Abstract
Lyme disease (Lyme borreliosis) is a tick-borne, zoonosis of adults and children caused by genospecies of the Borrelia burgdorferi sensu lato complex. The ailment, widespread throughout the Northern Hemisphere, continues to increase globally due to multiple environmental factors, coupled with increased incursion of humans into habitats that harbor the spirochete. B. burgdorferi sensu lato is transmitted by ticks from the Ixodes ricinus complex. In North America, B. burgdorferi causes nearly all infections; in Europe, B. afzelii and B. garinii are most associated with human disease. The spirochete’s unusual fragmented genome encodes a plethora of differentially expressed outer surface lipoproteins that play a seminal role in the bacterium’s ability to sustain itself within its enzootic cycle and cause disease when transmitted to its incidental human host. Tissue damage and symptomatology (i.e., clinical manifestations) result from the inflammatory response elicited by the bacterium and its constituents. The deposition of spirochetes into human dermal tissue generates a local inflammatory response that manifests as erythema migrans (EM), the hallmark skin lesion. If treated appropriately and early, the prognosis is excellent. However, in untreated patients, the disease may present with a wide range of clinical manifestations, most commonly involving the central nervous system, joints, or heart. A small percentage (~10%) of patients may go on to develop a poorly defined fibromyalgia-like illness, post-treatment Lyme disease (PTLD) unresponsive to prolonged antimicrobial therapy. Below we integrate current knowledge regarding the ecologic, epidemiologic, microbiologic, and immunologic facets of Lyme disease into a conceptual framework that sheds light on the disorder that healthcare providers encounter.
Introduction
Lyme disease is the prototype of an emerging infectious disease (Steere et al., 2004; Paules et al., 2018). The isolation of its etiologic agent, Borrelia burgdorferi, from humans in 1983 (Benach et al., 1983; Steere et al., 1983a; Barbour and Benach, 2019) capped an intensive hunt for a pathogen that just a short time before had been cultured from a black legged (deer) tick (Burgdorfer et al., 1982), initially named Ixodes dammini (Spielman et al., 1979) but subsequently found to belong to a species, I. scapularis, whose range had been expanding in the U.S. since it was first recognized since the 1920s (Burgdorfer and Gage, 1986; Eisen and Eisen, 2018). Critical to the chain of events that led to the discovery of the Lyme disease spirochete was the observation that many patients involved in an outbreak of oligoarthritis in Southeastern Connecticut also had a skin rash, erythema chronicum migrans (ECM; now erythema migrans, EM) (Steere et al., 1977a; Steere et al., 1977b), previously associated in Europe with the bite of the sheep tick Ixodes ricinus (Afzelius, 1910; Lipschütz, 1913; Afzelius, 1921; Lipschütz, 1923). The isolation of B. burgdorferi (Benach et al., 1983; Steere et al., 1983a; Barbour and Benach, 2019) sparked an explosive increase in our knowledge of the bacterium, the disease it causes, and the enzootic cycle that sustains and creates risk to humans who intrude upon it. We now know that Lyme disease (Lyme borreliosis) is the most prevalent tick-borne illness in the Palearctic region of the Northern Hemisphere and that its incidence continues to increase globally due to myriad demographic and environmental factors, including climate change (Mead, 2015; Ostfeld and Brunner, 2015; Schotthoefer and Frost, 2015; Semenza and Suk, 2018; Sharareh et al., 2019). Although the clinical manifestations of Lyme disease continue to be a source of considerable controversy, it is generally accepted that a relatively small number of syndromes dominate the clinical picture and that the vast majority of patients present with treatment-responsive acute illness (Steere et al., 2016; Stanek and Strle, 2018). Serologic surveys conducted in high prevalence areas indicate that asymptomatic infection also is relatively common (Hanrahan et al., 1984; Steere et al., 2003; Wilhelmsson et al., 2016; Carlsson et al., 2018); thus, despite the bacterium’s notorious reputation, benign outcomes often occur. The genomic sequence of B. burgdorferi revealed that the spirochete lacks genes encoding known toxigenic molecules as well as the secretory apparatus required to deliver them to the extracellular milieu it inhabits within its mammalian host (Fraser et al., 1997; Casjens et al., 2000). Whereas reservoir hosts are unaffected by lifelong infection with Lyme disease spirochetes (Oliver et al., 2003; Hersh et al., 2014) due to a poorly understood form of immunologic tolerance (Barbour, 2017), infected humans often mount local and systemic inflammatory responses that make them ill (Steere et al., 2016; Stanek and Strle, 2018). From this perspective, one can regard clinical Lyme disease in humans as an evolutionary “mismatch” between pathogen and the intolerant immune system of its incidental host. Beyond this reductionist view, however, we still have only a limited understanding of the microbial factors, pathogenic mechanisms, and immunologic responses that determine outcomes following the adventitious encounter of humans with this zoonotic microorganism.
Over the years, a number of excellent clinical reviews of Lyme disease have been published in journals and textbooks, and there is no need to reiterate all of this information herein (Radolf and Samuels, 2021; Stanek et al., 2012; Steere et al., 2016; Stanek and Strle, 2018). In addition, medical societies in both the United States and Europe have issued comprehensive guidelines for the diagnosis and management of this infection (Wormser et al., 2006; Halperin et al., 2007; Eldin et al., 2019). Rather, the primary objective of this review is to integrate current knowledge regarding the ecologic, epidemiologic, microbiologic, and immunologic facets of Lyme disease into a conceptual framework that sheds light on the disorder practitioners see and manage. As will be seen, many of the principal factors that determine the level of risk for populations and individuals lie outside the sphere of human activity. Since our goal is to develop a mechanistic picture of the human disorder, we intend to rely as much as possible upon data obtained from human studies; extrapolation from in vitro and animal models is necessary, indeed, unavoidable, given the constraints of human experimentation. At the same time, this review attempts to grapple with a vexing but fundamental issue—the extent to which infection in humans deviates from the infectious process in nature and that observed in experimental animal models.
Historical overview
The history of what we now call Lyme disease, dating back to the early part of the twentieth century, is instructive for contemporary understanding (Burgdorfer, 1986, 1993). However, the discoveries that ushered in our current understanding of the illness began in 1981, when Willy Burgdorfer, Jorge Benach and Alan Barbour identified a new Borrelia species from ticks collected on eastern Long Island (Burgdorfer et al., 1982; Barbour and Benach, 2019). As Burgdorfer (Burgdorfer, 1993) and, most recently, Barbour and Benach (Barbour and Benach, 2019) note in their colorful first-hand accounts, the finding was serendipitous and well illustrates Louis Pasteur’s famous dictum that “chance favors only the prepared mind”. Burgdorfer and Benach were seeking a vector to explain an outbreak of Rocky Mounted spotted fever on Eastern Long Island but stumbled across spirochetes when they dissected midguts from Ixodes scapularis ticks, recently recognized as the vector for Babesia microti (Spielman et al., 1979). Burgdorfer was aware that a possible spirochetal etiology for Lyme disease had been “in the air” since the late 1940s. Two other strokes of fortune contributed to the discovery: Benach possessed a bank of sera from convalescent Lyme disease patients, while Barbour, at the time studying relapsing fever at the Rocky Mountain Laboratory, had developed an improved medium for cultivation of Borrelia. They found that antibodies in the sera of Lyme disease patients reacted intensely with spirochetes in dissected tick midguts and spirochetes isolated from tick midguts using Barbour’s newly formulated (BSKII) medium (Burgdorfer et al., 1982). Within a year, groups separately led by Benach and Allen Steere isolated spirochetes from blood, skin, and cerebrospinal fluid (CSF), thereby establishing it as the etiologic agent (Burgdorfer et al., 1982; Burgdorfer, 1986). Using DNA-DNA hybridization, two groups (Hyde and Johnson, 1984; Schmid et al., 1984) subsequently showed that the spirochete was a new species of Borrelia, subsequently named B. burgdorferi (Johnson et al., 1984).
It soon became apparent that a variety of clinical syndromes described in the European medical literature were, in fact, manifestations of a tick-transmitted disorder caused by members of what eventually came to be known as the B. burgdorferi sensu lato complex (Belfaiza et al., 1993; Wang et al., 1999a; Cutler et al., 2017). EM, the classic skin lesion associated with this infectious disease, was first described by Afzelius, a Swedish dermatologist, in 1910 (Afzelius, 1910). Afzelius also correctly hypothesized that EM resulted from the tick-borne transmission to humans of a zoonotic pathogen (Afzelius, 1921); in 1921, Lipschütz identified Ixodes ricinus as the vector (Lipschütz, 1923). By the beginning of World War II, European investigators knew that EM was associated with several neurologic and dermatologic disorders and with musculoskeletal complaints (Garin, 1922; Bannwarth, 1941, 1944). After the war, Lenhoff (Lenhoff, 1948), a Swedish pathologist, described what he believed were spirochetes in biopsies of EM lesions, while Hollström (Hollstrom, 1951) demonstrated that penicillin was effective for its treatment.
In 1970, Rudolph J. Scrimenti, a Wisconsin dermatologist, reported the first case of Lyme disease acquired in the United States. The patient, by serendipity a physician, was bitten by a tick above his right iliac crest while grouse hunting in North Central Wisconsin. Three months later, he presented with an enormous EM rash extending from his right mid-chest to mid-back, encircling his right axilla and iliac crest, accompanied by hyperesthesia of the T12 and L1 dermatomes. Fortunately, Scrimenti knew of this “curious condition” from the European literature and of its responsiveness to penicillin; incredibly, the patient was symptom-free within 48 h of receiving what by today’s standards is considered a miniscule dose (1.2 MU) of intramuscular benzathine penicillin G. In 1976, Mast and Burrows (Mast and Burrows, 1976) reported the first cluster of cases from Southeastern Connecticut. Soon afterwards, Yale rheumatologists Allen Steere and Steven Malawista began investigating cases of arthritis in patients, many of whom were children, in and around Old Lyme, Connecticut. Mothers of afflicted children, skeptical of the diagnosis of juvenile rheumatoid arthritis made by local physicians, had informed the State Health Department about the outbreak and called it to the attention of Steere and Malawista. In their initial reports, they called the mysterious ailment Lyme arthritis (Steere et al., 1977a; Steere et al., 1977b). However, with the realization that most arthritis patients previously had EM and that nonarthritic manifestations (heart block, facial nerve palsy and/or meningitis) were associated with the rash, they subsequently changed the name to “Lyme disease” (Steere and Malawista, 1979). Shortly thereafter, they “closed the loop” by correlating cases of Lyme disease with the distribution of I. scapularis in the Northeast and I. pacificus in California and Oregon (Steere and Malawista, 1979). Of note, European authorities prefer Lyme borreliosis because, in their view, U.S. patients diagnosed with Lyme disease do not always have a disorder with a clear-cut infectious etiology (Stanek and Strle, 2018). Detailed narratives of the medical sleuthing that led to the discovery of B. burgdorferi can be found in Radolf and Samuels (2021), in Edlow’s entertaining book Bull’s Eye, Unraveling the Medical Mystery of Lyme Disease (Erdlow, 2003), and in the gripping narrative recently published by Barbour and Benach (2019).
Epidemiology
Please see Radolf and Samuels (2021) for a complete discussion of the epidemiology of Lyme disease. Lyme disease became a notifiable condition in the U.S. in 1991. Since 2008, a confirmed case has been defined as either (i) EM in a person with possible or known tick exposure in an endemic area or laboratory evidence of infection (almost always serological) or (ii) at least one recognized clinical manifestation other than EM along with confirmatory laboratory evidence (Schwartz et al., 2017). From 2008 to 2015, 208,834 confirmed cases were reported to the CDC with the highest number (29,959) in 2009 (Figure 1) (Schwartz et al., 2017). During this period, Lyme disease accounted for 82% of all tick-borne diseases and 63% of all vector-borne disease reported in the US, making it by far the most prevalent vector-borne illness in the United States and Ixodes scapularis the most important vector (Hamer et al., 2010; Mead, 2015; Rosenberg et al., 2018). Nationwide studies of health insurance claims (Nelson et al., 2015) and commercial laboratory diagnostic tests (Hinckley et al., 2014) suggest that underreporting is common and that the actual number of cases is closer to 300,000 per year (i.e., about tenfold higher than reported). Fourteen states, all located in the Northeast, mid-Atlantic, and upper Midwest (Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Virginia, and Wisconsin), accounted for 95.7% of confirmed cases; Delaware, Connecticut, and Vermont had the highest incidences (~65–69 cases per 100,000 population) (Figure 2) (Schwartz et al., 2017). For all years, confirmed and probable cases peaked during the first week in July, consistent with nymphs being the principal stage for transmission. The age distribution was bimodal, with peaks between 5–9 years and 50–55 years, a slight male predominance (56%), and whites representing the overwhelming majority (~90%) of cases (Schwartz et al., 2017). EM was the most common clinical manifestation, accounting for nearly three-fourths (72.2%) of patients, and carditis least common (1.5%); 27.5% had arthritis and 12.5% had a neurologic manifestation. Serologic surveys in the U.S. and Europe have revealed substantial rates of asymptomatic or subclinical infections among persons living in endemic areas (Hanrahan et al., 1984; Steere et al., 2003; Wilhelmsson et al., 2016; Carlsson et al., 2018). Not surprisingly, risk is proportional to time spent outdoors, whether recreationally or occupationally, in or near tick-infested woods and vegetation (Hengge et al., 2003; Finch et al., 2014).
Figure 1.
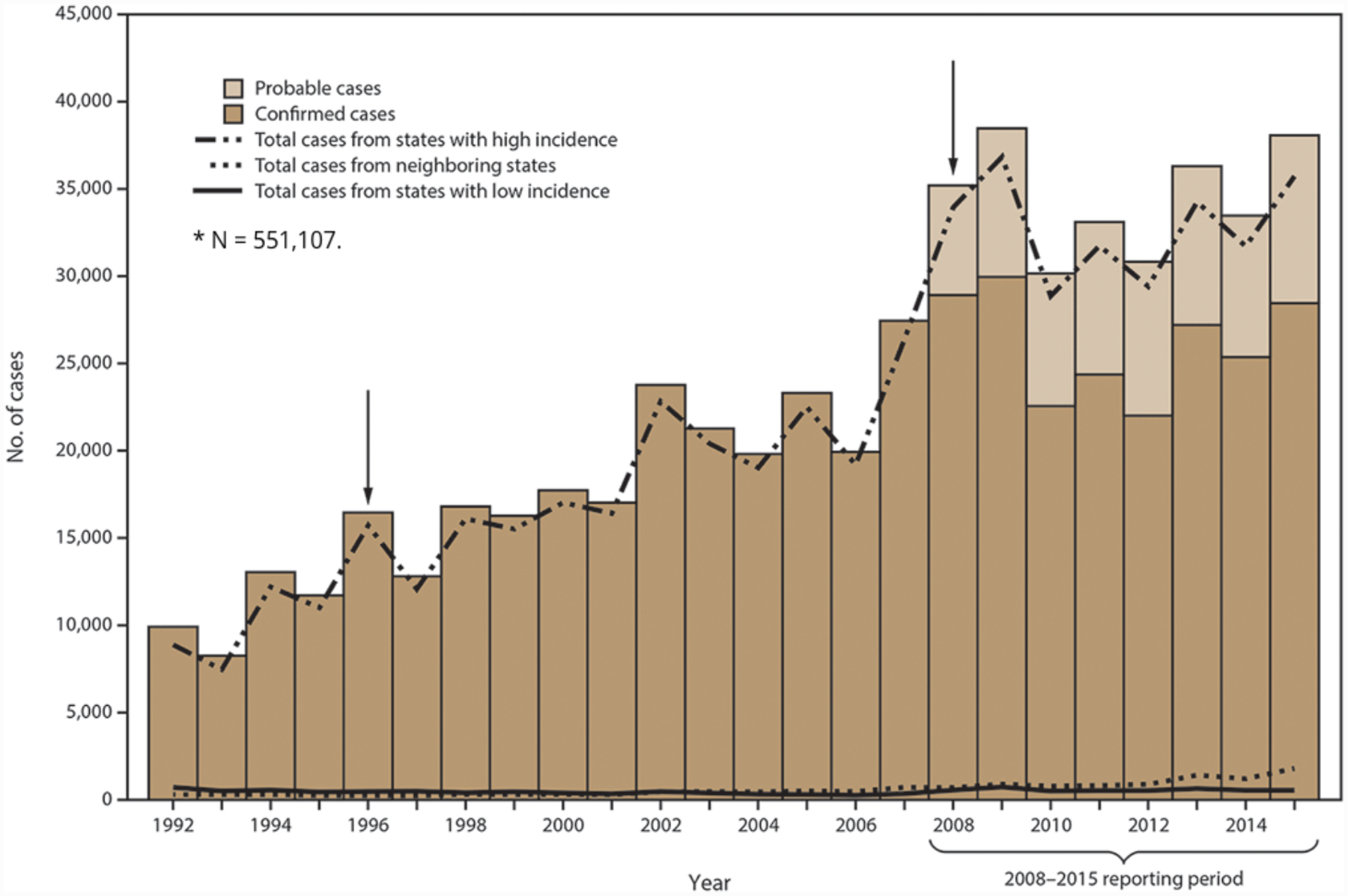
Number of confirmed and probable Lyme disease cases in the United States, 1992–2015. Arrows indicate notable changes in case definitions. The case definition was revised in 1996 to recommend a two-step testing method and in 2008 to increase specificity of laboratory evidence of infection and to include provision for report of probable cases (reproduced from Schwartz et al., 2017).
Figure 2.
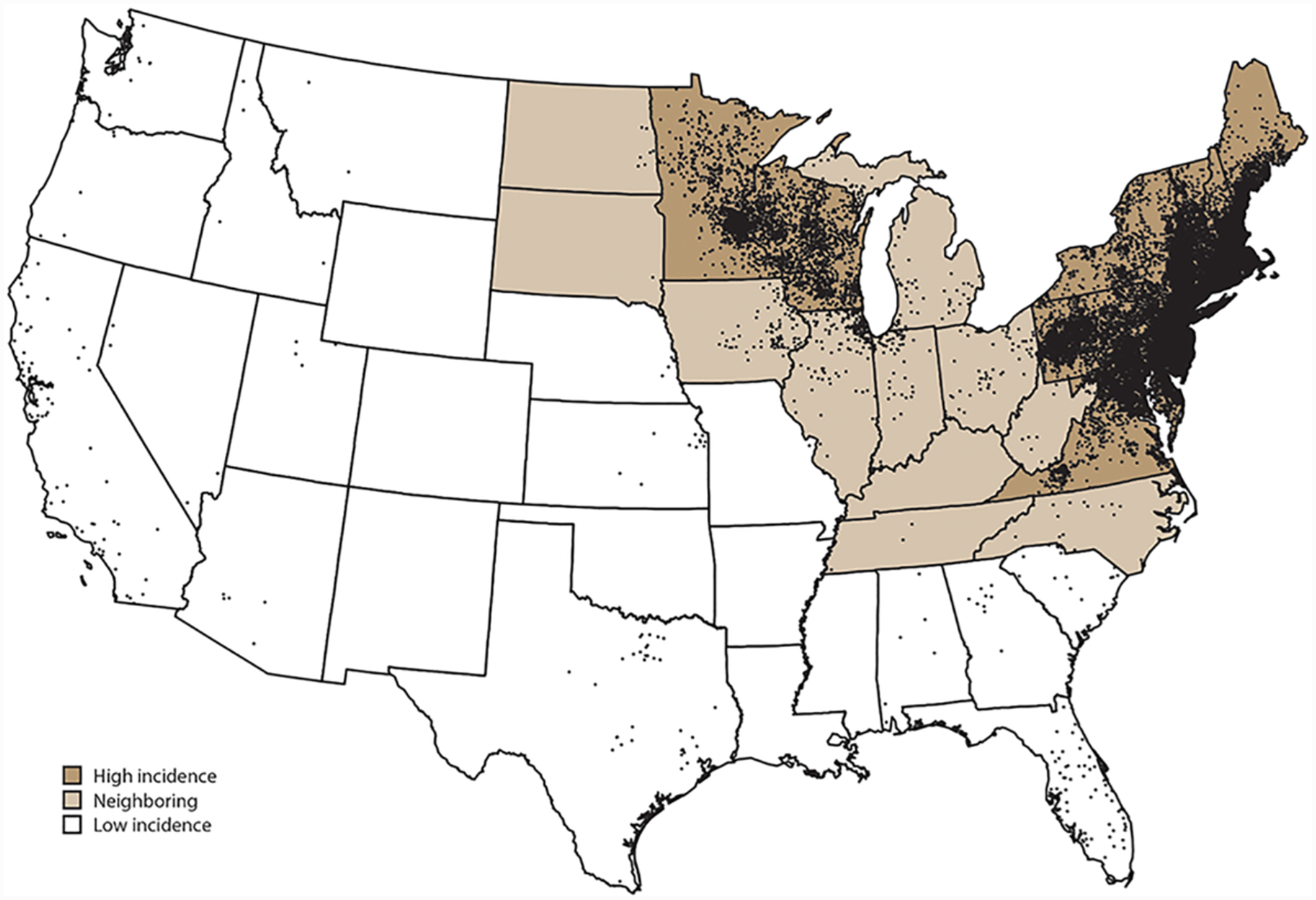
Average annual number of confirmed Lyme disease cases by county of residence in the United States, 2008–2015. Each dot represents one confirmed case (reproduced from Schwartz et al., 2017).
The last three decades have witnessed not only an impressive increase in the incidence of Lyme disease in North America but also a relentless expansion of its geographic range. Although historically associated with incursion into deciduous forests (Dennis and Hayes, 2002), Lyme disease now poses a threat to urban dwellers, as evidenced by cases acquired in New York City (VanAcker et al., 2019) and identification of B. burgdorferi-infected ticks in Chicago (Hamer et al., 2012). Moreover, the disease has expanded beyond the confines of the continental United States. It has emerged as a health threat in Southern Canada (Ogden et al., 2009; Gasmi et al., 2019), where the number of reported cases increased from 144 in 2005 to more than 2000 in 2017, and multiple surveys have reported identification of B. burgdorferi-infected I. scapularis ticks (Bouchard et al., 2015; Gasmi et al., 2016; Ogden et al., 2019). Although improved reporting and increased awareness are likely contributory factors (Orloski et al., 1998; Aenishaenslin et al., 2016), there is a strong consensus among entomologists that these epidemiologic trends reflect the collective impact of environmental drivers that increase the likelihood of human encounters with infected ticks (Eisen et al., 2016; Stone et al., 2017). Among the most important of these are (i) climate-mediated expansion of tick habitats (Ostfeld and Brunner, 2015; Dumic and Severnini, 2018), (ii) dispersal of infected I. scapularis by migratory birds (Olsen et al., 1995; Brinkerhoff et al., 2011; Hasle et al., 2011), (iii) increased densities of vertebrate reservoirs and deer populations upon which I. scapularis feed and mate as lands cleared for agriculture become reforested (Eisen and Eisen, 2018), and (iv) the increased risk of transmission associated with decreased biodiversity in endemic areas (LoGiudice et al., 2003; Granter et al., 2014; Ruyts et al., 2016). In short, one cannot divorce environmental factors fueling the proliferation of B. burgdorferi sensu lato in the wild (Gern, 2008) from their cumulative effects on human populations (Schwartz et al., 2017; Rosenberg et al., 2018).
Lyme disease also is the most prevalent vector-borne illness in Europe, where it is widely, though nonuniformly, distributed (Hubalek, 2009; Rizzoli et al., 2011; Sykes and Makiello, 2017). Remarkably, Lyme disease is not a mandatory notifiable disease in many European countries, complicating country by country comparison of epidemiologic data (van den Wijngaard et al., 2017). Although methods used to acquire surveillance and laboratory data vary greatly (van den Wijngaard et al., 2017), an estimated 85,000 cases occur each year throughout Europe (Sykes and Makiello, 2017). In Europe, as in the U.S., new cases peak in the summer months of June through August (Hubalek, 2009), and underreporting is believed to be common (van den Wijngaard et al., 2017). In Northern Europe, disease rates are highest in the Baltic states and Southern Sweden; in Central Europe, highest incidences are in Austria and Slovenia (Hubalek, 2009; Rizzoli et al., 2011; Sykes and Makiello, 2017). At the southern limits of the disease range (e.g., Italy and the Balkans), incidence decreases rapidly from north to south (Hubalek, 2009). Disease rates across the Continent parallel the densities of I. ricinus ticks affected with the pathogenic species most frequently detected in patients, B. afzelii and B. garinii (Coipan et al., 2016; Strnad et al., 2017; Estrada-Pena et al., 2018). Lyme disease rates are increasing in Europe for the same reasons as in North America—increased awareness (Smith and Takkinen, 2006), coupled with increasing distribution and abundance of I. ricinus due to the same environmental drivers, with climate change probably a major culprit (Medlock et al., 2013; Semenza and Suk, 2018).
Ecology
Although ticks capable of vectoring Lyme disease spirochetes often take their blood meals from humans, humans are not required for perpetuation of either ticks or spirochetes in nature. Humans are incidental, presumably “dead-end,” hosts that become infected when their lifestyles or activities intersect with habitats harboring spirochetes (Gern, 2009; Radolf et al., 2012; Eisen and Eisen, 2018). Only ticks belonging to the hard tick genus Ixodes are vector competent, that is, capable of acquiring and transmitting spirochetes (Lane et al., 1991; Gern, 2009; Eisen and Eisen, 2018). B. burgdorferi sensu lato is transmitted mainly by ticks of the Ixodes ricinus complex (Burgdorfer et al., 1991; Piesman and Gern, 2004) (also see Radolf and Samuels, 2021), I. scapularis in the Northeastern and Upper Midwestern United States; I. pacificus on the Pacific Coast; I. ricinus in Europe, Western Asia, and North Africa; and I. persulcatus in Eastern Europe and Asia (Gern, 2009; Mannelli et al., 2012; Franke et al., 2013). Ixodes ticks have a two-year life cycle with four life stages: egg, larva, nymph and adult (Figure 3). Ticks are born uninfected. Larvae acquire the spirochete by feeding on an infected reservoir host, and, after molting to the nymphal stage, transmit the pathogen when they feed on an uninfected reservoir or incidental host (Figure 4) (Gern, 2009; Radolf et al., 2012; Eisen and Eisen, 2018). The dependence of B. burgdorferi sensu lato on efficient transstadial transmission for long-term survival is an important distinction from relapsing fever spirochetes, which can be maintained within their argasid vectors by vertical or transovarial transmission (Barbour and Hayes, 1986; Rollend et al., 2013) (also see Radolf and Samuels, 2021).
Figure 3.
Ixodes scapularis stages.
Figure 4. The enzootic cycle of Borrelia burgdorferi.
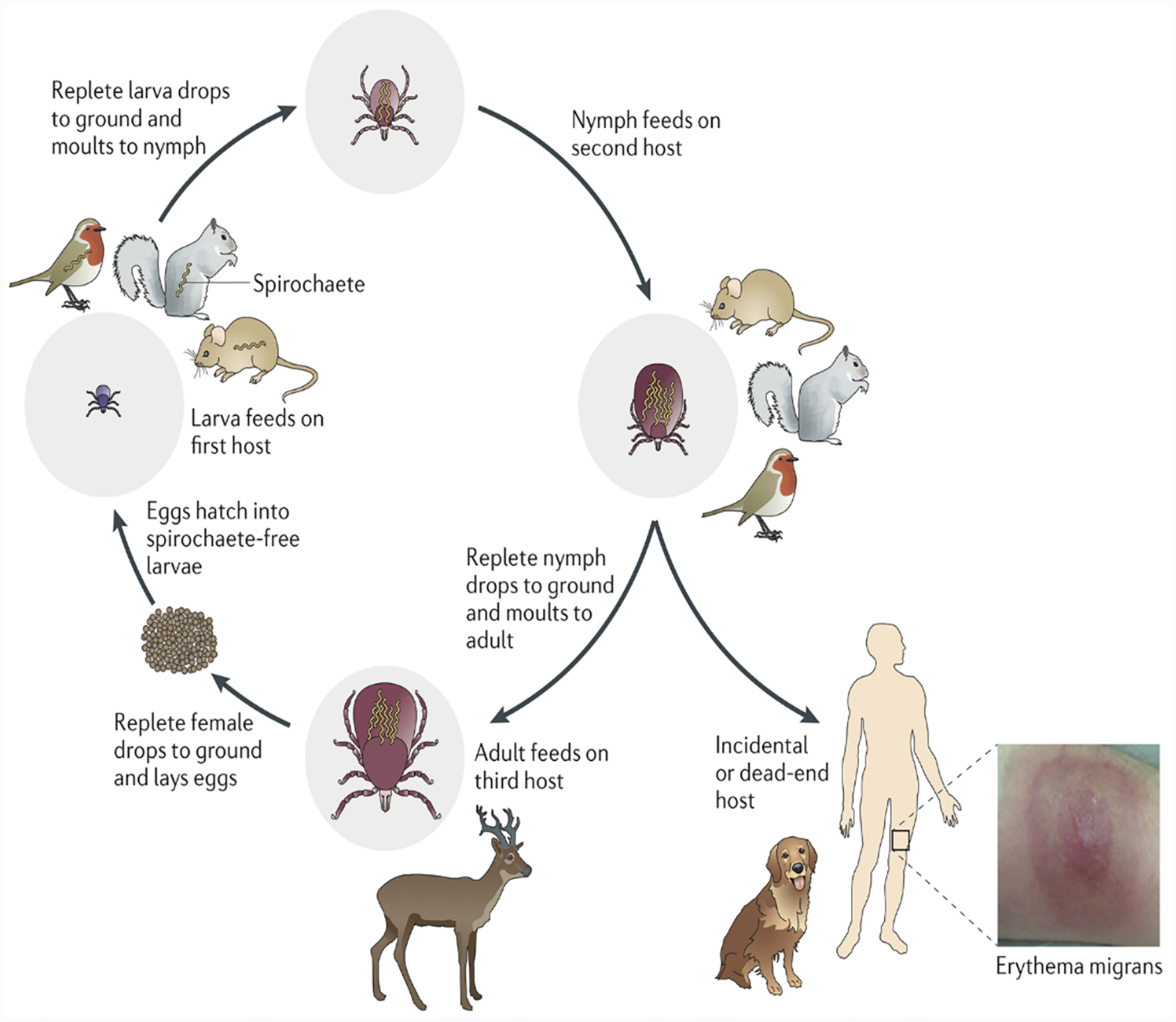
Ixodes ticks undergo a three-stage life cycle (larva, nymph and adult, with one blood meal per stage). Larval ticks acquire spirochetes by feeding on an infected reservoir animal, and the bacterium is retained during the subsequent stages. Transmission of spirochetes to a competent reservoir host by a feeding nymph perpetuates the enzootic cycle for the next generation of larval ticks. Adult ticks are not important for maintenance of B. burgdorferi in the wild; however, deer are important for maintenance of the tick population because adult ticks mate on them. Nymphs are responsible for the vast majority of spirochete transmission to humans, generally considered dead-end hosts, as are dogs. (Reprinted with permission from Radolf et al. 2012.)
Nymphal ticks are responsible for the majority of human infections (Piesman et al., 1987a; Falco et al., 1996; Dennis and Hayes, 2002). In addition to having high infection rates, nymphs quest during the summer months when humans are most likely to be outdoors and, because of their small size (approximately that of a poppy seed), are difficult to detect on body surfaces, clothing, and pets (Dennis and Hayes, 2002). Not surprisingly, the risk of infection for humans correlates with infection rates in vectors and reservoir hosts as well as tick density (Mather et al., 1996; Stafford et al., 1998; Falco et al., 1999; Pepin et al., 2012).
Immature ticks (larvae and nymphs) have a broad host range, including rodents, insectivores, birds, lagomorphs, and ungulates (LoGiudice et al., 2003; Ogden et al., 2008). Besides explaining how humans acquire infection, the aggressive feeding behaviors of these non-nidicolous (openly host-seeking) generalists enhance the opportunities for transmission of spirochetes amongst infection-competent vertebrates, linkage of ecological niches, and expansion of the geographic range of the disease (Kurtenbach et al., 2006). Although adult stage ticks have a twofold greater prevalence of infection than nymphs (Schwartz et al., 1997), they are much less important as vectors of human disease because adult males do not feed, and adult females usually feed on large reservoir-incompetent animals, typically white-tailed deer (Anderson, 1988; Kurtenbach et al., 2006). Furthermore, adults quest in late autumn through early spring when humans are less apt to encounter them and more apt to be wearing protective clothing (Falco et al., 1999; Dennis and Hayes, 2002). The western black-legged tick, Ixodes pacificus, is the primary vector of Lyme disease on the Pacific Coast (Campagna et al., 1983; Lane et al., 2007). Because I. pacificus larvae and nymphs preferentially feed on B. burgdorferi-refractory lizards (Lane and Loye, 1989; Lane and Quistad, 1998), infection rates in I. pacificus ticks are low (Lane et al., 2013; Rose et al., 2019), with a corresponding decrease in regional prevalence of human disease. Natural transmission cycles exist in non-endemic regions of the United States but are of lesser importance for humans because they involve reservoir hosts in remote geographic areas, less vector-competent Ixodes species, tick species with narrow host ranges that tend not to bite humans, and/or Borrelia species with limited infectivity for humans (Maupin et al., 1994; Dolan et al., 1997; Norris et al., 1999; Oliver et al., 2003; Franke et al., 2013).
Animals are reservoir competent if they become infected following the bite of an infected tick and can re-transmit the pathogen to a naïve vector (Mather et al., 1989; Hanincova et al., 2006; Brunner et al., 2008). In the case of Lyme disease, infection in a reservoir host must be of long enough duration to serve as a blood meal source for more than one tick life stage. As eloquently stated by Barbour (Barbour, 2017), what this (i.e., reservoir competence) “effectively means is usually a combination of resistance to and tolerance of infection in reservoir hosts of long-standing.” Peromyscus leucopus, the white-footed mouse, which thrives in habitats ranging from pristine forest to degraded woodlots, is a principal reservoir in the Northeast and North Central United States (Donahue et al., 1987; LoGiudice et al., 2003; Barbour, 2017). Once infected with B. burgdorferi, P. leucopus can remain infected for life without end-organ pathology (i.e., inflammatory response) or decreased longevity (Moody et al., 1994; Oliver et al., 2003; Schwanz et al., 2011; Voordouw et al., 2015) – dual indicators of a high degree of tolerance. Despite B. burgdorferi’s reputation as a “generalist” pathogen (Hanincova et al., 2006), not all I. scapularis blood meal hosts are equally competent reservoirs; moreover, evidence exists that tick hosts other than P. leucopus (e.g., shrews and chipmunks) can contribute to the maintenance of enzootic cycles in endemic areas (LoGiudice et al., 2003; Kurtenbach et al., 2006; Brisson et al., 2008; Franke et al., 2013). The degree of biodiversity in a given locale, more specifically, the relative proportions of competent and incompetent species, is a major determinant of the transmission risk for humans (LoGiudice et al., 2003; Granter et al., 2014; Ruyts et al., 2016). As noted earlier, in recent years, there has been growing appreciation of passerine birds as both reservoir hosts and vehicles for dissemination of infected ticks (Richter et al., 2000; Hasle et al., 2011; Norte et al., 2013). I. ricinus, the sheep tick, is the principal vector for Lyme disease spirochetes isolated from European patients (Gern, 2009). This tick is widely distributed throughout Europe with a range extending from Ireland to the Urals and from Southern Sweden to North Africa (McCoy et al., 2013; Cull et al., 2018; Estrada-Pena et al., 2018). The spatial prevalence of spirochete-infected I. ricinus ticks differs considerably and can be quite patchy even in areas with high overall densities (Estrada-Pena et al., 2018). According to a recent meta-analysis (Strnad et al., 2017), the highest rate of infected ticks was found in Central Europe and the lowest in the British Isles. B. afzelii and B. garinii, the genospecies most frequently associated with disease in European patients (Stanek and Strle, 2018), are the most commonly identified in questing I. ricinus nymphs (Rauter and Hartung, 2005; Estrada-Pena et al., 2018). The vector ecology of Lyme disease in Europe is even more complicated than in North America because of the greater diversity of European Borrelia populations (discussed below) and differences in reservoir host preferences (Gern, 2009; Mannelli et al., 2012; Franke et al., 2013). Small mammals, ground-foraging birds, and reptiles are common hosts for the larval and nymphal stages of I. ricinus, while adults (females) feed mostly on large mammals such as ungulates; both immature and adult stages will attach to humans (Gern, 2002). Only a small number of the more than 300 vertebrates serving as blood meal hosts for questing I. ricinus ticks are reservoir competent (Gern, 2009; Mannelli et al., 2012). Whereas both rodents and birds can serve as reservoirs for B. burgdorferi (Kurtenbach et al., 2006), B. afzelii depends mainly on rodents (mice and voles), while B. garinii depends on birds (Comstedt et al., 2011; Mannelli et al., 2012). Thus, I. ricinus can be likened to a “mixing vessel” for different Borrelia strains and species, with host associations driven by the filtering effect of spirochete selectivity for particular vertebrates, rather than adaptation of the bacterium to a vector with narrow feeding preferences (Margos et al., 2011). Two additional tick species, I. hexagonus and I. uriae, maintain the spirochete in transmission cycles separate from those of I. ricinus. I. hexagonus has a more restricted host range than I. ricinus, feeding primarily on carnivores, such as foxes and mustelids, occasionally on lagomorphs, and rarely birds (Gern, 2002). Transmission cycles involving I. uriae, a seabird specialist that feeds on a range of avian marine species, have been implicated in global dispersal of B. garinii (Olsen et al., 1993; Olsen et al., 1995; Comstedt et al., 2011; Munro et al., 2019).
