Key Points
Question
What is the prevalence of polypharmacy with central nervous system (CNS)–active medications among community-dwelling older adults with dementia in the US?
Findings
In this cross-sectional analysis of 1 159 968 older adults with dementia, traditional Medicare, and prescription coverage in 2018, 13.9% were prescribed CNS-active polypharmacy, defined as overlapping prescription fills for 3 or more medications from the following drug classes: antidepressants, antipsychotics, antiepileptics, benzodiazepines, nonbenzodiazepine benzodiazepine receptor agonist hypnotics, and opioids.
Meaning
In 2018, 13.9% of older adults with dementia in the US had filled prescriptions consistent with CNS-active polypharmacy.
Abstract
Importance
Community-dwelling older adults with dementia have a high prevalence of psychotropic and opioid use. In these patients, central nervous system (CNS)–active polypharmacy may increase the risk for impaired cognition, fall-related injury, and death.
Objective
To determine the extent of CNS-active polypharmacy among community-dwelling older adults with dementia in the US.
Design, Setting, and Participants
Cross-sectional analysis of all community-dwelling older adults with dementia (identified by International Classification of Diseases, Ninth Revision, Clinical Modification or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes; N = 1 159 968) and traditional Medicare coverage from 2015 to 2017. Medication exposure was estimated using prescription fills between October 1, 2017, and December 31, 2018.
Exposures
Part D coverage during the observation year (January 1-December 31, 2018).
Main Outcomes and Measures
The primary outcome was the prevalence of CNS-active polypharmacy in 2018, defined as exposure to 3 or more medications for longer than 30 days consecutively from the following classes: antidepressants, antipsychotics, antiepileptics, benzodiazepines, nonbenzodiazepine benzodiazepine receptor agonist hypnotics, and opioids. Among those who met the criterion for polypharmacy, duration of exposure, number of distinct medications and classes prescribed, common class combinations, and the most commonly used CNS-active medications also were determined.
Results
The study included 1 159 968 older adults with dementia (median age, 83.0 years [interquartile range {IQR}, 77.0-88.6 years]; 65.2% were female), of whom 13.9% (n = 161 412) met the criterion for CNS-active polypharmacy (32 139 610 polypharmacy-days of exposure). Those with CNS-active polypharmacy had a median age of 79.4 years (IQR, 74.0-85.5 years) and 71.2% were female. Among those who met the criterion for CNS-active polypharmacy, the median number of polypharmacy-days was 193 (IQR, 88-315 polypharmacy-days). Of those with CNS-active polypharmacy, 57.8% were exposed for longer than 180 days and 6.8% for 365 days; 29.4% were exposed to 5 or more medications and 5.2% were exposed to 5 or more medication classes. Ninety-two percent of polypharmacy-days included an antidepressant, 47.1% included an antipsychotic, and 40.7% included a benzodiazepine. The most common medication class combination included an antidepressant, an antiepileptic, and an antipsychotic (12.9% of polypharmacy-days). Gabapentin was the most common medication and was associated with 33.0% of polypharmacy-days.
Conclusions and Relevance
In this cross-sectional analysis of Medicare claims data, 13.9% of older adults with dementia in 2018 filled prescriptions consistent with CNS-active polypharmacy. The lack of information on prescribing indications limits judgments about clinical appropriateness of medication combinations for individual patients.
This pharmacoepidemiology study uses Medicare claims data to describe the prevalence of central nervous system–active polypharmacy among community-dwelling older adults with dementia in the US, including durations of exposure and number of drugs and drug classes.
Introduction
The number of persons with dementia in the US is projected to grow to 50 million people by 2050,1 but the US health care system is poorly equipped to deal with these patients and their complex medical and psychosocial needs.2 Although memory impairment is the cardinal feature of dementia, behavioral and psychological symptoms (eg, apathy, delusions, agitation) are common during all stages of illness and cause significant caregiver distress.3,4 Despite limited high-quality evidence of efficacy for pharmacological treatment,5 clinicians regularly prescribe psychotropic medications to community-dwelling persons with dementia6 in rates that far exceed use in the general older adult population.7
Central nervous system (CNS)–active polypharmacy (defined as combinations of multiple psychotropic or opioid medications or both psychotropic and opioid medications) has increased among older adults overall.8 The potential for such prescribing may be particularly high among persons with dementia because medications accumulate in response to pervasive but transient behavioral symptoms. The Beers Criteria from the American Geriatrics Society advise against such polypharmacy given its association with increased fall risk.9 These medications may also cause impaired cognition,10 cardiac conduction abnormalities,11 and respiratory suppression and death when involving opioids.12 Given the high degree of comorbid medical illness and frailty among persons with dementia, associated risks from drug-drug and drug-disease interactions may be even greater than among older adults overall. In addition, persons with dementia may have difficulty articulating any adverse effects they are experiencing.
The limited data on CNS-active polypharmacy among persons with dementia are from Europe and do not include antiepileptic medications or opioids, thereby significantly underestimating the true extent of exposure to such combinations.13,14,15 In light of pervasive psychotropic and opioid prescribing to persons with dementia in the US,6 as well as the extent of CNS-active polypharmacy among older adults overall,8 this analysis sought to determine the prevalence of polypharmacy among older adults with dementia living in the community, including the duration of exposure, the type of medication class combinations, and the most commonly prescribed individual medications.
Methods
The Michigan Medicine institutional review board approved this study. Informed consent was waived for this observational data analysis.
Cohort Identification
The study population included Medicare beneficiaries with continuous traditional fee-for-service (ie, Parts A and B but not Medicare Advantage) coverage between January 1, 2015, and December 31, 2017 (Figure).
Figure. Study Population of Older Adults With Dementia for Assessment of Central Nervous System (CNS)–Active Polypharmacy.
