Abstract
Introduction
Borrelia burgdorferi sensu lato (sl) is the causative agent of Lyme borreliosis. Currently there is no human vaccine against Lyme borreliosis, and most research focuses on recombinant protein vaccines. DNA tattoo vaccination with B. afzelii strain PKo OspC in mice has proven to be fully protective against B. afzelii syringe challenge and induces a favorable humoral immunity compared to recombinant protein vaccination. Alternatively, several recombinant protein vaccines based on tick proteins have shown promising effect in tick-bite infection models. In this study, we evaluated the efficacy of DNA vaccines against Borrelia OspC or tick antigens in a tick-bite infection model.
Method
We vaccinated C3H/HeN mice with OspC using a codon-optimized DNA vaccine or with recombinant protein. We challenged these mice with B. burgdorferi sensu stricto (ss)-infected Ixodes scapularis nymphs. Subsequently, we vaccinated C3H/HeN mice with DNA vaccines coding for tick proteins for which recombinant protein vaccines have previously resulted in interference with tick feeding and/or Borrelia transmission: Salp15, tHRF, TSLPI, and Tix-5. These mice were also challenged with B. burgdorferi ss infected Ixodes scapularis nymphs.
Results
DNA tattoo and recombinant OspC vaccination both induced total IgG responses. Borrelia cultures and DNA loads of skin and bladder remained negative in the mice vaccinated with OspC DNA vaccination, except for one culture. DNA vaccines against tick antigens Salp15 and Tix-5 induced IgG responses, while those against tHRF and TSLPI barely induced any IgG response. In addition, Borrelia cultures, and DNA loads from mice tattooed with DNA vaccines against tick proteins TSLPI, Salp15, tHRF, and Tix-5 were all positive.
Conclusion
A DNA tattoo vaccine against OspC induced high specific IgG titers and provided near total protection against B. burgdorferi ss infection by tick challenge. In contrast, DNA tattoo vaccines against tick proteins TSLPI, Salp15, tHRF, and Tix-5 induced low to moderate IgG titers and did not provide protection. Therefore, DNA tattoo vaccination does not seem a suitable vaccine strategy to identify, or screen for, tick antigens for anti-tick vaccines. However, DNA tattoo vaccination is a straightforward and effective vaccination platform to assess novel B. burgdorferi sl antigen candidates in a relevant tick challenge model.
Keywords: lyme disease, borrelia, DNA tattoo, DNA vaccination, OspC, tick proteins
Introduction
Lyme borreliosis is the most common vector-borne disease in the Northern hemisphere and is caused by the spirochete Borrelia burgdorferi sl, which is transmitted by Ixodes ticks. Vaccination would be an effective way to prevent Lyme disease. Currently there is no human vaccine available. Vaccines to prevent Borrelia burgdorferi sl infection could work in two ways: killing the pathogen to stop infection or targeting the vector to prevent successful transmission. Research therefore focuses on either protective antigens derived from the pathogen, B. burgdorferi sl, or from the vector, Ixodes ticks (1). When focusing on possible protective antigens from Borrelia, the most promising candidates in human vaccine studies are the outer surface proteins. Especially OspA, which is primarily expressed by Borrelia in unfed ticks, has been widely studied and was the primary component of the withdrawn human LYMErix™ vaccine (2–6). During transmission from tick to host, the Borrelia spirochete downregulates OspA and upregulates outer surface protein C, which is necessary to facilitate migration to the tick salivary glands and also plays a role in spirochete infection of the mammalian host. OspC was also shown to be an effective vaccine target, but has a high heterogenicity among different B. burgdorferi sl species and strains (7, 8).
In an alternative approach where the tick vector is targeted, tick saliva could play a pivotal role. Tick saliva contains several proteins that facilitate transmission and survival of tick-borne pathogens by using anti-inflammatory, anti-coagulant and immunosuppressive abilities (9, 10). Borrelia burgdorferi sl exploits tick salivary gland proteins to facilitate their transmission from tick to host, and vice versa to increase their chances of survival within the tick (11, 12). For example, OspC binds to Ixodes scapularis salivary protein Salp15 which protects the spirochete from antibody-mediated killing (12–14). In addition, Salp15 also has immunosuppressive properties in inhibiting CD4+ T cell and dendritic cell activation (15, 16). Interestingly, a vaccine directed against Salp15 has been shown to partially block B. burgdorferi ss infection (14, 17). Dai et al. also characterized tick histamine release factor, present in tick saliva and important to tick feeding (18). They showed significantly impaired tick feeding on mice when tHRF was silenced by RNA interference. Tick feeding and transmission of B. burgdorferi ss was also significantly diminished in tHRF immunized mice (18). Schuijt et al. identified Tick Salivary Lectin Pathway Inhibitor (TSLPI), an I. scapularis salivary protein, which was shown to impair complement-mediated killing of B. burgdorferi. B. burgdorferi transmission was impaired in mice that were injected with TSLPI rabbit antiserum (19). TIX-5 (tick inhibitor of factor Xa toward factor V) is another tick protein, with anticoagulant activity, that has been investigated in vaccination studies (20, 21). Adult I. scapularis tick engorgement weights from rTIX-5–immune rabbits were dramatically reduced compared to control rabbits. The effect on B. burgdorferi ss transmission has not been assessed. Thus, multiple promising tick salivary gland proteins have been identified and investigated as vaccine candidates to prevent tick feeding and/or transmission of B. burgdorferi sl from tick to host. It has been described in literature that these salivary gland antigens are expressed in infected ticks; Salp15 and TSLPI are even upregulated in infected ticks (13, 19). In addition, transcriptomic data from B. afzelii infected I. ricinus nymphs show that all these antigens are also expressed in infected I. ricinus salivary glands, indicating that the expression of these specific antigens appears particularly conserved even cross-species (22).
