Abstract
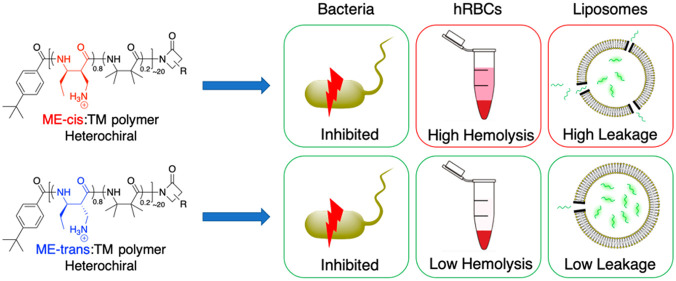
Amphiphilic nylon-3 polymers have been reported to mimic the biological activities of natural antimicrobial peptides, with high potency against bacteria and minimal toxicity toward eukaryotic cells. Amphiphilic balance, determined by the proportions of hydrophilic and lipophilic subunits, is considered one of the most important features for achieving this activity profile for nylon-3 polymers and many other antimicrobial polymers. Insufficient hydrophobicity often correlates with weak activities against bacteria, whereas excessive hydrophobicity correlates with high toxicity toward eukaryotic cells. To ask whether factors beyond amphiphilic balance influence polymer activities, we synthesized and evaluated new nylon-3 polymers with two stereoisomeric subunits, each bearing an ethyl side chain and an aminomethyl side chain. Subunits that differ only in stereochemistry are predicted to contribute equally to amphiphilic balance, but we observed that the stereochemical difference correlates with significant changes in biological activity profile. Antibacterial activities were not strongly affected by subunit stereochemistry, but the ability to disrupt eukaryotic cell membranes varied considerably. Experiments with planar lipid bilayers and synthetic liposomes suggested that eukaryotic membrane disruption results from polymer-mediated formation of large pores. Collectively, our results suggest that factors other than amphiphilic balance influence the membrane activity profile of synthetic polymers. Subunits that differ in stereochemistry are likely to have distinct conformational propensities, which could potentially lead to differences in the average shapes of polymer chains, even when the subunits are heterochiral. These findings highlight a dimension of polymer design that should be considered more broadly in efforts to improve specificity and efficacy of antimicrobial polymers.
Introduction
Eukaryotes deploy a wide array of antimicrobial peptides (AMPs) to control or prevent the growth of prokaryotes.1 Collectively, these AMPs manifest diverse conformations, including helix and sheet secondary structures, and some adopt discrete tertiary structures (usually enforced by internal disulfides). Many AMPs, however, do not appear to have a strong conformational preference.2,3 The heterogeneity in composition, sequence, and shape among AMPs has led many research groups to consider synthetic polymers as possible alternatives.4−14 These efforts have been motivated by the practical consideration that polymers are generally less costly to synthesize relative to sequence-specific peptides. From a fundamental perspective, the prospect of using sequence-random polymers to mimic functions performed in biology by sequence-specific polymers is of considerable interest.15,16
Natural AMPs have complex modes of action that are not yet fully understood, with mechanistic variations among specific peptides.15 Most AMPs appear to compromise bacterial membrane integrity, a property that could lead directly to antibacterial effects and/or enable AMPs to reach intracellular targets.17−19 AMPs are generally rich in residues with side chains that are cationic (Lys and Arg) or hydrophobic (such as Leu or Phe), with both functionalities contributing to membrane interaction.19,20 The complement of hydrophobic side chains in AMPs seems to be necessary for disruption of the lipid bilayer. The net positive charge that is prevalent among AMPs at neutral pH attracts them to the surfaces of bacterial cells, which have a net negative charge.2 In contrast, the surfaces of eukaryotic cells have little net charge as the outer leaflet of the plasma membrane is primarily composed of zwitterionic phospholipids.1
Common features among AMPs have inspired the design of antibacterial polymers with a wide range of backbones and appended functionality.6,10,21−27 Positive charges have typically been incorporated into side chains via protonated or quaternized nitrogen-based groups (amines or guanidines) or quaternized phosphorus; charged groups have also been incorporated directly into the backbone.10,28−30 Hydrophobicity has been incorporated via both side chains and the backbone.31−33 By combining hydrophobic and cationic groups in individual subunits or by mixing cationic and hydrophobic subunits, one can achieve the amphiphilicity necessary for an AMP-mimetic activity profile. The importance of maintaining balance between hydrophobic and cationic groups (“amphiphilic balance”) in antimicrobial polymer design, to ensure specificity toward prokaryotes, has been widely noted.31,34 Excessive hydrophobicity can lead to disruption of eukaryotic cell membranes, a trend that is manifested also among synthetic peptides and peptide mimics (such as peptoids) inspired by AMPs.35−37
This study asks whether factors other than amphiphilic balance can influence biological activity profiles of synthetic polymers. Specifically, we evaluated a new family of nylon-3 polymers based on two stereoisomeric subunits bearing a side chain amino group that should be protonated and therefore cationic near-neutral pH. This stereochemical difference had little effect on antimicrobial activity, but significant variations were observed in terms eukaryotic cell toxicity. We interpret these findings to suggest that the conformational propensities of polymer subunits, which can be altered by changes in backbone stereochemistry, influence membrane activity in a manner that is independent of and complementary to the influence of amphiphilic balance. Our results highlight a design variable that has received little attention so far in efforts to develop synthetic polymers with AMP-like activity profiles or other biomimetic functions.
The nylon-3 system is well-suited to exploration of stereochemistry-based strategies for tuning the polymer function, as demonstrated by the elegant work of Grinstaff et al. on carbohydrate-based nylon-3 polymers or “poly-amido-saccharides” (PAS). Grinstaff et al. used enantiopure carbohydrate-based β-lactams to generate unique synthetic polymers that display features of both polysaccharides and polypeptides. Homochiral polymers generated from diastereomeric subunits, glucose-based vs galactose-based, display substantial aqueous solubility differences.38−40 In addition, Grinstaff et al. found functional differences among macrosurfactants in which the hydrophilic “head groups” are short PAS chains. Macrosurfactants with a mixed headgroup containing galactose- and glucose-derived units inhibited bacterial biofilm formation, while analogous macrosurfactants with a pure galactose-derived PAS headgroup did not. The hydrophobic “tail” was essential for this activity; the PAS segments themselves were not active.9 It should be noted, however, that the PAS homopolymers are based on subunits that are rigid and enantiopure, which allows these chains to adopt distinct helical secondary structures depending on which diastereomeric subunit is employed.40 Even with this precedent, it was not obvious that membrane activities could be altered by varying between flexible diastereomeric subunits in a heterochiral format, as demonstrated in the work described below.
Results and Discussion
Polymer Design, Synthesis, and Characterization
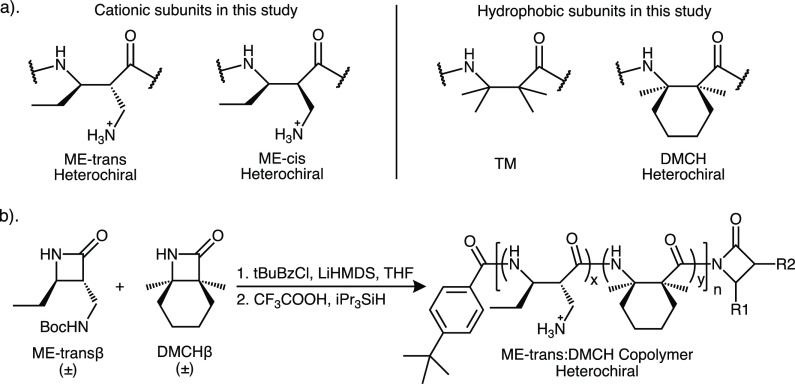
To examine the impact of cationic subunit stereochemistry on polymer activities, we focused on four new nylon-3 polymers generated by copolymerization of β-lactams that generate the subunits shown in Figure 1a. These β-lactams, and many others, are readily synthesized from the corresponding alkenes via cycloaddition of N-chlorosulfonyl isocyanate (CSI) followed by hydrolytic removal of the chlorosulfonyl group.41 The cycloaddition is stereospecific: alkene geometry (E vs Z) specifies the relative configuration of the β-lactam that gives rise to the ME-cis or ME-trans subunit. Base-catalyzed ring-opening polymerization of β-lactams provides nylon-3 materials, which feature a poly-β-amino acid backbone. Use of a strong acylating agent, such as p-tert-butylbenzoyl chloride, in the polymerization reaction leads to formation in situ of N-acyl-β-lactams that initiate the polymerization process. The identity of the acylating agent determines the identity of the acyl group at the N-terminus of each nylon-3 polymer chain. Copolymerization of a β-lactam pair generates chains containing two types of subunit.42
Figure 1.
(a) Cationic and hydrophobic subunits of nylon-3 polymers in this study. (b) Polymerization reaction for generating a cationic-hydrophobic nylon-3 copolymer from a β-lactam pair.
