Abstract
Background:
Serum vitamin B-12 is measured to evaluate vitamin B-12 status. Serum methylmalonic acid (MMA) is a specific functional indicator of vitamin B-12 status, however, concentrations increase with impaired renal function.
Objective:
Our main objective was to describe the distribution of serum vitamin B-12 and MMA in US adults, and estimate age-specific reference intervals for serum MMA in a healthy subpopulation with replete vitamin B-12 status and normal renal function.
Design:
We examined cross-sectional data for serum vitamin B-12 and MMA in adults participating in the National Health and Nutrition Examination Survey from 2011–2014. Vitamin B-12 was measured by electrochemiluminescence assay and MMA by isotope-dilution LC-MS/MS.
Results:
In both bivariate and multivariate analyses, age, race/Hispanic origin, and vitamin B-12 supplement use were generally significantly associated with serum vitamin B-12 and MMA concentrations. Serum MMA concentrations increased with age, particularly in persons ≥70 y. Non-Hispanic white persons had lower vitamin B-12 and higher MMA concentrations compared with non-Hispanic black persons. Shorter fasting times and impaired renal function were significantly associated with higher serum MMA concentrations, but not with serum vitamin B-12 concentrations after controlling for covariates. The central 95% reference intervals for serum vitamin B-12 and MMA concentrations were widest for persons ≥70 y compared with younger age groups. Compared with the overall population, the central 95% reference intervals for serum MMA concentrations were considerably narrower for a vitamin B-12 replete subpopulation with normal renal function, but still age-dependent. Serum vitamin B-12 showed little, while serum MMA showed notable increases with impaired renal function.
Conclusions:
The higher serum MMA concentrations throughout the entire distribution in older persons (especially persons ≥70 y) who are vitamin B-12 replete and have normal renal function indicate the need for age-specific MMA reference intervals to better interpret the vitamin B-12 status in epidemiologic research.
Keywords: National Health and Nutrition Examination Survey, NHANES, vitamin B-12, MMA, renal function
INTRODUCTION
Low vitamin B-12 status has been associated with anemia, various forms of neurological abnormalities, and impaired cognitive function [1]. Most commonly, serum vitamin B-12 concentrations are measured to assess vitamin B-12 status, but often a second indicator, such as serum methylmalonic acid (MMA), is assessed to improve problems with sensitivity and specificity associated with using serum vitamin B-12 alone [2,3]. MMA values tend to be higher in the elderly, but it was generally believed that this reflects inadequate vitamin B-12 status [4–6]. Furthermore, impaired renal function is recognized to lead to higher MMA concentrations [7–9]. Given the co-linearity of age and renal function and MMA, age-specific reference intervals are needed for MMA from a subpopulation pre-selected for vitamin B-12 status and renal function. Prior to this analysis, investigators mostly derived reference intervals from relatively small groups of healthy middle-aged adults, but some used elderly individuals before and/or after vitamin B-12 supplementation to derive so-called “health-related” reference intervals (Table 1). Vogiatzoglou et al. showed in a Norwegian population that the difference in MMA concentrations between middle-aged and elderly individuals was only partly explained by vitamin B-12 status and renal function [14], also suggesting the need for age-specific reference intervals.
Table 1.
Selected published reference intervals for serum or plasma methylmalonic acid
| Author (year) [Reference] | Population | Central 0.95 reference interval, nmol/L | Additional information |
|---|---|---|---|
| Allen 1990 [10] | Healthy U.S. middle-aged adults | 73–271 | Men and women, n=50; 18–65 y |
| Rasmussen 1990 [11] | Healthy Danish middle-aged adults | 50–370 | Men and women, n=58; 40–68 y (median: 53 y) |
| Rasmussen 1996 [4] | Healthy Danish middle-aged adults before and after vitamin B-12 supplementation for 1 wk (in a few cases for 2 wk) | 80–280 | Men (n=109) and women (n=126); 20–84 y (men; median: 50 y) and 20–85 y (women; median: 49 y); all but 1 subject had plasma creatinine concentrations within the reference interval for healthy subjects |
| Joosten 1996 [5] | Healthy Belgian, Dutch, and German middle-aged adults Healthy Dutch elderly living at home Healthy German elderly after B-vitamin supplementation for 3 wk |
62–247 72–476 55–278 |
Men and women, n=99; 19–55 y (mean: 30 y) Men and women, n=64; 65–88 y (mean: 76 y); no participant had creatinine clearance <30 mL/min Men and women, n=143; 65–96 y (mean: 75 y); no participant had creatinine clearance <30 mL/min |
| Lewerin 2003 [12] | Swedish elderly with and without B-vitamin supplementation Total study group at baseline Healthy elderly at baseline Healthy elderly after B-vitamin supplementation for 4 mo |
110–480 120–380 20–340 |
Men and women, n=209; 70–88 y (women) and 70–93 y (men) (overall median: 76 y) n=208 n=123 n=78 (vitamin B-12 replete) |
| Milman 2007 [13] | Healthy Danish pregnant women 18 wk gestation 32 wk gestation 39 wk gestation 8 wk post-partum |
40–290 50–340 60–360 80–350 |
Women (n=434) with a normal pregnancy ≥37 wk n=413 n=390 n=250 n=160 |
| Vogiatzoglou 2009 [14] | Norwegian middle-aged adults: Unselected Vitamin B-12 ≥200 pmol/L Vitamin B-12 ≥400 pmol/L Norwegian elderly: Unselected Vitamin B-12 ≥200 pmol/L Vitamin B-12 ≥400 pmol/L |
100–320 100–300 100–280 110–490 110–410 100–360 |
Men and women, n=3,684; 47–49 y n=3,684 n=3,568 n=1,306 (vitamin B-12 replete) Men and women, n=3,262; 71–74 y n=3,262 n=3,043 n=1,058 (vitamin B-12 replete) |
| Erdogan 2010 [6] | Healthy US adults US persons tested for MMA (unknown clinical history) 0–10 y 11–20 y 21–30 y 31–40 y 41–50 y 51–60 y 61–70 y ≥71 y |
60–360 0–510 30–260 50–330 50–400 50–400 50–420 50–440 50–480 |
Men (n=16) and women (n=24) Males and females (n=4,944); highest 10% of results disregarded (potentially unhealthy persons) n=28 n=39 n=165 n=287 n=545 n=813 n=918 n=2149 |
Using data from the National Health and Nutrition Examination Survey (NHANES) between 1991–1994 and 1999–2004, serum MMA concentrations have been described by demographic variables, use of dietary supplements, and by serum vitamin B-12 and serum creatinine concentrations [15–18]. However, previous analyses did not investigate the need for age-specific reference intervals. Using the most recent NHANES data from 2011–2014, our aim was to describe the distributions of serum vitamin B-12 and MMA concentrations in the adult US population by demographic and other relevant variables and to estimate central 95% reference intervals by age in the overall population compared with a subpopulation with replete vitamin B-12 status and/or normal renal function. The overarching goal of this paper was to bring awareness to the issue of properly defining the reference population when deriving reference intervals and not to investigate the complex relationship of vitamin B-12 status biomarkers, age, and renal function.
