Abstract
Nasopharyngeal carcinoma (NPC) is a head and neck cancer involving epithelial squamous-cell carcinoma of the nasopharynx that mainly occurs in individuals from East and Southeast Asia. We investigated whether Chinese herbal medicine (CHM) as a complementary therapy offers benefits to these patients. We retrospectively evaluated the Taiwan Cancer Registry (Long Form) database for patients with advanced NPC, using or not using CHM, between 2007–2013. Cox proportional-hazard model and Kaplan‒Meier survival analyses were applied for patient survival. CHM-users showed a lower overall and cancer-related mortality risk than non-users. For advanced NPC patients, the overall mortality risk was 0.799-fold for CHM-users, after controlling for age, gender, and Charlson comorbidity index (CCI) score (Cancer stages 3 + 4: adjusted hazard ratio [aHR]: 0.799, 95% confidence interval [CI]: 0.676–0.943, p = 0.008). CHM-users also showed a lower cancer-related mortality risk than non-users (aHR: 0.71, 95% CI: 0.53–0.96, p = 0.0273). Association rule analysis showed that CHM pairs were Ban-Zhi-Lian (BZL; Scutellaria barbata D.Don) and For single herbs, Bai-Hua-She-She-Cao (Herba Hedyotis Diffusae; Scleromitrion diffusum (Willd.) R.J.Wang (syn. Hedyotis diffusa Willd.) and Mai-Men-Dong (MMD; Ophiopogon japonicus (Thunb.) Ker Gawl.), and Gan-Lu-Yin (GLY) and BHSSC. Network analysis revealed that BHSSC was the core CHM, and BZL, GLY, and Xin-Yi-Qing-Fei-Tang (XYQFT) were important CHMs in cluster 1. In cluster 2, ShengDH, MMD, Xuan-Shen (XS; Scrophularia ningpoensis Hensl.), and Gua-Lou-Gen (GLG; Trichosanthes kirilowii Maxim.) were important CHMs. Thus, as a complementary therapy, CHM, and particularly the 8 CHMs identified, are important for the treatment of advanced NPC patients.
Keywords: advanced nasopharyngeal carcinoma, overall mortality, chinese herbal medicine, association rule, network analysis
Introduction
Nasopharyngeal carcinoma (NPC) is a head and neck cancer involving epithelial squamous-cell carcinoma of the nasopharynx (Ferlay et al., 2019), which mainly occurs in individuals from East and Southeast Asia (Cao et al., 2011; Ferlay et al., 2019). The global incidence of NPC is less than 1 per 100,000 person-years; however, in Taiwan, its incidence is 2.8–6.6 per 100,000 person-years (Hsu et al., 2011; Fan et al., 2018). Furthermore, in Taiwan, NPC is the most and second most common head and neck cancer in males and females, respectively (Huang et al., 2015). NPC treatment involves integration of radiotherapy, chemotherapy, and surgery (Perri et al., 2019). The main therapies for NPC are radiotherapy alone for early stage (T1-N0M0 stage) or combined with both chemotherapy and radiotherapy for advanced stages (T2N0‒T4N3M0) (Perri et al., 2019). With radiotherapy alone or chemotherapy in patients with early or advanced NPC, the 5-years survival rate approaches 90% (Blanchard et al., 2015). However, it may cause complications (Langendijk et al., 2008; Jensen et al., 2010), such as mucositis, dermatitis, xerostomia, dysphagia, hyposalivation, xerostomia, radiation caries, sensorineural hearing loss, radioactive osteonecrosis, triceps, temporal lobe injury, and hypothyroidism. Additionally, 8–10% of these patients develop therapeutic resistance and have recurrent disease and distant metastasis (Perri et al., 2011).
There is a need for alternative therapies that can be used in combination with conventional therapies (Salehi et al., 2019a; Salehi et al., 2019b). Chinese herbal medicine (CHM) is cost-effective and has relatively few side effects over long-term usage, and patients with cancer may choose CHM as their integrative, alternative, and complementary therapy to reduce complications from conventional therapies and to improve the overall survival rate in Taiwan (Ye et al., 2015; Hung et al., 2017; Kuo et al., 2018; Li et al., 2018). CHM shows anti-cancer activity via multiple specific targets, synergistic interactions with chemotherapy drugs, and minimal, acceptable side-effects (Aung et al., 2017). Furthermore, CHM and the related natural compounds exhibit protective effects against NPC (Kong et al., 2018; Song et al., 2019; Zhao et al., 2019; Guo et al., 2020). Consequently, these are investigated as alternative therapies for use, combined with conventional therapies, to improve the treatment of patients with NPC, particularly advance-stage NPC (Chen et al., 2019).
To evaluate the effect of CHM as a complementary therapy in patients with NPC, particularly advanced-stage NPC, we used a database in Taiwan to explore the effect of CHM on overall mortality. The CHM prescription pattern in NPC with lower overall mortality was also investigated.
Materials and Methods
Database Source
This study was performed using the Taiwan Cancer Registry (Long Form) database of the National Health Insurance Research Database (NHIRD) (http://tcr.cph.ntu.edu.tw/main.php?Page=N1) (Chiang et al., 2019). There were detailed TNM stage (TNM (tumor, lymph node, and metastasis), cancer stages and cause of death in this database. This database offered longitudinally linked data for each individual during the period between 2003 and 2016. All personal data were decoded. Informed consent was not required. This study was approved by the Institutional Review Board of the China Medical University Hospital (ethics approval number: CMUH107-REC3-074(CR1)).