Several groups have explored biological properties of nylon-3 materials, including antibacterial activity,9,43,44 antifungal activity,45 lung surfactant mimicry,46 cellular growth substrate activity,47 mucoadhesion,48 formation of low-adsorption hydrogels,49 inhibition of ice recrystallization,50 and protein stabilization during freeze-drying.38 Combining cationic subunits with hydrophobic subunits in which both backbone sp3 carbons have geminal substitution, such as the TM and DMCH subunits (Figure 1a; TM for “tetramethyl” and DMCH for “dimethycyclohexyl”), leads to favorable activity profiles, with good potency toward bacteria but little deleterious effect on eukaryotic cells.51,52
We used two new diastereomeric β-lactams to generate polymers containing the ME-cis or ME-trans subunits (Figure 1a; ME for “monoethyl”) as the source of the positive charges in the new nylon-3 polymers examined here. These β-lactams were synthesized via an approach previously used for related β-lactams bearing protected amino groups in side chains.43 The cis and trans configurations of these compounds were confirmed via X-ray crystal structures of synthetic intermediates (Figures S7 and S16, respectively). Comparison of these two β-lactams via reverse-phase UPLC suggested that they have very similar polarity (Figure S19), which supports our hypothesis that the ME-cis and ME-trans subunits in nylon-3 polymers have similar contributions to the amphiphilic balance of the polymer chains. These β-lactams and the one used to generate DMCH subunits were each prepared as a racemic mixture, and these racemates were used for all polymerization reactions. Therefore, all of the nylon-3 polymers described below are heterochiral because they contain subunits of both possible absolute configurations. In contrast, the relative configuration of the ethyl and aminomethyl side chains within an ME-cis or ME-trans subunit is fully controlled in each polymer based on the β-lactam precursor employed.
Distinctions between polymers containing ME-cis vs ME-trans subunits became evident during synthesis. Reactions to produce ME-trans:DMCH or ME-trans:TM copolymers proceeded smoothly in tetrahydrofuran (THF), but attempts to perform comparable reactions to generate copolymers containing ME-cis subunits were plagued by precipitation. Therefore, reactions to produce ME-cis-containing copolymers were performed in dimethylacetamide (DMAc). Even in this solvent, the reaction mixture for ME-cis:TM became turbid.
Polymers were analyzed before side-chain deprotection via gel permeation chromatography (GPC) in THF or DMAc and after side-chain deprotection via 1H NMR in D2O. Removal of the side-chain Boc protecting groups was accomplished by treatment with trifluoroacetic acid (TFA) with 5% (v/v) triisopropylsilane, and the deprotected polymers were therefore isolated as the trifluoroacetate salts. Table 1 summarizes polymer properties. GPC analysis was impossible for the ME-cis:TM copolymer because of solubility limitations, but the other three copolymers displayed low dispersity and relatively similar degrees of polymerization (DP). NMR analysis of the deprotected polymers allowed an independent determination of DP by using the unique aromatic resonances of the p-tert-butylbenzoyl end group as an internal integration reference. The ME-cis:TM copolymer was fully water-soluble after deprotection and could be included in this analysis. The results showed very similar DP values for all four polymers and reasonable consistency with GPC-derived DP values before deprotection in the three cases that allowed comparison. The NMR analysis also provided insight into average subunit proportions per chain, and the values were consistent among the four copolymers at ∼4:1 cationic:hydrophobic subunits. On the basis of these results, we conclude that amphiphilic balance was very similar between these samples of ME-cis:TM and ME-trans:TM and between these samples of ME-cis:DMCH and ME-trans:DMCH.
Table 1. Fundamental Characterizations of ME-Cis and ME-Trans Polymers.
| GPC characterization |
NMR characterization |
|||
|---|---|---|---|---|
| polymer | Đa | DPb (GPC) | DP (NMR) | subunit compositionc-NMR (cationic: hydrophobic) |
| ME-cis:TM | N/A | N/A | 18 | 14 ME-cis:4 TM |
| ME-trans:TM | 1.13 | 23 | 19 | 15 ME-trans:4 TM |
| ME-cis:DMCH | 1.19 | 15 | 19 | 15 ME-cis:4 DMCH |
| ME-trans:DMCH | 1.14 | 14 | 22 | 17 ME-trans:5 DMCH |
Dispersity (Đ), calculated by dividing the weight-averaged molecular weight by number-averaged molecular weight.
Degree of polymerization, the average number of repeat units in the polymer chains.
The average number of each subunit in the polymer chains.
Antibacterial Activities
The four copolymers were assessed for the ability to halt bacterial growth with a small panel of species, including one Gram-negative (Escherichia coli) and three Gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus, and Enterococcus faecium) (Table 2 and Figure S36). The activity profiles toward these four bacteria were quite similar among the four copolymers, as measured via minimum inhibitory concentration (MIC). The greatest variation was observed with E. faecium, for which DMCH-containing polymers were modestly more active than TM-containing polymers. Most of these studies were conducted in typical culture media that contain complex biologically derived components, brain heart infusion (BHI), or lysogeny broth (LB) medium. Moderate to good activities were observed in these media toward the Gram-positive species, but no activity toward E. coli was observed for any of the four copolymers in LB medium. We evaluated E. coli also in a chemically defined medium, EZRDM, which does not contain any biomacromolecular components.53,54 In EZRDM, all four copolymers were active against E. coli. We have previously reported similar medium-dependent variations in activity toward E. coli for other nylon-3 polymers, and this effect was traced to inhibitory interactions between polyanionic components of the complex medium and the polycationic polymer chains.54 On the basis of this precedent, it is noteworthy that the four copolymers studied here display significant activity toward Gram-positive species in complex media, which are presumably closer to conditions that would be encountered in vivo.55 We attempted to determine whether polymer inhibitory activities could be enhanced against S. aureus or E. faecium in EZRDM, but neither bacterium would grow in the chemically defined medium. Collectively, results from the bacterial growth assays show that there is no discernible difference between copolymers containing the ME-cis vs ME-trans subunits in terms of antibacterial activities.
Table 2. Bacteria Inhibitory Results for ME-Cis and ME-Trans Copolymers.
| MICa (μg/mL) of copolymers |
|||||
|---|---|---|---|---|---|
| polymer | E. coli (LB)b | E. coli (EZRDM)c | B. subtilis (LB) | S. aureus (LB) | E. faecium (BHI)d |
| ME-cis:TM | >200 | 31.3 | 3.1 | 12.5 | 50 |
| ME-trans:TM | >200 | 31.3 | 3.1 | 12.5 | 50 |
| ME-cis:DMCH | >200 | 31.3 | 3.1 | 12.5 | 12.5 |
| ME-trans:DMCH | >200 | 15.6 | 6.3 | 12.5 | 12.5 |
MIC = minimum inhibitory concentration for bacterial growth.
LB = Luria–Bertani medium.
EZRDM = EZ rich defined medium.
BHI = brain heart infusion medium.
Lytic Activities toward Eukaryotic Cells
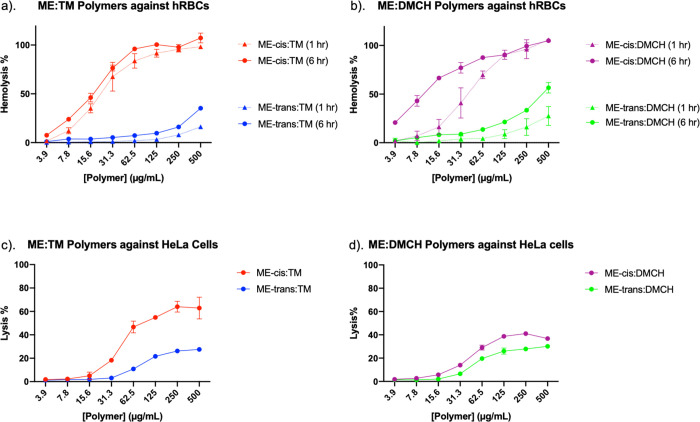
The most common approach to detecting deleterious effects of AMPs or AMP-inspired polymers on eukaryotic cells is to evaluate hemolytic activity, i.e., damage to human red blood cell membranes that allows hemoglobin (MW 65 kDa, 32 Å Stokes radius) to exit.34,56−58 Our hemolysis assays were conducted in Tris-buffered saline (TBS). We found that copolymers containing different stereoisomeric forms of the cationic subunit had significantly different effects on hemolytic activity. For copolymers containing either TM or DMCH as the hydrophobic component, hemolytic activity was high with the ME-cis cationic subunit but low with the ME-trans cationic subunit after a 1 h incubation (Figures 2a,b, dotted curves). The trend was similar when the exposure was lengthened to 6 h (Figures 2a,b, solid curves). Thus, the observed differences in hemolytic activity do not seem to reflect a difference in membrane disruption kinetics, at least on the time scale of several hours.
Figure 2.
Hemolysis activities in TBS buffer for (a) ME:TM copolymers or (b) ME:DMCH copolymers. Data obtained after 1 or 6 h incubation time are shown. HeLa cell membrane leakage activities in PBS buffer for (c) ME:TM copolymers or (d) ME:DMCH copolymers after 2 h incubation.
We wondered whether differences in hemolytic activity might be correlated to differences in polymer aggregation in aqueous solution. This concern was prompted by evidence that emerged during synthetic studies for aggregation in organic solvents of polymers containing ME-cis (but not ME-trans) with side chains protected. We used a standard assay format, based on the solubilization of a hydrophobic fluorophore, diphenylhexatriene (DPH), to ask whether any of the polymers formed micellar aggregates in TBS.59 No evidence for aggregation was detected up to the highest concentration tested, 500 μg/mL, for any of the polymers (Figure S38).