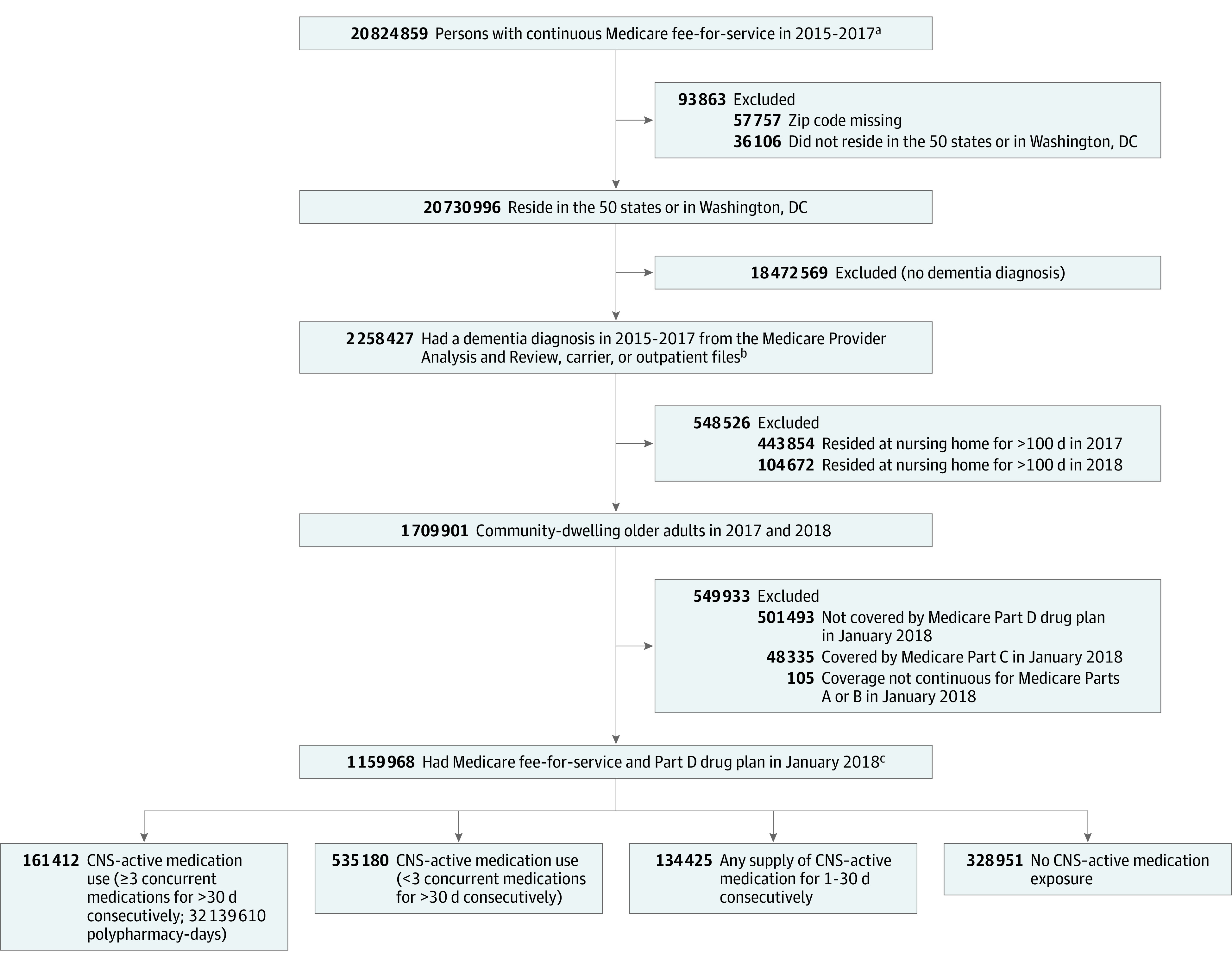
aFrom January 2015 to December 2017, persons had continuous Medicare Part A fee-for-service coverage (primarily covers inpatient and skilled nursing services) and Part B fee-for-service coverage (covers physician and outpatient services) but not Part C coverage (Medicare Advantage; beneficiaries enroll in a private health plan that delivers Medicare-covered Part A and Part B benefits).
bThe Medicare Provider Analysis and Review file contains billing records (ie, claims) for inpatient care encounters (acute and skilled nursing). Outpatient encounters are captured in the carrier file (claims from noninstitutional clinicians such as physicians) and the outpatient file (claims for outpatient services from institutional clinicians, some of whom may also provide hospital services through Part A coverage).
cA sensitivity analysis also was conducted that removed the 182 150 who died during 2018; among the 977 818 survivors, 142 602 experienced CNS-active polypharmacy (29 888 544 polypharmacy-days). Medicare beneficiaries do not have to enroll in Part D (prescription drug coverage) but in this study those without it were excluded (see prior box).
The cohort was limited to those with at least 1 International Classification of Diseases, Ninth Revision, Clinical Modification or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision dementia diagnosis code (eTable 1 in the Supplement) from a health care encounter that occurred from 2015 to 2017 using the inpatient (Medicare Provider Analysis and Review), carrier, and outpatient files; a period of 3 years was used for cohort identification to achieve greater sensitivity (0.80).16
The outcome observation period was calendar year 2018. Because of the requirement of 3 years of Medicare coverage to identify dementia, cohort members were aged 68 years or older at the start of the 2018 observation year. To limit the analysis to community-dwelling beneficiaries, those who had been long-stay nursing home residents (ie, >100 days in long-term care17) in 2017 or 2018 were excluded. To be included in the final study cohort, beneficiaries with dementia were required to have Part D prescription drug coverage on January 1, 2018. The study cohort was followed up until the earliest of the censoring events (death, loss of Medicare fee-for-service coverage, enrollment in Medicare Advantage, or loss of Part D coverage) or the end of the observation year (December 31, 2018).
Polypharmacy Assessment and Outcomes
Based on the Beers Criteria from the American Geriatrics Society, the primary outcome was the prevalence of CNS-active polypharmacy in 2018 that was defined as concurrent exposure to 3 or more medications for longer than 30 days consecutively from the following 6 classes: antidepressants, antipsychotics, antiepileptics, benzodiazepines, nonbenzodiazepine benzodiazepine receptor agonist hypnotics (ie, z-drugs), and opioids.9 The Beers Criteria from 2015 did not include antiepileptics, but the updated Beers Criteria from 2019 did and were used for this analysis. Medications within each class were identified using the American Hospital Formulary Service (eTable 2 in the Supplement).
Medication exposure was estimated using prescription fills between October 1, 2017, and December 31, 2018 (ie, the observation year plus the 3 preceding months). Daily exposure to each CNS-active medication was determined using the fill date and days’ supply; early fills (ie, October 1-December 31, 2017) were included to account for prescriptions received before the observation year began. Central nervous system–active polypharmacy was considered present when a beneficiary was simultaneously exposed to 3 or more CNS-active medications for longer than 30 days consecutively during the 2018 observation year. The medications did not have to be from different classes (eg, exposure to 3 antidepressants or exposure to 2 antipsychotics plus a benzodiazepine for >30 days consecutively; both combinations would meet the polypharmacy definition) per the Beers Criteria.
