Abstract
Beyond their historical role as the effector cells in allergic disorders, mast cells have been implicated in regulating both innate and adaptive immune responses. Possessing considerable functional plasticity, mast cells are abundant at mucosal surfaces, where the host and external environments interface. The purpose of this study was to evaluate the contribution of mast cells to allograft rejection at the ocular surface. Using a well-characterized murine model of corneal transplantation, we report that mast cells promote allosensitization. Our data show mast cell frequencies and activation are increased following transplantation. We demonstrate that mast cell inhibition (a) limits the infiltration of inflammatory cells and APC maturation at the graft site; (b) reduces allosensitization and the generation of Th1 cells in draining lymphoid tissues; (c) decreases graft infiltration of alloimmune-inflammatory cells; and (d) prolongs allograft survival. Our data demonstrate a novel function of mast cells in promoting allosensitization at the ocular surface.
Keywords: basic (laboratory) research/science, corneal transplantation/ophthalmology, rejection, sensitization
1 |. INTRODUCTION
The transplantation of tissues or organs from one individual to another is often the single viable treatment in end-stage organ dysfunction. Rapid development of new surgical techniques and immunosuppressives have both enhanced and prolonged the lives of millions of individuals over recent decades. Yet the major limitation of allogeneic transplantation, immune rejection, remains constant.1 The rejection of solid organ allografts involves a complex sequence of events orchestrated by both the innate and adaptive immune systems.2,3 The trauma and tissue injury that are associated with surgery provoke an initial innate response, with increased infiltration of inflammatory cells (including dendritic cells [DCs] and macrophages), and a concomitant release of pro-inflammatory cytokines. Antigen-presenting cells (APCs) migrate from the graft site to the recipient’s draining lymphoid organs, where they elicit an adaptive immune response by presenting donor antigens to naïve host T cells, resulting in the generation of graft-attacking type 1 T-helper (Th1) cells.4 Although much has been established regarding the cellular and molecular interactions that drive immune rejection, the induction of alloimmunity at the ocular surface has not been fully defined.
Mast cells are important effector cells of the immune system that are particularly abundant at mucosal surfaces.5 The critical role of mast cells in mediating pro-inflammatory responses to allergens and multicellular parasites is well recognized, but mast cells have been implicated in a much wider spectrum of inflammatory disorders including atherosclerosis, arthritis, and cancer.5–8 Recently, the capacity of mast cells to respond rapidly to environmental signals and modulate the adaptive immune response has attracted attention.5,9–12 Given the presence of mast cells at mucosal surfaces, and the array of grafts that involve these tissues (eg, cornea, lung, and intestine), it is remarkable that the contribution of mast cells to the induction of alloimmunity is not yet well established.
In this study, we conducted a series of experiments to determine how mast cells impact the generation of the alloimmune response. We investigated the function of mast cells using a well-characterized murine model of corneal transplantation.13–15 In this model of ocular alloimmunity, approximately half of the grafts are rejected within 3 weeks of transplantation, while the remaining half survive indefinitely. In comparison with other transplant models, this system permits the continuous evaluation of the alloimmune response (through the assessment of graft opacity) without necessitating host sacrifice.
Our data demonstrate that mast cell inhibition limits allosensitization in a murine model of transplantation. We clearly show that mast cell inhibition leads to decreased maturation of APCs and reduced generation of Th1 cells. Furthermore, mast cell inhibition attenuates graft infiltration of alloimmune-inflammatory cells, with an attendant delay in transplant rejection.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Six- to eight-week-old male wild type BALB/c and C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). The mice were housed in the Schepens Eye Research Institute animal vivarium and treated according to the guidelines set forth by the Association for Research in Vision and Ophthalmology. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee.
2.2 |. Orthotopic corneal transplantation
Orthotopic corneal transplantation was performed as previously described.16 Briefly, host beds were prepared by trephining a 1.5-mm site in the central cornea. In allogeneic transplantation, central 2.0-mm-diameter corneal grafts were excised by trephination from C57BL/6 mice and transplanted onto prepared BALB/c host beds with eight interrupted 11–0 nylon sutures (AB-0550S; MANI, Tochigi, Japan). In syngeneic transplantation, central 2.0-mm-diameter corneal grafts were excised by trephination from BALB/c mice and transplanted onto prepared BALB/c host beds with eight interrupted 11–0 nylon sutures. After surgery, tarsorrhaphy was performed to keep the eye closed for 3 days following transplantation. The tarsorrhaphy was positioned medially, thereby preventing spontaneous eye opening but permitting manual separation of the eyelids and the application of drops. Sutures were removed 7 days posttransplantation.
2.3 |. Evaluation of graft survival
Transplanted corneas were examined weekly in a blinded fashion for 6 weeks (or until sacrificed for analyses) using a slit-lamp microscope (n = 10 mice/group). A standardized opacity grading system was used.17 Grafts with opacity scores of >2 (ie, a level of opacity that obscures recognition of iris details) for at least two consecutive weeks at postoperative week 2 and onward were considered as immune-rejected. Eyes that underwent complications during or after surgery including intraoperative hemorrhage, cataract, infection, or synechia as well as grafts that became opaque in the first 2 weeks after transplantation (and never became clear) were excluded from the analysis.
2.4 |. Mast cell inhibitor administration
Three microliter of 2% cromolyn sodium (Sigma-Aldrich, St. Louis, MO) eye drops were administered topically to transplanted eyes at five time points on the day of transplantation (which were −3, −1, 0, 1, and 3 hours postoperatively). Furthermore, eye drops were administered 3×/day for up to 3 days postoperatively. Vehicle-treated mice were administered phosphate-buffered saline (PBS) eye drops.
2.5 |. Digestion of ocular surface tissue and lymph node cell preparation
Single cell suspensions were prepared from ocular surface tissues (corneas and conjunctivae) and ipsilateral draining submandibular lymph nodes as previously described.18 In brief, ocular surface tissues were digested in RPMI media (Lonza, Walkersville, MD) containing 4 mg/mL collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 2 mg/mL DNase I (Roche, Basel, Switzerland) for 60 min at 37°C, and then filtered through a 70-μm cell strainer. Draining lymph nodes (DLNs) in the cervical region were collected and single cell suspensions were prepared as previously described.19
2.6 |. Flow cytometry
Single cell suspensions were surface stained with fluorochrome-conjugated anti c-Kit (2B8), anti- FcεR1 (MAR-1), anti-CD11b (M1/70), anti-CD11c (N418), anti-MHCII (M5/114.15.2), anti-IAd (39–10-8), anti-IAb (KH74), anti-CD45 (30-F11), anti-CD3 (145–2C11), anti-CD4 (RM4–4) antibodies to evaluate c-Kit+ FcεR1+mast cells, CD11b+ myeloid immune cells, CD11c+ APCs, CD45+ total inflammatory cells, and CD3+CD4+T cells. Surface staining was performed by incubating cells with monoclonal antibodies or appropriate isotype controls for 30 minutes on ice in the dark. Thereafter, cells were washed twice using phosphate-buffered saline (PBS). Stained cells were subsequently suspended in PBS and analyzed using a LSR II flow cytometer (BD Biosciences, San Jose, CA) and Summit v4.3 software (Beckman Coulter, Indianapolis, IN) as described previously.19,20 Doublet discrimination was performed by gating concurrently on FSCarea versus FSCheight. Antibodies and matched isotypes were purchased from Biolegend, San Diego, CA.
