Abstract
Introduction:
Anterior Cruciate Ligament (ACL) reconstruction is an established surgical procedure. Synthetic ligaments represent an option for ACL reconstruction. Their popularity declined for the raising concerns due to re-ruptures, knee synovitis and early arthritis related to I and II generation artificial ligaments. The introduction of a III generation synthetic ligament (Ligament Advanced Reinforcement System-LARS) permitted renewed interest in the adoption of this kind of graft. Main purpose of our study was to describe the histological findings on samples obtained from a consecutive series of ACL revision surgeries due to LARS ACL reconstruction failures. Secondary aim was to determine the reason for LARS rupture.
Methods:
In a period between 2016 and 2018 eleven patients underwent ACL revision surgery due to LARS ACL reconstruction failure. At the time of the arthroscopic procedure, samples of synovial membrane and remnants of the torn LARS were sent to the Pathological-Anatomy Institute of our Hospital for a histological analysis.
Results:
Histological analysis of the synovial tissues confirmed the arthroscopic evidence of synovitis mainly characterized by chronic inflammation with predominance of multinucleated giant cells. The adoption of polarized light microscopy revealed the presence of brightly bi-refractive material (LARS wear particles) in the synovial tissue; at higher magnification wear debris were detected inside the cytoplasma of multi nucleated cells. The histological analysis of the removed LARS revealed a surrounding typical foreign body reaction with poor signs of fibrovascular ingrowth of the synthetic ligament.
Conclusions:
Our findings could not clearly advocate a unique mechanism of LARS-ACL reconstruction failure: biologic issues (poor tissue ingrowth) and mechanical issues (fibers properties and tunnel position) probably concur in a multi factorial manner. ACL reconstruction using artificial ligaments can not be considered a simple surgery. Artificial augments require some expertise and could therefore achieve better results if used by skilled sport surgeons other than trainees or low volume surgeons. The Authors believe that ACL reconstruction with synthetic devices still have restricted indications for selected patients (e.g. elderly patients who require a fast recovery, professional athlete, autologous tendons not available and/or refusing donor tendons). Our study arises additional suspicion on the unresponsiveness of synthetic fibers and claim some concern in the implantation of synthetic devices. (www.actabiomedica.it)
Keywords: anterior cruciate ligament, synthetic ligament, reconstruction, histology, failure
Introduction
Anterior Cruciate Ligament (ACL) reconstruction is an established surgical procedure performed to restore knee stability that can achieve optimal results in terms of both patients related outcomes (symptoms, return to pre-injury level) and objective clinical assessment (knee stability assessed by instrumentations) (23).
Several surgical techniques have been described and it has been postulated that the best option should be tailored on patients’ characteristics and expectations (22).
A crucial aspect regarding ACL reconstruction is the selection of the graft since each of the different possibilities have relative advantages and disadvantages. Autologous tendons harvested from a donor site (hamstring, patellar, quadriceps tendons) represent the graft of choice for many authors and the gold standard for younger patients (<40 years) (24). Allograft tendons (quadriceps, Achilles tendon, hamstring, patellar, anterior and posterior tibialis tendons and the fascia lata) represent an option in primary ACL reconstruction for older patients and in revision ACL surgery (21,24).
A third graft option for ACL reconstruction are synthetic ligaments. Supporters of artificial grafts advocate relative advantages compared to autografts and allografts: a quicker surgery in absence of donor site morbidity and a faster rehabilitation compared to autograft and the absence of potential disease transmission compared to allograft (21). Furthermore, the sterilization process and irradiation may contribute to the weakening of the allograft (26).
Despite the above assumptions, several failures in the early 80’s and 90’s - re-ruptures of the ligament, knee synovitis, clinical instability and early arthritis (1,2) - with I and II generation synthetic ligaments (1) lead to a progressive concern and their popularity declined.
