Abstract
There is a number of systemic diseases affecting the cornea. These include endocrine disorders (diabetes, Graves’ disease, Addison’s disease, hyperparathyroidism), infections with viruses (SARS-CoV-2, herpes simplex, varicella zoster, HTLV-1, Epstein-Barr virus) and bacteria (tuberculosis, syphilis and Pseudomonas aeruginosa), autoimmune and inflammatory diseases (rheumatoid arthritis, Sjögren’s syndrome, lupus erythematosus, gout, atopic and vernal keratoconjunctivitis, multiple sclerosis, granulomatosis with polyangiitis, sarcoidosis, Cogan’s syndrome, immunobullous diseases), corneal deposit disorders (Wilson’s disease, cystinosis Fabry disease, Meretoja’s syndrome, mucopolysaccharidosis, hyperlipoproteinemia), and genetic disorders (aniridia, Ehlers-Danlos syndromes, Marfan syndrome). Corneal manifestations often provide an insight to underlying systemic diseases and can act as the first indicator of an undiagnosed systemic condition. Routine eye exams can bring attention to potentially life-threatening illnesses. In this review, we provide a fairly detailed overview of the pathologic changes in the cornea described in various systemic diseases and also discuss underlying molecular mechanisms, as well as current and emerging treatments.
Keywords: diabetic cornea, Graves’ disease, Addison’s disease, SARS-CoV-2, herpes, zoster, tuberculosis, syphilis, Pseudomonas Aeruginosa, autoimmune disease, Sjögren’s syndrome, inflammation, keratoconjunctivitis, genetic corneal disease, corneal deposit disorder, aniridia, Ehlers-Danlos syndrome, Marfan syndrome, immunobullous disease
1. Introduction
Cornea is an integral part of the body and reacts to various diseases or genetic abnormalities at the systemic level. These insults may be infectious agents, metabolic disorders, autoimmune diseases or heritable changes in gene expression. Clinical aspects of corneal changes in various systemic disorders have been previously discussed (Mora et al. 2013; Consultant360 2014; Gillan 2015; Gomes et al. 2015; Patel, 2017; Dua et al. 2018; Wilkins et al. 2019; Patel et al. 2020). In this review, the authors tried to cover whenever possible the known mechanisms, signaling pathways, and emerging treatments for corneal alterations in systemic diseases. Because of the authors’ area of research and available evidence, diabetes is discussed in more detail. Attempts were made to cite newer reports. Although COVID-19 impact on the cornea is only beginning to be unraveled, pertinent literature is also covered as it is a rapidly expanding field. For certain diseases only a very limited clinical information on corneal involvement is available, mostly as case reports. Some other conditions can be cured by diet changes, e.g., vitamin deficiencies. For these reasons, such diseases were not discussed here. A summary of corneal manifestations of systemic diseases is provided in Table 1.
Table 1.
Corneal manifestations of human systemic diseases
| Endocrine Diseases | ||
|---|---|---|
| Systemic Disease | Pathophysiology | Corneal Manifestations |
| Diabetes Mellitus | Autoimmune loss of insulin-producing pancreatic cells (T1DM) or insulin resistance (T2DM) resulting in hyperglycemia. DM is associated with progressive macro- and micro angiopathy, neuropathy, and cardiovascular problems. | Keratopathy (compromised epithelial barrier function and wound healing, stem cell marker reduction, decreased p38 and EGFR/Akt signaling), edema; neuropathy (loss of subbasal corneal nerves), endothelial cell loss, increased stromal rigidity with altered biomechanics due to AGE accumulation, impaired tear film secretion. |
| Graves’ Disease | Autoimmune endocrine disease marked by hyperthyroidism and an enlarged thyroid gland. | Corneal inflammation, irritation, and dry eye due to corneal exposure caused by proptosis; changes in corneal biochemical properties. |
| Addison’s Disease | Primary adrenocortical insufficiency due to autoimmunity or infection (tuberculosis). | Corneal ulcers, keratoconjunctivitis, limbal stem cell deficiency, vision loss. |
| Hyperparathyroidism | Enlargement of parathyroid glands and abnormal secretion of parathyroid hormone, resulting in hypercalcemia. Secondary hyperparathyroidism is a common complication of chronic kidney failure | Band keratopathy due to calcium deposits in Bowman’s layer, conjunctiva, and peripheral cornea. Changes in endothelial morphology. |
| Infectious Diseases | ||
| Systemic Disease | Pathophysiology | Corneal Manifestations |
| Coronavirus Disease 2019 (COVID-19) | Multisystem infection with lung inflammation, fibrosis, respiratory failure, vasculitis, loss of smell, immune system problems with cytokine storm, coagulopathy. | Dry eye, blurred vision, itching, redness, tearing, discharge, foreign body sensation, conjunctivitis in a minority of patients. |
| Herpes Simplex Keratitis | Reactivation of the virus from the latent stage being the precursor to more severe manifestations on the ocular surface. | Corneal blindness, ulcers, corneal opacification, angiogenesis, and corneal nerve loss. |
| Shingles Caused by Varicella Zoster | Maculopapular or vesicular rash in different parts of the body due to reactivation of latent virus in the sensory nerve ganglia. | Reactivation in ophthalmic region of trigeminal cranial nerve (V) may cause conjunctivitis, anterior uveitis, episcleritis and keratitis. |
| Human T-Cell Leukemia Virus HTLV-1 | Adult T-cell leukemia / lymphoma, neurological disorder HTLV-1-associated myelopathy (tropical spastic paraparesis), HTLV-1-associated uveitis, bladder dysfunction. | Keratoconjunctivitis sicca, interstitial keratitis, corneal haze and opacities, thinning and scarring of the peripheral cornea, keratopathy and neovascularization. |
| Epstein-Barr Virus | Ubiquitous human herpes virus 4 that causes infectious mononucleosis. | Stromal keratitis with granular, ring-shaped opacities, delayed onset bilateral peripheral interstitial keratitis, corneal endotheliitis (also seen in CMV infection), epithelial-mesenchymal transition. |
| Tuberculosis | Primarily affects lungs and respiratory tract resulting in serve cough, fever, weight loss, and night sweats. | Lid vulgaris, conjunctivitis, scleritis, episcleritis, corneal phlycten, interstitial keratitis. |
| Syphilis | Painless sores and mild rashes. When left untreated, bacterium spreads and affects internal organs such as the eyes, brain, heart, nerves, bones, joints, and liver. | Uveitis and syphilis keratitis, which may lead to decreased visual acuity and even permanent blindness. |
| Pseudomonas aeruginosa Keratitis | Pneumonia, sepsis, ecthyma gangrenosum, osteomyelitis, otitis externa, urinary tract infections, skin infections. | Contact lens-related ulcers, biofilm formation, bacterial keratitis, corneal edema, liquefactive necrosis |
| Autoimmune and Inflammatory Diseases | ||
| Systemic Disease | Pathophysiology | Corneal Manifestations |
| Rheumatoid Arthritis | Autoimmune disease resulting in a chronic and painful inflammatory response, primarily in the joints. | Scleritis, episcleritis, peripheral ulcerative keratitis, keratoconjunctivitis sicca, and may be precursor to other rheumatic disease such as Sjögren’s syndrome. |
| Sjögren’s Syndrome | Rheumatic autoimmune disease in which the salivary and lacrimal glands become dysfunctional. | Moderate to severe ocular dryness, thus causing corneal melt/perforation, uveitis, scleritis, and in severe cases limbal stem cell deficiency. |
| Systemic Lupus Erythematosus | Inflammation of the joints, produces sensitive skin rashes and may even cause severe kidney and lung failure or damage to the central nervous system. | Inflammation may cause cataracts, keratoconjunctivitis sicca (via secondary Sjögren’s syndrome and rheumatoid arthritis), glaucoma, discoid lesions of eyelids, episcleritis, scleritis, keratitis, and uveitis. |
| Gout | Increased level of uric acid in the body that results in the accumulation of monosodium urate (MSU) crystals, mainly in the joints. | Keratitis and corneal endothelial dysfunction. |
| Atopic Keratoconjunctivitis | Allergic inflammatory disease associated with atopic dermatitis caused due to environmental allergens marked by itching, redness, and burning of the eyes, eczema of the eyelids, blepharitis along the lid margin, conjunctival inflammation, excessive tear production, and corneal complications. | Punctate keratitis, corneal erosions, corneal ulcerations, edema, epithelial defects, neovascularization, scarring, and vision loss. |
| Vernal Keratoconjunctivitis | Allergic inflammatory disease appearing during warm seasons. Marked by itching, redness, conjunctival and corneal inflammation, photophobia, foreign body sensation. | Punctate epithelial erosions, shield ulcers, stromal plaques, neovascularization, keratoconus, infectious keratitis, and LSCD. |
| Multiple Sclerosis | Apparently autoimmune demyelinating central nervous system disease with frequent optic neuritis. | Significant reduction of corneal nerve fiber density, branch density and length with axonal loss. |
| Granulomatosis with Polyangiitis | Idiopathic, multisystem inflammatory disease of the upper and lower respiratory tracts characterized by necrotizing granulomatous inflammation and vasculitis. | Bilateral peripheral ulcerative keratitis due to the presence of autoantibodies and inflammatory cells from limbal blood vessels, limbal edema, corneal thinning, endothelial cell loss. |
| Sarcoidosis | Inflammatory granulomatous disease affecting the lung and mediastinal lymphatic system characterized by the formation of non-caseating, giant cell granulomas with T lymphocyte and macrophage involvement. | Corneal small nerve fiber loss and damage, interstitial keratitis, band keratopathy from calcium deposits in the Bowman’s layer, dry eye. |
| Cogan’s Syndrome | Autoimmune disease characterized by inflammation in the eye and inner ear, with systemic vasculitis | Bilateral peripheral subepithelial keratitis with nummular lesions, deep stromal keratitis, granular infiltration in peripheral cornea, photophobia, excessive tear production, diminished visual acuity. |
| Immunobullous Diseases | Autoimmune diseases caused by specific autoantibodies that bind to epithelial cells, resulting in blistering lesions on the skin, mucous membranes, and oral cavity. | Punctate epithelial erosions, bilateral corneal perforations, corneal melting, decreased corneal nerve density, intraepithelial defects, anterior stromal fibrosis, corneal neovascularization. |
| Genetic Corneal Deposit Disorders | ||
| Systemic Disease | Pathophysiology | Corneal Manifestations |
| Wilson’s Disease | Excessive copper deposition in liver, brain, cornea, kidney due to mutation in gene encoding ATP7B protein. | Kayser-Fleischer ring and sunflower cataract formation due to copper accumulation. |
| Cystinosis | Intracellular accumulation of cysteine crystals in kidney, liver, spleen, eye, bone marrow, pancreas, thyroid, muscle, and brain due to mutations in the CTNS gene. | Formation of corneal crystals of cysteine deposits and photophobia, and recurrent corneal erosions in some cases. |
| Fabry Disease | Lysosomal accumulation of glycosphingolipids due to mutations in the GLA gene on the X-chromosome. | Cornea verticillata (vortex keratopathy) due to whorl-like deposits in the epithelial and sub-epithelial layers, with corneal haze and conjunctival vessel tortuosity. |
| Meretoja Syndrome | Amyloid deposition due to mutations in the gelsolin gene at chromosome 9q32–34. | Corneal lattice dystrophy, corneal ulcers, dry eye, photophobia, dysfunction of the meibomian glands, early development of cataract. |
| Mucopolysaccharidosis (7 subtypes known) | Lysosomal storage disorder characterized by the glycosaminoglycan (GAG) accumulation in bone, tendons, cartilage, cornea, skin, and connective tissue. | Corneal clouding that appears as yellowish-grey granules deposited in all layers of the cornea, but mainly in the stroma, increased keratocyte size and the displacement of collagen fibrils. |
| Hyperlipoproteinemia | Elevated levels of lipids and lipoproteins such as cholesterol in the blood. | Corneal arcus, a yellowish-grey ring of lipid deposits around the cornea and limbus. |
| Other Genetic Disorders Presenting Corneal Manifestations | ||
| Systemic Disease | Pathophysiology | Corneal Manifestations |
| Aniridia | Absence of iris, usually in both eyes and can be acquired through a haploinsufficiency truncating mutation in the PAX6 gene, congenitally, and by ocular injury. | Aniridia-associated keratopathy, conjunctival neovascularization, and corneal blindness caused by limbal stem cell insufficiency. Altered Notch1 and Wnt signaling. |
| Ehlers-Danlos Syndromes (EDS) | Autosomal recessive or dominant abnormalities of connective tissue due to mutations in a number of genes, in particular, in various collagen genes. Depending on the gene involved, clinical signs include fragile skin, skeletal dysmorphology with stunted growth, joint dislocation, vascular problems, etc. | Kyphoscoliotic EDS (mutated PLOD1 or FKBP14 genes) is associated with scleral fragility, microcornea. Brittle cornea syndrome (mutated ZNF469 or PRDM5 genes) can cause corneal rupture, scarring, keratoconus, keratoglobus. Classic EDS with mutations of COL5A1 or COL5A2 genes may result in thinner and steeper corneas. |
| Marfan syndrome | Autosomal dominant disorder of the connective tissue caused by mutation in gene encoding fibrillin and resulting in musculoskeletal symptoms. | Corneal flattening and thinning. |
2. Endocrine Diseases
2.1. Diabetes Mellitus
In the last decade, diabetes mellitus (DM) has reached epidemic proportions and is the leading cause of new blindness in adults between 25 and 64 years of age (Schmidt 2018; Lee and Mesfin 2020). DM affects all parts of the body including the eyes. This is true for both insulin-dependent diabetes (type 1, T1DM; an autoimmune disease leading to destruction of insulin-producing β cells in the pancreatic islets of Langerhans) and more common non-insulin-dependent diabetes (type 2, T2DM; characterized by insulin resistance in many organs leading to hyperglycemia and gradual decline in insulin production). The most severe ocular complication of DM is diabetic retinopathy (DR) (Aiello et al. 1998). It is the main contributor to new blindness cases in the United States (Aiello et al. 1998; Negi and Vernon 2003) and is a disease of retinal microvasculature.
Although retinal changes in diabetes can become vision-threatening over time and may lead to blindness as covered in detail previously (Stitt et al. 2016; Cabrera et al. 2020; Gui et al. 2020; Kutlutürk Karagöz et al. 2020), they are outside of the scope of this review.
Ocular surface including the cornea is also affected by DM. Corneal complications are relatively frequent and are observed in 45–70% of diabetic patients (Schultz et al. 1981; Abdelkader et al. 2011; Vieira-Potter et al. 2016; Ljubimov 2017; Zhao et al. 2019; Priyadarsini et al. 2020). These alterations may be symptomatically mild and are often underdiagnosed (Wylegała et al. 2006). For this reason, some studies have raised awareness of ophthalmologists to the necessity of assessing ocular surface changes during eye exams of diabetic patients (DeMill et al. 2016; Richdale et al. 2020). Although up to one-fourth of corneas harvested for transplantation in the United States originate from diabetic donors, convincing clinical studies have suggested that such corneas may present a risk to the graft recipients (Lass et al. 2019; Goldstein et al. 2020).
In recent years, several comprehensive reviews on clinical and experimental aspects of corneal DM have appeared (Calvo-Maroto et al. 2014; Misra et al. 2016; Vieira-Potter et al. 2016; Ljubimov 2017; Shih et al. 2017; Bikbova et al. 2018; Han et al. 2018; Zhao et al. 2019; Zhu et al. 2019; Mansoor et al. 2020; Priyadarsini et al. 2020; Roszkowska et al. 2020). For this reason, we will fairly briefly summarize key aspects of diabetic corneal disease, with emphasis on manifestations and mechanisms, as well as on promising approaches to treatment.
2.1.1. General Traits of Corneal Diabetes
DM leads to lasting alterations of corneal epithelium (keratopathy, DK), nerves (corneal neuropathy, DCN), stroma, endothelial cells, conjunctiva, corneal biomechanics and tear film (Ljubimov 2017; Priyadarsini et al. 2020; Mansoor et al. 2020). Symptomatically, epithelial and neural changes have been more important than others, and the bulk of the literature thus concerns these two aspects of diabetic cornea. The human diabetic cornea is also more susceptible than normal cornea to bacterial and viral infections (Wang et al. 2018).
2.1.1.1. Epithelial Abnormalities (Keratopathy)
Diabetic epitheliopathy/keratopathy, is manifested by various epithelial defects including fragility, stem cell dysfunction, altered basement membrane (BM) composition, delayed wound healing, impaired barrier function leading to edema, recurrent erosions, and non-healing ulcers (Cavallerano 1992; Saini and Khandalavla, 1995; Gekka et al. 2004; Quadrado et al. 2006; Bikbova et al. 2012; Vieira-Potter et al. 2016; Ljubimov 2017; Shih et al. 2017; Alfuraih et al. 2020; Jan et al. 2020; Priyadarsini et al. 2020). These signs (Figure 1) seem to be exacerbated with increasing DM duration and severity.
Figure 1.

Neurotrophic corneal ulcers in the right eye (A) and left eye (B) of a diabetic patient unresponsive to conventional treatments before starting topical insulin (25 IU/mL), with large persistent epithelial defects.
Reproduced with permission from: Tong, C.M., Iovieno, A., Yeung, S.N., 2020. Topical insulin for neurotrophic corneal ulcers. Can. J. Ophthalmol. 55, e170–e172.
DK is often associated with signs of DCN (Mocan et al. 2006; Bikbova et al. 2018) and may be developing as a consequence of DCN (Barsegian et al. 2018). However, some diabetic changes of the corneal epithelium do not appear to be related to DCN as shown in affected patients and animal models (Rosenberg et al. 2000; Saghizadeh et al. 2001a; 2005; 2011; Quadrado et al. 2006; Chikama et al. 2007). Additionally, animal and human cell and organ culture studies using hyperglycemic conditions showed direct and fast effects on normal corneal epithelium with diabetic-like changes in cell adhesion and impaired wound healing (Fujita et al. 2003; Tomomatsu et al. 2009; Xu et al. 2009; Yin and Yu 2010). Therefore, the cause-effect relationship between DK and DCN requires more in-depth studies.
2.1.1.1.1. Underlying Mechanisms of Epithelial Abnormalities
Molecular mechanisms of DK have been a subject of many recent studies. These will be discussed for both T1DM and T2DM because only few differences in corneal changes between DM types. The epithelial cell adhesion and BM structure are altered in human ex vivo diabetic corneas, with reduced BM immunostaining for laminins, nidogen-1, and limbal fibronectin (Hatchell et al. 1983; Tabatabay et al. 1988; Azar et al. 1992; Ljubimov et al. 1998; Sato et al. 1999; Fujita et al. 2003; Gül et al. 2008; Saghizadeh et al. 2011). The apparently degradative BM changes could affect epithelial cell migration and result in delayed and impaired wound healing. They may be due to increased expression of proteinases, such as MMP-3, MMP-10, and cathepsin F (Saghizadeh et al. 2001a; 2005). This suggestion was corroborated by studies of organ-cultured human corneas that recapitulate abnormalities seen in ex vivo and in vivo corneas (Kabosova et al. 2003). Adenovirus-driven increase in MMP-10 and cathepsin F in normal corneas resulted in the same BM changes as observed in diabetic corneas (Saghizadeh et al. 2010a). Conversely, proteinase silencing in diabetic corneas normalized BM patterns and improved wound healing (Saghizadeh et al. 2013; 2014; Ljubimov and Saghizadeh 2015; Kramerov et al. 2016).
Diabetic corneal stem cells also appear to be dysfunctional as evidenced by decreased expression of putative stem cell markers in ex vivo and organ-cultured human diabetic corneas. Such a decrease persists in 2D cultures of human diabetic limbal epithelium enriched in progenitor cells and could contribute to slow epithelial wound healing (Saghizadeh et al. 2011; Kramerov et al. 2015). These data were later corroborated in a mouse db/db model of T2DM (Ueno et al. 2014). The expression of these stem cell markers and slow wound healing in human diabetic organ-cultured corneas may be largely reverted to normal by gene therapy (Ljubimov and Saghizadeh 2015; Kramerov et al. 2021).
Various growth factors and cytokines, as well as their signaling pathways are altered in human diabetes as well as in animal models and 3D corneal organ cultures (Ljubimov 2017). Because growth factors and cytokines play important roles in corneal epithelial cell physiology and wound healing (Klenkler and Sheardown 2004; Ljubimov and Saghizadeh 2015), their abnormal expression and function may lead to DK. They may affect cell adhesion and wound healing, contribute to epithelial fragility and subbasal nerve loss in diabetic corneas. Some better studied growth factors that are altered in diabetic corneas are discussed below.
Opioid growth factor (OGF), or [Met5]-enkephalin, acting through its ζ receptor (OGFR) negatively regulates corneal epithelial proliferation and wound healing (Sassani et al. 2016). Levels of OGF are elevated in plasma of diabetic patients (McLaughlin et al. 2010). OGF and OGFR expression is increased in diabetic rat corneal epithelium (Zagon et al. 2020). Systemic or topical administration of opioid antagonist naltrexone normalized OGF blood levels as well as corneal epithelial wound healing in rats and rabbits with T1DM. Beneficial effect of naltrexone on epithelial wound healing may be due to its ability to increase cell proliferation.
Epidermal growth factor (EGF) activates through its receptor (EGFR) prosurvival signaling pathways of the phosphatidylinositol-3-kinase (PI3K) - Akt kinase axis, and extracellular regulated kinase (ERK) (Xu et al. 2009; Xu and Yu, 2011; Funari et al. 2013; Winkler et al. 2014). These pathways appear to be the major regulators of corneal epithelial wound healing as shown in animal models and human organ-cultured corneas (Zieske et al. 2000; Nakamura et al. 2001; Xu et al. 2009; Xu and Yu 2011; Ljubimov and Saghizadeh 2015; Ljubimov 2017).
In the in vivo animal diabetic corneas and in human 3D organ cultures, phosphorylation/activation of EGFR and its downstream signaling mediators Akt and ERK is diminished (Xu et al. 2009; Saghizadeh et al. 2010a; Xu and Yu 2011). This reduction has functional consequences. Human ex vivo diabetic corneas overexpress matrix metalloproteinase-10 (MMP-10), cathepsin F and miR-146a (Saghizadeh et al. 2001; 2005; Funari et al. 2013). Using adenoviral vectors or direct transduction (miR-146a) these agents were introduced into normal organ-cultured human corneas, leading to slowing of wound healing and decreased expression of phospho-EGFR and phospho-Akt (Saghizadeh et al. 2010a; Funari et al. 2013). Conversely, inhibition of MMP-10, cathepsin F or miR-146a in diabetic organ-cultured human corneas caused an increase of phospho-EGFR and phospho-Akt with significant wound healing acceleration (Funari et al. 2013; Saghizadeh et al. 2013; 2014). Stem cell marker patterns in treated diabetic corneas were also normalized (Ljubimov, 2017; Kramerov et al. 2021). Reduced activity of the EGFR-Akt axis may thus be an important mechanism of abnormally slow diabetic corneal epithelial wound healing and stem cell dysfunction. Akt activation in animal diabetic corneas by SIRT1 upregulation or PTEN inhibition also accelerates epithelial wound healing (Wang et al. 2013; Li et al. 2020).
Hepatocyte growth factor (HGF) and its receptor c-Met play a role in cell migration, proliferation, and apoptosis, and are expressed in all major corneal cell types (Wilson et al. 1993; Kakazu et al. 2004; Saghizadeh et al. 2010b; 2011). Corneal wound healing is accompanied by HGF increase in 2D cultured animal keratocytes and epithelial cells (Li et al. 1996; Kakazu et al. 2008). In ex vivo human diabetic corneas, HGF expression is increased but c-Met expression is decreased, suggesting impaired HGF signaling (Saghizadeh et al. 2005). Adenoviral-driven c-Met upregulation in organ-cultured human diabetic corneas could normalize diabetic and stem cell marker patterns and accelerate epithelial wound healing, confirming functional significance of c-Met changes in DK. these effects were mediated by phosphorylation/activation of p38 mitogen-activated protein kinase (Saghizadeh et al. 2010b; 2011; Kramerov et al. 2016). Similar effects of c-Met on p38 kinase activation were observed in cultured rabbit and human corneal cells, and in rabbit corneal organ cultures (Sharma et al. 2003).
Insulin-like growth factor-1 (IGF-1) acts in corneal epithelium through receptors to both insulin and IGF. It can influence cell proliferation, migration, and survival (Lee et al. 2006; Stuard et al. 2020a). In the ex vivo human diabetic corneal epithelium, IGF-1 is significantly elevated (Saghizadeh et al. 2001b). Although in a T1DM rat model, IGF-1 was shown to stimulate diabetic epithelial wound healing synergistically with a neuropeptide substance P (Nakamura et al. 2003), its own action may be attenuated by its binding proteins (IGFBPs). In human diabetic tears and in 2D corneal epithelial cells cultured in high glucose, IGFBP3 levels are increased, which can block phosphorylation of IGF-1R and prevent its activation (Wu et al. 2012; Stuard et al. 2020b). Adenovirus-driven SIRT1 overexpression in mouse T1DM corneas and in 2D cultures of human corneal epithelial cells positively influenced epithelial wound healing by downregulating IGFBP3 (Wang et al. 2013). Thus, IGFBP3 upregulation in diabetes may interfere with normalizing effect of increased IGF-1 on the diabetic corneal epithelial wound healing.
Several other less well studied factors are altered in the diabetic corneas, which may underlie epithelial wound healing abnormalities. A peptide thymosin β4 (Tβ4) is an actin-sequestering protein (Dedova et al. 2006) but can also regulate cell migration, angiogenesis, tissue regeneration and inflammation. Tβ4 can block tumor necrosis factor-α (TNF-α) induced inflammation and NF-κB activation (Sosne et al. 2010). It can promote corneal epithelial wound healing but is decreased in human diabetic corneas (Saghizadeh et al. 2005; Sosne 2018), which may contribute to slow wound closure.
Nerve growth factor (NGF) can accelerate diabetic corneal epithelial wound healing in mouse model of T2DM (Muangman et al. 2004) and reduce apoptosis in a rat model of T1DM (Park et al. 2016). However, whether its level or activity are changed in diabetic corneas, remains to be established.
Transforming growth factor (TGF)-β3 antifibrotic isoform is downregulated in healing diabetic corneal epithelium. Treatment of T1D diabetic rats with TGF-β3 improved wound healing through signaling via SMAD, PI3K-Akt and Serpine1 (Bettahi et al. 2014).
Changes in the levels of epithelial microRNAs (miRs), potent epigenetic regulators, have also been described in human diabetic ex vivo and organ-cultured corneas (Funari et al. 2013; Kulkarni et al. 2017). MiR-146a and miR-424 suppressed epithelial wound healing in 2D cultured human limbal epithelial cells and in organ-cultured corneas, and their specific inhibitors (antagomirs) expectedly accelerated healing (Funari et al. 2013; Winkler et al. 2014). This effect could be attributed to the inhibitory action of miR-146a on EGFR that was counteracted by the antagomir (Winkler et al. 2014). In the T1DM Akita mouse corneas and in 2D mouse limbal epithelial cell line grown in high glucose, an increase in miR-204–5p, an inhibitor of SIRT1, was observed. Concomitantly, SIRT1 was reduced in high glucose-treated cells. Subconjunctival injection of miR-204–5p antagomir in Akita mice upregulated SIRT1 and promoted wound healing (Gao et al. 2015). This could be due to SIRT1-induced downregulation of IGFBP3 that attenuates IGF-1 signaling (Wang et al. 2013). Overall, miR changes in diabetic corneas provide another important mechanism underlying epithelial abnormalities in DK.
Altered cell-cell interactions may also contribute to DK abnormalities. It was recently shown that extracellular vesicles from human normal but not diabetic 2D cultured limbal keratocytes can stimulate cultured epithelial cell migration and proliferation, as well as normal organ-cultured corneal epithelial wound healing and stem cell marker expression (Leszczynska et al. 2018).
Advanced glycation end products (AGEs) are formed through non-enzymatic protein glycosylation (glycation) and accumulate due to hyperglycemia in rat, monkey and human diabetic tissues including corneal cells and extracellular matrix (Zou et al. 2012; Madonna et al. 2017; del Buey et al. 2019). AGEs retard epithelial wound healing and cause apoptosis as shown using human 2D cultured immortalized corneal epithelial cells. This effect is dependent on AGE receptors and reactive oxygen species generated by activated NADPH oxidase (Shi et al. 2013a; 2013b). AGE accumulation in the human diabetic cornea could reduce epithelial and keratocyte attachment to the extracellular matrix, such as glycated type I collagen, fibronectin, and laminin (McDermott et al. 2003). They may also contribute to increased stromal collagen crosslinking and rigidity of diabetic corneas (del Buey et al. 2019).
2.1.1.2. Corneal Nerve Abnormalities (Neuropathy)
Systemic neuropathy is a hallmark of long-term DM of both types. Diabetic corneal neuropathy (DCN) was recognized over 40 years ago. Its primary clinical manifestation is reduction of corneal sensation (Bikbova et al. 2018; Mansoor et al. 2020). DCN and corneal nerve damage are observed early in human diabetes and even in pre-diabetes (Zhivov et al. 2013; Papanas and Ziegler, 2013; Petropoulos et al. 2015; Szalai et al. 2016; De Clerck et al. 2020). The severity of this pathological trait positively correlates with disease duration and DM stage, and is important for non-invasive diagnostics (Rosenberg et al. 2000; Saito et al. 2003; Cousen et al. 2007; Tavakoli et al. 2007; De Cillà et al. 2009; Zhivov et al. 2013; Cruzat et al. 2017; Pellegrini et al. 2020; Roszkowska et al. 2020; Salami et al. 2020). Loss of corneal sensation is thought to be the consequence of damage of corneal nerves, including reduced nerve fiber density and length (mostly in the inferior whorl), increased nerve tortuosity and thickness (Ljubimov 2017; Bikbova et al. 2018; Ferdousi et al. 2020). Most of the alterations concern the sub-basal (subepithelial) nerve plexus (Figure 2). This proximity and the insertion of nerve fiber terminals into the epithelial cell layer (Stepp et al. 2017) could explain the correlation between DK and DCN (De Cillà et al. 2009; He and Bazan 2012; Wang et al. 2012; Zhivov et al. 2013; Cai et al. 2014; Davidson et al. 2014; Stem et al. 2014). The subbasal corneal nerve reduction seen in human and animal diabetes alike correlates with alterations of dendritic cells that have neurotrophic functions (Leppin et al. 2014; Gao et al. 2016). The regeneration of subbasal nerves upon corneal epithelial wounding is significantly slower in diabetic animals (Wang et al. 2012; Gao et al. 2016). Although several factors have been implicated in the development of DCN following hyperglycemia including AGE that could reduce NGF and sphingolipids, oxidative stress, altered growth factors and signaling pathways, the mechanisms of diabetic neuropathy in general and DCN in particular still remain unclear (Markoulli et al. 2018; Barsegian et al. 2019).
Figure 2.

In-vivo confocal images of the subbasal nerve plexus of the (a) non-diabetic, (b) Type 1 diabetic and (c) Type 2 diabetic individuals. In both Type 1 and Type 2 diabetic patients, the corneal nerve fiber density, nerve fiber length, and total branch density are decreased compared to non-diabetic subjects. The nerves are more tortuous in patients with DM compared to controls.
Reproduced from: Mansoor, H., Tan, H.C., Lin, M.T., Mehta, J.S., Liu, Y.C., 2020. Diabetic corneal neuropathy. J. Clin. Med. 9, E3956.
2.1.1.3. Corneal Stromal Changes
Corneal stroma is also changed in DM. Deposition of abnormal collagen fibril bundles was observed in diabetic humans and monkeys (Rehany et al. 2000b; Zou et al. 2012), and keratocyte density appears to be reduced (Kalteniece et al. 2018). AGEs accumulate in the human diabetic corneal stroma leading to collagen crosslinking that could result in increased central corneal thickness and higher rigidity (Sady et al. 1995; del Buey et al. 2019). In diabetic rats, stromal edema was also observed (Gül et al. 2008). Two keratocyte MMPs, MMP-3 and MMP-10, are upregulated in the stroma of ex vivo human diabetic corneas and may contribute to its altered structure (Saghizadeh et al. 2001a). Diabetic corneal stroma also shows significant metabolic and lipidomic changes (Priyadarsini et al. 2016). It may be suggested that stromal alterations reflect some degenerative changes due to ECM remodeling by MMPs and collagen cross-linking due to AGE accumulation.