The cohort was split into 4 mutually exclusive groups based on exposure to CNS-active medications during the observation year: (1) 3 or more concurrent medications for longer than 30 days consecutively (met the criteria for a polypharmacy episode), (2) less than 3 concurrent medications for longer than 30 days consecutively, (3) any supply of medication for 1 to 30 days consecutively, and (4) no supply (no exposure). An episode of polypharmacy continued as long as 3 or more CNS-active medications were present, even if the constituent medications changed.
The polypharmacy-days of exposure formed the basis of the analysis. In addition to determining the overall prevalence, additional secondary outcomes included: (1) the total number of polypharmacy-days, (2) the number of distinct medications and distinct classes prescribed, (3) the most common combinations of CNS-active medication classes, and (4) the most-prescribed individual medications that contributed to polypharmacy.
Statistical Analysis
For descriptive purposes, baseline characteristics for the cohort overall and by CNS-active medication group were determined using demographic and clinical data derived from the 2017 Medicare Master Beneficiary Summary File, the Medicare Provider Analysis and Review files, carrier files (claims from noninstitutional clinicians such as physicians), and outpatient files (claims for outpatient services from institutional clinicians, some of whom may also provide hospital services through Part A coverage).
Race/ethnicity was included in the analysis given racial disparities in CNS-active prescribing7 and was identified using the Research Triangle Institute race code variable available in the Medicare Master Beneficiary Summary File, which was developed by combining Social Security Administration data and beneficiary name and validated against beneficiary self-report in the Consumer Assessment of Healthcare Providers and Systems survey.18
Clinical characteristics included dementia type, overall comorbidity defined using the Elixhauser comorbidity index, and specific diagnoses potentially associated with CNS-active medication use, including mental health, insomnia, pain, and seizure diagnoses. Characteristics across CNS-active medication groups were compared using the Kruskal-Wallis test and the Mann-Whitney test for continuous variables (eg, age) and the χ2 test for categorical variables (eg, depression).
Among those with CNS-active polypharmacy, the number of polypharmacy-days during the 2018 observation year were identified by summing the number of polypharmacy episodes for each person until the censoring event or end of the observation year, whichever came first. To address the possibility that the findings were related to medication use during end-of-life care, a sensitivity analysis was completed examining the primary outcomes with the cohort limited to those who survived through 2018.
This analysis was primarily descriptive and should be interpreted as exploratory. Limited statistical comparisons were completed to compare demographic and clinical characteristics across the 4 CNS-active polypharmacy exposure groups. The α level was set at .05 and the tests were 2-sided.
When reporting percentages related to CNS-active medication use (eg, percentage of polypharmacy-days involving ≥1 antidepressant), 95% CIs are not presented because the interval is not evident when reported to 1 significant figure after the decimal point. Data processing and analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
The study included 1 159 968 older adults with dementia; their median age was 83.0 years (interquartile range [IQR], 77.0-88.6 years) and 65.2% were female. In 2018, 28.4% were not exposed to any CNS-active medication; 11.6% were exposed to at least 1 CNS-active medication for 30 days consecutively or fewer; and 46.1% were exposed to at least 1 CNS-active medication for longer than 30 days consecutively but did not experience a CNS-active polypharmacy episode (Table 1). Central nervous system–active polypharmacy occurred in 13.9% (n = 161 412), yielding a total of 32 139 610 person-days of exposure (polypharmacy-days).
Table 1. Cohort Characteristics by Exposure to Central Nervous System (CNS)–Active Medications and Overall.
| Characteristic | Exposure to CNS-active medications during 2018 observation year | Overall | |||
|---|---|---|---|---|---|
| ≥3 concurrent medications for >30 d consecutively (polypharmacy) | <3 concurrent medications for >30 d consecutively | Any supply of medication for 1-30 d consecutively | No exposure | ||
| Community-dwelling older adults with dementia in 2018, No. (%) | 161 412 (13.9) | 535 180 (46.1) | 134 425 (11.6) | 328 951 (28.4) | 1 159 968 (100) |
| Sex, No. (%) | |||||
| Female | 114 920 (71.2) | 359 909 (67.3) | 82 132 (61.1) | 198 992 (60.5) | 755 953 (65.2) |
| Male | 46 492 (28.8) | 175 271 (32.7) | 52 293 (38.9) | 129 959 (39.5) | 404 015 (34.8) |
| Age group, median (IQR), y | 79.4 (74.0-85.5) | 82.9 (77.1-88.4) | 83.7 (77.8-89.2) | 84.7 (78.8-89.9) | 83.0 (77.0-88.6) |
| 68-69 | 12 231 (7.6) | 19 889 (3.7) | 4408 (3.3) | 8912 (2.7) | 45 440 (3.9) |
| 70-74 | 35 715 (22.1) | 72 876 (13.6) | 16 574 (12.3) | 34 317 (10.4) | 159 482 (13.7) |
| 75-79 | 36 946 (22.9) | 105 205 (19.7) | 24 313 (18.1) | 54 348 (16.5) | 220 812 (19.0) |
| 80-84 | 32 937 (20.4) | 121 669 (22.7) | 29 978 (22.3) | 72 363 (22.0) | 256 947 (22.2) |
| 85-89 | 26 079 (16.2) | 118 373 (22.1) | 30 391 (22.6) | 78 958 (24.0) | 253 801 (21.9) |
