Abstract
Recent evidence supports the notion that mitochondrial metabolism is necessary for stem cell fate. Historically, mitochondrial metabolism is linked to the production of ATP and TCA cycle metabolites to support stem cell survival and growth, respectively. However, it is now clear that beyond these canonical roles, mitochondria as signaling organelles dictate stem cell fate and function. In this review, we focus on key conceptual ideas on how mitochondria control mammalian stem cell fate and function through ROS generation, NAD+/NADH ratio regulation, pyruvate metabolism, TCA cycle metabolite production, and mitochondrial dynamics.
Keywords: TCA cycle, pyruvate, epigenetics, ROS, acetyl-CoA, L-2-HG, mitochondrial dynamics
eTOC statement:
There is a growing appreciation that beyond the canonical roles of producing ATP and biosynthetic intermediates, mitochondria as signaling organelles can dictate stem cell fate and function. Here we discuss multiple mechanisms by which mitochondrial metabolism controls mammalian stem cell fate.
Introduction
Stem cells undergo cell division to maintain the stem cell pool as well as differentiate into progenitor populations to generate and maintain tissue homeostasis. Many adult stem cells are in a quiescent state (Cheung and Rando, 2013). These different cellular fates of stem cells are accompanied by changes in their metabolism (Chandel et al., 2016; Intlekofer and Finley, 2019; Ito and Suda, 2014). Stem cells reside in different niches with unique nutrient availability that also is reflected in alterations of their metabolism. Traditionally, metabolism was thought to accompany changes in stem cell fate to support the differential metabolic needs of stem, progenitor, or differentiated cells. However, multiple studies in the past decade have demonstrated that changes in metabolism can dictate stem cell fate and function analogous to how transcriptional networks control stem cell fate (Bahat and Gross, 2019; Somasundaram et al., 2020). This has led to the appreciation that stem cell metabolism could be targeted to regenerate adult tissues after injury or rejuvenate tissues during normal aging.
In this review, we highlight what is known about the mechanisms by which mitochondria control mammalian stem cell fate. The review is not meant to be an exhaustive review of the literature on stem cell metabolism but to highlight key advances on how mitochondrial metabolism linked to reactive oxygen species (ROS) production, the ratio of oxidized to reduced nicotinamide adenine dinucleotide (NAD+/NADH ratio), pyruvate metabolism, tricarboxylic acid (TCA) cycle metabolite generation, as well as mitochondrial dynamics dictates mouse and human stem cell fate in vivo and maintenance of the pluripotent state of mouse and human stem cells.
Multiple functions of mitochondria
Mitochondrial metabolism supports cell survival and cell proliferation, as well as dictates different cellular states (Chandel, 2015). The canonical historical function ascribed to mitochondria as the “powerhouse of the cell” is the generation of adenosine triphosphate (ATP) by oxidative phosphorylation (OXPHOS), i.e., catabolism (Figure 1). An equally important function is to support anabolism through the production of TCA cycle metabolites such as citrate and oxaloacetate that generate macromolecules such as lipids and nucleotides, respectively (Figure 1). Oxidative TCA cycle function is linked to a functional electron transport chain (ETC). However, the TCA cycle can continue to generate metabolites by reductive mechanisms when ETC is impaired primarily by using pyruvate carboxylase (PC) to generate oxaloacetate from pyruvate for aspartate production and glutamine-dependent reductive carboxylation for citrate synthesis. The ETC complex III is also necessary for dihydroorotate dehydrogenase (DHODH) activity, a key rate-limiting enzyme in de novo pyrimidine synthesis (Sykes, 2018). It is important to note that both glycolytic and TCA cycle flux produce metabolites to fulfill anabolic demands (DeBerardinis and Chandel, 2020).
Figure 1: Mitochondria as bioenergetic and biosynthetic organelles support stem cell survival and growth.
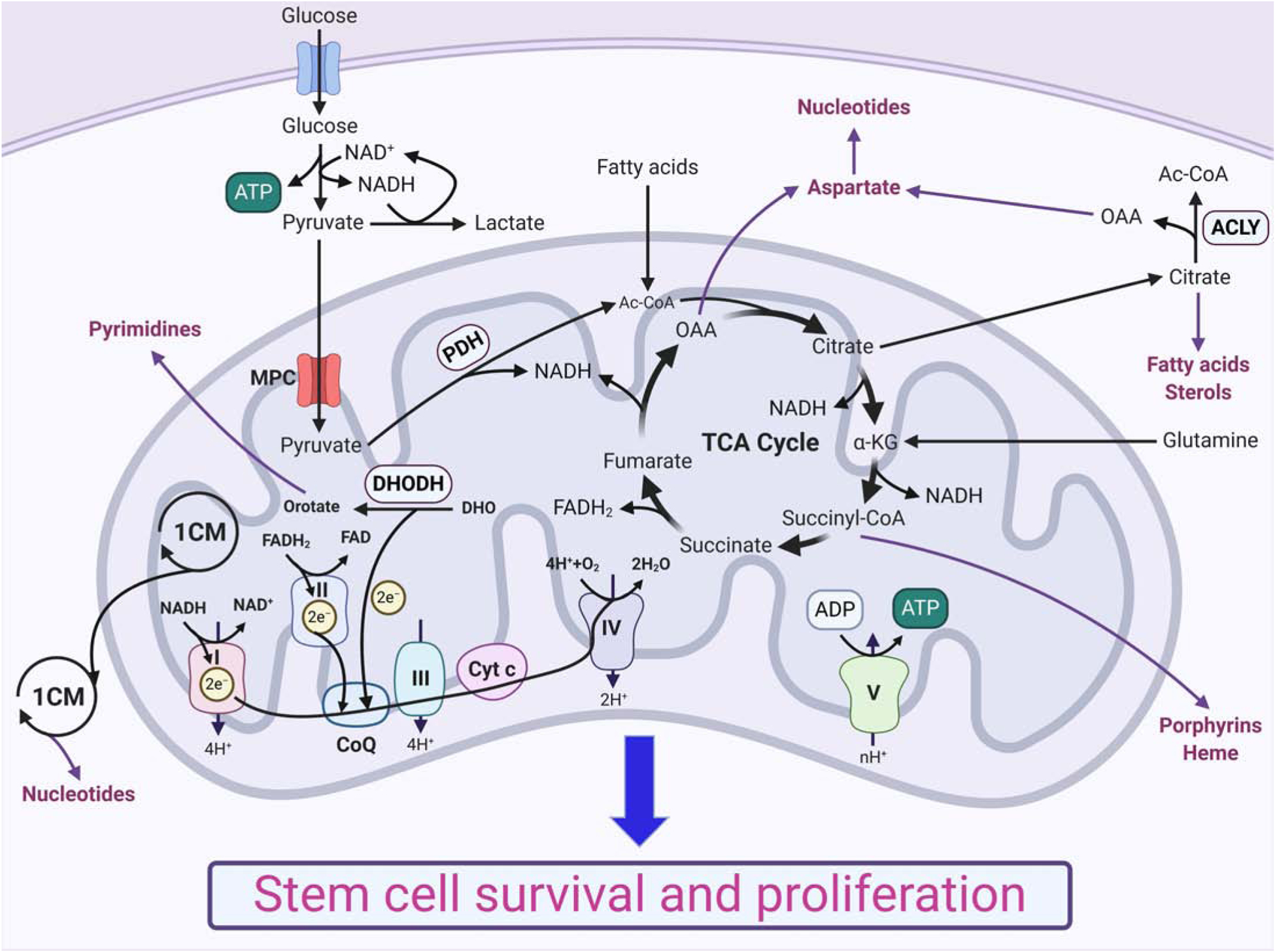
Glucose and multiple other substrates (e.g., fatty acids, glutamine) feed into the TCA cycle that generates biosynthetic intermediates and reducing equivalents, such as NADH and FADH2. Mitochondrial one-carbon metabolism (1CM) also provides biosynthetic intermediates. The intermediate substrates are used for the synthesis of macromolecules, such as lipids and nucleotides, and reducing equivalents fuel ATP generation by oxidative phosphorylation, which support stem cell survival and growth.
The third function of mitochondrial metabolism that emerged in the past two decades is that the release of disparate signaling moieties controls cell fate and function (Figure 2) (Martínez-Reyes and Chandel, 2020). Standard signaling pathways often involve post-translational modifications of amino acids within proteins, including phosphorylation, acetylation, and oxidation that require mitochondrial ATP, acetyl-CoA, and ROS, respectively (Baksh and Finley, 2020; Trefely et al., 2020). Mitochondria can release cytochrome c to initiate caspase-dependent cell death and mitochondrial DNA (mtDNA) to initiate multiple inflammatory cascades (Liu et al., 1996; West and Shadel, 2017). Mitochondria also serve as major regulators of free calcium (Ca2+), controlling various Ca2+-dependent signaling cascades and transcriptional networks (Raffaello et al., 2016). Mitochondrial outer membrane serves as a physical platform for different signaling complexes, such as mitochondrial antiviral signaling protein (MAVS) dependent innate immune responses and Bcl-2 (B cell lymphoma-2) dependent apoptosis (Tan and Finkel, 2020). Mitochondrial shape and dynamics also can serve as an important input in determining cell fate and function (Chen and Chan, 2017; Khacho and Slack, 2018). An emerging concept is that mitochondrial crosstalk with endoplasmic reticulum (ER) and lysosomes can regulate cell fate and function (Deus et al., 2020). Mitochondrial quality control by lysosomes also dictates stem cell fate and is covered in another review article in this issue. A key point is that alterations in mitochondrial function precede changes in transcription that are deterministic for stem cell fate (Bahat and Gross, 2019). Thus, mitochondria are signaling organelles, and we conceptually highlight this aspect of mitochondrial metabolism as a key node in control of stem cell fate and function.
Figure 2: Mitochondria as signaling organelles regulate stem cell fate and function.