2.1.1.4. Corneal Endothelial Abnormalities
Endothelial signs of diabetic corneal disease comprise increased cell pleomorphism (variation of cell shape) and polymegethism (variability of the cell area/size) (Shenoy et al. 2009; Módis et al. 2010; El-Agamy and Alsubaie 2017). A number of reports indicate decreased endothelial cell density in T1DM and T2DM patients that is more pronounced in patients with DR (Shenoy et al. 2009; Liaboe et al. 2017; El-Agamy and Alsubaie 2017; Çolak et al. 2020; Durukan 2020; Goldstein et al. 2020). Descemet’s membrane, the BM of corneal endothelium is also altered in DM. It contains abnormal wide-spaced collagen bundles that may result from excessive collagen glycation (Rehany et al. 2000a; 2000b; Akimoto et al. 2008). Diabetic changes in endothelial function remain unclear, although they might contribute to increased corneal thickness.
2.1.1.5. Conjunctival Involvement
Diabetic patients also present with conjunctival alterations including vascular dilation and reduction of capillaries with increased tortuosity, uneven vessel distribution, and decrease in goblet cell numbers (Cheung et al. 2001; Yoon et al. 2004; Owen et al. 2005; To et al. 2011; Gunay et al. 2016). Elevated proinflammatory cytokines are observed in patients with dry eye (Zhang et al. 2016). Conjunctival microbiome is also abnormal in diabetic patients; if treatment is needed it should involve vancomycin or other broad-spectrum antibiotics (Martins et al. 2004; Bilen et al. 2007).
2.1.1.6. Tear Film Changes
Tear film is mainly produced by the lacrimal gland. In DM, this gland suffers from inflammation, oxidative stress and AGE accumulation, resulting in reduced tear secretion with more frequent dry eye (Cousen et al. 2007; Manaviat et al. 2008; Beckman 2014). Decreased tear film stability in diabetic patients correlates with DCN, poor glycemic control, and reduced density of mucin-secreting conjunctival goblet cells (Dogru et al. 2001; Yoon et al. 2004). The severity of keratoconjunctivitis sicca correlates with the severity of diabetic retinopathy (Manaviat et al. 2008; Lv et al. 2014). Diabetic tear film alterations could also contribute to DK (Liu et al. 2015).
2.1.1.7. Biomechanical Abnormalities
Corneal biomechanics changes in diabetic patients have been documented. They concern corneal resistance factor (CRF) related to the tissue elasticity and corneal hysteresis (CH) as indicator of viscosity and biomechanical integrity (del Buey et al. 2019; Ramm et al. 2020; Wang et al. 2020). Diabetic corneas tend to have increased thickness and rigidity. Most recent studies agree that the human diabetic corneas have increased CH and CRF (Kotecha et al. 2010; Scheler et al. 2012; del Buey et al. 2019; Ramm et al. 2020; Wang et al. 2020). These biomechanical parameters correlate with patients’ Hb1Ac levels. Further studies are needed to understand the pathophysiological role of changed biomechanics in corneal diabetic disease.
Interestingly, increased viscosity and rigidity of the human diabetic cornea may be protective against keratoconus. Most of the available data suggest an inverse association of keratoconus development with DM (Seiler et al. 2000, Naderan et al. 2014; Woodward et al. 2016; McKay et al. 2019; Welchel et al. 2019), although one study reported positive association between T2DM and the presence and severity of keratoconus (Kosker et al. 2014). Negative association of keratoconus with DM is attributed to AGE-related accumulating collagen crosslinks in the diabetic corneal stroma mitigating its thinning (McKay et al. 2019; Welchel et al. 2019).
2.1.2. Surgical Complications with Diabetic Corneas
Structural and functional alterations in diabetic corneas pose a potential risk of complications upon eye surgery, mostly related to cataract and vitreoretinal surgery, and corneal or Descemet’s transplantation. Diabetic patients account for the majority of cases of corneal surgical complications with these methods (Bikbova et al. 2012; Vieira-Potter et al. 2016; Ljubimov 2017; Goldstein et al. 2020; Priyadarsini et al. 2020). Cases of DK development after ocular surgery have also been described (Sakamoto et al. 2004; Chen et al. 2009). There are data showing that contact lens wear also bears risks of corneal epithelial damage for diabetic patients, and eye practitioners should be aware of this (Bussan and Robertson 2019). Epithelial debridement before vitrectomy or retinal photocoagulation may result in abnormally slow recovery in corneal sensation (Chen et al. 2009; Mahgoub and Macky 2014). Cataract surgery in diabetic patients entails increased postoperative endothelial cell loss and increased corneal thickness with edema (Hugod et al. 2011; Yang et al. 2011; Dhasmana et al. 2014; Tsaousis et al. 2015; He et al. 2017; Elmekawey et al. 2020). Refractive corneal surgery may also be riskier in diabetic patients because of various DK-associated abnormalities and increased possibility of infections (Fraunfelder and Rich 2002; Jabbur et al. 2004). At the same time, eye surgeries may be safe in well-controlled DM (Cobo-Soriano et al. 2006; Goldstein et al. 2020; Labetoulle et al. 2020), although some authors call for caution in performing refractive surgeries in all diabetics (Mohammadpour 2007).
Human studies have also found that DM of corneal tissue donor was associated with increased chances of adverse effects (lower endothelial count, and graft dislocation and survival) on Descemet’s stripping automated endothelial keratoplasty (DSAEK) and Descemet’s membrane endothelial keratoplasty (DMEK). Additionally, the adhesion of Descemet’s membrane to the stroma is higher in diabetic corneas resulting in problems with graft preparation for DMEK (Greiner et al. 2014; Goldstein et al. 2020). It should be noted that DM donors may comprise up to one fourth of all corneal donors in the United States. This problem has been discussed in-depth in a recent review (Goldstein et al. 2020). Good glycemic control of the corneal donor and early DM stage appear to alleviate risks of graft rejection or further complications due to diabetic endothelial problems. However, late-stage DM may present significant risk to the recipient and such corneas should not be used (Lass et al. 2019; Goldstein et al. 2020).
2.1.3. Emerging Treatments
Diabetic complications are stable and once manifested do not usually recede with tight glucose control or available treatments. This may be due to long lasting epigenetic modifications (including DNA methylation and histone acetylation) that alter gene expression and are thought to be important in diabetic disease (Kowluru and Mohammad 2020). Currently, the treatment of DK and DCN remains symptomatic (Abdelkader et al. 2011; Priyadarsini et al. 2020). New experimental therapeutics have recently emerged, although most of them have been tested only in various in vitro and in vivo experimental models. Some of these promising treatments are discussed below.
2.1.3.1. Insulin
Insulin is a standard agent for DM treatment and has benefits for diabetic eye disease. Local insulin implants were shown to accelerate corneal wound healing in T1DM rats (Klocek et al. 2007). In T1DM mice, topical insulin could also prevent subbasal corneal nerve loss (Chen et al. 2013) and accelerate epithelial wound healing and corneal nerve repair after wounding by activating canonical Wnt signaling (Yang et al. 2020). Insulin eye drops significantly promoted corneal re-epithelialization after epithelial debridement for vitreoretinal surgeries in diabetic patients (Bastion and Ling 2013). Insulin in the diabetic cornea may improve cell proliferation and migration after wounding (Stuard et al. 2020a). However, accumulated diabetic epigenetic changes might counteract insulin action on the cornea. For instance, in human organ-cultured corneas and 2D cultured limbal epithelial cells from long-term diabetic donors, epithelial wound healing and stem cell marker expression are still impaired, although high insulin concentration is present in the medium (Kabosova et al. 2003; Kramerov et al. 2015). Overall, more human data are needed before recommending using insulin to treat DK and/or DCN.
2.1.3.2. Naltrexone
Naltrexone, a potent inhibitor of the OGF-OGFR interaction, is FDA approved for clinical treatment of alcohol and opioid dependence. In T1D and T2D mice and rats, topical naltrexone can normalize corneal epithelial wound healing, tear secretion, and corneal sensitivity/nerve function (Zagon et al. 2014; 2020; Sassani et al. 2016). Unlike many other agents mediating cell migration, naltrexone acts by stimulating cell proliferation inhibited by diabetes-elevated OGF (Zagon et al. 2020). Clinical trials of topical naltrexone for diabetic corneal disease are currently underway (McLaughlin et al. 2020).
2.1.3.3. Other Pharmacological Agents
A small human study has evaluated Tβ4 for facilitating corneal wound healing in diabetics with neurotrophic keratopathy. Significant improvement of epithelial defects with no side effects was observed, which makes Tβ4 treatment promising for DK therapy (Sosne et al. 2016). Similar effects are seen with autologous serum eye drops (Schulze et al. 2006) that are becoming popular with eye practitioners, although it is unclear which serum components are the most effective. Antidiabetic drugs nateglinide and glibenclamide can reduce changes of the Descemet’s membrane in Goto-Kakizaki T2DM rats (Akimoto et al. 2008). Some other agents were shown to reduce symptoms of DK and DCN in animal models. 1,5-isoquinolinediol [poly(ADP-ribose) polymerase inhibitor] could promote epithelial healing and increase corneal sensitivity in T1D diabetic rats (Byun et al. 2015). Topical lacritin fused with elastin-like polypeptide-based nanoparticles accelerated corneal epithelial wound healing in T1D NOD diabetic mice (Wang et al. 2014).
In animal T1DM and T2DM mouse and rat models, a number of treatments were recently shown to aid in restoring sensory innervation with increased corneal sensitivity and prevention of nerve fiber loss, thus alleviating DCN. Some of them could also promote epithelial wound healing. These treatments include diabetes-downregulated ciliary neurotrophic factor acting through STAT3 activation (Zhou et al. 2015; Guo et al. 2016), interleukin-1 (IL-1) receptor antagonist (Yan et al. 2016), substance P acting on neurokinin-1 receptor (Yang et al. 2014), curcumin in nanomicelles, pigment epithelium-derived factor, and resolvin D1 that can reduce reactive oxygen species (Guo et al. 2016; Zhang et al. 2018; Liu et al. 2020), omega-3 fatty acids (Coppey et al. 2020), mesencephalic astrocyte-derived neurotrophic factor that attenuates endoplasmic reticulum stress (Wang et al. 2020), neuropeptide VIP that activates Sonic Hedgehog signaling (Zhang et al. 2020), glycyrrhizin that attenuates the expression of inflammatory mediators and oxidative stress (Somayajulu et al. 2021), fenofibrate that restores PPARα downregulated in diabetes (Matlock et al. 2020), and leucine-rich α-2-glycoprotein-1 that activates JAK/STAT, TGF-β3 and EGFR-Akt signaling axes (Li et al. 2020). Topical NGF provided beneficial effects in T1DM and T2DM mouse corneas (Muangman et al. 2004; Park et al. 2016). Recombinant NGF (cenegermin, Oxervate™) is approved for neurotrophic keratitis treatment in the United States and Europe (Sheha et al. 2019). Given the promising animal data, cenegermin could be also beneficial for diabetic corneal disease but has not been used so far for this condition. Overall, the emerging treatments for DK and DCN need more efficacy and safety studies for possible clinical translation.
2.1.3.4. Gene and MicroRNA Therapy
Gene therapy approach is a powerful method to change the expression of a specific gene altered by the disease. In many gene therapy animal studies viruses are being used as delivery vehicles (Ljubimov and Saghizadeh 2015; Mohan et al. 2020). We have applied adenoviral (AV) gene therapy to normalize the expression of diabetes-altered genes c-Met, cathepsin F and MMP-10 in 3D organ-cultured human diabetic corneas and cultured limbal epithelial cells (Kramerov et al. 2016). Boosting the expression of c-Met and/or reducing cathepsin F and MMP-10 in diabetic corneas led to restoration of wound healing time and expression levels of diabetic and epithelial stem cell markers including laminins, nidogen-1 and integrin α3β1. Gene therapy effects were mediated by activation of EGFR-Akt axis and p38 kinase (Ljubimov 2017). Combination therapy was the most effective (Saghizadeh et al. 2014; Kramerov et al. 2016). Although efficient and safe in whole corneas, AV vectors showed marked toxicity in progenitor-enriched cultures of diabetic limbal epithelial cells (Kramerov et al. 2016; 2021). To circumvent this unwanted effect, we recently used nanoconstructs based on natural-derived and safe polymalic acid scaffold with attached antisense inhibitors to select genes. They exerted similar beneficial effects on diabetic corneas and cultured cells as AV gene therapy but completely lacked toxicity in a range of doses (Kramerov et al. 2021). This approach looks promising for future translation to the clinic.
Gene expression changes can be also achieved epigenetically using miRs. A number of miRs with altered expression in the human and mouse diabetic corneas have been described (Funari et al. 2013; Winkler et al. 2014; Hu et al. 2019; 2020). In the human corneas, miR-146a retarded epithelial wound healing by inhibiting EGFR. MiR-146a antagomir exerted an opposite effect (Funari et al. 2013; Winkler et al. 2014). Two other miRs, miR-34c and miR-181a, were elevated in trigeminal ganglia of T1DM mice. Subconjunctival injection of its antagomir accelerated corneal epithelial wound healing and promoted corneal nerve regeneration with stimulation of autophagy (Hu et al. 2019; 2020). It should be noted that miRs usually have more than one target even in the same cell, necessitating a careful validation of their use for therapy. Overall, current advances in the experimental use of gene therapy for corneal diabetes warrant its further development for clinical translation.
In summary, diabetic corneal disease presents as a serious but underestimated clinical problem that is important for millions of diabetic patients. The main concerns appear to be diabetic keratopathy and neuropathy. Whereas mechanisms of DK have been studied and a number of markers and affected signaling pathways described, pertinent studies of DCN are clearly lagging behind. The same concerns alterations of corneal stroma and endothelium. Currently known molecular signatures of this disease may be used as drug targets aimed at ameliorating corneal health. Experimental studies have provided promising drug candidates of different classes for treatment, although only insulin and naltrexone have been used so far in human patients. Future investigations should be aimed at developing combined therapies directed against both DK and DCN, and unraveling corneal molecular differences between diabetes types.
2.2. Graves’ Disease
Graves’ disease is an autoimmune endocrine disorder marked by hyperthyroidism and goiter, resulting in an enlarged thyroid gland. It can affect an individual of any age but is mostly seen in patients between the ages of 30–50 years. Weight loss, fatigue, heat intolerance, loss of appetite, tremor, and palpitations are the most common symptoms (Smith and Hegedüs 2016). Ocular abnormalities in Graves’ disease are common and often referred to as ophthalmic Graves’ disease, Graves’ orbitopathy, or thyroid eye disease (TED). It affects the orbit that causes eyelid retraction and proptosis (inability to close the eye), resulting in corneal exposure, which leads to redness, irritation, keratitis, dryness, and increased risk of infection (Sokol et al. 2010). Dry eye disease was reported in more than 66% of patients with moderate to severe TED (Kashkouli et al. 2018). A topographic analysis system revealed corneal changes in patients with Graves’ disease undergoing strabismus surgery (Kwitko et al. 1992). In another study, Karabulut et al. (2014) showed alterations in corneal biomechanical properties in patients with TED by decreasing corneal hysteresis, indicating a difference in structural and functional properties in the eyes of TED patients compared to control eyes. However, the central corneal thickness remains unchanged (Konuk et al. 2008). Microbial keratitis was also reported (Naik et al. 2019). Treatment with prednisone is prescribed to treat ocular inflammation (Prummel et al. 1989; Bartalena and Tanda 2009). Other systemic immunotherapies including rituxumab (anti-CD 20), TNF-α inhibitors, tocilizumab (anti-soluble IL-6 receptor) and cyclosporine (T lymphocyte inhibitor) have shown promising results (Strianese and Rossi 2019; Shin et al. 2009; Eid et al. 2020; Perez-Moreiras et al. 2019). Lubricating eye drops are also recommended for treatment of dry eye associated with Graves’ disease (Sokol et al. 2010).
2.3. Addison’s Disease
Addison’s disease, also known as primary adrenocortical insufficiency, is caused by infection (tuberculosis) or autoimmune reaction against the adrenal cortex. Symptoms include fatigue, nausea, weight loss, dizziness, and hyperpigmentation of the skin (Hellesen et al. 2018). Ocular manifestations are rare and include photophobia, ptosis, blepharitis and loss of eyelashes, keratoconjunctivitis, episcleritis, corneal ulcers, cataract, and papilloedema (Chopra et al. 2012). In two siblings with Addison’s disease, bilateral progressive vision loss and photophobia secondary to limbal stem cell deficiency (LSCD) were reported with diffuse corneal vascularization and delayed punctate fluorescein staining of corneal epithelium (Mohammadpour and Javadi 2006; Mohammadpour et al. 2006). Treatments involve hormone replacement therapy to restore the patient’s hormone levels, and corticosteroids to reduce inflammation (Hellesen et al. 2018).
2.4. Hyperparathyroidism
Hyperparathyroidism is caused by the abnormal secretion of the parathyroid hormone, resulting in hypercalcemia. It mainly affects postmenopausal women, but in 10–20% of the patients, it may be caused by an inherited parathyroid gland hyperfunction (Taniegra 2004). There are two forms of hyperparathyroidism, primary and secondary. Primary hyperparathyroidism develops from enlarged parathyroid glands, leading to hypercalcemia, whereas secondary hyperparathyroidism results from an underlying condition that causes hypocalcemia (Cordellat et al. 2012). Patients can present with skeletal, renal, gastrointestinal, ocular, cardiovascular, and neuromuscular manifestations associated with increased calcium and parathyroid hormone serum levels (Blackburn and Diamond 2007; Cordellat et al. 2012). A common cause of secondary hyperparathyroidism is chronic renal failure (Yuen et al. 2016).
In the cornea, hypercalcemia results in calcium deposition in the Bowman’s layer causing band keratopathy (Porter and Crombie 1973a; Golan et al. 1975; Petrohelos et al. 1977; Eom et al. 2013). The same manifestation occurs in chronic renal failure (Caldeira et al. 1970; Easterbrook and Mortimer 1970; Porter and Crombie 1973b; Klaassen-Broekema and van Bijsterveld 1993; Aktaş et al. 2007; Mullaem and Rosner 2012). Abeysiri and Sinha (2006) have reported in hyperparathyroidism an unusual pattern of Vogt white limbal girdle with adjacent flakelike subepithelial deposits of the conjunctiva, and peripheral white corneal deposits in the anterior stroma in a patient. Corneal endothelial changes in chronic renal failure, such as polymegethism and pleomorphism were also noted (Ohguro et al. 1999). Additionally, in an ovariectomized rat model, hypercalcemia significantly delayed corneal epithelial wound healing (Nagai et al. 2015). Primary hyperparathyroidism patients also show increased central corneal thickness and intraocular pressure (Baser et al. 2016; Sati et al. 2016). Treatments for hyperparathyroidism aim to control parathyroid hormone levels and consequently calcium levels, that can reduce corneal symptoms. When appropriate, surgery is recommended to remove the hyperfunctioning parathyroid tissue (Silva et al. 2018). Pharmacological options such as cinacalcet, a calcimimetic agent that binds to the calcium-sensing receptor to reduce serum calcium levels, has also been successfully used (Peacock et al. 2005).
3. Infectious Diseases
3.1. Viral Infections
3.1.1. Coronavirus Disease 2019 (COVID-19)
The severe acute respiratory syndrome caused by coronavirus-2 (SARS-CoV-2), a highly contagious new coronavirus, is responsible for coronavirus disease 2019 (COVID-19) pandemic which poses unprecedent challenges to the modern global health care system. Significant efforts were focused in most countries on lowering the community spread of SARS-CoV-2, which includes social distancing and the use of face masks. Besides the inhalation of aerosols from asymptomatic or COVID-19 patients, SARS-CoV-2 can gain entry via the mucosal surfaces present in the eye (Arora et al. 2020; Li et al. 2020) suggesting that the eye may be an important portal for SARS-CoV-2 entry and the manifestation of COVID-19. Therefore, eye protection (e.g., goggles or face shields) is strongly recommended for health care workers to reduce the risk of contracting COVID-19 (Chu et al. 2020). Importantly, better understanding of viral transmission and its pathogenesis at the ocular surface could help in the development of preventative and therapeutic measures to combat COVID-19.
The involvement of the eye in the transmission of infectious diseases has been documented since the 19th century (Maxcy 1919). Some recent studies have reported the presence of SARS-CoV-2 RNA in tears and conjunctiva of COVID-19 patients, as well as the expression of viral entry receptors, angiotensin-converting-enzyme-2 (ACE2) and TMPRSS2, in the cornea and conjunctiva (Zhou et al. 2020a; Sirakaya et al. 2020; Aiello et al. 2020; Roehrich et al. 2020; Guemes-Villahoz et al. 2020). However, the ocular surface involvement in SARS-CoV-2 transmission, either as a reservoir or as a target organ, is still unclear. Whereas anatomical and physiological characteristics render the eye a gateway for virus transmission to extraocular sites such as lungs (Belser et al. 2020), current evidence suggesting that the ocular surface is a possible entry route for the virus to cause COVID-19 remains scarce.
One report on various routes of SARS-CoV-2 entry in Rhesus monkey showed the presence of virus in the nasolacrimal and respiratory systems upon conjunctival inoculation (Makovoz et al. 2020). SARS-CoV-2 can also infect human ocular cells and eye organoids (Deng et al. 2020). Whereas these studies imply that SARS-CoV-2 can gain access to lungs and other tissues via ocular route, more studies are required to prove it unequivocally.
COVID-19 has increased severity and mortality in patients with some comorbidities, including type 2 diabetes, which is commonly associated with obesity (Belančić et al. 2020; Noor and Islam 2020; Wang and Meng 2020). The diabetic cornea presents with abnormalities that could facilitate viral infection including the breakdown of barrier function and tear film, as well as impaired wound healing and stem cell functions (Lee et al. 2019; Ljubimov 2017; Saghizadeh et al. 2017). Diabetic patients have significantly higher incidence of viral conjunctivitis (Ansari et al. 2017). Therefore, the abnormal diabetic ocular surface may present an easier entry site for SARS-CoV-2 than a non-diabetic one. Also, ocular transmission of SARS-CoV-2 can occur due its ability to infect and proliferate in ocular surface cells while DM may increase this response, in part, by modulating innate antiviral signaling (Graham et al. 2008; Lokugamage et al. 2020).
Studies have shown that SARS-CoV-2 may cause conjunctivitis and viral RNA has been detected in tears of COVID-19 patients (Wu et al. 2020; Xia et al. 2020; Colavita et al. 2020; Guan et al. 2020; Chen et al. 2020; Zhou et al. 2020b; Seah et al. 2020). Recent study by Sawant et al. (2020) showed a notable (13%) prevalence of SARS-CoV-2 in corneal and conjunctival tissues from COVID-19 donors (Figure 3).
Figure 3.

SARS-CoV-2 Spike (S) protein was detected in the corneal epithelium of the COVID-19 donors that were procured without any PVP-I disinfection treatment. OD (right) corneas from healthy and COVID-19 donors were fixed in formaldehyde and 10 μm thin sections were stained for IHC using antibody against SARS-CoV-2 Spike (S) protein (red color) while DAPI was used for nuclear staining (blue color). The image was captured at different magnifications (10X, 20X, and 60X) to visualize cellular location of the viral proteins. The region of interest has been highlighted using a yellow box and white arrows. E, corneal epithelium; S, corneal stroma. Sections stained with secondary antibody (anti-mouse Alexa Fluor 594) was used to assess the antibody specificity.
Reproduced with permission from: Sawant, O.B., Singh, S., Wright, R.E., Jones, K.M., Titus, M.S., Dennis, E., Hicks, E., Majmudar, P.A., Kumar, A., Mian, S.I., 2020. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul. Surf. S1542-0124(20)30168-3. doi: 10.1016/j.jtos.2020.11.002.
Although these studies indicate the possibility that ocular surface cells can be infected by SARS-CoV-2, the presence of viral RNA does not equate live viral infection, and to prove that, one needs to show viral antigens in the ocular tissue of COVID-19 patients (Sawant et al. 2020; Figure 3). Recently, the nucleocapsid protein antigen of SARS-CoV-2 was detected on the cells of the conjunctiva, trabecular, and iris of the patient infected with COVID-19 but not in the control participant (Yan et al. 2020).
Although published studies (Wang and Meng 2020; Makovoz et al. 2020; Wu et al. 2020; Xia et al. 2020; Colavita et al. 2020; Guan et al. 2020; Chen et al. 2020; Zhou et al. 2020b; Seah et al. 2020) indicate the possibility that SARS-CoV-2 could infect and replicate in corneal epithelial cells, some important aspects of virus-corneal cell interactions require thorough examination. These may include cytopathic effects of SARS-Cov-2 in corneal cells, or innate signaling pathways activated in infected cornea as compared to lung. Further studies would allow to examine specific interactions of SARS-CoV-2 with corneal epithelial cells, and induction of corneal innate antiviral mechanisms in COVID-19.
There is mounting evidence of the virus presence in ocular secretions and corneal cells of affected patients (Amesty et al. 2020; Ho et al. 2020; Sawant et al. 2020). The ocular surface tropism of SARS-CoV-2 and its potential to cause localized ocular disease should be considered as an opportunity for early diagnostics but also as a problem for corneal transplantation. The precise pathophysiological mechanisms of SARS-Cov-2 ocular infection and transmission remain to be elucidated.
3.1.2. Herpes Simplex Keratitis
Corneal herpetic keratitis results from viral infection most often caused by herpes simplex virus 1 (HSV-1). Although HSV-2 can also infect the eye, more than 90% of HSV ocular infections are caused by HSV-1. HSV-1 is known to be the leading infectious cause of corneal blindness and ulcers worldwide due to its facilitated acquisition via airborne droplet transmission (Liesegang 2001). Approximately 30,000 people in the United States suffer from recurrent ocular HSV infections annually, and, although medications exist to treat these infections, severe recurrent cases may require corneal transplants (Hill 1987; Liesegang 1999; 2001; Rajasagi and Rouse 2018). The most common contributing factor of HSV-1 ocular infection is inflammation of the ocular surface including the cornea (Figure 4), sclera, and conjunctiva (Chang et al. 2000). Additionally, immune system compromise as seen in HIV-infected people can significantly increase the risk of developing ocular HSV symptoms (Sobol et al. 2016). HSV-1-induced corneal scarring, also known as herpes stromal keratitis (HSK), can lead to blindness.
Figure 4.
Depiction of advanced herpetic stromal keratitis that has caused significant corneal opacity and neovascularization.
Reproduced with permission from: Rowe, A.M., St Leger, A.J., Jeon, S., Dhaliwal, D.K., Knickelbein, J.E., Hendricks, R.L., 2013. Herpes keratitis. Prog. Retin. Eye. Res. 32, 88–101.
Although a strong immune response is elicited following HSV-1 ocular infection, the virus has acquired several mechanisms to evade immune clearance (Kurt-Jones et al. 2017; Matundan et al. 2019; Tormanen et al. 2020). As a consequence, after an initial eye infection, HSV-1 travels to the sensory nerve ganglia, where it establishes a latent state and from where it can periodically reactivate and cause recurrent infections (Rock et al. 1987; Wechsler et al. 1988; Nicoll et al. 2012). The probability of recurrence increases after each reactivation event. These recurrent infections and prolonged inflammatory response after viral clearance lead to corneal scarring, opacification, neovascularization and loss of visual acuity (Lobo et al. 2020; Farooq and Shukla, 2012).
It is well established that corneal scarring is the result of inflammatory response to the pathogen (Brandt 2005; Koelle and Ghiasi 2005; Matundan et al. 2019; Lobo et al. 2020; Tormanen et al. 2020). Several attempts have been made to treat corneal HSV-1 using immune pathway to regulate the affect of stromal keratitis. Both lymphotoxin-α and -β are proinflammatory cytokines detectable up to 48 hours post viral infection in vitro and play a role in the induction of chemokines related to cornea-infiltrating proinflammatory cells. By using anti-lymphotoxins, stromal keratitis may be mitigated (Veiga-Parga et al. 2013). However, lymphotoxin-α knockout mice had increased corneal scarring, latency and mortality in response to HSV-1 ocular infection (Wang et al. 2019). Increased corneal opacity is mediated by IL-17, which is produced by CD4+ T-cells. Using anti-IL-17 in recurrent HSV-1 mouse models, downregulation of TNF-α expression in recurrent corneas with decreased herpetic stromal keratitis has been reported (Xia et al. 2012).
Typical treatment for HSV-1 ocular infections includes antiviral acyclovir and topical corticosteroids to suppress inflammation (Koelle and Ghiasi 2005). Most recently, topical treatments such as 2% cyclosporine-A and 1% prednisolone acetate eye drops have been found to improve corneal opacity from herpetic stromal keratitis (Peyman et al. 2018). Oral antivirals, e.g., acyclovir, are beneficial as prophylactic treatment reducing ocular recurrences (Young et al. 2010). Kim et al. (2018) described a novel treatment in which administration of prophylactic oral acyclovir and ascorbic acid has shown to reduce and prevent the recurrence of corneal epithelial herpetic keratitis. As many of these treatments are still being investigated, more work is needed in order to develop effective ways to treat and especially prevent ocular manifestations of HSV-1.
3.1.3. Shingles Caused by Varicella Zoster
A DNA virus Varicella zoster (chickenpox, herpesvirus type 3, VZV) causes shingles, or Herpes zoster, an inflammatory viral disease presenting as maculopapular or vesicular rash as a result of reactivation of latent virus acquired in childhood in the sensory nerve ganglia (Kalogeropoulos et al. 2015; Minor and Payne 2020). T-cell mediated responses are crucial for maintaining VZV in a latent state. Stress factors such a malignant disease, immunosuppressive treatments, HIV infection or even chemical or physical stressors may act as triggers for VZV reactivation (Kalogeropoulos et al. 2015; Depledge et al. 2018). When the latent virus becomes reactivated in ophthalmic region of trigeminal cranial nerve (V), it causes herpes zoster ophthalmicus (HZO). HZO represents 10–20% of all VZV cases. Ocular manifestations include common keratitis, uveitis, iritis, conjunctivitis, episcleritis, as well as rare retinal necrosis (Kalogeropoulos et al. 2015; Vrcek et al. 2017; Minor and Payne, 2020). The most severe eye-threatening complications of HZO are pan-uveitis and retinal necrosis (although it is rare). Studies have found that the amount of viral load in the aqueous humor of VZV patients is correlated to the manifestation and severity of anterior uveitis (Kido et al. 2008; Kalogeropoulos et al. 2015). Intravenous antiviral treatments, like acyclovir, may be recommended for 48 to 72 hours to reduce complications and decrease pain in severe cases, and oral antivirals may be taken if there are no complications (Kedar et al. 2019). Two vaccines Shingrix® (recombinant glycoprotein E; for adults over 50) and Zostavax® (live attenuated VZV) are in use in the United States. Centers for Disease Control guidelines state that Shingrix® is preferred because of higher efficacy (Lal et al. 2015; Warren-Gash et al. 2017; Kedar et al. 2019; Sullivan et al. 2019).
3.1.4. Human T-Cell Leukemia Virus HTLV-1
Human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus that propagates using its encoded reverse transcriptase to generate provirus DNA from viral RNA, which then is integrated into the host genome and primarily affects CD4+ T-cells (Terada et al. 2017). Aside from being known to cause adult T-cell leukemia, a neurological disorder tropical spastic paraparesis (TSP) and HTLV-1-associated myelopathy, HTLV-1-associated uveitis has been known to promote ocular complications, such as keratoconjunctivitis sicca, and interstitial keratitis. Patients with HTLV-1 present corneal abnormalities, such as corneal haze and opacities, thinning and scarring of the peripheral cornea, keratopathy, and neovascularization (Buggage et al. 2001). Treatments for HTLV-1-assocaited uveitis may include intraocular corticosteroids (Kamoi and Mochizuki 2012).
