk and improve locomotor function in spinal cord transection rats. 48 Furthermore, minocycline, antibody blockade of the CD95 (FAS) ligand and the blockade of glycosphingolipid‐induced iNOS have already proved to suppress the apoptosis of neurons and glia cells, and it is accompanied by improvement in neurological outcomes, and increase the efficacy of cell transplantation strategies. 49 In another recent study, CARD6, Caspase recruitment domain family member 6, was shown to be a key molecular which could inhibit apoptosis by decreasing Cyto‐c release to cytosol from mitochondria and inhibition of Caspase‐3 signalling pathway. 50 Zhang et al firstly performed a rat model of SCI, and upregulated p38 was related to inflammation and apoptosis after SCI. Zhang et al also suggested that p38 inhibitor SB203580 treatment could alleviate secondary SCI by suppressing inflammation and apoptosis. 51 The deficiency of progranulin, a 593 amino acid secreted glycoprotein, is not conducive to SCI recovery via promotion of cellular apoptosis and neuroinflammation. 52 In a recent study, metformin was found to increase the expressions of β‐catenin and brain derived neurotrophic factor (BDNF), inhibit neuron apoptosis and inflammatory response, and promote motor functional recovery in rats after SCI. 53
In the above research results, it has been shown that apoptosis contributed to the tissue damage following SCI. Finding the way to inhibit apoptosis after SCI may have important clinical implications for the further treatment. Although more and more studies focus on the intrinsic or extrinsic signals which are associated with apoptosis following SCI, there are more problems still need to be studied, including the detailed mechanisms causing apoptotic death of neurons, astrocytes, oligodendroglia and microglia after SCI.
3. THE ROLE OF NECROPTOSIS IN SPINAL CORD INJURY
Apoptosis was known as the only form of PCD traditionally, however, more and more studies showed that necrosis can be induced in a similar manner to apoptosis, which is termed ‘programmed necrosis’ or ‘necroptosis’. 54 Necroptosis can be regulated by the caspase‐independent pathway and shows the morphological characteristics of necrosis (early loss of plasma membrane integrity, gain in cell volume and swelling organelles). 55 , 56 In recent years, necroptosis has been found to contribute to many diseases including neurodegenerative diseases, ischaemic brain injury and viral myocardial infarction. 56 , 57 , 58 , 59
The most widely studied pathway causing necroptosis results from binding of Tumour necrosis factor‐α (TNF‐α) to TNF‐R1. 60 Various studies have shown that necroptosis are involved in intracellular signalling cascade transduced by receptor‐interacting protein 1/3 (RIP1/3) and mixed lineage kinase domain‐like protein (MLKL). 61 RIP1 kinase activity has been verified to be essential for the activation of necroptosis. 62 In recent research, the author found that overexpress and knockdown PSMB4 could regulate the MLKL and RIP3 pathway in TNF‐α induced necroptosis cell model. 63 Furthermore, Wang et al found that necrostatin‐1 (Nec‐1), inhibitor of necroptosis, could improve and protect the physiological function by inhibiting necroptosis via inhibition of RIP1/3–MLKL recruitment. 64 In another research, the results also revealed that necroptosis could contribute to neural cell death, and Nec‐1 could reduce histopathological and functional deficits after SCI in mice. 65 Therefore, Nec‐1 could be a potential target for treating SCI.
Moreover, accumulating evidences revealed that necroptosis is bound up with inflammation following SCI. 66 , 67 Fan et al found that endoplasmic reticulum of necroptotic microglia/macrophages could be manipulated for regulating inflammation following SCI. 68 Then their subsequent study showed that reactive astrocytes could undergo M1 microglia/macrophages‐induced necroptosis through TLR/MyD88 signalling pathway. 69 Shao et al found that Smurf1 could facilitate necroptosis of neurons after LPS‐induced neuroinflammation, and Smurf1 may be a potential target for treatment. 70
Necroptosis was already considered as a critical and emerging mode of PCD following SCI. It has been previously demonstrated that activation of necroptosis leads to neuronal and glial cell death after SCI. Activation of necroptosis has been found to lead to cell loss and tissue damage in central nervous system injury, including SCI. Although some work has been done to explore the molecular mechanism of necroptosis after SCI, the understanding of necroptosis is still inadequate. Therefore, identification of the role of necroptosis in the pathological processes of SCI is necessary to find the therapeutic target for secondary injury.