| 90-94 | 13 724 (8.5) | 72 722 (13.6) | 20 516 (15.3) | 55 936 (17.0) | 162 898 (14.0) |
| ≥95 | 3780 (2.3) | 24 446 (4.6) | 8245 (6.1) | 24 117 (7.3) | 60 588 (5.2) |
| Race/ethnicity, No. (%)a | |||||
| Non-Hispanic White | 138 465 (86.2) | 440 899 (82.7) | 104 666 (78.2) | 251 368 (76.7) | 935 398 (81.0) |
| Non-Hispanic Black | 8123 (5.1) | 37 933 (7.1) | 13 617 (10.2) | 34 387 (10.5) | 94 060 (8.1) |
| Hispanic | 10 007 (6.2) | 33 300 (6.3) | 9263 (6.9) | 21 381 (6.5) | 73 951 (6.4) |
| Other | 4022 (2.5) | 20 817 (3.9) | 6352 (4.7) | 20 513 (6.3) | 51 704 (4.5) |
| Low-income subsidy status, No. (%)b | 58 381 (36.2) | 147 672 (27.6) | 36 913 (27.5) | 85 271 (25.9) | 328 237 (28.3) |
| Rurality, No. (%)c | |||||
| Urban | 138 651 (86.0) | 458 654 (85.8) | 114 440 (85.2) | 283 468 (86.2) | 995 213 (85.8) |
| Rural | 22 669 (14.1) | 76 197 (14.3) | 19 916 (14.8) | 45 307 (13.8) | 164 089 (14.2) |
| Dementia type, No. (%) | |||||
| Alzheimer | 65 730 (40.7) | 220 917 (41.3) | 48 870 (36.4) | 124 990 (38.0) | 460 507 (39.7) |
| Vascular | 28 971 (17.9) | 81 860 (15.3) | 17 935 (13.3) | 40 889 (12.4) | 169 655 (14.6) |
| With Lewy bodies | 7065 (4.4) | 16 906 (3.2) | 3186 (2.4) | 6719 (2.0) | 33 876 (2.9) |
| Frontotemporal | 3640 (2.3) | 9480 (1.8) | 1854 (1.4) | 4268 (1.3) | 19 242 (1.7) |
| Unspecifiedd | 75 602 (46.8) | 256 492 (47.9) | 72 582 (54.0) | 174 041 (52.9) | 578 717 (49.9) |
| Comorbiditiese | |||||
| Elixhauser comorbidity score, median (IQR)f | 5 (3-7) | 4 (2-6) | 4 (2-6) | 3 (1-5) | 4 (2-6) |
| Noncancer pain | 138 123 (85.6) | 407 518 (76.1) | 101 050 (75.2) | 204 954 (62.3) | 851 645 (73.4) |
| Arthritis | 87 751 (54.4) | 216 065 (40.4) | 54 705 (40.7) | 90 896 (27.6) | 449 417 (38.7) |
| Back pain | 71 933 (44.6) | 159 720 (29.8) | 38 141 (28.4) | 55 932 (17.0) | 325 726 (28.1) |
| Neuropathic pain | 30 219 (18.7) | 66 579 (12.4) | 14 052 (10.5) | 22 220 (6.8) | 133 070 (11.5) |
| Headache | 12 719 (7.9) | 25 829 (4.8) | 5824 (4.3) | 7943 (2.4) | 52 315 (4.5) |
| Fibromyalgia | 12 173 (7.5) | 16 398 (3.1) | 3168 (2.4) | 4026 (1.2) | 35 765 (3.1) |
| Migraine headache | 4824 (3.0) | 6377 (1.2) | 1132 (0.8) | 1357 (0.4) | 13 690 (1.2) |
| Psychogenic | 511 (0.3) | 316 (0.1) | 56 (<0.1) | 57 (<0.1) | 940 (0.1) |
| Depression | 77 658 (48.1) | 144 351 (27.0) | 17 343 (12.9) | 19 734 (6.0) | 259 086 (22.3) |
| Anxiety | 71 510 (44.3) | 123 680 (23.1) | 19 709 (14.7) | 23 507 (7.1) | 238 406 (20.6) |
| Cancer | 21 011 (13.0) | 76 783 (14.3) | 23 261 (17.3) | 45 528 (13.8) | 166 583 (14.4) |
| Cancer-related pain | 327 (0.2) | 576 (0.1) | 279 (0.2) | 266 (0.1) | 1448 (0.1) |
| Insomnia | 24 134 (15.0) | 39 154 (7.3) | 7056 (5.2) | 8293 (2.5) | 78 637 (6.8) |
| Seizure disorder | 15 217 (9.4) | 23 803 (4.4) | 3110 (2.3) | 3510 (1.1) | 45 640 (3.9) |
| Bipolar disorder | 13 474 (8.3) | 8852 (1.7) | 1112 (0.8) | 1169 (0.4) | 24 607 (2.1) |
| Schizophrenia or schizoaffective disorder | 7492 (4.6) | 6756 (1.3) | 814 (0.6) | 986 (0.3) | 16 048 (1.4) |
| Other psychotic disorder | 8697 (5.4) | 11 144 (2.1) | 1936 (1.4) | 2643 (0.8) | 24 420 (2.1) |
| Substance use disorder | |||||
| Alcohol | 4129 (2.6) | 8225 (1.5) | 1948 (1.4) | 3674 (1.1) | 17 976 (1.5) |
| Drugs other than alcohol | 6856 (4.2) | 5544 (1.0) | 1031 (0.8) | 920 (0.3) | 14 351 (1.2) |
Abbreviation: IQR, interquartile range.
Classified using the Research Triangle Institute race variable. Of the overall cohort, 4855 (0.4%) were missing race/ethnicity. The percentages were calculated using denominators after excluding those with missing race.
Considered present if a given beneficiary was eligible for or enrolled in the Part D low-income subsidy for at least 1 month during the observation period.
Derived using beneficiary zip code and rural-urban commuting area codes. Urban areas comprise urban core and suburban areas; rural areas comprise large town, small town, and isolated rural areas. Of the overall cohort, 666 (0.1%) were missing rurality. The percentages were calculated using denominators after excluding those with missing rurality.
Did not receive 1 of the otherwise specified dementia diagnoses. The dementia subtypes were not mutually exclusive.
Data are expressed as No. (%) unless otherwise indicated. A comorbidity was considered present if a diagnosis indicating the given condition was present on at least 1 inpatient or 2 or more outpatient encounters on 2 separate days during 2017. Diagnosis codes for each comorbidity appear in eTable 1 in the Supplement.
Measures patient disease burden based on administrative data (range, 0-30; a score of 4 indicates the presence of 4 comorbidities based on claims data).
Characteristics of Those Who Met the Criterion for CNS-Active Polypharmacy
The persons exposed to polypharmacy were significantly younger (median age, 79.4 years [IQR, 74.0-85.5 years] vs 84.7 years [IQR, 78.8-89.9 years] in the no exposure group, P < .001) with larger percentages of non-Hispanic white (86.2% vs 76.7% in the no exposure group, P < .001) and low-income beneficiaries (36.2% vs 25.9% in the no exposure group, P < .001). In addition, the persons exposed to polypharmacy had a higher burden of medical comorbidity overall (median Elixhauser comorbidity score, 5 [IQR, 3-7] vs 3 [IQR, 1-5] in the no exposure group, P < .001). With the exception of cancer and cancer-related pain, the persons exposed to polypharmacy had a significantly higher prevalence of all individual clinical characteristics of interest, including noncancer pain, insomnia, psychiatric diagnoses, and seizure disorders (P < .001 for all).
