To the Editor: BNT162b2 is a nucleoside-modified RNA vaccine expressing the full-length prefusion spike glycoprotein (S) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In a randomized, placebo-controlled clinical trial involving approximately 44,000 participants, immunization conferred 95% efficacy against coronavirus disease 2019 (Covid-19).1
New, highly transmissible SARS-CoV-2 variants that were first detected in the United Kingdom (B.1.1.7 lineage), South Africa (B.1.351 lineage), and Brazil (P.1 lineage) with mutations in the S gene are spreading globally. To analyze effects on neutralization elicited by BNT162b2, we engineered S mutations from each of the three new lineages into USA-WA1/2020, a relatively early isolate of the virus from January 2020 (Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). We thereby produced three recombinant viruses representing each of these lineages and two additional ones in which we engineered subsets of mutations of the B.1.351 lineage. Thus, the first recombinant virus had all the mutations found in the S gene in the B.1.1.7 lineage (B.1.1.7-spike), the second had all the mutations found in the S gene in the P.1 lineage (P.1-spike), the third had all the mutations found in the S gene in the B.1.351 lineage (B.1.351-spike), the fourth had an N-terminal domain deletion found in the B.1.351 lineage and the globally dominant D614G substitution (B.1.351-∆242-244+D614G), and the fifth had the three mutations from the B.1.351 lineage affecting amino acids in the receptor-binding site (K417N, E484K, and N501Y) and a D614G substitution (B.1.351-RBD+D614G). The mutant amino acid residues in the B.1.351-RBD+D614G recombinant virus are also among those in the P.1 lineage virus, although in the P.1 lineage virus, K417 is mutated to threonine rather than asparagine. All the mutant viruses yielded infectious viral titers exceeding 107 plaque-forming units per milliliter. The B.1.1.7-spike and B.1.351-spike viruses formed plaques that were smaller than those formed by the other viruses (Fig. S2).
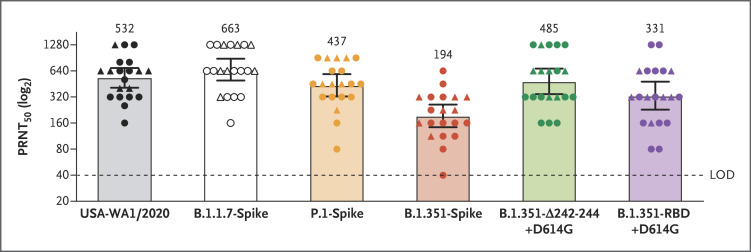
We performed 50% plaque reduction neutralization testing (PRNT50) using 20 serum samples that had been obtained from 15 participants in the pivotal trial1,2 2 or 4 weeks after the administration of the second dose of 30 μg of BNT162b2 (which occurred 3 weeks after the first immunization) (Fig. S3). All the serum samples efficiently neutralized USA-WA1/2020 and all the viruses with variant spikes. Almost all of them did so at titers higher than 1:40. Geometric mean neutralizing titers against USA-WA1/2020, B.1.1.7-spike, P.1-spike, B.1.351-spike, B.1.351-∆242-244+D614G, and B.1.351-RBD+D614G viruses were 532, 663, 437, 194, 485, and 331, respectively (Figure 1 and Table S1). Thus, as compared with neutralization of USA-WA1/2020, neutralization of B.1.1.7-spike and P.1-spike viruses was roughly equivalent, and neutralization of B.1.351-spike virus was robust but lower. Our data are also consistent with lower neutralization titers against the virus with the full set of B.1.351-spike mutations than against virus with either subset of mutations. Our findings also suggest that mutations that result in amino acid substitutions K417N, E484K, and N501Y in the receptor-binding site have a greater effect on neutralization than the 242–244 deletion affecting the N-terminal domain of the spike protein.
Figure 1. Serum Neutralization of Variant Strains of SARS-CoV-2 after the Second Dose of BNT162b2 Vaccine.
Shown are the results of 50% plaque reduction neutralization testing (PRNT50) with the use of 20 samples obtained from 15 trial participants 2 weeks (circles) or 4 weeks (triangles) after the administration of the second dose of the BNT162b2 vaccine. The mutant viruses were obtained by engineering the full set of mutations in the B.1.1.7, P.1., or B.1.351 lineage or subsets of the S gene mutations in the B.1.351 lineage (B.1.351-Δ242-244+D614G and B.1.351-RBD+D614G) into USA-WA1/2020. Each data point represents the geometric mean PRNT50 obtained with a serum sample against the indicated virus, including data from repeat experiments, as detailed in Table S1 in the Supplementary Appendix. The data for USA-WA1/2020 are from three experiments; for B.1.1.7-spike, B.1.351-Δ242-244+D614G, and B.1.351-RBD-D614G viruses from one experiment each; and for P.1-spike and B.1.351-spike viruses from two experiments each. In each experiment, the neutralization titer was determined in duplicate assays, and the geometric mean was taken. The heights of bars and the numbers over the bars indicate geometric mean titers. The 𝙸 bars indicate 95% confidence intervals. Statistical analysis was performed with the use of the Wilcoxon signed-rank test. The statistical significance of the difference between geometric mean titers in the USA-WA1/2020 neutralization assay and in each variant virus neutralization assay with the same serum samples are as follows: P=0.02 for B.1.1.7-spike; P=0.06 for P.1-spike; P<0.001 for B.1.351-spike; P=0.99 for B.1.351-Δ242-244+D614G; and P=0.005 for B.1.351-RBD+D614G. LOD denotes limit of detection.
Limitations of the study include the potential for mutations to alter neutralization by affecting spike function rather than antigenicity. Therefore, each neutralization assay with a different target virus is unique, and comparisons between neutralization titers from different assays should be interpreted with caution. Neutralizing activity against the B.1.351 lineage virus was robust at a geometric mean titer that was much higher than that obtained after one dose of BNT162b2, when strong efficacy was already observed in the C4591001 efficacy trial.1-3 T-cell immunity may also be involved in protection,4 and BNT162b2 immunization elicits CD8+ T-cell responses that recognize multiple variants.5 Ultimately, conclusions about vaccine-mediated protection that are extrapolated from neutralization or T-cell data must be validated by real-world evidence collected in regions where the SARS-CoV-2 variants are circulating.
Supplementary Appendix
Disclosure Forms
Preliminary Version
A preliminary version of this letter was published on February 17, 2021, and was updated on March 8, 2021, at NEJM.org.
Footnotes
Supported by Pfizer and BioNTech.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020;383:2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahin U, Muik A, Vogler I, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. December 11, 2020. (https://www.medrxiv.org/content/10.1101/2020.12.09.20245175v1). preprint.
- 4.Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020;26:842-844. [DOI] [PubMed] [Google Scholar]
- 5.Skelly DT, Harding AC, Gilbert-Jaramillo J, et al. Vaccine-induced immunity provides more robust heterotypic immunity than natural infection to emerging SARS-CoV-2 variants of concern. February 9, 2021. (https://www.researchsquare.com/article/rs-226857/v1). preprint. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.