Mitochondria generate ROS and different metabolites that can act as cellular signals to control stem cell maintenance, commitment into progenitor populations, and differentiation into different cell types. (SC: Stem Cell; PC: Progenitor Cell; DC: Differentiated Cell)
Reappraisal of mitochondria in stem cells
The resurgence of metabolism in the 21st century is linked to observations made by Otto Warburg in the 1920s that tumor slices take up glucose and secrete lactate in the presence of ample oxygen (aerobic glycolysis, or “The Warburg Effect”). Indeed, multiple studies reported that stem cells display the Warburg Effect (Intlekofer and Finley, 2019; Simsek et al., 2010). Moreover, hypoxiainducible factors (HIFs) that are known to be transcriptional activators of glycolysis were demonstrated to control stem cell fate (Krock et al., 2015; Mazumdar et al., 2010; Mohyeldin et al., 2010; Simsek et al., 2010; Takubo et al., 2010; Takubo et al., 2013). The use of dyes such as MitoTracker Green (MTG) to assess mitochondrial content in hematopoietic stem cells (HSCs) led to the conclusion that mitochondrial content declines during the transition from HSCs to multipotent progenitors (MPPs) (Filippi and Ghaffari, 2019). These studies led to the simplistic idea that glycolysis is the dominant metabolic phenotype that controls stem cell fate. However, recent studies have questioned the use of MTG as an appropriate dye to assess mitochondrial content since HSCs harbor xenobiotic efflux pumps that actively exclude MTG (de Almeida et al., 2017; Norddahl et al., 2011). The addition of verapamil, an inhibitor of efflux pumps, increased MTG staining in HSCs more than downstream progenitor hematopoietic populations (de Almeida et al., 2017; Norddahl et al., 2011). These results are corroborated by measuring mitochondrial versus nuclear DNA content (de Almeida et al., 2017). Competitive transplantation experiments revealed that HSCs in MTG-high fraction after verapamil treatment displayed long-term repopulation activity (de Almeida et al., 2017). Mice harboring mito-Dendra2, a fluorescent protein fused to the mitochondrial targeting signal of subunit VIII of cytochrome c oxidase as another measurement of mitochondrial content, in hematopoietic lineages displayed higher mito-Dendra2 fluorescent signal in HSCs compared to MPPs, and competitive transplantation assays revealed that HSCs with high fluorescent mito-Dendra2 signal possess long-term repopulation activity (de Almeida et al., 2017). Furthermore, pluripotent and adult quiescent stem cells that depend on glycolysis for ATP generation usually have abundant fully functional mitochondria (Schell et al., 2017; Varum et al., 2011; Wang et al., 2009; Wang et al., 2017; Zhang et al., 2011). Also, human pluripotent stem cells (hPSCs) exhibit similar oxygen consumption rates as differentiated cells (Varum et al., 2011; Zhang et al., 2011). Importantly, in the past few years, genetic studies in mice have demonstrated that mitochondrial ETC function, Ptpmt1 (PTEN-like mitochondrial phosphatase 1), pyruvate or fatty acid oxidation, and mitochondrial dynamics (fission/fusion) control stem cell fate in vivo (Ansó et al., 2017; Flores et al., 2017; Hamanaka et al., 2013; Ito et al., 2012; Kasahara et al., 2013; Khacho et al., 2016; Miranda et al., 2018; Prieto et al., 2016; Rodríguez-Colman et al., 2017; Schell et al., 2017; Son et al., 2013; Yu et al., 2013; Zhong et al., 2019). Thus, stem cells integrate distinct metabolites generated by both glycolysis and mitochondrial metabolism to regulate their fate and function (Bahat and Gross, 2019).
Embryonic development and adult tissue homeostasis and repair rely on the stem cell populations that undergo self-renewal and give rise to cells of different lineages. Declines in stem cell populations have been characteristic of aging and many degenerative diseases. Not surprisingly, mitochondrial dysfunctions have also been associated with these phenotypes (Gorman et al., 2016; Kauppila et al., 2017). For instance, mtDNA polymerase gamma (POLG) mutator mice, which carry proof-reading deficient POLG, accumulate mtDNA mutations and result in dysfunctions of different stem and progenitor cells, including hematopoietic progenitor cells (HPCs), neural stem cells (NSCs), and intestinal stem cells (ISCs) (Ahlqvist et al., 2012; Chen et al., 2009; Fox et al., 2012; Norddahl et al., 2011). Collectively, these studies highlight the importance of mitochondria in controlling stem cell fate and function in a variety of contexts.
Mitochondrial ROS regulate stem cell fate
Reactive oxygen species (ROS) refer to oxygen-containing free radicals or compounds that are more reactive than molecular oxygen. There are four main forms of intracellular ROS: superoxide radical anion (O2−), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and lipid hydroperoxides (LOOH). Mitochondrial ETC complex I and III, as well as cytosolic membrane-bound NADPH (reduced nicotinamide adenine dinucleotide phosphate) oxidases (NOXs), are major sites of O2− generation (Figure 3a) (Murphy, 2009; Wong et al., 2017). Immediately upon generation, O2− is reduced to more stable H2O2 by the action of superoxide dismutase 2 (SOD2) in the mitochondrial matrix and superoxide dismutase 1 (SOD1) in the mitochondrial intermembrane space and cytosol. H2O2 serves as a signaling molecule by directly oxidizing specific sulfur-containing amino acids (i.e., cysteine and methionine) that are critical for the functions, stability, and subcellular localization of a protein (Tan and Suda, 2018; Winterbourn, 2018). Such oxidations are usually reversible by the action of antioxidants. Peroxiredoxins are the main detoxifiers of H2O2 to water (H2O) (Winterbourn, 2018). H2O2 can also generate •OH in the presence of ferrous iron (Fe2+). Polyunsaturated fatty acids (PUFAs) react with •OH to produce LOOH that can initiate ferroptosis (Stockwell and Jiang, 2020; Zheng and Conrad, 2020). Glutathione peroxidase 4 (GPX4) and ferroptosis suppressor protein 1 (FSP1) dependent reduction of ubiquinone (Q) to ubiquinol (QH2) can detoxify LOOH (Stockwell and Jiang, 2020; Zheng and Conrad, 2020). The expression of the antioxidant genes is regulated by different transcription factors, including forkhead box protein O (FOXO) family members and nuclear factor (erythroid-derived 2)-like 2 (NRF2), to prevent the increase of ROS to levels that would be damaging to stem cells (Liang and Ghaffari, 2018; Tan and Suda, 2018). Here, we specifically review the role of mitochondrial ROS in the regulation of stem cell fate.
Figure 3: Reactive oxygen species regulate stem cell fate and function.
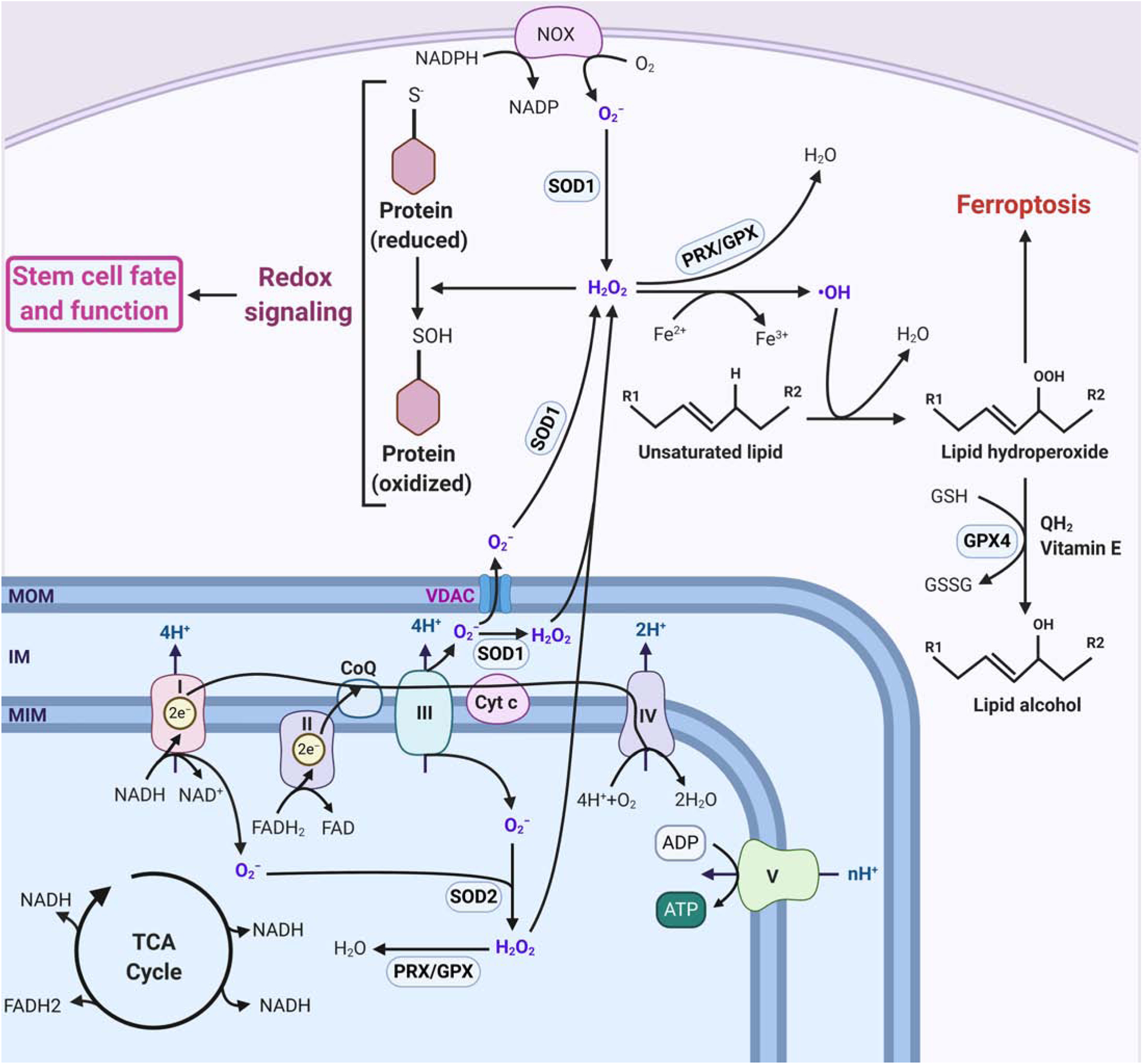

(3a) Mitochondrial ETC and cytosolic NOX are two major sites of superoxide radical (O2−) generation in a cell. Mitochondrial complex I generates O2− only in the mitochondrial matrix, whereas complex III generates O2− both in the matrix and intermembrane space. Immediately after generation, O2− gets converted into more stable and membrane-permeable hydrogen peroxide (H2O2) by superoxide dismutase (SOD2 in the matrix and SOD1 in the intermembrane space and cytosol). Mitochondrial O2− can also exit the mitochondria through voltage-dependent anion channels (VDAC) and get converted into H2O2 in the cytosol. H2O2 can oxidize specific sulfur-containing amino acids, primarily cysteine, of redox-sensitive proteins that are critical for stem cell fate and function and thereby control their functions, stability, and subcellular localization. Excess H2O2 can also generate hydroxyl radical (•OH) through Fenton reaction, which is coupled with Fe2+ oxidation. •OH can oxidize polyunsaturated fatty acids (PUFA) and generate lipid hydroperoxide that can induce cell death by ferroptosis. As excess ROS is toxic, cells have different ROS scavengers, such as peroxiredoxins (PRX) and glutathione peroxidases (GPX), as well, to maintain ROS homeostasis. (MOM: mitochondrial outer membrane; IM: intermembrane space; MIM: mitochondrial inner membrane)
(3b) Stem cells require a low basal level of ROS for the maintenance of self-renewal capacity. FOXO proteins help maintain low ROS levels by activating the expression of different ROS scavengers. A moderate physiological increase in ROS level is necessary for commitment to progenitor cells and differentiation into different cell types. However, excess ROS accumulation leads to stem cell exhaustion. (SC: Stem Cell, PC: Progenitor Cell, DC: Differentiated Cell)
A general theme with respect to mitochondrial ROS mediated regulation of mammalian pluripotent stem cells (PSCs) is that induced pluripotent stem cells (iPSCs), and embryonic stem cells (ESCs) have low levels of endogenous ROS to maintain genome integrity, which increases and is necessary for differentiation into different lineages (Tan and Suda, 2018). Of note, pluripotent stem cells (PSCs), which can give rise to cells of all three germ layers, can be isolated from inner cell mass (ICM) of preimplantation epiblast (embryonic stem cells (ESCs)) or the early post-implantation epiblast (epiblast stem cells (EpiSCs)). Also, PSCs can be generated from somatic cells by expressing pluripotency-associated transcription factors (induced PSCs, iPSCs). Pluripotent stem cells can exist in cultures in two different states: naive and primed (a more developmentally committed state), which primarily depends on cell culture conditions instead of the cell of origin (Weinberger et al., 2016). Naive and primed PSCs usually share molecular signatures with cells of the ICM and early post-implantation epiblasts, respectively (Martello and Smith, 2014). Inhibition of mitochondrial respiratory chain or depolarization of mitochondrial membrane potential (MMP) prevents PSC differentiation but maintains pluripotency (Mandal et al., 2011). Mutations in mtDNA increase mitochondrial H2O2 production and decrease iPSC reprogramming efficiency and self-renewal capacity (Hämäläinen et al., 2015). The addition of mitochondria-targeted ubiquinone (MitoQ), which decreases mitochondrial H2O2 production, rescues these defects (Hämäläinen et al., 2015). However, the addition of MitoQ causes toxicity to wild-type iPSCs and neural stem cells (NSCs) (Hämäläinen et al., 2015). Together these results indicate that a low basal level of mitochondrial H2O2 is necessary to maintain the viability of PSCs, and an increase in mitochondrial H2O2 triggers loss of stemness and is necessary for differentiation.