In addition to B. burgdorferi sl, Ixodes ticks also transmit other tick-borne diseases that can cause human infection such as Babesiosis and Anaplasmosis and several Flaviviruses. An anti-tick vaccine that would prevent the tick from feeding on a host could have the advantage of being able to provide protection against multiple tick-borne diseases (23).
Most research on new Lyme vaccines focuses on recombinant proteins, but DNA vaccination constitutes an alternative vaccination platform (24). DNA vaccines are easy to produce, highly stable and induce both humoral and cellular immune responses (25). While no human genomic vaccines targeting infectious diseases are currently on the market, the COVID pandemic might establish its mainstream acceptance in infectious diseases, as several genomic vaccines are currently being developed (26–28). A previous study by Wagemakers et al. has shown that DNA vaccination by tattoo with OspC from B. afzelii strain PKo as a model antigen was fully protective against B. afzelii syringe challenge in mice and induced favorable humoral immune responses compared to recombinant protein vaccination (29). In the current study, we have used OspC from B. burgdorferi ss strain N40, both as recombinant as well as DNA vaccine to evaluate whether DNA vaccination can also protect against Borrelia infection through tick challenge, more closely resembling the natural situation. The other goal of this study was to assess DNA vaccination as a modality to induce protective immune responses against tick antigens. We assessed tick salivary gland proteins TSLPI, Salp15, tHRF, and TIX-5 to test DNA vaccination as an easy screening vaccination platform for novel future candidates as an anti-tick vaccine. These described tick antigens were selected since they are known to be able to interfere with tick feeding and/or B. burgdorferi transmission when investigated in conventional vaccination approaches.
Materials and Methods
Ethics Statement
All experiments were reviewed and approved by the Animal Research Ethics Committee of the Academic Medical Center, Amsterdam, The Netherlands (protocol 208AI). Experiments have been conducted according to European and national guidelines.
Recombinant OspC Protein Generation
The OspC gene was amplified from genomic DNA from Borrelia burgdorferi ss strain N40 DNA and was cloned into pET-21b (Invitrogen), produced in E. coli and purified using Ni-NTA as detailed elsewhere (12).
Purity was checked using SDS-PAGE, and protein concentrations were measured using a Bradford assay.
Generation of DNA Vaccines
The DNA vaccines were designed as described before in Wagemakers et al. (29). From the B. burgdorferi N40 OspC gene sequence (NCBI reference DQ437463.1 and the respective tick salivary gland genes Salp15 (NCBI reference AAK97817.1), tHRF (NCBI reference DQ066335), TSLPI (NCBI reference AEE89466.1), and TIX-5 (NCBI reference AEE89467). The signal peptide (predicted by SignalP 4.0 web-based software, CBS, Lyngby, Denmark) was replaced with the human tissue plasminogen activator (hTPA) signal sequence (genbank AAA61213.1) (30). The resulting sequence was codon-optimized to mouse tRNA usage with Java Codon Adaptation tool (Braunschweig, Germany) (31). At the 5′ end a BamH1 and a Kozak sequence were added, and at the 3′ end a sequence encoding a double stop codon and a Xho1 were added. The insert was synthesized (BaseClear, Leiden, The Netherlands) and ligated into a BamH1/Xho1 restricted empty pVAX vector (Invitrogen, Carlsbad, CA, USA). The plasmid was amplified using a Nucleobond Xtra EF kit (Macherey-Nagel, Düren, Germany) and resuspended in DNase free water.