SUBJECTS AND METHODS
Participants and study design
NHANES is a series of cross-sectional probability samples of the civilian noninstitutionalized population in the United States. Each 2-y cycle collects information to assess the health and nutritional status of a nationally representative sample. Conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention (CDC), NHANES uses a complex, stratified, multistage probability sample. Participants receive a detailed in-home interview, followed by a physical examination, which includes the collection of a blood sample in a Mobile Examination Center. During 2011–2014, persons aged 80 y and over, non-Hispanic Asian, non-Hispanic black, and Hispanic individuals were oversampled to obtain reliable estimates for these population subgroups [19]. We used data from adult NHANES participants. The unweighted response rates for participants ≥20 y of age were 67% for the interview component and 64% for the examination component [20]. Participants provided consent and the NHANES protocol was approved by the National Center for Health Statistics Research Ethics Review Board.
Biomarker measurement
Serum vitamin B-12 and MMA have not been continuously monitored in NHANES. Since 2006, the biomarkers were only assessed during a 4-y period between 2011 and 2014. Serum from adult participants (≥20 y of age for 2011–2012 and ≥19 y of age for 2013–2014) was collected, processed, and shipped on dry ice to a CDC laboratory for analysis of serum vitamin B-12 and MMA. Vitamin B-12 was measured by use of the Roche cobas electrochemiluminescence assay on the Elecsys E-170 clinical analyzer; the assay achieved good long-term imprecision (CV) of 3–8% at 166–605 pmol/L [21]. Serum MMA was measured by isotope-dilution LC-MS/MS after butanol derivatization [22]; the assay achieved even lower long-term imprecision of 3–5% at 96–560 nmol/L [23].
Study variables
For our main analysis, we categorized the participant data into 4 age groups: 20–39, 40–59, 60–69 y and ≥70 y. Our analysis further categorized the demographic variables by sex (men and women) and by race/Hispanic origin (Hispanic [Mexican American + other Hispanic], non-Hispanic black, non-Hispanic white, and non-Hispanic Asian; other racial/Hispanic origin groups were included in overall estimates). We reported separate estimates for Mexican Americans to allow comparison to previous reports. We also examined physiologic and lifestyle variables previously shown to be associated with serum vitamin B-12 and MMA concentrations [14,24,25]: use of vitamin B-12-containing dietary supplements (mean daily intake consumed in the past 30 d) (self-reported; no and yes), fasting time (self-reported; <3, 3–<8, and ≥8 h), and renal function as an indicator of chronic kidney disease (CKD). The latter was determined by calculating the estimated glomerular filtration rate (eGFR [mL/(min x 1.73 m2)]) using the CKD-EPI creatinine equation [26]. We categorized eGFR according to the National Kidney Foundation classification system [27]: ≥60 and absence of albuminuria (normal renal function), ≥60 and presence of albuminuria (CKD stage 1–2), and <60 (CKD stage 3–5).
Statistical analysis
Statistical analyses were carried out using SAS for Windows software version 9.4 (SAS Institute, Cary, NC) and SAS callable SUDAAN software version 11 (RTI, Research triangle Park, NC) to account for the complex survey design. We used 2- and 4-y Mobile Examination Center survey weights to account for the unequal probabilities of selection and adjustment for non-response. SUDAAN uses Taylor series linearization to calculate variance estimates. This analysis was undertaken to examine whether serum MMA concentrations differed among subpopulations defined by demographic attributes, renal function and/or vitamin B-12 status. The research was performed following a pre-specified data analysis plan that was approved by all authors prior to conducting the statistical analysis. We excluded pregnant and/or lactating women from all analyses (n = 80 in 2011–2012 and n = 104 in 2013–2014). Of these women, some had missing serum vitamin B-12 and MMA measurements (n = 7 in 2011–2012 and n = 4 in 2013–2014). Our analytical sample of participants ≥20 y consisted of 4,804 (2011–2012) and 5,216 (2013–2014) participants with complete serum vitamin B-12 data and 4,807 (2011–2012) and 5,213 (2013–2014) participants with complete serum MMA data (Supplemental Figure 1). Since this is primarily a descriptive paper, we used available-case analysis (“pairwise deletion”) to handle missing or incomplete data (e.g., B-12 supplement use, renal function) to maximize the amount of information available. This means that a participant was deleted if at least 1 of a pair of variables was missing for that specific analysis (or association). Both serum B-12 and MMA concentrations were right skewed; therefore, a log transformation was used to make the data more symmetric to facilitate interpretation and statistical inference through the use of geometric mean. The weighted mean of the log-transformed data were back-transformed to the original scale to report the geometric mean concentration (and 95% CI) of serum B-12 and MMA for each study variable by survey period (2011–2012 and 2013–2014) for participants ≥20 y. The geometric means were compared across the categories of the study variables within each survey cycle on the basis of Wald F tests. In addition, Satterthwaite F tests were used to assess whether at least 1 of the adjusted geometric means across the categories was significantly different after controlling for additional covariates (age group, sex, race/Hispanic origin, vitamin B-12 supplement use, fasting time, and renal function). Lastly, the geometric means were also compared across the 2 survey cycles for each category of the study variables using Wald F test (PTrend value). Significance was defined as a 2-sided P value of <0.05 with no adjustment for multiple comparisons.
Since there were limited statistically significant differences for both serum B-12 and MMA across the 2 cycles, we combined the 4 y of NHANES data from 2011–2014 for participants ≥20 y. We calculated the median (95% CI) and central 95% reference intervals for serum vitamin B-12 and MMA for all participants and by age group. In addition, to demonstrate the effect of age, B-12 status, and renal function on serum MMA concentrations, we presented the weighted empirical cumulative distributions stratified by age group, as well as median and 95% reference intervals for the adult subpopulation with normal renal function (free of CKD) and/or vitamin B-12 replete (serum vitamin B-12 ≥300 pmol/L). We chose this vitamin B-12 cutoff based on previous work demonstrating that these concentrations likely indicate adequate vitamin B-12 status (>296 pmol/L [17] and >287 pmol/L [28]). We conducted an exploratory analysis to see how high serum MMA concentrations were on the other side of the spectrum, i.e., in persons with impaired renal function and/or vitamin B-12 deficiency (serum vitamin B-12 <200 pmol/L). This cutoff has been used to indicate low serum vitamin B-12 concentrations [29], “metabolically significant vitamin B-12 deficiency” [30,31], and is within the range of 148–221 pmol/L defined as “marginal vitamin B-12 deficiency” [32].
In order to provide relevant data to policy makers, we calculated the geometric mean and selected percentiles for serum vitamin B-12 and MMA by Dietary Reference Intake (DRI) age groups for participants ≥19 y (data on 19 y old persons were only available for 2013–2014).
RESULTS
Among the US population ≥20 y, approximately one-third each were young adults (20–39 y), middle-aged adults (40–59 y), and older adults (60–69 y and ≥70 y), half were women, and the race/Hispanic origin percentages were approximately 15%, 11%, 67%, and 5% for Hispanic, non-Hispanic black, non-Hispanic white, and non-Hispanic Asian, respectively (Table 2). Approximately 35% of the population used a dietary supplement containing vitamin B-12, approximately half fasted for ≥8 h, and approximately 80% had normal renal function.