3.1.5. Epstein-Barr Virus
Epstein-Barr virus (EBV) is a ubiquitous human herpes virus type 4 infecting more than 90% of the adult population involving B lymphocytes and epithelial abnormalities (Nowalk and Green 2016). It is a common cause of infectious mononucleosis. Several ocular symptoms such as keratitis, uveitis, granulomatous conjunctivitis, choroiditis, retinitis, and papillitis are linked to EBV infections (Slobod et al. 2000). In the cornea, stromal keratitis related to EBV infection with granular, ring-shaped opacities has been reported (Matoba 1990). Mononucleosis can also cause delayed onset bilateral peripheral interstitial keratitis (Iovieno et al. 2020). In vivo confocal microscopy revealed corneal endotheliitis in eyes infected with EBV; this is also observed in cases of cytomegalovirus (CMV) infection (Alfawaz et al. 2013; Peng et al. 2020). EBV infection in human corneal epithelial cell cultures caused epithelial-mesenchymal transition (EMT) via PI-3K/Akt/ERK activation induced by TGF-β1-dependent Syk and Src phosphorylation, which could lead to corneal fibrosis. Targeting these pathways may have therapeutic potential in treating EBV-induced EMT (Park et al. 2014). Due to its self-limiting nature, EBV treatment is mainly supportive. Antiviral drugs such as acyclovir, ganciclovir, cidofovir, and foscarnet, among others have been used to treat EBV and also CMV infection (Alfawaz et al. 2013; Yaro 2013). Acyclovir has shown significant reduction in EBV infected B lymphocytes, and systemic treatment has alleviated ocular symptoms (Rafailidis et al. 2010; Keorochana 2016). EBV-related inflammation can be treated with a corticosteroid regimen (Matoba 1990).
3.2. Bacterial Infections
3.2.1. Tuberculosis
Tuberculosis (TB), a communicable disease caused by Mycobacterium tuberculosis (Mtb), most commonly affects lungs. Nearly one-third of the world’s population is latently infected with TB, and more than nine million new cases are diagnosed each year, with 95% of infections in developing countries. When the airborne particles containing Mtb are inhaled, the bacteria reach the alveoli and are taken up by alveolar macrophages. Mtb can subvert antimicrobial macrophage machinery and avoid being degraded by phagolysosomes by blocking their maturation (Upadhyay et al. 2018). They also interfere with cellular trafficking and escape immune recognition (Zhai et al. 2019) allowing them to enter the lymphatic and circulatory system where they will travel systemically and affect multiple organs including the anterior and posterior segments of the eye. Corneal or anterior ocular manifestations of TB include lid vulgaris, conjunctivitis, scleritis, episcleritis, corneal phlycten, interstitial keratitis, and granulomatous uveitis (Oluleye 2013). Absence of Mtb in the lungs does not mean it cannot be found in the eye, as 60% of patients with extra-pulmonary manifestations of tuberculosis will not have the infection in their lungs (Alvarez and McCabe 1984).
Clinical case studies reported that chronic conjunctivitis caused by Mtb may present with approximately 2 × 2 mm gelatinous conjunctival lesions along the limbus, granulomas, nodule ulcerative lesions, or even subconjunctival nodular masses (Solmaz et al. 2018; Balyan et al. 2019; Chaurasia et al. 2019). Interstitial keratitis with corneal perforations has also been noted as a sign of systemic TB (Yangzes et al. 2019). Phlyctenular keratoconjunctivitis is a nodular inflammation of the limbus as an allergic response. According to a case study of 112 persons with this condition, in 86 patients (76.7%) it was associated with TB (Rohatgi and Dhaliwal 2000). These ocular manifestations of TB may lead to mild to pronounced vision loss depending on the severity. When anterior ocular manifestations of TB are diagnosed, treatment with isoniazid, rifampicin, pyrazinamide and ethambutol for a 6–9 months is recommended as a standard procedure (Figueira et al. 2017). In addition, corticosteroids are used along with anti-TB therapy to treat ocular complications. In severe cases, excision of nodule, granular, conjunctival, or lid masses may be necessary to prevent further ocular damage to avoid vision loss (Kee et al. 2016).
3.2.2. Syphilis
Syphilis is caused by Treponema pallidum, a spirochaete bacterium, and is commonly contracted via sexual interactions or congenitally from mother to child at birth, initially presenting itself in the form of painless sores and mild rashes. When left untreated for longer than 10 weeks, the bacterium will affect internal organs such as the eyes, brain, heart, nerves, bones, joints, and liver (Tudor et al. 2020). Syphilis can manifest on the ocular surface at any stage of the infection, anterior, intermediate, and posterior, with uveitis and syphilitic keratitis being the most common ocular manifestations. It may also cause interstitial keratitis, as well as chorioretinitis, retinitis, retinal vasculitis and cranial nerve and optic neuropathies (Barrow et al. 2020). Patients that are diagnosed with ocular syphilis are advised to get tested for human immunodeficiency virus (HIV) and neurosyphilis due to their similar risk factors and symptoms. Treatment for syphilis usually requires administration of antibiotics, most commonly parenteral penicillin G at any of the stages of bacterial infection (Kiss et al. 2005).
3.2.3. Pseudomonas Aeruginosa
Pseudomonas infection is caused by Pseudomonas aeruginosa, a Gram-negative aerobic rod-shaped bacterium, that is commonly found in water, soil, and plants (Norina and Raihan 2008). The bacterium generally does not cause infections in uncompromised individuals (Evans and Fleiszig 2013). In the healthy cornea, there are a number of defense mechanisms precluding infection that include antimicrobials such as β-defensins, cathelicidin LL-37, cytokeratin-derived antimicrobial peptides, and RNase7, and immunomodulators such as SP-D and ST2 Innate defenses of the cornea depend in part on MyD88, a key adaptor protein of toll-like receptors, and interpeukin-1R signaling (Evans and Fleiszig 2013). Severe infections with P. aeruginosa are typically seen in people who are immunocompromised or have preexisting conditions, such as cystic fibrosis and diabetes (Mulcahy et al. 2014). This bacterium is an important cause of hospital infections. P. aeruginosa is one of the most common cause of pneumonia in cystic fibrosis patients in their second and third decade of life (Burns et al. 2001) and wound infections in burn victims (Mayhall 2003). Infection can also lead to sepsis, ecthyma gangrenosum, osteomyelitis, otitis externa, urinary tract infections, and skin infections.
In the cornea, P. aeruginosa may cause contact lens-related ulcers and keratitis (Pinna et al. 2008). The bacteria produce reactive oxygen species, toxins and proteases, and can alter host immune responses (Duran and Refojo 1987; Kandasamy et al. 2010; Hilliam et al. 2020). Secreted proteases can cause corneal liquefactive necrosis leading to corneal damage and ulcer formation (Kreger and Gray 1978). The bacteria’s ability to form a biofilm allows irreversible surface adhesion. They adhere to contact lens surfaces more easily than many other pathogens (Marshall 1976). Treatment consists of antibiotics, most commonly fluoroquinolones and aminoglycosides. Emerging experimental therapies include cathelicidin peptides as adjuvants to vancomycin, topical flagellin (a ligand for toll-like receptor 5), and immunotherapeutics including monoclonal antibodies (Kumar et al. 2010; Mohammed et al. 2019; Hebert et al. 2020).
4. Autoimmune and Inflammatory Diseases
4.1. Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune disease resulting in a chronic and painful inflammatory response, primarily in the joints. Manifestations of RA that are non-articular are present in 10–20% of diagnosed patients. Systemic chronic inflammation in this disease may also cause inflammatory ocular diseases, leading to scleritis and episcleritis (Zlatanović et al. 2010; Sainz de la Maza et al. 2012). In a clinical correlational study, over 80% of RA participants with inflammatory ocular disease had scleritis, episcleritis, and peripheral ulcerative keratitis, and 62% had uveitis (Caimmi et al. 2018). RA may also result in secondary Sjögren’s syndrome, a severe ocular surface dryness due to desiccation or insufficient production of fluid or mucus in secretory glands as a result of the immune cell infiltration, which will negatively impact the eyes and ocular surface.
Therapy is still a major field of study as some mechanisms that cause RA are not fully understood. Rainsford et al. (2015) reviewed different treatments and pharmacological properties of hydroxychloroquine and chloroquine to aid in alleviating RA and related diseases. It was suggested that hydroxychloroquine and chloroquine are affecting MHC Class II expression and antigen presentation, and the production of pro-inflammatory cytokines like IL-1 and TNF-α, as well as controlling the generation of leucocyte reactive oxygen species. There are conclusive data supporting the efficacy and benefit of using systemic treatments in autoimmune disease to also resolve the ocular manifestations. Available treatments for RA include non-steroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying antirheumatic drugs (DMARD) including methotrexate, and various biologicals, such as TNF-α inhibitors, immunosuppressive drugs/antibodies inhibiting immune cell activity and pro-inflammatory interleukins (abatacept, rituximab, tocilizumab, sarilumab, cyclosporine A) and targeted synthetic DMARDs like Janus kinase inhibitors (Kaçmaz et al. 2009; Smolen et al. 2020). All of these treatments alleviate joint-related symptoms and consequentially may treat some ocular manifestations such as conjunctivitis, scleritis, episcleritis, keratitis and more (Burmester and Pope 2017).
4.2. Sjögren’s Syndrome
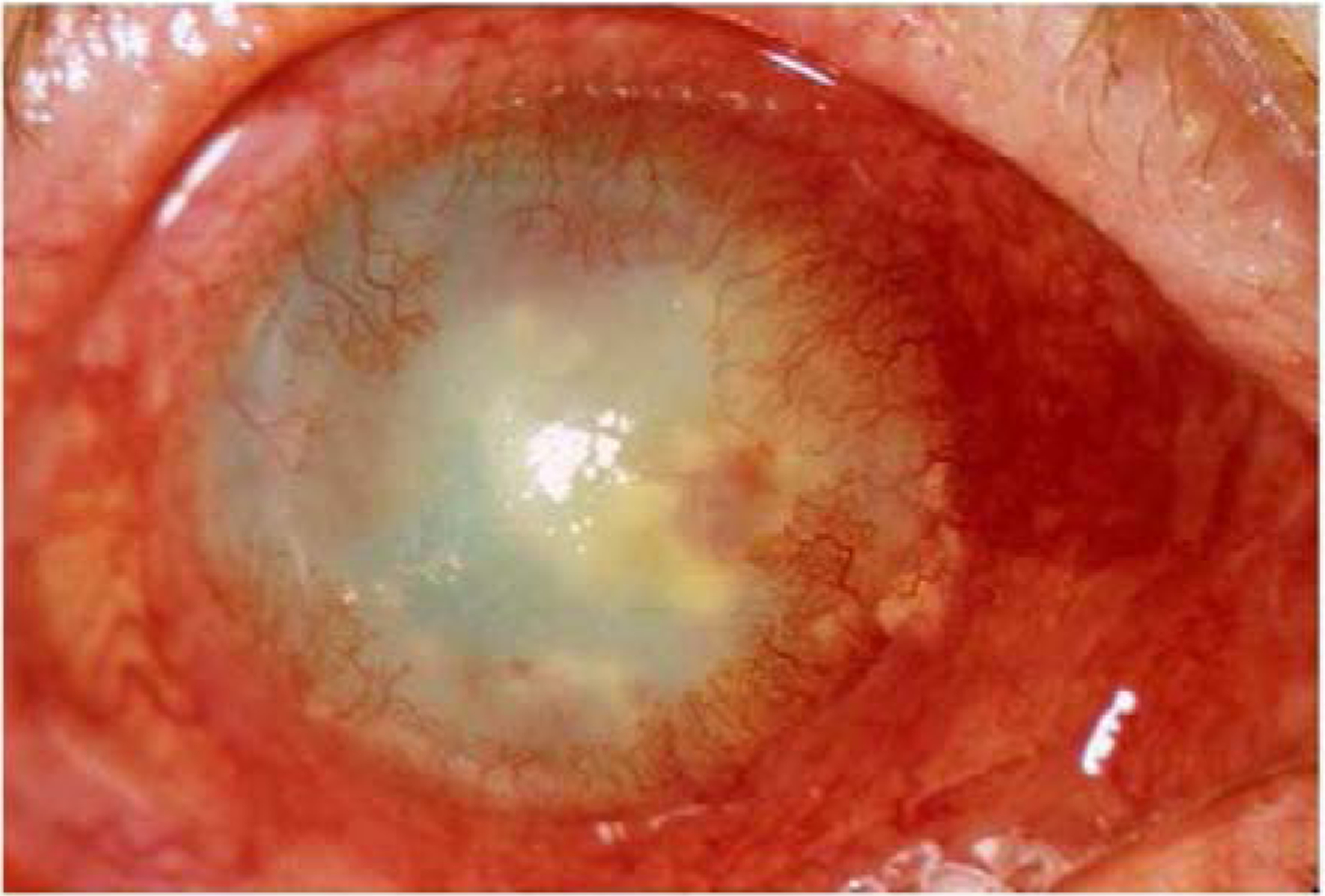
Sjögren’s syndrome is a rheumatoid autoimmune disease in which the salivary and lacrimal glands are compromised due to infiltration of immune cells including CD4+ helper T cells, CD8+ cytotoxic T cells, B cells, plasma cells, macrophages, dendritic and mast cells resulting in an immune-mediated secretory dysfunction (Rischmueller et al. 2016; Srivastava and Makarenkova 2020). This is a reaction of immune system to autoantigens and cytokines released by the epithelium of affected glands (Srivastava and Makarenkova 2020). This disease may be classified as primary if it occurs without any pre-existing rheumatoid disease, or secondary if the disease arises as a result of another rheumatoid disease such as RA, systemic lupus erythematosus, or scleroderma (Hernández-Molina et al. 2010). In Sjögren’s syndrome, the production of salivary, tear, and mucus secretions is inhibited, which detrimentally affects the ocular surface, because the dryness not only causes discomfort, but can also lead to corneal melt/perforation (Figure 5), uveitis, scleritis and more, due to the insufficient lubrication on the ocular surface promoting ulcers or inflammation, in addition to the internal ocular inflammatory response (Akpek et al. 2019).
Figure 5.

Corneal melt and perforation of patient diagnosed with Sjögren’s syndrome; image generated via slit lamp microscope.
Reproduced with permission from: Akpek, E.K., Bunya, V.Y., Saldanha, I.J., 2019. Sjögren’s syndrome: more than just dry eye. Cornea. 38, 658–661.
Evaluations to diagnose Sjögren’s syndrome based on ocular manifestations include Schirmer’s test which measures the amount of tear production in a period of time, serological testing for SS-A/Ro antibodies, and histological exam of a labial salivary gland biopsy (Stefanski et al. 2017). Despite many therapies available, there is yet to be a cure found for this syndrome, possibly due to the heterogeneity of the disease pathology (Srivastava and Makarenkova 2020). A number of treatments symptomatically address the dry eye disease including topical tear substitutes (e.g., amino acid enriched sodium hyaluronate, cyclosporine A, serum tear drops), corticosteroids, hydroxychloroquine, secretagogues, fatty acids, immunosuppressive agents, scleral contact lenses, occlusion of lacrimal puncta surgery, and tarsorrhaphy (Aragona et al. 2013; Valim et al. 2015; De Paiva et al. 2019; Ramos-Casals et al. 2020). More recently, emphasis was put on biological therapies that suppress T and B cell activation (e.g., lifitegrast, rituximab, abatacept and belimumab) and are also used to treat RA and lupus (Perez et al. 2016; Ramos-Casals et al. 2020). Life expectancy is not reduced by Sjögren’s syndrome; however, quality of life rapidly diminishes in patients who experience blurred vision, eye fatigue, and difficulty reading, in addition to all the other symptoms, regardless of having perfect visual acuity.
This autoimmune disease can also cause LSCD. Samoila and Gocan (2020) reviewed the clinical outcomes of transplanting cultivated allogeneic stem cells vs. oral mucosa epithelial cells in the total bilateral stem cells deficiency. Results suggest that autologous oral mucosa epithelial cells have some advantages over cultivated allogeneic limbal stem cells due to low or no risk of immune activation and no need to use immunosuppression. However, oral mucosa epithelial cells may entail a risk of persistent epithelial defects and graft failure, as compared to cultivated allogeneic limbal stem cells. Stem cell transplantation may be recommended in cases of severe disease with LSCD.
4.3. Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that causes inflammation of the joints, produces sensitive skin rashes and may even cause severe organ failure involving the kidneys, lungs, or damage to the central nervous system (Oates et al. 2003). SLE is a multi-factorial disease, which may develop from both endogenous genetic or immunoregulatory causes and exogenous acquired factors such as epigenetics, environmental and infectious diseases (Esen et al. 2012). Patients with SLE also have the risk of ocular manifestations such as disrupted mucocutaneous membranes and tissues, and of secondary diseases such as RA and Sjögren’s syndrome that lead to severe dry eye and can also cause retinal vascular and neuroophthalmic disease (Sivaraj et al. 2007). Ocular manifestations as a result of inflammatory responses include cataracts, keratoconjunctivitis sicca, glaucoma, discoid lesions of eyelids, episcleritis, scleritis, keratitis, and uveitis. Such ocular involvements were observed in 29 out of 98 SLE-diagnosed participants of a clinical study, and 20 out of those 29 had more than one ocular involvement (Dammacco et al. 2018).
Proinflammatory cytokines such as IL-17 and IL-23 are found in abundance in tear samples of patients with autoimmune diseases (Oh et al. 2011). This makes such genes a target for gene therapy since expression studies and clinical trials suggest that inhibition of IL-17 be a promising therapeutic approach for SLE (Koga et al. 2019). Research regarding significantly higher IFN-α activity in SLE-patients suggests that it is a heritable factor and may play a role in the development of the disease (Niewold et al. 2007; Lyn-Cook et al. 2014) Immunosuppressive treatments that target inflammation, which is the main cause of other manifestations at the ocular surface, include non-steroidal anti-inflammatory agents, anti-malarial drugs such as hydroxychloroquine, immunosuppressants like commonly used methotrexate and azathioprine, and biological drugs like belimumab and rituximab (Prados-Moreno et al. 2017; Felten et al. 2018; Plantone and Koudriavtseva 2018). These systemic SLE treatments will often have a beneficial effect on ocular manifestations of inflammation and secondary complications, but more investigations are needed to target the root causes of SLE.
4.4. Gout
Gout is a type of inflammatory arthritis that affects over nine million people in the United States, mostly middle-aged men and postmenopausal women (Singh et al. 2019). It is due to increased level of uric acid in the body that results in the accumulation of monosodium urate (MSU) crystals mainly in the joints. Subsequently, MSU crystals trigger an inflammatory cascade releasing pro-inflammatory cytokines, IL-1β, IL-6, and TNF-α (Dalbeth and Haskard 2005). Symptoms include pain, redness, warmth, swelling, and reduced range of motion in the affected joints. Gout is often associated with metabolic diseases such as obesity, hypertension, diabetes, and cardiovascular disease (Singh et al. 2011). Ocular abnormalities include gout keratitis in the cornea, scleritis, optic neuritis, glaucoma, and retinal hemorrhages and thrombosis (Sharon and Schlesinger 2016). Deposition of MSU crystals (tophi) have been reported in the cornea and conjunctiva in rare cases (Sarma et al. 2010; Lin et al. 2013; Lo, Broocker, and Grossniklaus 2015). Kösekahya et al. (2019) found that corneal endothelial function was impaired in gout patients, which was directly corelated to the duration of disease and uncontrolled uric acid levels. This may affect endothelial stability and increase corneal thickness by reducing Na,K-ATPase pump activity. Symptoms associated with gout can be treated with nonsteroidal anti-inflammatory drugs, oral and intravenous corticosteroids, colchicine, and diet modification by decreasing the consumption of foods high in purines (Hainer et al. 2014).
4.5. Atopic Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a non-infectious allergic inflammatory disease associated with atopic dermatitis. Environmental allergens contribute to immune system dysregulation with the involvement of mast cells, T-cells, eosinophils and basophils to cause atopic dermatitis, and subsequently, AKC. Typically, AKC starts in the early teens and can continue until the late 50s. It is marked by itching, redness, and burning of the eyes, eczema of the eyelids, blepharitis along the lid margin, conjunctival inflammation, excessive tear production, and corneal complications (Bielory and Bielory 2010; Chen et al. 2014). Both pro- and anti-inflammatory cytokines such as IFN-γ, TNF-α, IL-2, IL-4, and IL-10 have been reported to be elevated in the tears of AKC patients (Calder et al. 1999). Corneal complications arise from the exposure to inflammatory factors and lead to punctate keratitis and corneal erosions. More severe cases present with corneal ulcerations, edema, epithelial defects, neovascularization, and scarring that will eventually lead to blindness (Power et al. 1998; Messmer et al. 2002; Tanaka et al. 2004; Chen et al. 2013; Zemba et al. 2016; Jan et al. 2020). AKC patients show significantly lower basal epithelial cell density as compared to control eyes (Hu et al. 2008). Histopathologic investigations also revealed the presence of major basic protein, a cytotoxic eosinophil granule protein that impairs epithelial cell migration and proliferation, resulting in corneal erosions (Messmer et al. 2002; Utine et al. 2018).
Therapies for AKC are aimed at reducing eyelid, conjunctival, and corneal inflammation. Mast cell stabilizers, antihistamines, corticosteroids, and calcineurin inhibitors are the most commonly prescribed drugs for AKC. Mast cell stabilizers such as topical nedocromil sodium and sodium cromoglycate prevent the release of inflammatory mediators from mast cells and efficiently decrease inflammation but need re-application (Castillo et al. 2015). Antihistamines that block either or both H1 and H2 receptors are topically applied to reduce itching, redness, and eyelid swelling (Castillo et al. 2015; Ridolo et al. 2019). In severe cases of AKC with unsuccessful treatment with mast cell stabilizers and antihistamines, systemic and topical corticosteroids are recommended, but patients may develop serious side effects with long-term use such as increased risk of developing diabetes, glaucoma, and cataract (Jones and Rhee 2006; Penfornis and Kury-Paulin 2006; Pilkey et al. 2012; Rice et al. 2017; Kačmař and Cholevík 2019). To prevent such adverse lasting effects, immunosuppressive calcineurin inhibitors are considered. Ophthalmic suspension of 0.1% tacrolimus has been effective for the treatment of AKC in patients that do not respond to standard care (Yazu et al. 2019; Benaim et al. 2019). Studies have also demonstrated the effective use of topical 0.05% cyclosporin to reduce AKC-associated inflammation (Akpek et al. 2004).
4.6. Vernal Keratoconjunctivitis
Vernal keratoconjunctivitis (VKC) is a bilateral, chronic allergic inflammatory condition that affects the conjunctiva and the cornea during the warm seasons. Unlike AKC, disease onset is typically earlier in life between the ages of 8–12 years. Symptoms include palpebral and limbal conjunctivitis, itching, redness, photophobia, mucosal discharge along the palpebral conjunctiva, blurred vision, and foreign body sensation (Wong 1999). As with AKC, VKC is also caused by T cell-mediated hypersensitivity reaction resulting in hyperproduction of IgE and activation of eosinophils and mast cells (Leonardi 2013). Although conjunctival inflammation is the hallmark of VKC, visual loss occurs due to corneal complications often seen in the perennial form of the disease. Eosinophil secretions such as major basic protein and eosinophilic cationic protein (ECP), as well as other inflammatory cell secreted factors such as MMP-2, MMP-9, TGF-β1, FGF, and EGF cause corneal epithelial, BM, and stromal damage (Kumagai et al. 2006; Leonardi 2013; Feizi et al. 2020). 3–20% of VKC patients develop shield ulcers as a result of continuous untreated epithelial erosions and keratopathy that can further lead to plaque formation from protein and mucin secretions on the stroma (Cameron 1995; Arif et al. 2017). Other corneal manifestations include the more common keratoconus with corneal ectasia, corneal hydrops, and decreased corneal thickness observed in several VKC patients (Cameron et al. 1989; Totan et al. 2001; Feizi et al. 2020), and rare LSCD observed in 1.2% of cases (Sangwan et al. 2011). Patients with VKC showing a clinical evidence of LSCD revealed a presence of goblet cells in the cornea (Saboo et al. 2013).
Like in AKC, antihistamines such as H1 receptor blockers, and mast cell stabilizers are the first line of treatment for VKC (Leonardi 2013). In severe cases unresponsive to antihistamines, corticosteroids may be cautiously prescribed due to severe adverse effects including diabetes and infectious keratitis. Calcineurin inhibitors such as cyclosporine A and tacrolimus that block Th2 lymphocyte proliferation and IL-2 and IL-5 production have been used for the treatment of VKC with no serious side effects (Pucci et al. 2002; Keklikci et al. 2008; Leonardi 2013). Topical cyclosporine (0.05%) has been effectively used for the resolution of shield ulcers (Westland et al. 2018). In severe cases of vernal shield ulcers, surgical interventions have been considered. Complete debridement of shield ulcer and plaque with multilayered amniotic membrane transplantation have been successfully performed (Sharma et al. 2018). Cameron et al. (1995) reported the removal of central corneal lesions and inflammatory plaques by excimer laser phototherapeutic keratectomy (PTK), with rapid re-epithelialization. For VKC associated keratoconus, corneal cross-linking is effective (Abozaid et al. 2019; Alrobaian et al. 2019). Management and treatment of VKC was previously reviewed in more details (Leonardi 2013; Feizi et al. 2020).
4.7. Multiple Sclerosis
Multiple Sclerosis (MS) affects between 300,000 and 400,000 in The United States (Wallin et al. 2019). It is a demyelinating central nervous system disease and thought to be an autoimmune disorder (Graves and Balcer 2010). Alterations of vision are common in the affected individuals; about 50% of patients develop optic neuritis (Graves and Balcer 2010). This disorder mainly affects the retina and optic nerve but recently, corneal abnormalities have also been documented. Using in vivo corneal confocal microscopy, it was shown that MS results in significant reduction of corneal nerve fiber density, as well as branch density and length with axonal loss (Bitirgen et al. 2017; Mikolajczak et al. 2017; Petropoulos et al. 2017). There is also conflicting evidence about the changes in epithelial dendritic cell density (Bitirgen et al. 2017; Testa et al. 2020). These changes suggest corneal neuropathy development in MS, reminiscent of DCN. The mechanisms of these alterations as well as possible changes in corneal nerve myelination in MS remain to be established.
4.8. Granulomatosis with polyangiitis
Granulomatosis with polyangiitis is a rare, idiopathic, multisystem inflammatory disease characterized by necrotizing granulomatous inflammation and vasculitis. It mainly affects the upper and lower respiratory tract, lungs, and the kidney, but also the central nervous system, skin, joints, and the eye (Weeda and Coffey 2008). Ocular involvement is seen in 20–66% of patients with symptoms that include proptosis (it can provoke exposure keratopathy), conjunctivitis, scleritis, keratitis, dacryoadenitis, uveitis, optic nerve vasculitis, and retinal artery occlusion (Leavitt and Butrus 1991; Weeda and Coffey 2008; Almouhawis et al. 2013; Keorochana et al. 2017; Sfiniadaki et al. 2019). In the cornea, peripheral ulcerative keratitis (PUK) is a common feature of the disease. It is due to the presence of autoantibodies and inflammatory cells from limbal blood vessels (Messmer and Foster 1999; Ebrahimiadib et al. 2016; Lu et al. 2016; Cao et al. 2017). PUK is commonly unilateral but in up to 40% of patients may be bilateral and often associated with scleritis. Other corneal problems include peripheral keratitis without ulceration, peripheral corneal thinning, and more rarely, interstitial keratitis (Sfiniadaki et al. 2019). Reynolds et al. (1999) have shown that cytokeratin 3, a corneal epithelial-specific keratin, is an autoantigen associated with PUK in patients with this condition. Limbal edema and corneal defects with thinning have also been observed in some cases (Hood and Lowder 2010; Reddy et al. 2011). Similar to other systemic inflammatory diseases, corticosteroids (topical for eye problems) and immunosuppressive drugs are the main form of treatment that have increased patient survival to 95% at 5 years and 80% at 10 years. More recently, monoclonal antibodies to TNF-α and CD20 have also shown efficacy against scleritis and PUK (Weeda and Coffey 2008; Sfiniadaki et al. 2019).
4.9. Sarcoidosis
Sarcoidosis is an inflammatory granulomatous disease that affects the lung, mediastinal lymphatic system, skin, and the eye. It is characterized by the formation of non-caseating, giant cell granulomas with T lymphocyte and macrophage involvement (Salah et al. 2018). Ocular manifestations are presented in 20–50% of the cases and include scleritis, keratitis, uveitis, retinitis, dry eye, and conjunctival nodules (Heiligenhaus et al. 2011; Pasadhika and Rosenbaum 2015). In the cornea, there is small nerve fiber loss and damage due to decreased nerve fiber density (Dahan et al. 2013). One case report documented interstitial keratitis with posterior uveitis and optic nerve edema (Lennarson and Barney 1995), whereas another study reported peripheral ulcerative keratitis that progressed to corneal perforation (Siracuse-Lee and Saffra 2006). In some cases, patients show band keratopathy caused by calcium deposits in the Bowman’s layer (Crick et al. 1961). A multilobular, nodular, perilimbal mass has also been reported in a 16-year girl as a manifestation of sarcoidosis (Hegab et al. 1998). Treatment for ocular sarcoidosis includes topical and systemic corticosteroids to manage inflammation. In patients that are intolerant to corticosteroids, immunosuppressive anti-metabolites and calcineurin inhibitors have been considered (Pasadhika and Rosenbaum 2015).
4.10. Cogan’s Syndrome
Cogan’s syndrome is a rare autoimmune disorder that is characterized by systemic vasculitis of unknown etiology, and inflammation in the eye and the inner ear. Autoantibodies to a corneal antigen can be isolated from patients (Iliescu et al. 2015). Ocular symptoms include redness, irritation, photophobia, excessive tear production, and diminished visual acuity (Iliescu et al. 2015). Bilateral interstitial keratitis is the most common manifestation of the disease (Belhoucha et al. 2014). Other corneal findings involve bilateral peripheral subepithelial keratitis with faint, nummular lesions and deep stromal keratitis in some cases (Cobo and Haynes 1984). In other cases, irregular, granular corneal infiltration in the posterior cornea near the limbus was described (Grasland et al. 2004). Corticosteroids are usually the first line of treatment, followed by immunosuppressive agents if needed (Iliescu et al. 2015).
4.11. Immunobullous Diseases
Immunobullous diseases, also known as autoimmune blistering diseases, are a group of conditions caused by specific autoantibodies that bind to epithelial cells, resulting in blistering lesions on the skin, mucous membrane, and oral cavity by affecting the cell-cell and cell-matrix adhesion (Otten et al. 2014; Witte et al. 2018). They mainly include pemphigus, mucous membrane pemphigoid, epidermolysis bullosa acquisita, and linear IgA disease, among others.
Pemphigus is characterized by the presence of autoantibodies on skin keratinocytes that react to desmosomal component desmoglein. It comprises three forms, pemphigus vulgaris, pemphigus foliaceus, and paraneoplastic pemphigus (Buonavoglia et al. 2019). Ocular involvement is rare, and mainly includes non-cicatrizing bilateral conjunctivitis, blepharitis, dry eye, photophobia, redness, and tearing (Tan et al. 2015; Buonavoglia et al. 2019). Corneal abnormalities are observed only in paraneoplastic pemphigus with punctate epithelial erosions, bilateral corneal perforations, and corneal melting (Beele et al. 2001; Tam et al. 2009; Venkateswaran et al. 2020). Mucous membrane pemphigoid is triggered by autoantibodies to bullous pemphigoid (BP) 180, BP230, laminin-332, and the β4 subunit of α6β4 integrin on the epithelial BM. It causes blistering skin lesions and scarring along with conjunctivitis (Carey and Setterfield 2019). In vivo confocal microscopy revealed decreased corneal nerve density and elevated inflammatory dendritic cell density along with metaplasia of the corneal epithelial layers, intraepithelial defects, anterior stromal fibrosis, and hyperreflective endothelial deposits in patients with non-end-stage disease, whereas end-stage disease patients showed corneal neovascularization and scarring (Tepelus et al. 2017).
