Abstract
Background
Lymphedema is a common complication of breast cancer or its treatment. The gold standard treatment for lymphedema is complete decongestive therapy. There are few studies about the predictive factors for the effectiveness of complete decongestive therapy.
Aim
To evaluate the results of the intensive phase of complete decongestive therapy, and to determine the predictive factors for the response to treatment in patients with breast cancer‐related lymphedema.
Methods and Results
Fifty‐seven patients with breast cancer‐related lymphedema (mean age: 56.2 ± 11.2 years) who underwent complete decongestive therapy between 2014 and 2016 were evaluated retrospectively. Extremity volume was calculated using circumferential measurements and the truncated cone formula technique. Response to treatment was evaluated using the percentage reduction of excess volume formula, which was obtained by calculating the extremity volume before and after treatment. The median percentage reduction of excess volume was 27.7% (IQR,13.6‐50.3). The history of skin infection was related to lower percentage reduction of excess volume (P = 0.001). Although percentage reduction of excess volume was positively correlated with education level (r = 0.286, P = 0.031), percentage reduction of excess volume was negatively correlated with lymphedema duration (r = −0.361, P = 0.006), postoperative duration (r = −0.314, P = 0.018), percentage of excess volume (r = −0.398, P = 0.002), and number of complete decongestive therapy sessions (r = −0.436, P = 0.001). Univariate and multivariate analyses showed that the independent variables for percentage reduction of excess volume were percentage of excess volume (P = 0.009) and education level (P = 0.021).
Conclusion
Complete decongestive therapy is an effective method in patients with breast cancer related‐lymphedema. The most important predictive factors for the efficacy of treatment were found as percentage of excess volume and education level. Patients with breast cancer should be followed up regularly and receive complete decongestive therapy in the early stage of lymphedema.
Keywords: breast cancer, complete decongestive therapy, lymphedema, predictive factors
1. INTRODUCTION
Lymphedema is a soft tissue swelling resulting from the accumulation of protein rich fluid in the extracellular spaces 1, 2, 3. It is a common complication of breast cancer or its treatment 2, 4, 5. Breast cancer‐related lymphedema (BCRL) treatment involves physiotherapy, medical, surgical, and palliative care methods 6. The gold standard treatment for lymphedema is complete decongestive therapy (CDT)‐ 1, 3. This method consists of two phases: the intensive and maintenance phases 7, 8. The components of intensive phase are skin care, manual lymphatic drainage (MLD), multiple‐layer short‐stretch bandaging, and remedial exercises 1, 4, 7, 9, 10. The aims of the phase 2 are to conserve the results obtained in phase 1 7.
Numerous studies reported that CDT was an effective treatment method for BCRL 1, 4, 6, 7, 8, 9, 11, 12. In contrast, there are few studies about the predictive factors for the effectiveness of treatment 13, 14, 15, 16. Predicting the response to CDT and identifying factors that affect the efficacy of treatment could provide physicians a better clinical perspective on the management of BCRL treatment. The aim of this study was to evaluate the results of the intensive phase of CDT and to determine the predictive factors, including clinical and demographic features, breast cancer and treatment characteristics, and lymphedema and treatment characteristics of the response to treatment in patients with BCRL.
2. METHODS
2.1. Study population
The records of patients with BCRL who were treated in a single center between March 2014 and June 2016 were reviewed retrospectively. Ethics approval for the study was granted by the local Clinical Research Ethics Committee. Written informed consent was obtained from all patients. The patients, who were aged at least 18 years and had unilateral BCRL, were included in the study. The exclusion criteria were inadequate cognitive function, acute asthma, noncontrolled hypertension, decompensated heart failure, pregnancy, deep vein thrombosis, artery occlusion, active skin infection (erysipelas/cellulitis/lymphangitis), malignancy without treatment, and skin and/or subcutaneous malignancy. Sixty‐one patients with BCRL were treated with CDT. Two patients ceased the treatment at their request, one patient could not complete the treatment due to skin infection development, and one patient was excluded because of bilateral lymphedema. As a result, 57 female patients met our criteria.
The diagnosis of lymphedema was based on the circumferential technique and truncated cone formula. Lymphedema was defined as when the difference was at least 2 cm and the percentage of excess volume (PEV) was more than 5% between the upper extremities 13, 17, 18, 19.