Polypharmacy-Days, Medications, and Medication Classes for CNS-Active Polypharmacy
Of those who met the criterion for polypharmacy, the median number of polypharmacy-days was 193 (IQR, 88-315 polypharmacy-days). Of those with episodes of CNS-active polypharmacy, 57.8% were exposed for longer than 180 days and 6.8% for 365 days (Table 2). Of all polypharmacy-days, combinations with 3 CNS-active medications accounted for 55.3% of polypharmacy-days and 35.5% of exposed persons, 4 medications for 29.8% of polypharmacy-days and 35.1% of exposed persons, and 5 or more medications for 14.9% of polypharmacy-days and 29.4% of exposed persons. Combinations of medications from a single medication class accounted for 2.7% of polypharmacy-days and 1.8% of exposed persons; combinations of 3 classes, 48.3% of polypharmacy-days and 45.5% of exposed persons; 5 or more classes, 2.0% of polypharmacy-days and 5.2% of exposed persons.
Table 2. Central Nervous System (CNS)–Active Polypharmacy-Days, Number of Medications, and Number of Medication Classes Among Community-Dwelling Older Adults With Dementia in 2018.
| No. (%) | ||
|---|---|---|
| Exposure, polypharmacy-days (n = 32 139 610)a |
Exposed persons (n = 161 412)b |
|
| CNS-active polypharmacy-days | ||
| 31-180 | 6 081 538 (18.9) | 68 156 (42.2) |
| 181-270 | 6 292 042 (19.6) | 29 978 (18.6) |
| 271-364 | 15 737 525 (49.0) | 52 241 (32.4) |
| 365 | 4 028 505 (12.5) | 11 037 (6.8) |
| No. of unique CNS-active medications | ||
| 3 | 17 767 425 (55.3) | 57 253 (35.5) |
| 4 | 9 580 013 (29.8) | 56 656 (35.1) |
| 5 | 3 443 810 (10.7) | 29 954 (18.6) |
| ≥6 | 1 348 362 (4.2) | 17 549 (10.9) |
| No. of unique CNS-active medication classesc | ||
| 1 | 857 492 (2.7) | 2969 (1.8) |
| 2 | 10 538 694 (32.8) | 37 082 (23.0) |
| 3 | 15 512 267 (48.3) | 73 443 (45.5) |
| 4 | 4 597 057 (14.3) | 39 519 (24.5) |
| ≥5 | 634 100 (2.0) | 8399 (5.2) |
Defined as exposure to 3 or more CNS-active medications for longer than 30 days consecutively from the following classes: antidepressants, antipsychotics, antiepileptics, benzodiazepines, nonbenzodiazepine benzodiazepine receptor agonist hypnotics, and opioids.
Each person was assigned to a mutually exclusive group based on the maximum number of overlapping medications or classes during a polypharmacy episode.
Assigned using the American Hospital Formulary Service, which may not reflect the prescribing indication (eg, quetiapine is captured as an antipsychotic even though it may be prescribed for insomnia or anxiety).
Most Common Combinations and Medications for CNS-Active Polypharmacy
Of the medication classes associated with CNS-active polypharmacy, antidepressants accounted for 92.0% of polypharmacy-days, followed by antiepileptics (62.1%), antipsychotics (47.1%), benzodiazepines (40.7%), opioids (32.3%), and z-drugs (6.0%; Table 3). For polypharmacy combinations including the 5 nonantidepressant classes, antidepressants were the most commonly co-prescribed class. An opioid was co-prescribed with an antiepileptic for 19.8% of polypharmacy-days, with an antipsychotic for 9.1% of polypharmacy-days, with a benzodiazepine for 13.1% of polypharmacy-days, or with another opioid for 5.1% of polypharmacy-days.
Table 3. Medication Classes and Class Combinations Contributing to Central Nervous System (CNS)–Active Polypharmacy Among Community-Dwelling Older Adults With Dementia in 2018.
| Additional medication class | Overall | Medication class, % CNS-active polypharmacy-days (n = 32 139 610)a | |||||
|---|---|---|---|---|---|---|---|
| Antidepressant | Antiepileptic | Antipsychotic | Benzodiazepine | Opioid | Z-drugb | ||
| Antidepressant | 92.0 | 46.6 | 55.3 | 42.7 | 36.4 | 28.7 | 5.1 |
| Antiepileptic | 62.1 | 14.6 | 25.2 | 20.7 | 19.8 | 3.2 | |
| Antipsychotic | 47.1 | 5.3 | 17.8 | 9.1 | 1.8 | ||
| Benzodiazepine | 40.7 | 2.1 | 13.1 | 2.5 | |||
| Opioid | 32.3 | 5.1 | 2.4 | ||||
| Z-drugb | 6.0 | <0.1 | |||||
The percentages involve both the column and row for the medication classes (eg, where antidepressants and antiepileptics meet, the percentage represents CNS-active polypharmacy-days involving both an antidepressant and an antiepileptic). For cells where the row and column headings are the same (eg, antidepressants), the percentage represents CNS-active polypharmacy-days involving 2 or more medications from that class. Medication class was assigned using the American Hospital Formulary Service, which may not reflect the prescribing indication (eg, quetiapine is captured as an antipsychotic even though it may be prescribed for insomnia or anxiety).
Defined as nonbenzodiazepine benzodiazepine receptor agonist hypnotics.
The most common CNS-polypharmacy class combination included at least 1 antidepressant, 1 antiepileptic, and 1 antipsychotic, accounting for 12.9% of polypharmacy-days (eTable 3 in the Supplement). Among the 20 most frequent class combinations, 17 included an antidepressant and 12 included an antiepileptic. Antipsychotics and benzodiazepines were each part of 10 of the 20 most frequent combinations; opioids were part of 9 of the top 20. An opioid was prescribed with at least 1 CNS depressant in 8 of the 20 most frequent combinations: specifically, with an antiepileptic in 5, a benzodiazepine in 5, and an antipsychotic in 4.
Gabapentin was the individual medication that accounted for the largest percentage of polypharmacy-days (33.0%; Table 4) and accounted for 53.2% of all antiepileptic polypharmacy-days. The next most-prescribed medications were trazodone (26.0%) and quetiapine (24.4%). The remaining medications among the top 10 spots were antidepressants (mirtazapine [19.9%], sertraline [18.7%], escitalopram [14.7%], and duloxetine [14.5%]) and benzodiazepines (lorazepam [12.9%], clonazepam [12.0%], and alprazolam [12.0%]). The most common opioids were hydrocodone (11.5%) and tramadol (9.2%).