Study Subjects
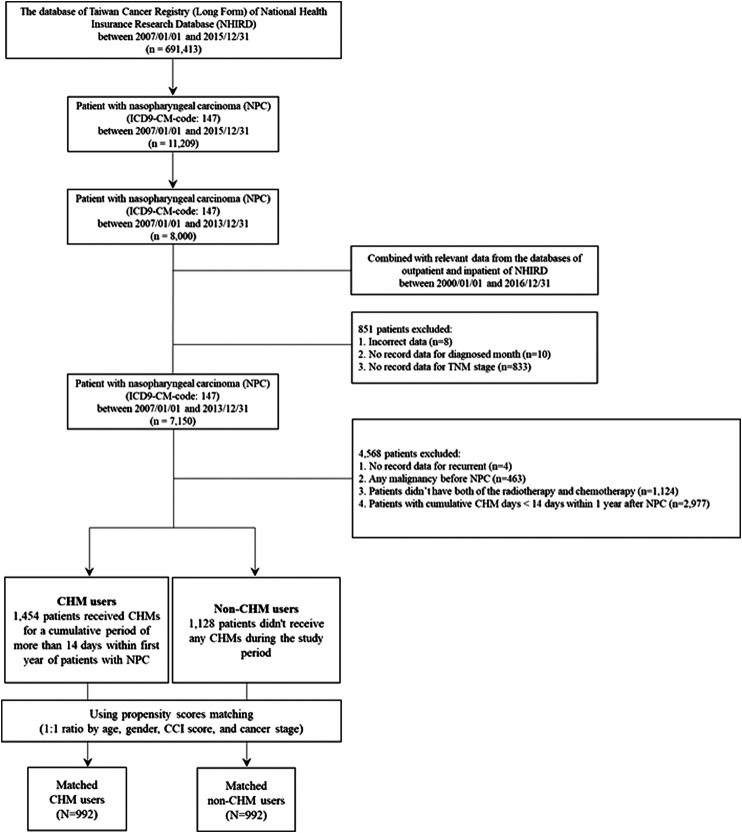
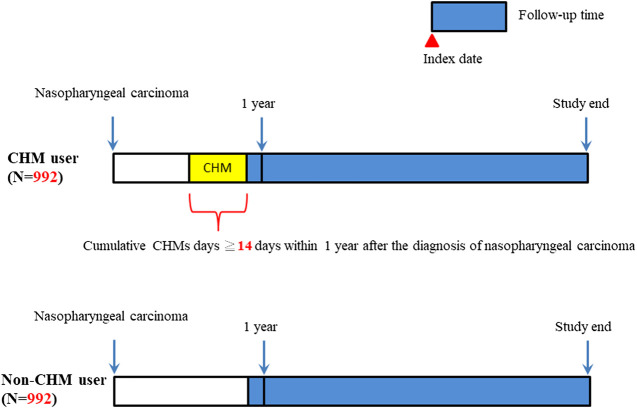
The International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) system was used to identify patients with nasopharyngeal carcinoma (NPC) (ICD-9-CM-code: 147). Overall, 7,150 patients with NPC were identified between 2007–2013 (Figure 1). After excluding patients with incorrect data, missing data, malignancy (ICD9-CM-code: 140–208), no radiotherapy or chemotherapy, and cumulative CHM use of <14 days, 1,454 patients were designated as CHM-users and 1,128 patients as non-users, who did not use CHMs during the follow-up period. To diminish potential bias due to confounders, age, gender, Charlson comorbidity index (CCI) score, and cancer stage CHM-users and non-users were applied to match the two groups for a 1:1 ratio using propensity score matching. After matching, there were 992 matched CHM/non-CHM-user pairs (Figure 1 and Table 1). The date was defined as the index date when 14 cumulative CHM days were completed. The CHM-users continued to use CHMs during the follow-up period. The date of death, the date of withdrawal from the NHIRD database, or the date of the end of follow-up (December 31, 2016) was defined as the study endpoint.
FIGURE 1.
Flowchart of nasopharyngeal carcinoma patient enrollment.
TABLE 1.
Demographic characteristics of patients with nasopharyngeal carcinoma.
| Characteristics | Total subjects | Matched subjects | ||||
|---|---|---|---|---|---|---|
| CHM users | Non- users | P-value | CHM users | Non- users | P-value | |
| N = 1,454 | N = 1,128 | N = 992 | N = 992 | |||
| N (%) | N (%) | N (%) | N (%) | |||
| Age (years old) | 0.015 | 0.945 | ||||
| Age<50 | 774 (53.23%) | 536 (47.52%) | 494 (49.80%) | 489 (49.29%) | ||
| 50≥Age<60 | 443 (30.47%) | 380 (33.69%) | 320 (32.26%) | 327 (32.96%) | ||
| Age≥60 | 237 (16.30%) | 212 (18.79%) | 178 (17.94%) | 176 (17.74%) | ||
| Gender | <0.001 | 0.901 | ||||
| Male | 1054 (72.49%) | 971 (86.08%) | 842 (84.88%) | 840 (84.68%) | ||
| Female | 400 (27.51%) | 157 (13.92%) | 150 (15.12%) | 152 (15.32%) | ||
| CCI score (Mean±SD) | 0.87± 1.22 | 0.67± 1.11 | <0.001 | 0.72± 1.06 | 0.71± 1.13 | 0.918 |
| T-stage | <0.001 | 1.000 | ||||
| T1-T2 | 845 (58.12%) | 576 (51.06%) | 539 (54.33%) | 539 (54.33%) | ||
| T3-T4 | 609 (41.88%) | 552 (48.94%) | 453 (45.67%) | 453 (45.67%) | ||
| N-stage | <0.001 | 0.529 | ||||
| N0 | 179 (12.31%) | 123 (10.90%) | 120 (12.10%) | 115 (11.59%) | ||
| N1-N2 | 1072 (73.73%) | 766 (67.91%) | 706 (71.17%) | 692 (69.76%) | ||
| N3-N4 | 203 (13.96%) | 239 (21.19%) | 166 (16.73%) | 185 (18.65%) | ||
| M-stage | <0.001 | 0.196 | ||||
| M0 | 1398 (96.15%) | 1048 (92.91%) | 945 (95.26%) | 932 (93.95%) | ||
| M1 | 56 (3.85%) | 80 (7.09%) | 47 (4.74%) | 60 (6.05%) | ||
| Cancer stage | <0.001 | 0.983 | ||||
| 1 | 47 (3.23%) | 31 (2.75%) | 32 (3.23%) | 31 (3.13%) | ||
| 2 | 392 (26.96%) | 231 (20.48%) | 229 (23.08%) | 223 (22.48%) | ||
| 3 | 568 (39.06%) | 381 (33.78%) | 359 (36.19%) | 366 (36.90%) | ||
| 4 | 447 (30.74%) | 485 (43.00%) | 372 (37.50%) | 372 (37.50%) | ||
| Surgery | ||||||
| No | 1401 (96.35%) | 1066 (94.50%) | 0.024 | 948 (95.56%) | 942 (94.96%) | 0.526 |
| Yes | 53 (3.65%) | 62 (5.50%) | 44 (4.44%) | 50 (5.04%) | ||
N, number; CHM, chinese herbal medicine; CCI, charlson comorbidity index; T-stage, tumor stage; N-stage, lymph nodes stage; M-stage, metastasis stage.
Age, gender, TNM stage, cancer stage, and surgery were expressed as categorical variable (number (%)).
Nasopharyngeal carcinoma (ICD-9-CM-code: 147).
P-values were obtained by chi-square test. Significant p-values (p < 0.05) were highlighted in bold italic.
Propensity score matching was performed for age, gender, CCI score, and cancer stage (1:1 ratio).
The Charlson comorbidities include congestive heart failure (ICD-9-CM: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4 - 425.9, 428.x), peripheral vascular disease (ICD-9-CM: 093.0, 437.3, 440.x, 441.x, 443.1 - 443.9, 447.1, 557.1, 557.9, V43.4), cerebrovascular disease (ICD-9-CM: 362.34, 430.x - 438.x), chronic pulmonary disease (ICD-9-CM: 416.8, 416.9, 490.x - 505.x, 506.4, 508.1, 508.8), rheumatic disease (ICD-9-CM: 446.5, 710.0 - 710.4, 714.0 - 714.2, 714.8, 725.x), peptic ulcer disease (ICD-9-CM: 531.x - 534.x), mild liver disease (ICD-9-CM: 070.22, 070.23, 070.32, 070.33, 070.44, 070.54, 070.6, 070.9, 570.x, 571.x, 573.3, 573.4, 573.8, 573.9, V42.7), diabetes without chronic complication (ICD-9-CM: 250.0 - 250.3, 250.8, 250.9), diabetes with chronic complication (ICD-9-CM: 250.4 - 250.7), hemiplegia or paraplegia (ICD-9-CM: 334.1, 342.x, 343.x, 344.0 - 344.6, 344.9), renal disease (ICD-9-CM: 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 582.x, 583.0 - 583.7, 585.x, 586.x, 588.0, V42.0, V45.1, V56.x), and any malignancy, including lymphoma and leukemia, except malignant neoplasm of skin (ICD-9-CM: 140.x - 172.x, 174.x - 195.8, 200.x - 208.x, 238.6). These comorbidities were recorded before the diagnosis of nasopharyngeal carcinoma.
