Abstract
The changes experienced by the arterial system due to the aging process have been extensively studied but are incompletely understood. Within-subject patterns of changes in regards to input impedance and wave reflection parameters have not been assessed. The Asklepios study is a longitudinal population study including healthy (at onset) middle-aged subjects, with 974 males and 1052 females undergoing two rounds of measurements of applanation tonometry and ultrasound, 10.15 ± 1.40 years apart. Carotid-femoral pulse wave velocity (PWV), aortic input impedance, and wave reflection parameters were assessed, and linear mixed-effects models were used to evaluate their longitudinal trajectories and determinants. Overall, the effective 10-year increase in PWV was less than expected from first round cross-sectional data, and PWV was found to accelerate more in women than in men. Interestingly, the increase in PWV was not paralleled by a decrease in arterial volume compliance, particularly in younger males. Aortic root characteristic impedance decreased with age in younger subjects while it increased for the older subjects in the study. These changes suggest that aortic dilation and elongation may play an important role determining the longitudinal age-related changes in impedance parameters in middle-age. Wave reflection decreased with aging, whereas resistance increased in women and decreased in men. We conclude that the effective impact of aging on arterial system properties, in a middle-aged population, is not well reflected by cross-sectional studies. Future studies should assess the interaction between geometric remodeling and wall stiffening as determinants of pulsatile hemodynamics.
Keywords: impedance, arterial stiffness, wave reflection, aging, longitudinal study, linear mixed-effects model
Graphical Abstract
Summary
Our results evidence that the effective impact of aging on arterial system properties is better reflected by longitudinal studies over cross-sectional approaches. We support the notion that more efforts are still needed to develop therapies aiming to influence directly arterial stiffness, whereas traditional risk factors together with socio-economic, genetic and environmental factors, deserve greater consideration in order to reduce the risk of cardiovascular events and achieve healthy vascular aging.
1. Introduction
The changes exhibited by the arterial system due to the aging process have been extensively studied [1-5], but are incompletely understood. With advancing age, the aorta and major arteries of the arterial tree may endure a loss/degradation of elastin resulting in wall stiffening on one hand, and geometric remodeling on the other. Aging has been reported to have a profound impact on aortic stiffness, dilatation and elongation, arterial input impedance and pulsatile hemodynamics. Adverse pulsatile hemodynamic changes associated with aging and various disease states play a central role in the pathogenesis of various cardiovascular diseases (CVD) [6-9].
Most of what is known about the impact of age on arterial hemodynamics and arterial system properties is based on cross-sectional studies performed over the past few decades [1, 10-13]. These studies have shown that arterial stiffness increases with age [1, 14-16], and that stiffening is accelerated by cardiovascular risk factors [10, 17, 18] (diabetes and hypertension being the most important). Arterial stiffness is considered to quantify the cumulative impact of exposure of the cardiovascular system to risk factors, and is a strong predictor of cardiovascular mortality and morbidity [17, 19].
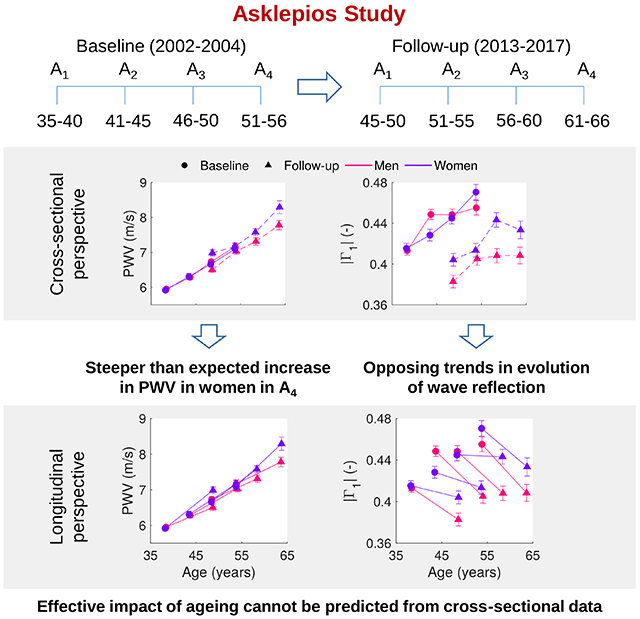
Although valuable, cross-sectional analyses do not account for cohort effects, which could arise in people born at different times, and could exert an impact on arterial phenotypes independent of age. Cohort effects may occur due to early and mid-life exposures, survival bias, and epidemiological transitions affecting life trajectories of morbidity through lifestyle, environmental and epigenetic changes [20]. If arterial stiffness is indeed an integrative marker of cardiovascular risk, the effective evolution of arterial stiffness within an individual or within the population may be quite different from what one would expect on the basis of historical cross-sectional data. Such data can only be obtained in longitudinal studies, where repeated measurements are acquired over time from the same subjects. Reference longitudinal studies such as the Framingham Heart Study (FHS) [19, 21], the Baltimore Longitudinal Study of Aging (BLSA) [22, 23], or the SardiNIA study [24], have previously reported on the impact of aging on arterial stiffness (mainly PWV) and its association with CV risk factors.
We previously reported and documented cross-sectional data on arterial input impedance, PWV and wave reflection in 2026 seemingly healthy middle-aged individuals (age 35 to 55), free from overt cardiovascular disease at study initiation (Asklepios study [25]). For both sexes, we found an increase with age in PWV, systemic vascular resistance, and parameters of wave reflection. Marked differences in aortic input impedance were found between men and women, while total aortic compliance [26] was found as the main determinant of carotid pulse pressure. The age-related increase in carotid-femoral PWV was not accompanied by an increase in arterial impedance, suggesting age-dependent mechanisms modulating the aortic cross-sectional area. After a period of about 10 years, follow-up measurements were repeated on the same individuals. The major aims of this study are: (1) to examine the 10-year longitudinal evolution of aortic input impedance, carotid-femoral PWV and wave reflection indices within the same cohort; (2) to assess how these effective changes compare to what was anticipated from the cross-sectional perspective.
2. Methods
The data that support the findings of this study are available from E.R.R. (ernst.rietzschel@ugent.be) upon reasonable request.