Table 4. Twenty Medications Most Frequently Contributing to Central Nervous System (CNS)–Active Polypharmacy Among Community-Dwelling Older Adults With Dementia in 2018.
| Rank | Generic name | Polypharmacy-days, % (n = 32 139 610)a |
Persons, % (n = 161 412)b |
Classc | Mechanism19 |
|---|---|---|---|---|---|
| 1 | Gabapentin | 33.0 | 36.7 | Antiepileptic | γ-aminobutyric acid analogue |
| 2 | Trazodone | 26.0 | 28.9 | Antidepressant | Inhibits serotonin reuptake; histamine and α1-adrenergic antagonist |
| 3 | Quetiapine | 24.4 | 27.1 | Antipsychotic | Dopamine, serotonin, histamine, and α1-adrenergic receptor antagonist |
| 4 | Mirtazapine | 19.9 | 22.0 | Antidepressant | α2-adrenergic and histamine antagonist; inhibits serotonin reuptake |
| 5 | Sertraline | 18.7 | 21.1 | Antidepressant | Serotonin reuptake inhibitor |
| 6 | Escitalopram | 14.7 | 16.9 | Antidepressant | Serotonin reuptake inhibitor |
| 7 | Duloxetine | 14.5 | 15.6 | Antidepressant | Serotonin and norepinephrine reuptake inhibitor |
| 8 | Lorazepam | 12.9 | 19.1 | Benzodiazepine | Enhances γ-aminobutyric acid activity |
| 9 | Clonazepam | 12.0 | 13.6 | Benzodiazepine | Enhances γ-aminobutyric acid activity |
| 10 | Alprazolam | 12.0 | 16.3 | Benzodiazepine | Enhances γ-aminobutyric acid activity |
| 11 | Citalopram | 11.7 | 13.1 | Antidepressant | Serotonin reuptake inhibitor |
| 12 | Divalproexd | 11.7 | 12.7 | Antiepileptic | Increases concentrations of γ-aminobutyric acid |
| 13 | Hydrocodonee | 11.5 | 20.1 | Opioid | μ-opiate agonist |
| 14 | Bupropion | 10.6 | 11.1 | Antidepressant | Norepinephrine and dopamine reuptake inhibitor |
| 15 | Risperidone | 9.4 | 10.9 | Antipsychotic | Dopamine and serotonin receptor antagonist |
| 16 | Tramadol | 9.2 | 18.2 | Opioid | μ-opiate agonist; serotonin and norepinephrine reuptake inhibitor |
| 17 | Oxycodonef | 8.4 | 12.6 | Opioid | μ-opiate agonist |
| 18 | Levetiracetam | 8.1 | 8.0 | Antiepileptic | Antiepileptic mechanism unclear20 |
| 19 | Venlafaxine | 8.0 | 8.2 | Antidepressant | Serotonin and norepinephrine reuptake inhibitor |
| 20 | Olanzapine | 7.7 | 8.4 | Antipsychotic | Dopamine, serotonin, histamine, and α1-adrenergic receptor antagonist |
Percentages sum to greater than 100 because a given beneficiary may take 3 or more medications each polypharmacy-day.
Limited to those who experienced an episode of CNS-active polypharmacy (defined as >30 days consecutively with concurrent exposure to ≥3 medications from the following 6 classes: antidepressants, antiepileptics, antipsychotics, benzodiazepines, opioids, and nonbenzodiazepine benzodiazepine receptor agonist hypnotics).
Assigned using the American Hospital Formulary Service, which may not reflect the prescribing indication (eg, quetiapine is captured as an antipsychotic even though it may be prescribed for insomnia or anxiety).
Divalproex, valproate, or valproic acid.
Includes combination medications (eg, hydrocodone/acetaminophen).
Includes combination medications (eg, oxycodone/acetaminophen).
Sensitivity Analysis
The prevalence of CNS-active polypharmacy among those who did not die during the 2018 observation year (n = 977 818) was slightly higher (14.6%) than among the cohort overall. Of those who met the criterion for polypharmacy in the sensitivity cohort, the median number of polypharmacy-days was 214 (IQR, 94-326 polypharmacy-days). Among those who were exposed to CNS-active medications, 57.6% were exposed for longer than 180 days and 7.7% were exposed for the entire year (eTable 4 in the Supplement). Combinations with 3 CNS-active medications accounted for 55.2% of polypharmacy-days, 4 medications for 29.8% of polypharmacy-days, and 5 or more medications for 15.0% of polypharmacy-days. Of all polypharmacy-days, 2.7% occurred with patients prescribed 3 medications from a single CNS-active class and 48.1% were accounted for by 3 medication classes.
Discussion
In this large Medicare sample of all community-dwelling older adults with dementia and Part D prescription drug coverage, 13.9% were exposed to CNS-active polypharmacy in 2018, defined as exposure to 3 or more medications (antidepressants, antipsychotics, antiepileptics, benzodiazepines, nonbenzodiazepine benzodiazepine receptor agonist hypnotics, and opioids) for longer than 30 days consecutively. Of those prescribed such a regimen, 57.8% were exposed for longer than 180 days and 6.8% for a full year. Antidepressants were the most common constituent medication class. The most common medications associated with CNS-active polypharmacy included an antiepileptic (gabapentin), an antidepressant (trazodone), and an antipsychotic (quetiapine).
To our knowledge, there are no prior studies among noninstitutionalized adults with dementia in the US with which to compare these results. Two analyses among older adults in the US overall (not limited to those with dementia) found that less than 1.5% took 3 or more CNS-active medications; both studies included opioid medications but not antiepileptics.8,21 However, international studies of polypharmacy have not included either. In international studies specifically among adults with dementia, the prevalence of psychotropic polypharmacy ranged from 13.8% to 50% across studies that used varying definitions of polypharmacy (ie, 2 vs 3 medications with different overlapping criteria) and included those receiving long-term care.13,14,15,22,23,24 Although this analysis excluded persons receiving federally regulated long-term care, Medicare beneficiaries in assisted living settings in the community were included because there is not a reliable way to identify them from Medicare claims; therefore, these persons may be overrepresented among those exposed to polypharmacy.25
This analysis used a relatively conservative definition of polypharmacy that required more than 30 days of overlap for at least 3 medications. A 30-day supply of a CNS-active medication that was either meant for short-term use or discontinued due to adverse effects would not contribute to polypharmacy—to do so, that medication (and ≥2 others) would have required refills to cross the polypharmacy threshold of longer than 30 days, suggesting ongoing therapy. Had a more conservative threshold been used, there still would have been a significant burden of polypharmacy: 77.7% of the polypharmacy sample was exposed for longer than 3 months (>90 days). In addition to relatively long periods, the number of medications frequently exceeded the Beers Criteria threshold in that 35.1% of those exposed to polypharmacy received 4 medications and 29.4% received 5 or more.