Generation of I. scapularis Nymphs with B. burgdorferi Strain N40
Low passage B. burgdorferi ss strain N40 spirochetes were cultured in MKP medium and counted by using a Petroff-Hausser counting chamber and dark-field microscopy. 1x106 spirochetes in 200 µl was injected subcutaneously between the shoulders of four six-to-eight-weeks-old female C3H/HeN mice, purchased from Charles River. Mice were checked for Borrelia infection positivity by qPCR after 14 days. Once infection was confirmed, approximately 500 I. scapularis larvae (kindly provided by Center for Disease Control and Prevention, BEI Resources, NIAID, NIH: Ixodes scapularis (Live), NR-44116) were placed on each mouse. In the following 6 days, the fully fed larvae that had fallen off the mice were collected and were allowed to mold into the nymphal stage during the next 6–8 weeks. Ticks were housed in an incubator (Panasonic) at room temperature and at a constant relative humidity of 90%. Once molted, nymphal infection rates were assessed by qPCR. To establish tick infection rate, DNA was extracted from 10 ticks using the Qiagen Blood and Tissue kit (Qiagen, Venlo, The Netherlands). Quantitative (q)PCR was used to quantify B. burgdorferi ss DNA in mouse tissues and was performed according to previously described protocol (29) and also in the section below “Borrelia detection and quantification by culture and qPCR”. Infection rate was > 90%.
Vaccination Experiments
Six-to-eight-weeks-old female C3H/HeN mice were purchased from Charles River. The vaccination experiment was carried out as previously described (29). Eight mice per experimental group were vaccinated at t=0, t=14, and t=28 days and sera were collected at each time point. For the recombinant OspC vaccine 10 μg protein was emulsified with complete Freund’s adjuvant at t=0 and 5 μg in incomplete Freund’s adjuvant at t=14 and t=28 days. All vaccinations were administered subcutaneously. For the DNA vaccines and the negative control, hair was removed from the mouse abdomen using hair removal cream. Using a Cheyenne Hawk tattoo machine carrying a Cheyenne 13-magnum tattoo needle (both MT.DERM, Berlin, Germany) 20 μg of the DNA vaccines was tattooed 0.5–1mm into the abdominal skin of the mice for 45 s at 100 Hz under isofluorane anesthesia. Two weeks after the third vaccination, at t=42, all mice were challenged with seven Ixodes scapularis nymphs, infected with B. burgdorferi ss strain N40. To determine tick attachment time and tick weights the B. burgdorferi ss strain N40-infected I. scapularis nymphs were placed in capsules on the vaccinated mice and allowed to feed to repletion. The nymphs were checked daily for attachment, collected and weighed when they had fallen off. Additional sera were collected at t=42 (pre-challenge) and at t=63 days mice were sacrificed and ear, skin, ankle, heart, bladder, and tissue was collected for analysis.
ELISA
To measure antigen-specific IgG, ELISAs were performed, as described previously (29). High-binding 96-well ELISA plates (Greiner Bio-one, Kremsmünster, Austria) were coated overnight at 4°C with 1 μg/ml recombinant protein (produced as described elsewhere (12, 18, 19, 21)), washed with PBS–Tween (phosphate-buffered saline–0.05% Tween) and incubated with blocking buffer (1% BSA in PBS, pH 6.5) for 2 h at room temperature. Mouse sera (collected at day 42 before tick challenge) were diluted in blocking buffer, added to the wells and incubated for 1 h at room temperature. Plates were washed and incubated for 1 h with horseradish peroxidase (HRP)-linked anti-mouse IgG (Cell Signaling, Beverly, MA, USA) diluted 1:1,000 in blocking buffer. The plates were washed again and developed using TMB substrate [50 µl TMB chromogene in 5 ml TMB substrate buffer (8,2 gr NaAc and 21 gr citric acid monohydrate dissolved in 1 liter H2O + 10 µl 3% H2O2, pH 5)] and optical density was measured in a Biotek (Winooski, VT, USA) ELISA plate reader at 450–655 nm.
Borrelia Detection and Quantification by Culture and qPCR
Cultures were carried out as described elsewhere (12). Murine bladder and skin samples were cultured in modified Kelly Pettenkofer (MKP) medium with rifampicin, 50 μg/ml and phosphomycin, 100 μg/ml) at 33°C. The cultures were checked weekly (for a total of 8 weeks) for the presence of motile spirochetes with dark field microscopy as described before (12). For all samples DNA was extracted using Qiagen Blood and Tissue kit (Qiagen, Venlo, The Netherlands). Quantitative (q)PCR was used to quantify B. burgdorferi ss DNA in mouse tissues and was performed according to previously described protocol (29). OspA primers were used for quantification; forward 5’-AAAAATATTTATTGGGAATAGGTCT-3’ and reverse 5’-CACCAGGCAAATCTACTGAA-3’, mouse Beta-actin forward 5’-AGCGGGAAATCGTGCGTG-3’ and reverse primer 5’-CAGGGTACATGGTGGTGCC-3’ were used for normalization. The qPCRs were performed on the LightCycler480 (Roche, Nutley, NJ, USA) using SYBR green dye (Roche) using the following PCR protocol: 95°C 6 min, and 60 cycles of 95°C 10 s, 60°C 20 s, and 72°C 20 s. Reactions were performed in triplicate. Results were analyzed using LinRegPCR software (Amsterdam, The Netherlands) (32). Negative and positive controls were included in each qPCR run.