For intracellular evaluation of IFN-γ in Th1 cells, lymph node single cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 20 ng/ml; Sigma-Aldrich) and ionomycin (1μg/mL; Sigma-Aldrich) for 4 hours in the presence of Golgistop (0.1 μL/100 μL media; BD Bioscience) at 37°C, as described previously.21 Intracellular staining was performed in order to evaluate the expression of IFN-γ in Th1 cells, Foxp3 in CD4+CD25+ cells (Tregs), and TNF-α in mast cells. Cells were washed twice with PBS following cell surface staining and resuspended in Fixation/Permeabilization Solution (eBioscience) for 60 minutes at 4°C. Fixed and permeabilized cells were then washed with Perm/Wash Buffer (eBioscience) and stained with fluorochrome conjugated anti-IFN-γ (XMG1.2), anti-Foxp3 (FJK-16 s, eBioscience), or anti-TNF-α (MP6-XT22) antibodies for 45 minutes on ice. Rat IgG2b, κ, Rat IgG2a k, Rat IgG1, κ, and Armenian Hamster IgG isotype controls were used. Antibodies and matched isotypes and were purchased from Biolegend, San Diego, CA, or as indicated. Cells were washed and suspended in PBS for flow cytometry analysis using a LSR II flow cytometer (BD Biosciences) and Summit v4.3 software (Beckman Coulter Life Sciences, Indianapolis, IN), as previously described.20,22
2.7 |. Cell sorting
T cells (CD90.2+ cells) and APCs (CD90.2− cells) from the lymph nodes of graft recipients, donors, and naive mice were isolated by magnetic sorting using CD90.2+ sorting kits as per the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA).
2.8 |. Direct and indirect allosensitization assays
Purified T cells (CD90.2+ magnetically-sorted) from allografted BALB/c mice (2 weeks following transplantation of C57BL/6 corneas) were incubated with donor C57BL/6 APCs (CD90.2−magnetically sorted splenocytes) for 48 hours in order to quantify frequencies of directly allosensitized T cells, or with syngeneic BALB/c APCs pulsed with sonicated donor antigen (2×107 C57BL/6 APCs/mL) to enumerate frequencies of indirectly allosensitized T cells. Total numbers of IFN-γ-secreting T cells were quantified using 96 well plates (Whatman Polyfiltronics, Rockland, MA) coated with 4 μg/mL primary anti-IFN-γ antibody (AN-18; BD Pharmingen, San Jose, CA) by ELISPOT as previously described.23,24 Frequencies of allosensitized Th1 cells (CD4+IFN-γ+) were evaluated by flow cytometry as described above.
2.9 |. β-hexosaminidase assay
The levels of β-hexosaminidase enzyme were estimated using a β-n-acetylglucosaminidase assay kit (Sigma-Aldrich), which is based on the hydrolysis of 4-Nitrophenyl N-acetyl-β-D-glucosaminide (NP-GlcNAc).25 Briefly, to evaluate mast cell activation, ocular surface tissues (cornea and conjunctiva) were lysed using 0.1% Triton X-100 (Sigma-Aldrich) for 20 minutes on ice. After centrifugation, supernatants were harvested and incubated with 0.1 mg/mL NP-GlcNAc (substrate) for 30 minutes at 37°C. Following this, the enzyme-substrate reaction was stopped with 5 mg/mL sodium carbonate. Absorbance was measured at 405 nm using a SpectraMax Plus 384 Microplate Reader (Molecular Devices, CA, USA). β-hexosaminidase levels were evaluated using the formula: Units/mL = (A405sample − A405blank) × 0.05 × 0.3 × DF/A405standard × time × volume of sample in mL.
2.10 |. Statistical analysis
Unpaired two-tailed Student’s t tests were used to compare means between two groups, and one-way ANOVA was used for the comparison of multiple groups. The significance level was set at P < .05. Kaplan-Meier analysis was adopted to construct survival curves, and the log-rank test was used to compare the rates of corneal graft survival. Results are presented as the mean ± standard error of mean of three independent experiments. In vivo evaluations of graft opacity were performed in a masked fashion. Samples sizes were estimated on the basis of previous experimental studies on corneal transplantation.16,23,26
3 |. RESULTS
3.1 |. Mast cell frequencies and activation increase following transplantation
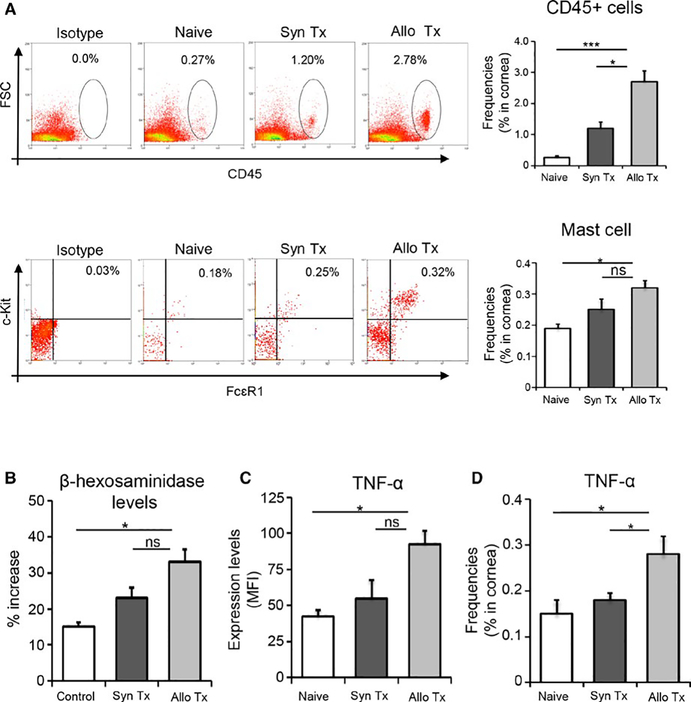
To investigate whether mast cell activation occurs during transplantation, we harvested corneal and conjunctival tissues at 6 hours posttransplantation, and stained single cell suspensions with fluorochrome-conjugated CD45, c-Kit, and FCεR1 monoclonal antibodies. Flow cytometry data were gated on CD45+ cells, and the frequencies of c-Kit+FCεR1+ mast cells were evaluated (Figure 1A). These data demonstrate a 68% increase in the frequencies of mast cells in the ocular surface tissues of allograft recipients relative to naïve mice (P < .006). Mast cell activation was evaluated by two methods: levels of β-hexosaminidase and expression of TNFα. β-hexosaminidase is highly abundant in mast cell granules, and is widely used as a marker of mast cell activation.27 Corneal and conjunctival cell lysates analyzed by β-hexosaminidase assay demonstrated a significantly greater increase in β-hexosaminidase levels in graft recipients compared to naïve controls (P < .01; Figure 1B). Ocular surface mast cells from transplant recipients exhibited a two-fold increase in TNFα expression relative to naïve mice (P < .004; Figure 1C), with higher frequencies of TNFα-expressing cells detected in the cornea (P < .05; Figure 1D). These results suggest that the frequency and activation of mast cells are upregulated following transplantation.