Table 2.
Sample sizes and percentages for serum vitamin B-12 and methylmalonic acid by variable categories in US persons ≥20 y of age, NHANES 2011–20141
| Serum vitamin B-12 | Serum MMA | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable categories | 2011–2012 | 2013–20142 | 2011–2012 | 2013–20142 | ||||
| n | % | n | % | n | % | n | % | |
| All | 4,804 | - | 5,216 | - | 4,807 | - | 5,213 | - |
| Age, y | ||||||||
| 20–39 | 1,670 | 35.2 | 1,705 | 35.2 | 1,670 | 35.1 | 1,703 | 35.2 |
| 40–59 | 1,608 | 38.8 | 1,823 | 37.4 | 1,608 | 38.8 | 1,821 | 37.4 |
| 60–69 | 800 | 14.0 | 866 | 14.8 | 800 | 14.0 | 867 | 14.8 |
| ≥70 | 726 | 12.0 | 822 | 12.6 | 729 | 12.1 | 822 | 12.6 |
| Sex | ||||||||
| Male | 2,424 | 49.1 | 2,535 | 49.3 | 2,424 | 49.1 | 2,534 | 49.3 |
| Female | 2,380 | 50.9 | 2,681 | 50.7 | 2,383 | 50.9 | 2,679 | 50.7 |
| Race-ethnicity | ||||||||
| Hispanic | 974 | 14.3 | 1,172 | 14.7 | 974 | 14.3 | 1,174 | 14.8 |
| Mexican American | 481 | 7.8 | 709 | 9.2 | 481 | 7.8 | 711 | 9.2 |
| Non-Hispanic Black | 1,220 | 10.8 | 1,026 | 10.8 | 1,220 | 10.8 | 1,024 | 10.8 |
| Non-Hispanic White | 1,805 | 67.4 | 2,279 | 66.6 | 1,807 | 67.4 | 2,275 | 66.6 |
| Non-Hispanic Asian | 670 | 5.0 | 588 | 5.2 | 671 | 5.0 | 589 | 5.3 |
| B-12 supplement use | ||||||||
| No | 3,303 | 66.1 | 3,514 | 64.1 | 3,304 | 66.0 | 3,515 | 64.1 |
| Yes | 1,467 | 33.9 | 1,672 | 35.9 | 1,469 | 34.0 | 1,668 | 35.9 |
| Fasting | ||||||||
| <3 h | 1,347 | 29.4 | 1,689 | 35.5 | 1,347 | 29.4 | 1,688 | 35.5 |
| 3–<8 h | 982 | 18.7 | 983 | 16.8 | 983 | 18.7 | 980 | 16.8 |
| ≥8 h | 2,475 | 51.9 | 2,544 | 47.7 | 2,477 | 51.9 | 2,545 | 47.7 |
| Renal function | ||||||||
| Normal | 3,730 | 82.2 | 3,989 | 80.1 | 3,731 | 82.2 | 3,987 | 80.1 |
| CKD stage 1–2 | 613 | 10.6 | 693 | 11.8 | 614 | 10.6 | 693 | 11.8 |
| CKD stage 3–5 | 424 | 7.3 | 482 | 8.1 | 425 | 7.3 | 481 | 8.1 |
Values are weighted percentages. Pregnant and lactating women were excluded; available-case analysis was used to handle missing or incomplete data (e.g., B-12 supplement use, renal function). CKD, chronic kidney disease; MMA, methylmalonic acid.
While NHANES 2013–2014 collected samples from participants ≥19 y of age, we only included data for participants ≥20 y of age.
Serum vitamin B-12 and MMA concentrations for 2011–2012 and 2013–2014 by variable categories
We observed no difference in serum vitamin B-12 (P = 0.47) or MMA (P = 0.06) concentrations for adults ≥20 y between 2011–2012 and 2013–2014 (Table 3). When we grouped data by demographic or other variables, we found no differences in serum vitamin B-12 or MMA concentrations between the 2 survey cycles, except for a small decrease in serum vitamin B-12 for 20–39 y olds (2.9%) and small increases in serum MMA for non-Hispanic blacks (5.6%), non-Hispanic whites (5.1%), and non-users of vitamin B-12 containing supplements (4.7%).
Table 3.
Serum vitamin B-12 and methylmalonic acid concentrations by variable categories in US persons ≥20 y of age, NHANES 2011–20141
| Serum vitamin B-12, pmol/L | Serum MMA, nmol/L | |||||
|---|---|---|---|---|---|---|
| Variables | 2011–2012 | 2013–20142 | PTrend value3 | 2011–2012 | 2013–20142 | PTrend value3 |
| All | 391 (382, 400) | 387 (380, 394) | 0.47 | 147 (143, 152) | 153 (149, 158) | 0.06 |
| Age, y | ||||||
| 20–39 | 381 (373, 388) | 370 (362, 378) | 0.0457 | 127 (123, 132) | 131 (127, 136) | 0.21 |
| 40–59 | 377 (365, 390) | 387 (374, 400) | 0.27 | 147 (141, 153) | 153 (146, 161) | 0.13 |
| 60–69 | 423 (390, 459) | 402 (380, 424) | 0.27 | 159 (149, 169) | 170 (162, 179) | 0.07 |
| ≥70 | 431 (411, 452) | 421 (396, 447) | 0.50 | 206 (199, 214) | 207 (198, 216) | 0.93 |
| Pcrude value4 | <0.0001 | 0.0034 | <0.0001 | <0.0001 | ||
| Padj value5 | 0.0464 | 0.12 | <0.0001 | <0.0001 | ||
| Sex | ||||||
| Male | 385 (371, 399) | 373 (364, 383) | 0.16 | 149 (145, 154) | 153 (150, 156) | 0.19 |
| Female | 397 (387, 406) | 400 (393, 408) | 0.49 | 145 (138, 152) | 153 (147, 160) | 0.08 |
| Pcrude value4 | 0.12 | 0.0001 | 0.22 | 0.93 | ||
| Padj value5 | 0.69 | 0.0024 | 0.08340 | 0.52 | ||
| Race-ethnicity6 | ||||||
| Hispanic | 396 (385, 407) | 393 (376, 411) | 0.81 | 128 (121, 135) | 127 (123, 132) | 0.91 |
| Mexican American | 406 (392, 421) | 404 (374, 436) | 0.89 | 120 (114, 127) | 123 (118, 128) | 0.41 |
| Non-Hispanic black | 425 (408, 442) | 408 (394, 423) | 0.12 | 124 (121, 128) | 131 (127, 136) | 0.0160 |
| Non-Hispanic white | 384 (371, 397) | 380 (371, 390) | 0.63 | 157 (152, 162) | 165 (159, 171) | 0.0277 |
| Non-Hispanic Asian | 407 (385, 431) | 401 (377, 426) | 0.70 | 134 (127, 141) | 138 (131, 146) | 0.38 |
| Pcrude value4 | 0.0012 | 0.0060 | <0.0001 | <0.0001 | ||
| Padj value5 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | ||
| B-12 supplement use7 | ||||||
| No | 350 (344, 357) | 344 (337, 351) | 0.18 | 149 (143, 154) | 156 (151, 162) | 0.0476 |
| Yes | 481 (458, 505) | 475 (463, 488) | 0.64 | 143 (138, 149) | 147 (143, 152) | 0.21 |
| Pcrude value4 | <0.0001 | <0.0001 | 0.08 | 0.0011 | ||
| Padj value5 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Fasting time, h | ||||||
| <3 | 400 (386, 415) | 390 (381, 399) | 0.18 | 158 (152, 164) | 164 (159, 170) | 0.12 |
| 3–<8 | 400 (379, 422) | 395 (380, 410) | 0.68 | 153 (146, 159) | 159 (153, 165) | 0.15 |
| ≥8 | 382 (374, 391) | 382 (371, 394) | 1.00 | 139 (134, 144) | 143 (138, 149) | 0.26 |
| Pcrude value4 | 0.0183 | 0.38 | <0.0001 | <0.0001 | ||
| Padj value5 | 0.25 | 0.38 | <0.0001 | <0.0001 | ||
| Renal function | ||||||
| Normal | 387 (376, 397) | 383 (376, 391) | 0.61 | 140 (136, 145) | 145 (140, 150) | 0.17 |
| CKD stage 1–2 | 388 (366, 411) | 388 (361, 418) | 0.98 | 152 (144, 161) | 159 (153, 165) | 0.21 |
| CKD stage 3–5 | 452 (423, 484) | 421 (390, 454) | 0.14 | 235 (219, 251) | 249 (234, 264) | 0.19 |
| Pcrude value4 | 0.0019 | 0.0420 | <0.0001 | <0.0001 | ||
| Padj value5 | 0.20 | 0.64 | <0.0001 | <0.0001 | ||
Values are weighted geometric means (95% CI). Serum vitamin B-12 was measured by Roche E-170 immunoassay and serum MMA by HPLC-MS/MS. See Table 2 for sample sizes. Pregnant and lactating women were excluded; available-case analysis was used to handle missing or incomplete data. CKD, chronic kidney disease; MMA, methylmalonic acid.