The patients' clinical and demographic variables (age, height, weight, occupation, education level, history of skin infection, breast cancer, and treatment characteristics [eg, date of surgery, type of surgery, number of positive and removed nodes, pathologic diagnosis, histologic grade of tumor, radiotherapy, chemotherapy, hormonotherapy]), complications (eg, shoulder pain, range of motion limitation of the shoulder, axillary web syndrome, cervical pain, myofascial pain, numbness, heaviness and tightness, pain, and weakness in the upper extremity), lymphedema characteristics (eg, lymphedematous extremity side, duration, and stage of lymphedema), and sessions of CDT were noted. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Patients whose BMI was under 18.50 kg/m2 were considered as underweight, 18.50 to 24.99 kg/m2 were defined as a normal weight, 25.00 to 29.99 kg/m2 were considered as pre‐obese, and ≥30.00 kg/m2 were defined as obese according to World Health Organization criteria 20. Lymphedema stage was recorded according to the criteria of International Society of Lymphology 7. Stage 0 is the subclinical phase, stage 1 signifies that tissue swelling diminishes with limb elevation, stage 2 means that the limb elevation alone is mostly inadequate for reducing swelling, and stage 3 encompasses lymphostatic elephantiasis 7.
The volumes of both extremities were measured prior to treatment; the affected arm was also measured at the end of the CDT. After both extremities were measured from the wrist to the top of the arm (axillary fold) at 4‐cm intervals, extremity volumes were calculated using the truncated cone formula.
(where V = extremity volume, h = height, C1 = circumference of the top of the cone, C2 = circumference of the base of the cone) 5, 15, 21, 22, 23.
The concurrent validity of this method was studied by Karges et al, and they reported that the reliability of the calculated volume measurements was comparable to the reliability of the water displacement volume measurements 24.
Excess volume was defined as the difference between the lymphedematous limb volume before treatment (VL) and the healthy limb volume (VH) 15. The percentage excess volume (PEV) was calculated with the formula as follows:
It is reported that PEV was better for defining lymphedema severity than absolute volume difference 3, 13. Response to treatment was evaluated using the percentage reduction of excess volume (PREV) formula:
PREV = 100% × (lymphedematous limb volume before treatment‐lymphedematous limb after treatment) / (VL‐VH) 13, 23.
2.2. Intensive phase of complete decongestive therapy
All patients were treated with CDT by a physiotherapist, who was specifically trained in lymphedema, five times per week, until the maximum volume reduction was achieved, as previously reported 1. The intensive phase of CDT involved MLD, multiple‐layer short‐stretch bandaging, skin care, and remedial exercises, in accordance with the recommendations of the International Society of Lymphology 7. The patients received MLD, which is a light manual massage technique for 45 minutes per session 16. The aim of MLD was referring the accumulated fluid to unaffected lymphatics, stimulating initial lymphatics, enhancing the superficial lymphatic contractions, and opening the inactive lymphatic anastomosis 10, 25. Multiple‐layer short‐stretch bandaging was applied after MLD to prevent the re‐accumulation of fluid in the lymphedematous extremity and to benefit from the muscle pump effect until the following day during the intensive phase of CDT. The characteristics of the short stretch bandages were high working pressure and low resting pressure 11, 26. Active, repetitive, and nonresistive exercises of the lymphedematous arm after bandaging were performed to the patients to increase fluid mobilization and enhance the muscle pump effect 8, 26. All patients were informed about skin care, which included skin cleaning, moisturizing, nail care, avoiding skin cuts, burns and insect bites, and preventing exposure to abrasive chemical products 8, 26, 27. In addition, weight control was suggested to the patients. After the maximum volume reduction was achieved, the maintenance phase was begun with due attention by the patients and their families 1. In addition, the self‐massage technique was taught to patients and/or their caregivers.
2.3. Statistical analysis
The results of the descriptive analysis are presented as mean ± standard deviation (SD) for data with normal distribution, median (interquartile range [IQR]) for data with non‐normal distribution, and number of cases with (%) for nominal variables. The Mann‐Whitney U test was used to compare the differences between two independent groups for non‐normally distributed variables. The Kruskal‐Wallis test was used for the comparison of the medians of variables among more than two independent groups. The Wilcoxon test was applied to compare the extremity volumes before and after CDT. While investigating the associations between non‐normally distributed or ordinal variables, correlation coefficients and their significance were calculated using the Spearman correlation test. In order to determine the final predictive factors of PREV, univariate and multivariate linear regression analyses were used. Data analyses were performed using the SPSS‐version 11.5 for Windows (SPSS Inc., Chicago, IL) statistical software. The statistical significance level was set at 0.05.