FIGURE 1.
Mast cell frequencies and activation increase following transplantation. Ocular surface mucosal tissues (corneas and conjunctivae) were harvested at 6 hours posttransplantation. (A) Single cell suspensions were analyzed by flow cytometry. Upper left panel: representative dot plots showing the frequencies of CD45+ cells in ocular surface tissues harvested from naïve mice, and syngeneic (Syn Tx) and allogeneic (Allo Tx) transplant recipients. Upper right panel: bar chart demonstrating the cumulative frequency data of ocular surface CD45+ cells in transplant recipients and naïve mice (***P < .001, t test). Lower left panel: representative dot plots showing the frequencies of CD45+c-Kit+FCεR1+ mast cells at ocular surface tissues (data shown are gated on CD45+ cells). Lower right panel: bar chart demonstrating the cumulative data of frequencies of ocular surface c-Kit+FCεR1+ mast cells in transplant recipients and naïve mice (*P < .006, t test). (B) Bar chart depicting the percentage increase in β-hexosaminidase expression in the control group (nontransplanted contralateral eyes), syngeneic graft recipients, and allogeneic graft recipients (*P < .005, t test). (C) Bar chart showing protein expression (mean fluorescence intensity, MFI) of TNF-α by ocular surface mast cells from allogeneic hosts, syngeneic hosts, and naïve mice (*P < .0045, t test). (D) Bar chart showing frequencies of TNF-α-expressing cells in the corneas of allogeneic hosts, syngeneic hosts, and naïve mice (*P < .05, t test). Representative data from four independent experiments are shown and each experiment consisted of five animals. Data are represented as mean ± SEM (error bar). One-way ANOVA was used to compare multiple groups (P < .05). ns = not significant
3.2 |. Topical administration of mast cell inhibitor reduces mast cell frequencies and activation at the ocular surface following transplantation
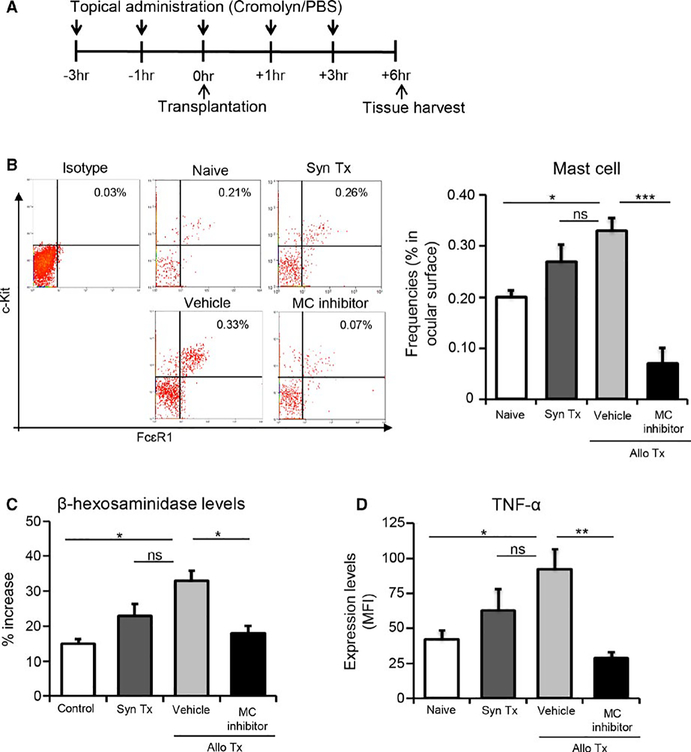
Next, we evaluated the efficacy of a clinically relevant pharmacological inhibitor of mast cells (cromolyn sodium) in suppressing mast cell function in our in vivo model of corneal transplantation.28 To determine this, we treated mice topically with mast cell inhibitor in the perioperative period and examined the frequencies and activation of mast cells. Two allogeneic transplant treatment groups were assessed in the experiment—one group was treated with mast cell inhibitor eye drops, and the other with vehicle eye drops (phosphate-buffered saline [PBS]). Eye drops were administered at 3 and 1 hour prior to transplantation, at the time of transplantation, as well as at 1 and 3 hours following transplantation (Figure 2A). Corneas and conjunctivae were harvested at 6 hours following surgery. Our flow cytometry analysis of single cell suspensions revealed that corneal transplantation increased the frequencies of mast cells in the ocular surface tissues, yet treatment with topical mast cell inhibitor substantially reduced (79%) the frequencies (P < .001; Figure 2B). Mast cell activation was also markedly inhibited, as demonstrated by a significant reduction in both the levels of β-hexosaminidase (P < .05) and expression of TNFα (P < .01) in the mast cell inhibitor-treated group relative to the vehicle-treated group (Figure 2C,D).
FIGURE 2.