4. THE ROLE OF AUTOPHAGY IN SPINAL CORD INJURY
The word ‘autophagy’ originally came from the Greek and means to eat (‘phagy’) oneself (‘auto’). 71 Autophagy is known as a non‐apoptotic form of PCD that ensures the maintenance of cellular homeostasis via the degradation and recycle of damaged organelles, toxic agents and long‐lived, needless proteins through an autophagosomal–lysosomal pathway. 72 , 73 , 74 During the course of autophagy, autophagosomes, which are double‐membrane vesicles, sequester cytoplasmic components including damaged organelles and toxic protein aggregates, then the autophagosomes can be combined with lysosomes to make lysosomal proteases degrade the cargo. 75 Previous studies have revealed that autophagy can promote cell death by inducing caspases‐dependent apoptosis in various cells. 18 , 76 , 77 , 78 The PI3K/Akt/mTOR is considered as the central signalling pathway involved in autophagy. 79 , 80 Elevated autophagy has been observed after SCI and accumulating evidences has revealed that autophagy can act as a pro‐survival mechanism by modulating the death of neural cells for neuroprotection after SCI. 75 , 81 Autophagosomes also have been found in cultured neurons or in vivo. 82 , 83 , 84 However, the role of autophagy in the subsequent neurodegeneration following SCI still remained controversial up to now, and both beneficial and detrimental outcomes are reported in previous studies about SCI.
Some researchers believe that autophagy plays an active role in confronting with stress after SCI, and it was considered as a potential therapeutic target of SCI. He et al demonstrated that, in cultured CNS neurons, boosting autophagy could stabilize the microtubules by degrading a microtubule destabilizing protein, SCG10 (superior cervical ganglia protein 10), and this was beneficial to promote axon growth. Moreover, Tat‐beclin1, a specific autophagy‐inducing peptide, could attenuate axon retraction, promote axon regeneration and improve functional recovery in mice with SCI. 85 Previous study demonstrated that the metformin have a protective effect on SCI which was through autophagy flux stimulation. 86 Furthermore, autophagy is thought to protect the neurons against endoplasmic reticulum (ER) stress, and its disruption after SCI could lead to ER‐stress‐induced neuronal apoptosis. 87 Recent studies showed that induction of astrocyte autophagy flux could enhance the viability of neurons, decrease neuronal apoptosis, and improve neurological outcomes. 88 The relation between autophagy and apoptosis was also discussed in some studies. The results of a previous research showed that induction of autophagy can produce neuroprotective effects in acute SCI in rats via inhibition of apoptosis. 89 Wang et al proved that autophagy may play a protective role against apoptosis in spinal cord neurons with mechanically‐injury. 90 Therefore, autophagy may be beneficial for the survival of neurons after SCI.
The other researchers, however, hold a different view. Bisicchia et al found that the inhibition of autophagosomes biogenesis could significantly attenuate remote degeneration and promote spontaneous functional recovery of SCI. 91 Furthermore, light chain 3 (LC3) is known as an important marker of autophagy, and a previous study proved that the number of the LC3‐positive cells increased significantly at the lesion site after hemisection of the spinal cord, and this result demonstrated that autophagic cell death could be commonly found in the damaged neural tissue following SCI. 81 In a recent study, ABT888, an inhibitor of Poly(ADP‐Ribose) Polymerase, was found to play a protective role after SCI through a reduction in the autophagy machinery. 92
Therefore, the effects of autophagy on the different period of SCI are still uncertain, and both protective and pathological functions of autophagy are possible. Therefore, in the future study, we should try to understand the mechanisms in more detail, and be fully aware of the role of autophagy in SCI may carry us a potential pleiotropic treatment that could target multiple cell types and pathways during the pathological process of SCI.
5. THE ROLE OF FERROPTOSIS IN SPINAL CORD INJURY
Ferroptosis is known as a newly discovered form of PCD and results from the accumulation of iron‐dependent lipid peroxide, and the term ‘ferroptosis’ was first used by Stockwell et al in 2012. 9 Ferroptosis is genetically, morphologically and biochemically differ from apoptosis. 93 Ferroptosis has the characteristic of iron‐dependent increase in reactive oxygen species (ROS), and ROS plays a critical role in ferroptosis. 93 , 94 Ferroptosis is considered to be involved in the pathological cell death associated with Alzheimer's diseases, Huntington's diseases, Parkinson's diseases, stroke, traumatic brain injury, ischaemia‐reperfusion injury and so on. 95 In recent years, more and more studies focus on the role of ferroptosis in SCI.
Ferroptosis is closely related to excitotoxicity‐induced neuron death. 93 Furthermore, previous study also showed that the neurons in forebrain were more likely affected by ferroptosis, and ferroptosis may be a critical neurodegenerative mechanism in some diseases including Alzheimer's disease. 96 Glutathione peroxidase 4 (GPX4) is a disincentive for ferroptosis, and ferroptosis inhibition by GPX4 is necessary for the health and survival of motor neuron in vivo. 97 Because Iron deposition is a key pathological event in ferroptosis, so deferoxamine (DFO), a drug used for treating iron overload, has been used as ferroptosis inhibitors in many disease models. 98 Previous study found that deferoxamine, a drug used for treating iron overload, could decrease total iron ion, tumour necrosis factor‐α, interleukin‐1β and caspase‐3 expression levels after SCI and inhibit apoptosis and formation of glial scar to improve function recovery. 99 Furthermore, a recent study focus on the effects of proanthocyanidins on SCI repair, and the results showed that intraperitoneal injections with proanthocyanidins could promote functional recovery of SCI via inhibiting ferroptosis. 100
These above studies showed that ferroptosis may play an important role in the serious consequences of secondary injury following SCI, and regulating this process contributed to function recovery after SCI. However, adequate studies on the role of ferroptosis in SCI are still lacking in this field. Therefore, research on ferroptosis is urgently needed to unravel the contribution of ferroptosis to neuronal demise and glial scar formation, and this may provide new therapeutic opportunities for SCI.