Epidermolysis bullosa acquisita (EBA) is mediated by autoantibodies to collagen type VII causing vesicle and bullae formation on the skin and mucous membrane erosions. Ocular features include severe cicatrizing conjunctivitis and scarring, symblephara formation, and abnormal positioning of the eyelashes (Kridin et al. 2019). In some cases, corneal involvement resulting in blindness has been reported with lesions, peripheral perforation, and advanced thinning (Dantas et al. 2001; Rousseau et al. 2020).
Linear IgA disease is a sub-epidermal blistering disease marked by linear deposits of BM IgA antibodies (Venning 2011). Like EBA, linear IgA disease also causes lesions associated with bullae or vesicles and presents with similar symptoms as other immunobullous diseases, such as dry eye, conjunctivitis and scarring, photophobia, foreign body sensation, and abnormal positioning of the eyelashes. For this reason, diagnosis must be confirmed with a skin biopsy showing linear IgA deposits between the dermis and epidermis (Aultbrinker et al. 1988; Ramos-Castellón et al. 2010). Cicatrizing conjunctivitis can lead to corneal perforation with epithelial defects, opacification, and superficial vascularization (Smith et al. 1999; Ramos-Castellón et al. 2010).
Topical and systemic therapies for treating immunobullous diseases have been reviewed in detail (Buonavoglia et al. 2019; Kridin et al. 2019). Corticosteroids as well as immunosuppressive drugs such as azathioprine, mycophenolate mofetil, and methotrexate are routinely used. Neutrophil targeting drugs like colchicine and dapsone have been prescribed to EBA patients, alone or in combination with immunosuppressants or corticosteroids (Hughes and Callen 2001; Kim et al. 2011; Adachi et al. 2016). Anti-CD20 antibody rituximab has also been tested (Kim et al. 2012; Herbert and Joly 2018; Lamberts et al. 2018). However, patients with linear IgA disease have been less responsive to rituximab, possibly due to continuous IgA-secreting B-cell population that is resistant to anti-CD20 therapy (Pinard et al. 2019; He et al. 2015).
5. Genetic Corneal Deposit Disorders
5.1. Wilson’s Disease
Wilson’s disease, also known as hepatolenticular degeneration is a rare autosomal recessive disease that results in excessive copper accumulation due to impaired copper metabolism (Ala et al. 2007). It is due to a mutation in the copper transporting gene encoding Wilson disease protein, or ATP7B on chromosome 13, and primarily affects the liver, but also the brain, cornea, and kidney (Bull et al. 1993; Petrukhin et al. 1993). In the cornea, this disease presents in the form of sunflower cataracts and Kayser-Fleischer ring (Figure 6) caused by the deposition of copper in the anterior capsule and Descemet’s membrane, respectively (Goel et al. 2019). Kayser-Fleischer ring appears as a reddish/greenish band of 1–3 mm in width, starting with an arc in the superior pole, followed by an arc in the inferior pole, and eventually forms a ring around the cornea (Ellis 1969; Suvarna 2008). These rings are detected by a slit lamp examination but can also be visible to the naked eye. Recently, anterior segment optical coherence tomography has been suggested for early detection as angle view is obscured by the corneal limbus during the early stages of the disease (Sridhar et al. 2017; Broniek-Kowalik et al. 2019). Apart from the Kayser-Fleischer ring, individuals with Wilson’s disease also show low blood ceruloplasmin levels (< 10 mg/dl) and other neurological symptoms, mainly affecting motor functions (Bandmann et al. 2015). Wilson’s disease can be managed with a low-copper diet and treated with chelating agents such as D-penicillamine (Van Caillie-Bertrand et al. 1985; Hedera 2019).
Figure 6.

Wilson’s disease. Kayser-Fleischer ring in peripheral cornea (white arrow) (A) and sunflower cataract (black arrow) on the anterior lens capsule in both eyes (A, B).
Reproduced with permission from: Jang, H.J., Kim, J.M., Choi, C.Y., 2014. Elemental analysis of sunflower cataract in Wilson’s disease: a study using scanning transmission electron microscopy and energy dispersive spectroscopy. Exp. Eye. Res. 121, 58–65.
5.2. Cystinosis
Cystinosis is a rare autosomal recessive lysosomal storage disorder caused by mutations in the CTNS gene that encodes the protein cystinosin, resulting in intracellular accumulation of cysteine crystals in several organs, including the kidney, liver, spleen, eye, bone marrow, pancreas, thyroid, muscle, and brain (Nesterova and Gahl 2012). It is diagnosed by measuring leucocyte cysteine levels (de Graaf-Hess, Trijbels, and Blom 1999; Levtchenko et al. 2004). There are three forms of cystinosis; infantile, adolescent, and adult, with the infantile form being the most severe (David et al. 2019). In the infantile form, cystinosis is the most common cause of Fanconi syndrome with impaired reabsorption in the proximal renal tubule. Polyuria, growth retardation, rickets, and progressive renal failure are other manifestations of infantile cystinosis that can be managed by dialysis and kidney transplantation (Nesterova and Gahl 2008; David et al. 2019). The adolescent form is less severe, whereas the adult form is benign and asymptomatic.
Ocular indicators include corneal cysteine crystals and photophobia, which is seen in all three forms of cystinosis (Biswas et al. 2018). Corneal crystals appear at the periphery and progress centripetally over time. They do not affect visual acuity until the crystals reach the central cornea. Foreign body sensation and inflammatory symptoms of recurrent corneal erosions are also reported when cysteine crystals enter the Bowman’s layer (Anikster et al. 2000; Fung et al. 2007; Tsilou et al. 2007). Beside the corneal involvement, deposition of cysteine crystals was also observed in the conjunctiva, iris, ciliary body, choroid, fundus, and optic nerve (Tsilou et al. 2007). A large cross-sectional study has reported that 75% patients aged 20–29 and 87% of patients over the age of 30 years with cystinosis showed anterior segment involvement separate from the corneal and conjunctival crystal deposits (Tsilou et al. 2002). Treatment mainly consists of oral cysteamine, however, topical administration of 0.5% cysteamine is required to effectively dissolve corneal crystals and reduce symptoms of photophobia (Iwata et al. 1998; Sham et al. 2014).
5.3. Fabry Disease
Fabry disease is an X-linked disorder caused by a deficiency of α-galactosidase enzyme due to mutations of the GLA gene, resulting in lysosomal accumulation of glycosphingolipids, mainly globotriaosylceramide over time (Schiffmann 2015). It affects both males and heterozygotic females but to a lesser extent (El-Abassi et al. 2014). Although the disease manifests at an early age, it usually remains undiagnosed until adulthood (Mastropasqua et al. 2006). Symptoms range from pain in arms and legs, reddish-purplish blemishes on the skin, and decreased sweating to myocardial infraction, stroke, and renal failure (El-Abassi et al. 2014).
Cornea verticillata (vortex keratopathy) is the most common ocular finding of this disease, which shows white or golden-brown whorl-like pattern of epithelial and subepithelial deposits in the cornea (Figure 7). It is reported to be more pronounced in affected females, although it is observed in both male (74%) and female carriers (66%) (Samiy 2008). Pitz et al. (2015) also demonstrated that ocular signs correlated well with disease severity, and patients with cornea verticillata appeared to have more severe disease than those without it. The group further reported that the prevalence of cornea verticillata was significantly higher in patients with null (male, 76.9%; female, 64.5%) and missense (male, 79.2%; female, 67.4%) mutations as compared to patients with mild missense (male, 17.1%; female, 23.1%) and the p.N215S (male, 15.0%; female, 15.6%) mutations (Pitz et al. 2015). Apart from cornea verticillata, patients also show conjunctival vessel tortuosity, corneal haze, lens opacity, retinal vessel tortuosity, anterior and posterior cataracts (Kalkum et al. 2016; Michaud 2019). Treatment for Fabry disease involves enzyme replacement therapy (ERT) with agalsidase α/Replagal and agalsidase β/Fabrazyme (Sirrs et al. 2014; Spada et al. 2019). ERT resulted in a decrease in globotriaosylceramide levels in the blood and reduced pain and the risk of other adverse effects such as renal failure and cardiac death (Schiffmann et al. 2003; Banikazemi et al. 2008; Rombach et al. 2014). ERT is also supplemented with other supportive medications such as angiotensin-converting enzyme (ACE) inhibitors, statins, and aspirin (Sirrs et al. 2014).
Figure 7.
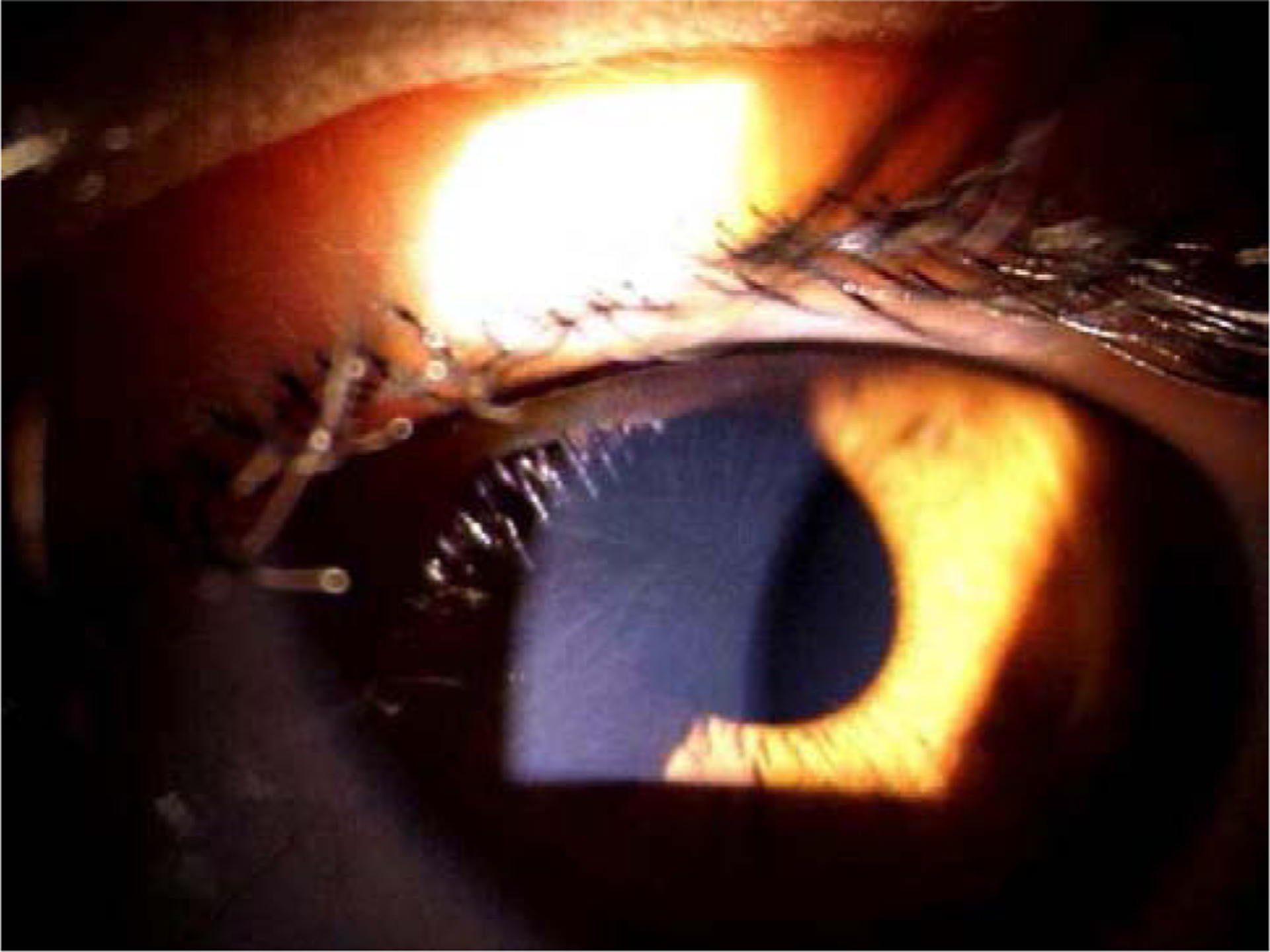
Cornea verticillata (vortex keratopathy) in a 7-year-old girl with Fabry disease.
Reproduced with permission from: Spada, M., Enea, A., Morrone, A., Fea, A., Porta, F., 2013. Cornea verticillata and Fabry disease. J. Pediatr. 163, 609.
5.4. Meretoja’s Syndrome
Meretoja’s syndrome, also known as familial amyloidosis Finnish type, or lattice corneal dystrophy type II, is a rare inherited autosomal dominant disease caused by mutations in the gelsolin gene (GSN) at chromosome 9q32–34. This mutation results in the substitution of asparagine by aspartate at residue 187 leading to extracellular amyloid deposition in several tissues such as cornea, skin, vascular walls, and perineurium (Casal et al. 2017; Friedhofer et al. 2017). Clinical manifestations have late onset, often appearing only between the third and fifth decades of life (Kiuru 1992). Corneal lattice dystrophy is often the first sign of the disease in most cases, although it is not considered a dystrophy anymore as it is caused by systemic amyloidosis (Nikoskinen et al. 2015; Friedhofer et al. 2017). Lattice formation is due to amyloid deposits under the Bowman’s layer, mainly in the anterior stroma resulting in subepithelial opacities (Kivelä et al. 1993; Huerva et al. 2007). Systemic features include facial paralysis, polyneuropathy, skin fragility, and hair loss (Casal et al. 2017; Carrwik and Stenevi 2009; Cabral-Macias et al. 2020). Facial paralysis and polyneuropathy lead to corneal ulcers, recurrent corneal erosions, and dry eye due to impaired eyelid closing and cranial nerve involvement. Patients with Meretoja syndrome also suffer from photophobia, dysfunction of the meibomian glands, early development of cataract, and higher risk of open-angle glaucoma (Kiuru, 1992; 1998; Carrwik and Stenevi, 2009; Starck et al. 1991). However, the mutated GSN gene in the trabecular muscle cells, but not the amyloid deposition appears to be responsible for increased intraocular pressure (IOP) in these patients (Kiuru, 1998).
Currently, there is no cure for Meretoja’s syndrome, and the treatment is mostly symptomatic. As most complications are ocular, ophthalmologists play an important role in the management and treatment of the symptoms. Eye examination, eyelid function test, and IOP measurements are highly recommended at regular intervals. In some cases, plastic surgery is considered for facial paralysis and impaired eyelid closure, which in turn also helps alleviate corneal abnormalities (Pihlamaa et al. 2011; Friedhofer et al. 2017). Pressure reducing topical treatments and lubricating eyedrops are prescribed to control the IOP and dry eye syndrome, respectively; however, care must be taken that they do not contain preservatives, as patients show low tolerance to preservatives. Keratoplasty is required for patients with vision loss due to amyloid deposits, although this will only prolong the natural process of the disease (Carrwik and Stenevi 2009). Phototherapeutic keratectomy (PTK) is performed to treat corneal opacities; however, it has been reported to cause delayed epithelial healing, and requires close monitoring until the epithelium is fully healed, to avoid ulceration, scarring, and infection (Das et al. 2005; Lee et al. 2018). Moreover, recurrence of the disease after PTK is a possibility, and may need retreatment (Dinh et al. 1999).
5.5. Mucopolysaccharidosis
Mucopolysaccharidosis (MPS) is a group of lysosomal storage disorders characterized by the accumulation of glycosaminoglycans (GAG) in bone, cartilage, tendons, cornea, skin, and connective tissue. It is due to inborn errors of GAG metabolism (Wraith et al. 1995). Seven different subtypes of the disease have been identified based on the enzyme defect with varying symptoms and severity (Ashworth et al. 2006; Khan et al. 2017). Ocular manifestations include corneal clouding, retinopathy, glaucoma, and optic nerve abnormalities (Del Longo et al. 2018). Corneal clouding is caused by increasing keratocyte size, the displacement of collagen fibrils, and progressive accumulation of GAG that appears as yellowish-grey granules deposited in all layers of the cornea, but mainly in the stroma (Fahnehjelm et al. 2012). Severity of corneal clouding has been reported to correlate with an increase in age and it subsequently results in loss of visual acuity (Couprie et al. 2010).
Evaluation of corneal transparency and corneal thickness by slit-lamp examination and optical coherence tomography (OCT), respectively, can be useful in the diagnosis of MPS. Several therapeutic approaches have been tested in different MPS subtypes including ERT, hematopoietic stem cell transplantation (HSCT), and gene therapy to replace or restore normal functional enzyme. In ERT, the enzymes are delivered to the lysosome by binding to the mannose-6-phosphate (M6P) receptors on the cell surface. Intravenous ERT has been effectively used for treating MPS I, MPS II, MPS IV, and MPS VI in visceral organs (Muenzer et al. 2002; Tomatsu et al. 2015; Brunelli et al. 2016; da Silva et al. 2016; Jameson et al. 2019). However, due to short half-life of the enzyme and its inability to cross the blood-brain barrier, there is limited penetration in the cornea, brain, bone, and heart, and hence, which renders it ineffective in alleviating symptoms in these tissues (Chen et al. 2019). Furthermore, patients require weekly and bi-weekly treatment that makes it considerably more expensive. Patients may also develop antibodies to the infused enzyme, resulting in an immune response that can block its effect (Ponder 2008). HSCT has been used for the treatment of MPS types I, II, IVA, VI, and VII and comprises transplantation of healthy donor cells. Unlike ERT, where only the deficient enzyme is infused that circulates with a short half-life, HSCT involves the circulation of the donor stem cells in the bloodstream (Taylor et al. 2019). HSCT is a one-time procedure, but finding an acceptable donor makes it a lengthy process (Noh and Lee, 2014) and has not shown any success in the treatment of corneal clouding (Guffon et al. 1998).
Gene therapy has shown promising results in restoring normal enzyme function in the cornea. Kamata et al. (2001) have reported that adenoviral injection of gene expressing human β-glucuronidase into the anterior chamber or intrastromal region of the cornea successfully treated corneal clouding of MPS VII in mice. Corneal clouding was also reduced in a model of canine MPS VII (Serratrice et al. 2014). Transduction of the gene encoding α-L-iduronidase (IUDA) via recombinant adeno-associated virus restored IUDA function in the cornea (Vance et al. 2016).
5.6. Hyperlipoproteinemia
Hyperlipoproteinemia is characterized by elevated levels of lipids and lipoproteins in the blood, mainly cholesterol (hypercholesterolemia). Diabetes, atherosclerosis, hypertension, and cardiovascular disease are comorbidities associated with hyperlipoproteinemia. Ocular manifestation is often the first sign of the disease involving the deposition of lipids in the cornea and limbus, known as corneal arcus or arcus senilis (Crispin, 1989; 2003; Hayasaka et al. 1989). It is a white, yellowish-grey ring in the corneal periphery caused by lipid infiltration of the stroma that does affect vision. Corneal arcus can also be a sign of familial hypercholesterolemia in children (Lock et al. 2019). Clinically, corneal arcus is asymptomatic and does not require specific therapy but can be improved by treating the underlying hyperlipidemia (Munjal and Kaufman 2020).
6. Other Genetic Disorders Presenting Corneal Manifestations
6.1. Aniridia
Aniridia is characterized by the absence of iris and may be an isolated ocular complication or associated with a syndrome. Usually, both eyes will have no iris, and the abnormality is mostly a result of a haploinsufficiency truncating mutation in the PAX6 gene, congenitally, and by ocular injury (Wawrocka and Krawczynski 2018). Aniridia is co-related with WAGRO syndrome, which stands for Wilms tumor, aniridia, genitourinary anomalies, mental retardation, and obesity. As mentioned, WAGRO syndrome is caused by a deletion on chromosome 11(11p) where the PAX6 gene is located. A proportion of deletion will often include several other genes, some of which include Wilms tumor gene (WT1) at location 11(13p) that mediates nephroblastoma development especially in children, and brain-derived neurotrophic factor gene (BDNF) at location 11(14.1p), which is the known candidate gene for obesity (Van Heyningen et al. 2007; Han et al. 2008; Ferreira et al. 2019).
Aniridia-associated keratopathy (AAK) causes damage to the cornea as a result of genetic factors described above, and may be also associated with dry eye due to meibomian gland dysfunction. It is linked to alterations of the epithelial phenotype with progressive conjunctivalization due to LSCD, decreased subbasal nerves, and invasion of immune cells (Lagali et al. 2018). In a recent study where 275 eyes were characterized based on corneal abnormalities from congenital aniridia, 13% of participants had central corneal opacity at birth, whereas 25% developed AAK with age (Lee et al. 2018). Most corneal damage associated with aniridia is related to the mutation of PAX6 gene as it is correlated to corneal blindness via LSCD and secondary glaucoma (Käsmann-Kellner et al. 2018). Change or damage of limbal epithelial palisades of Vogt in AAK causes ocular surface defects at different stages (Voskresenskaya et al. 2017). Latta et al. (2018) identified proteins that may be regulated by PAX6 and showed that SPINK7 mRNA, coding for a serine peptidase inhibitor, is downregulated in patients as well as in primary aniridia cell model. The results suggest that PAX6 gene controls corneal epithelial differentiation of retinoic acid signaling processes through ADH7 and ALDH1A1. Additionally, altered regulation in miR and mRNA levels supports that the conjunctiva in aniridia is maintained in pro-angiogenic and proliferative state, which mediates PAX6 mutation-related neovascularization (Latta et al. 2020). Downstream signaling in AAK is also altered, with suppression of Notch1 pathway but activation of Wnt pathways, both canonical and non-canonical, and Sonic hedgehog pathway (Vicente et al. 2018).
There is currently no cure for aniridia as it is mostly caused by LSCD, but in some cases limbal stem cell transplantation (LSCT) and Boston keratoprosthetics (KPro) done on patients at severe stages (III-Va) of AAK and LSCD resulted in significant improvement of visual acuity (Yazdanpanah et al. 2020). Management for aniridia may also include iris reconstruction by implanting an iris prosthesis (Mostafa et al. 2018). Although this is typically done for patients who have aniridia as a result of cataract, it may be a step towards AAK management. Gene therapy tools such as the CRISPR/Cas 9 system may be able to supply recombinant PAX6 protein as an efficient therapeutic approach for treating AAK (Roux et al. 2018). Anterior segment optical coherence tomography and in vivo confocal microscopy may be good diagnostic tools to monitor limbal stem cell progenitors and corneal epithelial changes in AAK (Voskresenskaya et al. 2017).
6.2. Ehlers-Danlos Syndromes
Ehlers-Danlos syndrome (EDS) is a group of genetic disorders caused by mutations in genes encoding fibrillar collagens. It comprises thirteen subtypes based on the affected gene with a wide range of musculoskeletal defects (Malfait et al. 2017). Corneal manifestations are seen mainly in brittle cornea syndrome (BCS), an autosomal recessive disorder caused by mutations in the genes ZNF469 or PRDM5 that play a role in corneal and extracellular matrix development, respectively (Walkden et al. 2019). Corneal problems involve thinning with central corneal thickness measuring often < 400 μm, which can subsequently cause keratoconus, keratoglobus, and high myopia. Loss of structural integrity can result in spontaneous rupture of these corneas that leads to vision loss (Burkitt Wright et al. 2013; Wan et al. 2018). There is also an increased risk for retinal detachment in these patients (Christensen et al. 2010). Non-ocular features of BCS include hyper-elasticity in skin and hearing impairment (Walkden et al. 2019). Significantly thinner and steeper corneas compared to the controls are also observed in some cases of classic EDS with mutated type V collagen genes, either COL5A1 or COL5A2 (Villani et al. 2013). Early diagnosis of BCS is important to prevent ocular rupture. However, due to its rarity and non-specific systemic symptoms BCS is often underdiagnosed, which may hamper proper disease management. Protective glasses have been prescribed to protect the corneas from thinning and perforation (Walkden et al. 2019). In some cases of progressive BCS, surgery is recommended including epikeratoplasty (host corneal epithelium is replaced with donor corneal disc) and penetrating keratoplasty (Kanellopoulos and Pe 2005). Collagen cross-linking was also tested to treat keratoconus with varying results (Caporossi et al. 2010; Caporossi et al. 2012; Kaufmann et al. 2015).
6.3. Marfan Syndrome
Marfan syndrome (MFS) is an autosomal dominant connective tissue disease caused by mutations in the fibrillin-1 gene (FBN-1) located on chromosome 15q15–21 (Yuan and Jing 2010). Fibrillin is the major component of 10 to 12 nm microfibrils. It plays a critical role in maintaining elasticity and contributes to the force-bearing capacity of ocular connective tissue (Robinson and Godfrey 2000). MFS is characterized by skeletal, cardiovascular, and ocular manifestations. Displacement of the crystalline lens from its normal location, known as ectopia lentis, is the main ocular feature of MFS (Bitterman and Sponseller 2017). Other abnormalities include flattened cornea, astigmatism, increased axial length, iris and ciliary body hypoplasia, and retinal detachment (Esfandiari et al. 2019). In a prospective case series, Sultan et al. (2002) reported decreased keratometry and pachymetry scores indicating flattening of the cornea and corneal thinning, respectively, in MFS patients as compared to control subjects. In vivo confocal microscopy also confirmed corneal thinning and showed opaque stromal matrix with an increased light backscattering in the stroma. Moreover, MFS patients also show increased corneal astigmatism associated with lens dislocation (Konradsen et al. 2012; Chen et al. 2018) and significant corneal deformation (Beene et al. 2016; Scheibenberger et al. 2018; Vanhonsebrouck et al. 2020).
Eye examinations with keratometry and pachymetry tests can be used for diagnosing the disease in addition to ectopia lentis, which does not present in all cases of MFS (Heur et al. 2008; Luebke et al. 2017). Blurred vision due to ectopia lentis can be corrected with eyeglasses that will also correct for corneal astigmatism (Esfandiari et al. 2019).
7. Conclusions
Cornea offers a unique window into a number of life-threatening systemic diseases. Although many of the corneal abnormalities associated with systemic pathology are not exclusive to a specific disorder, along with other symptoms they can reveal many systemic diseases. Therefore, it is important for eye health practitioners to recognize these manifestations for timely diagnosis, management, and treatment to prevent or retard the development of corneal blindness. It should also be noted that management and therapies for corneal complications of systemic diseases especially the emerging ones may be local and specific to the ocular surface (Antunes-Foschini et al. 2020). Future studies should also elucidate mechanisms of corneal alterations in systemic diseases, as this research area is currently underdeveloped.
Corneal involvement has been observed in a number of systemic diseases
Systemic diseases may affect the entire ocular surface
Corneal problems are seen in genetic, infectious, immune and endocrine diseases
Underlying mechanisms and emerging treatments are discussed
Supported by:
NIH grants R01 EY013431, EY031377 (Ljubimov), EY029160 (Ghiasi, Ljubimov), EY025377, EY029829 (Saghizadeh), EY013615, EY024649, EY026944 (Ghiasi), EY026964, EY027381 (Kumar), CIRM grant TRAN1COVID19-11975 (Arumugaswami) and grants from the Board of Governors Regenerative Medicine Institute (Saghizadeh, Ljubimov).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: AVL is an officer and stockholder of Arrogene Nanotechnology, Inc., 8560 West Sunset Boulevard, Suite 424, Los Angeles, CA 90069, USA.