Chinese Herbal Medicine
CHM products used in NPC patients contain two types: single herb and herbal formula (Supplementary Table S1). The herbal formula combines at least two single herbs. A single herb is a part of a plant, such as seeds, fruits, flowers, roots, stems, or leaves. Single herb CHM may also be organs of animals, insects, or minerals. In this study, NPC patients received CHM prescriptions from licensed Chinese medicine doctors, and these CHM prescriptions were produced by pharmaceutical manufacturers following Good Manufacturing Practice in Taiwan (Li et al., 2018; Tsai et al., 2019a; Cheng et al., 2019).
Association Rule
The CHM prescription profile was investigated using association rule mining (Yang et al., 2013). The association rule was also implemented as previously described for paired CHM combinations (Tsai et al., 2019b; Cheng et al., 2019; Tsai et al., 2019c; Chen et al., 2021; Tsai et al., 2020) using SAS software (version 9.4; SAS Institute, Cary, NC, USA). The strength of association between paired CHM combinations (CHM products X and Y) was shown using the support value (X) (%), confidence value (CHM_X→ CHM_Y; %), and lift value as previously described (Tsai et al., 2019c; Chen et al., 2021; Tsai et al., 2020) (Table 4).
TABLE 4.
Ten most commonly used pairs of CHM products for patients with nasopharyngeal carcinoma in Taiwan.
| CHM products (LHS, X) | Chinese name | Frequency of prescriptions of X product | CHM products (RHS, Y) | Chinese name | Frequency of prescriptions of Y product | Frequency of prescriptions of X and Y products | Support (X) (%) | Confidence (X →Y) (%) | Lift | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ban-Zhi-Lian (BZL) | 半枝蓮 | 2040 | → | Bai-Hua-She-She-Cao (BHSSC) | 白花蛇舌草 | 5719 | 1596 | 4.9 | 78.2 | 4.5 |
| Sheng-Di-Huang (ShengDH) | 生地黃 | 2232 | → | Mai-Men-Dong (MMD) | 麥門冬 | 3140 | 1041 | 3.2 | 46.6 | 4.9 |
| Gan-Lu-Yin (GLY) | 甘露飲 | 5185 | → | Bai-Hua-She-She-Cao (BHSSC) | 白花蛇舌草 | 5719 | 998 | 3.0 | 19.2 | 1.1 |
| Mai-Men-Dong (MMD) | 麥門冬 | 3140 | → | Xuan-Shen (XS) | 玄參 | 3323 | 930 | 2.8 | 29.6 | 2.9 |
| Sheng-Di-Huang (ShengDH) | 生地黃 | 2232 | → | Xuan-Shen (XS) | 玄參 | 3323 | 876 | 2.7 | 39.2 | 3.9 |
| Xin-Yi-Qing-Fei-Tang (XYQFT) | 辛夷清肺湯 | 3812 | → | Bai-Hua-She-She-Cao (BHSSC) | 白花蛇舌草 | 5719 | 823 | 2.5 | 21.6 | 1.2 |
| Gua-Lou-Gen (GLG) | 栝樓根 | 3156 | → | Bai-Hua-She-She-Cao (BHSSC) | 白花蛇舌草 | 5719 | 781 | 2.4 | 24.7 | 1.4 |
| Gua-Lou-Gen (GLG) | 栝樓根 | 3156 | → | Gan-Lu-Yin (GLY) | 甘露飲 | 5185 | 747 | 2.3 | 23.7 | 1.5 |
| Mai-Men-Dong (MMD) | 麥門冬 | 3140 | → | Bai-Hua-She-She-Cao (BHSSC) | 白花蛇舌草 | 5719 | 735 | 2.2 | 23.4 | 1.3 |
| Mai-Men-Dong (MMD) | 麥門冬 | 3140 | → | Gua-Lou-Gen (GLG) | 栝樓根 | 3156 | 729 | 2.2 | 23.2 | 2.4 |
CHM, Chinese herbal medicine; LHS, left-hand-side; RHS, right-hand-side.
Total prescriptions = 32842.
Support (X) (%) = Frequency of prescription of X and Y products / total prescriptions x 100%.
Confidence (X →Y) (%) = Frequency of prescription of X and Y products / Frequency of prescription of X product x 100%.
P (Y) (%) = Frequency of prescription of Y product / total prescriptions x 100%.
Lift = Confidence (X →Y) (%) / P (Y) (%).
Network Analysis
Network analysis for CHM clusters was accomplished as previously described (Tsai et al., 2019b; Cheng et al., 2019; Tsai et al., 2019c; Chen et al., 2021; Tsai et al., 2020) using Cytoscape (https://cytoscape.org/, version 3.7.0). The herbal formula is shown as a red circle, and a single herb is expressed as a green circle. The circle size indicates the prescription frequency of the CHM. The line size signifies the support value between paired CHM products. Line color displays the lift value between paired CHM products. The thicker and darker connection line shows a stronger connection strength between the paired CHM products.
Statistical Analysis
Categorical data (age, gender, TNM [tumor, lymph node, and metastasis] stage, cancer stage, and surgery) are shown as numbers (percentages), and the Chi-squared test was applied to evaluate the differences between CHM-users and non-users (Table 1). Crude and adjusted Cox proportional hazard models were used to estimate the risk of overall mortality (Table 2). The adjustment factors included age, gender, CHM use, CCI score, cancer stage, and surgery (Table 2). NPC patients were stratified according to cancer stage (Table 3 and Figure 4). For NPC patients in cancer stages 1 + 2, patients were stratified by age, gender, and CCI (Table 3 and Figure 4). For NPC patients in cancer stages 3 + 4, patients were also stratified by age, gender, and CCI (Table 3 and Figure 4). The adjustment factors included age, gender, CHM use, and CCI score (Table 3). Kaplan‒Meier curves and log-rank tests were performed to assess the cumulative incidence of overall mortality between the two groups (Figure 3). p-values of less than 0.05 were considered statistically significant. All analyses were completed using SAS software (version 9.4; SAS Institute).
TABLE 2.
Cox proportional hazard models for overall mortality in patients with nasopharyngeal carcinoma in Taiwan.
| Crude | Adjusted | |||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P-value | aHR | (95% CI) | P-value | |
| Age (years old) | ||||||
| Age<50 | Ref. | ND | ND | Ref. | ND | ND |
| 50≥Age<60 | 1.17 | (0.97–1.43) | 0.1067 | 1.20 | (0.98–1.46) | 0.0777 |
| Age≥60 | 2.37 | (1.95–2.89) | <.0001 | 2.36 | (1.9–2.93) | <.0001 |
| Gender | ||||||
| Male | Ref. | ND | ND | Ref. | ND | ND |
| Female | 0.77 | (0.6–0.98) | 0.0360 | 0.73 | (0.57–0.95) | 0.0173 |
| CHM use | ||||||
| No | Ref. | ND | ND | Ref. | ND | ND |
| Yes | 0.82 | (0.71–0.96) | 0.0125 | 0.78 | (0.67–0.92) | 0.0024 |
| CCI score (Mean±SD), per score | 1.21 | (1.13–1.29) | <.0001 | 1.07 | (1–1.16) | 0.0553 |
| Cancer stage | ||||||
| 1 | Ref. | ND | ND | Ref. | ND | ND |
| 2 | 1.732 | (0.76–3.94) | 0.1899 | 1.709 | (0.77–3.78) | 0.1865 |
| 3 | 2.869 | (1.29–6.39) | 0.0099 | 2.818 | (1.3–6.12) | 0.0088 |
| 4 | 6.75 | (3.07–14.86) | <.0001 | 6.857 | (3.19–14.73) | <.0001 |
| Surgery | ||||||
| No | Ref. | ND | ND | Ref. | ND | ND |
| Yes | 1.12 | (0.79–1.58) | 0.5239 | 1.00 | (0.69–1.43) | 0.9811 |
CHM, chinese herbal medicine; HR, hazard ratio; 95% CI, 95% confidence interval; ND, not determined; CCI, charlson comorbidity index.