The spirochete
The spirochete-host interface - the outer membrane Like all spirochetes (Holt, 1978), B. burgdorferi is a diderm consisting of an outer membrane (OM) that surrounds the periplasmic space, the peptidoglycan, the cytoplasmic membrane, and the protoplasmic cylinder (Figure 5) (Barbour and Hayes, 1986; Charon et al., 2009). The organelles of motility, the flagella, are contained entirely within the periplasmic compartment (Charon et al., 2012). In addition to propagating a planar wave that enables the spirochete to penetrate collagen matrices in connective tissue and endothelial junctions (Norman et al., 2008; Charon et al., 2012; Harman et al., 2012; Harman et al., 2013), the flagellar filaments also serve a cytoskeletal function (Charon et al., 2012). As they wind around the protoplasmic cylinder, they push against the elastic peptidoglycan sacculus, bending it to create the cell’s distinctive flat-wave morphology (Motaleb et al., 2000; Charon et al., 2009) (also see Radolf and Samuels, 2021).
Figure 5. The borrelial cell envelope.
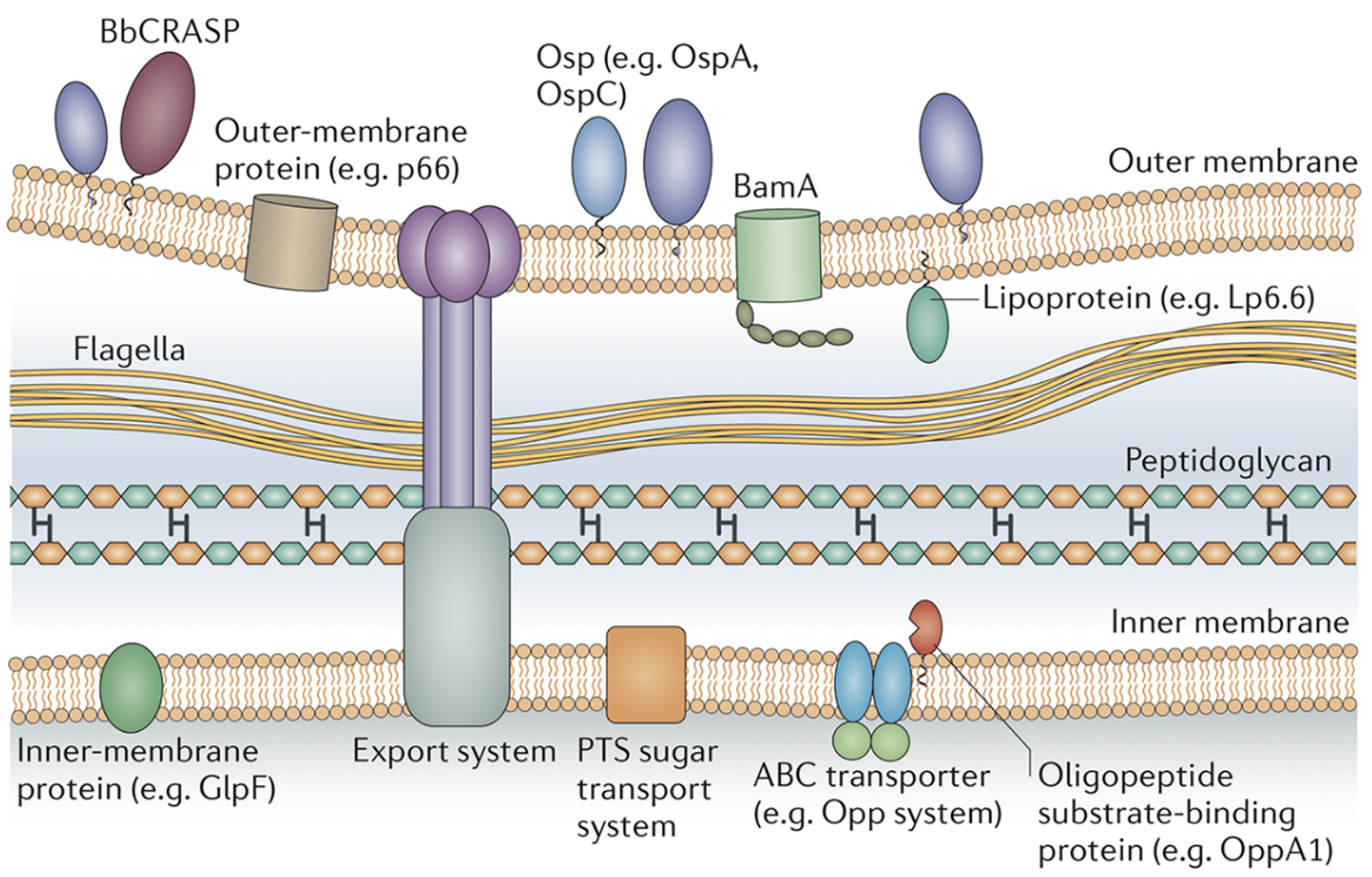
The outer membrane contains outer-surface lipoproteins (Osps) in high density and β-barrel outer-membrane-spanning proteins such as BamA in low density. The inner membrane is rich in integral membrane proteins, many of which are transporters. BbCRASP, complement regulator-acquiring surface protein; OppA1, oligopeptide permease A1; PTS, phosphotransferase system. Reprinted with permission from Radolf et al. 2012.
The B. burgdorferi OM comprises the host-pathogen interface in all milieus through which the spirochete transits or in which it takes up final residence; it is not surprising, therefore, that this structure has attracted great interest over the years (Barbour and Hayes, 1986; Kenedy et al., 2012; Radolf et al., 2012; Zuckert, 2019) (also see Radolf and Samuels, 2021). Because of its double-membrane architecture, B. burgdorferi often has been likened to Gram-negative bacteria. This analogy is inaccurate from the standpoints of phylogenetics (Paster et al., 1991; Daubin et al., 2002), ultrastructure (Radolf et al., 2012; Zuckert, 2019), composition (LaRocca et al., 2010; Radolf et al., 2012; LaRocca et al., 2013), and genomics (Fraser et al., 1997; Casjens et al., 2000; Stewart et al., 2005; Qiu and Martin, 2014). The OM of Gram-negative bacteria is an asymmetric bilayer composed of glycerophospholipids in the inner leaflet and the highly inflammatory glycolipid lipopolysaccharide (LPS) in the outer (Konovalova et al., 2017). Early reports that B. burgdorferi contains LPS (Beck et al., 1985; Habicht et al., 1986) were disproved, initially by chemical and immunological analysis (Takayama et al., 1987; Radolf et al., 1991), and subsequently by genomic sequencing (Fraser et al., 1997). The absence of LPS has important clinical ramifications inasmuch as spirochetemic Lyme disease patients rarely, if ever, manifest sepsis-like pathophysiology comparable to that seen in patients with Gram-negative bacteremia (Wormser et al., 2005; Wormser, 2006). Consequently, the presence of a sepsis syndrome in a Lyme disease patient should prompt a search for co-infections, such as babesiosis and anaplasmosis, also transmitted by ixodid ticks (Sanchez et al., 2016).
The OM of B. burgdorferi differs in other important respects from its Gram-negative counterparts: (i) It is much more easily damaged during routine laboratory manipulations (e.g., centrifugation and resuspension) and is far more susceptible to detergent solubilization (Brusca et al., 1991; Cox et al., 1996). (ii) It contains a much lower density of proteins with membrane-spanning domains, as assessed by freeze-fracture electron microscopy (Walker et al., 1991; Radolf et al., 1994). (iii) Although proteins with porin-like properties and function have been identified in the B. burgdorferi OM (Pinne et al., 2004; Pinne et al., 2007; Barcena-Uribarri et al., 2013; Kenedy et al., 2014), the bacterium does not contain orthologs for well-characterized Gram-negative porins (Fraser et al., 1997; Nikaido, 2003; Kenedy et al., 2016). (iv) The spirochete, however, does contain orthologs for TolC and the other components of an ATP-dependent efflux pump shown to contribute to the bacterium’s inherent antimicrobial resistance (Bunikis et al., 2008). (v) Though lacking LPS, B. burgdorferi OMs contain three abundant (comprising 50–60% of total lipids), immunogenic, but non-inflammatory, lower molecular weight glycolipids – cholesteryl-β-d-galactopyranoside, cholesteryl 6-O-acyl-β-d-galactopyranoside, and mono-α-galactosyl-diacylglycerol (Wheeler et al., 1993; Norgard et al., 1996; Ben-Menachem et al., 2003; Kinjo et al., 2006; Schroder et al., 2008; Huang et al., 2016). The cholesterol glycolipids spontaneously phase partition from the other lipids, forming lipid rafts or microdomains into which segregates a subset of outer surface lipoproteins (LaRocca et al., 2010; Toledo et al., 2014; Huang et al., 2016). Surprisingly, B. burgdorferi contains an ortholog for LptD, the outer membrane protein in Gram-negative bacteria that inserts newly exported LPS into the outer membrane (Botos et al., 2016); it is tempting to speculate that Borrelia appropriated the Gram-negative LPS transport pathway to serve its own needs – localization of glycolipids to the OM. (vi) Arguably, the most notable difference is the number and variety of lipoproteins that adorn the borrelial surface (Kenedy et al., 2012; Radolf et al., 2012; Dowdell et al., 2017). In Gram-negative microorganisms, lipoproteins typically are anchored to the inner leaflet of the outer membrane or the periplasmic leaflet of the cytoplasmic membrane and not exported to the bacterial surface (Zuckert, 2014; Konovalova et al., 2017). Although the tertiary structures of borrelial outer surface lipoproteins differ considerably (Li et al., 1997; Eicken et al., 2001; Kumaran et al., 2001; Eicken et al., 2002; Brangulis et al., 2018), their membrane topologies are identical. They are soluble polypeptides tethered to the external leaflet of the outer membrane by N-terminal lipids (Jones et al., 1995). This topological configuration presumably enables Borrelia to differentially express the enormous number of surface structures needed to sustain its dual-host lifestyle and helps explain its ability to infect a wide variety of vertebrate hosts (Wywial et al., 2009; Radolf et al., 2012; Brisson et al., 2013; Brissette and Gaultney, 2014; Caine and Coburn, 2016; Tufts et al., 2019).
Differential gene expression – tick transmission and back again
Because animals syringe-inoculated with in vitro-cultivated organisms develop manifestations indistinguishable from animals inoculated with ticks (Barthold et al., 2010), the arthropod phases of the bacterial life cycle might be considered irrelevant to pathogenesis. However, in the real world, Lyme disease spirochetes are transmitted by ticks and, therefore, the tick-mammal interface must be regarded as the starting point for the infectious process (Tilly et al., 2008; de Silva et al., 2009; Radolf et al., 2012; Steere et al., 2016) (also see Radolf and Samuels, 2021). Indeed, there is now overwhelming evidence that spirochetes in feeding ticks undergo complex alterations in their transcriptional and protein profiles that are not reproduced by in vitro culture conditions (Cugini et al., 2003; Iyer et al., 2015; Caimano et al., 2016; Stevenson and Seshu, 2018). Collectively, these changes enable the spirochete to adapt physiologically to the feeding midgut environment (He et al., 2011; Pappas et al., 2011; Dunham-Ems et al., 2012; Caimano et al., 2015; Bontemps-Gallo et al., 2016; Caimano et al., 2016), while, at the same time, preparing it for challenges looming during the mammalian phase (Grimm et al., 2004; Fisher et al., 2005; Dunham-Ems et al., 2012). They also promote binding to the bacterial surface of serum proteins that exploit mammalian proteolytic systems to facilitate dissemination from tick to mammal (Hu et al., 1995; Coleman et al., 1997; Onder et al., 2012) and protect against complement-mediated lysis (Kraiczy, 2016b; Zhi et al., 2018; Xie et al., 2019). The obligatory time course for this programmatic sequence explains why spirochetes are infrequently transmitted to mice (Piesman et al., 1987b; Ohnishi et al., 2001; Dunham-Ems et al., 2009) or humans (Berger et al., 1995; Falco et al., 1996; Sood et al., 1997; Nadelman et al., 2001) when ticks are attached for less than 48 h. It is critical for physicians to be aware of this time frame because it establishes the window of opportunity for antimicrobial prophylaxis (Nadelman et al., 2001; Wormser, 2006) and can be used to provide re-assurance to individuals who removed recently attached ticks that their risk of infection is low.
Our understanding (albeit still rather limited) of the genetic regulatory mechanisms that control the infectious process (Figure 6) dates back to the seminal discovery by Schwan and co-workers (Schwan et al., 1995) that OspC, an outer surface lipoprotein B. burgdorferi requires to establish mammalian infection (Grimm et al., 2004; Tilly et al., 2006; Dunham-Ems et al., 2012), is upregulated within the nymphal midgut during the blood meal. They also noted that this phenomenon can be mimicked by shifting from ambient to mammalian body temperature during in vitro cultivation. The Norgard group’s description of the RpoN/RpoS master regulatory pathway in a series of landmark papers (Hubner et al., 2001; Yang et al., 2003; Ouyang et al., 2009) provided mechanistic insight into these observations. Following a temperature shift in vitro, or at the outset of the nymphal blood meal in nature (Caimano et al., 2007), the spirochete’s alternative sigma factor RpoN works in concert with the response regulator Rrp2 and the Fur/PerR ortholog, BosR, to transcribe rpoS, the downstream “effector” sigma factor (for a complete discussion see Radolf and Samuels, 2021). Although RpoS was shown originally to transcribe just ospC and the dbpA gene encoding decorin-binding protein A (DbpA), another virulence determinant (Guo et al., 1995; Hagman et al., 1998; Fischer et al., 2003), we now know that the RpoS regulon encompasses approximately 10% of the B. burgdorferi genome, and includes many genes of unknown function (Caimano et al., 2007; Caimano et al., 2019). In Escherichia coli, activation of RpoS induces a complex, coordinated adaptive response involving a large cohort of genes that enables the bacterium to resist abiotic, physiological and environmental stresses (Chiang and Schellhorn, 2010; Hengge, 2011). The B. burgdorferi RpoS regulon, in contrast, contains only a handful of genes with discernible roles in physiology and stress responses (Caimano et al., 2007; Caimano et al., 2019). Organisms lacking RpoS are avirulent by tick- as well as needle-inoculation (Caimano et al., 2004; Fisher et al., 2005; Hyde et al., 2009; Ouyang et al., 2009; Xu et al., 2010; Dunham-Ems et al., 2012), implying that the RpoN/RpoS pathway regulates genes that promote dissemination within the tick as well as genes that function within the mammal. In contrast to the RpoS regulon in mammals, the cohort of genes controlled by the RpoN/RpoS pathway within feeding ticks is poorly defined. Gilmore’s group has identified two RpoS-dependent genes, bba64 and bba66, required for tick transmission (Gilmore et al., 2010; Patton et al., 2013).
Figure 6. B. burgdorferi gene regulatory programs throughout the enzootic cycle.
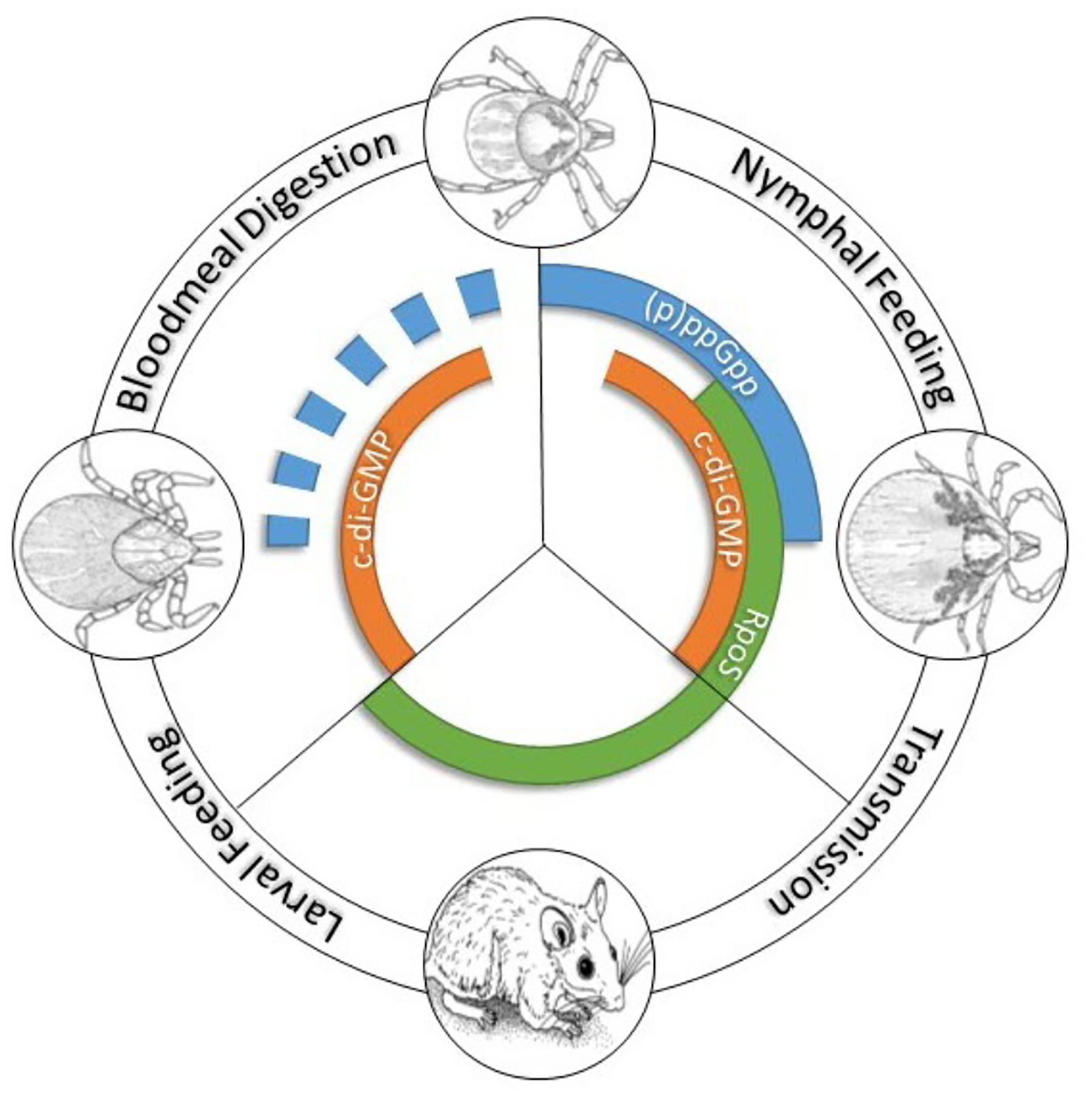
The second messengers (p)ppGpp and cyclic di-GMP regulate gene expression during the tick phases of the cycle. RpoS transcribes genes required for transmission (nymphal blood meal) and infection of the vertebrate reservoir. RpoS is OFF in unfed ticks and feeding larvae. (Figure courtesy of Dr. Ashley Groshong).
Following inoculation into the dermis of its naïve vertebrate host, the spirochete must solidify its foothold at the bite site via a poorly understood program for differential gene expression with multiple regulatory layers designated by the umbrella term “mammalian host adaptation” (Barthold et al., 1995; Montgomery et al., 1996; Akins et al., 1998) (see Radolf and Samuels, 2021). One involves turning OFF pathways that enable spirochetes to survive the many noxious aspects of the blood meal (Bontemps-Gallo et al., 2016); principal among these is the Hk1/Rrp1 two-component system that signals via the pleiotropic effector molecule cyclic-di-GMP (Hengge, 2009; He et al., 2011; Kostick et al., 2011; Caimano et al., 2015). Whether the absence of a tick-specific environmental signal or the appearance of a new mammalian host-derived cue(s) turns OFF synthesis of c-di-GMP by the diguanylate cyclase Rrp1 remains a matter of conjecture. The RpoN/RpoS pathway plays two critical, inter-dependent roles in mammalian host adaptation. First, it represses σ70-dependent tick-phase genes (Caimano et al., 2005; Caimano et al., 2007; Caimano et al., 2019), the midgut colonization factor OspA (de Silva et al., 1996; Pal et al., 2004a; Yang et al., 2004) being the prototype. This so-called “gatekeeper” repressor function of RpoS appears to work in tandem with the loss of c-di-GMP signaling to terminate expression of tick-phase genes (Caimano et al., 2019). For reasons that have yet to be determined, not all tick-phase genes (e.g., ospA and lp6.6) repressed by RpoS are upregulated by c-di-GMP. Although it is often stated that downregulation of OspA occurs within the tick in response to the blood meal (Pal et al., 2004a; Tilly et al., 2008; Caine et al., 2017), multiple lines of evidence argue that it is delayed until spirochetes reach the mammal (Belperron and Bockenstedt, 2001; Ohnishi et al., 2001; Mulay et al., 2009; Adams et al., 2017; Caimano et al., 2019). In other words, the reciprocal relationship between the expression of OspA and OspC is a mammalian host phase, not tick phase, phenomenon (Montgomery et al., 1996). RpoS-mediated downregulation of OspA and other immunogenic tick-phase lipoproteins explains why infection fails to generate antibodies against them (Gern et al., 1993; Golde et al., 1993; Brunet et al., 1995; Piesman et al., 1997; Vaz et al., 2001).
As noted already, the RpoN/RpoS pathway also upregulates expression of gene products required for infectivity. Of these, the lipoproteins OspC, DbpA/B, and BBK32 are by far the most extensively explored (Radolf et al., 2012; Groshong and Blevins, 2014; Caine and Coburn, 2016). The X-ray crystal structure of OspC, solved independently by two groups nearly twenty years ago, revealed an elongated, α-helical homodimer with putative ligand-binding sites at the distal “crown”of the dimer and at the interface between two opposing helices of the monomers (Eicken et al., 2001; Kumaran et al., 2001; Earnhart et al., 2010). The relationship(s) between this structure and OspC’s reported biological functions remains enigmatic. Along with directly recruiting the immunosuppressive tick salivary protein SALP15 to the bacterial surface (Anguita et al., 2002; Ramamoorthi et al., 2005; Hovius et al., 2008a), OspC interferes with innate clearance mechanisms (Stewart et al., 2006), putatively preventing phagocytosis by macrophages (Carrasco et al., 2015), and serves as a surface receptor for plasminogen (Lagal et al., 2006; Onder et al., 2012) and complement inhibitors (Caine et al., 2017) (see Radolf and Samuels, 2021). DbpA and B are adhesins for decorin, heparan, dermatan sulfate, heparan sulfate, and glycosoaminoglycans (GAGs) (Guo et al., 1998; Brown et al., 2001; Fischer et al., 2003; Pikas et al., 2003; Morgan and Wang, 2013). BBK32 is an adhesin, binding fibronectin and GAGs (Probert et al., 2001; Seshu et al., 2006; Moriarty et al., 2012; Lin et al., 2015), and an inhibitor of the classical complement pathway (Garcia et al., 2016; Xie et al., 2019). Whereas OspC functions primarily or exclusively at the bite site and is downregulated several weeks after infection due to the immune pressure exerted by the appearance of OspC antibodies (Liang et al., 2002; Tilly et al., 2006), DbpA/B and BBK32 function “downstream”, facilitating hematogenous dissemination and spirochete tropisms for heart and joints (Brown et al., 2001; Norman et al., 2008; Weening et al., 2008; Hyde et al., 2011; Moriarty et al., 2012; Fortune et al., 2014; Lin et al., 2014; Caine and Coburn, 2015; Lin et al., 2015; Ebady et al., 2016). Elegant studies by Moriarty’s group (Moriarty et al., 2012) revealed that BBK32 exerts its fibronectin and GAG binding activities in a sequential manner. Tethering to fibronectin on the endovascular surface recruits spirochetes from the circulating blood compartment, while binding to GAGs stabilizes interactions with endothelial cells, setting the stage for transmigration between endothelial cells (Szczepanski et al., 1990; Coleman et al., 1995). DbpA/B and BBK32 are just two elements of B. burgdorferi’s complicated adhesin story. Lyme disease spirochetes express a bewildering array of surface molecules with redundant ligand-binding activities; whether they work cooperatively or preferentially depending on the milieu and host infected is simply not understood (Caine and Coburn, 2016). This wide assortment of adhesins may help to determine the range of vertebrate hosts, including humans, that a borrelial species and, even strains within a species, can parasitize (Tufts et al., 2019).
Correct expression of adhesins in time and space is just one facet of the pathogenic process. Spirochetes deploy an arsenal to persist long enough in the reservoir to transit back to the vector when opportunity “knocks” in the form of a larva taking a blood meal. Such “persistence functionalities” include (i) directed motility to locate and penetrate dermal blood vessels following deposition and negotiate endovascular and tissue barriers at metastatic sites, all in response to chemotactic signals not even remotely understood (Charon et al., 2012; Motaleb et al., 2015; Hyde, 2017); (ii) expression of the proper combination of outer and inner membrane transporters to appropriate the huge spectrum of nutrients “generously” supplied by the vertebrate host to support the bacterium’s extremely limited biosynthetic capacity (Fraser et al., 1997; Gherardini et al., 2010; Corona and Schwartz, 2015); (iii) adjustment of central metabolism to take maximal advantage of the nutrients available within the various micro-environments in which it takes up residence (Corona and Schwartz, 2015; Iyer et al., 2015; Groshong et al., 2017), all while fending off innate and subsequently adaptive defenses, particularly complement (Kraiczy, 2016b; Tracy and Baumgarth, 2017) and antibodies (Norris, 2014; Stone and Brissette, 2017), as an “exposed” parasite within the extracellular milieu (Radolf et al., 2012). (Please see Radolf and Samuels, 2021, for comprehensive reviews of motility/chemotaxis, virulence, and immune evasion mechanisms.)
Upon larval acquisition, the RpoN/RpoS pathway rapidly shuts OFF, the HK1/Rrp1 pathway rapidly turns ON, and expression of tick-phase genes resumes, enabling successful colonization of the vector (Donahue et al., 1987; Yang et al., 2004; Caimano et al., 2015; Iyer et al., 2015; Caimano et al., 2019). How often do spirochetes execute this complex maneuver in humans? Although there is no question that Lyme disease spirochetes can persist in untreated humans and give rise to disease manifestations well after inoculation (Steere et al., 2016; Stanek and Strle, 2018), there are no reliable data as to whether persistence in humans is a common or rare occurrence. Are humans dead-end hosts because they are incompetent reservoirs or poorly accessible targets of opportunity for larvae? The biology underlying this question has clinical relevance. The technique called xenodiagnosis – allowing naïve larvae to feed on subjects to assess infection status (Telford et al., 2014) - is being used to determine whether individuals who remain symptomatic following treatment for Lyme disease (see below) harbor spirochete “persisters” (Marques et al., 2014). The rationale for xenodiagnosis as a diagnostic tool becomes cloudy if untreated individuals cannot infect ticks (Bockenstedt and Radolf, 2014).
Phylogenetic diversity and human disease Taxonomy and disease
Borrelia species fall into two major phyletic clusters, each with considerable heterogeneity (Bunikis et al., 2004; Cutler et al., 2017; Stone et al., 2017). One contains the relapsing fever spirochetes. With the notable exceptions of the louse-borne agent of endemic relapsing fever, B. recurrentis, and the I. ricinus complex-borne B. miyamotoi, relapsing fever spirochetes are transmitted by soft-bodied, argasid ticks (see Radolf and Samuels, 2021). The other cluster contains the agents of Lyme disease. The recognition by the early 1990s that multiple Borrelia species cause Lyme disease led to the designation of this cluster as the Borrelia burgdorferi sensu lato complex and the original isolate as B. burgdorferi sensu stricto (herein B. burgdorferi) Presently, the sensu lato complex comprises at least 20 proposed or confirmed species worldwide, nine of which have been found to cause human disease: B. burgdorferi, B. afzelii, B. garinii, B. bavariensis, B. spielmanii, B. lusitaniae, B. bissettii, B. valaisiana, and B. mayonii (Belfaiza et al., 1993; Wang et al., 1999a; Schotthoefer and Frost, 2015; Pritt et al., 2016; Cutler et al., 2017). However, the great majority of human Lyme disease cases are due to four pathogenic species, namely B. burgdorferi sensu stricto, B. afzelii, and B. garinii and the closely related B. bavariensis. Other species have been detected in specimens from only a few patients or, in single cases, which raises questions regarding their importance in the pathogenesis of human disease. In contrast to the United States, Lyme borreliosis in Europe is caused predominantly by B. afzelii, B. garinii, B. bavariensis (formerly B. garinii OspA type 4) and only rarely by B. burgdorferi (Stanek and Strle, 2018). As noted earlier, B. afzelii depends mainly on rodents as reservoir hosts, while B. garinii (as well as B. bavariensis) preferentially parasitizes birds (Comstedt et al., 2011; Mannelli et al., 2012). In vitro susceptibility to rodent versus avian complement has been invoked to explain these differences (Kraiczy, 2016b).
B. burgdorferi was thought to be the sole genospecies in the United States until 1995 when Marconi and associates (Marconi et al., 1995) isolated B. andersonii from cottontail rabbits and I. dentatus ticks. Subsequently, it became evident that enzootic cycles exist involving non-sensu stricto species and “specialist” ixodid ticks with strong, selective host preferences and little or no proclivity to bite humans. Perhaps the best characterized of these is B. bissettii (Bissett and Hill, 1987), transmitted by I. pacificus, I. affinis and I. spinipalpis and recovered and/or detected by PCR throughout the United States in ticks and a variety of vertebrates (Postic et al., 1998; Picken and Picken, 2000; Piesman, 2002; Oliver et al., 2003; Margos et al., 2016) and, in rare instances, humans (Picken et al., 1996; Girard et al., 2011; Golovchenko et al., 2016; Rudenko et al., 2016). Molecular evidence for infection of humans by B. americana and B. andersonii, particularly in Southern states, also has been reported (Clark et al., 2014). Moreover, B. mayonii was recently isolated from a few human specimens in upper midwestern U.S. (Pritt et al., 2016). However, in the United States, B. burgdorferi remains the primary agent of disease.
Recently, an already confusing taxonomic situation has become controversial. A comparative genomic search for molecular signatures to distinguish relapsing fever from Lyme disease spirochetes spawned a proposal that the two clusters be placed into separate genera (Adeolu and Gupta, 2014). The genus Borrelia would contain just the agents of relapsing fever, along with B. miyamotoi, while a new genus, Borreliella, would contain the agents of Lyme disease. The phylogenetic significance of these differences, and whether they justify the division, has been hotly debated (Barbour et al., 2017; Margos et al., 2017b; Margos et al., 2018; Estrada-Pena and Cabezas-Cruz, 2019). Opponents of this reclassification argue that it entails risks to public health and patient safety (Stevenson et al., 2019).
Comparison of B. burgdorferi sensu lato genospecies in United States and Europe
The most obvious clues that microbial genetics may be responsible for the differences in human disease have come from studies of the clinical presentation of Lyme borreliosis caused by different Borrelia genospecies and subspecies: in North America (B. burgdorferi) or Europe (namely B. afzelii, B. garinii, and B. bavariensis). With all four species, the first sign of infection is usually an expanding EM skin lesion (Nadelman et al., 1996; Strle et al., 1996; Steere, 2001). However, compared with B. afzelii or B. garinii infection in Europe, EM caused by B. burgdorferi in the U.S. is associated with a greater number of symptoms and more frequent hematogenous dissemination (Strle et al., 1999; Carlsson et al., 2003; Logar et al., 2004; Wormser et al., 2005; Wormser, 2006; Jones et al., 2008; Cerar et al., 2016). More pronounced differences are observed with later manifestations of disease that demonstrate B. burgdorferi is considerably more arthritogenic than B. afzelii, which usually remains localized to the skin, or B. garinii and B. bavariensis, which are predominantly associated with neurologic complications (van Dam et al., 1993; Balmelli and Piffaretti, 1995; Coipan et al., 2016; Jahfari et al., 2017; Gallais et al., 2018; Stanek and Strle, 2018; Grillon et al., 2019). An appreciation of these regional differences may help clinicians with the diagnosis and treatment of Lyme disease specific to a region. Differences in clinical presentation in North America and Europe also are observed by comparing patients infected on the two continents by B. burgdorferi. Infection with B. burgdorferi in the U.S. is associated with more symptomatic early infection compared to that in Europe, which resembles the milder infection seen with B. afzelii and B. garinii. Moreover, European B. burgdorferi strains appear to be more neurotropic than B. burgdorferi genotypes from North America and are associated with certain clinical manifestations, such as acrodermatitis chronica atrophicans, seldomly, if ever, found in the U.S. (Jungnick et al., 2015). These findings raise the intriguing possibility that strains in a region accrue similar characteristics by sharing genetic information; if so, then the feeding nymphal tick, where actively replicating strains can encounter each other, is the likely venue for such exchange.
Intraspecies genotypes are associated with distinct clinical phenotypes
B. burgdorferi can be divided into three genotypes, 16S-23S rRNA intergenic spacer types 1–3 (RST1–3), based on restriction fragment length polymorphisms of the 16S-23S rRNA intergenic spacer (IGS) (Wang et al., 1999a). OspC typing divides B. burgdorferi strains into ~30 genotypes (Liveris et al., 1999; Wang et al., 1999a; Wormser et al., 1999; Wang et al., 2001; Wang et al., 2002; Jones et al., 2006; Wormser et al., 2008a; Hanincova et al., 2013). The high degree of sequence variability among ospC genes is believed to reflect the selection of allelic variants by immunologic pressure exerted during infection of vertebrates (Wang et al., 1999b; Barbour and Travinsky, 2010; Baum et al., 2013). Individual vertebrate species serve as reservoir hosts for only a subset of OspC genotypes (Brisson and Dykhuizen, 2004; Hanincova et al., 2013; Vuong et al., 2014). A similar situation pertains to humans – only a subset of ospC genotypes circulating in ticks cause human disease (Seinost et al., 1999; Dykhuizen et al., 2008; Wormser et al., 2008a; Hanincova et al., 2013). These results imply that the tick serves as an incubator for ospC diversity, while the vertebrates upon which infected ticks feed exert a “filtering” effect, selecting for OspCs that “match” putative ligands in the blood meal host.
OspC and RST typing systems have been particularly useful in stratifying B. burgdorferi strains according to clinical presentation of disease (Liveris et al., 1999; Seinost et al., 1999; Wang et al., 1999a; Wormser et al., 1999; Bunikis et al., 2004; Alghaferi et al., 2005; Jones et al., 2006; Hanincova et al., 2008; Wormser et al., 2008a; Hanincova et al., 2013). All three RST types and 24 (of over 30) OspC types of B. burgdorferi have been recovered from patients with Lyme disease (Liveris et al., 1999; Wang et al., 1999a; Jones et al., 2006; Wormser et al., 2008b; Jones et al., 2009; Barbour and Travinsky, 2010). More recently, multilocus sequence typing (MLST) studies have been used to further sub-stratify the strains and provide additional insights into human infection that ultimately will require comparative genomics strategies to elucidate (Jungnick et al., 2015). According to the Borrelia MLST Database (https://pubmlst.org/borrelia/), >900 MLST sequence types have been identified, of which 220 are associated with disease in humans. Use of multiple typing systems together presumably can provide more information for clinical correlations than either system alone.
