Abstract
It was reported that histopathologic lesions are risk factors for the progression of IgA Nephropathy (IgAN). The aim of this study was to investigate the relationships between mesangial deposition of C1q and renal outcomes in IgAN. 1071 patients with primary IgAN diagnosed by renal biopsy were enrolled in multiple study centers form January 2013 to January 2017. Patients were divided into two groups: C1q-positive and C1q-negative. Using a 1: 4 propensity score matching (PSM) method identifying age, gender, and treatment modality to minimize confounding factors, 580 matched (out of 926) C1q-negative patients were compared with 145 C1q-positive patients to evaluate severity of baseline clinicopathological features and renal outcome. Kaplan–Meier and Cox proportional hazards analyses were performed to determine whether mesangial C1q deposition is associated with renal outcomes in IgAN. During the follow-up period (41.89 ± 22.85 months), 54 (9.31%) patients in the C1q negative group and 23 (15.86%) patients in C1q positive group reached the endpoint (50% decline of eGFR and/or ESRD or death) respectively (p = 0.01) in the matched cohort. Significantly more patients in C1q negative group achieved complete or partial remission during the follow up period (P = 0.003) both before and after PSM. Three, 5 and 7-year renal survival rates in C1q-positive patients were significantly lower than C1q-negative patients in either unmatched cohort or matched cohort (all p < 0.05). Furthermore, multivariate Cox regression analysis showed that independent risk factors influencing renal survival included Scr, urinary protein, T1-T2 lesion and C1q deposition. Mesangial C1q deposition is a predictor of poor renal survival in IgA nephropathy.
Trial registration TCTR, TCTR20140515001. Registered May 15, 2014, http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1074.
Subject terms: Nephrology, Kidney diseases, Glomerular diseases, IgA nephropathy
Introduction
Immunoglobulin A nephropathy (IgAN) is one of the most common glomerulonephritis (GN) worldwide, 20–40% of the patients progress to end stage renal disease (ESRD) within 10–20 years from the onset of the disease1,2. The diagnosis of IgAN is based on the preponderance of IgA deposits in the mesangium3,4. Low intensity C1q deposition may also be found2,5–8. Activation of the complement system locally via the classical, alternate and lectin pathways in glomeruli augments the inflammatory cascade and potentiates tissue injury in IgAN9,10. It was reported that mesangial C4d deposition, which is a marker of activation of the lectin pathway, is associated with progression to ESRD in IgAN patients11. However, whether the presence of C1q deposition, a critical protein in the classical complement pathway, is associated with IgAN remains largely unknown. Therefore, a multicenter, prospective, observational trial was performed to evaluate the relationships between mesangial C1q deposition and clinicopathological features as well as renal outcomes in IgAN patients.
Results
Demographic and clinicopathlogical features of the patients
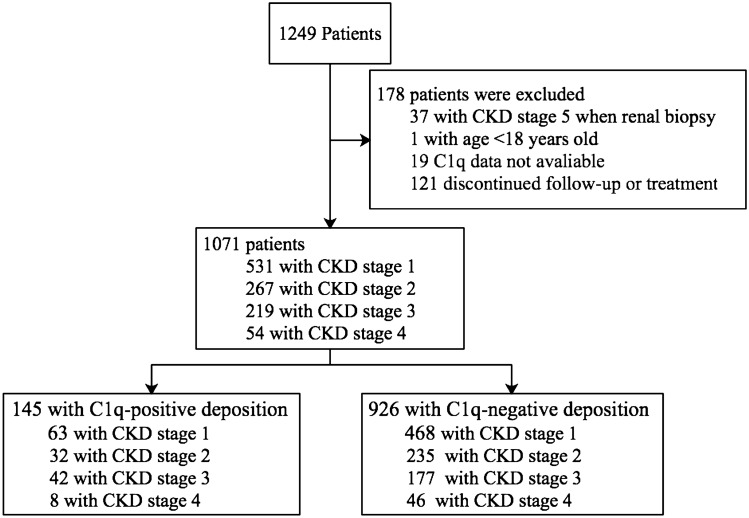
1249 IgAN patients from the study centers were enrolled. A total of 178 patients were excluded because of incomplete data, and 1071 patients were included in the analysis (Fig. 1). The average follow-up period was 40.90 ± 24.19 and 41.89 ± 22.85 months before and after adjustment for propensity scores, respectively. Comparison between C1q-positive (n = 145, 13.54%; 1 + , n = 114; 2 + , n = 30; 3 + , n = 1) and C1q-negative (n = 926, 86.46%) patients indicated that C1q deposition was associated with more severe clinical and pathologic features, such as higher level of mean arterial blood pressure (MAP), 24 h urine protein, and lower level of eGFR and serum albumin (Table 1). Corticosteroids were more frequently used in patients with C1q deposition (42.07% vs 35.53%; see Table 1). After 1:4 PS matching, 145 C1q-positive patients were matched with 580 C1q-negative patients. The standardized mean difference was 0.213 before matching, decreased to 0.065 after matching for age, gender and treatment, consistent with corrected bias between the C1q positive and negative patients. Significant differences in eGFR, IgG deposition, IgM deposition, C4 deposition, E1, T1/T2 and C1/C2 lesions were also observed between C1q positive and negative patients (Tables 1 and 2; Supplementary Tables S1 and S2).