The introduction on the market of a 3rd generation synthetic ligament (Ligament Advanced Reinforcement System-LARS) made of Polyethylene Terephtalate (PET) permitted renewed interest in the choice of this graft for primary (and revision) ACL reconstruction. The microscopic structure of the LARS and a particular purification mechanism of the fibers should lead to a better soft tissue ingrowth and reduce the risk of synovitis (3). Furthermore, the fibers of the synthetic ligament present two different arrangement: in the intraarticular portion, the ligament consists of longitudinal fibers that are twisted at 90° angles without transverse fibers while in the extra articular part the LARS is waved by longitudinal and transverse fibers (21). These peculiar issues of the LARS should minimize the shear stress to the device thus leading to a better ingrowth of tissues with an inferior risk of wearing, less spreading debris particles and, therefore, a minor risk of synovitis and other complications advocated to older synthetic devices (1,14).
Despite very satisfying results on short to mid-term follow-up (15,16,21) a recent study by Tulloch et al (20), reported a LARS-ACL reconstruction failure rate of 33.3% at a minimum of 6 year of follow-up and concluded that the LARS should not be considered as a graft option for primary ACL reconstruction.
Main purpose of our study was to describe the histological findings on samples obtained from a consecutive series of ACL revision surgeries due to synthetic (LARS) ACL reconstruction failures.
Secondary aim was to determine the cause of failure of LARS ACL reconstructions.
Materials and methods
In a period between 2016 and 2018 eleven patients (10 male, 1 female), mean age of 41 years-old (ranging from 24 to 49), presented at our Institution complaining for knee instability after ACL reconstruction performed with a synthetic ligament (LARS). All the patients had the primary ACL reconstruction in other hospitals and came at our clinics at a mean of 3 years (minimum 9 months, maximum 5 years) after the index surgery. All the patients were clinically tested (anterior drawer, Lachman and pivot shift test) and imaging investigations (X-Rays and MRI) were evaluated (Figure A-B-C).
Figure A-B-C-D.
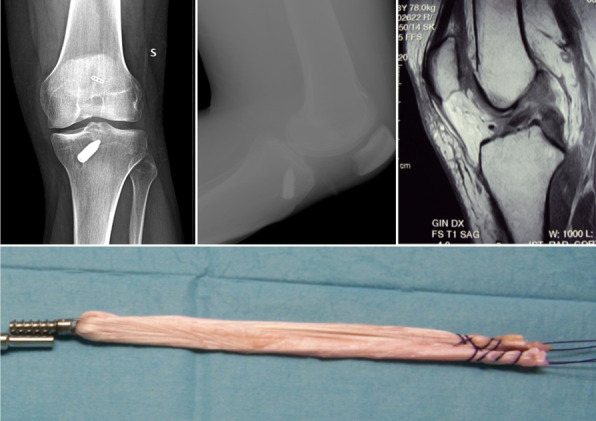
X-Rays showing fixation methods in primary ACL reconstruction (A-B). MRI demonstrating the rupture of the ACL (C). Allograft hamstring tendons quadruplicated and prepared with a TTS screw (D).
All the eleven patients were treated due to the failure (rupture) of the LARS-ACL reconstruction determining clinical instability; none of the patients were treated due other symptoms potentially related to the artificial ligament (e.g. swelling or synovitis) or associated lesions (e.g. meniscal tears, cartilage lesions, associated ligamentous injuries).
ACL revision surgeries were guided by the REVISE ACL classification (25) and in all the eleven patients we were able to perform a one-stage ACL revision surgery since previous tunnels well acceptable (7/11 revisions) or grossly malpositioned (4/11 revisions) such that they could be avoided during the drilling of new tunnels (26). In accordance with Di Benedetto et al (27), our preference in ACL revision surgery was towards allograft tendons – cryopreserved gracilis and semitendinosus - associated with a transtibial technique. In all the patients we utilized a femoral suspension system (Top Traction System-TTS) (28) (Figure D). At the time of the arthroscopic procedure, we withdrawed samples of periarticular tissues (synovial membrane) and remnants of the torn LARS (Figure E-F); when possible, the LARS stump was removed “en bloc”. Both the samples were sent to the Pathological-Anatomy Institute of our Hospital where the specimens were prepared on tissue slide sections and processed on Hematoxylin-Eosin staining. A histological analysis was performed on optical and polarized light microscopy at different magnification.
Figure E-F.