Topical administration of mast cell inhibitor reduces mast cell frequencies and activation at the ocular surface following corneal transplantation. (A) Schematic of experimental design showing the time points of eye drop administration relative to corneal transplantation and tissue harvesting. (B) Representative flow cytometric dot plots (left), and bar chart of cumulative data (right), showing frequencies of c-Kit+FCεR1+ mast cells at the ocular surface in naïve mice, syngeneic graft recipients (Syn Tx), and vehicle-treated and mast cell inhibitor-treated allotransplant recipients (data shown are gated on CD45+ cells). (C) Quantitation of percentage increase in β-hexosaminidase levels at ocular surface of indicated groups compared to control (nontransplanted contralateral eyes). (D) Bar chart showing protein expression (MFI) of TNF-α by ocular surface mast cells in naïve, syngeneic, as well as vehicle-treated and mast cell inhibitor-treated allogeneic groups. Representative data from four independent experiments are shown and each experiment consisted of 4–6 animals. Data are represented as mean ± SEM (error bar). t test, *P < .05, **P < .01, ***P < .001, ns = not significant. In addition, means among multiple groups were compared using one-way ANOVA (P < .05)
3.3 |. Mast cell inhibition reduces leukocyte infiltration and suppresses the maturation of antigen-presenting cells in graft
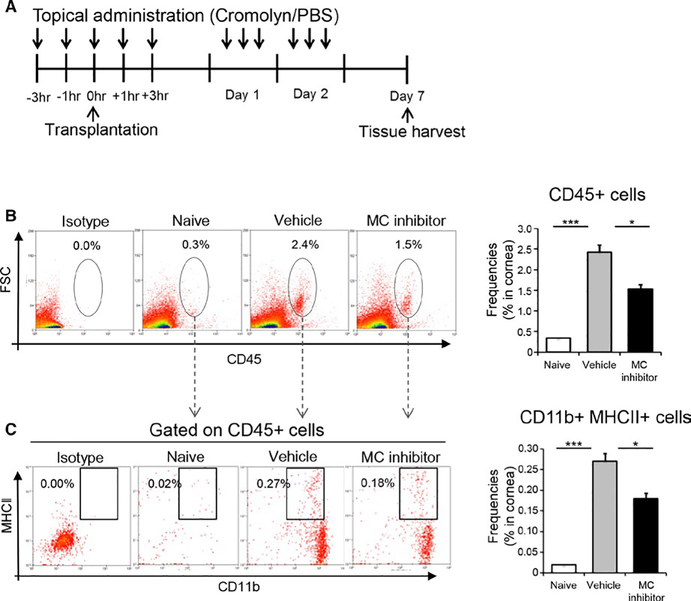
To delineate whether mast cell activation contributes to the induction of the allogeneic immune response, we administered topical mast cell inhibitor to mice perioperatively (Figure 3A), and evaluated leukocyte infiltration and the maturation of APCs resident in the graft following transplantation. Mice treated with vehicle eye drops (PBS) served as the control group. Corneas were harvested 7 days following transplantation. Frequencies of CD45+ leukocytes and CD11b+MHCII+mature APCs were assessed by flow cytometry. Our data demonstrate that CD45+ leukocyte infiltration of the cornea was substantially increased following transplantation in the vehicle-treated group, but this effect was reduced in the mast cell inhibitor-treated group (Figure 3B). Indeed, treatment with mast cell inhibitor resulted in a significant 38% decrease in corneal infiltration of CD45+ leukocytes (P < .05). Our data show that the frequencies of MHCII+CD11b+ mature APCs were markedly increased following transplantation in the vehicle-treated group, but treatment with mast cell inhibitor reduced this effect (P < .05) (Figure 3C). Additionally, to evaluate whether mast cell inhibitor (2% cromolyn) directly affects the maturation of APCs, we investigated the effect of cromolyn sodium on IFNγ-induced MHCII acquisition by bone marrow-derived immature DCs (BMDCs) using in vitro assays. Our data demonstrate that IFNγ significantly upregulates MHCII expression by BMDCs (MFI: 977 ± 39) relative to unstimulated BMDCs (MFI: 107 ± 57). The addition of cromolyn sodium to IFNγ-treated cultures did not significantly alter IFNγ-induced MHCII acquisition by BMDCs (MFI: 900 ± 86) (Figure S1). These findings suggest that, without directly affecting APCs, inhibition of mast cell function results in decreased CD45+ leukocytic infiltration and APC maturation in the allograft.
FIGURE 3.
Mast cell inhibition reduces leukocytic infiltration and suppresses the maturation of antigen-presenting cells in graft. (A) Schematic of experiment design showing the time points of eye drop administration in allotransplant recipients. After 7 days, corneas were harvested to evaluate the frequency of CD45+ leukocytes and mature APCs using flow cytometry. (B) Representative flow cytometric dot plots (left) and cumulative bar chart (right) showing total frequencies of CD45+ inflammatory cells in the corneas of naïve, vehicle-treated, and mast cell inhibitor-treated groups. (C) Representative flow cytometric dot plots (left) and cumulative bar chart (right) showing frequencies of CD11b+MHCII+mature APCs in the corneas of naïve, vehicle-treated, and mast cell inhibitor-treated groups. Representative data from three independent experiments are shown and each experiment consisted of six animals. Data are represented as mean ± SEM (error bar) (t test, *P < .01, ***P < .001)
3.4 |. Inhibition of mast cells limits activation of host- and donor-derived antigen-presenting cells and the generation of Th1 cells in lymphoid tissues
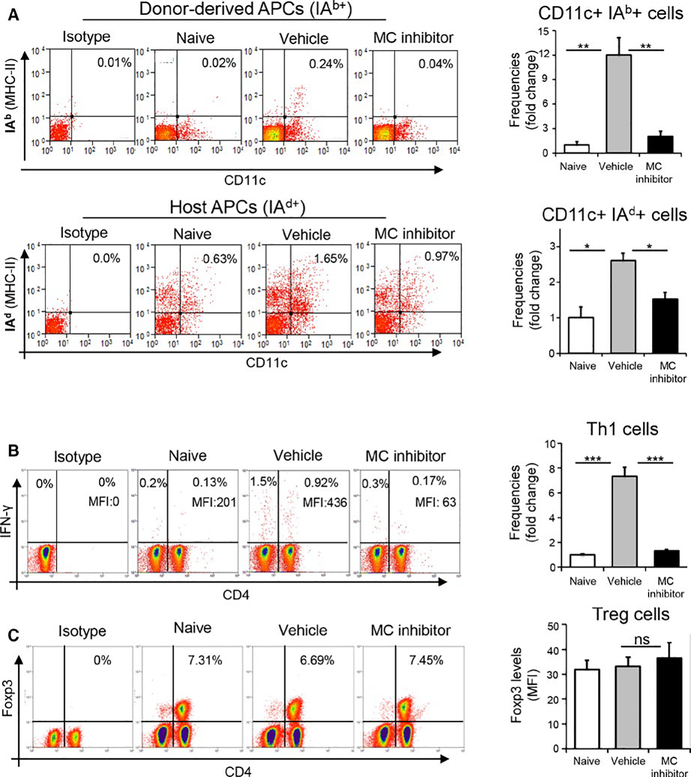
To further investigate the extent to which mast cells contribute toward alloimmunity, we evaluated the effects of mast cell blockade on the maturation of APCs and generation of IFNγ-producing CD4+ T cells (Th1 cells) in the draining lymph nodes (DLNs). Topical administration of mast cell inhibitor or vehicle eye drops followed the same treatment regimen as described previously (Figure 3A). DLNs were harvested at 14 days following transplantation. As both host-and donor-derived APCs contribute to the generation of Th1 cells,29 we enumerated the frequencies of IAb+ (donor-derived) and IAd+ (recipient) MHC II haplotypes by APCs derived from mast cell inhibitor-treated vs vehicle-treated allogeneic graft recipients (Figure 4A). Given the exclusive expression IAb+and IAd+MHC II haplotypes by donor (B6) and recipient APCs (BALB/c) respectively, our data demonstrate that the frequencies of both IAb+CD11c+ cells (donor APCs) and IAd+CD11c+ cells (host APCs) were substantially increased following transplantation in the vehicle-treated group, but this effect was abrogated in the mast cell inhibitor-treated group (Figure 4A). Specifically, mast cell inhibition decreased the frequencies of host-derived mature APCs by almost 50%, with a complete reduction in frequencies of donor-derived mature APCs relative to controls (P < .01).