While NHANES 2013–2014 collected samples from participants ≥19 y of age, we only included data for participants ≥20 y of age.
Test compares the 2 NHANES cycles with no covariate adjustment.
Wald F P value tests the null hypothesis of no difference for unadjusted geometric means for each variable separately.
Satterthwaite F P value tests the null hypothesis of no difference for covariate-adjusted (age, sex, race-ethnicity, vitamin B-12 supplement use, fasting time, and renal function) geometric means.
Hispanic sub-group represents the sum of MA and other Hispanic ethnicity; P values for race-ethnicity show comparison of Hispanic, NH White, NH Black, NH Asian, and other (not shown).
Vitamin B-12 containing dietary supplements.
Age and race/Hispanic origin were significantly associated with serum vitamin B-12 and MMA concentrations in both survey periods, while sex was only significantly associated with serum vitamin B-12 concentrations in 2013–2014 (Table 3). For the most part, these observations were unchanged after we controlled for other covariates (age group, sex, race/Hispanic origin, vitamin B-12 supplement use, fasting time, and renal function). However, during the 2013–2014 survey cycle, age was no longer significantly associated with serum vitamin B-12. We observed increasing serum vitamin B-12 and MMA concentrations with age, especially in persons ≥70 y. Serum vitamin B-12 concentrations were approximately 13% higher in persons ≥70 y compared with 20–39 y olds. For MMA, the age difference was more pronounced: persons ≥70 y had approximately 60% higher MMA concentrations compared with the youngest age group. Non-Hispanic white persons had lower vitamin B-12 (approximately 10%) and higher MMA (approximately 25%) concentrations compared with non-Hispanic black persons, while other race/Hispanic origin groups had intermediate concentrations.
Users of vitamin B-12 containing supplements had approximately 40% higher serum vitamin B-12 concentrations in both survey periods (statistically significant with and without controlling for other covariates), and approximately 5% lower MMA concentrations (statistically significant in 2013–2014 without and in both survey periods with controlling for other covariates) (Table 3). Shorter fasting time was significantly associated with approximately 10% higher MMA concentrations in both survey periods, but showed no association with serum vitamin B-12 after controlling for other covariates. Similarly, impaired renal function was significantly associated with approximately 10–15% higher serum vitamin B-12 and approximately 50–70% higher MMA concentrations in both survey periods, but for vitamin B-12 the association was no longer significant after controlling for other covariates.
Serum vitamin B-12 and MMA concentrations for 2011–2014 by DRI age groups
To provide data for policy makers interested in vitamin B-12 status by DRI age groups corresponding to intake categories, we calculated the geometric mean concentrations and percentile distributions for serum vitamin B-12 and MMA concentrations for participants ≥19 y (Table 4). The older age group (≥51 y) had higher serum vitamin B-12 and MMA concentrations compared with the younger age group (19–50 y), similar to what we observed with the 3 age group categories. We observed higher MMA concentrations for the older age group across the entire distribution and higher vitamin B-12 concentrations for the upper half of the distribution.
Table 4.
Serum vitamin B-12 and methylmalonic acid concentrations by Dietary Reference Intake age groups in US persons ≥19 y of age, NHANES 2011–20141
| Percentiles | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte Age group | n2 | Geometric mean (95% CI) | 2.5 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | 97.5 |
| B-12, pmol/L | |||||||||||
| 19–50 y | 5,457 | 374 (369, 380) | 163 | 190 | 219 | 281 | 369 | 489 | 633 | 752 | 885 |
| ≥51 y | 4,694 | 408 (399, 418) | 153 | 176 | 213 | 282 | 392 | 561 | 791 | 1040 | 1440 |
| P value | 0.0064 | ||||||||||
| MMA, nmol/L | |||||||||||
| 19–50 y | 5,455 | 133 (131, 136) | 67.5 | 74.4 | 83.0 | 103 | 129 | 165 | 214 | 263 | 338 |
| ≥51 y | 4,696 | 175 (171, 179) | 79.8 | 90.3 | 103 | 127 | 164 | 220 | 317 | 420 | 558 |
| P value | <0.0001 | ||||||||||
NHANES 2011–2012 collected samples from participants ≥20 y of age, while NHANES 2013–2014 collected samples from participants ≥19 y of age. Serum vitamin B-12 was measured by Roche E-170 immunoassay and serum MMA by HPLC-MS/MS. Pregnant and lactating women were excluded. P values are based on Wald F test. B-12, vitamin B-12; MMA, methylmalonic acid.