6. THE ROLE OF PARAPTOSIS AND PYROPTOSIS IN SPINAL CORD INJURY
Paraptosis and pyroptosis are two novel types of PCD, and they attract more and more researchers’ interest. Paraptosis has recently been implicated as a type of PCD which has the characteristic of dilation of mitochondria and/or ER swelling resulting from cytoplasmic vacuolation, and it does not depend on caspases and lacks apoptotic morphologies. 101 Paraptosis lacks the features of apoptosis and it is usually related to defective proteins in the ER, and previous study found a paraptotic response after what appears to involve nuclear targeting. 102 , 103 Some studies showed that mitogen‐activated protein kinases (MAPKs) might have key effects on paraptosis, and paraptosis can be supressed by AIP‐1/Alix and cycloheximide. 104 , 105 Furthermore, bortezomib/nutlin‐3 could perturb proteostasis, trigger ER/mitochondria stress and irrecoverable impairments in their structure and function, and ultimately lead to paraptotic cell death. 106 However, as a novel type of PCD, the determinants and consequences of paraptosis in SCI are not clear.
In CNS, it has been found that upregulation of p44 can activate IGF‐1R and neuronal death via paraptosis and autophagy. 107 In Alzheimer's disease, a previous study found that paraptosis was demonstrated in the early pathological stages of Alzheimer's disease, which might do harm to the mitochondria and lead to mitochondrial pathway‐mediated apoptosis, subsequently. 108 Furthermore, activated microglia can cause neuronal cell death with characteristic vacuolation following blocking the caspase cascade. 109 Microglia are known as a key component of the protective scar that forms after SCI, so in‐depth study on the role of activated microglia in paraptosis should be an interesting new direction. In summary, although some progresses have been made in the study of paraptosis, there are still many problems to be solved. Furthermore, the studies of the effects of paraptosis on SCI are insufficient, so there is an urgent need to identify the role of paraptosis in SCI.
Pyroptosis is known as a pro‐inflammatory form regulating cell death which results from caspase‐1 activation within the inflammasome complex and caspase‐11 (caspase‐4/5 in humans) activation following intracellular LPS recognition. 110 , 111 Pyroptosis mainly occurs in professional phagocytes of the myeloid lineage including macrophages, dendritic cells and neutrophils, although it has also been observed in keratinocytes, epithelial cells and neurons. 112 Pyroptosis has been found to make crucial contributions to inflammatory and anti‐microbial responses in infections. 112 Previous studies showed that the activation of NLRP1 could generate a functional caspase‐1‐containing inflammasome in vivo to regulate the pyroptosis, which has an important effect on the pathogenesis of neurological disorders, 113 and the rats with NLRP1 or caspase‐1 silencing could result in significantly reduced neuronal pyroptosis in the amygdala kindling‐induced rat model. 114 In a recent study, the results showed that microglial voltage‐gated proton channel deficiency could reduce NLRP3‐induced neuronal pyroptosis. 115 Furthermore, the neuroprotection of erythropoetin (EPO) against sevoflurane induced‐neuronal pyroptosis is related to Erk1/2‐Nrf2/Bach1 signal pathway. 116 A previous study demonstrated that S pneumoniae could induce pyroptosis in murine microglia and that NLRP3 inflammasome is critical for caspase‐1 activation during pyroptosis. 117
The involvement of pyroptosis in the pathological process of SCI has not yet been fully investigated. According to the previous study, pyroptosis has been shown to be closely associated with inflammation, so the relationship between pyroptosis and inflammatory response after SCI deserve our further in‐depth exploration.
7. CONCLUSION AND PERSPECTIVE
SCI and its devastating consequences brings more challenges to clinicians. Therefore, understanding the molecular basis of SCI may be beneficial for improved neuronal, glial survival and neurological deficits. PCD following primary SCI may has critical effect on the secondary injury mechanisms that produce the ultimate neurological deficit. In this review, we briefly summarized the relevant studies that discussed the involvement of PCD in SCI (Figure 1). We conclude that: (a) PCD, including apoptosis, necroptosis, autophagy, ferroptosis, pyroptosis and paraptosis, is generally involved in SCI, but the molecular mechanisms need further investigation; (b) insufficient experiments have confirmed the protective or pathological correlation between PCD and SCI, and this should be further explored; (c) determining the level of PCD in the patients with SCI might be potential tools for its diagnosis, treatment and prognosis.