Figure 1.
Study profile. CKD chronic kidney disease.
Table 1.
Demographic and clinical features of IgAN patients.
| Characteristics | All (n = 1071) | Groups | ||||
|---|---|---|---|---|---|---|
| C1q-positive (n = 145) | Unmatched cohort C1q-negative (n = 926) |
P value | Matched Cohort (1:4 PSM) C1q-negative (n = 580) |
P value | ||
| Clinical | ||||||
| Age (year) | 33.80 ± 10.96 | 33.18 ± 10.94 | 33.89 ± 10.97 | 0.466 | 33.99 ± 11.24 | 0.431 |
| Male (%) | 464(43.32) | 57(39.31) | 407(43.95) | 0.290 | 266 (45.86) | 0.156 |
| SBP (mmHg) | 130.63 ± 20.27 | 133.50 ± 21.36 | 130.18 ± 20.07 | 0.066 | 130.17 ± 19.38 | 0.070 |
| DBP (mmHg) | 83.58 ± 14.21 | 85.78 ± 14.92 | 83.24 ± 14.07 | 0.045 | 83.43 ± 14.21 | 0.078 |
| MAP (mmHg) | 99.26 ± 15.35 | 101.69 ± 16.45 | 98.88 ± 15.14 | 0.041 | 99.01 ± 15.04 | 0.060 |
| Hypertension (%) | 374 (34.92) | 58 (40.00) | 316 (34.13) | 0.168 | 198 (34.14) | 0.186 |
| Serum creatinine (µmol/L) | 100.76 ± 53.06 | 107.60 ± 53.70 | 99.69 ± 52.90 | 0.095 | 99.88 ± 53.49 | 0.121 |
| eGFR (ml/min per 1.73 m2) | 87.48 ± 49.72 | 81.03 ± 34.84 | 88.49 ± 51.60 | 0.027 | 87.26 ± 32.87 | 0.044 |
| Urinary Protein (g/24 h) | 2.67 ± 2.99 | 3.27 ± 2.74 | 2.58 ± 3.02 | 0.010 | 2.78 ± 3.22 | 0.092 |
| Serum albumin (g/L) | 38.15 ± 12.51 | 35.90 ± 7.96 | 38.50 ± 13.05 | 0.020 | 38.22 ± 15.62 | 0.084 |
| Uric acid (µmol/L) | 378.79 ± 158.08 | 377.05 ± 106.72 | 379.06 ± 164.72 | 0.887 | 380.11 ± 190.48 | 0.852 |
| CKD stage | 0.049 | 380.11 ± 190.48 | 0.059 | |||
| Stage 1 | 531 (49.58) | 63 (43.45) | 468 (50.54) | 294 (50.69) | ||
| Stage 2 | 267 (24.93) | 32 (22.07) | 235 (25.38) | 148 (25.52) | ||
| Stage 3 | 219 (20.45) | 42 (28.97) | 177 (19.11) | 110 (18.97) | ||
| Stage 4 | 54 (5.04) | 8 (5.52) | 46 (4.97) | 28 (4.83) | ||
| Treatment | 0.043 | 0.951 | ||||
| SC | 411 (38.38) | 42 (28.97) | 369 (39.85) | 168 (28.97) | ||
| CS | 390 (36.41) | 61 (42.07) | 329 (35.53) | 251 (43.28) | ||
| IT | 270 (25.21) | 42 (28.97) | 228 (24.62) | 161 (27.76) | ||
Values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation or median (interquartile range).
SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial pressure, eGFR estimated glomerular filtration rate, CKD chronic kidney disease, SC supportive care group, CS corticosteroids, IT immunosuppressive therapy.
Table 2.
Pathologic features of IgAN patients.
