To the Editor: Baden et al.1 report on a phase 3 clinical trial of the mRNA-1273 vaccine against SARS-CoV-2, and they provide information on immediate injection-site reactions, which were observed in 84.2% of the participants after the first dose. The trial also showed that delayed injection-site reactions (defined in that trial as those with an onset on or after day 8) occurred in 244 of the 30,420 participants (0.8%) after the first dose and in 68 participants (0.2%) after the second dose. These reactions included erythema, induration, and tenderness. The reactions typically resolved over the following 4 to 5 days. However, these reactions were not further characterized, and links between reactions after the first dose and those after the second dose were not provided to inform clinical care.
We have also observed delayed large local reactions to the mRNA-1273 vaccine, with a median onset on day 8 (range, 4 to 11) after the first dose. These reactions had a variable appearance (Figure 1). Here, we report on a series of 12 patients with these reactions, all of which appeared near the injection site after complete resolution of the initial local and systemic symptoms associated with vaccination. Five of the reactions were grade 3 plaques (≥10 cm in diameter) (Table 1). Some patients had concurrent systemic adverse effects, and among these patients, 2 had additional skin findings. Most patients received treatment for their symptoms (e.g., with ice and antihistamines). Some patients received glucocorticoids (topical, oral, or both), and 1 patient received antibiotic therapy for presumptive cellulitis. The symptoms resolved a median of 6 days after onset (range, 2 to 11).
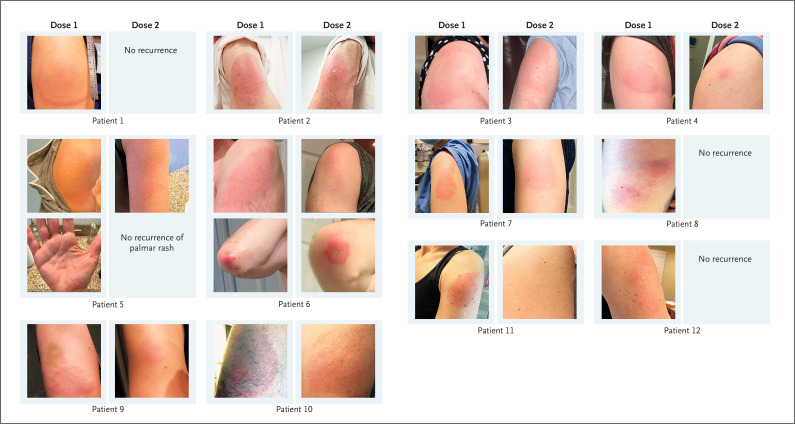
Figure 1. Delayed Cutaneous Reactions to mRNA-1273 Vaccine.
Shown are morphologic characteristics of delayed cutaneous reactions to mRNA-1273 vaccine, including annular plaques (in Patient 1), uniformly edematous plaques (in Patients 2, 6, and 11), and targetoid plaques (in Patient 3) near the site of vaccination. In several patients, there was considerable induration of the plaques (e.g., in Patients 8 and 9). In addition to a localized rash on the arm, two patients had other cutaneous symptoms, including papules on the palm and fingers (Patient 5) and urticarial plaques on the elbows (Patient 6). Patients 1, 5, 8, 9, 11, and 12 did not have a recurrence of large local reactions with the second dose, although some patients had minimal erythema. In Patients 2, 6, and 7, the reactions had an earlier onset and were lower grade after the second dose than after the first dose. In Patients 3, 4, and 10, the onset of the reactions after the second dose was earlier than after the first dose, but the reactions to the two doses were of a similar grade. Some photographs were taken by the patients using a mirror, so the images of the left and right arms may be transposed.