Bibliography
- Abdelkader H, Patel DV, McGhee CN, Alany RG, 2011. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin. Exp. Ophthalmol 39, 259–270. [DOI] [PubMed] [Google Scholar]
- Abeysiri P, Sinha A, 2006. An unusual pattern of corneal calcification in tertiary hyperparathyroidism. Arch. Ophthalmol 124, 138–139. [DOI] [PubMed] [Google Scholar]
- Abozaid MA, Hassan AA, Abdalla A, 2019. Intrastromal corneal ring segments implantation and corneal cross-linking for keratoconus in children with vernal keratoconjunctivitis - three-year results. Clin. Ophthalmol 13, 2151–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi A, Komine M, Suzuki M, Murata S, Hirano T, Ishii N, Hashimoto T, Ohtsuki M, 2016. Oral colchicine monotherapy for epidermolysis bullosa acquisita: Mechanism of action and efficacy. J. Dermatol 43, 1389–1391. [DOI] [PubMed] [Google Scholar]
- Aiello F, Gallo Afflitto G, Mancino R, Li JO, Cesareo M, Giannini C, Nucci C, 2020. Coronavirus disease 2019 (SARS-CoV-2) and colonization of ocular tissues and secretions: a systematic review. Eye (Lond). 34, 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL III, Klein R, 1998. Diabetic retinopathy. Diabetes Care. 21, 143–156. [DOI] [PubMed] [Google Scholar]
- Akimoto Y, Sawada H, Ohara-Imaizumi M, Nagamatsu S, Kawakami H, 2008. Change in long-spacing collagen in Descemet’s membrane of diabetic Goto-Kakizaki rats and its suppression by antidiabetic agents. Exp. Diabetes Res 2008, 818341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpek EK, Bunya VY, Saldanha IJ, 2019. Sjögren’s syndrome: more than just dry eye. Cornea. 38, 658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpek EK, Dart JK, Watson S, Christen W, Dursun D, Yoo S, O’Brien TP, Schein OD, Gottsch JD, 2004. A randomized trial of topical cyclosporin 0.05% in topical steroid-resistant atopic keratoconjunctivitis. Ophthalmology. 111, 476–482. [DOI] [PubMed] [Google Scholar]
- Aktaş Z, Ozdek S, Asli Dinç U, Akyürek N, Atalay V, Güz G, Hasanreisoglu B, 2007. Alterations in ocular surface and corneal thickness in relation to metabolic control in patients with chronic renal failure. Nephrology (Carlton). 12, 380–385. [DOI] [PubMed] [Google Scholar]
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML, 2007. Wilson’s disease. Lancet. 369, 397–408. [DOI] [PubMed] [Google Scholar]
- Alfuraih S, Barbarino A, Ross C, Shamloo K, Jhanji V, Zhang M, Sharma A, 2020. Effect of high glucose on ocular surface epithelial cell barrier and tight junction proteins. Invest. Ophthalmol. Vis. Sci 61, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almouhawis HA, Leao JC, Fedele S, Porter SR, 2013. Wegener’s granulomatosis: a review of clinical features and an update in diagnosis and treatment. J. Oral. Pathol. Med 42, 507–516. [DOI] [PubMed] [Google Scholar]
- Alrobaian M, Elsayed M, Alotaibi AK, AlHarbi M, May W, Stone DU, 2019. Safety and efficacy of corneal cross-linking in pediatric patients with keratoconus and vernal keratoconjunctivitis. Middle East Afr. J. Ophthalmol 26, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez S, McCabe WR, 1984. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine (Baltimore). 63, 25–55. [PubMed] [Google Scholar]
- Amesty MA, Alió Del Barrio JL, Alió JL, 2020. COVID-19 disease and ophthalmology: an update. Ophthalmol. Ther 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anikster Y, Lucero C, Guo J, Huizing M, Shotelersuk V, Bernardini I, McDowell G, Iwata F, Kaiser-Kupfer MI, Jaffe R, Thoene J, Schneider JA, Gahl WA, 2000. Ocular nonnephropathic cystinosis: clinical, biochemical, and molecular correlations. Pediatr. Res 47,17–23. [DOI] [PubMed] [Google Scholar]
- Ansari AS, de Lusignan S, Arrowsmith B, Hinton W, Munro N, McGovern A, 2017. Association between diabetes, level of glycemic control, and eye infection: a cohort study. Diabetes Care. 40, e30–e31. [DOI] [PubMed] [Google Scholar]
- Antunes-Foschini R, Adriano L, Murashima AAB, Barbosa AP, Nominato LF, Dias LC, Fantucci MZ, Garcia DM, Alves M, Rocha EM, 2020. Limitations and advances in new treatments and future perspectives of corneal blindness. Arq. Bras. Oftalmol 10.5935/0004-2749.20210042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif AS, Aaqil B, Siddiqui A, Nazneen Z, Farooq U, 2017. Corneal complications and visual impairment in vernal keratoconjunctivitis patients. J. Ayub. Med. Coll. Abbottabad 29, 58–60. [PubMed] [Google Scholar]
- Arora R, Goel R, Kumar S, Chhabra M, Saxena S, Manchanda V, Pumma P, 2020. Evaluation of SARS-CoV-2 in tears of moderate to severe COVID-19 patients. Ophthalmology. S0161-6420(20)30847-2. doi: 10.1016/j.ophtha.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth JL, Biswas S, Wraith E, Lloyd IC, 2006. The ocular features of the mucopolysaccharidoses. Eye (Lond). 20, 553–563. [DOI] [PubMed] [Google Scholar]
- Aultbrinker EA, Starr MB, Donnenfeld ED, 1988. Linear IgA disease. The ocular manifestations. Ophthalmology. 95, 340–343. [DOI] [PubMed] [Google Scholar]
- Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK, 1992. Altered epithelial-basement membrane interactions in diabetic corneas. Arch. Ophthalmol 110, 537–540. [DOI] [PubMed] [Google Scholar]
- Balyan M, Malhotra C, Jain AK, 2019. Multifocal phlyctenular conjunctivitis in association with pulmonary tuberculosis. Indian. J. Ophthalmol 67, 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandmann O, Weiss KH, Kaler SG, 2015. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 14, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ, 2007. Fabry Disease Clinical Trial Study Group. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann. Intern. Med 146, 77–86. [DOI] [PubMed] [Google Scholar]
- Barrow RY, Ahmed F, Bolan GA, Workowski KA, 2020. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR. Recomm. Rep 68, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegian A, Lee J, Salifu MO, McFarlane SI, 2018. Corneal neuropathy: an underrated manifestation of diabetes mellitus. J. Clin. Endocrinol. Diabetes 2, JCED–111.31372600 [Google Scholar]
- Bartalena L, Tanda ML, 2009. Clinical practice. Graves’ ophthalmopathy. N. Engl. J. Med 360, 994–1001. [DOI] [PubMed] [Google Scholar]
- Baser H, Cuhaci N, Topaloglu O, Yulek F, Ugurlu N, Ersoy R, Cagil N, Cakir B, 2016. Is there any association between primary hyperparathyroidism and ocular changes, such as central corneal thickness, retinal thickness, and intraocular pressure? Endocrine. 51, 545–550. [DOI] [PubMed] [Google Scholar]
- Bastion ML, Ling KP, 2013. Topical insulin for healing of diabetic epithelial defects: A retrospective review of corneal debridement during vitreoretinal surgery in Malaysian patients. Med. J. Malaysia 68, 208–216. [PubMed] [Google Scholar]
- Beckman KA, 2014. Characterization of dry eye disease in diabetic patients versus nondiabetic patients. Cornea. 33, 851–854. [DOI] [PubMed] [Google Scholar]
- Beele H, Claerhout I, Kestelyn P, Dierckxens L, Naeyaert JM, De Laey JJ, 2001. Bilateral corneal melting in a patient with paraneoplastic pemphigus. Dermatology. 202, 147–150. [DOI] [PubMed] [Google Scholar]
- Beene LC, Traboulsi EI, Seven I, Ford MR, Sinha Roy A, Butler RS, Dupps WJ Jr., 2016. Corneal deformation response and ocular geometry: a noninvasive diagnostic strategy in Marfan syndrome. Am. J. Ophthalmol 161, 56–64.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belančić A, Kresović A, Rački V, 2020. Potential pathophysiological mechanisms leading to increased COVID-19 susceptibility and severity in obesity. Obes. Med 19, 100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhoucha B, Belghmidi S, Benbaddan I, Rochdi Y, Hajji I, Nouri H, Aderdour I, Hattaoui M, Moutaouakil A, Raji A, 2014. Atypical Cogan’s syndrome: case report of an oculoaudiovestibular disease. Am. J. Med. Case Rep 2, 139–142. [Google Scholar]
- Belser JA, Rota PA, Tumpey TM, 2013. Ocular tropism of respiratory viruses. Microbiol. Mol. Biol. Rev 77,144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaim D, Tétart F, Bauvin O, Delcampe A, Joly P, Muraine M, Gueudry J, 2019. Tacrolimus ointment in the management of atopic keratoconjunctivitis. J. Fr. Ophtalmol 42, e147–e151. [DOI] [PubMed] [Google Scholar]
- Bettahi I, Sun H, Gao N, Wang F, Mi X, Chen W, Liu Z, Yu FS, 2014. Genome-wide transcriptional analysis of differentially expressed genes in diabetic, healing corneal epithelial cells: hyperglycemia-suppressed TGFβ3 expression contributes to the delay of epithelial wound healing in diabetic corneas. Diabetes. 63, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers EM, Galli M, Barbui T, Comfurius P, Zwaal RF, 1991. Lupus anticoagulant IgG’s (LA) are not directed to phospholipids only, but to a complex of lipid-bound human prothrombin. Thromb. Haemost 66, 629–632. [PubMed] [Google Scholar]
- Bielory B, Bielory L, 2010. Atopic dermatitis and keratoconjunctivitis. Immunol. Allergy Clin. North Am 30, 323–336. [DOI] [PubMed] [Google Scholar]
- Bikbova G, Oshitari T, Baba T, Bikbov M, Yamamoto S, 2018. Diabetic corneal neuropathy: clinical perspectives. Clin. Ophthalmol 12, 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikbova G, Oshitari T, Tawada A,Yamamoto S, 2012. Corneal changes in diabetes mellitus. Curr. Diabetes Rev 8, 294–302. [DOI] [PubMed] [Google Scholar]
- Bilen H, Ates O, Astam N, Uslu H Akcay G, Baykal O, 2007. Conjunctival flora in patients with type 1 or type 2 diabetes mellitus. Adv. Ther 24, 1028–1035. [DOI] [PubMed] [Google Scholar]
- Biswas S, Gaviria M, Malheiro L, Marques JP, Giordano V, Liang H, 2018. Latest clinical approaches in the ocular management of cystinosis: a review of current practice and opinion from the Ophthalmology Cystinosis Forum. Ophthalmol. Ther 7, 307–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitirgen G, Akpinar Z, Malik RA, Ozkagnici A, 2017. Use of corneal confocal microscopy to detect corneal nerve loss and increased dendritic cells in patients with multiple sclerosis. JAMA Ophthalmol. 135, 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman AD, Sponseller PD, 2017. Marfan Syndrome: A clinical update. J. Am. Acad. Orthop. Surg 25, 603–609. [DOI] [PubMed] [Google Scholar]
- Blackburn M, Diamond T, 2007. Primary hyperparathyroidism and familial hyperparathyroid syndromes. Aust. Fam. Physician 36, 1029–1033. [PubMed] [Google Scholar]
- Brandt CR, 2005. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp. Eye. Res 80, 607–621. [DOI] [PubMed] [Google Scholar]
- Broniek-Kowalik K, Dzieżyc K, Litwin T, Członkowska A, Szaflik JP, 2019. Anterior segment optical coherence tomography (AS-OCT) as a new method of detecting copper deposits forming the Kayser-Fleischer ring in patients with Wilson disease. Acta. Ophthalmol 97, e757–e760. [DOI] [PubMed] [Google Scholar]
- Brunelli MJ, Atallah ÁN, da Silva EM, 2016. Enzyme replacement therapy with galsulfase for mucopolysaccharidosis type VI. Cochrane Database Syst. Rev 3, CD009806. [DOI] [PubMed] [Google Scholar]
- Buggage RR, Levy-Clarke GA, Smith JA, 2001. New corneal findings in human T-cell lymphotrophic virus type 1 infection. Am. J. Ophthalmol 131, 309–313. [DOI] [PubMed] [Google Scholar]
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW, 1993. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet 5, 327–37. [DOI] [PubMed] [Google Scholar]
- Buonavoglia A, Leone P, Dammacco R, Di Lernia G, Petruzzi M, Bonamonte D, Vacca A, Racanelli V, Dammacco F, 2019. Pemphigus and mucous membrane pemphigoid: An update from diagnosis to therapy. Autoimmun. Rev 18, 349–358. [DOI] [PubMed] [Google Scholar]
- Burkitt Wright EM, Porter LF, Spencer HL, Clayton-Smith J, Au L, Munier FL, Smithson S, Suri M, Rohrbach M, Manson FD, Black GC, 2013. Brittle cornea syndrome: recognition, molecular diagnosis and management. Orphanet. J. Rare. Dis 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, Hiatt P, McCoy K, Castile R, Smith AL, Ramsey BW, 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis 183, 444–452. [DOI] [PubMed] [Google Scholar]
- Bussan KA, Robertson DM, 2019. Contact lens wear and the diabetic corneal epithelium: A happy or disastrous marriage? J. Diabetes Complications 33, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun YS, Kang B, Yoo YS, Joo CK, 2015. Poly(ADP-ribose) polymerase inhibition improves corneal epithelial innervation and wound healing in diabetic rats. Invest. Ophthalmol. Vis. Sci 56, 1948–1955. [DOI] [PubMed] [Google Scholar]
- Cabral-Macias J, Garcia-Montaño LA, Pérezpeña-Díazconti M, Aguilar MC, Garcia G, Vencedor-Meraz CI, Graue-Hernandez EO, Chacón-Camacho OF, Zenteno JC, 2020. Clinical, histopathological, and in silico pathogenicity analyses in a pedigree with familial amyloidosis of the Finnish type (Meretoja syndrome) caused by a novel gelsolin mutation. Mol. Vis 26, 345–354. [PMC free article] [PubMed] [Google Scholar]
- Cabrera AP, Mankad RN, Marek L, Das R, Rangasamy S, Monickaraj F, Das A, 2020. Genotypes and phenotypes: a search for influential genes in diabetic retinopathy. Int. J. Mol. Sci 21, 2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Zhu M, Petroll WM, Koppaka V, Robertson DM, 2014. The impact of type 1 diabetes mellitus on corneal epithelial nerve morphology and the corneal epithelium. Am. J. Pathol 184, 2662–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimmi C, Crowson CS, Smith WM, Matteson EL, Makol A, 2018. Clinical correlates, outcomes, and predictors of inflammatory ocular disease associated with rheumatoid arthritis in the biologic era. J. Rheumatol 45, 595–603. [DOI] [PubMed] [Google Scholar]
- Caldeira JA, Sabbaga E, Ianhez LE, 1970. Conjunctival and corneal changes in renal failure. Influence of renal transplantation. Br. J. Ophthalmol 54, 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder VL, Jolly G, Hingorani M, Adamson P, Leonardi A, Secchi AG, Buckley RJ, Lightman S, 1999. Cytokine production and mRNA expression by conjunctival T-cell lines in chronic allergic eye disease. Clin. Exp. Allergy 29, 1214–1222. [DOI] [PubMed] [Google Scholar]
- Calvo-Maroto AM, Perez-Cambrodí RJ, Albarán-Diego C, Pons A, Cerviño A, 2014. Optical quality of the diabetic eye: a review. Eye (Lond). 28, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JA, 1995. Shield ulcers and plaques of the cornea in vernal keratoconjunctivitis. Ophthalmology. 102, 985–993. [DOI] [PubMed] [Google Scholar]
- Cameron JA, Al-Rajhi AA, Badr IA, 1989. Corneal ectasia in vernal keratoconjunctivitis. Ophthalmology. 96, 1615–1623. [DOI] [PubMed] [Google Scholar]
- Cameron JA, Antonios SR, Badr IA, 1995. Excimer laser phototherapeutic keratectomy for shield ulcers and corneal plaques in vernal keratoconjunctivitis. J. Refract. Surg 11, 31–35. [DOI] [PubMed] [Google Scholar]
- Cao Y, Zhang W, Wu J, Zhang H, Zhou H, 2017. Peripheral ulcerative keratitis associated with autoimmune disease: pathogenesis and treatment. J. Ophthalmol 2017, 7298026. doi: 10.1155/2017/7298026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, 2010. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am. J. Ophthalmol 149, 585–593. [DOI] [PubMed] [Google Scholar]
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R, Balestrazzi A, 2012. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 31, 227–231. [DOI] [PubMed] [Google Scholar]
- Carey B, Setterfield J, 2019. Mucous membrane pemphigoid and oral blistering diseases. Clin. Exp. Dermatol 44, 732–739. [DOI] [PubMed] [Google Scholar]
- Carrwik C, Stenevi U, 2009. Lattice corneal dystrophy, gelsolin type (Meretoja’s syndrome). Acta. Ophthalmol 87, 813–819. [DOI] [PubMed] [Google Scholar]
- Casal I, Monteiro S, Abreu C, Neves M, Oliveira L, Beirão M, 2017. Meretoja’s syndrome: lattice corneal dystrophy, gelsolin type. Case. Rep. Med 2017, 2843417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A, 2015. Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis. Cochrane Database Syst. Rev 6, CD009566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallerano J, 1992. Ocular manifestations of diabetes mellitus. Optom. Clin 2, 93–116. [PubMed] [Google Scholar]
- Chang E, Galle L, Maggs D, Estes DM, Mitchell WJ, 2000. Pathogenesis of herpes simplex virus type 1-induced corneal inflammation in perforin-deficient mice. J. Virol 74, 11832–11840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S, McCrae KR, 2017. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. 31, 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia S, Ramappa M, Murthy SI, Vemuganti GK, Fernandes M, Sharma S, Sangwan V, 2014. Chronic conjunctivitis due to Mycobacterium tuberculosis. Int. Ophthalmol 34, 655–660. [DOI] [PubMed] [Google Scholar]
- Chen DK, Frizzi KE, Guernsey LS, Ladt K, Mizisin AP, Calcutt NA, 2013. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J. Peripher. Nerv. Syst 18, 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Sawamoto K, Mason RW, Kobayashi H, Yamaguchi S, Suzuki Y, Orii K, Orii T, Tomatsu S, 2019. Enzyme replacement therapy for mucopolysaccharidoses; past, present, and future. J. Hum. Genet 64, 1153–1171. [DOI] [PubMed] [Google Scholar]
- Chen J, Jing Q, Tang Y, Qian D, Lu Y, Jiang Y, 2018. Corneal curvature, astigmatism, and aberrations in Marfan Syndrome with lens subluxation: evaluation by Pentacam HR System. Sci. Rep 8, 4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Applebaum DS, Sun GS, Pflugfelder SC, 2014. Atopic keratoconjunctivitis: A review. J. Am. Acad. Dermatol 70, 569–575. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, Xin N, Huang Z, Liu L, Zhang G, Wang J, 2020. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br. J. Ophthalmol 104, 748–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WL, Lin CT, Ko PS, Yeh PT, Kuan YH, Hu FR, Yang CM, 2009. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology. 116, 1038–1047. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Ramanujam S, Greer DA, Kumagai LF, Aoki TT, 2001. Microvascular abnormalities in the bulbar conjunctiva of patients with type 2 diabetes mellitus. Endocr. Pract 7, 358–363. [DOI] [PubMed] [Google Scholar]
- Chikama T, Wakuta M, Liu Y, Nishida T, 2007. Deviated mechanism of wound healing in diabetic corneas. Cornea. 26(9 Suppl 1), S75–S81. [DOI] [PubMed] [Google Scholar]
- Chopra R, Chander A, Jacob JJ, 2012. The eye as a window to rare endocrine disorders. Indian J. Endocrinol. Metab 16, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AE, Knappskog PM, Midtbø M, Gjesdal CG, Mengel-From J, Morling N, Rødahl E, Boman H, 2010. Brittle cornea syndrome associated with a missense mutation in the zinc-finger 469 gene. Invest. Ophthalmol. Vis. Sci 51, 47–52. [DOI] [PubMed] [Google Scholar]
- Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, 2020. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet (Lond). 395, 1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo-Soriano R, Beltrán J, Baviera J 2006. LASIK outcome in patients with underlying systemic contraindications: a preliminary study. Ophthalmology. 113, 1118.e1–e8. [DOI] [PubMed] [Google Scholar]
- Cobo LM, Haynes BF, 1984. Early corneal findings in Cogan’s syndrome. Ophthalmology. 91, 903–907. [DOI] [PubMed] [Google Scholar]
- Çolak S, Kazanci B, Ozçelik Soba D, Ozdamar Erol Y, Yilmazbas P, 2020. Effects of diabetes duration and HgA1C level on corneal endothelial morphology. Eur. J. Ophthalmol 1120672120914812. [DOI] [PubMed] [Google Scholar]
- Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, Nicastri E, Bevilacqua N, Giancola ML, Corpolongo A, Ippolito G, Capobianchi MR, Castilletti C, 2020. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann. Intern. Med 173, 242–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consultant360., 2014. Corneal manifestations of systemic diseases. 54, issue 6. https://www.consultant360.com/articles/corneal-manifestations-systemic-diseases (accessed 14 December 2020). [Google Scholar]
- Coppey L, Davidson E, Shevalye H, Obrosov A, Torres M, Yorek MA, 2020. Progressive loss of corneal nerve fibers and sensitivity in rats modeling obesity and type 2 diabetes is reversible with omega-3 fatty acid intervention: supporting cornea analyses as a marker for peripheral neuropathy and treatment. Diabetes Metab. Syndr. Obes 13, 1367–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordellat IM, 2012. Hyperparathyroidism: primary or secondary disease? Reumatol. Clin 8, 287–291. [DOI] [PubMed] [Google Scholar]
- Couprie J, Denis P, Guffon N, Reynes N, Masset H, Beby F, 2010. Manifestations ophtalmologiques de la maladie de Morquio [Ocular manifestations in patients affected by Morquio syndrome (MPS IV)]. J. Fr. Ophtalmol 33, 617–622. French. [DOI] [PubMed] [Google Scholar]
- Cousen P, Cackett P, Bennett H, Swa K, Dhillon B, 2007. Tear production and corneal sensitivity in diabetes. J. Diabetes Complications 21, 371–373. [DOI] [PubMed] [Google Scholar]
- Crick RP, Hoyle C, Smellie H, 1961. The eyes in sarcoidosis. Br. J. Ophthalmol 45, 461–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispin S, 2002. Ocular lipid deposition and hyperlipoproteinaemia. Prog. Retin. Eye Res 21, 169–224. [DOI] [PubMed] [Google Scholar]
- Crispin SM, 1989. Lipid deposition at the limbus. Eye (Lond). 3(Pt 2), 240–250. [DOI] [PubMed] [Google Scholar]
- Cruzat A, Qazi Y, Hamrah P, 2017. In vivo confocal microscopy of corneal nerves in health and disease. Ocul. Surf 15, 15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva EM, Strufaldi MW, Andriolo RB, Silva LA, 2016. Enzyme replacement therapy with idursulfase for mucopolysaccharidosis type II (Hunter syndrome). Cochrane Database Syst. Rev 2, CD008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Dunne A, Swartjes M, Proto PL, Heij L, Vogels O, van Velzen M, Sarton E, Niesters M, Tannemaat MR, Cerami A, Brines M, 2013. ARA 290 improves symptoms in patients with sarcoidosis-associated small nerve fiber loss and increases corneal nerve fiber density. Mol. Med 19, 334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbeth N, Haskard DO, 2005. Mechanisms of inflammation in gout. Rheumatology (Oxford). 44,1090–1096. [DOI] [PubMed] [Google Scholar]
- Dammacco R, Procaccio P, Racanelli V, Vacca A, Dammacco F, 2018. Ocular involvement in systemic lupus erythematosus: the experience of two tertiary referral centers. Ocul. Immunol. Inflamm 26, 1154–1165. [DOI] [PubMed] [Google Scholar]
- Dantas PE, Nishiwaki-Dantas MC, Seguim MH, Cursino JW, 2001. Bilateral corneal involvement in epidermolysis bullosa acquisita. Cornea. 20, 664–667. [DOI] [PubMed] [Google Scholar]
- Das S, Langenbucher A, Seitz B, 2005. Delayed healing of corneal epithelium after phototherapeutic keratectomy for lattice dystrophy. Cornea. 24, 283–287. [DOI] [PubMed] [Google Scholar]
- David D, Princiero Berlingerio S, Elmonem MA, Oliveira Arcolino F, Soliman N, van den Heuvel B, Gijsbers R, Levtchenko E, 2019. Molecular basis of cystinosis: geographic distribution, functional consequences of mutations in the CTNS Gene, and potential for repair. Nephron. 141,133–146. [DOI] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Kardon RH, Yorek MA, 2014. Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Invest. Ophthalmol. Vis. Sci 55, 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cillà S, Ranno S, Carini E, Fogagnolo P, Ceresara G, Orzalesi N, Rossetti L, 2009. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Invest. Ophthalmol. Vis. Sci 50, 5155–5158. [DOI] [PubMed] [Google Scholar]
- De Clerck EEB, Schouten JSAG, Berendschot TTJM, Koolschijn RS, Nuijts RMMA, Schram MT, Schaper NC, Henry RMA, Dagnelie PC, Ruggeri A, Guimarães P, Stehouwer CDA, Webers CAB, 2020. Reduced corneal nerve fibre length in prediabetes and type 2 diabetes: The Maastricht Study. Acta. Ophthalmol 98, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf-Hess A, Trijbels F, Blom H, 1999. New method for determining cystine in leukocytes and fibroblasts. Clin. Chem 45, 2224–2228. [PubMed] [Google Scholar]
- De Paiva CS, Pflugfelder SC, Ng SM, Akpek EK, 2019. Topical cyclosporine A therapy for dry eye syndrome. Cochrane Database Syst. Rev 9, CD010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedova IV, Nikolaeva OP, Safer D, De La Cruz EM, dos Remedios CG, 2006. Thymosin β4 induces a conformational change in actin monomers. Biophys. J 90, 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Buey MA, Casas P, Caramello C, López N, de la Rica M, Subirón AB, Lanchares E, Huerva V, Grzybowski A, Ascaso FJ, 2019. An update on corneal biomechanics and architecture in diabetes. J. Ophthalmol 2019, 7645352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Longo A, Piozzi E, Schweizer F, 2018. Ocular features in mucopolysaccharidosis: diagnosis and treatment. Ital. J. Pediatr 44(Suppl 2), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMill DL, Hussain M, Pop-Busui R, Shtein RM 2016. Ocular surface disease in patients with diabetic peripheral neuropathy. Br. J. Ophthalmol 100, 924–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Bao L, Gao H, Xiang Z, Qu Y, Song Z, Gong S, Liu J, Liu J, Yu P, Qi F, Xu Y, Li F, Xiao C, Lv Q, Xue J, Wei Q, Liu M, Wang G, Wang S, Yu H, Chen T, Liu X, Zhao W, Han Y, Qin C, 2020. Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat. Commun 11, 4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depledge DP, Sadaoka T, Ouwendijk WJD, 2018. Molecular aspects of varicella-zoster virus latency. Viruses. 10, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhasmana R, Singh IP, Nagpal RC, 2014. Corneal changes in diabetic patients after manual small incision cataract surgery. J. Clin. Diagn. Res 8, VC03–VC06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh R, Rapuano CJ, Cohen EJ, Laibson PR, 1999. Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy. Ophthalmology. 106, 1490–1497. [DOI] [PubMed] [Google Scholar]
- Dogru M, Katakami C, Inoue M, 2001. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 108, 586–592. [DOI] [PubMed] [Google Scholar]
- Dua HS, Said DG, Messmer EM, Rolando M, Benitez-Del-Castillo JM, Hossain PN, Shortt AJ, Geerling G, Nubile M, Figueiredo FC, Rauz S, Mastropasqua L, Rama P, Baudouin C, 2018. Neurotrophic keratopathy. Prog. Retin. Eye Res 66, 107–131. [DOI] [PubMed] [Google Scholar]
- Duran JA, Refojo MF, 1987. Pseudomonas attachment to new hydrogel contact lenses. Arch. Ophthalmol 105, 106–109. [DOI] [PubMed] [Google Scholar]
- Durukan I, 2020. Corneal endothelial changes in type 2 diabetes mellitus relative to diabetic retinopathy. Clin. Exp. Optom 103, 474–478. [DOI] [PubMed] [Google Scholar]
- Easterbrook M, Mortimer CB, 1970. Ocular signs in chronic renal failure. Br. J. Ophthalmol 54, 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimiadib N, Modjtahedi BS, Roohipoor R, Anesi SD, Foster CS, 2016. Successful treatment strategies in granulomatosis with polyangiitis-associated peripheral ulcerative keratitis. Cornea. 35, 1459–1465. [DOI] [PubMed] [Google Scholar]
- Eid L, Coste-Verdier V, Longueville E, Ribeiro E, Nicolescu-Catargi B, Korobelnik JF, 2020. The effects of Rituximab on Graves’ orbitopathy: A retrospective study of 14 patients. Eur. J. Ophthalmol 30, 1008–1013. [DOI] [PubMed] [Google Scholar]
- El-Abassi R, Singhal D, England JD, 2014. Fabry’s disease. J. Neurol. Sci 344, 5–19. [DOI] [PubMed] [Google Scholar]
- El-Agamy A, Alsubaie S, 2017. Corneal endothelium and central corneal thickness changes in type 2 diabetes mellitus. Clin. Ophthalmol 11, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis PP, 1969. Ocular deposition of copper in hypercupremia. Am. J. Ophthalmol 68, 423–427. [DOI] [PubMed] [Google Scholar]
- Elmekawey H, Abdelaziz M, El Baradey M, Kotb M, 2020. Epithelial remodeling following phacoemulsification in diabetic patients using anterior-segment optical coherence tomography: a comparative study. Clin. Ophthalmol 14, 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom Y, Han JY, Kang SY, Kim HM, Huh K, Song JS, 2013. Calcium hydroxyapatite crystals in the anterior chamber of the eye in a patient with renal hyperparathyroidism. Cornea. 32, 1502–1504. [DOI] [PubMed] [Google Scholar]
- Esen BA, Yilmaz G, Uzun S, Ozdamar M, Aksözek A, Kamalı S, Türkoğlu S, Gül A, Ocal L, Aral O, Inanç M, 2012. Serologic response to Epstein-Barr virus antigens in patients with systemic lupus erythematosus: a controlled study. Rheumatol. Int 32, 79–83. [DOI] [PubMed] [Google Scholar]
- Esfandiari H, Ansari S, Mohammad-Rabei H, Mets MB, 2019. Management strategies of ocular abnormalities in patients with Marfan Syndrome: current perspective. J. Ophthalmic. Vis. Res 14, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DJ, Fleiszig SMJ, 2013. Why does the healthy cornea resist Pseudomonas aeruginosa infection? Am. J. Ophthalmol 155, 961–970.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnehjelm KT, Ashworth JL, Pitz S, Olsson M, Törnquist AL, Lindahl P, Summers CG, 2012. Clinical guidelines for diagnosing and managing ocular manifestations in children with mucopolysaccharidosis. Acta. Ophthalmol 90, 595–602. [DOI] [PubMed] [Google Scholar]
- Farooq AV, Shukla D, 2012. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv. Ophthalmol 57, 448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi S, Javadi MA, Alemzadeh-Ansari M, Arabi A, Shahraki T, Kheirkhah A, 2020. Management of corneal complications in vernal keratoconjunctivitis: A review. Ocul. Surf 24, S1542-0124(20)30161-0. doi: 10.1016/j.jtos.2020.10.005. [DOI] [PubMed] [Google Scholar]
- Felten R, Dervovic E, Chasset F, Gottenberg JE, Sibilia J, Scher F, Arnaud L, 2018. The 2018 pipeline of targeted therapies under clinical development for systemic lupus erythematosus: a systematic review of trials. Autoimmun. Rev 17, 781–790. [DOI] [PubMed] [Google Scholar]
- Ferdousi M, Kalteniece A, Petropoulos I, Azmi S, Dhage S, Marshall A, Boulton AJM, Efron N, Faber CG, Lauria G, Soran H, Malik RA, 2020. Diabetic neuropathy is characterized by progressive corneal nerve fiber loss in the central and inferior whorl regions. Invest. Ophthalmol. Vis. Sci 61, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MAT, Almeida Júnior IG, Kuratani DK, Rosa RFM, Gonzales JFO, Telles LEB, Ferrão YA, Zen PRG, 2019. WAGRO syndrome: a rare genetic condition associated with aniridia and additional ophthalmologic abnormalities. Arq. Bras. Oftalmol 82, 336–338. [DOI] [PubMed] [Google Scholar]
- Figueira L, Fonseca S, Ladeira I, Duarte R, 2017. Ocular tuberculosis: Position paper on diagnosis and treatment management. Rev. Port. Pneumol 23, 31–38. [DOI] [PubMed] [Google Scholar]
- Fraunfelder FW, Rich LF 2002. Laser-assisted in situ keratomileusis complications in diabetes mellitus. Cornea. 21, 246–248. [DOI] [PubMed] [Google Scholar]