Study population
This is a substudy of the Asklepios cohort study, which has been previously described in detail [27]. Non-invasive measurements of carotid blood pressure (applanation tonometry) and aortic flow (ultrasound) were performed on 2524 middle-aged subjects aged 35-55 years at baseline. Measurements were repeated on the same individuals after a follow-up time of 10.15 ± 1.40 years (91.0% returning volunteers). Of the 2026 subjects that formed the baseline cohort for our study on arterial hemodynamics and impedance [25], 33 died before follow-up, 94 were lost to follow-up, moved or withdrew from follow-up, and 83 declined re-exam because of loss of interest, illness or logistical difficulties; an additional 59 presented incomplete data sets (n=54 inability and/or technical failure to accurately assess carotid tonometry, and n=5 missing flow data). Supplemental Figure S1 shows a detailed flowchart of participants with available data at baseline and follow-up. Basic analyses were limited to 1757 subjects (920 women) with analyzable datasets on both occasions (complete case analysis). The study protocol was approved by the ethical committee of the Ghent University Hospital, and all subjects gave written informed consent.
Statistical methods
Analyses of variance and covariance were performed for all the variables of interest, accounting for the repeated measures by subjects, with categorical variables age, sex, visit, and their interaction terms included in the models (and covariates when applicable – see online supplement and results). In addition, for a better comprehension of the longitudinal relationships among variables, linear mixed-effects (LME) analyses were performed [28-30] and models describing the effects on PWV, blood pressure (BP) variables, impedance and wave reflection parameters were constructed. We refer to the online Data Supplement for details. In the results and figures, observed data (and analyses of (co)variance) as well as data predicted from the mixed-effects model analysis will be presented side by side when relevant.
An expanded version of the Methods section can be found in the online Data Supplement, under the headings Study population, Measured and derived variables, and Statistical methods.
3. Results
Basic clinical data and hemodynamic parameters of the study cohort at baseline and follow-up, stratified by sex and age group, are shown in Table 1. For further details, see the online Data Supplement.
Table 1.
Basic clinical data and hemodynamic parameters of the complete case cohort at baseline (V1) and follow-up (V2).
| Parameter | Men (N=837) | Women (N=920) | ||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A1 | A2 | A3 | A4 | |
| N | 226 | 230 | 212 | 169 | 269 | 256 | 214 | 181 |
| Follow-up time, y | 9.8 (9.1-11.1) | 10 (9.3-11.2) | 9.6 (8.9-10.5) | 9.6 (9-10.3) | 10.1 (9.-11.8) | 9.9 (9.2-11) | 9.7 (9-10.6) | 9.8 (9.1-10.6) |
| Age, y | ||||||||
| V1 | 38.4 (37-39.9) | 43.7 (42.4-45) | 48.4 (47.1-49.5) | 53.9 (52.3-55.3) | 38.4 (36.9-39.7) | 43.4 (42-44.8) | 48.3 (47.1-49.6) | 53.5 (52.2-55) |
| V2 | 48.7 (46.9-50.2)* | 54.1 (52.4-55.3)* | 58.2 (56.8-59.6)* | 63.7 (61.9-65.1)* | 48.8 (47-50.4)* | 53.6 (52-54.9)*† | 58.2 (56.9-59.6)* | 63.9 (62-65.2)* |
| Height, cm | ||||||||
| V1 | 177.6±6.63 | 175.9±6.10 | 175.4±6.73 | 174.5±6.23 | 164.8±5.89† | 164.3±6.15† | 162.8±5.65† | 161.7±5.47† |
| V2 | 177.0±6.55* | 175.4±5.98* | 174.7±6.55* | 173.5±6.28* | 164.6±5.81*† | 163.7±6.14*† | 161.9±5.55*† | 160.7±5.49*† |
| Weight, kg | ||||||||
| V1 | 79.5 (72.2-87) | 80 (71.8-87) | 81 (72.5-88.7) | 79.2 (74.1-84.3) | 63.2 (58-70)† | 62.9 (57.8-69.5)† | 63.4 (58.4-70.5)† | 63.3 (56.7-71.1)† |
| V2 | 82.9 (74.6-90.5)* | 82.5 (75-89.7)* | 82.1 (75.2-91.6)* | 80.7 (74.4-87.2)* | 68.3 (60.5-76.6)*† | 66.6 (60.3-73.7)*† | 66.1 (59.8-73.9)*† | 66 (59.6-73.3)*† |
| BMI, kg/m2 | ||||||||
| V1 | 25 (23.2-27.1) | 25.6 (23.6-27.7) | 26.2 (24.1-28.6) | 26 (24-27.8) | 23 (21-25.7)† | 23.3 (21.5-25.6)† | 23.9 (22.3-26.1)† | 24 (22.2-27.2)† |
| V2 | 26.2 (24.1-28.6)* | 26.5 (24.4-29)* | 26.9 (24.9-29.1)* | 26.6 (24.6-29.1)* | 24.5 (22.3-28.2)*† | 28.4 (22.3-27.2)*† | 24.7 (22.6-28.3)*† | 25.4 (22.9-28)*† |
| BSA, m2 | ||||||||
| V1 | 1.98±0.15 | 1.96±0.15 | 1.97±0.15 | 1.94±0.13 | 1.71±0.14† | 1.70±0.15† | 1.70±0.14† | 1.69±0.14† |
| V2 | 2.01±0.17* | 1.99±0.15* | 1.99±0.16* | 1.95±0.14 | 1.76±0.16*† | 1.74±0.15*† | 1.72±0.14*† | 1.70±0.14*† |
| SBP, mmHg | ||||||||
| V1 | 130 (123-137) | 132 (124-140) | 134 (124-143) | 134 (124.8-144.3) | 121 (114-132)† | 125 (116-135)† | 128 (120-138)† | 134 (123-146) |
| V2 | 132 (124-143.6)* | 136 (128-146)* | 137 (128-145.5)* | 135.8 (128-144) | 132 (119.8-146)* | 131.8 (120.7-145)*† | 137 (124-147.8)* | 139 (126-151.5)* |
| DBP, mmHg | ||||||||