3. RESULTS
A total of 57 patients with BCRL who received CDT were included in the study. The clinical and demographic characteristics, breast cancer and treatment characteristics, and lymphedema treatment characteristics are presented in Table 1.
Table 1.
Patient characteristics
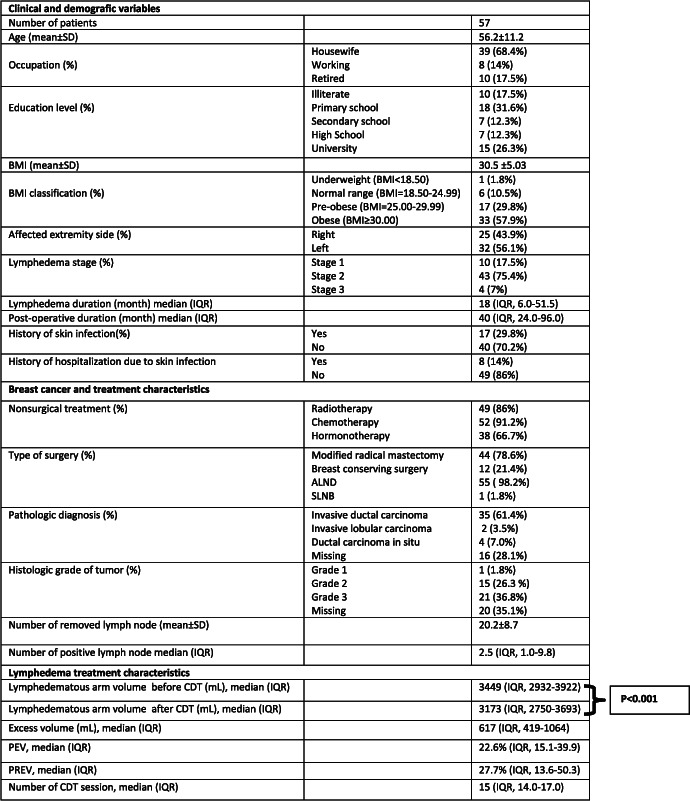
|
Abbreviations: ALND, axillary lymph node dissection; BMI, body mass index; CDT, complete decongestive therapy; IQR, interquartile range; PEV, percentage of excess volume; PREV, percentage reduction of excess volume; SD, standard deviation; SLNB, sentinel lymph node biopsy.
The median volume of the affected arm was 3449 mL (IQR, 2932‐3922), and the median PEV was 22.6% (IQR, 15.1‐39.9) before CDT. The median lymphedematous limb volume reduced to 3173 mL (IQR, 2750‐3693), which was statistically significant, after 15 (IQR, 14.0‐17.0) sessions of intensive phase of CDT (P < 0.001). The median PREV was 27.7% (IQR, 13.6‐50.3).
PEV was positively correlated with lymphedema duration (r = 0.282, P = 0.034), postoperative duration (r = 0.265, P = 0.048), lymphedema stage (r = 0.567, P < 0.001), and number of CDT sessions (r = 0.423, P = 0.001). In contrast, PEV was negatively correlated with PREV (r = −0.398, P = 0.002). Although PREV was positively correlated with education level (r = 0.286, P = 0.031), it was negatively correlated with lymphedema duration (r = −0.361, P = 0.006), postoperative duration (r = −0.314, P = 0.018), lymphedema stage (r = −0.370, P = 0.005), PEV (r = −0.398, P = 0.002), and the number of CDT sessions (r = −0.436, P = 0.001). Age, BMI, the number of removed lymph nodes, and the number of positive lymph nodes were not correlated with PEV and PREV. The clinical and demographic characteristics and their correlation with PEV and PREV are presented in Table 2.
Table 2.