Mitochondrial ROS has also been implicated as a critical regulator of quiescence, activation, proliferation, differentiation, and exhaustion of adult stem cells (Liang and Ghaffari, 2018; Tan and Suda, 2018). Quiescent, self-renewing adult stem cells usually maintain a low basal level of ROS, which moderately increases during commitment, proliferation, and differentiation (Liang and Ghaffari, 2018; Tan and Suda, 2018). HSCs with the lowest ROS levels have the highest self-renewal potential (Jang and Sharkis, 2007). FOXO family transcription factors activate the expression of different antioxidant genes to keep the ROS levels low in adult stem cells (Ito and Suda, 2014). FOXO3 deficient or FOXO1/3/4 deficient murine NSCs and neural progenitor cells (NPCs) exhibit rapid proliferation and increase in brain size immediately after birth, followed by a precipitous decline in the self-renewal capacity and the number of both NSCs and NPCs (Paik et al., 2009; Renault et al., 2009). Murine HSCs and skeletal muscle stem cells (MuSCs) or “satellite cells” show a similar requirement of FOXO3 for the maintenance of self-renewal capacity (Gopinath et al., 2014; Miyamoto et al., 2007; Miyamoto et al., 2008; Tothova et al., 2007). It is important to note that beyond regulating oxidative stress, FOXOs regulate mitochondrial oxidative metabolism (Rimmelé et al., 2015). A unifying concept emerging is that a low level of mitochondrial ROS is required for adult stem cell self-renewal and quiescence, and an increase in mitochondrial H2O2 production is necessary for normal stem cell proliferation and differentiation (Figure 3b). By contrast, aberrantly high mitochondrial H2O2 levels trigger stem cell exhaustion. For instance, loss of mitochondrial carrier homolog 2 (MTCH2) triggers an increase in mitochondrial mass, ATP, and ROS levels and promotes entry of hematopoietic stem and progenitor cells (HSPCs) into the cell cycle (Maryanovich et al., 2012; Maryanovich et al., 2015). Multiple other studies have shown that the low basal level of ROS is essential for maintaining the self-renewal capacity of different adult stem cells, including spermatogonial stem cells, NSCs, and airway basal stem cells (Le Belle et al., 2011; Morimoto et al., 2013; Paul et al., 2014). It is unknown what factors control the production of mitochondrial ETC generated ROS in stem cells. One clue comes from the observation that HSCs require mitochondrial fatty acid oxidation (FAO) for HSC maintenance and function (Ito et al., 2012). FAO might provide electrons to ETC through NADH and dihydroflavine-adenine dinucleotide (FADH2) to generate ROS for HSC fate and function.
A moderate physiological increase in mitochondrial H2O2 level is necessary for the commitment, proliferation, and differentiation of different adult stem cells, including HSCs, keratinocyte stem cells, airway basal stem cells, MuSCs, NSCs, and mesenchymal stem cells (MSCs) (Bhattacharya and Scimè, 2020; Hamanaka et al., 2013; Juntilla et al., 2010; Khacho et al., 2016; Paul et al., 2014; Tormos et al., 2011). Decreased mitochondrial H2O2 levels in AKT1/2 double deficient HSCs cause increased quiescence and defects in differentiation, which can be rescued by pharmacologically increasing mitochondrial H2O2 levels in those cells (Juntilla et al., 2010). Increased mitochondrial H2O2 induces differentiation of murine HSCs primarily through modulating the activities of p38 MAPK and FOXO family of transcription factors. Besides, an increase in mitochondrial H2O2 levels is necessary for HSC homing and initial proliferation after transplantation into lethally irradiated mice, indicating that ROS might be essential for HSC homing in the bone marrow microenvironment (Golan et al., 2012).
Excessive ROS accumulation leads to loss of quiescence and stem cell exhaustion, as well as senescence and apoptosis of the stem cells (Tan and Suda, 2018). For instance, an increase in ROS levels due to loss of FOXO3 results in loss of HSC quiescence (Miyamoto et al., 2007; Tothova et al., 2007). Similarly, increased ROS levels in ATM-deficient mice cause HSC exhaustion by activating the p38-MAPK pathway, which can be reversed by prolonged treatment with an antioxidant or a p38-MAPK inhibitor (Ito et al., 2004). The microenvironment of stem cells can also maintain the balance of ROS between quiescence and differentiation (Itkin et al., 2016; Ludin et al., 2012; Taniguchi Ishikawa et al., 2012). For example, a rare population of α-smooth muscle actin (α-SMA) expressing monocytes and macrophages in bone marrow has been found to protect HSPCs under steady-state conditions and sublethal irradiation-induced stress (Ludin et al., 2012). α-SMA+ monocytes and macrophages express cyclooxygenase 2 (COX2) at a high level, and COX2-derived prostaglandin E2 (PGE2) decreases ROS generation through inhibition of protein kinase Akt in HSCs (Ludin et al., 2012). Of note, HSPCs with a low ROS level are usually quiescent and have superior long-term repopulation ability, whereas HSPCs with a high level of ROS have short term repopulation ability and exhibit a bias toward myeloid differentiation. PGE2 also augments the expression of chemokines CXCR4 on HSPCs and CXCL12 in nearby stromal cells, and CXCR4-CXCL2 interaction is essential for HSC quiescence (Ludin et al., 2012). Another study shows that connexin-43 (Cx43), a connexin constituent of gap junctions (GJs) expressed in HSCs, protects HSCs from oxidative stress-induced senescence by transferring ROS from HSCs to stromal cells (Taniguchi Ishikawa et al., 2012). Also, the low oxygen tension of the microenvironment helps keep the ROS level in HSC low. For instance, perisinusoidal bone marrow regions contain less permeable arterial blood vessels, which help maintain ROS levels low in murine quiescent HSCs (Itkin et al., 2016). On the other hand, more permeable sinusoids increase ROS levels and induce activation, migration, and differentiation of HSPCs (Itkin et al., 2016). Therefore, cells must prevent aberrant ROS levels in the stem cells to protect their self-renewal capacity and function. It is likely that LOOH is the major toxic ROS in stem cells. But this needs to be validated with genetic interventions that prevent the generation of LOOH. Going forward, the details of how H2O2 or other ROS specifically targets pathways to maintain stem cell fate and function needs to be elucidated.
Regulation of stem cell fate by TCA cycle metabolites
Stem cell identity and functions, like other cells, are determined primarily by the gene expression profiles. In eukaryotic cells, gene transcriptions are controlled through sophisticated coordination between transcription factors and chromatin remodelers and modifiers (Dai et al., 2020). DNA and distinct histone methylations often promote the self-renewal capacity of adult stem cells, whereas demethylation results in stem cell activation, proliferation, and differentiation (Beerman and Rossi, 2015). Different TCA cycle metabolites are the substrates of many chromatin-modifying enzymes and can regulate gene expression by controlling chromatin modifications (Figure 4) (Martínez-Reyes and Chandel, 2020; Schvartzman et al., 2018). For instance, histone acetylation, an active chromatin modification, is catalyzed by histone acetyltransferases (HAT) and requires acetyl-CoA as the substrate. Similarly, DNA and histone methylations and demethylations are also associated with gene expression regulation. The Jumonji C domain-containing ten-eleven translocation (TET) enzymes involved in the reversal of cytosine methylation through stepwise oxidation, and histone demethylases (KDMs) are α-ketoglutarate (α-KG) dependent dioxygenases and key regulators of stem cell fate and function (Avgustinova and Benitah, 2016; Ly et al., 2020). Besides, being structurally similar to α-KG, succinate, fumarate, and 2-hydroxyglutarate (both D- and L- isoforms) can competitively inhibit TETs and KDMs and thereby regulate gene expression (Chowdhury et al., 2011; Schvartzman et al., 2018; Xu et al., 2011). Furthermore, mitochondrial one-carbon metabolism contributes to the cellular pool of S-adenosylmethionine (SAM) that is necessary for histone and DNA methylation (Dai et al., 2020). Similarly, RNA N6-methyladenosine methylation and demethylation, which regulates gene expression post-transcriptionally, can be regulated by SAM and α-KG, respectively (Yue et al., 2015). Here, we focus on the role of two TCA cycle intermediates, acetyl-CoA, and α-KG, in the regulation of mammalian embryonic and adult stem cell fate.
Figure 4: TCA cycle metabolites and NAD+/NADH ratio regulate stem cell fate and function through chromatin modifications.

Citrate derived acetyl-CoA is a substrate for histone acetyltransferase (HAT). Sirtuins, which are NAD+ dependent histone deacetylases, can remove histone acetyl marks. Mitochondria is a key regulator of the cellular NAD+/NADH ratio that regulates the activities of sirtuins. Similarly, mitochondrial one-carbon metabolism (1CM) contributes to the production of S-adenosyl methionine (SAM), which is a substrate for histone and DNA methyltransferases (KMTs, and DNMTs, respectively). Also, Jumonji C domain-containing ten-eleven translocation (TET) enzymes involved in the reversal of cytosine methylation and histone demethylases (KDMs) require α-KG, and these enzymes can be competitively inhibited by succinate, fumarate, and L-2-HG.