FIGURE 1.
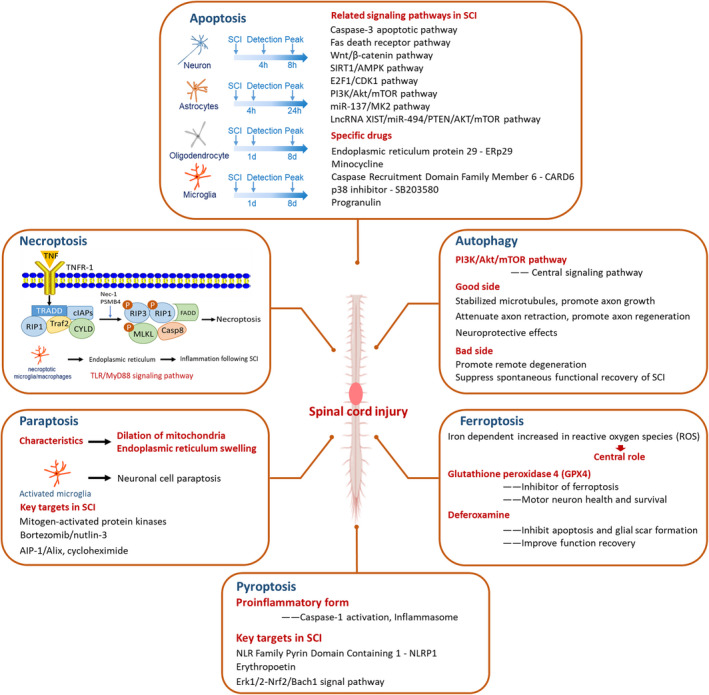
Different types of programmed cell death play different roles in spinal cord injury
Based on these conclusions, we make some suggestions concerning the future studies: (a) the correlation among apoptosis, necroptosis, autophagy, ferroptosis, pyroptosis and paraptosis in SCI should be investigated, comprehensively, and it is essential to illuminate which type of PCD play the dominant role in SCI; (b) the relationship between different types of PCD and other molecular mechanisms underlying SCI should be explored; (c) the regulation ways of PCD in SCI should be explored, especially the ways to regulate the expression levels of PCD‐associated genes. (d) the identification of new agents targeting specific PCD pathways in different phases of SCI is needed in the future work.
Although it has been shown that PCD play important roles in different models of SCI, we stress that the complexity of central nervous system injury cannot be reduced to a single physiopathological mechanism or to inhibition or activation of a single cell death type. Therefore, specific combination therapies targeting several PCD modalities could be a more promising strategy for the treatment of SCI. Furthermore, more effort on translating the current findings to post‐injury administration of drugs to block or activate cell death modalities is necessary.
In conclusion, this review can help us to understand the various functions of PCD in the pathological process of SCI. Thoughtful consideration and more detailed findings about the roles of PCD will contribute to our novel understanding of SCI of unknown aetiology in the near future.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
ZS and SF designed the review, ZS, SY, JL and LS collected the articles, ZS wrote the paper, ZS drafted the figures, ZS, GN, XK and SF revised the manuscript. All authors reviewed the final version of manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (81620108018, 82002309), the Tianjin Medical University General Hospital Funding (ZYYFY2019019, 209060401201).
Shi Z, Yuan S, Shi L, et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54:e12992. 10.1111/cpr.12992
DATA AVAILABILITY STATEMENT
The data of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kramer JL, Lammertse DP, Schubert M, Curt A, Steeves JD. Relationship between motor recovery and independence after sensorimotor‐complete cervical spinal cord injury. Neurorehabil Neural Repair. 2012;26(9):1064‐1071. [DOI] [PubMed] [Google Scholar]
- 2. Saunders LL, Clarke A, Tate DG, Forchheimer M, Krause JS. Lifetime prevalence of chronic health conditions among persons with spinal cord injury. Arch Phys Med Rehabil. 2015;96(4):673‐679. [DOI] [PubMed] [Google Scholar]
- 3. Shi Z, Zhou H, Lu L, et al. The roles of microRNAs in spinal cord injury. Int J Neurosci. 2017;127(12):1104‐1115. [DOI] [PubMed] [Google Scholar]
- 4. Bai L, Mei X, Wang Y, et al. The Role of Netrin‐1 in Improving functional recovery through autophagy stimulation following spinal cord injury in rats. Front Cell Neurosci. 2017;11:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Y, Wang Z, Li J, Li X, Xiao J. Fibroblast growth factors in the management of spinal cord injury. J Cell Mol Med. 2018;22(1):25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tardivo V, Crobeddu E, Pilloni G, et al. Say “no” to spinal cord injury: is nitric oxide an option for therapeutic strategies? Int J Neurosci. 2015;125(2):81‐90. [DOI] [PubMed] [Google Scholar]
- 7. Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell‐type dependent response. J Neurochem. 2007;102(4):1242‐1255. [DOI] [PubMed] [Google Scholar]
- 8. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75(1):15‐26. [DOI] [PubMed] [Google Scholar]
- 9. Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21(4):648‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ranjan A, Iwakuma T. Non‐canonical cell death induced by p53. Int J Mol Sci. 2016;17(12): 2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu YJ, Zheng L, Hu YW, Wang Q. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 2017;476:28‐37. [DOI] [PubMed] [Google Scholar]
- 12. Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907‐1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fulda S. Apoptosis pathways and their therapeutic exploitation in pancreatic cancer. J Cell Mol Med. 2009;13(7):1221‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Lee CG. MicroRNA and cancer–focus on apoptosis. J Cell Mol Med. 2009;13(1):12‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cabon L, Martinez‐Torres AC, Susin SA. Programmed cell death comes in many flavors. Med Sci (Paris). 2013;29(12):1117‐1124. [DOI] [PubMed] [Google Scholar]