- Friedhofer H, Vassiliadis AH, Scarpa MB, Luitgards BF, Gemperli R, 2017. Meretoja syndrome: general considerations and contributions of plastic surgery in surgical treatment. Aesthet. Surg. J 38, NP10–NP15. [DOI] [PubMed] [Google Scholar]
- Fujita H, Morita I, Takase H, Ohno-Matsui K, Mochizuki M 2003. Prolonged exposure to high glucose impaired cellular behavior of normal human corneal epithelial cells. Curr. Eye Res 27, 197–203. [DOI] [PubMed] [Google Scholar]
- Funari VA, Winkler M, Brown J, Dimitrijevich SD, Ljubimov AV, Saghizadeh M, 2013. Differentially expressed wound healing-related microRNAs in the human diabetic cornea. PLoS One. 8, e84425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung AT, Fraser-Bell S, Ojaimi E, Sutton G, 2007. In vivo confocal microscopy and polarizing microscopy of the cornea in a patient with nephropathic cystinosis. Clin. Exp. Ophthalmol 35, 292–293. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang Y, Zhao X, Chen P, Xie L, 2015. MicroRNA-204–5p-mediated regulation of SIRT1 contributes to the delay of epithelial cell cycle traversal in diabetic corneas. Invest. Ophthalmol. Vis. Sci 56, 1493–1504. [DOI] [PubMed] [Google Scholar]
- Gao N, Yan C, Lee P, Sun H, Yu F, 2016. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J. Clin. Invest 126, 1998–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekka M, Miyata K, Nagai Y, Nemoto S, Sameshima T, Tanabe T, Maruoka S, Nakahara M, Kato S, Amano S, 2004. Corneal epithelial barrier function in diabetic patients. Cornea. 23, 35–37. [DOI] [PubMed] [Google Scholar]
- Gillan WDH, 2015. Corneal manifestations of selected systemic diseases: A review. Afr. Vision Eye Health 74, 287. [Google Scholar]
- Goel S, Sahay P, Maharana PK, Titiyal JS, 2019. Ocular manifestations of Wilson’s disease. B.M.J. Case Rep 12, e229662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan A, Savir H, Bar-Meir S, Oliver I, De Vries A, 1975. Band keratopathy due to hyperparathyroidism. Ophthalmologica. 171, 119–122. [DOI] [PubMed] [Google Scholar]
- Goldstein AS, Janson BJ, Skeie JM, Ling JJ, Greiner MA, 2020. The effects of diabetes mellitus on the corneal endothelium: A review. Surv. Ophthalmol 65, 438–450. [DOI] [PubMed] [Google Scholar]
- Gomes BA, Santhiago MR, Jorge PA, Kara-José N, Moraes HV Jr, Kara-Junior N, 2015. Corneal involvement in systemic inflammatory diseases. Eye Contact Lens. 41, 141–144. [DOI] [PubMed] [Google Scholar]
- Graham RL, Sparks JS, Eckerle LD, Sims AC, Denison MR, 2008. SARS coronavirus replicase proteins in pathogenesis. Virus Res. 133, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasland A, Pouchot J, Hachulla E, Blétry O, Papo T, Vinceneux P, Study Group for Cogan’s Syndrome, 2004. Typical and atypical Cogan’s syndrome: 32 cases and review of the literature. Rheumatology (Oxford). 43, 1007–1015. [DOI] [PubMed] [Google Scholar]
- Graves J, Balcer LJ, 2010. Eye disorders in patients with multiple sclerosis: natural history and management. Clin. Ophthalmol 4, 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner MA, Rixen JJ, Wagoner MD, Schmidt GA, Stoeger CG, Straiko MD, Zimmerman MB, Kitzmann AS, Goins KM, 2014. Diabetes mellitus increases risk of unsuccessful graft preparation in Descemet membrane endothelial keratoplasty: a multicenter study. Cornea. 33, 1129–1133. [DOI] [PubMed] [Google Scholar]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19, 2020. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guemes-Villahoz N, Burgos-Blasco B, Vidal-Villegas B, Garcia-Feijoo J, Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Konstas AG, 2020. Novel insights into the transmission of SARS-CoV-2 through the ocular surface and its detection in tears and conjunctival secretions: a review. Adv. Ther 37, 4086–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffon N, Souillet G, Maire I, Straczek J, Guibaud P, 1998. Follow-up of nine patients with Hurler syndrome after bone marrow transplantation. J. Pediatr 133, 119–125. [DOI] [PubMed] [Google Scholar]
- Gui F, You Z, Fu S, Wu H, Zhang Y, 2020. Endothelial dysfunction in diabetic retinopathy. Front. Endocrinol. (Lausanne) 11, 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gül M, Emre S, Eşrefoğlu M, Vard N, 2008. Protective effects of melatonin and aminoguanidine on the cornea in streptozotocin-induced diabetic rats. Cornea. 27, 795–801. [DOI] [PubMed] [Google Scholar]
- Gunay M, Celik G, Yildiz E, Bardak H, Koc N, Kirmizibekmez H, Gunay BO, Yesiltepe Mutlu RG, 2016. Ocular surface characteristics in diabetic children. Curr. Eye Res 41, 1526–1531. [DOI] [PubMed] [Google Scholar]
- Guo C, Li M, Qi X, Lin G, Cui F, Li F, Wu X, 2016. Intranasal delivery of nanomicelle curcumin promotes corneal epithelial wound healing in streptozotocin-induced diabetic mice. Sci. Rep 6, 29753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer BL, Matheson E, Wilkes RT, 2014. Diagnosis, treatment, and prevention of gout. Am. Fam. Physician 90, 831–836. [PubMed] [Google Scholar]
- Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA, 2008. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N. Engl. J. Med 359, 918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SB, Yang HK, Hyon JY, 2018. Influence of diabetes mellitus on anterior segment of the eye. Clin. Interv. Aging 14, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchell DL, Magolan JJ Jr, Besson MJ, Goldman AI, Pederson HJ, Schultz KJ, 1983. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch. Ophthalmol 101, 469–471. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Honda M, Kitaoka M, Chiba R, 1984. Corneal arcus in Japanese family with type IIa hyperlipoproteinemia. Jpn. J. Ophthalmol 28, 254–258. [PubMed] [Google Scholar]
- He J, Bazan HE, 2012. Mapping the nerve architecture of diabetic human corneas. Ophthalmology. 119, 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Diakonis VF, Alavi Y, Yesilirmak N, Waren D, Donaldson K, 2017. Endothelial cell loss in diabetic and nondiabetic eyes after cataract surgery. Cornea. 36, 948–951. [DOI] [PubMed] [Google Scholar]
- He Y, Shimoda M, Ono Y, Villalobos IB, Mitra A, Konia T, Grando SA, Zone JJ, Maverakis E, 2015. Persistence of autoreactive IgA-secreting B cells despite multiple immunosuppressive medications including rituximab. JAMA Dermatol. 151(6), 646–50. [DOI] [PubMed] [Google Scholar]
- Hebert V, Joly P, 2018. Rituximab in pemphigus. Immunotherapy. 10, 27–37. [DOI] [PubMed] [Google Scholar]
- Hebert W, DiGiandomenico A, Zegans M, 2020. Multifunctional monoclonal antibody targeting Pseudomonas aeruginosa keratitis in mice. Vaccines (Basel). 8, 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedera P, 2019. Wilson’s disease: A master of disguise. Parkinsonism Relat. Disord 59, 140–145. [DOI] [PubMed] [Google Scholar]
- Hegab SM, al-Mutawa SA, Sheriff SM, 1998. Sarcoidosis presenting as multilobular limbal corneal nodules. J. Pediatr. Ophthalmol. Strabismus 35, 323–326. [DOI] [PubMed] [Google Scholar]
- Heiligenhaus A, Wefelmeyer D, Wefelmeyer E, Rösel M, Schrenk M, 2011. The eye as a common site for the early clinical manifestation of sarcoidosis. Ophthalmic Res. 46, 9–12. [DOI] [PubMed] [Google Scholar]
- Hellesen A, Bratland E, Husebye ES, 2018. Autoimmune Addison’s disease - An update on pathogenesis. Ann. Endocrinol. (Paris) 79, 157–163. [DOI] [PubMed] [Google Scholar]
- Hernández-Molina G, Avila-Casado C, Cárdenas-Velázquez F, Hernández-Hernández C, Calderillo ML, Marroquín V, Soto-Abraham V, Recillas-Gispert C, Sánchez-Guerrero J, 2010. Similarities and differences between primary and secondary Sjögren’s syndrome. J. Rheumatol 37, 800–808. [DOI] [PubMed] [Google Scholar]
- Heur M, Costin B, Crowe S, Grimm RA, Moran R, Svensson LG, Traboulsi EI, 2008. The value of keratometry and central corneal thickness measurements in the clinical diagnosis of Marfan syndrome. Am. J. Ophthalmol 145, 997–1001. [DOI] [PubMed] [Google Scholar]
- Hill TJ, 1987. Ocular pathogenicity of herpes simplex virus. Curr. Eye. Res 6, 1–7. [DOI] [PubMed] [Google Scholar]
- Hilliam Y, Kaye S, Winstanley C, 2020. Pseudomonas aeruginosa and microbial keratitis. J Med Microbiol. 69, 3–13. [DOI] [PubMed] [Google Scholar]
- Ho D, Low R, Tong L, Gupta V, Veeraraghavan A, Agrawal R, 2020. COVID-19 and the ocular surface: a review of transmission and manifestations. Ocul. Immunol. Inflamm 28, 726–734. [DOI] [PubMed] [Google Scholar]
- Hood CT, Lowder CY, 2010. Images in clinical medicine. Peripheral ulcerative keratitis in Wegener’s granulomatosis. N. Engl. J. Med 363, e2. [DOI] [PubMed] [Google Scholar]
- Hu J, Hu XY, Kan T, 2019. MiR-34c participates in diabetic corneal neuropathy via regulation of autophagy. Invest. Ophthalmol. Vis. Sci 60, 16–25. [DOI] [PubMed] [Google Scholar]
- Hu J, Huang Y, Lin Y, Lin J, 2020. Protective effect inhibiting the expression of miR-181a on the diabetic corneal nerve in a mouse model. Exp. Eye. Res 192, 107925. [DOI] [PubMed] [Google Scholar]
- Hu Y, Matsumoto Y, Adan ES, Dogru M, Fukagawa K, Tsubota K, Fujishima H, 2008. Corneal in vivo confocal scanning laser microscopy in patients with atopic keratoconjunctivitis. Ophthalmology. 115, 2004–20012. [DOI] [PubMed] [Google Scholar]
- Huerva V, Velasco A, Sánchez MC, Mateo AJ, Matías-Guiu X, 2007. Lattice corneal dystrophy type II: clinical, pathologic, and molecular study in a Spanish family. Eur. J. Ophthalmol 17, 424–429. [DOI] [PubMed] [Google Scholar]
- Hughes AP, Callen JP, 2001. Epidermolysis bullosa acquisita responsive to dapsone therapy. J. Cutan. Med. Surg 5, 397–399. [DOI] [PubMed] [Google Scholar]
- Hugod M, Storr-Paulsen A, Norregaard JC, Nicolini J, Larsen AB, Thulesen J, 2011. Corneal endothelial cell changes associated with cataract surgery in patients with type 2 diabetes mellitus. Cornea. 30, 749–753. [DOI] [PubMed] [Google Scholar]
- Iliescu DA, Timaru CM, Batras M, De Simone A, Stefan C, 2015. Cogan’s syndrome. Rom. J. Ophthalmol 59, 6–13. [PMC free article] [PubMed] [Google Scholar]
- Iovieno A, Coassin M, Viscogliosi F, Adani C, Cimino L, Fontana L, 2020. Delayed-onset bilateral peripheral posterior interstitial keratitis associated with Epstein-Barr virus-induced infectious mononucleosis. Ocul. Immunol. Inflamm 1–4. doi: 10.1080/09273948.2020.1811351. [DOI] [PubMed] [Google Scholar]
- Iwata F, Kuehl EM, Reed GF, McCain LM, Gahl WA, Kaiser-Kupfer MI, 1998. A randomized clinical trial of topical cysteamine disulfide (cystamine) versus free thiol (cysteamine) in the treatment of corneal cystine crystals in cystinosis. Mol. Genet. Metab 64, 237–242. [DOI] [PubMed] [Google Scholar]
- Jabbur NS, Chicani CF, Kuo IC, O’Brien TP 2004. Risk factors in interface epithelialization after laser in situ keratomileusis. J. Refract. Surg 20, 343–348. [DOI] [PubMed] [Google Scholar]
- Jameson E, Jones S, Remmington T, 2019. Enzyme replacement therapy with laronidase (Aldurazyme®) for treating mucopolysaccharidosis type I. Cochrane Database Syst. Rev 6, CD009354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan RL, Tai MC, Ho CH, Chu CC, Wang JJ, Tseng SH, Chang YS, 2020. Risk of recurrent corneal erosion in patients with diabetes mellitus in Taiwan: a population-based cohort study. B.M.J. Open 10, e035933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan RL, Weng SF, Wang JJ, Tseng SH, Chang YS, 2020. Association between atopic keratoconjunctivitis and the risk of corneal ulcer. Br. J. Ophthalmol 2020, 316206. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kim JM, Choi CY, 2014. Elemental analysis of sunflower cataract in Wilson’s disease: a study using scanning transmission electron microscopy and energy dispersive spectroscopy. Exp. Eye. Res 121, 58–65. [DOI] [PubMed] [Google Scholar]
- Jones R 3rd, Rhee DJ, 2006. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr. Opin. Ophthalmol 17, 163–167. [DOI] [PubMed] [Google Scholar]
- Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zieske JD, Ljubimov AV, 2003. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp. Eye Res 77, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kačmař J, Cholevík D, 2019. Corticosteroid induced posterior subcapsular cataract. Cesk. Slov. Oftalmol 74, 226–232. [DOI] [PubMed] [Google Scholar]
- Kaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Jabs DA, Levy-Clarke GA, Foster CS, 2010. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 117, 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakazu A, Chandrasekher G, Bazan HE, 2004. HGF protects corneal epithelial cells from apoptosis by the PI-3K/Akt-1/Bad-but not the ERK1/2-mediated signaling pathway. Invest. Ophthalmol. Vis. Sci 45, 3485–3492. [DOI] [PubMed] [Google Scholar]
- Kakazu A, Sharma G, Bazan HE, 2008. Association of protein tyrosine phosphatases (PTPs)-1B with c-Met receptor and modulation of corneal epithelial wound healing. Invest. Ophthalmol. Vis. Sci 49, 2927–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkum G, Pitz S, Karabul N, Beck M, Pintos-Morell G, Parini R, Rohrbach M, Bizjajeva S, Ramaswami U, 2016. Paediatric Fabry disease: prognostic significance of ocular changes for disease severity. BMC. Ophthalmol 16, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeropoulos CD, Bassukas ID, Moschos MM, Tabbara KF, 2015. Eye and periocular skin involvement in herpes zoster infection. Med. Hypothesis. Discov. Innov. Ophthalmol 4, 142–156. [PMC free article] [PubMed] [Google Scholar]
- Kalteniece A, Ferdousi M, Azmi S, Marshall A, Soran H, Malik RA, 2018. Keratocyte density is reduced and related to corneal nerve damage in diabetic neuropathy. Invest. Ophthalmol. Vis. Sci 59, 3584–3590. [DOI] [PubMed] [Google Scholar]
- Kamata Y, Okuyama T, Kosuga M, O’hira A, Kanaji A, Sasaki K, Yamada M, Azuma N, 2001. Adenovirus-mediated gene therapy for corneal clouding in mice with mucopolysaccharidosis type VII. Mol. Ther 4, 307–312. [DOI] [PubMed] [Google Scholar]
- Kamoi K, Mochizuki M, 2012. HTLV-1 uveitis. Front. Microbiol 3, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Thirumalmuthu K, Prajna NV, Lalitha P, Mohankumar V, Devarajan B, 2020. Comparative genomics of ocular Pseudomonas aeruginosa strains from keratitis patients with different clinical outcomes. Genomics. August 31:S0888-7543(20)30520-6. doi: 10.1016/j.ygeno.2020.08.032. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos AJ, Pe LH, 2005. An alternative surgical procedure for the management of keratoglobus. Cornea. 24, 1024–1026. [DOI] [PubMed] [Google Scholar]
- Karabulut GO, Kaynak P, Altan C, Ozturker C, Aksoy EF, Demirok A, Yılmaz OF, 2014. Corneal biomechanical properties in thyroid eye disease. Kaohsiung. J. Med. Sci 30, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkouli MB, Alemzadeh SA, Aghaei H, Pakdel F, Abdolalizadeh P, Ghazizadeh M, Moradpasandi F, 2018. Subjective versus objective dry eye disease in patients with moderate-severe thyroid eye disease. Ocul. Surf 16, 458–462. [DOI] [PubMed] [Google Scholar]
- Käsmann-Kellner B, Latta L, Fries FN, Viestenz A, Seitz B, 2018. Diagnostic impact of anterior segment angiography of limbal stem cell insufficiency in PAX6-related aniridia. Clin. Anat 31, 392–397. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Schubiger G, Thiel MA, 2015. Corneal cross-linking for brittle cornea syndrome. Cornea. 34, 1326–1328. [DOI] [PubMed] [Google Scholar]
- Kedar S, Jayagopal LN, Berger JR, 2019. Neurological and ophthalmological manifestations of Varicella zoster virus. J. Neuroophthalmol 39, 220–231. [DOI] [PubMed] [Google Scholar]
- Kee AR, Gonzalez-Lopez JJ, Al-Hity A, Gupta B, Lee CS, Gunasekeran DV, Jayabalan N, Grant R, Kon OM, Gupta V, Westcott M, Pavesio C, Agrawal R, 2016. Anti-tubercular therapy for intraocular tuberculosis: A systematic review and meta-analysis. Surv. Ophthalmol 61, 628–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keklikci U, Soker SI, Sakalar YB, Unlu K, Ozekinci S, Tunik S, 2008. Efficacy of topical cyclosporin A 0.05% in conjunctival impression cytology specimens and clinical findings of severe vernal keratoconjunctivitis in children. Jpn. J. Ophthalmol 2, 357–362. [DOI] [PubMed] [Google Scholar]
- Keorochana N, 2016. A case report of Epstein-Barr virus-associated retinal vasculitis: successful treatment using only acyclovir therapy. Int. Med. Case Rep. J 9, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keorochana N, Klanarongran K, Satayasoontorn K, Chaiamnuay S, 2017. Necrobiotic xanthogranuloma scleritis in a case of granulomatosis with polyangiitis (Wegener’s granulomatosis). Int. Med. Case. Rep. J 10, 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Peracha H, Ballhausen D, Wiesbauer A, Rohrbach M, Gautschi M, Mason RW, Giugliani R, Suzuki Y, Orii KE, Orii T, Tomatsu S, 2017. Epidemiology of mucopolysaccharidoses. Mol. Genet. Metab 121, 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido S, Sugita S, Horie S, Miyanaga M, Miyata K, Shimizu N, Morio T, Mochizuki M, 2008. Association of varicella zoster virus load in the aqueous humor with clinical manifestations of anterior uveitis in herpes zoster ophthalmicus and zoster sine herpete. Br. J. Ophthalmol 92, 505–508. [DOI] [PubMed] [Google Scholar]
- Kim GN, Yoo WS, Park MH, Chung JK, Han YS, Chung IY, Seo SW, Yoo JM, Kim SJ, 2018. Clinical features of herpes simplex keratitis in a Korean tertiary referral center: efficacy of oral antiviral and ascorbic acid on recurrence. Korean J. Ophthalmol 32, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim YH, Kim SC, 2011. Epidermolysis bullosa acquisita: a retrospective clinical analysis of 30 cases. Acta. Derm. Venereol 91, 307–312. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SE, Kim SC, 2012. Successful treatment of epidermolysis bullosa acquisita with rituximab therapy. J. Dermatol 39, 477–479. [DOI] [PubMed] [Google Scholar]
- Kiss S, Damico FM, Young LH, 2005. Ocular manifestations and treatment of syphilis. Semin. Ophthalmol 20, 161–167. [DOI] [PubMed] [Google Scholar]
- Kiuru S, 1992. Familial amyloidosis of the Finnish type (FAF). A clinical study of 30 patients. Acta. Neurol. Scand 86, 346–353. [DOI] [PubMed] [Google Scholar]
- Kiuru S, 1998. Gelsolin-related familial amyloidosis, Finnish type (FAF), and its variants found worldwide. Amyloid. 5, 55–66. [DOI] [PubMed] [Google Scholar]
- Kivelä T, Tarkkanen A, McLean I, Ghiso J, Frangione B, Haltia M, 1993. Immunohistochemical analysis of lattice corneal dystrophies types I and II. Br. J. Ophthalmol 77, 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen-Broekema N, van Bijsterveld OP, 1993. Limbal and corneal calcification in patients with chronic renal failure. Br. J. Ophthalmol 77, 569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenkler B, Sheardown H, 2004. Growth factors in the anterior segment: role in tissue maintenance, wound healing and ocular pathology. Exp. Eye Res 79, 677–688. [DOI] [PubMed] [Google Scholar]
- Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS, 2007. Topically applied naltrexone restores corneal reepithelialization in diabetic rats. J. Ocul. Pharmacol. Ther 23, 89–102. [DOI] [PubMed] [Google Scholar]
- Koelle DM, Ghiasi H, 2005. Prospects for developing an effective vaccine against ocular herpes simplex virus infection. Curr. Eye Res 30, 929–942. [DOI] [PubMed] [Google Scholar]
- Koga T, Ichinose K, Kawakami A, Tsokos GC, 2019. The role of IL-17 in systemic lupus erythematosus and its potential as a therapeutic target. Expert Rev. Clin. Immunol 15, 629–637. [DOI] [PubMed] [Google Scholar]
- Konradsen TR, Koivula A, Kugelberg M, Zetterström C, 2012. Corneal curvature, pachymetry, and endothelial cell density in Marfan syndrome. Acta. Ophthalmol 90, 375–379. [DOI] [PubMed] [Google Scholar]
- Konuk O, Aktas Z, Aksoy S, Onol M, Unal M, 2008. Hyperthyroidism and severity of orbital disease do not change the central corneal thickness in Graves’ ophthalmopathy. Eur. J. Ophthalmol 18, 125–127. [PubMed] [Google Scholar]
- Kösekahya P, Üçgül Atılgan C, Atılgan KG, Koç M, Tekin K, Çağlayan M, Göker YŞ, 2019. Corneal endothelial morphology and thickness changes in patients with gout. Turk. J. Ophthalmol 49, 178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosker M, Suri K, Hammersmith KM, Nassef AH, Nagra PK, Rapuano CJ, 2014. Another look at the association between diabetes and keratoconus. Cornea. 33, 774–779. [DOI] [PubMed] [Google Scholar]
- Kotecha A, Oddone F, Sinapis C, Elsheikh A, Sinapis D, Sinapis A, Garway-Heath DF., 2010. Corneal biomechanical characteristics in patients with diabetes mellitus. J. Cataract Refract. Surg 36, 1822–1828. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Mohammad G, 2020. Epigenetics and mitochondrial stability in the metabolic memory phenomenon associated with continued progression of diabetic retinopathy. Sci. Rep 10, 6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerov AA, Saghizadeh M, Ljubimov AV, 2016. Adenoviral gene therapy for diabetic keratopathy: effects on wound healing and stem cell marker expression in human organ-cultured corneas and limbal epithelial cells. J. Vis. Exp 7, e54058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerov AA, Saghizadeh M, Maguen E, Rabinowitz YS, Ljubimov AV, 2015. Persistence of reduced expression of putative stem cell markers and slow wound healing in cultured diabetic limbal epithelial cells. Mol. Vis 21, 1357–1367. [PMC free article] [PubMed] [Google Scholar]
- Kramerov AA, Shah R, Ding H, Holler E, Turjman S, Rabinowitz YS, Ghiam S, Maguen E, Svendsen CN, Saghizadeh M, Ljubimova JY, Ljubimov AV, 2021. Novel nanopolymer RNA therapeutics normalize human diabetic corneal wound healing and epithelial stem cells. Nanomedicine. 32, 102332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger AS, Gray LD, 1978. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect. Immun 19, 630–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kridin K, Kneiber D, Kowalski EH, Valdebran M, Amber KT, 2019. Epidermolysis bullosa acquisita: A comprehensive review. Autoimmun. Rev 18, 786–795. [DOI] [PubMed] [Google Scholar]
- Kulkarni M, Leszczynska A, Wei G, Winkler MA, Tang J, Funari VA, Deng N, Liu Z, Punj V, Deng SX, Ljubimov AV, Saghizadeh M, 2017. Genome-wide analysis suggests a differential microRNA signature associated with normal and diabetic human corneal limbus. Sci. Rep 7, 3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai N, Fukuda K, Fujitsu Y, Yamamoto K, Nishida T, 2006. Role of structural cells of the cornea and conjunctiva in the pathogenesis of vernal keratoconjunctivitis. Prog. Retin. Eye Res 25, 165–187. [DOI] [PubMed] [Google Scholar]
- Kumar A, Gao N, Standiford TJ, Gallo RL, Yu FS, 2010. Topical flagellin protects the injured corneas from Pseudomonas aeruginosa infection. Microbes Infect. 12, 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Orzalli MH, Knipe DM, 2017. Innate immune mechanisms and herpes simplex virus infection and disease. Adv. Anat. Embryol. Cell Biol 223, 49–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlutürk Karagöz I, Allahverdiyev A, Bağırova M, Abamor EŞ, Dinparvar S, 2020. Current approaches in treatment of diabetic retinopathy and future perspectives. J. Ocul. Pharmacol. Ther 36, 487–496. [DOI] [PubMed] [Google Scholar]
- Kwitko S, Feldon S, McDonnell PJ, 1992. Corneal topographic changes following strabismus surgery in Grave’s disease. Cornea. 11, 36–40. [DOI] [PubMed] [Google Scholar]
- Labetoulle M, Behndig A, Tassignon MJ, Nuijts R, Mencucci R, Güell JL, Pleyer U, Szaflik J, Rosen P, Bérard A, Chiambaretta F, Cochener-Lamard B, Intracameral Mydrane (ICMA) Ethics Group, 2020. Safety and efficacy of a standardized intracameral combination of mydriatics and anesthetic for cataract surgery in type-2 diabetic patients. BMC. Ophthalmol 20, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagali N, Wowra B, Dobrowolski D, Utheim TP, Fagerholm P, Wylegala E, 2018. Stage-related central corneal epithelial transformation in congenital aniridia-associated keratopathy. Ocul. Surf 16, 163–172. [DOI] [PubMed] [Google Scholar]
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC, ZOE-50 Study Group, 2015. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med 372, 2087–2096. [DOI] [PubMed] [Google Scholar]
- Lamberts A, Euverman HI, Terra JB, Jonkman MF, Horváth B, 2018. Effectiveness and safety of rituximab in recalcitrant pemphigoid diseases. Front. Immunol 9, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass JH, Benetz BA, Patel SV, Szczotka-Flynn LB, O’Brien R, Ayala AR, Maguire MG, Daoud YJ, Greiner MA, Hannush SB, Lee WB, Mauger TF, Menegay HJ, Mifflin MD, Raizman MB, Rose-Nussbaumer J, Schultze RL, Schmidt GA, Sugar A, Terry MA, Verdier DD, Cornea Preservation Time Study Group, 2019. Donor, recipient, and operative factors associated with increased endothelial cell loss in the Cornea Preservation Time Study. JAMA Ophthalmol. 137, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta L, Ludwig N, Krammes L, Stachon T, Fries FN, Mukwaya A, Szentmáry N, Seitz B, Wowra B, Kahraman M, Keller A, Meese E, Lagali N, Käsmann-Kellner B, 2020. Abnormal neovascular and proliferative conjunctival phenotype in limbal stem cell deficiency is associated with altered microRNA and gene expression modulated by PAX6 mutational status in congenital aniridia. Ocul. Surf S1542-0124(20)30077-X. doi: 10.1016/j.jtos.2020.04.014. [DOI] [PubMed] [Google Scholar]
- Latta L, Nordström K, Stachon T, Langenbucher A, Fries FN, Szentmáry N, Seitz B, Käsmann-Kellner B, 2019. Expression of retinoic acid signaling components ADH7 and ALDH1A1 is reduced in aniridia limbal epithelial cells and a siRNA primary cell based aniridia model. Exp. Eye Res 179, 8–17. [DOI] [PubMed] [Google Scholar]
- Leavitt JA, Butrus SI, 1991. Wegener’s granulomatosis presenting as dacryoadenitis. Cornea. 10, 542–545. [DOI] [PubMed] [Google Scholar]
- Lee HK, Kim MK, Oh JY, 2018. Corneal abnormalities in congenital aniridia: congenital central corneal opacity versus aniridia-associated keratopathy. Am. J. Ophthalmol 185, 75–80. [DOI] [PubMed] [Google Scholar]
- Lee HK, Lee JH, Kim M, Kariya Y, Miyazaki K, Kim EK, 2006. Insulin-like growth factor-1 induces migration and expression of laminin-5 in cultured human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci 47, 873–882. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim JH, Lee D, Chang JW, Shin JY, Seo JW, Seo MH, Moon NJ, 2018. Long-term clinical outcome of femtosecond laser-assisted lamellar keratectomy with phototherapeutic keratectomy in anterior corneal stromal dystrophy. Br. J. Ophthalmol 102, 31–36. [DOI] [PubMed] [Google Scholar]
- Lee PS-Y, Gao N, Dike M, Shkilnyy O, Me R, Zhang Y, Yu F-SX, 2019. Opposing effects of neuropilin-1 and -2 on sensory nerve regeneration in wounded corneas: role of Sema3C in ameliorating diabetic neurotrophic keratopathy. Diabetes. 68, 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Mesfin FB, 2020. Blindness. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. [Google Scholar]
- Lennarson P, Barney NP, 1995. Interstitial keratitis as presenting ophthalmic sign of sarcoidosis in a child. J. Pediatr. Ophthalmol. Strabismus 32, 194–196. [DOI] [PubMed] [Google Scholar]
- Leonardi A, 2013. Management of vernal keratoconjunctivitis. Ophthalmol. Ther 2, 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppin K, Behrendt AK, Reichard M, Stachs O, Guthoff RF, Baltrusch S, Eule JC, Vollmar B, 2014. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest. Ophthalmol. Vis. Sci 55, 3603–3615. [DOI] [PubMed] [Google Scholar]
- Leszczynska A, Kulkarni M, Ljubimov AV, Saghizadeh M, 2018. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci. Rep 8, 15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levtchenko E, de Graaf-Hess A, Wilmer M, van den Heuvel L, Monnens L, Blom H, 2004. Comparison of cystine determination in mixed leukocytes vs polymorphonuclear leukocytes for diagnosis of cystinosis and monitoring of cysteamine therapy. Clin. Chem 50, 1686–1688. [DOI] [PubMed] [Google Scholar]
- Li J, Qi X, Wang X, Li W, Li Y, Zhou Q, 2020. PTEN inhibition facilitates diabetic corneal epithelial regeneration by reactivating Akt signaling pathway. Transl. Vis. Sci. Technol 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Weng J, Mohan RR, Bennett GL, Schwall R, Wang ZF, Tabor K, Kim J, Hargrave S, Cuevas KH, Wilson SE, 1996. Hepatocyte growth factor and hepatocyte growth factor receptor in the lacrimal gland, tears, and cornea. Invest. Ophthalmol. Vis. Sci 37, 727–739. [PubMed] [Google Scholar]
- Li W, Wang X, Cheng J, Li J, Wang Q, Zhou Q, Li H, Xue J, Zhang Y, Yang L, Xie L, 2020. Leucine-rich α-2-glycoprotein-1 promotes diabetic corneal epithelial wound healing and nerve regeneration via regulation of matrix metalloproteinases. Exp. Eye Res 196, 108060. [DOI] [PubMed] [Google Scholar]
- Li X, Chan JF, Li KK, Tso EY, Yip CC, Sridhar S, Chung TW, Chiu KH, Hung DL, Wu AK, Chau SK, Liu R, Lung KC, Tam AR, Cheng VC, To KK, Chan KH, Hung IF, Yuen KY, 2020. Detection of SARS-CoV-2 in conjunctival secretions from patients without ocular symptoms. Infection. 1–9. doi: 10.1007/s15010-020-01524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaboe CA, Aldrich BT, Carter PC, Skeie JM, Burckart KA, Schmidt GA, Reed CR, Zimmerman MB, Greiner MA, 2017. Assessing the impact of diabetes mellitus on donor corneal endothelial cell density. Cornea. 36:561–566. [DOI] [PubMed] [Google Scholar]
- Liesegang T, 1999. Herpes simplex. Cornea. 18, 739. [PubMed] [Google Scholar]
- Liesegang TJ, 2001. Herpes simplex virus epidemiology and ocular importance. Cornea. 20, 1–13. [DOI] [PubMed] [Google Scholar]
- Lin J, Zhao GQ, Che CY, Yang SS, Wang Q, Li CG, 2013. Characteristics of ocular abnormalities in gout patients. Int. J. Ophthalmol 6, 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Sheng M, Liu Y, Wang P, Chen Y, Chen L, Wang W, Li B, 2015. Expression of SIRT1 and oxidative stress in diabetic dry eye. Int. J. Clin. Exp. Pathol 8, 7644–7653. [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu H, Lu X, Tombran-Tink J, Zhao S, 2020. PEDF attenuates ocular surface damage in diabetic mice model through its antioxidant properties. Curr. Eye Res 30, 1–7. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, 2017. Diabetic complications in the cornea. Vis. Res 139, 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimov AV, Huang ZS, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC, 1998. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J. Histochem. Cytochem 46, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M, 2015. Progress in corneal wound healing. Prog. Retin. Eye Res 49, 17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WR, Broocker G, Grossniklaus HE, 2005. Histopathologic examination of conjunctival tophi in gouty arthritis. Am. J. Ophthalmol 140, 1152–1154. [DOI] [PubMed] [Google Scholar]