Factors and their correlation with lymphedema severity and response to treatment
| Age | BMI | Education Level | LE Duration | Postoperative Duration | Number of Skin Infection | Number of Removed LN | Number of Positive LN | Tumor Grade | LE Stage | Number of Session | PREV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEV | r = 0.226 | r = 0.005 | r = − 0.087 | r = 0.282 | r = 0.265 | r = −0.016 | r = 0.236 | r = −0.183 | r = 0.002 | r = 0.567 | r = 0.423 | r = − 0.398 |
| P = 0.091 | P = 0.972 | P = 0.522 | P = 0.034* | P = 0.048* | P = 0.950 | P = 0.201 | P = 0.317 | P = 0.992 | P < 0.001*** | P = 0.001** | P = 0.002** | |
| PREV | r = −0.207 | r = −0.064 | r = 0.286 | r = −0.361 | r = − 0.314 | r = −0.056 | r = −0.116 | r = 0.156 | r = 0.039 | r = − 0.370 | r = − 0.436 | |
| P = 0.122 | P = 0.635 | P = 0.031** | P = 0.006** | P = 0.018* | P = 0.831 | P = 0.534 | P = 0.395 | P = 0.817 | P = 0.005** | P = 0.001** |
Abbreviations: LE, lymphedema; LN, lymph node; PEV, percentage of excess volume; PREV, percentage reduction of excess volume.
The history of skin infection was related to the greater lymphedema severity (PEV) (P = 0.003) and also related to worse CDT efficacy (PREV) (P = 0.001). In contrast, there was no correlation between the number of skin infection and PEV (P = 0.950) or PREV (P = 0.831). While a history of chemotherapy was not related to PEV (P = 0.632), but was related to higher PREV (P = 0.007). Occupation, the affected extremity side, the range of motion limitation, pain, heaviness and tightness or numbness in the affected extremity, mastectomy vs breast‐conserving surgery, radiotherapy, hormonotherapy, cancer grade, and pathologic diagnosis were not related with PEV and PREV. Due to only one patient undergoing sentinel lymph node biopsy (SLNB), the efficacy of CDT in patients with SLNB and axillary lymph node dissection (ALND) were not compared. The clinical and demographic characteristics affecting PEV and PREV are presented in Table 3.
Table 3.
Factors associated with PEV and PREV
| PEV | PREV | |||||
|---|---|---|---|---|---|---|
| Median | IQR | P | Median | IQR | P | |
| Occupation Housewife | 22.40 | 15.5‐40.1 | 26.92 | 13.5‐49.8 | ||
| Working | 22.20 | 14.0‐32.8 | 0.938 | 34.89 | 20.3‐101.2 | 0.609 |
| Retired | 26.10 | 14.1‐38.8 | 32.97 | 8.9‐47.6 | ||
| Affected extremity Right | 21.10 | 12.5‐43.5 | 0.579 | 27.85 | 16.4‐44.3 | 0.910 |
| Left | 25.30 | 17.0‐39.1 | 26.59 | 12.4‐52.8 | ||
| History of skin infection Yes | 39.80 | 22.9‐49.5 | 0.003** | 13.50 | 8.8‐24.3 | 0.001** |
| No | 19.85 | 12.2‐31.9 | 34.80 | 22.3‐56.2 | ||
| Type of surgery MRM | 22.80 | 14.9‐43.1 | 0.690 | 27.39 | 11.9‐49.3 | 0.632 |
| BCS | 21.85 | 13.9‐31.5 | 26.42 | 20.9‐52.8 | ||
| Pathologic diagnosis IDC | 23.20 | 12.8‐36.6 | 27.72 | 14.7‐53.8 | ||
| ILC | 11.75 | 5.5‐..... | 0.131 | 39.29 | 20.1‐ … . | 0.788 |
| DCIS | 33.65 | 20.6‐44.9 | 17.59 | 10.2‐55.8 | ||
| Radiotherapy Yes | 23.20 | 17.0‐42.1 | 0.071 | 26.92 | 13.6‐48.8 | 0.848 |
| No | 14.35 | 10.0‐26.7 | 28.03 | 11.3‐54.1 | ||
| Chemotherapy Yes | 22.50 | 15.6‐39.0 | 0.632 | 28.19 | 19.0‐53.0 | 0.007** |
| No | 27.40 | 13.3‐55.8 | 7.79 | 4.5‐20.6 | ||
| Hormonotherapy Yes | 22.90 | 14.2‐34.8 | 0.483 | 28.19 | 18.5‐51.5 | 0.426 |
| No | 30.00 | 15.6‐44.2 | 21.18 | 12.4‐53.1 | ||
| Heaviness and tightness Yes | 22.80 | 14.9‐43.1 | 0.887 | 25.68 | 12.4‐50.5 | 0.209 |
| No | 22.60 | 14.9‐33.6 | 37.73 | 22.5‐53.1 | ||
| Numbness Yes | 23.20 | 16.0‐39.9 | 0.845 | 27.72 | 13.5‐47.8 | 0.948 |
| No | 22.25 | 12.8‐39.9 | 27.57 | 13.3‐53.9 | ||
| Limitation of ROM Yes | 27.90 | 17.0‐40.1 | 0.446 | 23.30 | 12.1‐49.8 | 0.456 |
| No | 21.75 | 12.8‐34.9 | 28.03 | 17.9‐51.6 | ||
| Extremity pain Yes | 20.55 | 9.6‐35.7 | 0.181 | 27.05 | 14.4‐55.0 | 0.724 |
| No | 23.20 | 17.0‐44.1 | 27.72 | 13.2‐44.6 | ||
Abbreviations: BCS, breast conserving surgery; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; IQR, interquartile range; MRM, modified radical mastectomy; PEV, percentage of excess volume; PREV, percentage reduction of excess volume; ROM, range of motion.