Nasopharyngeal carcinoma (ICD-9-CM-code: 147).
CCI score (Mean±SD) was expressed as a continuous variable. The risk of overall mortality increased with CCI score(HR 1.07/score) in our study.
Models adjusted for age, gender, CHM use, CCI score, cancer stage, and surgery.
P-value (p < 0.05) was shown in bold italic font.
TABLE 3.
Subgroup analysis for the risk of overall mortality in patients with nasopharyngeal carcinoma when stratified by cancer stages.
| Subgroup | CHM users N=992 | Non- users N=992 | Crude | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event | All | Event | All | HR | (95%CI) | P-value | aHR | (95%CI) | P-value | |
| Cancer stages (all) | ||||||||||
| Overall | 280 | 992 | 323 | 992 | 0.822 | (0.705–0.959) | 0.0125 | 0.813 | (0.697–0.947) | 0.0081 |
| Age (Mean±SD) | 280 | 992 | 323 | 992 | 1.035 | (1.027–1.043) | <.0001 | 1.032 | (1.024–1.040) | <.0001 |
| Gender | ||||||||||
| Male | 249 | 842 | 278 | 840 | Ref. | ND | ND | Ref. | ND | ND |
| Female | 31 | 150 | 45 | 152 | 0.766 | (0.597–0.983) | 0.036 | 0.768 | (0.598–0.985) | 0.0379 |
| CCI (Mean±SD) | 280 | 992 | 323 | 992 | 1.206 | (1.128–1.290) | <.0001 | 1.099 | (1.022–1.181) | 0.0105 |
| Cancer stages 1+2 | ||||||||||
| Overall | 35 | 261 | 42 | 254 | 0.791 | (0.506–1.236) | 0.3031 | 0.84 | (0.534–1.323) | 0.4527 |
| Age (Mean±SD) | 35 | 261 | 42 | 254 | 1.035 | (1.008–1.062) | 0.0116 | 1.027 | (1.000–1.054) | 0.0481 |
| Gender | ||||||||||
| Male | 30 | 222 | 35 | 207 | Ref. | ND | ND | Ref. | ND | ND |
| Female | 5 | 39 | 7 | 47 | 0.86 | (0.438–1.688) | 0.6612 | 0.816 | (0.419–1.589) | 0.549 |
| CCI (Mean±SD) | 35 | 261 | 42 | 254 | 1.369 | (1.155–1.622) | 0.0003 | 1.277 | (1.084–1.503) | 0.0034 |
| Cancer stages 3+4 | ||||||||||
| Overall | 245 | 731 | 281 | 738 | 0.825 | (0.699–0.973) | 0.0223 | 0.799 | (0.676–0.943) | 0.008 |
| Age (Mean±SD) | 245 | 731 | 281 | 738 | 1.036 | (1.028–1.044) | <.0001 | 1.034 | (1.026–1.042) | <.0001 |
| Gender | ||||||||||
| Male | 219 | 620 | 243 | 633 | Ref. | ND | ND | Ref. | ND | ND |
| Female | 26 | 111 | 38 | 105 | 0.771 | (0.587–1.011) | 0.0603 | 0.791 | (0.604–1.036) | 0.0891 |
| CCI (Mean±SD) | 245 | 731 | 281 | 738 | 1.172 | (1.094–1.255) | <.0001 | 1.057 | (0.978–1.141) | 0.1597 |
CHM, Chinese herbal medicine; HR, hazard ratio; aHR, adjusted hazard ratio; 95% CI, 95% confidence interval; ND, not determined; CCI, Charlson comorbidity index. Nasopharyngeal carcinoma (ICD-9-CM-code: 147).
Models adjusted for age, gender, and CCI score. P-value (p < 0.05) was shown in bold italic font.
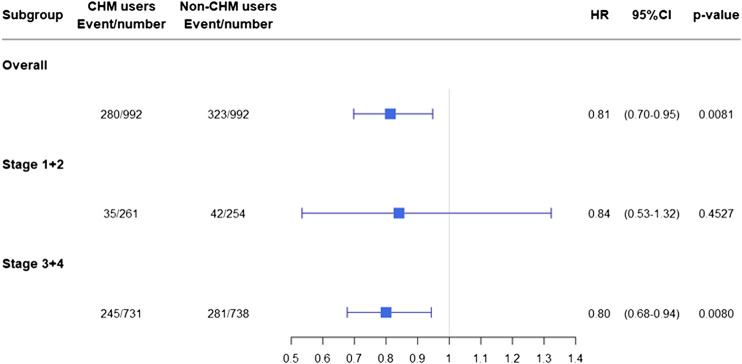
FIGURE 4.
Risk of overall mortality in NPC patients when stratified by cancer stages. Abbreviations: NPC, nasopharyngeal carcinoma; CHM, chinese herbal medicine; HR, hazard ratio; CI, confidence interval.
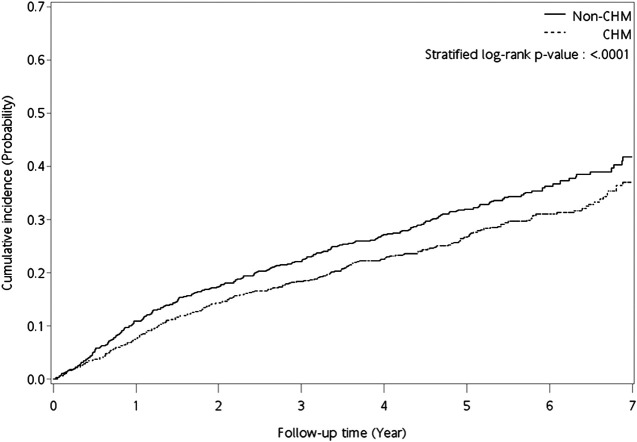
FIGURE 3.
Kaplan‒Meier curves for overall mortality for NPC patients. Abbreviations: CHM, chinese herbal medicine; NPC, nasopharyngeal carcinoma.
Results
Demographic Characteristics
For CHM-users and non-users, age, gender, CCI score, TNM stage, cancer stage, and surgery differed significantly (total subjects; p-value < 0.05; Table 1). To decrease confounding effects, propensity score matching was performed, after which there were no significant differences in demographic characteristics between the two matched users (p-value > 0.05).