| V1 | 74.4±9.39 | 77.4±10.7 | 79.2±9.82 | 79.1±12.1 | 73.5±10.9 | 75.3±10.7† | 76.0±10.1† | 77.9±10.9 |
| V2 | 81.8±10.6* | 83.7±10.2* | 81.7±10.6* | 79.4±9.44 | 81.2±10.2* | 80.0±9.96*† | 80.7±9.09* | 78.6±10.0* |
| MAP, mmHg | ||||||||
| V1 | 97.1±9.29 | 100.0±11.3 | 101.9±10.9 | 102.7±13.6 | 95.4±11.7 | 98.0±11.8 | 100.0±11.5† | 103.1±12.6 |
| V2 | 102.8±12.0* | 105.4±12.3* | 104.2±12.2* | 102.1±11.0 | 103.0±12.5* | 101.8±12.2*† | 104.2±11.8* | 103.8±12.3 |
| SBPCA, mmHg | ||||||||
| V1 | 126.4±11.6 | 128.9±13.9 | 131.0±13.9 | 134.1±17.9 | 122.7±14.8† | 126.9±16.4 | 130.9±16.4 | 137.0±19.7 |
| V2 | 131.9±16.1* | 136.1±18.0* | 135.9±16.5* | 135.7±16.8 | 133.0±18.6* | 132.8±18.8* | 137.9±18.9* | 140.3±20.5† |
| PPCA, mmHg | ||||||||
| V1 | 52.0±9.93 | 51.5±8.50 | 51.8±9.49 | 55.1±11.8 | 49.3±9.15† | 51.7±11.0 | 54.9±12.4† | 59.1±15.6† |
| V2 | 50.1±10.6* | 52.3±12.6 | 54.3±10.8* | 56.3±12.8 | 51.8±12.4* | 52.8±13.2 | 57.2±14.1*† | 61.7±17.2† |
| HR, bpm | ||||||||
| V1 | 60.8±8.39 | 60.2±8.68 | 62.5±9.49 | 61.1±9.33 | 65.1±9.24† | 65.9±8.93† | 64.7±8.46† | 65.1±7.94† |
| V2 | 61.8±9.51 | 60.9±8.66 | 62.1±9.28 | 60.6±9.52 | 65.4±8.55† | 64.5±8.86*† | 63.1±7.91* | 63.7±7.72*† |
| SV, mL | ||||||||
| V1 | 87.0±17.8 | 87.8±19.4 | 85.6±17.7 | 86.7±18.1 | 70.5±13.0† | 70.8±14.4† | 70.5±13.0† | 69.5±15.3† |
| V2 | 91.6±18.6* | 92.4±18.9* | 91.6±20.5* | 90.6±20.2 | 74.5±14.8*† | 73.2±15.7*† | 72.3±15.3† | 73.3±17.2*† |
| CO, L/min | ||||||||
| V1 | 5.23±1.04 | 5.23±1.17 | 5.30±1.13 | 5.26±1.16 | 4.55±0.91† | 4.65±1.07† | 4.53±0.90† | 4.49±0.98† |
| V2 | 5.59±1.13* | 5.58±1.14* | 5.66±1.44* | 5.44±1.31 | 4.83±0.98*† | 4.68±1.01† | 4.54±1.02† | 4.63±1.07† |
| LVOT area, cm2 | ||||||||
| V1 | 3.80±0.60 | 3.73±0.60 | 3.70±0.59 | 3.70±0.59 | 2.87±0.44† | 2.85±0.49† | 2.82±0.42† | 2.82±0.43† |
| V2 | 4.02±0.63* | 3.91±0.67* | 3.86±0.69* | 3.79±0.62* | 3.05±0.47*† | 2.99±0.49*† | 2.98±0.49*† | 2.99±0.48*† |
Data are expressed as mean ± SD or median (interquartile range). BMI: body mass index; BSA: body surface area; SBP and DBP: systolic and diastolic brachial pressure; MAP: mean arterial pressure; SBPCA and PPCA: carotid systolic and pulse pressure; HR: heart rate; SV: stroke volume; CO: cardiac output; LVOT: left ventricular outflow tract.
P<0.05 testing whether the difference between visits was statistically significant per sex and age category.
P<.05 testing whether the difference between sexes was statistically significant per visit and age category.
Pulse Wave Velocity and Blood Pressure indices
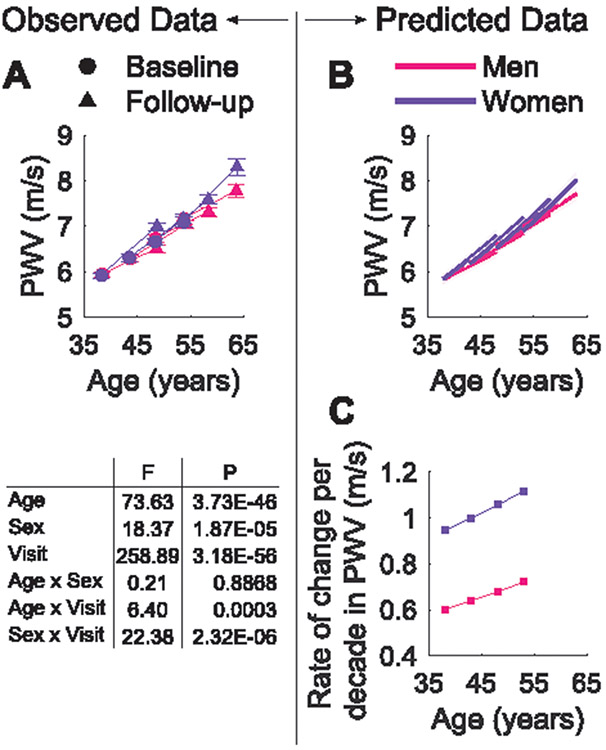
PWV, adjusted for mean arterial pressure (MAP), increased significantly between examination visits with differences by age group and sex (P<0.001; Figure 1A). Basic mixed-effects models for the effects on PWV were first constructed for all subjects, including entry-age, (entry-age)2, time, sex, and their interaction terms (see Table S1 in the Data Supplement). The average effect of entry-age on PWV was sex-independent, but longitudinally there were significant differences by sex that accelerate at older age, as shown for the significant interaction term (Sex×(Entry-Age)2×Time). Models were then constructed for men and women separately (Table S2). From these models, the predicted longitudinal trajectories with 95% confidence intervals and rates of change of PWV per decade were plotted by sex and age group at baseline (Figure 1B-C); the predicted rate of change in PWV for subjects between 35 and 55 years increased around 18%, with higher values for women in all age categories. In women, the 10 year change in PWV increases from 0.95 m/s/10yr in the youngest group to 1.12 m/s/10yr in the oldest group.
Figure 1.
Observed (A) and predicted (B) longitudinal trajectories in PWV per sex and age strata. Shaded area represents the non-simultaneous 95% confidence intervals. (C) Model-predicted rate of change per decade in PWV for men and women. The plots of the predictions refer to models in Supplemental Table S2. The P and F values in the table indicate the statistical significance of the factors age, sex, visit, and their interaction in the ANCOVA, where PWV was adjusted for MAP. Values of the observations are mean ± SEM.
Supplemental Table S3 shows the estimates for final fitted generalized linear mixed-effects (GLME) models of the effects on PWV after the inclusion of potential covariates (HR, weight, height and BP variables). There was a cross-sectional independent association of SBPCA, DBP or MAP with PWV, but longitudinal associations were not significant. However, PPCA was associated with the longitudinal increase in PWV over time in women. Additional models (Table S4) explore the effects of PWV on the longitudinal changes of BP indices. Higher PWV was associated with lower longitudinal trajectories in all BP variables except for PPCA, for both men and women.