Univariate analysis showed that the education level (P = 0.014), history of skin infection (P = 0.015), stage of lymphedema (P = 0.002), PEV (P = 0.006), and excess volume (P = 0.025) were independent variables for PREV (Table 4). However, PEV (P = 0.009) and education level (P = 0.021) were the only predictive variables for CDT efficacy in multivariate linear regression analysis (R2 = 0.183) (Table 5).
Table 4.
Univariate analysis of factors associated with PREV
| B | 95% CI | P | |
|---|---|---|---|
| Education level | 7.884 | 1.687–14.080 | 0.014* |
| History of skin infection | −25.130 | (−45.222)‐(−5.038) | 0.015* |
| Stage of lymphedema | −29.845 | (−48.185)‐(−11.504) | 0.002** |
| PEV | −0.744 | (−1.263)‐(−0.225) | 0.006 ** |
| Excess volume | −0.019 | (−0.035)‐(−0.003) | 0.025 * |
| Age | −0.640 | (−1.495)‐(0.216) | 0.140 |
| Lymphedema duration | −0.120 | (−0.288)‐(0.048) | 0.159 |
| Postoperative duration | −0.106 | (−0.239)‐(0.027) | 0.116 |
| Chemotherapy | 27.876 | (−5.587)‐(61.338) | 0.101 |
| VL | −0.007 | (−0.017)‐(0.004) | 0.196 |
Abbreviations: PEV, percentage of excess volume; PREV, percentage reduction of excess volume; VL, lymphedematous limb volume before CDT.
Table 5.
Multivariate analysis of factors associated with PREV
| B | Standard Error | P | |
|---|---|---|---|
| Education level | 6.999 | 2.946 | 0.021* |
| PEV | −0.678 | 0.250 | 0.009** |
Abbreviations: PEV, percentage of excess volume; PREV, percentage reduction of excess volume.
4. DISCUSSION
In this study, the results of the intensive phase of CDT and the predictive factors affecting treatment were evaluated. The median lymphedematous limb volume reduced from 3449 to 3173 mL with 15 sessions of CDT, which shows that patients were successfully treated, statistically. PEV was used instead of excess volume for determining lymphedema severity, as in previous studies 3, 13, 14, 15. The median PEV before CDT was 22.6%. The median PREV, which shows the efficacy of CDT, was 27.7%. The higher PREV ratio signified better treatment response.
There was a negative correlation between PEV and PREV. Furthermore, PEV was a predictive factor for PREV in univariate and multivariate analyses. Lymphedema stage was positively correlated with PEV and was negatively correlated with PREV. These findings showed that treatment response was better in patients with lower lymphedema severity. Similar to these data, previous studies indicated that initial lymphedema severity was the most important predictive factor for the efficacy of CDT 3, 13, 16, 28. In view of these results, it is important to consider that early treatment before the development of fibrosis and skin changes could be beneficial for better CDT response.
The median number of treatment sessions in our study was 15. Forner‐Cordero et al performed a prospective multicenter cohort study to identify independent predictive factors of CDT 3. They reported that the mean number of sessions was 16.8 3. The number of sessions was positively correlated with PEV and was negatively correlated with PREV in the current study. These findings showed that, patients with severe lymphedema required more treatment sessions, and their treatment response was worse due to lymphedema severity. Thus, treating patients in the early stage of the disease may be more effective and also cost‐effective.