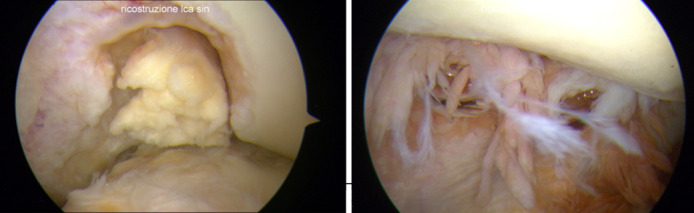
Arthroscopic findings. Femoral-side LARS stump with intercondylar and medial condyle signs of cartilage damage (E). Intra-articular tissues demonstrating villonodular synovitis (F).
Results
At the time of the ACL revision surgery we documented the arthroscopic findings in all the patients.
In eleven revision surgery (100% of the patients) the clinical diagnosis of rupture of the synthetic ACL reconstruction was confirmed intra operatively and in seven of the eleven patients we were able to remove “en bloc” one of the two stumps of the LARS, usually on the proximal (femoral) side (Figure G-H).
Figure G-H.
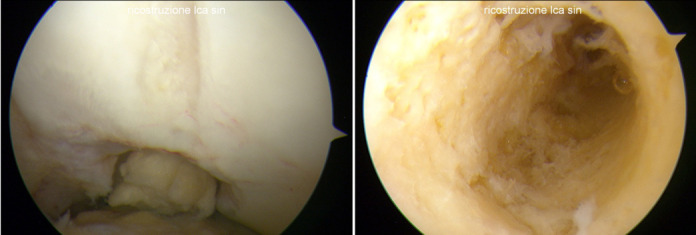
Rupture of the LARS at the opening of the femoral tunnel (G). (H) Femoral Tunnel after the removal of the LARS. Figure H demonstrates the absence of macroscopic signs of ligament bone ingrowth
As expected, the removal of the LARS remnant showed the absence of macroscopic integration of the ligament at the bone-ligament interface; according to other authors (20,21), in all the eleven revision surgeries we have not found signs of osteolysis and/or widening of the tibial or femoral tunnels. Furthermore, in six of the eleven patients (54.5%) we found a wrong position of the tunnel and in 5 of them, according to Samitier et al and recent literature (26,29,30), the misplaced tunnel was more often on the femoral side and usually in a too vertical and anterior position. The roughly malposition of the tunnel led us to drill a new tunnel without a coalescence or weakening of the bone (Figure X-Y). In all the 11 revision surgeries we were able to perform a one-stage procedure without the need of staged procedure.
Figure X-Y.
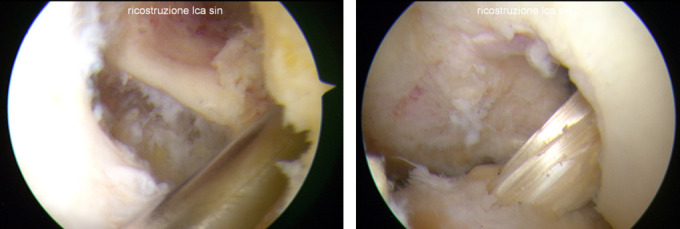
The examination tool show the direction of the new femoral tunnel without interfering with the previous anterior tunnel (X). Allograft hamstring ACL reconstruction in situ. The previous tunnel is still visible (Y).
In all the patients we found severe widespread villonodular synovitis to every compartment of the knee joint. The synovitis was macroscopically characterized by evident hyperemic and redundant intra-articular tissue (Figure I-J).
Figure I-J.
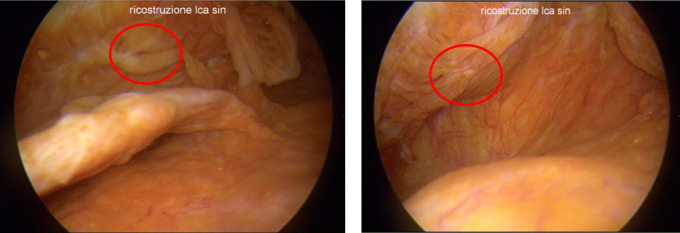
Severe widespread synovitis with hyperemic and hypertrophic tissues of the knee. Roughly PET debris are visible (red circles).
During the ACL revision surgeries we removed arthroscopically the failed LARS and we took samples of synovial tissue; both the specimens were preserved in formalin and sent to the pathological-anatomy institute for the analysis.