Overall Mortality
In the investigation of overall mortality in patients with NPC (Table 2), the crude Cox proportional hazard model revealed significant differences in age, gender, CHM use, CCI score, and cancer stage. After adjusting for these variances, the adjusted Cox proportional hazard model showed that patients aged over 60 years had a higher overall mortality risk than those aged below 50 years (Table 2; adjusted hazard ratio [aHR]: 2.36, 95% CI: 1.90–2.93, p < 0.0001). Females showed a lower risk of overall mortality than males (Table 2; aHR: 0.73, 95% CI: 0.57–0.95, p = 0.0173). Patients with cancer stage 3 had a higher risk of overall mortality than those with cancer stage 1 (Table 2; aHR: 2.82, 95% CI: 1.30‒6.12, p = 0.0088). Patients with cancer stage 4 had a higher risk of overall mortality than those with cancer stage 1 (Table 2; aHR: 6.86, 95% CI: 3.19‒14.73, p < 0.0001).
CHM-users showed a lower overall mortality risk than non-users (aHR: 0.78, 95% CI: 0.67–0.92, p = 0.0024; Table 2). Kaplan‒Meier survival plots revealed the difference in the cumulative incidence of overall mortality between the two groups of users (Figure 2; p < 0.0001, log-rank test). The cumulative incidence of overall mortality was significantly higher in non-users.
FIGURE 2.
Diagram of follow-up time for NPC patients. Abbreviations: NPC, nasopharyngeal carcinoma.
The hazard ratios for overall mortality in these NPC patients were separated into subgroups according to cancer stage. Among these subgroups, a lower overall mortality risk was observed in CHM-users in patients with cancer stages 3 + 4 (advanced stages; aHR: 0.799, 95% CI: 0.676–0.943, p = 0.008) (Table 3 and Figure 4).
Chinese Herbal Medicine Prescription Pattern
The herbal composition and related prescription frequency information for patients with NPC are listed in Table S1. Based on prescription frequency, Gan-Lu-Yin (GLY) was the most commonly prescribed herbal formula, Xin-Yi-Qing-Fei-Tang (XYQFT) was the second herbal formula. For single herbs, Bai-Hua-She-She-Cao (Herba Hedyotis Diffusae; Hedyotis diffusa Willd.) was most prescribed single herb, followed by Xuan-Shen (Radix Scrophulariae; Scrophularia ningpoensis Hensl.) and Gua-Lou-Gen (Radix Trichosanthis; Trichosanthes kirilowii Maxim.).
Association rule analysis revealed the CHM product pairs most used for patients with NPC (Table 4). Higher levels of support, confidence, and lift indicated stronger associations for paired CHM products. The most commonly used paired CHM products were Ban-Zhi-Lian (BZL)→Bai-Hua-She-She-Cao (BHSSC) (first co-prescription frequency: 1,596, support: 4.9%, confidence: 78.2%, lift: 4.5), followed by Sheng-Di-Huang (ShengDH)→Mai-Men-Dong (MMD) (second co-prescription frequency: 1,041, support: 3.2%, confidence: 46.6%, lift: 4.9), and GLY→BHSSC (third co-prescription frequency: 998, support: 3.0%, confidence: 19.2%, lift: 1.1) (Table 4).
Network analysis revealed the CHM prescription network for patients with NPC (Figure 5). There were 992 patients who used 32,842 prescriptions by traditional Chinese medicine doctors (Table 4). Network analysis revealed two clusters (Figure 5). In cluster 1, BHSSC showed the core CHM. BZL, GLY, and XYQFT were nearby CHMs. In cluster 2, ShengDH, MMD, XS, and GLG were important CHMs. Our results show that these 8 CHMs are important for patients with NPC.
FIGURE 5.
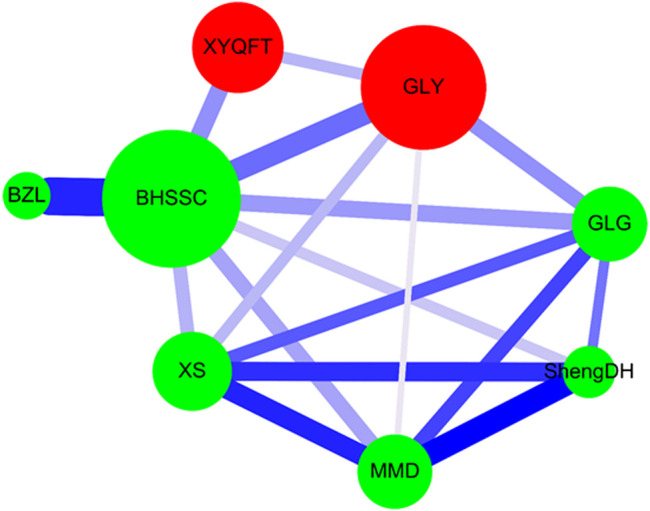
CHM network analysis in NPC patients. Herbal formula is shown as the red circle, and single herb is expressed as the green circle. The circle size indicates prescription frequency of the CHM. The line size and color signify the support value and the lift value between paired CHM products, respectively. The thicker and darker connection line shows the stronger strength of connection between the paired CHM products. Abbreviations: NPC, nasopharyngeal carcinoma; CHM, chinese herbal medicine.
Discussion
The long-term therapeutic effects of CHM in patients with NPC, particularly advanced-stage NPC, remain to be elucidated (Kim et al., 2015; Song et al., 2019). In our study, with the TNM and cancer stage information and NPC patients identified from the database of the Taiwan Cancer Registry (Long Form) of the NHIRD, we were able to assess the CHM effects for long-term use in NPC patients with advanced-stage disease (cancer stages 3 + 4). NPC patients who used CHM had lower overall and cancer-related mortality than those who did not use CHM after a 7-years follow-up. There was 88.2% of NPC patients who had died from the various forms of cancer (malignancies; ICD9-CM-code: 140–208; Supplementary Figure S2). There were 81.3% of NPC patients who had died from NPC cancer (ICD9-CM-code: 147; Supplementary Figure S2). Only 6.9% of NPC patients who had died from other than NPC cancer (malignancies; ICD9-CM-code: 140–208, except for 147; Supplementary Figure S2). There was only 1.5% of NPC patients who had died from cardiocerebrovascular diseases (ICD9-CM-code: 390–459; Supplementary Figure S2). For NPC patients, CHM-users showed a lower cancer-related mortality risk than non-users (Supplementarys Table S9; Figure S6). Also, the NPC-related mortality risk was lower for CHM users after controlling for age, gender, and CCI score for these NPC patients (Supplementarys Table S10; Figure S7).
For advanced NPC patients, the overall mortality risk was 0.799-fold (95%CI: 0.676–0.943) for CHM-users after controlling for age, gender, and CCI score. The dose and duration of using CHM was associated with a reduced risk of overall mortality among patients with NPC (Supplementarys Tables S3–S8; Figures S1–S3). There was 88.6% of advanced NPC patients who had died from the various forms of cancer (malignancies; ICD9-CM-code: 140–208; Supplementary Figure S5). There were 82.9% of advanced NPC patients who had died from NPC cancer (ICD9-CM-code: 147; Supplementary Figure S5). Only 5.7% of advanced NPC patients who had died from other than NPC cancer (malignancies; ICD9-CM-code: 140–208, except for 147; Supplementary Figure S5). There was only 1.7% of advanced NPC patients who had died from cardiocerebrovascular diseases (ICD9-CM-code: 390–459; Supplementary Figure S5). For advanced NPC patients, CHM-users showed a lower cancer-related mortality risk than non-users (Supplementarys Table S11; Figure S8). Similar result was also observed in the NPC-related mortality. The NPC-related mortality risk was lower for CHM users after controlling for age, gender, and CCI score for these advanced NPC patients (Supplementarys Table S13; Figure S9). Furthermore, we found that eight CHMs were important for these advanced NPC patients by association rules and network analyses. These results provide the utility of clinical CHM as a complementary therapy for patients with advanced NPC.