To obtain additional understanding of polymer effects on eukaryotic cells, we used the CytoTox One assay to evaluate interactions of the four nylon-3 copolymers with HeLa cells.60 This assay measures the leakage of lactate dehydrogenase (LDH; tetramer MW 140 kDa, 42 Å Stokes radius) from cells, which indicates membrane damage.61 When these assays were conducted with cells in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), lytic activity was negligible. However, when the assays were conducted in phosphate-buffered saline (PBS), significant cell lysis was observed (Figures 2c,d). The suppression of cell lysis in the medium containing biomacromolecular components (DMEM-FBS) parallels observations reported above and previously regarding polymer effects on E. coli.54 The identity of the hydrophobic subunit had a large impact on copolymer HeLa cell lytic activity. ME-cis:TM induced considerably more LDH leakage from HeLa cells in PBS than did ME-trans:TM, which mirrors the trend observed in hemolysis assays. However, ME-cis:DMCH and ME-trans:DMCH were similar in their abilities to induce LDH leakage in PBS.
Collectively, the eukaryotic cell lysis experiments reveal that amphiphilic balance cannot be the only factor influencing membrane activity because polymers that differ only in the stereoisomeric form of the cationic subunits (ME-cis vs ME-trans) should be similar or identical in their amphiphilic balance. Moreover, the observation of different trends in HeLa cell lysis as a result of hydrophobic subunit identity, TM vs DMCH (Figures 2c,d), suggests that the impact of amphiphilic balance may be modulated by copolymer composition.
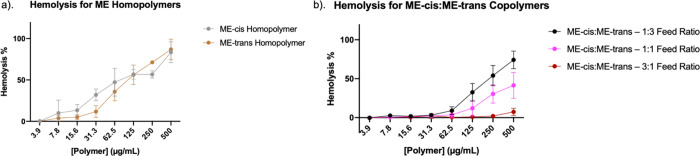
Because hemolytic activity was the most sensitive among all the cell-based assays to the stereochemical variation between ME-cis and ME-trans, we conducted additional hemolysis studies on polymers containing only these two cationic subunits (see the Supporting Information for GPC characterization). Homopolymers of ME-cis or ME-trans were highly hemolytic and similar to one another (Figure 3a). Comparing these data to those in Figures 2a,b indicates that introduction of ∼20% of the hydrophobic subunit, either TM or DMCH, causes a profound decline in hemolytic activity for polymers containing ME-trans, while the effect on polymers containing ME-cis is more subtle. Although it might seem surprising that addition of hydrophobic subunits could lead to a diminution of hemolytic activity, we note that this trend has been observed previously with nylon-3 polymers containing the DM cationic unit (Figure 4).51,52 The ME-cis and ME-trans homopolymers displayed substantially reduced antibacterial activities (Table S1 and Figure S37) relative to the copolymers containing these subunits (Table 2), which demonstrates that the small proportion of TM or DMCH in the copolymers is critical for antibacterial efficacy.
Figure 3.
(a) Hemolysis activities for ME-cis and ME-trans homopolymers. (b) Hemolysis activities for copolymers containing ME-cis and ME-trans subunits, with varying proportions of ME-cis and ME-trans subunits.
Figure 4.
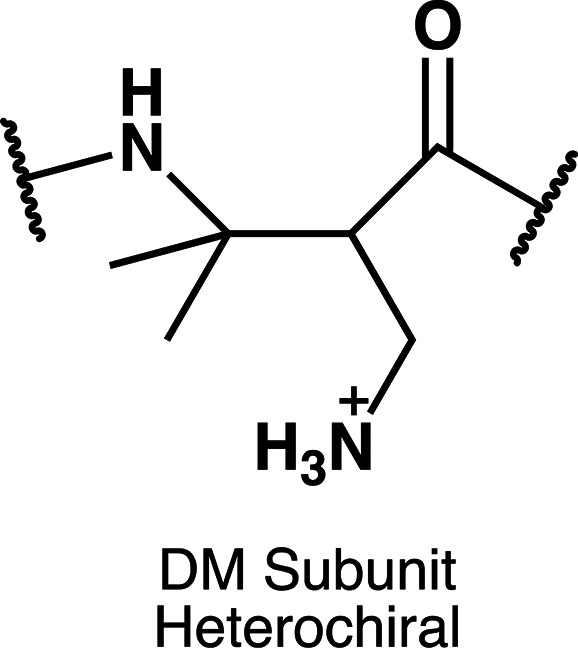
Structure of the DM subunit.
Figure 3b shows hemolysis data obtained for three polymers generated via copolymerization of β-lactams leading to ME-cis or ME-trans subunits in varying proportions. These three copolymers have similar or identical amphiphilic balance, and they are similar or identical to the homopolymers in this regard. Varying the proportion of ME-cis and ME-trans subunits results in substantial differences in hemolytic activity. When ME-trans is dominant, hemolytic activity is most pronounced. In contrast, when ME-cis is dominant, little hemolytic activity is detected. It seems puzzling that inclusion of a small proportion of the ME-trans subunit in a polymer largely composed of ME-cis subunits can lead to a significant decrease of hemolytic activity at 500 μg/mL, when the ME-cis and ME-trans homopolymers are each highly hemolytic at this concentration. Collectively, the data in Figure 3 support the general conclusion that factors other than amphiphilic balance can exert a substantial effect on membrane activity, at least for eukaryotic cells.
Experiments with Synthetic Vesicles
AMPs and their synthetic mimics exert their effects on cells by interacting with and destabilizing the membrane bilayer and possibly other mechanisms.1,2,19,43 To learn more about how the nylon-3 polymers affect lipid bilayers, we undertook studies with synthetic vesicles containing 90% 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 10% 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (DOPS).62 DOPC has a zwitterionic headgroup; more than half of the lipids in an RBC or HeLa cell membrane have a zwitterionic phosphocholine headgroup. The DOPS headgroup is anionic. The proportion of zwitterionic and anionic phospholipids in our vesicles is similar to the proportion in RBC or HeLa cell membranes.63,64 The RBC membrane contains ∼30% cholesterol,65 but preliminary studies indicated that inclusion of cholesterol in our vesicles did not affect the trends reported below (Figure S43).
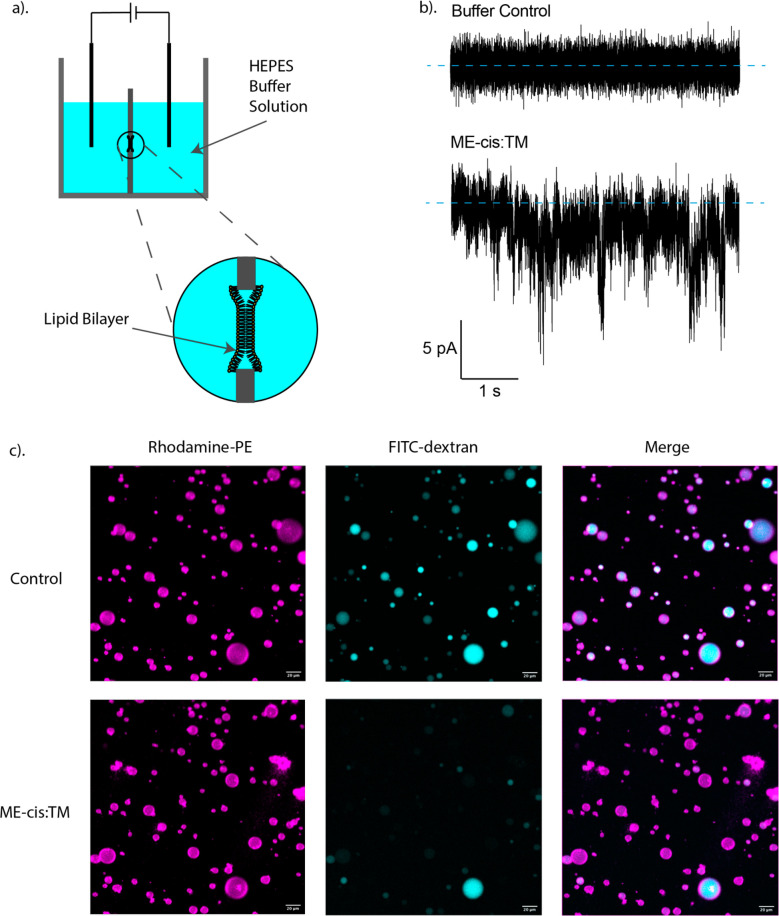
Our initial in vitro studies focused on ME-cis:TM, which was a potent inducer of leakage with both RBCs and HeLa cells. To establish whether ME-cis:TM causes formation of pores, we employed the black lipid membrane (BLM) method to evaluate bilayers formed by 9:1 DOPC:DOPS (Figure 5a). As shown in Figure 5b, addition of ME-cis:TM to the aqueous solution on one side of the bilayer led to transient and irregular ion conduction across the bilayer. The nature of the conductance observed in the presence of the polymer is consistent with transient formation of irregular/chaotic pores with variable dimensions. These observations are not consistent with formation of channels that have a discrete structure, such as those formed by protegrin-166,67 or the influenza M2 protein.68,69 The latter conclusion is consistent with our expectations: the polymer sample contains a wide array of chains that vary in length, composition, subunit identity sequence, and ME-cis subunit stereochemistry sequence, and it therefore seems exceedingly unlikely that discrete channel structures could form.
Figure 5.