FIGURE 4.
Inhibition of mast cells limits APC maturation and Th1 generation in lymphoid tissues. (A) Representative flow cytometric dot plots (left) and cumulative bar chart (right) of fold change in the frequencies of donor-derived MHCII IAb+ (upper panel) and host-derived MHCII IAd+ (lower panel) APCs in the draining lymph nodes of naïve mice, and vehicle-treated and mast cell inhibitor-treated allograft recipients at 2 weeks posttransplantation. (B) Representative flow cytometric dot plots (left) and cumulative bar chart of fold change in the frequencies of IFNγ+CD4+ Th1 cells in the draining lymph nodes (right) of naïve, vehicle-treated, and mast cell inhibitor-treated groups at 2 weeks posttransplantation. (C) Representative flow cytometric dot plots (left) and cumulative bar chart showing protein expression (MFI) of Foxp3 by Tregs (right) in the draining lymph nodes of naïve, vehicle-treated, and mast cell inhibitor-treated groups at 2 weeks posttransplantation. Representative data from three independent experiments are shown and each experiment consisted of five animals. Data are represented as mean ± SEM (error bar). t test, *P < .05, **P < .01, ***P < .001, ns = not significant
Our data show that both the frequencies of CD4+IFNγ+ Th1 cells and expression levels of IFNγ (MFI) in the DLNs of the vehicle-treated group increased following transplantation relative to the naïve group (Figure 4B). Treatment with mast cell inhibitor resulted in a substantial decrease in both the frequencies of Th1 cells (82%) and protein expression (85%) of IFNγ compared to controls (P < .001; Figure 4B). Given the critical role of CD4+Foxp3+ regulatory T cells (Tregs) in suppressing the Th1 immune response, we evaluated the impact of mast cell inhibition on Treg frequencies following transplantation.30 Interestingly, mast cell inhibition did not significantly alter either the frequency of CD4+Foxp3+ Tregs or expression of Foxp3 (MFI) by Tregs between the groups (Figure 4C). These findings strongly suggest that mast cells promote allosensitization without affecting Tregs.
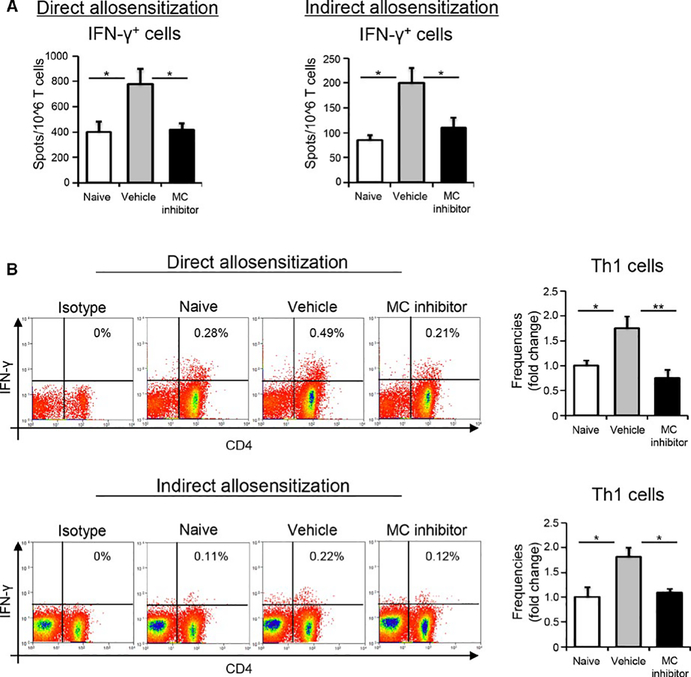
3.5 |. Inhibition of mast cell function suppresses both direct and indirect allosensitization
Having observed decreased generation of the Th1 immune response following mast cell inhibition, as well as reduced frequencies of both host- and donor-derived APCs, we subsequently assessed whether blockade of mast cell activity limits donor APC-mediated direct allosensitization or host APC-induced indirect allosensitization. We investigated both: (a) direct allosensitization by co-culturing donor APCs (B6) with recipient T cells (BALB/c), and (b) indirect allosensitization by co-culturing recipient APCs (BALB/c) (pulsed with the lysate of donor splenocytes) with recipient T cells (BALB/c). In the direct allosensitization assays, mast cell inhibition resulted in significantly decreased frequencies of CD4+IFNγ+ Th1 cells (≈50%) compared to vehicle control (P < .05; Figure 5A left panel, P < .01; 5B top panel). Similarly, in the indirect allosensitization assays, significantly decreased frequencies of CD4+IFNγ+ Th1 cells were observed in the mast cell inhibitor-treated group relative to the vehicle-treated group (P < .05; Figure 5A right panel & 5B lower panel). These results imply that mast cell inhibition suppresses Th1 immune responses by inhibiting both the direct and indirect pathways of allosensitization.
FIGURE 5.
