ABSTRACT
Tsetse flies of the palpalis group, particularly Glossina fuscipes, are the main vectors of human African trypanosomiasis or sleeping sickness in Congo-Brazzaville. They transmit the deadly human parasite, Trypanosoma brucei gambiense and other trypanosomes that cause animal trypanosomiasis. Knowledge on diversity, population structure, population size, and gene flow is a prerequisite for designing effective tsetse control strategies. There is limited published information on these parameters including migration patterns of G. fuscipes in Congo-Brazzaville. We genotyped 288 samples of G. fuscipes from Bomassa (BMSA), Bouemba (BEMB), and Talangai (TLG) locations at 10 microsatellite loci and determined levels of genetic diversity, differentiation, structuring, and gene flow among populations. We observed high genetic diversity in all three localities. Mean expected heterozygosity was 0.77 ± 0.04, and mean allelic richness was 11.2 ± 1.35. Deficiency of heterozygosity was observed in all populations with positive and significant FIS values (0.077–0.149). Structure analysis revealed three clusters with genetic admixtures, evidence of closely related but potentially different taxa within G. fuscipes. Genetic differentiation indices were low but significant (FST = 0.049, P < 0.05), indicating ongoing gene flow countered with a stronger force of drift. We recorded significant migration from all the three populations, suggesting exchange of genetic information between and among locations. Ne estimates revealed high and infinite population sizes in BEMB and TLG. These critical factors should be considered when planning area-wide tsetse control interventions in the country to prevent resurgence of tsetse from relict populations and/or reinvasion of cleared habitats.
INTRODUCTION
Tsetse flies (Diptera: Glossinidae) are sole vectors of human African trypanosomiasis (HAT) and animal African trypanosomiasis (AAT) in sub-Saharan Africa.1 Tsetse flies of the palpalis group are the most important vectors of HAT2,3 as they transmit Trypanosoma brucei gambiense (Tbg) and Trypanosoma brucei rhodesiense, deadly parasites that cause chronic and acute infections, respectively.4,5 For the period 2004–2018, it is estimated that approximately 54 million people were at different levels of risk of contracting HAT with 95% of the population being at risk of Tbg infection.2,3,6 Fortunately, HAT is nearing elimination. Only < 1,000 HAT cases (including both gambiense and rhodesiense HAT) were reported in 2018, with gambiense HAT representing the main burden of the disease at 98% of the total.3 Majority of the population at risk of gambiense HAT is found in central Africa, with the Democratic Republic of Congo (DRC) taking the largest share (74%). Congo-Brazzaville (the country where the current study was conducted) has approximately 5% of the population at risk of gambiense HAT. In addition to the human disease, tsetse flies also transmit nagana, a debilitating disease that severely affects animal husbandry and productivity in rural settings, thus affecting rural economies and livelihoods of millions of people living in such areas. The situation is further aggravated by political crises such as civil wars, which cause displacement of populations and increase risk of HAT transmission as populations move through tsetse-infested areas, in some cases with their livestock.3
Studies conducted during colonial era showed that among tsetse species and subspecies reported in former French colonies in Africa, four predominant vectors of the palpalis group (Glossina fuscipes fuscipes, Glossina fuscipes quanzensis, Glossina fuscipes martinii, and Glossina palpalis palpalis) played an important role in the transmission of gambiense-type HAT and AAT. The subject of this study was G. fuscipes (s.l.) hereinafter will be referred to as G. fuscipes. A recent report based on crude combination of numbers of HAT cases and tsetse distribution maps6,7 indicates that > 90% of HAT cases were transmitted by G. f. fuscipes (Uganda, Sudan, Congo-Brazzaville, and Central African Republic) and G. f. quanzensis (the DRC, Angola, and Congo-Brazzaville). A smaller number of HAT cases were transmitted by G. palpalis gambiensis and G. p. palpalis in West Africa (Guinea and Cote d’Ivoire). Glossina palpalis palpalis could also be responsible for some low-level transmission of gambiense HAT in the DRC and Congo-Brazzaville.
Most HAT and AAT control experts agree that tsetse control remains one of the most effective and sustainable ways of managing African trypanosomiasis. Unfortunately, for Congo-Brazzaville, the last reported vector control operation took place in the late 1980s, where the trapping method8 was introduced in areas infested with G. p. palpalis.9 These trapping efforts led to significant reduction in tsetse apparent densities9,10 and to a decline in infection rates in tsetse midguts and in sentinel herds of domestic livestock.11 Unfortunately, because of the political instability witnessed in the country in the early to late 1990s, tsetse control operations in the country broke down. Plans are only being made now, by the national control program under the Ministry of Health, to revive such operations with the view to eradicating tsetse flies from the country in the long-run by using integrated area-wide approaches. Successful implementation of such area-wide control operations will require generation of empirical evidence on tsetse population dynamics.
Evidence-based control is important because local tsetse eradication efforts, though successful, have not achieved the intended absolute (100%) eradication, and in many cases, fly populations have re-emerged.9,10 The failure to clear treated areas of tsetse flies has been attributed to either resurgence of the suppressed residual populations that survive during the eradication campaigns or to reinvasion from neighboring infested but untreated areas.12 The threat of reinvasion from untreated tsetse habitats can be effectively addressed through integrated area-wide vector control approaches, including the use of sterile insect technique13 where applicable.
For effective area-wide management and/or eradication of tsetse vectors to be realized, a thorough understanding of the genetic diversity, differentiation, population size, and structure of the target vector species is required. A prerequisite to the success of any vector control campaign aiming at eradication is to identify and target isolated populations to minimize the risk of reinvasion.14,15 Population genetic studies have proved useful in identifying genetically and/or geographically isolated tsetse populations. For example, studies on population genetics of G. p. palpalis in West and central Africa provided evidence that any sustainable control efforts of such riverine tsetse should be undertaken through transboundary projects16,17 to mitigate against re-infestation from untreated tsetse habitats across boundaries. Other studies conducted in Burkina Faso, Guinea, and Senegal indicated that riverine tsetse populations are sufficiently isolated to warrant attempts at complete eradication.18,19 Whereas there have been several studies on the genetic diversity and population structure of morsitans and palpalis group tsetse in eastern, Southern, Western, and some parts of central Africa, such data are lacking in Congo-Brazzaville. A recent study based on mitochondrial DNA (mtDNA) sequence revealed an inconclusive picture on the genetic diversity of G. fuscipes (s.l.) populations collected from three geographical areas in Congo-Brazzaville.20 Genetic diversity was high in some G. fuscipes (s.l.) populations, but low in others, and genetic differentiation ranged from moderate to high among subpopulations.20 Although mtDNA markers evolve rapidly, they are nonrecombining, are maternally inherited, and exhibit high rates of mutation, and mtDNA is not a neutral marker in the strict sense. Directional selection on mtDNA sequence has been reported in a number of insect species.21,22 In the present study, we genotyped G. fuscipes flies from three geographical locations at a set of 10 autosomal microsatellite loci to gain a deeper understanding of genetic diversity and differentiation, rates and patterns of gene flow, population structure, and effective population size of this important species in Congo-Brazzaville. We envisaged that the findings of this study will help guide national tsetse control operations.
MATERIALS AND METHODS
Study area, tsetse collection, and DNA extraction.