Sample size
Reference intervals for serum vitamin B-12 and MMA concentrations for 2011–2014 by age groups
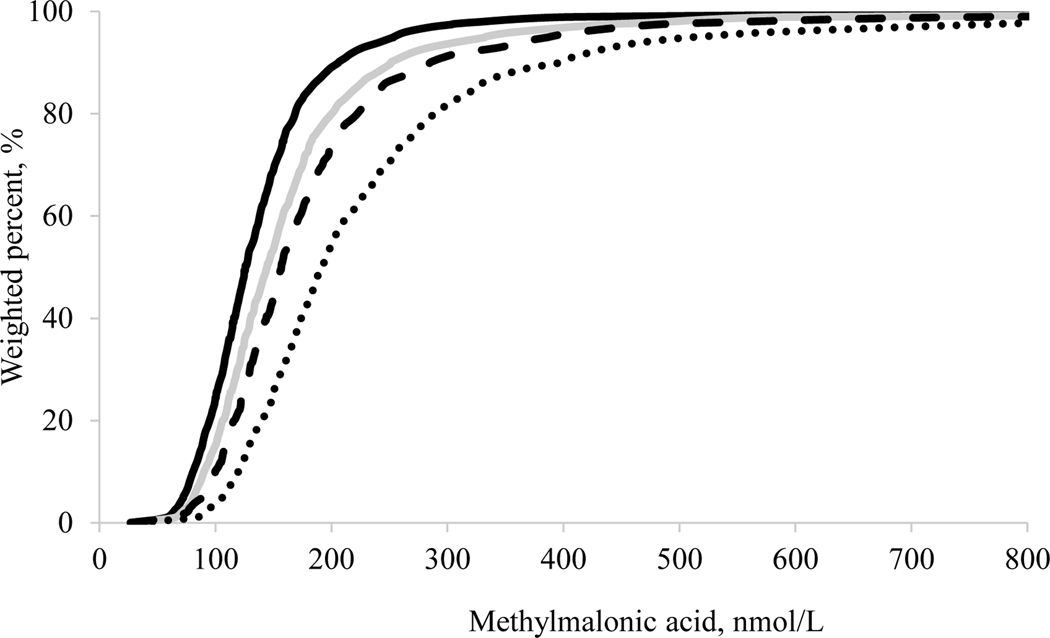
The central 95% reference interval for serum vitamin B-12 concentrations (overall group) was wider for persons 60–69 y (152–1,530 pmol/L, n = 1,166) and ≥70 y (137–1,720 pmol/L, n = 1,548) compared with the 2 younger age groups (20–39 y: 167–840 pmol/L, n = 3,375; 40–59 y: 160–1,150 pmol/L, n = 3,431) (Table 5). We observed the same pattern for serum MMA concentrations (overall group); participants ≥70 y showed a reference interval of 92.5–758 nmol/L (n = 1,551). However, as we limited this group to a subpopulation with normal renal function (85.9–571 nmol/L, n = 672) and further to a subpopulation also being vitamin B-12 replete (84.3–317 nmol/L, n = 491), the central 95% reference intervals narrowed considerably. Nonetheless, even in the subpopulation with replete vitamin B-12 status and normal renal function, the reference interval for the 20–39 y age group was lower compared with the reference intervals for the 40–59 y, 60–69 y, and ≥70 y age groups. We noted similar patterns for the medians, with higher MMA concentrations with increasing age even in the subpopulation with replete vitamin B-12 status and normal renal function. We also noted similar patterns for the entire distribution of MMA concentrations (Figure 1).
Table 5.
Serum MMA and vitamin B-12 median, central 95% reference intervals, and corresponding samples sizes by age group in US persons ≥20 y of age, NHANES 2011–20141
| Serum B-12, pmol/L | Serum MMA, nmol/L | ||||
|---|---|---|---|---|---|
| Age group | Overall2 | Overall2 | Normal renal function3 | B-12 replete4 | B-12 replete & normal renal function5 |
| Median (95% CI) | |||||
| All | 378 (372, 384) | 144 (141, 147) | 138 (134, 141) | 135 (132, 139) | 130 (127, 133) |
| Age, y | |||||
| 20–39 | 373 (364, 381) | 126 (123, 128) | 125 (123, 128) | 121 (118, 124) | 120 (118, 124) |
| 40–59 | 365 (357, 378) | 144 (140, 148) | 142 (138, 146) | 133 (129, 139) | 133 (128, 138) |
| 60–69 | 398 (375, 420) | 157 (150, 164) | 151 (143, 158) | 149 (143, 154) | 143 (134, 149) |
| ≥70 | 416 (401, 428) | 191 (186, 197) | 168 (162, 175) | 180 (175, 184) | 161 (151, 167) |
| 2.5th – 97.5th Percentile | |||||
| All | 158 – 1,140 | 70.6 – 451 | 69.6 – 391 | 67.9 – 328 | 67.2 – 281 |
| Age, y | |||||
| 20–39 | 167 – 840 | 65.1 – 304 | 65.5 – 297 | 63.3 – 256 | 63.5 – 254 |
| 40–59 | 160 – 1,150 | 72.8 – 442 | 73.0 – 403 | 70.5 – 324 | 70.5 – 293 |
| 60–69 | 152 – 1,530 | 77.0 – 497 | 76.4 – 430 | 74.0 – 327 | 72.4 – 281 |
| ≥70 | 137 – 1,720 | 92.5 – 758 | 85.9 – 571 | 90.1 – 447 | 84.3 – 317 |
| Sample size | |||||
| All | 10,020 | 10,020 | 7,718 | 7,109 | 5,481 |
| Age, y | |||||
| 20–39 | 3,375 | 3,373 | 3,038 | 2,420 | 2,178 |
| 40–59 | 3,431 | 3,429 | 2,835 | 2,393 | 1,988 |
| 60–69 | 1,666 | 1,667 | 1,173 | 1,170 | 824 |
| ≥70 | 1,548 | 1,551 | 672 | 1,126 | 491 |
Values are weighted percentiles and sample sizes. While NHANES 2013–2014 collected samples from participants ≥19 y of age, we only included data for participants ≥20 y of age. Serum vitamin B-12 was measured by Roche E-170 immunoassay and serum MMA by HPLC-MS/MS. B-12, vitamin B-12; CKD, chronic kidney disease; MMA, methylmalonic acid.
Pregnant and lactating women were excluded.
Pregnant and lactating women, participants with CKD stage 1–5, and participants with missing or incomplete CKD stage information were excluded.
Pregnant and lactating women, participants with serum B-12 <300 pmol/L, and participants with missing serum vitamin B-12 data were excluded.
Pregnant and lactating women, participants with CKD stage 1–5 and serum B-12 <300 pmol/L, and participants with missing/incomplete CKD stage information and/or missing serum vitamin B-12 data were excluded.
Figure 1.
Weighted empirical cumulative distributions (ECDF) for serum methylmalonic acid concentrations in US persons ≥20 y of age who are vitamin B-12 replete and have normal renal function by age group, NHANES 2011–2014. Black solid line: 20–39 y (n = 2,178); grey solid line: 40–59 y (n = 1,988); large dashed line: 60–69 y (n = 824); small dashed line: ≥70 y (n = 491).
We observed a selective impact of vitamin B-12 status vs. renal function by age group (Supplemental Figure 2). In the 20–39 y (panel A) and 40–59 y (panel B) age groups, excluding persons with low vitamin B-12 status shifted the MMA distributions lower compared to excluding persons with impaired renal function. In the 60–69 y (panel C) and ≥70 y age groups (panel D), both of these approaches resulted in a similar shift of the MMA distributions, but the combined approach (excluding persons with low vitamin B-12 status and impaired renal function) had an additive effect and resulted in an even bigger shift to lower MMA concentrations, especially in the oldest age group.