| Characteristics | All (n = 1071) | Groups | ||||
|---|---|---|---|---|---|---|
| C1q-positive (n = 145) | Unmatched cohort C1q-negative (n = 926) |
P value | Matched Cohort (1:4 PSM) C1q-negative (n = 580) |
P value | ||
| Pathologic | ||||||
| Oxford classification | ||||||
| M1 | 805 (75.16) | 118 (81.38) | 687 (74.19) | 0.062 | 441 (76.03) | 0.171 |
| E1 | 56 (5.23) | 19 (13.10) | 37 (4.00) | < 0.001 | 28 (4.83) | < 0.001 |
| S | 542 (50.61) | 66 (45.52) | 476 (51.40) | 0.187 | 307 (52.93) | 0.110 |
| T0 | 836 (78.06) | 102 (70.34) | 734 (79.27) | 0.007 | 456 (78.62) | 0.044 |
| T1 | 190 (17.74) | 39 (26.90) | 151(16.31) | 103 (17.76) | ||
| T2 | 45 (4.20) | 4 (2.76) | 41 (4.43) | 21 (3.62) | ||
| C0 | 762 (71.15) | 92 (63.45) | 670 (72.35) | < 0.001 | 407 (70.17) | 0.004 |
| C1 | 243 (22.69) | 33 (22.76) | 210 (22.68) | 140 (24.14) | ||
| C2 | 66 (6.16) | 20 (13.79) | 46 (4.97) | 33 (5.69) | ||
| IgA deposition | 0.530 | 0.440 | ||||
| 1 + | 116 (10.83) | 20 (13.79) | 96 (10.37) | 58 (10.00) | ||
| 2 + | 495 (46.22) | 62 (42.76) | 433 (46.76) | 276 (47.59) | ||
| 3 + | 454 (42.39) | 62 (42.76) | 392 (42.33) | 244 (42.07) | ||
| 4 + | 6 (0.56%) | 1 (0.69) | 5 (0.54) | 2 (0.34) | ||
| IgG deposition | 97 (9.06) | 32 (22.07) | 65 (7.02) | < 0.001 | 40 (6.90) | < 0.001 |
| IgM deposition | 521 (48.65) | 127 (87.59) | 394 (42.55) | < 0.001 | 251 (43.28) | < 0.001 |
| C3 deposition | 876 (81.79) | 124 (85.52) | 752 (81.21) | 0.211 | 458 (78.97) | 0.076 |
| C4 deposition | 52 (4.86) | 33 (22.76) | 19 (2.05) | < 0.001 | 11 (1.90) | < 0.001 |
Values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation or median (interquartile range).
M mesangial proliferation, E endocapillary proliferation, S segmental sclerosis, T tubular atrophy/interstitial fibrosis, C crescents.
Clinical outcomes in IgAN patients
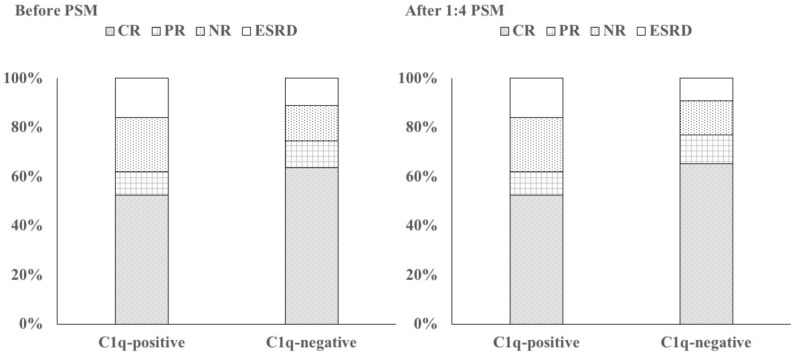
The clinical outcomes in IgAN patients are shown in Table 3; Supplementary Table S3, Fig. 2 and supplementary Fig. S1. Of all the 1071 patients, 62.09% (665) patients achieved CR, 10.83% (116) patients reached PR, 15.41% (165) patients ended in NR, and 11.67% (125) patients progressed to ESRD. Patients in the C1q-positive group had considerably lower incidence of CR and PR and higher incidence of ESRD than is observed in the unmatched C1q negative (p = 0.05) and matched C1q negative (p = 0.003) groups (Table 3 and Supplementary Table S3).
Table 3.
Clinical outcome of IgAN patients.
| CR | PR | NR | ESRD/death | |
|---|---|---|---|---|
| Before PS matching (n = 1071) | ||||
| C1-positive (n = 145) | 76 (52.4%) | 14 (9.7%) | 32 (22.1%) | 23 (15.9%) |
| C1-negative (n = 926) | 589 (63.6%) | 102 (11.0%) | 133 (14.4%) | 102 (11.0%) |
| After PS matching (n = 725, 1:4PSM) | ||||
| C1-positive (n = 145) | 76 (52.4%) | 14 (9.7%) | 32 (22.1%) | 23 (15.9%) |
| C1-negative (n = 580) | 378 (65.2%) | 69 (11.9%) | 79 (13.6%) | 54 (9.3%) |
Values for categorical variables are given as number (percentage).
CR complete remission, PR partial remission, NR no response, ESRD end stage renal disease.
Figure 2.
Treatment response and renal outcomes in IgAN patients. CR complete remission, PR partial remission, NR no response, ESRD end stage renal disease.