Several studies have now demonstrated differential pathogenicity among various genotypes within B. burgdorferi (Seinost et al., 1999; Wormser et al., 1999; Wang et al., 2001; Wang et al., 2002; Jones et al., 2006; Wormser et al., 2008b; Jones et al., 2009; Strle et al., 2011b). As a generalization, RST1 genotypes contain ospC alleles associated with invasive disease, whereas RST3 genotypes contain predominantly noninvasive ospC alleles (Hanincova et al., 2008; Wormser et al., 2008a). For example, RST1 strains are more often detectable in blood in mice and humans (Wormser et al., 1999; Wang et al., 2001; Wang et al., 2002; Jones et al., 2006; Dykhuizen et al., 2008), suggesting that they disseminate more readily and/or reach higher numbers in blood. Moreover, RST1 OspC type A strains are associated with more symptomatic early infection in patients with EM, and they more frequently cause antibiotic-refractory Lyme arthritis than other strains (Jones et al., 2009; Strle et al., 2011b). The greater disease severity and propensity for dissemination of RST1 vs RST3 genotypes was corroborated experimentally in the murine model (Wang et al., 2001; Wang et al., 2002). The data also suggest that RST1 strains are likely a contributing factor in the prevalence and severity of Lyme disease in the Northeastern U.S. (Derdakova et al., 2004; Margos et al., 2008; Hoen et al., 2009). In the mouse model, RST1 OspC type A strains have higher transmission efficiency from mice to ticks than other strains (Derdakova et al., 2004). In addition, they appear to be a recently evolved clonal lineage that may be an important factor in the emergence of the Lyme disease in the northeastern U.S. (Margos et al., 2008; Qiu et al., 2008; Hoen et al., 2009).
Like B. burgdorferi, only certain subsets of B. afzelii or B. garinii ospC types could be linked to human infection. Of the 14 ospC groups in B. afzelii, nine were found in human isolates and only two associated with invasive disease. Gallais et al. (Gallais et al., 2018) as well as Coipan et al. (Coipan et al., 2016) found a small number of B. afzelii sequence types associated with localized or disseminated infection. Similarly, only nine of the 22 ospC groups identified in B. garinii were isolated from humans, and four contained all invasive isolates. In a PCR-based comparison of tick and EM B. afzelii strains, Tijsee-Klasen et al. (Tijsse-Klasen et al., 2013) also found a correlation between specific IGS and ospC haplotypes in the patient samples. These results were extended by MLST analyses that indicated that only a small number of B. afzelii sequence types associated with localized or disseminated infection (Derdakova et al., 2004; Margos et al., 2008; Qiu et al., 2008; Hoen et al., 2009; Gallais et al., 2018).
Plasmid-encoded variable lipoproteins and host specificity
The B. burgdorferi genome encodes over 120 lipoproteins (Setubal et al., 2006), the large majority of which make their way to the spirochete’s surface at some point during the enzootic cycle via a poorly understood secretory pathway (Kenedy et al., 2012; Dowdell et al., 2017; Zuckert, 2019); many exhibit high degrees of sequence polymorphisms presumably driven by adaptive pressures exerted by vertebrates (Roberts et al., 1998; Wywial et al., 2009; Haven et al., 2011; Casjens et al., 2012; Brisson et al., 2013; Mongodin et al., 2013). Among these sequence variable lipoproteins are the lp56-encoded DbpA/B paralogs; the cp32-endoded OspE/OspF/Elp, Mlp, and RevA paralogous families; and the PFam54 paralogs, many, but not all, encoded on lp54. In addition to sequence variability at specific loci, for some of these families, the mix of paralogs varies between strains (Wywial et al., 2009; Brisson et al., 2013; Caimano et al., 2019). Importantly, variants of proteins within a family can differ functionally. For example, isogenic B. burgdorferi mutants expressing strain-specific DbpA variants exhibit pronounced differences in binding to GAGs and cultured kidney epithelial cells (Benoit et al., 2011) and marked differences in tissue tropisms in the murine model (Lin et al., 2014). Despite the high degree of structural similarity among Pfam54 paralogs, only one, BBA68 (CspA), binds complement inhibitory factors (Wywial et al., 2009; Brangulis et al., 2019). Although a challenging hypothesis to test experimentally, it is now widely believed that variability in the repertoires of these host-interactive Osps is a principal determinant of differential infectivity for vertebrate species, including humans (Tufts et al., 2019). As noted above, work with OspC establishes a paradigm for this line of thinking. Another, pioneered by Kurtenbach (Kurtenbach et al., 1998; Kurtenbach et al., 2002), is that serum resistance/susceptibility determines host-range; this appealing concept has prompted investigation of an ever expanding array of complement inhibitory proteins (Garcia et al., 2016; Kraiczy, 2016a, b; Caine et al., 2017; Marcinkiewicz et al., 2019; Tufts et al., 2019; Xie et al., 2019). One can integrate these paradigms by positing that dissemination and long-term survival of B. burgdorferi in a particular host requires an “appropriate” combination of early survival/host adaptation Osps (e.g., OspC and anticomplementary lipoproteins) and adhesins/invasins that function during later stages of infection. It then follows that (i) whether an inoculated strain has the correct assortment of Osps to cause local or systemic human disease is a chance event and (ii) variations in Osp repertoires may give rise to different clinical manifestations. It is even conceivable that some clinical manifestations without known counterparts in nature (e.g., neuroborreliosis) are the inadvertent consequence of adaptive changes at loci functionally downstream of OspC.
Novel sequencing approaches to characterize Borrelia genetic diversity and its impact on phenotypic heterogeneity
Recent advances in sequencing technologies raise the possibility that deep genetic characterization of clinical isolates may elucidate strain- and species-specific differences that promote virulence, immunogenicity, organ tropism, or persistent infection. Second-generation technologies, particularly those commercialized by Illumina, excel at “resequencing” and can produce very high-quality genomic sequences. However, the sequence reads are relatively short (36–250 bases) and cannot resolve longer genomic fragments or areas with extensive diversity. Thus, while short read approaches have been used with success to study the genome sequence of B. burgdorferi chromosome and conserved plasmids cp26 and lp54 (Castillo-Ramirez et al., 2016; Margos et al., 2017a; Tyler et al., 2018), they are poorly suited to the study of the less-conserved plasmids. Recently, technologies that produce longer reads have been introduced by Oxford Nanopore Technologies (ONT) and Pacific Biosciences. These methods can generate much longer sequence reads, albeit at lower sequence quality, making them unsuitable for assembly in the absence of additional high-accuracy sequence (Bashir et al., 2012; Antipov et al., 2016; Wick et al., 2017). However, the combination of these short- and long-read sequencing approaches is poised to open the full landscape of B. burgdorferi genomic analyses for clinical correlations. Early successes include demonstration of the utility of long-read approaches for obtaining finished sequence of plasmids (Kingry et al., 2016; Margos et al., 2017a; Jabbari et al., 2018) and a detailed study, carried out by Chaconas and colleagues, of the mutation rate and switching kinetics at the vlsE locus (Verhey et al., 2018).
Infection of humans
The bite site
The seminal event in the natural history of Lyme disease is, of course, the deposition of spirochetes into the skin when an infected tick, usually a nymph, feeds on a human instead of a vertebrate in the wild. Although spirochetes undergo dramatic expansion (≥ 300-fold) within the midgut during the blood meal, only a remarkably small number manage to complete the journey from tick to vertebrate (De Silva and Fikrig, 1995; Coleman et al., 1997; Dunham-Ems et al., 2009). Along the way, they encounter myriad anatomical, biochemical, and immunological barriers that passively and actively reduce their numbers (Pal et al., 2004b; Fisher et al., 2005; Bontemps-Gallo et al., 2016; Sonenshine and Macaluso, 2017; Shaw et al., 2018). qPCR and immunofluorescence analysis yielded values of approximately 20 spirochetes per salivary gland at peak infectivity (Ohnishi et al., 2001; Piesman et al., 2001). While this number is well below published ID50 values determined using in vitro-cultivated organisms (Sadziene et al., 1993; Xu et al., 1996), it must be remembered, per above, that spirochetes delivered by ticks undergo dramatic transcriptomic and proteomic changes during feeding (Cugini et al., 2003; Pal and Fikrig, 2003; Drecktrah et al., 2015; Iyer et al., 2015; Caimano et al., 2016; Bernard et al., 2018; Stevenson and Seshu, 2018) in addition to exploiting the battery of pharmacologic, hemostatic, anticomplementary, and immunosuppressive factors in the tick salivary cocktail (“saliva-assisted transmission”) (Hovius, 2009; Chmelar et al., 2016; Simo et al., 2017; Nuttall, 2019) (also see Radolf and Samuels, 2021). In recent years, tick salivary proteins that contribute to pathogen transmission have been identified (Fikrig and Narasimhan, 2006; Hovius et al., 2008b; Murfin and Fikrig, 2017; Simo et al., 2017). Of these, the multi-functional Salp15 has been the most extensively studied. In addition to inhibiting activation of naïve CD4+ T cells by binding to CD4 (Anguita et al., 2002; Tomas-Cortazar et al., 2017) and dendritic cells through interactions with DC-SIGN (Hovius et al., 2008a), Salp15 binds to OspC on spirochetes within saliva, protecting against antibody-mediated killing (Ramamoorthi et al., 2005). Even so, the spirochetes that do complete the journey from midgut to dermis initially have a precarious existence. In mice, dissemination does not occur if the inoculation site is excised within the first several days of tick detachment (Shih et al., 1992). Moreover, spirochetes inoculated into skin are antigenically heterogeneous and do not appear to have a uniform ability to establish infection (Ohnishi et al., 2001). At some point, they overcome the local bottleneck to colonization of their new mammalian host and dissemination (Troy et al., 2013; Rego et al., 2014). The importance of the RpoN/RpoS-regulated genes (Figure 6) and anti-complementary surface lipoproteins for early survival in the mammal was discussed above (also see Radolf and Samuels, 2021).
With their foothold established, spirochetes begin to replicate and migrate outwards along the plane of the skin and downward towards the dermal microvasculature (Figure 7) (Skare et al., 2016; Hyde, 2017). In ex vivo gelatin matrices that mimic the extracellular collagenous matrix spirochetes traverse in vivo (Zambrano et al., 2004; Dunham-Ems et al., 2009), the pathogen moves at a rapid clip, ~4 microns per second (Harman et al., 2012), far faster than pursuing phagocytes (Vig and Wolgemuth, 2014). Motility, however, is not uniform; spirochetes transition between a variety of motility states determined by transient adhesions, although the specific adhesins involved have yet to be identified (Harman et al., 2012). Presumably, these movements, along with PAMPs shed by live and dead organisms, trigger local danger signals that result in the accumulation of resident innate immune cells, primarily macrophages and dendritic cells, as well as the recruitment of circulating immune cells with skin-homing capacity (Mullegger et al., 2000; Salazar et al., 2003; Petzke and Schwartz, 2015; Marques et al., 2017). The ensuing inflammatory response gives rise to the hallmark skin lesion, erythema migrans (EM) (Wormser, 2006; Nadelman, 2015; Stanek and Strle, 2018). Eventually, organisms reach densities easily detected by culture, PCR (Nowakowski et al., 2001; Aguero-Rosenfeld et al., 2005; Ruzic-Sabljic and Cerar, 2017), and even histopathology (Duray, 1989b). Not surprisingly, detection of spirochetes by culture or PCR correlates with lesion size (Stupica et al., 2015). The rate of expansion of EM lesions (~20 cm2 per day) is thought to reflect the speed at which spirochetes migrating away from the bite site are trailed by the local inflammatory response (Dandache and Nadelman, 2008). Along these lines, a mathematical model predicted that the rates of bacterial replication and dissemination within the dermis are the primary determinants of the rate of EM progression (Vig and Wolgemuth, 2014). A cardinal difference between Lyme disease in humans and mice is that the latter do not develop EM (Barthold, 1996); in other words, development of EM can be considered an indication of the lack of local tolerance of humans to the presence of B. burgdorferi. Like humans, tick or needle-inoculated non-human primates develop EM (Philipp et al., 1993; Pachner et al., 2001; Embers et al., 2017), as do rabbits (Wheeler et al., 1989; Foley et al., 1995), another non-reservoir host species.
Figure 7.
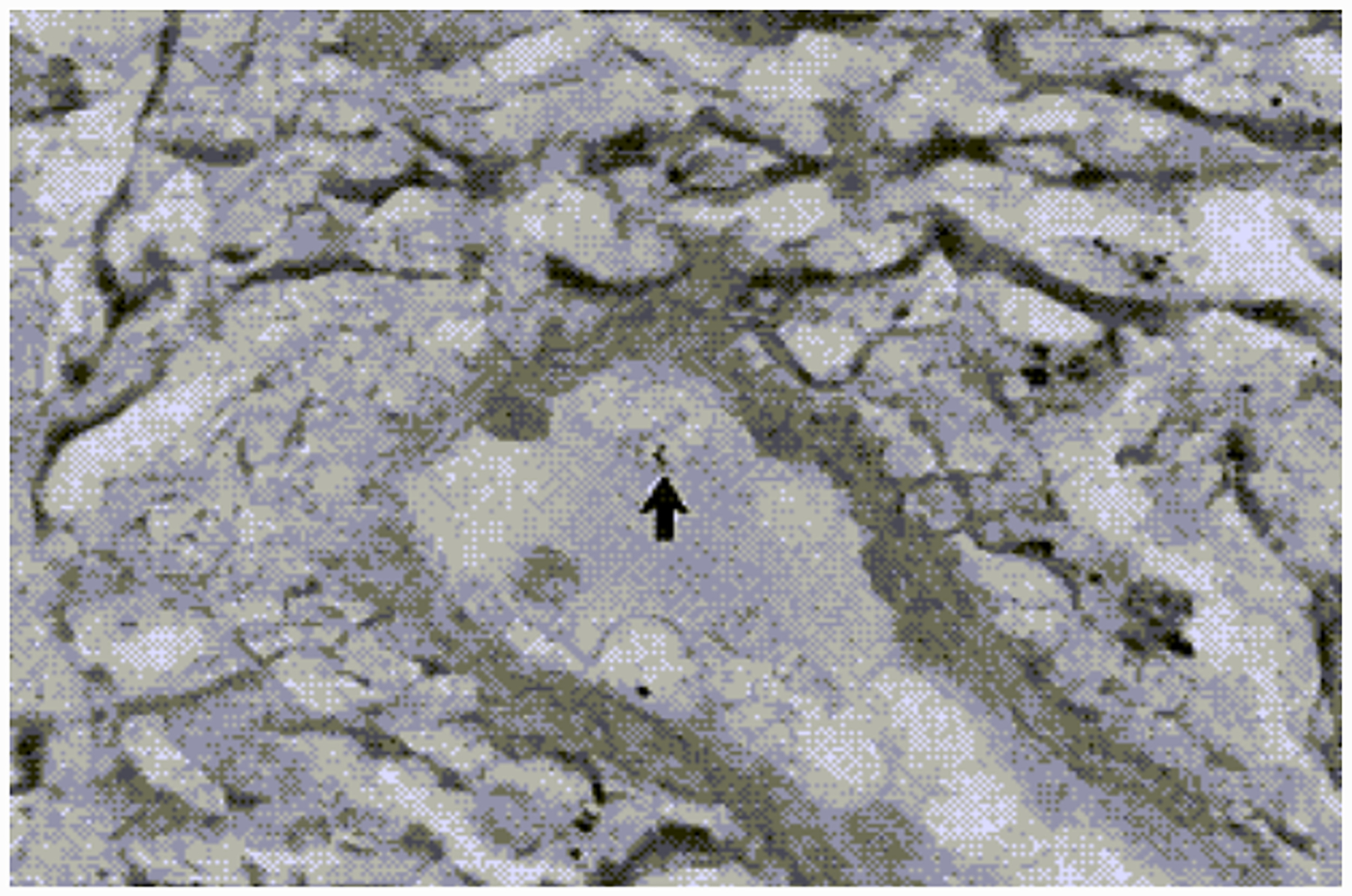
Silver stained biopsy from an erythema migrans lesion showing a spirochete that has penetrated a dermal venule. (Reproduced with permission from Duray, 1989b).
Asymptomatic infection
Clinical manifestations are not an inevitable outcome of tick inoculation with B. burgdorferi. As noted previously, seroepidemiologic studies in Lyme disease endemic areas have shown that substantial proportions of persons with antibodies to B. burgdorferi are asymptomatic. In Europe, asymptomatic or subclinical infection may be as common as clinically apparent disease, whereas in the U.S. only a small subset of infected patients are asymptomatic (Hanrahan et al., 1984; Steere et al., 2003; Wilhelmsson et al., 2016; Carlsson et al., 2018). In the OspA vaccine trial, 30 of the 269 patients who met the criteria for Lyme disease were classified as having asymptomatic IgG seroconversion to B. burgdorferi (Steere et al., 1998). Upon subsequent investigation, 14 of these individuals were found to have had symptoms and/or a rash compatible with EM that was not appreciated during their participation in the study. Eight patients, however, were truly asymptomatic and went untreated. Surprisingly, only one of these eight individuals developed a late complication – arthritis (Steere et al., 2003). Wormser and co-workers (Wormser et al., 2001b) proposed that some asymptomatic infections may be attributed to noninvasive strains. This is another way of stating that at least some uneventful outcomes reflect the general non-permissiveness of humans as hosts for Lyme disease spirochetes. Unfortunately, methodologies to distinguish patients who have cleared “benign” asymptomatic infection from those who may be persistently infected and at risk for subsequent late complications do not exist. The management of asymptomatic persons found to be seroreactive for Lyme disease is an important unresolved issue for practitioners in endemic areas (Wormser et al., 2006). A comparison with syphilis, caused by Treponema pallidum, a spirochete that has evolved to persist in humans, is instructive: It is well recognized that patients with latent syphilitic infection are at risk of recrudescent disease, and, therefore, must be treated (Radolf et al., 2019).
Hematogenous dissemination and organ system invasion
That spirochetes disseminate hematogenously during early infection has been known since B. burgdorferi was first isolated in the early 1980s (Benach et al., 1983; Steere et al., 1983a). Wormser and colleagues (Wormser et al., 2001a) showed that spirochetemia occurs in approximately 40% of patients with EM but that the extremely low spirochete concentrations in blood (estimated to be ~1 bacterium per 10 ml) necessitate culturing large volumes of plasma (Wormser et al., 2001a) (also see Radolf and Samuels, 2021). The low spirochete burdens in blood during early Lyme disease also explains why PCR analysis of blood, a volume-limited technique, has poor diagnostic yield (Aguero-Rosenfeld et al., 2005; Lohr et al., 2018). Since untreated patients cannot be followed prospectively, it is not known whether spirochetemia occurs constantly at low levels or at varying levels intermittently. In a large retrospective review of spirochetemic EM patients evaluated using the large-volume culture technique, hematogenous dissemination was not associated with duration or size of EM (Wormser et al., 2005); these results are in accord with data that borrelial genotypic factors are the predominant determinants of whether invasive infection occurs. Approximately 20% of EM patients have secondary EM-like skin lesions, a clinical indicator of hematogenous dissemination analogous to the rash of secondary syphilis (Wormser et al., 2005). Interestingly, blood cultures were positive in only five of 26 patients with extracutaneous manifestations of Lyme disease, four of whom had concomitant erythema migrans (Nowakowski et al., 2009); these results suggest that spirochetemia occurs predominantly during early infection. Even though spirochetemia may be difficult to detect, the high proportion of constitutional symptoms strongly points to the systemic nature of Lyme disease even when it appears microbiologically localized (Wormser, 2006; Steere et al., 2016). The discovery of metabolic (Molins et al., 2017) and proteomic (Zhou et al., 2020) signatures in the blood of early Lyme disease patients further indicates the systemic nature of earlier illness regardless of whether clinical signs of dissemination are present.
Little is known about the mechanisms by which circulating spirochetes recognize and invade target organs. Studies with cultured human umbilical vein endothelial cells have shown that organisms rapidly attach to vascular endothelium and negotiate their way through intercellular junctions (transmigration), subsequently attaching to subendothelial matrix components (Comstock and Thomas, 1989; Szczepanski et al., 1990). Real-time intravital confocal microscopy revealed that transmigration through capillaries and post-capillary venules in vivo is a multi-step process engaged in by only a small percentage of GFP-expressing spirochetes introduced intravenously into mice (Moriarty et al., 2008; Norman et al., 2008). The role of the adhesin BBK32 in trans-endothelial migration was described above.
Aside from skin, the heart, joints, and nervous system are the most affected metastatic sites (see below). How frequently do blood-borne spirochetes gain access to these organ systems? Unlike with experimental animal models, obtaining precise values for the incidence rates of disseminated manifestations of Lyme disease in humans is a major challenge. For example, while some authorities cite incidence rates for carditis as high as 10% (Scheffold et al., 2015), these values are almost certainly overestimates, perhaps reflecting the era when early Lyme disease often went unrecognized and, therefore, untreated. Based on surveillance data obtained from 2001–2010, the CDC determined that cardiac involvement occurs in approximately 1% of reported cases (Forrester et al., 2014). On the other hand, cardiac involvement occurs in diverse strains of laboratory mice and in most inoculated animals (Barthold et al., 1990) (also see Radolf and Samuels, 2021). Thus, the comparatively low percentage of carditis in humans suggests that B. burgdorferi does not have as strong a tropism for human cardiac tissue. This conclusion is supported by studies with rhesus macaques that found that carditis was absent or mild unless animals were immunosuppressed (Philipp et al., 1993; Cadavid et al., 2004).
In an early report describing 12 U.S. patients with acute disseminated Lyme disease, six with acute cranial neuritis, three with multiple EM, and three with both cranial neuritis and multiple EM, eight patients had spirochetal DNA in their cerebrospinal fluid (CSF) detectable by PCR (Luft et al., 1992). More recent, large European studies involving patients infected with neurotropic Borrelia genospecies indicate that these findings greatly overestimated the incidence of CNS invasion during early Lyme disease. During a 5.5-year period, Strle and colleagues (Ogrinc et al., 2013) found neurologic symptoms in only 161 of 2751 (6%) patients with erythema migrans; of these, only 31 (19%) had CSF abnormalities, and spirochetes were isolated from only 6 of 127 untreated individuals (Ogrinc et al., 2013). In a subsequent study, they isolated spirochetes from only 12 of 177 persons with Bannwarth Syndrome (15%) (Ogrinc et al., 2016). These latter results bear out Halperin’s contention that neurologic complications in Lyme disease often result from inflammation of blood vessels supplying nerve roots and peripheral nerves as opposed to penetration of the blood-brain barrier and/or invasion of brain parenchyma (Halperin, 2008).
Clinical Manifestations
Early studies divided Lyme disease into three distinct stages analogous to those of syphilis (Steere, 2001): Stage 1, erythema migrans; Stage 2, neurologic or cardiac involvement, and Stage 3, arthritis. This staging system is still in use today (Steere et al., 2016). It is particularly important for caregivers on the front lines to think in terms of localized infection, EM, and early and late consequences of dissemination (Trayes et al., 2018; Schoen, 2020). However, only a minority of untreated patients develops all clinical manifestations, although in some patients signs of early and disseminated Lyme disease are present at the same time.
Skin
Numerous clinical series have established that EM is the most common manifestation of B. burgdorferi infection in the United States and Europe (Berglund et al., 1995; Steere and Sikand, 2003; Wormser et al., 2006; Enkelmann et al., 2018; Stanek and Strle, 2018). Most patients presenting with this rash have primary EM, the lesion that develops at the site of tick inoculation, typically within 7 to 14 days after detachment. Primary EM can be located anywhere but in adults occurs most commonly below the waist. In North American studies, only 14–32% of individuals presenting with EM recall a tick bite. To distinguish EM from the transient and more localized inflammatory reactions that often develop following an insect bite, the Centers for Disease Control and Prevention designated 5 cm as the minimum lesion diameter required for clinical diagnosis (Figure 8). While this size criterion improves the specificity of clinical diagnosis, it must be emphasized that bona fide EM lesions can be less than 5 cm, particularly when detected shortly after tick detachment. EM is traditionally described as an expanding, annular, erythematous skin lesion with central clearing, the so-called classic “bull’s eye rash” (Steere, 2001). Central clearing, however, is not as characteristic as was once thought: two large North American studies found only 37% (Nadelman et al., 1996) and 9% (Smith et al., 2002) of patients had central clearing. Central clearing appears to be more common in Europe, where most cases are due to B. afzelii and have a longer duration prior to treatment (Strle et al., 1999; Dandache and Nadelman, 2008). EM-associated systemic illness can vary from none to moderate constitutional symptoms consisting of arthralgia, malaise, fatigue, headache, and low-grade fever and chills (Berglund et al., 1995; Wormser et al., 2006; Dandache and Nadelman, 2008; Stanek and Strle, 2018). Studies of EM patients have reported a difference in the incidence of extracutaneous manifestations in America and Europe. Less than 50% of European patients have extracutaneous manifestations as opposed to more than 75% of patients in the United States (Strle et al., 1999; Dandache and Nadelman, 2008). Regional lymphadenopathy is the most common physical finding associated with EM in both Europe and North America. Differences in EM and associated symptoms caused by B. garinii and B. afzelii also have been noted (Logar et al., 2004; Strle et al., 2011a). Patients with B. garinii had shorter incubation periods, faster evolution of their EM, and more symptomatic lesions (burning, itching, and pain) as well as modestly increased systemic symptomatology.
Figure 8. Erythema migrans (EM) lesions:
(A) Diffuse EM expanding centripetally over the right quadriceps. (B) EM lesion on the right torso forming a classic “bulls-eye” pattern; note the central eschar (the tick bite site). (C) Ulcerated necrotic and EM lesion. Black lines demarcate area of receding erythema following antibiotic therapy. (D) Multiple EM lesions of disseminated early Lyme disease over the posterior trunk and lower back.
The most common histological pattern of EM is a superficial and deep dermal infiltrate consisting mostly of lymphocytes, but also containing neutrophils, macrophages, and plasma cells, often in a perivascular distribution (Figure 9) (Duray, 1989b; de Koning, 1993). Over the years, efforts to dissect this cellular response have been predicated on the assumption that it plays a pivotal role in determining the outcome of infection, possibly helping to initiate the ill-defined, treatment-recalcitrant symptomatic state known as post-treatment Lyme disease syndrome (PTLDS) (Crowder et al., 2014; Weitzner et al., 2015; Bouquet et al., 2016) (also see Radolf and Samuels, 2021). Using an epidermal blister fluid suction technique to extract dermal cells for immunophenotypic analysis by flow cytometry, Salazar and co-workers (Salazar et al., 2003) showed that EM infiltrates contain diverse cellular elements derived from both the innate and adaptive arms of the immune system, including plasmacytoid dendritic cells and memory-effector T cells. Greater than 80% of lesional T cells expressed CCR5 and/or CXR3, suggesting a strongly polarized Th1 response, confirmed by high levels of IFN-γ in the blister (interstitial) fluids. Th1 polarization of skin-recruited, antigen-sensitized T cells was supported by Glickstein et al (Glickstein et al., 2003), who found IFN-γ to be the predominant cytokine produced by peripheral blood leukocytes from culture-confirmed EM incubated with borrelial lysates, and by Jones et al. (Jones et al., 2008), who found high levels of transcripts for IFN-γ and Th1-associated chemokines in RNAs extracted from EM lesions. The latter study also was noteworthy for its comparison of the chemokine and cytokine mRNA profiles of EM lesions from European and U.S. patients infected with B. afzelii and B. burgdorferi, respectively. Consistent with the generally milder course of EM in Europe (Strle et al., 1999; Dandache and Nadelman, 2008), they found that lesions from U.S. patients had significantly higher mRNA levels for chemokines associated with macrophage activation and Th1 cell recruitment. Similarly, studies in serum from EM patients, or in macrophage cell cultures stimulated with Borrelia isolates from these patients, demonstrated that infection with U.S. B. burgdorferi strains elicits higher levels of cytokines and chemokines associated with innate and Th1 adaptive responses than European B. afzelii or B. garinii strains (Strle et al., 2009).
Figure 9.
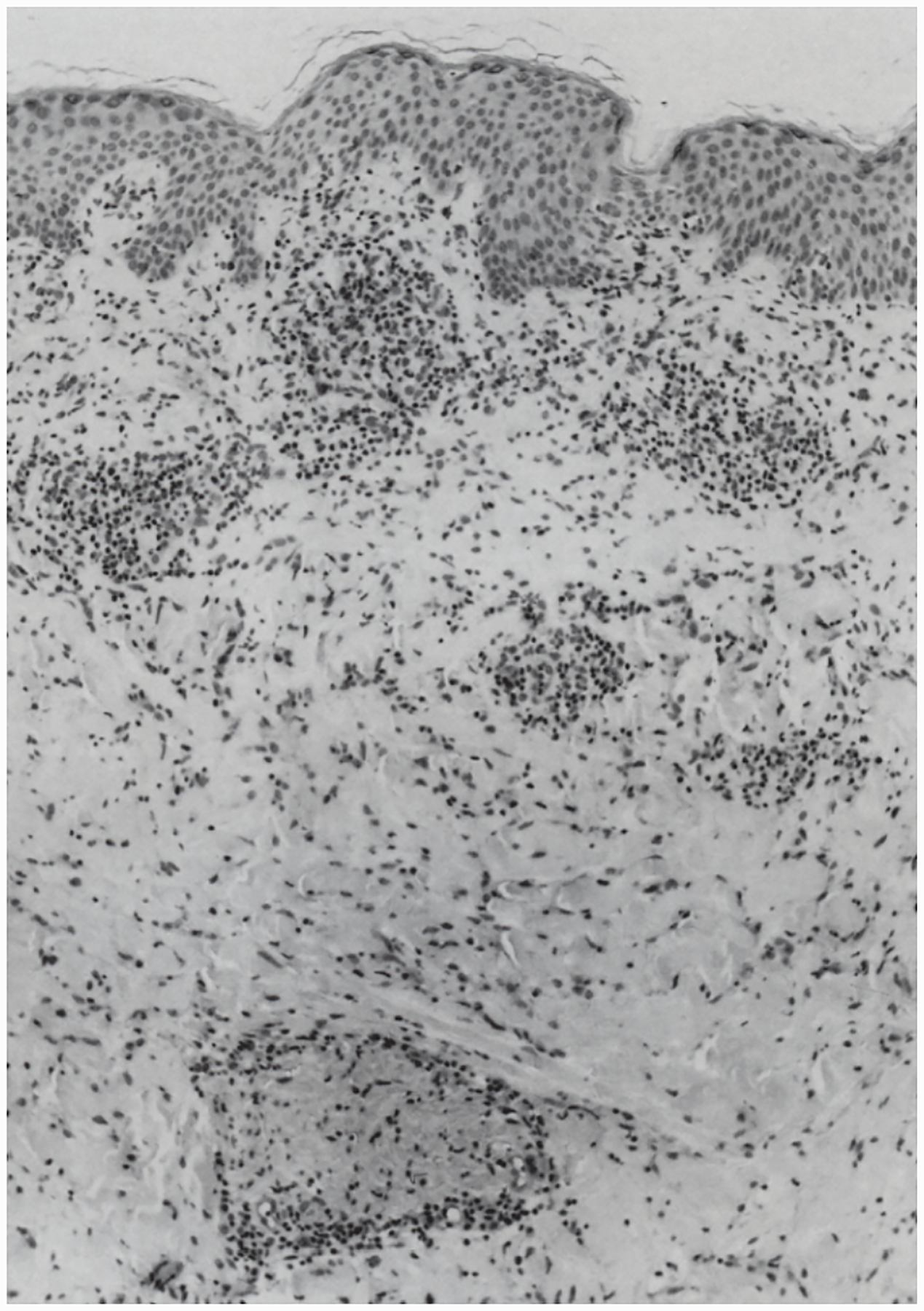
Biopsy taken from the center of an EM lesion showing a superficial and deep perivascular and interstitial infiltrate of lymphocytes and histiocytes. H&E, × 100 (Reprinted with permission from de Koning et al.)
A recent comparative microarray analysis of mRNAs extracted from 18 EM biopsies and 27 controls has provided an unprecedented global assessment of the transcriptional response elicited by B. burgdorferi infection of human skin (Marques et al., 2017). CXCL9, CXCL10, and CXCL11, chemokines strongly implicated in early and late Lyme disease pathogenesis (Lepej et al., 2005; Mullegger et al., 2007; Shin et al., 2010), were the most highly induced genes. Interferon signaling was the top activated pathway, followed by pathways for pattern recognition receptors of bacteria and viruses and DC maturation. Based upon this comprehensive dataset, Marques et al. (Marques et al., 2017) proposed a model for the immunopathogenesis of cutaneous B. burgdorferi infection that explains the genesis and amplification of the local inflammatory response along with a novel mechanism spirochetes deploy for evading it. Briefly, TLR-mediated activation of local and recruited phagocytes upon ingestion and degradation of spirochetes elicits numerous inflammatory mediators, including Th1-polarizing chemokines. Production of IFN-γ by T cells and type I IFNs by macrophages and plasmacytoid dendritic cells bolsters phagocytosis and elicits myriad interferon-responsive genes; the latter include genes encoding enzymes involved in tryptophan catabolism. The authors hypothesized that spirochetes exploit the inhibition of T cell priming and enhanced development of FoxP3+ Tregs resulting from tryptophan depletion.
In Europe, but not North America, Lyme disease is associated with two additional dermatologic disorders: borrelial lymphocytoma (BL) and acrodermatitis chronica atrophicans (ACA) (Steere et al., 2016; Stanek and Strle, 2018). BL may occur concurrently with EM or a few days before or up to several weeks after EM (Strle et al., 1992; Brehmer-Andersson et al., 1998; Glatz et al., 2015; Maraspin et al., 2016), generally manifesting as solitary, bluish-red nodule on the ear lobe or the nipple area (Figure 10) (Strle et al., 1992; Colli et al., 2004). Histopathologically, BL is characterized by a dense polyclonal lymphocytic infiltrate in the dermis or subcutaneous tissue; differentiation from lymphoma is usually not very difficult since lymphocytic infiltration in BL is polyclonal, while in lymphoma it is oligoclonal or monoclonal (Colli et al., 2004). Maraspin et al. (Maraspin et al., 2016) reported an isolation rate of one in three from BL tissue and that B. afzelii is the main causative agent; of 13 typed strains, 11 were B. afzelii, one was B. garinii, and one was B. bissettii. Although systemic symptoms are rarely associated with lymphocytoma, most patients report mild symptoms localized to the site of the lesion.
Figure 10. Borrelial lymphocytoma.