(a) Setup of black lipid membrane (BLM) experiments. (b) BLM results with HEPES buffer control (upper graph) and ME-cis:TM (lower graph), with 2 μL of 250 μg/mL ME-cis:TM in PBS added to one side of the BLM chamber. (c) Giant unilamellar vesicles (GUVs) formed from 9:1 DOPC:DOPS encapsulating 4 kDa FITC-labeled dextran, before (control panel) and after (ME-cis:TM panel) the addition of 20 μL of a 1 mg/mL ME-cis:TM solution to 100 μL of GUV suspension. Cyan color came from FITC-labeled dextran, and magenta color came from rhodamine-labeled GUV membranes. The disappearance of cyan color from GUVs in the FITC-channel of panel c indicates leakage of FITC-labeled dextran.
Additional studies were conducted with liposomes to ask whether hemolytic activity arises from pores in the cell membrane or wholesale membrane lysis. We prepared giant unilamellar vesicles (GUVs) with 9:1 DOPC:DOPS that were labeled with 0.1% rhodamine-tagged phosphatidylethanolamine (PE) to enable vesicle imaging via fluorescence microscopy. Dextran with average molecular weight 4 kDa and bearing a fluorescein tag was encapsulated within the GUVs (Figure 5c). When these vesicles in PBS were treated with ME-cis:TM copolymer (167 μg/mL), the cyan fluorescence inside the vesicles slowly dissipated, which suggests that the dextran exited the vesicles through large, copolymer-induced pores. The disappearance of cyan fluorescence presumably reflects dilution of the fluorescein-labeled dextran in the large extravesicular volume. Even after the cyan fluorescence had dissipated, the vesicles themselves remained visible, as indicated by fluorescence in the rhodamine channel (Figure 5c and Video S1). This observation indicates that although pores were generated in the bilayer, the GUV structure remained intact. In contrast, after the dextran-loaded GUVs were treated with the detergent Triton X-100, the GUVs were no longer visible, suggesting wholesale vesicle dissolution (Video S2). Collectively, these initial GUV studies support the hypothesis that ME-cis:TM forms large pores in the lipid bilayer that enable the entrapped 4 kDaA dextran to diffuse out and that ME-cis:TM does not completely destroy the GUVs.
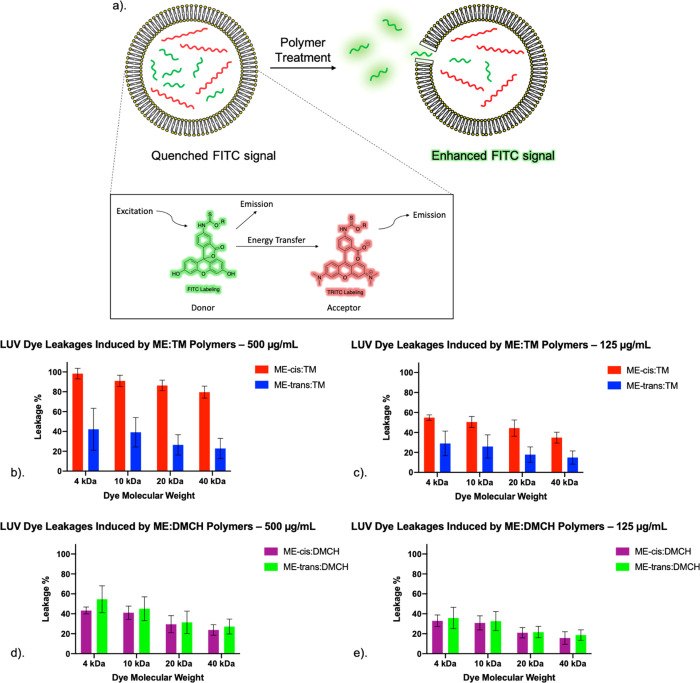
We then used a quantitative dye-release assay to compare the effects of the four nylon-3 copolymers on a lipid bilayer. These studies employed large unilamellar vesicles (LUVs) formed from 9:1 DOPC:DOPS. The LUVs enclosed two fluorophore-bearing dextrans: one of 155 kDa bearing tetramethylrhodamine (TRITC) and the other of 4, 10, 20, or 40 kDa bearing fluorescein (FITC) (see Table S2 for average Stokes radii). The 20 kDa FITC-dextran has an average Stokes radius similar to that of hemoglobin, and the 40 kDa FITC-dextran has an average Stokes radius similar to that of LDH.58,61 When a TRITC-dextran is coentrapped with a FITC-dextran, FITC emission is not observed because of energy transfer to TRITC.70 If a polymer damages the lipid bilayer, however, the smaller dextran can escape, and this process is detected via an increase in fluorescence with excitation at 490 nm and emission at 520 nm (Figure 6a). In these experiments, “100%” dextran release is defined as the fluorescence value measured after exposure of LUVs to the lytic peptide melittin for the same incubation time as was used for the polymers.
Figure 6.
(a). Design of large unilamellar vesicle (LUV) experiments. Before polymer treatment, fluorescence signal from entrapped FITC-labeled dextran is quenched by entrapped TRITC-labeled dextran via FRET. TRITC-labeled dextran (155 kDa) has an average Stokes radius of 85 Å, making it difficult to escape from the pores of liposomes. If polymer treatment causes leakage of vesicle contents, the FITC fluorescence signal will no longer be quenched. (b) LUV dye leakage for four different FITC-labeled dextran sizes caused by TM-containing copolymers at 500 μg/mL. (c) LUV dye leakage caused by TM-containing copolymers at 125 μg/mL. (d) LUV dye leakage caused by DMCH-containing copolymers at 500 μg/mL. (e) LUV dye leakage caused by DMCH-containing copolymers at 125 μg/mL. In these studies, the FITC fluorescence signal is measured immediately after addition of polymer to the LUV preparation.
For experiments conducted with TM-containing copolymers, at either 125 or 500 μg/mL, there was a clear distinction between the copolymer containing ME-cis and that containing ME-trans, with ME-cis:TM causing a greater extent of dextran escape than ME-trans:TM (Figures 6b,c). For each polymer, the extent of dextran escape seemed to be inversely correlated to FITC-dextran size, although the variations were small. The greater ability of ME-cis:TM relative to ME-trans:TM to induce dextran leakage from the LUVs is consistent with greater ability of ME-cis:TM relative to ME-trans:TM to induce protein leakage from RBCs or HeLa cells (Figures 2a,c and Figures S41a,b).
The behavior of DMCH-containing polymers differed from that of TM-containing polymers in the LUV dye leakage assays in that the extent of dextran leakage was similar for ME-cis:DMCH and ME-trans:DMCH (Figures 6e,f). This similarity correlates with the similarity between these two polymers in causing LDH leakage from HeLa cells (Figures 2d and Figures S41c,d) and does not correlate with the significant difference between the two polymers in hemolytic activity (Figure 2b).
The disparity between the trends in hemolysis activities and the trends in liposome dye leakage activities for ME-cis:DMCH and ME-trans:DMCH could potentially be related to differences in membrane composition. The liposome bilayer contains no protein molecules, whereas the hRBC membrane is approximately half protein by weight.71 RBC membrane integrity is maintained by the cytoskeleton protein network, a cellular feature that may prevent membrane damage from toxins.72 Given the difference in membrane compositions between synthetic vesicles and RBCs, we speculate that the impact on cellular membranes exerted by polymers containing ME-cis vs ME-trans may be differentially affected by membrane proteins, which leads to different hemolytic activities. The membranes of HeLa cells and hRBCs have different protein compositions, and these compositional differences might underlie the differences between trends in LDH leakage compared to hemoglobin release.65,73,74 In addition, although our GUV results suggest that the polymers primarily act as pore-forming agents, it is possible that the large tendency of ME-cis:DMCH to induce hemoglobin release from hRBCs arises because a subset of the cells have their membranes fully dissolved (as would be caused by Triton X-100 and other detergents).
It has long been known that natural antimicrobial peptides, composed of l-α-amino acid residues, and their enantiomers (d-residues) display identical or nearly identical antibacterial and mammalian cell-lytic activities.75−77 These observations indicate that the mechanism underlying antibacterial and mammalian cell-lytic activities does not involve binding to specific biomacromolecules, such as proteins. Such targets are chiral and occur in only one enantiomeric form; therefore, a protein target will respond in distinct ways to the two enantiomers of a binding partner.78,79 The precedents involving antimicrobial peptide enantiomers are consistent with our conclusion that activity differences we observe as a function of subunit stereochemistry, ME-cis vs ME-trans, do not arise from differences in engagement of specific cellular targets by the polymers but rather from differences in the physical properties of polymer chains containing the diastereomeric subunits.