The study area and method of tsetse sample collection were previously described in detail.20 A map showing the sample collection sites is provided in Supplemental Figure S1. In brief, we sampled G. fuscipes in three areas of Congo-Brazzaville, that is, Bomassa (BMSA) in the north (Sangha region), at the Cameroon border, in Bouemba (BEMB) in Plateaux region, and in Talangai (TLG) in Ngabe corridor of the Congo River, in plateau-Batéké (northern Pool region). Bomassa was selected as a study site; this area is located just at the periphery of a national park that is located right at the border with Cameroon and Central African Republic. Therefore, the data from this area are important for transboundary tsetse control strategies for G. fuscipes spp. In addition, we selected BEMB and TLG because both areas are located in the Ngabe corridor and are of strategic relevance because they are located directly opposite the tsetse-infested and sleeping sickness–endemic foci of Bolobo and Kwamouth in the DRC.
We used non-baited biconical traps, and fly catches were collected after 24 hours. Genomic DNA was extracted from individual tsetse flies by using a QIAgen DNeasy® Blood and Tissues extraction kit (QIagen, Qiagen, Valencia, CA), as per the manufacturer’s instructions and as previously described.20 In brief, legs obtained from both male and female tsetse flies that had been preserved in 95% ethanol were air-dried for 24 hours to enable total evaporation of ethanol. Thereafter, 1.5-mL Eppendorf tubes (Shanghai, China) containing legs of individual flies were briefly dipped in liquid nitrogen to facilitate crushing and grinding using micro-pestles. The resulting finely ground tissue was lysed, precipitated, and resuspended in buffer before proceeding with final DNA extraction and purification were carried out as per the manufacturer’s instructions.
Microsatellite loci amplification and genotyping.
Ten specific microsatellite markers previously validated and applied in genotyping G. f. fuscipes populations23 were used to assess the genetic diversity and differentiation as well as population structure between and among three populations (nine subpopulations) in Congo-Brazzaville. All the genotyped flies had previously been identified morphologically as G. f. fuscipes and through mtDNA sequencing as G. fuscipes (s.l.), with all samples showing ≥ 98–100% identity to G. f. fuscipes sequences derived from the National Center for Biotechnology Information database.20 The full list of microsatellite loci used in this study, their primer sequences, and authors are provided in Supplemental Appendix Table S5.
PCR amplification of DNA was performed using fluorescently labeled (with FAM, HEX, or TAM) forward primers in 20 µL final reaction volumes containing a Qiagen Multiplex24 Kit (Cat. 206143), H2O, and 20 ng/µL DNA template. PCR amplifications were carried out by using an Applied Biosystems Veriti 96-well thermal cycler (Foster City, CA) at the Biotechnology laboratory, Kenyan Forestry Research Institute, Muguga Kenya. Fluorescently labeled primers were included at a final concentration of 0.4 µM. Four sets of three multiplex primers oriented by the marker sizes and by the choice of fluorescent dyes were used in the PCR analysis. The amplifications were performed using multiplex PCR as described25,26 taking into consideration differences in annealing temperature and PCR conditions for the 10 microsatellite markers as recently used by Opiro et al.23 We also bore in mind the fact that some forward primer was labeled with Tetramethylrhodamine, which is the same as a reference marker dye for capillary electrophoresis standard.
Amplifications were performed under the following conditions: initial denaturation at 94°C for 15 minutes, followed by denaturation at 94°C for 30 seconds, annealing at 57°C for 90 seconds, an initial extension at 72°C for 60 seconds, and a final extension at 60°C for 30 minutes, all for 35 cycles, and a final hold at 4°C. Microsatellite genotypic data were generated on an ABI 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA). PCR products were diluted 1/10 in double-distilled sterile water, and 0.5 µL of diluted product was transferred to a new plate containing 9.5 µL of HIDI formamide and 0.125 µL GeneScan 600 LIZ size standard (Applied Biosystems, Foster City, CA). Denaturation was performed on a thermocycler for 95°C for 3 minutes and immediately placed on ice before being subjected to capillary electrophoresis on an ABI 3500 automated sequencer (Applied Biosystems-Hitachi). Genotypes scoring and marker validation were performed using GeneMapper version 3.7 (Applied Biosystems).
Data analyses.
Marker validation and genetic diversity estimates.
Genepop v. 4.6 (Laboratiore de Genetique et Environment, Montpellier, France)27 web version was used to validate microsatellite markers using the neutrality and independence test, important precursors to population structure assessments.28 Departures from Hardy Weinberg equilibrium (HWE) proportions in each sample and microsatellite locus were determined using an approximation of an exact test based on a Markov chain iteration using a burn-in of 10,000 and 1,000 batches with 10,000 iterations per batch. We obtained significance values following Fisher’s method that combines probabilities of exact tests.29 We evaluated microsatellite genotypic linkage disequilibrium (LD) among pairs of loci using the Fisher exact test of Hardy–Weinberg proportion for multiple alleles.29 Pairwise tests for LD were estimated to obtain chi-squared (χ2) distribution per locus pair, degrees of freedom, and original P-values for the test of significance. Multiple tests in the detection of LD and HWE were corrected using the false discovery rate approach by using Benjamini–Hochberg correction30 (Supplemental Appendix Tables S2 and S3), which in contrast to the Bonferroni correction, has lower incidences of false negatives.30 Complementarily, Bonferroni28,30,31 correction was used to confirm the significance tests of LD among the 45 pairs of loci (P-values are under LD [P < 0.001]).
Fixation index (FIS) values were evaluated after testing for deviations from Hardy–Weinberg equilibrium (HWE). The global test for heterozygote deficiency and heterozygote excess was performed using genetic diversity statistics by estimating allele frequencies, expected (He) and observed (Ho) heterozygosities, and allelic richness (AR) for each population by using the program Arlequin32 v. 3.5.2 (Bern, Switzerland) and confirmed in FSTAT v2.9.3 (Lausanne, Switzerland).
Population structure and genetic differentiation.
Unbiased estimators of F-statistics33 were obtained by using FSTAT v. 2.9.4 (Lausanne, Switzerland)34 and GENALEX.6 (Canberra, Australia).35 We also calculated Wright’s F-statistics36 following the variance method using 10,000 permutations over loci for 95% CI and Markov chain Monte Carlo 100,000 dememorizations as implemented in Arlequin.32 The technique minimizes the departure from HWE and LD. We used Genepop27 online-based program to conduct Fisher’s exact test on genotypes to assess the significance of genetic differentiation.
The population structure of the 288 samples from three populations (nine subpopulations) with more than 10 loci was inferred by using Bayesian program Structure37 v. 2.3.4 (Pritchard Lab, Stanford, CA).38 Structure analyses were conducted with K = 2 as the most likely number of genetically distinct population groups based on ΔK42 (Supplemental Appendix Figure S2). Simulations were run with K ranging from one to 10, with 20 iterations per value of K. For each run, we assumed an admixture model and independent allele frequencies using a “burn-in” value of 50,000 and thereafter, 250,000 iterations. In addition to Structure, we performed discriminant analysis of principal components (DAPC) using R version 3.5.239 with the “adegenet” package v. 1.4.2 (Oxford, United Kingdom).40 Analysis of principal components is a multivariate, model-free method that makes no assumptions about deviations from Hardy–Weinberg and LD, designed to describe patterns of genetic clustering among groups of individual samples.41 To get insights into partitioning of microsatellite variance within and between genetic units, we performed analysis of molecular variance (AMOVA) in Arlequin v. 3.5.32 Nine subpopulations grouped into three populations were considered.
To test the correlation between the genetic distances (FST) and geographic distances for the three tsetse populations, the Mantel test for isolation by distance (IBD) was performed in GenAlex version 6.503.35
Relatedness and migration.