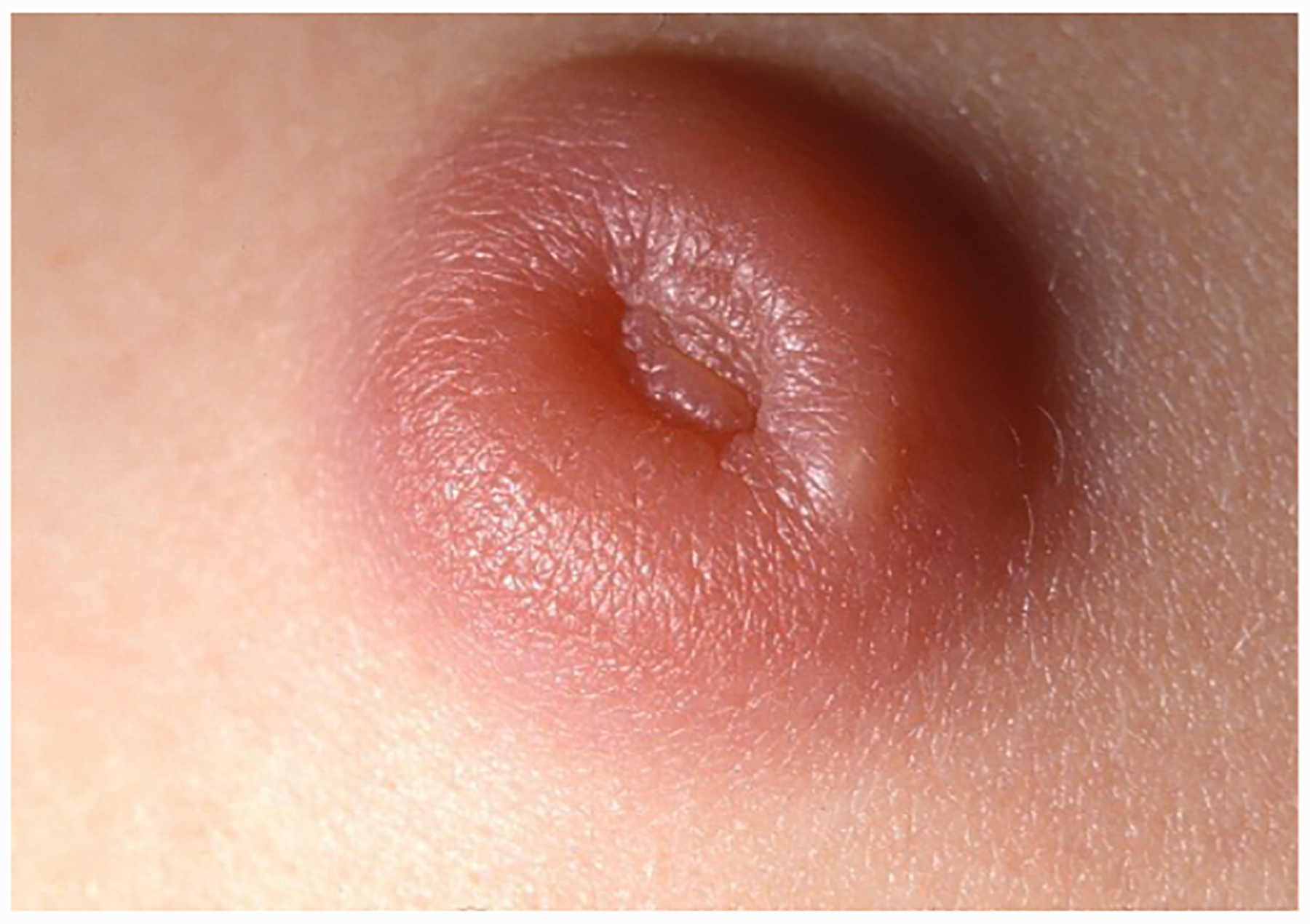
Image shows a firm, indolent swelling of the left nipple of an eight-year-old boy with prior history of a tick bite and serological evidence of Lyme disease. Photograph courtesy of Ulrich Heininger, University Children’s Hospital, Basel, Switzerland.
ACA is an extremely late manifestation, typically developing months to years after the onset of untreated infection (Brehmer-Andersson et al., 1998; Brandt et al., 2015). Occurring throughout Northern, Central, and Eastern Europe, ACA is associated primarily with B. afzelii infection. Initially the skin lesions are distinguished by inflammation and edema. With time, the lesions become increasingly atrophic. ACA typically involves the distal extensor part of extremities and, less commonly the trunk, sparing the face. Polyneuropathy, small joint arthritis with subluxation, arthritis of the large joints, and periosteal thickening of the bones may occur in the same extremity as ACA.
Lyme neuroborreliosis
The neurologic manifestations of Lyme disease, often referred to as Lyme neuroborrelisosis, reflect the capacity of B. burgdorferi sensu lato to invade diverse targets within the peripheral and central nervous system and to cause neurological complications weeks to months after infection (Halperin, 2003; Koedel et al., 2015; Halperin, 2018; Garcia-Monco and Benach, 2019). Early nervous system involvement is usually manifested by the involvement of cranial and/or peripheral nerves or nerve roots typically associated with lymphocytic meningitis. Classical Bannwarth syndrome (BS) is defined as meningoradiculoneuritis; cranial neuritis may be or may not be present (Halperin, 2015; Ogrinc et al., 2016; Shah et al., 2018; Garcia-Monco and Benach, 2019). BS is the most typical manifestation of Lyme neuroborreliosis in adult European populations (Ogrinc et al., 2016); although rare in the U.S., a cluster of five cases recently was reported from the upper Midwest (Shah et al., 2018). Currently, peripheral facial palsy and an isolated aseptic (viral-like) meningitis picture are the most common early neurologic manifestation in the U.S. (Garcia-Monco and Benach, 2019). Initial studies of North American patients with untreated EM found that approximately 15% developed meningitis or cranial neuritis within the first three months after presentation (Steere et al., 1983b; Halperin et al., 1989). It is now believed that early neurologic involvement is much more common in Europe than in the United States, reflecting the prevalence of neurotropic strains of B. garinii (Koedel et al., 2015; Steere et al., 2016; Stanek and Strle, 2018).
Although late disseminated B. burgdorferi infection has been linked to a variety of nervous system disorders over the years, the neurologic entities now generally accepted as causally associated with late Lyme disease – encephalomyelitis, encephalopathy, and axonal polyneuropathy (Logigian et al., 1990; Logigian and Steere, 1992; Wormser et al., 2006) – are quite rare. As documented in the 2006 IDSA Lyme disease guidelines (Wormser et al., 2006), only one case of encephalomyelitis, nine patients with peripheral neuropathy and seven patients with encephalopathy were seen by the panel members in the 5 years preceding their publication. Peripheral nerve involvement in late Lyme disease is an axonopathy that may be distributed symmetrically or asymmetrically, and in some cases, resemble polyneuritis multiplex (Halperin et al., 1990; Logigian et al., 1990). Patients with this entity complain of paresthesias and hyperthesias and have abnormal nerve conduction. In Europe, peripheral neuropathy may be found in association with ACA but rarely, if ever, occurs in patients without ACA or other Lyme disease manifestations (Kristoferitsch et al., 1988; Mygland et al., 2006). Halperin (Halperin, 2008) has voiced considerable skepticism that Lyme disease is a significant cause of stroke.
Not surprisingly, the inflammatory mechanisms underlying CNS neuroborreliosis are complex, involving both innate and adaptive immune systems. Building upon their in vivo work with the primate model, Philipp and co-workers (Ramesh et al., 2003; Bernardino et al., 2008; Ramesh et al., 2008) demonstrated in an elegant series of studies that spirochetes induce profound proinflammatory and apoptotic changes in rhesus macaque glial cells and astroctyes and, most recently, isolated brain slices, that involve diverse chemokines, cytokines, and TLR-mediated signals. Analysis of cerebrospinal fluids from neuroborreliosis patients has identified borrelial antigen-specific CD4+ T cells, CD8+ T cells, and B cells (Cepok et al., 2003; Jacobsen et al., 2003; Rupprecht et al., 2005; Lunemann et al., 2007), as well as unusual subsets of dendritic cells (Pashenkov et al., 2001). Collectively, these results underscore how greatly CNS infection by Lyme disease spirochetes differs from meningitis caused by pyogenic bacteria.
Lyme carditis
As with all forms of disseminated Lyme disease, the incidence of symptomatic cardiac involvement in the United States appears to have decreased dramatically and is now considered to be approximately 1% (Forrester et al., 2014). Available evidence indicates that carditis does not occur if early Lyme disease patients without cardiac manifestations are appropriately treated (Robinson et al., 2015). When first described, concurrent EM and other forms of early Lyme disease was the rule (van der Linde, 1991); in recent years, however, one-half or less of patients present with concurrent EM (Forrester and Mead, 2014; Marcos et al., 2019). In their recent review, Forrester and Mead (Forrester and Mead, 2014) noted a striking male predominance (84%), a finding supported by a retrospective chart review conducted at Stony Brook, Long Island (Marcos et al., 2019).
The principal manifestation of Lyme carditis is atrioventricular block proximal to the bundle of His, which can fluctuate and progress rapidly (typically within hours, occasionally within minutes) (Fish et al., 2008; Robinson et al., 2015). Other forms of conduction system disease, such as atrial fibrillation (Zainal et al., 2019) and sick sinus syndrome (Gazendam et al., 2020), also have been reported. Although diffuse myocardial involvement has been seen in cases that have come to autopsy (Duray, 1989a; Tavora et al., 2008; Muehlenbachs et al., 2016), clinically apparent acute myocarditis is uncommon (Robinson et al., 2015). Valvular heart disease also occurs but is extremely rare, with only a handful of microbiologically confirmed cases reported (Nikolić et al., 2020). Two PCR-confirmed cases occurred in the United States (Paim et al., 2018; Haddad et al., 2019), while one European case was attributed to B. bissettii (Rudenko et al., 2008). Early European studies reported an etiologic association between Lyme disease and chronic dilated cardiomyopathy, but these have not subsequently been reproduced on either side of the Atlantic (Robinson et al., 2015). Even without therapy, Lyme carditis may be self-limited (Steere et al., 1980). With appropriate therapy, the prognosis is excellent, with a medium time of 6 days to improvement or restoration of normal sinus rhythm.
With only 11 cases in the literature, sudden death is an extremely rare, though, tragically, now well-recognized outcome of Lyme carditis (Marx et al., 2019). In 2013, the CDC called attention to the potential for Lyme disease to cause cardiac fatalities when it reported three cases occurring from November, 2012 to July, 2013 in young individuals (26 to 38 years) from Connecticut, Massachusetts, and New Hampshire (Centers for Disease and Prevention, 2013). Muehlenbachs et al. (Muehlenbachs et al., 2016) evaluated autopsy tissue samples from these, plus two additional cases, by light microscopy, immunohistochemistry (IHC), and PCR. Pancarditis with a characteristic “road map” distribution of infiltrates consisting of lymphocytes (T cell predominant but also B cells), histiocytes, and plasma cells were seen in all five. The conduction system was specifically evaluated in two cases; the presence of severe inflammation in the sinoatrial and AV nodes, including intense necrotizing inflammation of the AV node in one, led them to speculate that involvement of the conduction system caused the deaths. However, arrhythmias also seem likely in some. In all five, spirochetes were widely detected by Warthin-Starry silver stain, IHC, and PCR and co-localized with collagen and decorin (Figure 11). The ease of detecting spirochetes in heart samples contrasted with the paucity of bacteria detected by IHC and PCR in numerous other organs, indicating that in these patients B. burgdorferi clearly possessed a tropism for cardiac tissue.
Figure 11.
Localization of spirochetes (left panel, immunohistochemistry, arrowhead) with collagen (middle panel, blue, trichrome, arrowhead) and decorin (right panel, red, immunohistochemistry) in the myocardium of a patient with fatal myocarditis. (Reproduced with permission from Muehlenbachs et al., 2016).
Lyme arthritis
Arthritis is the most common and serious rheumatologic consequence of spirochetal infection and dissemination. Although Lyme arthritis was once believed to be a North American phenomenon because of the defining epidemic in the 1970s (Steere et al., 1977b) and the paucity of reports of tick-related arthritis in the European literature of the day (Stanek and Strle, 2018), this entity also occurs in European patients without a clear genospecies predominance (Herzer, 1993; Steere, 1997; Haugeberg et al., 2014; Enkelmann et al., 2018). The incubation period for joint involvement can vary from days to years, usually 3 to 6 months, from the time of infection (Steere et al., 1987; Herzer, 1991), and it is presumed that spirochetes typically invade joints well before the onset of arthritic symptoms (Bockenstedt and Wormser, 2014). In some patients, episodes of periarticular pain may precede frank arthritis by weeks to months (Stanek and Strle, 2018). The presence of high-titer antibodies to multiple B. burgdorferi antigens in the sera of Lyme arthritis patients (Akin et al., 1999; Wormser et al., 2006) is consistent with the concept of a period of quiescence within the joint prior to the development of clinically evident arthritis (Bockenstedt and Wormser, 2014).
Lyme arthritis is a monoarticular or oligoarticular large joint arthritis characterized by episodes of inflammation with swelling, large effusions and surprisingly little pain (Arvikar and Steere, 2015). The knee is the most commonly affected joint, but other large or small joints, such as the ankle, shoulder, elbow or wrist, may be affected (Figure 12); exclusive involvement of small joints is unusual (Arvikar and Steere, 2015; Stanek and Strle, 2018). In the original study defining the arthritic complications of Lyme disease, prior to use of antibiotics for treatment of the infection, 34 of 55 patients who presented with EM went on to develop arthritis (Steere et al., 1987). Twenty-eight of the 34 patients experienced intermittent arthritis with the episodes of joint swelling lasting from a few days to several months before spontaneously resolving. Over time, the interval between episodes gradually increased before the arthritis spontaneously remitted. Six patients, however, had a year or more of unremitting arthritis. According to a more recent report by the CDC, of ~150,000 newly reported cases of Lyme disease in the U.S. (reported on from 1992–2006) up to 30% present as Lyme arthritis. In contrast, in Europe, the percentage of B. burgdorferi-infected individuals who develop arthritis is less than 10% (Enkelmann et al., 2018; Stanek and Strle, 2018). Synovial biopsy of untreated Lyme arthritis shows synovial hypertrophy with vascular proliferation, a mixed infiltrate including T cells, B cells, and follicular dendritic cells (Steere et al., 1979; Duray and Steere, 1986). Consistent with findings in tissue, joint fluid in Lyme arthritis patients contains markedly elevated levels of cytokines and chemokines associated with innate and adaptive immune responses, with particularly high levels of IFNγ-inducible chemokines CXCL9 and CXCL10. The levels of these mediators are nominally lower in serum supporting the idea that these immune responses are occurring locally in joints (Jones et al., 2008; Strle et al., 2017). In addition to inflammatory mediators, matrix-degrading enzymes such as matrix metalloproteinases (MMPs), which degrade both collagen and proteoglycans (Murphy and Nagase, 2008), are believed to contribute to the erosion of cartilage and bone. MMPs have been detected by zymography in the synovial fluids of Lyme arthritis patients (Hu et al., 2001), and in ex vivo experiments, B. burgdorferi elicits their production by cartilage explants (Lin et al., 2001), chondrocytes (Behera et al., 2004) and monocytes (Gebbia et al., 2004).
Figure 12.
Lyme arthritis manifested as left knee swelling in a young adult. Clinically there was no redness, few constitutional symptoms, and little pain. The patient recovered after a 4-week course of oral antibiotics. Photgraph courtesy of Dr. Jeffrey Thompson, Connecticut Children’s Medical Center.
The pathogenesis of Lyme arthritis is enigmatic from several standpoints. Though undoubtedly arising from hematogenous dissemination of spirochetes into joints or periarticular tissues, with only two documented exceptions (Snydman et al., 1986; Schmidli et al., 1988), efforts to recover live spirochetes from synovial fluid as a rule have been unsuccessful. In 1994, Nocton and co-workers (Nocton et al., 1994) made two seminal observations: (i) B. burgdorferi DNA can be detected in synovial fluids from the large majority (75 of 88) of Lyme arthritis patients and (ii) PCR-positive patients usually respond well to antimicrobial therapy. In PCR-positive patients, resolution of arthritis with antibiotics confirms that spirochete-elicited inflammation drives the arthritic process (Bockenstedt and Wormser, 2014). However, where the spirochetes producing the DNA are hiding in these individuals and what triggers the inflammation after a presumptive period of quiescence remain mysteries (Bockenstedt and Wormser, 2014). The murine model offers some help with the first conundrum. In mice, spirochetes do not infect the joint spaces per se but are visualized in collagen-rich, periarticular structures (Barthold et al., 1991; Bockenstedt and Wormser, 2014).
Although Lyme arthritis usually resolves following appropriate antibiotic therapy, some patients (estimated to be ~10%) have a persistent proliferative synovitis, characterized by synovial hyperplasia, vascular damage, intense inflammation, and fibrosis, termed post-infectious, antibiotic-refractory Lyme arthritis (Steere and Angelis, 2006; Chan and Pollock, 2015). Patients diagnosed with this entity are strongly seropositive but usually lack a PCR signal for B. burgdorferi DNA in joints (Wormser et al., 2006; Li et al., 2011). The central mechanistic question is how spirochetes initiate a localized, dysregulated inflammatory process that persists after they have been eradicated (Singh and Girschick, 2004; Shin et al., 2007; Shen et al., 2010; Strle et al., 2012). The increased prevalence of certain HLA alleles that are also associated with rheumatoid arthritis (Steere et al., 1990) spawned the hypothesis that antibiotic-refractory Lyme arthritis is a form of infection-induced autoimmunity caused by molecular mimicry (Klempner and Huber, 1999; Bolz and Weis, 2004). Subsequent studies identified a sequence in OspA as the molecular mimic for a sequence in hLFA-1 (Gross et al., 1998; Chen et al., 1999) and reported that the HLA-DR alleles overrepresented in antibiotic-refractory arthritis patients bind the OspA mimotope (Steere et al., 2006). The public health consequences of these publications were enormous since OspA was the antigenic component of the Lyme disease vaccine released at approximately the same time (Sigal et al., 1998; Steere et al., 1998). Unsubstantiated concerns about autoimmunity due to vaccination with OspA contributed to the commercial failure of the vaccine and its withdrawal from the market (Plotkin, 2011; Willyard, 2014). In subsequent publications, Steere’s group backed away from the association of persistent synovitis with T cell reactivity to either OspA or LFA-1 (Kannian et al., 2007; Drouin et al., 2013). However, in support of the concept of spirochete-induced autoimmunity, the Steere group has now identified four novel self-antigens, ECGF, ApoB-100, Annexin A2, and MMP10, which are targets of T and B cell responses in patients with refractory Lyme arthritis (Drouin et al., 2013; Pianta et al., 2015; Crowley et al., 2016; Arvikar et al., 2017; Strle et al., 2017; Steere, 2020).
Although the etiology remains unclear, much has been learned in recent years about the dysregulated inflammatory processes that underlie post-infectious Lyme arthritis (Steere, 2020). For example, patients with a particular single nucleotide polymorphism in TLR1, particularly those who are infected with B. burgdorferi RST1 strains, appear to be predisposed to excessive Th1-associated inflammation (Strle et al., 2012). Compared with antibiotic-responsive Lyme arthritis patients, persons with post-infectious arthritis had lower levels of CD4+CD25+ regulatory T cells in their synovial fluids and their regulatory T cells were less effective at downregulating Th1 effector responses (Vudattu et al., 2013). High throughput RNA sequencing revealed a robust IFNγ response in synovium that correlates inversely with expression of genes involved in tissue repair and drives differentiation of fibroblast-like synoviocytes into immune effector cells that are thought to amplify and perpetuate these dysregulated Th1 responses (Lochhead et al., 2019a; Lochhead et al., 2019b). An alternative, or perhaps complementary, mechanism for persistent inflammation based on innate immunity recently has been proposed. Jutras et al. (Jutras et al., 2019) reported that B. burgdorferi cannot recycle peptidoglycan and sheds peptidoglycan fragments (muropeptides) into its environment. They postulated that peptidoglycan fragments shed into synovial fluid prior to the eradication of bacteria causes chronic activation of NOD2-dependent arthritogenic pathways. Thus, the infection with certain Borrelia strains in genetically predisposed individuals appears to set the stage for dysregulated immune responses with particularly high levels of IFNγ that promote synovial hypertrophy, inhibit tissue repair, and are permissive to the development of autoreactive T and B cell responses in joints. Microbial remnants such as peptidoglycan, which can persist in the absence of active spirochete infection, may further perpetuate these responses.
Lyme Disease in Children
As noted earlier, Lyme disease began as an affliction of children (Steere et al., 1977b) and it continues so to this day. The clinical manifestations of Lyme disease in children, as in adults, mainly involve the skin, central nervous system, joints, and heart (Feder, 2008; Sood, 2015). The primary EM lesion in children is most commonly found on the head and neck (26%), arms or legs (25%), or lower back (24%) (Salazar et al., 1993; Gerber et al., 1996). Awareness of EM in the head and neck is important because such lesions can be hidden by the hairline. Less frequently lesions can be found over the abdomen (9%), the axilla (8%), the groin (5%), or the chest (3%) (Gerber et al., 1996). Most single EM lesions in children are uniformly erythematous; however, they also can have central erythema or clearing and, in rare instances, painful central ulcerations (Figure 8C). Children also may present with constitutional symptoms, including fever (24%), fatigue (58%), headache (42%) and arthralgias (33%) (Gerber et al., 1996). The presence of multiple EMs has been documented in up to 40% of children (Gerber et al., 1996; Arnez et al., 2003) and most have associated fever (45%), fatigue (80%), and headache (70%). Similar to adults with Lyme disease, ~20% of children may present with constitutional symptoms as the only manifestation (Feder et al., 1993; Gerber et al., 1996). Practitioners in endemic areas need to have a high index of suspicion to avoid missing the diagnosis in such children.
Of course, arthritis also may be the presenting manifestation of Lyme disease in children (Steere et al., 1977b; Szer et al., 1991). As in adults, the incubation period is highly variable with a mean of about 4 months (Szer et al., 1991). In contrast to the early era, most children who present with Lyme arthritis today do not have a history of EM. In these children, the rash was either unseen, or perhaps they never had it at all. Lyme arthritis in children usually involves single large joints, most commonly the knee (Gerber et al., 1998). However, series describing monoarticular arthritis of the hips and elbows in children have appeared of late (Cruz et al., 2017; Gendelberg and Hennrikus, 2018). Fever occurs in 25–50% of children with Lyme arthritis, as opposed to adults who are usually afebrile. Joint fluid obtained from these children may have large numbers of leukocytes (> 50,000 cells/mm3) with a predominance of neutrophils. If not suspected, the disease can be mistaken for septic arthritis caused by pyogenic bacteria (e.g., Staphylococcus aureus). A recent series comparing Lyme arthritis of the hip to septic arthritis emphasized that the former is more likely to occur in children who are afebrile and without leukocytosis (Cruz et al., 2017). If treated appropriately, the prognosis is excellent and with rare exceptions a full recovery is the outcome. Antibiotic-refractory Lyme arthritis is unusual in children (Daikh et al., 2013; Sood, 2015).
Lyme disease in children also may present with neurologic manifestations (Cook et al., 1997; Eppes et al., 1999; Sood, 2015). Most children with neuroborreliosis do not provide a history of EM. The most common neurologic presentations in children include facial nerve palsy and lymphocytic meningitis (Cook et al., 1997; Eppes et al., 1999; Sood, 2015). Children with Lyme meningitis also may have increased intracranial pressure, and some have optic neuritis and papilledema (Kan et al., 1998); increased intracranial pressure may occur in the absence of meningitis (Kan et al., 1998; Sood, 2006). Though rare, BS should be considered in children in Europe or who have traveled to Europe who present with characteristic radicular pain (Sood, 2015). The CSF pleocytosis is predominantly lymphocytic; in one study, a CSF containing 10% or more neutrophils had a negative predictive value of 99% for Lyme disease compared to viral meningitis (Shah et al., 2005). More severe neurologic manifestations, such as myelitis and severe encephalitis, are exceedingly rare in young children. Though rare, Lyme carditis may also occur in children (Bolourchi et al., 2019). When it occurs, the most common manifestation in children is transient high-grade AV block (Silver et al., 2007). Less commonly, children may present in heart failure because of myocarditis. The prognosis of both neurologic and cardiac Lyme disease in children is excellent following appropriate therapy (Wormser et al., 2006).
Maternal-fetal transmission of B. burgdorferi has never been definitively documented, and epidemiologic investigations have failed to establish an association between infection and adverse outcomes of pregnancy (Strobino et al., 1993; Gerber and Zalneraitis, 1994; Sood, 2015).
Reinfection
Recurrent episodes of Lyme disease can theoretically be due to either relapse or reinfection (Nadelman and Wormser, 2007). For EM to be due to relapse, the lesion must occur at the same site as the original infection and within a relatively short time interval. Reinfection is defined as EM occurring at a site distant from the initial lesion, often with a punctum or eschar indicative of a recent tick bite, months to years after the initial treated episode. Evidence for the relapse scenario is scant. Two groups have reported their inability to re-isolate spirochetes from the site of EM following therapy, although administration of antibiotics at the time of re-culture confounds interpretation of these data (Berger et al., 1992; Nadelman et al., 1993). In contrast, longitudinal studies conducted in highly endemic areas, Westchester, NY and Block Island, RI, found that up to 20% of patients treated for EM met criteria for reinfection (Nowakowski et al., 2003; Krause et al., 2006). An impressive report provided compelling molecular evidence based on ospC genotyping of isolates from 17 patients with 22 paired episodes of EM that recurrences are caused by different B. burgdorferi strains (Nadelman and Wormser, 2013). Using statistical modeling, this group subsequently argued that elicitation of durable strain-specific immunity explains why patients were reinfected with different strains (Khatchikian et al., 2014). Without serological data, however, this hypothesis remains unproven, and there is evidence from the mouse model that protective immunity following tick inoculation wanes within a year (Piesman et al., 1997). Furthermore, Barthold and Bockenstedt (Barthold and Bockenstedt, 1993) showed that levels of antibodies capable of conferring passive protection fall off much more rapidly than antibodies detected by ELISA with whole cell lysates. The implication of this finding is that high reactivity in serodiagnostic tests may not be an accurate indicator of immune status. One also must remember that a substantial percentage of persons with EM do not have detectable antibodies, even after treatment (Wormser, 2006). Such individuals will be susceptible to reinfection within a relatively short time if they live in an endemic area or frequently engage in high-risk activities.
Serologic response
The need for accurate serologic tests for diagnosis of Lyme disease has been a major driving force behind efforts to elucidate the humoral response to the spirochete. Consequently, the antibody response to B. burgdorferi sensu lato has been extensively dissected over the years with increasingly sophisticated methodologies in order to identify borrelial polypeptides that are not only highly immunogenic in a large percentage of patients but also specific for B. burgdorferi (Craft et al., 1986; Dressler et al., 1993; Engstrom et al., 1995; Aguero-Rosenfeld et al., 2005; Nowalk et al., 2006; Lohr et al., 2018) (also see Radolf and Samuels, 2021). This combination has not been easy to find. Cross-reactivity between B. burgdorferi FlaB, the major flagellar sheath protein, and flagellar antigens of commensal bacteria is the primary reason why this polypeptide, which induces a robust antibody response early in infection (Dressler et al., 1993), fell out of favor as a diagnostic antigen (Aguero-Rosenfeld et al., 2005; Lohr et al., 2018). OspC also induces a strong antibody response during early infection (Fung et al., 1994), consistent with the murine studies demonstrating its critical role for the establishment of infection following tick inoculation, but immunodominant epitopes of OspC proteins from different isolates are extremely heterogeneous (Lohr et al., 2018). A number of other borrelial proteins, many, but not all, lipoproteins, are strong immunogens but are hypervariable, expressed relatively late in the course of disease, and/or recognized by an insufficient percentage of patients (Aguero-Rosenfeld et al., 2005; Wilske et al., 2007; Lohr et al., 2018). Ironically, the antigenically variable VlsE protein, which in the murine model facilitates evasion of antibody responses, has a region with a conserved epitope that is highly immunogenic in early as well as late Lyme disease patients and has been exploited for improved serodiagnosis in Europe as well as North America (Liang et al., 1999) (Liang et al., 1999; Wormser et al., 2013; Lohr et al., 2018; Stanek and Strle, 2018; Zannoli et al., 2020).
Because approximately one-half of patients with EM do not mount detectable antibody responses to the pathogen, lack of seroreactivity cannot be used to rule out the diagnosis of EM (Wormser et al., 2006; Dandache and Nadelman, 2008). There is evidence that serodiagnostic sensitivity is improved by employing borrelial antigens known to be expressed in vivo during early infection (Brandt et al., 2019). Seroreactivity increases substantially following therapy for EM (Vaz et al., 2001; Dandache and Nadelman, 2008), indicating that killing of organisms causes liberation of spirochetal antigens and enhances processing for antibody production. Most untreated patients have detectable antibodies within a month of infection. Accordingly, clinical manifestations caused by the dissemination of spirochetes, are for the most part, accompanied by seroreactivity (Wormser et al., 2006; Lohr et al., 2018; Stanek and Strle, 2018). The flip side of this coin is that IgG antibodies can be present for life and, therefore, cannot be used by themselves as indicators of active infection. While assays using whole cell lysates are less affected by strain variability and the heterogeneity in patient responses than those using single antigens, they can yield false-positives due to the presence of cross-reactive, background antibodies. This problem has been circumvented by the development of the two-tiered testing algorithm in which immunoblotting is used to confirm serologic reactivity by ELISA (Aguero-Rosenfeld et al., 2005; Wormser et al., 2006; Lohr et al., 2018; Stanek and Strle, 2018). The success of the two-tiered approach rests upon the implementation of strict criteria for the interpretation of immunoblots based on reactivity with a small subset of borrelial polypeptides defined by their SDS-PAGE mobilities (i.e., apparent molecular masses) (Dressler et al., 1993). It is noteworthy, considering the T cell reactivity studies discussed below, that reactivity with OspA is not a criterion for immunoblot reactivity.
The general picture that has emerged over the years is that IgG and IgM antibodies to the spirochete develop slowly and are directed against an increasingly diverse array of proteins as infection progresses (Craft et al., 1986; Dressler et al., 1993; Lohr et al., 2018). The earliest responses are to flagellin B (FlaB), OspC (25 kDa) and BmpA (39 kDa) with responses to a number of additional antigens, such as VlsE, fibronectin-binding protein (BBK32), and decorin-binding protein A (DbpA), developing as B. burgdorferi disseminates (Bacon et al., 2003; Aguero-Rosenfeld et al., 2005; Wilske et al., 2007; Lohr et al., 2018). This temporal pattern is consistent with the notion that the bacterium draws upon an expanding repertoire of differentially expressed proteins once within its vertebrate host, including the phased expression of paralogous surface-exposed lipoproteins (Caimano et al., 2019). Carroll and co-workers (Nowalk et al., 2006) completed an extensive proteomics-based survey of murine and human antibody responses to B. burgdorferi proteins, concluding that the contours of the two antibody profiles share many similarities during early infection.
What role, if any, do antibodies play in control of the disease process in humans? This question is far more difficult to answer for humans than in mice because humans cannot be followed serially without treatment and they cannot be passively immunized prior to experimental infection. The presence of intense and diverse antibody responses in patients with late manifestations of disease (Steere, 2001) is clear-cut evidence that, as in mice, spirochetes can persist despite high titers of circulating antibodies (see below). Nevertheless, there is in vitro evidence that the antibodies produced during human infection have bactericidal activity (Pavia et al., 1997; Callister et al., 2002) and can passively protect animals against inoculation with in vitro cultivated spirochetes (Fikrig et al., 1994). Robinson and coworkers (Blum et al., 2018) dramatically advanced this notion using single-cell paired-chain and bulk heavy-chain antibody repertoire sequencing to investigate circulating B cell responses during early Lyme disease. They demonstrated expanded memory B cell and plasmablast populations that produce antibodies with specificities for several B. burgdorferi antigens (e.g., VlsE, DbpA, DbpB, and OspC) and that recombinant mAbs recognizing several of these inhibit spirochete growth in vitro. They also noted that robust plasmablast responses encoding B. burgdorferi-inhibitory antibodies were associated with more rapid disease resolution. As stated earlier, infection with less invasive borrelial strains has been proposed as one explanation for the high prevalence of asymptomatic B. burgdorferi infection in endemic areas (Wormser et al., 2001b). The capacity of humoral responses to contain and even eliminate spirochetes provides a second, non-mutually exclusive explanation, which also is in accord with the idea that humans lack reservoir-competence for the bacterium (Bockenstedt and Radolf, 2014).
Chronic Lyme disease and post-treatment Lyme disease syndrome
Please see the comprehensive discussion by Marques and Hu in Radolf and Samuels (2021). At one time, the term “chronic Lyme disease” (CLD) referred to late stage manifestations of untreated B. burgdorferi sensu lato infection, such as encephalomyelitis, encephalo-pathy, neuropathy, arthritis, and ACA (Koedel et al., 2015). Over the years, however, this term has come to denote persons with chronic, subjective complaints (e.g., neurocognitive syndromes, mood disorders, fibromyalgia-type symptoms, and chronic fatigue), in the absence of a documented history of Lyme disease or objective physical or laboratory evidence of infection, who some practitioners maintain are afflicted by treatment-recalcitrant Lyme disease spirochetes (Cameron et al., 2004; Feder et al., 2007; Marques, 2008; Baker, 2010; Stricker and Johnson, 2014; Lantos, 2015). Few “mainstream” authorities believe there is convincing evidence for persistent infection (or any infection, for that matter) in these individuals (Feder et al., 2007; Koedel et al., 2015; Baker and Wormser, 2017; Shapiro et al., 2017). Clinicians who routinely diagnose CLD contend that the conventional laboratory tests mainstream practitioners rely upon are notoriously unreliable (Cameron et al., 2004; Stricker and Johnson, 2014). To further support their position, these self-designated “Lyme literate” physicians and their staunch supporters in advocacy groups cite myriad studies purporting to show that during chronic infection B. burgdorferi assumes unusual morphologies (variously termed “L forms”, “round bodies”, “L forms”, etc.) and/or metabolic states resistant to standard treatment regimens (Stricker and Johnson, 2011; Lantos, 2015; Sharma et al., 2015; Timmaraju et al., 2015; Feng et al., 2016b; Baker and Wormser, 2017). Even so, the pathophysiologic rationale is difficult to grasp (Halperin, 2008; Baker and Wormser, 2017). As noted by Halperin, for spirochetes to persist and cause disease without provoking discernable inflammation or an immunologic response manifested by detectable serum antibodies, B. burgdorferi would have to differ from virtually every other known chronic, systemic bacterial pathogen. The outcome of this questionable line of thinking is prolonged antimicrobial therapy of uninfected (though truly unwell) individuals, sometimes involving unusual regimens and agents of unproven efficacy for Lyme disease (Feder et al., 2007; Lantos et al., 2015). The potential dangers of these treatment regimens have been well documented (Lantos, 2015). While historically a North American phenomenon (Barbour and Fish, 1993), CLD appears to be gaining traction in Europe and, understandably, generating increasing concern among European investigators (Koedel et al., 2015; Gentilini and Bricaire, 2019; Peri et al., 2019).
Almost 30 years ago, clinicians began to report that some Lyme disease patients have persistent nonspecific symptoms after receiving an adequate course of therapy (Dinerman and Steere, 1992; Asch et al., 1994; Shadick et al., 1994). In its 2006 guidelines, the Infectious Diseases Society of America created a working definition for this entity, now called “post-treatment Lyme disease syndrome” or PTLDS: clinical symptoms persisting at least six months after treatment for Lyme disease in persons who lack objective evidence of treatment failure, reinfection, or relapse (Wormser et al., 2006; Aucott, 2015; Lantos, 2015; Strle and Strle, 2020). PTLDS differs from CLD in one particularly important respect – patients with PTLDS have unequivocal documentation for appropriately treated Lyme disease. PTLDS occurs in only a small percentage of treated patients. As noted by Lantos in an excellent review (Lantos, 2015), only 222 (3.8%) of 5846 patients screened to participate in clinical trials for PTLDS had credible evidence for past Lyme disease, while less than 10% of patients in ten prospective studies of EM and early disseminated Lyme disease described persistent symptoms such as myalgias or fatigue 9 or more months following treatment. Fortunately, multiple longitudinal studies in the U.S. and Europe report that functional impairment by PTLDS diminishes over time (Cerar et al., 2010; Wormser et al., 2015; Stupica et al., 2018a; Stupica et al., 2018b; Wormser et al., 2020).