Antidepressants were the most common constituent medication class, consistent with their place as the psychotropic class most commonly prescribed both to older adults overall and those with dementia.6,7 There is minimal high-quality evidence to support the efficacy of antidepressants to treat depression in patients with dementia.26 Antidepressants may be prescribed for a variety of other indications based on varying levels of evidence; for example, to treat agitation, a use supported by the Citalopram for Agitation in Alzheimer Disease Study (CitAD) trial,11 or to treat apathy, which resembles depression and is distressing to family but does not benefit from use of antidepressants.27,28 Additional potential indications are pain or insomnia and these diagnoses were higher among those experiencing polypharmacy. Trazodone and mirtazapine, which are the top 2 antidepressants that contributed to polypharmacy-days, are both commonly prescribed off-label for insomnia.29,30
As with antidepressants, other medications that contributed to polypharmacy may have been prescribed for multiple indications, including gabapentin and quetiapine, which are among the top individual medications. Even though gabapentin is captured by the Beers Criteria as an antiepileptic, and seizure disorders were more common among those exposed to polypharmacy, the majority of its use has been found to be for off-label psychiatric or pain disorders.31 Similarly, quetiapine is an antipsychotic but it is frequently prescribed off-label for anxiety or insomnia.32
Apart from cancer and cancer-related pain, the prevalence of all other clinical conditions including insomnia, mental disorders, and noncancer pain was higher among those with CNS-active polypharmacy. However, it is not possible to directly link the medications prescribed to their multiple potential indications.
There are several specific risks related to CNS-active polypharmacy and the constituent medications. First, there is a risk of respiratory suppression and death from combinations of opioids and CNS depressants or drugs termed opioid potentiators, including benzodiazepines, antipsychotics, and gabapentinoids, which are subject to a black-box warning from the US Food and Drug Administration.33 In this analysis, 41% of opioid polypharmacy-days included a benzodiazepine and 28% included an antipsychotic.
Second, several antipsychotics and citalopram cause QT-interval prolongation, which increases the risk for cardiac arrhythmia.11,34 Third, fall-related injury is associated with many CNS-active medications, both individually and in combination,21,35,36 and this increased fall risk associated with CNS-active polypharmacy is the reason it was added to the Beers Criteria as potentially inappropriate.37
Fourth, many of these medications adversely affect cognition, an undesired side effect in those who have received a dementia diagnosis, a diagnosis applied in clinical practice to patients who have experienced significant declines in cognition and function. Among older adults with dementia in the Clinical Antipsychotic Trial of Intervention Effectiveness–Alzheimer’s Disease trial, patients who received an antipsychotic experienced a score decline on the Mini-Mental State Examination that was nearly 2.5 points (on a 30-point scale) higher than among those treated with placebo over 36 weeks of treatment.38 Benzodiazepines also impair cognition, with more pronounced effects among older patients.39 In the CitAD trial of citalopram for agitation in dementia,11 those receiving citalopram experienced worsening of cognition relative to those receiving placebo. In addition to class-specific effects, additional burden of CNS-active polypharmacy overall has been associated with additional cognitive decline.10
Limitations
This study has several limitations. First, the prescription medication claims may have overestimated the actual exposure to polypharmacy if the prescriptions were filled but not taken or only taken on an as-needed basis.
Second, without knowing the indication for the medications or examining the range of prescribed dosages, it is not possible to assess the appropriateness of the particular combinations used. Third, the specific harms associated with CNS-active polypharmacy in this cohort were not examined.
Fourth, to limit the cohort to those with dementia, the analysis was limited to those with traditional fee-for-service Medicare and Part D prescription coverage, which limits generalizability to all older adults. Fifth, cohort identification relied on a dementia diagnosis in Medicare claims data, which may mean some individuals with dementia ended up being excluded, whereas others who had been identified did not have the illness. However, 3 years of data were used to maximize sensitivity of the algorithm to identify those with dementia.16 Sixth, these data did not provide information on dementia severity.
Conclusions
In this cross-sectional analysis of Medicare claims data, 13.9% of older adults with dementia in 2018 filled prescriptions consistent with CNS-active polypharmacy. The lack of information on prescribing indications limits judgments about clinical appropriateness of medication combinations for individual patients.
eTable 1. ICD-10 codes for dementia and other clinical characteristics
eTable 2. Central nervous system-active classes and medications
eTable 3. Class combinations contributing to central nervous system-active polypharmacy among community-dwelling older adults with dementia in 2018
eTable 4. Central nervous system-active polypharmacy days, number of medications, and number of medication classes among community-dwelling older adults with dementia in 2018 and who survived through 2018
References
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778-1783. doi: 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bynum JP. The long reach of Alzheimer’s disease: patients, practice, and policy. Health Aff (Millwood). 2014;33(4):534-540. doi: 10.1377/hlthaff.2013.1247 [DOI] [PubMed] [Google Scholar]
- 3.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475-1483. doi: 10.1001/jama.288.12.1475 [DOI] [PubMed] [Google Scholar]
- 4.Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46(2):210-215. doi: 10.1111/j.1532-5415.1998.tb02542.x [DOI] [PubMed] [Google Scholar]
- 5.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maust DT, Strominger J, Bynum JPW, et al. Prevalence of psychotropic and opioid prescription fills among community-dwelling older adults with dementia in the US. JAMA. 2020;324(7):706-708. doi: 10.1001/jama.2020.8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore TJ, Mattison DR. Adult utilization of psychiatric drugs and differences by sex, age, and race. JAMA Intern Med. 2017;177(2):274-275. doi: 10.1001/jamainternmed.2016.7507 [DOI] [PubMed] [Google Scholar]