In all the patients a partial synovectomy rather than a radical synovectomy was performed in order to limit post-operative bleeding and swelling of the knees thus to facilitate an early rehabilitation protocol.
Histological analysis of the synovial tissue confirmed the arthroscopic evidence of synovitis; confocal microscopy revealed at different magnification the hyperplasia and the hypertrophy of the synovial tissue characterized by a typical cellularity of chronic inflammation with predominance of multinucleated giant cells typical of foreign body reaction (Figure K-L).
Figure K-L.
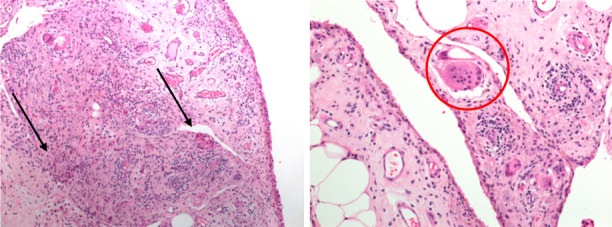
Hematoxylin-Eosin staining of Synovial Tissue. 2X magnification microscopy demonstrate multiple multi-nucleated giant cells (black arrows) (H). 40X magnification shows a macrophage cell close to a foreign body (red circle) (I).
The adoption of polarized light microscopy - on the same histological slide - revealed at 2X magnification microscopy the presence of brightly polarizable - bi-refractive – material spread in the synovial tissue; at higher magnification (40X) polarized light microscopy detected the bi-refractive material inside the cytoplasm of multi nucleated cells (Figure M-N).
Figure L-M.
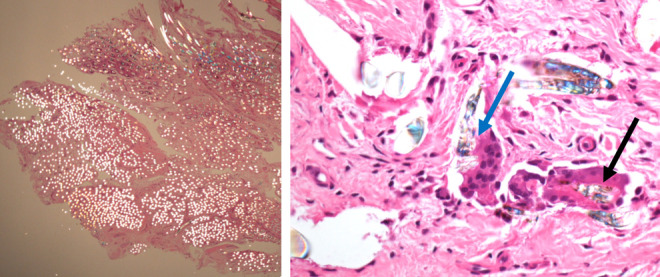
Polarized light microscopy. 2X magnification showing spread debris in the synovial tissue (J). 40X magnification keeping a minimal polarization: black arrows on the right shows PET debris of the LARS inside the cytoplasm of a multinucleated cell. Blue arrow indicates a macrophage in the act of phagocyting a PET particle (K).
Assuming that bi-refractivity under polarized light is a characteristic of the synthetic fibers - poly ethylene terephthalate (PET) - we were able to —demonstrate the widespread diffusion of wear particles in the synovial tissue and the consequent reaction of the organism.
None of the synovial tissue samples presented signs of malignancy or local aggressivity. The same histological analysis was performed on sections of the stump or sections of the remnant of the ligament prepared on hematoxylin-eosin staining.
Light microscopy at different magnification well documented the arrangement of synthetic fibers disposed in a parallel and regular layout surrounded by a poor, dense fibrous - scar-like -tissue with multinucleated giant cell interposed (Figure N-O). Furthermore, the histological description made on the LARS revealed a typical foreign body reaction with poor signs of fibrovascular ingrowth of the synthetic ligament.
Figure N-O.
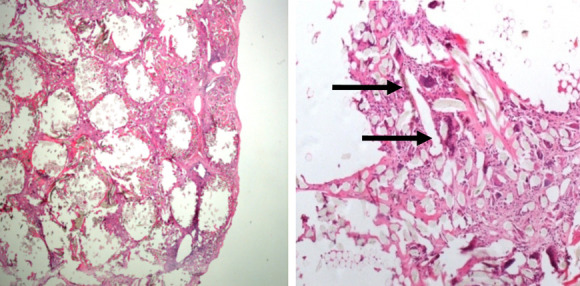
Section of the LARS specimen demonstrates the regular arrangement of the fibers of the ligament with multinucleated giant cells (arrows) interposed between the fibers.
Polarized light microscopy applied on LARS sections (Figure P-Q) confirmed the brightly polarizable aspect of the synthetic PET fibers thus to confirm the nature of the wear particles demonstrated in the synovial tissue and inside the cytoplasm of multi nucleated cells.
Figure P-Q.