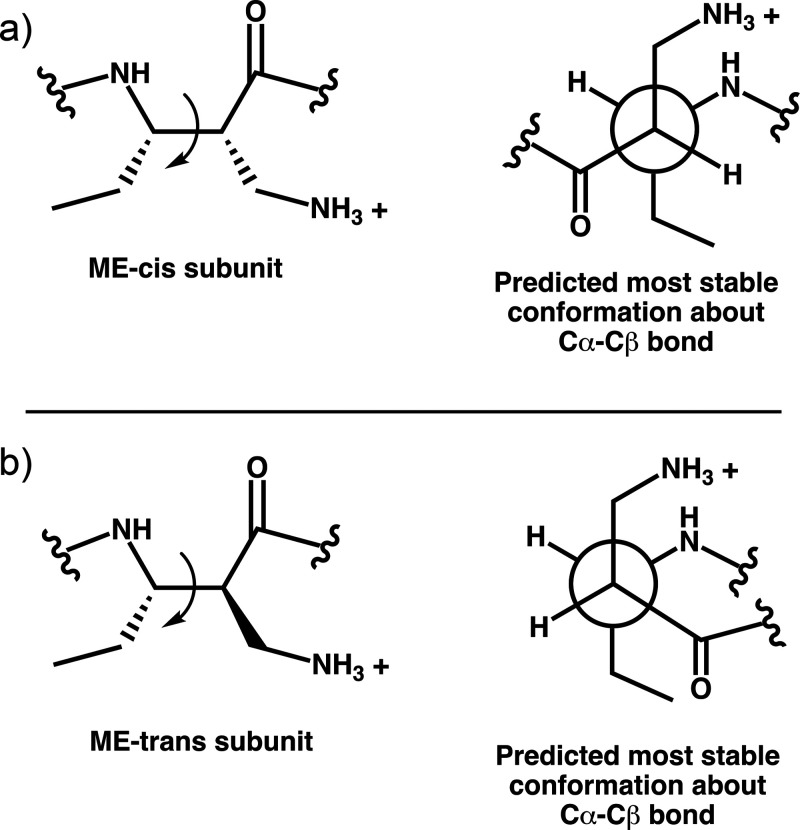
We propose that activity differences among polymers containing ME-cis vs ME-trans subunits arise from variations in the conformational propensities of these two subunits. Figure 7 shows the conformation that is predicted to be most stable about the central Cα-Cβ bond of an ME-cis or ME-trans subunit. These predictions are based on consideration of A-values (energy difference between conformers of monosubstituted cyclohexane derivatives with the substituent axial vs equatorial); A-values are well-established indicators of the steric bulk of a substituent.80,81 Unbranched substituents with an sp3 atom attached to the cyclohexane ring tend to have larger A-values (i.e., are more sterically demanding) relative to substituents with an sp2 atom attached to the ring.80 Therefore, the conformers we anticipate to be most stable have the ethyl and aminomethyl substituents anti to one another. This expected preference causes the C=O and NH of the ME-cis subunit to be anti to one another, but these groups are gauche to one another for ME-trans. Near room temperature, all three of the staggered conformations about the central Cα–Cβ bond of an ME-cis or ME-trans subunit should be populated to some degree, and interchange among these conformers should be rapid. However, the expected preference for one conformer in each case means that, on average, an ME-cis subunit favors extended conformations of the nylon-3 backbone (C=O anti to NH), while an ME-trans subunit favors more compact conformations (C=O gauche to NH). The expected difference in favored conformations for ME-cis and ME-trans subunits could, in principle, lead to different global shapes of polymer chains and thereby affect the physical and biological properties of the polymers. These predictions may explain the substantial differences in solubility we observed before side-chain deprotection for polymers containing ME-cis vs ME-trans in organic solvents like THF. Early work on discrete β-peptide oligomers revealed that incorporation of β-amino acid residues that favor an extended conformation tended to cause insolubility, while use of β-amino acid residues that favor a helical conformation (C=O and NH gauche) generated soluble β-peptides.82−84
Figure 7.
Newman projections showing the predicted most stable conformations about the Cα–Cβ bond for an ME-cis subunit (a) and an ME-trans subunit (b).
Variations in physical properties among nylon-3 polymers containing ME-cis vs ME-trans subunits can be viewed in the context of research on vinyl polymers with variable tacticity. Changes in tacticity can cause profound changes in polymer physical properties, which presumably arise at least in part from changes in the conformational properties of the polymer chains. When polypropylene (PP) is atactic, for example, the material is amorphous, but syndiotactic and isotactic PP display crystalline domains that affect physical properties, such as melting temperature and Young’s modulus.85,86 Because our nylon-3 polymers are prepared from racemic β-lactams, these polymers are necessarily atactic, whether the subunits are ME-cis, ME-trans, or a combination of the two.
The observations reported here emphasize the importance of varying subunit stereochemistry in antibacterial polymer design. Stereochemistry of subunits has largely been overlooked as a design variable in the development of antibacterial or other bioactive polymers; some previously developed polymers completely lack stereochemical elements.87 Many antibacterial materials have been produced from achiral precursors (e.g., methacrylate esters or cyclic carbonate esters) via reactions that generate sp3 stereogenic centers within the polymer chain but without control of subunit configuration. The resulting polymers are therefore heterochiral; that is, they contain subunits of both possible absolute configurations, presumably with random distribution of the two possible absolute configurations along each chain.88,89 Other antibacterial polymers, such as most nylon-3 polymers, have been generated in heterochiral form because racemic chiral precursors have been used.42,90
Conclusions
The data presented here show that altering the relative configuration of the ethyl and aminomethyl side chains within an ME-cis or ME-trans subunit exerts a substantial effect on the biological activities of nylon-3 polymers. The antibacterial activities we measured were not strongly affected by ME subunit relative configuration, but activities toward mammalian cell membranes and toward liposomes with lipid composition similar to that of mammalian cell membranes displayed considerable sensitivity to the presence of ME-cis vs ME-trans. Because the diastereomeric ME subunits presumably make similar or identical contributions to the polymer amphiphilic balance, our findings show that factors beyond amphiphilic balance can play a significant role in determining the biological activity profiles of synthetic polymers. This conclusion is significant, given that each copolymer sample we evaluated contained many different chains that varied in length, subunit composition, sequence of subunits, and sequence of subunit configurations. The functional differences were manifested despite this profound chain heterogeneity.
Tailoring amphiphilic balance is a critical factor in developing synthetic polymers that are intended to display potent inhibition of bacterial growth without harming eukaryotic cells or to perform other biological functions without manifesting toxic side effects.34,90 However, the data reported here reveal that polymer properties other than amphiphilic balance can exert substantial effects on polymer performance, even when the polymer chains are highly heterogeneous. This conclusion is based on functional differences we observed between or among copolymer samples that were comparable in all characteristics except the identity of the ME subunits, which varied between the diastereomeric ME-cis and ME-trans forms. These discoveries suggest that a greater focus on harnessing subunit stereoisomerism will be productive in future efforts directed toward design of polymers with precisely defined bioactivity profiles.
Acknowledgments
We thank I. Guzei for X-ray crystal structure determination. This research was supported in part by the U.S. National Institutes of Health (R01 GM093265 and R33 AI121684 for S.H.G.; R35 NS097362 and R01 MH061876 for E.R.C.). E.R.C. is an Investigator of the Howard Hughes Medical Institute.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c12731.
Synthesis and characterizations of β-lactam monomers and resulting nylon-3 polymers, UPLC characterization of β-lactam monomers, assays for nylon-3 polymers including aggregation studies, bacteria inhibitory studies, and liposome dye leakage studies (PDF)
Video S1: 20 μL of 1 mg/mL ME-cis:TM polymer solution in PBS was added to 100 μL of GUV suspension in PBS buffer, with GUV composed of 90% DOPC and 10% DOPS; FITC-dextran (4 kDa) was encapsulated in GUVs (MOV)
Video S2: 10 μL of 10% Triton X-100 solution in PBS was added to 100 μL of GUV suspension in PBS, where 40 kDa FITC-labeled dextran is encapsulated in the GUVs (MOV)
Video S3: 20 μL of 1 mg/mL ME-trans:TM solution in PBS added to 100 μL of GUV suspension in PBS buffer, with GUV composed of 90% DOPC and 10% DOPS; FITC-dextran (4 kDa) was encapsulated in GUVs (MOV)
Video S4: 50 μL of 200 μg/mL melittin solution in PBS was added to 100 μL of GUV suspension in PBS, with 40 kDa FITC-dextran encapsulated (MOV)
Accession Codes
CCDC 2033849–2033850 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Present Address
S.W.H.: Department of Chemistry, Princeton University, Princeton, NJ 08540.
Author Present Address
L.A.R.: Johnson & Johnson Co., Skillman, NJ 08558.
Author Contributions
⊥ L.L. and K.C.C. contributed equally to this work as co-first authors.
The authors declare the following competing financial interest(s): S.H.G. is an inventor on patents and patent applications covering antimicrobial nylon-3 polymers. L.A.R. is an inventor on a patent application covering antimicrobial nylon-3 polymers.