Table 1. Patients with Remarkable, Delayed, Large Local Reactions to the mRNA-1273 Vaccine.*.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic and clinical variables | ||||||||||||
| Age — yr | 37 | 61 | 45 | 31 | 40 | 43 | 38 | 49 | 31 | 47 | 52 | 46 |
| Sex | Female | Female | Female | Female | Female | Male | Female | Female | Female | Male | Female | Female |
| Race or ethnic group | Asian, non-Hispanic | White, non-Hispanic | White, non-Hispanic | White, non-Hispanic | White, non-Hispanic | White, non-Hispanic | White, non-Hispanic | White, non-Hispanic | White, non-Hispanic | White, Black, Native American, Hispanic | White, non-Hispanic | White, non-Hispanic |
| Allergy history | None | Contrast allergy (hives) |
Rhinitis, penicillin allergy (hives), large local reaction to influenza vaccine | Urticaria, rhinitis | None | None | Wasp allergy (hives) | Idiopathic urticaria (none in 5 yr) | None | Almond allergy (hives), rhinitis | Isolated episode of facial angioedema approximately 40 yr previously | Penicillin allergy (rash), sulfasalazine (drug fever) |
| Dose 1 | ||||||||||||
| Day of reaction onset | 8 | 8 | 8 | 8 | 4 | 9 | 9 | 8 | 10 | 11 | 8 | 9 |
| Local symptoms near injection site | Pruritus | Pain, warmth | Pruritus, pain | Pruritus | Pruritus, pain | Pruritus, pain, warmth | Pain | Pruritus, burning, pain, warmth, erythema, induration, hyperpigmentation | Pruritus, warmth | Pain | Swelling, pain | Pruritus |
| Maximum lesion diameter — cm | 9.0 | 10.0 | 14.0 | 5.0 | 13.0 | 12.5 | 7.0 | Two separate lesions, each 3.0–4.0 cm | 7.5 | 7.0 | 19.5 | 7.0 |
| Symptoms concurrent with delayed large local reaction | None | None | Fatigue, myalgias, headache, chills | Lymphadenopathy (days 6–8) | Headache, fatigue, fever (maximum temperature, 100.1°F), palmar rash | Rash near elbow (day 11) | None | None | Fatigue | Fatigue, myalgias |
Postural tachycardia, hypertension (heart rate, 130 bpm; blood pressure, 140–156 mm Hg systolic, 90–112 mm Hg diastolic) | Headache |
| Treatment for reaction | Cetirizine 10 mg once daily, hydrocortisone 1% topical (days 9–12) | Cetirizine 10 mg, famotidine 20 mg, diphenhydramine 25–50 mg, clobetasol propionate 0.05% topical (all as needed) | Diphenhydramine 25–50 mg (as needed) | Fexofenadine at high doses (180–360 mg twice daily) | Cetirizine 10 mg, diphenhydramine 25–50 mg (as needed), triamcinolone 0.1% topical, prednisone (started on day 6 at 20 mg with 5-day taper) | Cetirizine 10 mg, diphenhydramine 25–50 mg, famotidine 20 mg (as needed), prednisone (started on day 11 at 40 mg daily with 12 day taper) | Loratadine 10 mg (as needed) | Ice packs, one dose of diphenhydramine 50 mg | Hydrocortisone 1% topical (as needed) | None | Amoxicillin (875 mg)–clavulanic acid (125 mg) twice daily (started on day 9 for 7 days) | None |
| Day of resolution | 14 | 14 | 14 | 15 | 14 | 16 | 13 | 19 | 12 | 17 | 14 | 11 |
| Resolution status before dose 2 | Complete resolution | Hyperpigmentation, change in sensation (“tingling,” “dullness”) | Hyperpigmentation, burning sensation | Pain, itching continued through dose 2 | Complete resolution | Mild symptoms in elbow area but otherwise resolved | Complete resolution | Hyperpigmentation | Complete resolution | Complete resolution | Complete resolution | Complete resolution |
| Dose 2 | ||||||||||||
| Location | Opposite arm | Opposite arm | Opposite arm | Opposite arm | Same arm | Opposite arm | Opposite arm | Opposite arm | Same arm | Opposite arm | Opposite arm | Same arm |
| Premedication | Cetirizine 10 mg (one dose) | Cetirizine 10 mg (one dose) | Diphenhydramine 25 mg (one dose) | Fexofenadine 180 mg twice daily | Cetirizine 10 mg twice daily starting 4 days before vaccination–day 3 after vaccination | Diphenhydramine 25 mg (one dose), 4 hr before vaccination | Loratadine 10 mg (one dose) | Fexofenadine 180 mg (one dose) the day of and day after vaccination | None | None | None | None |
| Initial systemic symptoms | Myalgias, chills, fatigue |
Fever, chills, headache | Fever, chills, fatigue, headache | Fever, chills | Headache, fever, chills, myalgias, lymphadenitis | Fever, headache lymphadenitis |