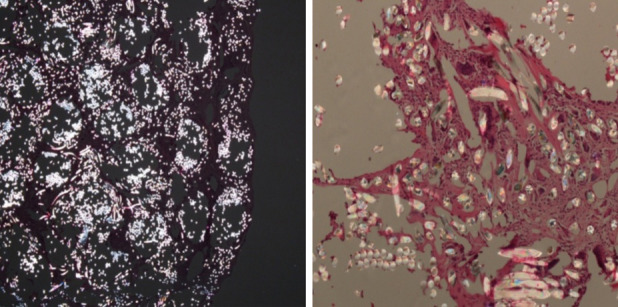
same sections of picture L and M on polarizable light microscopy. LARS fibers show the typical bi refractivity.
Histological analysis performed both on the synovial tissues and on the synthetic ligament did not show any suspect cellularity for knee infection.
Discussion
ACL reconstruction is the gold standard to treat knee instability and to prevent meniscal tears and cartilage damage after an ACL injury: autograft tendons appear the graft of choice especially in young patients (4, 5).
Early artificial ligaments yielded poor results in terms of clinical outcomes and incidence of complications including mechanical failures, synovitis and early arthritis thus the majority of the orthopedic community ceased this graft option till the early 90’s (12).
The introduction of LARS as a possible device for ACL reconstruction gained renewed interest among synthetic devices. Bianchi et al compared the LARS to hamstring tendon autograft in a 8 years follow-up concluding that both the grafts dramatically improved the knee functional outcome with LARS being superior in terms of achieved joint stability (14). In contrast Jia et al compared the LARS and autograft ACL reconstruction in a large meta-analysis and concluded that the two grafts are not different in terms of patient-oriented outcomes and complications but instrumented knee laxity was more evident after synthetic ligaments, especially for early generation devices (14).
Jia demonstrated a low failure rate at a mid-term follow-up (seven years) with an incidence of 4.4% confirming an overall failure incidence of 4.75% in all the studies whit a mean follow-up longer than 3 years (15).
Despite good mid-term results in the cited studies (12-15), no authors postulated LARS as the graft of choice and still recommended caution in its use: some surgeons advocated the LARS as a suitable option for faster recovery after ACL reconstruction (15), others indicate LARS as an alternative for carefully selected cases, especially in older patients (12-16).
Tiefenbok, in contrast, after a minimum follow-up of 10 years, concluded that LARS system should not be currently suggested as a potential graft for primary ACL reconstruction due to a re-rupture failure rate of 27.8% and a low percentage of patient satisfaction (55.6%)( 17). Furthermore, Li et al reported the case of a 26 years-old patient with rare severe knee synovitis 3 years after the operation (18); some concerns arise from the young age of the patient, probably not respecting the correct indications for ACL reconstruction previously mentioned. Same author during revision arthroscopy observed a large amount of synovial hyperplasia in the knee joint and found the femoral tunnel placed too anteriorly. Moreover, the author performed a histological analysis of the LARS and detached a thick fibrous scar tissue around the graft and a poorly organized fibrous scar tissue infiltrated into the graft fibers (18).
Although these results came from a single patient case report, the histological analysis completely agree with our findings on eleven consecutive patients thus to supporting the hypothesis that without an appropriate tissue ingrowth, the LARS could progressively lose its structural integrity with an eventual graft fatigue failure.
Similar findings were reported by Norsworthy: the author during “second-look” surgeries after LARS ACL reconstruction demonstrated variable fibrous tissue incorporation with the LARS device (only 1 of the 21 patients) and frequent chronic synovitis with giant cell foreign body reaction (9 of the 21 patients) (1).
Recently Tulloch et al (19) took 12 second-look arthroscopies after primary LARS-ACL reconstruction due to mechanical symptoms (meniscal tears, cyclops lesions, cartilage damage) and/or knee instability: as described in our series, none of the patients underwent surgery for symptomatic synovitis. Interestingly the Author histologically demonstrated the presence of a hypertrophic synovial tissue more often than the arthroscopic appearance of synovitis among patient with a ruptured LARS; moreover, the author demonstrated the presence of synovitis also in one of the six patients with an entire LARS and this is in accordance also with previous studies (7). The population enrolled in our study consisted only of patient with a rupture LARS and could therefore represent a bias: the confirmation of synovial tissue inflammation with an entire LARS or even in patients with normal arthroscopic intra-articular tissue aspect, reinforce the importance of our findings.