Supplementary Material
References
- Hancock R. E. W.; Sahl H.-G. Antimicrobial and Host-Defense Peptides as New Anti-Infective Therapeutic Strategies. Nat. Biotechnol. 2006, 24 (12), 1551–1557. 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415 (6870), 389–395. 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Mahlapuu M.; Håkansson J.; Ringstad L.; Björn C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K.; DeGrado W. F. Amphiphilic Polymethacrylate Derivatives as Antimicrobial Agents. J. Am. Chem. Soc. 2005, 127 (12), 4128–4129. 10.1021/ja044205+. [DOI] [PubMed] [Google Scholar]
- Nederberg F.; Zhang Y.; Tan J. P. K.; Xu K.; Wang H.; Yang C.; Gao S.; Guo X. D.; Fukushima K.; Li L.; Hedrick J. L.; Yang Y.-Y. Biodegradable Nanostructures with Selective Lysis of Microbial Membranes. Nat. Chem. 2011, 3 (5), 409–414. 10.1038/nchem.1012. [DOI] [PubMed] [Google Scholar]
- Ilker M. F.; Nüsslein K.; Tew G. N.; Coughlin E. B. Tuning the Hemolytic and Antibacterial Activities of Amphiphilic Polynorbornene Derivatives. J. Am. Chem. Soc. 2004, 126 (48), 15870–15875. 10.1021/ja045664d. [DOI] [PubMed] [Google Scholar]
- Mankoci S.; Kaiser R. L.; Sahai N.; Barton H. A.; Joy A. Bactericidal Peptidomimetic Polyurethanes with Remarkable Selectivity against Escherichia Coli. ACS Biomater. Sci. Eng. 2017, 3 (10), 2588–2597. 10.1021/acsbiomaterials.7b00309. [DOI] [PubMed] [Google Scholar]
- Gelman M. A.; Weisblum B.; Lynn D. M.; Gellman S. H. Biocidal Activity of Polystyrenes That Are Cationic by Virtue of Protonation. Org. Lett. 2004, 6 (4), 557–560. 10.1021/ol036341+. [DOI] [PubMed] [Google Scholar]
- Dane E. L.; Ballok A. E.; O’Toole G. A.; Grinstaff M. W. Synthesis of Bioinspired Carbohydrate Amphiphiles That Promote and Inhibit Biofilms. Chem. Sci. 2014, 5 (2), 551–557. 10.1039/C3SC52777H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert T. J.; Hisey B.; Harrison T. D.; Trant J. F.; Gillies E. R.; Ragogna P. J. Surprising Antibacterial Activity and Selectivity of Hydrophilic Polyphosphoniums Featuring Sugar and Hydroxy Substituents. Angew. Chem., Int. Ed. 2018, 57 (39), 12707–12710. 10.1002/anie.201806412. [DOI] [PubMed] [Google Scholar]
- Fukushima K.; Tan J. P. K.; Korevaar P. A.; Yang Y. Y.; Pitera J.; Nelson A.; Maune H.; Coady D. J.; Frommer J. E.; Engler A. C.; Huang Y.; Xu K.; Ji Z.; Qiao Y.; Fan W.; Li L.; Wiradharma N.; Meijer E. W.; Hedrick J. L. Broad-Spectrum Antimicrobial Supramolecular Assemblies with Distinctive Size and Shape. ACS Nano 2012, 6 (10), 9191–9199. 10.1021/nn3035217. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Qian Y.; Xie J.; Zhang W.; Jiang W.; Xiao X.; Chen S.; Dai C.; Cong Z.; Ji Z.; Shao N.; Liu L.; Wu Y.; Liu R. Poly(2-Oxazoline)-Based Functional Peptide Mimics: Eradicating MRSA Infections and Persisters While Alleviating Antimicrobial Resistance. Angew. Chem., Int. Ed. 2020, 59 (16), 6412–6419. 10.1002/anie.202000505. [DOI] [PubMed] [Google Scholar]
- Judzewitsch P. R.; Nguyen T.-K.; Shanmugam S.; Wong E. H. H.; Boyer C. Towards Sequence-Controlled Antimicrobial Polymers: Effect of Polymer Block Order on Antimicrobial Activity. Angew. Chem., Int. Ed. 2018, 57 (17), 4559–4564. 10.1002/anie.201713036. [DOI] [PubMed] [Google Scholar]
- Namivandi-Zangeneh R.; Sadrearhami Z.; Bagheri A.; Sauvage-Nguyen M.; Ho K. K. K.; Kumar N.; Wong E. H. H.; Boyer C. Nitric Oxide-Loaded Antimicrobial Polymer for the Synergistic Eradication of Bacterial Biofilm. ACS Macro Lett. 2018, 7 (5), 592–597. 10.1021/acsmacrolett.8b00190. [DOI] [PubMed] [Google Scholar]
- Jenssen H.; Hamill P.; Hancock R. E. W. Peptide Antimicrobial Agents. Clin. Microbiol. Rev. 2006, 19 (3), 491–511. 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo E. F.; Kuroda K. Structural Determinants of Antimicrobial Activity in Polymers Which Mimic Host Defense Peptides. Appl. Microbiol. Biotechnol. 2010, 87 (5), 1605–1615. 10.1007/s00253-010-2687-z. [DOI] [PubMed] [Google Scholar]
- Brogden N. K.; Brogden K. A. Will New Generations of Modified Antimicrobial Peptides Improve Their Potential as Pharmaceuticals?. Int. J. Antimicrob. Agents 2011, 38 (3), 217–225. 10.1016/j.ijantimicag.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.; Mohapatra S.; Weisshaar J. C. Rigidification of the Escherichia Coli Cytoplasm by the Human Antimicrobial Peptide LL-37 Revealed by Superresolution Fluorescence Microscopy. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (3), 1017–1026. 10.1073/pnas.1814924116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chongsiriwatana N. P.; Patch J. A.; Czyzewski A. M.; Dohm M. T.; Ivankin A.; Gidalevitz D.; Zuckermann R. N.; Barron A. E. Peptoids That Mimic the Structure, Function, and Mechanism of Helical Antimicrobial Peptides. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (8), 2794–2799. 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B.; Wang G. The Importance of Amino Acid Composition in Natural AMPs: An Evolutional, Structural, and Functional Perspective. Front. Immunol. 2012, 3, 221. 10.3389/fimmu.2012.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikula P.; Mlnarikova M.; Takahashi H.; Babica P.; Kuroda K.; Blaha L.; Sovadinova I. Branched Poly(Ethylene Imine)s as Anti-Algal and Anti-Cyanobacterial Agents with Selective Flocculation Behavior to Cyanobacteria over Algae. Macromol. Biosci. 2018, 18 (10), 1800187. 10.1002/mabi.201800187. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Jiang W.; Xie J.; Zhang W.; Ji Z.; Zou J.; Cong Z.; Xiao X.; Gu J.; Liu R. Peptide-Mimicking Poly(2-Oxazoline)s Displaying Potent Antimicrobial Properties. ChemMedChem 2021, 16 (2), 309–315. 10.1002/cmdc.202000530. [DOI] [PubMed] [Google Scholar]
- Mortazavian H.; Foster L. L.; Bhat R.; Patel S.; Kuroda K. Decoupling the Functional Roles of Cationic and Hydrophobic Groups in the Antimicrobial and Hemolytic Activities of Methacrylate Random Copolymers. Biomacromolecules 2018, 19 (11), 4370–4378. 10.1021/acs.biomac.8b01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C.; Zhang T.; Ortiz-Ortiz D. N.; Vishwakarma A.; Barton H. A.; Joy A. Modification of Narrow-Spectrum Peptidomimetic Polyurethanes with Fatty Acid Chains Confers Broad-Spectrum Antibacterial Activity. Polym. Int. 2019, 68 (7), 1255–1262. 10.1002/pi.5773. [DOI] [Google Scholar]
- Qi F.; Qian Y.; Shao N.; Zhou R.; Zhang S.; Lu Z.; Zhou M.; Xie J.; Wei T.; Yu Q.; Liu R. Practical Preparation of Infection-Resistant Biomedical Surfaces from Antimicrobial β-Peptide Polymers. ACS Appl. Mater. Interfaces 2019, 11 (21), 18907–18913. 10.1021/acsami.9b02915. [DOI] [PubMed] [Google Scholar]
- Peng C.; Vishwakarma A.; Li Z.; Miyoshi T.; Barton H. A.; Joy A. Modification of a Conventional Polyurethane Composition Provides Significant Anti-Biofilm Activity against Escherichia Coli. Polym. Chem. 2018, 9 (23), 3195–3198. 10.1039/C8PY00492G. [DOI] [Google Scholar]
- Yang Y.; Cai Z.; Huang Z.; Tang X.; Zhang X. Antimicrobial Cationic Polymers: From Structural Design to Functional Control. Polym. J. 2018, 50 (1), 33–44. 10.1038/pj.2017.72. [DOI] [Google Scholar]
- Zhang H.; Liu Y.; Luo T.; Zhao Q.; Cui K.; Huang J.; Jiang T.; Ma Z. Synthesis of Novel Guanidine-Based ABA Triblock Copolymers and Their Antimicrobial Honeycomb Films. Polym. Chem. 2018, 9 (28), 3922–3930. 10.1039/C8PY00732B. [DOI] [Google Scholar]
- Xue Y.; Xiao H.; Zhang Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16 (2), 3626–3655. 10.3390/ijms16023626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Z.; Finn M. G. Thiabicyclononane-Based Antimicrobial Polycations. J. Am. Chem. Soc. 2017, 139 (43), 15401–15406. 10.1021/jacs.7b07596. [DOI] [PubMed] [Google Scholar]