Patient and renal survival
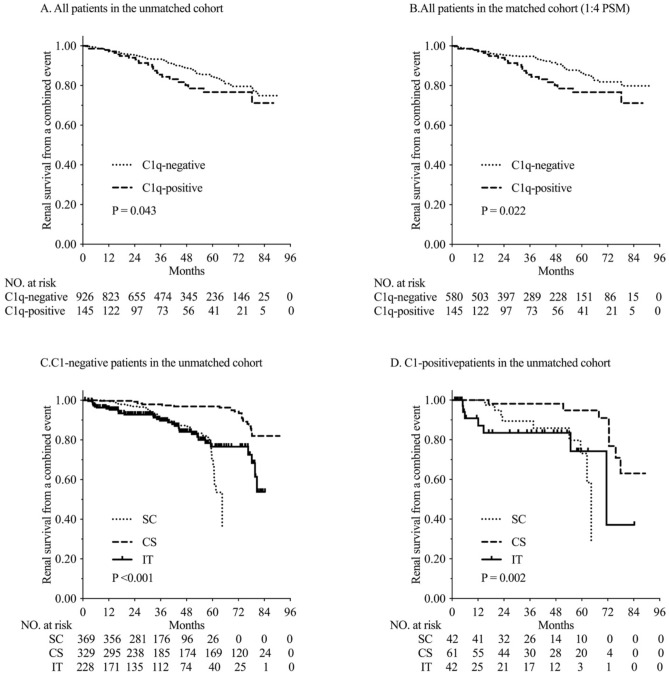
One patient with C1q deposition died from pneumonia after 2 month of corticosteroids treatment. No patient without C1q deposition died during follow up. More patients with C1q deposition (15.86%) reached the combined endpoint (50% decline of eGFR and/or ESRD or death) during follow up period than patients without C1q deposition before (11.01%) and after (9.31%) PS matching (all p < 0.05). The renal survival rates based on a 50% decline of eGFR, ESRD or death were higher in the C1q-negative patients than in the C1q-positive patients, before (p = 0.04) and after PS matching (p = 0.02). Furthermore, the 3, 5 and 7-year renal survival rates, assessed in terms of combined endpoints, were significantly lower in the C1q-positive patients (88.28%, 84.14% and 83.45%, respectively) compared with the unmatched C1q negative (94.82%, 91.79% and 88.98%, respectively) and matched C1q negative (95.52%, 92.07% and 90.69%, respectively) patients (Fig. 3 and Supplementary Fig. S2).
Figure 3.
Kaplan–Meier analysis for the probability of composite endpoints.
For C1q-positive and C1q-nepative patients before PS matching, we found that CS could both improve the renal survival compared with SC and IT groups (P < 0.001; Fig. 3C,D); while there was no significant difference between SC and IT groups. Further analysis revealed that kidney survival rates in C1q-negative patients received corticosteroids (CS group) were remarkably higher than that in C1q-positive patients (93.01% vs 86.89%, P < 0.05; Fig. 3C,D).
Renal failure predictors
With 50% decline in eGFR and/or ESRD as the combined endpoint, multivariate Cox regression analysis of the unmatched C1q-negative and C1q-positive patients suggested that independent risk factors of poor renal survival include Scr, urinary protein level, T1 or T2 lesion, C1q deposition and C3 deposition. After PS matching, Scr, urinary protein level, T1 or T2 lesion, C1q deposition were also recognized as independent risk factors (Table 4 and Supplementary Table S4).
Table 4.
Cox proportional hazard model for the primary endpoint in IgA nephropathy patients with CKD.
| Unmatched cohort | Matched cohort | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Hypertension | 1.226 (0.792–1.898) | 0.361 | 4.397 (2.485–7.782) | < 0.001 |
| Serum creatinine | 1.007 (1.004–1.009) | < 0.001 | 1.008 (1.004–1.012) | < 0.001 |
| Urinary protein | 1.093 (1.023–1.168) | 0.009 | 1.100 (1.039–1.164) | 0.001 |
| Pathologic | ||||
| Oxford | ||||
| M1 | 0.463 (0.298–0.721) | 0.001 | 2.146 (0.833–5.527) | 0.114 |
| E1 | 1.877 (0.883–3.991) | 0.102 | 3.210 (1.277–8.069) | 0.013 |
| S | 0.921 (0.637–1.331) | 0.662 | 1.209 (0.722–2.025) | 0.470 |
| T0 | 1 | – | 1 | – |
| T1 | 4.305 (2.577–7.192) | < 0.001 | 3.915 (2.010–7.627) | < 0.001 |
| T2 | 6.543 (3.581–11.955) | < 0.001 | 9.677 (3.963–23.631) | < 0.001 |
| C1/C2 | 0.835 (0.540–1.291) | 0.417 | 0.639 (0.366–1.115) | 0.115 |
| C1q deposition | 1.659 (1.039–2.650) | 0.034 | 1.948 (1.120–3.387) | 0.018 |
| C3 deposition | 1.965 (1.186–3.305) | 0.011 | 0.960 (0.463–1.988) | 0.912 |
| C4 deposition | 0.792 (0.335–1.876) | 0.597 | 0.422 (0.155–1.149) | 0.091 |
HR hazard ratio, 95% CI 95% confidence interval, M mesangial proliferation, E endocapillary proliferation, S segmental sclerosis, T tubular atrophy/interstitial fibrosis, C crescents.
Discussion
Activation of the complement system is one of the key participants in the injuries related to IgAN. Activation of the classical complement pathway is initiated after binding of C1q to immune complexes or charged molecules. However, because C1q deposits are not essential for the definitive diagnosis of IgAN, little attention has been paid to its clinical significance. Thus, further research is needed to explore the utility of C1q as a diagnostic or prognostic tool in IgAN patients. C1q deposition rates vary in IgAN patients11–16, and possible explanations for the discrepancies among studies may be related to differences in race, age, gender ratio, urine protein and methods of analysis.