We enrolled primary NPC patients who received both radiotherapy and chemotherapy. Approximately 80% of these patients were <60 years old and about 80% were male. Our results are similar to those of previous studies (Song et al., 2019). NPC patients in Taiwan were characterized by more males, and 80% of them were under 60 years old in another study (Song et al., 2019). The 5-years overall mortality for NPC patients was approximately 30% in our study, which is in agreement with previous studies (Ji et al., 2019; Zhu et al., 2019). We found that the 5-years overall mortality for patients with NPC was about 25% when patients used CHM. Several Chinese herbs and compounds exhibit protective effects against NPC (Kong et al., 2018; Song et al., 2019; Zhao et al., 2019; Guo et al., 2020). Our results showed the protective effects of the clinical use of CHMs against overall mortality in NPC patients, particularly those with advanced stages.
Our association rule analysis showed that the most commonly used CHM pairs were BZL →BHSSC, followed by ShengDH→MMD, and GLY→BHSSC. Our network analysis showed that, in cluster 1, BHSSC showed the core CHM, and BZL, GLY, and XYQFT were nearby CHMs. In cluster 2, ShengDH, MMD, XS, and GLG were important CHMs.
We identified single herbs, including BZL, BHSSC, ShengDH, MMD, XS, and GLG. Among these single herbs, BZL and BHSSC show anti-cancer and anti-inflammatory activities (Perez et al., 2010; Zhang et al., 2017). BZL is the entire plant of Scutellaria barbata D. Don (the Lamiaceae family). Interestingly, natural compounds of BZL, including scutebarbatines, barbatin D, barbatin E, 3′,4′,5,7-tetrahydroxyflavone, 5,7,4′-trihydroxyflavone (apigenin), and quercetin show significant cytotoxic activities against NPC cells (Ong et al., 2004; Dai et al., 2008; Dai et al., 2011; Daker et al., 2012; Li et al., 2014; Wu et al., 2017). Notably, apigenin also inhibits Epstein‒Barr virus (EBV) reactivation (Wu et al., 2017). Scleromitrion diffusum (Willd.) R.J.Wang (syn. Hedyotis diffusa Willd. (Rubiaceae family)). Natural compounds of BHSSC, including shecaocerenoside A, shecaoiridoidside C, coumarin, and quercetin, exhibit anti-tumor activity, including human NPC cells (Wang et al., 2017; Peng et al., 2018).
GLY contains ten single herbs. GLY has shown anti-NPC activity in NPC patients (Song et al., 2019). The GLY extract also shows anti-angiogenic effects (Pan et al., 2010). Our advanced NPC patients also used GLY, with a better survival rate. The natural compound epigallocatechin-3-gallate (EGCG) is from GLY and inhibits human NPC cell migration by suppressing MMP-2 expression (Ho et al., 2019). EGCG also suppresses NPC cell growth by attenuating STAT3 activation (Lin et al., 2014). 5,7-dihydroxyflavone promotes human NPC cell apoptosis via tumor necrosis factor-related apoptosis-inducing ligands (Li et al., 2011). Genistein induces NPC cell growth inhibition and G2/M arrest (Han et al., 2010). Genistein also suppresses NPC stem cell growth via sonic hedgehog signaling (Zhang et al., 2019). Other natural compounds of GLY, including apigenin, coumarin, and quercetin show significant cytotoxic activities against NPC cells (Ong et al., 2004; Daker et al., 2012; Peng et al., 2018).
XYQFT contains 10 single herbs and is prescribed to treat respiratory-related diseases, including asthma and allergic rhinitis (Yen et al., 2015; Lo et al., 2020). XYQFT also contains natural compounds, including EGCG, apigenin, coumarin, genistein, and quercetin, which exhibit anti-NPC activities (Ong et al., 2004; Han et al., 2010; Daker et al., 2012; Lin et al., 2014; Peng et al., 2018; Ho et al., 2019; Zhang et al., 2019). Furthermore, 1,3,8-trihydroxy-6-methylanthraquinone (emodin) from XYQFT inhibits EBV reactivation and suppresses NPC cell proliferation (Ma et al., 2017; Wu et al., 2019). The 3-phenyl-2-propenal (trans-cinnamaldehyde) inhibits NPC cells (Daker et al., 2013).
This study demonstrated that complementary CHM therapy may reduce overall and cancer related mortality among advanced NPC patients. There are eight clinically used CHM products that are potentially useful for advanced NPC patients. However, the actual dose of specific CHMs in this prescription for patients was unknown, and the metabolism of the co-prescription pattern in humans, as well as potential confounders (i.e., body mass index, fatty tissue, lifestyle, personalized treatments, social-economic status, and cigarette smoking etc.), were not clarified in this study. Therefore, further randomized controlled trials and functional investigations of these potentially useful CHM products are necessary to validate their efficacy and safety in these patients.
Acknowledgments
We are grateful to the Health Data Science Center at the China Medical University Hospital for providing administrative, technical, and funding support.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Institutional Review Board of the China Medical University Hospital (ethics approval number: CMUH107-REC3-074(CR1)). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
C-YW, T-CW, T-ML, and Y-JL wrote the manuscript and interpreted the data. W-ML, C-HH, J-SC, C-JC, T-HL, C-CL, and S-MH collected, assembled, and analyzed the data. W-ML, F-JT, S-TH, T-YC, T-ML, and Y-JL provided the study materials. T-ML and Y-JL designed, conceived the study, and amended the manuscript.
Funding
This study was supported by grants from the China Medical University, Taiwan (CMU108-MF-32, CMU108-S-15, and CMU108-S-17), the China Medical University Hospital, Taiwan (DMR-109-145, DMR-109-188, and DMR-109-192), and the Ministry of Science and Technology, Taiwan (MOST 106-2320-B-039 -017 -MY3, MOST 108-2314-B-039-044-MY3, and MOST 109-2320-B-039-035-MY3). The funding entities for this study had no role in the study design, data collection, data analysis, interpretation, or authorship of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.607413/full#supplementary-material.