- Palermo E. F.; Lienkamp K.; Gillies E. R.; Ragogna P. J. Antibacterial Activity of Polymers: Discussions on the Nature of Amphiphilic Balance. Angew. Chem., Int. Ed. 2019, 58 (12), 3690–3693. 10.1002/anie.201813810. [DOI] [PubMed] [Google Scholar]
- Ergene C.; Palermo E. F. Cationic Poly(Benzyl Ether)s as Self-Immolative Antimicrobial Polymers. Biomacromolecules 2017, 18 (10), 3400–3409. 10.1021/acs.biomac.7b01062. [DOI] [PubMed] [Google Scholar]
- Kurowska M.; Eickenscheidt A.; Guevara-Solarte D.-L.; Widyaya V. T.; Marx F.; Al-Ahmad A.; Lienkamp K. A Simultaneously Antimicrobial, Protein-Repellent, and Cell-Compatible Polyzwitterion Network. Biomacromolecules 2017, 18 (4), 1373–1386. 10.1021/acs.biomac.7b00100. [DOI] [PubMed] [Google Scholar]
- Takahashi H.; Caputo G. A.; Vemparala S.; Kuroda K. Synthetic Random Copolymers as a Molecular Platform To Mimic Host-Defense Antimicrobial Peptides. Bioconjugate Chem. 2017, 28 (5), 1340–1350. 10.1021/acs.bioconjchem.7b00114. [DOI] [PubMed] [Google Scholar]
- Stark M.; Liu L.-P.; Deber C. M. Cationic Hydrophobic Peptides with Antimicrobial Activity. Antimicrob. Agents Chemother. 2002, 46 (11), 3585–3590. 10.1128/AAC.46.11.3585-3590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.-R.; Raman N.; Gellman S. H.; Lynn D. M.; Palecek S. P. Hydrophobicity and Helicity Regulate the Antifungal Activity of 14-Helical β-Peptides. ACS Chem. Biol. 2014, 9 (7), 1613–1621. 10.1021/cb500203e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Kang D.; Choi J.; Huang W.; Wadman M.; Barron A. E.; Seo J. Effect of Side Chain Hydrophobicity and Cationic Charge on Antimicrobial Activity and Cytotoxicity of Helical Peptoids. Bioorg. Med. Chem. Lett. 2018, 28 (2), 170–173. 10.1016/j.bmcl.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Stidham S. E.; Chin S. L.; Dane E. L.; Grinstaff M. W. Carboxylated Glucuronic Poly-Amido-Saccharides as Protein Stabilizing Agents. J. Am. Chem. Soc. 2014, 136 (27), 9544–9547. 10.1021/ja5036804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R.; Dane E. L.; Zeng J.; McKnight C. J.; Grinstaff M. W. Synthesis of Altrose Poly-Amido-Saccharides with β- N -(1→2)- D -Amide Linkages: A Right-Handed Helical Conformation Engineered in at the Monomer Level. J. Am. Chem. Soc. 2017, 139 (40), 14217–14223. 10.1021/jacs.7b07405. [DOI] [PubMed] [Google Scholar]
- Chin S. L.; Lu Q.; Dane E. L.; Dominguez L.; McKnight C. J.; Straub J. E.; Grinstaff M. W. Combined Molecular Dynamics Simulations and Experimental Studies of the Structure and Dynamics of Poly-Amido-Saccharides. J. Am. Chem. Soc. 2016, 138 (20), 6532–6540. 10.1021/jacs.6b01837. [DOI] [PubMed] [Google Scholar]
- Barrett A. G. M.; Betts M. J.; Fenwick A. Acyl and Sulfonyl Isocyanates in. Beta.-Lactam Synthesis. J. Org. Chem. 1985, 50 (2), 169–175. 10.1021/jo00202a006. [DOI] [Google Scholar]
- Zhang J.; Kissounko D. A.; Lee S. E.; Gellman S. H.; Stahl S. S. Access to Poly-β-Peptides with Functionalized Side Chains and End Groups via Controlled Ring-Opening Polymerization of β-Lactams. J. Am. Chem. Soc. 2009, 131 (4), 1589–1597. 10.1021/ja8069192. [DOI] [PubMed] [Google Scholar]
- Mowery B. P.; Lee S. E.; Kissounko D. A.; Epand R. F.; Epand R. M.; Weisblum B.; Stahl S. S.; Gellman S. H. Mimicry of Antimicrobial Host-Defense Peptides by Random Copolymers. J. Am. Chem. Soc. 2007, 129 (50), 15474–15476. 10.1021/ja077288d. [DOI] [PubMed] [Google Scholar]
- Si Z.; Lim H. W.; Tay M. Y. F.; Du Y.; Ruan L.; Qiu H.; Zamudio-Vazquez R.; Reghu S.; Chen Y.; Tiong W. S.; Marimuthu K.; De P. P.; Ng O. T.; Zhu Y.; Gan Y.-H.; Chi Y. R.; Duan H.; Bazan G. C.; Greenberg E. P.; Chan-Park M. B.; Pethe K. A Glycosylated Cationic Block Poly(β-Peptide) Reverses Intrinsic Antibiotic Resistance in All ESKAPE Gram-Negative Bacteria. Angew. Chem., Int. Ed. 2020, 59 (17), 6819–6826. 10.1002/anie.201914304. [DOI] [PubMed] [Google Scholar]
- Liu R.; Chen X.; Hayouka Z.; Chakraborty S.; Falk S. P.; Weisblum B.; Masters K. S.; Gellman S. H. Nylon-3 Polymers with Selective Antifungal Activity. J. Am. Chem. Soc. 2013, 135 (14), 5270–5273. 10.1021/ja4006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm M. T.; Mowery B. P.; Czyzewski A. M.; Stahl S. S.; Gellman S. H.; Barron A. E. Biophysical Mimicry of Lung Surfactant Protein B by Random Nylon-3 Copolymers. J. Am. Chem. Soc. 2010, 132 (23), 7957–7967. 10.1021/ja909734n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.-R.; Stahl S. S.; Gellman S. H.; Masters K. S. Nylon-3 Copolymers That Generate Cell-Adhesive Surfaces Identified by Library Screening. J. Am. Chem. Soc. 2009, 131 (46), 16779–16789. 10.1021/ja9050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balijepalli A. S.; Sabatelle R. C.; Chen M.; Suki B.; Grinstaff M. W. A Synthetic Bioinspired Carbohydrate Polymer with Mucoadhesive Properties. Angew. Chem., Int. Ed. 2020, 59 (2), 704–710. 10.1002/anie.201911720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Chen Q.; Zhang W.; Liu H.; Wan J.; Qian Y.; Li B.; Tang S.; Liu Y.; Chen S.; Liu R. Silk-Inspired β-Peptide Materials Resist Fouling and the Foreign-Body Response. Angew. Chem., Int. Ed. 2020, 59 (24), 9586–9593. 10.1002/anie.202000416. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J.; Cornejo N. R.; Gellman S. H. Inhibition of Ice Recrystallization by Nylon-3 Polymers. ACS Macro Lett. 2017, 6 (7), 695–699. 10.1021/acsmacrolett.7b00396. [DOI] [PubMed] [Google Scholar]
- Liu R.; Chen X.; Chakraborty S.; Lemke J. J.; Hayouka Z.; Chow C.; Welch R. A.; Weisblum B.; Masters K. S.; Gellman S. H. Tuning the Biological Activity Profile of Antibacterial Polymers via Subunit Substitution Pattern. J. Am. Chem. Soc. 2014, 136 (11), 4410–4418. 10.1021/ja500367u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank L. A.; Agrawal A.; Liu L.; Zhu Y.; Mustafi M.; Weisshaar J. C.; Gellman S. H. Diverse Impacts on Prokaryotic and Eukaryotic Membrane Activities from Hydrophobic Subunit Variation Among Nylon-3 Copolymers. ACS Chem. Biol. 2021, 16 (1), 176–184. 10.1021/acschembio.0c00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C.; Bloch P. L.; Smith D. F. Culture Medium for Enterobacteria. J. Bacteriol. 1974, 119 (3), 736–747. 10.1128/JB.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.; Chakraborty S.; Liu R.; Gellman S. H.; Weisshaar J. C. Medium Effects on Minimum Inhibitory Concentrations of Nylon-3 Polymers against E. Coli. PLoS One 2014, 9 (8), e104500 10.1371/journal.pone.0104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossling J.; Slack J. M. Predominant Gram-Positive Bacteria in Human Feces: Numbers, Variety, and Persistence. Infect. Immun. 1974, 9 (4), 719–729. 10.1128/IAI.9.4.719-729.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. Y.; Yang S.-T.; Park E. J.; Eom S. H.; Song W. K.; Kim J. I.; Lee S.-H.; Lee M. K.; Lee D. G.; Hahm K.-S.; Kim Y. Antibacterial, Antitumor and Hemolytic Activities of α-Helical Antibiotic Peptide, P18 and Its Analogs. J. Pept. Res. 2001, 58 (6), 504–514. 10.1034/j.1399-3011.2001.00934.x. [DOI] [PubMed] [Google Scholar]
- Amin K.; Dannenfelser R.-M. In Vitro Hemolysis: Guidance for the Pharmaceutical Scientist. J. Pharm. Sci. 2006, 95 (6), 1173–1176. 10.1002/jps.20627. [DOI] [PubMed] [Google Scholar]
- Armstrong J. K.; Wenby R. B.; Meiselman H. J.; Fisher T. C. The Hydrodynamic Radii of Macromolecules and Their Effect on Red Blood Cell Aggregation. Biophys. J. 2004, 87 (6), 4259–4270. 10.1529/biophysj.104.047746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A.; London E. Fluorometric Determination of Critical Micelle Concentration Avoiding Interference from Detergent Charge. Anal. Biochem. 1984, 139 (2), 408–412. 10.1016/0003-2697(84)90026-5. [DOI] [PubMed] [Google Scholar]