Statistical Methods
Differences between experimental groups between B. burgdorferi ss loads in qPCR were statistically tested by two-sided nonparametric tests (Mann-Whitney, GraphPad Prism software version 5.0, San Diego, CA, USA). Differences between experimental groups in tick weight were statistically tested by one-way ANOVA.
Results
Osp C Vaccination
Vaccination with recombinant OspC as a positive control was compared to vaccination with OspC as a DNA vaccine to determine the efficacy of the DNA vaccine strategy ( Figure 1 ). Both the rOspC and OspC DNA vaccine were able to induce robust IgG responses, although the titer of rOspC was significantly higher ( Figure 1A ). As expected from vaccination with a Borrelia protein, the OspC vaccinated groups did not show decreased tick weight or duration of attachment ( Figures 1C, D ).
Figure 1.
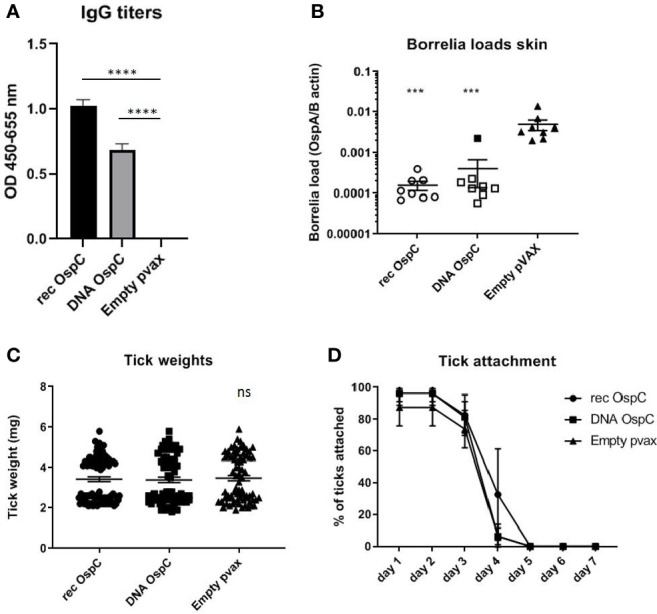
(A–D) rOspC vaccination versus vaccination with OspC DNA vaccine in a 0-14-28 day immunization protocol. (A) Specific total IgG titers were measured in an Enzyme-linked immunosorbent assay (ELISA). Plates were coated with recombinant protein and incubated with mouse sera collected at timepoint 42 days pre-challenge. Sera was diluted in steps of 3 starting with 100 times and ending with 218,700 times dilution Presented are the total IgG titers incubated with mouse sera 1:8,100 diluted for OspC. Statistical significance was calculated for each experimental group compared to the control, Empty pvax, using a one-way ANOVA statistical test (p< 0.05, **p<0.01, ***p<0.001, ****p<0.0001). (B) Borrelia loads in skin samples of the tick attachment site as determined by qPCR. Closed dots depict positive Borrelia loads, open dots depict PCR negative samples for which the OspA detection limit was divided by the sample’s mouse beta actin load. The Borrelia loads were compared to the negative control Empty pvax using a two-sided non parametric test (Mann-Whitney *P < 0.05, **P<0.01, ***P<0.001). (C) Tick weights in mg. Immunized mice were challenged with B. burgdorferi N40-infected I. scapularis nymphs. Nymphs were placed in a collection capsule on the back of each mice. The capsules were checked daily and the ticks were allowed to feed to repletion. Once they had fallen off their weight was measured. (D) Tick attachment is presented as percentage of ticks that are still attached per day. ns, not significant.
We also assessed whether the conventional and DNA OspC vaccines were able to provide protection against infection with B. burgdorferi ss strain N40 transmitted by I. scapularis nymphs. For this purpose we performed qPCR and culture of several tissues we obtained by sacrificing the mice 21 days after the challenge. B. burgdorferi ss DNA loads in the skin challenge site were negative in all of the rOspC vaccinated mice and in seven out of eight of OspC DNA vaccinated mice ( Figure 1B ). B. burgdorferi ss culture data of the skin challenge site and bladder, corresponded with the qPCR data; seven out of eight mice were Borrelia negative ( Table 1 ).
Table 1.
Culture positivity 8 weeks after challenge for OspC vaccination.
| Skin | Bladder | |
|---|---|---|
| Rec OspC | 0/8 | 0/8 |
| DNA OspC | 1/8 | 1/8 |
| Empty pvax | 8/8 | 8/8 |
Cultures of skin and bladder were checked weekly for growth of Borrelia.