Previous studies showed that lymphedema duration was predictive for the response to treatment 15, 16. Lymphedema duration and postoperative duration were positively correlated with PEV and were negatively correlated with PREV in the present study. However, they were excluded in the univariate and multivariate linear regression analyses. Vignes et al analyzed the factors associated with lymphedema volume in a cross‐sectional study 5. In contrast to our study, they reported that the duration of lymphedema and delay from cancer to onset of lymphedema were associated with lymphedema volume. 5 Our data support those of Liao et al who, in a retrospective cohort study, found that, although lymphedema duration was predictive for PEV, but could not directly predict the efficacy of CDT. Moreover, they proposed that the duration of lymphedema could affect lymphedema severity, and PEV could be a predictor for PREV 13. In light of these results, it is important to consider the pathogenesis of lymphedema. Chronic inflammation, which includes lymphocytes, macrophages, dendritic cells, and the cytokines of these cells, cause cellular proliferation, migration of fibroblasts, and finally fibrosis and skin changes 29. Based on the pathogenesis of lymphedema and the results of our study, patients receiving treatment for breast cancer should be followed up regularly. If lymphedema is identified, it should be treated without delay.
Due to the decrease of lymphatic capacity with age, lymphedema risk might be greater in older patients 30. However, the relationship with age and the occurrence of lymphedema or efficacy of CDT are still controversial 4, 5, 13, 14. In this study, age was not found as a predictive factor for lymphedema severity or CDT efficacy.
Even though previous studies demonstrated that ALND, lymph node metastasis, radiotherapy, higher cancer grade, a high number of positive lymph nodes, and adjuvant chemotherapy were risk factors for development or severity of lymphedema, these results could not be supported in our study 2, 8, 31. This may have been caused by the unavailability of medical reports and pathology reports of all patients due to their undergoing surgery at different hospitals. The efficacy of CDT in patients who underwent ALND and SLNB could not be compared, because only one patient had SLNB. Current study showed that surgical method, radiotherapy, and the number of removed lymph nodes were not associated with CDT efficacy, which was confirmed by Liao et al 13. While Eyigor et al found similar results to our study; they also added that tamoxifen use was positively correlated with PEV 14. In contrast, hormonotherapy was not a predictor in the current study. Vignes et al noted that lymphedema volume was greater in patients who underwent mastectomy versus lumpectomy 5. Forner‐Cordero et al demonstrated that the efficacy of treatment was worse in patients who received chemotherapy 3. Interestingly the response to treatment was found better in patients with a history of chemotherapy in our study, but it was excluded in linear regression analysis. The reason of these findings could be that 91.2% of the participants had received chemotherapy in this study. Further studies with large patient populations may enlighten the relationship between chemotherapy and the response to treatment.
Previous studies showed that obesity was a risk factor for developing lymphedema and lymphedema severity 2, 4, 5, 32. Moreover, Vignes et al conducted a prospective cohort study to determine predictors for CDT efficacy 15. They found that BMI and duration of lymphedema were predictive for absolute volume reduction with CDT. Also, they stated that other clinical variables or characteristics of cancer treatment did not indicate the efficacy of CDT 15. Our results were unable to support the relationship between BMI and lymphedema severity or efficacy. Because the majority of participants were pre‐obese and obese, which might account for the lack of statistical significance in the current study. Therefore, further studies that involve BMI with normal distribution may give a better point of view for the effect of obesity on treatment response. All patients with lymphedema should be informed about weight control and be referred to a dietitian if required.
Although, there was no relationship between occupation and PEV or PREV, academic education level was found as an independent variable for response to treatment in our study. To the best of our knowledge, the effect of education level on the efficacy of CDT has not been reported previously. A higher education level may provide a better understanding of recommendations, skin care, and exercises. Further studies with a larger sample sizes are needed to support the effect of education level on the response to CDT.
Infection and adipogenesis are risk factors for exacerbating lymphedema 29. Lymphedema severity was greater, and the efficacy of CDT was worse in patients who had a history of skin infection in the present study. Although history of infection was an independent variable in the univariate analysis, it was excluded in the multivariate analysis. Furthermore, the number of skin infections was not a predictor for PEV or PREV. Similarly, Vignes et al demonstrated that lymphedema volume was higher in patients with a history of cellulitis 5. To the best of our knowledge, the effect of skin infection history on the response to CDT has not been reported before. Although history of infection was excluded in the multivariate analysis in the current study, skin care, preventing patients from getting skin infections, and effective infection treatment when required are very important for avoiding the vicious cycle between lymphedema and infection. CDT in the early stage may reduce the risk of skin infection. In addition, preventing patients from getting infections might provide better lymphedema control and greater efficacy of CDT. The limitations of this study are that it was a retrospective cohort study, and not all the medical reports of all participants were available.