- Korzeniewski C.; Callewaert D. M. An Enzyme-Release Assay for Natural Cytotoxicity. J. Immunol. Methods 1983, 64 (3), 313–320. 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Dams T.; Ostendorp R.; Ott M.; Rutkat K.; Jaenicke R. Tetrameric and Octameric Lactate Dehydrogenase from the Hyperthermophilic Bacterium Thermotoga Maritima. Eur. J. Biochem. 1996, 240 (1), 274–279. 10.1111/j.1432-1033.1996.0274h.x. [DOI] [PubMed] [Google Scholar]
- Som A.; Tew G. N. Influence of Lipid Composition on Membrane Activity of Antimicrobial Phenylene Ethynylene Oligomers. J. Phys. Chem. B 2008, 112 (11), 3495–3502. 10.1021/jp077487j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge J. T.; Phillips G. B. Composition of Phospholipids and of Phospholipid Fatty Acids and Aldehydes in Human Red Cells. J. Lipid Res. 1967, 8 (6), 667–675. 10.1016/S0022-2275(20)38890-8. [DOI] [PubMed] [Google Scholar]
- Lorizate M.; Sachsenheimer T.; Glass B.; Habermann A.; Gerl M. J.; Kräusslich H.-G.; Brügger B. Comparative Lipidomics Analysis of HIV-1 Particles and Their Producer Cell Membrane in Different Cell Lines. Cell. Microbiol. 2013, 15 (2), 292–304. 10.1111/cmi.12101. [DOI] [PubMed] [Google Scholar]
- Mohandas N.; Gallagher P. G. Red Cell Membrane: Past, Present, and Future. Blood 2008, 112 (10), 3939–3948. 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Weiss T. M.; Lehrer R. I.; Huang H. W. Crystallization of Antimicrobial Pores in Membranes: Magainin and Protegrin. Biophys. J. 2000, 79 (4), 2002–2009. 10.1016/S0006-3495(00)76448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langham A. A.; Ahmad A. S.; Kaznessis Y. N. On the Nature of Antimicrobial Activity: A Model for Protegrin-1 Pores. J. Am. Chem. Soc. 2008, 130 (13), 4338–4346. 10.1021/ja0780380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L. H.; Holsinger L. J.; Lamb R. A. Influenza Virus M2 Protein Has Ion Channel Activity. Cell 1992, 69 (3), 517–528. 10.1016/0092-8674(92)90452-I. [DOI] [PubMed] [Google Scholar]
- Acharya R.; Carnevale V.; Fiorin G.; Levine B. G.; Polishchuk A. L.; Balannik V.; Samish I.; Lamb R. A.; Pinto L. H.; DeGrado W. F.; Klein M. L. Structure and Mechanism of Proton Transport through the Transmembrane Tetrameric M2 Protein Bundle of the Influenza A Virus. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (34), 15075–15080. 10.1073/pnas.1007071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trón L.; Szöllósi J.; Damjanovich S.; Helliwell S. H.; Arndt-Jovin D. J.; Jovin T. M. Flow Cytometric Measurement of Fluorescence Resonance Energy Transfer on Cell Surfaces. Quantitative Evaluation of the Transfer Efficiency on a Cell-by-Cell Basis. Biophys. J. 1984, 45 (5), 939–946. 10.1016/S0006-3495(84)84240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A.; Guidotti G. The Protein of Human Erythrocyte Membranes I. PREPARATION, SOLUBILIZATION, AND PARTIAL CHARACTERIZATION. J. Biol. Chem. 1968, 243 (8), 1985–1992. 10.1016/S0021-9258(18)93538-6. [DOI] [PubMed] [Google Scholar]
- Brito C.; Cabanes D.; Sarmento Mesquita F.; Sousa S. Mechanisms Protecting Host Cells against Bacterial Pore-Forming Toxins. Cell. Mol. Life Sci. 2019, 76 (7), 1319–1339. 10.1007/s00018-018-2992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. The Membrane Skeleton of Human Erythrocytes and Its Implications for More Complex Cells. Annu. Rev. Biochem. 1985, 54 (1), 273–304. 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Mangeat P. H.; Burridge K. Immunoprecipitation of Nonerythrocyte Spectrin within Live Cells Following Microinjection of Specific Antibodies: Relation to Cytoskeletal Structures. J. Cell Biol. 1984, 98 (4), 1363–1377. 10.1083/jcb.98.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade D.; Boman A.; Wahlin B.; Drain C. M.; Andreu D.; Boman H. G.; Merrifield R. B. All-D Amino Acid-Containing Channel-Forming Antibiotic Peptides. Proc. Natl. Acad. Sci. U. S. A. 1990, 87 (12), 4761–4765. 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurgan K. W.; Kleman A. F.; Bingman C. A.; Kreitler D. F.; Weisblum B.; Forest K. T.; Gellman S. H. Retention of Native Quaternary Structure in Racemic Melittin Crystals. J. Am. Chem. Soc. 2019, 141 (19), 7704–7708. 10.1021/jacs.9b02691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z.; Cary B. P.; Bingman C. A.; Wang C.; Kreitler D. F.; Satyshur K. A.; Forest K. T.; Gellman S. H. Use of a Stereochemical Strategy To Probe the Mechanism of Phenol-Soluble Modulin A3 Toxicity. J. Am. Chem. Soc. 2019, 141 (19), 7660–7664. 10.1021/jacs.9b00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton R. C.; Milton S. C.; Kent S. B. Total Chemical Synthesis of a D-Enzyme: The Enantiomers of HIV-1 Protease Show Reciprocal Chiral Substrate Specificity [Corrected]. Science 1992, 256 (5062), 1445–1448. 10.1126/science.1604320. [DOI] [PubMed] [Google Scholar]
- Uppalapati M.; Lee D. J.; Mandal K.; Li H.; Miranda L. P.; Lowitz J.; Kenney J.; Adams J. J.; Ault-Riché D.; Kent S. B. H.; Sidhu S. S. A Potent D -Protein Antagonist of VEGF-A Is Nonimmunogenic, Metabolically Stable, and Longer-Circulating in Vivo. ACS Chem. Biol. 2016, 11 (4), 1058–1065. 10.1021/acschembio.5b01006. [DOI] [PubMed] [Google Scholar]
- Winstein S.; Holness N. J. Neighboring Carbon and Hydrogen. XIX. t-Butylcyclohexyl Derivatives. Quantitative Conformational Analysis. J. Am. Chem. Soc. 1955, 77 (21), 5562–5578. 10.1021/ja01626a037. [DOI] [Google Scholar]
- Boiadjiev S. E.; Lightner D. A. Steric Size in Conformational Analysis. Steric Compression Analyzed by Circular Dichroism Spectroscopy. J. Am. Chem. Soc. 2000, 122 (46), 11328–11339. 10.1021/ja002069c. [DOI] [Google Scholar]
- Appella D. H.; Christianson L. A.; Klein D. A.; Powell D. R.; Huang X.; Barchi J. J.; Gellman S. H. Residue-Based Control of Helix Shape in β-Peptide Oligomers. Nature 1997, 387 (6631), 381–384. 10.1038/387381a0. [DOI] [PubMed] [Google Scholar]
- Appella D. H.; Christianson L. A.; Karle I. L.; Powell D. R.; Gellman S. H. β-Peptide Foldamers: Robust Helix Formation in a New Family of β-Amino Acid Oligomers. J. Am. Chem. Soc. 1996, 118 (51), 13071–13072. 10.1021/ja963290l. [DOI] [Google Scholar]
- Seebach D.; Ciceri P. E.; Overhand M.; Jaun B.; Rigo D.; Oberer L.; Hommel U.; Amstutz R.; Widmer H. Probing the Helical Secondary Structure of Short-Chain β-Peptides. Helv. Chim. Acta 1996, 79 (8), 2043–2066. 10.1002/hlca.19960790802. [DOI] [Google Scholar]
- Teator A. J.; Leibfarth F. A. Catalyst-Controlled Stereoselective Cationic Polymerization of Vinyl Ethers. Science 2019, 363 (6434), 1439–1443. 10.1126/science.aaw1703. [DOI] [PubMed] [Google Scholar]
- Glüge R.; Altenbach H.; Kolesov I.; Mahmood N.; Beiner M.; Androsch R. On the Effective Elastic Properties of Isotactic Polypropylene. Polymer 2019, 160 (3), 291–302. 10.1016/j.polymer.2018.10.061. [DOI] [Google Scholar]
- Mankoci S.; Ewing J.; Dalai P.; Sahai N.; Barton H. A.; Joy A. Bacterial Membrane Selective Antimicrobial Peptide-Mimetic Polyurethanes: Structure-Property Correlations and Mechanisms of Action. Biomacromolecules 2019, 20 (11), 4096–4106. 10.1021/acs.biomac.9b00939. [DOI] [PubMed] [Google Scholar]
- Takahashi H.; Nadres E. T.; Kuroda K. Cationic Amphiphilic Polymers with Antimicrobial Activity for Oral Care Applications: Eradication of S. Mutans Biofilm. Biomacromolecules 2017, 18 (1), 257–265. 10.1021/acs.biomac.6b01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippius C.; Bütün V.; Erel-Goktepe I. Bacterial Anti-Adhesive Properties of a Monolayer of Zwitterionic Block Copolymer Micelles. Mater. Sci. Eng., C 2014, 41, 354–362. 10.1016/j.msec.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Mowery B. P.; Lindner A. H.; Weisblum B.; Stahl S. S.; Gellman S. H. Structure-activity Relationships among Random Nylon-3 Copolymers That Mimic Antibacterial Host-Defense Peptides. J. Am. Chem. Soc. 2009, 131 (28), 9735–9745. 10.1021/ja901613g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.