Tick Salivary Gland DNA Vaccines
DNA vaccines against TSLPI, Salp15, tHRF, and Tix-5 did not induce robust IgG responses ( Figure 2A ). Although moderate antibody response against Salp 15 and Tix-5 compared to Empty pvax could be observed, TSLPI and tHRF did not elicit any noteworthy IgG response. When assessing tick attachment time and tick weight, the DNA vaccines against tick antigens also did not demonstrate a difference compared to the Empty pvax control ( Figures 2C, D ). As described above, OspC DNA vaccination protected all but one mouse against Borrelia infection. In stark contrast, B. burgdorferi ss DNA loads in TSLPI, Salp15, tHRF, and Tix-5 skin samples, were all positive ( Figure 2B ). Also, B. burgdorferi ss cultures from mice tattooed with DNA vaccines against tick proteins TSLPI, Salp15, tHRF, and TIX-5 were all positive and almost all within 2 weeks time ( Table 2 ).
Figure 2.
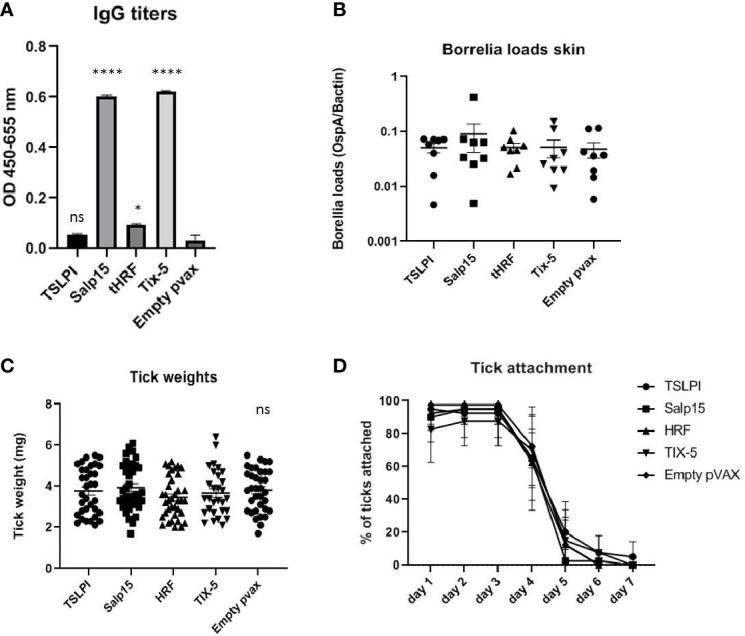
(A–D) DNA vaccination with tick salivary gland genes TSLPI, tHRF, Salp15, and Tix-5 in a 0-14-28 day immunization protocol. (A) Specific total IgG titers were measured in an Enzyme-linked immunosorbent assay (ELISA). Plates were coated with recombinant protein and incubated with mouse sera collected at timepoint 42 days pre-challenge. Sera was diluted in steps of 3 starting with 100 times and ending with 218,700 times dilution. Presented are the total IgG titers incubated with mouse sera 1:300 diluted for the tick salivary gland gene DNA vaccines. Statistical significance was calculated for each experimental group compared to the control, Empty pvax, using a one-way ANOVA statistical test (p< 0.05, **p<0.01, ***p<0.001, ****p<0.0001). (B) Borrelia loads in skin samples of the tick attachment site as determined by qPCR. Closed dots depict positive Borrelia loads, open dots depict PCR negative samples for which the OspA detection limit was divided by the sample’s mouse beta actin load. The Borrelia loads were compared to the negative control Empty pvax using a two-sided non parametric test (Mann-Whitney *P < 0.05, **P<0.01, ***P<0.001). (C) Tick weights in mg. Immunized mice were challenged with B. burgdorferi N40-infected I. scapularis nymphs. Nymphs were placed in a collection capsule on the back of each mice. The capsules were checked daily and the ticks were allowed to feed to repletion. Once they had fallen off their weight was measured. (D) Tick attachment is presented as percentage of ticks that are still attached per day. ns, not significant.
Table 2.
Culture positivity 8 weeks after challenge for tick salivary gland DNA vaccination.
| Skin | Bladder | |
|---|---|---|
| TSLPI | 8/8 | 8/8 |
| Salp15 | 8/8 | 8/8 |
| tHRF | 8/8 | 8/8 |
| Tix-5 | 8/8 | 8/8 |
| Empty pvax | 8/8 | 8/8 |
Cultures of skin and bladder were checked weekly for growth of Borrelia.