Input and Characteristic Impedance, Systemic Vascular Resistance and Total Arterial Compliance
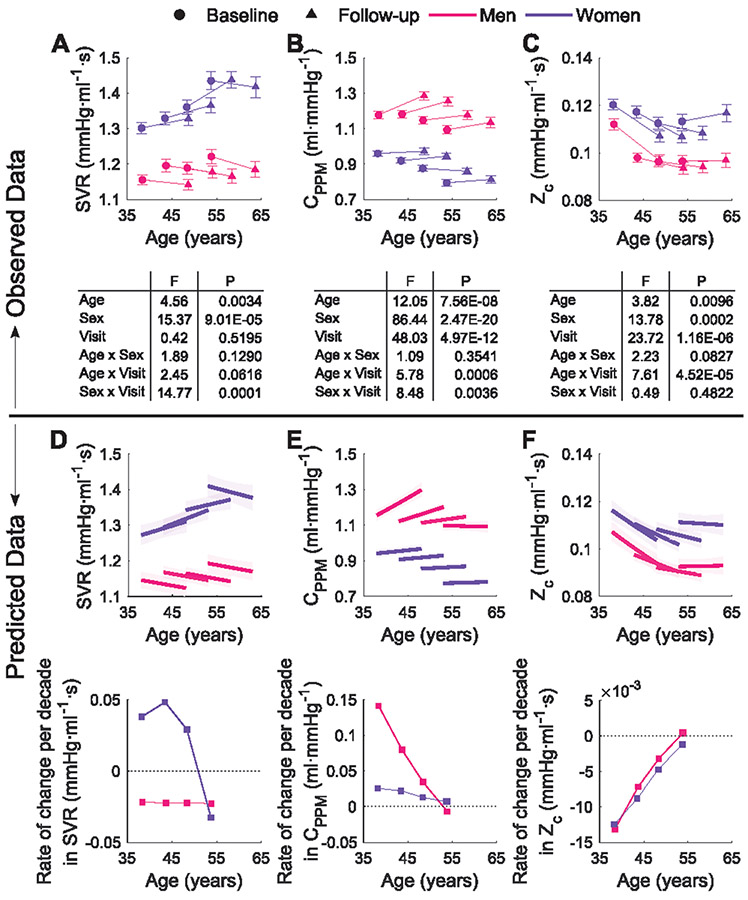
Figure 2 shows the modulus (logarithmic scale) and phase angle of input impedance (Zin), divided by age category and sex. The observed changes between examination visits, of parameters describing the impedance patterns (i.e. compliance CPPM, systemic vascular resistance, SVR and characteristic impedance, Zc) are shown in Figure 3A-C. In men, SVR decreased with time independent of the entry-age. Women showed a nonlinear pattern in the longitudinal changes in SVR over time, with an accelerated decrease at older age. The average rates of change in SVR over ten years increased for females in the younger groups and had a steeper decrease in older women. For males, it decreased with aging at a constant rate over the entire entry-age range (Figure 3D).
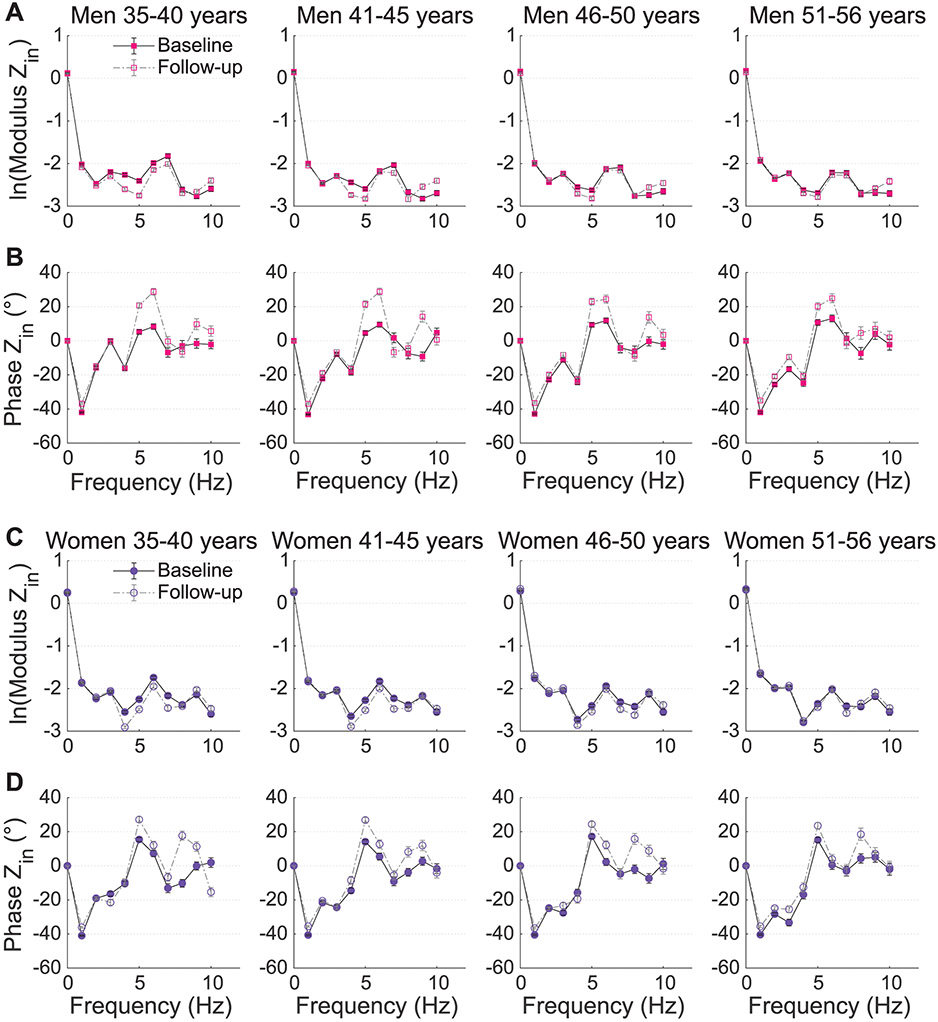
Figure 2.
Logarithmic transformed modulus (A and C) and phase angle (B and D) of Zin for males (top) and females (bottom) at both visits. Data are presented in stratum per age category.
Figure 3.