We used ML-Relate42 to estimate allele frequencies in the population from individuals whose relatedness is being estimated and to assess relatedness between individuals in sampled populations over microsatellite marker loci. In addition, we used GeneClass 2.0 (Montpellier, France)43 to detect first-generation migrants, that is, individuals born in a population other than the one in which they were sampled. A threshold alpha level of 0.05 was used to determine the critical values. Detection of first-generation migrants and progeny of successful mating of very recent migrants between genetic regions was evaluated by calculating likelihood of finding an individual in the locality in which it was sampled (Lh) versus the greatest likelihood among all sampled localities (Lmax), with their ratio (Lh/Lmax) identifying the migrants.26,44 The relationship between each pair of individuals that has the highest likelihood among the four following relationships was tested using ML-Relate: relatedness, unrelated (U), half siblings (HSs), full siblings (FSs), and parent offspring (PO).
Effective size and population bottlenecks.
Effective population size (Ne), approximate number of adult flies which transmit their genes to next generation, was estimated using the molecular co-ancestry method45 as implemented in NeEstimator v. 2.1 (Molecular Fisheries Laboratory, Brisbane, Australia). We used Genepop pipeline to estimate contemporary effective population size (Ne) using multi-locus diploid genotypes from population samples.
We used Bottleneck software (INRA, Montpellier, France)46 to test for recent bottlenecks in population size. The two-phase mutation model (TPM) was applied to test for disequilibrium between heterozygosities and allele numbers in populations because neither the infinite allele model (IAM) nor the stepping stone model of mutation strictly apply to microsatellite loci.47
RESULTS
Microsatellites validation.
Deviations from Hardy–Weinberg equilibrium (HWE).
Two loci (A03b and B05) of 10 showed significant departures from HWE across all the three populations sampled, and two others (GmH09 and pg17) showed departures from random mating in two (BEMB and TLG) of the three populations. We obtained high polymorphism over all the 10 markers (AR ranged from 11.1 to 14.4; Supplemental Appendix Table S1). Fixation index is a measure of departures from random mating within populations and is the correlation of alleles within individuals relative to the population. After Bonferroni correction for multiple testing, FIS values showed significant (P < 0.001) departures from random mating at six microsatellites loci (A03, pg28, B05, GmH09, pg17, and GmmL11) in the three populations. Mean FIS values across all loci were 0.077 ± 0.002, 0.078 ± 0.002, and 0.147 ± 0.000 in BMSA, BEMB, and TLG, respectively. These results point to moderate deficit of heterozygosity, particularly in TLG (Supplemental Appendix Table S2).
Linkage disequilibrium.
Significant LD was detected in five of 45 (11%) Bonferroni pairwise comparisons. This is above 5% expected by chance alone. However, when we applied Bonferroni correction for multiple tests (at critical value of P = 0.05/45 = 0.001), none of the loci pairwise tests were significantly in LD (Supplemental Appendix Tables S3), indicating that the microsatellite loci used in this study are independently inherited in the three populations sampled.
Genetic diversity indices and relatedness.
Genetic diversity indices were high and nearly similar in all sites (Table 1). Ho varied from 0.65 to 0.72 (mean Ho = 0.65 ± 0.3), with the highest value being observed in BEMB and BMSA (0.72) and the lowest (0.65) in TLG. Similarly, He was high in all localities ranging from 0.76 in BEMB and TLG to 0.78 in BMSA. Mean He was 0.77 ± 0.04. It was observed that in all cases, Ho differed significantly (P < 0.05) from He, showing that the populations were at equilibrium as He > Ho. In addition, AR was high and homogeneous (AR > 11; mean AR = 11.4 ± 1.46 in BMSA, 11.1 ± 1.15 in BEMB, and 11.1 ± 1.43 in TLG). Altogether, these results show that the three populations each harbor high levels of genetic diversity, pointing to ongoing immigration and random mating among populations.
Table 1.
Summary of diversity statistics showings sampling site name and identity, latitude, longitude, number of samples analyzed (N), mean allelic richness (AR) across loci, observed (Ho) and expected heterozygosity (He), inbreeding coefficient (FIS), and FIS P-value (P < 0.05)
| Sites | GPS coordinates | Diversity indices | Relatedness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site name | ID | Latitude | Longitude | N | AR | Ho | He | FIS | FIS P-value | U | HS (%) | FS (%) | PO (%) |
| Bomassa | BMSA | 2°12′13.89″N | 16°11′07.77″E | 96 | 11.4 | 0.72 | 0.78 | 0.085 | 0.002 | 85.03 | 11.51 | 1.28 | 2.18 |
| Bouemba | BEMB | 2°09′41.56″S | 16°08′10.67″E | 96 | 11.1 | 0.71 | 0.76 | 0.077 | 0.002 | 85.07 | 12.68 | 1.18 | 1.07 |
| Talangai | TLG | 3°17′12.84″S | 16°11′44.05″E | 96 | 11.1 | 0.65 | 0.76 | 0.149 | 0.002 | 85.13 | 12.33 | 1.32 | 1.22 |
FIS P-value (P = 0.002, P < 0.05) and FIS significant values of fixation index indicating gene flow between individual of the populations are indicated in bold.
Four categories of relatedness indices were generated from ML-Relate analysis. These were U, HS, FS, and PO. Results from these analyses showed that on average, > 85% of individuals sampled were unrelated, 12.2% were HSss, 1.3% were FSss, and ∼1.5% were parent offspring (Table 1). Generally, there were no significant differences in relatedness observed within the different categories (U, HS, FS, and PO; Table 1) across the three locations (BMSA, BEMB, and TLG) from where samples were obtained. It was however noted that BMSA had the highest parent offspring (∼2.2%), followed by TLG (1.2%) and BEMB (1.1%) (Table 1).
Population structure and genetic differentiation.
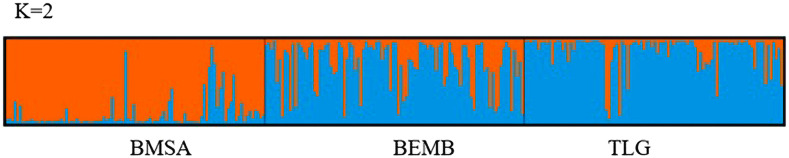
Bayesian assignment of microsatellite genotypes in Structure (Figure 1) and DAPC (Figure 2) identified three clusters (BMSA, BEMB, and TLG) with evidence of admixture of flies between the clusters, suggesting ongoing gene flow.
Figure 1.
Structure results for K = 1–10. Each bar represents a single fly with the proportion of colors representing the Bayesian probability of assignment (q-value) of an individual. Black vertical lines separate sampling sites: Bomassa (BMSA), Bouemba (BEMB), and TLG.
Figure 2.
Discriminant analysis of principle component inferred in the R (R Development core team) “adegenet” package using 80 principle components based on Glossina fuscipes microsatellite data for three sampling sites.
Although admixture was observed in all the three populations, BMSA stood out as a more distinct population as opposed to BEMB and TLG, which seemed more homogenous with only very few flies from these two populations being admixed with BMSA population. The Structure admixture shows closely related species but seemingly different taxa.
Pairwise FST values (data not shown) ranged from 0.037 to 0.064. Although the values indicated little genetic differentiation, they were all significant (P = 0.000). The highest differentiation (FST = 0.064) was observed between BEMB and BMSA, followed by BEMB and TLG (FST = 0.046) and BMSA and TLG (FST = 0.037). The little genetic differentiation indicates that allele frequencies are nearly homogenous, and rates of gene flow are high among the populations sampled in the current study. Additional insights into how the genetic variation was partitioned across the different populations were obtained by an AMOVA. The AMOVA of all 10 microsatellite loci was consistent with the Structure and differentiation estimates. Analysis of molecular variance showed that most of the variations (89.5%) were found within individuals in the sampled populations. Variation among population groups and among populations within the groups accounted for 4.9% and 5.6% of the variance, respectively (Table 2). The IBD test using showed that there was no significant linear correlation between geographic and genetic distances with R2 = 0.9494 (P = 0.19) (Figure 2), indicating that there was no IBD.