- 16. Foller M, Huber SM, Lang F. Erythrocyte programmed cell death. IUBMB Life. 2008;60(10):661‐668. [DOI] [PubMed] [Google Scholar]
- 17. Lekli I, Haines DD, Balla G, Tosaki A. Autophagy: an adaptive physiological countermeasure to cellular senescence and ischaemia/reperfusion‐associated cardiac arrhythmias. J Cell Mol Med. 2017;21(6):1058‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song CY, Guo JF, Liu Y, Tang BS. Autophagy and Its comprehensive impact on ALS. Int J Neurosci. 2012;122(12):695‐703. [DOI] [PubMed] [Google Scholar]
- 19. Kolb JP, Oguin TH 3rd, Oberst A, Martinez J. Programmed cell death and inflammation: winter is coming. Trends Immunol. 2017;38(10):705‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Godlewski M, Kobylinska A. Programmed cell death ‐ strategy for maintenance cellular organisms homeostasis. Postepy Hig Med Dosw (Online). 2016;70:1229‐1244. [PubMed] [Google Scholar]
- 21. Felder KM, Hoelzle K, Ritzmann M, et al. Hemotrophic mycoplasmas induce programmed cell death in red blood cells. Cell Physiol Biochem. 2011;27(5):557‐564. [DOI] [PubMed] [Google Scholar]
- 22. Song B, Zhou T, Liu J, Shao L. Involvement of programmed cell death in neurotoxicity of metallic nanoparticles: recent advances and future perspectives. Nanoscale Res Lett. 2016;11(1):484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen HC, Hsu PW, Tzaan WC, Lee AW. Effects of the combined administration of vitamins C and E on the oxidative stress status and programmed cell death pathways after experimental spinal cord injury. Spinal Cord. 2014;52(1):24‐28. [DOI] [PubMed] [Google Scholar]
- 24. Zha J, Smith A, Andreansky S, Bracchi‐Ricard V, Bethea JR. Chronic thoracic spinal cord injury impairs CD8+ T‐cell function by up‐regulating programmed cell death‐1 expression. J Neuroinflammation. 2014;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self‐eating and self‐killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741‐752. [DOI] [PubMed] [Google Scholar]
- 26. Iorga A, Dara L, Kaplowitz N. Drug‐induced liver injury: cascade of events leading to cell death, apoptosis or necrosis. Int J Mol Sci. 2017;18(5):1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thornton C, Leaw B, Mallard C, Nair S, Jinnai M, Hagberg H. Cell death in the developing brain after Hypoxia‐Ischemia. Front Cell Neurosci. 2017;11:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma D, Kanneganti TD. Inflammatory cell death in intestinal pathologies. Immunol Rev. 2017;280(1):57‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu J, Ashwell KWS, Waite P. Advances in secondary spinal cord injury: role of apoptosis. Spine. 2000;25(14):1859‐1866. [DOI] [PubMed] [Google Scholar]
- 30. Yong C, Arnold PM, Zoubine MN, et al. Apoptosis in cellular compartments of rat spinal cord after severe contusion injury. J Neurotrauma. 1998;15(7):459‐472. [DOI] [PubMed] [Google Scholar]
- 31. Li GL, Brodin G, Farooque M, et al. Apoptosis and expression of Bcl‐2 after compression trauma to rat spinal cord. J Neuropathol Exp Neurol. 1996;55(3):280‐289. [DOI] [PubMed] [Google Scholar]
- 32. Liu XZ, Xu XM, Hu R, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17(14):5395‐5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50(5):798‐808. [DOI] [PubMed] [Google Scholar]
- 34. Emery E, Aldana P, Bunge MB, et al. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89(6):911‐920. [DOI] [PubMed] [Google Scholar]
- 35. Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94(6):695‐698. [DOI] [PubMed] [Google Scholar]
- 36. Springer JE, Azbill RD, Knapp PE. Activation of the caspase‐3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5(8):943‐946. [DOI] [PubMed] [Google Scholar]
- 37. Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103(1):203‐218. [DOI] [PubMed] [Google Scholar]
- 38. Yoshino O, Matsuno H, Nakamura H, et al. The role of Fas‐mediated apoptosis after traumatic spinal cord injury. Spine. 2004;29(13):1394‐1404. [DOI] [PubMed] [Google Scholar]
- 39. Zurita M, Vaquero J, Zurita I. Presence and significance of CD‐95 (Fas/APO1) expression after spinal cord injury. J Neurosurg. 2001;94(2 Suppl):257‐264. [DOI] [PubMed] [Google Scholar]
- 40. Nagata S, Golstein P. The Fas death factor. Science. 1995;267(5203):1449‐1456. [DOI] [PubMed] [Google Scholar]
- 41. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. [DOI] [PubMed] [Google Scholar]