Top: Observed changes between baseline and follow-up measurements in SVR (A), CPPM (B), and Zc (C) per sex and age strata. Values are mean ± SEM. In the ANCOVA, the three parameters were adjusted for height and weight; CPPM and Zc additionally for MAP. Bottom: Model-predicted longitudinal trajectories and rates of change per decade in SVR (D), CPPM (E), and Zc (F) per sex and age strata. Shaded area represents the non-simultaneous 95% confidence intervals. The plots refer to models in Supplemental Table S5.
Total arterial compliance increased between visits mainly among men, particularly among those who were younger at entry (significant negative Entry-Age×Time interaction; Table S5). The predicted average rate of change in CPPM was thus mainly positive for men, decreasing as age increased. Women on the other hand, had less variation in the rate of change, with slight differences by age group (Figure 3E).
The fitted model for Zc evidenced, on average, a longitudinal decrease over time in both men and women, which however was dependent on the entry-age, with older subjects (particularly men) having an increase. Predicted rates of change over 10 years for both sexes (Figure 3F), decrease for entry ages between 35 and 50 years and show a slight increase for entry ages older than 50 years.
We refer to the online Data Supplement for additional details on the basic analysis of the complete dataset and analysis of differences in Zin between visits and by age and sex at specific harmonics. GLME models of the longitudinal effects on SVR, CPPM and Zc for men and women can be found in Supplemental Table S5 with plots of residuals and correlations in Figures S2-S4.
Indices of Wave Reflection: RWTT, ∣Γ1∣ and amplitudes of Pf and Pb
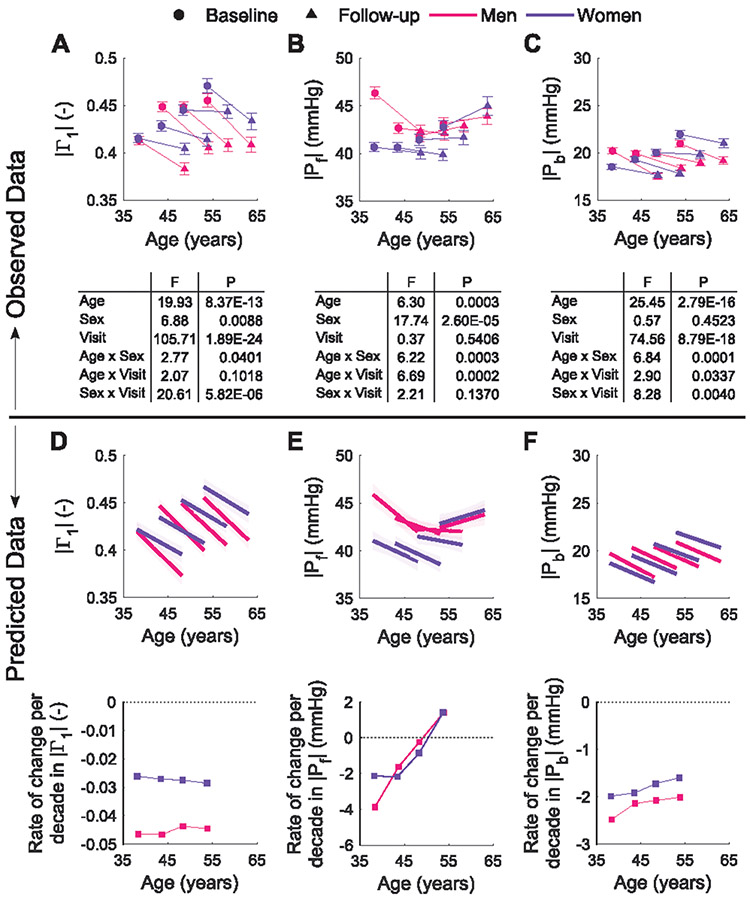
Observed longitudinal changes for the amplitude of the reflection coefficient at the heart frequency (∣Γ1∣), and the amplitudes of the forward (∣Pf∣) and the backward (∣Pb∣) pressure waves are shown in Figure 4A-C. Both reflection measures, ∣Γ1∣ and the reflection magnitude (∣Pb∣/∣Pf∣, data not shown), decreased between examination visits for all age categories and both sexes, showing an opposite trend as observed from cross-sectional data. The longitudinal trajectories of the reflected wave transit time (RWTT) are displayed in supplemental Figure S5. RWTT decreased between visits, and was shorter, with steeper and more uniform decreases in women (P<0.01). Additional details can be found in the online Data Supplement. Figure 4D-F and supplemental Table S6 show the LME models for ∣Γ1∣, ∣Pf∣ and ∣Pb∣, modelled using height, weight, HR and MAP as potential covariates. ∣Pb∣ decreased with aging for both sexes; the longitudinal change in ∣Pb∣ was associated with height in men and with MAP in both men and women. On the other hand, ∣Pf∣ differed by sex and showed a nonlinear dependence with age. Body weight, MAP, and HR in women, also determined the longitudinal changes in ∣Pf∣ over time.
Figure 4.
Top: Observed changes between baseline and follow-up measurements in the wave reflection coefficient derived from impedance analysis, ∣Γ1∣ (A), and the amplitudes of the forward and backward pressure waves, ∣Pf∣ (B) and ∣Pb∣ (C), per sex and age strata. Bottom: Model-predicted longitudinal trajectories and rates of change per decade in ∣Γ1∣ (D), ∣Pf∣ (E) and ∣Pb∣ (F) per sex and age strata. The plots refer to models in Supplemental Table S6.