The presence of substantial C1q deposition raises the possibility of lupus nephritis with conspicuous IgA deposition. Therefore, patients with lupus nephritis were excluded in this study if endothelial tubuloreticular inclusions were observed on electron microscopy and/or the presence of positive antinuclear antibodies, as per the Kidney Disease Improving Global Outcomes (KDIGO) and American College of Rheumatology (ACR) guidelines. On one hand, similar results were seen in our study no matter the 1: 4 propensity score matching (PSM) or 1:1 PSM was used in this study after calculating a propensity score (PS) for each study patient. On the other hand, in the present study, we demonstrate that C1q positive patients had worse clinical features than both matched and unmatched C1q negative patients. Patients with C1q deposition presented with lower baseline eGFR, rather than severer proteinuria, consistent with activation of the classic complement pathway as a critical determinant in the pathogenesis and clinical outcomes in IgAN. Some prior studies suggested no association between C1q deposition and clinical features in IgAN patients11, while other studies have reported that mesangial C1q deposition is associated with poor renal outcomes in patients with IgAN11,15, and the slope of eGFR (ΔeGFR/Month) decline was steeper in the C1q-positive patients over a 4-year follow-up11. Other studies have also suggested that patients with C1q or IgM deposition had heavier proteinuria than patients without C1q deposition15,16, and in agreement with our study, it was suggested that mesangial C1q deposition was associated with higher Lee’s glomerular grade of IgAN (i.e. severer pathologic changes)11. Collectively, our observations and those of others suggest that mesangial C1q deposition portends poor renal survival in IgAN patients.
To our knowledge, our study is the first to report association between C1q deposition and Oxford classification of renal pathological changes (MESTC) in IgAN. We observe higher rates of endocapillary hypercellularity (E1), tubular atrophy or interstitial fibrosis (T1 or T2), cellular or fibrocellular crescents (C1 or C2), IgG deposition, IgM deposition and C4 deposition in the C1q positive patients compared with the C1q negative patients (p < 0.05), consistent with worse pathological features in C1q-positive patients. Formation of glycan-specific IgG or IgM autoantibodies, which recognize undergalactosylated IgA1 molecules leads to C1q deposition and is crucial for the pathogenesis of IgAN9,17. Furthermore, this study indicates that C4 deposits in the absence of C1q are rare in IgAN (2% of biopsies), suggesting that C4 is activated through the classical pathway. Based on these findings, we speculate that mesangial C1q deposition may induce persistent inflammatory response, leading to endothelial proliferation and cellular crescent formation. Absent effective therapy, these pathological lesions transform into chronic renal fibrosis. Therefore, mesangial C1q deposition in IgAN patients should be considered as an indicator of more aggressive disease, and thus warrants treatment with corticosteroids or immunosuppression.
We found that patients in the C1q-negative group achieved more clinical remission (CR and PR), and had less progression to ESRD than C1q-positive patients (p < 0.05). Furthermore, 15.86% C1q-positive patients reached with combined endpoint (50% decline of eGFR and/or ESRD or death) at a median of about 3.5 years, which would equate to 84.14% of the cohort at 22 years. Even patients without C1q staining showed severe disease with a predicted 65.32% of the cohort reaching the combined endpoint at 22 years. Besides, in terms of Kaplan–Meier survival analysis, the 3, 5 and 7-year renal survival rates in C1q-negative patients were significantly better than in the C1q-positive patients (p < 0.05). Furthermore, corticosteroids were more frequently used in patients with C1q deposition and corticosteroids could improve the renal survival of them.
Cumulative evidence suggests an association between pathological changes and clinical prognosis in IgAN. Several studies have already reported that impaired kidney function, sustained hypertension and renal tubular interstitial atrophy (T1/ T2) were independent risk factors for poor prognosis in IgAN15,18,19. Consistently, COX multivariate analysis in our study suggested that Scr, urinary protein, T1 or T2 lesion and C1q deposition were independent indicators of worse renal outcome in IgAN patients. However, few studies reported about the significance of C1q deposition in the pathogenesis of IgAN. In addition, our study recruited more patients and the follow-up was longer. Lastly, PS matching method was used in the current study to minimize bias between patients, which improved the accuracy and reliability of our results.
Based on the findings of our study, we speculate that activation of the classical pathway of complement system plays an important role in the pathogenesis of IgAN. IgG/IgM and C1q co-deposition may initiate the classical complement pathway through activation of C2, C4 and C3—to form the membrane attack complex (MAC), which eventually causes renal inflammation and fibrosis. Although activation of the alternative or lectin pathways of complement were thought to be more important in the pathogenesis of IgAN1,9, our results suggest that focused investigations on activation of the classical complement pathway may be warranted—to better understand the pathogenesis of IgAN.