Table 2.
Analysis of molecular variance between three populations of Glossina fuscipes showing levels of differentiation and based the two ecosystems (savannah/plateau vs rain forest).
| Source of variation | Degrees of freedom | Sum of squares | Variance components | Percentage of variation |
|---|---|---|---|---|
| Among groups | 2 | 28.908 | 0.06797Va | 4.88 |
| Among populations within groups | 295 | 399.870 | 0.07826Vb | 5.62 |
| Within populations | 288 | 359.000 | 1.24653Vc | 89.50 |
| Total | 575 | 787.778 | 1.39276 |
Migration patterns.
GeneClass analysis to detect first-generation migrants and migration analysis showed significant migration from all the three populations, indicating ongoing exchange of genetic material among the three sampled locations (Table 3), thus confirming the admixed genetic structure revealed by Structure and DPCA as described above. Considering all the 288 samples collected, 29 (10%) were immigrants (Table 3). Twelve (12) of these individuals migrated from TLG to BEMB (12/29) and two from TLG to BMSA (2/29). Eight of the fly samples collected in BMSA were found to have migrated from BEMB (8/29), and three (3/29) of the flies sampled in BMSA were observed to have originated from TLG (3/29). Four migrant flies were collected from BEMB, of which two (2/29) had originated from TLG and two (2/29) from BMSA. Overall, majority (20/29; 69%) of the immigrants shown to have originated from BEMB were collected from either BMSA or TLG. Bouemba had only 13.8% (4/29), whereas BMSA and TLG had 37.9% and 48.3% immigrants, respectively (Table 3), as shown in Supplemental Appendix Figure S3. This implies that BEMB was the most stable population, whereas TLG is the most unstable population.
Table 3.
Migration of individuals showing sites of origin and site migrated from
| Home (sampling site) | Sample ID | Migrated from (site) | P-value |
|---|---|---|---|
| BMSA | BMSA4 | BEMB | 0.001 |
| BMSA | BMSA30 | BEMB | 0.005 |
| BMSA | BMSA32 | BEMB | 0.006 |
| BMSA | BMSA33 | TLG | 0.006 |
| BMSA | BMSA77 | BEMB | 0.011 |
| BMSA | BMSA79 | TLG | 0.019 |
| BMSA | BMSA80 | TLG | 0.003 |
| BMSA | BMSA83 | BEMB | 0.021 |
| BMSA | BMSA84 | BEMB | 0.002 |
| BMSA | BMSA47 | BEMB | 0.043 |
| BMSA | BMSA85 | BEMB | 0.036 |
| BEMB | BEMB170 | BMSA | 0.018 |
| BEMB | BEMB173 | TLG | 0.040 |
| BEMB | BEMB210 | BMSA | 0.009 |
| BEMB | BEMB401 | TLG | 0.030 |
| TLG | TLG89 | BEMB | 0.027 |
| TLG | TLG103 | BMSA | 0.014 |
| TLG | TLG106 | BEMB | 0.037 |
| TLG | TLG107 | BMSA | 0.038 |
| TLG | TLG110 | BEMB | 0.027 |
| TLG | TLG436 | BEMB | 0.014 |
| TLG | TLG438 | BEMB | 0.004 |
| TLG | TLG439 | BEMB | 0.015 |
| TLG | TLG440 | BEMB | 0.001 |
| TLG | TLG443 | BEMB | 0.000 |
| TLG | TLG444 | BEMB | 0.047 |
| TLG | TLG449 | BEMB | 0.003 |
| TLG | TLG453 | BEMB | 0.001 |
| TLG | TLG455 | BEMB | 0.003 |
BEMB = Bouemba; BMSA = Bomassa; TLG = Talangai.
Effective population size and bottleneck.
We estimated effective population size (Table 4) based on the IAM and the TPM. All the three populations (BEMB, TLG, and BMSA) showed significant heterozygote deficiency when compared with the expected equilibrium under the IAM model. However, results generated under the TPM model indicated that the populations are not significantly deficient of heterozygosity when compared with the expected equilibrium after Bonferroni correction (Table 4).
Table 4.
Estimates of effective population size (Ne), bottleneck site, Ne estimates, the Ne 95% CI; Ne was estimated with the linkage disequilibrium method in NeEstimator48 tests for population bottlenecks were run in Bottleneck.46
| Population | Pop ID | Sample size | Ne | Ne 95% CI | P-value (two-phase mutation) | P-value (infinite allele model) | AFD* |
|---|---|---|---|---|---|---|---|
| Bomassa | BMSA | 96 | 275.4 | 180.5–539.6 | 0.25 | 0.005 | Normal L-shaped |
| Bouemba | BEMB | 96 | 6,981.6 | 526–∞ | 0.42 | 0.012 | Normal L-shaped |
| Talangai | TLG | 96 | 443.2 | 162.5–∞ | 0.78 | 0.0015 | Normal L-shaped |
AFD = allele frequency distribution.
We observed L-shaped allele frequency distribution and a significant heterozygosity deficiency in all populations, indicating the absence of any genetic bottleneck among the sampled G. fuscipes population in the recent past. The TPM model showed no significant heterozygosity deficiency (TPM, P value > 0.05) compared with the expected equilibrium after correction, but significant heterozygote deficiency was detected in the IAM model in all populations.
Ne (number of reproductive migrants per generation) estimates revealed high to infinite population size in BEMB (6,981.6; 95% CI = 526, infinity), moderate size in TLG (443.2; 95% CI = 162.5, infinity), and low population size in BMSA (275.4; 95% CI = 180.5–539.6) (Table 4). No significant reduction in effective population size was observed in any of the sampled sites using the TPM. However, the IAM model showed a significant population bottlenecks in all populations (P < 0.05). We consider the results generated under TPM as this model is more appropriate for microsatellite DNA markers. No deviations from the normal L-shaped allele frequency distribution were observed, further providing evidence of duality of no population bottlenecks and mutation drift equilibrium.
DISCUSSION
Hardy–Weinberg and LD.
The present study evaluated genetic diversity, differentiation, population structure, and size of G. fuscipes in Congo-Brazzaville (at microsatellite loci), where this tsetse vector plays an important role in the transmission of sleeping sickness and nagana. Although nine of the 30 (30%) FIS values showed significant departures from random mating (Supplemental Appendix Table S2) as previously observed in G. f. fuscipes population,26 none of the values was significant after Bonferroni correction (results not shown).
All FIS values, except one, were positive, indicating heterozygote deficiency. However, only two loci (A03b and B05) showed significant departures from HWE across all the sampled populations, with two other loci (GmH09 and pg17) only exhibiting departures from random mating in two (BEMB and TLG) of the three sampled populations. These incidences of departure from HWE are likely because of the presence of null alleles. Unfortunately, we were unable to estimate the frequency of null alleles as some of the microsatellite markers used in this study did not have any published information on the number of motifs.49–51 However, all these markers have previously been amplified successfully among field samples of G. fuscipes.26 In addition, all loci were polymorphic (> 10 alleles) and therefore useful for population genetic studies. Apart from the null allele hypothesis, the observed deficiency of heterozygotes could have been caused by population subdivision (Table 1), considering that G. fuscipes samples were collected from different geographical locations and during dry season when tsetse populations seem to retreat into refugia that could transiently behave as independently breeding demes, a phenomenon previously reported in Glossina pallidipes.52 We do not associate these departures with differences in selective forces operating on the loci as microsatellite markers are generally considered to be selectively neutral53 therefore are not influenced by natural selection and are instead influenced by gene flow, genetic drift, and mutation.