Effects of antihypertensive and lipid-lowering medications
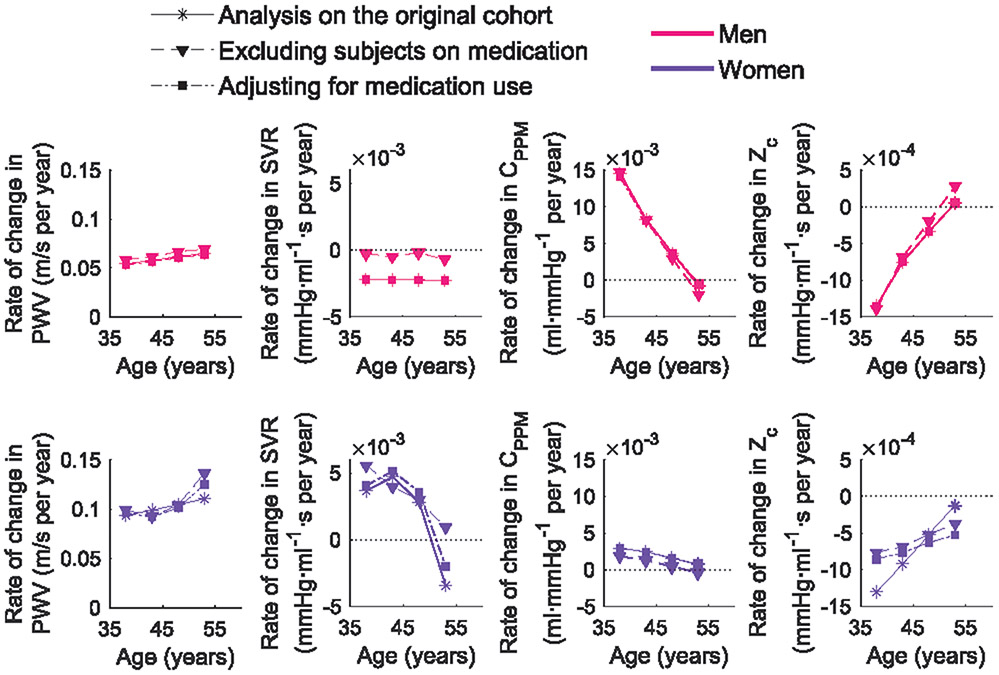
An important factor that might have influenced the observed longitudinal patterns is the start of medication use between visits. From baseline to follow-up, 165 subjects started taking antihypertensive medications only, 281 lipid-lowering medications only, and 133 both drug treatments. Further analyses were performed after exclusion of these subjects, which resulted in a population of N=1447 (54% women, 59% of excluded subjects were from A3 and A4). Besides excluding data, we also followed a second approach where the starting use of medication was controlled for in the statistical models, allowing their interaction with time and entry-age. A comparison of the modelled rates of change of the original cohort with both approaches, in average subjects not on medications, is shown in Figure 5. Models were adjusted for time, entry-age, entry-age2, HR, weight, height, and MAP when applicable. Overall, longitudinal trajectories in PWV, CPPM, and Zc were minimally affected when accounting for medication use. Women showed slight differences in the patterns of change, with a steeper increase in PWV for older subjects, and a slower decrease in Zc for younger. In these models, the use of lipid-lowering drugs had a decreasing effect on PWV and Zc that reduces over time. As for SVR, analysis of the reduced cohort (excluding subjects who started medication) removed the decrease in older women (although the trend remained) while for men, this analysis resulted in a minimal decrease in the annual rate of change.
Figure 5.
Annual rate of change of PWV and impedance parameters for average subjects (men and women) not on medications and per age group, in the analysis of the original cohort, after excluding subjects taking antihypertensive and lipid-lowering medications, and in models adjusting for the use of medications. Models included entry-age, time, HR, height, weight, MAP, and interaction terms with time and entry-age, as possible covariates.
4. Discussion
In this study, we have shown the effective impact of aging on the arterial system properties and dynamics in the middle-aged. To our knowledge, this is the first study to report and analyze the longitudinal evolution of impedance and wave reflection parameters (besides blood pressure and arterial stiffness) over a span of about a decade. A major observation to notice from our longitudinal data is that impedance and wave reflection parameters have a different evolution, and in some cases even opposed, from results of cross-sectional analyses. Indeed, while cross-sectional data show a decrease with age in total arterial compliance and an increase in resistance, our longitudinal data demonstrate an increase in CPPM and a decrease in SVR, mainly for men and for the older age groups. Similar opposite behavior is found for wave reflection parameters, such as the reflection coefficient and the reflection magnitude. Although PWV increased with age, concordant with cross-sectional analyses, it increased more rapidly than expected in women, while the opposite was true for men; besides it was not paralleled by a decrease in arterial compliance, mainly in younger subjects.
Longitudinal trajectories of PWV and BP variables
While the overall consensus is that PWV increases with age, the modelled age-dependency of the relation between PWV and time is more complex than this and depends on the chosen models. Our results, as well as previously published results [23, 24], show no baseline differences in PWV between men and women in the age range of study. In the models accounting only for entry-age and time (Figure 1 and Table S2), the longitudinal trajectories of PWV over time were independent of the baseline age and their rates of change, although higher for women, showed a similar increasing pattern with age for both sexes, in agreement with reports from the SardiNIA longitudinal study [24]. However, in models including covariates (Table S3), the longitudinal increase in PWV over time was associated with higher entry-age mainly in women, thus implying that the older the subject entering into the study, the higher was the increase in PWV in ten years. Similarly to the present study, in the BLSA [23], sex differences in PWV arise and accelerate with aging; however, in contrast to our study, men had a steeper longitudinal increase in PWV than women. Controversial results when referring to sex differences in PWV values with age have been previously reported [5, 31-33], with a lack of consensus on whether PWV is higher in women or in men, which may be influenced by study design factors such as the age range of participants, ethnicity, body weight, the variety of methods used when calculating PWV, or whether the data are cross-sectional or longitudinal. It has been also suggested that this divergence may be associated to menopause [33], a factor not accounted for in our study.
Previous studies [21, 24] have shown a dissociation of BP variables with longitudinal changes in PWV. Our results are in line with these previous findings, where none of the BP variables was longitudinally associated with PWV trajectories over time (except PPCA in women). Vice versa, PWV was inversely associated with the longitudinal changes in all BP variables except PPCA for both sexes (Supplemental Tables S3-S4). Results from the FHS [21] and the BLSA [22] showed an association of higher PWV with the longitudinal increase in SBP, supporting the premise that accelerated arterial stiffness is one of the causes of hypertension in older adults and not vice versa. Our outcomes on the other hand, show that the longitudinal increase in SBPCA over time is lower for higher PWV among middle aged-adults. However, when subjects taking medications were excluded from the analyses, increased SBPCA was associated with the longitudinal increase in PWV. Similar results were obtained in [23], with models adjusting for medications use. What is clear from these results is that medication has an impact on the relationship between blood pressure and arterial stiffness, and that this relation is rather complex. Note that a direct comparison of our results with previous longitudinal studies is limited by differences in the statistical approaches, location where blood pressure was measured, the age range and characteristics of the study population, and duration of the follow-up (the Asklepios study has the longest mean follow-up compared to [21, 22, 24]).