- 42. Gao K, Shen Z, Yuan Y, et al. Simvastatin inhibits neural cell apoptosis and promotes locomotor recovery via activation of Wnt/beta‐catenin signaling pathway after spinal cord injury. J Neurochem. 2016;138(1):139‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu J, Kharebava G, Piao C, et al. Inhibition of E2F1/CDK1 pathway attenuates neuronal apoptosis in vitro and confers neuroprotection after spinal cord injury in vivo. PLoS One. 2012;7(7):e42129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao H, Chen S, Gao K, et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience. 2017;348:241‐251. [DOI] [PubMed] [Google Scholar]
- 45. Gao L, Dai C, Feng Z, Zhang L, Zhang Z. MiR‐137 inhibited inflammatory response and apoptosis after spinal cord injury via targeting of MK2. J Cell Biochem. 2018;119(4):3280‐3292. [DOI] [PubMed] [Google Scholar]
- 46. Gu S, Xie R, Liu X, Shou J, Gu W, Che X. Long coding RNA XIST contributes to neuronal apoptosis through the downregulation of AKT phosphorylation and is negatively regulated by miR‐494 in rat spinal cord injury. Int J Mol Sci. 2017;18(4):732 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Wang Z, Zhou L, Zheng X, et al. Autophagy protects against PI3K/Akt/mTOR‐mediated apoptosis of spinal cord neurons after mechanical injury. Neurosci Lett. 2017;656:158‐164. [DOI] [PubMed] [Google Scholar]
- 48. Liu R, Zhao W, Zhao Q, et al. Endoplasmic reticulum protein 29 protects cortical neurons from apoptosis and promoting corticospinal tract regeneration to improve neural behavior via caspase and Erk signal in rats with spinal cord transection. Mol Neurobiol. 2014;50(3):1035‐1048. [DOI] [PubMed] [Google Scholar]
- 49. Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004;10(12):580‐583. [DOI] [PubMed] [Google Scholar]
- 50. Wang JL, Luo X, Liu L. Targeting CARD6 attenuates spinal cord injury (SCI) in mice through inhibiting apoptosis, inflammation and oxidative stress associated ROS production. Aging. 2019;11(24):12213‐12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang HW, Ding JD, Zhang ZS, et al. Critical role of p38 in spinal cord injury by regulating inflammation and apoptosis in a rat model. Spine. 2020;45(7):E355‐E363. [DOI] [PubMed] [Google Scholar]
- 52. Wang C, Zhang L, Ndong JDLC, et al. Progranulin deficiency exacerbates spinal cord injury by promoting neuroinflammation and cell apoptosis in mice. J Neuroinflammation. 2019;16(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang T, Wang F, Li K, Lv C, Gao K, Lv C. Therapeutic effect of metformin on inflammation and apoptosis after spinal cord injury in rats through the Wnt/beta‐catenin signaling pathway. Neurosci Lett. 2020;739:135440. [DOI] [PubMed] [Google Scholar]
- 54. Su Z, Yang Z, Xu Y, Chen Y, Yu Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6(11):8474‐8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hitomi J, Christofferson DE, Ng A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dunai Z, Bauer PI, Mihalik R. Necroptosis: biochemical, physiological and pathological aspects. Pathol Oncol Res. 2011;17(4):791‐800. [DOI] [PubMed] [Google Scholar]
- 57. Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112‐119. [DOI] [PubMed] [Google Scholar]
- 58. Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21(4):227‐233. [DOI] [PubMed] [Google Scholar]
- 59. Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14(4):469‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Andera L. Signaling activated by the death receptors of the TNFR family. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153(3):173‐180. [DOI] [PubMed] [Google Scholar]
- 61. Sun L, Wang H, Wang Z, et al. Mixed lineage kinase domain‐like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1‐2):213‐227. [DOI] [PubMed] [Google Scholar]
- 62. Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase‐8‐independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489‐495. [DOI] [PubMed] [Google Scholar]
- 63. Wu C, Chen J, Liu Y, et al. Upregulation of PSMB4 is associated with the necroptosis after spinal cord injury. Neurochem Res. 2016;41(11):1‐10. [DOI] [PubMed] [Google Scholar]
- 64. Wang Y, Wang H, Tao Y, Zhang S, Wang J, Feng X. Necroptosis inhibitor necrostatin‐1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience. 2014;266:91‐101. [DOI] [PubMed] [Google Scholar]
- 65. Liu M, Wu W, Li H, et al. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J Spinal Cord Med. 2015;38(6):745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Newton K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 2015;25(6):347‐353. [DOI] [PubMed] [Google Scholar]