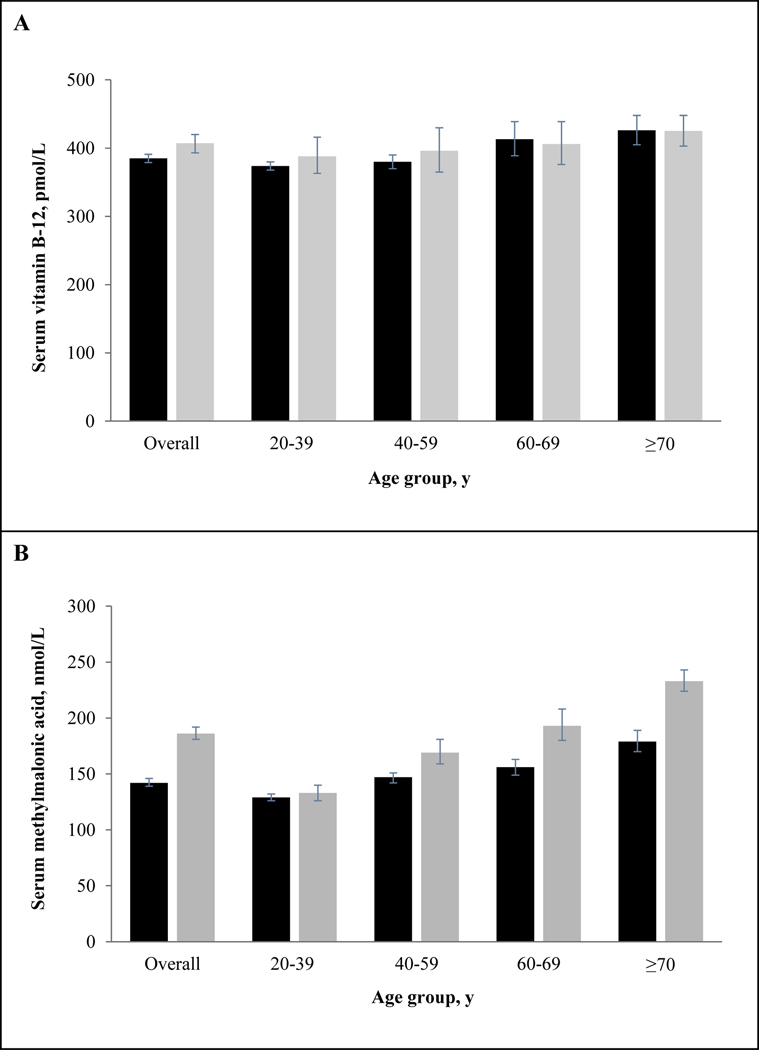
We stratified our analytic sample by age and renal function to illustrate the effect of impaired renal function on the relationship between age and biomarker concentrations (Figure 2). At the geometric mean, serum vitamin B-12 (panel A) showed small and consistent age differences for persons with normal vs. impaired renal function: 40–59 y olds had 1.6% (normal) and 2.1% (impaired) higher concentrations, 60–69 y olds had 9.4% (normal) and 4.4% (impaired) higher concentrations, and ≥70 y olds had 12% (normal) and 9% (impaired) higher concentrations compared with 20–39 y olds. Serum vitamin B-12 concentrations for people with normal vs. impaired renal function were significantly different in the overall population (P = 0.0072), but not when stratified by age, 20–39 y (P = 0.30), 40–59 y (P = 0.39), 60–69 y (P = 0.74), and ≥70 y (P = 0.97). On the other hand, serum MMA (panel B) showed large and inconsistent age differences for persons with normal vs. impaired renal function: compared with 20–39 y olds, 40–59 y olds had 14% (normal) and 27% (impaired) higher concentrations, 60–69 y olds had 21% (normal) and 45% (impaired) higher concentrations, and ≥70 y olds had 39% (normal) and 75% (impaired) higher concentrations. Serum MMA concentrations were significantly different in persons with normal vs. impaired renal function (P ≤0.0001), with the exception of those aged 20–39 y (P = 0.30).
Figure 2.
Serum vitamin B-12 (panel A) and methylmalonic acid (panel B) geometric mean concentrations by age group and renal function in US persons ≥20 y of age, NHANES 2011–2014. Black bars represent normal renal function and grey bars represent impaired renal function (stage 1–5 chronic kidney disease). Error bars represent 95% CI. Sample sizes for B-12 and normal vs. impaired renal function: overall (n = 7,719 vs. 2,212); 20–39 y (n = 3,040 vs. 309); 40–59 y (n = 2,837 vs. 575); 60–69 y (n = 1,172 vs. 479), and ≥70 y (n = 670 vs. 849). Sample sizes for MMA and normal vs. impaired renal function: overall (n = 7,718 vs. 2,213); 20–39 y (n = 3,038 vs. 309); 40–59 y (n = 2,835 vs. 575); 60–69 y (n = 1,173 vs. 479); and ≥70 y (n = 672 vs. 850).
MMA concentrations in persons with impaired renal function and/or vitamin B-12 deficiency
Not surprisingly, MMA concentrations were much higher at the opposite side of the spectrum where participants showed unhealthy characteristics (Supplemental Table 1). MMA median concentrations were generally higher in vitamin B-12 deficient persons compared with persons with impaired renal function. We also observed increasing concentrations with increasing age group. However, most of the 97.5th percentile estimates were subject to greater uncertainty due to the small cell size, as can be seen by the large 95% CIs.
DISCUSSION
Serum MMA concentrations have been described by age and/or vitamin B-12 status in several moderate sample size studies of healthy middle-aged and elderly Americans and Europeans (mostly from Scandinavian countries) [4–6,10–17]. Earlier work from NHANES 1999–2004 using a large, nationally representative sample of the racially-diverse US population concluded that the “age-related increase in serum MMA concentrations is likely to be due to a concomitant decline in kidney function and vitamin B-12 status” [18]. Our analysis extends this work by better defining these 2 variables relative to MMA concentrations and thus providing helpful information to both epidemiologists as well as clinicians. We confirmed the previously reported positive association between serum MMA and age [4–6,15,16,18] and the inverse association between serum MMA and renal function [7–9]. While serum vitamin B-12 also showed a weak inverse association with renal function, the association was no longer significant after we adjusted for other covariates. The important finding of our study was that serum MMA concentrations were higher as age increased even in a reference population of vitamin B-12 replete persons with normal renal function, confirming the observations made by Vogiatzoglou et al. in the Norwegian population [14], and suggesting that age-specific reference intervals should be used when interpreting serum MMA concentrations. However, since the reference intervals in the 40–59 y and 60–69 y age groups are similar, it may suggest these 2 age groups do not need separate reference intervals for MMA.
In contrast to studies in European populations that found similar or lower serum vitamin B-12 concentrations in older compared with younger persons [5,8,14], when combining the 2 cycles together, we found slightly higher serum vitamin B-12 concentrations in persons ≥60–69 y and ≥70 y compared with the 2 younger age groups, even when controlling for covariates like vitamin B-12 supplement use (data not shown). However, when we stratified our NHANES 2011–2014 sample by vitamin B-12 supplement use, we observed increasing serum vitamin B-12 concentrations with age (20–39, 40–59, 60–69, and ≥70 y) in supplement users (429, 466, 533, and 543 pmol/L), but similar concentrations in non-users (357, 342, 338, and 341 pmol/L) (data not shown). A previous NHANES analysis showed increased consumption frequency of dietary supplements containing vitamin B-12 by older persons [33], supporting our observation.