In conclusion, the intensive phase of CDT is an effective method in patients with BCRL. Also the most important predictive factors are the percentage of excess volume and education level for the intensive phase of CDT efficacy, according to our study.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
AUTHORS' CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conceptualization, M.D., D.K.; Methodology, M.D., S.U.D.; Investigation, D.K., U.D.O.; Writing—Original Draft, D.K.; Writing—Review and Editing, M.D., S.U.D., U.D.O; Supervision, M.D.
ACKNOWLEDGEMENTS
The authors received no financial support for the research and/or authorship of this article.
Keskin D, Dalyan M, Ünsal‐Delialioğlu S, Düzlü‐Öztürk Ü. The results of the intensive phase of complete decongestive therapy and the determination of predictive factors for response to treatment in patients with breast cancer related‐lymphedema. Cancer Reports. 2020;e1225. 10.1002/cnr2.1225
There is no conflict of interest with respect to the authorship and/or publication of this article.
There is no financial support for the research and/or authorship of this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. Lasinski BB, Thrift KM, Squire D, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM&R. 2012;4(8):580‐601. [DOI] [PubMed] [Google Scholar]
- 2. Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. The Breast Journal. 2010. Jan;16(1):48‐54. [DOI] [PubMed] [Google Scholar]
- 3. Forner‐Cordero I, Muñoz‐Langa J, Forner‐Cordero A, DeMiguel‐Jimeno JM. Predictive factors of response to decongestive therapy in patients with breast‐cancer‐related lymphedema. Annals of Surgical Oncology. 2010;17(3):744‐751. [DOI] [PubMed] [Google Scholar]
- 4. Koul R, Dufan T, Russell C, et al. Efficacy of complete decongestive therapy and manual lymphatic drainage on treatment‐related lymphedema in breast cancer. International Journal of Radiation Oncology, Biology, Physics. 2007. Mar 1;67(3):841‐846. [DOI] [PubMed] [Google Scholar]
- 5. Vignes S, Arrault M, Dupuy A. Factors associated with increased breast cancer‐related lymphedema volume. Acta Oncologica. 2007. Jan 8;46(8):1138‐1142. [DOI] [PubMed] [Google Scholar]
- 6. Buragadda S, Alhusaini AA, Melam GR, Arora N. Effect of complete decongestive therapy and a home program for patients with post mastectomy lymphedema. Journal of Physical Therapy Science. 2015. Sep;27(9):2743‐2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. International Society of Lymphology . The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology. 2013. Mar;46(1):1‐11. [PubMed] [Google Scholar]
- 8. Ezzo J, Manheimer E, McNeely ML, et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database of Systematic Reviews. 2015. May 21;5(5):CD003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stamatakos M, Stefanaki C, Kontzoglou K. Lymphedema and breast cancer: a review of the literature. Breast Cancer. 2011;18(3):174‐180. [DOI] [PubMed] [Google Scholar]
- 10. Huang T‐W, Tseng S‐H, Lin C‐C, et al. Effects of manual lymphatic drainage on breast cancer‐related lymphedema: a systematic review and meta‐analysis of randomized controlled trials. World Journal of Surgical Oncology. 2013. Jan 24;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang Y, Jang D‐H, Jeon JY, et al. Pressure monitoring of multilayer inelastic bandaging and the effect of padding in breast cancer‐related lymphedema patients. American Journal of Physical Medicine & Rehabilitation. 2012. Sep;91(9):768‐773. [DOI] [PubMed] [Google Scholar]
- 12. King M, Deveaux A, White H, Rayson D. Compression garments versus compression bandaging in decongestive lymphatic therapy for breast cancer‐related lymphedema: a randomized controlled trial. Support Care Cancer. 2012. May;20(5):1031‐1036. [DOI] [PubMed] [Google Scholar]
- 13. Liao S‐F, Li S‐H, Huang H‐Y, et al. The efficacy of complex decongestive physiotherapy (CDP) and predictive factors of lymphedema severity and response to CDP in breast cancer‐related lymphedema (BCRL). Breast. 2013. Oct;22(5):703‐706. [DOI] [PubMed] [Google Scholar]