Significant LD was detected in 11% (5% above expected random occurrence) of pairwise tests (Supplemental Appendix Table S3). However, after Bonferroni correction, no pairwise loci test showed evidence of LD. This result is consistent with findings from other studies where these markers (except pg17) were previously tested and found to be selectively neutral and independently inherited26 when used to genotype G. f. fuscipes populations for which the markers were developed by Dyer et al.,13 Abila et al.,50 and Hyseni et al.54. Our results therefore confirm that the microsatellite loci used in this study are independently inherited in the three populations sampled.
Genetic diversity estimates and relatedness.
We observed high genetic diversity in all populations sampled based on AR, Ho, He, and FIS values (Table 1). Such high genetic diversity is indicative of ongoing immigration and large effective population sizes of the sampled populations. It would seem that previous tsetse control efforts in Congo-Brazzaville, a post-conflict country, were not sufficiently intense to affect population size and genetic diversity indices. The high genetic variability observed in this study is similar to the levels of microsatellite diversities (Ho, He, and AR) previously reported among some G. f. fuscipes populations in Uganda.26,55,56 However, diversity values recorded in Uganda were slightly lower than the genetic diversity values reported in Congo-Brazzaville. This minor difference in diversity levels could be attributed to differences in effective population sizes of G. f. fuscipes in Uganda as compared with Congo-Brazzaville. It is reported that in Uganda, there have been intense tsetse control operations such as the Farming in Tsetse Controlled Areas that took place57 in 1999–2010 and the Stamp Out Sleeping Sickness campaign in the 2000s in southeastern and northeastern regions of the country, respectively.26 Such control operations might have affected the diversity of G. f. fuscipes populations in Uganda. On the contrary, most tsetse control operations in Congo-Brazzaville may have been hampered by the intermittent civil conflict in the country, particularly in the late 1990s. Civil conflict adversely impacts tsetse and trypanosomiasis control operations because of breakdown in national vector and disease control systems.58
Positive and significant FIS values (Table 1) observed in BMSA, BEMB, and TLG indicate that individuals in subpopulations are more related (inbreeding) than would be expected under random mating patterns. Such deficit of heterozygosity could also be attributed to the Wahlund effect (departures from random mating) caused by divergent or subdivided populations,59,60 characterized by high to moderate genetic variability.51 The low, but significant, overall FIS value (0.057) provides evidence of modest population subdivision, which, as explained above, could indicate that tsetse samples collected during the dry season could be behaving as independently breeding units.52
Population structure and genetic differentiation.
Both Structure (Figure 1) and DAPC (Figure 2) analyses identified three clusters (BMSA, BEMB, and TLG). The clusters were however not entirely distinct as there was evidence of admixture, suggesting ongoing but somewhat limited gene flow among them. The high levels of admixture are evident from Structure and DAPC were corroborated with low pairwise FST values (Table 2). Bomassa seemed relatively more distinct than BEMB and TLG that appeared to be regularly exchanging genetic material between them and thus more genetically homogenous. The relatively higher genetic admixture between BEMB and TLG was previously reported based on mitochondrial haplotype frequencies20 and could be attributed to the relative proximity between the two locations (130 km apart) as opposed to the larger geographical distance (400 km) between BEMB and BMSA. Apart from the geographical isolation which was not significant as determined by Mantel’s IBD test, the picture revealed by the Structure analysis could also be interpreted to mean that we could be dealing with closely related but different taxa. It was recently reported from phylogenetic analysis based on mtDNA cox1 gene sequences that BEMB was inhabited by G. f. fuscipes (based on 100% similarity to GenBank G. f. fuscipes sequences originating from Uganda).20 The same study reported that BMSA and TLG were likely populated with a G. fuscipes complex comprising G. f. quanzensis, G. f. martini, and G. f. fuscipes, a probable reason why a significant proportion (∼85%) of haplotypes in the three localities (BMSA, BEMB, and TLG) were private, with BEMB and TLG sharing the greatest proportion of the shared haplotypes.20 Given the high levels of admixture observed between BEMB and TLG in the current study (Figure 1), it would seem that there is no significant reproductive barrier between members of the G. fuscipes species complex. This is a subject for further investigation.
Pairwise estimates of genetic differentiation revealed little (FST ≥ 0.037) but significant genetic differentiation (P < 0.001) between BMSA and TLG. The little but significant differentiation points to high rates of gene flow and homogeneity in microsatellite allele frequencies among the sampled G. fuscipes populations. Significant FST in the face of ongoing gene flow is consistent with findings of previous studies on G. f. fuscipes populations in Uganda that concluded that genetic drift was acting as a stronger force than gene flow.50,61
The highest differentiation (FST = 0.064) was recorded between BEMB and BMSA, whereas the lowest (FST = 0.037) was between BMSA and TLG. These results are consistent with the findings of a recent study based on mtDNA cox1 marker20 which also showed that BEMB and BMSA were the most genetically differentiated populations. In addition to the large geographical distance that separates BEMB and BMSA, the low rate of gene flow between them is also attributed to the fact that tributaries of different rivers pass through the two locations. It is noteworthy however that the mtDNA-based differentiation index (FST = 0.152) between the two locations reported by Ref. 20 was more than two orders of magnitude the value of the microsatellite-based FST (0.064) recorded in the current study. This incongruence in the magnitude of FST values based on mitochondrial and microsatellite DNA markers is not surprising, given that the two markers have different properties in terms of evolutionary rates and inheritance patterns. Contrasting indices of genetic differentiation based on microsatellites versus those based on mitochondrial variation have previously been reported in tsetse flies.47,62,63 Mitochondrial variation is known to show greater degree of genetic differentiation because it represents an effective population size of roughly one-quarter that afforded by sexually reproducing, diploid variation and has high sensitivity to demographic forces.64
The lower indices of genetic differentiation between BEMB and TLG (FST = 0.046) or between BMSA and TLG (FST = 0.037) could be due to sympatry among these populations, given that they share the same river drainage systems (rivers Kadei, Congo, and Sangha). Elsewhere, sympatry among Kenyan and Ugandan G. f. fuscipes populations sharing same river drainage formation (Sio–Malaba basin) was previously suggested as an explanation of the observed pattern of differentiation in this riverine species of tsetse.61 More recently, it was reported that indeed, ecological and geographic features, especially river systems, played a major role in keeping G. f. fuscipes populations genetically connected in northern Uganda.26 The authors suggested that this fact should be taken into consideration when designing vector control operations in the region.
Analysis of molecular variance results was consistent with the Structure and differentiation estimates. The global FST (0.048) showed low but significant differentiation among G. fuscipes populations in Congo-Brazzaville, with only ∼4.8% of the variation being attributed to differences among the three population groups. Even more interesting was the variance among subpopulations which contributed a mere 0.4% of the total variation with a correspondingly F-statistic (FSC ∼ 0.004). Altogether, these results indicate limited gene flow among populations, but high rates of gene flow among subpopulations (specific trapping sites) within populations. However, the Mantel test for isolation by distance (Figure 3)65 did not show any significant correlation between genetic (FST) and geographic distances (R2 = 0.9494, P = 0.19) for the three G. fuscipes populations. This IBD result lends credence to the hypothesis that the low but significant genetic differentiation observed among populations could be because of other barriers to gene flow, for example, reproductive or physical features such as different river systems. As stated earlier, the level of admixture observed in the Structure analysis may rule out reproductive barrier. Nevertheless, the role of these alternative (non-geographical distance) barriers to gene flow in shaping the population structure of G. fuscipes in Congo-Brazzaville clearly needs to be investigated further.