Although we have found several important findings, limitations of our study should also be noted. First, because of the observational design of this study, it is difficult to determine whether prospective intervention and treatment will affect the prognosis of patients with C1q deposition. Second, all patients included in this study were from a single ethnicity (Han Chinese) and the levels of serum C3 and C4 weren't shown in the study. Finally, although we performed PS matching analysis to minimize bias between C1q positive and negative patients, we could only include age, gender, and treatment modality in the PS model; therefore, other confounding factors may exist. Hence, the association between C1q deposition and renal outcomes in IgAN patients must be interpreted with caution.
In conclusion, glomerular deposition of C1q is associated with worse clinicopathologic features and renal outcomes in IgAN patients. Mesangial C1q deposition is an independent risk factor for poor renal prognosis in IgAN.
Methods
Patients
Biopsy-proven primary IgAN from patients aged 18 to 75 years were recruited from 4 nephrology centers (West China Hospital, Affiliated Hospital of Zunyi Medical University, Zigong Third People’s Hospital and People’s Hospital of Mianzu city) between January 2013 and January 2017. Inclusion criteria were: (1) Biopsy-proven diagnosis of IgAN and no less than 10 glomeruli the biopsy specimen; (2) the patient had no history of kidney transplant surgery. Exclusion criteria were: (1) secondary IgAN due to systemic diseases such as systemic lupus erythematosus, Henoch–Schönlein purpura and liver cirrhosis, etc.; (2) C1q nephropathy; (3) pregnancy; (4) hepatitis B virus carriers and other chronic liver diseases; (5) baseline estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73 m2. The levels of autoimmune antibodies (such as ANA, anti-dsDNA, anti-ENA and anti-Smith) in all biopsy-proven IgAN patients enrolled in the study were negative, thus, these patients were not diagnosed with systemic lupus erythematosus (SLE) according to American College of Rheumatology Guidelines.
Patients were followed up for at least 12 months or shorter if they reached endpoint of study. Patients were followed up monthly in study centers. This research complies with the principles of the Helsinki Declaration and was approved by The Ethics Committee of West China Hospital of Sichuan University, Affiliated Hospital of Zunyi Medical University, Zigong Third People’s Hospital and People’s Hospital of Mianzu city. Written informed consent was obtained from all patients before enrollment into the study.
Measurements and renal pathology data
Clinical indexes (gender, age, medical history, blood pressure) and laboratory data such as serum albumin (Alb), serum creatinine (Scr), uric acid (UA), 24 h urinary protein at the time of biopsy were collected. Estimated GFR (eGFR) was evaluated according to CKD-EPI (CKD Epidemiology Collaboration) formula20.
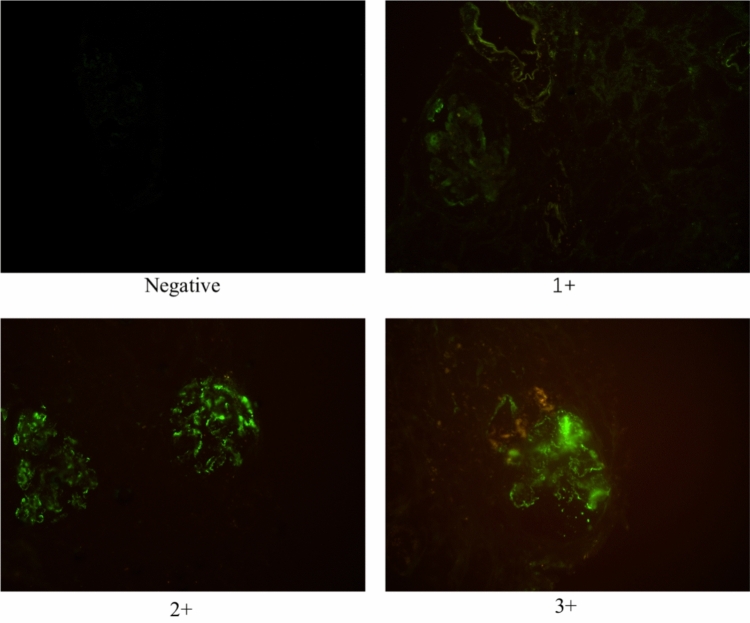
All patients included in the study underwent ultrasound-guided percutaneous renal biopsy. All kidney specimens were obtained by percutaneous needle biopsy and routinely processed for light microscopy, immunofluorescence, and electron microscopy to detect pathologic alterations in the kidney. Serial 2–3 µm thick sections were stained with hematoxylin and eosin (H&E), periodic acid-Schiff, Masson’s trichrome, and periodic acid-methenamine for light microscopy. For immunofluorescence, we used antibodies against IgA, IgG, IgM, C3, C4, C1q, kappa and lambda light chains. The sections were washed thrice with PBS for 5 min each time, incubated with monoclonal rat anti-human C1q antibody (1:100) at 37 °C for 60 min, and washed thrice with PBS for 5 min each time. Then incubated with Alexa Fluor 488 polyclonal goat anti-rat secondary antibody (1:200), and imaged using fluorescence microscope. C1q-positive glomerular deposition was defined as diffuse mesangial and scored as (1 +), (2 +), (3 +); C1q-negative glomerular staining was defined as segmental deposition or absence of staining (shown in Fig. 4).