- 14. Eyigör S, Cinar E, Caramat I, Unlu BK. Factors influencing response to lymphedema treatment in patients with breast cancer‐related lymphedema. Support Care Cancer. 2015. Sep 8;23(9):2705‐2710. [DOI] [PubMed] [Google Scholar]
- 15. Vignes S, Porcher R, Champagne A, Dupuy A. Predictive factors of response to intensive decongestive physiotherapy in upper limb lymphedema after breast cancer treatment: a cohort study. Breast Cancer Research and Treatment. 2006. Jul 3;98(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 16. Haghighat S, Lotfi‐Tokaldany M, Maboudi AAK, Karami M, Bahadori A, Weiss J. Predictive factors of response to phase I complete decongestive therapy in upper extremity lymphedema following breast carcinoma in Iran. Lymphology. 2013. Jun;46(2):97‐104. [PubMed] [Google Scholar]
- 17. Johansson K, Branje E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncologica. 2010. Jan 26;49(2):166‐173. [DOI] [PubMed] [Google Scholar]
- 18. Korpan MI, Crevenna R, Fialka‐Moser V. Lymphedema: a therapeutic approach in the treatment and rehabilitation of cancer patients. American Journal of Physical Medicine & Rehabilitation. 2011;90(5 Suppl 1):S69‐S75. [DOI] [PubMed] [Google Scholar]
- 19. Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. American Journal of Surgery. 2004. Jan;187(1):69‐72. [DOI] [PubMed] [Google Scholar]
- 20. WHO . BMI classification. p. http://apps.who.int/bmi/index.jsp?introPage=intro_.
- 21. Deltombe T, Jamart J, Recloux S, et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007. Mar;40(1):26‐34. [PubMed] [Google Scholar]
- 22. Katz‐Leurer M, Bracha J. Test‐retest reliability of arm volume measurement in women with breast cancer‐ related lymphoedema. J Lymphoedema. 2012;7(2). [Google Scholar]
- 23. Yamamoto T, Todo Y, Kaneuchi M, Handa Y, Watanabe K, Yamamoto R. Study of edema reduction patterns during the treatment phase of complex decongestive physiotherapy for extremity lymphedema. Lymphology. 2008;41(2):80‐86. [PubMed] [Google Scholar]
- 24. Karges JR, Mark BE, Stikeleather SJ, Worrell TW. Concurrent validity of upper‐extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Physical Therapy. 2003;83(2):134‐145. [PubMed] [Google Scholar]
- 25. Zimmermann A, Wozniewski M, Szklarska A, Lipowicz A, Szuba A. Efficacy of manual lymphatic drainage in preventing secondary lymphedema after breast cancer surgery. Lymphology. 2012. Sep;45(3):103‐112. [PubMed] [Google Scholar]
- 26.DeLisa's Physical Medicine and Rehabilitation Principles and PracticeAndrews KL, Oderich GS, Bjarnason H, Gamble GL. Vascular diseases. In: Frontera WR, Gans BM, Walsh NE, Robinson LR, eds. . 5th ed. ; 2010:1179‐1205.
- 27. Quirion E. Recognizing and treating upper extremity lymphedema in postmastectomy/lumpectomy patients: a guide for primary care providers. Journal of the American Academy of Nurse Practitioners. 2010. Sep;22(9):450‐459. [DOI] [PubMed] [Google Scholar]
- 28. Ramos SM, O'Donnell LS, Knight G. Edema volume, not timing, is the key to success in lymphedema treatment. American Journal of Surgery. 1999. Oct;178(4):311‐315. [DOI] [PubMed] [Google Scholar]
- 29. Saito Y, Nakagami H, Kaneda Y, Morishita R. Lymphedema and therapeutic lymphangiogenesis. BioMed Research International. 2013;2013:804675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bergmann A, da Costa Leite Ferreira MG, de Aguiar SS, et al. Physiotherapy in upper limb lymphedema after breast cancer treatment: a randomized study. Lymphology. 2014. Jun;47(2):82‐91. [PubMed] [Google Scholar]
- 31. Kim M, Shin KH, Jung S‐Y, et al. Identification of prognostic risk factors for transient and persistent lymphedema after multimodal treatment for breast cancer. Cancer Research and Treatment. 2016. Oct;48(4):1330‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bar Ad V, Dutta PR, Solin LJ, et al. Time‐course of arm lymphedema and potential risk factors for progression of lymphedema after breast conservation treatment for early stage breast cancer. The Breast Journal. 2012. May;18(3):219‐225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