- 67. Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16(7):689‐697. [DOI] [PubMed] [Google Scholar]
- 68. Fan H, Tang HB, Kang J, et al. Involvement of endoplasmic reticulum stress in the necroptosis of microglia/macrophages after spinal cord injury. Neuroscience. 2015;311:362‐373. [DOI] [PubMed] [Google Scholar]
- 69. Fan H, Zhang K, Shan L, et al. Reactive astrocytes undergo M1 microglia/macrohpages‐induced necroptosis in spinal cord injury. Mol Neurodegener. 2016;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shao L, Liu X, Zhu S, Liu C, Gao Y, Xu X. The role of Smurf1 in neuronal necroptosis after lipopolysaccharide‐induced neuroinflammation. Cell Mol Neurobiol. 2018;38(4):809‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang HL, Mei J, Chang KK, Zhou WJ, Huang LQ, Li MQ. Autophagy in endometriosis. Am J Transl Res. 2017;9(11):4707‐4725. [PMC free article] [PubMed] [Google Scholar]
- 72. Shin HJ, Kim H, Oh S, et al. AMPK‐SKP2‐CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534(7608):553‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Denton D, Kumar S. Autophagy‐dependent cell death. Cell Death Differ. 2019;26(4):605‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lipinski MM, Wu J, Faden AI, Sarkar C. Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal. 2015;23(6):565‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Boya P, Gonzalez‐Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25(3):1025‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Diaz M, Gonzalez R, Plano D, Palop JA, Sanmartin C, Encio I. A diphenyldiselenide derivative induces autophagy via JNK in HTB‐54 lung cancer cells. J Cell Mol Med. 2018;22(1):289‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li W, Sultana N, Siraj N, et al. Autophagy dysfunction and regulatory cystatin C in macrophage death of atherosclerosis. J Cell Mol Med. 2016;20(9):1664‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584(7):1287‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lu X, Fan Q, Xu L, et al. Ursolic acid attenuates diabetic mesangial cell injury through the up‐regulation of autophagy via miRNA‐21/PTEN/Akt/mTOR suppression. PLoS One. 2015;10(2):e0117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine. 2011;36(22):E1427‐1434. [DOI] [PubMed] [Google Scholar]
- 82. Maday S, Holzbaur EL. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell. 2014;30(1):71‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang Y, Coleman M, Zhang L, Zheng X, Yue Z. Autophagy in axonal and dendritic degeneration. Trends Neurosci. 2013;36(7):418‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang D, Han S, Wang S, Luo Y, Zhao L, Li J. cPKCgamma‐mediated down‐regulation of UCHL1 alleviates ischaemic neuronal injuries by decreasing autophagy via ERK‐mTOR pathway. J Cell Mol Med. 2017;21(12):3641‐3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. He M, Ding Y, Chu C, Tang J, Xiao Q, Luo ZG. Autophagy induction stabilizes microtubules and promotes axon regeneration after spinal cord injury. Proc Natl Acad Sci USA. 2016;113(40):11324‐11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang D, Xuan J, Zheng BB, et al. Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Mol Neurobiol. 2017;54(5):3327‐3341. [DOI] [PubMed] [Google Scholar]
- 87. Liu S, Sarkar C, Dinizo M, et al. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis. 2015;6:e1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu X, Tian F, Wang S, Wang F, Xiong L. Astrocyte autophagy flux protects neurons against oxygen‐glucose deprivation and ischemic/reperfusion injury. Rejuvenation Res. 2018;21(5):405‐415. [DOI] [PubMed] [Google Scholar]
- 89. Tang P, Hou H, Zhang L, et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49(1):276‐287. [DOI] [PubMed] [Google Scholar]
- 90. Wang ZY, Lin JH, Muharram A, Liu WG. Beclin‐1‐mediated autophagy protects spinal cord neurons against mechanical injury‐induced apoptosis. Apoptosis. 2014;19(6):933‐945. [DOI] [PubMed] [Google Scholar]
- 91. Bisicchia E, Latini L, Cavallucci V, et al. Autophagy inhibition favors survival of rubrospinal neurons after spinal cord hemisection. Mol Neurobiol. 2017;54(7):4896‐4907. [DOI] [PubMed] [Google Scholar]
- 92. Casili G, Campolo M, Lanza M, et al. Role of ABT888, a Novel Poly(ADP‐Ribose) Polymerase (PARP) inhibitor in countering autophagy and apoptotic processes associated to spinal cord injury. Mol Neurobiol. 2020;57(11):4394‐4407. [DOI] [PubMed] [Google Scholar]