References
- Aung T. N., Qu Z., Kortschak R. D., Adelson D. L. (2017). Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 18, 656. 10.3390/ijms18030656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard P., Lee A., Marguet S., Leclercq J., Ng W. T., Ma J., et al. (2015). Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 16, 645–655. 10.1016/S1470-2045(15)70126-9 [DOI] [PubMed] [Google Scholar]
- Cao S. M., Simons M. J., Qian C. N. (2011). The prevalence and prevention of nasopharyngeal carcinoma in China. Chin. J. Cancer 30, 114–119. 10.5732/cjc.010.10377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. P., Chan A. T. C., Le Q. T., Blanchard P., Sun Y., Ma J. (2019). Nasopharyngeal carcinoma. Lancet 394, 64–80. 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- Chen C. J., Liu X., Chiou J. S., Hang L. W., Li T. M., Tsai F. J., et al. (2021). Effects of chinese herbal medicines on dementia risk in patients with sleep disorders in Taiwan. J. Ethnopharmacol. 264, 113267. 10.1016/j.jep.2020.113267 [DOI] [PubMed] [Google Scholar]
- Cheng C. F., Lin Y. J., Tsai F. J., Li T. M., Lin T. H., Liao C. C., et al. (2019). Effects of Chinese herbal medicines on the risk of overall mortality, readmission, and reoperation in hip fracture patients. Front. Pharmacol. 10, 629. 10.3389/fphar.2019.00629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. J., Wang Y. W., Lee W. C. (2019). Taiwan’s nationwide cancer Registry system of 40 years: past, present, and future. J. Formos. Med. Assoc. 118, 856–858. 10.1016/j.jfma.2019.01.012 [DOI] [PubMed] [Google Scholar]
- Dai S. J., Shen L., Ren Y. (2008). Two new neo-clerodane diterpenoids from Scutellaria barbata. J. Integr. Plant Biol. 50, 699–702. 10.1111/j.1744-7909.2008.00672.x [DOI] [PubMed] [Google Scholar]
- Dai S. J., Peng W. B., Shen L., Zhang D. W., Ren Y. (2011). New norditerpenoid alkaloids from Scutellaria barbata with cytotoxic activities. Nat. Prod. Res. 25, 1019–1024. 10.1080/14786410903132464 [DOI] [PubMed] [Google Scholar]
- Daker M., Ahmad M., Khoo A. S. (2012). Quercetin-induced inhibition and synergistic activity with cisplatin-a chemotherapeutic strategy for nasopharyngeal carcinoma cells. Cancer Cell Int. 12, 34. 10.1186/1475-2867-12-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daker M., Lin V. Y., Akowuah G. A., Yam M. F., Ahmad M. (2013). Inhibitory effects of Cinnamomum burmannii Blume stem bark extract and trans-cinnamaldehyde on nasopharyngeal carcinoma cells; synergism with cisplatin. Exp. Ther. Med. 5, 1701–1709. 10.3892/etm.2013.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H. C., Chen C. Y., Hsu Y. C., Chou R. H., Teng C. J., Chiu C. H., et al. (2018). Increased risk of incident nasopharyngeal carcinoma with exposure to air pollution. PLoS One 13, e0204568. 10.1371/journal.pone.0204568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D. M., Piñeros M., et al. (2019). Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953. 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- Guo S., Zhang J., Wei C., Lu Z., Cai R., Pan D., et al. (2020). Anticancer effects of brusatol in nasopharyngeal carcinoma through suppression of the Akt/mTOR signaling pathway. Cancer Chemother. Pharmacol. 85, 1097–1108. 10.1007/s00280-020-04083-3 [DOI] [PubMed] [Google Scholar]
- Han H., Zhong C., Zhang X., Liu R., Pan M., Tan L., et al. (2010). Genistein induces growth inhibition and G2/M arrest in nasopharyngeal carcinoma cells. Nutr. Cancer 62, 641–647. 10.1080/01635581003605490 [DOI] [PubMed] [Google Scholar]
- Ho H. C., Huang C. C., Lu Y. T., Yeh C. M., Ho Y. T., Yang S. F., et al. (2019). Epigallocatechin-3-gallate inhibits migration of human nasopharyngeal carcinoma cells by repressing MMP-2 expression. J. Cell. Physiol. 234, 20915–20924. 10.1002/jcp.28696 [DOI] [PubMed] [Google Scholar]
- Hsu W. L., Yu K. J., Chien Y. C., Chiang C. J., Cheng Y. J., Chen J. Y., et al. (2011). Familial tendency and risk of nasopharyngeal carcinoma in taiwan: effects of covariates on risk. Am. J. Epidemiol. 173, 292–299. 10.1093/aje/kwq358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. Y., Lin C. L., Lin C. Y., Jen Y. M., Lo C. H., Sung F. C., et al. (2015). Survival outcome of patients with nasopharyngeal carcinoma: a nationwide analysis of 13 407 patients in Taiwan. Clin. Otolaryngol. 40, 327–334. 10.1111/coa.12371 [DOI] [PubMed] [Google Scholar]
- Hung K. F., Hsu C. P., Chiang J. H., Lin H. J., Kuo Y. T., Sun M. F., et al. (2017). Complementary Chinese herbal medicine therapy improves survival of patients with gastric cancer in Taiwan: a nationwide retrospective matched-cohort study. J. Ethnopharmacol. 199, 168–174. 10.1016/j.jep.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Jensen S. B., Pedersen A. M., Vissink A., Andersen E., Brown C. G., Davies A. N., et al. (2010). A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Supportive Care Cancer 18, 1039–1060. 10.1007/s00520-010-0827-8 [DOI] [PubMed] [Google Scholar]
- Ji M. F., Sheng W., Cheng W. M., Ng M. H., Wu B. H., Yu X., et al. (2019). Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO-NPC-001) in southern China. Ann. Oncol. 30, 1630–1637. 10.1093/annonc/mdz231 [DOI] [PubMed] [Google Scholar]
- Kim W., Lee W. B., Lee J., Min B. I., Lee H., Cho S. H. (2015). Traditional herbal medicine as adjunctive therapy for nasopharyngeal cancer: a systematic review and meta-analysis. Integr. Cancer Ther. 14, 212–220. 10.1177/1534735415572881 [DOI] [PubMed] [Google Scholar]
- Kong L., Li X., Wang H., He G., Tang A. (2018). Calycosin inhibits nasopharyngeal carcinoma cells by influencing EWSAT1 expression to regulate the TRAF6-related pathways. Biomed. Pharmacother. 106, 342–348. 10.1016/j.biopha.2018.06.143 [DOI] [PubMed] [Google Scholar]
- Kuo Y. T., Liao H. H., Chiang J. H., Wu M. Y., Chen B. C., Chang C. M., et al. (2018). Complementary Chinese herbal medicine therapy improves survival of patients with pancreatic cancer in taiwan: a nationwide population-based cohort study. Integr. Cancer Ther. 17, 411–422. 10.1177/1534735417722224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langendijk J. A., Doornaert P., Verdonck-De Leeuw I. M., Leemans C. R., Aaronson N. K., Slotman B. J. (2008). Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J. Clin. Oncol. 26, 3770–3776. 10.1200/JCO.2007.14.6647 [DOI] [PubMed] [Google Scholar]
- Li R., Zhao Y., Chen J., Shao S., Zhang X. (2014). Fisetin inhibits migration, invasion and epithelial-mesenchymal transition of LMP1-positive nasopharyngeal carcinoma cells. Mol. Med. Rep. 9, 413–418. 10.3892/mmr.2013.1836 [DOI] [PubMed] [Google Scholar]
- Li T. M., Yu Y. H., Tsai F. J., Cheng C. F., Wu Y. C., Ho T. J., et al. (2018). Characteristics of Chinese herbal medicine usage and its effect on survival of lung cancer patients in Taiwan. J. Ethnopharmacol. 213, 92–100. 10.1016/j.jep.2017.10.031 [DOI] [PubMed] [Google Scholar]