Figure 3.
Mantel test for isolation by distance performed65 using GenAlEx ver. 6.50335 to test the correlation between the genetic distances (FST) and geographic distances for the three tsetse populations.
Relatedness and migration pattern.
Results on GeneClass showed significant migration from all the three populations (BMSA, BEMB, and TLG), indicating ongoing gene flow between the sampled locations. The connectivity shown between BEMB and TLG is attributed first to their geographical proximity, and second, it seems plateau Batéké hills do not pose a significant barrier to migration between the two locations. In addition, it seems that the suitability and near similarity of their ecological environments could be favoring such migration. The migration observed from BMSA to TLG could be due to passive dispersal of tsetse pupae along riverine habitats caused by seasonal flooding of river systems such as Kadei and Congo, a phenomenon previously observed for G. f. fuscipes in Uganda.63 These results are consistent with and support previously reported findings based on mtDNA cox1 sequence variation.20 The migration pattern observed from BMSA to TLG and BEMB based on haplotype distribution observed by Ref. 20 is now clearly defined and confirmed based on microsatellites (Supplemental Appendix Figure S3).
Results presented in this study show that there are more first-generation migrants from BMSA to BEMB (8/11) than from BMSA to TLG (3/11). We also observed massive migration from TLG to BEMB (12/14 first-generation migrants), whereas only 2/14 first-generation migrants from TLG to BMSA. Bouemba provided the least number of first-generation migrants to BMSA (2/14) and TLG (2/14) (Table 3). These results indicate that BEMB is the most stable habitat among the three populations/localities and could be serving as a reservoir of gene pool of G. fuscipes species (20/29 of total migrants) in Congo-Brazzaville, particularly in the areas sampled in this study. On the other hand, TLG (14/29), which is located downstream in the river systems, toward Brazzaville city represents the most unstable population, with 48.3% of total migration.
The foregoing migration patterns corroborate with the observed genetic structure pattern of three clusters with admixture (Figures 1 and 2) and are consistent with high rates of gene flow among the populations, regardless of the geographical distance between them. The migration (Table 3) among G. fuscipes populations in Congo-Brazzaville seems to be taking place in all directions, thus contributing to the little differentiation evidenced even between populations that are geographically isolated (e.g., 400 km from BMSA to BEMB). The detection of first-generation migrants (Table 3) in all the three population groups demonstrates successful mating of individuals between regions and leading to viable offspring (Table 1 and Supplemental Appendix Table S4). This can be explained by the fact that physical barriers in these areas do not seem to affect the migration process.
Effective population size and bottleneck.
NeEstimator48 tests for population bottlenecks have shown infinite population size in BEMB and TLG at 95% CI, and the allele frequency distribution analysis revealed a normal L-shaped distribution, evidence of no recent bottleneck66 in all populations including BMSA. This seemingly large effective population size is consistent with signatures of recent and rapid population expansion reported20 and may further explain the high genetic variability observed in the three populations. The apparently large effective population size of G. fuscipes obtained in this study indicate fairly stable genetic populations, a phenomenon that has previously been reported on the G. f. fuscipes and G. pallidipes52,55,67,68 and more recently by Ciosi et al.26 and Okeyo et al.69. The large Ne estimates and resulting genetic stability should be taken into consideration when planning and setting optimal control of this riverine tsetse species in the Congo. These Ne estimates would, for example, be helpful in determining the number of sterile males to be released and the density of insecticide-impregnated traps to be deployed for population suppression. It is clear from the Ne data that tsetse control in BEMB for instance would require much more dense deployment of traps or targets.
CONCLUSION
Microsatellite genotypic data presented in this study revealed high genetic diversities among G. fuscipes populations sampled in Congo-Brazzaville, a clear indication of ongoing random mating among them. In addition, there was moderate deficit of heterozygosity among the three G. fuscipes populations, a pointer to population subdivision, consistent with the genetic structuring observed among the same populations based on mtDNA haplotype frequencies and suggesting the presence of closely related but different taxa. Our results show clustering and admixture of the G. fuscipes populations sampled in Congo-Brazzaville. The populations are also moderately but significantly differentiated genetically, indicating that there is ongoing gene flow that is strongly countered by genetic drift. As recently demonstrated using mtDNA sequence data,20 this study has confirmed that the G. fuscipes sampled in Congo-Brazzaville harbor high genetic diversity, indicative of a large effective population corroborated by large Ne estimates that depict temporally and genetically stable populations. The rates of gene flow observed in this study indicate that rivers may serve as a conduit for passive dispersal of G. fuscipes between BMSA and TLG.
Implications for vector control.
The low genetic differentiation observed between the three populations of G. fuscipes and lack of isolation between them and the diversity indicators observed point to ongoing gene flow among them. More importantly, there is much higher rates of gene flow among subpopulations within the three populations. Thus, in any vector control study in the country, the moderately but significantly genetically differentiated populations should be targeted as a unit in an integrated area-wide vector control strategy. The same would not apply to subpopulations among which we detected significantly high rates of gene flow (data not shown) that would lead to reinvasion of treated/cleared tsetse habitats from untreated/uncleared areas. In addition, the high levels of admixture observed among the genetic clusters further confirm the potential risk of reinvasion from neighboring clusters. Because the main populations of G. fuscipes in the country seem to be extensively connected genetically, despite the geographical distances between them, perhaps a rolling carpet approach akin to the one proposed by Kaba et al.,70 Bouyer et al.,71 and Vreysen et al.72 for other riverine species in West Africa would be the most suitable control strategy to minimize chances of reinvasion. Furthermore, the relatively isolated populations should be targeted as separate breeding units between which barriers could be set up to prevent reinvasion. Future studies should focus on gaining a better understanding on the potential existence of a species complex and assessing temporal aspects of genetic diversity and structure of G. fuscipes in Congo-Brazzaville under a more elaborate sampling scheme.
Supplemental Appendix tables, and figures
ACKNOWLEDGMENTS
We gratefully acknowledge the support provided by the management of PAUSTI. We also thank the current and former directors of Biotechnology Research Institute–Kenya Agriculture and Livestock Research Organization (BioRI-KALRO), Raymond E. Mdachi and Grace A. Murilla, respectively, for providing administrative support, particularly with the procurement of reagents and for permission to use the BioRI-KALRO research facilities. We thank John Gicheru of the Kenya Forestry Research Institute (KEFRI), Department of Biotechnology, for his assistance while the first author worked at the genotyping platform. John provided excellent help with marker optimization and genotyping services for which we are most grateful. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Note: Supplemental Appendix tables, and figures appear at www.ajtmh.org.