Figure 4.
C1q deposition in the mesangium from negative to 3 + (original magnification × 40).
Renal biopsy specimens of all patients were reviewed by at least two experienced pathologists and two nephrologists. The discrepancy in diagnosis between pathologists and nephrologists was resolved by reviewing the biopsies and coming to a consensus. When there were inconsistencies or doubts among pathologists and clinical investigators, these were submitted to a higher-level pathologist for review. Pathological lesions were graded according to the updated Oxford classification21: mesangial hypercellularity (M0/M1); endocapillary hypercellularity (E0/E1); segmental glomerulosclerosis (S0/S1); tubular atrophy / interstitial fibrosis (T0/T1/T2)21 and cellular or fibrocellular crescents (C0/C1/C2)22.
Treatment
Treatment modalities were recorded. Patients were treated with 3 different treatment strategies as described previously19: supportive care (SC, full dose angiotensin-converting-enzyme inhibitor or angiotensin receptor blockers), supportive care combined with corticosteroids (CS, 0.5–1 mg/kg prednisone daily, tapering down within 6–8 months) and supportive care plus corticosteroids and immunosuppressant therapy (IT, cyclophosphamide 2 mg/kg daily for 3 months, or MMF 1-2 g daily for 6–8 months, or cyclosporine 3–5 mg/kg daily for 6–8 months, or tacrolimus 0.03–0.05 mg/kg daily for 6–8 months).
Outcomes
Responses to therapy included complete remission (CR), partial remission (PR), no response (NR) and end stage renal disease (ESRD)19. CR was defined as urinary protein excretion < 0.5 g/24 h, with < 10% decrease in eGFR relative to baseline. PR was defined as > 50% decrease in proteinuria relative to baseline, with < 10% decrease in eGFR relative to baseline. NR was defined as < 50% decrease in proteinuria relative to baseline, or > 10% decrease in eGFR relative to baseline. ESRD was defined as eGFR < 15 mL/min/1.73 m2 or requiring maintenance renal replacement treatment. The primary endpoint was the combined endpoint of a 50% decline of eGFR and/or ESRD or death.
Statistical analysis
Categorical data were analyzed using Chi-square tests or Fischer’s exact test. Continuous variables were analyzed with unpaired t test or nonparametric Mann–Whitney U test. Categorical variables are presented as frequencies (percentages). Continuous variables are presented as mean ± standard deviation (SD) or median [interquartile range (IQR)]. Multivariate Cox regression analysis was used to evaluate the crude effect of clinical and pathological variables on the renal outcomes before and after adjusting for propensity scores. All statistical analyses were performed using R version 3.3.4 statistical software (The R Foundation for Statistical Computing) and Graphpad Prism7.0. Statistical significance was determined as p < 0.05.
Propensity score matching
Propensity score matching (PSM) is a statistical matching technique that attempts to estimate the effectiveness of treatments, policies, or other interventions by taking covariates into account23,24. PSM reduces the bias due to confounding variables including age, gender, and treatment modality. Therefore, a PSM approach based on the presence of C1q deposition in IgAN patients was used to minimize the effects of confounding factors24. The propensity score is calculated by the following conditional probability.
The caliper is defined by the maximum propensity score difference within the matched pair. Three methods of matching individuals with similar propensity scores are presented based on the concept of the caliper in the R package MatchIt25. PSM was applied to the KARE data to ensure homogeneity of demographic variables (covariates) between the C1q-positive and C1q-negative groups, using the R package MatchIt.
Since it was necessary to ensure balanced matches between the C1q-positive and C1q-negative groups, we manipulated the caliper (from 0 to 1) by increments of 0.01. We checked the p-values using the paired t-test and the Wilcoxon test to evaluate the homogeneity of the C1q-positive and C1q-negative propensity scores at each caliper increment and for each method of caliper choice. To ensure demographic homogeneity of the C1q-positive and C1q-negative groups, we only considered calipers for which the p-values of both the paired t-test and the Wilcoxon test were larger than 0.0526. To ensure balanced matches, a caliper and maximum allowable difference between two groups was defined as 0.1, resulting in a relatively narrow difference between matched subjects24. Standardized mean differences were estimated for all baseline covariates before and after matching to assess pre-match imbalance and post-match balance. Although a 1:1 ratio between matched subjects is most commonly used. However, a matching ratio of 1:4 was used for a larger number of control subjects than test subjects in order to improve study power. Finally, after calculating a propensity score (PS) for each study patient, all patients were assigned a 1:4 propensity score matching (PSM) with the occurrence of C1q deposition as the dependent variable to adjust for the observed characteristics of non-randomly assigned patients.
Ethical approval
The Ethics Committee of West China Hospital of Sichuan University, Affiliated Hospital of Zunyi Medical University, Zigong Third People’s Hospital and People’s Hospital of Mianzu city approved this prospective study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all patients before enrollment into the study.