Inhibition of mast cell function suppresses both direct and indirect allosensitization. In vitro studies were conducted to further evaluate the effect of mast cell inhibition on direct versus indirect allosensitization. Specifically, direct allosensitization was assessed by co-culturing donor APCs (B6) with recipient T cells (BALB/c) purified from either vehicle- or mast cell inhibitor-treated groups. Indirect allosensitization was evaluated by co-culturing recipient APCs (BALB/c) (pulsed with the lysate of donor splenocytes) with recipient T cells (BALB/c) from the indicated groups. (A) Bar chart quantitating the number of IFNγ+ cells using ELISPOT assay. (B) Representative flow cytometry dot plots (left) and cumulative bar charts (right) showing the frequencies of Th1 cells (gated on CD3+ cells) in vehicle- vs. mast cell inhibitor-treated groups. t test, *P < .05, **P < .01
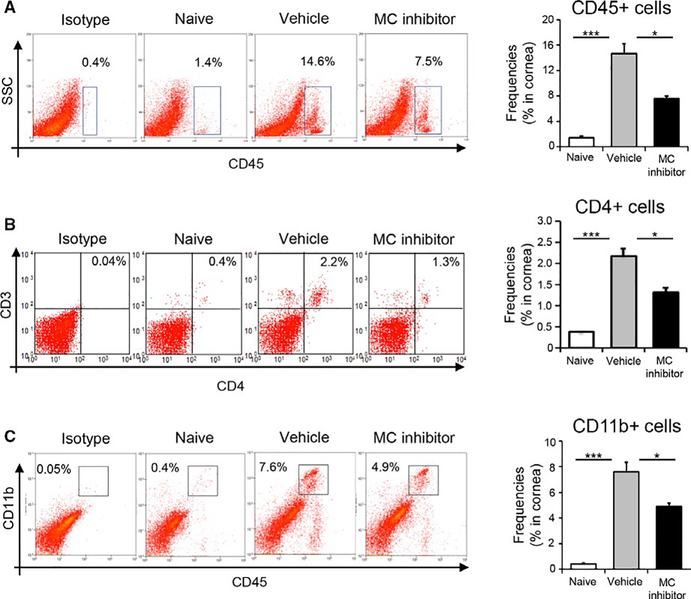
3.6 |. Inhibition of mast cell function attenuates infiltration of alloimmune-inflammatory cells into the graft
To examine whether mast cell-induced allosensitization leads to an increased infiltration of inflammatory cells into the graft, we evaluated the infiltration of CD45+ inflammatory cells, CD4+ T cells, and CD11b+ myeloid immune cells. Topical administration of mast cell inhibitor or vehicle eye drops followed the same treatment regimen as described previously (Figure 3A). Corneas were harvested 14 days following transplantation. Our flow cytometric data demonstrate that treatment with mast cell inhibitor reduced the graft infiltration of total CD45+ inflammatory cells by 49% relative to controls (P < .01; Figure 6A). Treatment with mast cell inhibitor also resulted in decreased infiltration of CD4+ T cells immune cells (P < .01; Figure 6B) and CD11b+ myeloid immune cells (P < .01; Figure 6C) by 41% and 36%, respectively relative to controls. Taken together, our results indicate that mast cell inhibition suppresses the infiltration of graft-damaging T cells and reduces tissue inflammation at the graft site.
FIGURE 6.
Inhibition of mast cell function reduces the infiltration of alloimmune-inflammatory cells into the graft. (A) Representative flow cytometric dot plots (left) and cumulative bar chart (right) showing frequencies of CD45+ inflammatory cells in the corneas of naïve, vehicle-treated, and mast cell inhibitor-treated groups at 2 weeks posttransplantation. (B) Representative flow cytometric dot plots (left) and cumulative bar chart (right) showing frequencies of CD4+ T cells in the corneas of naïve, vehicle-treated, and mast cell inhibitor-treated allograft recipients at 2 weeks posttransplantation. (C) Representative flow cytometric dot plots (left) and cumulative bar chart (right) showing frequencies of CD11b+ myeloid immune cells in the corneas of naïve, vehicle-treated, and mast cell inhibitor-treated groups at 2 weeks posttransplantation. Representative data from three independent experiments are shown and each experiment consisted of five animals. Data are represented as mean ± SEM (error bar). t test, *P < .05, ***P < .001
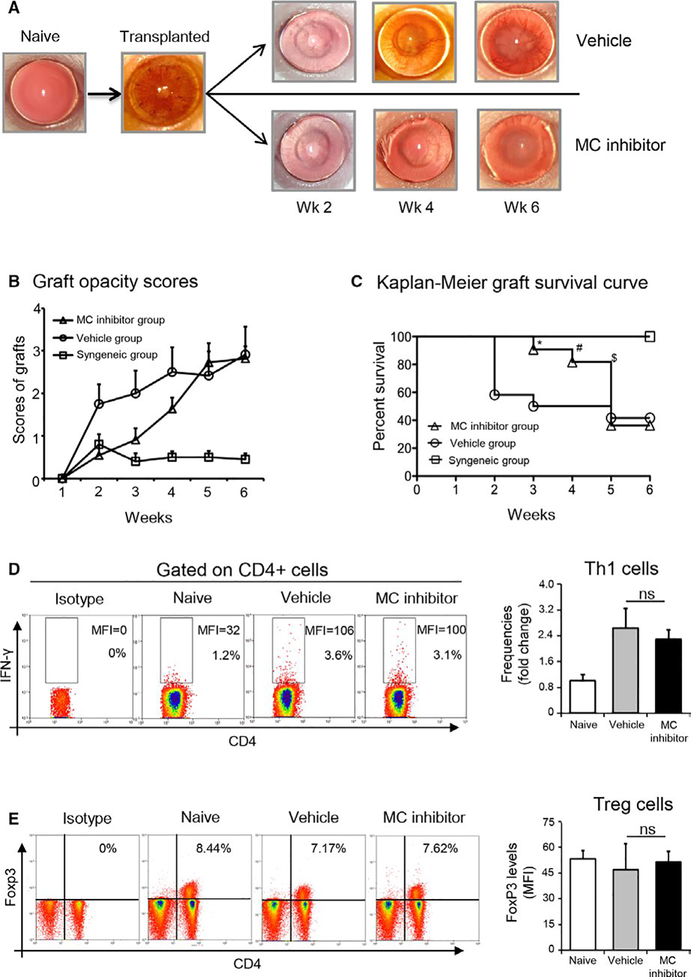
3.7 |. Inhibition of mast cells prolongs corneal allograft survival
To assess whether mast cell inhibition influences corneal allograft rejection, we evaluated graft survival in three treatment groups (n = 10 mice/group): (a) topical administration of mast cell inhibitor to allogeneic graft recipients according to treatment regimen described previously (Figure 3A); (b) topical administration of vehicle to allogeneic graft recipients according to treatment regimen described previously (Figure 3A); (c) untreated syngeneic graft recipients. Grafted eyes were examined once weekly using slit lamp microscopy for a follow-up period of 6 weeks to monitor graft survival. The mast cell inhibitor-treated allogeneic transplantation group demonstrated lower graft opacity scores compared to the vehicle-treated allogeneic transplantation group between postoperative weeks 1 and 4. After postoperative week 5, graft opacity scores were comparable between mast cell inhibitor-treated and vehicle-treated groups (Figure 7A,B). Treatment with mast cell inhibitor in the allogeneic transplantation group resulted in significantly prolonged graft survival relative to controls (Figure 7C). Having established that mast cells contribute to the induction of allosensitization at weeks 1 and 2, we investigated whether mast cells continued to promote alloimmunity at later time points. Our data show that at 6 weeks, there was no difference in either the frequencies of CD4+IFNγ+ Th1 cells or CD4+Foxp3+ Tregs in the DLNs of mast cell inhibitor-treated allogeneic graft recipients relative to vehicle-treated controls (Figure 7D,E). Furthermore, no difference in the expression (MFI) of IFNγ or Foxp3 was observed between the groups. Taken together, these results suggest that mast cell inhibition delays allosensitization and prolongs graft survival following corneal transplantation.
FIGURE 7.