- Lobo AM, Agelidis AM, Shukla D, 2019. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul. Surf 17, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JH, Ross CA, Flaherty M, 2018. Corneal arcus as the presenting sign of familial hypercholesterolemia in a young child. J. AAPOS 22, 467–468. [DOI] [PubMed] [Google Scholar]
- Lokugamage KG, Hage A, de Vries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, Rajsbaum R, Menachery VD, 2020. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J. Virol 94, e01410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CW, Zhou DD, Wang J, Hao JL, 2016. Surgical treatment of peripheral ulcerative keratitis and necrotizing scleritis in granulomatosis with polyangiitis. Saudi Med. J 37, 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke J, Boehringer D, Eberwein P, Reinhard T, 2017. Corneal K-values as a diagnostic screening tool for Marfan syndrome. Cornea. 36, 700–703. [DOI] [PubMed] [Google Scholar]
- Lv H, Li A, Zhang X, Xu M, Qiao Y, Zhang J, Yu L, 2014. Meta-analysis and review on the changes of tear function and corneal sensitivity in diabetic patients. Acta Ophthalmol. 92, e96–e104. [DOI] [PubMed] [Google Scholar]
- Madonna R, Balistreri CR, Geng YJ, De Caterina R, 2017. Diabetic microangiopathy: Pathogenetic insights and novel therapeutic approaches. Vascul. Pharmacol 90, 1–7. [DOI] [PubMed] [Google Scholar]
- Mahgoub MM, Macky TA, 2014. Changes in corneal sensation following 20 and 23 G vitrectomy in diabetic and non-diabetic patients. Eye (Lond). 28, 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovoz B, Moeller R, Zebitz Eriksen A, tenOever BR, Blenkinsop TA, 2020. SARS-CoV-2 infection of ocular cells from human adult donor eyes and hESC-derived eye organoids. SSRN. 3650574.32742243 [Google Scholar]
- Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bowen JM, Brady AF, Burrows NP, Castori M, Cohen H, Colombi M, Demirdas S, De Backer J, De Paepe A, Fournel-Gigleux S, Frank M, Ghali N, Giunta C, Grahame R, Hakim A, Jeunemaitre X, Johnson D, Juul-Kristensen B, Kapferer-Seebacher I, Kazkaz H, Kosho T, Lavallee ME, Levy H, Mendoza-Londono R, Pepin M, Pope FM, Reinstein E, Robert L, Rohrbach M, Sanders L, Sobey GJ, Van Damme T, Vandersteen A, van Mourik C, Voermans N, Wheeldon N, Zschocke J, Tinkle B, 2017. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C. Semin. Med. Genet 175, 8–26. [DOI] [PubMed] [Google Scholar]
- Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR, 2008. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmology. 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor H, Tan HC, Lin MT, Mehta JS, Liu YC, 2020. Diabetic corneal neuropathy. J. Clin. Med 9, E3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M, 2018. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul. Surf 16, 45–57. [DOI] [PubMed] [Google Scholar]
- Martins EN, Alvarenga LS, Höfling-Lima AL, Freitas D, Zorat-Yu MC, Farah ME, Mannis MJ, 2004. Aerobic bacterial conjunctival flora in diabetic patients. Cornea. 23, 136–142. [DOI] [PubMed] [Google Scholar]
- Mastropasqua L, Nubile M, Lanzini M, Carpineto P, Toto L, Ciancaglini M, 2006. Corneal and conjunctival manifestations in Fabry disease: in vivo confocal microscopy study. Am. J. Ophthalmol 141, 709–718. [DOI] [PubMed] [Google Scholar]
- Matlock HG, Qiu F, Malechka V, Zhou K, Cheng R, Benyajati S, Whelchel A, Karamichos D, Ma JX, 2020. Pathogenic role of PPARα downregulation in corneal nerve degeneration and impaired corneal sensitivity in diabetes. Diabetes. 69, 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba AY, 1990. Ocular disease associated with Epstein-Barr virus infection. Surv. Ophthalmol 35, 145–150. [DOI] [PubMed] [Google Scholar]
- Matundan H, Ghiasi H, 2019. Herpes simplex virus 1 ICP22 suppresses CD80 expression by murine dendritic cells. J. Virol 93, e01803–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxcy KF, 1919. The transmission of infection through the eye. J. Am. Med. Assoc 72, 636–639. [Google Scholar]
- Mayhall CG, 2003. The epidemiology of burn wound infections: Then and now. Clin. Infect. Dis 37, 543–550. [DOI] [PubMed] [Google Scholar]
- McDermott AM, Xiao TL, Kern TS, Murphy CJ, 2003. Non-enzymatic glycation in corneas from normal and diabetic donors and its effects on epithelial cell attachment in vitro. Optometry. 74, 443–452. [PubMed] [Google Scholar]
- McKay TB, Priyadarsini S, Karamichos D, 2019. Mechanisms of collagen crosslinking in diabetes and keratoconus. Cells. 8, 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Sassani JW, Klocek MS, Zagon IS, 2010. Diabetic keratopathy and treatment by modulation of the opioid growth factor (OGF)-OGF receptor (OGFr) axis with naltrexone: a review. Brain Res. Bull 81, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Sassani JW, Zagon IS, 2020. Naltrexone as a novel therapeutic for diabetic corneal complications. J. Cell Immunol 2, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer EM, Foster CS, 1999. Vasculitic peripheral ulcerative keratitis. Surv. Ophthalmol 43, 379–396. [DOI] [PubMed] [Google Scholar]
- Messmer EM, May CA, Stefani FH, Welge-Luessen U, Kampik A, 2002. Toxic eosinophil granule protein deposition in corneal ulcerations and scars associated with atopic keratoconjunctivitis. Am. J. Ophthalmol 134, 816–821. [DOI] [PubMed] [Google Scholar]
- Michaud L, 2019. Longitudinal study on ocular manifestations in a cohort of patients with Fabry disease. PLoS One. 14, e0213329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczak J, Zimmermann H, Kheirkhah A, Kadas EM, Oberwahrenbrock T, Muller R, Ren A, Kuchling J, Dietze H, Prüss H, Paul F, Hamrah P, Brandt AU, 2017. Patients with multiple sclerosis demonstrate reduced subbasal corneal nerve fibre density. Mult. Scler 23, 1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor M, Payne E, 2020. Herpes zoster ophthalmicus. In: StatPearls. Treasure Island (FL): StatPearls Publishing. [PubMed] [Google Scholar]
- Misra SL, Braatvedt GD, Patel DV, 2016. Impact of diabetes mellitus on the ocular surface: a review. Clin. Exp. Ophthalmol 44, 278–288. [DOI] [PubMed] [Google Scholar]
- Mocan MC, Durukan I, Irkec M, Orhan M, 2006. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 25, 769–773. [DOI] [PubMed] [Google Scholar]
- Módis L Jr., Szalai E, Kertész K, Kemény-Beke A, Kettesy B, Berta A, 2010. Evaluation of the corneal endothelium in patients with diabetes mellitus type I and II. Histol. Histopathol 25. 1531–1537. [DOI] [PubMed] [Google Scholar]
- Mohammadpour M 2007. LASIK and systemic contraindications. Ophthalmology. 114, 1032–1033. [DOI] [PubMed] [Google Scholar]
- Mohammadpour M, Javadi MA, 2006. Keratitis associated with multiple endocrine deficiency. Cornea. 25, 112–114. [DOI] [PubMed] [Google Scholar]
- Mohammadpour M, Javadi MA, Karimian F, 2006. Limbal stem cell deficiency in the context of autoimmune polyendocrinopathy. Eur. J. Ophthalmol 16, 870–872. [DOI] [PubMed] [Google Scholar]
- Mohammed I, Said DG, Nubile M, Mastropasqua L, Dua HS, 2019. Cathelicidin-derived synthetic peptide improves therapeutic potential of vancomycin against Pseudomonas aeruginosa. Front. Microbiol 10, 2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Martin LM, Sinha NR, 2020. Novel insights into gene therapy in the cornea. Exp. Eye. Res 108361. doi: 10.1016/j.exer.2020.108361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora ML, Smith RE, 2013. Corneal and systemic diseases. In: Duane’s Ophthalmology, Tasman W, Jaeger EA, eds., Lippincott Williams & Wilkins, Philadelphia, v 4. Ch 15. [Google Scholar]
- Mostafa YS, Osman AA, Hassanein DH, Zeid AM, Sherif AM, 2018. Iris reconstruction using artificial iris prosthesis for management of aniridia. Eur. J. Ophthalmol 28, 103–107. [DOI] [PubMed] [Google Scholar]
- Muangman P, Muffley LA, Anthony JP, Spenny ML, Underwood RA, Olerud JE, Gibran NS, 2004. Nerve growth factor accelerates wound healing in diabetic mice. Wound Repair Regen. 12, 44–52. [DOI] [PubMed] [Google Scholar]
- Muenzer J, Lamsa JC, Garcia A, Dacosta J, Garcia J, Treco DA, 2002. Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta. Paediatr. Suppl 91, 98–99. [DOI] [PubMed] [Google Scholar]
- Mulcahy LR, Isabella VM, Lewis K, 2014. Pseudomonas aeruginosa biofilms in disease. Microb. Ecol 68:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaem G, Rosner MH, 2012. Ocular problems in the patient with end-stage renal disease. Semin. Dial 25, 403–407. [DOI] [PubMed] [Google Scholar]
- Munjal A, Kaufman EJ, 2020. Arcus senilis. In: StatPearls. Treasure Island (FL): StatPearls Publishing. From: https://www.ncbi.nlm.nih.gov/books/NBK554370/. [PubMed] [Google Scholar]
- Nagai N, Ogata F, Kawasaki N, Ito Y, Funakami Y, Okamoto N, Shimomura Y, 2015. Hypercalcemia Leads to Delayed Corneal Wound Healing in Ovariectomized Rats. Biol. Pharm. Bull 38, 1063–1069. [DOI] [PubMed] [Google Scholar]
- Naik MN, Vasanthapuram VH, Joseph J, Murthy SI, 2019. Microbial keratitis in thyroid eye disease: clinical features, microbiological profile, and treatment outcome. Ophthalmic Plast. Reconstr. Surg 35, 543–548. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kawahara M, Morishige N, Chikama T, Nakata K, Nishida T, 2003. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia 46, 839–842. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Sotozono C, Kinoshita S, 2001. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp. Eye Res 72, 511–517. [DOI] [PubMed] [Google Scholar]
- Negi A, Vernon SA, 2003. An overview of the eye in diabetes. J. R. Soc. Med 96, 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterova G, Gahl W, 2008. Nephropathic cystinosis: late complications of a multisystemic disease. Pediatr. Nephrol 23, 863–878. [DOI] [PubMed] [Google Scholar]
- Nesterova G, Gahl WA, 2013. Cystinosis: the evolution of a treatable disease. Pediatr. Nephrol 28, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll MP, Proença JT, Connor V, Efstathiou S, 2012. Influence of herpes simplex virus 1 latency-associated transcripts on the establishment and maintenance of latency in the ROSA26R reporter mouse model. J. Virol 86, 8848–8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK, 2007. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 8, 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoskinen T, Schmidt EK, Strbian D, Kiuru-Enari S, Atula S, 2015. Natural course of Finnish gelsolin amyloidosis. Ann. Med 47, 506–511. [DOI] [PubMed] [Google Scholar]
- Noh H, Lee JI, 2014. Current and potential therapeutic strategies for mucopolysaccharidoses. J. Clin. Pharm. Ther 39, 215–224. [DOI] [PubMed] [Google Scholar]
- Noor FM, Islam MM, 2020. Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis. J. Community Health 45, 1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norina TJ, Raihan S, 2008. Microbial keratitis: aetiological diagnosis and clinical features in patients admitted to Hospital Universiti Sains Malaysia. Singapore Med. J 49:67–71. [PubMed] [Google Scholar]
- Nowalk A, Green M, 2016. Epstein-Barr virus. Microbiol. Spectr 4. [DOI] [PubMed] [Google Scholar]
- Oates JC, Levesque MC, Hobbs MR, Smith EG, Molano ID, Page GP, Hill BS, Weinberg JB, Cooper GS, Gilkeson GS, 2003. Nitric oxide synthase 2 promoter polymorphisms and systemic lupus erythematosus in african-americans. J. Rheumatol 30, 60–67. [PubMed] [Google Scholar]
- Oh JY, Kim MK, Choi HJ, Ko JH, Kang EJ, Lee HJ, Wee WR, Lee JH, 2011. Investigating the relationship between serum interleukin-17 levels and systemic immune-mediated disease in patients with dry eye syndrome. Korean J. Ophthalmol 25, 73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohguro N, Matsuda M, Fukuda M, 1999. Corneal endothelial changes in patients with chronic renal failure. Am. J. Ophthalmol 128, 234–236. [DOI] [PubMed] [Google Scholar]
- Oluleye TS, 2013. Tuberculous uveitis. J. Multidiscip. Healthc 6, 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten JV, Hashimoto T, Hertl M, Payne AS, Sitaru C, 2014. Molecular diagnosis in autoimmune skin blistering conditions. Curr. Mol. Med 14, 69–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CG, Newsom RSB, Rudnicka AR, Ellis TJ, Woodward EG, 2005. Vascular response of the bulbar conjunctiva to diabetes and elevated blood pressure. Ophthalmology. 112, 1801–1808. [DOI] [PubMed] [Google Scholar]
- Papanas N, Ziegler D, 2013. Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Curr. Diab. Rep 13, 488–499. [DOI] [PubMed] [Google Scholar]
- Park GB, Kim D, Kim YS, Kim S, Lee HK, Yang JW, Hur DY, 2014. The Epstein-Barr virus causes epithelial-mesenchymal transition in human corneal epithelial cells via Syk/src and Akt/Erk signaling pathways. Invest. Ophthalmol. Vis. Sci 55, 1770–1779. [DOI] [PubMed] [Google Scholar]
- Park JH, Kang SS, Kim JY, Tchah H, 2016. Nerve growth factor attenuates apoptosis and inflammation in the diabetic cornea. Invest. Ophthalmol. Vis. Sci 57, 6767–6775. [DOI] [PubMed] [Google Scholar]
- Pasadhika S, Rosenbaum JT, 2015. Ocular sarcoidosis. Clin. Chest. Med 36, 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DV, 2017. Systemic associations of corneal deposits: a review and photographic guide. Clin. Exp. Ophthalmol 45, 14–23. [DOI] [PubMed] [Google Scholar]
- Patel S, Hwang J, Mehra D, Galor A, 2020. Corneal nerve abnormalities in ocular and systemic diseases. Exp. Eye Res October 10:108284. doi: 10.1016/j.exer.2020.108284. [DOI] [PubMed] [Google Scholar]
- Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D, 2005. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J. Clin. Endocrinol. Metab 90, 135–141. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Sebastiani S, Tucci L, Giannaccare G, Moscatiello S, Laffi G, Pagotto U, Di Dalmazi G, Versura P, 2020. Association between alterations of corneal sub-basal nerve plexus analyzed with in vivo confocal microscopy and long-term glycemic variability. Eur. J. Ophthalmol 1120672120964126. [DOI] [PubMed] [Google Scholar]
- Penfornis A, Kury-Paulin S, 2006. Immunosuppressive drug-induced diabetes. Diabetes Metab. 32(5 Pt 2), 539–546. [DOI] [PubMed] [Google Scholar]
- Peng RM, Guo YX, Xiao GG, Li CD, Hong J, 2019. Characteristics of corneal endotheliitis among different viruses by in vivo confocal microscopy. Ocul. Immunol. Inflamm 1–9. doi: 10.1080/09273948.2019.1678648 [DOI] [PubMed] [Google Scholar]
- Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R, 2016. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul. Surf 14, 207–215. [DOI] [PubMed] [Google Scholar]
- Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez FM, Castillo Laguarta JM, Del Estad Cabello A, Gessa Sorroche M, España Gregori E, Sales-Sanz M, Tocilizumab in Graves Orbitopathy Study Group, 2018. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant Graves orbitopathy: a randomized clinical trial. Am. J. Ophthalmol 195, 181–190. [DOI] [PubMed] [Google Scholar]
- Petrohelos M, Ticoulis D, Diamantacos P, 1977. Band keratopathy with bilateral deafness as a presenting sign of hyperparathyroidism. Br. J. Ophthalmol 61, 494–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos IN, Green P, Chan AW, Alam U, Fadavi H, Marshall A, Asghar O, Efron N, Tavakoli M, Malik RA, 2015. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PLoS One. 10, e0123517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos IN, Kamran S, Li Y, Khan A, Ponirakis G, Akhtar N, Deleu D, Shuaib A, Malik RA, 2017. Corneal confocal microscopy: an imaging endpoint for axonal degeneration in multiple sclerosis. Invest. Ophthalmol. Vis. Sci 58, 3677–3681. [DOI] [PubMed] [Google Scholar]
- Petrukhin K, Fischer SG, Pirastu M, Tanzi RE, Chernov I, Devoto M, Brzustowicz LM, Cayanis E, Vitale E, Russo JJ, Matseoane D, Boukhgalter B, Wasco W, Figus AL, Loudianos J, Cao A, Sternlieb I, Evgrafov O, Parano E, Pavone L, Warburton D, Ott J, Penchaszadeh GK, Scheinberg IH, Gilliam TC, 1993. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat. Genet 5, 338–343. [DOI] [PubMed] [Google Scholar]
- Peyman A, Nayebzadeh M, Peyman M, Afshari NA, Pourazizi M, 2019. Topical cyclosporine-A versus prednisolone for herpetic stromal keratitis: a randomized controlled trial. Acta. Ophthalmol 97, e194–e198. [DOI] [PubMed] [Google Scholar]
- Pihlamaa T, Rautio J, Kiuru-Enari S, Suominen S, 2011. Gelsolin amyloidosis as a cause of early aging and progressive bilateral facial paralysis. Plast. Reconstr. Surg 127, 2342–2351. [DOI] [PubMed] [Google Scholar]
- Pilkey J, Streeter L, Beel A, Hiebert T, Li X, 2012. Corticosteroid-induced diabetes in palliative care. J. Palliat. Med 15, 681–689. [DOI] [PubMed] [Google Scholar]
- Pinard C, Hebert V, Lecuyer M, Sacre L, Joly P, 2019. Linear IgA bullous dermatosis treated with rituximab. JAAD Case Rep. 5, 124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna A, Usai D, Sechi LA, Molicotti P, Zanetti S, Carta A, 2008. Detection of virulence factors in Pseudomonas aeruginosa strains isolated from contact lens-associated corneal ulcers. Cornea. 27, 320–326. [DOI] [PubMed] [Google Scholar]
- Pitz S, Kalkum G, Arash L, Karabul N, Sodi A, Larroque S, Beck M, Gal A, 2015. Ocular signs correlate well with disease severity and genotype in Fabry disease. PLoS One. 10, e0120814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantone D, Koudriavtseva T, 2018. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin. Drug Investig 38, 653–671. [DOI] [PubMed] [Google Scholar]
- Ponder KP, 2008. Immune response hinders therapy for lysosomal storage diseases. J. Clin. Invest 118, 2686–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Crombie AL, 1973a. Corneal calcification as a presenting and diagnostic sign in hyperparathyroidism. Br. J. Ophthalmol 57, 665–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Crombie AL, 1973b. Corneal and conjunctival calcification in chronic renal failure. Br. J. Ophthalmol 57, 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power WJ, Tugal-Tutkun I, Foster CS, 1998. Long-term follow-up of patients with atopic keratoconjunctivitis. Ophthalmology. 105, 637–642. [DOI] [PubMed] [Google Scholar]
- Prados-Moreno S, Sabio JM, Pérez-Mármol JM, Navarrete-Navarrete N, Peralta-Ramírez MI, 2018. Adherence to treatment in patients with systemic lupus erythematosus. Med. Clin. (Barc) 150, 8–15. [DOI] [PubMed] [Google Scholar]
- Priyadarsini S, McKay TB, Sarker-Nag A, Allegood J, Chalfant C, Ma JX, Karamichos D, 2016. Complete metabolome and lipidome analysis reveals novel biomarkers in the human diabetic corneal stroma. Exp. Eye Res 153, 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini S, Whelchel A, Nicholas S, Sharif R, Riaz K, Karamichos D, 2020. Diabetic keratopathy: Insights and challenges. Surv. Ophthalmol 65, 513–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prummel MF, Mourits MP, Berghout A, Krenning EP, van der Gaag R, Koornneef L, Wiersinga WM, 1989. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N. Engl. J. Med 321, 1353–1359. [DOI] [PubMed] [Google Scholar]
- Pucci N, Novembre E, Cianferoni A, Lombardi E, Bernardini R, Caputo R, Campa L, Vierucci A, 2002. Efficacy and safety of cyclosporine eyedrops in vernal keratoconjunctivitis. Ann. Allergy Asthma Immunol 89, 298–303. [DOI] [PubMed] [Google Scholar]
- Quadrado MJ, Popper M, Morgado AM, Murta JN, Van Best JA, 2006. Diabetes and corneal cell densities in humans by in vivo confocal microscopy. Cornea. 25, 761–768. [DOI] [PubMed] [Google Scholar]
- Rafailidis PI, Mavros MN, Kapaskelis A, Falagas ME, 2010. Antiviral treatment for severe EBV infections in apparently immunocompetent patients. J. Clin. Virol 49, 151–157. [DOI] [PubMed] [Google Scholar]
- Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF, 2015. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 23, 231–269. [DOI] [PubMed] [Google Scholar]
- Rajasagi NK, Rouse BT, 2018. Application of our understanding of pathogenesis of herpetic stromal keratitis for novel therapy. Microbes Infect. 20, 526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L, Herber R, Spoerl E, Pillunat LE, Terai N, 2020. Factors influencing corneal biomechanics in diabetes mellitus. Cornea. 39, 552–557. [DOI] [PubMed] [Google Scholar]
- Ramos-Casals M, Brito-Zerón P, Bombardieri S, Bootsma H, De Vita S, Dörner T, Fisher BA, Gottenberg JE, Hernandez-Molina G, Kocher A, Kostov B, Kruize AA, Mandl T, Ng WF, Retamozo S, Seror R, Shoenfeld Y, Sisó-Almirall A, Tzioufas AG, Vitali C, Bowman S, Mariette X; EULAR-Sjögren Syndrome Task Force Group, 2020. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis 79, 3–18. [DOI] [PubMed] [Google Scholar]
- Ramos-Castellón C, Ortiz-Nieva G, Fresán F, Villalvazo L, Garfias Y, Navas A, Jiménez-Martínez MC, 2010. Ocular involvement and blindness secondary to linear IgA dermatosis. J. Ophthalmol 2010, 280396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SC, Tajunisah I, Rohana T, 2011. Bilateral scleromalacia perforans and peripheral corneal thinning in Wegener’s granulomatosis. Int. J. Ophthalmol 4, 439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehany U, Ishii Y, Lahav M, Rumelt S, 2000a. Collagen pleomorphism in Descemet’s membrane of streptozotocin-induced diabetic rats: an electron microscopy study. Cornea. 19, 390–392. [DOI] [PubMed] [Google Scholar]
- Rehany U, Ishii Y, Lahav M, Rumelt S, 2000b. Ultrastructural changes in corneas of diabetic patients: an electron-microscopy study. Cornea. 19, 534–538. [DOI] [PubMed] [Google Scholar]
- Reynolds I, Tullo AB, John SL, Holt PJ, Hillarby MC, 1999. Corneal epithelial-specific cytokeratin 3 is an autoantigen in Wegener’s granulomatosis-associated peripheral ulcerative keratitis. Invest. Ophthalmol. Vis. Sci 40, 2147–2151. [PubMed] [Google Scholar]
- Rice JB, White AG, Scarpati LM, Wan G, Nelson WW, 2017. Long-term systemic corticosteroid exposure: a systematic literature review. Clin. Ther 39, 2216–2229. [DOI] [PubMed] [Google Scholar]
- Richdale K, Chao C, Hamilton M, 2020. Eye care providers’ emerging roles in early detection of diabetes and management of diabetic changes to the ocular surface: a review. B.M.J. Open Diabetes Res. Care 8, e001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridolo E, Kihlgren P, Pellicelli I, Nizi MC, Pucciarini F, Incorvaia C, 2019. Atopic keratoconjunctivitis: pharmacotherapy for the elderly. Drugs Aging. 36, 581–588. [DOI] [PubMed] [Google Scholar]
- Rischmueller M, Tieu J, Lester S, 2016. Primary Sjögren’s syndrome. Best Pract. Res. Clin. Rheumatol 30, 189–220. [DOI] [PubMed] [Google Scholar]
- Robinson PN, Godfrey M, 2000. The molecular genetics of Marfan syndrome and related microfibrillopathies. J. Med. Genet 37(1), 9–25. doi: 10.1136/jmg.37.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock DL, Nesburn AB, Ghiasi H, Ong J, Lewis TL, Lokensgard JR, Wechsler SL, 1987. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol 61, 3820–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrich H, Yuan C, Hou JH, 2020. Immunohistochemical study of SARS-CoV-2 viral entry factors in the cornea and ocular surface. Cornea. 39, 1556–1562. [DOI] [PubMed] [Google Scholar]
- Rohatgi J, Dhaliwal U, 2000. Phlyctenular eye disease: a reappraisal. Jpn. J. Ophthalmol 44, 146–50. [DOI] [PubMed] [Google Scholar]
- Rombach SM, Smid BE, Linthorst GE, Dijkgraaf MG, Hollak CE, 2014. Natural course of Fabry disease and the effectiveness of enzyme replacement therapy: a systematic review and meta-analysis: effectiveness of ERT in different disease stages. J. Inherit. Metab. Dis 37, 341–352. [DOI] [PubMed] [Google Scholar]
- Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH, 2000. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest. Ophthalmol. Vis. Sci 41, 2915–2921. [PubMed] [Google Scholar]
- Roszkowska AM, Licitra C, Tumminello G, Postorino EI, Colonna MR, Aragona P, 2020. Corneal nerves in diabetes - The role of the in vivo corneal confocal microscopy of the subbasal nerve plexus in the assessment of peripheral small fiber neuropathy. Surv. Ophthalmol S0039-6257(20)30133-30138. doi: 10.1016/j.survophthal.2020.09.003. [DOI] [PubMed] [Google Scholar]
- Rousseau A, Prost-Squarcioni C, Doan S, Leroux-Villet C, Caux F, Hoang-Xuan T, Cochereau I, Gabison E, 2020. Ocular involvement in epidermolysis bullosa acquisita with long-term follow-up. Br. J. Ophthalmol 104, 235–240. [DOI] [PubMed] [Google Scholar]
- Roux LN, Petit I, Domart R, Concordet JP, Qu J, Zhou H, Joliot A, Ferrigno O, Aberdam D, 2018. Modeling of aniridia-related keratopathy by CRISPR/Cas9 genome editing of human limbal epithelial cells and rescue by recombinant PAX6 protein. Stem Cells. 36, 1421–1429. [DOI] [PubMed] [Google Scholar]
- Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL, 2013. Herpes keratitis. Prog. Retin. Eye. Res 32, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboo US, Basu S, Tiwari S, Mohamed A, Vemuganti GK, Sangwan VS, 2013. Clinical and cytologic evidence of limbal stem cell deficiency in eyes with long-standing vernal keratoconjunctivitis. Asia Pac. J. Ophthalmol. (Phila) 2, 88–93. [DOI] [PubMed] [Google Scholar]
- Sady C, Khosrof S, Nagaraj R, 1995. Advanced Maillard reaction and crosslinking of corneal collagen in diabetes. Biochem. Biophys. Res. Commun 214, 793–797. [DOI] [PubMed] [Google Scholar]
- Saghizadeh M, Brown DJ, Castellon R, Chwa M, Huang GH, Ljubimova JY, Rosenberg S, Spirin KS, Stolitenko RB, Adachi W, Kinoshita S, Murphy G, Windsor LJ, Kenney MC, Ljubimov AV, 2001a. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: a possible mechanism of basement membrane and integrin alterations. Am. J. Pathol 158, 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Chwa M, Aoki A, Lin B, Pirouzmanesh A, Brown DJ, Ljubimov AV, Kenney MC, 2001b. Altered expression of growth factors and cytokines in keratoconus, bullous keratopathy and diabetic human corneas. Exp. Eye Res 73, 179–189. [DOI] [PubMed] [Google Scholar]
- Saghizadeh M, Dib CM, Brunken WJ, Ljubimov AV, 2014. Normalization of wound healing and stem cell marker patterns in organ-cultured human diabetic corneas by gene therapy of limbal cells. Exp. Eye Res 129, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Epifantseva I, Hemmati DM, Ghiam CA, Brunken WJ, Ljubimov AV, 2013. Enhanced wound healing, kinase and stem cell marker expression in diabetic organ-cultured human corneas upon MMP-10 and cathepsin F gene silencing. Invest. Ophthalmol. Vis. Sci 54, 8172–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Svendsen CN, Ljubimov AV, 2017. Concise review: stem cells for corneal wound healing. Stem Cells. 35, 2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Tajbakhsh J, Aoki AM, Wang C, Chai NN, Ljubimova JY, Sasaki T, Sosne G, Carlson MR, Nelson SF, Ljubimov AV, 2005. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest. Ophthalmol. Vis. Sci 46, 3604–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Yaghoobzadeh Y, Hu J, Ljubimova JY, Black KL, Castro MG, Ljubimov AV, 2010a. Adenovirus-driven overexpression of proteinases in organ-cultured normal human corneas leads to diabetic-like changes. Brain Res. Bull 81, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Yu FS, Castro MG, Ljubimov AV, 2010b. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Invest. Ophthalmol. Vis. Sci 51, 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Soleymani S, Harounian A, Bhakta B, Troyanovsky SM, Brunken WJ, Pellegrini G, Ljubimov AV, 2011. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol. Vis 17, 2177–2190. [PMC free article] [PubMed] [Google Scholar]
- Sah RP, Paudel N, Shrestha JB, Marfan’s syndrome: a refractive challenge for optometrists. Clin. Exp. Optom 96(6), 581–3. doi: 10.1111/cxo.12026. [DOI] [PubMed] [Google Scholar]
- Saini JS, Khandalavla B, 1995. Corneal epithelial fragility in diabetes mellitus. Can. J. Ophthalmol 30, 142–146. [PubMed] [Google Scholar]
- Sainz de la Maza M, Molina N, Gonzalez-Gonzalez LA, Doctor PP, Tauber J, Foster CS, 2012. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology. 119, 43–50. [DOI] [PubMed] [Google Scholar]
- Saito J, Enoki M, Hara M, Morishige N, Chikama T, Nishida T, 2003. Correlation of corneal sensation, but not of basal or reflex tear secretion, with the stage of diabetic retinopathy. Cornea. 22, 15–18. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Sasaki H, Kitagawa K, 2004. Successful treatment of diabetic keratopathy with punctal occlusion. Acta Ophthalmol. Scand 82, 115–117. [DOI] [PubMed] [Google Scholar]
- Salah S, Abad S, Monnet D, Brézin AP, 2018. Sarcoidosis. J. Fr. Ophtalmol 41, e451–e467. [DOI] [PubMed] [Google Scholar]
- Salami MO, Aribaba OT, Musa KO, Rotimi-Samuel A, Onakoya AO, 2020. Relationship between corneal sensitivity and diabetic retinopathy among diabetics attending a Nigerian Teaching Hospital. Int. Ophthalmol 40, 2707–2716. [DOI] [PubMed] [Google Scholar]
- Samiy N, 2008. Ocular features of Fabry disease: diagnosis of a treatable life-threatening disorder. Surv. Ophthalmol 53, 416–423. [DOI] [PubMed] [Google Scholar]
- Samoila O, Gocan D, 2020. Clinical outcomes from cultivated allogenic stem cells vs. oral mucosa epithelial transplants in total bilateral stem cells deficiency. Front. Med. (Lausanne) 7, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan VS, Jain V, Vemuganti GK, Murthy SI, 2011. Vernal keratoconjunctivitis with limbal stem cell deficiency. Cornea. 30, 491–496. [DOI] [PubMed] [Google Scholar]
- Sarma P, Das D, Deka P, Deka AC, 2010. Subconjunctival urate crystals: a case report. Cornea. 29, 830–832. [DOI] [PubMed] [Google Scholar]
- Sassani JW, Mc Laughlin PJ, Zagon IS, 2016. The Yin and Yang of the opioid growth regulatory system: focus on diabetes-The Lorenz E. Zimmerman Tribute Lecture. J. Diabetes Res 2016, 9703729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sati A, Jha A, Moulick PS, Shankar S, Gupta S, Khan MA, Dogra M, Sangwan VS, 2016. Corneal endothelial alterations in chronic renal failure. Cornea. 35, 1320–1325. [DOI] [PubMed] [Google Scholar]
- Sato N, Nakamura M, Chikama T, Nishida T, 1999. Abnormal deposition of laminin and type IV collagen at corneal epithelial basement membrane during wound healing in diabetic rats. Jpn. J. Ophthalmol 43, 343–347. [DOI] [PubMed] [Google Scholar]
- Sawant OB, Singh S, Wright RE, Jones KM, Titus MS, Dennis E, Hicks E, Majmudar PA, Kumar A, Mian SI, 2020. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul. Surf S1542-0124(20)30168-3. doi: 10.1016/j.jtos.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibenberger D, Frings A, Steinberg J, Schüler H, Druchkiv V, Katz T, von Kodolitsch Y, Linke S, 2018. Ocular manifestation in Marfan syndrome: corneal biomechanical properties relate to increased systemic score points. Graefes. Arch. Clin. Exp. Ophthalmol 256(6), 1159–1163. doi: 10.1007/s00417-018-3946-4. [DOI] [PubMed] [Google Scholar]