Supplementary Information
Acknowledgements
Sincere thanks should be given to all of the participants and attending physicians for their contributions. This study was partly supported by the Science and Technology Planning Project of Sichuan Province (No. 2019YFS0280).
Author contributions
Conception and design: L.T., W.Q., Y.T.; administrative support: W.Q., Y.T.; collection and assembly of data L.T., G.-Q.P., Z.-X.Z., J.-X.T., L.Z., D.-M.W.; data analysis and interpretation: L.T.; manuscript writing: L.T., D.S.H., W.Q. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84715-7.
References
- 1.Wyatt RJ, Julian BA. IgA nephropathy. N. Engl. J. Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 2.Glassock RJ. The pathogenesis of IgA nephropathy. Curr. Opin. Nephrol. Hypertens. 2011;20:153–160. doi: 10.1097/MNH.0b013e3283436f5c. [DOI] [PubMed] [Google Scholar]
- 3.Yu HH, Chiang BL. Diagnosis and classification of IgA nephropathy. Autoimmun. Rev. 2014;13:556–559. doi: 10.1016/j.autrev.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S, et al. Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J. Am. Soc. Nephrol. JASN. 2016;27:1278–1287. doi: 10.1681/asn.2015060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, et al. Pathological demography of native patients in a nephrology center in China. Chin. Med. J. 2003;116:1377–1381. [PubMed] [Google Scholar]
- 6.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–923. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, et al. Decreased serum C3 levels in immunoglobulin A (IgA) nephropathy with chronic kidney disease: A propensity score matching study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017;23:673–681. doi: 10.12659/MSM.903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roufosse CA, Cook HT. Pathological predictors of prognosis in immunoglobulin A nephropathy: a review. Curr. Opin. Nephrol. Hypertens. 2009;18:212–219. doi: 10.1097/MNH.0b013e328329605c. [DOI] [PubMed] [Google Scholar]
- 9.Berger SP, Roos A, Daha MR. Complement and the kidney: What the nephrologist needs to know in 2006? Nephrol. Dial. Transplant. 2005;20:2613–2619. doi: 10.1093/ndt/gfi166. [DOI] [PubMed] [Google Scholar]
- 10.Maillard N, et al. Current understanding of the role of complement in IgA nephropathy. J. Am. Society Nephrol. JASN. 2015;26:1503–1512. doi: 10.1681/asn.2014101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, et al. Association of C1q deposition with renal outcomes in IgA nephropathy. Clin. Nephrol. 2013;80:98–104. doi: 10.5414/cn107854. [DOI] [PubMed] [Google Scholar]
- 12.Jennette JC. The immunohistology of IgA nephropathy. Am. J. Kidney Dis. 1988;12:348–352. doi: 10.1016/S0272-6386(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 13.Woodroffe AJ, et al. Immunologic studies in IgA nephropathy. Kidney Int. 1980;18:366–374. doi: 10.1038/ki.1980.147. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura M, et al. Significance of IgA deposits on the glomerular capillary walls in IgA nephropathy. Am. J. Kidney Dis. 1987;9:404–409. doi: 10.1016/S0272-6386(87)80143-9. [DOI] [PubMed] [Google Scholar]
- 15.Nishiwaki H, et al. Absence of mesangial C1q deposition is associated with resolution of proteinuria and hematuria after tonsillectomy plus steroid pulse therapy for immunoglobulin a nephropathy. Nephron. 2015;130:1–7. doi: 10.1159/000381217. [DOI] [PubMed] [Google Scholar]
- 16.Thomas R. W. & James. M. Immunoglobulin M and C1q Mesangial Labeling in IgA Nephropathy. Am. J. Kidney Dis.32, pp 589–592 (1998). [DOI] [PubMed]
- 17.Lai KN. Pathogenesis of IgA nephropathy. Nat. Rev. Nephrol. 2012;8:275–283. doi: 10.1038/nrneph.2012.58. [DOI] [PubMed] [Google Scholar]
- 18.Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis Work GroupKDIGO Clinical Practice Guideline for Glomerulonephritis. Immunoglobulin A nephropathy. Kidney Int Suppl2, 139–274 (2012).
- 19.Tan L, Tang Y, Peng W, Mathew BS, Qin W. Combined immunosuppressive treatment may improve short-term renal outcomes in chinese patients with advanced IgA nephropathy. Kidney Blood Press. Res. 2018;43:1333–1343. doi: 10.1159/000492592. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J. Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cattran DC, et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 22.Trimarchi H, et al. Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu. Rev. Public Health. 2000;21:121–145. doi: 10.1146/annurev.publhealth.21.1.121. [DOI] [PubMed] [Google Scholar]
- 24.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998;17:2265–2281. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Ho D, Imai K, King G, Stuart EA. MatchIt: Nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011;42:1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 26.Park C, Jiang N, Park T. Pure additive contribution of genetic variants to a risk prediction model using propensity score matching: application to type 2 diabetes. Genom. Inf. 2019;17:e47. doi: 10.5808/GI.2019.17.4.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.