Longitudinal trajectories of impedance and wave reflection parameters
The longitudinal trajectories of Zc decreased for the younger subjects (A1 to A3). On the other hand, CPPM increased over the 10-year period and this increase declined with older age. Different factors may explain why age-related changes in volume compliance differ from previous reports [25, 34]. First, most reports are cross-sectional and when looking at our data from a cross-sectional perspective, compliance does decrease with age in both rounds. Second, we are studying a large cohort in a narrow age range that is not often considered. Our main rationale to include apparently healthy middle-aged subjects at baseline was to specifically start studying the cardiovascular system at a moment where, presumably, major cardiovascular adaptations still need to take place. The pattern of an increase in volume compliance concomitant with an increase in PWV suggests that aortic geometric remodeling (such as dilation and elongation) may be involved. Indeed, earlier studies report that the aorta undergoes marked changes with aging, becoming more tortuous with increased elongation [4] and increased aortic root diameter which is larger in men compared to women [35]. The former may also explain the decrease in aortic Zc for all but the oldest age category. Characteristic impedance is known to be very sensitive to aortic size, as quantified by the water hammer equation [10, 36]; therefore, aortic dilation may be able to overcome the hemodynamic impact of aortic stiffening. Besides, it has been already suggested that the effective cross-sectional area (Aeff) plays an important role in the arterial function, particularly as a determinant of aortic Zc [10].
In the design of our population study, we did not account for a direct measure of change in aortic size (dilatation or elongation). In the previous report of the Asklepios study [25], a relation between age and the cross-sectional area of the LV outflow tract (which can be seen as an indication of further changes in Aeff) could not be established. In contrast, in our present longitudinal study, LVOT cross-sectional area increased significantly between visits (see Table 1). In the younger middle-aged males, while PWV increased a 9% between visits, Zc decreased a 13%, which suggest from the water hammer equation a 25% increase in Aeff. Total arterial compliance, via the distensibility coefficient, could be assumed to be proportional to Aeff/PWV2, thus the increase in both parameters would imply an increase in arterial compliance of ~5%, which corresponds with the order of magnitude of the increase observed in CPPM for this group of subjects.
Contrary to what has been observed by cross-sectional studies including the baseline Asklepios study [1, 25, 37], in the transition from middle-aged to older of our sample population wave reflection did not increase but decreased with aging for men and women. There was a nonlinear relation in ∣Γ1∣ with the cross-sectional age in men, but longitudinal trajectories over time were independent of the entry-age. The longitudinal trajectories in ∣Pf∣ had a marked dependency with aging, whereas ∣Pb∣ decreased albeit with less age-dependency. The conventional understanding of wave reflection shows that with advancing age, increased aortic stiffness results in larger reflected waves returning earlier in the aorta and elevating blood pressure [38]. On the other hand, Mitchell et al. [19, 39] have suggested that the increase in characteristic impedance due to increasing aortic stiffness with aging, leads to an impedance matching between the aorta and large peripheral arteries, which in turns lead to a reduction in wave reflection. The patterns of change for the older subjects in our study are consistent with these findings. However, it is important to consider that impedance matching is also highly sensitive to the geometry of proximal and distal vessels. Future studies should examine the determinants of wave reflection in more detail, including the role of segmental stiffening and conduit artery geometry.
Cardiovascular risk factors do not fully explain the dissociation between baseline and follow-up observations
A major observation from our study is the dissociation between our longitudinal outcomes and what was expected based on our previous cross-sectional analysis [25]. We cannot exclude that secular trends due to a change of lifestyle, diet, socio-economic factors or environmental exposures may be affecting our results, since they are likely to occur in the long observation period. Such factors were not fully accounted for in our study and in general are considered a limitation of long period longitudinal studies. Nonetheless, we performed additional analyses testing for the association between the studied parameters and traditional cardiovascular risk factors such as obesity, diabetes, smoking status, alcohol intake, hypertension, use of lipid-lowering treatments, and the education level. Although the longitudinal effect of medication use was significant for some parameters, overall the patterns of change remained consistent (Figure 5). When accounting for the other cardiovascular risk factors, the patterns of change persisted in general. Further information is discussed in the Data Supplement (see Tables S7-S9 and Figures S6-S7). Significant differences were also observed when comparing parameters in subjects of a certain age at baseline with subjects that reached the same age during follow-up. Speculatively, this may be an effect of subjects of a same age experiencing a different lifestyle history and different environmental exposures. Additional details are discussed in the Data Supplement (Comparison of subjects of the same age but distinct generation controlling for lifestyle factors). It was also verified by a multiple regression analysis for the rate of change of some variables (data not shown), adjusting for age, sex, and the baseline measurement of the variable, that our results are not affected by regression to the mean.
Our study has some limitations that should be discussed. Our noninvasive data of central pressure were estimated at the carotid artery as a surrogate for aortic pressure, this combined with central aortic flow might have influenced the derived impedance and reflection parameters. The evaluated population was narrowed down to white middle-aged subjects that transitioned to an older cohort in the span of the study, thus our results cannot be generalized to younger or elder subjects, or to other races and ethnicities. Besides, our longitudinal study includes only one follow-up measurement, limiting the detection power of complex interactions that may have occurred between both examination visits. Derived PWV at follow-up was not corrected for the changes in the path length between visits.
5. Perspectives
In middle-aged adults, the evolution of arterial parameters over a 10-year period differ from cross-sectional trends, leading to differences in subjects of similar ages but a decade apart. Overall, the arterial system of subjects that reached a certain age in the span of the study had higher compliance, body size measures, CO, SV, PWV (in women) than subjects that were the same age at baseline, but also presented lower wave reflection parameters, SVR and characteristic impedance. These differences may be conditioned by different socio-economic factors, environmental exposures, and changes of subjects’ lifestyle, rather than aging alone. Although PWV rises with age indicating stiffening of the arterial tree, this increase was not paralleled by a decrease in volume compliance or by an increase in characteristic impedance. This suggest that dilation and/or elongation of the aorta plays an important role determining impedance. Our results evidence the importance of performing longitudinal studies over cross-sectional studies, which do not properly reflect the effective impact of aging on arterial system properties. More efforts are still needed to develop therapies aiming to influence directly arterial stiffness, whereas traditional risk factors together with socio-economic, genetic, and environmental factors, deserve greater consideration in order to reduce the risk of cardiovascular events and achieve healthy vascular aging.
Supplementary Material
Novelty and Significance:
What Is New?
This is the first study to report longitudinal changes on impedance and wave reflection parameters over a decade of age, in middle-aged men and women.
The 10-years longitudinal changes on arterial compliance, systemic vascular resistance, the reflection coefficient, and the reflected pressure wave, oppose to the cross-sectional observations.
What Is Relevant?
In white middle-aged individuals, PWV accelerates at older age more in women than in men, and this increase in PWV with aging is not paralleled by a decrease in arterial compliance.
A discrepancy in the longitudinal evolution over a decade of time, of PWV and impedance parameters (arterial compliance and characteristic impedance), could be explained by aortic dilatation and/or elongation.