Discussion
In this study, we assessed the utility of DNA vaccination against Lyme borreliosis using a known Borrelia antigen - OspC - that has been shown to elicit protection when used as a recombinant protein vaccine. Both rOspC and OspC DNA vaccination resulted in robust antibody production in mice and subsequently protected against B. burgdorferi ss transmission by tick challenge. DNA vaccination therefore embodies a promising vaccination strategy, as it allows for rapid vaccination schedules, they are easy to produce, and besides humoral immunity, are also capable of inducing cellular immunity. Especially humoral immunity is described to be very important for clearance of Borrelia (33, 34). Interestingly, as the OspC DNA vaccine was able to induce moderate to high antibody titers and provided near total protection against B. burgdorferi ss infection by tick challenge. Therefore, immunization with plasmid DNA for this particular Borrelia antigen is, as described previously, an alternative platform for vaccination, as opposed to recombinant protein vaccination (35). It also shows that DNA vaccination against B. burgdorferi ss antigens in itself is effective in protecting against infection in a tick challenge model. Given the heterogeneity of OspC protein sequences among different B. burgdorferi sl species and strains, DNA vaccination could be an interesting platform, as multiple OspC sequence for a multivalent vaccine can be relatively easily combined (36). Future studies should focus on the effectiveness of a DNA vaccine targeting various OspC variants on protection against a range of B. burgdorferi sl isolates.
Secondly, we examined several anti-tick vaccine candidates as DNA vaccines. The ability of Borrelia spirochetes to establish an infection in mammals is partly dependent on tick (salivary gland) proteins, which makes these proteins interesting candidates for an anti-tick vaccine. Our goal was to determine the protective abilities of four tick salivary gland proteins used as DNA vaccines. In contrast to their recombinant protein vaccines described in literature, the tick salivary gland antigen DNA vaccines induced low (Salp15 and Tix-5) or hardly any (TSLPI and tHRF) IgG titers and did not provide protection against B. burgdorferi ss infection by tick challenge. It could therefore be speculated that the low or absent IgG titers are responsible for the fact that there was no protection observed and an adequate humoral immune response is essential to neutralize the function of these tick proteins and preventing transmission of B. burgdorferi ss. The low expression of these antigens by murine cells could be one reason for the low immunogenicity. Perhaps this could be circumvented by stronger adjuvants. Indeed, in addition to our strategy - i.e., addition of a hTPA signal and Kozak sequence and codon-optimization - adjuvant modifications can be made to DNA vaccines, such as genetic adjuvant strategies to improve the immune response induced by DNA vaccines (37). Regardless, both Salp15 and TIX5 were able to elicit a moderate IgG response, yet no protection against B. burgdorferi infection was observed. It should be mentioned that, although it has been established that an anti-tick vaccine based on TIX-5 impairs tick-feeding the effect of such a vaccine on Borrelia transmission has never been investigated (21). In contrast, for Salp15, for which an adjuvanted recombinant protein vaccine was able to interfere with B. burgdorferi transmission, the observed low IgG titers induced by our DNA vaccine are likely to cause for the lack of protection.
In this study, we have assessed DNA vaccination as a tool for two different vaccination approaches to protect against B. burgdorferi ss infection: targeting the spirochete with OspC or targeting the tick vector using Salp15, Tix-5, TSLPI, or tHRF. Although IgG levels are important for both approaches, the mechanism that leads to protection as a result of these antibodies differ greatly. Antibodies bound to OspC not only neutralize the ability of OspC to interact with Salp15 and shield against complement-mediated killing, they facilitate complement-mediated killing and phagocytosis of the spirochete. Antibodies against tick salivary gland proteins that are not directly bound to Borrelia can only neutralize the function of these proteins. As such high IgG levels against tick salivary gland proteins might possibly even be more important in protection compared to Borrelia antigens. Regardless, and as discussed above, it is clear that IgG levels induced by DNA vaccination against these tick proteins are insufficient (14).
In conclusion, we have shown that a successful vaccine against Borrelia is not restricted to conventional recombinant vaccination strategies and also works against tick-mediated transmission. This implies that DNA tattoo vaccination can be used to as a rapid and relatively easily screening tool to assess immunogenicity and efficacy of future novel B. burgdorferi sl vaccine candidates. In contrast, DNA vaccination appears not to be a suitable method to induce adequate immune responses against tick antigens and subsequent protection against B. burgdorferi ss; at least not for the selected tick salivary gland antigens Salp15, tHRF, TSLPI, and TIX-5.
Data Availability Statement
The raw data supporting the conclusions of this article could be made available upon reasonable request.
Ethics Statement
The animal study was reviewed and approved by Animal Research Ethics Committee of the Academic Medical Center, Amsterdam, The Netherlands.
Author Contributions
MK, JT, and AW designed the study. JE, JT, and MK performed the study procedures. AB and AW provided information about the DNA vaccine design. JH supervised the study progress. All authors contributed to the article and approved the submitted version.