- 8.Maust DT, Gerlach LB, Gibson A, Kales HC, Blow FC, Olfson M. Trends in central nervous system-active polypharmacy among older adults seen in outpatient care in the United States. JAMA Intern Med. 2017;177(4):583-585. doi: 10.1001/jamainternmed.2016.9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.2019 American Geriatrics Society Beers Criteria Update Expert Panel . American Geriatrics Society 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 10.Wright RM, Roumani YF, Boudreau R, et al. ; Health, Aging and Body Composition Study . Effect of central nervous system medication use on decline in cognition in community-dwelling older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57(2):243-250. doi: 10.1111/j.1532-5415.2008.02127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porsteinsson AP, Drye LT, Pollock BG, et al. ; CitAD Research Group . Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691. doi: 10.1001/jama.2014.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nørgaard A, Jensen-Dahm C, Gasse C, Hansen ES, Waldemar G. Psychotropic polypharmacy in patients with dementia: prevalence and predictors. J Alzheimers Dis. 2017;56(2):707-716. doi: 10.3233/JAD-160828 [DOI] [PubMed] [Google Scholar]
- 14.Orsel K, Taipale H, Tolppanen A-M, et al. Psychotropic drugs use and psychotropic polypharmacy among persons with Alzheimer’s disease. Eur Neuropsychopharmacol. 2018;28(11):1260-1269. doi: 10.1016/j.euroneuro.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 15.Breining A, Bonnet-Zamponi D, Zerah L, et al. Exposure to psychotropics in the French older population living with dementia: a nationwide population-based study. Int J Geriatr Psychiatry. 2017;32(7):750-760. doi: 10.1002/gps.4517 [DOI] [PubMed] [Google Scholar]
- 16.Taylor DH Jr, Fillenbaum GG, Ezell ME. The accuracy of Medicare claims data in identifying Alzheimer’s disease. J Clin Epidemiol. 2002;55(9):929-937. doi: 10.1016/S0895-4356(02)00452-3 [DOI] [PubMed] [Google Scholar]
- 17.Wei Y-J, Simoni-Wastila L, Zuckerman IH, Brandt N, Lucas JA. Algorithm for identifying nursing home days using Medicare claims and Minimum Data Set assessment data. Med Care. 2016;54(11):e73-e77. doi: 10.1097/MLR.0000000000000109 [DOI] [PubMed] [Google Scholar]
- 18.Eicheldinger C, Bonito A. More accurate racial and ethnic codes for Medicare administrative data. Health Care Financ Rev. 2008;29(3):27-42. [PMC free article] [PubMed] [Google Scholar]
- 19.National Library of Medicine, National Center for Biotechnology Information . PubChem. Accessed February 4, 2021. https://pubchem.ncbi.nlm.nih.gov
- 20.Deshpande LS, Delorenzo RJ. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front Neurol. 2014;5:11. doi: 10.3389/fneur.2014.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanlon JT, Boudreau RM, Roumani YF, et al. Number and dosage of central nervous system medications on recurrent falls in community elders: the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2009;64(4):492-498. doi: 10.1093/gerona/gln043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafsson M, Karlsson S, Gustafson Y, Lövheim H. Psychotropic drug use among people with dementia—a six-month follow-up study. BMC Pharmacol Toxicol. 2013;14:56. doi: 10.1186/2050-6511-14-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasudev A, Shariff SZ, Liu K, et al. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004-2013. Am J Geriatr Psychiatry. 2015;23(12):1259-1269. doi: 10.1016/j.jagp.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 24.Walsh KA, O’Regan NA, Byrne S, Browne J, Meagher DJ, Timmons S. Patterns of psychotropic prescribing and polypharmacy in older hospitalized patients in Ireland: the influence of dementia on prescribing. Int Psychogeriatr. 2016;28(11):1807-1820. doi: 10.1017/S1041610216001307 [DOI] [PubMed] [Google Scholar]
- 25.Abrahamson K, Davila H, Kirk L, Garavito GA, Mueller C. Can a nursing home psychotropic reduction project be successfully implemented in assisted living? J Appl Gerontol. 2020;733464820948328. [DOI] [PubMed] [Google Scholar]
- 26.Dudas R, Malouf R, McCleery J, Dening T. Antidepressants for treating depression in dementia. Cochrane Database Syst Rev. 2018;8:CD003944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maust DT, Langa KM, Blow FC, Kales HC. Psychotropic use and associated neuropsychiatric symptoms among patients with dementia in the USA. Int J Geriatr Psychiatry. 2017;32(2):164-174. doi: 10.1002/gps.4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landes AM, Sperry SD, Strauss ME, Geldmacher DS. Apathy in Alzheimer’s disease. J Am Geriatr Soc. 2001;49(12):1700-1707. doi: 10.1046/j.1532-5415.2001.49282.x [DOI] [PubMed] [Google Scholar]
- 29.Iaboni A, Bronskill SE, Reynolds KB, et al. Changing pattern of sedative use in older adults: a population-based cohort study. Drugs Aging. 2016;33(7):523-533. doi: 10.1007/s40266-016-0380-3 [DOI] [PubMed] [Google Scholar]
- 30.Bain KT. Management of chronic insomnia in elderly persons. Am J Geriatr Pharmacother. 2006;4(2):168-192. doi: 10.1016/j.amjopharm.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 31.Kesselheim AS, Darby D, Studdert DM, Glynn R, Levin R, Avorn J. False Claims Act prosecution did not deter off-label drug use in the case of Neurontin. Health Aff (Millwood). 2011;30(12):2318-2327. doi: 10.1377/hlthaff.2011.0370 [DOI] [PubMed] [Google Scholar]
- 32.McKean A, Monasterio E. Off-label use of atypical antipsychotics: cause for concern? CNS Drugs. 2012;26(5):383-390. doi: 10.2165/11632030-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration . FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. Published August 31, 2016. Accessed May 10, 2019. https://www.fda.gov/media/99761/download
- 34.Lambiase PD, de Bono JP, Schilling RJ, et al. British Heart Rhythm Society clinical practice guidelines on the management of patients developing QT prolongation on antipsychotic medication. Arrhythm Electrophysiol Rev. 2019;8(3):161-165. doi: 10.15420/aer.2019.8.3.G1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray SL, Marcum ZA, Dublin S, et al. Association between medications acting on the central nervous system and fall-related injuries in community-dwelling older adults: a new user cohort study. J Gerontol A Biol Sci Med Sci. 2020;75(5):1003-1009. doi: 10.1093/gerona/glz270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960. doi: 10.1001/archinternmed.2009.357 [DOI] [PubMed] [Google Scholar]
- 37.American Geriatrics Society 2015 Beers Criteria Update Expert Panel . American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 38.Vigen CL, Mack WJ, Keefe RS, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer’s disease: outcomes from CATIE-AD. Am J Psychiatry. 2011;168(8):831-839. doi: 10.1176/appi.ajp.2011.08121844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639-658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-10 codes for dementia and other clinical characteristics
eTable 2. Central nervous system-active classes and medications
eTable 3. Class combinations contributing to central nervous system-active polypharmacy among community-dwelling older adults with dementia in 2018
eTable 4. Central nervous system-active polypharmacy days, number of medications, and number of medication classes among community-dwelling older adults with dementia in 2018 and who survived through 2018