- Scheler A, Spoeri E, Boehm AG, 2012. Effect of diabetes mellitus on corneal biomechanics and measurement of intraocular pressure. Acta. Ophthalmol 90, e447–e451. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, 2015. Fabry disease. Handb. Clin. Neurol 132, 231–248. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Floeter MK, Dambrosia JM, Gupta S, Moore DF, Sharabi Y, Khurana RK, Brady RO, 2003. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 28, 703–710. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, 2018. Highlighting diabetes mellitus: the epidemic continues. Arterioscler. Thromb. Vasc. Biol 38, e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH, 1981. Diabetic keratopathy. Trans. Am. Ophthalmol. Soc 79, 180–199. [PMC free article] [PubMed] [Google Scholar]
- Schulze SD, Sekundo W, Kroll P, 2006. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am. J. Ophthalmol 142, 207–211. [DOI] [PubMed] [Google Scholar]
- Seah IYJ, Anderson DE, Kang AEZ, Wang L, Rao P, Young BE, Lye DC, Agrawal R, 2020. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 127, 977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler T, Huhle S, Spoeri E, Kunath E, 2000. Manifest diabetes and keratoconus: a retrospective case-control study. Graefes Arch. Clin. Exp. Ophthalmol 238, 822–825. [DOI] [PubMed] [Google Scholar]
- Serratrice N, Cubizolle A, Ibanes S, Mestre-Francés N, Bayo-Puxan N, Creyssels S, Gennetier A, Bernex F, Verdier JM, Haskins ME, Couderc G, Malecaze F, Kalatzis V, Kremer EJ, 2014. Corrective GUSB transfer to the canine mucopolysaccharidosis VII cornea using a helper-dependent canine adenovirus vector. J. Control. Release 181, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfiniadaki E, Tsiara I, Theodossiadis P, Chatziralli I, 2019. Ocular manifestations of granulomatosis with polyangiitis: a review of the literature. Ophthalmol. Ther 8, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams F, Livingstone I, Oladiwura D, Ramaesh K, 2014. Treatment of corneal cystine crystal accumulation in patients with cystinosis. Clin. Ophthalmol 8, 2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma GD, He J, Bazan HE, 2003. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J. Biol. Chem 278, 21989–21997. [DOI] [PubMed] [Google Scholar]
- Sharma N, Singhal D, Maharana PK, Jain R, Sahay P, Titiyal JS, 2018. Continuous intraoperative optical coherence tomography-guided shield ulcer debridement with tuck in multilayered amniotic membrane transplantation. Indian J. Ophthalmol 66, 816–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon Y, Schlesinger N, 2016. Beyond Joints: a review of ocular abnormalities in gout and hyperuricemia. Curr. Rheumatol. Rep 18, 37. [DOI] [PubMed] [Google Scholar]
- Sheha H, Tighe S, Hashem O, Hayashida Y, 2019. Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin. Ophthalmol 13, 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy R, Khandekar R, Bialasiewicz A, Al Muniri A, 2009. Corneal endothelium in patients with diabetes mellitus: a historical cohort study. Eur. J Ophthalmol 19, 369–375. [DOI] [PubMed] [Google Scholar]
- Shi L, Chen H, Yu X, Wu X, 2013a. Advanced glycation end products delay corneal epithelial wound healing through reactive oxygen species generation. Mol. Cell. Biochem 383, 253–259. [DOI] [PubMed] [Google Scholar]
- Shi L, Yu X, Yang H, Wu X, 2013b. Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways. PLoS One. 8, e66781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih KC, Lam KS, Tong L, 2017. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr. Diabetes 7, e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JI, Kim MJ, Lee JS, 2009. Graves’ disease, rheumatoid arthritis, and anti-tumor necrosis factor-α therapy. J. Rheumatol 36, 449–450. [DOI] [PubMed] [Google Scholar]
- Silva BC, Cusano NE, Bilezikian JP, 2018. Primary hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab 32, 593–607. [DOI] [PubMed] [Google Scholar]
- Singh G, Lingala B, Mithal A, 2019. Gout and hyperuricaemia in the USA: prevalence and trends. Rheumatology (Oxford). 58, 2177–2180. [DOI] [PubMed] [Google Scholar]
- Singh JA, Reddy SG, Kundukulam J, 2011. Risk factors for gout and prevention: a systematic review of the literature. Curr. Opin. Rheumatol 23, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracuse-Lee D, Saffra N, 2006. Peripheral ulcerative keratitis in sarcoidosis: a case report. Cornea. 25, 618–620. [DOI] [PubMed] [Google Scholar]
- Sirakaya E, Sahiner M, Sirakaya HA, 2020. A patient with bilateral conjunctivitis positive for SARS-Cov-2 RNA in conjunctival sample. Cornea 10.1097/ICO.0000000000002485. doi: 10.1097/ICO.0000000000002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirrs SM, Bichet DG, Casey R, Clarke JT, Lemoine K, Doucette S, West ML, CFDI investigators, 2014. Outcomes of patients treated through the Canadian Fabry disease initiative. Mol. Genet. Metab 111, 499–506. [DOI] [PubMed] [Google Scholar]
- Sivaraj RR, Durrani OM, Denniston AK, Murray PI, Gordon C, 2007. Ocular manifestations of systemic lupus erythematosus. Rheumatology (Oxford). 46, 1757–1762. [DOI] [PubMed] [Google Scholar]
- Slobod KS, Sandlund JT, Spiegel PH, Haik B, Hurwitz JL, Conley ME, Bowman LC, Benaim E, Jenkins JJ, Stocks RM, Gan Y, Sixbey JW, 2000. Molecular evidence of ocular Epstein-Barr virus infection. Clin. Infect. Dis 31, 184–188. [DOI] [PubMed] [Google Scholar]
- Smith JR, Kupa A, Coster DJ, 1999. Linear IgA disease. Aust. N. Z. J. Ophthalmol 27, 443–446. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Hegedüs L, 2016. Graves’ disease. N. Engl. J. Med 375, 1552–1565. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Müller-Ladner U, Mysler EF, da Silva JAP, Poór G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D, 2020. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis 79, 685–699. [DOI] [PubMed] [Google Scholar]
- Sobol EK, Fargione RA, Atiya M, Diaz JD, Powell JA, Gritz DC, 2016. Case-control study of herpes simplex eye disease: Bronx epidemiology of human immunodeficiency virus eye studies. Cornea. 35, 801–806. [DOI] [PubMed] [Google Scholar]
- Sokol JA, Foulks GN, Haider A, Nunery WR, 2010. Ocular surface effects of thyroid disease. Ocul. Surf 8, 29–39. [DOI] [PubMed] [Google Scholar]
- Solmaz N, Önder F, Demir N, Altuntaş Aydın Ö, 2018. Primary conjunctival tuberculosis. Turk. J. Ophthalmol 48, 39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somayajulu M, McClellan SA, Pitchaikannu A, Bessert D, Liu L, Steinle J, Hazlett LD, 2021. Effects of glycyrrhizin treatment on diabetic cornea. J. Ocul. Pharmacol. Ther 21 December 2020. doi: 10.1089/jop.2020.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosne G, 2018. Thymosin beta 4 and the eye: the journey from bench to bedside. Expert Opin. Biol. Ther 18(sup1), 99–104. [DOI] [PubMed] [Google Scholar]
- Sosne G, Qiu P, Kurpakus-Wheater M, Matthew H, 2010. Thymosin β4 and corneal wound healing: visions of the future. Ann. N. Y. Acad. Sci 1194, 190–198. [DOI] [PubMed] [Google Scholar]
- Spada M, Baron R, Elliott PM, Falissard B, Hilz MJ, Monserrat L, Tøndel C, Tylki-Szymańska A, Wanner C, Germain DP, 2019. The effect of enzyme replacement therapy on clinical outcomes in paediatric patients with Fabry disease - A systematic literature review by a European panel of experts. Mol. Genet. Metab 126, 212–223. [DOI] [PubMed] [Google Scholar]
- Spada M, Enea A, Morrone A, Fea A, Porta F, 2013. Cornea verticillata and Fabry disease. J. Pediatr 163, 609. [DOI] [PubMed] [Google Scholar]
- Sridhar MS, Rangaraju A, Anbarasu K, Reddy SP, Daga S, Jayalakshmi S, Shaik B, 2017. Evaluation of Kayser-Fleischer ring in Wilson disease by anterior segment optical coherence tomography. Indian J. Ophthalmol 65, 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Makarenkova HP, 2020. Innate immunity and biological therapies for the treatment of Sjögren’s syndrome. Int. J. Mol. Sci 21, 9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck T, Kenyon KR, Hanninen LA, Beyer-Machule C, Fabian R, Gorn RA, McMullan FD, Baum J, McAdam KP, 1991. Clinical and histopathologic studies of two families with lattice corneal dystrophy and familial systemic amyloidosis (Meretoja syndrome). Ophthalmology. 98, 1197–206. [DOI] [PubMed] [Google Scholar]
- Stefanski AL, Tomiak C, Pleyer U, Dietrich T, Burmester GR, Dörner T, 2017. The diagnosis and treatment of Sjögren’s syndrome. Dtsch. Arztebl. Int 114, 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stem MS, Hussain M, Lentz SI, Raval N, Gardner TW, Pop-Busui R, Shtein RM, 2014. Differential reduction of corneal nerve fiber length in patients with type 1 or type 1 diabetes mellitus. J. Diabetes Complications 28, 658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Tadvalkar G, Hakh R, Pal-Ghosh S, 2017. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia. 65, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simó R, Lois N, 2016. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res 51, 156–86. [DOI] [PubMed] [Google Scholar]
- Strianese D, Rossi F, 2019. Interruption of autoimmunity for thyroid eye disease: B-cell and T-cell strategy. Eye (Lond). 33, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuard WL, Titone R, Robertson DM, 2020a. The IGF/insulin-IGFBP axis in corneal development, wound healing, and disease. Front. Endocrinol. (Lausanne) 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuard WL, Titone R, Robertson DM, 2020b. Tear levels of IGFBP-3: a potential biomarker for diabetic nerve changes in the cornea. Eye Contact Lens. 46, 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan NL, Eberhardt CS, Wieland A, Vora KA, Pulendran B, Ahmed R, 2019. Understanding the immunology of the Zostavax shingles vaccine. Curr. Opin. Immunol 59, 25–30. [DOI] [PubMed] [Google Scholar]
- Sultan G, Baudouin C, Auzerie O, De Saint Jean M, Goldschild M, Pisella PJ, Marfan Study Group, 2002. Cornea in Marfan disease: Orbscan and in vivo confocal microscopy analysis. Invest. Ophthalmol. Vis. Sci 43(6), 1757–64. [PubMed] [Google Scholar]
- Suvarna JC, 2008. Kayser-Fleischer ring. J. Postgrad. Med 54, 238–240. [DOI] [PubMed] [Google Scholar]
- Szalai E, Deák E, Módis L Jr, Németh G, Berta A, Nagy A, Felszeghy E, Káposzta R, Malik RA, Csutak A, 2016. Early corneal cellular and nerve fiber pathology in young patients with type 1 diabetes mellitus identified using corneal confocal microscopy. Invest. Ophthalmol. Vis. Sci 57, 853–858. [DOI] [PubMed] [Google Scholar]
- Tabatabay CA, Bumbacher M, Baumgartner B, Leuenberger PM, 1988. Reduced number of hemidesmosomes in the corneal epithelium of diabetics with proliferative vitreoretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol 226, 389–392. [DOI] [PubMed] [Google Scholar]
- Tam PM, Cheng LL, Young AL, Lam PT, 2009. Paraneoplastic pemphigus: an uncommon cause of chronic cicatrising conjunctivitis. BMJ Case Rep. 2009, bcr12.2008.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JC, Tat LT, Francis KB, Mendoza CG, Murrell DF, Coroneo MT, 2015. Prospective study of ocular manifestations of pemphigus and bullous pemphigoid identifies a high prevalence of dry eye syndrome. Cornea. 34, 443–448. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Dogru M, Takano Y, Miyake-Kashima M, Asano-Kato N, Fukagawa K, Tsubota K, Fujishima H, 2004. The relation of conjunctival and corneal findings in severe ocular allergies. Cornea. 23, 464–467. [DOI] [PubMed] [Google Scholar]
- Taniegra ED, 2004.Hyperparathyroidism. Am. Fam. Physician 69, 333–339. [PubMed] [Google Scholar]
- Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Malik RA, 2007. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care. 30, 1895–1897. [DOI] [PubMed] [Google Scholar]
- Taylor M, Khan S, Stapleton M, Wang J, Chen J, Wynn R, Yabe H, Chinen Y, Boelens JJ, Mason RW, Kubaski F, Horovitz DDG, Barth AL, Serafini M, Bernardo ME, Kobayashi H, Orii KE, Suzuki Y, Orii T, Tomatsu S, 2019. Hematopoietic stem cell transplantation for mucopolysaccharidoses: past, present, and future. Biol. Blood Marrow Transplant 25, e226–e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepelus TC, Huang J, Sadda SR, Lee OL, 2017. Characterization of corneal involvement in eyes with mucous membrane pemphigoid by in vivo confocal microscopy. Cornea. 36, 933–941. [DOI] [PubMed] [Google Scholar]
- Terada Y, Kamoi K, Komizo T, Miyata K, Mochizuki M, 2017. Human T cell leukemia virus type 1 and eye diseases. J. Ocul. Pharmacol. Ther 33, 216–223. [DOI] [PubMed] [Google Scholar]
- Testa V, De Santis N, Scotto R, Della Giustina P, Ferro Desideri L, Cellerino M, Cordano C, Inglese M, Uccelli A, Vagge A, Traverso CE, Iester M, 2020. Corneal epithelial dendritic cells in patients with multiple sclerosis: An in vivo confocal microscopy study. J. Clin. Neurosci 81, 139–143. [DOI] [PubMed] [Google Scholar]
- To WJ, Telander DG, Lloyd ME, Chen PC, Cheung AT, 2011. Correlation of conjunctival microangiopathy with retinopathy in type-2 diabetes mellitus (T2DM) patients. Clin. Hemorheol. Microcirc 47, 131–141. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Sawamoto K, Alméciga-Díaz CJ, Shimada T, Bober MB, Chinen Y, Yabe H, Montaño AM, Giugliani R, Kubaski F, Yasuda E, Rodríguez-López A, Espejo-Mojica AJ, Sánchez OF, Mason RW, Barrera LA, Mackenzie WG, Orii T, 2015. Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome. Drug Des. Devel. Ther 9, 1937–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomomatsu T, Takamura Y, Kubo E, Akagi Y, 2009. Aldose reductase inhibitor counteracts the attenuated adhesion of human corneal epithelial cells induced by high glucose through modulation of MMP-10 expression. Diabetes Res. Clin. Pract 86, 16–23. [DOI] [PubMed] [Google Scholar]
- Tong CM, Iovieno A, Yeung SN, 2020. Topical insulin for neurotrophic corneal ulcers. Can. J. Ophthalmol 55:e170–e172. [DOI] [PubMed] [Google Scholar]
- Tormanen K, Wang S, Ghiasi H, 2020. CD80 plays a critical role in increased inflammatory responses in herpes simplex virus 1-infected mouse corneas. J. Virol 94, e01511–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totan Y, Hepşen IF, Cekiç O, Gündüz A, Aydin E, 2001. Incidence of keratoconus in subjects with vernal keratoconjunctivitis: a videokeratographic study. Ophthalmology. 108, 824–827. [DOI] [PubMed] [Google Scholar]
- Tsaousis KT, Panagiotou DZ, Kostopoulou E, Vlatsios V, Stampouli D, 2015. Corneal oedema after phacoemulsification in the early postoperative period: A qualitative comparative case-control study between diabetics and non-diabetics. Ann. Med. Surg (Lond) 5, 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilou E, Zhou M, Gahl W, Sieving PC, Chan CC, 2007. Ophthalmic manifestations and histopathology of infantile nephropathic cystinosis: report of a case and review of the literature. Surv. Ophthalmol 52, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilou ET, Rubin BI, Reed GF, Iwata F, Gahl W, Kaiser-Kupfer MI, 2002. Age-related prevalence of anterior segment complications in patients with infantile nephropathic cystinosis. Cornea.21, 173–176. [DOI] [PubMed] [Google Scholar]
- Ueno H, Hattori T, Kumagai Y, Suzuki N, Ueno S, Takagi H, 2014. Alterations in the corneal nerve and stem/progenitor cells in diabetes: preventive effects of insulin-like growth factor-1 treatment. Int. J. Endocrinol 2014, 312401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S, Mittal E, Philips JA, 2018. Tuberculosis and the art of macrophage manipulation. Pathog. Dis 76, fty037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utine CA, Stern M, Akpek EK., 2019. Immunopathological features of severe chronic atopic keratoconjunctivitis and effects of topical cyclosporine treatment. Ocul. Immunol. Inflamm 27, 1184–1193. [DOI] [PubMed] [Google Scholar]
- Valim V, Trevisani VF, de Sousa JM, Vilela VS, Belfort R Jr., 2015. Current approach to dry eye disease. Clin. Rev. Allergy Immunol 2015;49, 288–297. [DOI] [PubMed] [Google Scholar]
- Van Caillie-Bertrand M, Degenhart HJ, Luijendijk I, Bouquet J, Sinaasappel M, 1985. Wilson’s disease: assessment of D-penicillamine treatment. Arch. Dis. Child 60, 652–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen V, Hoovers JM, de Kraker J, Crolla JA, 2007. Raised risk of Wilms tumour in patients with aniridia and submicroscopic WT1 deletion. J. Med. Genet 44, 787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance M, Llanga T, Bennett W, Woodard K, Murlidharan G, Chungfat N, Asokan A, Gilger B, Kurtzberg J, Samulski RJ, Hirsch ML, 2016. AAV gene therapy for MPS1-associated corneal blindness. Sci. Rep 6, 22131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhonsebrouck E, Consejo A, Coucke PJ, Leroy BP, Kreps EO, 2020. The corneoscleral shape in Marfan syndrome. Acta. Ophthalmol doi: 10.1111/aos.14636. [DOI] [PubMed] [Google Scholar]
- Veiga-Parga T, Giménez F, Mulik S, Chiang EY, Grogan JL, Rouse BT, 2013. Controlling herpetic stromal keratitis by modulating lymphotoxin-alpha-mediated inflammatory pathways. Microbes Infect. 15, 677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran N, Klavdianou O, Kondylis G, Kosmidis I, Palioura S, 2020. Paraneoplastic pemphigus associated with bilateral corneal perforations in follicular dendritic cell sarcoma. Ocul. Immunol. Inflamm 13, 1–3. [DOI] [PubMed] [Google Scholar]
- Venning VA, 2011. Linear IgA disease: clinical presentation, diagnosis, and pathogenesis. Dermatol. Clin 29, 453–458. [DOI] [PubMed] [Google Scholar]
- Vicente A, Byström B, Pedrosa Domellöf F, 2018. Altered signaling pathways in aniridia-related keratopathy. Invest. Ophthalmol. Vis. Sci 59, 5531–5541. [DOI] [PubMed] [Google Scholar]
- Vieira-Potter VJ, Karamichos D, Lee DJ, 2016. Ocular complications of diabetes and therapeutic approaches. Biomed. Res. Int 2016, 3801570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani E, Garoli E, Bassotti A, Magnani F, Tresoldi L, Nucci P, Ratiglia R, 2013. The cornea in classic type Ehlers-Danlos syndrome: macro- and microstructural changes. Invest. Ophthalmol. Vis. Sci 54, 8062–8068. [DOI] [PubMed] [Google Scholar]
- Voskresenskaya A, Pozdeyeva N, Vasilyeva T, Batkov Y, Shipunov A, Gagloev B, Zinchenko R, 2017. Clinical and morphological manifestations of aniridia-associated keratopathy on anterior segment optical coherence tomography and in vivo confocal microscopy. Ocul. Surf 15, 759–769. [DOI] [PubMed] [Google Scholar]
- Vrcek I, Choudhury E, Durairaj V, 2017. Herpes zoster ophthalmicus: A Review for the internist. Am. J. Med 130, 21–26. [DOI] [PubMed] [Google Scholar]
- Walkden A, Burkitt-Wright E, Au L, 2019. Brittle cornea syndrome: current perspectives. Clin. Ophthalmol 13, 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, Cutter GR, Kaye WE, Wagner L, Tremlett H, Buka SL, Dilokthornsakul P, Topol B, Chen LH, LaRocca NG, US Multiple Sclerosis Prevalence Workgroup, 2019. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 92, e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q, Tang J, Han Y, Xiao Q, Deng Y, 2018. Brittle cornea syndrome: a case report and review of the literature. BMC. Ophthalmol 18, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Yang S, Zhai HL, Zhang YY, Cui CX, Wang JY, Xie LX, 2018. A comparative study of risk factors for corneal infection in diabetic and non-diabetic patients. Int. J. Ophthalmol 11, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gao N, Yin J, Yu FS, 2012. Reduced innervation and delayed re-innervation after epithelial wounding in type 2 diabetic Goto-Kakizaki rats. Am. J. Pathol 181, 2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Meng W, 2020. COVID-19 and diabetes: the contributions of hyperglycemia. J. Mol. Cell Biol mjaa054. doi: 10.1093/jmcb/mjaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Despanie J, Shi P, Edman-Woolcott MC, Lin YA, Cui H, Heur JM, Fini ME, Hamm-Alvarez SF, MacKay JA, 2014. Lacritin-mediated regeneration of the corneal epithelia by protein polymer nanoparticles. J. Mater. Chem. B. Mater. Biol. Med 2, 8131–8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li W, Zhou Q, Li J, Wang X, Zhang J, Li D, Qi X, Liu T, Zhao X, Li S, Yang L, Xie L, 2020. MANF promotes diabetic corneal epithelial wound healing and nerve regeneration by attenuating hyperglycemia-induced endoplasmic reticulum stress. Diabetes. 69, 1264–1278. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu G, Wang W, Wang J, Chen L, He M, Chen Z, 2020. Changes in corneal biomechanics in patients with diabetes mellitus: a systematic review and meta-analysis. Acta. Diabetol 57, 973–981. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao X, Shi D, Chen P, Yu Y, Yang L, Xie L, 2013. Overexpression of SIRT1 promotes high glucose-attenuated corneal epithelial wound healing via p53 regulation of the IGFBP3/IGF-1R/AKT pathway. Invest. Ophthalmol. Vis. Sci 54, 3806–3814. [DOI] [PubMed] [Google Scholar]
- Warren-Gash C, Forbes H, Breuer J, 2017. Varicella and herpes zoster vaccine development: lessons learned. Expert Rev. Vaccines 16, 1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrocka A, Krawczynski MR, 2018. The genetics of aniridia - simple things become complicated. J. Appl. Genet 59, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler SL, Nesburn AB, Watson R, Slanina SM, Ghiasi H, 1988. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J. Virol 62, 4051–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda LW Jr., Coffey SA, 2008. Wegener’s granulomatosis. Oral Maxillofac. Surg. Clin. North. Am 20, 643–649. [DOI] [PubMed] [Google Scholar]
- Westland T, Patryn EK, Nieuwendaal CP, van der Meulen IJE, Mourits MP, Lapid-Gortzak R, 2018. Vernal shield ulcers treated with frequently installed topical cyclosporine 0.05% eyedrops. Int. Ophthalmol 38, 363–368. [DOI] [PubMed] [Google Scholar]
- Whelchel AE, McKay TB, Priyadarsini S, Rowsey T, Karamichos D, 2019. Association between diabetes and keratoconus: a retrospective analysis. Sci. Rep 9, 13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins NA, Munir S, Levin MR, 2019. Corneal manifestations of systemic diseases. Review Optometry. April 15, 34–41. [Google Scholar]
- Wilson SE, Walker JW, Chwang EL, He YG, 1993. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest. Ophthalmol. Vis. Sci 34, 2544–2561. [PubMed] [Google Scholar]
- Winkler MA, Dib C, Ljubimov AV, Saghizadeh M, 2014. Targeting miR-146a to treat delayed wound healing in human diabetic organ-cultured corneas. PLoS One. 9, e114692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte M, Zillikens D, Schmidt E, 2018. Diagnosis of autoimmune blistering diseases. Front. Med. (Lausanne) 5, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong NN, 1999. Vernal vs. atopic keratoconjunctivitis. Clin. Eye Vis. Care 11, 13–16. [Google Scholar]
- Woodward MA, Blachley TS, Stein JD, 2016. The association between sociodemographic factors, common systemic diseases, and keratoconus: an analysis of a nationwide heath care claims database. Ophthalmology. 123, 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith JE, 1995. The mucopolysaccharidoses: a clinical review and guide to management. Arch. Dis. Child 72, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K, 2020. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol, 138, 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Buckner BR, Zhu M, Cavanagh HD, Robertson DM, 2012. Elevated IGFBP3 levels in diabetic tears: a negative regulator of IGF-1 signaling in the corneal epithelium. Ocul. Surf 10, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylegała E, Moćko L, Woyna-Orlewicz A, Teper S, Orzhechowska-Wylegała B, 2006. [Diabetic complications within ocular surface] (in Polish). Pol. Merkur Lekarski 21, 495–497. [PubMed] [Google Scholar]
- Xia J, Tong J, Liu M, Shen Y, Guo D, 2020. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol 92, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Zhang S, Cao Z, Hu Y, Yang H, Wang D, 2013. Interleukin-17 enhanced immunoinflammatory lesions in a mouse model of recurrent herpetic keratitis. Microbes Infect. 15, 126–39. [DOI] [PubMed] [Google Scholar]
- Xu K, Yu FS, 2011. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest. Ophthalmol. Vis. Sci 52, 3301–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KP, Li Y, Ljubimov AV, Yu FX, 2009. High glucose suppresses EGFR-PI3K-AKT signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 58, 1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Gao N, Sun H, Yin J, Lee P, Zhou L, Fan X, Yu FS, 2016. Targeting imbalance between IL-1β and IL-1 receptor antagonist ameliorates delayed epithelium wound healing in diabetic mouse corneas. Am. J. Pathol 186, 1466–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Diao B, Liu Y, Zhang W, Wang G, Chen X, 2020. Severe acute respiratory syndrome coronavirus 2 nucleocapsid protein in the ocular tissues of a patient previously infected with coronavirus disease 2019. JAMA Ophthalmol. 138, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Di G, Qi X, Qu M, Wang Y, Duan H, Danielson P, Xie L, Zhou Q, 2014. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes. 63, 4262–4274. [DOI] [PubMed] [Google Scholar]
- Yang R, Sha X, Zeng M, Tan Y, Zheng Y, Fan F, 2011. The influence of phacoemulsification on corneal endothelial cells at varying blood glucose levels. Eye Sci. 26, 91–95. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang Y, Zhang Z, Dan J, Zhou Q, Wang X, Li W, Zhou L, Yang L, Xie L, 2020. Insulin promotes corneal nerve repair and wound healing in type 1 diabetic mice by enhancing Wnt/β-catenin signaling. Am. J. Pathol 190, 2237–2250. [DOI] [PubMed] [Google Scholar]
- Yangzes S, Dogra M, Ram J, 2020. Interstitial keratitis with corneal perforation as the presenting sign of systemic tuberculosis. Ocul. Immunol. Inflamm 28, 421–423. [DOI] [PubMed] [Google Scholar]
- Yaro A, 2013. Epstein-Barr infection: Current treatment options. Ann. Trop. Med. Pub. Health 6, 10. [Google Scholar]
- Yazdanpanah G, Bohm KJ, Hassan OM, Karas FI, Elhusseiny AM, Nonpassopon M, Niparugs M, Tu EY, Sugar J, Rosenblatt MI, Cortina MS, Djalilian AR, 2020. Management of congenital aniridia-associated keratopathy: long-term outcomes from a tertiary referral center. Am. J. Ophthalmol 210, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazu H, Shimizu E, Aketa N, Dogru M, Okada N, Fukagawa K, Fujishima H, 2019. The efficacy of 0.1% tacrolimus ophthalmic suspension in the treatment of severe atopic keratoconjunctivitis. Ann. Allergy Asthma Immunol 122, 387–392. [DOI] [PubMed] [Google Scholar]
- Yin J, Yu FS, 2010. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Invest. Ophthalmol. Vis. Sci 51, 1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KC, Im SK, Seo MS, 2004. Changes of tear film and ocular surface in diabetes mellitus. Korean J. Ophthalmol 18, 168–174. [DOI] [PubMed] [Google Scholar]
- Young RC, Hodge DO, Liesegang TJ, and Keith H Baratz KH, 2010. The incidence, recurrence and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976 through 2007: The impact of oral antiviral prophylaxis. Arch. Ophthalmol 128, 1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SM, Jing H, 2010. Marfan’s syndrome: an overview. Sao Paulo Med. J 128, 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen NK, Ananthakrishnan S, Campbell MJ, 2016. Hyperparathyroidism of renal disease. Perm. J 20, 15–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, Immonen JA, McLaughlin PJ, 2014. Ocular surface abnormalities related to type 2 diabetes are reversed by the opioid antagonist naltrexone. Clin. Exp. Ophthalmol 42, 159–168. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, Purushothaman I, McLaughlin PJ, 2020. Blockade of OGFr delays the onset and reduces the severity of diabetic ocular surface complications. Exp. Biol. Med November 17:1535370220972060. doi: 10.1177/1535370220972060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemba M, Burcea M, Camburu G, 2016. Atopic keratoconjunctivitis with corneal ulcer. Case report. Rom. J. Ophthalmol 60, 200–206. [PMC free article] [PubMed] [Google Scholar]
- Zhai W, Wu F, Zhang Y, Fu Y, Liu Z, 2019. The immune escape mechanisms of Mycobacterium tuberculosis. Int. J. Mol. Sci 20, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Xi L, Zhao S, Wei R, Huang Y, Yang R, Su L, Liu X, 2016. Interleukin-1β and tumour necrosis factor-α levels in conjunctiva of diabetic patients with symptomatic moderate dry eye: case-control study. BMJ Open. 6, e010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao N, Wu L, Lee PSY, Me R, Dai C, Xie L, Yu FX, 2020. Role of VIP and Sonic Hedgehog signaling pathways in mediating epithelial wound healing, sensory nerve regeneration, and their defects in diabetic corneas. Diabetes. 69, 1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hu X, Qi X, Di G, Zhang Y, Wang Q, Zhou Q, 2018. Resolvin D1 promotes corneal epithelial wound healing and restoration of mechanical sensation in diabetic mice. Mol. Vis 24, 274–285. [PMC free article] [PubMed] [Google Scholar]
- Zhao H, He Y, Ren YR, Chen BH, 2019. Corneal alteration and pathogenesis in diabetes mellitus. Int. J. Ophthalmol 12, 1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivov A, Winter K, Hovakimyan M, Peschel S, Harder V, Schober HC, Kundt G, Baltrusch S, Guthoff RF, Stachs O, 2013. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS One. 8, e52157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ, 2020a. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 Infection. Ocul. Surf 18, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Chen P, Di G, Zhang Y, Wang Y, Qi X, Duan H, Xie L, 2015. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells. 33, 1566–1576. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Duan C, Zeng Y, Tong Y, Nie Y, Yang Y, Chen Z, Chen C, 2020b. Ocular findings and proportion with conjunctival SARS-COV-2 in COVID-19 patients. Ophthalmology.127, 982–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Titone R, Robertson DM, 2019. The impact of hyperglycemia on the corneal epithelium: Molecular mechanisms and insight. Ocul. Surf 17, 644–654. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Takahashi H, Hutcheon AEK, Dalbone AC, 2000. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest. Ophthalmol. Vis. Sci 41, 1346–1355. [PubMed] [Google Scholar]
- Zlatanović G, Veselinović D, Cekić S, Zivković M, Dorđević-Jocić J, Zlatanović M, 2010. Ocular manifestation of rheumatoid arthritis-different forms and frequency. Bosn. J. Basic. Med. Sci 10, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C, Wang S, Huang F, Zhang YA, 2012. Advanced glycation end products and ultrastructural changes in corneas of long-term streptozotocin-induced diabetic monkeys. Cornea. 31, 1455–1459. [DOI] [PubMed] [Google Scholar]