Cytosolic acetyl-CoA required for histone acetylation is generated by ATP citrate lyase (ACLY) and acyl-CoA synthetase short-chain family member 2 (ACSS2) that use citrate and acetate as substrates, respectively (Dai et al., 2020; Wellen et al., 2009). Mouse and human PSCs have open chromatin structure characterized by DNA hypomethylation, abundant active histone modifications (e.g., H3 and H4 acetylation, H3K4 trimethylations, etc.), and low heterochromatin (Gaspar-Maia et al., 2011). To maintain this unique chromatin state, PSCs have distinct metabolic requirements compared to differentiated cells. For instance, PSCs require high acetyl-CoA levels for maintaining enriched histone acetylation marks and pluripotency and self-renewal capacity (Moussaieff et al., 2015; Wang et al., 2009). However, the sources of acetyl-CoA differ between hPSCs and mouse ESCs (mESCs). In mESCs, glucose and threonine catabolism contribute to high acetyl-CoA levels (Shyh-Chang et al., 2013; Wang et al., 2009). Of note, threonine catabolism generates acetyl-CoA and glycine in mitochondria, and mESCs critically depend on this process (Shyh-Chang et al., 2013). On the other hand, hPSCs lack threonine dehydrogenase (TDH) enzyme, and acetyl-CoA is primarily produced from glucose-derived pyruvate (Moussaieff et al., 2015). In hPSCs, mitochondrial acetyl-CoA is exported to cytosol in the form of citrate (Moussaieff et al., 2015). Citrate is then converted back into acetyl-CoA by ACLY enzyme and is necessary for H3K9/K27 acetylation (Moussaieff et al., 2015). By contrast, during early differentiation of hPSCs, glycolysis-derived acetyl-CoA is not exported to the cytosol via citrate and is associated with a loss of H3K9/K27 acetylation marks (Moussaieff et al., 2015). Importantly, treatment with acetate, a precursor of acetyl-CoA, increases H3K9/K27 acetylation marks and delays early differentiation in hPSCs and mESCs, indicating that decline in acetyl-CoA levels drives the early differentiation in PSCs, possibly through reducing histone acetylation marks and thereby making chromatin less accessible (Moussaieff et al., 2015). Conversely, acetyl-CoA generated by ACLY in adult stem cells is necessary for differentiation in part through histone acetylation mediated regulation of gene expression (Das et al., 2017; Zhou et al., 2019). Thus, TCA cycle generated acetyl-CoA controls stemness of mESCs and hPSCs and differentiation of adult stem cells.
DNA and histone methylations are driven by SAM as the methyl donor. Naive hPSCs control SAM levels to regulate H3K4 trimethylation, characteristic marks of active chromatin (Shiraki et al., 2014). However, SAM is maintained at a low level in naive hPSCs to keep the repressive H3K27 trimethylation marks at low levels as well (Sperber et al., 2015). Nicotinamide N-methyltransferase (NNMT) enzyme, which consumes SAM, is upregulated in hPSCs and necessary for maintaining SAM and H3K27 trimethylation marks at low levels (Sperber et al., 2015). Knockdown of NNMT increases SAM level and H3K27 trimethylation marks, activates HIF, represses Wnt signaling, and induces a switch from naive to primed state (Sperber et al., 2015). On the other hand, KDMs and TETs use α-KG as a substrate. α-KG has differential effects on the fate of naive and primed mammalian PSCs. Naive mESCs generally maintain high levels of α-KG through both glutamine and glucose catabolism (Carey et al., 2015). Naive mESCs exhibit elevated α-KG/succinate ratio and DNA and histone hypomethylation to maintain a highly accessible genome. Although glutamine is dispensable for naive PSC culture, naive mESCs cultured in glutamine-free media show reduced α-KG levels, and increases in total trimethylations, and a decrease in total monomethylations on H3K9, H3K27, H3K36, and H4K20 residues (Carey et al., 2015; Vardhana et al., 2019). Importantly, treatment with cell-permeable dimethyl α-KG (DMKG) reverses the increases in the H3K27 and H4K20 trimethylation marks in the mESCs, confirming that the increases in trimethylations are actually caused by a decline in α-KG levels in the cells (Carey et al., 2015). Furthermore, during spontaneous differentiation of naive mESCs, α-KG level declines, and DMKG treatment delays the differentiation possibly by slowing down repression of pluripotency factor expression through activating DNA and histone demethylation at their loci (Carey et al., 2015). DMKG has also been shown to promote mESC pluripotency and self-renewal capacity. Cellular α-KG levels are regulated by the phosphoserine aminotransferase 1 (Psat1) enzyme in naive mESCs to maintain pluripotency (Hwang et al., 2016). The expression of Psat1 is regulated by core pluripotency factors Oct4, Sox2, and Nanog. Psat1 is an enzyme of de novo serine synthesis pathway that couples the conversion of 3-phosphohydroxy-pyruvate and glutamate to 3-phosphoserine and α-KG. In contrast, increasing the α-KG levels promotes differentiation of primed hPSCs and mouse EpiSCs (mEpiSCs), whereas inhibition of α-KG production from glutamine delays primed hPSC differentiation (TeSlaa et al., 2016). Furthermore, succinate accumulation and thereby, decrease in α-KG/succinate ratio induced by pharmacological inhibition or genetic knockdown of succinate dehydrogenase A (SDHA) delays primed hPSC differentiation (TeSlaa et al., 2016). This effect can be rescued by DMKG treatment, which increases the α-KG/succinate ratio. It also appears that an increase or decrease in α-KG/succinate ratio regulates primed PSC fate through activating or inhibiting TET and KDM enzymes, respectively (TeSlaa et al., 2016).
Self-renewing adult stem cells often reside in hypoxic niches and are usually glycolytic (Mohyeldin et al., 2010). Hypoxia decreases mitochondrial ETC function, causing an increase in L-2-hydroxyglutarate (L-2-HG) and succinate accumulation (Intlekofer et al., 2015; Oldham et al., 2015). As a result, high succinate/α-KG or high L-2-HG/α-KG ratio might help maintain the self-renewal capacity of adult stem cells by suppressing DNA demethylation through inhibiting TET and KDM enzymes. By contrast, elevated OXPHOS, as observed in activated adult stem cells, usually increases α-KG levels and decreases succinate or L-2-HG levels leading to histone and DNA demethylation, thus enabling stem cell activation, proliferation, and differentiation. A key question not fully understood in the field is how changes in these metabolites (acetyl-CoA, aKG, succinate, fumarate, and L- or D-2HG) modify specific histone acetylation and methylation marks, and DNA methylation marks at specific loci to modulate gene expression necessary to determine stem cell fate.
NAD+/NADH ratio regulates the stem cell fate and function
TCA cycle generates reducing equivalents NADH and FADH2 that are subsequently oxidized into NAD+ and FAD by ETC complex I and II, respectively, to fuel OXPHOS (Chandel, 2015). Thus, mitochondria can act as critical regulators of cellular NAD+/NADH ratio that can dictate the fate and function of different cell types, including stem cells. PSCs and adult stem cell fate and function can be regulated by sirtuins, a class of NAD+ dependent deacylases that remove various acyl groups (e.g., acetyl, succinyl, malonyl, glutaryl, or long-chain acyl groups) from different proteins (Fang et al., 2019). There are seven sirtuins (SIRT1 to SIRT7) in mammals, and they reside in specific subcellular locations (Fang et al., 2019). SIRT1 and SIRT2 can reside in the nucleus or cytoplasm, whereas SIRT6 and SIRT7 are nuclear proteins (Fang et al., 2019). SIRT3, SIRT4, and SIRT5 are found in the mitochondrial matrix (Fang et al., 2019). Both mESCs and hPSCs have elevated SIRT1 protein level, which declines during differentiation (Saunders et al., 2010; Williams et al., 2016; Zhang et al., 2014). SIRT1 maintains naive mESC pluripotency (Williams et al., 2016; Zhang et al., 2014). Both SIRT2 and SIRT6 are necessary for proper lineage commitment of PSCs (Cha et al., 2017; Etchegaray et al., 2015; Si et al., 2013). However, to date, it is not clear whether changes in mitochondrial NAD+/NADH ratio control these nuclear SIRTs to regulate stem cell fate.
Mitochondrial complex I has the ability to regenerate NAD+ and thereby regulates SIRT3 dependent acetylation of proteins in the mitochondrial matrix (Karamanlidis et al., 2013). HSCs are enriched in SIRT3 (Brown et al., 2013). SIRT3 expression reduces with age, and SIRT3 loss in aged mice reduces the HSC pool and compromises HSC self-renewal upon serial transplantation stress (Brown et al., 2013). Furthermore, SIRT3 overexpression reduces oxidative stress and rescues functional defects in aged HSCs (Brown et al., 2013). Similarly, MuSCs in aged mice exhibit mitochondrial dysfunction and low NAD+ levels (Zhang et al., 2016). Treatment with nicotinamide riboside (NR), a precursor of NAD+, improves mitochondrial function in MuSCs, protects them from senescence, increases their number, and improves muscle regeneration capacity (Zhang et al., 2016). NR treatment also protects NSCs and melanocyte stem cells from senescence (Zhang et al., 2016). However, the beneficial effect of NR treatment disappears in mice lacking SIRT1 in MuSCs, indicating the necessity of SIRT1 for this process (Zhang et al., 2016). Activation of quiescent MuSCs is accompanied by a switch from FAO to glycolysis, resulting in a decrease in cellular NAD+ levels and SIRT1 activity (Ryall et al., 2015). Deletion of the deacetylase domain of SIRT1 in mice leads to premature MuSC differentiation and impairs muscle regeneration (Ryall et al., 2015). It is important to note that total NAD+ levels in part are controlled by NAMPT (nicotinamide phosphoribosyltransferase), the rate-limiting enzyme of the NAD+ salvage pathway, which declines with age in multiple tissues, including the mouse hippocampus (Stein and Imai, 2014). Deletion of NAMPT in adult neural stem and progenitor cells reduces their ability of self-renewal, proliferation, and oligodendrocyte generation, which can be rescued by nicotinamide mononucleotide (NMN; a precursor of NAD+) supplementation (Stein and Imai, 2014). These studies highlight that NAD+ is a key regulator of stem cell fate during the normal aging process.