Race/Hispanic origin was significantly associated with serum vitamin B-12 and MMA concentrations, and it remained significant after we adjusted for other covariates. Previous reports from earlier NHANES surveys [16,34] also showed that non-Hispanic blacks and Mexican Americans had higher serum vitamin B-12 concentrations compared to non-Hispanic whites. The mean serum MMA concentrations were significantly lower in non-Hispanic blacks compared with non-Hispanic whites, as noted both in the pre-folic acid fortification [15] and post-fortification periods [16,18]. A new enzymatic polymorphism that showed a strong association with elevated MMA concentrations was recently reported in the white Irish population [35] that may partially explain the higher MMA concentrations in non-Hispanic whites. Our study also reports the first nationally representative serum vitamin B-12 and MMA concentrations for non-Hispanic Asians. Similar to Hispanics, this population group showed intermediate concentrations for both biomarkers, between those observed for non-Hispanic blacks and non-Hispanic whites. An earlier investigation in an elderly community-dwelling multiethnic population also found higher serum vitamin B-12 concentrations in Asian Americans compared with Whites [36]. A recent study conducted in a convenience sample of healthy young Canadian women reported similar serum vitamin B-12 and plasma MMA concentrations for women of South Asian vs. European ethnicity [37].
The upper end of the age-specific central 95% reference interval for serum MMA in persons who were vitamin B-12 replete and had normal renal function (254, 293, 281, 317 nmol/L in 20–39, 40–59, 60–69, and ≥70 y, respectively) was much lower compared with the “apparently healthy” overall population (304, 442, 497, 758 nmol/L in 20–39, 40–59, 60–69, and ≥70 y, respectively). This shows that MMA cutoffs derived from epidemiologic data are too high if parameters such as renal function and vitamin B-12 status are not considered. As such, the study of Erdogan et al. [6] found a higher reference interval of 60–360 nmol/L in “apparently healthy” US adults with unknown clinical history. Moreover, when the authors derived representative ranges by age group from close to 5,000 MMA tests performed in 2006 using a nonparametric approach following exclusion of the top 10% of values, they also arrived at much higher upper end MMA cutoffs (from 260 nmol/L for 11–20 y to 480 nmol/L for ≥71 y) compared with our study. Our age-specific MMA reference intervals for vitamin B-12 replete persons with normal renal function were in agreement with some of the published reference intervals in other populations [4,5,10,14], but not with all [5,11,12,14]. The higher reference intervals found in some studies may be due to the lack of consideration for impaired renal function.
There is still debate on what the right serum vitamin B-12 cutoff is to denote adequate vitamin B-12 status [17,28,38]. Vogiatzoglou et al. defined individuals with serum vitamin B-12 ≥400 pmol/L as vitamin B-12 replete, while individuals with serum vitamin B-12 <200 pmol/L and ≥200 pmol/L but <400 pmol/L were considered as low/low-normal and intermediate, respectively [14]. A frequently used serum vitamin B-12 cutoff to separate possible “subclinical deficiency” from repletion is 296 pmol/L [39]. In a modeling analysis of a MMA-derived change point for serum vitamin B-12, Bailey et al. found that serum vitamin B-12 concentrations >287 pmol/L likely indicate adequate vitamin B-12 status [28]. Thus, we chose the rounded cutoff of 300 pmol/L to define vitamin B-12 repletion.
Our exploratory analysis in a unhealthy subgroup of US adults with impaired renal function and/or vitamin B-12 deficiency provides new insights that are helpful to clinicians, however the results have to be interpreted with caution due to the small sample size. Nonetheless, we observed the same kind of age pattern in this subgroup as in the healthy subgroup.
Our group previously demonstrated that serum vitamin B-12 concentrations did not change appreciably from pre- (NHANES 1988–1994) to post-folic acid fortification (NHANES 1999–2004) [40]. In this current analysis, we verified that during the 15-y post-fortification period (NHANES 1999–2014) serum vitamin B-12 concentrations continued to be similar (Supplemental Figure 3 and Supplemental Text 1). Serum MMA concentrations showed a small but significant increase over the same time period. The mean increase was 9.5%, but it varied by age group: 20–39 y: 4.8%; 40–59 y: 14.2%; 60–69 y: 14.9% and ≥70 y: 7.8%. Whether this small increase is due to secular changes in intake of foods rich in vitamin B-12, fortification practices, or dietary supplements, other confounding factors such as renal function, or unknown sampling biases between the NHANES surveys, is not known. However, this small change is not considered to be of public health concern.
Our analysis is subject to some limitations. While NHANES is a large probability sample designed to estimate information on the nutritional status in selected groups of the U.S. population, it is a cross-sectional study. For this reason, any associations identified between serum vitamin B-12 or MMA concentrations with other variables need to be interpreted with caution as there may be other factors that were not investigated and that may impact the relationship. While we had limited missing data, any missing values, if they are not at random, can bias the reported estimates. The strengths of our paper are the large sample size that allowed us to provide age-specific MMA reference intervals as well as nationally representative estimates for different race-Hispanic origin groups and the first data for non-Hispanic Asians.
In summary, the age-specific MMA reference intervals reported in this study will be a useful tool for epidemiologists and public health officials to monitor the vitamin B-12 status of the population over time or to compare the vitamin B-12 status of different populations.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Gwendolyn Gabey and Donna LaVoie (CDC’s National Center for Environmental Health) for performing the serum MMA and vitamin B-12 analysis. Author contributions: CMP developed the study concept and design. MRS performed the statistical data analysis. EMM, CMP, and YA conducted the data interpretation. EMM wrote the initial draft, which was modified based on critical review from all coauthors. CMP has primary responsibility for all content. All authors read and approved the final manuscript. None of the authors declared any personal or financial conflict of interest.