As with CLD, the central questions surrounding PTLDS are the underlying mechanism(s) of persistent symptomatology and whether extended antimicrobial therapy, or indeed any antimicrobial treatment in addition to that initially administered, is beneficial (Delong et al., 2012; Fallon et al., 2012; Klempner et al., 2013). Convincing evidence for Borrelia infection in PTLDS patients has not been obtained using PCR, culture, or xenodiagnosis of blood and/or spinal fluid (Klempner et al., 2001; Fallon et al., 2008; Marques et al., 2014). In the absence of direct microbiologic evidence, response to extended therapy has come to be regarded as an alternative means of addressing the persistence question (Klempner et al., 2013). Five double-blind, placebo-controlled treatment trials in the U.S. and Europe have failed to demonstrate lasting benefit for extended therapy (Klempner et al., 2001; Kaplan et al., 2003; Krupp et al., 2003; Fallon et al., 2008; Berende et al., 2016). “Lyme literate” skeptics reject these results on methodologic grounds (Delong et al., 2012; Fallon et al., 2012; Klempner et al., 2013), also pointing to the evidence, per above, that spirochetes in vivo become “persisters” refractory to conventionally used agents (Stricker and Johnson, 2011; Sharma et al., 2015; Timmaraju et al., 2015; Feng et al., 2016b). Proponents of the persister theory were heartened by a highly publicized study maintaining, based on scant data for recovery of viable organisms, that spirochetes persisted in treated rhesus macaques (Embers et al., 2012). Agents that purportedly kill persister spirochetes in vitro have been identified (Sharma et al., 2015; Theophilus et al., 2015; Feng et al., 2016a; Feng et al., 2016c), while one group has reported that the combination of daptomycin plus doxycycline eradicated persister (aggregated, stationary phase) organisms from mice, whereas doxycycline plus ceftriaxone did not (Feng et al., 2019). One can safely predict that at some time in the future clinical trials of regimens believed capable of eliminating persister forms will be done.
In the absence of compelling evidence for bacterial persistence, other investigators lean towards the notion that spirochetes initiate an inflammatory process that is not turned off once the organisms have been eradicated. Using intravital microscopy in mice, Bockenstedt et al. (Bockenstedt et al., 2012) showed that B. burgdorferi antigens, but not infectious spirochetes, remain adjacent to cartilage for extended periods after antibiotic treatment; they proposed that this residual debris is the cause of ongoing inflammation. Investigations in humans also provide some, but not entirely consistent, support for a post-infectious inflammatory process. Strle et al. (Strle et al., 2014) reported that high IL-23 levels at presentation with early Lyme disease correlated with development of persistent symptoms, while Aucott et al. (Aucott et al., 2016) found elevated levels of the T cell chemokine CCL19 in PTLDS patients. Wormser’s group found that PTLDS patients have significantly higher C-reactive protein (CRP) levels than treated patients who become asymptomatic (Uhde et al., 2016) or persons with chronic fatigue/fibromyalgia (Uhde et al., 2018). RNA-seq analysis of early Lyme disease patients revealed a differential gene expression signature that continued for at least six months post-treatment in symptomatic, but not asymptomatic, treated individuals (Bouquet et al., 2016). In contrast, another group recently reported that transcriptional signatures associated with early disseminated Lyme disease normalized within six months of antibiotic treatment (Petzke et al., 2020). And so it goes….
Future Directions
Lyme disease is now regarded as one of the first in a series of explosive infectious disease phenomena that gave rise to the contemporary concept of emerging infectious diseases - the notion that the infectious agents that impact human welfare are dynamic and strongly influenced by factors both within and outside the normal sphere of human activity. In fact, since the first description of the disease, we have come to appreciate the global dimensions of the threat to human health posed by B. burgdorferi sensu lato and its arthropod vectors. Over the years, the notoriety and novelty of the disease have attracted the attention of numerous investigators; as this article and other reviews (see Radolf and Samuels, 2021) attest, our knowledge of the disorder, its etiologic agent, and the natural world that shape them has expanded at a meteoric pace. So, what remains to be done from the perspective of disease in humans? Vaccine development is an obvious answer, although pharmaceutical companies have shown at best muted enthusiasm for the development of second-generation vaccines after the commercial failure and precipitous withdrawal of the first-generation vaccine based on OspA. Clearly, alternative prophylactic strategies that focus on preventing enzootic transmission and spread of the pathogen warrant great emphasis. But the major thrust needs to be in the realms of cellular and molecular pathogenesis.
While genetic and bioinformatics breakthroughs undoubtedly will be made, the ultimate question is how to interpret this information in the context of human disease. As any syphilologist will affirm, Lyme disease researchers are blessed not only with a cultivatable organism but an excellent and extremely versatile animal model whose full potential has not yet been realized. However, to quote a now clichéd maxim, “humans are not mice”, and extrapolation is always fraught with danger. Nor are all the major features of human disease recapitulated by the murine model. As examples one need only point to erythema migrans and central nervous system complications, both of which underscore the existence of unique interactions between the spirochete and human cells. With the exception of AIDS, and, perhaps, COVID-19 most recently, no infectious disease presents a stronger paradigm for translational research. With the advent of systems biology and related genomics-based technologies, investigators need to build upon findings made with surrogate systems rather than regarding them as ends in themselves. The stakes are high, judging by the intensity of the Lyme disease wars currently being waged on the medical and legal fronts (see Radolf and Samuels, 2021). We not only need to understand how B. burgdorferi accomplishes its parasitic strategy once introduced into humans, but also how the outcomes of this interaction differ from those seen in experimental systems and how engagement of the human immune system by the bacterium appears to leave debilitating footprints after its progress has been arrested.
Acknowledgements
This work was supported by grants R01 AI-29735 and R21 AI-139940 from the National Institute of Allergy and Infectious Diseases to JDR, by funds generously provided by the Department of Research of Connecticut Children’s Medical Center to JDR, by grants R21 AI14491 and R21 AI149278 from the National Institute of Allergy and Infectious Diseases to KS and grant P3-0296 from the Slovenian Research Agency to FS.
References
- Adams PP, Flores Avile C, and Jewett MW (2017). A dual luciferase reporter system for B. burgdorferi measures transcriptional activity during tick-pathogen interactions. Front Cell Infect Microbiol 7, 225. 10.3389/fcimb.2017.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeolu M, and Gupta RS (2014). A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 105, 1049–1072. 10.1007/s10482-014-0164-x [DOI] [PubMed] [Google Scholar]
- Aenishaenslin C, Bouchard C, Koffi JK, Pelcat Y, and Ogden NH (2016). Evidence of rapid changes in Lyme disease awareness in Canada. Ticks Tick Borne Dis 7, 1067–1074. 10.1016/j.ttbdis.2016.09.007 [DOI] [PubMed] [Google Scholar]
- Afzelius A (1910). Verhandlungen der dermatologischen Gesellschaft zu Stockholm. Arch Dermatol Syph, 405–406. [Google Scholar]
- Afzelius A (1921). Erythema chronicum migrans. Acta Derm Venereol, 120–125. [Google Scholar]
- Aguero-Rosenfeld ME, Wang G, Schwartz I, and Wormser GP (2005). Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18, 484–509. 10.1128/CMR.18.3.484-509.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin E, McHugh GL, Flavell RA, Fikrig E, and Steere AC (1999). The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect Immun 67, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins DR, Bourell KW, Caimano MJ, Norgard MV, and Radolf JD (1998). A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest 101, 2240–2250. 10.1172/JCI2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghaferi MY, Anderson JM, Park J, Auwaerter PG, Aucott JN, Norris DE, and Dumler JS (2005). Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J Clin Microbiol 43, 1879–1884. 10.1128/JCM.43.4.1879-1884.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JF (1988). Mammalian and avian reservoirs for Borrelia burgdorferi. Ann N Y Acad Sci 539, 180–191. 10.1111/j.1749-6632.1988.tb31852.x [DOI] [PubMed] [Google Scholar]
- Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, et al. (2002). Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity 16, 849–859. [DOI] [PubMed] [Google Scholar]
- Antipov D, Korobeynikov A, McLean JS, and Pevzner PA (2016). hybridSPAdes: an algorithm for hybrid assembly of short and long reads. Bioinformatics 32, 1009–1015. 10.1093/bioinformatics/btv688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnez M, Pleterski-Rigler D, Luznik-Bufon T, Ruzic-Sabljic E, and Strle F (2003). Solitary and multiple erythema migrans in children: comparison of demographic, clinical and laboratory findings. Infection 31, 404–409. 10.1007/s15010-003-4007-3 [DOI] [PubMed] [Google Scholar]
- Arvikar SL, and Steere AC (2015). Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 29, 269–280. 10.1016/j.idc.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvikar SL, Crowley JT, Sulka KB, and Steere AC (2017). Autoimmune arthritides, rheumatoid arthritis, psoriatic arthritis, or peripheral spondyloarthritis following Lyme disease. Arthritis & Rheumatology 69, 194–202. 10.1002/art.39866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch ES, Bujak DI, Weiss M, Peterson MG, and Weinstein A (1994). Lyme disease: an infectious and postinfectious syndrome. J Rheumatol 21, 454–461. [PubMed] [Google Scholar]
- Aucott JN (2015). Posttreatment Lyme disease syndrome. Infect Dis Clin North Am 29, 309–323. 10.1016/j.idc.2015.02.012 [DOI] [PubMed] [Google Scholar]
- Aucott JN, Soloski MJ, Rebman AW, Crowder LA, Lahey LJ, Wagner CA, Robinson WH, and Bechtold KT (2016). CCL19 as a chemokine risk factor for posttreatment Lyme disease syndrome: a prospective clinical cohort study. Clin Vaccine Immunol 23, 757–766. 10.1128/CVI.00071-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon RM, Biggerstaff BJ, Schriefer ME, Gilmore RD Jr., Philipp MT, Steere AC, Wormser GP, Marques AR, and Johnson BJ (2003). Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J Infect Dis 187, 1187–1199. 10.1086/374395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PJ (2010). Chronic Lyme disease: in defense of the scientific enterprise. FASEB J 24, 4175–4177. 10.1096/fj.10-167247 [DOI] [PubMed] [Google Scholar]
- Baker PJ, and Wormser GP (2017). The clinical relevance of studies on Borrelia burgdorferi persisters. Am J Med 130, 1009–1010. 10.1016/j.amjmed.2017.04.014 [DOI] [PubMed] [Google Scholar]
- Balmelli T, and Piffaretti JC (1995). Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res Microbiol 146, 329–340. 10.1016/0923-2508(96)81056-4 [DOI] [PubMed] [Google Scholar]
- Bannwarth A (1941). Chronische lymphocytare Meningitis, entzundliche Polyneuritis und “Rheumatismus”. Ein Beitrag zum Problem “Allergie und Nervensystem”. Arch Psychiatri, 284–376. [Google Scholar]
- Bannwarth A (1944). Zur klinik und pathogenese de “chronischen lymphocytaren meningitis”. Arch Psychiatr Nervenkr, 161–185. [Google Scholar]
- Barbour AG, and Hayes SF (1986). Biology of Borrelia species. Microbiol Rev 50, 381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, and Fish D (1993). The biological and social phenomenon of Lyme disease. Science 260, 1610–1616. [DOI] [PubMed] [Google Scholar]
- Barbour AG, and Travinsky B (2010). Evolution and distribution of the ospC gene, a transferable serotype determinant of Borrelia burgdorferi. MBio 1. 10.1128/mBio.00153-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG (2017). Infection resistance and tolerance in Peromyscus spp., natural reservoirs of microbes that are virulent for humans. Semin Cell Dev Biol 61, 115–122. 10.1016/j.semcdb.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Adeolu M, and Gupta RS (2017). Division of the genus Borrelia into two genera (corresponding to Lyme disease and relapsing fever groups) reflects their genetic and phenotypic distinctiveness and will lead to a better understanding of these two groups of microbes (Margos et al. (2016) There is inadequate evidence to support the division of the genus Borrelia. Int. J. Syst. Evol. Microbiol. doi: 10.1099/ijsem.0.001717). Int J Syst Evol Microbiol 67, 2058–2067. 10.1099/ijsem.0.001815 [DOI] [PubMed] [Google Scholar]
- Barbour AG, and Benach JL (2019). Discovery of the Lyme Disease Agent. MBio 10. 10.1128/mBio.02166-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcena-Uribarri I, Thein M, Maier E, Bonde M, Bergstrom S, and Benz R (2013). Use of nonelectrolytes reveals the channel size and oligomeric constitution of the Borrelia burgdorferi P66 porin. PLoS One 8, e78272. 10.1371/journal.pone.0078272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Beck DS, Hansen GM, Terwilliger GA, and Moody KD (1990). Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis 162, 133–138. [DOI] [PubMed] [Google Scholar]
- Barthold SW, Persing DH, Armstrong AL, and Peeples RA (1991). Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol 139, 263–273. [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, and Bockenstedt LK (1993). Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun 61, 4696–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Fikrig E, Bockenstedt LK, and Persing DH (1995). Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun 63, 2255–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW (1996). Lyme borreliosis in the laboratory mouse. J Spirochet Tick-Borne Dis, 22–44. [Google Scholar]
- Barthold SW, Cadavid D, and Philipp MT (2010). Animal Models of Lyme Borreliosis. In Borrelia: Molecular Biology, Host Interaction, and Pathogenesis, Samuels DS, and Radolf JD, eds. (Norfolk, UK: Caister Academic Press; ), pp. 359–411. [Google Scholar]
- Bashir A, Klammer A, Robins WP, Chin CS, Webster D, Paxinos E, Hsu D, Ashby M, Wang S, Peluso P, et al. (2012). A hybrid approach for the automated finishing of bacterial genomes. Nat Biotechnol 30, 701–707. 10.1038/nbt.2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum E, Randall AZ, Zeller M, and Barbour AG (2013). Inferring epitopes of a polymorphic antigen amidst broadly cross-reactive antibodies using protein microarrays: a study of OspC proteins of Borrelia burgdorferi. PLoS One 8, e67445. 10.1371/journal.pone.0067445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck G, Habicht GS, Benach JL, and Coleman JL (1985). Chemical and biologic characterization of a lipopolysaccharide extracted from the Lyme disease spirochete (Borrelia burgdorferi). J Infect Dis 152, 108–117. [DOI] [PubMed] [Google Scholar]
- Behera AK, Thorpe CM, Kidder JM, Smith W, Hildebrand E, and Hu LT (2004). Borrelia burgdorferi-induced expression of matrix metalloproteinases from human chondrocytes requires mitogen-activated protein kinase and Janus kinase/signal transducer and activator of transcription signaling pathways. Infect Immun 72, 2864–2871. 10.1128/iai.72.5.2864-2871.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfaiza J, Postic D, Bellenger E, Baranton G, and Girons IS (1993). Genomic fingerprinting of Borrelia burgdorferi sensu lato by pulsed-field gel electrophoresis. J Clin Microbiol 31, 2873–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belperron AA, and Bockenstedt LK (2001). Natural antibody affects survival of the spirochete Borrelia burgdorferi within feeding ticks. Infect Immun 69, 6456–6462. 10.1128/IAI.69.10.6456-6462.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, and Schneerson R (2003). A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci U S A 100, 7913–7918. 10.1073/pnas.1232451100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, et al. (1983). Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med 308, 740–742. 10.1056/NEJM198303313081302 [DOI] [PubMed] [Google Scholar]
- Benoit VM, Fischer JR, Lin YP, Parveen N, and Leong JM (2011). Allelic variation of the Lyme disease spirochete adhesin DbpA influences spirochetal binding to decorin, dermatan sulfate, and mammalian cells. Infect Immun 79, 3501–3509. 10.1128/IAI.00163-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berende A, ter Hofstede HJ, Vos FJ, van Middendorp H, Vogelaar ML, Tromp M, van den Hoogen FH, Donders AR, Evers AW, and Kullberg BJ (2016). Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N Engl J Med 374, 1209–1220. 10.1056/NEJMoa1505425 [DOI] [PubMed] [Google Scholar]
- Berger BW, Johnson RC, Kodner C, and Coleman L (1992). Failure of Borrelia burgdorferi to survive in the skin of patients with antibiotic-treated Lyme disease. J Am Acad Dermatol 27, 34–37. 10.1016/0190-9622(92)70152-6 [DOI] [PubMed] [Google Scholar]
- Berger BW, Johnson RC, Kodner C, and Coleman L (1995). Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol 32, 184–187. [DOI] [PubMed] [Google Scholar]
- Berglund J, Eitrem R, Ornstein K, Lindberg A, Ringer A, Elmrud H, Carlsson M, Runehagen A, Svanborg C, and Norrby R (1995). An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med 333, 1319–1327. 10.1056/NEJM199511163332004 [DOI] [PubMed] [Google Scholar]
- Bernard Q, Smith AA, Yang X, Koci J, Foor SD, Cramer SD, Zhuang X, Dwyer JE, Lin YP, Mongodin EF, et al. (2018). Plasticity in early immune evasion strategies of a bacterial pathogen. Proc Natl Acad Sci U S A 115, E3788–E3797. 10.1073/pnas.1718595115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino AL, Myers TA, Alvarez X, Hasegawa A, and Philipp MT (2008). Toll-like receptors: insights into their possible role in the pathogenesis of lyme neuroborreliosis. Infect Immun 76, 4385–4395. 10.1128/IAI.00394-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett ML, and Hill W (1987). Characterization of Borrelia burgdorferi strains isolated from Ixodes pacificus ticks in California. J Clin Microbiol 25, 2296–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum LK, Adamska JZ, Martin DS, Rebman AW, Elliott SE, Cao RRL, Embers ME, Aucott JN, Soloski MJ, and Robinson WH (2018). Robust B cell responses predict rapid resolution of Lyme disease. Front Immunol 9, 1634. 10.3389/fimmu.2018.01634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt LK, Gonzalez DG, Haberman AM, and Belperron AA (2012). Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest 122, 2652–2660. 10.1172/JCI58813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt LK, and Radolf JD (2014). Xenodiagnosis for posttreatment Lyme disease syndrome: resolving the conundrum or adding to it? Clin Infect Dis 58, 946–948. 10.1093/cid/cit942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt LK, and Wormser GP (2014). Unraveling Lyme disease. Arthritis Rheumatol 66, 2313–2323. 10.1002/art.38756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolourchi M, Silver ES, and Liberman L (2019). Advanced heart block in children with Lyme disease. Pediatr Cardiol 40, 513–517. 10.1007/s00246-018-2003-8 [DOI] [PubMed] [Google Scholar]
- Bolz DD, and Weis JJ (2004). Molecular mimicry to Borrelia burgdorferi: pathway to autoimmunity? Autoimmunity 37, 387–392. 10.1080/08916930410001713098 [DOI] [PubMed] [Google Scholar]
- Bontemps-Gallo S, Lawrence K, and Gherardini FC (2016). Two different virulence-related regulatory pathways in Borrelia burgdorferi are directly affected by osmotic fluxes in the bllood meal of feeding Ixodes ticks. PLoS Pathog 12, e1005791. 10.1371/journal.ppat.1005791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botos I, Majdalani N, Mayclin SJ, McCarthy JG, Lundquist K, Wojtowicz D, Barnard TJ, Gumbart JC, and Buchanan SK (2016). Structural and functional characterization of the LPS transporter LptDE from Gram-negative pathogens. Structure 24, 965–976. 10.1016/j.str.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Leonard E, Koffi JK, Pelcat Y, Peregrine A, Chilton N, Rochon K, Lysyk T, Lindsay LR, and Ogden NH (2015). The increasing risk of Lyme disease in Canada. Can Vet J 56, 693–699. [PMC free article] [PubMed] [Google Scholar]
- Bouquet J, Soloski MJ, Swei A, Cheadle C, Federman S, Billaud JN, Rebman AW, Kabre B, Halpert R, Boorgula M, et al. (2016). Longitudinal transcriptome analysis reveals a sustained differential gene expression signature in patients treated for acute Lyme disease. MBio 7, e00100–00116. 10.1128/mBio.00100-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt FC, Ertas B, Falk TM, Metze D, and Boer-Auer A (2015). Histopathology and immunophenotype of acrodermatitis chronica atrophicans correlated with ospA and ospC genotypes of Borrelia species. J Cutan Pathol 42, 674–692. 10.1111/cup.12550 [DOI] [PubMed] [Google Scholar]
- Brandt KS, Ullmann AJ, Molins CR, Horiuchi K, Biggerstaff BJ, and Gilmore RD (2019). Evaluation of in vivo expressed Borrelia burgdorferi antigens for improved IgM serodiagnosis of early Lyme disease. Diagn Microbiol Infect Dis 93, 196–202. 10.1016/j.diagmicrobio.2018.09.012 [DOI] [PubMed] [Google Scholar]
- Brangulis K, Akopjana I, Petrovskis I, Kazaks A, Kraiczy P, and Tars K (2018). Crystal structure of the membrane attack complex assembly inhibitor BGA71 from the Lyme disease agent Borrelia bavariensis. Sci Rep 8, 11286. 10.1038/s41598-018-29651-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangulis K, Akopjana I, Petrovskis I, Kazaks A, and Tars K (2019). Crystal structure of Borrelia burgdorferi outer surface protein BBA69 in comparison to the paralogous protein CspA. Ticks Tick Borne Dis 10, 1135–1141. 10.1016/j.ttbdis.2019.06.009 [DOI] [PubMed] [Google Scholar]
- Brehmer-Andersson E, Hovmark A, and Asbrink E (1998). Acrodermatitis chronica atrophicans: histopathologic findings and clinical correlations in 111 cases. Acta Derm Venereol 78, 207–213. [DOI] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Folsom-O’Keefe CM, Tsao K, and Diuk-Wasser MA (2011). Do birds affect Lyme disease risk? Range expansion of the vector-borne pathogen Borrelia burgdorferi. 9, 103–110. 10.1890/090062 [DOI] [Google Scholar]
- Brissette CA, and Gaultney RA (2014). That’s my story, and I’m sticking to it--an update on B. burgdorferi adhesins. Front Cell Infect Microbiol 4, 41. 10.3389/fcimb.2014.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, and Dykhuizen DE (2004). ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168, 713–722. 10.1534/genetics.104.028738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE, and Ostfeld RS (2008). Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc Biol Sci 275, 227–235. 10.1098/rspb.2007.1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Zhou W, Jutras BL, Casjens S, and Stevenson B (2013). Distribution of cp32 prophages among Lyme disease-causing spirochetes and natural diversity of their lipoprotein-encoding erp loci. Appl Environ Microbiol 79, 4115–4128. 10.1128/AEM.00817-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EL, Wooten RM, Johnson BJ, Iozzo RV, Smith A, Dolan MC, Guo BP, Weis JJ, and Hook M (2001). Resistance to Lyme disease in decorin-deficient mice. J Clin Invest 107, 845–852. 10.1172/JCI11692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet LR, Sellitto C, Spielman A, and Telford SR 3rd (1995). Antibody response of the mouse reservoir of Borrelia burgdorferi in nature. Infect Immun 63, 3030–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner JL, LoGiudice K, and Ostfeld RS (2008). Estimating reservoir competence of Borrelia burgdorferi hosts: prevalence and infectivity, sensitivity, and specificity. J Med Entomol 45, 139–147. 10.1603/0022-2585(2008)45[139:ercobb]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Brusca JS, McDowall AW, Norgard MV, and Radolf JD (1991). Localization of outer surface proteins A and B in both the outer membrane and intracellular compartments of Borrelia burgdorferi. J Bacteriol 173, 8004–8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis I, Denker K, Ostberg Y, Andersen C, Benz R, and Bergstrom S (2008). An RND-type efflux system in Borrelia burgdorferi is involved in virulence and resistance to antimicrobial compounds. PLoS Pathog 4, e1000009. 10.1371/journal.ppat.1000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J, Garpmo U, Tsao J, Berglund J, Fish D, and Barbour AG (2004). Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 150, 1741–1755. 10.1099/mic.0.26944-0 [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, and Davis JP (1982). Lyme disease-a tick-borne spirochetosis? Science 216, 1317–1319. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W (1986). Discovery of the Lyme disease spirochete: a historical review. Zentralbl Bakteriol Mikrobiol Hyg A 263, 7–10. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, and Gage KL (1986). Susceptibility of the black-legged tick, Ixodes scapularis, to the Lyme disease spirochete, Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A 263, 15–20. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Anderson JF, Gern L, Lane RS, Piesman J, and Spielman A (1991). Relationship of Borrelia burgdorferi to its arthropod vectors. Scand J Infect Dis Suppl 77, 35–40. [PubMed] [Google Scholar]
- Burgdorfer W (1993). How the discovery of Borrelia burgdorferi came about. Clin Dermatol 11, 335–338. [DOI] [PubMed] [Google Scholar]
- Cadavid D, Bai Y, Hodzic E, Narayan K, Barthold SW, and Pachner AR (2004). Cardiac involvement in non-human primates infected with the Lyme disease spirochete Borrelia burgdorferi. 84, 1439–1450. 10.1038/labinvest.3700177 [DOI] [PubMed] [Google Scholar]
- Caimano MJ, Eggers CH, Hazlett KR, and Radolf JD (2004). RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun 72, 6433–6445. 10.1128/IAI.72.11.6433-6445.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Eggers CH, Gonzalez CA, and Radolf JD (2005). Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J Bacteriol 187, 7845–7852. 10.1128/JB.187.22.7845-7852.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, and Radolf JD (2007). Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol 65, 1193–1217. 10.1111/j.1365-2958.2007.05860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, and Radolf JD (2015). Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission. Infect Immun 83, 3043–3060. 10.1128/IAI.00315-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Drecktrah D, Kung F, and Samuels DS (2016). Interaction of the Lyme disease spirochete with its tick vector. Cell Microbiol 18, 919–927. 10.1111/cmi.12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Groshong AM, Belperron A, Mao J, Hawley K, Luthra A, Graham DE, Earnhart CG, Marconi RT, Bockenstedt LK, et al. (2019). The RpoS gatekeeper in Borrelia burgdorferi: an invariant regulatory scheme that promotes spirochete persistence in reservoir hosts and niche diversity. Front Microbiol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine JA, and Coburn J (2015). A short-term Borrelia burgdorferi infection model identifies tissue tropisms and bloodstream survival conferred by adhesion proteins. Infect Immun 83, 3184–3194. 10.1128/IAI.00349-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine JA, and Coburn J (2016). Multifunctional and redundant roles of Borrelia burgdorferi outer surface proteins in tissue adhesion, colonization, and eomplement evasion. Front Immunol 7, 442. 10.3389/fimmu.2016.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine JA, Lin YP, Kessler JR, Sato H, Leong JM, and Coburn J (2017). Borrelia burgdorferi outer surface protein C (OspC) binds complement component C4b and confers bloodstream survival. Cell Microbiol 19. 10.1111/cmi.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister SM, Jobe DA, Agger WA, Schell RF, Kowalski TJ, Lovrich SD, and Marks JA (2002). Ability of the borreliacidal antibody test to confirm Lyme disease in clinical practice. Clin Diagn Lab Immunol 9, 908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D, Gaito A, Harris N, Bach G, Bellovin S, Bock K, Bock S, Burrascano J, Dickey C, Horowitz R, et al. (2004). Evidence-based guidelines for the management of Lyme disease. Expert Rev Anti Infect Ther 2, S1–13. https://doi.org/ERI0201S [pii] [DOI] [PubMed] [Google Scholar]
- Campagna J, Lavoie PE, Birnbaum NS, and Furman DP (1983). Lyme disease in northern California. West J Med 139, 319–323. [PMC free article] [PubMed] [Google Scholar]
- Carlsson H, Ekerfelt C, Henningsson AJ, Brudin L, and Tjernberg I (2018). Subclinical Lyme borreliosis is common in south-eastern Sweden and may be distinguished from Lyme neuroborreliosis by sex, age and specific immune marker patterns. Ticks Tick Borne Dis 9, 742–748. 10.1016/j.ttbdis.2018.02.011 [DOI] [PubMed] [Google Scholar]
- Carlsson SA, Granlund H, Jansson C, Nyman D, and Wahlberg P (2003). Characteristics of erythema migrans in Borrelia afzelii and Borrelia garinii infections. Scand J Infect Dis 35, 31–33. 10.1080/0036554021000026978 [DOI] [PubMed] [Google Scholar]
- Carrasco SE, Troxell B, Yang Y, Brandt SL, Li H, Sandusky GE, Condon KW, Serezani CH, and Yang XF (2015). Outer surface protein OspC is an antiphagocytic factor that protects Borrelia burgdorferi from phagocytosis by macrophages. Infect Immun 83, 4848–4860. 10.1128/IAI.01215-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, et al. (2000). A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 35, 490–516. [DOI] [PubMed] [Google Scholar]
- Casjens SR, Mongodin EF, Qiu WG, Luft BJ, Schutzer SE, Gilcrease EB, Huang WM, Vujadinovic M, Aron JK, Vargas LC, et al. (2012). Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 7, e33280. 10.1371/journal.pone.0033280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Ramirez S, Fingerle V, Jungnick S, Straubinger RK, Krebs S, Blum H, Meinel DM, Hofmann H, Guertler P, Sing A, et al. (2016). Trans-Atlantic exchanges have shaped the population structure of the Lyme disease agent Borrelia burgdorferi sensu stricto. Sci Rep 6, 22794. 10.1038/srep22794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease, C., and Prevention (2013). Three sudden cardiac deaths associated with Lyme carditis - United States, November 2012-July 2013. MMWR Morb Mortal Wkly Rep 62, 993–996. [PMC free article] [PubMed] [Google Scholar]
- Cepok S, Zhou D, Vogel F, Rosche B, Grummel V, Sommer N, and Hemmer B (2003). The immune response at onset and during recovery from Borrelia burgdorferi meningoradiculitis. Arch Neurol 60, 849–855. 10.1001/archneur.60.6.849 [DOI] [PubMed] [Google Scholar]
- Cerar D, Cerar T, Ruzic-Sabljic E, Wormser GP, and Strle F (2010). Subjective symptoms after treatment of early Lyme disease. Am J Med 123, 79–86. 10.1016/j.amjmed.2009.05.011 [DOI] [PubMed] [Google Scholar]
- Cerar T, Strle F, Stupica D, Ruzic-Sabljic E, McHugh G, Steere AC, and Strle K (2016). Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg Infect Dis 22, 818–827. 10.3201/eid2205.151806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SS, and Pollock AN (2015). Lyme arthritis. Pediatr Emerg Care 31, 680–681. 10.1097/PEC.0000000000000576 [DOI] [PubMed] [Google Scholar]
- Charon NW, Goldstein SF, Marko M, Hsieh C, Gebhardt LL, Motaleb MA, Wolgemuth CW, Limberger RJ, and Rowe N (2009). The flat-ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J Bacteriol 191, 600–607. 10.1128/JB.01288-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon NW, Cockburn A, Li C, Liu J, Miller KA, Miller MR, Motaleb MA, and Wolgemuth CW (2012). The unique paradigm of spirochete motility and chemotaxis. Annu Rev Microbiol 66, 349–370. 10.1146/annurev-micro-092611-150145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Field JA, Glickstein L, Molloy PJ, Huber BT, and Steere AC (1999). Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer surface protein A of Borrelia burgdorferi. Arthritis Rheum 42, 1813–1822. [DOI] [PubMed] [Google Scholar]
- Chiang SM, and Schellhorn HE (2010). Evolution of the RpoS regulon: origin of RpoS and the conservation of RpoS-dependent regulation in bacteria. J Mol Evol 70, 557–571. 10.1007/s00239-010-9352-0 [DOI] [PubMed] [Google Scholar]
- Chmelar J, Kotal J, Kopecky J, Pedra JHF, and Kotsyfakis M (2016). All for one and one for all on the tick-host battlefield. Trends Parasitol 32, 368–377. 10.1016/j.pt.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Leydet BF, and Threlkeld C (2014). Geographical and genospecies distribution of Borrelia burgdorferi sensu lato DNA detected in humans in the USA. J Med Microbiol 63, 674–684. 10.1099/jmm.0.073122-0 [DOI] [PubMed] [Google Scholar]
- Coipan EC, Jahfari S, Fonville M, Oei GA, Spanjaard L, Takumi K, Hovius JW, and Sprong H (2016). Imbalanced presence of Borrelia burgdorferi s.l. multilocus sequence types in clinical manifestations of Lyme borreliosis. Infect Genet Evol 42, 66–76. 10.1016/j.meegid.2016.04.019 [DOI] [PubMed] [Google Scholar]
- Coleman JL, Sellati TJ, Testa JE, Kew RR, Furie MB, and Benach JL (1995). Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun 63, 2478–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Gebbia JA, Piesman J, Degen JL, Bugge TH, and Benach JL (1997). Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89, 1111–1119. [DOI] [PubMed] [Google Scholar]
- Colli C, Leinweber B, Mullegger R, Chott A, Kerl H, and Cerroni L (2004). Borrelia burgdorferi-associated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol 31, 232–240. [DOI] [PubMed] [Google Scholar]
- Comstedt P, Jakobsson T, and Bergstrom S (2011). Global ecology and epidemiology of Borrelia garinii spirochetes. Infect Ecol Epidemiol 1. 10.3402/iee.v1i0.9545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock LE, and Thomas DD (1989). Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun 57, 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, Macartney KK, Rose CD, Hunt PG, Eppes SC, and Reilly JS (1997). Lyme disease and seventh nerve paralysis in children. Am J Otolaryngol 18, 320–323. [DOI] [PubMed] [Google Scholar]
- Corona A, and Schwartz I (2015). Borrelia burgdorferi: carbon metabolism and the tick-mammal enzootic cycle. Microbiol Spectr 3. 10.1128/microbiolspec.MBP-0011-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DL, Akins DR, Bourell KW, Lahdenne P, Norgard MV, and Radolf JD (1996). Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci U S A 93, 7973–7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft JE, Fischer DK, Shimamoto GT, and Steere AC (1986). Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest 78, 934–939. 10.1172/JCI112683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder LA, Yedlin VA, Weinstein ER, Kortte KB, and Aucott JN (2014). Lyme disease and post-treatment Lyme disease syndrome: the neglected disease in our own backyard. Public Health 128, 784–791. 10.1016/j.puhe.2014.06.016 [DOI] [PubMed] [Google Scholar]
- Crowley JT, Strle K, Drouin EE, Pianta A, Arvikar SL, Wang Q, Costello CE, and Steere AC (2016). Matrix metalloproteinase-10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic-refractory Lyme arthritis. J Autoimmun 69, 24–37. 10.1016/j.jaut.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AI Jr., Aversano FJ, Seeley MA, Sankar WN, and Baldwin KD (2017). Pediatric Lyme arthritis of the hip: The great imitator? J Pediatr Orthop 37, 355–361. 10.1097/BPO.0000000000000664 [DOI] [PubMed] [Google Scholar]
- Cugini C, Medrano M, Schwan TG, and Coburn J (2003). Regulation of expression of the Borrelia burgdorferi b3-chain integrin ligand, P66, in ticks and in culture. Infect Immun 71, 1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull B, Pietzsch ME, Hansford KM, Gillingham EL, and Medlock JM (2018). Surveillance of British ticks: An overview of species records, host associations, and new records of Ixodes ricinus distribution. Ticks Tick Borne Dis 9, 605–614. 10.1016/j.ttbdis.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Cutler SJ, Ruzic-Sabljic E, and Potkonjak A (2017). Emerging borreliae - Expanding beyond Lyme borreliosis. Mol Cell Probes 31, 22–27. 10.1016/j.mcp.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Daikh BE, Emerson FE, Smith RP, Lucas FL, and McCarthy CA (2013). Lyme arthritis: a comparison of presentation, synovial fluid analysis, and treatment course in children and adults. Arthritis Care Res (Hoboken) 65, 1986–1990. 10.1002/acr.22086 [DOI] [PubMed] [Google Scholar]