One direct link of how mitochondrial NAD+/NADH ratio could impact stem cell function is through the generation of L-2-HG. Mitochondrial ETC impairment, acidity, or hypoxia decreases NAD+ regeneration, resulting in a decrease in NAD+/NADH ratio, and triggers an increase in L-2-HG level. Unlike D-2-HG, an oncometabolite produced by mutant isocitrate dehydrogenases (IDH1/2), lactate dehydrogenases (LDHA, LDHC), and cytosolic and mitochondrial malate dehydrogenases (MDH1 and MDH2, respectively) can use α-KG as a promiscuous substrate and NADH as a coenzyme to generate L-2-HG (Intlekofer et al., 2017; Nadtochiy et al., 2016; Teng et al., 2016). Being structurally similar to α-KG, L-2-HG can competitively inhibit α-KG dependent dioxygenases, including Jumonji C domain-containing KDMs and TETs. Since α-KG can dictate stem cell fate and function through regulating histone and DNA methylations, L-2-HG might also be a key regulator of stem cell biology. Loss of the mitochondrial ETC complex III subunit Rieske iron-sulfur protein (RISP) in fetal mouse HSCs increases NADH/NAD+ ratio and triggers L-2-HG levels to impair their differentiation into MPPs without affecting their ability to self-renew, resulting in embryonic lethality (Ansó et al., 2017). Loss of RISP in adult mouse HSCs disrupts their quiescence and causes severe pancytopenia and lethality (Ansó et al., 2017). HSCs deficient in RISP display DNA and histone hypermethylation as well as histone hypoacetylation (Ansó et al., 2017). Interestingly, loss of mitochondrial complex III function in oncogenically transformed HSCs decreases proliferation and tumor progression, thus highlighting differential mitochondria dependent proliferative pathways between normal HSCs and oncogenic transformed HSCs (Martínez-Reyes et al., 2020). Collectively, these studies indicate that mitochondrial complex III is dispensable for the proliferation of HSCs but a dominant regulator of HSC differentiation and quiescence. It is not known whether the elevated L-2-HG impairs differentiation or loss of quiescence through epigenetic modifications.
Pyruvate metabolism dictates stem cell fate and function
Pyruvate can have two primary fates: reduction into lactate in the cytosol and oxidation through the TCA cycle in mitochondria. Pyruvate metabolism has been shown to regulate the fate and function of both PSCs and adult stem cells. In culture, pluripotent, self-renewing stem cells are usually glycolytic, whereas a switch from cytosolic pyruvate reduction to mitochondrial pyruvate oxidation occurs during differentiation (Figure 5). Uncoupling protein 2 (UCP2), which shunts glucose-derived pyruvate away from mitochondria, is elevated in hPSCs and repressed during differentiation (Zhang et al., 2011). Also, UCP2 overexpression impairs hPSC differentiation (Zhang et al., 2011). Pyruvate dehydrogenase (PDH) activity, which oxidizes pyruvate into acetyl-CoA, is low in hPSCs. Also, during somatic cell reprogramming into iPSCs, a switch from OXPHOS to glycolysis occurs, and consequently, blocking glycolysis impairs reprogramming (Folmes et al., 2011). Similarly, quiescent HSCs display high glycolytic activity, as evidenced by high Fructose 1,6-bisphosphate and pyruvate levels and low phosphoenolpyruvate levels (Takubo et al., 2013). PDK2/4 expression, which inhibits mitochondrial pyruvate oxidation into acetyl-CoA by pyruvate dehydrogenase (PDH) in the mitochondrial matrix and thereby promotes glycolysis, is also elevated in quiescent HSCs (Takubo et al., 2013). HIF, which promotes glycolysis by upregulating PDK2/4 expression, has been shown to be essential for maintaining quiescence and reconstitution capacity of HSCs in transplantation assay (Takubo et al., 2010). Another study also shows the necessity of aryl hydrocarbon receptor nuclear translocator (ARNT), the HIF-α dimerization partner, and HIF-α for the HSC survival and homeostasis (Krock et al., 2015). However, other studies have reported the dispensability of HIF for HSC quiescence and reconstitution capacity (Guitart et al., 2013; Vukovic et al., 2016). By contrast, mitochondrial OXPHOS is essential for HSC differentiation into different blood cells but not for HSC maintenance (Ansó et al., 2017; Yu et al., 2013). It remains unknown why diminished PDH activity is necessary for HSC quiescence. Does low PDH activity decrease acetylation or ROS production to maintain quiescence? A recent study shows that primed HSCs characterized by a high MMP uptake more glucose and exhibit both increased glucose consumption and mitochondrial respiration compared to quiescent HSCs characterized by a low MMP (Liang et al., 2020). The study also shows that glucose consumption and mitochondrial pyruvate transport are necessary for the survival of primed HSCs, but not quiescent HSCs (Liang et al., 2020).
Figure 5: Pyruvate metabolism regulates stem cell fate and function.

Stem cells usually reduce glucose-derived pyruvate into lactate in the cytosol, whereas differentiated cells oxidize pyruvate through TCA cycle. In contrast to differentiated cells, stem cells have low levels of mitochondrial pyruvate carriers (MPC) that transports pyruvate from the cytosol into the mitochondria, and increased levels of uncoupling protein 2 (UCP2) that shunts away pyruvate from mitochondria into the cytosol, and pyruvate dehydrogenase kinases (PDKs) that inhibit pyruvate to acetyl-CoA conversion in the mitochondria. Stem cells also produce less H2O2 compared to their differentiated counterparts.
Mouse intestinal stem cells (ISCs) exhibit a reduced level of MPC1/2 expression (Schell et al., 2017). MPC1/2 encode mitochondrial pyruvate carrier (MPC) (Bricker et al., 2012; Herzig et al., 2012). MPC resides in the mitochondrial inner membrane and is necessary for transporting glucose-derived pyruvate from the cytosol into the mitochondrial matrix, where it gets oxidized by PDH to acetyl Co-A. In differentiated cells, MPC1 expression increases (Schell et al., 2017). MPC1 overexpression or substituting glucose with galactose to decrease glycolytic flux impairs leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) expression and promotes differentiation (Schell et al., 2017). By contrast, loss of MPC1 in Lgr5+ ISCs promotes maintenance and proliferation of murine ISCs both in vivo and in vitro and delays ISC differentiation in vitro through mechanisms that are not fully understood (Schell et al., 2017). Loss of MPC1 forces these cells to use FAO (Schell et al., 2017). It is not fully understood how the loss of pyruvate oxidation increases the ISC pool. It could be due to controlling the production of mitochondrial acetyl-CoA that affects histone acetylation. Conversely, promoting mitochondrial pyruvate oxidation increases ISC differentiation through mtROS mediated p38 MAPK activation (Rodríguez-Colman et al., 2017). Blocking glycolysis and promoting OXPHOS by substituting glucose with galactose in the media promotes ISC differentiation and mid-gut organoid formation (Rodríguez-Colman et al., 2017). By contrast, impairing ETC function pharmacologically or treatment with mtROS scavengers impairs the mid-gut organoid forming capacity of ISCs (Rodríguez-Colman et al., 2017). An interesting observation is that Paneth cells in the intestinal crypt, which are usually glycolytic, supply lactate as a source of pyruvate for ISCs, which is necessary for mid-gut organoid formation (Rodríguez-Colman et al., 2017).
An exciting observation with a potential clinical application of manipulating pyruvate metabolism comes from the observation that genetic deletion of MPC1 or LDHA promotes or impairs, respectively, hair follicle stem cells (HFSCs) activation (Flores et al., 2017). MPC1 or LDHA loss increases or decreases, respectively, lactate production (Flores et al., 2017). Topical treatment of resting mouse epidermis during a resting phase of the hair cycle (when HFSCs are quiescent) with different ETC inhibitors to increase lactate production results in activation of HFSCs and hair cycle (Miranda et al., 2018). Hair cycle in aged mice is usually protracted. However, topical treatment with ETC inhibitors or MPC inhibitors in aged mice increases the lactate pools and makes the hair cycle similar to that of younger mice (Miranda et al., 2018). Mechanisms underlying how an increase in lactate production stimulates HFSCs remain unknown. LDHA dependent generation of lactate due to MPC or ETC inhibition is linked to the cytosolic conversion of NADH to NAD+ accompanied by changes in pH. Lactate can directly modify histones to regulate gene expression in macrophages, while an elevation in intracellular pH promotes non-enzymatic β-catenin acetylation downstream of Wnt signaling in the tailbud (Oginuma et al., 2020; Zhang et al., 2019). Changes in NAD+/NADH ratio, as discussed above, can also control gene expression. It is not clear whether an increase in lactate, changes in NAD+/NADH ratio, or a decrease in mitochondrial pyruvate oxidation is the essential step in regulating HFSC activation. It is unlikely that pyruvate as a metabolite directly functions as a modulator of stem cell fate.
Mitochondrial dynamics regulate stem cell fate
Mitochondria are highly dynamic organelles that continuously change their morphology through opposing membrane fission and fusion processes (Friedman and Nunnari, 2014). Mitochondrial fission is mediated by DRP1 (dynamin-related protein 1), a large GTPase. DRP1 is a cytosolic protein that, upon activation, is recruited on the mitochondrial membrane in collaboration with accessory proteins such as mitochondrial fission factor (MFF), mitochondrial fission protein 1 (FIS1), and mitochondrial elongation factor 1 and 2 (Mief1/MiD51, and Mief2/Mid49). On the other hand, mitochondrial fusion is mediated by three large GTPases- MFN1 (mitofusin 1), MFN2 (mitofusin 2), and OPA1 (optic atrophy 1). MFN1 and MFN2 are localized on the mitochondrial outer membrane, whereas OPA1 is localized on the mitochondrial inner membrane. PSCs usually contain fragmented, globular mitochondria, whereas their differentiated counterparts contain elongated, tubular mitochondria (Chen and Chan, 2017). By contrast, mitochondrial fission is necessary for the commitment and differentiation of NSCs and Lgr5+ crypt-based columnar cells (CBCs) (Khacho et al., 2016; Ludikhuize et al., 2020).
Naive mESCs in vitro display fragmented and perinuclear mitochondrial morphology, while the primed pluripotent state represented by mEpiSC have elongated mitochondria (Zhou et al., 2012). Mitochondrial fission is necessary for the reprogramming of somatic cells into pluripotent stem cells (iPSCs) (Friedman and Nunnari, 2014; Prieto et al., 2016; Son et al., 2013). For instance, mouse embryonic fibroblasts (MEFs) usually have tubular mitochondrial morphology, whereas expression of Yamanaka factors (OCT4, SOX2, KLF4, and MYC (OSKM)) in MEFs induces mitochondrial fragmentation during early reprogramming (Prieto et al., 2016). Inhibition of DRP1 function by RNA interference or overexpression of the dominant-negative DRP1K38A impairs OSKM induced mitochondrial fission and significantly reduces the number of pluripotent alkaline peroxidase (AP) positive colonies (Prieto et al., 2016). Of note, mitochondrial fragmentation decreases during the later stage of reprogramming, and mitochondria in cells regain tubular morphology (Prieto et al., 2016). Similarly, DRP1 and REX1 (reduced expression 1), which promotes DRP1 phosphorylation and mitochondrial fission, are essential for maintaining pluripotency and self-renewal capacity of hPSCs (Son et al., 2013). Knockdown of DRP1 or REX1 induces a change in mitochondrial morphology from globular to complex elongated network and promotes differentiation (Son et al., 2013). REX1 is also essential for reprogramming of somatic cells into iPSCs, and overexpression of REX1 enhances reprogramming efficiency (Son et al., 2013).