Headache, myalgias | Chills, myalgias |
Chills, myalgias |
Fatigue, fever, chills |
Fever, chills, nausea, myalgias, lymphadenopathy | Myalgias, headache, fever |
| Skin reaction after initial symptoms | None | Rash (5 cm in diameter) on day 3; increased to 8 cm in diameter and dark red by day 5 | Rash on day 2; increased to >13 cm in diameter | Rash on day 2; increased to 5 cm in diameter (same size as with dose 1, but much fainter) | Slight erythema at injection site on days 0–1 | Minor erythema at injection site on day 1, with flare of rash that occurred near elbow with dose 1 | Rash and itching at injection site on day 3 (lasted 24 hr); lingering itching through day 5 | Slight erythema on day 2–3; idiopathic urticaria recurred on day 12 | Small area of erythema on day 2–3 | Rash (similar to that after dose 1) on days 3–4; increased to approximately 7 cm in diameter | Slight erythema on days 2–3 | None |
| Additional treatment after reaction | NA | Clobetasol propionate 0.05% topical (as needed) | Diphenhydramine 25 mg, hydrocortisone 1% topical (both as needed) | Cetirizine 10 mg, diclofenac 1% topical gel, triamcinolone 0.1% topical (all as needed) | NA | Diphenhydramine 25 mg (one dose), famotidine 20 mg (one dose) | Loratadine 10 mg (as needed) | NA | NA | NA | NA | NA |
| Large local reaction (dose 2 vs. dose 1) | ||||||||||||
| Onset | None | Earlier | Earlier | Earlier | None | Earlier | Earlier | None | None | Earlier | None | None |
| Grade | None | Lower | Similar | Similar | Erythema only | Lower | Lower | Erythema only | Erythema only | Similar | Erythema only | None |
None of the patients had known previous SARS-CoV-2 infection. Clinical data were reported by the patients. NA denotes not applicable (i.e., the patient had no reaction or had mild symptoms that did not warrant treatment).
Our suspicion of delayed-type or T-cell–mediated hypersensitivity was supported by skin-biopsy specimens obtained from a patient with a delayed large local reaction who was not among the 12 patients described here. Those specimens showed superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells (see Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org).
Given that neither local injection-site reactions nor delayed-type hypersensitivity reactions are contraindications to subsequent vaccination,2 all 12 patients were encouraged to receive the second dose and completed their mRNA-1273 vaccination course. Although half the patients did not have a recurrence of large local reactions, three patients had recurrent reactions that were similar to those after the initial dose, and three patients had recurrent reactions that were of a lower grade than those after the initial dose. The median onset of cutaneous symptoms after the second dose (day 2; range, 1 to 3) was earlier than that after the first dose (Table 1).
Clinicians may not be prepared to address delayed local reactions to the mRNA-1273 vaccine. Given the scale-up of mass vaccination campaigns across the world, these reactions are likely to generate concerns among patients and requests for evaluation. These reactions have not been consistently recognized, guidance regarding the second dose of vaccine has varied, and many patients have unnecessarily received antibiotic agents. We hope this letter encourages additional reporting and communication regarding the epidemiologic characteristics, causes, and implications of these delayed cutaneous reactions, since this information might allay the concerns of patients, encourage completion of vaccination, and minimize the unnecessary use of antibiotic agents.
Supplementary Appendix
Disclosure Forms
The content of this letter is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or Massachusetts General Hospital.
This letter was published on March 3, 2021, at NEJM.org.
Footnotes
Supported by a grant (K01AI125631, to Dr. Blumenthal) from the NIH and a grant (to Dr. Blumenthal) from the Department of Medicine Transformative Scholar Program at Massachusetts General Hospital.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol 2012;130:25-43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.