- Dandache P, and Nadelman RB (2008). Erythema migrans. Infect Dis Clin North Am 22, 235–260, vi. 10.1016/j.idc.2007.12.012 [DOI] [PubMed] [Google Scholar]
- Daubin V, Gouy M, and Perriere G (2002). A phylogenomic approach to bacterial phylogeny: evidence of a core of genes sharing a common history. Genome Res 12, 1080–1090. 10.1101/gr.187002de [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning J (1993). Histopathologic patterns of erythema migrans and borrelial lymphocytoma. Clin Dermatol 11, 377–383. [DOI] [PubMed] [Google Scholar]
- De Silva AM, and Fikrig E (1995). Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg 53, 397–404. [DOI] [PubMed] [Google Scholar]
- de Silva AM, Telford SR 3rd, Brunet LR, Barthold SW, and Fikrig E (1996). Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med 183, 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Tyson KR, and Pal U (2009). Molecular characterization of the tick-Borrelia interface. Front Biosci (Landmark Ed) 14, 3051–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong AK, Blossom B, Maloney EL, and Phillips SE (2012). Antibiotic retreatment of Lyme disease in patients with persistent symptoms: a biostatistical review of randomized, placebo-controlled, clinical trials. Contemp Clin Trials 33, 1132–1142. 10.1016/j.cct.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Dennis DT, and Hayes EB (2002). Epidemiology of Lyme Borreliosis. In Lyme Borreliosis Biology, Epidemiology, and Control, Gray JS, Kahl O, Lane RS, and Stanek G, eds. (New York: CABI Publishing; ), pp. 251–280. [Google Scholar]
- Derdakova M, Dudioak V, Brei B, Brownstein JS, Schwartz I, and Fish D (2004). Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl Environ Microbiol 70, 6783–6788. 10.1128/AEM.70.11.6783-6788.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinerman H, and Steere AC (1992). Lyme disease associated with fibromyalgia. Ann Intern Med 117, 281–285. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Maupin GO, Panella NA, Golde WT, and Piesman J (1997). Vector competence of Ixodes scapularis, I. spinipalpis, and Dermacentor andersoni (Acari:Ixodidae) in transmitting Borrelia burgdorferi, the etiologic agent of Lyme disease. J Med Entomol 34, 128–135. 10.1093/jmedent/34.2.128 [DOI] [PubMed] [Google Scholar]
- Donahue JG, Piesman J, and Spielman A (1987). Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg 36, 92–96. [DOI] [PubMed] [Google Scholar]
- Dowdell AS, Murphy MD, Azodi C, Swanson SK, Florens L, Chen S, and Zuckert WR (2017). Comprehensive spatial analysis of the Borrelia burgdorferi lipoproteome reveals a compartmentalization bias toward the bacterial surface. J Bacteriol 199. 10.1128/JB.00658-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D, Lybecker M, Popitsch N, Rescheneder P, Hall LS, and Samuels DS (2015). The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLoS Pathog 11, e1005160. 10.1371/journal.ppat.1005160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler F, Whalen JA, Reinhardt BN, and Steere AC (1993). Western blotting in the serodiagnosis of Lyme disease. J Infect Dis 167, 392–400. [DOI] [PubMed] [Google Scholar]
- Drouin EE, Seward RJ, Strle K, McHugh G, Katchar K, Londono D, Yao C, Costello CE, and Steere AC (2013). A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum 65, 186–196. 10.1002/art.37732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumic I, and Severnini E (2018). “Ticking Bomb”: the impact of climate change on the incidence of Lyme disease. Can J Infect Dis Med Microbiol 2018, 5719081. 10.1155/2018/5719081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, and Radolf JD (2009). Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest 119, 3652–3665. 10.1172/JCI39401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Eggers CH, and Radolf JD (2012). Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog 8, e1002532. 10.1371/journal.ppat.1002532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duray PH, and Steere AC (1986). The spectrum of organ and systems pathology in human Lyme disease. Zentralbl Bakteriol Mikrobiol Hyg A 263, 169–178. [DOI] [PubMed] [Google Scholar]
- Duray PH (1989a). Clinical pathologic correlations of Lyme disease. Rev Infect Dis 11 Suppl 6, S1487–1493. [DOI] [PubMed] [Google Scholar]
- Duray PH (1989b). Histopathology of clinical phases of human Lyme disease. Rheum Dis Clin North Am 15, 691–710. [PubMed] [Google Scholar]
- Dykhuizen DE, Brisson D, Sandigursky S, Wormser GP, Nowakowski J, Nadelman RB, and Schwartz I (2008). The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am J Trop Med Hyg 78, 806–810. [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Leblanc DV, Alix KE, Desrosiers DC, Radolf JD, and Marconi RT (2010). Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Mol Microbiol 76, 393–408. 10.1111/j.1365-2958.2010.07103.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebady R, Niddam AF, Boczula AE, Kim YR, Gupta N, Tang TT, Odisho T, Zhi H, Simmons CA, Skare JT, et al. (2016). Biomechanics of Borrelia burgdorferi vascular interactions. Cell Rep 16, 2593–2604. 10.1016/j.celrep.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicken C, Sharma V, Klabunde T, Owens RT, Pikas DS, Hook M, and Sacchettini JC (2001). Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J Biol Chem 276, 10010–10015. 10.1074/jbc.M010062200 [DOI] [PubMed] [Google Scholar]
- Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, and Sacchettini JC (2002). Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem 277, 21691–21696. 10.1074/jbc.M201547200 [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Ogden NH, and Beard CB (2016). Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J Med Entomol 53, 250–261. 10.1093/jme/tjv199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, and Eisen L (2018). The blacklegged tick, Ixodes scapularis : an increasing public health concern. Trends in Parasitology 34, 295–309. 10.1016/j.pt.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldin C, Raffetin A, Bouiller K, Hansmann Y, Roblot F, Raoult D, and Parola P (2019). Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med Mal Infect 49, 121–132. 10.1016/j.medmal.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, Jacobs MB, Hasenkampf NR, Martin DS, Narasimhan S, et al. (2012). Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One 7, e29914. 10.1371/journal.pone.0029914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embers ME, Hasenkampf NR, Jacobs MB, Tardo AC, Doyle-Meyers LA, Philipp MT, and Hodzic E (2017). Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLOS ONE 12, e0189071. 10.1371/journal.pone.0189071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom SM, Shoop E, and Johnson RC (1995). Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol 33, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkelmann J, Bohmer M, Fingerle V, Siffczyk C, Werber D, Littmann M, Merbecks SS, Helmeke C, Schroeder S, Hell S, et al. (2018). Incidence of notified Lyme borreliosis in Germany, 2013–2017. Sci Rep 8, 14976. 10.1038/s41598-018-33136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppes SC, Nelson DK, Lewis LL, and Klein JD (1999). Characterization of Lyme meningitis and comparison with viral meningitis in children. Pediatrics 103, 957–960. 10.1542/peds.103.5.957 [DOI] [PubMed] [Google Scholar]
- Erdlow JA (2003). Bull’s Eye. Unraveling the Medical Mystery of Lyme Disease (New Haven: Yale University Press; ). [PubMed] [Google Scholar]
- Estrada-Pena A, Cutler S, Potkonjak A, Vassier-Tussaut M, Van Bortel W, Zeller H, Fernandez-Ruiz N, and Mihalca AD (2018). An updated meta-analysis of the distribution and prevalence of Borrelia burgdorferi s.l. in ticks in Europe. Int J Health Geogr 17, 41. 10.1186/s12942-018-0163-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Pena A, and Cabezas-Cruz A (2019). Phyloproteomic and functional analyses do not support a split in the genus Borrelia (phylum Spirochaetes). BMC Evol Biol 19, 54. 10.1186/s12862-019-1379-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco RC, Fish D, and Piesman J (1996). Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol 143, 187–192. [DOI] [PubMed] [Google Scholar]
- Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D, and Wormser GP (1999). Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am J Epidemiol 149, 771–776. [DOI] [PubMed] [Google Scholar]
- Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, Slavov I, Cheng J, Dobkin J, Nelson DR, et al. (2008). A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 70, 992–1003. 10.1212/01.WNL.0000284604.61160.2d [DOI] [PubMed] [Google Scholar]
- Fallon BA, Petkova E, Keilp JG, and Britton CB (2012). A reappraisal of the U.S. Clinical trials of post-treatment lyme disease syndrome. Open Neurol J 6, 79–87. 10.2174/1874205X01206010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder HM Jr., Gerber MA, Krause PJ, Ryan R, and Shapiro ED (1993). Early Lyme disease: a flu-like illness without erythema migrans. Pediatrics 91, 456–459. [PubMed] [Google Scholar]
- Feder HM Jr., Johnson BJ, O’Connell S, Shapiro ED, Steere AC, Wormser GP, Ad Hoc International Lyme Disease, G., Agger WA, Artsob H, Auwaerter P, et al. (2007). A critical appraisal of “chronic Lyme disease”. N Engl J Med 357, 1422–1430. 10.1056/NEJMra072023 [DOI] [PubMed] [Google Scholar]
- Feder HM Jr. (2008). Lyme disease in children. Infect Dis Clin North Am 22, 315–326, vii. 10.1016/j.idc.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Feng J, Shi W, Zhang S, Sullivan D, Auwaerter PG, and Zhang Y (2016a). A drug combination screen identifies drugs active against amoxicillin-induced round bodies of in vitro Borrelia burgdorferi persisters from an FDA drug library. Front Microbiol 7, 743. 10.3389/fmicb.2016.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Weitner M, Shi W, Zhang S, and Zhang Y (2016b). Eradication of biofilm-like microcolony structures of Borrelia burgdorferi by daunomycin and daptomycin but not mitomycin C in combination with doxycycline and cefuroxime. Front Microbiol 7, 62. 10.3389/fmicb.2016.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zhang S, Shi W, and Zhang Y (2016c). Ceftriaxone pulse dosing fails to eradicate biofilm-like microcolony B. burgdorferi persisters which are sterilized by daptomycin/doxycycline/cefuroxime without pulse dosing. Front Microbiol 7, 1744. 10.3389/fmicb.2016.01744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Li T, Yee R, Yuan Y, Bai C, Cai M, Shi W, Embers M, Brayton C, Saeki H, et al. (2019). Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: implications for understanding persistence, Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discov Med 27, 125–138. [PubMed] [Google Scholar]
- Fikrig E, Bockenstedt LK, Barthold SW, Chen M, Tao H, Ali-Salaam P, Telford SR, and Flavell RA (1994). Sera from patients with chronic Lyme disease protect mice from Lyme borreliosis. J Infect Dis 169, 568–574. [DOI] [PubMed] [Google Scholar]
- Fikrig E, and Narasimhan S (2006). Borrelia burgdorferi--traveling incognito? Microbes Infect 8, 1390–1399. 10.1016/j.micinf.2005.12.022 [DOI] [PubMed] [Google Scholar]
- Finch C, Al-Damluji MS, Krause PJ, Niccolai L, Steeves T, O’Keefe CF, and Diuk-Wasser MA (2014). Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLoS One 9, e84758. 10.1371/journal.pone.0084758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JR, Parveen N, Magoun L, and Leong JM (2003). Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci U S A 100, 7307–7312. 10.1073/pnas.1231043100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish AE, Pride YB, and Pinto DS (2008). Lyme carditis. Infect Dis Clin North Am 22, 275–288, vi. 10.1016/j.idc.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, and Gherardini FC (2005). Borrelia burgdorferi s54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A 102, 5162–5167. 10.1073/pnas.0408536102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley DM, Gayek RJ, Skare JT, Wagar EA, Champion CI, Blanco DR, Lovett MA, and Miller JN (1995). Rabbit model of Lyme borreliosis: erythema migrans, infection-derived immunity, and identification of Borrelia burgdorferi proteins associated with virulence and protective immunity. J Clin Invest 96, 965–975. 10.1172/JCI118144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester JD, and Mead P (2014). Third-degree heart block associated with lyme carditis: review of published cases. Clin Infect Dis 59, 996–1000. 10.1093/cid/ciu411 [DOI] [PubMed] [Google Scholar]
- Forrester JD, Meiman J, Mullins J, Nelson R, Ertel SH, Cartter M, Brown CM, Lijewski V, Schiffman E, Neitzel D, et al. (2014). Notes from the field: update on Lyme carditis, groups at high risk, and frequency of associated sudden cardiac death--United States. MMWR Morb Mortal Wkly Rep 63, 982–983. [PMC free article] [PubMed] [Google Scholar]
- Fortune DE, Lin YP, Deka RK, Groshong AM, Moore BP, Hagman KE, Leong JM, Tomchick DR, and Blevins JS (2014). Identification of lysine residues in the Borrelia burgdorferi DbpA adhesin required for murine infection. Infect Immun 82, 3186–3198. 10.1128/IAI.02036-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke J, Hildebrandt A, and Dorn W (2013). Exploring gaps in our knowledge on Lyme borreliosis spirochaetes--updates on complex heterogeneity, ecology, and pathogenicity. Ticks Tick Borne Dis 4, 11–25. 10.1016/j.ttbdis.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, et al. (1997). Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390, 580–586. 10.1038/37551 [DOI] [PubMed] [Google Scholar]
- Fung BP, McHugh GL, Leong JM, and Steere AC (1994). Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun 62, 3213–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallais F, De Martino SJ, Sauleau EA, Hansmann Y, Lipsker D, Lenormand C, Talagrand-Reboul E, Boyer PH, Boulanger N, Jaulhac B, et al. (2018). Multilocus sequence typing of clinical Borreliella afzelii strains: population structure and differential ability to disseminate in humans. Parasit Vectors 11, 374. 10.1186/s13071-018-2938-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Monco JC, and Benach JL (2019). Lyme Neuroborreliosis: clinical outcomes, controversy, pathogenesis, and polymicrobial infections. Ann Neurol 85, 21–31. 10.1002/ana.25389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia BL, Zhi H, Wager B, Hook M, and Skare JT (2016). Borrelia burgdorferi BBK32 inhibits the classical pathway by blocking activation of the C1 complement complex. PLoS Pathog 12, e1005404. 10.1371/journal.ppat.1005404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin C, and Bujadoux C (1922). Paralysie par les tiques. J Med Lyon 3, 765–767. [Google Scholar]
- Gasmi S, Ogden NH, Leighton PA, Lindsay LR, and Thivierge K (2016). Analysis of the human population bitten by Ixodes scapularis ticks in Quebec, Canada: Increasing risk of Lyme disease. Ticks Tick Borne Dis 7, 1075–1081. 10.1016/j.ttbdis.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Gasmi S, Ogden NH, Ripoche M, Leighton PA, Lindsay RL, Nelder MP, Rees E, Bouchard C, Vrbova L, Rusk R, et al. (2019). Detection of municipalities at-risk of Lyme disease using passive surveillance of Ixodes scapularis as an early signal: A province-specific indicator in Canada. PLoS One 14, e0212637. 10.1371/journal.pone.0212637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazendam N, Yeung C, and Baranchuk A (2020). Lyme carditis presenting as sick sinus syndrome. Journal of Electrocardiology 59, 65–67. 10.1016/j.jelectrocard.2020.01.007 [DOI] [PubMed] [Google Scholar]
- Gebbia JA, Coleman JL, and Benach JL (2004). Selective induction of matrix metalloproteinases by Borrelia burgdorferi via toll-like receptor 2 in monocytes. J Infect Dis 189, 113–119. 10.1086/380414 [DOI] [PubMed] [Google Scholar]
- Gendelberg D, and Hennrikus WL (2018). Lyme arthritis of the pediatric elbow: A case series. Orthopedics 41, e511–e515. 10.3928/01477447-20180511-01 [DOI] [PubMed] [Google Scholar]
- Gentilini M, and Bricaire F (2019). Chronic Lyme disease: A scam that should be condemned! Med Mal Infect 49, 83–84. 10.1016/j.medmal.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Gerber MA, and Zalneraitis EL (1994). Childhood neurologic disorders and Lyme disease during pregnancy. Pediatr Neurol 11, 41–43. 10.1016/0887-8994(94)90088-4 [DOI] [PubMed] [Google Scholar]
- Gerber MA, Shapiro ED, Burke GS, Parcells VJ, and Bell GL (1996). Lyme disease in children in southeastern Connecticut. Pediatric Lyme Disease Study Group. N Engl J Med 335, 1270–1274. 10.1056/NEJM199610243351703 [DOI] [PubMed] [Google Scholar]
- Gerber MA, Zemel LS, and Shapiro ED (1998). Lyme arthritis in children: clinical epidemiology and long-term outcomes. Pediatrics 102, 905–908. [DOI] [PubMed] [Google Scholar]
- Gern L, Schaible UE, and Simon MM (1993). Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J Infect Dis 167, 971–975. 10.1093/infdis/167.4.971 [DOI] [PubMed] [Google Scholar]
- Gern L (2008). Borrelia burgdorferi sensu lato, the agent of lyme borreliosis: life in the wilds. Parasite 15, 244–247. [DOI] [PubMed] [Google Scholar]
- Gern L (2009). Life cycle of Borrelia burgdorferi sensu lato and transmission to humans. Curr Probl Dermatol 37, 18–30. 10.1159/000213068 [DOI] [PubMed] [Google Scholar]
- Gern L, and Humair P-F (2002). Ecology of Borrelia burgdorferi in Europe. In Lyme Borreliosis Biology, Epidemiology, and Control, Gray JS, Kahl O, Lane RS, and Stanek G, ed. (New York, NY: CABI Publishing; ), pp. 149–174. [Google Scholar]
- Gherardini F, Boylan J, Lawrence K, and Skare J (2010). Metabolism and Physiology of Borrelia. In Borrelia Molecular Biology, Host Interaction and Pathogenesis, Samuels DS, and Radolf JD, eds. (Norfolk, UK: Caister Academic Press; ), pp. 103–138. [Google Scholar]
- Gilmore RD Jr., Howison RR, Dietrich G, Patton TG, Clifton DR, and Carroll JA (2010). The bba64 gene of Borrelia burgdorferi, the Lyme disease agent, is critical for mammalian infection via tick bite transmission. Proc Natl Acad Sci U S A 107, 7515–7520. 10.1073/pnas.1000268107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard YA, Fedorova N, and Lane RS (2011). Genetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north-coastal California residents. J Clin Microbiol 49, 945–954. 10.1128/JCM.01689-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz M, Resinger A, Semmelweis K, Ambros-Rudolph CM, and Mullegger RR (2015). Clinical spectrum of skin manifestations of Lyme borreliosis in 204 children in Austria. Acta Derm Venereol 95, 565–571. 10.2340/00015555-2000 [DOI] [PubMed] [Google Scholar]
- Glickstein L, Moore B, Bledsoe T, Damle N, Sikand V, and Steere AC (2003). Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect Immun 71, 6051–6053. 10.1128/iai.71.10.6051-6053.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde WT, Burkot TR, Sviat S, Keen MG, Mayer LW, Johnson BJ, and Piesman J (1993). The major histocompatibility complex-restricted response of recombinant inbred strains of mice to natural tick transmission of Borrelia burgdorferi. J Exp Med 177, 9–17. 10.1084/jem.177.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovchenko M, Vancova M, Clark K, Oliver JH Jr., Grubhoffer L, and Rudenko N (2016). A divergent spirochete strain isolated from a resident of the southeastern United States was identified by multilocus sequence typing as Borrelia bissettii. Parasit Vectors 9, 68. 10.1186/s13071-016-1353-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granter SR, Bernstein A, and Ostfeld RS (2014). Of mice and men: Lyme disease and biodiversity. Perspect Biol Med 57, 198–207. 10.1353/pbm.2014.0015 [DOI] [PubMed] [Google Scholar]
- Grillon A, Scherlinger M, Boyer PH, De Martino S, Perdriger A, Blasquez A, Wipff J, Korganow AS, Bonnard C, Cantagrel A, et al. (2019). Characteristics and clinical outcomes after treatment of a national cohort of PCR-positive Lyme arthritis. Semin Arthritis Rheum 48, 1105–1112. 10.1016/j.semarthrit.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, and Rosa PA (2004). Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A 101, 3142–3147. 10.1073/pnas.0306845101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groshong AM, and Blevins JS (2014). Insights into the biology of Borrelia burgdorferi gained through the application of molecular genetics. Adv Appl Microbiol 86, 41–143. 10.1016/B978-0-12-800262-9.00002-0 [DOI] [PubMed] [Google Scholar]
- Groshong AM, Dey A, Bezsonova I, Caimano MJ, and Radolf JD (2017). Peptide uptake is essential for Borrelia burgdorferi viability andiInvolves structural and regulatory complexity of its oligopeptide transporter. mBio 8. 10.1128/mBio.02047-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, Field JA, Steere AC, and Huber BT (1998). Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281, 703–706. [DOI] [PubMed] [Google Scholar]
- Guo BP, Norris SJ, Rosenberg LC, and Hook M (1995). Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun 63, 3467–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BP, Brown EL, Dorward DW, Rosenberg LC, and Hook M (1998). Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol 30, 711–723. [DOI] [PubMed] [Google Scholar]
- Habicht GS, Beck G, Benach JL, and Coleman JL (1986). Borrelia burgdorferi lipopolysaccharide and its role in the pathogenesis of Lyme disease. Zentralbl Bakteriol Mikrobiol Hyg A 263, 137–141. [DOI] [PubMed] [Google Scholar]
- Haddad O, Gillinov M, Fraser T, Shrestha N, and Pettersson GB (2019). Mitral valve endocarditis: A rare manifestation of Lyme disease. Ann Thorac Surg 108, e85–e86. 10.1016/j.athoracsur.2018.12.046 [DOI] [PubMed] [Google Scholar]
- Hagman KE, Lahdenne P, Popova TG, Porcella SF, Akins DR, Radolf JD, and Norgard MV (1998). Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun 66, 2674–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin J, Luft BJ, Volkman DJ, and Dattwyler RJ (1990). Lyme neuroborreliosis. Peripheral nervous system manifestations. Brain 113 (Pt 4), 1207–1221. 10.1093/brain/113.4.1207 [DOI] [PubMed] [Google Scholar]
- Halperin JJ, Luft BJ, Anand AK, Roque CT, Alvarez O, Volkman DJ, and Dattwyler RJ (1989). Lyme neuroborreliosis: central nervous system manifestations. Neurology 39, 753–759. [DOI] [PubMed] [Google Scholar]
- Halperin JJ (2003). Lyme disease and the peripheral nervous system. Muscle Nerve 28, 133–143. 10.1002/mus.10337 [DOI] [PubMed] [Google Scholar]
- Halperin JJ, Shapiro ED, Logigian E, Belman AL, Dotevall L, Wormser GP, Krupp L, Gronseth G, Bever CT Jr., and Quality Standards Subcommittee of the American Academy of, N. (2007). Practice parameter: treatment of nervous system Lyme disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 69, 91–102. 10.1212/01.wnl.0000265517.66976.28 [DOI] [PubMed] [Google Scholar]
- Halperin JJ (2008). Nervous system Lyme disease. Infect Dis Clin North Am 22, 261–274, vi. 10.1016/j.idc.2007.12.009 [DOI] [PubMed] [Google Scholar]
- Halperin JJ (2015). Nervous system Lyme disease. Infect Dis Clin North Am 29, 241–253. 10.1016/j.idc.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Halperin JJ (2018). Neuroborreliosis. Neurol Clin 36, 821–830. 10.1016/j.ncl.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Hamer SA, Tsao JI, Walker ED, and Hickling GJ (2010). Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. Ecohealth 7, 47–63. 10.1007/s10393-010-0287-0 [DOI] [PubMed] [Google Scholar]
- Hamer SA, Goldberg TL, Kitron UD, Brawn JD, Anderson TK, Loss SR, Walker ED, and Hamer GL (2012). Wild birds and urban ecology of ticks and tick-borne pathogens, Chicago, Illinois, USA, 2005–2010. Emerg Infect Dis 18, 1589–1595. 10.3201/eid1810.120511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K, Kurtenbach K, Diuk-Wasser M, Brei B, and Fish D (2006). Epidemic spread of Lyme borreliosis, northeastern United States. Emerg Infect Dis 12, 604–611. 10.3201/eid1204.051016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K, Liveris D, Sandigursky S, Wormser GP, and Schwartz I (2008). Borrelia burgdorferi sensu stricto is clonal in patients with early Lyme borreliosis. Appl Environ Microbiol 74, 5008–5014. 10.1128/AEM.00479-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K, Mukherjee P, Ogden NH, Margos G, Wormser GP, Reed KD, Meece JK, Vandermause MF, and Schwartz I (2013). Multilocus sequence typing of Borrelia burgdorferi suggests existence of lineages with differential pathogenic properties in humans. PLoS One 8, e73066. 10.1371/journal.pone.0073066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan JP, Benach JL, Coleman JL, Bosler EM, Morse DL, Cameron DJ, Edelman R, and Kaslow RA (1984). Incidence and cumulative frequency of endemic Lyme disease in a community. J Infect Dis 150, 489–496. [DOI] [PubMed] [Google Scholar]
- Harman M, Vig DK, Radolf JD, and Wolgemuth CW (2013). Viscous dynamics of Lyme disease and syphilis spirochetes reveal flagellar torque and drag. Biophys J 105, 2273–2280. 10.1016/j.bpj.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman MW, Dunham-Ems SM, Caimano MJ, Belperron AA, Bockenstedt LK, Fu HC, Radolf JD, and Wolgemuth CW (2012). The heterogeneous motility of the Lyme disease spirochete in gelatin mimics dissemination through tissue. Proc Natl Acad Sci U S A 109, 3059–3064. 10.1073/pnas.1114362109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasle G, Bjune GA, Midthjell L, Røed KH, and Leinaas HP (2011). Transport of Ixodes ricinus infected with Borrelia species to Norway by northward-migrating passerine birds. 2, 37–43. 10.1016/j.ttbdis.2010.10.004 [DOI] [PubMed] [Google Scholar]
- Haugeberg G, Hansen IJ, Skarpaas T, Noraas S, and Kjelland V (2014). Lyme arthritis in southern Norway--an endemic area for Lyme borreliosis. BMC Infect Dis 14, 185. 10.1186/1471-2334-14-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haven J, Vargas LC, Mongodin EF, Xue V, Hernandez Y, Pagan P, Fraser-Liggett CM, Schutzer SE, Luft BJ, Casjens SR, et al. (2011). Pervasive recombination and sympatric genome diversification driven by frequency-dependent selection in Borrelia burgdorferi, the Lyme disease bacterium. Genetics 189, 951–966. 10.1534/genetics.111.130773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, Norgard MV, Gomelsky M, and Yang XF (2011). Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog 7, e1002133. 10.1371/journal.ppat.1002133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R (2009). Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7, 263–273. [DOI] [PubMed] [Google Scholar]
- Hengge R (2011). Stationary-phase gene regulation in Escherichia coli EcoSal Plus 4. 10.1128/ecosalplus.5.6.3 [DOI] [PubMed] [Google Scholar]
- Hengge UR, Tannapfel A, Tyring SK, Erbel R, Arendt G, and Ruzicka T (2003). Lyme borreliosis. Lancet Infect Dis 3, 489–500. [DOI] [PubMed] [Google Scholar]
- Hersh MH, LaDeau SL, Previtali MA, and Ostfeld RS (2014). When is a parasite not a parasite? Effects of larval tick burdens on white-footed mouse survival. Ecology 95, 1360–1369. [DOI] [PubMed] [Google Scholar]
- Herzer P (1991). Joint manifestations of Lyme borreliosis in Europe. Scand J Infect Dis Suppl 77, 55–63. [PubMed] [Google Scholar]
- Herzer P (1993). Rheumatic manifestations in Lyme borreliosis. Clin Dermatol 11, 401–406. 10.1016/0738-081x(93)90096-u [DOI] [PubMed] [Google Scholar]
- Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, and Mead PS (2014). Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59, 676–681. 10.1093/cid/ciu397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen AG, Margos G, Bent SJ, Diuk-Wasser MA, Barbour A, Kurtenbach K, and Fish D (2009). Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc Natl Acad Sci U S A 106, 15013–15018. 10.1073/pnas.0903810106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstrom E (1951). Successful treatment of erythema migrans Afzelius. Acta Derm Venereol 31, 235–243. [PubMed] [Google Scholar]
- Holt SC (1978). Anatomy and chemistry of spirochetes. Microbiol Rev 42, 114–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius JW, de Jong MA, den Dunnen J, Litjens M, Fikrig E, van der Poll T, Gringhuis SI, and Geijtenbeek TB (2008a). Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog 4, e31. 10.1371/journal.ppat.0040031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius JW, Levi M, and Fikrig E (2008b). Salivating for knowledge: potential pharmacological agents in tick saliva. PLoS Med 5, e43. 10.1371/journal.pmed.0050043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius JW (2009). Spitting image: tick saliva assists the causative agent of Lyme disease in evading host skin’s innate immune response. J Invest Dermatol 129, 2337–2339. 10.1038/jid.2009.202 [DOI] [PubMed] [Google Scholar]
- Hu LT, Perides G, Noring R, and Klempner MS (1995). Binding of human plasminogen to Borrelia burgdorferi. Infect Immun 63, 3491–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Eskildsen MA, Masgala C, Steere AC, Arner EC, Pratta MA, Grodzinsky AJ, Loening A, and Perides G (2001). Host metalloproteinases in Lyme arthritis. Arthritis Rheum 44, 1401–1410. [DOI] [PubMed] [Google Scholar]
- Huang Z, Toledo AM, Benach JL, and London E (2016). Ordered nembrane domain-forming properties of the lipids of Borrelia burgdorferi. Biophys J 111, 2666–2675. 10.1016/j.bpj.2016.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubalek Z (2009). Epidemiology of Lyme borreliosis. Curr Probl Dermatol 37, 31–50. 10.1159/000213069 [DOI] [PubMed] [Google Scholar]
- Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, and Norgard MV (2001). Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A 98, 12724–12729. 10.1073/pnas.231442498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde FW, and Johnson RC (1984). Genetic relationship of Lyme disease spirochetes to Borrelia, Treponema, and Leptospira spp. J Clin Microbiol 20, 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Shaw DK, Smith Iii R, Trzeciakowski JP, and Skare JT (2009). The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol Microbiol 74, 1344–1355. 10.1111/j.1365-2958.2009.06951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Weening EH, Chang M, Trzeciakowski JP, Hook M, Cirillo JD, and Skare JT (2011). Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol Microbiol 82, 99–113. 10.1111/j.1365-2958.2011.07801.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA (2017). Borrelia burgdorferi keeps moving and carries on: a review of borrelial dissemination and invasion. Front Immunol 8, 114. 10.3389/fimmu.2017.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Caimano MJ, Luthra A, Axline D Jr., Corona A, Iacobas DA, Radolf JD, and Schwartz I (2015). Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Mol Microbiol 95, 509–538. 10.1111/mmi.12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari N, Glusman G, Joesch-Cohen LM, Reddy PJ, Moritz RL, Hood L, and Lausted CG (2018). Whole genome sequence and comparative analysis of Borrelia burgdorferi MM1. PLoS One 13, e0198135. 10.1371/journal.pone.0198135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen M, Zhou D, Cepok S, Nessler S, Happel M, Stei S, Wilske B, Sommer N, and Hemmer B (2003). Clonal accumulation of activated CD8+ T cells in the central nervous system during the early phase of neuroborreliosis. J Infect Dis 187, 963–973. 10.1086/368131 [DOI] [PubMed] [Google Scholar]
- Jahfari S, Krawczyk A, Coipan EC, Fonville M, Hovius JW, Sprong H, and Takumi K (2017). Enzootic origins for clinical manifestations of Lyme borreliosis. Infect Genet Evol 49, 48–54. 10.1016/j.meegid.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Johnson RC, Schmid GP, Hyde FW, Steigerwalt AG, and Brenner DJ (1984). Borrelia burgdorferi sp. nov: Etiologic agent of Lyme disease. Int J Syst Bacteriol 34, 496–497. [Google Scholar]
- Jones JD, Bourell KW, Norgard MV, and Radolf JD (1995). Membrane topology of Borrelia burgdorferi and Treponema pallidum lipoproteins. Infect Immun 63, 2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Glickstein LJ, Damle N, Sikand VK, McHugh G, and Steere AC (2006). Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J Clin Microbiol 44, 4407–4413. 10.1128/JCM.01077-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Muellegger RR, Means TK, Lee M, Glickstein LJ, Damle N, Sikand VK, Luster AD, and Steere AC (2008). Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis 46, 85–92. 10.1086/524022 [DOI] [PubMed] [Google Scholar]
- Jones KL, McHugh GA, Glickstein LJ, and Steere AC (2009). Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: High frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum 60, 2174–2182. 10.1002/art.24812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnick S, Margos G, Rieger M, Dzaferovic E, Bent SJ, Overzier E, Silaghi C, Walder G, Wex F, Koloczek J, et al. (2015). Borrelia burgdorferi sensu stricto and Borrelia afzelii: population structure and differential pathogenicity. Int J Med Microbiol 305, 673–681. 10.1016/j.ijmm.2015.08.017 [DOI] [PubMed] [Google Scholar]
- Jutras BL, Lochhead RB, Kloos ZA, Biboy J, Strle K, Booth CJ, Govers SK, Gray J, Schumann P, Vollmer W, et al. (2019). Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc Natl Acad Sci U S A 116, 13498–13507. 10.1073/pnas.1904170116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L, Sood SK, and Maytal J (1998). Pseudotumor cerebri in Lyme disease: a case report and literature review. Pediatr Neurol 18, 439–441. 10.1016/s0887-8994(97)00215-4 [DOI] [PubMed] [Google Scholar]
- Kannian P, Drouin EE, Glickstein L, Kwok WW, Nepom GT, and Steere AC (2007). Decline in the frequencies of Borrelia burgdorferi OspA161 175-specific T cells after antibiotic therapy in HLA-DRB1*0401-positive patients with antibiotic-responsive or antibiotic-refractory lyme arthritis. J Immunol 179, 6336–6342. [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Trevino RP, Johnson GM, Levy L, Dornbush R, Hu LT, Evans J, Weinstein A, Schmid CH, and Klempner MS (2003). Cognitive function in post-treatment Lyme disease: do additional antibiotics help? Neurology 60, 1916–1922. [DOI] [PubMed] [Google Scholar]
- Kenedy MR, Lenhart TR, and Akins DR (2012). The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol Med Microbiol 66, 1–19. 10.1111/j.1574-695X.2012.00980.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenedy MR, Luthra A, Anand A, Dunn JP, Radolf JD, and Akins DR (2014). Structural modeling and physicochemical characterization provide evidence that P66 forms a b-barrel in the Borrelia burgdorferi outer membrane. J Bacteriol 196, 859–872. 10.1128/JB.01236-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenedy MR, Scott EJ 2nd, Shrestha B, Anand A, Iqbal H, Radolf JD, Dyer DW, and Akins DR (2016). Consensus computational network analysis for identifying candidate outer membrane proteins from Borrelia spirochetes. BMC Microbiol 16, 141. 10.1186/s12866-016-0762-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatchikian CE, Nadelman RB, Nowakowski J, Schwartz I, Wormser GP, and Brisson D (2014). Evidence for strain-specific immunity in patients treated for early Lyme disease. Infect Immun 82, 1408–1413. 10.1128/IAI.01451-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingry LC, Batra D, Replogle A, Rowe LA, Pritt BS, and Petersen JM (2016). Whole genome sequence and comparative genomics of the novel Lyme borreliosis causing pathogen, Borrelia mayonii. PLoS One 11, e0168994. 10.1371/journal.pone.0168994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. (2006). Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol 7, 978–986. 10.1038/ni1380 [DOI] [PubMed] [Google Scholar]
- Klempner MS, and Huber BT (1999). Is it thee or me?--autoimmunity in Lyme disease. Nat Med 5, 1346–1347. 10.1038/70907 [DOI] [PubMed] [Google Scholar]
- Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, Norton D, Levy L, Wall D, McCall J, et al. (2001). Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med 345, 85–92. 10.1056/NEJM200107123450202 [DOI] [PubMed] [Google Scholar]
- Klempner MS, Baker PJ, Shapiro ED, Marques A, Dattwyler RJ, Halperin JJ, and Wormser GP (2013). Treatment trials for post-Lyme disease symptoms revisited. Am J Med 126, 665–669. 10.1016/j.amjmed.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedel U, Fingerle V, and Pfister HW (2015). Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol 11, 446–456. 10.1038/nrneurol.2015.121 [DOI] [PubMed] [Google Scholar]