A balance between mitochondrial fusion and fission is also essential for maintaining the full pluripotency and developmental potential of iPSCs (Zhong et al., 2019). Of note, iPSCs can have two pluripotent states- full and partial. Fully pluripotent iPSCs maintain a balance between mitochondrial fission and fusion, form embryoid bodies (EB) efficiently, and develop into live pups by tetraploid complementation (Zhong et al., 2019). On the other hand, partially pluripotent iPSCs exhibit upregulation of fission promoting proteins (e.g., DRP1, FIS1, and MFF), are less efficient in EB formation, and do not produce live pups by tetraploid complementation (Zhong et al., 2019). The levels of DRP1, FIS1, and MFF proteins decrease during EB differentiation (Zhong et al., 2019). Disruption of mitochondrial dynamics by forced activation of fission through overexpression of MFF protein in fully pluripotent mESCs and iPSCs affects their in vivo development and impairs their self-renewal capacity and neuronal differentiation potential (Zhong et al., 2019). By contrast, inhibition of excess fission by MFF knockdown in partially pluripotent iPSCs restores their EB formation capacity and neuronal differentiation potential (Zhong et al., 2019). A key question not fully understood in the field is how changes in fusion and fission in mESCs or iPSCs alter metabolites or ROS production to determine stem cell fate.
Quiescent, non-transplanted murine HSCs usually contain evenly dispersed, small, motile, immature mitochondria with low mitochondrial membrane potential (MMP) and exhibit high regenerative potential (Hinge et al., 2020). By contrast, transplanted HSCs, which have undergone consecutive cell divisions, usually accumulate elongated and swollen mitochondria with irregular cristae and high MMP through asymmetric segregation and have high ROS levels and low regenerative potential (Hinge et al., 2020). However, the mitochondria in transplanted HSCs fail to maintain MMP under stress (Hinge et al., 2020). Genetic ablation of DRP1 in quiescent non-transplanted murine HSCs does not affect their quiescence but results in mitochondrial aggregation and reduced motility, as well as reduced reconstitution potential upon transplantation (Hinge et al., 2020). This finding indicates that mitochondrial fission is necessary to maintain mitochondrial morphology and regenerative potential of HSCs. Asymmetric segregation of lysosomes, a crucial organelle for mitophagy, also plays an important role in maintaining mitochondrial morphology and regenerative potential of HSCs (Liang et al., 2020). The findings also suggest that the accumulation of defective mitochondria with elevated ROS levels might be responsible for the accumulation of DNA damage in HSCs following stress-induced proliferation. Accumulation of defective mitochondria through asymmetric cell division may also underlie HSC aging and clonal hematopoiesis.
Mitochondrial fusion appears to be essential for the differentiation of stem cells. For instance, MFN2 knockdown impairs the differentiation of human iPSCs into cortical neurons, while MFN2 overexpression improves mitochondrial respiration and promotes this process (Fang et al., 2016). Similarly, loss of MFN1/2 in the mouse embryonic heart and loss of MFN2/OPA1 in mESCs impair cardiac development and mESC differentiation into cardiomyocytes, respectively (Kasahara et al., 2013). The defects result from Ca2+/calcineurin-dependent activation of Notch1 signaling (Kasahara et al., 2013). One caution in the interpretation of these studies is that MFN2 and OPA1, in contrast to MFN1, have other roles beyond controlling fusion. MFN2 and OPA1 regulate tethering to ER and cristae remodeling, respectively (Giacomello et al., 2020). Loss of MFN1, which exclusively controls fusion, does not show an overt phenotype compared to the loss of MFN2 or OPA1 in similar biological contexts. For example, MFN2, independent of its role in fusion, facilitate tethering of mitochondria to the ER, which is necessary for maintaining HSCs with long-term lymphoid potential by buffering Ca2+ to preclude aberrant activation of the Ca2+/calcineurin dependent NFAT (nuclear factor of activated T cells) activity (Luchsinger et al., 2016).
The effect of mitochondrial fission and fusion on the fate of NSCs and LRG5+ crypt-based CBCs is divergent compared to mESCs or iPSCs. Mitochondrial fission regulates neuronal development (Khacho et al., 2016). At mid-neurogenesis, uncommitted stem cells in the ventricular zone (VZ) that contain NSCs usually have elongated mitochondria, whereas committed NPCs contain fragmented mitochondria (Khacho et al., 2016). The change in morphology is also associated with a physiological, moderate increase in ROS levels (Khacho et al., 2016). Similarly, deletion of OPA1 or MFN1/2 in uncommitted NSCs induces excessive mitochondrial fragmentation and a physiological increase in ROS levels, which, through NRF2 mediated retrograde signaling, suppresses self-renewal as evidenced by decreased neurosphere formation and promote differentiation (Khacho et al., 2016). Importantly, treatment with ROS scavengers rescues the defect in neurosphere formation in OPA1 deficient cells. On the other hand, loss of DRP1 in uncommitted NSCs causes mitochondrial elongation, decreases mtROS levels, and promotes NSC self-renewal capacity, as evidenced by a modest increase in neurosphere numbers (Khacho et al., 2016). Of note, such acute disruptions of mitochondrial dynamics do not result in a detectable change in mitochondrial ATP levels, suggesting that the effects mentioned above are not the result of ATP level change (Khacho et al., 2016). The mechanisms underlying the increase in ROS production by fission is not fully understood. Differentiation of LRG5+ CBCs into Paneth and Goblet cells requires mitochondrial fission (Ludikhuize et al., 2020). FOXOs maintain mitochondrial function in LRG5+ CBCs, and their loss triggers fission and induces differentiation (Ludikhuize et al., 2020). It would be of interest to know whether downregulation of FOXOs or manipulating fission triggers changes in TCA cycle metabolites that could impact epigenetics resulting in differentiation. Thus, mitochondrial dynamics are regulators of stem cell fate though the underlying mechanisms are not fully understood.
Recent studies have also suggested important roles of mitochondrial clearance in dictating stem cell fate. For instance, ATG5-independent noncanonical autophagy, which reduces mtDNA content and induces lactate formation, has been shown to be necessary for iPSC reprogramming, especially during_the early stage (Ma et al., 2015). Similarly, autophagy is necessary for the maintenance of stemness and regenerative capacity of HSCs by clearing mitochondria (Ho et al., 2017). Induction of mitophagy, a special form of autophagy that usually clears damaged mitochondria, promotes the self-renewing expansion of murine and human HSCs that are atop the HSC hierarchy and preferentially undergo symmetric cell division (Ito et al., 2016; Vannini et al., 2016). Importantly, inhibition of mitophagy through siRNA-mediated knockdown of Parkin 2 abrogates their self-renewing expansion and maintenance (Ito et al., 2016). However, Parkin 2 deficient murine HSCs display normal function in transplantation experiments suggesting possible off-target effects of Parkin 2 siRNAs (Ho et al., 2017). These findings indicate that the basal autophagy machinery but not the stress-induced Parkin 2 dependent mitophagy is likely important for mitochondrial turnover. Asymmetric sorting of aged and young mitochondria during cell division also plays an important role in regulating stem cell fates. For instance, daughter cells that receive more young mitochondria during asymmetric division of human mammary stemlike cells usually maintain stem cell properties, whereas daughter cells that receive more old mitochondria undergo differentiation (Katajisto et al., 2015). The age-dependent segregation of mitochondria depends on normal mitochondrial quality-control mechanisms, such as fission and mitophagy (Katajisto et al., 2015).
Conclusion
Historically, a combination of transcription factors, chromatin-modifying factors, and signaling pathways are the key players in regulating stem cell fate and function. In the past two decades, changes in metabolism have also been shown to be dominant inputs in controlling stem cell fate and function. Mitochondrial TCA cycle metabolism and ETC function have emerged as key inputs in regulating stem cell fate. To date, in vitro studies have shown that carbons from glucose and glutamine are necessary to generate TCA cycle intermediates. However, a caveat is that these studies are done in media that is not physiologic, and thus studies in vitro using human or mouse plasma-like media might come to a different conclusion about what are the sources of carbon fuels for the production of TCA cycle intermediates. For example, lactate is devoid in most cell culture media, but human and mouse plasma levels are in the low mM levels (Cantor et al., 2017). Moreover, the carbon fuels that are necessary to maintain stem cell quiescence or sustain differentiated phenotype in vivo is not fully understood. Although it is clear that mitochondria regulate stem cell fate and function in vitro and in vivo in multiple contexts, the specific mechanisms by which changes in mitochondrial TCA cycle metabolism and ETC function drive stem cell fate is missing. Understanding these mechanisms could provide therapeutic potential in the rejuvenation of tissues during normal aging or a particular disease (Navarro Negredo et al., 2020).
Acknowledgments
This work was supported by NIH (5R35CA197532 and 5R01AI148190) to N.S.C. and Northwestern University Pulmonary and Critical Care Department’s Cugell Predoctoral Fellowship to R.P.C. Figures are created with BioRender (https://biorender.com/).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no competing interests.
References
- Ahlqvist KJ, Hämäläinen RH, Yatsuga S, Uutela M, Terzioglu M, Götz A, Forsström S, Salven P, Angers-Loustau A, Kopra OH, et al. (2012). Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell metabolism 15, 100–109. [DOI] [PubMed] [Google Scholar]
- Ansó E, Weinberg SE, Diebold LP, Thompson BJ, Malinge S, Schumacker PT, Liu X, Zhang Y, Shao Z, Steadman M, et al. (2017). The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nature cell biology 19, 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgustinova A, and Benitah SA (2016). Epigenetic control of adult stem cell function. Nature reviews. Molecular cell biology 17, 643–658. [DOI] [PubMed] [Google Scholar]
- Bahat A, and Gross A (2019). Mitochondrial plasticity in cell fate regulation. The Journal of biological chemistry 294, 13852–13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksh SC, and Finley LWS (2020). Metabolic Coordination of Cell Fate by α-Ketoglutarate-Dependent Dioxygenases. Trends in cell biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman I, and Rossi DJ (2015). Epigenetic Control of Stem Cell Potential during Homeostasis, Aging, and Disease. Cell stem cell 16, 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, and Scimè A (2020). Mitochondrial Function in Muscle Stem Cell Fates. Frontiers in cell and developmental biology 8, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, et al. (2012). A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science (New York, N.Y.) 337, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, and Chen D (2013). SIRT3 reverses aging-associated degeneration. Cell reports 3, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JR, Abu-Remaileh M, Kanarek N, Freinkman E, Gao X, Louissaint A Jr., Lewis CA, and Sabatini DM (2017). Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell 169, 258–272.e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey BW, Finley LW, Cross JR, Allis CD, and Thompson CB (2015). Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y, Han MJ, Cha HJ, Zoldan J, Burkart A, Jung JH, Jang Y, Kim CH, Jeong HC, Kim BG, et al. (2017). Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis. Nature cell biology 19, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS (2015). Navigating metabolism.