Inhibition of mast cells prolongs corneal allograft survival. (A) Representative slit lamp biomicroscope photographs of a normal murine cornea, corneal allograft on the day of transplant surgery, and at 2, 4, and 6 weeks postoperative surgery in vehicle-treated allogeneic group and the mast cell inhibitor-treated allogeneic group. (B) Line diagram showing corneal graft opacity scores of the vehicle-treated allogeneic group and the mast cell inhibitor-treated allogeneic group. (C) Kaplan-Meier graft survival curves for the syngeneic group, the vehicle-treated allogeneic group and the mast cell inhibitor-treated allogeneic group (n = 10 in each group; log-rank test; *P < .015, **P < .03, ***P < .05). (D) Representative flow cytometric dot plots showing frequencies and expression levels (MFI) of IFNγ in CD4+ T cells (left panel). Cumulative bar chart showing fold change in the frequencies of IFNγ+CD4+ Th1 cells in the draining lymph nodes of naïve, vehicle-treated, and mast cell inhibitor-treated groups at 6 weeks posttransplantation (right panel). (E) Representative flow cytometric dot plots showing frequencies of CD4+Foxp3+ Tregs (left panel). Bar chart showing protein expression (MFI) of Foxp3 by Tregs in the draining lymph nodes of naïve, vehicle-treated, and mast cell inhibitor-treated groups at 6 weeks posttransplantation (right panel). Representative data from two independent experiments are shown and each experiment consisted of 10 animals. Data are represented as mean ± SEM (error bar). t test, ns =not significant
4 |. DISCUSSION
This study advances our knowledge of the contribution of mast cells to allosensitization at the ocular surface. Our data demonstrate that the frequencies and activation of mast cells are upregulated following corneal transplantation. Furthermore, we show that mast cell inhibition: (a) limits the infiltration of inflammatory cells and APC maturation at the graft site; (b) suppresses both direct and indirect allosensitization in draining lymphoid tissues; (c) reduces graft infiltration of alloimmune-inflammatory cells; and (d) prolongs allograft survival.
Beyond their well-characterized role in allergic inflammation, recent evidence has implicated mast cells in a wide array of protective and pathological roles.5 Mast cells are now recognized as important mediators of innate immunity, triggering the first and fastest immune response, with the rapid release of presynthesized cytokines (eg, tumor necrosis factor [TNF]-α), and proteases that drive inflammation.31 As an example of the critical role played by mast cells in generating the innate immune response, mast cells have been shown to contribute to the recruitment of neutrophils to sites of bacterial infection.32 The capacity of mast cells to modulate adaptive immunity has also been established.10 Given the diverse functional contributions of mast cells to innate and adaptive immunity, it is unsurprising that these cells are relevant to tissue transplantation.31 Previous studies have identified anti-inflammatory functions of mast cells in murine models of transplantation, yet are characterized by a lack of consensus, with some investigators reporting that mast cells reduce effector T cell proliferation via an IL-10-mediated mechanism33 and others detailing the critical contribution of mast cells toward CD4+CD25+Foxp3+ Treg cell-dependent peripheral tolerance.34 Our study advances the current knowledge by demonstrating the role of mast cells in promoting allosensitization at the ocular surface.
Increased mast cell frequencies in mucosal tissues have been reported in a number of inflammatory disorders, including asthma, and inflammatory bowel disease.35,36 In addition to previous studies documenting the distribution of mast cells at the ocular surface37,38 (and our own immunofluorescence imaging shown in Figure S2), a study by Yamagami et al has reported mast cell infiltration into corneal allografts.39 Our study is consistent with these observations, demonstrating significantly higher frequencies of mast cells in the ocular surface tissues of transplanted animals relative to naïve controls. Furthermore, we demonstrate enhanced mast cell activation in grafted mice compared to naïve controls; with heightened expression of TNF-α exhibited, and a greater fold change in β-hexosaminidase levels. TNF-α is an important marker of mast cell activation: it exerts significant effects on both the innate and adaptive immune systems, and functions in an autocrine manner to maintain the survival and activation of mast cells.9,40,41 The β-hexosaminidase assay has emerged as a sensitive assay for evaluating mast cell activation.42
APC maturation at the graft site is critical for productive presentation of transplant antigens to host T cells.43 However, the factors that regulate APC maturation following transplantation have not been fully delineated. Our study demonstrates that, in addition to immature APCs, the grafted site contains mast cells. We show that mast cells promote APC maturation and the initiation of allosensitization, leading to the generation of Th1 cells in the draining lymph nodes. Indeed, we observed elevated secretion of TNF-α by mast cells following transplantation, which has been shown to foster APC maturation and promote APC-CD4+ T-helper cell interactions.44–46 We propose that treatment with mast cell inhibitor altered the local inflammatory milieu and dampened allosensitization, which was observed as a decrease in mature APCs and Th1 effector cells in the lymphoid tissues. Since graft survival is determined by the balance between the effector and regulatory arms of the alloimmune response, we investigated the effect of mast cell inhibition on Tregs.16 Importantly, our data demonstrate no difference in either Treg frequencies or protein expression of Foxp3 at week 2. This observation suggests that mast cells alter the allogeneic immune response predominately via the effector, rather than regulatory, arm.
The consequences of diminished allosensitization were observed at the graft site, with decreased infiltration of inflammatory cells and prolonged graft survival. Although mast cell inhibition resulted in decreased graft opacity from postoperative week 1 until week 4, no difference was seen in opacity (or graft survival) after this time. This finding can be explained by our analysis of immune cells in the draining lymph nodes at 6 weeks posttransplantation, with no difference observed in either the frequencies of Th1 effector cells or protein expression of IFNγ+ in the mast cell inhibitor-treated group compared to control. Furthermore, no difference was seen in Treg frequencies or protein expression of Foxp3, which is critical for long-term allotolerance. Taken together, these data suggest that mast cell inhibition modulates the initial stage of allosensitization, with decreased inflammation and prolonged graft survival observed, but does not impact allosensitization at later time points.
In sum, our findings clearly demonstrate the novel contribution of mast cells to allosensitization at the ocular surface. Furthermore, our data suggest that mast cell inhibition has translational potential as a therapeutic strategy to prolong corneal allograft survival.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (EY024602 to S.K.C. and core grant P30EY003790).