- 93. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron‐dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ma S, Dielschneider RF, Henson ES, et al. Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLoS One. 2017;12(8):e0182921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hambright WS, Fonseca RS, Chen L, Na R, Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen L, Hambright WS, Na R, Ran Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J Biol Chem. 2015;290(47):28097‐28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang Y, Fan BY, Pang YL, et al. Neuroprotective effect of deferoxamine on erastininduced ferroptosis in primary cortical neurons. Neural Regen Res. 2020;15(8):1539‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hao J, Li B, Duan HQ, et al. Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats. Neural Regen Res. 2017;12(6):959‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhou H, Yin C, Zhang Z, et al. Proanthocyanidin promotes functional recovery of spinal cord injury via inhibiting ferroptosis. J Chem Neuroanat. 2020;107:101807. [DOI] [PubMed] [Google Scholar]
- 101. Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA. 2000;97(26):14376‐14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pierroz V, Rubbiani R, Gentili C, et al. Dual mode of cell death upon the photo‐irradiation of a Ru(II) polypyridyl complex in interphase or mitosis. Chem Sci. 2016;7(9):6115‐6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xue J, Li R, Zhao X, et al. Morusin induces paraptosis‐like cell death through mitochondrial calcium overload and dysfunction in epithelial ovarian cancer. Chem Biol Interact. 2018;283:59‐74. [DOI] [PubMed] [Google Scholar]
- 104. Sperandio S, Poksay K, de Belle I, et al. Paraptosis: mediation by MAP kinases and inhibition by AIP‐1/Alix. Cell Death Differ. 2004;11(10):1066‐1075. [DOI] [PubMed] [Google Scholar]
- 105. Wang WB, Feng LX, Yue QX, et al. Paraptosis accompanied by autophagy and apoptosis was induced by celastrol, a natural compound with influence on proteasome, ER stress and Hsp90. J Cell Physiol. 2012;227(5):2196‐2206. [DOI] [PubMed] [Google Scholar]
- 106. Lee DM, Kim IY, Seo MJ, Kwon MR, Choi KS. Nutlin‐3 enhances the bortezomib sensitivity of p53‐defective cancer cells by inducing paraptosis. Exp Mol Med. 2017;49(8):e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pehar M, O'Riordan KJ, Burns‐Cusato M, et al. Altered longevity‐assurance activity of p53:p44 in the mouse causes memory loss, neurodegeneration and premature death. Aging Cell. 2010;9(2):174‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jia DP, Wang S, Zhang BC, Fang F. Paraptosis triggers mitochondrial pathway‐mediated apoptosis in Alzheimer's disease. Exp Ther Med. 2015;10(2):804‐808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109. Tanabe K, Nakanishi H, Maeda H, et al. A predominant apoptotic death pathway of neuronal PC12 cells induced by activated microglia is displaced by a non‐apoptotic death pathway following blockage of caspase‐3‐dependent cascade. J Biol Chem. 1999;274(22):15725‐15731. [DOI] [PubMed] [Google Scholar]
- 110. Shi J, Gao W, Shao F. Pyroptosis: gasdermin‐mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245‐254. [DOI] [PubMed] [Google Scholar]
- 111. Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 2019;52(2):e12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vande Walle L, Lamkanfi M. Pyroptosis. Curr Biol. 2016;26(13):R568‐572. [DOI] [PubMed] [Google Scholar]
- 113. Tan MS, Yu JT, Jiang T, Zhu XC, Tan L. The NLRP3 inflammasome in Alzheimer's disease. Mol Neurobiol. 2013;48(3):875‐882. [DOI] [PubMed] [Google Scholar]
- 114. Tan CC, Zhang JG, Tan MS, et al. NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling‐induced rat model. J Neuroinflammation. 2015;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li X, Yu Z, Zong W, et al. Deficiency of the microglial Hv1 proton channel attenuates neuronal pyroptosis and inhibits inflammatory reaction after spinal cord injury. J Neuroinflammation. 2020;17(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Li R, Zhang LM, Sun WB. Erythropoietin rescues primary rat cortical neurons from pyroptosis and apoptosis via Erk1/2‐Nrf2/Bach1 signal pathway. Brain Res Bull. 2017;130:236‐244. [DOI] [PubMed] [Google Scholar]
- 117. Kim JY, Paton JC, Briles DE, Rhee DK, Pyo S. Streptococcus pneumoniae induces pyroptosis through the regulation of autophagy in murine microglia. Oncotarget. 2015;6(42):44161‐44178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available from the corresponding author upon reasonable request.