Tulloch et al in another paper (20) reported an elevated LARS ACL reconstruction failure rate of 33.3% at a median of 3.9 years after reconstruction: the reported data is high end unexpected for the Author himself. Several key points are in common between our findings and this study: the failure of the LARS mainly on the opening of the femoral tunnel, the absence of evidence of tunnel widening and the detection of frequent synovitis.
Whilst our results cannot conclude on a direct relation between LARS failure and the development of synovitis, our study supports previous research about the risk to expose the knee to synthetic material due to the risk of developing a foreign body reaction.
Although these objective considerations, our study has several limitations.
First, a low number of enrolled patients.
We don’t use synthetic ligaments neither for primary and revision ACL surgery; all the patients had primary ACL reconstruction in other hospitals and the use of synthetic ligaments still has few indications compared to autologous and heterologous tendons.
Second, the lack of a control group.
Our study only had a descriptive purpose of the histological findings on cases of LARS-ACL reconstruction failure (rupture of the ligament); in the period of the study we did not have any patients who underwent to a second-look arthroscopy in presence of an intact LARS neo-ACL so it was not possible to provide a control group.
Third, all the primary ACL reconstruction were performed in other hospitals.
Preoperative (X-Rays) and intraoperative findings demonstrated in several patients a wrong position of the synthetic ligament as shown in Figure R-S: this issue is detrimental for the synthetic ligament (8,9) due to the impingement in the intercondylar notch and the consequent weakening of the fibers (mechanical failure?). Furthermore, some essential key point (8,9) in the use of the LARS at the time of the primary reconstruction (e.g. ACL stumps preserve) could not be evaluated.
Figure R-S.
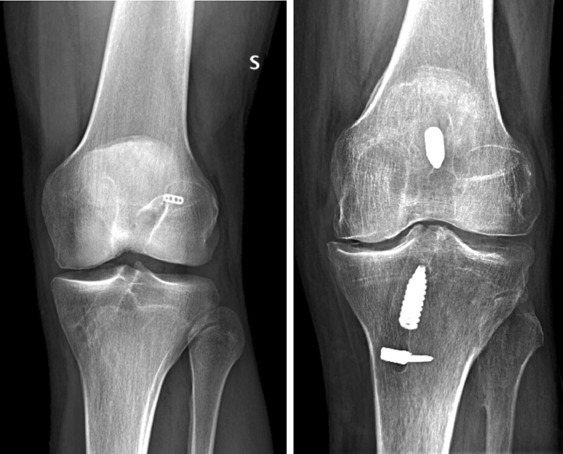
Pre operative X-Rays show two cases of failed ACL reconstructions using the LARS: both cases demonstrate a vertical ligament in the intercondylar notch.
Fourth, the histhological analysis were performed only on patients with a rupture LARS.
Although we demonstrated a poor ingrowth inside the LARS, the isthopathological findings could be influenced by the spreading of the synthetic particles inside the knee joint (6) due to the rupture of the ligament and so our results are not definitive to advocate a biological failure of the graft.
Conclusions
Despite objective results of our study (histologic analysis) we cannot clearly advocate a unique mechanism of LARS-ACL reconstruction failures: biologic issues (poor tissue ingrowth) and mechanical issues (fibers properties and tunnel position) probably concur in a multi factorial manner.
Although synthetic devices have some advantages compared to other grafts, ACL reconstruction using artificial ligaments cannot be considered a straightforward surgery. Artificial augments require some expertise in ligament reconstruction surgery and is therefore a demanding procedure that can achieve better results in skilled sport surgeon hands other than trainees or low volume surgeon.
The Authors continue not to use synthetic ligaments for primary (or revision) ACL reconstruction and believe that ACL reconstruction with synthetic devices still have restricted indications in selected patients (e.g. elderly patients who require a fast recovery, professional athlete, autologous tendons not available and/or refusing donor tendons).