Funding information
National Eye Institute, Grant/Award
Number: EY024602; National Institutes of Health, Grant/Award Number: EY024602 and P30EY003790
Abbreviations:
- APCs
antigen-presenting cells
- BMDCs
bone marrow-derived immature dendritic cells
- DCs
dendritic cells
- DLNs
draining lymph nodes
- NP-GlcNAc
4-Nitrophenyl N-acetyl-β-D-glucosaminide
- PBS
phosphate-buffered saline
- Th1
type 1 T-helper
- TNF
tumor necrosis factor
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–2729. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SP, Porrett PM, Turka LA. Innate immunity in transplant tolerance and rejection. Immunol Rev. 2011;241(1):39–48. [DOI] [PubMed] [Google Scholar]
- 3.Magil AB. Monocytes/macrophages in renal allograft rejection. Transplant Rev (Orlando). 2009;23(4):199–208. [DOI] [PubMed] [Google Scholar]
- 4.Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. J Immunol. 2016;196(10):3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voehringer D Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13(5):362–375. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13(6):719–724. [DOI] [PubMed] [Google Scholar]
- 7.Hueber AJ, Asquith DL, Miller AM, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184(7):3336–3340. [DOI] [PubMed] [Google Scholar]
- 8.Khazaie K, Blatner NR, Khan MW, et al. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30(1):45–60. [DOI] [PubMed] [Google Scholar]
- 9.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams C, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23(1):749–786. [DOI] [PubMed] [Google Scholar]
- 10.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–142. [DOI] [PubMed] [Google Scholar]
- 11.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol. 2011;31(6):475–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Féger F, Varadaradjalou S, Gao Z, Abraham SN, Arock M. The role of mast cells in host defense and their subversion by bacterial pathogens. Trends Immunol. 2002;23(3):151–158. [DOI] [PubMed] [Google Scholar]
- 13.Inomata T, Mashaghi A, Di Zazzo A, Dana R. Ocular surgical models for immune and angiogenic responses. J Biol methods. 2015;2(3):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahvildari M, Omoto M, Chen Y, et al. In vivo expansion of regulatory T cells by low-dose interleukin-2 treatment increases allograft survival in corneal transplantation. Transplantation. 2016;100(3):525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiZazzo A, Tahvildari M, Subbarayal B, et al. Proangiogenic function of T cells in corneal transplantation. Transplantation. 2017;101(4):778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice–evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54(4):694–704. [DOI] [PubMed] [Google Scholar]
- 18.Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J Immunol. 2007;179(6):3672–3679. [DOI] [PubMed] [Google Scholar]
- 19.Dohlman TH, Ding J, Dana R, Chauhan SK. T cell-derived granulocyte-macrophage colony-stimulating factor contributes to dry eye disease pathogenesis by promoting CD11b+ myeloid cell maturation and migration. Invest Ophthalmol Vis Sci. 2017;58(2):1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahu SK, Mittal SK, Foulsham W, Li M, Sangwan VS, Chauhan SK. Mast cells initiate the recruitment of neutrophils following ocular surface injury. Investig Ophthalmol Vis Sci. 2018;59(5):1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan SK, ElAnnan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182(3):1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittal SK, Mashaghi A, Amouzegar A, et al. Mesenchymal stromal cells inhibit neutrophil effector functions in a murine model of ocular inflammation. Investig Ophthalmol Vis Sci. 2018;59(3):1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omoto M, Katikireddy KR, Rezazadeh A, Dohlman TH, Chauhan SK. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Invest Ophthalmol Vis Sci. 2014;55(10):6631–6638. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, Chauhan SK, Saban DR, Dana R. Role of CCR7 in facilitating direct allosensitization and regulatory T-cell function in high-risk corneal transplantation. Investigative Opthalmology & Visual Science. 2010;51(2):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf AJ, Reyes CN, Liang W, et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell. 2016;166(3):624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chauhan SK, Saban DR, Dohlman TH, Dana R. CCL-21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J Immunol. 2014;192(2):817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuishi N, Murakami S, Ohno A, et al. Does β-hexosaminidase function only as a degranulation indicator in mast cells? The primary role of β-hexosaminidase in mast cell granules. J Immunol. 2014;193(4):1886–1894. [DOI] [PubMed] [Google Scholar]
- 28.Storms W, Kaliner MA. Cromolyn sodium: Fitting an old friend into current asthma treatment. J Asthma. 2005;42(2):79–89. [PubMed] [Google Scholar]
- 29.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004;173(7):4464–4469. [DOI] [PubMed] [Google Scholar]
- 30.Vignali D, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahanyar J, Koerner MM, Loebe M, Youker KA, Torre-Amione G, Noon GP. The role of mast cells after solid organ transplantation. Transplantation. 2008;85(10):1365–1371. [DOI] [PubMed] [Google Scholar]
- 32.Thakurdas SM, Melicoff E, Sansores-Garcia L, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282(29):20809–20815. [DOI] [PubMed] [Google Scholar]
- 33.Leveson-Gower DB, Sega EI, Kalesnikoff J, et al. Mast cells suppress murine GVHD in a mechanism independent of CD4+CD25+ regulatory T cells. Blood. 2013;122(22):3659–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu L-F, Lind EF, Gondek DC, et al. Mast cells are essen-tial intermediaries in regulatory T-cell tolerance. Nature. 2006;442(7106):997–1002. [DOI] [PubMed] [Google Scholar]
- 35.Amin K, Lúdvíksdóttir D, Janson C, et al. Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. BHR Group. Am J Respir Crit Care Med. 2000;162(6):2295–2301. [DOI] [PubMed] [Google Scholar]
- 36.De Winter BY, van den Wijngaard RM, de Jonge WJ. Intestinal mast cells in gut inflammation and motility disturbances. Biochim Biophys Acta - Mol Basis Dis. 2012;1822(1):66–73. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Fu T, Song F, et al. Mast cells participate in corneal development in mice. Sci Rep. 2015;5(1):17569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamagami S, Hamrah P, Zhang Q, Liu Y, Huq S, Dana MR. Early ocular chemokine gene expression and leukocyte infiltration after high-risk corneal transplantation. Mol Vis. 2005;11:632–640. [PubMed] [Google Scholar]
- 40.Nakae S, Suto H, Iikura M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176(4):2238–2248. [DOI] [PubMed] [Google Scholar]
- 41.Coward WR, Okayama Y, Sagara H, Wilson SJ, Holgate ST, Church MK. NF-kappa B and TNF-alpha: a positive autocrine loop in human lung mast cells? J Immunol. 2002;169(9):5287–5293. [DOI] [PubMed] [Google Scholar]
- 42.Kuehn HS, Radinger M, Gilfillan AM. Measuring mast cell mediator release. Curr Protoc Immunol. 2010;Chapter;7:Unit7.38. 10.1002/0471142735.im0738s91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alegre M-L, Lakkis FG, Morelli AE. Antigen presentation in transplantation. Trends Immunol. 2016;37(12):831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trevejo JM, Marino MW, Philpott N, et al. TNF-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc Natl Acad Sci. 2001;98(21):12162–12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.deLuca LS, Gommerman JL. Fine-tuning of dendritic cell biology by the TNF superfamily. Nat Rev Immunol. 2012;12(5):339–351. [DOI] [PubMed] [Google Scholar]
- 46.Bulfone-Paus S, Bahri R. Mast cells as regulators of T cell responses. Front Immunol. 2015;6:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.