REFERENCES
- 1.Leak SGA, 1998. Tsetse Biology and Ecology: Their Role in the Epidemiology and Control of Trypanosomiasis. Wallingford, United Kingdom: CABI publishing in Association with ILRI, 3–16. [Google Scholar]
- 2.WHO , 2020. The Elimination of Human African Trypanosomiasis Is in Sight: Report from the Third WHO Stakeholders Meeting on Elimination of Gambiense Human African Trypanosomiasis, Vol. 12. Geneva, Switzerland: World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco J, Cecchi G, Priotto G, Paone M, Diarra A, Grout L, Simarro PP, Zhao W, Argaw D, 2020. Monitoring the elimination of human African trypanosomiasis at continental and country level: update to 2018. PLoS Negl Trop Dis 14: e0008261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simarro P, Jannin J, Cattand P, 2008. Eliminating human African trypanosomiasis: where do we stand and what comes next? PLoS Med 5: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solano P, Torr SJ, Lehane MJ, 2013. Is vector control needed to eliminate gambiense human African trypanosomiasis? Front Cell Infect Microbiol 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simarro P, Cecchi G, Franco JR, Paone M, Diarra A, Ruiz-Postigo JA, Fèvre EM, Mattioli RC, Jannin JG, 2012. Estimating and mapping the population at risk of sleeping sickness. PLoS Negl Trop Dis 6: e1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simarro PP, Cecchi G, Paone M, Franco JR, Diarra A, Ruiz JA, Fèvre EM, Courtin F, Mattioli RC, Jannin JG, 2010. The Atlas of human African trypanosomiasis: a contribution to global mapping of neglected tropical diseases. Int J Health Geogr 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duteurtre JP, Gouteux JP, 1986. Les strategies de lutte antisommeilleuse en republique populaire du Congo. Med Trop 46: 375–380. [PubMed] [Google Scholar]
- 9.Gouteux JP, Toudic A, Sinda D, 1988. Utilisation D’animaux sentinelles dans L’evaluation de La lutte contre les vecteurs de La Maladie du sommeil. Premiers resultats dans un foyer congolais. Acta Trop 45: 331–338. [PubMed] [Google Scholar]
- 10.Gouteux JP, Noireau F, 1986. Un nouvel écran‐piège pour la lutte anti‐tsétsé: description et essais dans un foyer congolais de trypanosomiase humaine. Entomol Exp Appl 41: 291–297. [Google Scholar]
- 11.Noireau F, Okamba-Osseke F, Gouteux JP, 1990. Impact immédiat d’une lutte antivectorielle par piégeage sur I’enzootie de trypanosomose au Sud-Congo. Revue Revue Élev Méd Vét Pays Trop 43: 93–96. [PubMed] [Google Scholar]
- 12.Bouyer J, Dicko AH, Cecchi G, Ravel S, Guerrini L, Solano P, Vreysen MJ, De Meeus T, Lancelot R, 2015. Mapping landscape friction to locate isolated tsetse populations that are candidates for elimination. Proc Natl Acad Sci U S A 112: 14575–14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyer NA, et al. 2011. Cryptic diversity within the major trypanosomiasis vector Glossina fuscipes revealed by molecular markers. PLoS Negl Trop Dis 5: e1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato AB, Hyseni C, Okedi LM, Ouma JO, Aksoy S, Caccone A, Masembe C, 2015. Mitochondrial DNA sequence divergence and diversity of Glossina fuscipes fuscipes in the Lake Victoria basin of Uganda: implications for control. Parasit Vectors 8: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaboyo JP, 2002. Aiming to eliminate tsetse from Africa. Trends Parasitol 18: 473–475. [DOI] [PubMed] [Google Scholar]
- 16.Meeûs TDe, Ravel S, Rayaisse J, Kaba D, Courtin F, Bouyer J, Dayo GK, Camara M, Solano P, 2014. Genetic correlations within and between isolated tsetse populations. Acta Trop 138: S6–S11. [DOI] [PubMed] [Google Scholar]
- 17.De Meeus T, Bouyer J, Ravel S, Solano P, 2015. Ecotype evolution in Glossina palpalis subspecies, major vectors of sleeping sickness. PLoS Negl Trop Dis 9: e0003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyer NA, et al. 2009. Evidence for a discrete evolutionary lineage within equatorial Guinea suggests that the tsetse fly Glossina palpalis palpalis exists as a species complex. Mol Ecol 18: 3268–3282. [DOI] [PubMed] [Google Scholar]
- 19.De Meeûs T, Ravel S, Rayaisse JB, Kaba D, Courtin F, Bouyer J, Dayo GK, Camara M, Solano P, 2014. Genetic correlations within and between isolated tsetse populations: what can we learn? Acta Trop 138: S6–S11. [DOI] [PubMed] [Google Scholar]
- 20.Mayoke A, Muya SM, Bateta R, Mireji PO, Okoth SO, Onyoyo SG, Auma JE, Ouma JO, 2020. Genetic diversity and phylogenetic relationships of tsetse flies of the palpalis group in Congo Brazzaville based on mitochondrial cox1 gene sequences. Parasit Vectors 13: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira DCSG, Raychoudhury R, Lavrov DV, Werren JH, 2008. Rapidly evolving mitochondrial genome and directional selection in mitochondrial genes in the parasitic wasp Nasonia (Hymenoptera: Pteromalidae). Mol Biol Evol 25: 2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun JT, Jin PY, Hoffmann AA, Duan XZ, Dai J, Hu G, Xue XF, Hong XY, 2018. Evolutionary divergence of mitochondrial genomes in two Tetranychus species distributed across different climates. Insect Mol Biol 27: 698–709. [DOI] [PubMed] [Google Scholar]
- 23.Opiro R, Saarman NP, Echodu R, Opiyo EA, Dion K, Halyard A, Dunn AW, Aksoy S, Caccone A, 2017. Genetic diversity and population structure of the tsetse fly Glossina fuscipes fuscipes (Diptera: Glossinidae) in northern Uganda: implications for vector control. PLoS Negl Trop Dis 11: e0005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaac C, Ciosi M, Hamilton A, Scullion KM, Dede P, Igbinosa IB, Patrick O, Nmorsi G, Masiga D, Turner CMR, 2016. Molecular identification of different trypanosome species and subspecies in tsetse flies of northern Nigeria. Parasit Vectors 9: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omondi SF, Machua J, Muturi GM, Gicheru JM, Hanaoka S, 2019. Evidence of high genetic diversity and significant population structuring in Vachellia tortilis (Forsk.) Galasso & Banfi population in Kenya. Ann For Sci 76: 47. [Google Scholar]
- 26.Ciosi M, Masiga DK, Turner CMR, 2014. Laboratory colonisation and genetic bottlenecks in the tsetse fly Glossina pallidipes. PLoS Negl Trop Dis 8: e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rousset F, 2008. GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour 8: 103–106. [DOI] [PubMed] [Google Scholar]
- 28.Hauser S, Wakeland K, Leberg P, 2018. Inconsistent use of multiple comparison corrections in studies of population genetic structure: are some type I errors more tolerable than others? 19: 144–148. [DOI] [PubMed] [Google Scholar]
- 29.Guo SW, Thompson EA, 1992. Performing the exact test of Hardy-Weinberg proportion for for multiple alleles. Biometrical J 48: 361–372. [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300. [Google Scholar]
- 31.Sethuraman A, Wulf GK, Gonzalez NM, Grenier CE, Kansagra KS, Mey KK, Nunez SB, Bryce Z, 2019. Continued misuse of multiple testing correction methods in population genetics‐a wake‐up call? 19: 23–26. [DOI] [PubMed] [Google Scholar]
- 32.Schneider S, Excoffier L, Laval G, Schneider S, 2005. Arlequin: an integrated software package for population genetics data analysis. Evol Bioinform Online 1: 47–50. [PMC free article] [PubMed] [Google Scholar]
- 33.Weir BS, Cockerham CC, 1984. Estimating F-statistics for the analysis of population structure. Evolution 38: 1358–1370. [DOI] [PubMed] [Google Scholar]
- 34.Goudet J, Raymond M, De Meeüs T, Rousset F, 1996. Testing differentiation in diploid populations. Genetics 144: 1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peakall R, Smouse PE, 2012. GenALEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28: 2537–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright S, 1950. The genetical structure of populations. The Genetical Structure of Populations. Chicago, IL: University of Chicago Galh Lecture at University College, 323–354. [Google Scholar]
- 37.Pritcharda KJ, Wen X, Falushb D, 2010. Structure software version 2.3. Statistics 10: 1–39. [Google Scholar]
- 38.Pritchard J, Stephens M, 2000. Association mapping in structured populations. Am J Hum Genet 67: 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R Core Team , 2008. R: A Language and Environment for Statistical Computing. Available at: https://www.r-project.org/. Accessed December 27, 2020.