- Chandel NS, Jasper H, Ho TT, and Passegué E (2016). Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nature cell biology 18, 823–832. [DOI] [PubMed] [Google Scholar]
- Chen H, and Chan DC (2017). Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells. Cell metabolism 26, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Logan TD, Hochberg ML, Shelat SG, Yu X, Wilding GE, Tan W, Kujoth GC, Prolla TA, Selak MA, et al. (2009). Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood 114, 4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, and Rando TA (2013). Molecular regulation of stem cell quiescence. Nature reviews. Molecular cell biology 14, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR, Leung IK, Li XS, Woon EC, Yang M, et al. (2011). The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO reports 12, 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Ramesh V, and Locasale JW (2020). The evolving metabolic landscape of chromatin biology and epigenetics. Nature reviews. Genetics 21, 737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Morvan F, Morozzi G, Jourde B, Minetti GC, Kahle P, Rivet H, Brebbia P, Toussaint G, Glass DJ, et al. (2017). ATP Citrate Lyase Regulates Myofiber Differentiation and Increases Regeneration by Altering Histone Acetylation. Cell reports 21, 3003–3011. [DOI] [PubMed] [Google Scholar]
- de Almeida MJ, Luchsinger LL, Corrigan DJ, Williams LJ, and Snoeck HW (2017). Dye-Independent Methods Reveal Elevated Mitochondrial Mass in Hematopoietic Stem Cells. Cell stem cell 21, 725–729.e724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, and Chandel NS (2020). We need to talk about the Warburg effect. Nature metabolism 2, 127–129. [DOI] [PubMed] [Google Scholar]
- Deus CM, Yambire KF, Oliveira PJ, and Raimundo N (2020). Mitochondria-Lysosome Crosstalk: From Physiology to Neurodegeneration. Trends in molecular medicine 26, 71–88. [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Chavez L, Huang Y, Ross KN, Choi J, Martinez-Pastor B, Walsh RM, Sommer CA, Lienhard M, Gladden A, et al. (2015). The histone deacetylase SIRT6 controls embryonic stem cell fate via TET-mediated production of 5-hydroxymethylcytosine. Nature cell biology 17, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Yan S, Yu Q, Chen D, and Yan SS (2016). Mfn2 is Required for Mitochondrial Development and Synapse Formation in Human Induced Pluripotent Stem Cells/hiPSC Derived Cortical Neurons. Scientific reports 6, 31462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Tang S, and Li X (2019). Sirtuins in Metabolic and Epigenetic Regulation of Stem Cells. Trends in endocrinology and metabolism: TEM 30, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi MD, and Ghaffari S (2019). Mitochondria in the maintenance of hematopoietic stem cells: new perspectives and opportunities. Blood 133, 1943–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Schell J, Krall AS, Jelinek D, Miranda M, Grigorian M, Braas D, White AC, Zhou JL, Graham NA, et al. (2017). Lactate dehydrogenase activity drives hair follicle stem cell activation. Nature cell biology 19, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, and Terzic A (2011). Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell metabolism 14, 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RG, Magness S, Kujoth GC, Prolla TA, and Maeda N (2012). Mitochondrial DNA polymerase editing mutation, PolgD257A, disturbs stem-progenitor cell cycling in the small intestine and restricts excess fat absorption. American journal of physiology. Gastrointestinal and liver physiology 302, G914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, and Nunnari J (2014). Mitochondrial form and function. Nature 505, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Maia A, Alajem A, Meshorer E, and Ramalho-Santos M (2011). Open chromatin in pluripotency and reprogramming. Nature reviews. Molecular cell biology 12, 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Pyakurel A, Glytsou C, and Scorrano L (2020). The cell biology of mitochondrial membrane dynamics. Nature reviews. Molecular cell biology 21, 204–224. [DOI] [PubMed] [Google Scholar]
- Golan K, Vagima Y, Ludin A, Itkin T, Cohen-Gur S, Kalinkovich A, Kollet O, Kim C, Schajnovitz A, Ovadya Y, et al. (2012). S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood 119, 2478–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SD, Webb AE, Brunet A, and Rando TA (2014). FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem cell reports 2, 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR, Zeviani M, and Turnbull DM (2016). Mitochondrial diseases. Nature reviews. Disease primers 2, 16080. [DOI] [PubMed] [Google Scholar]
- Guitart AV, Subramani C, Armesilla-Diaz A, Smith G, Sepulveda C, Gezer D, Vukovic M, Dunn K, Pollard P, Holyoake TL, et al. (2013). Hif-2α is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood 122, 1741–1745. [DOI] [PubMed] [Google Scholar]
- Hämäläinen RH, Ahlqvist KJ, Ellonen P, Lepistö M, Logan A, Otonkoski T, Murphy MP, and Suomalainen A (2015). mtDNA Mutagenesis Disrupts Pluripotent Stem Cell Function by Altering Redox Signaling. Cell reports 11, 1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, Getsios S, Gottardi CJ, DeBerardinis RJ, Lavker RM, et al. (2013). Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Science signaling 6, ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, and Martinou JC (2012). Identification and functional expression of the mitochondrial pyruvate carrier. Science (New York, N.Y.) 337, 93–96. [DOI] [PubMed] [Google Scholar]
- Hinge A, He J, Bartram J, Javier J, Xu J, Fjellman E, Sesaki H, Li T, Yu J, Wunderlich M, et al. (2020). Asymmetrically Segregated Mitochondria Provide Cellular Memory of Hematopoietic Stem Cell Replicative History and Drive HSC Attrition. Cell stem cell 26, 420–430.e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, and Passegué E (2017). Autophagy maintains the metabolism and function of young and old stem cells. Nature 543, 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IY, Kwak S, Lee S, Kim H, Lee SE, Kim JH, Kim YA, Jeon YK, Chung DH, Jin X, et al. (2016). Psat1-Dependent Fluctuations in α-Ketoglutarate Affect the Timing of ESC Differentiation. Cell metabolism 24, 494–501. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Dematteo RG, Venneti S, Finley LW, Lu C, Judkins AR, Rustenburg AS, Grinaway PB, Chodera JD, Cross JR, et al. (2015). Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell metabolism 22, 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, and Finley LWS (2019). Metabolic signatures of cancer cells and stem cells. Nature metabolism 1, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Wang B, Liu H, Shah H, Carmona-Fontaine C, Rustenburg AS, Salah S, Gunner MR, Chodera JD, Cross JR, et al. (2017). L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nature chemical biology 13, 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP, Ledergor G, Jung Y, Milo I, Poulos MG, et al. (2016). Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, et al. (2012). A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nature medicine 18, 1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. (2004). Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002. [DOI] [PubMed] [Google Scholar]
- Ito K, and Suda T (2014). Metabolic requirements for the maintenance of self-renewing stem cells. Nature reviews. Molecular cell biology 15, 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Turcotte R, Cui J, Zimmerman SE, Pinho S, Mizoguchi T, Arai F, Runnels JM, Alt C, Teruya-Feldstein J, et al. (2016). Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science (New York, N.Y.) 354, 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YY, and Sharkis SJ (2007). A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110, 3056–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, and Koretzky GA (2010). AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115, 4030–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr., Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, and Tian R (2013). Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell metabolism 18, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara A, Cipolat S, Chen Y, Dorn GW 2nd, and Scorrano L (2013). Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science (New York, N.Y.) 342, 734–737. [DOI] [PubMed] [Google Scholar]
- Katajisto P, Döhla J, Chaffer CL, Pentinmikko N, Marjanovic N, Iqbal S, Zoncu R, Chen W, Weinberg RA, and Sabatini DM (2015). Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science (New York, N.Y.) 348, 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppila TES, Kauppila JHK, and Larsson NG (2017). Mammalian Mitochondria and Aging: An Update. Cell metabolism 25, 57–71. [DOI] [PubMed] [Google Scholar]
- Khacho M, Clark A, Svoboda DS, Azzi J, MacLaurin JG, Meghaizel C, Sesaki H, Lagace DC, Germain M, Harper ME, et al. (2016). Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell stem cell 19, 232–247. [DOI] [PubMed] [Google Scholar]
- Khacho M, and Slack RS (2018). Mitochondrial dynamics in the regulation of neurogenesis: From development to the adult brain. Developmental dynamics : an official publication of the American Association of Anatomists 247, 47–53. [DOI] [PubMed] [Google Scholar]
- Krock BL, Eisinger-Mathason TS, Giannoukos DN, Shay JE, Gohil M, Lee DS, Nakazawa MS, Sesen J, Skuli N, and Simon MC (2015). The aryl hydrocarbon receptor nuclear translocator is an essential regulator of murine hematopoietic stem cell viability. Blood 125, 3263–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, and Kornblum HI (2011). Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell stem cell 8, 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Arif T, Kalmykova S, Kasianov A, Lin M, Menon V, Qiu J, Bernitz JM, Moore K, Lin F, et al. (2020). Restraining Lysosomal Activity Preserves Hematopoietic Stem Cell Quiescence and Potency. Cell stem cell 26, 359–376.e357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, and Ghaffari S (2018). Stem Cells Seen Through the FOXO Lens: An Evolving Paradigm. Current topics in developmental biology 127, 23–47. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, and Wang X (1996). Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157. [DOI] [PubMed] [Google Scholar]
- Luchsinger LL, de Almeida MJ, Corrigan DJ, Mumau M, and Snoeck HW (2016). Mitofusin 2 maintains haematopoietic stem cells with extensive lymphoid potential. Nature 529, 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludikhuize MC, Meerlo M, Gallego MP, Xanthakis D, Burgaya Julià M, Nguyen NTB, Brombacher EC, Liv N, Maurice MM, Paik JH, et al. (2020). Mitochondria Define Intestinal Stem Cell Differentiation Downstream of a FOXO/Notch Axis. Cell metabolism 32, 889–900.e887. [DOI] [PubMed] [Google Scholar]
- Ludin A, Itkin T, Gur-Cohen S, Mildner A, Shezen E, Golan K, Kollet O, Kalinkovich A, Porat Z, D’Uva G, et al. (2012). Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nature immunology 13, 1072–1082. [DOI] [PubMed] [Google Scholar]
- Ly CH, Lynch GS, and Ryall JG (2020). A Metabolic Roadmap for Somatic Stem Cell Fate. Cell metabolism 31, 1052–1067. [DOI] [PubMed] [Google Scholar]
- Ma T, Li J, Xu Y, Yu C, Xu T, Wang H, Liu K, Cao N, Nie BM, Zhu SY, et al. (2015). Atg5-independent autophagy regulates mitochondrial clearance and is essential for iPSC reprogramming. Nature cell biology 17, 1379–1387. [DOI] [PubMed] [Google Scholar]
- Mandal S, Lindgren AG, Srivastava AS, Clark AT, and Banerjee U (2011). Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem cells (Dayton, Ohio) 29, 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martello G, and Smith A (2014). The nature of embryonic stem cells. Annual review of cell and developmental biology 30, 647–675. [DOI] [PubMed] [Google Scholar]
- Martínez-Reyes I, Cardona LR, Kong H, Vasan K, McElroy GS, Werner M, Kihshen H, Reczek CR, Weinberg SE, Gao P, et al. (2020). Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature 585, 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Reyes I, and Chandel NS (2020). Mitochondrial TCA cycle metabolites control physiology and disease. Nature communications 11, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanovich M, Oberkovitz G, Niv H, Vorobiyov L, Zaltsman Y, Brenner O, Lapidot T, Jung S, and Gross A (2012). The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nature cell biology 14, 535–541. [DOI] [PubMed] [Google Scholar]
- Maryanovich M, Zaltsman Y, Ruggiero A, Goldman A, Shachnai L, Zaidman SL, Porat Z, Golan K, Lapidot T, and Gross A (2015). An MTCH2 pathway repressing mitochondria metabolism regulates haematopoietic stem cell fate. Nature communications 6, 7901. [DOI] [PubMed] [Google Scholar]
- Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, and Simon MC (2010). O2 regulates stem cells through Wnt/β-catenin signalling. Nature cell biology 12, 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Christofk H, Jones DL, and Lowry WE (2018). Topical Inhibition of the Electron Transport Chain Can Stimulate the Hair Cycle. The Journal of investigative dermatology 138, 968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. (2007). Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell stem cell 1, 101–112. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyamoto T, Kato R, Yoshimura A, Motoyama N, and Suda T (2008). FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging. Blood 112, 4485–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohyeldin A, Garzón-Muvdi T, and Quiñones-Hinojosa A (2010). Oxygen in stem cell biology: a critical component of the stem cell niche. Cell stem cell 7, 150–161. [DOI] [PubMed] [Google Scholar]
- Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, and Shinohara T (2013). ROS are required for mouse spermatogonial stem cell self-renewal. Cell stem cell 12, 774–786. [DOI] [PubMed] [Google Scholar]
- Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M, et al. (2015). Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell metabolism 21, 392–402. [DOI] [PubMed] [Google Scholar]
- Murphy MP (2009). How mitochondria produce reactive oxygen species. The Biochemical journal 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Schafer X, Fu D, Nehrke K, Munger J, and Brookes PS (2016). Acidic pH Is a Metabolic Switch for 2-Hydroxyglutarate Generation and Signaling. The Journal of biological chemistry 291, 20188–20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro Negredo P, Yeo RW, and Brunet A (2020). Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell stem cell 27, 202–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, Sigvardsson M, and Bryder D (2011). Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell stem cell 8, 499–510. [DOI] [PubMed] [Google Scholar]
- Oginuma M, Harima Y, Tarazona OA, Diaz-Cuadros M, Michaut A, Ishitani T, Xiong F, and Pourquié O (2020). Intracellular pH controls WNT downstream of glycolysis in amniote embryos. Nature 584, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham WM, Clish CB, Yang Y, and Loscalzo J (2015). Hypoxia-Mediated Increases in L-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress. Cell metabolism 22, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J, et al. (2009). FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell stem cell 5, 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, et al. (2014). Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell stem cell 15, 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto J, León M, Ponsoda X, Sendra R, Bort R, Ferrer-Lorente R, Raya A, López-García C, and Torres J (2016). Early ERK1/2 activation promotes DRP1-dependent mitochondrial fission necessary for cell reprogramming. Nature communications 7, 11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, Mammucari C, Gherardi G, and Rizzuto R (2016). Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends in biochemical sciences 41, 1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, et al. (2009). FoxO3 regulates neural stem cell homeostasis. Cell stem cell 5, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelé P, Liang R, Bigarella CL, Kocabas F, Xie J, Serasinghe MN, Chipuk J, Sadek H, Zhang CC, and Ghaffari S (2015). Mitochondrial metabolism in hematopoietic stem cells requires functional FOXO3. EMBO reports 16, 1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, et al. (2017). Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543, 424–427. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Dell’Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, et al. (2015). The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell stem cell 16, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, Willenbring H, and Verdin E (2010). miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging 2, 415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell JC, Wisidagama DR, Bensard C, Zhao H, Wei P, Tanner J, Flores A, Mohlman J, Sorensen LK, Earl CS, et al. (2017). Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nature cell biology 19, 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartzman JM, Thompson CB, and Finley LWS (2018). Metabolic regulation of chromatin modifications and gene expression. The Journal of cell biology 217, 2247–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki N, Shiraki Y, Tsuyama T, Obata F, Miura M, Nagae G, Aburatani H, Kume K, Endo F, and Kume S (2014). Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell metabolism 19, 780–794. [DOI] [PubMed] [Google Scholar]
- Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S, Zhang J, Onder T, Unternaehrer JJ, Zhu H, et al. (2013). Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science (New York, N.Y.) 339, 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Chen W, Guo X, Chen L, Wang G, Xu Y, and Kang J (2013). Activation of GSK3β by Sirt2 is required for early lineage commitment of mouse embryonic stem cell. PloS one 8, e76699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, and Sadek HA (2010). The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell stem cell 7, 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram L, Levy S, Hussein AM, Ehnes DD, Mathieu J, and Ruohola-Baker H (2020). Epigenetic metabolites license stem cell states. Current topics in developmental biology 138, 209–240. [DOI] [PubMed] [Google Scholar]
- Son MY, Choi H, Han YM, and Cho YS (2013). Unveiling the critical role of REX1 in the regulation of human stem cell pluripotency. Stem cells (Dayton, Ohio) 31, 2374–2387. [DOI] [PubMed] [Google Scholar]
- Sperber H, Mathieu J, Wang Y, Ferreccio A, Hesson J, Xu Z, Fischer KA, Devi A, Detraux D, Gu H, et al. (2015). The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nature cell biology 17, 1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, and Imai S (2014). Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. The EMBO journal 33, 1321–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell BR, and Jiang X (2020). The Chemistry and Biology of Ferroptosis. Cell chemical biology 27, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes DB (2018). The emergence of dihydroorotate dehydrogenase (DHODH) as a therapeutic target in acute myeloid leukemia. Expert opinion on therapeutic targets 22, 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, et al. (2010). Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell stem cell 7, 391–402. [DOI] [PubMed] [Google Scholar]
- Takubo K, Nagamatsu G, Kobayashi CI, Nakamura-Ishizu A, Kobayashi H, Ikeda E, Goda N, Rahimi Y, Johnson RS, Soga T, et al. (2013). Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell stem cell 12, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DQ, and Suda T (2018). Reactive Oxygen Species and Mitochondrial Homeostasis as Regulators of Stem Cell Fate and Function. Antioxidants & redox signaling 29, 149–168. [DOI] [PubMed] [Google Scholar]
- Tan JX, and Finkel T (2020). Mitochondria as intracellular signaling platforms in health and disease. The Journal of cell biology 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Ishikawa E, Gonzalez-Nieto D, Ghiaur G, Dunn SK, Ficker AM, Murali B, Madhu M, Gutstein DE, Fishman GI, Barrio LC, et al. (2012). Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proceedings of the National Academy of Sciences of the United States of America 109, 9071–9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng X, Emmett MJ, Lazar MA, Goldberg E, and Rabinowitz JD (2016). Lactate Dehydrogenase C Produces S-2-Hydroxyglutarate in Mouse Testis. ACS chemical biology 11, 2420–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeSlaa T, Chaikovsky AC, Lipchina I, Escobar SL, Hochedlinger K, Huang J, Graeber TG, Braas D, and Teitell MA (2016). α-Ketoglutarate Accelerates the Initial Differentiation of Primed Human Pluripotent Stem Cells. Cell metabolism 24, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, and Chandel NS (2011). Mitochondrial complex III ROS regulate adipocyte differentiation. Cell metabolism 14, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. (2007). FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339. [DOI] [PubMed] [Google Scholar]
- Trefely S, Lovell CD, Snyder NW, and Wellen KE (2020). Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Molecular metabolism 38, 100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini N, Girotra M, Naveiras O, Nikitin G, Campos V, Giger S, Roch A, Auwerx J, and Lutolf MP (2016). Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nature communications 7, 13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhana SA, Arnold PK, Rosen BP, Chen Y, Carey BW, Huangfu D, Carmona Fontaine C, Thompson CB, and Finley LWS (2019). Glutamine independence is a selectable feature of pluripotent stem cells. Nature metabolism 1, 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley C.A.t., Ramalho-Santos J, Van Houten B, and Schatten G (2011). Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PloS one 6, e20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic M, Sepulveda C, Subramani C, Guitart AV, Mohr J, Allen L, Panagopoulou TI, Paris J, Lawson H, Villacreces A, et al. (2016). Adult hematopoietic stem cells lacking Hif-1α self-renew normally. Blood 127, 2841–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Alexander P, Wu L, Hammer R, Cleaver O, and McKnight SL (2009). Dependence of mouse embryonic stem cells on threonine catabolism. Science (New York, N.Y.) 325, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang T, Wang L, Cai Y, Zhong X, He X, Hu L, Tian S, Wu M, Hui L, et al. (2017). Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission. The EMBO journal 36, 1330–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger L, Ayyash M, Novershtern N, and Hanna JH (2016). Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nature reviews. Molecular cell biology 17, 155–169. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, and Thompson CB (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science (New York, N.Y.) 324, 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, and Shadel GS (2017). Mitochondrial DNA in innate immune responses and inflammatory pathology. Nature reviews. Immunology 17, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EO, Taylor AK, Bell EL, Lim R, Kim DM, and Guarente L (2016). Sirtuin 1 Promotes Deacetylation of Oct4 and Maintenance of Naive Pluripotency. Cell reports 17, 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC (2018). Biological Production, Detection, and Fate of Hydrogen Peroxide. Antioxidants & redox signaling 29, 541–551. [DOI] [PubMed] [Google Scholar]
- Wong HS, Dighe PA, Mezera V, Monternier PA, and Brand MD (2017). Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. The Journal of biological chemistry 292, 16804–16809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, et al. (2011). Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer cell 19, 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Liu X, Shen J, Jovanovic O, Pohl EE, Gerson SL, Finkel T, Broxmeyer HE, and Qu CK (2013). Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell stem cell 12, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu J, and He C (2015). RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes & development 29, 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al. (2019). Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al. (2016). NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (New York, N.Y.) 352, 1436–1443. [DOI] [PubMed] [Google Scholar]
- Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K, Wang G, Do A, et al. (2011). UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. The EMBO journal 30, 4860–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZN, Chung SK, Xu Z, and Xu Y (2014). Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through Sirt1-mediated deacetylation. Stem cells (Dayton, Ohio) 32, 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, and Conrad M (2020). The Metabolic Underpinnings of Ferroptosis. Cell metabolism 32, 920–937. [DOI] [PubMed] [Google Scholar]
- Zhong X, Cui P, Cai Y, Wang L, He X, Long P, Lu K, Yan R, Zhang Y, Pan X, et al. (2019). Mitochondrial Dynamics Is Critical for the Full Pluripotency and Embryonic Developmental Potential of Pluripotent Stem Cells. Cell metabolism 29, 979–992.e974. [DOI] [PubMed] [Google Scholar]
- Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C, et al. (2012). HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. The EMBO journal 31, 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhao T, Du J, Ji G, Li X, Ji S, Tian W, Wang X, and Hao A (2019). TIGAR promotes neural stem cell differentiation through acetyl-CoA-mediated histone acetylation. Cell death & disease 10, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]