Our study arises additional suspicion on the unresponsiveness of synthetic fibers and even if the results cannot support a definitive relation between LARS failure and synovitis, we claim some concerns to expose the knee to artificial ligament implantation.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Cameron J, Norsworthy MB. BS., FRACS (Australia) My experience with the LARS ACL device at minimum 5 year follow up. 2017 ISAKOS Congress [Google Scholar]
- 2.Ventura A, Terzaghi C, Legnani C, Borgo E, Albisetti W. Synthetic grafts for anterior cruciate ligament rupture: 19-year outcome study. The Knee. 2010;17-2:108–13. doi: 10.1016/j.knee.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Newman SD, Atkinson HD, Willis-Owen CA. Anterior cruciate ligament reconstruction with the ligament augmentation and reconstruction system: a systematic review. Int Orthop. 2013 Feb;37(2):321–6. doi: 10.1007/s00264-012-1654-y. doi: 10.1007/s00264-012-1654-y. Epub 2012 Sep 14. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roe J, Pincewski L, Russell V, et al. A 7-Year Follow-up of Patellar Tendon and Hamstring Tendon Grafts for Arthroscopic Anterior Cruciate Ligament Reconstruction. AJSM. 2005;33(9):1337–1345. doi: 10.1177/0363546504274145. [DOI] [PubMed] [Google Scholar]
- 5.Yasen S, Borton Z, Eyre-Brook A, et al. Clinical outcomes of anatomic, all inside, anterior cruciate ligament (ACL) reconstruction. The Knee. 2017;24:55–62. doi: 10.1016/j.knee.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Glezos CM1, Waller A, Bourke HE, Salmon LJ, Pinczewski LA. Disabling synovitis associated with LARS artificial ligament use in anterior cruciate ligament reconstruction: a case report. Am J Sports Med. 2012 May;40(5):1167–71. doi: 10.1177/0363546512438510. doi: 10.1177/0363546512438510. Epub 2012 Mar 9. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Yao Z, Jiang J, Hua Y, Chen J, Li Y, Gao K, Chen S. Biologic failure of a ligament advanced reinforcement system artificial ligament in anterior cruciate ligament reconstruction: a report of serious knee synovitis. Arthroscopy. 2012 Apr;28(4):583–6. doi: 10.1016/j.arthro.2011.12.008. doi: 10.1016/j.arthro.2011.12.008. Epub 2012 Feb 22. [DOI] [PubMed] [Google Scholar]
- 8.Guidoin MF1, Marois Y, Bejui J, Poddevin N, King MW. Analysis of retrieved polymer fiber based replacements for the ACL. Biomaterials. 2000 Dec;21(23):2461–74. doi: 10.1016/s0142-9612(00)00114-9. [DOI] [PubMed] [Google Scholar]
- 9.Bugelli , et al. LARS in ACL reconstruction: evaluation of 60 cases with 5-year minimum follow-up (2017) Musculoskeletal Surg. doi: 10.1007/s12306-017-0499-3. DOI 10.1007//s12306-017-0499-3. [DOI] [PubMed] [Google Scholar]
- 10.Viateau V1, Manassero M, Anagnostou F, Guérard S, Mitton D, Migonney V. Biological and biomechanical evaluation of the ligament advanced reinforcement system (LARS AC) in a sheep model of anterior cruciate ligament replacement: a 3-month and 12-month study. doi: 10.1016/j.arthro.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Zhenyu J, Chenchen X, Wei W, et al. Clinical outcomes of anterior cruciate ligament reconstruction using LARS artificial graft with an at least 7-year follow-up. Medicine Open. 2017;96(14):e6568. doi: 10.1097/MD.0000000000006568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parchi P.D, et al. Anteriori Cruciate Ligament reconstruction with LARS Artificial Ligament-Clinical results after long-term follow-up. Joints. 2018;6:75–79. doi: 10.1055/s-0038-1653950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia , et al. Comparison of artificial graft versus autograft in anterior cruciate ligament reconstruction: a meta-analysis. BMC Musculoskeletal Disorders. 2017;18:309. doi: 10.1186/s12891-017-1672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi , et al. LARS versus hamstring tendon autograft in anterior cruciate ligament reconstruction: a single-centre, single surgeon retrospective study with 8 years of follow-up. European Journal of Orthopaedic Surgery & Traumatology. doi: 10.1007/s00590-018-2304-x. https://doi.org/10.1007/s00590-018-2304-x. [DOI] [PubMed] [Google Scholar]