- Konovalova A, Kahne DE, and Silhavy TJ (2017). Outer membrane biogenesis. Annu Rev Microbiol 71, 539–556. 10.1146/annurev-micro-090816-093754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, and Marconi RT (2011). The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol Microbiol 81, 219–231. 10.1111/j.1365-2958.2011.07687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiczy P (2016a). Travelling between two worlds: complement as a gatekeeper for an expanded host range of Lyme disease spirochetes. Vet Sci 3. 10.3390/vetsci3020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiczy P (2016b). Hide and seek: how Lyme disease spirochetes overcome complement attack. Front Immunol 7, 385. 10.3389/fimmu.2016.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Foley DT, Burke GS, Christianson D, Closter L, Spielman A, and Tick-Borne Disease Study G (2006). Reinfection and relapse in early Lyme disease. Am J Trop Med Hyg 75, 1090–1094. [PubMed] [Google Scholar]
- Kristoferitsch W, Sluga E, Graf M, Partsch H, Neumann R, Stanek G, and Budka H (1988). Neuropathy associated with acrodermatitis chronica atrophicans. Clinical and morphological features. Ann N Y Acad Sci 539, 35–45. 10.1111/j.1749-6632.1988.tb31836.x [DOI] [PubMed] [Google Scholar]
- Krupp LB, Hyman LG, Grimson R, Coyle PK, Melville P, Ahnn S, Dattwyler R, and Chandler B (2003). Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology 60, 1923–1930. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Eswaramoorthy S, Luft BJ, Koide S, Dunn JJ, Lawson CL, and Swaminathan S (2001). Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. EMBO J 20, 971–978. 10.1093/emboj/20.5.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach K, Sewell HS, Ogden NH, Randolph SE, and Nuttall PA (1998). Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun 66, 1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, and Kraiczy P (2002). Host association of Borrelia burgdorferi sensu lato--the key role of host complement. Trends Microbiol 10, 74–79. [DOI] [PubMed] [Google Scholar]
- Kurtenbach K, Hanincova K, Tsao JI, Margos G, Fish D, and Ogden NH (2006). Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol 4, 660–669. 10.1038/nrmicro1475 [DOI] [PubMed] [Google Scholar]
- Lagal V, Portnoi D, Faure G, Postic D, and Baranton G (2006). Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes Infect 8, 645–652. 10.1016/j.micinf.2005.08.017 [DOI] [PubMed] [Google Scholar]
- Lane RS, and Loye JE (1989). Lyme disease in California: interrelationship of Ixodes pacificus (Acari: Ixodidae), the western fence lizard (Sceloporus occidentalis), and Borrelia burgdorferi. J Med Entomol 26, 272–278. 10.1093/jmedent/26.4.272 [DOI] [PubMed] [Google Scholar]
- Lane RS, Piesman J, and Burgdorfer W (1991). Lyme borreliosis: relation of its causative agent to Its vectors and hosts in North America and Europe. 36, 587–609. 10.1146/annurev.en.36.010191.003103 [DOI] [PubMed] [Google Scholar]
- Lane RS, and Quistad GB (1998). Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis). J Parasitol 84, 29–34. [PubMed] [Google Scholar]
- Lane RS, Mun J, Peribanez MA, and Stubbs HA (2007). Host-seeking behavior of Ixodes pacificus (Acari: Ixodidae) nymphs in relation to environmental parameters in dense-woodland and woodland-grass habitats. J Vector Ecol 32, 342–357. [DOI] [PubMed] [Google Scholar]
- Lane RS, Fedorova N, Kleinjan JE, and Maxwell M (2013). Ecoepidemiological factors contributing to the low risk of human exposure to ixodid tick-borne borreliae in southern California, USA. Ticks Tick Borne Dis 4, 377–385. 10.1016/j.ttbdis.2013.02.005 [DOI] [PubMed] [Google Scholar]
- Lantos PM (2015). Chronic Lyme disease. Infect Dis Clin North Am 29, 325–340. 10.1016/j.idc.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos PM, Shapiro ED, Auwaerter PG, Baker PJ, Halperin JJ, McSweegan E, and Wormser GP (2015). Unorthodox alternative therapies marketed to treat Lyme disease. Clin Infect Dis 60, 1776–1782. 10.1093/cid/civ186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Crowley JT, Cusack BJ, Pathak P, Benach J, London E, Garcia-Monco JC, and Benach JL (2010). Cholesterol lipids of Borrelia burgdorferi form lipid rafts and are required for the bactericidal activity of a complement-independent antibody. Cell Host Microbe 8, 331–342. 10.1016/j.chom.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Pathak P, Chiantia S, Toledo A, Silvius JR, Benach JL, and London E (2013). Proving lipid rafts exist: membrane domains in the prokaryote Borrelia burgdorferi have the same properties as eukaryotic lipid rafts. PLoS Pathog 9, e1003353. 10.1371/journal.ppat.1003353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhoff C (1948). Spirochaetes in aetiologically obscure diseases. Acta Dermatologica Venereologica 295–324. [PubMed] [Google Scholar]
- Lepej SZ, Rode OD, Jeren T, Vince A, Remenar A, and Barsic B (2005). Increased expression of CXCR3 and CCR5 on memory CD4+ T-cells migrating into the cerebrospinal fluid of patients with neuroborreliosis: the role of CXCL10 and CXCL11. J Neuroimmunol 163, 128–134. 10.1016/j.jneuroim.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Li H, Dunn JJ, Luft BJ, and Lawson CL (1997). Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci U S A 94, 3584–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, and Steere AC (2011). Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or lyme arthritis. Arthritis Rheum 63, 2238–2247. 10.1002/art.30384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Steere AC, Marques AR, Johnson BJ, Miller JN, and Philipp MT (1999). Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J Clin Microbiol 37, 3990–3996. 10.1128/JCM.37.12.3990-3996.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Jacobs MB, Bowers LC, and Philipp MT (2002). An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med 195, 415–422. 10.1084/jem.20011870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kidder JM, Noring R, Steere AC, Klempner MS, and Hu LT (2001). Differences in synovial fluid levels of matrix metalloproteinases suggest separate mechanisms of pathogenesis in Lyme arthritis before and after antibiotic treatment. J Infect Dis 184, 174–180. 10.1086/322000 [DOI] [PubMed] [Google Scholar]
- Lin YP, Benoit V, Yang X, Martinez-Herranz R, Pal U, and Leong JM (2014). Strain-specific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the lyme disease spirochete. PLoS Pathog 10, e1004238. 10.1371/journal.ppat.1004238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Chen Q, Ritchie JA, Dufour NP, Fischer JR, Coburn J, and Leong JM (2015). Glycosaminoglycan binding by Borrelia burgdorferi adhesin BBK32 specifically and uniquely promotes joint colonization. Cell Microbiol 17, 860–875. 10.1111/cmi.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipschütz B (1913). Uber eine seltene Erythemform (Erythema chronicum migrans) Arch Dermatol Syph 118, 349–356. [Google Scholar]
- Lipschütz B (1923). Weiter Beitgrag zur Kenngtnis des “Erythema chronicum migrans”. Arch Dermatol Re 143, 365–374. [Google Scholar]
- Liveris D, Varde S, Iyer R, Koenig S, Bittker S, Cooper D, McKenna D, Nowakowski J, Nadelman RB, Wormser GP, et al. (1999). Genetic diversity of Borrelia burgdorferi in lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol 37, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead RB, Arvikar SL, Aversa JM, Sadreyev RI, Strle K, and Steere AC (2019a). Robust interferon signature and suppressed tissue repair gene expression in synovial tissue from patients with postinfectious, Borrelia burgdorferi-induced Lyme arthritis. Cell Microbiol 21, e12954. 10.1111/cmi.12954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead RB, Ordonez D, Arvikar SL, Aversa JM, Oh LS, Heyworth B, Sadreyev R, Steere AC, and Strle K (2019b). Interferon-gamma production in Lyme arthritis synovial tissue promotes differentiation of fibroblast-like synoviocytes into immune effector cells. Cell Microbiol 21, e12992. 10.1111/cmi.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logar M, Ruzic-Sabljic E, Maraspin V, Lotric-Furlan S, Cimperman J, Jurca T, and Strle F (2004). Comparison of erythema migrans caused by Borrelia afzelii and Borrelia garinii. Infection 32, 15–19. 10.1007/s15010-004-3042-z [DOI] [PubMed] [Google Scholar]
- Logigian EL, Kaplan RF, and Steere AC (1990). Chronic neurologic manifestations of Lyme disease. N Engl J Med 323, 1438–1444. 10.1056/NEJM199011223232102 [DOI] [PubMed] [Google Scholar]
- Logigian EL, and Steere AC (1992). Clinical and electrophysiologic findings in chronic neuropathy of Lyme disease. Neurology 42, 303–311. [DOI] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, and Keesing F (2003). The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A 100, 567–571. 10.1073/pnas.0233733100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr B, Fingerle V, Norris DE, and Hunfeld KP (2018). Laboratory diagnosis of Lyme borreliosis: Current state of the art and future perspectives. Crit Rev Clin Lab Sci 55, 219–245. 10.1080/10408363.2018.1450353 [DOI] [PubMed] [Google Scholar]
- Luft BJ, Steinman CR, Neimark HC, Muralidhar B, Rush T, Finkel MF, Kunkel M, and Dattwyler RJ (1992). Invasion of the central nervous system by Borrelia burgdorferi in acute disseminated infection. JAMA 267, 1364–1367. [PubMed] [Google Scholar]
- Lunemann JD, Gelderblom H, Sospedra M, Quandt JA, Pinilla C, Marques A, and Martin R (2007). Cerebrospinal fluid-infiltrating CD4+ T cells recognize Borrelia burgdorferi lysine-enriched protein domains and central nervous system autoantigens in early lyme encephalitis. Infect Immun 75, 243–251. 10.1128/IAI.01110-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli A, Bertolotti L, Gern L, and Gray J (2012). Ecology of Borrelia burgdorferi sensu lato in Europe: transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol Rev 36, 837–861. 10.1111/j.1574-6976.2011.00312.x [DOI] [PubMed] [Google Scholar]
- Maraspin V, Nahtigal Klevisar M, Ruzic-Sabljic E, Lusa L, and Strle F (2016). Borrelial Lymphocytoma in Adult Patients. Clin Infect Dis 63, 914–921. 10.1093/cid/ciw417 [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz AL, Dupuis AP 2nd, Zamba-Campero M, Nowak N, Kraiczy P, Ram S, Kramer LD, and Lin YP (2019). Blood treatment of Lyme borreliae demonstrates the mechanism of CspZ-mediated complement evasion to promote systemic infection in vertebrate hosts. Cell Microbiol 21, e12998. 10.1111/cmi.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi RT, Liveris D, and Schwartz I (1995). Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol 33, 2427–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos LA, Castle PM, Smith K, Khoo T, Morley EJ, Bloom M, and Fries BC (2019). Risk factors for Lyme carditis: A case-control study. European Journal of Preventive Cardiology, 204748731987604. 10.1177/2047487319876046 [DOI] [PubMed] [Google Scholar]
- Margos G, Gatewood AG, Aanensen DM, Hanincova K, Terekhova D, Vollmer SA, Cornet M, Piesman J, Donaghy M, Bormane A, et al. (2008). MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc Natl Acad Sci U S A 105, 8730–8735. 10.1073/pnas.0800323105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Vollmer SA, Ogden NH, and Fish D (2011). Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect Genet Evol 11, 1545–1563. 10.1016/j.meegid.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Lane RS, Fedorova N, Koloczek J, Piesman J, Hojgaard A, Sing A, and Fingerle V (2016). Borrelia bissettiae sp. nov. and Borrelia californiensis sp. nov. prevail in diverse enzootic transmission cycles. Int J Syst Evol Microbiol 66, 1447–1452. 10.1099/ijsem.0.000897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Hepner S, Mang C, Marosevic D, Reynolds SE, Krebs S, Sing A, Derdakova M, Reiter MA, and Fingerle V (2017a). Lost in plasmids: next generation sequencing and the complex genome of the tick-borne pathogen Borrelia burgdorferi. BMC Genomics 18, 422. 10.1186/s12864-017-3804-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Marosevic D, Cutler S, Derdakova M, Diuk-Wasser M, Emler S, Fish D, Gray J, Hunfeldt KP, Jaulhac B, et al. (2017b). There is inadequate evidence to support the division of the genus Borrelia. Int J Syst Evol Microbiol 67, 1081–1084. 10.1099/ijsem.0.001717 [DOI] [PubMed] [Google Scholar]
- Margos G, Gofton A, Wibberg D, Dangel A, Marosevic D, Loh SM, Oskam C, and Fingerle V (2018). The genus Borrelia reloaded. PLoS One 13, e0208432. 10.1371/journal.pone.0208432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A (2008). Chronic Lyme disease: a review. Infect Dis Clin North Am 22, 341–360, vii–viii. 10.1016/j.idc.2007.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A, Telford SR 3rd, Turk SP, Chung E, Williams C, Dardick K, Krause PJ, Brandeburg C, Crowder CD, Carolan HE, et al. (2014). Xenodiagnosis to detect Borrelia burgdorferi infection: a first-in-human study. Clin Infect Dis 58, 937–945. 10.1093/cid/cit939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A, Schwartz I, Wormser GP, Wang Y, Hornung RL, Demirkale CY, Munson PJ, Turk SP, Williams C, Lee CR, et al. (2017). Transcriptome assessment of erythema migrans skin lesions in patients with early Lyme disease reveals predominant interferon signaling. J Infect Dis 217, 158–167. 10.1093/infdis/jix563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx GE, Leikauskas J, Lindstrom K, Mann E, Reagan-Steiner S, Matkovic E, Read JS, Kelso P, Kwit NA, Hinckley AF, et al. (2019). Fatal Lyme carditis in New England: Two case reports. Annals of Internal Medicine. 10.7326/l19-0483 [DOI] [PubMed] [Google Scholar]
- Mast WE, and Burrows WM Jr. (1976). Erythema chronicum migrans in the United States. JAMA 236, 859–860. [PubMed] [Google Scholar]
- Mather TN, Wilson ML, Moore SI, Ribeiro JM, and Spielman A (1989). Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am J Epidemiol 130, 143–150. 10.1093/oxfordjournals.aje.a115306 [DOI] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, and Matyas BT (1996). Entomologic index for human risk of Lyme disease. Am J Epidemiol 144, 1066–1069. [DOI] [PubMed] [Google Scholar]
- Maupin GO, Gage KL, Piesman J, Montenieri J, Sviat SL, VanderZanden L, Happ CM, Dolan M, and Johnson BJ (1994). Discovery of an enzootic cycle of Borrelia burgdorferi in Neotoma mexicana and Ixodes spinipalpis from northern Colorado, an area where Lyme disease is nonendemic. J Infect Dis 170, 636–643. 10.1093/infdis/170.3.636 [DOI] [PubMed] [Google Scholar]
- McCoy KD, Leger E, and Dietrich M (2013). Host specialization in ticks and transmission of tick-borne diseases: a review. Front Cell Infect Microbiol 3, 57. 10.3389/fcimb.2013.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS (2015). Epidemiology of Lyme disease. Infect Dis Clin North Am 29, 187–210. 10.1016/j.idc.2015.02.010 [DOI] [PubMed] [Google Scholar]
- Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Pena A, George JC, Golovljova I, Jaenson TG, Jensen JK, Jensen PM, et al. (2013). Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 6, 1. 10.1186/1756-3305-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molins CR, Ashton LV, Wormser GP, Andre BG, Hess AM, Delorey MJ, Pilgard MA, Johnson BJ, Webb K, Islam MN, et al. (2017). Metabolic differentiation of early Lyme disease from southern tick-associated rash illness (STARI). Sci Transl Med 9. 10.1126/scitranslmed.aal2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongodin EF, Casjens SR, Bruno JF, Xu Y, Drabek EF, Riley DR, Cantarel BL, Pagan PE, Hernandez YA, Vargas LC, et al. (2013). Inter- and intra-specific pan-genomes of Borrelia burgdorferi sensu lato: genome stability and adaptive radiation. BMC Genomics 14, 693. 10.1186/1471-2164-14-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RR, Malawista SE, Feen KJ, and Bockenstedt LK (1996). Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med 183, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody KD, Terwilliger GA, Hansen GM, and Barthold SW (1994). Experimental Borrelia burgdorferi infection in Peromyscus leucopus. J Wildl Dis 30, 155–161. 10.7589/0090-3558-30.2.155 [DOI] [PubMed] [Google Scholar]
- Morgan A, and Wang X (2013). The novel heparin-binding motif in decorin-binding protein A from strain B31 of Borrelia burgdorferi explains the higher binding affinity. Biochemistry 52, 8237–8245. 10.1021/bi401376u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty TJ, Norman MU, Colarusso P, Bankhead T, Kubes P, and Chaconas G (2008). Real-time high resolution 3D imaging of the Lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog 4, e1000090. 10.1371/journal.ppat.1000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty TJ, Shi M, Lin YP, Ebady R, Zhou H, Odisho T, Hardy PO, Salman-Dilgimen A, Wu J, Weening EH, et al. (2012). Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules. Mol Microbiol 86, 1116–1131. 10.1111/mmi.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Corum L, Bono JL, Elias AF, Rosa P, Samuels DS, and Charon NW (2000). Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc Natl Acad Sci U S A 97, 10899–10904. 10.1073/pnas.200221797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Liu J, and Wooten RM (2015). Spirochetal motility and chemotaxis in the natural enzootic cycle and development of Lyme disease. Curr Opin Microbiol 28, 106–113. 10.1016/j.mib.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbachs A, Bollweg BC, Schulz TJ, Forrester JD, DeLeon Carnes M, Molins C, Ray GS, Cummings PM, Ritter JM, Blau DM, et al. (2016). Cardiac tropism of Borrelia burgdorferi: An autopsy study of sudden cardiac death associated with Lyme carditis. Am J Pathol 186, 1195–1205. 10.1016/j.ajpath.2015.12.027 [DOI] [PubMed] [Google Scholar]
- Mulay VB, Caimano MJ, Iyer R, Dunham-Ems S, Liveris D, Petzke MM, Schwartz I, and Radolf JD (2009). Borrelia burgdorferi bba74 is expressed exclusively during tick feeding and is regulated by both arthropod- and mammalian host-specific signals. J Bacteriol 191, 2783–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullegger RR, McHugh G, Ruthazer R, Binder B, Kerl H, and Steere AC (2000). Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J Invest Dermatol 115, 1115–1123. 10.1046/j.1523-1747.2000.00198.x [DOI] [PubMed] [Google Scholar]
- Mullegger RR, Means TK, Shin JJ, Lee M, Jones KL, Glickstein LJ, Luster AD, and Steere AC (2007). Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect Immun 75, 4621–4628. 10.1128/IAI.00263-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro HJ, Ogden NH, Mechai S, Lindsay LR, Robertson GJ, Whitney H, and Lang AS (2019). Genetic diversity of Borrelia garinii from Ixodes uriae collected in seabird colonies of the northwestern Atlantic Ocean. Ticks Tick Borne Dis 10, 101255. 10.1016/j.ttbdis.2019.06.014 [DOI] [PubMed] [Google Scholar]
- Murfin KE, and Fikrig E (2017). Tick bioactive molecules as novel therapeutics: beyond vaccine targets. Front Cell Infect Microbiol 7, 222. 10.3389/fcimb.2017.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, and Nagase H (2008). Progress in matrix metalloproteinase research. Mol Aspects Med 29, 290–308. 10.1016/j.mam.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mygland A, Skarpaas T, and Ljostad U (2006). Chronic polyneuropathy and Lyme disease. Eur J Neurol 13, 1213–1215. 10.1111/j.1468-1331.2006.01395.x [DOI] [PubMed] [Google Scholar]
- Nadelman RB, Nowakowski J, Forseter G, Bittker S, Cooper D, Goldberg N, McKenna D, and Wormser GP (1993). Failure to isolate Borrelia burgdorferi after antimicrobial therapy in culture-documented Lyme borreliosis associated with erythema migrans: report of a prospective study. Am J Med 94, 583–588. [DOI] [PubMed] [Google Scholar]
- Nadelman RB, Nowakowski J, Forseter G, Goldberg NS, Bittker S, Cooper D, Aguero-Rosenfeld M, and Wormser GP (1996). The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med 100, 502–508. [DOI] [PubMed] [Google Scholar]
- Nadelman RB, Nowakowski J, Fish D, Falco RC, Freeman K, McKenna D, Welch P, Marcus R, Aguero-Rosenfeld ME, Dennis DT, et al. (2001). Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med 345, 79–84. 10.1056/NEJM200107123450201 [DOI] [PubMed] [Google Scholar]
- Nadelman RB, and Wormser GP (2007). Reinfection in patients with Lyme disease. Clin Infect Dis 45, 1032–1038. 10.1086/521256 [DOI] [PubMed] [Google Scholar]
- Nadelman RB, and Wormser GP (2013). Reinfection versus relapse in Lyme disease. N Engl J Med 368, 1063–1064. 10.1056/NEJMc1215615 [DOI] [PubMed] [Google Scholar]
- Nadelman RB (2015). Erythema migrans. Infect Dis Clin North Am 29, 211–239. 10.1016/j.idc.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, and Mead PS (2015). Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis 21, 1625–1631. 10.3201/eid2109.150417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H (2003). Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67, 593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolić A, Boljević D, Bojić M, Veljković S, Vuković D, Paglietti B, Micić J, and Rubino S (2020). Lyme endocarditis as an emerging infectious disease: A review of the literature. Frontiers in Microbiology 11. 10.3389/fmicb.2020.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, and Steere AC (1994). Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 330, 229–234. 10.1056/NEJM199401273300401 [DOI] [PubMed] [Google Scholar]
- Norgard MV, Arndt LL, Akins DR, Curetty LL, Harrich DA, and Radolf JD (1996). Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-kB. Infect Immun 64, 3845–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MU, Moriarty TJ, Dresser AR, Millen B, Kubes P, and Chaconas G (2008). Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog 4, e1000169. 10.1371/journal.ppat.1000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DE, Johnson BJ, Piesman J, Maupin GO, Clark JL, and Black W.C.t. (1999). Population genetics and phylogenetic analysis of Colorado Borrelia burgdorferi. Am J Trop Med Hyg 60, 699–707. 10.4269/ajtmh.1999.60.699 [DOI] [PubMed] [Google Scholar]
- Norris SJ (2014). vls antigenic variation systems of Lyme disease Borrelia: eluding host immunity through both random, segmental gene conversion and framework heterogeneity. Microbiol Spectr 2. 10.1128/microbiolspec.MDNA3-0038-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norte AC, Ramos JA, Gern L, Nuncio MS, and Lopes de Carvalho I (2013). Birds as reservoirs for Borrelia burgdorferi s.l. in western Europe: circulation of B. turdi and other genospecies in bird-tick cycles in Portugal. Environ Microbiol 15, 386–397. 10.1111/j.1462-2920.2012.02834.x [DOI] [PubMed] [Google Scholar]
- Nowakowski J, Schwartz I, Liveris D, Wang G, Aguero-Rosenfeld ME, Girao G, McKenna D, Nadelman RB, Cavaliere LF, Wormser GP, et al. (2001). Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin Infect Dis 33, 2023–2027. 10.1086/324490 [DOI] [PubMed] [Google Scholar]
- Nowakowski J, Nadelman RB, Sell R, McKenna D, Cavaliere LF, Holmgren D, Gaidici A, and Wormser GP (2003). Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med 115, 91–96. [DOI] [PubMed] [Google Scholar]
- Nowakowski J, McKenna D, Nadelman RB, Bittker S, Cooper D, Pavia C, Holmgren D, Visintainer P, and Wormser GP (2009). Blood cultures for patients with extracutaneous manifestations of Lyme disease in the United States. Clin Infect Dis 49, 1733–1735. 10.1086/648076 [DOI] [PubMed] [Google Scholar]
- Nowalk AJ, Gilmore RD Jr., and Carroll JA (2006). Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect Immun 74, 3864–3873. 10.1128/IAI.00189-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall PA (2019). Wonders of tick saliva. Ticks Tick Borne Dis 10, 470–481. 10.1016/j.ttbdis.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Hanincova K, Barker IK, Bigras-Poulin M, Charron DF, Heagy A, Francis CM, O’Callaghan CJ, Schwartz I, et al. (2008). Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl Environ Microbiol 74, 1780–1790. 10.1128/AEM.01982-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Morshed M, Sockett PN, and Artsob H (2009). The emergence of Lyme disease in Canada. CMAJ 180, 1221–1224. 10.1503/cmaj.080148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Bouchard C, Badcock J, Drebot MA, Elias SP, Hatchette TF, Koffi JK, Leighton PA, Lindsay LR, Lubelczyk CB, et al. (2019). What is the real number of Lyme disease cases in Canada? BMC Public Health 19. 10.1186/s12889-019-7219-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrinc K, Lotric-Furlan S, Maraspin V, Lusa L, Cerar T, Ruzic-Sabljic E, and Strle F (2013). Suspected early Lyme neuroborreliosis in patients with erythema migrans. Clin Infect Dis 57, 501–509. 10.1093/cid/cit317 [DOI] [PubMed] [Google Scholar]
- Ogrinc K, Lusa L, Lotric-Furlan S, Bogovic P, Stupica D, Cerar T, Ruzic-Sabljic E, and Strle F (2016). Course and outcome of early European Lyme neuroborreliosis (Bannwarth Syndrome): clinical and laboratory findings. Clin Infect Dis 63, 346–353. 10.1093/cid/ciw299 [DOI] [PubMed] [Google Scholar]
- Ohnishi J, Piesman J, and de Silva AM (2001). Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci U S A 98, 670–675. 10.1073/pnas.98.2.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JH Jr., Lin T, Gao L, Clark KL, Banks CW, Durden LA, James AM, and Chandler FW Jr. (2003). An enzootic transmission cycle of Lyme borreliosis spirochetes in the southeastern United States. Proc Natl Acad Sci U S A 100, 11642–11645. 10.1073/pnas.1434553100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B, Jaenson TG, Noppa L, Bunikis J, and Bergstrom S (1993). A Lyme borreliosis cycle in seabirds and Ixodes uriae ticks. Nature 362, 340–342. 10.1038/362340a0 [DOI] [PubMed] [Google Scholar]
- Olsen B, Duffy DC, Jaenson TG, Gylfe A, Bonnedahl J, and Bergstrom S (1995). Transhemispheric exchange of Lyme disease spirochetes by seabirds. J Clin Microbiol 33, 3270–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder O, Humphrey PT, McOmber B, Korobova F, Francella N, Greenbaum DC, and Brisson D (2012). OspC is potent plasminogen receptor on surface of Borrelia burgdorferi. J Biol Chem 287, 16860–16868. 10.1074/jbc.M111.290775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloski KA, Campbell GL, Genese CA, Beckley JW, Schriefer ME, Spitalny KC, and Dennis DT (1998). Emergence of Lyme disease in Hunterdon County, New Jersey, 1993: a case-control study of risk factors and evaluation of reporting patterns. Am J Epidemiol 147, 391–397. 10.1093/oxfordjournals.aje.a009462 [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, and Brunner JL (2015). Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci 370. 10.1098/rstb.2014.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Kumar M, Kariu T, Haq S, Goldberg M, Pal U, and Norgard MV (2009). BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol Microbiol 74, 1331–1343. 10.1111/j.1365-2958.2009.06945.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachner AR, Gelderblom H, and Cadavid D (2001). The rhesus model of Lyme neuroborreliosis. Immunol Rev 183, 186–204. [DOI] [PubMed] [Google Scholar]
- Paim AC, Baddour LM, Pritt BS, Schuetz AN, and Wilson JW (2018). Lyme endocarditis. Am J Med 131, 1126–1129. 10.1016/j.amjmed.2018.02.032 [DOI] [PubMed] [Google Scholar]
- Pal U, and Fikrig E (2003). Adaptation of Borrelia burgdorferi in the vector and vertebrate host. Microbes Infect 5, 659–666. [DOI] [PubMed] [Google Scholar]
- Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, Desilva AM, Bao F, Yang X, Pypaert M, Pradhan D, et al. (2004a). TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119, 457–468. 10.1016/j.cell.2004.10.027 [DOI] [PubMed] [Google Scholar]
- Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, Norgard MV, and Fikrig E (2004b). OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest 113, 220–230. 10.1172/JCI19894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas CJ, Iyer R, Petzke MM, Caimano MJ, Radolf JD, and Schwartz I (2011). Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog 7, e1002102. 10.1371/journal.ppat.1002102 PPATHOGENS-D-11–00446 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, and Link H (2001). Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain 124, 480–492. [DOI] [PubMed] [Google Scholar]
- Paster BJ, Dewhirst FE, Weisburg WG, Tordoff LA, Fraser GJ, Hespell RB, Stanton TB, Zablen L, Mandelco L, and Woese CR (1991). Phylogenetic analysis of the spirochetes. J Bacteriol 173, 6101–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton TG, Brandt KS, Nolder C, Clifton DR, Carroll JA, and Gilmore RD (2013). Borrelia burgdorferi bba66 gene inactivation results in attenuated mouse infection by tick transmission. Infect Immun 81, 2488–2498. 10.1128/IAI.00140-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules CI, Marston HD, Bloom ME, and Fauci AS (2018). tickborne diseases - confronting a growing threat. N Engl J Med 379, 701–703. 10.1056/NEJMp1807870 [DOI] [PubMed] [Google Scholar]
- Pavia CS, Wormser GP, and Norman GL (1997). Activity of sera from patients with Lyme disease against Borrelia burgdorferi. Clin Infect Dis 25 Suppl 1, S25–30. [DOI] [PubMed] [Google Scholar]
- Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, and Diuk-Wasser MA (2012). Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am J Trop Med Hyg 86, 1062–1071. 10.4269/ajtmh.2012.11-0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri F, Nistico D, Morabito G, Occhipinti A, Ventura A, Barbi E, and Cozzi G (2019). Somatic symptom disorder should be suspected in children with alleged chronic Lyme disease. Eur J Pediatr 178, 1297–1300. 10.1007/s00431-019-03416-6 [DOI] [PubMed] [Google Scholar]
- Petzke M, and Schwartz I (2015). Borrelia burgdorferi pathogenesis and the immune response. Clin Lab Med 35, 745–764. 10.1016/j.cll.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Petzke MM, Volyanskyy K, Mao Y, Arevalo B, Zohn R, Quituisaca J, Wormser GP, Dimitrova N, and Schwartz I (2020). Global transcriptome analysis identifies a diagnostic signature for early disseminated Lyme disease and its resolution. mBio 11. 10.1128/mBio.00047-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp MT, Aydintug MK, Bohm RP Jr., Cogswell FB, Dennis VA, Lanners HN, Lowrie RC Jr., Roberts ED, Conway MD, Karacorlu M, et al. (1993). Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun 61, 3047–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianta A, Drouin EE, Crowley JT, Arvikar S, Strle K, Costello CE, and Steere AC (2015). Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fibroblast proliferation in patients with antibiotic-refractory Lyme arthritis. Clin Immunol 160, 336–341. 10.1016/j.clim.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken RN, Cheng Y, Strle F, and Picken MM (1996). Patient isolates of Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities of strain 25015. J Infect Dis 174, 1112–1115. [DOI] [PubMed] [Google Scholar]
- Picken RN, and Picken MM (2000). Molecular characterization of Borrelia spp. isolates from greater metropolitan Chicago reveals the presence of Borrelia bissettii. Preliminary report. J Mol Microbiol Biotechnol 2, 505–507. [PubMed] [Google Scholar]
- Piesman J, Mather TN, Dammin GJ, Telford SR 3rd, Lastavica CC, and Spielman A (1987a). Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol 126, 1187–1189. [DOI] [PubMed] [Google Scholar]
- Piesman J, Mather TN, Sinsky RJ, and Spielman A (1987b). Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol 25, 557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Dolan MC, Happ CM, Luft BJ, Rooney SE, Mather TN, and Golde WT (1997). Duration of immunity to reinfection with tick-transmitted Borrelia burgdorferi in naturally infected mice. Infect Immun 65, 4043–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Schneider BS, and Zeidner NS (2001). Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J Clin Microbiol 39, 4145–4148. 10.1128/JCM.39.11.4145-4148.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J (2002). Ecology of Borrelia burgdorferi sensu lato in North America. In Lyme Borreliosis Biology, Epidemiology, and Control, Gray JS, Kahl O, Lane RS, and Stanek G, eds. (New York: CABI Publishing; ), pp. 223–250. [Google Scholar]
- Piesman J, and Gern L (2004). Lyme borreliosis in Europe and North America. Parasitology 129 Suppl, S191–220. [DOI] [PubMed] [Google Scholar]
- Pikas DS, Brown EL, Gurusiddappa S, Lee LY, Xu Y, and Hook M (2003). Decorin-binding sites in the adhesin DbpA from Borrelia burgdorferi: a synthetic peptide approach. J Biol Chem 278, 30920–30926. 10.1074/jbc.M303979200 [DOI] [PubMed] [Google Scholar]
- Pinne M, Ostberg Y, Comstedt P, and Bergstrom S (2004). Molecular analysis of the channel-forming protein P13 and its paralogue family 48 from different Lyme disease Borrelia species. Microbiology 150, 549–559. 10.1099/mic.0.26728-0 [DOI] [PubMed] [Google Scholar]
- Pinne M, Thein M, Denker K, Benz R, Coburn J, and Bergstrom S (2007). Elimination of channel-forming activity by insertional inactivation of the p66 gene in Borrelia burgdorferi. FEMS Microbiol Lett 266, 241–249. 10.1111/j.1574-6968.2006.00529.x [DOI] [PubMed] [Google Scholar]
- Plotkin SA (2011). Correcting a public health fiasco: The need for a new vaccine against Lyme disease. Clin Infect Dis 52 Suppl 3, s271–275. 10.1093/cid/ciq119 [DOI] [PubMed] [Google Scholar]
- Postic D, Ras NM, Lane RS, Hendson M, and Baranton G (1998). Expanded diversity among Californian borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127). J Clin Microbiol 36, 3497–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, et al. (2016). Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis 16, 556–564. 10.1016/S1473-3099(15)00464-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert WS, Kim JH, Hook M, and Johnson BJ (2001). Mapping the ligand-binding region of Borrelia burgdorferi fibronectin-binding protein BBK32. Infect Immun 69, 4129–4133. 10.1128/IAI.69.6.4129-4133.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WG, Bruno JF, McCaig WD, Xu Y, Livey I, Schriefer ME, and Luft BJ (2008). Wide distribution of a high-virulence Borrelia burgdorferi clone in Europe and North America. Emerg Infect Dis 14, 1097–1104. 10.3201/eid1407.070880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WG, and Martin CL (2014). Evolutionary genomics of Borrelia burgdorferi sensu lato: findings, hypotheses, and the rise of hybrids. Infect Genet Evol 27, 576–593. 10.1016/j.meegid.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Norgard MV, Brandt ME, Isaacs RD, Thompson PA, and Beutler B (1991). Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol 147, 1968–1974. [PubMed] [Google Scholar]
- Radolf JD, Bourell KW, Akins DR, Brusca JS, and Norgard MV (1994). Analysis of Borrelia burgdorferi membrane architecture by freeze-fracture electron microscopy. J Bacteriol 176, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, and Hu LT (2012). Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10, 87–99. 10.1038/nrmicro2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Tramont EC, and Salazar JC (2019). Syphilis (Treponema pallidum). In Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases, Mandell GL, Dolin R, and Blaser MJ, eds. (Philadelphia: Churchill Livingtone Elsevier; ), pp. 2865–2892. [Google Scholar]
- Radolf JD and Samuels DS (2021). Lyme Disease and Relapsing Fever Spirochetes: Genomics, Molecular Biology, Host Interactions and Disease Pathogenesis (Norfolk, UK: Caister Academic Press; ). 10.21775/9781913652616 [DOI] [Google Scholar]
- Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, et al. (2005). The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436, 573–577. 10.1038/nature03812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Alvarez AL, Roberts ED, Dennis VA, Lasater BL, Alvarez X, and Philipp MT (2003). Pathogenesis of Lyme neuroborreliosis: Borrelia burgdorferi lipoproteins induce both proliferation and apoptosis in rhesus monkey astrocytes. Eur J Immunol 33, 2539–2550. 10.1002/eji.200323872 [DOI] [PubMed] [Google Scholar]
- Ramesh G, Borda JT, Dufour J, Kaushal D, Ramamoorthy R, Lackner AA, and Philipp MT (2008). Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am J Pathol 173, 1415–1427. 10.2353/ajpath.2008.080483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauter C, and Hartung T (2005). Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl Environ Microbiol 71, 7203–7216. 10.1128/AEM.71.11.7203-7216.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego RO, Bestor A, Stefka J, and Rosa PA (2014). Population bottlenecks during the infectious cycle of the Lyme disease spirochete Borrelia burgdorferi. PLoS One 9, e101009. 10.1371/journal.pone.0101009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Spielman A, Komar N, and Matuschka FR (2000). Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg Infect Dis 6, 133–138. 10.3201/eid0602.000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, and Rosa R (2011). Lyme borreliosis in Europe. Euro Surveill 16. [PubMed] [Google Scholar]
- Roberts WC, Mullikin BA, Lathigra R, and Hanson MS (1998). Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect Immun 66, 5275–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, Kobayashi T, Higgins Y, Calkins H, and Melia MT (2015). Lyme carditis. Infect Dis Clin North Am 29, 255–268. 10.1016/j.idc.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Rollend L, Fish D, and Childs JE (2013). Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis 4, 46–51. 10.1016/j.ttbdis.2012.06.008 [DOI] [PubMed] [Google Scholar]