Funding
This project has received funding from the European Union’s Seventh Programme for research, technological development, and demonstration under grant agreement No. 602272 of which JH was the scientific coordinator.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The following reagent was provided by Centers for Disease Control and Prevention for distribution by BEI Resources, NIAID, NIH: Ixodes scapularis (Live), NR-44116.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.615011/full#supplementary-material
Specific total IgG titers were measured in an Enzyme-linked immunosorbent assay (ELISA). Plates were coated with recombinant protein OspC 1µg/ml and incubated with specific mouse sera collected at timepoint 42 days pre-challenge. Sera were diluted to 218700 times.
Specific total IgG titers were measured in an Enzyme-linked immunosorbent assay (ELISA). Plates were coated with recombinant protein (TSLPI, Salp15, tHRF, Tix-5 1µg/ml) and incubated with mouse sera collected at timepoint 42 days pre-challenge. Sera were diluted to 218700 times.
References
- 1. Gomes-Solecki M, Arnaboldi PM, Backenson PB, Benach JL, Cooper CL, Dattwyler RJ, et al. Protective Immunity and New Vaccines for Lyme Disease. Clin Infect Dis (2020) 70:1768–73. 10.1093/cid/ciz872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wressnigg N, Barrett PN, Pollabauer EM, O’Rourke M, Portsmouth D, Schwendinger MG, et al. A Novel multivalent OspA vaccine against Lyme borreliosis is safe and immunogenic in an adult population previously infected with Borrelia burgdorferi sensu lato. Clin Vaccine Immunol (2014) 21:1490–9. 10.1128/cvi.00406-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nayak A, Schüler W, Seidel S, Gomez I, Meinke A, Comstedt P, et al. Broadly Protective Multivalent OspA Vaccine against Lyme Borreliosis, Developed Based on Surface Shaping of the C-Terminal Fragment. Infect Immun (2020) 88(4):e00917–19. 10.1128/iai.00917-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao H, Bao FF, Liu A. Safety, immunogenicity, and efficacy of Borrelia burgdorferi outer surface protein A (OspA) vaccine: A meta-analysis. J Infect Dev Ctries (2017) 11:1–9. 10.3855/jidc.7999 [DOI] [PubMed] [Google Scholar]
- 5. Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med (1998) 339:209–15. 10.1056/nejm199807233390401 [DOI] [PubMed] [Google Scholar]
- 6. Sigal LH, Zahradnik JM, Lavin P, Patella SJ, Bryant G, Haselby R, et al. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium. N Engl J Med (1998) 339:216–22. 10.1056/nejm199807233390402 [DOI] [PubMed] [Google Scholar]
- 7. Wilske B, Busch U, Fingerle V, Jauris-Heipke S, Preac Mursic V, Rössler D, et al. Immunological and molecular variability of OspA and OspC. Implications for Borrelia vaccine development. Infection (1996) 24:208–12. 10.1007/bf01713341 [DOI] [PubMed] [Google Scholar]
- 8. Earnhart CG, Buckles EL, Dumler JS, Marconi RT. Demonstration of OspC type diversity in invasive human lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect Immun (2005) 73:7869–77. 10.1128/iai.73.12.7869-7877.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci (Landmark Ed) (2009) 14:2051–88. 10.2741/3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hovius JW, Levi M, Fikrig E. Salivating for knowledge: potential pharmacological agents in tick saliva. PLoS Med (2008) 5:e43. 10.1371/journal.pmed.0050043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hovius JW, Ramamoorthi N, Van’t Veer C, de Groot KA, Nijhof AM, Jongejan F, et al. Identification of Salp15 homologues in Ixodes ricinus ticks. Vector Borne Zoonotic Dis (Larchmont NY) (2007) 7:296–303. 10.1089/vbz.2006.0624 [DOI] [PubMed] [Google Scholar]
- 12. Hovius JW, Schuijt TJ, de Groot KA, Roelofs JJ, Oei GA, Marquart JA, et al. Preferential protection of Borrelia burgdorferi sensu stricto by a Salp15 homologue in Ixodes ricinus saliva. J Infect Dis (2008) 198:1189–97. 10.1086/591917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature (2005) 436:573–7. 10.1038/nature03812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai J, Wang P, Adusumilli S, Booth CJ, Narasimhan S, Anguita J, et al. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe (2009) 6:482–92. 10.1016/j.chom.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hovius JW, de Jong MA, den Dunnen J, Litjens M, Fikrig E, van der Poll T, et al. Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog (2008) 4:e31. 10.1371/journal.ppat.0040031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, et al. Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity (2002) 16:849–59. 10.1016/s1074-7613(02)00325-4 [DOI] [PubMed] [Google Scholar]