- 15.Jia , et al. Clinical outcomes aof anterior cruciate ligament reconstruction using LARS artificial graft with at least 7-year follow-up. Medicine. 2017;96(14):e6568. doi: 10.1097/MD.0000000000006568. http://dx.doi.org/10.1097/MD.0000000000006568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bugelli , et al. LARS in ACL reconstruction: evaluation of 60 cases with 5-year minimum follw-up. Musculoskeletal Surg. 2017 doi: 10.1007/s12306-017-0499-3. DOI 10.1007/s12306-017-0499-3. [DOI] [PubMed] [Google Scholar]
- 17.Tiefenboeck , et al. Clinical and functional outcome after anterior cruciate ligament reconstruction using the LARS system at a minimum follow-up of 10 years. Knee. 2015;22(6):565–568. doi: 10.1016/j.knee.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Li , et al. Biologic failure of a ligament advanced reinforcement system artificial ligament in anterior cruciate ligament reconstruction: a report of serious knee synovitis. Arthroscopy. 2012;28:583–6. doi: 10.1016/j.arthro.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Tulloch , et al. Synovitis following anterior cruciate ligament reconstruction using the LARS device. Knee Surg Sports Traumatol Arthrosc. Aug 2019;27(8):2592–2598. doi: 10.1007/s00167-018-5280-0. [DOI] [PubMed] [Google Scholar]
- 20.Tulloch , et al. Primary ACL reconstruction using the LARS device is associated with a high failure rate at minimum of 6-year follow-up. European Society of Sports Traumatology, Knee Surgery, Arthroscopy. doi: 10.1007/s00167-019-05478-3. https://doi.org/10.1007/s00167-019-05478-3. [DOI] [PubMed] [Google Scholar]
- 21.Satora , et al. Synthetic grafts in the treatment of ruptured anterior cruciate ligament of the knee joint. Polym Med. 2017;47(1):55–59. doi: 10.17219/pim/76819. [DOI] [PubMed] [Google Scholar]
- 22.Filbay , et al. Evidence-based recommendations for the management of anterior cruciate ligament (ACL) rupture. Best Pract Res Clin Rheumatol. 2019 Feb;33(1):33–47. doi: 10.1016/j.berh.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mall , et al. Results After Anterior Cruciate Ligament Reconstruction in Patients Older Than 40 Years: How Do They Compare With Younger Patients? A Systematic Review and Comparison With Younger Populations. Sports Health. 2016 Mar-Apr;8(2):177–81. doi: 10.1177/1941738115622138. doi: 10.1177/1941738115622138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kan , et al. Autograft Versus Allograft in Anterior Cruciate Ligament Reconstruction: A Meta-Analysis With Trial Sequential Analysis, Medicine (Baltimore) Sep 2016;95(38):e4936. doi: 10.1097/MD.0000000000004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De SA, et al. The REVision using imaging to guide staging and evaluation (REVISE) in ACL reconstruction classification. J Knee Surg. 2019 Sep 30 doi: 10.1055/s-0039-1697902. doi: 10.1055/s-0039-1697902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilde , et al. Revision anterior cruciate ligament reconstruction, Sports Health: a multidisciplinary approach. 2014;6:504. doi: 10.1177/1941738113500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Benedetto P, et al. Causes of failure of anterior cruciate ligament reconstruction and revision surgical strategies. Knee Surg Relat Res. 2016;28(4):319–324. doi: 10.5792/ksrr.16.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Benedetto P, et al. New System for femoral fixation (TTS) in anterior cruciate ligament reconstruction with hamstring tendons-5 year follow-up. Orthopaedic Proceedings. 2009;91-B(SUPP_I):86–86. [Google Scholar]
- 29.Dini , et al. Multiple ACL revision: failure analysis and clinical outcome. The Journal of Knee Surgery. doi: 10.1055/s-0039-3400741. DOI https://doi.org/10.1055/s-0039-3400741. [DOI] [PubMed] [Google Scholar]
- 30.Samitier , et al. Failure of anterior cruciate ligament reconstruction. Arch Bone Jt Surg. 2015;3(4):220–240. [PMC free article] [PubMed] [Google Scholar]