REFERENCES
- 1.Stabler SP. Vitamin B12. In: Present Knowledge in Nutrition. 9th ed. Washington, DC: International Life Sciences Institute Press, 2006;302–13. [Google Scholar]
- 2.Green R. Vitamin B12 deficiency from the perspective of practicing hematologist. Blood 2017;129:2603–11. [DOI] [PubMed] [Google Scholar]
- 3.Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Curtin LR, Durazo-Arvizo RA et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94:313S–21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen K, Møller J, Lyngbak M, Pedersen AMH, Dybkjær L. Age- and gender-specific reference intervals for total homocysteine and methylmalonic acid in plasma before and after vitamin supplementation. Clin Chem 1996;42:630–6. [PubMed] [Google Scholar]
- 5.Joosten E, Lesaffre R, Riezler R. Are different reference intervals for methylmalonic acid and total homocysteine necessary in elderly people? Eur J Haematol 1996;57:222–6. [DOI] [PubMed] [Google Scholar]
- 6.Erdogan E, Nelson GJ, Rockwood AL, Frank EL. Evaluation of reference intervals for methylmalonic acid in plasma/serum and urine. Clin Chim Acta 2010;411:1827–9. [DOI] [PubMed] [Google Scholar]
- 7.Lindgren A. Elevated serum methylmalonic acid: how much comes from cobalamin deficiency and how much comes from the kidneys? Scand J Clin Lab Invest 2002;62:15–20. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R, Refsum H, Birks J, Evans JG, Johnston C, Sherliker P, Ueland PM, Schneede J, McPartlin J, Nexo E et al. Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr 2003;77:1241–7. [DOI] [PubMed] [Google Scholar]
- 9.Valente E, Scott JM, Ueland PM, Cunningham C, Casey M, Molloy AM. Diagnostic accuracy of hologranscobalamin, methylmalonic acid, serum cobalamin, and other indicators of tissue vitamin B12 status in the elderly. Clin Chem 2011;57:856–63. [DOI] [PubMed] [Google Scholar]
- 10.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Diagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol 1990;34:90–8. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen K, Møller J, Østergaard K, Kristensen MØ, Jensen J. Methylmalonic acid concentrations in serum of normal subjects: biological variability and effect of oral L-isoleucine loads before and after intramuscular administration of cobalamin. Clin Chem 1990;36:1295–9. [PubMed] [Google Scholar]
- 12.Lewerin C, Nilsson-Ehle H, Matousek M, Lindstedt G, Steen B. Reduction of plasma homocysteine and serum methylmalonate concentrations in apparently healthy elderly subjects after treatment with folic acid, vitamin B12 and vitamin B6: a randomized trial. Eur J Clin Nutr 2003;57:1426–36. [DOI] [PubMed] [Google Scholar]
- 13.Milman N, Bergholt T, Byg K-E, Erikson L, Hvas A-M. Reference intervals for haematological variables during normal pregnancy and postpartum in 434 healthy Danish women. Eur J Haematol 2007;79:39–46. [DOI] [PubMed] [Google Scholar]
- 14.Vogiatzoglou A, Oulhaj A, Smith DA, Nurk E, Drevon CA, Ueland PM, Vollset SE, Tell GS, Refsum H. Determinants of plasma methylmalonic acid in a large population; implications for assessment of vitamin B12 status. Clin Chem 2009;55:2198–206. [DOI] [PubMed] [Google Scholar]
- 15.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Elevated serum methylmalonic acid concentrations are common among elderly Americans. J Nutr 2002;132:2799–803. [DOI] [PubMed] [Google Scholar]
- 16.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr 2005;82:442–50. [DOI] [PubMed] [Google Scholar]
- 17.Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, Sempos CT, Yetley EA. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr 2011;94:552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandji V, Kafai MR. Population preference values for serum methylmalonic acid concentrations and its relationship with age, sex, race-ethnicity, supplement use, kidney function and serum vitamin B-12 in the post-folic acid fortification period. Nutrients 2018;10(74);doi: 10.3390/nu10010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: Sample design, 2011–2014. National Center for Health Statistics. Vital Health Stat 2 (162). 2014. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. NHANES response rates and population totals. Washington, DC: Centers for Disease Control and Prevention, 2014. Available from: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx. (cited 31 October 2018). [Google Scholar]
- 21.Centers for Disease Control and Prevention. Laboratory procedure manual for vitamin B12 in serum by Roche E-170 Vitamin B12 “ECLIA”. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/vitb12_g_met.pdf (cited 31 October 2018).
- 22.Mineva EM, Zhang M, Rabinowitz DJ, Phinney KW, Pfeiffer CM. An LC-MS/MS method for serum methylmalonic acid suitable for monitoring vitamin B12 status in population surveys. Anal Bional Chem 2015;407:2955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Laboratory procedure manual for methylmalonic acid in serum by LC-MS/MS. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/MMA_G_MET.pdf (cited 31 October 2018).
- 24.Pfeiffer CM, Sternberg MR, Schleicher RL, Rybak ME. Dietary supplement use and smoking were strongly related to biomarkers of water-soluble vitamin status after adjusting for socioeconomic and lifestyle factors in a representative sample of US adults. J Nutr 2013;143:957S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes BMH, Pfeiffer CM, Sternberg MR, Schleicher RL. Selected pre-analytical and physiological factors are weakly to moderately associated with twenty-nine biomarkers of diet and nutrition, NHANES 2003–2006. J Nutr 2013;143:1001S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Balk E, Kausz A, Levin A, Steffes M, Hogg RJ, Perrone RD, Lau J, Eknoyan G et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47. [DOI] [PubMed] [Google Scholar]
- 28.Bailey RL, Durazo-Arvizu RA, Carmel R, Green R, Pfeiffer CM, Sempos CT, Carriquiry A, Yetley EA. Modeling a methylmalonic acid-derived change point for serum vitamin B-12 for adults in NHANES. Am J Clin Nutr 2013;98:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahlin A, Hill R, Winblad B, Bäckman L. Effects of serum vitamin B12 and folate status on episodic memory performance in very old age: a population-based study. Psychology and Aging 1996;11:487–96. [DOI] [PubMed] [Google Scholar]
- 30.Clarke R, Evans JG, Schneede J, Nexo E, Bates C, Fletcher A, Prentice A, Johnston C, Ueland PM, Refsum H et al. Vitamin B12 and folate deficiency on later life. Age and Aging 2004; 33:34–41. [DOI] [PubMed] [Google Scholar]
- 31.Hva A-M, Nexo E. Holotranscobalamine as predictor of vitamin B12 status. Clin Chem Lab Med 2003;41:1489–92. [DOI] [PubMed] [Google Scholar]
- 32.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr 2009;89(suppl):693S-6S. [DOI] [PubMed] [Google Scholar]
- 33.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr 2011;141:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright JD, Bialostosky K, Gunter EW, Carroll MD, Najjar MF, Browman BA, Johnson CL. Blood folate and vitamin B12: United States, 1988–94. Vital Health Stat 1998;243:1–78. [PubMed] [Google Scholar]
- 35.Molly AM, Pangilinan F, Mills JL, Shane B, O’Neil MB, McGaughey DM, Velkova A, Abaan HO, Ueland PM, McNulty H. et al. A common polymorphism in HIBCH influences methylmalonic acid concentrations in blood independently of cobalamin. Am J Hum Genet 2016;98:869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmel R, Green R, Jacobsen DW, Rasmussen K, Florea M, Azen C. Serum cobalamin, homocysteine, and methylmalonic acid concentrations in a multiethnic elderly population: ethnic and sex differences in cobalamin and metabolite abnormalities. Am J Clin Nutr 1999;70:904–10. [DOI] [PubMed] [Google Scholar]
- 37.Quay TAW, Schroder TH, Jeruszka-Bielak M, Li W, Devlin AM, Barr SI, Lamers Y. High prevalence of suboptimal vitamin B12 status in young adult women of South Asian and European ethnicity. Appl Physiol Nutr Metab 2015;40:1279–86. [DOI] [PubMed] [Google Scholar]
- 38.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 2011;94:348S–58S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stabler SP. Screening the older population for cobalamin (vitamin B12) deficiency. J Am Geriatr Soc 1995;43:1290–7. [DOI] [PubMed] [Google Scholar]
- 40.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am J Clin Nutr 2007;86:718–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.