- Li X., Wang J. N., Huang J. M., Xiong X. K., Chen M. F., Ong C. N., et al. (2011). Chrysin promotes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced apoptosis in human cancer cell lines. Toxicol. Vitro 25, 630–635. 10.1016/j.tiv.2010.12.013 [DOI] [PubMed] [Google Scholar]
- Lin C. H., Chao L. K., Hung P. H., Chen Y. J. (2014). EGCG inhibits the growth and tumorigenicity of nasopharyngeal tumor-initiating cells through attenuation of STAT3 activation. Int. J. Clin. Exp. Pathol. 7, 2372–2381. [PMC free article] [PubMed] [Google Scholar]
- Lo P. C., Lin S. K., Lai J. N. (2020). Long-term use of Chinese herbal medicine therapy reduced the risk of asthma hospitalization in school-age children: a nationwide population-based cohort study in Taiwan. J. Tradit. Complement Med. 10, 141–149. 10.1016/j.jtcme.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Yang Y., Yin Z., Liu M., Wang L., Chen L., et al. (2017). Emodin suppresses the nasopharyngeal carcinoma cells by targeting the chloride channels. Biomed. Pharmacother. 90, 615–625. 10.1016/j.biopha.2017.03.088 [DOI] [PubMed] [Google Scholar]
- Ong C. S., Tran E., Nguyen T. T., Ong C. K., Lee S. K., Lee J. J., et al. (2004). Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol. Rep. 11, 727–733. 10.3892/or.11.3.727 [DOI] [PubMed] [Google Scholar]
- Pan C. H., Hsieh I. C., Liu F. C., Hsieh W. T., Sheu M. J., Koizumi A., et al. (2010). Effects of a Chinese herbal health formula, “Gan-Lu-Yin”, on angiogenesis. J. Agric. Food Chem. 58, 7685–7692. 10.1021/jf1002254 [DOI] [PubMed] [Google Scholar]
- Peng L., Huang Y. T., Chen J., Zhuang Y. X., Zhang F., Chen J. Y., et al. (2018). Osthole sensitizes with radiotherapy to suppress tumorigenesis of human nasopharyngeal carcinoma in vitro and in vivo . Cancer Manag. Res. 10, 5471–5477. 10.2147/CMAR.S182798 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Perez A. T., Arun B., Tripathy D., Tagliaferri M. A., Shaw H. S., Kimmick G. G., et al. (2010). A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res. Treat. 120, 111–118. 10.1007/s10549-009-0678-5 [DOI] [PubMed] [Google Scholar]
- Perri F., Bosso D., Buonerba C., Lorenzo G. D., Scarpati G. D. (2011). Locally advanced nasopharyngeal carcinoma: current and emerging treatment strategies. World J. Clin. Oncol. 2, 377–383. 10.5306/wjco.v2.i12.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri F., Della Vittoria Scarpati G., Caponigro F., Ionna F., Longo F., Buonopane S., et al. (2019). Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther. 12, 1583–1591. 10.2147/OTT.S188148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B., Ata A., Nanjangud V. A. K., Sharopov F., Ramirez-Alarcon K., Ruiz-Ortega A., et al. (2019a). Antidiabetic potential of medicinal plants and their active components. Biomolecules 9, 551. 10.3390/biom9100551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B., Upadhyay S., Erdogan Orhan I., Kumar Jugran A., S L. D. J., D A. D., et al. (2019b). Therapeutic potential of alpha- and beta-pinene: a miracle gift of nature. Biomolecules 9, 738. 10.3390/biom9110738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. C., Hung K. F., Liang K. L., Chiang J. H., Huang H. C., Lee H. J., et al. (2019). Adjunctive Chinese herbal medicine therapy for nasopharyngeal carcinoma: clinical evidence and experimental validation. Head Neck 41, 2860–2872. 10.1002/hed.25766 [DOI] [PubMed] [Google Scholar]
- Tsai F. J., Cheng C. F., Chen C. J., Lin C. Y., Wu Y. F., Li T. M., et al. (2019a). Effects of Chinese herbal medicine therapy on survival and hepatic outcomes in patients with hepatitis C virus infection in Taiwan. Phytomedicine 57, 30–38. 10.1016/j.phymed.2018.09.237 [DOI] [PubMed] [Google Scholar]
- Tsai F. J., Cheng C. F., Chen C. J., Lin C. Y., Wu Y. F., Li T. M., et al. (2019b). Effects of Chinese herbal medicine therapy on survival and hepatic outcomes in patients with hepatitis C virus infection in Taiwan. Phytomedicine 57, 30–38. 10.1016/j.phymed.2018.09.237 [DOI] [PubMed] [Google Scholar]
- Tsai F. J., Liu X., Chen C. J., Li T. M., Chiou J. S., Chuang P. H., et al. (2019c). Chinese herbal medicine therapy and the risk of overall mortality for patients with liver cancer who underwent surgical resection in Taiwan. Complement Ther. Med. 47, 102213. 10.1016/j.ctim.2019.102213 [DOI] [PubMed] [Google Scholar]
- Tsai F. J., Yang P. Y., Chen C. J., Li J. P., Li T. M., Chiou J. S., et al. (2020). Decreased overall mortality rate with Chinese herbal medicine usage in patients with decompensated liver cirrhosis in Taiwan. BMC Complement Med. Ther. 20, 221. 10.1186/s12906-020-03010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhou X., Wang Y., Wei D., Deng C., Xu X., et al. (2017). The antitumor constituents from Hedyotis diffusa Willd. Molecules 22, 2101. 10.3390/molecules22122101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. C., Fang C. Y., Cheng Y. J., Hsu H. Y., Chou S. P., Huang S. Y., et al. (2017). Inhibition of Epstein-Barr virus reactivation by the flavonoid apigenin. J. Biomed. Sci. 24, 2. 10.1186/s12929-016-0313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. C., Chen M. S., Cheng Y. J., Ko Y. C., Lin S. F., Chiu I. M., et al. (2019). Emodin inhibits EBV reactivation and represses NPC tumorigenesis. Cancers 11, 1795. 10.3390/cancers11111795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. H., Kang J. H., Park Y. B., Park Y. J., Oh H. S., Kim S. B. (2013). Association rule mining and network analysis in oriental medicine. PLoS One 8, e59241. 10.1371/journal.pone.0059241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Jia Y., Ji K. E., Sanders A. J., Xue K., Ji J., et al. (2015). Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol. Lett. 10, 1240–1250. 10.3892/ol.2015.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. R., Liang K. L., Huang T. P., Fan J. Y., Chang T. T., Sun M. F. (2015). Characteristics of traditional Chinese medicine use for children with allergic rhinitis: a nationwide population-based study. Int. J. Pediatr. Otorhinolaryngol. 79, 591–597. 10.1016/j.ijporl.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liang Y., He C. (2017). Anticancer activities and mechanisms of heat-clearing and detoxicating traditional Chinese herbal medicine. Chin. Med. 12, 20. 10.1186/s13020-017-0140-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Cao W. S., Wang X. Q., Zhang M., Lu X. M., Chen J. Q., et al. (2019). Genistein inhibits nasopharyngeal cancer stem cells through sonic hedgehog signaling. Phytother Res. 33, 2783–2791. 10.1002/ptr.6464 [DOI] [PubMed] [Google Scholar]
- Zhao W., Tang L., Gao S., Xin L., Zhang H., Li Y. (2019). Junduqing extractive promotes the apoptosis of nasopharyngeal carcinoma cells through down-regulating Mcl-1 and Bcl-xL and up-regulating Caspase-3, Caspase-8 and Caspase-9. Artif. Cells Nanomed. Biotechnol. 47, 3904–3912. 10.1080/21691401.2019.1667815 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Song X., Li R., Quan H., Yan L. (2019). Assessment of nasopharyngeal cancer in young patients aged </= 30 years. Front. Oncol. 9, 1179. 10.3389/fonc.2019.01179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.