Wave reflection indices decreased with aging in the studied age spectrum and for both sexes, supporting the hypothesis of impedance matching between the aorta and large peripheral arteries with aging.
Acknowledgments
The authors thank the Asklepios Study staff for assistance in the data preparation. The authors acknowledge all the participants from the Asklepios Study who voluntarily contributed to the study.
Sources of Funding
The Asklepios Study was partly funded by an FWO research grant (G.0427.03). This work was supported by the Special Research Fund of Ghent University (01W03117) (sandwich doctoral scholarship D.C.A.).
Footnotes
Disclosures
J.A.C. has recently consulted for Bayer, Sanifit, Fukuda-Denshi, Bristol-Myers Squibb, JNJ, Edwards Life Sciences, Merck and the Galway-Mayo Institute of Technology. He is named as inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. He has received honoraria for editorial roles from the American Heart Association and the American College of Cardiology.
E.R.R. reports grants from Ghent University, has received unrestricted educational grants from Amgen, MSD, Astra-Zeneca, Sanofi and Unilever, speakers' fees from Novo Nordisk, Boehringer Ingelheim, Amgen, Sanofi-Aventis, Novartis and Teva. These grants were payed to Ghent University and are outside submitted work. E.R.R. reports device loans from ResMed and GE Healthcare (to Ghent University, Asklepios study).
6. References
- 1.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women. The Framingham heart study. Hypertension. 2004;43(6):1239–1245. [DOI] [PubMed] [Google Scholar]
- 2.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O'rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71(2):202–210. [DOI] [PubMed] [Google Scholar]
- 3.Franklin SS, Gustin IV W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation. 1997;96(1):308–315. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC: Cardiovascular Imaging. 2008;1(6):739–748. [DOI] [PubMed] [Google Scholar]
- 5.Lee HY, Oh BH. Aging and Arterial Stiffness. Circulation Journal. 2010;74(11):2257–2262. [DOI] [PubMed] [Google Scholar]
- 6.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–3390. [DOI] [PubMed] [Google Scholar]
- 7.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. European Heart Journal. 2010;31(15):1865–1871. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen C-H, Cruickshank JK, et al. Aortic pulse wave velocity improves cardiovascular event prediction. An individual participant meta-analysis of prospective observational data from 17635 subjects. Journal of the American College of Cardiology. 2014;63(7):636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell GF, Lacourcière Y, Ouellet J-P, Izzo JL, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension. The role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108(13):1592–1598. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: The Framingham Heart Study. Circulation. 2007;115(20):2628–2636. [DOI] [PubMed] [Google Scholar]
- 12.Nichols WW, O'Rourke MF, Avolio AP, Yaginuma T, Murgo JP, Pepine CJ, Conti CR. Effects of age on ventricular-vascular coupling. The American Journal of Cardiology. 1985;55(9):1179–1184. [DOI] [PubMed] [Google Scholar]
- 13.The Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: "establishing normal and reference values". European Heart Journal. 2010;31(19):2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68(1):50–58. [DOI] [PubMed] [Google Scholar]
- 15.Weber T, O’Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. American Journal of Hypertension. 2008;21(11):1194–1202. [DOI] [PubMed] [Google Scholar]
- 16.McEniery CM, Yasmin Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, on behalf of the ACCT Investigators. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). Journal of the American College of Cardiology. 2005;46(9):1753–1760. [DOI] [PubMed] [Google Scholar]
- 17.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. European Heart Journal. 2006;27(21):2588–2605. [DOI] [PubMed] [Google Scholar]
- 18.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. Journal of the American College of Cardiology. 2004;43(8):1388–1395. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121(4):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirinos JA. Discerning the age-related heterogeneity in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. [Editorial]. 2019;74(5):613–616. [DOI] [PubMed] [Google Scholar]
- 21.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. Journal of the American College of Cardiology. 2008;51(14):1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62(5):934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scuteri A, Morrell CH, Orrù M, Strait JB, Tarasov KV, Ferreli LAP, Loi F, Pilia MG, Delitala A, Spurgeon H, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension. 2014;64(6):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR, on behalf of the Asklepios investigators. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007;49(6):1248–1255. [DOI] [PubMed] [Google Scholar]
- 26.Stergiopulos N, Segers P, Westerhof N. Use of pulse pressure method for estimating total arterial compliance in vivo. American Journal of Physiology-Heart and Circulatory Physiology. 1999;276(2):H424–H428. [DOI] [PubMed] [Google Scholar]
- 27.Rietzschel ER, De Buyzere ML, Bekaert S, Segers P, De Bacquer D, Cooman L, Van Damme P, Cassiman P, Langlois M, van Oostveldt P, et al. Rationale, design, methods and baseline characteristics of the Asklepios Study. European Journal of Cardiovascular Prevention & Rehabilitation. 2007;14(2):179–191. [DOI] [PubMed] [Google Scholar]
- 28.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New-York: Springer; 2000. [Google Scholar]
- 29.Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2009;64(2):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa S, Johnson PC, Schielzeth H. The coefficient of determination R^2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface. 2017;14(134):20170213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsao CW, Washington F, Musani SK, Cooper LL, Tripathi A, Hamburg NM, Benjamin EJ, Vasan RS, Mitchell GF, Fox ER. Clinical Correlates of Aortic Stiffness and Wave Amplitude in Black Men and Women in the Community. Journal of the American Heart Association. 2018;7(21):e008431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Rourke MF, Hayward CS. Arterial stiffness, gender and heart rate. Journal of Hypertension. [Editorial]. 2003;21(3):487–490. [DOI] [PubMed] [Google Scholar]
- 33.Subramanya V, Ambale-Venkatesh B, Ohyama Y, Zhao D, Nwabuo CC, Post WS, Guallar E, Ouyang P, Shah SJ, Allison MA, et al. Relation of sex hormone levels with prevalent and 10-year change in aortic distensibility assessed by MRI: the multi-ethnic study of atherosclerosis. American Journal of Hypertension. 2018;31(7):774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA, Bluemke DA. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [MESA]). The American Journal of Cardiology. 2008;102(4):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, Mitchell GF, Benjamin EJ, Vasan RS. Aortic root remodeling over the adult life course: longitudinal data from the Framingham Heart Study. Circulation. 2010;122(9):884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chirinos JA, Segers P, Hughes T, Townsend R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2019;74(9):1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K-L, Cheng H-M, Sung S-H, Chuang S-Y, Li C-H, Spurgeon HA, Ting C-T, Najjar SS, Lakatta EG, Yin FC. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55(3):799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45(4):652–658. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. Journal of Applied Physiology. 2008;105(5):1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.