- 40.Jombart T, 2008. Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24: 1403–1405. [DOI] [PubMed] [Google Scholar]
- 41.Jombart T, Devillard S, Balloux F, 2010. Discriminant analysis of principal components: a new method for the analysis of geneticallystructured populations 11: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalinowski S, 2008. User ’ s manual for ML-relate input files null alleles. Mol Ecol Notes 6: 576–579. [Google Scholar]
- 43.Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A, 2004. GENECLASS2: a software for genetic assignment and first-generation migrant detection. J Hered 95: 536–539. [DOI] [PubMed] [Google Scholar]
- 44.Okeyo WA, et al. 2018. Genetic differentiation of Glossina pallidipes tsetse flies in southern Kenya. Am J Trop Med Hyg 99: 945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura T, 2008. Estimation of effective number of breeders from molecular coancestry of single cohort sample. Evol Appl 1: 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piry S, Luikart G, 1999. BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Heredit 90: 502–503. [Google Scholar]
- 47.Ouma JO, Marquez JG, Krafsur ES, 2005. Macrogeographic population structure of the tsetse fly, Glossina pallidipes (Diptera: Glossinidae). Bull Entomol Res 95: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Do C, Waples RS, Peel D, Macbeth GM, Tillett BJ, Ovenden JR, 2014. NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne ) from genetic data. Mol Ecol Resour 14: 209–214. [DOI] [PubMed] [Google Scholar]
- 49.Brown JE, Komatsu KJ, Abila PP, Robinson AS, Okedi LMA, Dyer N, Donnelly MJ, Slotman MA, Caccone A, 2008. Polymorphic microsatellite markers for the tsetse fly Glossina fuscipes fuscipes (Diptera: Glossinidae), a vector of human African trypanosomiasis. Mol Ecol Resour 8: 1506–1508. [DOI] [PubMed] [Google Scholar]
- 50.Abila PP, Slotman MA, Parmakelis A, Dion KB, Robinson AS, Muwanika VB, Enyaru JCK, Lokedi LM, Aksoy S, Caccone A, 2008. High levels of genetic differentiation between Ugandan Glossina fuscipes fuscipes populations separated by Lake Kyoga. PLoS Negl Trop Dis 2: e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luna C, Bonizzoni M, Cheng Q, Robinson AS, Aksoy S, Zheng L, 2001. Microsatellite polymorphism in tsetse flies (Diptera: Glossinidae). J Med Entomol 38: 376–381. [DOI] [PubMed] [Google Scholar]
- 52.Ouma J, Krafsur E, 2010. The influence of temporal and seasonal changes on genetic diversity and population structure of the tsetse fly, Glossina pallidipes in Kenya. East Afr Agric For J 77: 59–68. [Google Scholar]
- 53.Kirk H, Freeland JR, 2011. Applications and implications of neutral versus non-neutral markers in Molecular Ecology. Int J Mol Sci 12: 3967–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hyseni C, Beadell JS, Ocampo ZG, Ouma JO, Okedi LM, Gaunt W, Caccone A, 2011. The G. m. morsitans (Diptera: Glossinidae) genome as a source of microsatellite markers for other tsetse fly (Glossina) species. Mol Ecol Resour 11: 1–11.21429158 [Google Scholar]
- 55.Echodu R, Beadell JS, Okedi LM, Hyseni C, Aksoy S, Caccone A, 2011. Temporal stability of Glossina fuscipes fuscipes populations in Uganda. Parasit Vectors 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Echodu R, Sistrom M, Hyseni C, Enyaru J, Okedi L, Aksoy S, Caccone A, 2013. Genetically distinct Glossina fuscipes fuscipes populations in the Lake Kyoga region of Uganda and its relevance for human African trypanosomiasis. Biomed Res Int 2013: 614721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer A, Holt HR, Selby R, Guitian J, 2016. Past and ongoing tsetse and animal trypanosomiasis control operations in five African countries: a systematic review. PLoS Negl Trop Dis 10: e0005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berrang Ford L, 2007. Civil conflict and sleeping sickness in Africa in general and Uganda in particular. Confl Health 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mélachio TTT, Njiokou F, Ravel S, Simo G, Solano P, De Meeûs T, 2015. Effect of sampling methods, effective population size and migration rate estimation in Glossina palpalis palpalis from Cameroon. Infect Genet Evol 33: 150–157. [DOI] [PubMed] [Google Scholar]
- 60.Ravel S, de Meeus T, Dujardin JP, Zézé DG, Gooding RH, Dusfour I, Sané B, Cuny G, Solano P, 2007. The tsetse fly Glossina palpalis palpalis is composed of several genetically differentiated small populations in the sleeping sickness focus of Bonon, Côte d’Ivoire. Infect Genet Evol 7: 116–125. [DOI] [PubMed] [Google Scholar]
- 61.Krafsur ES, Marquez JG, Ouma JO, 2008. Structure of some east African Glossina fuscipes fuscipes populations. Med Vet Entomol 22: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouma J, Beadell JS, Hyseni C, Okedi LM, Krafsur ES, Aksoy S, Caccone A, 2011. Genetic diversity and population structure of Glossina pallidipes in Uganda and western Kenya. Parasit Vectors 4: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beadell JS, Hyseni C, Abila PP, Azabo R, Enyaru JCK, Ouma JO, Mohammed YO, Okedi LM, Aksoy S, Caccone A, 2010. Phylogeography and population structure of Glossina fuscipes fuscipes in Uganda: implications for control of tsetse. PLoS Negl Trop Dis 4: e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Avise JC, 2004. Molecular Markers, Natural History and Evolution 2nd ed. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 65.Jensen JL, Bohonak AJ, Kelley ST, 2005. Isolation by distance, web service. BMC Genet 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bateta R, et al. 2020. Phylogeography and population structure of the tsetse fly Glossina pallidipes in Kenya and the serengeti ecosystem. PLoS Negl Trop Dis 14: e0007855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ouma JO, Marquez JG, Krafsur ES, 2006. Microgeographical breeding structure of the tsetse fly, Glossina pallidipes in south-western Kenya. Med Vet Entomol 20: 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hyseni C, Kato AB, Okedi LM, Masembe C, Ouma JO, 2012. The population structure of Glossina fuscipes fuscipes in the Lake Victoria basin in Uganda: implications for vector control. Parasit Vectors 5: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okeyo WA, et al. 2017. Temporal genetic differentiation in Glossina pallidipes tsetse fly populations in Kenya. Parasit Vectors 10: 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaba D, Ravel S, Solano P, Allou K, Bosson-vanga H, Gardes L, Goran EKN, Schofield CJ, Kon M, 2012. Phenetic and genetic structure of tsetse fly populations (Glossina palpalis palpalis) in southern Ivory Coast. Parasit Vectors 5: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bouyer J, Balenghien T, Ravel S, Vial L, Sidibé I, Thévenon S, Solano P, De Meeûs T, 2009. Population sizes and dispersal pattern of tsetse flies: rolling on the river? Mol Ecol 18: 2787–2797. [DOI] [PubMed] [Google Scholar]
- 72.Vreysen MJB, Saleh KM, Ali MY, Abdulla AM, Zhu ZR, Juma KG, Dyck VA, Msangi AR, Mkonyi PA, Feldmann FU, 2000. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J Econ Entomol 93: 123–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.