- 17. Hovius JW, van Dam AP, Fikrig E. Tick-host-pathogen interactions in Lyme borreliosis. Trends Parasitol (2007) 23:434–8. 10.1016/j.pt.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 18. Dai J, Narasimhan S, Zhang L, Liu L, Wang P. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the lyme disease agent. PLoS Pathog (2010) 6:e1001205. 10.1371/journal.ppat.1001205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, et al. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe (2011) 10:136–46. 10.1016/j.chom.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schuijt TJ, Narasimhan S, Daffre S, DePonte K, Hovius JW, Van’t Veer C, et al. Identification and characterization of Ixodes scapularis antigens that elicit tick immunity using yeast surface display. PLoS One (2011) 6:e15926. 10.1371/journal.pone.0015926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schuijt TJ, Bakhtiari K, Daffre S, Deponte K, Wielders SJ, Marquart JA, et al. Factor Xa activation of factor V is of paramount importance in initiating the coagulation system: lessons from a tick salivary protein. Circulation (2013) 128:254–66. 10.1161/circulationaha.113.003191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trentelman JJA, Sima R, Krezdorn N, Tomás-Cortázar J, Barriales D, Takumi K, et al. A combined transcriptomic approach to identify candidates for an anti-tick vaccine blocking B. afzelii transmission. Sci Rep (2020) 10:20061. 10.1038/s41598-020-76268-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Labuda M, Trimnell AR, Licková M, Kazimírová M, Davies GM, Lissina O, et al. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog (2006) 2:e27. 10.1371/journal.ppat.0020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bins AD, Jorritsma A, Wolkers MC, Hung CF, Wu TC, Schumacher TN, et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med (2005) 11:899–904. 10.1038/nm1264 [DOI] [PubMed] [Google Scholar]
- 25. Srivastava IK, Liu MA. Gene vaccines. Ann Intern Med (2003) 138:550–9. 10.7326/0003-4819-138-7-200304010-00011 [DOI] [PubMed] [Google Scholar]
- 26. Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science (New York NY) (2020) 369:806–11. 10.1126/science.abc6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med (2020) 383(20):1920–31. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature (2020) 586(7830):589–93. 10.1038/s41586-020-2639-4 [DOI] [PubMed] [Google Scholar]
- 29. Wagemakers A, Mason LM, Oei A, de Wever B, van der Poll T, Bins AD, et al. Rapid outer-surface protein C DNA tattoo vaccination protects against Borrelia afzelii infection. Gene Ther (2014) 21:1051–7. 10.1038/gt.2014.87 [DOI] [PubMed] [Google Scholar]
- 30. Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods (2011) 8:785–6. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 31. Grote A, Hiller K, Scheer M, Münch R, Nörtemann B, Hempel DC, et al. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res (2005) 33:W526–531. 10.1093/nar/gki376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res (2009) 37:e45. 10.1093/nar/gkp045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhong W, Stehle T, Museteanu C, Siebers A, Gern L, Kramer M, et al. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc Natl Acad Sci U S A (1997) 94:12533–8. 10.1073/pnas.94.23.12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhong W, Gern L, Stehle T, Museteanu C, Kramer M, Wallich R, et al. Resolution of experimental and tick-borne Borrelia burgdorferi infection in mice by passive, but not active immunization using recombinant OspC. Eur J Immunol (1999) 29:946–57. [DOI] [PubMed] [Google Scholar]
- 35. Scheiblhofer S, Weiss R, Dürnberger H, Mostböck S, Breitenbach M, Livey I, et al. A DNA vaccine encoding the outer surface protein C from Borrelia burgdorferi is able to induce protective immune responses. Microbes Infect (2003) 5:939–46. 10.1016/s1286-4579(03)00182-5 [DOI] [PubMed] [Google Scholar]
- 36. Dhama K, Mahendran M, Gupta PK, Rai A. DNA vaccines and their applications in veterinary practice: current perspectives. Vet Res Commun (2008) 32:341–56. 10.1007/s11259-008-9040-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scheerlinck JY. Genetic adjuvants for DNA vaccines. Vaccine (2001) 19:2647–56. 10.1016/s0264-410x(00)00495-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specific total IgG titers were measured in an Enzyme-linked immunosorbent assay (ELISA). Plates were coated with recombinant protein OspC 1µg/ml and incubated with specific mouse sera collected at timepoint 42 days pre-challenge. Sera were diluted to 218700 times.
Specific total IgG titers were measured in an Enzyme-linked immunosorbent assay (ELISA). Plates were coated with recombinant protein (TSLPI, Salp15, tHRF, Tix-5 1µg/ml) and incubated with mouse sera collected at timepoint 42 days pre-challenge. Sera were diluted to 218700 times.
Data Availability Statement
The raw data supporting the conclusions of this article could be made available upon reasonable request.