- Rose I, Yoshimizu MH, Bonilla DL, Fedorova N, Lane RS, and Padgett KA (2019). Phylogeography of Borrelia spirochetes in Ixodes pacificus and Ixodes spinipalpis ticks highlights differential acarological risk of tick-borne disease transmission in northern versus southern California. PLoS One 14, e0214726. 10.1371/journal.pone.0214726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, et al. (2018). Vital signs: trends in reported vectorborne disease cases - United States and territories, 2004–2016. MMWR Morb Mortal Wkly Rep 67, 496–501. 10.15585/mmwr.mm6717e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Mokracek A, Piskunova N, Ruzek D, Mallatova N, and Grubhoffer L (2008). Detection of Borrelia bissettii in cardiac valve tissue of a patient with endocarditis and aortic valve stenosis in the Czech Republic. 46, 3540–3543. 10.1128/jcm.01032-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Vancova M, Clark K, Grubhoffer L, and Oliver JH Jr. (2016). Isolation of live Borrelia burgdorferi sensu lato spirochaetes from patients with undefined disorders and symptoms not typical for Lyme borreliosis. Clin Microbiol Infect 22, 267 e269–215. 10.1016/j.cmi.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Rupprecht TA, Pfister HW, Angele B, Kastenbauer S, Wilske B, and Koedel U (2005). The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology 65, 448–450. 10.1212/01.wnl.0000171349.06645.79 [DOI] [PubMed] [Google Scholar]
- Ruyts SC, Ampoorter E, Coipan EC, Baeten L, Heylen D, Sprong H, Matthysen E, and Verheyen K (2016). Diversifying forest communities may change Lyme disease risk: extra dimension to the dilution effect in Europe. Parasitology 143, 1310–1319. 10.1017/S0031182016000688 [DOI] [PubMed] [Google Scholar]
- Ruzic-Sabljic E, and Cerar T (2017). Progress in the molecular diagnosis of Lyme disease. Expert Rev Mol Diagn 17, 19–30. 10.1080/14737159.2016.1246959 [DOI] [PubMed] [Google Scholar]
- Sadziene A, Barbour AG, Rosa PA, and Thomas DD (1993). An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect Immun 61, 3590–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar JC, Gerber MA, and Goff CW (1993). Long-term outcome of Lyme disease in children given early treatment. J Pediatr 122, 591–593. [DOI] [PubMed] [Google Scholar]
- Salazar JC, Pope CD, Sellati TJ, Feder HM Jr., Kiely TG, Dardick KR, Buckman RL, Moore MW, Caimano MJ, Pope JG, et al. (2003). Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol 171, 2660–2670. 10.4049/jimmunol.171.5.2660 [DOI] [PubMed] [Google Scholar]
- Sanchez E, Vannier E, Wormser GP, and Hu LT (2016). Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA 315, 1767–1777. 10.1001/jama.2016.2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffold N, Herkommer B, Kandolf R, and May AE (2015). Lyme carditis--diagnosis, treatment and prognosis. Dtsch Arztebl Int 112, 202–208. 10.3238/arztebl.2015.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid GP, Steigerwalt AG, Johnson SE, Barbour AG, Steere AC, Robinson IM, and Brenner DJ (1984). DNA characterization of the spirochete that causes Lyme disease. J Clin Microbiol 20, 155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidli J, Hunziker T, Moesli P, and Schaad UB (1988). Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J Infect Dis 158, 905–906. 10.1093/infdis/158.4.905 [DOI] [PubMed] [Google Scholar]
- Schoen RT (2020). Challenges in the diagnosis and treatment of Lyme disease. Curr Rheumatol Rep 22, 3. 10.1007/s11926-019-0857-2 [DOI] [PubMed] [Google Scholar]
- Schotthoefer AM, and Frost HM (2015). Ecology and epidemiology of Lyme borreliosis. Clin Lab Med 35, 723–743. 10.1016/j.cll.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Schroder NW, Eckert J, Stubs G, and Schumann RR (2008). Immune responses induced by spirochetal outer membrane lipoproteins and glycolipids. Immunobiology 213, 329–340. 10.1016/j.imbio.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, and Rosa PA (1995). Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A 92, 2909–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanz LE, Voordouw MJ, Brisson D, and Ostfeld RS (2011). Borrelia burgdorferi has minimal impact on the Lyme disease reservoir host Peromyscus leucopus. Vector Borne Zoonotic Dis 11, 117–124. 10.1089/vbz.2009.0215 [DOI] [PubMed] [Google Scholar]
- Schwartz AM, Hinckley AF, Mead PS, Hook SA, and Kugeler KJ (2017). Surveillance for Lyme Disease - United States, 2008–2015. MMWR Surveill Summ 66, 1–12. 10.15585/mmwr.ss6622a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz I, Fish D, and Daniels TJ (1997). Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. N Engl J Med 337, 49–50. 10.1056/NEJM199707033370111 [DOI] [PubMed] [Google Scholar]
- Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Wormser GP, Schriefer ME, and Luft BJ (1999). Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun 67, 3518–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza JC, and Suk JE (2018). Vector-borne diseases and climate change: a European perspective. FEMS Microbiol Lett 365. 10.1093/femsle/fnx244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Hook M, and Skare JT (2006). Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol 59, 1591–1601. 10.1111/j.1365-2958.2005.05042.x [DOI] [PubMed] [Google Scholar]
- Setubal JC, Reis M, Matsunaga J, and Haake DA (2006). Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152, 113–121. 10.1099/mic.0.28317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, Duray PH, Larson MG, Wright EA, Ginsburg KS, et al. (1994). The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med 121, 560–567. [DOI] [PubMed] [Google Scholar]
- Shah A, O’Horo JC, Wilson JW, Granger D, and Theel ES (2018). An unusual cluster of neuroinvasive Lyme disease cases presenting with Bannwarth syndrome in the Midwest United States. Open Forum Infect Dis 5, ofx276. 10.1093/ofid/ofx276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SS, Zaoutis TE, Turnquist J, Hodinka RL, and Coffin SE (2005). Early differentiation of Lyme from enteroviral meningitis. Pediatr Infect Dis J 24, 542–545. 10.1097/01.inf.0000164767.73746.6e [DOI] [PubMed] [Google Scholar]
- Shapiro ED, Baker PJ, and Wormser GP (2017). False and misleading information about Lyme disease. Am J Med 130, 771–772. 10.1016/j.amjmed.2017.01.030 [DOI] [PubMed] [Google Scholar]
- Sharareh N, Behler RP, Roome AB, Shepherd J, Garruto RM, and Sabounchi NS (2019). Risk factors of Lyme disease: an intersection of environmental ecology and systems science. Healthcare (Basel) 7. 10.3390/healthcare7020066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Brown AV, Matluck NE, Hu LT, and Lewis K (2015). Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob Agents Chemother 59, 4616–4624. 10.1128/AAC.00864-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DK, Tate AT, Schneider DS, Levashina EA, Kagan JC, Pal U, Fikrig E, and Pedra JHF (2018). Vector immunity and evolutionary ecology: the harmonious dissonance. Trends Immunol 39, 862–873. 10.1016/j.it.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Shin JJ, Strle K, McHugh G, Li X, Glickstein LJ, Drouin EE, and Steere AC (2010). Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis Rheum 62, 2127–2137. 10.1002/art.27468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih CM, Pollack RJ, Telford SR 3rd, and Spielman A (1992). Delayed dissemination of Lyme disease spirochetes from the site of deposition in the skin of mice. J Infect Dis 166, 827–831. [DOI] [PubMed] [Google Scholar]
- Shin JJ, Glickstein LJ, and Steere AC (2007). High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum 56, 1325–1335. 10.1002/art.22441 [DOI] [PubMed] [Google Scholar]
- Shin JJ, Strle K, Glickstein LJ, Luster AD, and Steere AC (2010). Borrelia burgdorferi stimulation of chemokine secretion by cells of monocyte lineage in patients with Lyme arthritis. Arthritis Res Ther 12, R168. 10.1186/ar3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal LH, Zahradnik JM, Lavin P, Patella SJ, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, et al. (1998). A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant outer-surface protein A Lyme disease vaccine study consortium. N Engl J Med 339, 216–222. 10.1056/NEJM199807233390402 [DOI] [PubMed] [Google Scholar]
- Silver E, Pass RH, Kaufman S, Hordof AJ, and Liberman L (2007). Complete heart block due to Lyme carditis in two pediatric patients and a review of the literature. Congenit Heart Dis 2, 338–341. 10.1111/j.1747-0803.2007.00122.x [DOI] [PubMed] [Google Scholar]
- Simo L, Kazimirova M, Richardson J, and Bonnet SI (2017). The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front Cell Infect Microbiol 7, 281. 10.3389/fcimb.2017.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, and Girschick HJ (2004). Lyme borreliosis: from infection to autoimmunity. Clin Microbiol Infect 10, 598–614. 10.1111/j.1469-0691.2004.00895.x [DOI] [PubMed] [Google Scholar]
- Skare JT, Shaw DK, Trzeciakowski JP, and Hyde JA (2016). in vivo imaging demonstrates that Borrelia burgdorferi ospC is uniquely expressed temporally and spatially throughout experimental infection. PLoS One 11, e0162501. 10.1371/journal.pone.0162501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, and Takkinen J (2006). Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Euro Surveill 11, E060622060621. [DOI] [PubMed] [Google Scholar]
- Smith RP, Schoen RT, Rahn DW, Sikand VK, Nowakowski J, Parenti DL, Holman MS, Persing DH, and Steere AC (2002). Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 136, 421–428. [DOI] [PubMed] [Google Scholar]
- Snydman DR, Schenkein DP, Berardi VP, Lastavica CC, and Pariser KM (1986). Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann Intern Med 104, 798–800. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, and Macaluso KR (2017). Microbial invasion vs. tick immune regulation. Front Cell Infect Microbiol 7, 390. 10.3389/fcimb.2017.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood SK, Salzman MB, Johnson BJ, Happ CM, Feig K, Carmody L, Rubin LG, Hilton E, and Piesman J (1997). Duration of tick attachment as a predictor of the risk of Lyme disease in an area in which Lyme disease is endemic. J Infect Dis 175, 996–999. [DOI] [PubMed] [Google Scholar]
- Sood SK (2006). What we have learned about Lyme borreliosis from studies in children. Wien Klin Wochenschr 118, 638–642. 10.1007/s00508-006-0689-8 [DOI] [PubMed] [Google Scholar]
- Sood SK (2015). Lyme disease in children. Infect Dis Clin North Am 29, 281–294. 10.1016/j.idc.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Spielman A, Clifford CM, Piesman J, and Corwin MD (1979). Human babesiosis on Nantucket Island, USA: description of the vector, Ixodes (Ixodes) dammini, n. sp. (Acarina: Ixodidae). J Med Entomol 15, 218–234. [DOI] [PubMed] [Google Scholar]
- Stafford KC 3rd, Cartter ML, Magnarelli LA, Ertel SH, and Mshar PA (1998). Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol 36, 1240–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek G, Wormser GP, Gray J, and Strle F (2012). Lyme borreliosis. Lancet 379, 461–473. 10.1016/S0140-6736(11)60103-7 [DOI] [PubMed] [Google Scholar]
- Stanek G, and Strle F (2018). Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol Rev 42, 233–258. 10.1093/femsre/fux047 [DOI] [PubMed] [Google Scholar]
- Steere AC, Malawista SE, Hardin JA, Ruddy S, Askenase W, and Andiman WA (1977a). Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med 86, 685–698. [DOI] [PubMed] [Google Scholar]
- Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, and Steele FM (1977b). Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum 20, 7–17. [DOI] [PubMed] [Google Scholar]
- Steere AC, Gibofsky A, Patarroyo ME, Winchester RJ, Hardin JA, and Malawista SE (1979). Chronic Lyme arthritis. Clinical and immunogenetic differentiation from rheumatoid arthritis. Ann Intern Med 90, 896–901. [DOI] [PubMed] [Google Scholar]
- Steere AC, and Malawista SE (1979). Cases of Lyme disease in the United States: locations correlated with distribution of Ixodes dammini. Ann Intern Med 91, 730–733. [DOI] [PubMed] [Google Scholar]
- Steere AC, Batsford WP, Weinberg M, Alexander J, Berger HJ, Wolfson S, and Malawista SE (1980). Lyme carditis: cardiac abnormalities of Lyme disease. Ann Intern Med 93, 8–16. [DOI] [PubMed] [Google Scholar]
- Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, and Malawista SE (1983a). The spirochetal etiology of Lyme disease. N Engl J Med 308, 733–740. 10.1056/NEJM198303313081301 [DOI] [PubMed] [Google Scholar]
- Steere AC, Pachner AR, and Malawista SE (1983b). Neurologic abnormalities of Lyme disease: successful treatment with high-dose intravenous penicillin. Ann Intern Med 99, 767–772. [DOI] [PubMed] [Google Scholar]
- Steere AC, Schoen RT, and Taylor E (1987). The clinical evolution of Lyme arthritis. Ann Intern Med 107, 725–731. [DOI] [PubMed] [Google Scholar]
- Steere AC, Dwyer E, and Winchester R (1990). Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med 323, 219–223. 10.1056/NEJM199007263230402 [DOI] [PubMed] [Google Scholar]
- Steere AC (1997). Diagnosis and treatment of Lyme arthritis. Med Clin North Am 81, 179–194. 10.1016/s0025-7125(05)70510-1 [DOI] [PubMed] [Google Scholar]
- Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Nowakowski J, Schmid CH, Laukamp S, Buscarino C, et al. (1998). Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med 339, 209–215. 10.1056/NEJM199807233390401 [DOI] [PubMed] [Google Scholar]
- Steere AC (2001). Lyme disease. N Engl J Med 345, 115–125. 10.1056/NEJM200107123450207 [DOI] [PubMed] [Google Scholar]
- Steere AC, and Sikand VK (2003). The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med 348, 2472–2474. 10.1056/NEJM200306123482423 [DOI] [PubMed] [Google Scholar]
- Steere AC, Sikand VK, Schoen RT, and Nowakowski J (2003). Asymptomatic infection with Borrelia burgdorferi. Clin Infect Dis 37, 528–532. 10.1086/376914 [DOI] [PubMed] [Google Scholar]
- Steere AC, Coburn J, and Glickstein L (2004). The emergence of Lyme disease. J Clin Invest 113, 1093–1101. 10.1172/JCI21681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, and Angelis SM (2006). Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum 54, 3079–3086. 10.1002/art.22131 [DOI] [PubMed] [Google Scholar]
- Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, and Baxter-Lowe LA (2006). Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med 203, 961–971. 10.1084/jem.20052471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, and Mead PS (2016). Lyme borreliosis. Nat Rev Dis Primers 2, 16090. 10.1038/nrdp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC (2020). Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 10.1172/JCI138062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, and Seshu J (2018). Regulation of gene and protein expression in the Lyme disease spirochete. Curr Top Microbiol Immunol 415, 83–112. 10.1007/82_2017_49 [DOI] [PubMed] [Google Scholar]
- Stevenson B, Fingerle V, Wormser GP, and Margos G (2019). Public health and patient safety concerns merit retention of Lyme borreliosis-associated spirochetes within the genus Borrelia, and rejection of the genus novum Borreliella. Ticks Tick Borne Dis 10, 1–4. 10.1016/j.ttbdis.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Stewart PE, Byram R, Grimm D, Tilly K, and Rosa PA (2005). The plasmids of Borrelia burgdorferi: essential genetic elements of a pathogen. Plasmid 53, 1–13. 10.1016/j.plasmid.2004.10.006 [DOI] [PubMed] [Google Scholar]
- Stewart PE, Wang X, Bueschel DM, Clifton DR, Grimm D, Tilly K, Carroll JA, Weis JJ, and Rosa PA (2006). Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect Immun 74, 3547–3553. 10.1128/IAI.00158-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BL, and Brissette CA (2017). Host immune evasion by Lyme and relapsing fever Borreliae: findings to lead future studies for Borrelia miyamotoi. Front Immunol 8, 12. 10.3389/fimmu.2017.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BL, Tourand Y, and Brissette CA (2017). Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis 17, 619–629. 10.1089/vbz.2017.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker RB, and Johnson L (2011). Lyme disease: the next decade. Infect Drug Resist 4, 1–9. 10.2147/IDR.S15653idr-4-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker RB, and Johnson L (2014). Lyme disease: call for a “Manhattan Project” to combat the epidemic. PLoS Pathog 10, e1003796. 10.1371/journal.ppat.1003796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle F, Pleterski-Rigler D, Cimperman J, Pejovnik-Pustinek A, Ruzic E, and Stanek G (1992). Solitary borrelial lymphocytoma: Report of 36 cases. Infection 20, 20`–206. 10.1007/BF02033059 [DOI] [PubMed] [Google Scholar]
- Strle F, Nelson JA, Ruzic-Sabljic E, Cimperman J, Maraspin V, Lotric-Furlan S, Cheng Y, Picken MM, Trenholme GM, and Picken RN (1996). European Lyme borreliosis: 231 culture-confirmed cases involving patients with erythema migrans. Clin Infect Dis 23, 61–65. [DOI] [PubMed] [Google Scholar]
- Strle F, Nadelman RB, Cimperman J, Nowakowski J, Picken RN, Schwartz I, Maraspin V, Aguero-Rosenfeld ME, Varde S, Lotric-Furlan S, et al. (1999). Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York State and by Borrelia afzelii in Slovenia. Ann Intern Med 130, 32–36. [DOI] [PubMed] [Google Scholar]
- Strle F, Ruzic-Sabljic E, Logar M, Maraspin V, Lotric-Furlan S, Cimperman J, Ogrinc K, Stupica D, Nadelman RB, Nowakowski J, et al. (2011a). Comparison of erythema migrans caused by Borrelia burgdorferi and Borrelia garinii. Vector Borne Zoonotic Dis 11, 1253–1258. 10.1089/vbz.2010.0230 [DOI] [PubMed] [Google Scholar]
- Strle K, Drouin EE, Shen S, El Khoury J, McHugh G, Ruzic-Sabljic E, Strle F, and Steere AC (2009). Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis 200, 1936–1943. 10.1086/648091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Jones KL, Drouin EE, Li X, and Steere AC (2011b). Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol 178, 2726–2739. 10.1016/j.ajpath.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Shin JJ, Glickstein LJ, and Steere AC (2012). Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum 64, 1497–1507. 10.1002/art.34383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Stupica D, Drouin EE, Steere AC, and Strle F (2014). Elevated levels of IL-23 in a subset of patients with post-Lyme disease symptoms following erythema migrans. Clin Infect Dis 58, 372–380. 10.1093/cid/cit735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Sulka KB, Pianta A, Crowley JT, Arvikar SL, Anselmo A, Sadreyev R, and Steere AC (2017). T-Helper 17 cell cytokine responses in Lyme disease correlate with Borrelia burgdorferi antibodies during early infection and with autoantibodies late in the illness in patients with antibiotic-refractory Lyme arthritis. Clin Infect Dis 64, 930–938. 10.1093/cid/cix002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, and Strle F (2020). Posttreatment symptoms in Lyme borreliosis. Clin Infect Dis. 10.1093/cid/ciz1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad M, Hönig V, Růžek D, Grubhoffer L, and Rego ROM (2017). Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Applied and Environmental Microbiology 83. 10.1128/aem.00609-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobino BA, Williams CL, Abid S, Chalson R, and Spierling P (1993). Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol 169, 367–374. 10.1016/0002-9378(93)90088-z [DOI] [PubMed] [Google Scholar]
- Stupica D, Lusa L, Maraspin V, Bogovic P, Vidmar D, O’Rourke M, Traweger A, Livey I, and Strle F (2015). Correlation of culture positivity, PCR positivity, and burden of Borrelia burgdorferi sensu lato in skin samples of erythema migrans patients with clinical findings. PLoS One 10, e0136600. 10.1371/journal.pone.0136600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupica D, Maraspin V, Bogovic P, Ogrinc K, Blagus R, Cerar T, and Strle F (2018a). Comparison of clinical course and treatment outcome for patients with early disseminated or early localized Lyme borreliosis. JAMA Dermatol 154, 1050–1056. 10.1001/jamadermatol.2018.2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupica D, Veluscek M, Blagus R, Bogovic P, Rojko T, Cerar T, and Strle F (2018b). Oral doxycycline versus intravenous ceftriaxone for treatment of multiple erythema migrans: an open-label alternate-treatment observational trial. J Antimicrob Chemother 73, 1352–1358. 10.1093/jac/dkx534 [DOI] [PubMed] [Google Scholar]
- Sykes RA, and Makiello P (2017). An estimate of Lyme borreliosis incidence in Western Europe. J Public Health (Oxf) 39, 74–81. 10.1093/pubmed/fdw017 [DOI] [PubMed] [Google Scholar]
- Szczepanski A, Furie MB, Benach JL, Lane BP, and Fleit HB (1990). Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest 85, 1637–1647. 10.1172/JCI114615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer IS, Taylor E, and Steere AC (1991). The long-term course of Lyme arthritis in children. N Engl J Med 325, 159–163. 10.1056/NEJM199107183250304 [DOI] [PubMed] [Google Scholar]
- Takayama K, Rothenberg RJ, and Barbour AG (1987). Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun 55, 2311–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavora F, Burke A, Li L, Franks TJ, and Virmani R (2008). Postmortem confirmation of Lyme carditis with polymerase chain reaction. Cardiovasc Pathol 17, 103–107. 10.1016/j.carpath.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Telford SR 3rd, Hu LT, and Marques A (2014). Is there a place for xenodiagnosis in the clinic? Expert Rev Anti Infect Ther 12, 1307–1310. 10.1586/14787210.2014.966084 [DOI] [PubMed] [Google Scholar]
- Theophilus PA, Victoria MJ, Socarras KM, Filush KR, Gupta K, Luecke DF, and Sapi E (2015). Effectiveness of Stevia rebaudiana whole leaf extract against the various morphological forms of Borrelia Burgdorferi in vitro. Eur J Microbiol Immunol (Bp) 5, 268–280. 10.1556/1886.2015.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijsse-Klasen E, Pandak N, Hengeveld P, Takumi K, Koopmans MP, and Sprong H (2013). Ability to cause erythema migrans differs between Borrelia burgdorferi sensu lato isolates. Parasit Vectors 6, 23. 10.1186/1756-3305-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, et al. (2006). Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74, 3554–3564. 10.1128/IAI.01950-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Rosa PA, and Stewart PE (2008). Biology of infection with Borrelia burgdorferi. Infect Dis Clin North Am 22, 217–234, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmaraju VA, Theophilus PA, Balasubramanian K, Shakih S, Luecke DF, and Sapi E (2015). Biofilm formation by Borrelia burgdorferi sensu lato. FEMS Microbiol Lett 362, fnv120. 10.1093/femsle/fnv120 [DOI] [PubMed] [Google Scholar]
- Toledo A, Crowley JT, Coleman JL, LaRocca TJ, Chiantia S, London E, and Benach JL (2014). Selective association of outer surface lipoproteins with the lipid rafts of Borrelia burgdorferi. MBio 5, e00899–00814. 10.1128/mBio.00899-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas-Cortazar J, Martin-Ruiz I, Barriales D, Pascual-Itoiz MA, de Juan VG, Caro-Maldonado A, Merino N, Marina A, Blanco FJ, Flores JM, et al. (2017). The immunosuppressive effect of the tick protein, Salp15, is long-lasting and persists in a murine model of hematopoietic transplant. Sci Rep 7, 10740. 10.1038/s41598-017-11354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy KE, and Baumgarth N (2017). Borrelia burgdorferi manipulates innate and adaptive immunity to establish persistence in rodent reservoir hosts. Front Immunol 8, 116. 10.3389/fimmu.2017.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayes KP, Savage K, and Studdiford JS (2018). Annular lesions: diagnosis and treatment. Am Fam Physician 98, 283–291. [PubMed] [Google Scholar]
- Troy EB, Lin T, Gao L, Lazinski DW, Camilli A, Norris SJ, and Hu LT (2013). Understanding barriers to Borrelia burgdorferi dissemination during infection using massively parallel sequencing. Infect Immun 81, 2347–2357. 10.1128/IAI.00266-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufts DM, Hart TM, Chen GF, Kolokotronis SO, Diuk-Wasser MA, and Lin YP (2019). Outer surface protein polymorphisms linked to host-spirochete association in Lyme borreliae. Mol Microbiol 111, 868–882. 10.1111/mmi.14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler S, Tyson S, Dibernardo A, Drebot M, Feil EJ, Graham M, Knox NC, Lindsay LR, Margos G, Mechai S, et al. (2018). Whole genome sequencing and phylogenetic analysis of strains of the agent of Lyme disease Borrelia burgdorferi from Canadian emergence zones. Sci Rep 8, 10552. 10.1038/s41598-018-28908-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde M, Ajamian M, Li X, Wormser GP, Marques A, and Alaedini A (2016). Expression of C-reactive protein and serum amyloid A in early to late manifestations of Lyme disease. Clin Infect Dis 63, 1399–1404. 10.1093/cid/ciw599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde M, Indart A, Fallon BA, Wormser GP, Marques AR, Vernon SD, and Alaedini A (2018). C-reactive protein response in patients with post-treatment Lyme disease symptoms versus those with myalgic encephalomyelitis/chronic fatigue syndrome. Clin Infect Dis 67, 1309–1310. 10.1093/cid/ciy299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam AP, Kuiper H, Vos K, Widjojokusumo A, de Jongh BM, Spanjaard L, Ramselaar AC, Kramer MD, and Dankert J (1993). Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis 17, 708–717. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard CC, Hofhuis A, Simoes M, Rood E, van Pelt W, Zeller H, and Van Bortel W (2017). Surveillance perspective on Lyme borreliosis across the European Union and European Economic Area. Euro Surveill 22. 10.2807/1560-7917.ES.2017.22.27.30569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde MR (1991). Lyme carditis: clinical characteristics of 105 cases. Scand J Infect Dis Suppl 77, 81–84. [PubMed] [Google Scholar]
- VanAcker MC, Little EAH, Molaei G, Bajwa WI, and Diuk-Wasser MA (2019). Enhancement of risk for Lyme disease by landscape connectivity, New York, New York, USA. Emerg Infect Dis 25, 1136–1143. 10.3201/eid2506.181741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz A, Glickstein L, Field JA, McHugh G, Sikand VK, Damle N, and Steere AC (2001). Cellular and humoral immune responses to Borrelia burgdorferi antigens in patients with culture-positive early Lyme disease. Infect Immun 69, 7437–7444. 10.1128/IAI.69.12.7437-7444.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey TB, Castellanos M, and Chaconas G (2018). Antigenic Variation in the Lyme spirochete: insights into recombinational wwitching with a suggested role for error-prone repair. Cell Rep 23, 2595–2605. 10.1016/j.celrep.2018.04.117 [DOI] [PubMed] [Google Scholar]
- Vig DK, and Wolgemuth CW (2014). Spatiotemporal evolution of erythema migrans, the hallmark rash of Lyme disease. Biophys J 106, 763–768. 10.1016/j.bpj.2013.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voordouw MJ, Lachish S, and Dolan MC (2015). The Lyme disease pathogen has no effect on the survival of its rodent reservoir host. PLoS One 10, e0118265. 10.1371/journal.pone.0118265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vudattu NK, Strle K, Steere AC, and Drouin EE (2013). Dysregulation of CD4+CD25(high) T cells in the synovial fluid of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheum 65, 1643–1653. 10.1002/art.37910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HB, Canham CD, Fonseca DM, Brisson D, Morin PJ, Smouse PE, and Ostfeld RS (2014). Occurrence and transmission efficiencies of Borrelia burgdorferi ospC types in avian and mammalian wildlife. Infect Genet Evol 27, 594–600. 10.1016/j.meegid.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EM, Borenstein LA, Blanco DR, Miller JN, and Lovett MA (1991). Analysis of outer membrane ultrastructure of pathogenic Treponema and Borrelia species by freeze-fracture electron microscopy. J Bacteriol 173, 5585–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, van Dam AP, Schwartz I, and Dankert J (1999a). Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin Microbiol Rev 12, 633–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ojaimi C, Iyer R, Saksenberg V, McClain SA, Wormser GP, and Schwartz I (2001). Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect Immun 69, 4303–4312. 10.1128/IAI.69.7.4303-4312.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ojaimi C, Wu H, Saksenberg V, Iyer R, Liveris D, McClain SA, Wormser GP, and Schwartz I (2002). Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis 186, 782–791. 10.1086/343043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, and Luft BJ (1999b). Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151, 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening EH, Parveen N, Trzeciakowski JP, Leong JM, Hook M, and Skare JT (2008). Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect Immun 76, 5694–5705. 10.1128/IAI.00690-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzner E, McKenna D, Nowakowski J, Scavarda C, Dornbush R, Bittker S, Cooper D, Nadelman RB, Visintainer P, Schwartz I, et al. (2015). Long-term assessment of post-treatment symptoms in patients with culture-confirmed early Lyme disease. Clin Infect Dis 61, 1800–1806. 10.1093/cid/civ735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CM, Coleman JL, Habicht GS, and Benach JL (1989). Adult Ixodes dammini on rabbits: development of acute inflammation in the skin and immune responses to salivary gland, midgut, and spirochetal components. J Infect Dis 159, 265–273. [DOI] [PubMed] [Google Scholar]
- Wheeler CM, Garcia Monco JC, Benach JL, Golightly MG, Habicht GS, and Steere AC (1993). Nonprotein antigens of Borrelia burgdorferi. J Infect Dis 167, 665–674. [DOI] [PubMed] [Google Scholar]
- Wick RR, Judd LM, Gorrie CL, and Holt KE (2017). Completing bacterial genome assemblies with multiplex MinION sequencing. Microb Genom 3, e000132. 10.1099/mgen.0.000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson P, Fryland L, Lindblom P, Sjowall J, Ahlm C, Berglund J, Haglund M, Henningsson AJ, Nolskog P, Nordberg M, et al. (2016). A prospective study on the incidence of Borrelia burgdorferi sensu lato infection after a tick bite in Sweden and on the Aland Islands, Finland (2008–2009). Ticks Tick Borne Dis 7, 71–79. 10.1016/j.ttbdis.2015.08.009 [DOI] [PubMed] [Google Scholar]
- Willyard C (2014). Resurrecting the ‘yuppie vaccine’. Nat Med 20, 698–701. 10.1038/nm0714-698 [DOI] [PubMed] [Google Scholar]
- Wilske B, Fingerle V, and Schulte-Spechtel U (2007). Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol Med Microbiol 49, 13–21. 10.1111/j.1574-695X.2006.00139.x [DOI] [PubMed] [Google Scholar]
- Wormser GP, Liveris D, Nowakowski J, Nadelman RB, Cavaliere LF, McKenna D, Holmgren D, and Schwartz I (1999). Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis 180, 720–725. 10.1086/314922 [DOI] [PubMed] [Google Scholar]
- Wormser GP, Bittker S, Cooper D, Nowakowski J, Nadelman RB, and Pavia C (2001a). Yield of large-volume blood cultures in patients with early Lyme disease. J Infect Dis 184, 1070–1072. 10.1086/323424 [DOI] [PubMed] [Google Scholar]
- Wormser GP, Nadelman RB, Nowakowski J, and Schwartz I (2001b). Asymptomatic Borrelia burgdorferi infection. Med Hypotheses 57, 435–438. 10.1054/mehy.2001.1338 [DOI] [PubMed] [Google Scholar]
- Wormser GP, McKenna D, Carlin J, Nadelman RB, Cavaliere LF, Holmgren D, Byrne DW, and Nowakowski J (2005). Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 142, 751–755. [DOI] [PubMed] [Google Scholar]
- Wormser GP (2006). Clinical practice. Early Lyme disease. N Engl J Med 354, 2794–2801. 10.1056/NEJMcp061181 [DOI] [PubMed] [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, et al. (2006). The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43, 1089–1134. 10.1086/508667 [DOI] [PubMed] [Google Scholar]
- Wormser GP, Brisson D, Liveris D, Hanincova K, Sandigursky S, Nowakowski J, Nadelman RB, Ludin S, and Schwartz I (2008a). Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis 198, 1358–1364. 10.1086/592279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP, Liveris D, Hanincova K, Brisson D, Ludin S, Stracuzzi VJ, Embers ME, Philipp MT, Levin A, Aguero-Rosenfeld M, et al. (2008b). Effect of Borrelia burgdorferi genotype on the sensitivity of C6 and 2-tier testing in North American patients with culture-confirmed Lyme disease. Clin Infect Dis 47, 910–914. 10.1086/591529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP, Schriefer M, Aguero-Rosenfeld ME, Levin A, Steere AC, Nadelman RB, Nowakowski J, Marques A, Johnson BJ, and Dumler JS (2013). Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis 75, 9–15. 10.1016/j.diagmicrobio.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP, Weitzner E, McKenna D, Nadelman RB, Scavarda C, Molla I, Dornbush R, Visintainer P, and Nowakowski J (2015). Long-term assessment of health-related quality of life in patients with culture-confirmed early Lyme disease. Clin Infect Dis 61, 244–247. 10.1093/cid/civ277 [DOI] [PubMed] [Google Scholar]
- Wormser GP, McKenna D, Karmen CL, Shaffer KD, Silverman JH, Nowakowski J, Scavarda C, Shapiro ED, and Visintainer P (2020). Prospective evaluation of the frequency and severity of symptoms in Lyme disease patients with erythema migrans compared with matched controls at baseline, 6 months, and 12 months. Clin Infect Dis. 10.1093/cid/ciz1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wywial E, Haven J, Casjens SR, Hernandez YA, Singh S, Mongodin EF, Fraser-Liggett CM, Luft BJ, Schutzer SE, and Qiu WG (2009). Fast, adaptive evolution at a bacterial host-resistance locus: the PFam54 gene array in Borrelia burgdorferi. Gene 445, 26–37. 10.1016/j.gene.2009.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Zhi H, Garrigues RJ, Keightley A, Garcia BL, and Skare JT (2019). Structural determination of the complement inhibitory domain of Borrelia burgdorferi BBK32 provides insight into classical pathway complement evasion by Lyme disease spirochetes. PLoS Pathog 15, e1007659. 10.1371/journal.ppat.1007659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Caimano MJ, Lin T, He M, Radolf JD, Norris SJ, Gherardini F, Wolfe AJ, and Yang XF (2010). Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog 6, e1001104. 10.1371/journal.ppat.1001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Kodner C, Coleman L, and Johnson RC (1996). Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect Immun 64, 3870–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Alani SM, and Norgard MV (2003). The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci U S A 100, 11001–11006. 10.1073/pnas.1834315100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Pal U, Alani SM, Fikrig E, and Norgard MV (2004). Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med 199, 641–648. 10.1084/jem.20031960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal A, Hanafi A, Nadkarni N, Mubasher M, Lingutla D, and Hoefen R (2019). Lyme carditis presenting as atrial fibrillation. BMJ Case Rep 12. 10.1136/bcr-2018-228975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano MC, Beklemisheva AA, Bryksin AV, Newman SA, and Cabello FC (2004). Borrelia burgdorferi binds to, invades, and colonizes native type I collagen lattices. Infect Immun 72, 3138–3146. 10.1128/IAI.72.6.3138-3146.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannoli S, Fantini M, Semprini S, Marchini B, Ceccarelli B, Sparacino M, Schiavone P, Belgrano A, Ruscio M, Gobbetti M, et al. (2020). Multicenter evaluation of the C6 Lyme ELISA kit for the diagnosis of Lyme disease. Microorganisms 8, 457. 10.3390/microorganisms8030457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi H, Xie J, and Skare JT (2018). The classical complement pathway Is required to control Borrelia burgdorferi levels during experimental infection. Front Immunol 9, 959. 10.3389/fimmu.2018.00959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Qin S, Sun M, Tang L, Yan X, Kim TK, Caballero J, Glusman G, Brunkow ME, Soloski MJ, et al. (2020). Measurement of organ-specific and acute-phase blood protein levels in early Lyme disease. J Proteome Res 19, 346–359. 10.1021/acs.jproteome.9b00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckert WR (2014). Secretion of bacterial lipoproteins: through the cytoplasmic membrane, the periplasm and beyond. Biochim Biophys Acta 1843, 1509–1516. 10.1016/j.bbamcr.2014.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckert WR (2019). Protein secretion in spirochetes. Microbiol Spectr 7. 10.1128/microbiolspec.PSIB-0026-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]


